Abstract
Exposure to World Trade Center (WTC) dust/fumes and traumas on 11 September 2001 has been reported as a risk factor for post-traumatic stress disorder (PTSD) and other mental/physical health symptoms in WTC-affected populations. Increased systemic inflammation and oxidative stress from the exposure and subsequent illnesses have been proposed as contributors to the underlying biological processes. Many blood-based biomarkers of systemic inflammation, including C-reactive protein (CRP), are useful for non-invasive diagnostic and monitoring of disease process, and also potential targets for therapeutic interventions. Twenty years after 9/11, however, the relationships between WTC exposure, chronic PTSD, and systemic inflammation are only beginning to be systematically investigated in the WTC-affected civilian population despite the fact that symptoms of PTSD and systemic inflammation are still common and persistent. This paper aims to address this knowledge gap, using enrollees of the WTC Environmental Health Center (EHC), a federally designated treatment and surveillance program for community members (WTC Survivors) exposed to the 9/11 terrorist attack. We conducted a mediation analysis to investigate the association between acute WTC dust cloud traumatic exposure (WDCTE) on 9/11, chronic PTSD symptoms, and levels of systemic inflammation. The data indicate that the chronic PTSD symptoms and some specific symptom clusters of PTSD significantly mediate the WDCTE on systemic inflammation, as reflected by the CRP levels. As both chronic PTSD and systemic inflammation are long-term risk factors for neurodegeneration and cognitive decline, further research on the implications of this finding is warranted.
Keywords: C-reactive protein, systemic inflammation, PTSD symptom cluster, partial mediation, PCL score, cognitive impairment, WTC dust cloud
1. Introduction
The collapse of the World Trade Center (WTC) towers on 11 September 2001 due to a terrorist attack resulted in an enormous environmental disaster and negatively impacted a large number of local community members, including local residents, local workers, students, and those passing by the area on 9/11. Many community members experienced acute physical and psychological exposures, such as inhalation of dust from the dust clouds that were created as the WTC towers collapsed, and fear for their lives as they ran from and were engulfed by those massive dust clouds. Other acute psychological exposures included witnessing destruction, death, dismemberment, and escaping from the collapsing towers. For easy exposition, we subsequently refer to the WTC dust cloud traumatic exposure on 11 September 2001 as WDCTE. Chronic exposures included those from re-suspended dust and fumes, and witnessing months of rescue and recovery efforts, and displacement from work and homes [1,2,3,4]. In short, acute or chronic 9/11-related exposures for the WTC Survivors, including children and pregnant women, can be quite substantial. In fact, even among the children in the WTC-affected community, 12 years after 9/11, we and others have identified increased serum dioxins and furans in those who experienced WTC dust at home [5,6,7,8,9,10,11,12,13,14]. Well-described adverse health effects in the WTC disaster-exposed survivor and first responder populations include persistent aerodigestive disorders [15,16,17,18,19,20,21,22,23,24,25], cancers [26,27,28,29,30,31,32], and a high rate of post-traumatic stress disorder (PTSD) [16,19,24,33,34,35,36,37,38,39].
The WTC Environmental Health Center (WTC EHC) was established to treat and monitor community members’ health conditions that resulted from exposure to the terrorist attack and its aftermath [40]. The WTC EHC is a CDC/NIOSH-designated Center of Excellence in the WTC Health Program (WTCHP). All of the participants in the WTC EHC are required to have at least one CDC/NIOSH-certified mental health or medical condition related to specific WTC exposures. Initial and monitoring evaluations include assessments of both physical and mental health symptoms, including PTSD Checklist (PCL) scores. Based on these data, we previously reported high rates and persistence of PTSD symptoms in the WTC EHC population [37]. The high rates may be in part due to the diversity of the population, including many with low income and education level, factors previously reported to be associated with the development of PTSD after traumatic exposures [41,42]. Indeed, compared to the general WTC Responders, who are predominantly white male professionals, the members of the WTC EHC are civilians untrained for disasters, with an equal gender distribution, representation of diverse age, races, and ethnicities, as well as a wide range of social economic statuses. In addition, the presence of co-morbid mental–physical symptoms, including depression, anxiety, lower respiratory symptoms, and cancers, have also been associated with probable PTSD, reinforcing the need to consider co-morbid presentations [16,39,43].
The environmental exposures from WTC dust and fumes, as well as from general air pollution have been linked to a wide range of adverse health effects [2,44], including increased risk of mental health disorders [45,46,47], respiratory and cardiovascular diseases [14,23,48,49,50,51,52,53], neurotoxicity and neuropathy [54,55,56], cancers [26,27,28,29,30,31,32], and cognitive decline [57,58]. The biological mechanisms contributing to this wide range of adverse health effects have been hypothesized to involve systemic inflammation resulting from oxidative stress [51,59,60]. Indeed, measures of the inflammatory markers in the blood, including C-reactive protein (CRP) [61,62,63,64,65,66], white blood cell (WBC) counts [67], and Interleukin 6 (IL-6) [68], were shown to be elevated in populations with high particulate air pollution exposure. Importantly, most of the community members who were caught in the WTC dust cloud on 9/11 also had a traumatic life-threatening experience, thus, we and others have reported that elevated levels of CRP were associated with symptoms of PTSD and depression among the members of the WTC EHC [34,69,70,71]. Moreover, some studies further provide evidence that PTSD may induce systemic inflammation [72,73,74,75]. The ongoing systemic inflammation (observed many years after 9/11) is unlikely to be triggered directly by the original WDCTE, given the known short half-life of CRP. Therefore, we set out to test the alternative hypothesis that the chronic PTSD symptoms actually can induce systemic inflammation, by assessing whether the chronic PTSD symptoms mediate the effects of the WDCTE on the elevated CRP. Furthermore, the heterogeneous symptoms of PTSD can generally be clustered into four categories reflecting diverse components (re-experiencing; avoidance; negative alterations in cognitions and mood; and alterations in arousal and reactivity) [34,76,77]. We previously established that re-experiencing is significantly associated with elevated CRP levels among the members of the WTC EHC [34]. To extend our previous findings, this current paper further examines the differences in the effects of the four PTSD symptom clusters on mediating the association of WDCTE on the CRP level. This relationship might have further implications for aging and other long term health issues at the WTC EHC. In particular, PTSD is a well-known risk factor of cognitive decline [57,58,78,79,80], and also found as a mediator of the association between WTC exposure and subjective concerns of cognitive decline among the WTC-exposed firefighters [81]. Cognitive decline beyond normal aging has become a significant health concern in the aging 9/11 cohorts. Importantly, twenty years after 9/11, more than 75% of members of the WTC EHC are now more than 55 years old. Thus, in the long run, it is of interest to better understand the relationship between WTC dust cloud exposure, systemic inflammation, chronic PTSD symptoms, and cognitive decline in the WTC EHC for the affected community members.
2. Methods
2.1. Study Subjects
The patients from the WTC EHC at Bellevue Hospital Center with information on the CRP measures were included in the current study. The WTC EHC is a federally designated treatment and monitoring program for WTC Survivors including local workers, local residents, students, and those passing by the area of WTC on 9/11. All of the enrollees of the WTC EHC are required to have CDC/NIOSH-certified psychiatric or medical conditions related to specific WTC exposures. The inclusion criteria for patients to enroll in the WTC EHC were previously reported [82], and include presence as a local worker, resident, student or passer-by in the disaster area on 9/11, or presence as a local worker, resident, student between 11 September 2001 and 31 July 2002. The criteria were codified by the WTCHP and can be found at https://www.cdc.gov/wtc/eligiblegroups.html#nycSurvivor. CRP measurements were conducted for the patients at the initial or monitoring visits between August 2007 and January 2018. We included all of the patients with CRP data at the WTC EHC Bellevue Hospital and complete exposure information, and physical and mental health records. We excluded the patients who did not have CRP measurements in that time period. All of the data were recorded in the Institutional Review Board of New York University Grossman School of Medicine’s approved research database (NCT00404898), and only data from the patients who signed informed consent forms were utilized for analysis. The exposure information includes WTC dust cloud and exposure category classification [83], and the physical and mental health data include persistent lower respiratory symptoms (LRS), and symptoms of PTSD, depression, and anxiety [15,16,17,19,20,33,34,37]. A total of 731 patients from the WTC EHC were included in our analysis.
2.2. WTC Exposures and Medical Assessment
The patients completed a comprehensive, interviewer-administered questionnaire upon enrollment in the WTC EHC, that included demographic information and characterizations of WTC-related exposure [22]. Individuals who reported having been in the blinding dust cloud caused by the collapse of WTC buildings on 11 September 2001 were classified as having WTC dust cloud traumatic exposure. The potential for WTC acute/chronic exposures was also characterized by four categories: local worker; local resident (resident); clean-up worker; and other. The patients who reported more than one pack-year history of tobacco use were defined as ever smokers. The body mass index (BMI) of the patients was calculated, using information gathered during the initial medical visit. The presence and severity of LRS, of cough, wheeze, chest tightness, and dyspnea at rest, were measured by standardized health questionnaires [22]. The patients with symptoms more than twice per week during the month preceding enrollment were considered as persistent LRS.
