Abstract
Breast cancer is one of the top causes of death, particularly among women, and it affects many women. Cancer can also be caused by various factors, including acquiring genetic alteration. Doctors use radiation to detect and treat breast cancer. As a result, breast cancer becomes radiation-resistant, necessitating a new strategy for its treatment. The approach discovered by the researchers is a flavonoid, which is being researched to see if it might help treat radiation-resistant breast cancer more safely than an approved medicine already being used in the field. As a result, this study focuses on the role of flavonoids in breast cancer suppression, breast cancer gene anomalies, and the resulting apoptotic mechanism.
Keywords: breast cancer, apoptosis, flavonoid
1. Introduction
Cancer is a condition in which some cells in the body grow out of control and spread to other parts of the body. Cancer can begin anywhere in the trillions of cells that make up the human body [1]. Various cancers such as lung, stomach, esophageal, and breast cancer exist all over the body without our knowledge. These cancers initially disappear due to the body’s immune response, but if not, the tumor grows and causes damage to the body and develops into cancer. These cancers have the following several symptoms: weight fluctuations, such as unexpected weight loss or increase; yellowing, darkening, or redness of the skin, unhealed wounds, alterations to existing moles are all signs of skin changes; as well as, coughing that will not go away or breathing problems [2].
Among cancers that are a global issue, breast cancer is the most common cancer in the world [3]. Breast cancer is a frequent condition among women in their forties and fifties. It has a 20% mortality rate and a 30% morbidity rate. Furthermore, the cancer incidence rate is increasing daily due to developing human physiological instability and current nutritional eating habits [4]. Premature menstruation and delayed menopause, for example, increase the risk of breast cancer [5]. In addition, many side effects often exist even after overcoming cancer. The main side effects of breast cancer are fatigue, loss of appetite, nausea, pain, and weight loss [6].
A cancerous cell or tissue differs significantly from a physiologically normal cell or tissue. For starters, cancer starts with a single cell. In other words, when a cell obtains several mutations and transforms into a cancer cell, it continues to divide and increase to create cancer [7]. Second, once cancer has developed, it cannot control its growth. Normal tissue stops growing when it reaches a particular size, while cancer continues to grow, often forming huge masses. Invasive growth has the following attributes: as cancer spreads, it burrows into the normal tissue around it, making surgical resection difficult [8]. Furthermore, cancer is characterized by an undifferentiated condition. Each organ’s normal tissue has unique histological traits, while cancer does not [9].
Metastasis is another trait. The spread of cancer to tissues that are not directly associated with it is known as metastasis. When metastasis is discovered, the cancer is usually categorized as terminal [10]. As a result, the existence or absence of metastasis is critical in selecting a treatment strategy.
Surgery, chemotherapy, hormone therapy, biological therapy, and radiation therapy are mainly used to treat breast cancer. Surgery is the medical practice of cutting cancerous tissue, and chemotherapy is a treatment method using particular drugs to kill cancer cells [11]. Hormone therapy is a way to block cancer cells from getting the hormones they need to grow. Biological treatments work with the body’s immune system to help fight cancer cells or control the side effects of other cancer treatments [12]. Radiation therapy uses light rays similar to X-rays to kill cancer cells [13]. There are methods of such treatment, but there are cases in which people are harmed by such treatment. Among them, side effects occur, such as loss of appetite, nausea and vomiting, fatigue, mouth pain, hair loss, weight gain, and a higher risk of infection by other diseases [14]. In addition, as a reaction to these side effects, there are cases where there is a resistance to the treatment method, so even if other treatments are used, there are cases where it is meaningless [15].
When cells grow and divide more than they should, or do not die when they should, an abnormal tissue mass arises [14]. They are called tumors. Tumors can be benign or malignant. Benign tumors grow slowly and expand and have a capsule to prevent spread to surrounding tissues. Moreover, it is well-differentiated and the cells are mature [16]. When surgically removed, recurrence is rare, and there is no metastasis. Benign tumors are almost harmless to the human body, but they become problematic when pressure is applied to significant organs or when they are closed. There is virtually no harm to the human body, and the prognosis is good. Conversely, malignant tumors proliferate and grow while infiltrating into surrounding tissues [17]. Because there is no capsule, it penetrates the surrounding tissue, making removing the tumor difficult even with surgery. They have poor differentiation and immature cells since it spreads to surrounding tissues, recurrence is common after surgery [17]. Metastasis is common because it spreads to surrounding and moving issues. If left untreated with surgery, radiation therapy, or chemotherapy, it can cause death. For malignant tumors, the prognosis depends on the time of diagnosis, degree of progression, and metastasis [18].
Flavonoids are a class of natural substances that have phenolic structures in diverse forms and are found in plants [19]. Flavones, flavanones, flavanols, flavonols, isoflavones, and anthocyanidins are the six subclasses of flavonoids [20]. According to a study, flavonoids have anti-inflammatory [21], antiviral [22], anti-allergic [23], antioxidant [24], and anti-tumor [25] properties. According to a study, flavonoids also inhibit tumor growth by causing death in cancer cells [26]. Therefore, it is possible to treat breast cancer more safely than a dangerous method with side effects by inducing the death of cancer cells and receiving radiation treatment.
There is much information about flavonoids and their numerous routes in breast cancer. As a result, we would like to summarize flavonoids’ anticancer properties and the links between flavonoids and breast cancer.
2. Flavonoid and Anti-Cancer Effect
Flavonoids are natural compounds that belong to a group of polyphenolic plant secondary metabolites found in various fruits, vegetables, and beverages [27,28]. Flavonoids are anticancer because they reduce the symptoms of cancer. Flavonoid applications include proliferation inhibition, cell cycle arrest, apoptosis, antioxidants, and anti-metastasis. The caspase-9, mitochondrial-driven apoptosis, extrinsic, caspase-8, and death receptor-driven apoptosis signaling pathways, among others, are involved in flavonoids’ anticancer activities [27].
Bcl-2 (B-cell lymphoma 2), PARP (poly ADP-ribose polymerase), FLIP (FADD (Fas-associated death domain)-like interleukin 1-converting enzyme) inhibitory protein), as well as regulatory genes such as p53, can all be affected [29]. Flavonoids such as quercetin can inhibit PKB (protein kinase B (also referred to as Akt)) [30], the protein implicated in apoptosis avoidance [31].
c-FLIP is a master regulator of anti-apoptotic mechanisms that have been found in abundance in a variety of cancer cells [32]. The overexpression of c-FLIP interferes with the caspase-8 protein, preventing caspase-8 from cleaving and terminating the apoptotic mechanism [33]. Thymoquinone reduced c-FLIP expression at the translational level but did not influence c-FLIP transcriptional regulation. However, the literature has well documented that ROS plays a role in the post-translational modification of c-FLIP via increased proteasomal activity [34,35]. Numerous researchers have recently looked into the relationship between NF-кB and Bcl-2; for example, the transcriptional expression of the NF-B target BCL-2 [36,37].
One of the fundamental mechanisms behind flavonoids’ anti-tumor action has been discovered as a cell cycle arrest. Both natural and synthetic flavonoids have been found to limit cancer cell proliferation at the G2/M phase, principally through regulating cyclin expression levels [38,39,40].
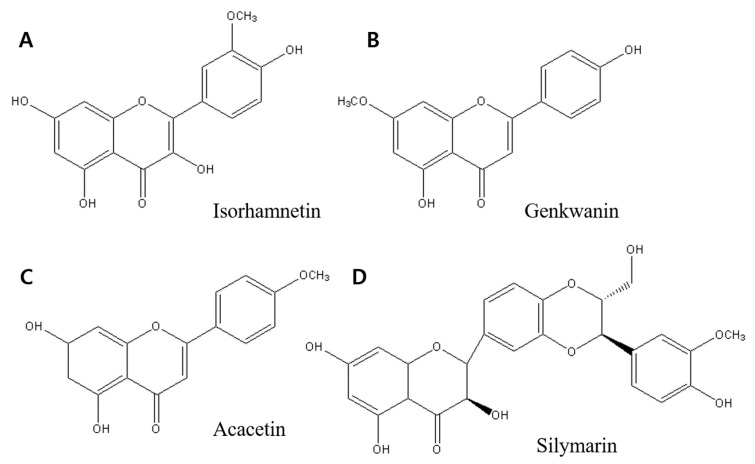
Flavonoids (Figure 1) can modulate proliferation [5,39,41,42,43,44], invasion [45,46], and inflammatory signals through a variety of pathways associated with their qualities as antioxidants [24,47], estrogen agonists, or CYP1 (Cytochrome P450) inhibitors [39]. Flavonoids have also been found to influence inflammatory signaling via systems such as NF-кB (nuclear factor B), which regulates cell proliferation and survival [21,23].
Figure 1.
Structure of flavonoid. They were drawn by ChemDraw Pro 8.0.
