Summary
Background
Inflammatory bowel disease (IBD) is a chronic inflammatory immune‐mediated disorder of the gut with frequent extra‐intestinal complications. Pancreatic involvement in IBD is not uncommon and comprises a heterogeneous group of conditions, including acute pancreatitis (AP), chronic pancreatitis (CP), autoimmune pancreatitis (AIP) and pancreatic exocrine insufficiency (PEI); however, data on such an association remain sparse and heterogeneous.
Method
PubMed/MEDLINE and EMBASE databases were searched for studies investigating pancreatic involvement in patients with IBD.
Results
Four thousand one hundred and twenty‐one records were identified and 547 screened; finally, 124 studies were included in the review. AP is the most frequent pancreatic manifestation in IBD; the majority of AP cases in IBD are due to gallstones and drugs but cases of idiopathic AP are increasingly reported. AIP is a rare disease, but a strong association with IBD has been demonstrated, especially for type 2 and ulcerative colitis. The pathogenetic link between IBD and AIP remains unclear, but an immune‐mediated pathway seems plausible. An association between CP and PEI with IBD has also been suggested, but data are to date scarce and conflicting.
Conclusion
This is the first systematic review of the association between IBD and pancreatic diseases. Gallstones and drugs should be considered the most probable causes of AP in IBD, with type 2 AIP also being possible.
Abstract
Systematic review. Pancreatic involvement in inflammatory bowel disease (IBD). The wide spectrum of pancreatic involvement in patients with IBD may represent a challenge. From the 124 studies analyzed, acute pancreatitis (AP) is the most frequent pancreatic manifestation in IBD; the majority of AP cases in IBD are due to gallstones and drugs, but cases of idiopathic AP are increasingly reported. Autoimmune pancreatisis is a rare disease, but a strong association with IBD has been demonstrated, especially for type 2 and ulcerative colitis.
1. INTRODUCTION
Inflammatory bowel disease (IBD), which includes Crohn's disease (CD) and ulcerative colitis (UC), is a chronic inflammatory immune‐mediated disorder of the gut. It is considered a multisystemic disorder since up to 50% of patients experience at least one extra‐intestinal manifestation. 1 Extra‐intestinal presentations may virtually involve any organ and system with a potentially detrimental impact on the patient's functional status and quality of life.
Pancreatic involvement is not uncommon but is often underestimated and neglected.
Several pancreatic conditions have been reported with increased prevalence in CD and UC compared to the general population. 2 Pancreatic abnormalities in IBD include acute pancreatitis (AP), chronic pancreatitis (CP), autoimmune pancreatitis (AIP), pancreatic exocrine insufficiency (PEI) and asymptomatic abnormalities, comprising both imaging and laboratory findings. 3 As for causality, the involvement of the pancreas in IBD may be framed in the autoimmune process itself or be iatrogenic.
We, therefore, aimed to investigate pancreatic involvement in patients with IBD by performing a systematic review with the objective to evaluate the aetiology, prevalence and impact of pancreatic diseases in patients with IBD.
2. METHODS
2.1. Data sources
This systematic review was performed and reviewed according to the updated Preferred Reporting Items for Systematic Reviews and MetaAnalyses (PRISMA) statement. 4 A computerised literature search was performed in PubMed/MEDLINE and EMBASE databases (from January 1970 to January 2022) to retrieve pertinent primary studies.
The search terms “Inflammatory bowel disease”, “Crohn's Disease”, “Ulcerative colitis” “Idiopathic acute pancreatitis”, “drug‐induced pancreatitis”, “Autoimmune pancreatitis”, “pancreatitis”, “chronic pancreatitis”, “hyperamylasemia”, “Cholelithiasis”, “exocrine pancreatic insufficiency”, “Pancreatic ductal adenocarcinoma”, “asymptomatic pancreatic abnormalities”, “pancreatic diseases” with synonyms were combined. The search strategy included both medical subject headings (MeSH) terms and free language words.
Specific search terms were defined as detailed in Appendix S1. The titles of all identified articles were screened to evaluate their relevance, and the abstracts and/or full texts of selected potentially relevant papers were further evaluated. With a snowball method, additional articles were searched by hand‐searching reference lists of all the articles retrieved to identify potentially relevant studies. Non‐English language papers were excluded. The protocol of this systematic review has been submitted in the International Prospective Register of Systematic Reviews (PROSPERO, ID 314688) and is available on request from the corresponding author.
2.2. Study selection criteria
Both retrospective and prospective studies, single‐arm, cross‐sectional (cohort or case–control), case‐series and reports, registry‐based studies, controlled and randomised studies were included. Editorials, reviews, systematic reviews, meta‐analysis, preprint, conference abstracts, study register entries, clinical study reports, dissertations, unpublished manuscripts, government reports or any other document providing relevant information were also retrieved. The research included studies of patients with both CD and UC. Articles published as abstracts were included, whereas non‐English language papers were excluded.
2.3. Quality assessment of primary studies
All of the included studies were evaluated according to their methodological quality, study design (case‐series and reports, single‐arm, cross‐sectional, registry‐based studies, controlled and randomised studies), patient selection (consecutive or non‐consecutive), data collection (prospective, retrospective or unknown), statistical methods, endpoints and length of follow‐up. The quality of included studies was assessed according to Newcastle–Ottawa scale. 5
2.4. Methods of the review of the literature
Six reviewers (S.M., I.F., C.V., L.P., L.C. and M.F.) identified all the articles divided by topic (two reviewers by each topic). The reviewers screened all the articles based on the title article and abstract. Duplicates were identified and removed. The remaining studies were assessed by examining the full‐text papers for adherence to the topic. The data concerning the types of participants and outcome measures were independently extracted by the reviewers, who openly discussed any discrepancies. Only in the case of disagreement was the further and definitive judgement of an independent clinical expert (G.C.) applied. The excluded studies and the reasons for exclusion were recorded.
2.5. Ethics approval
Ethical approval was not required as data are not individualised, and primary data were not collected.
3. RESULTS
A total number of 4121 studies were identified and 547 screened. After filtering for English language, human studies, year range, article type and removing duplicates, 202 full‐text articles were considered. Out of these, 124 studies constituted the final dataset as containing pertinent data. Figure 1 represents the study selection process in the PRISMA 2020 diagram. 4
FIGURE 1.
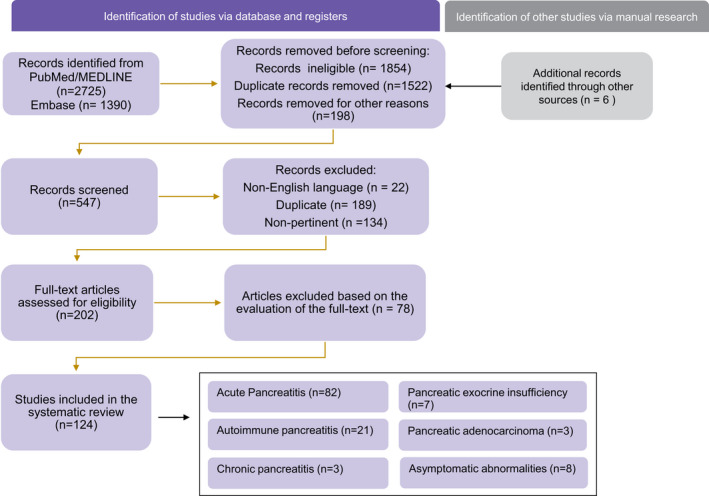
PRISMA 2020 diagram showing the study selection process, with the results divided by topics
Considering the clinical heterogeneity of all the studies, no quantitative synthesis was possible and the results of the review are presented and discussed being organised by topics.
3.1. Acute pancreatitis
AP is the most frequent pancreatic disorder associated with IBD and it is characterised by acute inflammation of the pancreatic parenchyma. We identified 82 studies regarding AP in IBD (Figure 1), eight concerning ‘epidemiology’, 74 concerning ‘aetiology’, of which five regarding ‘gallstone disease’, 46 ‘drug‐induced AP’, two ‘idiopathic AP’, 21 ‘other causes’.
3.2. Epidemiology
In the general population, the incidence rate of AP ranges from 10 to 44 per 100,000/year, 6 being one of the leading causes of hospital admission and expenses for digestive diseases. 7 Patients with IBD seem to be at increased risk for acute pancreatitis.
We identified eight studies specifically dealing with the epidemiology of AP in patients with IBD. 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 A population‐based cohort study in Taiwan reported the overall incidence of AP in IBD to be 3.56‐fold higher than in patients without IBD. 8 In a retrospective study by Bermejo et al. 82 episodes of AP were observed in 67 patients (53 CD and 14 UC), in a total of 5073 IBD patients, with a cumulative incidence of AP in IBD of 1.6% during a mean follow‐up period of 14 years. 9 Similarly, in a study of 852 patients with CD, the incidence rate of AP was 1.4%, over a follow‐up period of 10 years. 10 A Danish 16‐year nationwide follow‐up study demonstrated an elevated risk of AP with an incidence rate of 4.3% and 2.1%, in CD and UC respectively. 11 Moreover, in a recent retrospective cohort study conducted at nine Spanish IBD referral centres, 185 patients with IBD (68.7% CD) were identified with a first episode of AP, between 1998 and 2018. 12 A retrospective study analysing paediatric and adult patients presenting with AP as the first symptom of IBD demonstrated that AP preceded the diagnosis of IBD in 2.17% (10/460) of paediatric patients with IBD, compared to only 0.06% (2/3500) of adult patients with IBD. 13
In a recent meta‐analysis, the risk of AP was increased in patients with IBD and particularly higher in patients with CD. The overall estimated risk ratio for AP was 2.78 in patients with IBD and 3.62 and 2.24 for CD and UC respectively. Due to the observational design of the studies included, the mechanisms underlying the increased risk of pancreatitis are unknown and remain to be investigated. 14 Another meta‐analysis confirmed that IBD elevates the risk of AP with a pooled annual incidence of AP in IBD of 210/100,000 person‐years (95% CI, 84–392/100,000 person‐years). 15
While not all of these studies clearly defined AP following the revised Atlanta classification of AP, 16 in the large majority of them the diagnosis was made on clinical basis; therefore, asymptomatic cases of hyperamylasaemia/lipasemia alone should not affect the results. Moreover, the results of these studies and the two retrieved meta‐analyses, uniformly document a significantly increased risk of AP in patients with IBD.