2.3. Markers of Systemic Inflammation
A wide-range CRP (wr-CRP) assay (Siemen’s Diagnostic Center, Tarrytown, NY, USA) was used to measure the CRP. The wr-CRP assay is a clinically used measurement, with a wide range of sensitivity and a lower limit of detection of 0.12 mg/L. The wr-CRP correlates significantly with the high sensitivity CRP (hs-CRP) measurements and quantitation of microinflammatory activity in individuals [84]. A value > 3 mg/L was considered to be “High” [85]. The white blood cell (WBC) counts (per 103 cells/mL) were also obtained for the patients from the lab studies, in the electronic medical records for the initial visit [86].
2.4. Mental Health Symptoms
PTSD symptom presence and severity were measured by the Post-traumatic Checklist-17 (PCL) [87]. Designation as positive for probable PTSD was defined as a PCL score ≥ 44 [19]. The questions from the PCL were also matched to the DSM-5 diagnostic criteria for characterization into four clusters, as previously described [34], reflecting symptoms of re-experiencing, avoidance, negative cognition/mood, and arousal. An average score for each patient was calculated for each cluster, ranging between 1–5.
The Hopkins Symptom Checklist (HSCL-25) was used for detecting depression and anxiety [88]. These scales provided scores of depression (HSCL-D) and anxiety (HSCL-A) severity, where a score ≥ 1.75 is considered to suggest probable depression or probable anxiety.
2.5. Statistical Methods and Mediation Analyses
The descriptive statistics were calculated for systemic inflammation biomarkers (e.g., CRP and WBC count), demographic characteristics, exposures, LRS, and mental health symptoms, in which the continuous variables were summarized using mean and standard deviation (SD) or median and interquartile range (IQR) depending on the normality of the data. The categorical variables were summarized using count and percentage. The difference between the independent groups was assessed by two-sample t-tests or Mann–Whitney tests for continuous variables and by Chi-squared test for categorical variables.
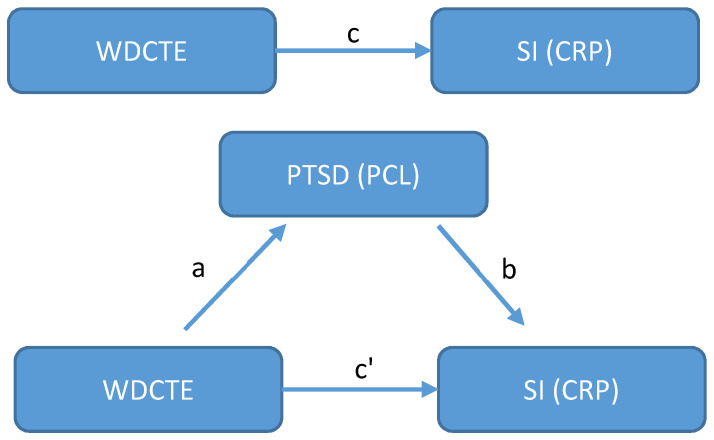
To investigate whether PTSD (measured by PCL score) mediates the effect of WDCTE on systemic inflammation (measured by CRP level), we first performed mediation analysis using the regression method [89,90,91]. Figure 1 presents the basic concept of the general approach. Let n be the total number of study participants for and denote W, C, P, and V as WDCTE, CRP level, PCL score, and some vector of other relevant covariates, respectively. Three multiple linear regression models are given as follows to assess the potential mediation effects:
| (1) |
| (2) |
| (3) |
where denotes the regression coefficients. Model (1) assesses whether the WDCTE affects the CRP level with adjustment for relevant covariates V. (i.e., path c in Figure 1) is called total effect of WDCTE on CRP level. A significant total effect () suggests a further step to assess whether the PCL score is a mediator of the association between the WDCTE and CRP level. Model (2) regards the effect of the WDCTE on the PCL score (i.e., , the path a in Figure 1). If is significant, the PCL score could be a potential mediator and Model (3) can be conducted. In Model (3), both the WDCTE and PCL scores are treated as predictors of the CRP level. To establish that the PCL score mediates the effect of the WDCTE on the CRP level, the effect of the PCL score on the CRP level after controlling for the WDCTE (i.e., , path b in Figure 1) needs to be significant. The strength of mediation further depends on the effect size of the WDCTE on the CRP level, after controlling for the PCL score (i.e., , the path c’ in Figure 1, also called direct effect) and its significance. Although the conventional regression method for mediation analysis, as proposed by Baron and Kenny in 1986, used zero and non-zero effects instead of requiring statistical significance to determine the associations, in the above models, due to the concern of a small sample size [89], we used statistical significance in our case, in order to obtain more conclusive results. The causal variable WDCTE used in the above models is a binary variable (Yes/No). The CRP was log-transformed due to the skewed distribution, and the PCL score was used to reflect PTSD symptoms. All of the above models were adjusted for a vector of covariates V, including demographic variables (i.e., gender, race/ethnicity, age on 9/11, education, income, BMI, and smoking history), LRS (i.e., cough, wheeze, chest tightness, and dyspnea at rest), and WTC exposure category. To further obtain the amount of mediation effect or indirect effect, we used the formula [89]. The 95% confidence interval (CI) and the related p-value of the mediation effect were calculated by a quasi-Bayesian Monte Carlo method, based on normal approximation [92]. We implemented this method using the “mediation” R package [93]. The same approaches were applied for the investigation of the mediation effect of each PTSD symptom cluster in the association between WDCTE and CRP levels. A value of p < 0.05 was used to test for two-sided statistical significance. All of the statistical analyses were conducted using R, version 4.1.3 (R Core Team, Vienna, Austria).
Figure 1.
Diagrams for mediation analysis. We consider the effect of WTC dust cloud traumatic exposure (WDCTE) on systemic inflammation (SI), i.e., CRP level, is potentially mediated by PTSD (PCL score). Path c and c′ are the total effect and direct effect of WDCTE on CRP level; path a is the effect of WDCTE on PTSD (PCL); path b is the effect of PTSD (PCL) on CRP level.
3. Results
3.1. Patient Characteristics
Table 1 presents the characteristics of the study group, which included 731 patients. Similar to our previous studies [22,34,94], half of the diverse population was female, 40% identified as Hispanic, the overall mean age on 9/11 was 43, about 66% had greater than high school education, and the majority were non-smokers and reported a low annual individual income (≤$30K). Half of the population reported being exposed to WDCTE, and the majority were local workers. The patients reported high proportions of persistent LRS and overall high positive rates of mental health symptoms, in which 43% had probable PTSD, 58% had depression, and 51% had anxiety. High CRP levels were also found, with levels > 3 mg/L in 37%. The median WBC count was reported as 6900 cells/mL with an IQR between 5700 cells/mL and 8300 cells/mL.
Table 1.
Patient characteristics (n = 731).
| Demographic Characteristics | |
|---|---|
| Gender, n (%) | |
| Female | 373 (51.0) |
| Male | 358 (49.0) |
| Age on 9/11 in year, mean (SD) a | 42.8 (11.5) |
| Race/ethnicity, n (%) | |
| Hispanic | 289 (39.5) |
| Non-Hispanic white | 221 (30.2) |
| Non-Hispanic Black | 143 (19.6) |
| Other | 78 (10.7) |
| Education, n (%) | |
| ≤High school | 252 (34.5) |
| >High school | 479 (65.5) |
| Income, n (%) | |
| ≤$30,000/year | 465 (63.6) |
| >$30,000/year | 266 (36.4) |
| BMI, mean (SD) | 28.54 (6.2) |
| Ever smoker, n (%) | |
| Yes | 230 (31.5) |
| No | 501 (68.5) |
| WTC Exposures | |
| WTC dust cloud traumatic exposure, n (%) | |
| Yes | 368 (50.3) |
| No | 363 (49.7) |
| WTC exposure category classification, n (%) | |
| Local Worker | 372 (50.9) |
| Resident | 130 (17.8) |
| Clean-up worker | 138 (18.9) |
| Other | 91 (12.5) |
| Lower respiratory symptoms within a month prior to enrollment | |
| Cough, n (%) | |
| Yes | 499 (68.2) |
| No | 232 (31.7) |
| Wheezing, n (%) | |
| Yes | 378 (51.7) |
| No | 353 (48.3) |
| Chest tightness, n (%) | |
| Yes | 463 (63.3) |
| No | 268 (36.7) |
| Dyspnea at rest, n (%) | |
| Yes | 296 (40.5) |
| No | 435 (59.5) |
| Positive mental health score | |
| PTSD, n (%) | |
| Yes (PCL ≥ 44) | 316 (43.2) |
| No (PCL < 44) | 415 (56.8) |
| Depression, n (%) | |
| Yes (HSCL-D ≥ 1.75) | 427 (58.4) |
| No (HSCL-D < 1.75) | 304 (41.6) |
| Anxiety, n (%) | |
| Yes (HSCL-A ≥ 1.75) | 370 (50.6) |
| No (HSCL-A < 1.75) | 361 (49.4) |
| Any of above mental health issues, n (%) | |
| Yes | 476 (65.1) |
| No | 255 (34.9) |
| CRP | |
| CRP in mg/L, median [IQR] b | 1.6 [0.4, 5.3] |
| CRP > 3 mg/L, n (%) | |
| Yes | 269 (36.8) |
| No | 462 (63.2) |
| WBC | |
| WBC count in 103 cells/mL, median [IQR] | 6.9 [5.7, 8.3] |
a SD, standard deviation; b IQR, inter-quartile range.
