Abstract
Background
The optimal treatment strategy for complex aortic arch and proximal descending aortic pathologies remains controversial. Despite the frozen elephant trunk (FET) technique's increasing popularity, its use over the conventional elephant trunk (CET) remains a matter of physician preference and outcomes are varied.
Methods
This meta‐analysis of available comparative studies of FET versus CET sought to examine differences in survival, reintervention, and adverse events. The following databases were searched from inception—May 2020: Ovid MEDLINE, Ovid EMBASE, and The Cochrane Library. Studies retrieved were then screened for eligibility against predefined inclusion/exclusion criteria with a protocol registered on Open Science Framework at https://osf.io/hrfze/.
Results
The search identified 1911 citations, with five studies included. The resultant meta‐analysis included 313 CET and 292 FET cases. FET had lower perioperative mortality (risk ratio [RR]: 0.50, 95% confidence interval [CI]: [0.42; 0.60], p < .001) and improved 1‐year survival compared to CET (hazard ratio: 0.63, 95% CI: [0.42; 0.95], p = .03). There were no significant differences in rates of overall or open reinterventions following FET versus CET, but FET did yield a significantly higher rate of endovascular reintervention (RR: 2.32, 95% CI: [1.17; 4.61], p = .03). No significant differences were observed in the incidences of postoperative stroke, spinal cord injury, or renal failure between groups.
Conclusions
The FET technique yields superior rates of perioperative and medium‐term survival with no significant increase in overall reinterventions. There was no significant difference in the rate of spinal cord injury between groups, providing further large‐scale evidence that the FET is an acceptable, safe alternative to the CET.
Keywords: aortic arch, elephant trunk, frozen elephant trunk, reintervention
1. INTRODUCTION
Aortic pathologies involving the aortic arch and descending thoracic aorta pose unique treatment challenges and have historically required advanced, technically demanding open surgical repair. In 1983, Borst and colleagues described what is now known as the conventional elephant trunk (CET) technique, a two‐intervention approach to aortic repair defined by the placement of a free‐floating extension of an aortic arch prosthesis into the proximal descending aorta at the conclusion of the first arch surgery via open sternotomy. This allows for extension of the prosthetic trunk to its final desired level in a second, either open or endovascular, approach that addresses concomitant descending thoracic aortic disease. 1 , 2 Advantages of the CET technique include reduced dissection and surgical preparation of the distal arch segment, thereby decreasing the risk of neighboring structure injury, a shortened clamping time during thoracoabdominal aortic repair, and the lack of a need to clamp proximally to the left subclavian artery. 2 However, extensive open repair of the aorta is challenging and carries a high‐risk burden due to the necessity for cardiopulmonary bypass and hypothermic circulatory arrest (with or without cerebral perfusion). 3 , 4 , 5
In an effort to reduce the morbidity and mortality associated with these two separate open procedures, Kato and colleagues reported their early experience with a one stage, hybrid‐repair in which a self‐expanding stent was deployed in the descending aorta and saw successful thrombosis of aneurysms or false lumens in all 10 cases, seven of which were thoracic aneurysms and three of which were dissections of the distal arch. 6 In 2003, Kark et al. modified this technique with the introduction of a custom‐made, hybrid prosthesis, thereby introducing the “frozen elephant trunk” (FET) technique, a one‐stage, hybrid procedure in which an endovascular stent graft is placed antegrade, attached proximally to the arch graft, and securely anchored to its final desired level in the descending aorta during open total arch repair. 7 This technique is becoming increasingly common, in no small part due to encouraging results. 8 , 9 , 10 Yet, the FET is not without its potential complications, notably a non‐negligible incidence of spinal cord injury most often attributed to ischemia or occlusion of the thoracic intercostal arteries. 11 , 12 , 13 While previous work has compared outcomes of the FET to a heterogeneous group of conventional techniques for aortic arch surgery, none has directly compared the use of FET to the CET. 14
Here, we systematically review studies involving repair of the extensive aortic pathology using either the CET or FET, highlighting operative strategies, postoperative outcomes, and reintervention rates. Using these studies, we provide recommendations and potential cautions for cardiac surgeons considering utilizing the FET in aortic repair.
2. METHODS
2.1. Human subjects research
As a systematic review and meta‐analysis, Institutional Review Board (IRB) approval, consent of human subjects, or clinical trial registration were not applicable for this study.
2.2. Literature search strategy
This study was performed following the preferred reporting items for systematic reviews and meta‐analyses (PRISMA) statement, with the completed PRISMA checklist available in Supporting Information. 15 A protocol was registered on Open Science Framework and made publicly available at: https://osf.io/hrfze/. A medical librarian performed comprehensive searches to identify studies that evaluated the relative efficacy and safety of the frozen/antegrade stent grafts with respect to the conventional surgical elephant trunk graft in the treatment of acute or chronic aortic dissection.