3.3. Aetiology
A wide variety of factors may cause AP in IBD (Table 1), including gallstones, adverse effects of drugs, duodenal inflammatory lesions, iatrogenic harm accompanying endoscopic procedures, primary sclerosing cholangitis (PSC) and autoimmunity. Not infrequently, a precise cause cannot be identified, thus defining also a group of idiopathic AP (IAP). 12
TABLE 1.
Causes of acute pancreatitis in inflammatory bowel disease (IBD)
| Cholelithiasis |
| Drug‐induced |
| Higher Likelihood of association |
| Azathioprine (AZA) and its active metabolite 6‐mercaptopurine |
| Salazopyrine and 5‐ASA‐derived drugs |
| Antibacterial agents (Metronidazole) |
| Lower Likelihood of association |
| Corticosteroids |
| Biological agents (infliximab and vedolizumab) |
| Autoimmune pancreatitis (AIP) (Type 2) |
| Idiopathic acute pancreatitis (IAP) |
| Duodenal inflammatory lesions (stenosis, fistula, direct infiltration of inflammation) |
| Post‐procedural (small bowel endoscopy) |
| Primary sclerosing cholangitis (PSC) |
3.3.1. Cholelithiasis
Cholelithiasis is one of the most common causes of AP in IBD and a strong association between gallstones formation and CD has been demonstrated.
We identified five studies dealing with the association between IBD and gallstone disease. 17 , 18 , 19 , 20 , 21 According to a meta‐analysis specifically investigating this association, patients with IBD had a significantly higher prevalence of gallstones compared to the control group [odds ratio (OR) 1.72, 95% confidence interval (CI) 1.40–2.12, P < 0.0001]; the subgroup analyses showed that the risk of cholelithiasis was increased in CD patients (OR 2.05, 95% CI 1.61–2.63, P < 0.0001) but not in UC patients (OR 1.12, 95% CI 0.75–1.68, P = 0.585). 17 Many independent factors in CD have been related to gallstones development, namely site of disease at diagnosis (ileo‐colonic location), lifetime surgery, the extent of ileal resections (>30 cm), number of clinical recurrences (>3), total parenteral nutrition and frequency and duration of hospitalisations. 18 , 19 Indeed gallstones development is mainly due to the malabsorption of bile salts in the ileum, which leads to impaired enterohepatic circulation. 20 Also, total parenteral nutrition and a prolonged fasting state may reduce gallbladder emptying, and this may further increase the risk of the development of gallstones and biliary sludge. 18 , 21
3.3.2. Drug‐induced acute pancreatitis
Many drugs used to treat IBD are potentially pancreato‐toxic and drug‐induced AP is particularly frequent in the IBD population. 2 , 22 , 23 , 24
The occurrence of AP due to thiopurines, namely azathioprine (AZA) and its active metabolite 6‐mercaptopurine has been described since the 1970s. 9 , 25 , 26 , 27 , 28 , 29 , 30 , 31 Since then, at least 16 studies explored this topic and were included in this systematic review. 9 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39
This side effect is reported in up to 7.3% of patients with IBD taking AZA in longitudinal prospective and retrospective studies, 27 , 28 while in a recent meta‐analysis focusing on AZA and 6‐mercaptopurine for maintenance of remission in ulcerative colitis a lower frequency, below 3%, was reported. 32 However, this meta‐analysis was not focused on drug‐induced AP, so it may underestimate its frequency; moreover, it seems that the incidence is higher in patients with CD compared to UC; the female gender is also associated with a 3.4‐fold higher risk and smoking seems to be the strongest risk factor for thiopurine‐induced AP. 33 It is a likely idiosyncratic, dose‐independent and unpredictable adverse drug reaction, usually occurring in the first month of therapy. 33 The course of thiopurine‐induced AP is usually mild with rapid clinical improvement on withdrawal of the offending drug. In a prospective study, 24% presented nausea and vomiting, and 14% had fever, 43% of patients required hospitalisation with a median inpatient period of 5 days; only 10% of patients developed peripancreatic fluid collections, yet none required surgical/endoscopic intervention. 28 In a recent retrospective study on 787 IBD patients on AZA, the rate of abdominal pain was 6.9%, but only 3.3% of patients had AP, typically within the first 2 months of treatment, with active smoking being the only independent risk factor for AZA‐induced AP (OR = 3.2). 34
Many pathophysiological mechanisms have been proposed, including immunologic reactions and direct toxic effects. Genetic polymorphisms have been strongly associated with the development of thiopurine‐induced pancreatitis: a genome‐wide association study (GWAS) identified a strong association between the Class II HLA gene region polymorphism (rs2647087) and thiopurine‐induced AP, 35 with the estimated risk being 9% in patients heterozygous at rs2647087 and 17% in homozygotes. 36 On the other hand, polymorphisms in the thiopurine S‐methyltransferase (TMPT) gene, which are known to be associated with other dose‐independent side effects, such as hepatotoxicity and myelotoxicity, showed no association with thiopurine‐induced pancreatitis. 37
Wilson and colleagues screened their patients with IBD who were candidates for treatment with AZA for the haplotype HLADQA1‐HLADRB1*07:01A > C haplotype that has been associated with increased risk of AP after AZA and obtained an 11‐fold reduction of this adverse event, suggesting that a personalised treatment strategy may reduce the risk. 38 Furthermore, in a more recent retrospective series, azathioprine‐induced AP in patients with IBD was associated with AB0 blood group B (OR 3.17), which is an established risk factor for AP in general; this remained significant even after adjustment for known risk factors such as CD and active smoking. 39
However, apart from these genetic analyses, it is often unpredictable which individuals are at risk for thiopurines‐induced AP in clinical practice. Even other drugs could contribute to or precipitate AZA toxicity such as budesonide which has been reported as a risk factor of azathioprine‐induced pancreatitis. 34 In very selected cases with mild pancreatitis, a re‐challenge test has been attempted, 38 although the availability of many other treatments has rendered this approach unnecessarily risky.
5‐ASA compounds, including sulphasalazine, mesalazine and olsalazine, have been less frequently involved in drug‐induced AP. Ten of the selected studies addressed this association. 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49
AP as an adverse reaction to 5‐ASA derivatives has been first described by Block et al, 40 and subsequently by several other authors, 41 although reports are sometimes conflicting. 42 It is reported to occur more frequently with mesalazine (7.5 per million prescriptions) compared to sulphasalazine (1.1 per million prescriptions) (OR 7.0; 95% CI 2.6–18.6; p < 0.001). 43 AP has been reported also with olsalazine 44 and even as a consequence of rectal 5‐ASA enema administration. 45 A retrospective case–control study demonstrated that the risk of AP does not differ among patients using the mesalazine Multi Matrix System (MMX) or a comparator. 46
The incidence has been estimated to be 1/million days of treatment. 49 However, the frequency of AP with the above medications is not clear and may be underestimated, as most evidence comes from case reports, 40 , 44 , 45 , 46 , 47 , 48 narrative reviews, 2 , 49 another systematic review, which collected 42 patients. 41 Only one population‐based case–control study 42 evaluated 1590 incident cases of AP from the Hospital Discharge Registry of the North Jutland County of Denmark from 1991 to 2002 and among them, 21 patients had IBD and 5 were taking 5‐ASA compounds. Most cases of 5‐ASA‐related AP occurred within the first 6 weeks of therapy, even if it can occur at any time point. 47 The course is mild, although rare cases of severe necrotising pancreatitis have been reported. 48 Clinical improvement usually occurs within 4 days after drug withdrawal. 47 , 49
Regarding biological agents, nine studies were selected. 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 Drug‐induced AP is an extremely rare adverse effect of biological agents with only a few cases reported for infliximab 50 and vedolizumab. 51 On the other hand, anti‐TNF agents were also used to improve AP course in animal studies, 52 , 53 , 54 whether they may be of benefit in humans is unknown.
Multiple mechanisms have been hypothesised in the causative process of drug‐induced pancreatitis, including pancreatic duct constriction, arteriolar thrombosis and an immune‐mediated mechanism (similar to what was reported for hepatitis) 55 , 56 ; tofacitinib, and to a lesser extent, infliximab are also known to cause lipid profile abnormalities, which theoretically could lead to hypertriglyceridemia‐induced pancreatitis. 57 , 58
Other medications could be involved in drug‐induced AP in IBD. Antibacterials, such as metronidazole, are commonly employed as primary therapy for perianal Crohn's disease, with few reports of drug‐induced AP. 59 A recent epidemiological study showed an increased risk of AP within 1 month of exposure to a single or combined regimen of oral metronidazole; however, a direct causality link, as well as the possible pathogenetic mechanism were not so clear. 60 It has been hypothesised that metronidazole may promote the formation of hydrogen peroxide, superoxide and other free radicals, which are toxic for pancreatic β‐cells; other suggested mechanisms include immune‐mediated inflammatory response and pancreatic duct constriction. 61
AP has also been reported in association with steroids, as an extremely rare and debatable side effect. 62 , 63 , 64 , 65 One case–control study found a nearly threefold increased risk of AP in patients taking betamethasone and slightly lower for those taking prednisolone. 63 A recent meta‐analysis, in the setting of systemic lupus erythematosus, reported a cumulative incidence of 5% of corticosteroid‐associated AP, 64 with the risk reaching its highest level in the first 2–14 days after steroid administration and gradually decreasing thereafter. 62 , 63 , 64 On the other hand, another study in the setting of optic neuritis 65 did not show any increased risk.
To sum up, thiopurines, 5‐aminosalicylic acid (5‐ASA) compounds, and metronidazole are considered class Ia drugs, for which there is convincing evidence for the association with AP. 66 Indeed, they are among the drugs most commonly associated with drug‐induced AP. 67 The evidence for an association with corticosteroids, and biological agents is weaker, the latter being exclusively based on case reports. At any rate, reports of drug‐induced AP are often hampered by a lack of exclusion of all other possible causes and it has to be taken into account that, especially in older studies, the definition of AP was not standardised and an AP diagnosis may have been reported merely based on the elevation of pancreatic enzymes and abdominal pain (that may occur for many other reasons in patients with IBD), without radiological confirmation.