3.2. Characteristics Associated with PTSD Symptoms at Initial Visit
Demographic characteristics, WTC exposures, LRS symptoms, CRP, and WBC count were univariately compared between the PTSD (PCL ≥ 44) and non-PTSD (PCL < 44) groups (Table 2). Similar to what we found before, the community members who self-reported as Hispanic (p = 0.002), had ≤ high school education (p = 0.002), or had income ≤ $30,000/year (p = 0.001), were more likely to have probable PTSD. The presence of any LRS symptoms (cough: p = 0.011; wheeze: p = 0.002; chest tightness: p < 0.001; and dyspnea at rest: p < 0.001) was related to a positive score for PTSD. The patients who self-reported as cleanup workers (p = 0.04) or being caught in the WTC dust cloud (p = 0.006) were also more likely to have PTSD symptoms. We also found significant elevations of CRP levels in the PTSD group compared to the non-PTSD group (p = 0.004). Another inflammatory marker, WBC count, also showed increased levels in the PTSD group.
Table 2.
Univariate analyses for association of PTSD with each of the predictors (n = 731).
| PTSD | p-Value c | ||
|---|---|---|---|
| No (PCL < 44) n = 415 |
Yes (PCL ≥ 44) n = 316 |
||
| Demographics | |||
| Gender, n (%) | 0.432 | ||
| Female | 206 (49.6) | 167 (52.8) | |
| Male | 209 (50.4) | 149 (47.2) | |
| Age on 911 in year, mean (SD) a | 42.9 (12.6) | 42.6 (9.7) | 0.725 |
| Race/ethnicity, n (%) | 0.002 | ||
| Hispanic | 139 (33.5) | 150 (47.5) | |
| Non-Hispanic white | 139 (33.5) | 82 (25.9) | |
| Non-Hispanic Black | 91 (21.9) | 52 (16.5) | |
| Other | 46 (11.1) | 32 (10.1) | |
| Education, n (%) | 0.002 | ||
| ≤High school | 123 (29.6) | 129 (40.8) | |
| >High school | 292 (70.4) | 187 (59.2) | |
| Income, n (%) | 0.001 | ||
| ≤$30,000/year | 242 (58.3) | 223 (70.6) | |
| >$30,000/year | 173 (41.7) | 93 (29.4) | |
| BMI, mean (SD) | 28.35 (6.3) | 28.79 (6.1) | 0.344 |
| Ever smoker, n (%) | 0.638 | ||
| Yes | 134 (32.3) | 96 (30.4) | |
| No | 281 (67.7) | 220 (69.6) | |
| Exposures | |||
| WTC dust cloud traumatic exposure, n (%) | 0.006 | ||
| Yes | 190 (45.8) | 178 (56.3) | |
| No | 225 (54.2) | 138 (43.7) | |
| Exposure classification, n (%) | 0.042 | ||
| Worker | 212 (51.1) | 160 (50.6) | |
| Resident | 82 (19.8) | 48 (15.2) | |
| Clean-up worker | 65 (15.0) | 73 (23.1) | |
| Other | 56 (13.5) | 35 (11.1) | |
| Lower respiratory symptoms | |||
| Cough, n (%) | 0.011 | ||
| Yes | 267 (64.3) | 232 (73.4) | |
| No | 148 (35.7) | 84 (26.6) | |
| Wheeze, n (%) | 0.002 | ||
| Yes | 193 (46.5) | 185 (58.5) | |
| No | 222 (53.5) | 131 (41.5) | |
| Chest tightness, n (%) | <0.001 | ||
| Yes | 232 (55.9) | 231 (73.1) | |
| No | 183 (44.1) | 85 (26.9) | |
| Dyspnea at rest, n (%) | <0.001 | ||
| Yes | 144 (34.7) | 152 (48.1) | |
| No | 271 (65.3) | 164 (51.9) | |
| CRP | |||
| CRP in mg/L, median [IQR] b | 1.3 [0.3, 4.5] | 1.9 [0.5, 5.8] | 0.004 |
| CRP > 3 mg/L, n (%) | 0.041 | ||
| Yes | 139 (33.5) | 130 (41.1) | |
| No | 276 (66.5) | 186 (58.9) | |
| WBC | |||
| WBC in 103 cells/mL, median [IQR] | 6.7 [5.6, 8.2] | 6.9 [5.8, 8.4] | 0.214 |
a SD, standard deviation; b IQR, inter-quartile range; c p values were computed based on chi-squared tests for categorical predictors, and two-sample t-tests or Mann–Whitney tests for continuous predictors; bold numbers under p-value column indicate significant at 0.05 level.
3.3. PTSD Symptoms (PCL Score) Mediates the Association between WDCTE and Systemic Inflammation (CRP Level)
In this section, we report the mediation effect of the PTSD (PCL score) on the effect of WDCTE on systemic inflammation, as reflected in the CRP level. The regression-based method for mediation analysis has been described in the Statistical Methods and Mediation Analyses section, with Figure 1. Table 3 presents the results of the three regression models used for establishing the mediations and the summary of the mediation effect. Model (1) reflects the total effect of the WDCTE on the CRP level () after adjusting for demographic variables and LRS. The WDCTE is significantly related to elevated CRP levels ( = 0.27, p = 0.02). In addition, the WDCTE is also significantly associated to the PCL score ( = 5.06, p < 0.01), with adjustment of the other factors according to Model (2). The significances of and imply that PTSD, as measured by the PCL score, may mediate the effect of the WDCTE on the CRP level. In Model (3), the PCL score was still significantly associated with CRP level, while controlling for the WDCTE and adjusting for other factors ( = 0.01, p = 0.01), but the WDCTE is no longer significantly associated with the CRP level while controlling for the PCL scores ( = 0.22, p = 0.06). The effect of the WDCTE on the CRP levels decreased from = 0.27 of Model (1) to = 0.22 of Model (3), which suggests that the total effect of the WDCTE on the CRP level was strongly mediated by the PCL score. The mediation effect or indirect effect of the PCL score was computed as = 0.27 − 0.22 = 0.05, with the 95% CI ranging from 0.01 to 0.09 and p = 0.01, which yields about 17% of total effect of the WDCTE on the CRP level being mediated by PCL score.
Table 3.
Multiple linear regression models for assessing mediation effect of PTSD (PCL score) in the association of WDCTE and CRP level (log(CRP)), adjusted for demographic characteristics and lower respiratory symptoms (n = 731).