Searches were run on May 20, 2020 in the following databases: Ovid MEDLINE (ALL—1946–present); Ovid EMBASE (1974–present); and The Cochrane Library (Wiley). Searches included all appropriate subject headings and keywords for the concepts of “aortic dissection,” “aortic aneurysm,” “elephant trunk graft,” and “frozen/antegrade stent.” The full search strategy for Ovid MEDLINE is available in Supporting Information. To limit publication bias, there were no publication date, or article type restrictions on the search strategy. For articles selected for inclusion in this study, reference lists and citing articles were pulled from Scopus (Elsevier) and also screened.
2.3. Eligibility criteria
Titles and abstracts were reviewed against predefined inclusion/exclusion criteria. Articles considered for inclusion were: (1) English language articles; (2) patient cohorts larger than 10; (3) studies reporting either reintervention rates or at least 1‐year survival data. Excluded studies were: (1) studies lacking a comparison or control group; (2) non‐English; (3) patient cohorts smaller than 10; (4) review articles, meeting abstracts, editorials, animal studies, or commentaries.
2.4. Data extraction and critical appraisal
After duplicate studies were removed, reviewers screened citations using Covidence systematic review software. Each study was screened by two independent reviewers; discrepancies were resolved by consensus or consultation with a third reviewer. Titles and abstracts were reviewed against predefined inclusion/exclusion criteria as above.
After article selection, two investigators performed data extraction independently, and the extracted data were verified by a third investigator for accuracy. The following variables were included: study demographics (sample size, publication year, and design), patient demographics (age, sex, comorbidities [hypertension, diabetes, smoking, and chronic renal failure]), and procedural and postoperative factors (30‐day and 1‐year mortality, rate and method of reintervention, prevalence of post‐op complications [ischemic post‐op stroke, defined as a cerebrovascular accident resulting in a permanent neurologic deficit, spinal cord injury, defined as permanent paraparesis or paraplegia, and renal failure, defined as permanent indication for dialysis at discharge], antegrade cerebral perfusion time [in minutes], cardiopulmonary bypass time [in min], cross‐clamp time [in min], and duration of circulatory arrest [in min]). Study quality was assessed with Downs and Black Checklist for Quality Assessment, with the full checklist for each study available in Supporting Information.
2.5. Statistical analysis
P values for baseline patient characteristics were generated via unpaired t testing or χ 2 testing of weighted averages and standard deviations as appropriate (GraphPad). Log hazard ratios comparing 1‐year survival outcomes between FET and CET patients were estimated from the reported number at risk and the percent survival according to the estimation formulas outlined in Tierney et al. (2007). 16 Random‐effects meta‐analysis models with a continuity correction of 0.1 were computed for each outcome. Pooled results of 1‐year survival were reported as a hazard ratio and all other complication outcomes were reported as risk ratios. The inverse‐variance method was implemented to pool hazard ratios, while the Mantel–Haenszel method was used to pool risk ratios. Between‐study heterogeneity was assessed using Cochran's Q, I 2, and Tau‐squared measures. Funnel plots were used to examine publication bias and funnel plot asymmetry was quantified using Egger's test. Sensitivity analysis was conducted when influential outliers were detected. A significance level of α = .05 was used and all statistical analyses were performed with R statistical software, version 4.0.3 (R Foundation for Statistical Computing).
3. RESULTS
Database searches and included studies' reference lists and citing articles retrieved 2934 results. After duplicate studies were removed, reviewers screened a total of 1911 citations using Covidence systematic review software. Full text was then pulled for 469 selected studies for a second round of eligibility screening. A total of six studies were selected for inclusion in meta‐analysis. However, due to heterogeneity demonstrated by Egger's regression testing for funnel plot asymmetry, Hirano et al. were ultimately excluded from all analyses, as the data reported in their study consistently fell outside the limits of the funnel plots for each primary and secondary endpoint. A version of the results inclusive of this study has been included as a sensitivity analysis in Supporting Information. Due to the relative weight of the data from Shrestha et al., a sensitivity analysis exclusive of this study was performed and also included in Supporting Information. No additional studies were included from review of reference lists. Thus, five studies comprising 605 patients were ultimately included in meta‐analysis (Table 1). A total of 292 patients (48.3%) underwent CET procedures, while 313 patients (51.7%) had undergone FET procedures. Pooled patient demographic data is presented in Table 2. The full PRISMA flow diagram outlining the study selection process is available in Figure 1.
Table 1.