3.3.3. Idiopathic acute pancreatitis
Cases of idiopathic acute pancreatitis (IAP) are increasingly reported both as initial presentation and as extra‐intestinal manifestations in the course of IBD, without specific aetiological factors being documented. The present systematic review retrieved two studies. 12 , 68 According to a recent publication, IAP represents the second cause of AP in patients with IBD. 12 Among 185 patients with IBD (68.7% CD) and a first episode of AP, 38 (20.6%) fulfilled the criteria for IAP. Differently from other causes of AP, IAP seemed more frequent in UC, with a mild course but with a high risk of recurrence. 12 In the same study, IAP patients also presented a significantly higher 5‐year risk of developing chronic pancreatitis (5.2%). 12 Moreover, the 5‐year risk of being diagnosed with autoimmune pancreatitis was higher in IAP patients (14.1% vs. 0.7%, log‐rank p < 0.001). Finally, the course of IBD during the year that followed the first episode of IAP did not differ from the groups without IAP. 12 Therefore, in this category of patients, there are some cases of undiagnosed autoimmune pancreatitis type 2 and probably overlapping cases with drug‐induced acute pancreatitis.
3.3.4. Other causes
Several studies retrieved in the present systematic review reported other possible causes of AP, represented by duodenal and/or papillary lesions, procedural accidents due to either endoscopic balloons, or endoscopic retrograde cholangiopancreatography (ERCP), and primary sclerosing cholangitis (PSC). 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89
In duodenal manifestations of CD, it has been reported that, even rarely, fistulas in the duodenal papilla, 71 as well as stenosis of the duodenum 72 can cause reflux pancreatitis; even cases of direct inflammatory infiltration from the duodenum into the pancreas have been described. 73
Small bowel enteroscopy (either single‐balloon or double‐balloon) is an endoscopic procedure that enables direct visualisation and histological sampling of the small bowel mucosa and is therefore useful in CD diagnosis and management. 74 Isolated post‐procedure hyperamylasaemia, without pancreatitis, has been reported in 17–75% of patients who underwent peroral double‐balloon enteroscopy (DBE) 75 , 76 , 77 ; whereas post‐procedural AP was reported in 0.7%–3.2% of cases. 75 , 76 In a prospective trial involving 48 patients undergoing peroral DBE, hyperamylasaemia, and hyperlipasemia after peroral DBE occurred in 12 of 48 patients (25%), whereas the incidence of AP was reported to be 12.5% when clinically diagnosed. 16 On the other hand, in another paper post‐small bowel enteroscopy hyperamylasaemia was reported in 13 patients (16%), but none had complaints suggesting acute pancreatitis. 75 Total insertion length, duration and time between the first and second inflations of the balloon were risk factors for AP. 78 No differences were observed between single and double balloon endoscopies in a randomised multicentre trial including 130 patients 79 as well as in two recent meta‐analyses. 80 , 81 Aetiology has not been defined yet, but the most likely mechanism seems to be related to vascular distress causing a hypoxic state, as supported by the experimental evidence of hypoxic areas and necrotic zones in the pancreatic tissue of pigs. 82
PSC is an idiopathic disease in which multiple diffuse stenoses in the intrahepatic and extrahepatic bile ducts lead to progressive cholestasis. 83 The incidence and prevalence rates of PSC are not negligible in the United States and Northern Europe, with a reported incidence of 0.4–1.22 per 100,000 inhabitants/year and a prevalence of 4.15–16.2 per 100,000 inhabitants. 84 Both genetic and environmental factors are reported to be involved in its onset. It is a condition strongly associated with IBD: in the West 50–80% of patients with PSC develop complicating IBD, and common disease susceptibility genes with IBD have been found. 85 The association is stronger for UC than CD. 86 Although the exact mechanism remains unknown, it has been reported that PSC patients may develop AP. 87 , 88 It may be caused by the bile and sludge reflux into the pancreatic duct, possibly due to strictures of the distal part of the common bile and/or pancreatic ducts. Indeed, endoscopic biliary stent placement was reported to be effective to prevent recurrent pancreatitis. 87 Furthermore, PSC has been reported to be an independent risk factor for post‐ERCP pancreatitis. 89
3.4. Autoimmune pancreatitis
Autoimmune pancreatitis (AIP) is a chronic benign pancreatic disorder characterised by painless obstructive jaundice (with or without a pancreatic mass), evidence of peculiar histology pattern of lymphoplasmacytic infiltrate and fibrosis, and a dramatic response to steroids. 90 Two distinct subtypes of AIP have been identified and specific diagnostic criteria have been detailed for both, assessing five main features, which are shown in Table 2.
TABLE 2.
Main features of type 1 and type 2 autoimmune pancreatitis (AIP)
| Type 1 AIP | Type 2 AIP | |
|---|---|---|
| Median age at onset (years) | 60 | 45 |
| Sex difference | M > F | M = F |
| Clinical onset | Jaundice | Acute Pancreatitis |
| IgG–IgG4 elevated | Yes | No |
| Autoantibodies positive | Yes | No |
| Pancreatic histology | Lymphoplasmacytic sclerosing pancreatitis | Granulocytic epithelial lesion |
| Other organs involvement |
IgG4 systemic disease Sclerosing cholangitis, sialadenitis. Retroperitoneal fibrosis |
IBD (UC > CD) |
| Diagnostic elementsa |
Elevated IgG4 suggestive, Histology not mandatory |
Concomitant IBD suggestive, Histology mandatory |
| Treatment strategy | Steroids | Steroids (IBD‐therapy) |
| Recurrence risk |
High (40%–60%) Maintenance therapy |
Low (9%–25%) No maintenance therapy |
According to ICDC.
Twenty‐one studies on AIP in IBD were analysed in this systematic review 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 (Figure 1). AIP occurring in association with IBD is rather rare, yet the prevalence in IBD seems to be significantly higher than in the general population and, usually, it is of type 2. 91 , 92 AIP, especially type 2, appears to be more frequent in Western countries, with an estimated prevalence rate of 4.6–6% among acute and chronic pancreatitis cases and around 0.001–0.004% in the general population. 93 , 94 , 95 Only two studies conducted in Asia examined the prevalence of AIP in patients with IBD, reporting a prevalence of 0.3–0.5%, 96 , 97 which is approximately 100‐fold higher than in the general population. 94 , 95 However, this rate may be underestimated, as suggested by studies demonstrating a frequency of pancreatic duct abnormalities in up to 10% of patients with IBD 112 and considering that type 2 AIP more often requires a challenging histological confirmation of diagnosis.
On the other hand, the prevalence of IBD in AIP patients has been reported to be 12–15‐fold higher than in the general population 97 , 99 , 100 : an international multicentre survey found a prevalence of 16% UC and 1% CD among 64 patients with type 2 AIP, while IBD prevalence in type 1 AIP was much lower (1% among 153 patients). 101 As for isolated AIP, the prevalence of concomitant IBD‐AIP varies widely worldwide, being less frequent in Asian Countries. In Western countries, the reported prevalence of UC among AIP patients was 30%–35%, 102 while studies from Korea and Japan reported a UC prevalence of 5.8% and 16% respectively. 96 , 97 Overall, there is an increased prevalence of AIP occurring in UC than in CD patients, since more than 60% of patients with concomitant IBD‐AIP are affected by UC. 103
IBD diagnosis usually precedes AIP diagnosis by 2–5 years 103 and a cumulative increasing probability of AIP after UC diagnosis has been reported (0.2% after 1 year, 0.8% after 10 years). 96 , 97 Yet, AIP can be the first manifestation of or even precede IBD in 25% and 20% of patients respectively. 103 In patients with pre‐existing IBD, at the time of AIP diagnosis, most patients had active intestinal disease. 96 , 103 The mean age at AIP onset is 35 years and no gender predominance has been reported. 103
The clinical presentation of AIP in IBD is rather similar to that of the isolated disease. Acute pancreatitis is the most common clinical presentation, up to 80% of patients in the largest series of AIP‐IBD. In 15% of cases, associated inflammatory cholangitis was observed, which on the contrary is more common in type 1 AIP. 103
The impact of AIP on the natural history of IBD has not been clearly defined yet, as available data are discordant. According to some authors, IBD associated with AIP carries a worse prognosis compared to IBD alone, with a higher proportion of extensive colitis and increased risk of colectomy reported both for UC and CD. 97 , 99 Other studies, however, found no differences in UC extent or activity in patients with or without AIP. 96 Interestingly, in the largest multicentric retrospective series published to date, comprising 91 patients with AIP‐IBD, in UC patients, AIP was independently associated with a history of colectomy (OR, 7.1; 95% CI, 2.5–20, P < 0.05) but also with rectal location (OR, 2.9; 95% CI, 1.3–6.3; P < 0.05). One may infer that two different groups of UC patients seem at the highest risk of AIP: a) patients with extensive and refractory UC, carrying a higher risk of colectomy, and b) patients with mild distal location. 103 In the same series, CD‐AIP patients had less stricturing–penetrating behaviour, including less perianal disease and again a higher risk of colectomy. 103
The typical imaging finding of AIP, especially of type II disease, is a diffuse pancreatic enlargement, which gives the gland a sausage‐shaped appearance, in some cases with a low‐attenuating capsule‐like rim 90 , 91 (Figure 2), associated with main pancreatic duct (MPD) diffuse or focal stricture with possible upstream dilatation. However, AIP (more frequently type I) may also present as focal and subtle changes; in this case, the diagnosis is challenging and the possibility of malignancy has to be ruled out.
FIGURE 2.