| Model (1) (Path c) |
Model (2) (Path a) |
Model (3) (Path b and c’) |
||||
|---|---|---|---|---|---|---|
| Log (CRP) | PTSD (PCL Score) | Log (CRP) | ||||
| β | p-Value | β | p-Value | β | p-Value | |
| (Intercept) | −10.95 | <0.01 | 43.15 | <0.01 | −11.35 | <0.01 |
| PCL score | 0.01 | 0.01 | ||||
| WDCTE | 0.27 | 0.02 | 5.06 | <0.01 | 0.22 | 0.06 |
| Adjusted factors: | ||||||
| Sex—Male | −0.20 | 0.07 | −1.78 | 0.12 | −0.18 | 0.10 |
| Age on 9/11 | 0.01 | 0.21 | 0.001 | 0.99 | 0.01 | 0.21 |
| Race/ethnicity (ref = Hispanic) | ||||||
| NH-White | −0.14 | 0.39 | −4.04 | 0.02 | −0.10 | 0.53 |
| NH-Black | −0.003 | 0.98 | −5.77 | <0.01 | 0.05 | 0.77 |
| Other | 0.09 | 0.68 | −3.98 | 0.07 | 0.12 | 0.55 |
| Education > high school | −0.02 | 0.87 | −1.31 | 0.35 | −0.01 | 0.95 |
| Income > $30,000/year | −0.04 | 0.74 | −4.56 | <0.01 | 0.002 | 0.99 |
| log(BMI) | 3.17 | <0.01 | −0.48 | 0.87 | 3.17 | <0.01 |
| Ever smoker (>1 p-y) | 0.44 | <0.01 | −1.16 | 0.36 | 0.45 | <0.01 |
| Exposure category (ref = Clean-up Worker) | ||||||
| Resident | 0.08 | 0.71 | −4.34 | 0.06 | 0.08 | 0.68 |
| Local Worker | 0.06 | 0.75 | −1.89 | 0.33 | 0.12 | 0.58 |
| Other | 0.12 | 0.59 | −3.92 | 0.08 | 0.15 | 0.48 |
| Lower respiratory symptoms | ||||||
| Cough | 0.33 | 0.01 | 1.38 | 0.29 | 0.32 | 0.01 |
| Wheezing | 0.12 | 0.33 | 1.35 | 0.29 | 0.11 | 0.38 |
| Chest tightness | −0.08 | 0.49 | 4.03 | <0.01 | −0.12 | 0.32 |
| Dyspnea at rest | 0.15 | 0.21 | 3.43 | 0.01 | 0.12 | 0.32 |
| Mediation Effect of PCL score | ||||||
| β | 95%CI Lower * | 95%CI Upper * | p-value * | |||
| Total effect (c) | 0.27 | 0.03 | 0.49 | 0.03 | ||
| Direct effect c’) | 0.22 | −0.02 | 0.45 | 0.07 | ||
| Mediation effect (c-c’ = a * b) | 0.05 | 0.01 | 0.09 | 0.01 | ||
| Proportion Mediated ((c-c’)/c) | 17.0% | 2.1% | 85% | 0.03 | ||
* The 95% confidence interval (CI) and p-value of mediation effect of PCL score were estimated based on a quasi-Bayesian approximation; path a, b, c, and c’ refer to the paths indicated in Figure 1; bold numbers under P columns indicate significant at 0.05 level.
3.4. Mediation Effects of PTSD Symptom Clusters in the Association between WDCTE and Systemic Inflammation
Table 4 shows the results of the mediation effects of each PTSD symptom cluster (sub-PCL score) in the association between the WDCTE and systemic inflammation as measured by the CRP level. The impact of the WDCTE on the CRP level was significantly mediated by re-experiencing (β = 0.05, p < 0.01 and 17.5% of the total effect of WDCTE on CRP level were mediated), avoidance (β = 0.03, p = 0.02), and negative cognition/mood symptoms (β = 0.04, p = 0.02).
Table 4.
Mediation effect of each PTSD symptom cluster (sub-PCL score) in the association of WDCTE and CRP level (log(CRP)), adjusted for demographic characteristics and lower respiratory symptoms (n = 731).
| Re-Experiencing | Avoidance | Negative Cognitions/Mood | Arousal | |||||
|---|---|---|---|---|---|---|---|---|
| β | p-Value | β | p-Value | β | p-Value | β | p-Value | |
| Total effect | 0.27 | 0.03 | 0.27 | 0.03 | 0.27 | 0.03 | 0.27 | 0.03 |
| Direct effect | 0.22 | 0.07 | 0.24 | 0.05 | 0.23 | 0.06 | 0.24 | 0.05 |
| Mediation effect | 0.05 | <0.01 | 0.03 | 0.02 | 0.04 | 0.02 | 0.03 | 0.12 |
| Proportion Mediated | 17.5% | 0.03 | 10.1% | 0.05 | 13.1% | 0.05 | 9.0% | 0.14 |
p-values were estimated based on a quasi-Bayesian approximation; bold numbers under p-value columns indicate significant at 0.05 level.
4. Discussion
The current study assessed the relationships between chronic PTSD symptoms and specific PTSD symptom clusters and ongoing systemic inflammation, as measured by the CRP level in the context of the traumatic WTC dust cloud exposure. The WTC Survivors studied were enrolled in the WTC Environmental Health Center (WTC EHC) and many of the individuals had acute traumatic exposures to the initial dust clouds created as the WTC buildings collapsed (WTC dust cloud) on 11 September 2001, as well as having witnessed death and dismemberment, and often experienced their own fear of death as they escaped collapsing buildings or were engulfed in blinding dust clouds. The adverse health effects included chronic symptoms of PTSD, depression, anxiety, upper and lower respiratory symptoms, cancers and cognitive decline [15,16,17,18,19,20,22,26,27,28,29,30,31,33,34,35,36,37,40,57,58].
Our findings enhance and expand upon our previous studies, suggesting an important association between the traumatic WTC dust cloud exposure, systemic inflammation, and post-traumatic stress pathology and PTSD symptom clusters [34]. In our study cohort, persistent symptoms of PTSD and depression have emerged as two highly prevalent and comorbid post-traumatic stress responses to the WTC disaster exposures. The existing literature on air pollution studies indicates that air pollution can lead to systemic inflammation and elevated CRP [61,62,63,64,65,66], and systemic inflammation has emerged in the literature as one of the plausible biological mechanisms in the pathogenesis of depression and PTSD [95,96]. Note that we have previously reported that most of the subjects with symptoms of PTSD experience chronic or persistent symptoms [37]. The possibility exists that the physiologic corollary to the re-experiencing cluster of PTSD symptoms is a mechanism that may actually trigger systemic inflammation responses, including CRP and acute inflammatory cytokines. Indeed, the elevated CRP levels have been reported in association with the chronic re-experiencing symptoms of PTSD after traumatic exposures [72,73,97], and with arousal symptoms [98]. Given the short half-life of CRP, the observed elevated CRP many years after 9/11 in these subjects with PTSD symptoms might be triggered by the re-experiencing symptoms of PTSD. We thus conducted further mediation analysis and found that chronic PTSD symptoms and PTSD symptom clusters were significant mediators of the traumatic WTC dust cloud exposure on the elevated levels of CRP.
Newly published studies in the WTC firefighter first-responder cohort suggest that PTSD completely mediates the subjective cognitive complaints in WTC-affected first responders [81]. Our data are consistent with this report and suggest that chronic PTSD symptoms similarly mediate the ongoing systemic inflammation, as measured in CRP. Thus, persistent PTSD symptoms are accompanied by chronic systemic inflammation, and treatment of the PTSD may reduce the inflammation. Chronic systemic inflammation and PTSD are known risk factors for neuroinflammation, neurodegeneration, and cognitive decline [58]. Thus, a better understanding of the roles of systemic inflammation and of how to reduce this inflammation would have a significant health impact on the cognitive issues for the patients in the WTC EHC, and generally for WTC Survivors. This gains increasing importance, since more than 75% of the WTC Survivors in the WTC EHC are now over 55 years old, and cognitive decline is thus an increasing health issue for this group.
The current study has multiple limitations. First, the study participants were selected based on the inclusion criteria stated in the Methods section which are, in turn, based on the available patient information from existing clinical databases. Additional exclusion criteria may be helpful for future studies, for example, including the presence of co-existing inflammatory disease before 9/11 and/or those who had traumatic experiences other than WTC exposure. Second, both the levels of the CRP and the symptoms of PTSD are cross-sectional data. Longitudinal data would allow more formal causal inference. We are currently planning longitudinal studies to further investigate and validate these preliminary findings on the relationship between the symptoms of PTSD and the levels of serum CRP. This study used patients with available CRP levels measured for evaluation purposes at the WTC EHC during a limited time frame. Third, we adjusted for co-morbid LRS and obesity, but future studies should consider randomly sampling patients and then obtain levels of systemic inflammation markers in order to reduce the impact of comorbidities. Respiratory conditions and obesity are not the only types of WTC-related comorbidities associated with inflammation; it is desirable to adjust for other possible WTC-related co-morbidities in future studies. Additionally, the serum CRP is known to lack specificity as a measure of systemic inflammation and may be upregulated by additional co-morbid conditions. Our previous study evaluated the presence of co-morbid LRS, reduced spirometry, and increased forced oscillation measurements. Our findings highlight the importance of understanding the interaction between the traumatic exposures, PTSD, and inflammation, and reinforce the need for future studies including those involving investigation of this hypothesis via random sampling of patients and by including measures of additional blood-based biomarkers, such as IL-6 and other cytokines [96,99,100].