Many flavonoids have been shown to have anticancer properties. In human breast cancer cells, certain flavonoids (isorhamnetin, genkwanin, acacetin, silymarin, eriocitrin, icariin, kaempferol, silibinin, apigenin, and luteolin) suppress cell growth and induce apoptosis, autophagy, or cell cycle arrest [5,20,38,43,44,48,49]. Table 1 shows the anticancer properties of introduced flavonoids.
Table 1.
Relationships between flavonoid and anticancer activity.
| Flavonoid | Anti-Cancer Activity from the Study | Cancer | Reference |
|---|---|---|---|
| Isorhamnetin, Genkwanin, and Acacetin | Inhibit cell proliferation and induce apoptosis and autophagy | Breast cancer | [43] |
| Silymarin | Suppresses proliferation inducing apoptosis | Ovarian cancer | [55] |
| Kaempferol | Induce proliferation and induce cell cycle arrest, apoptosis, and DNA damage | Breast cancer | [38] |
| Eriocitrin | Inhibited cell growth and promoted death | Breast cancer | [5] |
| Apigenin and luteolin | Inhibit proliferation | Breast cancer | [20] |
| Icariin | Apoptosis and improve anti-tumor immunity | Breast cancer | [48] |
| Silibinin | Autophagy is triggered by ROS-dependent mitochondrial failure. | Breast cancer | [49] |
Furthermore, the anticancer action of flavonoids has been discovered to be broad (affecting most types of cancer) and cancer-specific, with levels that cause cancer cells to proapoptotic and have no effect on normal cells [29]. The flavonoids’ capacity to target common pathways in cancer cells is most likely to blame. The CYP1 enzyme family, for example, is expressed primarily in tumor cells and premalignant tissue, with no evidence of expression in normal surrounding tissues [50]. This suggests that developing medicines targeted exclusively to aberrant tissue has a lot of promise. Its specificity could be further improved because many of the chemicals employed are only bioactivated when they come into contact with these enzymes.
Flavonoids decrease cancer cell proliferation and invasiveness via modulating ROS-scavenging enzyme activities, participating in cell cycle arrest, inducing apoptosis and autophagy, and suppressing cancer cell proliferation and invasiveness [51].
Because of their ability to stabilize free radicals due to the presence of phenolic hydroxyl groups, flavonoids can directly scavenge ROS and chelate metal ions [52,53]. Activating antioxidant enzymes, repressing pro-oxidant enzymes, and stimulating antioxidant enzymes and phase 2 detoxification enzymes are indirect flavonoid antioxidant effects [51]. Flavonoid anticancer actions entail both antioxidant and pro-oxidant activity [54].
Flavonoids also have an additive effect. As a result, one of the potential ways to apply flavonoids to cancer treatment has been suggested as follows: a mixture of various polyphenols administered concurrently with anticancer medicines. According to a study flavonoids do not influence other natural anticancer activities (such as phase II detoxification enzymes’ influence). A flavonoid is a good natural cancer prevention product due to its accessibility, demonstrated efficacy, and few side effects.
3. Breast Cancer
Cancer cells have DNA and RNA that are very similar (but not identical) to cells from the organism from whence they came. This is why they are not always noticed by the immune system, even significantly when it is compromised [56]. Cancer arises when the immune system malfunctions and/or the quantity of cells created exceeds the immune system’s ability to eradicate [57]. Under certain circumstances, such as an unfavorable environment, the rate of DNA and RNA mutations might be excessive (due to radiation, chemicals, etc.) [8].
Tumors are abnormal tissue masses that occur when cells divide and expand faster than they should or do not die when they should. Tumors can be benign (non-cancerous) or malignant (cancerous) [58]. Benign tumors can grow quite large but do not spread to surrounding tissues or other body sections [58].
In both industrialized and developing countries, breast cancer is one of the most frequent cancers among women. Around 2 million new cases of breast cancer were reported worldwide, according to the American Cancer Society [59]. In 2017, more than 250,000 new breast cancer cases were identified in the United States, and 12 percent of all women in the country will be diagnosed with the disease at some point throughout their lives [32]. Promote cell proliferation, reduce cell death, or have biological changes leading to breast cancer [20]. Breast cancer develops in the breast tissue, most usually in the inner lining of milk ducts or the lobules that supply milk to the vents. Breast cancer is 100 times more common in women than in men, yet males have a worse prognosis due to detection delays [60].
Breast cancer can be treated with various options, such as surgical resection with or without lymph node dissection, radiation, and chemotherapy. For breast cancer patients, adjuvant chemotherapy, radiation therapy, or hormone therapy is applied after surgery. If surgery is not possible, radiation therapy and chemotherapy are administered [61]. Chemotherapy is widely used before and after surgery for curing and reducing recurrence [62]. In this case, hormone therapy is used to lower the risk of recurrence of hormone-sensitive breast cancer [61]. At this time, in the case of systemic treatment such as chemotherapy or hormone therapy, it may cause a systemic reaction and, in the case of women of childbearing age, it may affect fertility. On the other hand, radiation therapy, a local treatment method, is applied to all patients who have had a partial mastectomy. Radiation therapy is one of the essential local and regional therapies for breast cancer treatment. Most breast cancer patients receive radiotherapy after surgical resection; however, not all patients benefit equally because some have a locoregional relapse. The leading cause (Table 2) of this relapse is radio resistance [63]. Radio-resistance is the adaptability of malignant cells or tissues to radiotherapy-induced damage and irradiation survival (IR) [64,65].
Table 2.
Relationship between breast cancer side effect case and radiation therapy.
| Side Effect | Explanation | Reference |
|---|---|---|
| Breast changes | Radiation may cause the breasts to shrink or become denser. | [67] |
| Brachial plexopathy | Breast or chest wall radiation can damage the nerves that travel through the arm, wrist, and hand. Damage to the nerves can result in numbness, discomfort, or weakness in the affected area. | [68] |
| Sore throat | Radiation to the lymph nodes around the collarbone might produce a painful throat or make swallowing difficult. Once the treatment is over, these symptoms should go away. | [69] |
| Lymphedema | Lymphedema is a condition in which the arm, hand, or chest swells. Radiation can sometimes harm neighboring lymph nodes, resulting in lymph fluid accumulation. | [70] |
| Nausea | Radiation can produce nausea; however, this is an infrequent side effect. | [71] |
| Rib fracture | Radiation therapy can weaken the ribs, making them more prone to breaking or fracturing. However, with the use of new treatment regimens, this is a relatively rare occurrence. | [72] |
| Heart problems | The heart can be damaged if a doctor uses radiation on the left side of the chest. This is rare now that new protocols have been implemented. | [2] |
| Lung problems | Radiation can induce inflammation in the lungs on an infrequent occasions. Radiation pneumonitis is the medical word for this condition, which causes shortness of breath, coughing, and a low-grade fever that will go away with time. | [73] |
| Swelling | Swelling or inflammation of the breast or surrounding tissue is possible. Swelling should subside after a few weeks of the treatment’s completion. | [74] |
| A second cancer | In scarce situations, radiation exposure can raise the risk of developing second cancer. | [75] |
Radiotherapy usually causes severe side effects in women undergoing breast cancer treatment. Radiation therapy can cause exhaustion and a red, sunburn-like rash where radiation is being delivered. Breast tissue may appear larger or stiffer as well [63]. These negative consequences might be debilitating. Radiation-resistant breast cancer cells cannot receive radiation therapy or helpful treatment. Therefore, breast cancer should be treated using other methods, and one of those methods is to treat cancer using flavonoids [66]. In particular, using adjuvant flavonoids can lower the risk of breast cancer and systemic side effects, which can improve breast cancer survivors’ quality of life. By noting that these flavonoids have these actions in vivo, the possibility of flavonoids being treated for breast cancer is increased (Table 3 and Table 4).
Table 3.
The principal effect of flavonoid in vivo.
| Flavonoid | Function In Vivo in Breast Cancer | Reference |
|---|---|---|
| Kaempferol | Osteoprotective effect | [76] |
| Apigenin | Inhibit tumorigenesis | [77] |
| Icariin | Prevent glucocorticoid-induced osteocyte apoptosis | [78] |
| Luteolin | Anticarcinogenic effect Antioxidant |
[79,80] |
| Acacetin | Exhibit anticancer activity Inhibit angiogenesis |
[81,82] |
| Scutellarein | Inhibition of cell proliferation and Metastasis | [83] |
Table 4.
Relationship between flavonoid and plant.
| Flavonoid | Plant | Reference |
|---|---|---|
| Scutellarein | Scutellaria baicalensis | [83] |
| Prunetinoside | Prunus yedoensis | [84] |
| Naringin | Citrus grandis | [85] |
| Apigetrin | Teucrium gnaphalodes | [86] |
| Anthocyanins | Vitis cognitive Pulliat | [87] |
| Luteolin | Cichorium endivia | [88] |
| Kaempferol | Vitis vinifera L. | [89] |
| Silibinin | Cirsium japonicum var. ussuriense (Regel) Kitam. | [90] |
4. Suppose Pathway
Flavonoids induce apoptosis as a usual way of acting on cells in radiation-resistant breast cancer. We examined simple anticancer activity from many flavonoid effects (Table 1, Table 3 and Table 4). Using such products, a non-harmful therapeutic agent can be developed to select and kill cancer cells in the field safely. An essential aspect of this treatment is that flavonoids induce apoptosis, and thus the cancer cells disappear. Except for cancer, it can be a safe therapeutic strategy for the human body. Therefore, we are going to focus on apoptosis and breast cancer.