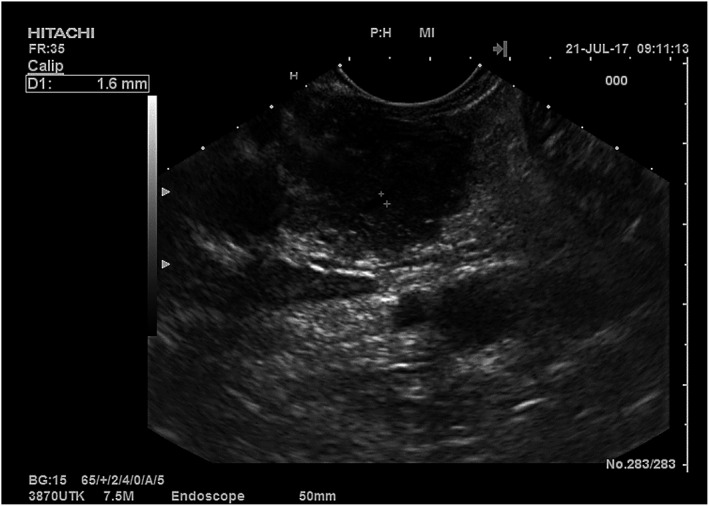
Endoscopic ultrasound appearance of autoimmune pancreatitis (AIP). The image shows the head of the pancreas examined from the duodenum with a diffuse coarse and hypoechoic appearance (sausage‐shaped) in a patient with type 2 AIP. The main pancreatic duct was normal (calliper 1.6 mm)
The pathogenetic link which correlates IBD and AIP is still unclear, but an immune‐mediated pathway seems reasonable. In the largest series published to date, at the time of AIP diagnosis, more than 70% of patients had an active intestinal disease, thus suggesting a role for systemic inflammation in the pathogenesis of AIP. 103 A shared lymphocyte homing mechanism has been proposed: it has been reported that in AIP marked lymphoid infiltration produces tertiary lymphoid tissues, that resemble gut‐associated lymphoid tissue (GALT) with over‐expression of mucosal addressin cell adhesion molecule 1 (MadCAM‐1) and peripheral lymph node addressins, as observed in active UC. 104 Indeed, from a histological point of view, type 2 AIP diagnostic characteristic is the granulocyte epithelial lesion caused by neutrophil infiltration of pancreatic duct epithelium 105 , 106 , 107 which resembles the crypt abscesses observed in the colonic mucosa of patients with UC. 106 , 108
On the other hand, a possible link between IBD and type 1 AIP has also been hypothesised, as part of an IgG4 systemic disease and in a study by Ravi et al. patients with a previous diagnosis of UC were reported to have an increased number of IgG4‐positive cells (10/HPF) on colon samples. 99 Yet, other authors also found that up to 4% of patients with IBD had elevated IgG4 serum levels with the possible diagnosis of IgG4‐related systemic disease, even if none of them had evidence of autoimmune pancreatitis. 109
For what concerns treatment, both type 1 and 2 AIP show a dramatic response to steroid therapy; high dose prednisolone (0.6 or 40 mg/day) for 4 weeks is the recommended treatment, then gradually tapered by decrements of 5 mg over 2–3 months. 91 Relapse rates are significantly higher in type 1 AIP patients, ranging between 40 and 60% compared with 9–25% in type 2 AIP. 93 , 110 Treatment response and recurrence rate in AIP‐IBD patients seem to be similar to type 2 isolated AIP. In the largest published cohort, 34% of patients had at least one recurrence, whereas 20% had steroid‐dependent AIP, which was successfully treated with azathioprine in the majority of them. 103
Nowadays, although many patients with concomitant AIP‐IBD may already be receiving immunomodulators or biologics at AIP diagnosis, published data on the effect of these drugs on type 2 AIP are still lacking and steroid therapy continues to be the mainstay of AIP treatment.
Recently, successful treatment of type 2 AIP with colchicine has been reported, with its rationale based on its inhibitory effect on neutrophils, the most characteristic immune cells infiltrating type 2 AIP. 111
3.5. Chronic pancreatitis
Chronic pancreatitis (CP) is a chronic inflammatory and fibrotic disease leading to progressive loss of exocrine (acinar cells) and endocrine (islets) tissue. 113 CP can be a result of recurrent flares of AP, especially when the aetiological factors, such as smoking, alcohol consumption, are not treated. CP is usually diagnosed based on clinical features: chronic or relapsing pancreatic‐type pain (epigastric pain radiating to the back), even if there is currently no diagnostic reference standard; thus the combination of clinical signs, diagnostic imaging findings usually lead to the diagnosis of CP.
An association between IBD and CP has also been suggested in at least three studies, included in this systematic review 98 , 114 , 115 ; however, high‐quality studies are lacking, therefore evidence remains limited, 69 and further studies are warranted. 14
A multicentric French study, conducted between 1981 and 1996, retrieved six patients presenting with features of idiopathic CP and UC and two patients with concomitant CP and Crohn's disease; a review of the literature performed in the same paper identified six additional cases of CP associated with UC and 14 associated with Crohn's disease. 98 In the same study, CP was associated with extensive disease and the risk of total colectomy in UC, suggesting a correlation between disease severity and progression. 98 Moreover, weight loss and pancreatic duct stenosis were also more frequent in UC compared to Crohn's disease (41% vs 12% and 50% vs 23% respectively). 98 Pathological specimens were analysed in five patients and demonstrated the presence of inter‐ and intra‐lobular fibrosis with marked acinar regression in three and the presence of granulomas in two patients, both with Crohn's disease. 98 A recent nationwide population‐based cohort study in Taiwan demonstrated that the incidence of CP in patients with IBD was 10.3 higher than that in non‐IBD patients (5.75 vs 0.56 per 10,000 person‐year). On the other hand, the CP cohort exhibited a higher risk of developing IBD, with a significantly higher risk for Crohn's disease (adjusted hazard ratio = 12.9) as compared to UC (adjusted hazard ratio = 2.80). 115 There are several possible aetiological factors for CP in IBD. AIP can progress to CP and may justify at least some of these cases. Recurrent AP episodes caused by drugs or other causes may also result in CP. Also, environmental factors associated with Crohn's disease such as smoking also cause CP. 69 , 113 In patients with IBD, CP symptoms, such as abdominal pain or steatorrhoea, may actually worsen IBD‐related symptoms, further reducing the quality of life. CP pain is, unfortunately, difficult to manage, often requiring a multidisciplinary approach with a pain management team. 113
3.6. Pancreatic exocrine insufficiency
Pancreatic exocrine insufficiency (PEI) refers to the presence of maldigestion and malabsorption of nutrients as a consequence of a severely reduced pancreatic enzyme output, usually less than 10% of that necessary to sustain normal digestion. 116
Data regarding PEI during the course of IBD are scarce and conflicting. We identified seven studies addressing this topic. 117 , 118 , 119 , 120 , 121 , 122 , 123 In an Italian cross‐sectional study, PEI was frequently demonstrated in patients with IBD, when screened by the faecal elastase‐1 test and, in this study, using a cut‐off of ≤200 μg/g, PEI was found in 22% of UC and 14% of CD patients, with an overall OR for patients with IBD of 10.5 compared to controls. The risk of PEI was related to loose stools, a larger number of bowel movements per day, and previous surgery 117 ; PEI was reversible in most patients and persistent PEI was not associated with clinically active disease. 117 Possible mechanisms for the development of PEI in CD include pancreatic autoantibodies, duodenal reflux or duodenal–pancreatic anatomical alterations, 118 and reduced hormone secretion from the gut resulting in reduced stimulation of pancreatic juice. 119 The presence of pancreatic autoantibodies directed against the exocrine portion of the pancreas has been reported in about one‐third of CD patients. 120 , 121 However, the association between CD and PEI is not fully elucidated. A recent study failed to demonstrate a substantial association between PEI and CD, using the faecal elastase‐1 test. 122 As regards UC, data are even more limited. 123 A critical point is the diagnostic method used to diagnose PEI. Among indirect pancreatic function tests, faecal elastase‐1 is the most commonly employed. 124 It is commonly accepted that a faecal elastase‐1 level ≤200 μg/g stool indicates PEI, with levels of 100–200 μg/g typically indicating mild to moderate impairment and levels <100 μg/g reflecting severe impairment. 125 , 126 However, faecal elastase test has limitations as a definitive reference standard for PEI diagnosis 127 and poor diagnostic accuracy has been reported in patients with diarrhoea as dilution can lead to false‐positive results. 128 This should be considered carefully in patients with active IBD.
3.7. Pancreatic ductal adenocarcinoma
Data regarding the risk factors of hepato‐pancreato‐biliary neoplasms in IBD patients with or without PSC are scanty and conflicting. Three large studies were selected. 70 , 129 , 130 In a large Korean study, evaluating 5595 CD and 10,049 UC from 2011 to 2014 to explore the overall cancer risk in patients with IBD, a significantly increased PDAC risk (OR 8.6, 95% CI 1.0–31.0) was reported only in women with CD. 129 In another multicentric cohort study, including 5506 patients with CD and 5522 patients with UC (of whom 2% were affected by PSC), an association with neoplastic lesions was related to concomitant PSC. 70 In this study, the incidence of PDAC was higher (OR 11.22, 95% CI 4.11–30.62) in patients with IBD and PSC compared to patients with IBD and without PSC. 70 More recently, in a population‐based cohort study from Norway and Sweden, among the 141,960 IBD patients (3.2% with PSC), 282 pancreatic cancers were diagnosed, during a median follow‐up of 10.0 years (standardised incidence ratio 1.3, 95% CI 1.2–1.5). The relative risk of PDAC was considerably higher in PSC‐IBD patients, with a standardised incidence ratio of 9.0; however, the standardised incidence ratio was still slightly increased also in non‐PSC‐IBD patients, compared to the general population. 130
Several hypotheses have been made on the etiopathogenetic mechanisms underlying this possible association between IBD and PDAC. The immunosuppressive treatment, used to control disease activity, might have a role by impairing immune surveillance; another possible hypothesis is that chronic inflammation (particularly in the context of PSC) could be associated with an increased risk of cancer. 70 Environmental factors associated both with the risk of PDAC and CD such as smoking may also contribute to the association.
However, a clear association has not yet been demonstrated, and further studies are needed.
3.8. Asymptomatic abnormalities
Asymptomatic hyperamylasaemia or hyperlipasemia and abnormalities of pancreas morphology have been reported in variable percentages in IBD. We reviewed five studies facing this topic. 131 , 132 , 133 , 134 , 135
Elevation of serum pancreatic enzymes, in the absence of any clinical symptoms and morphological alteration, has been demonstrated in 11%–14% of patients with IBD after excluding other possible causes, such as renal impairment, familial pancreatic hyperenzymemia, macroamylasemia and salivary gland disease. 131 , 132 , 134 The prevalence seems to be slightly higher in UC compared to CD (11 vs 7% respectively) and in patients with concomitant PSC. 134 However, clinical relevance and association with IBD remain unclear and even if these abnormalities are relatively common in IBD, they are usually harmless, and even their role in the development of exocrine pancreatic insufficiency has not been confirmed. 112 , 133 Therefore, the measurement of lipase and amylase should be avoided in patients with IBD and without evidence of AP, as lipase and amylase are only of diagnostic value in AP, but not in any other pancreatic disease.