Summary of studies
| Author (year) and study type | Study period | Patients | Aortic disease (%) | FET stent‐graft length | CSF drain? | Treatment outcomes | Adverse effects | Limitations/Downs and Black Quality Assessment score |
|---|---|---|---|---|---|---|---|---|
| Leontyev (2013) | 1/2003‐12/2011 | CET: 125 | Aneurysm: | Stent‐graft sized according to the dimension of the native nondiseased aorta or true aortic lumen in patients with acute or chronic dissection, with 5%–10% oversizing | Yes for all FET cases (could not confirm first 20 FET cases, confirmed use in all others) | In‐hospital mortality was 21.6% versus 8.7% for CET and FET patients, respectively (p = .1) | Stroke occurred in 16% versus 13% of CET versus FET patients (p = .4) | Limitations: Retrospective, nonrandomized, single center study; patients with heterogenous aortic pathology |
| CET: 42 (33.6) | ||||||||
| Spinal cord injury was significantly higher in the FET group (21.7% vs. 4.0%, p < .001) | ||||||||
| D&B score: 20 | ||||||||
| FET: 27 (58.7) | Estimated 1‐,3‐, and 5‐year survival were 70 ± 4%, 70 ± 4%, and 68 ± 4% (CET) and 74 ± 7%, 60 ± 9%, and 40 ± 1% (FET) | |||||||
| Renal failure occurred in 184% versus 23.9% of CET versus FET pts (p = .4) | ||||||||
| Acute type A: | ||||||||
| CET: 67 (53.6) | ||||||||
| FET:8 (17.4) | ||||||||
| Acute type B: | ||||||||
| FET: 46 | CET: 6 (4.8) | |||||||
| FET: 7 (16.2) | ||||||||
| Chronic type A: | ||||||||
| CET:3 (2.4) | ||||||||
| FET: 1 (2.2) | ||||||||
| Chronic type B: | ||||||||
| CET: 2 (1.6) | ||||||||
| RCS | FET: 1 (2.2) | |||||||
| DiEusanio (2014) | 2003‐2011 | CET: 36 | Reoperation: | Not reported | No | No significant difference was found for in‐hospital mortality (13.9% vs. 4.8% for CET and FET patients, respectively (p = .2)) | No significant difference was found for permanent neurologic dysfunction (elephant trunk: 5.7% vs. 9.5% for CET and FET (p = .4)) or paraplegia (2.9% vs. 4.8% for CET and FET, respectively (p = .6)) | Limitations: Retrospective, nonrandomized, small sample size; patients with heterogenous aortic pathology |
| CET: 4 (11.1) | ||||||||
| FET: 6 (28.6) | ||||||||
| D&B score: 19 | ||||||||
| Urgent/emergent: | ||||||||
| Endovascular second‐stage procedures were successfully performed in all FET patients with residual aneurysmal disease (n = 3); 9/11 CET patients with second‐stage procedures required conventional surgical replacement through lateral thoracotomy | ||||||||
| CET: 11 (30.6) | ||||||||
| FET: 3 (14.3) | ||||||||
| FET: 21 | ||||||||
| RCS | ||||||||
| Kaplan–Meier estimate of 4‐year survival was 75.8 ± 7.6 and 72.8 ± 10.6 in CET and FET patients, respectively (log‐rank p = .8) | ||||||||
| Shrestha (2015) | 8/2001‐3/2013 | CET: 97 | Aneurysm: | Stent‐graft length was chosen depending on the distance of the landing zone from the left subclavian artery | Yes for all FET cases; performed in CET cases “according to anatomic needs” | In‐hospital mortality was 24.7% versus 12.2% for CET and FET, respectively | Postoperative stroke rate was 12.4% versus 13.3% for CET and FET, respectively | Limitations: Retrospective, nonrandomized, single center study; heterogenous aortic pathology |
| CET: 43 (44) | ||||||||
| FET: 62 (34) | ||||||||
| D&B score: 19 | ||||||||
| During follow‐up, 27.8% of CET cases underwent a second‐stage procedure vs 27.7% in FET group | ||||||||