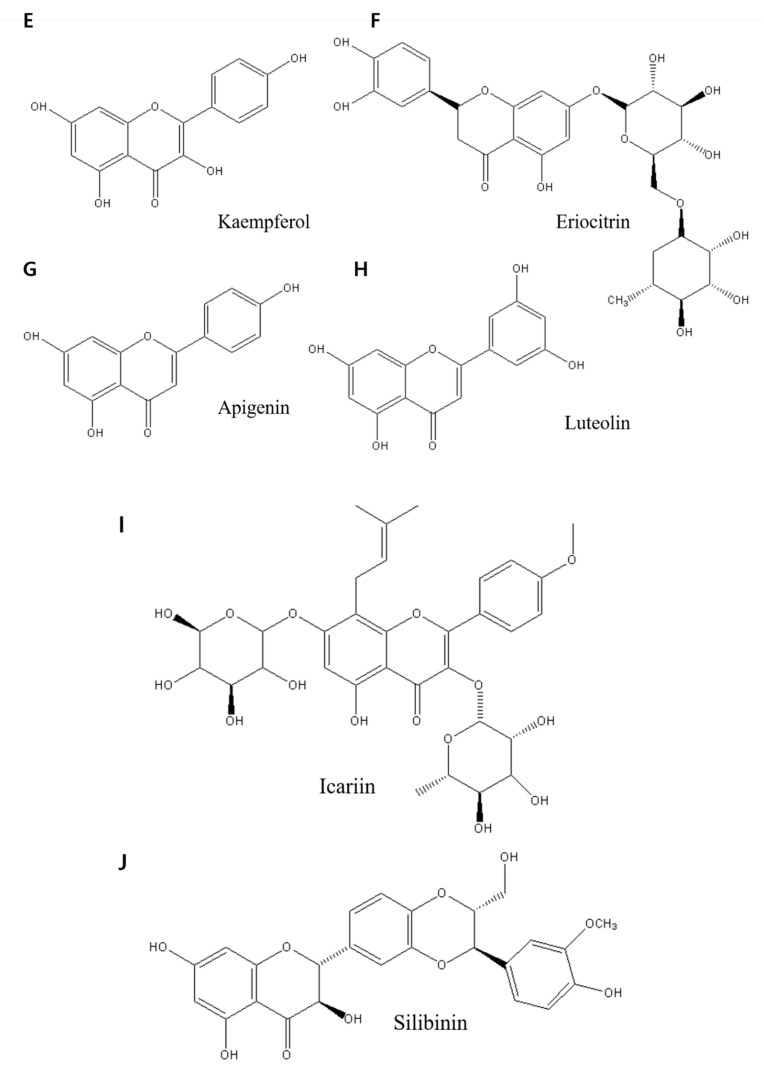
When a cell stops growing and dividing, it enters apoptosis, a sort of cell death [55]. Within physiological conditions, in other words, apoptosis (Figure 2) is a critical mechanism that results in the eradication of unwanted cells [27]. There are those whose flavonoids can affect each of those pathways and in which layer the pathway they may exert their role. Extrinsic and intrinsic apoptosis pathways are two types of apoptosis pathways. The extrinsic route, which includes caspase-8, -10, -3, -7, and BID, is one of the apoptotic pathways (BH3-interacting domain death agonist). Apoptosis can be triggered by extracellular death receptors such as TNF-related apoptosis-inducing ligand (TRAIL), tumor necrosis factor (TNF), and Fas cell surface death receptor (FAS), as well as intracellular stimuli such as hunger, irreversible genetic damage, osmotic stress, and hypoxia [91]. The intrinsic pathway, also called the mitochondrial apoptosis pathway, comprises many stimuli that act on different cell targets. Caspase-8 and caspase-10 can BID shortened BID in the extrinsic route. The tBID (to be decided or to be determined) activates BAK (BCL2-antagonist/killer 1) and BAX (Bcl-2-associated X protein) [55]. Inherent lethal stimuli can activate BAX and BAK via stimulating BH3-only proteins. Activated BAK and BAX can form MPT (mitochondrial permeability transition) pores on mitochondrial outer walls, allowing cytochrome c to leak into the cytoplasm due to MOMP induction and act as a signaling molecule in the cytoplasm, facilitating the formation of apoptosomes (proapoptotic proteins such as Smac/Diablo, HrtA2/Om, and cytochrome c). When an apoptosome forms, caspase-9 is the first to be activated, followed by caspase-3 and caspase-7. Activating caspase-3 and caspase-7 causes apoptosis, killing cellular components [55,92].
Figure 2.
There are two pathways of apoptosis. There are extrinsic and intrinsic pathways, and both induce apoptosis.
The extrinsic pathway contains genes from the superfamily and includes interactions mediated by transmembrane death receptors, TNF receptors, and the extrinsic pathway. The creation of a “death-included signaling complex” (DISC) comprised of TNF receptor 1 (TNFR1), Fas-Fas-L, death receptor 3 (DR3), death receptor 4 (DR4), and tumor necrosis factor superfamily 10 (known as TRAIL/Apo2L) is triggered by binding between death receptors and ligands [92]. Various death receptors and ligands are found in the extrinsic route. As examples, we will look at Fas-L and TNF receptors. When FAS is combined with Fas-L, it forms a trimer. As a result, the FAS death effector domain becomes visible, which can be linked to the adaptor protein FAS-associated death domain (FADD) [93]. Procaspase-8 and procaspase-10 can bind to this domain, cleaving them to activate caspase-8 and caspase-10, which subsequently activate caspase-3 and caspase-7 as effector caspases, breaking the target protein and producing apoptosis [55].
Flavonoids have been utilized to treat cancer by inducing apoptosis in several trials. In Hep3B cells, scutellarein can trigger the Fas-mediated extrinsic apoptotic pathway [94]. Apigenin increases cell death in MDA MB-231 and MCF-7 human breast cancer cells, causing significant toxicity and, most critically, apoptosis [95]. Apoptosis is also induced by GL-V9 in human breast cancer cell lines [96]. By reducing miR-27a expression, isoliquiritigenin, a flavonoid, can also block melanoma cells from growing and migrating [55,91].
Normal breast development is controlled by a balance of cell proliferation and apoptosis, and there is evidence that tumor growth is caused by excessive proliferation and decreased apoptosis [97]. The balance of proliferation and apoptosis is critical in deciding whether a tumor will grow or reduce in response to chemotherapy, radiation, or hormone therapy [63,98]. All of these things work by causing apoptosis in some way. Investigating apoptosis and its control and treatment makes it feasible to characterize the biology of specific tumors at the molecular and biochemical levels and use this information to assist patients [99]. It can be said that this is an effect that can be obtained by inducing apoptosis by flavonoids to such an extent that the patient does not feel uncomfortable even after treatment in the direction of fewer sequelae than helping the patient. Certain drawbacks to these trials could make it difficult to say definitively that they have an anticancer impact.
Genetic mutations accumulate in epithelial tissue during carcinogenesis, and cellular functions are lost. The phenotypic characteristics of cells alter as they progress from average to malignant lesions, superficial tumors, and finally, invasive illness [100]. During the premalignant stage, apoptosis, proliferation, and cell cycle regulatory markers differ significantly. Apoptosis is enhanced in both ductal carcinomas in situ and invasive breast cancers [101]. Invasive breast cancer apoptosis appears lower than proliferation in normal breast epithelium [27,99].
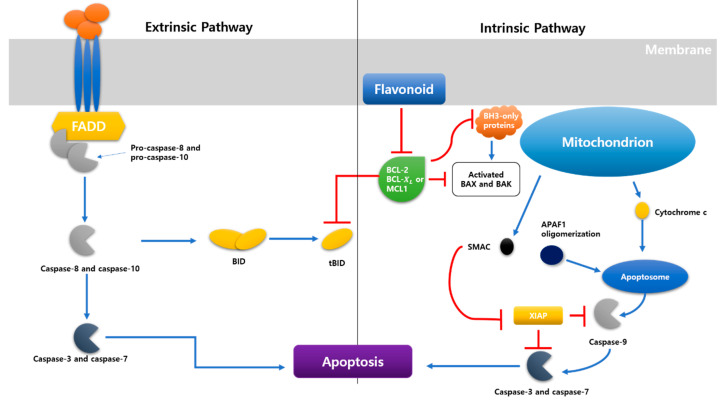
The cell death pathway’s fundamental mechanism could be simplified to just a few essential proteins that a conserved across species. In humans, these regulators have been discovered with many homologs expressed in various tissues [99,102]. In the context of breast cancer, the Bcl-2 family of apoptosis inhibitors and promoters and the p53 tumor suppressor gene have been intensively researched [103]. The image demonstrates the relationship between these oncogenes and apoptotic pathway proteins (Figure 3).