A possible cause of hyperenzymemia is the presence of pancreatic antibodies, found in up to 39% of CD patients compared with 4%–23% of UC patients and 3% of healthy controls, 135 and directed against exocrine pancreas, glycoprotein 2 and CUB/zona pellucida‐like domain‐containing protein (CUZD1) antigens. They seem to correlate with CD location and behaviour, being more prevalent in the case of ileitis and previous surgical intervention, 136 stricturing behaviour and perianal disease 137 and in case of early IBD onset (age at diagnosis ≤16 years). 138 However, none of these appears to be specific and they have been found in many other autoimmune diseases, 139 such as refractory celiac disease, PSC without IBD and patients with cholangiocarcinoma. 140 , 141 Finally, also some asymptomatic radiological abnormalities are described. We found three studies addressing radiological abnormalities. 112 , 123 , 142
In a radiological study with magnetic resonance cholangiopancreatography (MRCP), pancreatic abnormalities of the ductal system have been reported in 16.4% of asymptomatic UC patients with no history of alcohol intake or previous episodes of acute pancreatitis. Such abnormalities seem to be more prevalent in patients with concomitant PSC. 123 , 142 A more recent study showed a rate of pancreatic duct abnormalities of 10.8% in patients with IBD; however, no distinction has been reported regarding patients with a previous history of pancreatitis. 112
Diagnostic workup is generally similar to the general population, even if in patients with IBD, diagnosis and management may represent a challenge for the clinicians, because of the aforementioned possible bias of interpretation of the clinical test for pancreatic function.
4. CONCLUSION
Our study systematically reviewed the association between IBD and pancreatic diseases, which resulted consistent for AP, mainly due to gallstones and drugs. Even if rare, AIP shows a strong association with IBD, especially between type 2 AIP and UC. Asymptomatic abnormalities may be as frequent as 11%–14%. The wide spectrum of pancreatic involvement in patients with IBD (Figure 3) may represent a challenge to the clinician facing patients with IBD. In fact in these patients, acute abdominal pain may occur due to the IBD itself or its acute intra‐abdominal complications. The biochemical tests to examine the pancreatic function, including amylase and lipase in patients with IBD, can be affected by a high fraction of false positives that do not exactly reflect an actual AP.
FIGURE 3.

The spectrum of pancreatic manifestation in inflammatory bowel disease (IBD)
On the other hand, the possibility of pancreatic involvement should not be overlooked and the clinicians facing patients with IBD should be aware of it. A collaborative approach with a pancreas specialist may be the most productive route to manage these patients.
AUTHORSHIP
Guarantor of the article: Sara Massironi.
AUTHOR CONTRIBUTIONS
Sara Massironi: Conceptualization (lead); data curation (equal); investigation (equal); methodology (equal); writing – original draft (equal); writing – review and editing (lead). Ilaria Fanetti: Data curation (equal); investigation (equal); methodology (equal); writing – original draft (equal); writing – review and editing (equal). Chiara Viganò: Data curation (equal); investigation (equal); writing – original draft (equal); writing – review and editing (equal). Lorena Pirola: Data curation (equal); methodology (equal); writing – original draft (equal). Maria Fichera: Data curation (equal); methodology (equal); writing – original draft (equal). Laura Cristoferi: Data curation (equal); methodology (equal); writing – original draft (equal). Gabriele Capurso: Data curation (equal); methodology (equal); writing – original draft (equal); writing – review and editing (equal). Pietro Invernizzi: Writing – review and editing (equal). Silvio Danese: Conceptualization (lead); methodology (equal); supervision (equal); writing – review and editing (equal).
Supporting information
Appendix S1
ACKNOWLEDGEMENTS
Pietro Invernizzi and Sara Massironi are members of the European Reference Network on Hepatological Diseases (ERN RARE LIVER), and they thank AMAF Monza ONLUS and AIRCS for the unrestricted research funding.
Declaration of personal interests: Silvio Danese reports consultancy fees from AbbVie, Alimentiv, Allergan, Amgen, AstraZeneca, Athos Therapeutics, Biogen, Boehringer Ingelheim, Celgene, Celltrion, Eli Lilly, Enthera, Ferring Pharmaceuticals Inc., Gilead, Hospira, Inotrem, Janssen, Johnson & Johnson, MSD, Mundipharma, Mylan, Pfizer, Roche, Sandoz, Sublimity Therapeutics, Takeda, TiGenix, UCB Inc., and Vifor Silvio Danese reports lecture fees from Abbvie, Amgen, Dr Falk Pharma, Ferring Pharmaceuticals Inc., Gilead, Janssen, Mylan, Pfizer, Takeda.
No potential competing interest was reported by the other authors.
Massironi S, Fanetti I, Viganò C, Pirola L, Fichera M, Cristoferi L, et al. Systematic review—pancreatic involvement in inflammatory bowel disease. Aliment Pharmacol Ther. 2022;55:1478–1491. 10.1111/apt.16949
The Handling Editor for this article was Dr Mike Burkitt, and this uncommissioned review was accepted for publication after full peer‐review.
REFERENCES
- 1. Vavricka SR, Schoepfer A, Scharl M, Lakatos PL, Navarini A, Rogler G. Extraintestinal manifestations of inflammatory bowel disease. Inflamm Bowel Dis. 2015;21(8):1982–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fousekis FS, Theopistos VI, Katsanos KH, Christodoulou DK. Pancreatic involvement in inflammatory bowel disease: a review. J Clin Med Res. 2018;10(10):743–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ramos LR, Sachar DB, DiMaio CJ, Colombel JF, Torres J. Inflammatory bowel disease and pancreatitis: a review. J Crohns Colitis. 2016;10(1):95–104. [DOI] [PubMed] [Google Scholar]
- 4. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wells GASB, O'Connell D. The Newcastle‐Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. Ottawa: Ottawa Hospital Research Institute; 2014. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Google Scholar]
- 6. Spanier BW, Dijkgraaf MG, Bruno MJ. Epidemiology, aetiology and outcome of acute and chronic pancreatitis: an update. Best Pract Res Clin Gastroenterol. 2008;22(1):45–63. [DOI] [PubMed] [Google Scholar]
- 7. Mathews SC, Izmailyan S, Brito FA, Yamal JM, Mikhail O, Revere FL. Prevalence and financial burden of digestive diseases in a commercially insured population. Clin Gastroenterol Hepatol. 2021;S1542‐3565(21):00711‐4. [DOI] [PubMed] [Google Scholar]
- 8. Chen YT, Su JS, Tseng CW, Chen CC, Lin CL, Kao CH. Inflammatory bowel disease on the risk of acute pancreatitis: a population‐based cohort study. J Gastroenterol Hepatol. 2016;31(4):782–7. [DOI] [PubMed] [Google Scholar]
- 9. Bermejo F, Lopez‐Sanroman A, Taxonera C, Gisbert JP, Pérez‐Calle JL, Vera I, et al. Acute pancreatitis in inflammatory bowel disease, with special reference to azathioprine‐induced pancreatitis. Aliment Pharmacol Ther. 2008;28(5):623–8. [DOI] [PubMed] [Google Scholar]
- 10. Weber P, Seibold F, Jenss H. Acute pancreatitis in Crohn's disease. J Clin Gastroenterol. 1993;17(4):286–91. [DOI] [PubMed] [Google Scholar]
- 11. Rasmussen HH, Fonager K, Sørensen HT, Pedersen L, Dahlerup JF, Steffensen FH. Risk of acute pancreatitis in patients with chronic inflammatory bowel disease. A Danish 16‐year nationwide follow‐up study. Scand J Gastroenterol. 1999;34(2):199–201. [DOI] [PubMed] [Google Scholar]
- 12. G G, de Paredes A, et al. Idiopathic acute pancreatitis in patients with inflammatory bowel disease: a multicenter cohort study. Pancreatology. 2020;20:331–7. [DOI] [PubMed] [Google Scholar]
- 13. Broide E, Dotan I, Weiss B, Wilschanski M, Yerushalmi B, Klar A, et al. Idiopathic pancreatitis preceding the diagnosis of inflammatory bowel disease is more frequent in pediatric patients. J Pediatr Gastroenterol Nutr. 2011;52(6):714–7. [DOI] [PubMed] [Google Scholar]
- 14. Pedersen JE, Ängquist LH, Jensen CB, Kjærgaard JS, Jess T, Allin KH. Risk of pancreatitis in patients with inflammatory bowel disease ‐ a meta‐analysis. Dan Med J. 2020;67(3):A08190427. [PubMed] [Google Scholar]
- 15. Tél B, Stubnya B, Gede N, Varjú P, Kiss Z, Márta K, et al. Inflammatory bowel diseases elevate the risk of developing acute pancreatitis: a meta‐analysis. Pancreas. 2020;49(9):1174–81. [DOI] [PubMed] [Google Scholar]
- 16. Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis‐‐2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102–11. [DOI] [PubMed] [Google Scholar]
- 17. Zhang FM, Xu CF, Shan GD, Chen HT, Xu GQ. Is gallstone disease associated with inflammatory bowel diseases? A meta‐analysis. J Dig Dis. 2015;16(11):634–41. [DOI] [PubMed] [Google Scholar]
- 18. Parente F, Pastore L, Bargiggia S, Cucino C, Greco S, Molteni M, et al. Incidence and risk factors for gallstones in patients with inflammatory bowel disease: a large case‐control study. Hepatology. 2007;45(5):1267–74. [DOI] [PubMed] [Google Scholar]
- 19. Fagagnini S, Heinrich H, Rossel JB, Biedermann L, Frei P, Zeitz J, et al. Risk factors for gallstones and kidney stones in a cohort of patients with inflammatory bowel diseases. PLoS One. 2017;12(10):e0185193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maurer P, Haag K, Roth M, Kuder C, Schölmerich J. No evidence for abnormal gallbladder emptying in Crohn's disease. Hepatogastroenterology. 1996;43(10):807–12. [PubMed] [Google Scholar]