| Acute dissection: | ||||||||
| CET: 47 (48) | ||||||||
| FET: 67 (37) | ||||||||
| Chronic dissection: CET: 7 (7) | ||||||||
| FET: 180 | ||||||||
| FET: 51 (28) | ||||||||
| Reoperation: | ||||||||
| CET: 20 (21) | ||||||||
| FET: 54 (30) | ||||||||
| RCS | ||||||||
| Mkalaluh (2018) | 2001‐2017 | CET: 25 | Aneurysm: | Not reported | Not reported | In‐hospital mortality was statistically similar: 32% versus 20% for CET and FET, respectively (p = .52) | No significant difference between the incidence of stroke, acute renal failure or postoperative bleeding between CET and FET | Limitations: Retrospective, nonrandomized, single‐center study; small sample size |
| CET: 16 (64) | ||||||||
| FET: 12 (48) | D&B score: 20 | |||||||
| Dissection: | One‐year survival rates were higher with FET vs CET (60% vs. 38%), but not statistically significant | |||||||
| CET: 13 (52) | ||||||||
| FET: 15 (60) | ||||||||
| FET: 25 | ||||||||
| CCS | ||||||||
| Furitachi (2019) | 1/2010‐8/2018 | CET: 30 | Acute type A dissection: | Length selected by measuring the distance along an aortic centerline from the left carotid artery and the left subclavian artery to the descending aorta at the level of T4–6 | Not reported | No significant different was found for perioperative (30‐day) mortality between FET and CET patients (10% vs. 5%, respectively (p = .64)) | 6.7% of patients in the CET group experienced recurrent nerve palsy, and 6.7% of pts experienced paraplegia, whereas no patients in the FET group experienced either recurrent nerve palsy or paraplegia | Limitations: Retrospective, nonrandomized, single‐center study; small sample size |
| CET: 30 (100) | ||||||||
| D&B score: 18 | ||||||||
| FET: 20 (100) | ||||||||
| FET: 20 | ||||||||
| RCS | ||||||||
| 6.7% of pts in the CET group experienced a cerebrovascular event, while no patients in the FET group experienced an event | ||||||||
| Graft diameter was selected to be 90% of the outer aortic diameter at the level of the distal landing zone | ||||||||
| Stent‐graft induced new entry occurred in 15.8% of FET cases, with no cases in the CET group |
Abbreviations: CCS, case‐control matching study; CET, conventional elephant trunk; CSF, cerebrospinal fluid; D&B, Downs and Black; FET, frozen elephant trunk; RCS, retrospective cohort study.
Table 2.
Summative demographic and operative data
| Cohort size | Mean age (years) | Female N (%) | Mean ACP time (min) | Mean CA time (min) | Mean bypass time (min) | Mean cross‐clamp time (min) | Minimum temperature (°C) | |
|---|---|---|---|---|---|---|---|---|
| CET | 313 | 61.8 ± 1.85 | 123 (39.3) | 51.08 ± 15.9 | 47.6 ± 7.60 | 229.1 ± 13.8 | 126.8 ± 22.1 | 23.6 ± 1.4 |
| FET | 292 | 64.3 ± 2.93 | 96 (32.8) | 69.2 ± 12.2 | 53.3 ± 4.38 | 226.1 ± 7.06 | 114.9 ± 28.7 | 25.0 ± 0.02 |
| p‐value | ‐ | <.0001 | .119 | <.0001 | <.0001 | .0006 | <.0001 | p = .06 |
| X² | ‐ | ‐ | 2.43, 1 df | ‐ | ‐ | ‐ | ‐ | ‐ |
Note: Summary of pooled demographic and operative data for the included studies.
Abbreviations: ACP, antegrade cerebral perfusion; CA, circulatory arrest; CET, conventional elephant trunk; df, degrees of freedom; FET, frozen elephant trunk.
Figure 1.
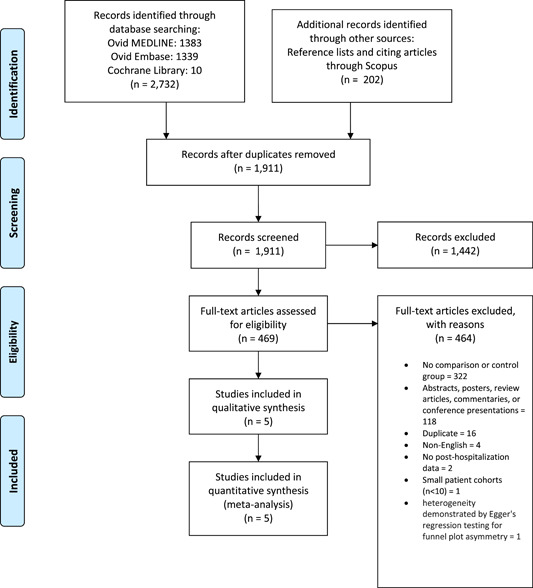
Preferred reporting items for systematic reviews and meta‐analyses (PRISMA) flow diagram. PRISMA flow diagram outlining the process of study identification and selection.