Figure 3.
Between oncogenes and proteins involved in apoptosis. It was discovered that there is a link between chemicals that inhibit apoptosis and the consequences that cause it.
Proteins produced by the Bcl-2 gene family can either promote or inhibit apoptosis. Pro-apoptotic proteins include Bax, Bak, Bad, and Bcl-xs, whereas anti-apoptotic proteins include Bcl-2 and Bcl-xL (B-cell lymphoma-extra-large). Bcl-2 is connected to the expression of estrogen and progesterone receptors, both favorable prognostic markers in breast cancer, and is expressed in about 75% of primary breast cancer [104] malignancies. Patients with Bcl-2 positive tumors have a better prognosis than those with Bcl-2 negative cancers, suggesting an unexpected link between an apoptotic inhibitor and a favorable result. BAG-1 (BAG family molecular chaperone regulator 1), a multifunctional protein that controls apoptosis, has recently been linked to better survival in women with early-stage breast cancer [104]. BAG 1 interacts with other members of the Bcl-2 family, as well as heat shock proteins and estrogen receptors, in what appears to be a contradictory finding in a protein.
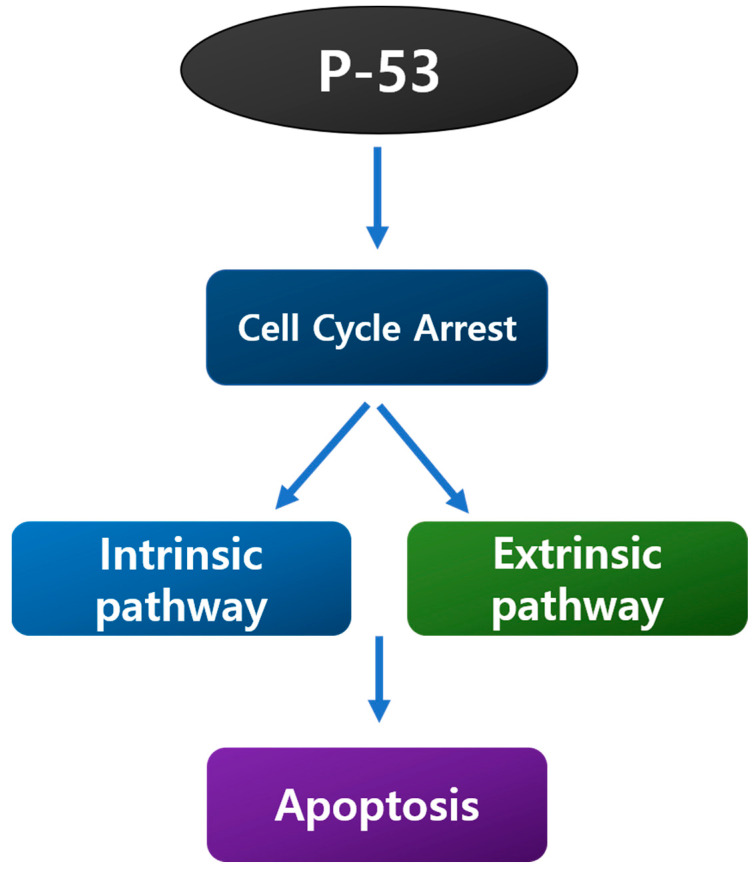
P53 (Figure 4) is a protein that regulates various biological processes and cell cycles, including apoptosis, biological processes, and DNA repair [105]. The most prevalent mutational event in cancer is mutations in this gene. Several studies have connected gene mutations or increased p53 protein synthesis (an indirect indicator of conversion because it typically leads to protein stabilization) to a poor prognosis in breast cancer [106,107]. Chemotherapy, tamoxifen, and radiotherapy have been shown to cause apoptosis in cells via p53-dependent and p53-independent routes [108,109].
Figure 4.
Biological processes involving P53.
5. Conclusions
Flavonoids are a broad group of physiologically active water-soluble plant compounds (such as anthocyanins and flavones) that are prevalent in fruits, vegetables, and herbs and come in various colors ranging from yellow to red to blue [110]. Flavonoids have many anticancer functions, such as proliferation, invasion, and inflammatory signals through various pathways associated with their qualities as antioxidants [24]. These flavonoids can treat cancer cells by targeting their anticancer effects.
Breast cancer is one of the most common malignancies in women, both in terms of incidence and death, and it is also one of the deadliest diseases on the planet [111]. Radiation is often used when examining or treating breast cancer. The radiation makes breast cancer cells resistant to radiation. In this case, a treatment method other than radiation should be chosen, and we think that flavonoids in combination with other treatments is suitable among those methods. Not only is it safe, but because it induces the cell’s function, flavonoids can be a safe method unlike regular anticancer drugs that attack other parts of the cell.
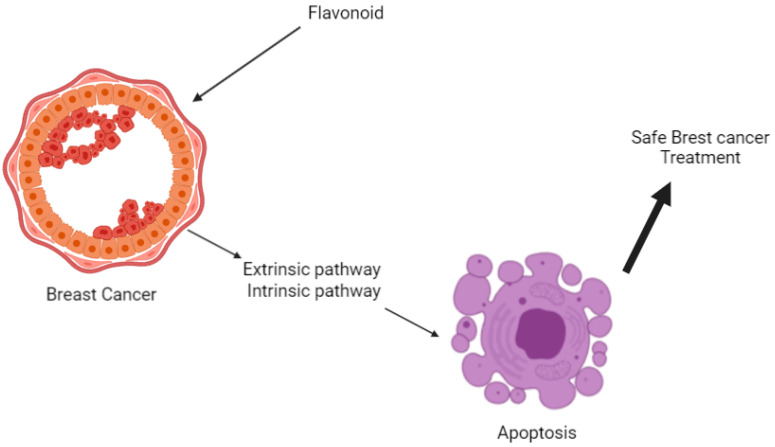
Apoptosis (Figure 5) in breast cancer has been studied using the Bcl-2 family of tumor suppressors and the p53 tumor suppressors. Bcl-2 proteins are members of the Bcl-2 family of proteins. Bcl-2 and Bcl-xL are antiapoptotic, whereas Bax, Bak, Bad, and Bcl-xs are proapoptotic [55]. BAG-1, a multifunctional apoptosis-controlling protein, has lately been related to an improved prognosis in patients with early-stage breast cancer [104]. Apoptosis, the cell cycle, and DNA repair are all regulated by the protein p53. It causes apoptosis in both p53-dependent and non-dependent cells [112,113].
Figure 5.
Flavonoid-induced apoptosis of breast cancer cells.
Women who have had breast cancer up to this point have experienced side effects even after being cured by treatment (Table 2). To avoid these side effects, if flavonoids were commercially available as a drug without side effects in an environmentally friendly way and used for various cancer patients, many cancer patients would be able to recover and take care of their lives fully. Flavonoids are necessary for you to be as comfortable as possible while avoiding any adverse effects. Moreover, to affirm the antitumor impact, we believe it is vital to identify limitations that may occur in this research.
The most frequent malignancy among women is breast cancer. It may cause discomfort if the breast develops resistance to radiation therapy and the tumor is treated by sectioning the breast. Therefore, this review paper proposes a method for treating cancers, including those that have acquired radiation resistance, in combination with flavonoids without causing inconvenience to patients with these cancers, especially breast cancer, as in this review paper.
Abbreviations
FLIP: (FADD (Fas-associated death domain)-like interleukin 1—converting enzyme, BcL-2: B cell lymphoma 2, PKB: protein kinase B, CYP1: cytochrome p450, NF-кB: nuclear factor B, PCD: programmed cell death, BID-BH3-interacting domain death agonist, PARP: poly-ADP-ribose polymerase, TNF: tumor necrosis factor, TRAIL: TNF-related apoptosis inducing ligand, FAS: Fas cell surface death receptor, tBID: to be decided or to be determined, BAK: Bcl2-antagonist/killer 1, BAX: Bcl 2-associated X protein, MPT: mitochondrial permeability transition, MOMP: mitochondrial outer membrane permeability, B cell lymphoma-extra-large, BAG1: BAG family molecular chaperone regulator 1.
Author Contributions
M.Y.P. and Y.K. contributed equally to this work. Conceptualization, M.Y.P. and Y.K.; methodology, S.E.H.; software, H.H.K.; validation, P.B.B. and A.A.; investigation, S.E.H.; resources, P.B.B.; writing—original draft preparation, S.H.J.; writing—review and editing, A.A.; supervision, G.S.K.; project administration, G.S.K. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors clearly declare that they have no conflict of interest in this study.