- 21. Pitt HA, King W III, Mann LL, Roslyn JJ, Berquist WE, Ament ME, et al. Increased risk of cholelithiasis with prolonged total parenteral nutrition. Am J Surg. 1983;145(1):106–12. [DOI] [PubMed] [Google Scholar]
- 22. Antonini F, Pezzilli R, Angelelli L, Macarri G. Pancreatic disorders in inflammatory bowel disease. World J Gastrointest Pathophysiol. 2016;7(3):276–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lankisch PG, Droge M, Gottesleben F. Drug induced acute pancreatitis: incidence and severity. Gut. 1995;37(4):565–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Trivedi CD, Pitchumoni CS. Drug‐induced pancreatitis: an update. J Clin Gastroenterol. 2005;39(8):709–16. [DOI] [PubMed] [Google Scholar]
- 25. Weersma RK, Peters FTM, Oostenbrug LE, van den Berg AP, van Haastert M, Ploeg RJ, et al. Increased incidence of azathioprine‐induced pancreatitis in Crohn's disease compared with other diseases. Aliment Pharmacol Ther. 2004;20(8):843–50. [DOI] [PubMed] [Google Scholar]
- 26. Floyd A, Pedersen L, Nielsen GL, Thorlacius‐Ussing O, Sorensen HT. Risk of acute pancreatitis in users of azathioprine: a population‐based case‐control study. Am J Gastroenterol. 2003;98(6):1305–8. [DOI] [PubMed] [Google Scholar]
- 27. Peixoto A et al. Azathioprine‐induced acute pancreatitis in inflammatory bowel disease : natural history and severity spectrum. Acta Gastroenterol Belg. 2017;80(1):87–8. [PubMed] [Google Scholar]
- 28. Teich N, Mohl W, Bokemeyer B, Bündgens B, Büning J, Miehlke S, et al. Azathioprine‐induced acute pancreatitis in patients with inflammatory bowel diseases—a prospective study on incidence and severity. J Crohns Colitis. 2016;10(1):61–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sturdevant RA et al. Azathioprine‐related pancreatitis in patients with Crohn's disease. Gastroenterology. 1979;77(4 Pt 2):883–6. [PubMed] [Google Scholar]
- 30. Present DH, Korelitz BI, Wisch N, Glass JL, Sachar DB, Pasternack BS. Treatment of Crohn's disease with 6‐mercaptopurine. A long‐term, randomized, double‐blind study. N Engl J Med. 1980;302(18):981–7. [DOI] [PubMed] [Google Scholar]
- 31. Nogueira JR, Freedman MA. Acute pancreatitis as a complication of Imuran therapy in regional enteritis. Gastroenterology. 1972;62(5):1040–1. [PubMed] [Google Scholar]
- 32. Timmer A et al. Azathioprine and 6‐mercaptopurine for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2016;5:Cd000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ledder O, Lemberg DA, Day AS. Thiopurine‐induced pancreatitis in inflammatory bowel diseases. Expert Rev Gastroenterol Hepatol. 2015;9(4):399–403. [DOI] [PubMed] [Google Scholar]
- 34. Eskazan T, Bozcan S, Atay K, Yildirim S, Demir N, Celik S, et al. Frequency, predisposing factors, and clinical outcome of azathioprine‐induced pancreatitis among patients with inflammatory bowel disease: results from a tertiary referral center. Pancreas. 2021;50(9):1274–80. [DOI] [PubMed] [Google Scholar]
- 35. Wilson A, Jansen LE, Rose RV, Gregor JC, Ponich T, Chande N, et al. HLA‐DQA1‐HLA‐DRB1 polymorphism is a major predictor of azathioprine‐induced pancreatitis in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2018;47(5):615–20. [DOI] [PubMed] [Google Scholar]
- 36. Heap GA et al. HLA‐DQA1‐HLA‐DRB1 variants confer susceptibility to pancreatitis induced by thiopurine immunosuppressants. Nat Genet. 2014;46(10):1131–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu YP, Wu HY, Yang X, Xu HQ, Li YC, Shi DC, et al. Association between thiopurine S‐methyltransferase polymorphisms and thiopurine‐induced adverse drug reactions in patients with inflammatory bowel disease: a meta‐analysis. PLoS One. 2015;10(3):e0121745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wilson A, Wang Q, Choi YH, Ponich T, Gregor JC, Chande N, et al. Pretreatment HLADQA1‐HLADRB1 testing for the prevention of azathioprine‐induced pancreatitis in inflammatory bowel disease: a prospective cohort study. Clin Transl Gastroenterol. 2021;12(4):e00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Teich N, Bokemeyer B, Mohl W, Walldorf J, Bruns T, Schmidt C, et al. Blood group B is associated with azathioprine‐induced acute pancreatitis in patients with IBD. Gut. 2017;66(8):1531–2. [DOI] [PubMed] [Google Scholar]
- 40. Block MB, Genant HK, Kirsner JB. Pancreatitis as an adverse reaction to salicylazosulfapyridine. N Engl J Med. 1970;282(7):380–2. [DOI] [PubMed] [Google Scholar]
- 41. Meczker A, Miko A, Hegyi P. 5‐ASA induces mild acute pancreatitis. Case report and review of the literature. J Gastrointestin Liver Dis. 2018;27(2):189–94. [DOI] [PubMed] [Google Scholar]
- 42. Munk EM, Pedersen L, Floyd A, Norgard B, Rasmussen HH, Sorensen HT. Inflammatory bowel diseases, 5‐aminosalicylic acid and sulfasalazine treatment and risk of acute pancreatitis: a population‐based case‐control study. Am J Gastroenterol. 2004;99(5):884–8. [DOI] [PubMed] [Google Scholar]
- 43. Ransford RA, Langman MJ. Sulphasalazine and mesalazine: serious adverse reactions re‐evaluated on the basis of suspected adverse reaction reports to the committee on safety of medicines. Gut. 2002;51(4):536–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Garau P, Orenstein SR, Neigut DA, Kocoshis SA. Pancreatitis associated with olsalazine and sulfasalazine in children with ulcerative colitis. J Pediatr Gastroenterol Nutr. 1994;18(4):481–5. [DOI] [PubMed] [Google Scholar]
- 45. Isaacs KL, Murphy D. Pancreatitis after rectal administration of 5‐aminosalicylic acid. J Clin Gastroenterol. 1990;12(2):198–9. [DOI] [PubMed] [Google Scholar]
- 46. Russo L, Schneider G, Gardiner MH, Lanes S, Streck P, Rosen S. Role of pharmacoepidemiology studies in addressing pharmacovigilance questions: a case example of pancreatitis risk among ulcerative colitis patients using mesalazine. Eur J Clin Pharmacol. 2014;70(6):709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fernandez J et al. Acute pancreatitis after long‐term 5‐aminosalicylic acid therapy. Am J Gastroenterol. 1997;92(12):2302–3. [PubMed] [Google Scholar]
- 48. Faintuch J, Mott CB, Machado MC. Pancreatitis and pancreatic necrosis during sulfasalazine therapy. Int Surg. 1985;70(3):271–2. [PubMed] [Google Scholar]
- 49. Pitchumoni CS, Rubin A, Das K. Pancreatitis in inflammatory bowel diseases. J Clin Gastroenterol. 2010;44(4):246–53. [DOI] [PubMed] [Google Scholar]
- 50. Werlang, M.E. , Lewis M.D., and Bartel M.J., Tumor necrosis factor alpha inhibitor‐induced acute pancreatitis, In ACG Case Rep J. 2017: United States p e103. [DOI] [PMC free article] [PubMed]
- 51. Picardo S, So K, Venugopal K, Chin M. Vedolizumab‐induced acute pancreatitis: the first reported clinical case. BMJ Case Rep. 2018; bcr2017222554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Li WD, Jia L, Ou Y, Jiang SM, Qiu JH, Huang YX et al. Infliximab: protective effect to intestinal barrier function of rat with acute necrosis pancreatitis at early stage. Pancreas. 2013;42(2):366–7. [DOI] [PubMed] [Google Scholar]
- 53. Huang YX, Li WD, Jia L, Qiu JH, Jiang SM, Ou Y, et al. Infliximab enhances the therapeutic effectiveness of octreotide on acute necrotizing pancreatitis in rat model. Pancreas. 2012;41(6):849–54. [DOI] [PubMed] [Google Scholar]
- 54. Norman JG, Fink GW, Messina J, Carter G, Franz MG. Timing of tumor necrosis factor antagonism is critical in determining outcome in murine lethal acute pancreatitis. Surgery. 1996;120(3):515–21. [DOI] [PubMed] [Google Scholar]
- 55. Thiéfin G, Morelet A, Heurgué A, Diebold MD, Eschard JP. Infliximab‐induced hepatitis: absence of cross‐toxicity with etanercept. Joint Bone Spine. 2008;75(6):737–9. [DOI] [PubMed] [Google Scholar]
- 56. Simons‐Linares CR, Elkhouly MA, Salazar MJ. Drug‐induced acute pancreatitis in adults: an update. Pancreas. 2019;48(10):1263–73. [DOI] [PubMed] [Google Scholar]
- 57. Koutroubakis IE, Oustamanolakis P, Malliaraki N, Karmiris K, Chalkiadakis I, Ganotakis E, et al. Effects of tumor necrosis factor alpha inhibition with infliximab on lipid levels and insulin resistance in patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2009;21(3):283–8. [DOI] [PubMed] [Google Scholar]
- 58. Spanakis E, Sidiropoulos P, Papadakis J, Ganotakis E, Katsikas G, Karvounaris S, et al. Modest but sustained increase of serum high density lipoprotein cholesterol levels in patients with inflammatory arthritides treated with infliximab. J Rheumatol. 2006;33(12):2440–6. [PubMed] [Google Scholar]
- 59. Youssef I et al. Metronidazole‐induced pancreatitis: is there Underrecognition? A case report and systematic review of the literature. Case Rep Gastrointest Med. 2019;2019:4840539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Barbulescu A, Oskarsson V, Lindblad M, Ljung R, Brooke HL. Oral metronidazole use and risk of acute pancreatitis: a population‐based case‐control study. Clin Epidemiol. 2018;10:1573–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sura ME, Heinrich KA, Suseno M. Metronidazole‐associated pancreatitis. Ann Pharmacother. 2000;34(10):1152–5. [DOI] [PubMed] [Google Scholar]