In 2013, Leontyev and colleagues published a single‐center study comparing the use of CET and FET in 171 patients at their institution between 2003 and 2011, reporting an in‐hospital mortality of 21.6% versus 8.7% for CET and FET patients, respectively (p = .1). 17 Stroke occurred in 16% versus 13% of CET versus FET patients (p = .4). Of note, the occurrence of new‐onset permanent neurologic deficit was significantly higher in the FET group (21.7 vs. 4.0%, p < .001). Importantly, the authors utilized cerebrospinal fluid (CSF) drains in nearly all FET cases; they were only unable to confirm use of a CSF drain in the first 20 FET patients included in the analysis. The estimated 1‐, 3‐, and 5‐year survival were 70 ± 4%, 70 ± 4%, and 68 ± 4% (CET), and 74 ± 7 and 60 ± 9, 40 ± 1% (FET), with a mean survival time of 5.2 ± 0.3 versus 3.8 ± 0.5 years (CET vs. FET, log‐rank p = .9). 17
The following year, DiEusanio et al. conducted a two‐site, prospective cohort study comparing the success of CET and FET in 57 patients. 18 The authors did not utilize CSF drainage in either the CET or FET group. The study found no significant difference in hospital mortality or permanent neurologic deficit between the two groups. In the CET group, 68.4% of patients did not undergo a second‐stage procedure during follow‐up. Endovascular second‐stage procedures were successfully performed in all FET cases. Four‐year survival was estimated to be 75.8 ± 7.6% and 72.8 ± 10.6% in CET and FET patients, respectively (log‐rank p = .8). 18
Shrestha and colleagues published a 2015 prospective cohort study evaluating the two techniques in 277 patients from 2001 through 2014. 19 For individuals undergoing a CET procedure, in‐hospital mortality was 24.7%, the postoperative stroke rate was 12.4%, and during follow‐up, 27.8% underwent a second‐stage procedure. In individuals undergoing a FET procedure, in‐hospital mortality was 12.2%, the postoperative stroke rate was 13.3% and during follow‐up, 27.7% patients underwent further interventions. 19 Of note, the authors report that CSF drainage with a target pressure <12 mmHg was employed in all patients undergoing FET, whereas drainage was employed in CET patients only “according to anatomic needs.” 19
In 2018, Mkalaluh and colleagues published a prospective, single‐center, case‐control study of 25 patient pairs who underwent either CET or FET procedures between 2001 and 2017. 20 In‐hospital mortality, as well as the incidence of stroke and acute renal failure were comparable between the groups, although the authors did not report use of a CSF drain. While the 1‐year survival rates were higher in the FET cohort compared to the CET approach (60% vs. 38%), these results were reported without statistical significance. 20
In 2019, Furitachi et al. published a prospective, single‐center study comparing outcomes of CET and FET procedures in 50 patients between 2010 and 2018. Interestingly, there was no case of permanent neurologic deficit in the FET group, whereas four patients in the CET group experienced permanent neurologic deficits. 21 Of note, the authors do not report use of a CSF drain.
3.1. Meta‐analysis
A summary of relevant findings has been summarized in Table 3. Primary endpoints included 1‐year survival, both open and endovascular reintervention rates, and overall reintervention rates. Patients who had undergone FET had higher rates of 1‐year survival with respect to those who had undergone CET (random‐effects hazard ratio [HR]: 0.63, 95% confidence interval [CI]: [0.42; 0.95], p = .03) (Figure 2), as well as lower rates of open reintervention (random‐effects risk ratio [RR]: 0.33, 95% CI: [0.05; 2.37], p = .17), although random‐effects modeling for open reintervention failed to produce significant results (Figure 3A). Of note, Mkalaluh et al. did not report open or endovascular reintervention results, and thus n = 4 for each of those endpoints. There was no statistically significant difference found between rates of overall reinterventions as well (random‐effects RR = 0.92, 95% CI = [0.55; 1.55], p = .66) (Figure 3B). Importantly, the FET resulted in a significantly higher rate of endovascular reintervention when compared to that of the CET (random‐effects RR: 2.32, 95% CI: [1.17; 4.61], p = .03) (Figure 3C).
Table 3.
Summary of findings
| Outcome | Random‐effects model RR/HR | 95% CI | z | p‐value |
|---|---|---|---|---|
| Perioperative mortality | 0.5 | (0.42; 0.60) | 11.07 | .0004 |
| 1‐year survival | 0.63 | (0.42; 0.95) | −2.2 | .0279 |
| Overall reintervention | 0.92 | (0.55; 1.55) | −0.49 | .6604 |
| Open reintervention | 0.33 | (0.05; 2.37) | −1.79 | .1706 |
| Endovascular reintervention | 2.32 | (1.17; 4.61) | 3.92 | .0296 |
| Stroke | 1.11 | (0.62; 2.00) | 0.49 | .6522 |
| Spinal cord injury | 0.94 | (0.25; 3.53) | −0.15 | .8938 |
| Renal failure | 1.19 | (0.95; 1.50) | 2.43 | .0935 |
Note: Results of meta‐analysis for all outcomes of interest. Pooled results of 1‐year survival were reported as an HR, pooled with the inverse‐variance method, and all other outcomes were reported as RRs, pooled with the Mantel–Haenszel method. FET is providing the RR/HR for all above data.
Abbreviations: CET, conventional elephant trunk; CI, confidence interval; FET, frozen elephant trunk; HR, hazard ratio; RRs, risk ratios.
Figure 2.