Funding Statement
This study was supported by the National Research Foundation of Korea funded by Ministry of Science and ICT (grant no. 2020R1A2B5B01001807).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Steck S.E., Murphy E.A. Dietary patterns and cancer risk. Nat. Rev. Cancer. 2020;20:125–138. doi: 10.1038/s41568-019-0227-4. [DOI] [PubMed] [Google Scholar]
- 2.Nardin S., Mora E., Varughese F.M., D’Avanzo F., Vachanaram A.R., Rossi V., Saggia C., Rubinelli S., Gennari A. Breast Cancer Survivorship, Quality of Life, and Late Toxicities. Front. Oncol. 2020;10:864. doi: 10.3389/fonc.2020.00864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferlay J., Colombet M., Soerjomataram I., Parkin D.M., Pineros M., Znaor A., Bray F. Cancer statistics for the year 2020: An overview. Int. J. Cancer. 2021;149:778–789. doi: 10.1002/ijc.33588. [DOI] [PubMed] [Google Scholar]
- 4.Petroni G., Buqué A., Zitvogel L., Kroemer G., Galluzzi L. Immunomodulation by targeted anticancer agents. Cancer Cell. 2021;39:310–345. doi: 10.1016/j.ccell.2020.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Yuan C., Chen G., Jing C., Liu M., Liang B., Gong G., Yu M. Eriocitrin, a dietary flavonoid suppressed cell proliferation, induced apoptosis through modulation of JAK2/STAT3 and JNK/p38 MAPKs signaling pathway in MCF-7 cells. J. Biochem. Mol. Toxicol. 2021;36:e22943. doi: 10.1002/jbt.22943. [DOI] [PubMed] [Google Scholar]
- 6.Britt K.L., Cuzick J., Phillips K.-A. Key steps for effective breast cancer prevention. Nat. Rev. Cancer. 2020;20:417–436. doi: 10.1038/s41568-020-0266-x. [DOI] [PubMed] [Google Scholar]
- 7.McKinney S.M., Sieniek M., Godbole V., Godwin J., Antropova N., Ashrafian H., Back T., Chesus M., Corrado G.S., Darzi A. International evaluation of an AI system for breast cancer screening. Nature. 2020;577:89–94. doi: 10.1038/s41586-019-1799-6. [DOI] [PubMed] [Google Scholar]
- 8.Fahad Ullah M. Breast cancer: Current perspectives on the disease status. Breast Cancer Metastasis Drug Resist. 2019;1152:51–64. doi: 10.1007/978-3-030-20301-6_4. [DOI] [PubMed] [Google Scholar]
- 9.de Ruijter T.C., Veeck J., de Hoon J.P.J., van Engeland M., Tjan-Heijnen V.C. Characteristics of triple-negative breast cancer. J. Cancer Res. Clin. Oncol. 2011;137:183–192. doi: 10.1007/s00432-010-0957-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suhail Y., Cain M.P., Vanaja K., Kurywchak P.A., Levchenko A., Kalluri R., Kshitiz. Systems Biology of Cancer Metastasis. Cell Syst. 2019;9:109–127. doi: 10.1016/j.cels.2019.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strobel O., Neoptolemos J., Jäger D., Büchler M.W. Optimizing the outcomes of pancreatic cancer surgery. Nat. Rev. Clin. Oncol. 2019;16:11–26. doi: 10.1038/s41571-018-0112-1. [DOI] [PubMed] [Google Scholar]
- 12.Waks A.G., Winer E.P. Breast cancer treatment: A review. JAMA. 2019;321:288–300. doi: 10.1001/jama.2018.19323. [DOI] [PubMed] [Google Scholar]
- 13.Wu Y., Su L., Yuan M., Chen T., Ye J., Jiang Y., Song J., Yang H. In Vivo X-ray Triggered Catalysis of H2 Generation for Cancer Synergistic Gas Radiotherapy. Angew. Chem. 2021;133:12978–12985. doi: 10.1002/ange.202100002. [DOI] [PubMed] [Google Scholar]
- 14.Shedden-Mora M.C., Pan Y., Heisig S.R., von Blanckenburg P., Rief W., Witzel I., Albert U.-S., Nestoriuc Y. Optimizing expectations about endocrine treatment for breast cancer: Results of the randomized controlled psy-breast trial. Clin. Psychol. Eur. 2020;2:1–20. doi: 10.32872/cpe.v2i1.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cai F., Luis M.A.F., Lin X., Wang M., Cai L., Cen C., Biskup E. Anthracycline-induced cardiotoxicity in the chemotherapy treatment of breast cancer: Preventive strategies and treatment (Review) Mol. Clin. Oncol. 2019;11:15–23. doi: 10.3892/mco.2019.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu P., Gao W., Su M., Nice E.C., Zhang W., Lin J., Xie N. Adaptive mechanisms of tumor therapy resistance driven by tumor microenvironment. Front. Cell Dev. Biol. 2021;9:641469. doi: 10.3389/fcell.2021.641469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y., Lin B., Liang S., Tao J., Zhang L., Wang J., Zheng J. Epidemiology and Survival of Patients with Malignant Carotid Body Tumors in the SEER Database. J. Vasc. Surg. 2022 doi: 10.1016/j.jvs.2022.04.039. in press . [DOI] [PubMed] [Google Scholar]
- 18.Ji Y., Zhang W. Th17 cells: Positive or negative role in tumor? Cancer Immunol. Immunother. 2010;59:979–987. doi: 10.1007/s00262-010-0849-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar S., Pandey A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013;2013:162750. doi: 10.1155/2013/162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin C.H., Chang C.Y., Lee K.R., Lin H.J., Chen T.H., Wan L. Flavones inhibit breast cancer proliferation through the Akt/FOXO3a signaling pathway. BMC Cancer. 2015;15:958. doi: 10.1186/s12885-015-1965-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Owor R.O., Bedane K.G., Openda Y.I., Zühlkec S., Derese S., Ong’amo G., Ndakala A., Spiteller M. Synergistic anti-inflammatory activities of a new flavone and other flavonoids from Tephrosia hildebrandtii vatke. Nat. Prod. Res. 2021;35:4486–4493. doi: 10.1080/14786419.2020.1736065. [DOI] [PubMed] [Google Scholar]
- 22.Kim C.H., Kim J.E., Song Y.J. Antiviral Activities of Quercetin and Isoquercitrin Against Human Herpesviruses. Molecules. 2020;25:2379. doi: 10.3390/molecules25102379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sugiura Y., Katsuzaki H., Imai K., Amano H. The Anti-Allergic and Anti-Inflammatory Effects of Phlorotannins from the Edible Brown Algae, Ecklonia sp. and Eisenia sp. Nat. Prod. Commun. 2021;16:12. doi: 10.1177/1934578X211060924. [DOI] [Google Scholar]
- 24.Khongkaew P., Wattanaarsakit P., Papadopoulos K.I., Chaemsawang W. Antioxidant Effects and in vitro Cytotoxicity on Human Cancer Cell Lines of Flavonoid-Rich Flamboyant (Delonix regia (Bojer) Raf.) Flower Extract. Curr. Pharm. Biotechnol. 2021;22:1821–1831. doi: 10.2174/1389201021666201029154746. [DOI] [PubMed] [Google Scholar]
- 25.Hatono M., Ikeda H., Suzuki Y., Kajiwara Y., Kawada K., Tsukioki T., Kochi M., Suzawa K., Iwamoto T., Yamamoto H., et al. Effect of isoflavones on breast cancer cell development and their impact on breast cancer treatments. Breast Cancer Res. Treat. 2021;185:307–316. doi: 10.1007/s10549-020-05957-z. [DOI] [PubMed] [Google Scholar]
- 26.Yan W., Ma X., Zhao X., Zhang S. Baicalein induces apoptosis and autophagy of breast cancer cells via inhibiting PI3K/AKT pathway in vivo and vitro. Drug Des. Devel. 2018;12:3961–3972. doi: 10.2147/DDDT.S181939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raina R., Hussain A., Sharma R. Molecular insight into apoptosis mediated by flavones in cancer (Review) World Acad. Sci. J. 2020;2:6. doi: 10.3892/wasj.2020.47. [DOI] [Google Scholar]
- 28.Panche A.N., Diwan A.D., Chandra S.R. Flavonoids: An overview. J. Nutr. Sci. 2016;5:e47. doi: 10.1017/jns.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez-Perez C., Ward C., Cook G., Mullen P., McPhail D., Harrison D.J., Langdon S.P. Novel flavonoids as anti-cancer agents: Mechanisms of action and promise for their potential application in breast cancer. Biochem. Soc. Trans. 2014;42:1017–1023. doi: 10.1042/BST20140073. [DOI] [PubMed] [Google Scholar]
- 30.Lee D.H., Szczepanski M., Lee Y.J. Role of Bax in quercetin-induced apoptosis in human prostate cancer cells. Biochem. Pharm. 2008;75:2345–2355. doi: 10.1016/j.bcp.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khorsandi L., Orazizadeh M., Niazvand F., Abbaspour M.R., Mansouri E., Khodadadi A. Quercetin induces apoptosis and necroptosis in MCF-7 breast cancer cells. Bratisl Lek Listy. 2017;118:123–128. doi: 10.4149/BLL_2017_025. [DOI] [PubMed] [Google Scholar]