- 62. Caplan A, Fett N, Rosenbach M, Werth VP, Micheletti RG. Prevention and management of glucocorticoid‐induced side effects: a comprehensive review: gastrointestinal and endocrinologic side effects. J Am Acad Dermatol. 2017;76(1):11–6. [DOI] [PubMed] [Google Scholar]
- 63. Sadr‐Azodi O, Mattsson F, Bexlius TS, Lindblad M, Lagergren J, Ljung R. Association of oral glucocorticoid use with an increased risk of acute pancreatitis: a population‐based nested case‐control study. JAMA Intern Med. 2013;173(6):444–9. [DOI] [PubMed] [Google Scholar]
- 64. Dwivedi P, Kumar RR, Dhooria A, Adarsh MB, Malhotra S, Kakkar N, et al. Corticosteroid‐associated lupus pancreatitis: a case series and systematic review of the literature. Lupus. 2019;28(6):731–9. [DOI] [PubMed] [Google Scholar]
- 65. Chrousos GA et al. Side effects of glucocorticoid treatment. Experience of the optic neuritis treatment trial. JAMA. 1993;269(16):2110–2. [PubMed] [Google Scholar]
- 66. Wolfe D, Kanji S, Yazdi F, Barbeau P, Rice D, Beck A, et al. Drug induced pancreatitis: a systematic review of case reports to determine potential drug associations. PLoS One. 2020;15(4):e0231883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Meczker Á, Hanák L, Párniczky A, Szentesi A, Erőss B, Hegyi P, et al. Analysis of 1060 cases of drug‐induced acute pancreatitis. Gastroenterology. 2020;159(5):1958–1961.e8. [DOI] [PubMed] [Google Scholar]
- 68. Almarri, N.M. , Alobaidli A.J., Almarhabi A.A., Alshammari M.A., Acute pancreatitis as an initial presentation of Crohn's disease: a case report, in J Family Med Prim Care. 2019, Copyright: (c) 2019 Journal of Family Medicine and Primary Care.: India. p. 3752–3754, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Iida T, Wagatsuma K, Hirayama D, Yokoyama Y, Nakase H. The etiology of pancreatic manifestations in patients with inflammatory bowel disease. J Clin Med. 2019;8(7):916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ananthakrishnan AN, Cagan A, Gainer VS, Cheng SC, Cai T, Szolovits P, et al. Mortality and extraintestinal cancers in patients with primary sclerosing cholangitis and inflammatory bowel disease. J Crohns Colitis. 2014;8(9):956–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Legge DA, Hoffman HN 2nd, Carlson HC. Pancreatitis as a complication of regional enteritis of the duodenum. Gastroenterology. 1971;61(6):834–7. [PubMed] [Google Scholar]
- 72. Meltzer SJ, Korelitz BI. Pancreatitis and duodenopancreatic reflux in Crohn's disease. Case report and review of the literature. J Clin Gastroenterol. 1988;10(5):555–8. [DOI] [PubMed] [Google Scholar]
- 73. Gschwantler M, Kogelbauer G, Klose W, Bibus B, Tscholakoff D, Weiss W. The pancreas as a site of granulomatous inflammation in Crohn's disease. Gastroenterology. 1995;108(4):1246–9. [DOI] [PubMed] [Google Scholar]
- 74. Spiceland CM, Lodhia N. Endoscopy in inflammatory bowel disease: role in diagnosis, management, and treatment. World J Gastroenterol. 2018;24(35):4014–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Aktas H, Mensink P, Haringsma J, Kuipers E. Low incidence of hyperamylasemia after proximal double‐balloon enteroscopy: has the insertion technique improved? Endoscopy. 2009;41(8):670–3. [DOI] [PubMed] [Google Scholar]
- 76. Zepeda‐Gomez S et al. Risk of hyperamylasemia and acute pancreatitis after double‐balloon enteroscopy: a prospective study. Endoscopy. 2011;43(9):766–70. [DOI] [PubMed] [Google Scholar]
- 77. Feng N, Dai J, Lu H, Li XB, Gao YJ, Ge ZZ. Hyperamylasemia is associated with increased intestinal permeability in patients undergoing diagnostic oral double‐balloon enteroscopy. World J Gastroenterol. 2014;20(2):539–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Pata C, Akyüz U, Erzin Y, Mutlu N, Mercan A, Dirican A. Post‐procedure elevated amylase and lipase levels after double‐balloon enteroscopy: relations with the double‐balloon technique. Dig Dis Sci. 2010;55(7):1982–8. [DOI] [PubMed] [Google Scholar]
- 79. Domagk D, Mensink P, Aktas H, Lenz P, Meister T, Luegering A, et al. Single‐ vs. double‐balloon enteroscopy in small‐bowel diagnostics: a randomized multicenter trial. Endoscopy. 2011;43(6):472–6. [DOI] [PubMed] [Google Scholar]
- 80. Kim TJ, Kim ER, Chang DK, Kim YH, Hong SN. Comparison of the efficacy and safety of single‐ versus double‐balloon Enteroscopy performed by Endoscopist experts in single‐balloon Enteroscopy: a single‐center experience and meta‐analysis. Gut Liver. 2017;11(4):520–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lipka S, Rabbanifard R, Kumar A, Brady P. Single versus double balloon enteroscopy for small bowel diagnostics: a systematic review and meta‐analysis. J Clin Gastroenterol. 2015;49(3):177–84. [DOI] [PubMed] [Google Scholar]
- 82. Latorre R, López‐Albors O, Soria F, Morcillo E, Esteban P, Pérez‐Cuadrado‐Robles E, et al. Evidences supporting the vascular etiology of post‐double balloon enteroscopy pancreatitis: study in porcine model. World J Gastroenterol. 2017;23(34):6201–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Fricker ZP, Lichtenstein DR. Primary Sclerosing cholangitis: a concise review of diagnosis and management. Dig Dis Sci. 2019;64(3):632–42. [DOI] [PubMed] [Google Scholar]
- 84. Boonstra K, Beuers U, Ponsioen CY. Epidemiology of primary sclerosing cholangitis and primary biliary cirrhosis: a systematic review. J Hepatol. 2012;56(5):1181–8. [DOI] [PubMed] [Google Scholar]
- 85. Karlsen TH, Folseraas T, Thorburn D, Vesterhus M. Primary sclerosing cholangitis ‐ a comprehensive review. J Hepatol. 2017;67(6):1298–323. [DOI] [PubMed] [Google Scholar]
- 86. Ji SG et al. Genome‐wide association study of primary sclerosing cholangitis identifies new risk loci and quantifies the genetic relationship with inflammatory bowel disease. Nat Genet. 2017;49(2):269–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Goldin E, Libson E, Wengrower D, Antal S, Kovacs Z, Rachmilewitz D. Severe acute pancreatitis as the presenting symptom of primary sclerosing cholangitis: treatment by endoscopic insertion of a biliary stent. Int Surg. 1990;75(1):58–60. [PubMed] [Google Scholar]
- 88. Matsushita M, Nagasawa M, Sato Y, Souda KI, Kobayashi Y. Primary sclerosing cholangitis associated with limy bile and acute pancreatitis. Pancreatology. 2005;5(4–5):466–9. [DOI] [PubMed] [Google Scholar]
- 89. von Seth E, Arnelo U, Enochsson L, Bergquist A. Primary sclerosing cholangitis increases the risk for pancreatitis after endoscopic retrograde cholangiopancreatography. Liver Int. 2015;35(1):254–62. [DOI] [PubMed] [Google Scholar]
- 90. Shimosegawa T, Chari ST, Frulloni L, Kamisawa T, Kawa S, Mino‐Kenudson M, et al. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas. 2011;40(3):352–8. [DOI] [PubMed] [Google Scholar]
- 91. Majumder S, Takahashi N, Chari ST. Autoimmune Pancreatitis. Dig Dis Sci. 2017;62(7):1762–9. [DOI] [PubMed] [Google Scholar]
- 92. Tsen A, Alishahi Y, Rosenkranz L. Autoimmune pancreatitis and inflammatory bowel disease: an updated review. J Clin Gastroenterol. 2017;51(3):208–14. [DOI] [PubMed] [Google Scholar]
- 93. Sah, R.P. , Chari S.T., Pannala R., Sugumar A., Clain J.E., Levy M.J., Pearson R.K., Smyrk T.C., Petersen B.T., Topazian M.D., Takahashi N., Farnell M.B., Vege S.S., Differences in clinical profile and relapse rate of type 1 versus type 2 autoimmune pancreatitis. Gastroenterology, 2010. 139(1): p. 140–8; quiz e12‐3. [DOI] [PubMed] [Google Scholar]
- 94. Hart PA, Zen Y, Chari ST. Recent advances in autoimmune pancreatitis. Gastroenterology. 2015;149(1):39–51. [DOI] [PubMed] [Google Scholar]
- 95. Kanno A, Masamune A, Okazaki K, Kamisawa T, Kawa S, Nishimori I, et al. Nationwide epidemiological survey of autoimmune pancreatitis in Japan in 2011. Pancreas. 2015;44(4):535–9. [DOI] [PubMed] [Google Scholar]
- 96. Ueki T, Kawamoto K, Otsuka Y, Minoda R, Maruo T, Matsumura K, et al. Prevalence and clinicopathological features of autoimmune pancreatitis in Japanese patients with inflammatory bowel disease. Pancreas. 2015;44(3):434–40. [DOI] [PubMed] [Google Scholar]
- 97. Park SH, Kim D, Ye BD, Yang SK, Kim JH, Yang DH, et al. The characteristics of ulcerative colitis associated with autoimmune pancreatitis. J Clin Gastroenterol. 2013;47(6):520–5. [DOI] [PubMed] [Google Scholar]
- 98. Barthet M, Hastier P, Bernard JP, Bordes G, Frederick J, Allio S, et al. Chronic pancreatitis and inflammatory bowel disease: true or coincidental association? Am J Gastroenterol. 1999;94(8):2141–8. [DOI] [PubMed] [Google Scholar]
- 99. Ravi K, Chari ST, Vege SS, Sandborn WJ, Smyrk TC, Loftus EV Jr. Inflammatory bowel disease in the setting of autoimmune pancreatitis. Inflamm Bowel Dis. 2009;15(9):1326–30. [DOI] [PubMed] [Google Scholar]
- 100. Schneider A, Hirth M, Weiss C, Weidner P, Antoni C, Thomann A, et al. Prevalence of inflammatory bowel disease in alcoholic, non‐alcoholic and autoimmune pancreatitis. Z Gastroenterol. 2018;56(5):469–78. [DOI] [PubMed] [Google Scholar]
- 101. Kamisawa T, Chari ST, Giday SA, Kim MH, Chung JB, Lee KT, et al. Clinical profile of autoimmune pancreatitis and its histological subtypes: an international multicenter survey. Pancreas. 2011;40(6):809–14. [DOI] [PubMed] [Google Scholar]
- 102. Srinath AI, Gupta N, Husain SZ. Probing the Association of Pancreatitis in inflammatory bowel disease. Inflamm Bowel Dis. 2016;22(2):465–75. [DOI] [PubMed] [Google Scholar]