5. Conclusions
The goal of this study was to understand the relationship between the WTC exposures, chronic PTSD symptoms with specific symptom clusters, and ongoing systemic inflammation in a cohort of community members with acute and traumatic WTC exposures. The identification of chronic PTSD symptom clusters as potential mediators of exposure on ongoing systemic inflammation biomarkers may have long-term implications for the health of those exposed to the WTC disaster, especially as the population ages. Specifically, the identification of PTSD clusters as mediators of inflammation suggest potential treatment targets to reduce inflammation. Moreover, this finding is salient relative to the risk of cognitive decline in the WTC-affected community members, as chronic systemic inflammation is a known risk factor of neuroinflammation, which, in turn, can be a risk factor for neurodegeneration and cognitive decline [58]. The current study is limited by the available cross-sectional CRP readings collected for the purpose of treatment; thus, well-designed longitudinal studies of a more comprehensive set of blood biomarkers, to further investigate the potential causal relationships, are warranted.
Acknowledgments
We graciously appreciate Michelle Hyde and Angeles Pai, for their tireless administration of the program. We thank all of the mental health team and medical staff of the WTC Environmental Health Center for their dedication to the treatment and support of our patients. We also thank members of the WTC Health Program Survivor Steering Committee for their invaluable advice and efforts on behalf of the local community. We thank HHC for hosting WTC EHC and administrative support. The authors also would like to thank three anonymous reviewers for their careful reading and constructive suggestions.
Author Contributions
Conceptualization, Y.S. and Y.Z.; methodology, Y.S. and Y.Z.; formal analysis, Y.Z. and Y.S.; data curation, Y.Z.; writing—original draft preparation, Y.Z. and Y.S.; writing—review and editing, Y.S., Y.Z., R.R. and J.R.; funding acquisition, Y.S. and J.R. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used in this paper are from the clinical databases at the WTC EHC Data Center. We only used de-identified and anonymized information. The datasets are not publically available, but the de-identified and anonymized information is potentially available upon reasonable request to the WTC EHC Data Center.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was partially funded by National Institute of Occupational Safety and Health (NIH/NIOSH), Centers of Disease Control and Prevention (CDC) 200-2017-93327, 200-2017-93427, U01 OH012486, National Institute of Environmental Health Sciences (NIEHS) Grant 5P30ES000260.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lioy P.J., Georgopoulos P. The anatomy of the exposures that occurred around the World Trade Center site: 9/11 and beyond. Ann. N.Y. Acad. Sci. 2006;1076:54–79. doi: 10.1196/annals.1371.002. [DOI] [PubMed] [Google Scholar]
- 2.Lippmann M., Cohen M.D., Chen L.C. Health effects of World Trade Center (WTC) Dust: An unprecedented disaster’s inadequate risk management. Crit. Rev. Toxicol. 2015;45:492–530. doi: 10.3109/10408444.2015.1044601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maslow C.B., Friedman S.M., Pillai P.S., Reibman J., Berger K.I., Goldring R., Stellman S.D., Farfel M. Chronic and acute exposures to the world trade center disaster and lower respiratory symptoms: Area residents and workers. Am. J. Public Health. 2012;102:1186–1194. doi: 10.2105/AJPH.2011.300561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reibman J., Levy-Carrick N., Miles T., Flynn K., Hughes C., Crane M., Lucchini R.G. Destruction of the World Trade Towers: Lessons Learned from an Environmental Health Disaster. Ann. Am. Thorac. Soc. 2016;13:577–583. doi: 10.1513/AnnalsATS.201509-572PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kahn L.G., Han X., Koshy T.T., Shao Y., Chu D.B., Kannan K., Trasande L. Adolescents exposed to the World Trade Center collapse have elevated serum dioxin and furan concentrations more than 12 years later. Environ. Int. 2018;111:268–278. doi: 10.1016/j.envint.2017.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolff M.S., Teitelbaum S.L., Lioy P.J., Santella R.M., Wang R.Y., Jones R.L., Caldwell K.L., Sjödin A., Turner W.E., Li W., et al. Exposures among pregnant women near the World Trade Center site on 11 September 2001. Environ. Health Perspect. 2005;113:739–748. doi: 10.1289/ehp.7694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trasande L., Koshy T.T., Gilbert J., Burdine L.K., Attina T.M., Ghassabian A., Honda M., Marmor M., Chu D.B., Han X., et al. Serum perfluoroalkyl substances in children exposed to the world trade center disaster. Environ. Res. 2017;154:212–221. doi: 10.1016/j.envres.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koshy T.T., Attina T.M., Ghassabian A., Gilbert J., Burdine L.K., Marmor M., Honda M., Chu D.B., Han X., Shao Y., et al. Serum perfluoroalkyl substances and cardiometabolic consequences in adolescents exposed to the World Trade Center disaster and a matched comparison group. Environ. Int. 2017;109:128–135. doi: 10.1016/j.envint.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spratlen M.J., Perera F.P., Sjodin A., Wang Y., Herbstman J.B., Trasande L. Understanding the Role of Persistent Organic Pollutants and Stress in the Association between Proximity to the World Trade Center Disaster and Birth Outcomes. Int. J. Environ. Res. Public Health. 2022;19:2008. doi: 10.3390/ijerph19042008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spratlen M.J., Perera F.P., Lederman S.A., Rauh V.A., Robinson M., Kannan K., Trasande L., Herbstman J. The association between prenatal exposure to perfluoroalkyl substances and childhood neurodevelopment. Environ. Pollut. 2020;263:114444. doi: 10.1016/j.envpol.2020.114444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spratlen M.J., Perera F.P., Lederman S.A., Robinson M., Kannan K., Trasande L., Herbstman J. Cord blood perfluoroalkyl substances in mothers exposed to the World Trade Center disaster during pregnancy. Environ. Pollut. 2019;246:482–490. doi: 10.1016/j.envpol.2018.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trye A., Berger K.I., Naidu M., Attina T.M., Gilbert J., Koshy T.T., Han X., Marmor M., Shao Y., Giusti R., et al. Respiratory health and lung function in children exposed to the World Trade Center disaster. J. Pediatr. 2018;201:134–140.e6. doi: 10.1016/j.jpeds.2018.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trasande L., Koshy T.T., Gilbert J., Burdine L.K., Marmor M., Han X., Shao Y., Chemtob C., Attina T.M., Urbina E.M. Cardiometabolic profiles of adolescents and young adults exposed to the World Trade Center Disaster. Environ. Res. 2018;160:107–114. doi: 10.1016/j.envres.2017.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trasande L., Fiorino E.K., Attina T., Berger K., Goldring R., Chemtob C., Levy-Carrick N., Shao Y., Liu M., Urbina E., et al. Associations of World Trade Center exposures with pulmonary and cardiometabolic outcomes among children seeking care for health concerns. Sci. Total Environ. 2013;444:320–326. doi: 10.1016/j.scitotenv.2012.11.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin S., Jones R., Reibman J., Bowers J., Fitzgerald E.F., Hwang S.A. Reported respiratory symptoms and adverse home conditions after 9/11 among residents living near the World Trade Center. J. Asthma Off. J. Assoc. Care Asthma. 2007;44:325–332. doi: 10.1080/02770900701344181. [DOI] [PubMed] [Google Scholar]
- 16.Brackbill R.M., Hadler J.L., DiGrande L., Ekenga C.C., Farfel M.R., Friedman S., Perlman S.E., Stellman S.D., Walker D.J., Wu D., et al. Asthma and posttraumatic stress symptoms 5 to 6 years following exposure to the World Trade Center terrorist attack. JAMA. 2009;302:502–516. doi: 10.1001/jama.2009.1121. [DOI] [PubMed] [Google Scholar]
- 17.Friedman S.M., Farfel M.R., Maslow C.B., Cone J.E., Brackbill R.M., Stellman S.D. Comorbid persistent lower respiratory symptoms and posttraumatic stress disorder 5–6 years post-9/11 in responders enrolled in the World Trade Center Health Registry. Am. J. Ind. Med. 2013;56:1251–1261. doi: 10.1002/ajim.22217. [DOI] [PubMed] [Google Scholar]