- 32.Park E.J., Chauhan A.K., Min K.-J., Park D.C., Kwon T.K. Thymoquinone induces apoptosis through downregulation of c-FLIP and Bcl-2 in renal carcinoma Caki cells. Oncol. Rep. 2016;36:2261–2267. doi: 10.3892/or.2016.5019. [DOI] [PubMed] [Google Scholar]
- 33.Yu X., Ye X., Gao Q. Infrared handprint image restoration algorithm based on apoptotic mechanism. IEEE Access. 2020;8:47334–47343. doi: 10.1109/ACCESS.2020.2979018. [DOI] [Google Scholar]
- 34.Safa A. c-FLIP, a master anti-apoptotic regulator. Exp. Oncol. 2012;34:176. [PMC free article] [PubMed] [Google Scholar]
- 35.Hughes M.A., Powley I.R., Jukes-Jones R., Horn S., Feoktistova M., Fairall L., Schwabe J.W., Leverkus M., Cain K., MacFarlane M. Co-operative and hierarchical binding of c-FLIP and caspase-8: A unified model defines how c-FLIP isoforms differentially control cell fate. Mol. Cell. 2016;61:834–849. doi: 10.1016/j.molcel.2016.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chipuk J.E., Moldoveanu T., Llambi F., Parsons M.J., Green D.R. The BCL-2 family reunion. Mol. Cell. 2010;37:299–310. doi: 10.1016/j.molcel.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Warren C.F., Wong-Brown M.W., Bowden N.A. BCL-2 family isoforms in apoptosis and cancer. Cell Death Dis. 2019;10:1–12. doi: 10.1038/s41419-019-1407-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu L., Xue L. Kaempferol Suppresses Proliferation and Induces Cell Cycle Arrest, Apoptosis, and DNA Damage in Breast Cancer Cells. Oncol. Res. 2019;27:629–634. doi: 10.3727/096504018X15228018559434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Androutsopoulos V.P., Mahale S., Arroo R.R., Potter G. Anticancer effects of the flavonoid diosmetin on cell cycle progression and proliferation of MDA-MB 468 breast cancer cells due to CYP1 activation. Oncol. Rep. 2009;21:1525–1528. doi: 10.3892/or_00000384. [DOI] [PubMed] [Google Scholar]
- 40.Huang L., Jin K., Lan H. Luteolin inhibits cell cycle progression and induces apoptosis of breast cancer cells through downregulation of human telomerase reverse transcriptase. Oncol. Lett. 2019;17:3842–3850. doi: 10.3892/ol.2019.10052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang N., Wang J., Sheng A., Huang S., Tang Y., Ma S., Hong G. Emodin Inhibits the Proliferation of MCF-7 Human Breast Cancer Cells Through Activation of Aryl Hydrocarbon Receptor (AhR) Front. Pharmacol. 2021;11:622046. doi: 10.3389/fphar.2020.622046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pham T.H., Page Y.L., Percevault F., Ferrière F., Flouriot G., Pakdel F. Apigenin, a Partial Antagonist of the Estrogen Receptor (ER), Inhibits ER-Positive Breast Cancer Cell Proliferation through Akt/FOXM1 Signaling. Int. J. Mol. Sci. 2021;22:470. doi: 10.3390/ijms22010470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang H.-W., Hu J.-J., Fu R.-Q., Liu X., Zhang Y.-H., Li J., Liu L., Li Y.-N., Deng Q., Luo Q.-S., et al. Flavonoids inhibit cell proliferation and induce apoptosis and autophagy through downregulation of PI3Kγ mediated PI3K/AKT/mTOR/p70S6K/ULK signaling pathway in human breast cancer cells. Sci. Rep. 2018;8:11255. doi: 10.1038/s41598-018-29308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim S.-H., Choo G.-S., Yoo E.-S., Woo J.-S., Lee J.-H., Han S.-H., Jung S.-H., Kim H.-J., Jung J.-Y. Silymarin inhibits proliferation of human breast cancer cells via regulation of the MAPK signaling pathway and induction of apoptosis. Oncol. Lett. 2021;21:492. doi: 10.3892/ol.2021.12753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jin H., Lee W.S., Eun S.Y., Jung J.H., Park H.-S., Kim G., Choi Y.H., Ryu C.H., Jung J.M., Hong S.C., et al. Morin, a flavonoid from Moraceae, suppresses growth and invasion of the highly metastatic breast cancer cell line MDA-MB-231 partly through suppression of the Akt pathway. Int. J. Oncol. 2014;45:1629–1637. doi: 10.3892/ijo.2014.2535. [DOI] [PubMed] [Google Scholar]
- 46.Wang L., Ling Y., Chen Y., Li C.-L., Feng F., You Q.-D., Lu N., Guo Q.-L. Flavonoid baicalein suppresses adhesion, migration and invasion of MDA-MB-231 human breast cancer cells. Cancer Lett. 2010;297:42–48. doi: 10.1016/j.canlet.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 47.Seufi A.M., Ibrahim S.S., Elmaghraby T.K., Hafez E.E. Preventive effect of the flavonoid, quercetin, on hepatic cancer in rats via oxidant/antioxidant activity: Molecular and histological evidences. J. Exp. Clin. Cancer Res. 2009;28:80. doi: 10.1186/1756-9966-28-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song L., Chen X., Mi L., Liu C., Zhu S., Yang T., Luo X., Zhang Q., Lu H., Liang X. Icariin-induced inhibition of SIRT6/NF-kappaB triggers redox mediated apoptosis and enhances anti-tumor immunity in triple-negative breast cancer. Cancer Sci. 2020;111:4242–4256. doi: 10.1111/cas.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang K., Wang W., Jin X., Wang Z., Ji Z., Meng G. Silibinin, a natural flavonoid, induces autophagy via ROS-dependent mitochondrial dysfunction and loss of ATP involving BNIP3 in human MCF7 breast cancer cells. Oncol. Rep. 2015;33:2711–2718. doi: 10.3892/or.2015.3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Naif H.M., Al-Obaide M.A.I., Hassani H.H., Hamdan A.S., Kalaf Z.S. Association of Cytochrome CYP1A1 Gene Polymorphisms and Tobacco Smoking With the Risk of Breast Cancer in Women From Iraq. Front Public Health. 2018;6:96. doi: 10.3389/fpubh.2018.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kopustinskiene D.M., Jakstas V., Savickas A., Bernatoniene J. Flavonoids as Anticancer Agents. Nutrients. 2020;12:457. doi: 10.3390/nu12020457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Youn H.S., Lee J.Y., Saitoh S.I., Miyake K., Kang K.W., Choi Y.J., Hwang D.H. Suppression of MyD88- and TRIF-dependent signaling pathways of toll-like receptor by (−)-epigallocatechin-3-gallate, a polyphenol component of green tea. Biochem. Pharmacol. 2006;72:850–859. doi: 10.1016/j.bcp.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 53.Fraga C.G., Galleano M., Verstraeten S.V., Oteiza P.I. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol. Asp. Med. 2010;31:435–445. doi: 10.1016/j.mam.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 54.Raffa D., Maggio B., Raimondi M.V., Plescia F., Daidone G. Recent discoveries of anticancer flavonoids. Eur. J. Med. Chem. 2017;142:213–228. doi: 10.1016/j.ejmech.2017.07.034. [DOI] [PubMed] [Google Scholar]
- 55.Park M.Y., Ha S.E., Vetrivel P., Kim H.H., Bhosale P.B., Abusaliya A., Kim G.S. Differences of Key Proteins between Apoptosis and Necroptosis. BioMed Res. Int. 2021;2021:3420168. doi: 10.1155/2021/3420168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cox T.R. The matrix in cancer. Nat. Rev. Cancer. 2021;21:217–238. doi: 10.1038/s41568-020-00329-7. [DOI] [PubMed] [Google Scholar]
- 57.Baxevanis C.N., Fortis S.P., Perez S.A. The balance between breast cancer and the immune system: Challenges for prognosis and clinical benefit from immunotherapies. Semin. Cancer Biol. 2021;72:76–89. doi: 10.1016/j.semcancer.2019.12.018. [DOI] [PubMed] [Google Scholar]
- 58.Turashvili G., Brogi E. Tumor Heterogeneity in Breast Cancer. Front. Med. 2017;4:227. doi: 10.3389/fmed.2017.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Selvakumar P., Badgeley A., Murphy P., Anwar H., Sharma U., Lawrence K., Lakshmikuttyamma A. Flavonoids and Other Polyphenols Act as Epigenetic Modifiers in Breast Cancer. Nutrients. 2020;12:30761. doi: 10.3390/nu12030761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sharma G.N., Dave R., Sanadya J., Sharma P., Sharma K.K. Various types and management of breast cancer: An overview. J. Adv. Pharm. Technol. Res. 2010;1:109–126. [PMC free article] [PubMed] [Google Scholar]