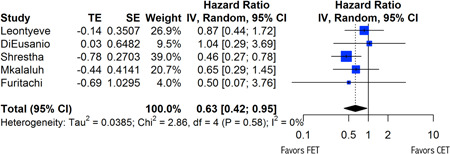
Forest plot for 1‐year survival. Forest plot demonstrating variable heterogeneity. CI, confidence interval; df, degrees of freedom; IV, inverse variance.
Figure 3.
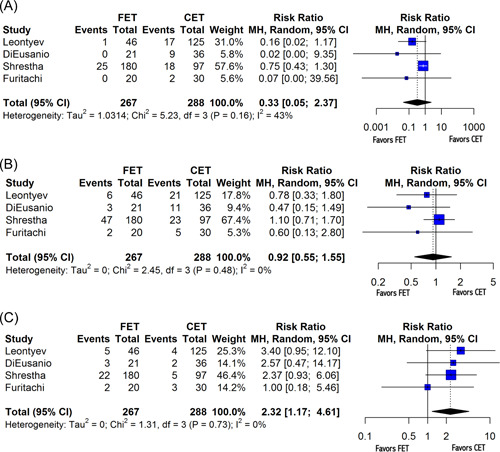
Forest plot for reintervention rates. (A) Forest plot demonstrating variable heterogeneity for open reintervention. (B) Forest plot for overall reintervention. (C) Forest plot for endovascular reintervention. CI, confidence interval; df, degrees of freedom; MH, Mantel–Haenszel test.
Secondary endpoints included perioperative (30‐day) mortality as well as postoperative incidence of stroke, spinal cord injury, and renal failure. FET procedures were associated with a lower perioperative mortality rate than that from CET procedures (random‐effects RR: 0.50, 95% CI: [0.42; 0.60], p < .001) (Figure 4). No significant difference was observed in the incidence of postoperative stroke (random‐effects RR: 1.11, 95% CI: [0.62; 2.00], p = .65) (Figure 5A), spinal cord injury (random‐effects RR: 1.37, 95% CI: [0.25; 7.43], p = .63) (Figure 5B), or renal failure (random‐effects RR: 1.19, 95% CI: [0.95; 1.50], p = .09) (Figure 5C) between cET and FET cases. Furitachi et al. did not present data on the incidence of postoperative renal failure, and thus n = 4 for that analysis.
Figure 4.
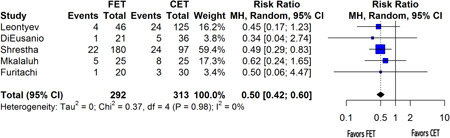
Forest plot for perioperative mortality. Forest plot demonstrating variable heterogeneity. CI, confidence interval; df, degrees of freedom; MH, Mantel–Haenszel test.
Figure 5.
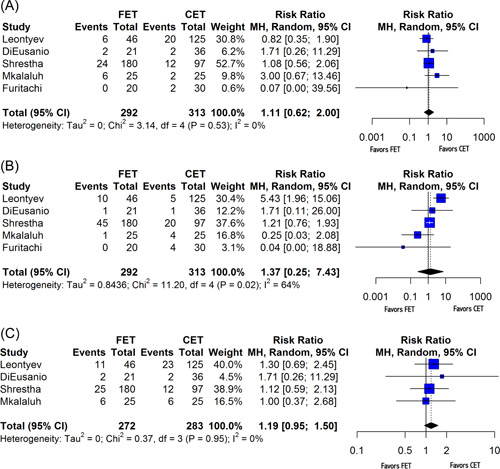
Forest plots for adverse events. (A) Forest plot demonstrating variable heterogeneity for stroke. (B) Forest plot for spinal cord injury. (C) Forest plot for renal failure. CI, confidence interval; df, degrees of freedom; MH, Mantel–Haenszel test.
4. DISCUSSION
This study is the first to systematically assess differences in both survival and reintervention rates between the CET and FET procedures. With regard to survival, our aggregated data suggest that FET procedures are superior to CET, as FET procedures demonstrated significantly increased rates of survival at both the 30‐day and 1‐year time mark. While an a priori power analysis was not conducted, the finding of a statistically significant difference between groups with regard to the primary outcome of 1‐year survival provided sufficient evidence that the study was adequately powered to detect differences for this outcome.
While no difference was ascertained with regard to open reintervention between the two techniques, the FET was associated with a higher rate of endovascular reintervention, which is consistent with previous literature. 2 Importantly, previous work has demonstrated that endovascular completion procedures carry less risk and shorter operative times than do secondary open repairs. 22 Considering that our study suggests FET facilitates the use of endovascular completion techniques over interval open procedures, likely because FET provides a more secure landing zone than CET, this may explain why overall survival is improved with the FET approach.