- 18.Reibman J., Lin S., Hwang S.-A.A., Gulati M., Bowers J.A., Rogers L., Berger K.I., Hoerning A., Gomez M., Fitzgerald E.F. The World Trade Center residents’ respiratory health study: New-onset respiratory symptoms and pulmonary function. Environ. Health Perspect. 2005;113:406–411. doi: 10.1289/ehp.7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farfel M., DiGrande L., Brackbill R., Prann A., Cone J., Friedman S., Walker D.J., Pezeshki G., Thomas P., Galea S., et al. An overview of 9/11 experiences and respiratory and mental health conditions among World Trade Center Health Registry enrollees. J. Urban Health. 2008;85:880–909. doi: 10.1007/s11524-008-9317-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin S., Reibman J., Bowers J.A., Hwang S.-A., Hoerning A., Gomez M.I., Fitzgerald E.F. Upper Respiratory Symptoms and Other Health Effects among Residents Living Near the World Trade Center Site after September 11, 2001. Am. J. Epidemiol. 2005;162:499–507. doi: 10.1093/aje/kwi233. [DOI] [PubMed] [Google Scholar]
- 21.Banauch G.I., Alleyne D., Sanchez R., Olender K., Cohen H.W., Weiden M., Kelly K.J., Prezant D.J. Persistent hyperreactivity and reactive airway dysfunction in firefighters at the World Trade Center. Am. J. Respir. Crit. Care Med. 2003;168:54–62. doi: 10.1164/rccm.200211-1329OC. [DOI] [PubMed] [Google Scholar]
- 22.Jordan H.T., Friedman S.M., Reibman J., Goldring R.M., Archie S.A.M., Ortega F., Alper H., Shao Y., Maslow C.B., Cone J.E. Risk factors for persistence of lower respiratory symptoms among community members exposed to the 2001 World Trade Center terrorist attacks. Occup. Environ. Med. 2017;74:449–455. doi: 10.1136/oemed-2016-104157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alper H.E., Yu S., Stellman S.D., Brackbill R.M. Injury, intense dust exposure, and chronic disease among survivors of the World Trade Center terrorist attacks of 11 September 2001. Inj. Epidemiol. 2017;4:17. doi: 10.1186/s40621-017-0115-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luft B., Schechter C., Kotov R., Broihier J., Reissman D., Guerrera K., Udasin I., Moline J., Harrison D., Friedman-Jimenez G., et al. Exposure, probable PTSD and lower respiratory illness among World Trade Center rescue, recovery and clean-up workers. Psychol. Med. 2012;42:1069–1079. doi: 10.1017/S003329171100256X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rafael E., Christie J., Teamer J.A., Bienenfeld L.A., Afilaka A.A., Crane M., Levin S.M., Herbert R. Reflux symptoms and disorders and pulmonary disease in former World Trade Center rescue and recovery workers and volunteers. J. Occup. Environ. Med. 2008;50:1351–1354. doi: 10.1097/JOM.0b013e3181845f9b. [DOI] [PubMed] [Google Scholar]
- 26.Shao Y., Durmus N., Zhang Y., Pehlivan S., Fernandez-Beros M.-E., Umana L., Corona R., Addessi A., Abbott S.A., Smyth-Giambanco S., et al. The development of a WTC environmental health center pan-cancer database. Int. J. Environ. Res. Public Health. 2021;18:1646. doi: 10.3390/ijerph18041646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J., Cone J.E., Kahn A.R., Brackbill R.M., Farfel M.R., Greene C.M., Hadler J.L., Stayner L.T., Stellman S.D. Association between World Trade Center exposure and excess cancer risk. JAMA. 2012;308:2479–2488. doi: 10.1001/jama.2012.110980. [DOI] [PubMed] [Google Scholar]
- 28.Li J., Yung J., Qiao B., Takemoto E., Goldfarb D.G., Zeig-Owens R., Cone J.E., Brackbill R.M., Farfel M.R., Kahn A.R., et al. Cancer incidence in World Trade Center rescue and recovery workers: 14 years of follow-up. JNCI J. Natl. Cancer Inst. 2022;114:210–219. doi: 10.1093/jnci/djab165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moline J.M., Herbert R., Crowley L., Troy K., Hodgman E., Shukla G., Udasin I., Luft B., Wallenstein S., Landrigan P., et al. Multiple myeloma in World Trade Center responders: A case series. J. Occup. Environ. Med. 2009;51:896–902. doi: 10.1097/JOM.0b013e3181ad49c8. [DOI] [PubMed] [Google Scholar]
- 30.Solan S., Wallenstein S., Shapiro M., Teitelbaum S.L., Stevenson L., Kochman A., Kaplan J., Dellenbaugh C., Kahn A., Biro F.N., et al. Cancer incidence in world trade center rescue and recovery workers, 2001–2008. Environ. Health Perspect. 2013;121:699–704. doi: 10.1289/ehp.1205894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li J., Brackbill R.M., Liao T.S., Qiao B., Cone J.E., Farfel M.R., Hadler J.L., Kahn A.R., Konty K.J., Stayner L.T., et al. Ten-year cancer incidence in rescue/recovery workers and civilians exposed to the 11 September 2001 terrorist attacks on the World Trade Center. Am. J. Ind. Med. 2016;59:709–721. doi: 10.1002/ajim.22638. [DOI] [PubMed] [Google Scholar]
- 32.Shapiro M.Z., Wallenstein S.R., Dasaro C.R., Lucchini R.G., Sacks H.S., Teitelbaum S.L., Thanik E.S., Crane M.A., Harrison D.J., Luft B.J., et al. Cancer in general responders participating in World Trade Center health programs, 2003–2013. JNCI Cancer Spectr. 2020;4:pkz090. doi: 10.1093/jncics/pkz090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DiGrande L., Perrin M.A., Thorpe L.E., Thalji L., Murphy J., Wu D., Farfel M., Brackbill R.M. Posttraumatic stress symptoms, PTSD, and risk factors among lower Manhattan residents 2–3 years after the 11 September 2001 terrorist attacks. J. Trauma Stress. 2008;21:264–273. doi: 10.1002/jts.20345. [DOI] [PubMed] [Google Scholar]
- 34.Rosen R.L., Levy-Carrick N., Reibman J., Xu N., Shao Y., Liu M., Ferri L., Kazeros A., Caplan-Shaw C.E., Pradhan D.R., et al. Elevated C-reactive protein and posttraumatic stress pathology among survivors of the 9/11 World Trade Center attacks. J. Psychiatr. Res. 2017;89:14–21. doi: 10.1016/j.jpsychires.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 35.Perrin M.A., DiGrande L., Wheeler K., Thorpe L., Farfel M., Brackbill R. Differences in PTSD prevalence and associated risk factors among World Trade Center disaster rescue and recovery workers. Am. J. Psychiatry. 2007;164:1385–1394. doi: 10.1176/appi.ajp.2007.06101645. [DOI] [PubMed] [Google Scholar]
- 36.Feder A., Mota N., Salim R., Rodriguez J., Singh R., Schaffer J., Schechter C.B., Cancelmo L.M., Bromet E.J., Katz C.L., et al. Risk, coping and PTSD symptom trajectories in World Trade Center responders. J. Psychiatr. Res. 2016;82:68–79. doi: 10.1016/j.jpsychires.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 37.Rosen R., Zhu Z., Shao Y., Liu M., Bao J., Levy-Carrick N., Reibman J. Longitudinal change of PTSD symptoms in community members after the World Trade Center destruction. Int. J. Environ. Res. Public Health. 2019;16:1215. doi: 10.3390/ijerph16071215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bromet E., Hobbs M., Clouston S., Gonzalez A., Kotov R., Luft B. DSM-IV post-traumatic stress disorder among World Trade Center responders 11–13 years after the disaster of 11 September 2001 (9/11) Psychol. Med. 2016;46:771–783. doi: 10.1017/S0033291715002184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kotov R., Bromet E.J., Schechter C., Broihier J., Feder A., Friedman-Jimenez G., Gonzalez A., Guerrera K., Kaplan J., Moline J., et al. Posttraumatic stress disorder and the risk of respiratory problems in World Trade Center responders: Longitudinal test of a pathway. Psychosom Med. 2015;77:438–448. doi: 10.1097/PSY.0000000000000179. [DOI] [PubMed] [Google Scholar]
- 40.Kazeros A., Zhang E., Cheng X., Shao Y., Liu M., Qian M., Caplan-Shaw C., Berger K.I., Goldring R.M., Ghumman M., et al. Systemic Inflammation Associated With World Trade Center Dust Exposures and Airway Abnormalities in the Local Community. J. Occup. Environ. Med./Am. Coll. Occup. Environ. Med. 2015;57:610–616. doi: 10.1097/JOM.0000000000000458. [DOI] [PubMed] [Google Scholar]
- 41.Norris F.H., Friedman M.J., Watson P.J., Byrne C.M., Diaz E., Kaniasty K. 60,000 disaster victims speak: Part I. An empirical review of the empirical literature, 1981–2001. Psychiatry. 2002;65:207–239. doi: 10.1521/psyc.65.3.207.20173. [DOI] [PubMed] [Google Scholar]