- 61.Corey B., Smania M.A., Spotts H., Andersen M. Young Women With Breast Cancer: Treatment, Care, and Nursing Implications. Clin. J. Oncol. Nurs. 2020;24:139–147. doi: 10.1188/20.CJON.139-147. [DOI] [PubMed] [Google Scholar]
- 62.Bauersfeld S.P., Kessler C.S., Wischnewsky M., Jaensch A., Steckhan N., Stange R., Kunz B., Bruckner B., Sehouli J., Michalsen A. The effects of short-term fasting on quality of life and tolerance to chemotherapy in patients with breast and ovarian cancer: A randomized cross-over pilot study. BMC Cancer. 2018;18:476. doi: 10.1186/s12885-018-4353-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paramanantham A., Jung E.J., Go S.I., Jeong B.K., Jung J.M., Hong S.C., Kim G.S., Lee W.S. Activated ERK Signaling Is One of the Major Hub Signals Related to the Acquisition of Radiotherapy-Resistant MDA-MB-231 Breast Cancer Cells. Int. J. Mol. Sci. 2021;22:4940. doi: 10.3390/ijms22094940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Luo M., Ding L., Li Q., Yao H. miR-668 enhances the radioresistance of human breast cancer cell by targeting IkappaBalpha. Breast Cancer. 2017;24:673–682. doi: 10.1007/s12282-017-0756-1. [DOI] [PubMed] [Google Scholar]
- 65.Yadav P., Shankar B.S. Radio resistance in breast cancer cells is mediated through TGF-β signalling, hybrid epithelial-mesenchymal phenotype and cancer stem cells. Biomed. Pharmacother. 2019;111:119–130. doi: 10.1016/j.biopha.2018.12.055. [DOI] [PubMed] [Google Scholar]
- 66.Berk Ş., Kaya S., Akkol E.K., Bardakçı H. A comprehensive and current review on the role of flavonoids in lung cancer–Experimental and theoretical approaches. Phytomedicine. 2022;98:153938. doi: 10.1016/j.phymed.2022.153938. [DOI] [PubMed] [Google Scholar]
- 67.Brunt A.M., Haviland J.S., Sydenham M., Agrawal R.K., Algurafi H., Alhasso A., Barrett-Lee P., Bliss P., Bloomfield D., Bowen J., et al. Ten-Year Results of FAST: A Randomized Controlled Trial of 5-Fraction Whole-Breast Radiotherapy for Early Breast Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020;38:3261–3272. doi: 10.1200/JCO.19.02750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morris B.A., Burr A.R., Anderson B.M., Howard S.P. Late Radiation Related Brachial Plexopathy After Pulsed Reduced Dose Rate Reirradiation of an Axillary Breast Cancer Recurrence. Pract. Radiat. Oncol. 2021;11:319–322. doi: 10.1016/j.prro.2021.06.003. [DOI] [PubMed] [Google Scholar]
- 69.Teclegeorgish Z.W., Mokgalaka N.S., Vukea N., de la Mare J.-A., Tembu V.J. Cytotoxicity of triterpenoids from Clerodendrum glabrum against triple negative breast cancer cells in vitro. S. Afr. J. Bot. 2020;133:144–150. doi: 10.1016/j.sajb.2020.07.009. [DOI] [Google Scholar]
- 70.Baumann F.T., Reike A., Hallek M., Wiskemann J., Reimer V. Does Exercise Have a Preventive Effect on Secondary Lymphedema in Breast Cancer Patients Following Local Treatment—A Systematic Review. Breast Care. 2018;13:380–385. doi: 10.1159/000487428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aybar D.O., Kılıc S.P., Çınkır H.Y. The effect of breathing exercise on nausea, vomiting and functional status in breast cancer patients undergoing chemotherapy. Complementary Ther. Clin. Pract. 2020;40:101213. doi: 10.1016/j.ctcp.2020.101213. [DOI] [PubMed] [Google Scholar]
- 72.Lee S.M., Lee J.W., Kim W.C., Min C.K., Kim E.S., Jo I.Y. Effects of Tumor-Rib Distance and Dose-Dependent Rib Volume on Radiation-Induced Rib Fractures in Patients with Breast Cancer. J. Pers. Med. 2022;12:240. doi: 10.3390/jpm12020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Koyama Y., Adachi K., Yagi M., Go Y., Orimoto K., Kawai S., Uenaka N., Okazaki M., Asaoka M., Teraoka S., et al. Successful treatment of G-CSF-related aortitis with prednisolone during preoperative chemotherapy for breast cancer: A case report. Surg. Case Rep. 2021;7:23. doi: 10.1186/s40792-021-01111-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fu M.R., Axelrod D., Guth A., McTernan M.L., Qiu J.M., Zhou Z., Ko E., Magny-Normilus C., Scagliola J., Wang Y. The Effects of Obesity on Lymphatic Pain and Swelling in Breast Cancer Patients. Biomedicines. 2021;9:818. doi: 10.3390/biomedicines9070818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brett J.O., Spring L.M., Bardia A., Wander S.A. ESR1 mutation as an emerging clinical biomarker in metastatic hormone receptor-positive breast cancer. Breast Cancer Res. 2021;23:85. doi: 10.1186/s13058-021-01462-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wong S.K., Chin K.Y., Ima-Nirwana S. The Osteoprotective Effects Of Kaempferol: The Evidence From In Vivo And In Vitro Studies. Drug Des. Devel. 2019;13:3497–3514. doi: 10.2147/DDDT.S227738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen X., Xu H., Yu X., Wang X., Zhu X., Xu X. Apigenin inhibits in vitro and in vivo tumorigenesis in cisplatin-resistant colon cancer cells by inducing autophagy, programmed cell death and targeting m-TOR/PI3K/Akt signalling pathway. J. Buon. 2019;24:488–493. [PubMed] [Google Scholar]
- 78.Feng R., Feng L., Yuan Z., Wang D., Wang F., Tan B., Han S., Li T., Li D., Han Y. Icariin protects against glucocorticoid-induced osteoporosis in vitro and prevents glucocorticoid-induced osteocyte apoptosis in vivo. Cell Biochem. Biophys. 2013;67:189–197. doi: 10.1007/s12013-013-9533-8. [DOI] [PubMed] [Google Scholar]
- 79.Seelinger G., Merfort I., Wolfle U., Schempp C.M. Anti-carcinogenic effects of the flavonoid luteolin. Molecules. 2008;13:2628–2651. doi: 10.3390/molecules13102628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Seelinger G., Merfort I., Schempp C.M. Anti-oxidant, anti-inflammatory and anti-allergic activities of luteolin. Planta Med. 2008;74:1667–1677. doi: 10.1055/s-0028-1088314. [DOI] [PubMed] [Google Scholar]
- 81.Kim H.R., Park C.G., Jung J.Y. Acacetin (5,7-dihydroxy-4’-methoxyflavone) exhibits in vitro and in vivo anticancer activity through the suppression of NF-κB/Akt signaling in prostate cancer cells. Int. J. Mol. Med. 2014;33:317–324. doi: 10.3892/ijmm.2013.1571. [DOI] [PubMed] [Google Scholar]
- 82.Bhat T.A., Nambiar D., Tailor D., Pal A., Agarwal R., Singh R.P. Acacetin inhibits in vitro and in vivo angiogenesis and downregulates Stat signaling and VEGF expression. Cancer Prev. Res. (Phila) 2013;6:1128–1139. doi: 10.1158/1940-6207.CAPR-13-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ha S.E., Kim S.M., Vetrivel P., Kim H.H., Bhosale P.B., Heo J.D., Lee H.J., Kim G.S. Inhibition of Cell Proliferation and Metastasis by Scutellarein Regulating PI3K/Akt/NF-kappaB Signaling through PTEN Activation in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2021;22:8841. doi: 10.3390/ijms22168841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Abusaliya A., Bhosale P.B., Kim H.H., Ha S.E., Park M.Y., Jeong S.H., Vetrivel P., Park J.-S., Kim G.S. Prunetinoside Inhibits Lipopolysaccharide-Provoked Inflammatory Response via Suppressing NF-κB and Activating the JNK-Mediated Signaling Pathway in RAW264.7 Macrophage Cells. Int. J. Mol. Sci. 2022;23:5442. doi: 10.3390/ijms23105442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Raha S., Kim S.M., Lee H.J., Yumnam S., Saralamma V.V., Ha S.E., Lee W.S., Kim G.S. Naringin Induces Lysosomal Permeabilization and Autophagy Cell Death in AGS Gastric Cancer Cells. Am. J. Chin. Med. 2020;48:679–702. doi: 10.1142/S0192415X20500342. [DOI] [PubMed] [Google Scholar]
- 86.Kim S.M., Vetrivel P., Ha S.E., Kim H.H., Kim J.A., Kim G.S. Apigetrin induces extrinsic apoptosis, autophagy and G2/M phase cell cycle arrest through PI3K/AKT/mTOR pathway in AGS human gastric cancer cell. J. Nutr. Biochem. 2020;83:108427. doi: 10.1016/j.jnutbio.2020.108427. [DOI] [PubMed] [Google Scholar]