It is also possible that these improved survival data result not from an increase in endovascular reintervention in the FET, but rather a harmful decrease in completion procedure rates in the CET group. The CET was designed to facilitate a second intervention, whereas completion procedure rates for the FET have been generally accepted as ranging from 4% to 15%. 22 Previous work has acknowledged that one of the major risks of the CET procedure is the propensity for patients to be lost to follow‐up and never undergo their necessary completion procedure. This further supports the superiority of the FET over the CET, as the former frequently does not require a secondary procedure; however, in the aforementioned instances in which reintervention is necessary, there rarely exists a contraindication. 2 In our study, indications for FET reintervention included stent‐graft‐induced new entry, which occurred in patients with chronic dissection, enlargement of the distal aortic diameter in patients with chronic dissection due to sustained blood flow from the reentry into the false lumen, migration of the FET, pseudoaneurysm formation, and stent‐graft infection. 17 , 23
Secondary to death, perhaps the most feared complication of the FET procedure is that of spinal cord injury. Interestingly, our study reports that FET procedures do not significantly increase the risk of suffering a permanent neurologic deficit when compared to the CET. It is important to note that two of the five studies report selective use of a CSF drain in FET groups, which while serving as a potential confounder, also highlights a potential role for CSF drainage in all ET procedures as a means of decreasing neurological complication. Along with CSF drainage, hypothermia has also proven crucial to reducing postoperative neurologic deficits. In this meta‐analysis, while the average minimum temperature of the FET group was lower than that of the CET group, the difference failed to reach statistical significance (Table 2). Finally, there were also no significant differences in the postoperative rates of stroke or renal failure as well, thereby suggesting that the FET does not pose any additional or unique morbidity burden upon patients.
5. LIMITATIONS
Our study has several limitations. First, the number of included studies is notably small, and heterogeneity in the data required further study exclusion as described above. The retrospective nature of the study also precluded rigorous controls with regard to operative technique and graft selection, although of note each of the included studies maintained cerebral perfusion in an antegrade fashion. While frequently overlapping with one another, the long study period, often longer than a decade, may also introduce intra‐study variation with regard to technique as they each continued to evolve. Additionally, the lack of randomization may introduce treatment allocation bias, which is a strong limitation of this study. Another important limitation is the heterogeneity of indications for aortic arch procedures in these patients. This known confounder underlines the need for more rigorous clinical trials. In spite of this, this analysis supports the FET as a reasonable and safe alternative to CET for all indications.
6. CONCLUSIONS AND FUTURE DIRECTIONS
The present study is the first of its kind to systematically aggregate and assess the relative reintervention rates in individuals undergoing CET and FET procedures in a heterogeneous population of patients with complex aortic pathology. It suggests that the FET technique yields superior rates of both perioperative and 1‐year survival. Importantly, the study found no significant difference in the rate of spinal cord injury between individuals undergoing CET and FET. This supports FET as an acceptable, safe alternative to the CET while awaiting the higher level of data and recommendations a randomized, controlled trial comparing CET and FET could provide.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
Supporting information
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Vernice NA, Wingo ME, Walker PB, et al. The great vessel freeze‐out: a meta‐analysis of conventional versus frozen elephant trunks in aortic arch surgery. J Card Surg. 2022;37:2397‐2407. 10.1111/jocs.16596
REFERENCES
- 1. Borst HG, Walterbusch G, Schaps D. Extensive aortic replacement using “elephant trunk” prosthesis. Thorac Cardiovasc Surg. 1983;31(1):37‐40. [DOI] [PubMed] [Google Scholar]
- 2. Di Bartolomeo R, Murana G, Di Marco L, et al. Frozen versus conventional elephant trunk technique: application in clinical practice. European Journal of Cardiothoracic Surgery. 2017;51(suppl 1):i20‐i28. [DOI] [PubMed] [Google Scholar]