- 42.Kessler R.C., Sonnega A., Bromet E., Hughes M., Nelson C.B. Posttraumatic stress disorder in the National Comorbidity Survey. Arch. Gen. Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 43.Li J., Zweig K.C., Brackbill R.M., Farfel M.R., Cone J.E. Comorbidity amplifies the effects of post-9/11 posttraumatic stress disorder trajectories on health-related quality of life. Qual. Life Res. 2018;27:651–660. doi: 10.1007/s11136-017-1764-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thurston G.D., Kipen H., Annesi-Maesano I., Balmes J., Brook R.D., Cromar K., De Matteis S., Forastiere F., Forsberg B., Frampton M.W., et al. A joint ERS/ATS policy statement: What constitutes an adverse health effect of air pollution? An analytical framework. Eur. Respir. J. 2017;49:1600419. doi: 10.1183/13993003.00419-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pun Vivian C., Manjourides J., Suh H. Association of Ambient Air Pollution with Depressive and Anxiety Symptoms in Older Adults: Results from the NSHAP Study. Environ. Health Perspect. 2017;125:342–348. doi: 10.1289/EHP494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kioumourtzoglou M.-A., Power M.C., Hart J.E., Okereke O.I., Coull B.A., Laden F., Weisskopf M.G. The Association Between Air Pollution and Onset of Depression Among Middle-Aged and Older Women. Am. J. Epidemiol. 2017;185:801–809. doi: 10.1093/aje/kww163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wisnivesky J.P., Teitelbaum S.L., Todd A.C., Boffetta P., Crane M., Crowley L., De la Hoz R.E., Dellenbaugh C., Harrison D., Herbert R., et al. Persistence of multiple illnesses in World Trade Center rescue and recovery workers: A cohort study. Lancet. 2011;378:888–897. doi: 10.1016/S0140-6736(11)61180-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohen A.J., Brauer M., Burnett R., Anderson H.R., Frostad J., Estep K., Balakrishnan K., Brunekreef B., Dandona L., Dandona R., et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. Lancet. 2017;389:1907–1918. doi: 10.1016/S0140-6736(17)30505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim K.-H., Kabir E., Kabir S. A review on the human health impact of airborne particulate matter. Environ. Int. 2015;74:136–143. doi: 10.1016/j.envint.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 50.Shao Y., Zhang Y., Liu M., Fernandez-Beros M.E., Qian M., Reibman J. Gene-Environment Interaction between the IL1RN Variants and Childhood Environmental Tobacco Smoke Exposure in Asthma Risk. Int. J. Environ. Res. Public Health. 2020;17:2036. doi: 10.3390/ijerph17062036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lim C.C., Thurston G.D. Air Pollution, Oxidative Stress, and Diabetes: A Life Course Epidemiologic Perspective. Curr. Diabetes Rep. 2019;19:58. doi: 10.1007/s11892-019-1181-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thurston G.D., Ahn J., Cromar K.R., Shao Y., Reynolds H.R., Jerrett M., Lim C.C., Shanley R., Park Y., Hayes R.B. Ambient particulate matter air pollution exposure and mortality in the NIH-AARP diet and health cohort. Environ. Health Perspect. 2016;124:484–490. doi: 10.1289/ehp.1509676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu S., Alper H.E., Nguyen A.-M., Brackbill R.M. Risk of stroke among survivors of the September 11, 2001, World Trade Center Disaster. J. Occup. Environ. Med. 2018;60:e371–e376. doi: 10.1097/JOM.0000000000001361. [DOI] [PubMed] [Google Scholar]
- 54.MohanKumar S.M.J., Campbell A., Block M., Veronesi B. Particulate matter, oxidative stress and neurotoxicity. NeuroToxicology. 2008;29:479–488. doi: 10.1016/j.neuro.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 55.Marmor M., Thawani S., Cotrina M.L., Shao Y., Wong E.S., Stecker M.M., Wang B., Allen A., Wilkenfeld M., Vinik E.J., et al. Case-Control Study of Paresthesia Among World Trade Center-Exposed Community Members. J. Occup. Environ. Med. 2020;62:307–316. doi: 10.1097/JOM.0000000000001828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thawani S., Wang B., Shao Y., Reibman J., Marmor M. Time to onset of paresthesia among community members exposed to the World Trade Center disaster. Int. J. Environ. Res. Public Health. 2019;16:1429. doi: 10.3390/ijerph16081429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosen R., Shao Y., Zhang Q., Bao J., Zhang Y., Masurkar A., Wisniewski T., Urban N., Reibman J. Cognitive function among World Trade Center-exposed community members with mental health symptoms. Int. J. Environ. Res. Public Health. 2022;19:3440. doi: 10.3390/ijerph19063440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Clouston S.A., Hall C.B., Kritikos M., Bennett D.A., DeKosky S., Edwards J., Finch C., Kreisl W.C., Mielke M., Peskind E.R., et al. Cognitive impairment and World Trade Centre-related exposures. Nat. Rev. Neurol. 2022;18:103–116. doi: 10.1038/s41582-021-00576-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mostafavi N., Vlaanderen J., Chadeau-Hyam M., Beelen R., Modig L., Palli D., Bergdahl I.A., Vineis P., Hoek G., Kyrtopoulos S., et al. Inflammatory markers in relation to long-term air pollution. Environ. Int. 2015;81:1–7. doi: 10.1016/j.envint.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 60.Zeka A., Sullivan J.R., Vokonas P.S., Sparrow D., Schwartz J. Inflammatory markers and particulate air pollution: Characterizing the pathway to disease. Int. J. Epidemiol. 2006;35:1347–1354. doi: 10.1093/ije/dyl132. [DOI] [PubMed] [Google Scholar]
- 61.Dabass A., Talbott E.O., Venkat A., Rager J., Marsh G.M., Sharma R.K., Holguin F. Association of exposure to particulate matter (PM2.5) air pollution and biomarkers of cardiovascular disease risk in adult NHANES participants (2001–2008) Int. J. Hyg. Environ. Health. 2016;219:301–310. doi: 10.1016/j.ijheh.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 62.Lee P.-C., Talbott E.O., Roberts J.M., Catov J.M., Sharma R.K., Ritz B. Particulate air pollution exposure and C-reactive protein during early pregnancy. Epidemiology. 2011;22:524–531. doi: 10.1097/EDE.0b013e31821c6c58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shima M. Air pollution and serum C-reactive protein concentration in children. J. Epidemiol. 2007;17:169–176. doi: 10.2188/jea.17.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Viehmann A., Hertel S., Fuks K., Eisele L., Moebus S., Möhlenkamp S., Nonnemacher M., Jakobs H., Erbel R., Jöckel K.-H. Long-term residential exposure to urban air pollution, and repeated measures of systemic blood markers of inflammation and coagulation. Occup. Environ. Med. 2015;72:656–663. doi: 10.1136/oemed-2014-102800. [DOI] [PubMed] [Google Scholar]
- 65.Calderon-Garciduenas L., Villarreal-Calderon R., Valencia-Salazar G., Henriquez-Roldan C., Gutiérrez-Castrellón P., Torres-Jardon R., Osnaya-Brizuela N., Romero L., Torres-Jardón R., Solt A., et al. Systemic inflammation, endothelial dysfunction, and activation in clinically healthy children exposed to air pollutants. Inhal. Toxicol. 2008;20:499–506. doi: 10.1080/08958370701864797. [DOI] [PubMed] [Google Scholar]
- 66.Elbarbary M., Oganesyan A., Honda T., Morgan G., Guo Y., Guo Y., Negin J. Systemic inflammation (C-Reactive Protein) in older Chinese adults is associated with long-term exposure to ambient air pollution. Int. J. Environ. Res. Public Health. 2021;18:3258. doi: 10.3390/ijerph18063258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen J.-C., Schwartz J. Metabolic Syndrome and Inflammatory Responses to Long-Term Particulate Air Pollutants. Environ. Health Perspect. 2008;116:612–617. doi: 10.1289/ehp.10565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hajat A., Allison M., Diez-Roux A.V., Jenny N.S., Jorgensen N.W., Szpiro A.A., Vedal S., Kaufman J.D. Long-term exposure to air pollution and markers of inflammation, coagulation, and endothelial activation: A repeat-measures analysis in the Multi-Ethnic Study of Atherosclerosis (MESA) Epidemiology. 2015;26:310–320. doi: 10.1097/EDE.0000000000000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gill J.M., Saligan L., Woods S., Page G. PTSD is associated with an excess of inflammatory immune activities. Perspect. Psychiatr. Care. 2009;45:262–277. doi: 10.1111/j.1744-6163.2009.00229.x. [DOI] [PubMed] [Google Scholar]
- 70.Howren M.B., Lamkin D.M., Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosom. Med. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 71.Valkanova V., Ebmeier K.P., Allan C.L. CRP, IL-6 and depression: A systematic review and meta-analysis of longitudinal studies. J. Affect. Disord. 2013;150:736–744. doi: 10.1016/j.jad.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 72.Miller R.J., Sutherland A.G., Hutchison J.D., Alexander D.A. C-reactive protein and interleukin 6 receptor in post-traumatic stress disorder: A pilot study. Cytokine. 2001;13:253–255. doi: 10.1006/cyto.2000.0825. [DOI] [PubMed] [Google Scholar]