- 87.Paramanantham A., Kim M.J., Jung E.J., Kim H.J., Chang S.H., Jung J.M., Hong S.C., Shin S.C., Kim G.S., Lee W.S. Anthocyanins Isolated from Vitis coignetiae Pulliat Enhances Cisplatin Sensitivity in MCF-7 Human Breast Cancer Cells through Inhibition of Akt and NF-kappaB Activation. Molecules. 2020;25:3623. doi: 10.3390/molecules25163623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stagos D., Balabanos D., Savva S., Skaperda Z., Priftis A., Kerasioti E., Mikropoulou E.V., Vougogiannopoulou K., Mitakou S., Halabalaki M., et al. Extracts from the Mediterranean Food Plants Carthamus lanatus, Cichorium intybus, and Cichorium spinosum Enhanced GSH Levels and Increased Nrf2 Expression in Human Endothelial Cells. Oxid. Med. Cell Longev. 2018;2018:6594101. doi: 10.1155/2018/6594101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Harbeoui H., Hichami A., Wannes W.A., Lemput J., Tounsi M.S., Khan N.A. Anti-inflammatory effect of grape (Vitis vinifera L.) seed extract through the downregulation of NF-κB and MAPK pathways in LPS-induced RAW264.7 macrophages. S. Afr. J. Bot. 2019;125:1–8. doi: 10.1016/j.sajb.2019.06.026. [DOI] [Google Scholar]
- 90.Han H.-S., Shin J.-S., Lee S.-B., Park J.C., Lee K.-T. Cirsimarin, a flavone glucoside from the aerial part of Cirsium japonicum var. ussuriense (Regel) Kitam. ex Ohwi, suppresses the JAK/STAT and IRF-3 signaling pathway in LPS-stimulated RAW 264.7 macrophages. Chem.-Biol. Interact. 2018;293:38–47. doi: 10.1016/j.cbi.2018.07.024. [DOI] [PubMed] [Google Scholar]
- 91.Mishra A.P., Salehi B., Sharifi-Rad M., Pezzani R., Kobarfard F., Sharifi-Rad J., Nigam M. Programmed Cell Death, from a Cancer Perspective: An Overview. Mol. Diagn. Ther. 2018;22:281–295. doi: 10.1007/s40291-018-0329-9. [DOI] [PubMed] [Google Scholar]
- 92.D’Arcy M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019;43:582–592. doi: 10.1002/cbin.11137. [DOI] [PubMed] [Google Scholar]
- 93.Pfeffer C.M., Singh A.T. Apoptosis: A target for anticancer therapy. Int. J. Mol. Sci. 2018;19:448. doi: 10.3390/ijms19020448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ha S.E., Kim S.M., Lee H.J., Vetrivel P., Venkatarame Gowda Saralamma V., Heo J.D., Kim E.H., Lee S.J., Kim G.S. Scutellarein induces fas-mediated extrinsic apoptosis and G2/M cell cycle arrest in Hep3B hepatocellular carcinoma cells. Nutrients. 2019;11:263. doi: 10.3390/nu11020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vrhovac Madunić I., Madunić J., Antunović M., Paradžik M., Garaj-Vrhovac V., Breljak D., Marijanović I., Gajski G. Apigenin, a dietary flavonoid, induces apoptosis, DNA damage, and oxidative stress in human breast cancer MCF-7 and MDA MB-231 cells. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2018;391:537–550. doi: 10.1007/s00210-018-1486-4. [DOI] [PubMed] [Google Scholar]
- 96.Guo Y., Wei L., Zhou Y., Lu N., Tang X., Li Z., Wang X. Flavonoid GL-V9 induces apoptosis and inhibits glycolysis of breast cancer via disrupting GSK-3β-modulated mitochondrial binding of HKII. Free Radic. Biol. Med. 2020;146:119–129. doi: 10.1016/j.freeradbiomed.2019.10.413. [DOI] [PubMed] [Google Scholar]
- 97.Huang W., Guo X., Wang C., Alzhan A., Liu Z., Ma X., Shu Q. α-Linolenic acid induces apoptosis, inhibits the invasion and metastasis, and arrests cell cycle in human breast cancer cells by inhibiting fatty acid synthase. J. Funct. Foods. 2022;92:105041. doi: 10.1016/j.jff.2022.105041. [DOI] [Google Scholar]
- 98.Paramanantham A., Jung E.J., Kim H.J., Jeong B.K., Jung J.-M., Kim G.S., Hong S.C., Lee W.S. Doxorubicin-Resistant TNBC Cells Exhibit Rapid Growth with Cancer Stem Cell-like Properties and EMT Phenotype, Which Can Be Transferred to Parental Cells through Autocrine Signaling. Int. J. Mol. Sci. 2021;22:12438. doi: 10.3390/ijms222212438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Parton M., Dowsett M., Smith I. Studies of apoptosis in breast cancer. BMJ. 2001;322:1528–1532. doi: 10.1136/bmj.322.7301.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Guo L., Li Y., Xing Z., Zhang J., Zhang J. Role of VEGFB in electrical pulse stimulation inhibits apoptosis in C2C12 myotubes. Peptides. 2022;154:170823. doi: 10.1016/j.peptides.2022.170823. [DOI] [PubMed] [Google Scholar]
- 101.Mehraj U., Aisha S., Sofi S., Mir M.A. Expression pattern and prognostic significance of baculoviral inhibitor of apoptosis repeat-containing 5 (BIRC5) in breast cancer: A comprehensive analysis. Adv. Cancer Biol.-Metastasis. 2022;4:100037. doi: 10.1016/j.adcanc.2022.100037. [DOI] [Google Scholar]
- 102.Siddiqui W.A., Ahad A., Ahsan H. The mystery of BCL2 family: Bcl-2 proteins and apoptosis: An update. Arch Toxicol. 2015;89:289–317. doi: 10.1007/s00204-014-1448-7. [DOI] [PubMed] [Google Scholar]
- 103.Volkmann N., Marassi F.M., Newmeyer D.D., Hanein D. The rheostat in the membrane: BCL-2 family proteins and apoptosis. Cell Death Differ. 2014;21:206–215. doi: 10.1038/cdd.2013.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Papadakis E.S., Reeves T., Robson N.H., Maishman T., Packham G., Cutress R.I. BAG-1 as a biomarker in early breast cancer prognosis: A systematic review with meta-analyses. Br. J. Cancer. 2017;116:1585–1594. doi: 10.1038/bjc.2017.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang X., Simpson E.R., Brown K.A. p53: Protection against Tumor Growth beyond Effects on Cell Cycle and Apoptosis. Cancer Res. 2015;75:5001–5007. doi: 10.1158/0008-5472.CAN-15-0563. [DOI] [PubMed] [Google Scholar]
- 106.Pan Y., Yuan Y., Liu G., Wei Y. P53 and Ki-67 as prognostic markers in triple-negative breast cancer patients. PLoS ONE. 2017;12:e0172324. doi: 10.1371/journal.pone.0172324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yang P., Du C.W., Kwan M., Liang S.X., Zhang G.J. The impact of p53 in predicting clinical outcome of breast cancer patients with visceral metastasis. Sci. Rep. 2013;3:2246. doi: 10.1038/srep02246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tavana O., Benjamin C.L., Puebla-Osorio N., Sang M., Ullrich S.E., Ananthaswamy H.N., Zhu C. Absence of p53-dependent apoptosis leads to UV radiation hypersensitivity, enhanced immunosuppression and cellular senescence. Cell Cycle. 2010;9:3328–3336. doi: 10.4161/cc.9.16.12688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dabiri Y., Abu El Maaty M.A., Chan H.Y., Wolker J., Ott I., Wolfl S., Cheng X. p53-Dependent Anti-Proliferative and Pro-Apoptotic Effects of a Gold(I) N-Heterocyclic Carbene (NHC) Complex in Colorectal Cancer Cells. Front Oncol. 2019;9:438. doi: 10.3389/fonc.2019.00438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Attari F., Keighobadi F., Abdollahi M., Arefian E., Lotfizadeh R., Sepehri H., Moridi Farimani M. Inhibitory effect of flavonoid xanthomicrol on triple-negative breast tumor via regulation of cancer-associated microRNAs. Phytother Res. 2021;35:1967–1982. doi: 10.1002/ptr.6940. [DOI] [PubMed] [Google Scholar]
- 111.Ferlay J., Colombet M., Soerjomataram I., Mathers C., Parkin D.M., Pineros M., Znaor A., Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer. 2019;144:1941–1953. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 112.Kim S.M., Ha S.E., Lee H.J., Rampogu S., Vetrivel P., Kim H.H., Venkatarame Gowda Saralamma V., Lee K.W., Kim G.S. Sinensetin Induces Autophagic Cell Death through p53-Related AMPK/mTOR Signaling in Hepatocellular Carcinoma HepG2 Cells. Nutrients. 2020;12:2462. doi: 10.3390/nu12082462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xu Y., Wang S., Chan H.F., Lu H., Lin Z., He C., Chen M. Dihydromyricetin Induces Apoptosis and Reverses Drug Resistance in Ovarian Cancer Cells by p53-mediated Downregulation of Survivin. Sci. Rep. 2017;7:46060. doi: 10.1038/srep46060. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.