- 3. Kavanagh EP, Jordan F, Hynes N, et al. Hybrid repair versus conventional open repair for aortic arch dissection. Cochrane Database Syst Rev. 2018;2018(1):CD012920. 10.1002/14651858.CD012920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Murphy EH, Stanley GA, Ilves M, et al. Thoracic endovascular repair (TEVAR) in the management of aortic arch pathology. Ann Vasc Surg. 2012;26(1):55‐66. [DOI] [PubMed] [Google Scholar]
- 5. Rampoldi V, Trimarchi S, Eagle KA, et al. Simple risk models to predict surgical mortality in acute type A aortic dissection: the International Registry of Acute Aortic Dissection score. Ann Thorac Surg. 2007;83(1):55‐61. [DOI] [PubMed] [Google Scholar]
- 6. Kato M, Ohnishi K, Kaneko M, et al. New graft‐implanting method for thoracic aortic aneurysm or dissection with a stented graft. Circulation. 1996;94(suppl 9):II188‐II193. [PubMed] [Google Scholar]
- 7. Karck M, Chavan A, Hagl C, Friedrich H, Galanski M, Haverich A. The frozen elephant trunk technique: a new treatment for thoracic aortic aneurysms. J Thorac Cardiovasc Surg. 2003;125(6):1550‐1553. [DOI] [PubMed] [Google Scholar]
- 8. Weiss G, Tsagakis K, Jakob H, et al. The frozen elephant trunk technique for the treatment of complicated type B aortic dissection with involvement of the aortic arch: multicentre early experience. Eur J Cardiothorac Surg. 2015;47:106‐114. 10.1093/ejcts/ezu067 [DOI] [PubMed] [Google Scholar]
- 9. Di Eusanio M, Armaro A, Di Marco L, et al. Short‐ and midterm results after hybrid treatment of chronic aortic dissection with the frozen elephant trunk technique. Eur J Cardiothorac Surg. 2011;40:875‐880. [DOI] [PubMed] [Google Scholar]
- 10. Poon SS, Tian DH, Yan T, et al. Frozen elephant trunk does not increase incidence of paraplegia in patients with acute type A aortic dissection. J Thorac Cardiovasc Surg. 2020;159(4):1189‐1196.e1. 10.1016/j.jtcvs.2019.03.097 [DOI] [PubMed] [Google Scholar]
- 11. Di Eusanio M, Castrovinci S, Tian DH, et al. Antegrade stenting of the descending thoracic aorta during DeBakey type 1 acute aortic dissection repair. Eur J Cardiothorac Surg. 2014;45(6):967‐975. 10.1093/ejcts/ezt493 [DOI] [PubMed] [Google Scholar]
- 12. Di Marco L, Pantaleo A, Leone A, Murana G, Di Bartolomeo R, Pacini D. The frozen elephant trunk technique: European Association for cardio‐thoracic surgery position and bologna experience. Korean J Thorac Cardiovasc Surg. 2017;50(1):1‐7. 10.5090/kjtcs.2017.50.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lin HH, Liao SF, Wu CF, Li PC, Li ML. Outcome of frozen elephant trunk technique for acute type A aortic dissection: as systematic review and meta‐analysis. Medicine (Baltimore). 2015;94(16):e694. 10.1097/MD.0000000000000694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hanif H, Dubois L, Ouzounian M, et al. Canadian thoracic aortic collaborative (CTAC) investigators. Aortic arch reconstructive surgery with conventional techniques vs frozen elephant trunk: a systematic review and meta‐analysis. Can J Cardiol. 2018;34(3):262‐273. 10.1016/j.cjca.2017.12.020 [DOI] [PubMed] [Google Scholar]
- 15. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials. 2007;8(1):16. 10.1186/1745-6215-8-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leontyev S, Borger MA, Etz CD, et al. Experience with the conventional and frozen elephant trunk techniques: a single‐centre study. Eur J Cardiothorac Surg. 2013;44(6):1076‐1082. 10.1093/ejcts/ezt252 [DOI] [PubMed] [Google Scholar]
- 18. Di Eusanio M, Borger M, Petridis FD, et al. Conventional versus frozen elephant trunk surgery for extensive disease of the thoracic aorta. J Cardiovasc Med (Hagerstown). 2014;15(11):803‐809. 10.2459/JCM.0b013e328364559c [DOI] [PubMed] [Google Scholar]
- 19. Shrestha M, Beckmann E, Krueger H, et al. The elephant trunk is freezing: the Hannover experience. J Thorac Cardiovasc Surg. 2015;149(5):1286‐1293. 10.1016/j.jtcvs.2015.01.044 [DOI] [PubMed] [Google Scholar]
- 20. Mkalaluh S, Szczechowicz M, Mashhour A, et al. Total aortic arch replacement using elephant trunk or frozen elephant trunk technique: a case‐control matching study. J Thorac Dis. 2018;10(11):6192‐6200. 10.21037/jtd.2018.10.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Furutachi A, Takamatsu M, Nogami E, et al. Early and mid‐term outcomes of total arch replacement with the frozen elephant trunk technique for type A acute aortic dissection. Interact Cardiovasc Thorac Surg. 2019;29(5):753‐760. 10.1093/icvts/ivz154 [DOI] [PubMed] [Google Scholar]
- 22. Rustum S, Beckmann E, Wilhelmi M, et al. Is the frozen elephant trunk procedure superior to the conventional elephant trunk procedure for completion of the second stage? Eur J Cardiothorac Surg. 2017;52(4):725‐732. 10.1093/ejcts/ezx199 [DOI] [PubMed] [Google Scholar]
- 23. Hirano K, Tokui T, Nakamura B, et al. Impact of the frozen elephant trunk technique on total aortic arch replacement. Ann Vasc Surg. 2020;65:206‐216. 10.1016/j.avsg.2019.10.075 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.
Supporting information.
Supporting information.