- 73.von Känel R., Hepp U., Kraemer B., Traber R., Keel M., Mica L., Schnyder U. Evidence for low-grade systemic proinflammatory activity in patients with posttraumatic stress disorder. J. Psychiatr. Res. 2007;41:744–752. doi: 10.1016/j.jpsychires.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 74.Solomon Z., Levin Y., Assayag E.B., Furman O., Shenhar-Tsarfaty S., Berliner S., Ohry A. The implication of combat stress and PTSD trajectories in metabolic syndrome and elevated C-reactive protein levels: A longitudinal study. J. Clin. Psychiatry. 2017;78:e1180–e1186. doi: 10.4088/JCP.16m11344. [DOI] [PubMed] [Google Scholar]
- 75.Sumner J.A., Chen Q., Roberts A.L., Winning A., Rimm E.B., Gilsanz P., Glymour M.M., Tworoger S.S., Koenen K.C., Kubzansky L.D. Cross-sectional and longitudinal associations of chronic posttraumatic stress disorder with inflammatory and endothelial function markers in women. Biol. Psychiatry. 2017;82:875–884. doi: 10.1016/j.biopsych.2017.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cox B.J., Clara I.P., Enns M.W. Posttraumatic stress disorder and the structure of common mental disorders. Depress. Anxiety. 2002;15:168–171. doi: 10.1002/da.10052. [DOI] [PubMed] [Google Scholar]
- 77.Marshall G.N., Schell T.L., Miles J.N. A multi-sample confirmatory factor analysis of PTSD symptoms: What exactly is wrong with the DSM-IV structure? Clin. Psychol. Rev. 2013;33:54–66. doi: 10.1016/j.cpr.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bhattarai J.J., Oehlert M.E., Multon K.D., Sumerall S.W. Dementia and Cognitive Impairment among U.S. Veterans With a History of MDD or PTSD: A Retrospective Cohort Study Based on Sex and Race. J. Aging Health. 2018;31:1398–1422. doi: 10.1177/0898264318781131. [DOI] [PubMed] [Google Scholar]
- 79.Schuitevoerder S., Rosen J.W., Twamley E.W., Ayers C.R., Sones H., Lohr J.B., Goetter E.M., Fonzo G.A., Holloway K.J., Thorp S.R. A meta-analysis of cognitive functioning in older adults with PTSD. J. Anxiety Disord. 2013;27:550–558. doi: 10.1016/j.janxdis.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 80.Alper H.E., Tuly R.A., Seil K., Brite J. Post-9/11 Mental Health Comorbidity Predicts Self-Reported Confusion or Memory Loss in World Trade Center Health Registry Enrollees. Int. J. Environ. Res. Public Health. 2020;17:7330. doi: 10.3390/ijerph17197330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Singh A., Zeig-Owens R., Rabin L., Schwartz T., Webber M.P., Appel D., Prezant D.J., Hall C.B. PTSD and depressive symptoms as potential mediators of the association between World Trade Center exposure and subjective cognitive concerns in rescue/recovery workers. Int. J. Environ. Res. Public Health. 2020;17:5683. doi: 10.3390/ijerph17165683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Durmus N., Shao Y., Arslan A.A., Zhang Y., Pehlivan S., Fernandez-Beros M.-E., Umana L., Corona R., Smyth-Giambanco S., Abbott S.A., et al. Characteristics of cancer patients in the world Trade center environmental health center. Int. J. Environ. Res. Public Health. 2020;17:7190. doi: 10.3390/ijerph17197190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rom W.N., Reibman J., Rogers L., Weiden M.D., Oppenheimer B., Berger K., Goldring R., Harrison D., Prezant D. Emerging exposures and respiratory health: World Trade Center dust. Proc. Am. Thorac. Soc. 2010;7:142–145. doi: 10.1513/pats.200908-092RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rogowski O., Vered Y., Shapira I., Hirsh M., Zakut V., Berliner S. Introducing the wide range C-reactive protein (wr-CRP) into clinical use for the detection of microinflammation. Clin. Chim. Acta. 2005;358:151–158. doi: 10.1016/j.cccn.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 85.Yeh E.T., Willerson J.T. Coming of age of C-reactive protein: Using inflammation markers in cardiology. Circulation. 2003;107:370–371. doi: 10.1161/01.CIR.0000053731.05365.5A. [DOI] [PubMed] [Google Scholar]
- 86.Kazeros A., Maa M.-T., Patrawalla P., Liu M., Shao Y., Qian M., Turetz M., Parsia S., Caplan-Shaw C., Berger K.I., et al. Elevated peripheral eosinophils are associated with new-onset and persistent wheeze and airflow obstruction in world trade center-exposed individuals. J. Asthma. 2013;50:25–32. doi: 10.3109/02770903.2012.743149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Weathers F.W.L.B., Herman D.S., Huska J.A., Keane T.M. The PTSD Checklist (PCL): Reliability, Validity, and Diagnostic Utility. International Society for Truamatic Stress Studies; Chicago, IL, USA: 1993. [Google Scholar]
- 88.Derogatis L.R., Lipman R.S., Rickels K., Uhlenhuth E.H., Covi L. The Hopkins Symptom Checklist (HSCL): A self-report symptom inventory. Behav. Sci. 1974;19:1–15. doi: 10.1002/bs.3830190102. [DOI] [PubMed] [Google Scholar]
- 89.Baron R.M., Kenny D.A. The moderator–mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J. Personal. Soc. Psychol. 1986;51:1173–1182. doi: 10.1037/0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 90.Judd C.M., Kenny D.A. Process analysis: Estimating mediation in treatment evaluations. Eval. Rev. 1981;5:602–619. doi: 10.1177/0193841X8100500502. [DOI] [Google Scholar]
- 91.James L.R., Brett J.M. Mediators, moderators, and tests for mediation. J. Appl. Psychol. 1984;69:307–321. doi: 10.1037/0021-9010.69.2.307. [DOI] [Google Scholar]
- 92.Imai K., Keele L., Tingley D. A general approach to causal mediation analysis. Psychol. Methods. 2010;15:309–334. doi: 10.1037/a0020761. [DOI] [PubMed] [Google Scholar]
- 93.Tingley D., Teppei H., Mit Y., Keele L.J., State P., Imai K. Mediation: R package for causal mediation analysis. J. Stat. Softw. 2014;59:1–38. doi: 10.18637/jss.v059.i05. [DOI] [Google Scholar]
- 94.Liu M., Qian M., Cheng Q., Berger K.I., Shao Y., Turetz M., Kazeros A., Parsia S., Goldring R.M., Caplan-Shaw C., et al. Longitudinal spirometry among patients in a treatment program for community members with World Trade Center-related illness. J. Occup. Environ. Med./Am. Coll. Occup. Environ. Med. 2012;54:1208–1213. doi: 10.1097/JOM.0b013e31826bb78e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Baker D.G., Nievergelt C.M., O’Connor D.T. Biomarkers of PTSD: Neuropeptides and immune signaling. Neuropharmacology. 2012;62:663–673. doi: 10.1016/j.neuropharm.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 96.Passos I.C., Vasconcelos-Moreno M.P., Costa L.G., Kunz M., Brietzke E., Quevedo J., Salum G., Magalhães P.V., Kapczinski F., Kauer-Sant’Anna M. Inflammatory markers in post-traumatic stress disorder: A systematic review, meta-analysis, and meta-regression. Lancet Psychiatry. 2015;2:1002–1012. doi: 10.1016/S2215-0366(15)00309-0. [DOI] [PubMed] [Google Scholar]
- 97.Canetti D., Russ E., Luborsky J., Gerhart J.I., Hobfoll S.E. Inflamed by the flames? The impact of terrorism and war on immunity. J. Trauma. Stress. 2014;27:345–352. doi: 10.1002/jts.21920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Michopoulos V., Rothbaum A.O., Jovanovic T., Almli L.M., Bradley B., Rothbaum B.O., Gillespie C.F., Ressler K.J. Association of CRP genetic variation and CRP level with elevated PTSD symptoms and physiological responses in a civilian population with high levels of trauma. Am. J. Psychiatry. 2015;172:353–362. doi: 10.1176/appi.ajp.2014.14020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim T.D., Lee S., Yoon S. Inflammation in post-traumatic stress disorder (PTSD): A review of potential correlates of PTSD with a neurological perspective. Antioxidants. 2020;9:107. doi: 10.3390/antiox9020107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Del Giudice M., Gangestad S.W. Rethinking IL-6 and CRP: Why they are more than inflammatory biomarkers, and why it matters. Brain Behav. Immun. 2018;70:61–75. doi: 10.1016/j.bbi.2018.02.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used in this paper are from the clinical databases at the WTC EHC Data Center. We only used de-identified and anonymized information. The datasets are not publically available, but the de-identified and anonymized information is potentially available upon reasonable request to the WTC EHC Data Center.