- 103. Lorenzo D, Maire F, Stefanescu C, Gornet JM, Seksik P, Serrero M, et al. Features of autoimmune pancreatitis associated with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2018;16(1):59–67. [DOI] [PubMed] [Google Scholar]
- 104. Sakai Y, Kobayashi M. Lymphocyte 'homing' and chronic inflammation. Pathol Int. 2015;65(7):344–54. [DOI] [PubMed] [Google Scholar]
- 105. Notohara K, Burgart LJ, Yadav D, Chari S, Smyrk TC. Idiopathic chronic pancreatitis with periductal lymphoplasmacytic infiltration: clinicopathologic features of 35 cases. Am J Surg Pathol. 2003;27(8):1119–27. [DOI] [PubMed] [Google Scholar]
- 106. Zamboni G, Luettges J, Capelli P, Frulloni L, Cavallini G, Pederzoli P, et al. Histopathological features of diagnostic and clinical relevance in autoimmune pancreatitis: a study on 53 resection specimens and 9 biopsy specimens. Virchows Arch. 2004;445(6):552–63. [DOI] [PubMed] [Google Scholar]
- 107. Song TJ, Kim JH, Kim MH, Jang JW, Park DH, Lee SS, et al. Comparison of clinical findings between histologically confirmed type 1 and type 2 autoimmune pancreatitis. J Gastroenterol Hepatol. 2012;27(4):700–8. [DOI] [PubMed] [Google Scholar]
- 108. Kloppel G et al. Autoimmune pancreatitis: the clinicopathological characteristics of the subtype with granulocytic epithelial lesions. J Gastroenterol. 2010;45(8):787–93. [DOI] [PubMed] [Google Scholar]
- 109. Topal F, Saritas Yuksel E, Ekinci N, Pekdiker M, Cakalagaoglu F, Alper E, et al. The prevalence of IgG4‐positive plasma cell infiltrates in inflammatory bowel disease patients without autoimmune pancreatitis. Turk J Gastroenterol. 2014;25(5):558–62. [DOI] [PubMed] [Google Scholar]
- 110. Sandanayake NS, Church NI, Chapman MH, Johnson GJ, Dhar DK, Amin Z, et al. Presentation and management of post‐treatment relapse in autoimmune pancreatitis/immunoglobulin G4‐associated cholangitis. Clin Gastroenterol Hepatol. 2009;7(10):1089–96. [DOI] [PubMed] [Google Scholar]
- 111. Chiabrando F, Lanzillotta M, Palumbo D, Pedica F, Caruso M, Capurso G, et al. Treating type 2 autoimmune pancreatitis with colchicine: a case series. Ann Intern Med. 2021;174(12):1775–6. [DOI] [PubMed] [Google Scholar]
- 112. Barthet M, Lesavre N, Desplats S, Panuel M, Gasmi M, Bernard JP, et al. Frequency and characteristics of pancreatitis in patients with inflammatory bowel disease. Pancreatology. 2006;6(5):464–71. [DOI] [PubMed] [Google Scholar]
- 113. Gupte A, Goede D, Tuite R, Forsmark CE. Chronic pancreatitis. BMJ. 2018;361:k2126. [DOI] [PubMed] [Google Scholar]
- 114. Axon AT, Ashton MG, Lintott DJ. Chronic pancreatitis and inflammatory bowel disease. Clin Radiol. 1979;30(2):179–82. [DOI] [PubMed] [Google Scholar]
- 115. Chen YL, Hsu CW, Cheng CC, Yiang GT, Lin CS, Lin CL, et al. Increased subsequent risk of inflammatory bowel disease association in patients with chronic pancreatitis: a nationwide population‐based cohort study. Curr Med Res Opin. 2017;33(6):1077–82. [DOI] [PubMed] [Google Scholar]
- 116. Singh VK, Haupt ME, Geller DE, Hall JA, Diez PMQ. Less common etiologies of exocrine pancreatic insufficiency. World J Gastroenterol. 2017;23(39):7059–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Maconi G, Dominici R, Molteni M, Ardizzone S, Bosani M, Ferrara E, et al. Prevalence of pancreatic insufficiency in inflammatory bowel diseases. Assessment by fecal elastase‐1. Dig Dis Sci. 2008;53(1):262–70. [DOI] [PubMed] [Google Scholar]
- 118. Piontek M, Hengels KJ, Strohmeyer G. Crohn's disease: what about the pancreas? J Clin Gastroenterol. 1990;12(5):491–3. [PubMed] [Google Scholar]
- 119. Hegnhoj J, Hansen CP, Rannem T, Sobirk H, Andersen LB, Andersen JR. Pancreatic function in Crohn's disease. Gut. 1990;31(9):1076–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Seibold F, Weber P, Jenss H, Wiedmann KH. Antibodies to a trypsin sensitive pancreatic antigen in chronic inflammatory bowel disease: specific markers for a subgroup of patients with Crohn's disease. Gut. 1991;32(10):1192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Seibold F, Mork H, Tanza S, Muller A, Holzhuter C, Weber P, et al. Pancreatic autoantibodies in Crohn's disease: a family study. Gut. 1997;40(4):481–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Hedstrom A et al. Pancreatic exocrine insufficiency and Crohn's disease. Minerva Gastroenterol Dietol. 2019;19:S52. [DOI] [PubMed] [Google Scholar]
- 123. Toda N, Akahane M, Kiryu S, Matsubara Y, Yamaji Y, Okamoto M, et al. Pancreas duct abnormalities in patients with ulcerative colitis: a magnetic resonance pancreatography study. Inflamm Bowel Dis. 2005;11(10):903–8. [DOI] [PubMed] [Google Scholar]
- 124. Capurso G, Traini M, Piciucchi M, Signoretti M, Arcidiacono PG. Exocrine pancreatic insufficiency: prevalence, diagnosis, and management. Clin Exp Gastroenterol. 2019;12:129–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Loser C, Mollgaard A, Folsch UR. Faecal elastase 1: a novel, highly sensitive, and specific tubeless pancreatic function test. Gut. 1996;39(4):580–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Partelli S, Frulloni L, Minniti C, Bassi C, Barugola G, D’Onofrio M, et al. Faecal elastase‐1 is an independent predictor of survival in advanced pancreatic cancer. Dig Liver Dis. 2012;44(11):945–51. [DOI] [PubMed] [Google Scholar]
- 127. Dominguez‐Munoz JE et al. Potential for screening for pancreatic exocrine insufficiency using the fecal Elastase‐1 test. Dig Dis Sci. 2017;62(5):1119–30. [DOI] [PubMed] [Google Scholar]
- 128. Keller J, Aghdassi AA, Lerch MM, Mayerle JV, Layer P. Tests of pancreatic exocrine function ‐ clinical significance in pancreatic and non‐pancreatic disorders. Best Pract Res Clin Gastroenterol. 2009;23(3):425–39. [DOI] [PubMed] [Google Scholar]
- 129. Jung YS, Han M, Park S, Kim WH, Cheon JH. Cancer risk in the early stages of inflammatory bowel disease in Korean patients: a Nationwide population‐based study. J Crohns Colitis. 2017;11(8):954–62. [DOI] [PubMed] [Google Scholar]
- 130. Yu J, Refsum E, Helsingen LM, Folseraas T, Ploner A, Wieszczy P, et al. Risk of hepato‐pancreato‐biliary cancer is increased by primary sclerosing cholangitis in patients with inflammatory bowel disease: a population‐based cohort study. United European Gastroenterol J. 2022;10:212–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Katz S, Bank S, Greenberg RE, Lendvai S, Lesser M, Napolitano B. Hyperamylasemia in inflammatory bowel disease. J Clin Gastroenterol. 1988;10(6):627–30. [DOI] [PubMed] [Google Scholar]
- 132. Bokemeyer B. Asymptomatic elevation of serum lipase and amylase in conjunction with Crohn's disease and ulcerative colitis. Z Gastroenterol. 2002;40(1):5–10. [DOI] [PubMed] [Google Scholar]
- 133. Seibold F, Scheurlen M, Müller A, Jenss H, Weber P. Impaired pancreatic function in patients with Crohn's disease with and without pancreatic autoantibodies. J Clin Gastroenterol. 1996;22(3):202–6. [DOI] [PubMed] [Google Scholar]
- 134. Heikius B, Niemelä S, Lehtola J, Karttunen TJ. Elevated pancreatic enzymes in inflammatory bowel disease are associated with extensive disease. Am J Gastroenterol. 1999;94(4):1062–9. [DOI] [PubMed] [Google Scholar]
- 135. Stöcker W, Otte M, Ulrich S, Normann D, Finkbeiner H, Stöcker K, et al. Autoimmunity to pancreatic juice in Crohn's disease. Results of an autoantibody screening in patients with chronic inflammatory bowel disease. Scand J Gastroenterol Suppl. 1987;139:41–52. [DOI] [PubMed] [Google Scholar]
- 136. Pavlidis P et al. Ileal inflammation may trigger the development of GP2‐specific pancreatic autoantibodies in patients with Crohn's disease. Clin Dev Immunol. 2012;2012:640835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Bogdanos DP, Rigopoulou EI, Smyk DS, Roggenbuck D, Reinhold D, Forbes A, et al. Diagnostic value, clinical utility and pathogenic significance of reactivity to the molecular targets of Crohn's disease specific‐pancreatic autoantibodies. Autoimmun Rev. 2011;11(2):143–8. [DOI] [PubMed] [Google Scholar]
- 138. Bogdanos DP, Roggenbuck D, Reinhold D, Wex T, Pavlidis P, von Arnim U, et al. Pancreatic‐specific autoantibodies to glycoprotein 2 mirror disease location and behaviour in younger patients with Crohn's disease. BMC Gastroenterol. 2012;12:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Smyk DS et al. Autoantibodies in autoimmune pancreatitis. Int J Rheumatol. 2012;2012:940831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Gross S, Bakker SF, van Bodegraven AA, van Hoogstraten IMW, Gelderman KA, Bouma G, et al. Increased IgA glycoprotein‐2 specific antibody titres in refractory celiac disease. J Gastrointestin Liver Dis. 2014;23(2):127–33. [DOI] [PubMed] [Google Scholar]
- 141. Tornai T, Tornai D, Sipeki N, Tornai I, Alsulaimani R, Fechner K, et al. Loss of tolerance to gut immunity protein, glycoprotein 2 (GP2) is associated with progressive disease course in primary sclerosing cholangitis. Sci Rep. 2018;8(1):399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Heikius B, Niemelä S, Lehtola J, Karttunen T, Lähde S. Hepatobiliary and coexisting pancreatic duct abnormalities in patients with inflammatory bowel disease. Scand J Gastroenterol. 1997;32(2):153–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


