Abstract
Simkania negevensis, a novel microorganism belonging to the family Simkaniaceae in the order Chlamydiales, has an intracellular developmental cycle during which two morphological entities, elementary bodies (EB) and reticulate bodies (RB), are seen by electron microscopy. Rates of seropositivity to the organism are high in certain population groups, and S. negevensis has been associated with respiratory illness in humans. This study reports for the first time the ability of S. negevensis to survive and grow inside Acanthamoeba polyphaga in addition to its known ability to grow in cell cultures of human or simian origin. Infectivity of S. negevensis and growth in amoebae were monitored by immunoperoxidase assays. Long-term persistence and exponential growth of S. negevensis in amoebal trophozoites were demonstrated by infectivity assays and by electron microscopy. EB and dividing RB of S. negevensis were observed within inclusion bodies inside A. polyphaga. When S. negevensis-infected A. polyphaga amoebae were exposed to adverse conditions resulting in encystation of the amoebae, several possible outcomes were observed: cysts containing both normal amoebic cytoplasm and S. negevensis; cysts in which S. negevensis cells were relegated to the space between cyst walls; and cysts containing S. negevensis, but apparently lacking amoebal cytoplasm. S. negevensis within dried amoebal cysts was capable of long-term survival. The possibility that amoebae may have a role in natural transmission of S. negevensis needs to be investigated.
Simkania negevensis is a recently discovered chlamydia-like intracellular microorganism (17, 18, 20) that has been associated with bronchiolitis in infants (19) and with community-acquired pneumonia in adults (23). Exposure to the organism, formerly referred to as “the microorganism Z” or “Simkania Z,” is widespread in the Negev region of Israel (12), in Vancouver, British Columbia, Canada, in Brooklyn, N.Y., and in Lima, Peru (unpublished data).
S. negevensis has been phylogenetically assigned to a new family, Simkaniaceae, in the order Chlamydiales, based on ribosomal DNA (rDNA) sequence comparisons (8). The recently proposed reorganization of the taxonomy of the order Chlamydiales includes two other new families, Waddliaceae and Parachlamydiaceae (8, 28). Microorganisms of the latter family have been shown to be able to grow as endocytobionts in protozoa of the genus Acanthamoeba (1, 16), and evidence for their possible association with respiratory illness has been reported (5). Detection of parachlamydia-related 16S rDNA sequences in respiratory specimens and specimens of aortic tissue has also been reported (13, 27).
Free-living amoebae such as Acanthamoeba are commonly found in natural water sources and usually feed on bacteria; however, some bacteria, such as Legionella pneumophila, are capable of surviving within amoebae, amoebal cysts, or amoebal respirable vesicles, rendering the bacteria highly resistant to adverse conditions, such as elevated temperature, chlorination, and biocidal compounds (3, 21). At the molecular level, legionellae interact with their protozoan hosts much as they do with mammalian cells (14). In this study, we wished to determine whether S. negevensis, which was first discovered as a cell culture contaminant, is able to grow in trophozoites of acanthamoebae and survive within amoebal cysts.
MATERIALS AND METHODS
Growth of S. negevensis, standard purification procedure, and determination of IFU.
Vero cells were grown in RPMI medium supplemented with 15% fetal calf serum, 1% glucose, 100 U of penicillin per ml, 100 μg of streptomycin per ml, 1.2 μg of nystatin per ml, 8 μg of gentamicin per ml, and 50 μg of vancomycin per ml. S. negevensis (ATCC VR 1471T) was grown in Vero cell cultures in the presence of 1 μg of cycloheximide per ml. For the standard purification procedure, cultures were harvested with glass beads between 7 and 15 days after infection and mildly sonicated. Cell debris was removed by centrifugation for 10 min at 1,000 × g. S. negevensis reticulate bodies (RB) and elementary bodies (EB) were purified on Urografin (76%; Schering, AG, Berlin, Germany) gradients as described by Caldwell et al. (6) for purification of Chlamydia trachomatis. Briefly, after removal of cell debris by low-speed centrifugation, the bacterial suspension was centrifuged through a 35% (vol/vol) Urografin “cushion” (30 min at 54,000 × g) in a Beckman SW28 rotor. The pellet was resuspended in HEPES (25 mM)-saline buffer and layered onto a discontinuous (40% to 44% to 52% [vol/vol]) Urografin gradient in HEPES-saline buffer. After centrifugation for 45 min at 54,000 × g, RB and EB bands were removed by puncturing the gradient tube and diluted in HEPES-saline; particles were sedimented by further centrifugation (30 min at 54,000 × g). The number of infectious-center-forming units (IFU) was determined by 10-fold dilution and infection of Vero cells cultured in 96-well plates. Three days postinfection, the plates were fixed in 95% ethanol for 10 min at room temperature and examined for IFU by the microtiter plate immunoperoxidase assay (PIPA), performed as described previously (19). Briefly, after fixation, plates were incubated for 1 h at 37°C with S. negevensis-specific antisera raised in rabbits, washed, and reincubated (1 h, 37°C) with swine anti-rabbit horseradish peroxidase-conjugated antibodies, and, after another wash, stained with diaminobenzidine as a substrate. Infectious centers were counted under a magnification of ×200 with an inverted microscope and averaged for triplicate wells, and the number of IFU per milliliter in the original sample was calculated.
Cultivation of amoebae and estimation of number of amoebae in closed culture bottles.
Acanthamoeba polyphaga strain Linc Ap-1, described by Fallon and Rowbotham (10) and kindly provided by R. J. Birtles (Unité des Rickettsies, CNRS EP J 0054, Faculté de Médecine, Marseille, France) was grown at 25°C under axenic conditions in PYG medium (32) supplemented with 100 U of penicillin ml−1, 100 μg of streptomycin ml−1, 1.2 μg of nystatin ml−1, 8 μg of gentamicin ml−1, and 50 μg of vancomycin ml−1.
Growth of A. polyphaga in 25-cm2 flasks (in 5 ml of PYG medium) was monitored by observation under an inverted phase-contrast microscope. The amoebae in 10 microscope fields (magnification, ×200) were counted, and the average number per field was calculated. Preliminary calibration of amoebal counts per microscope field against hemocytometer counts, by using uninfected amoebae, made determination of the number of amoebae in closed culture flasks possible. A calibration factor of 2.6 × 103 to 3.0 × 103 amoebae per ml for each amoeba seen in the field (at ×200 magnification) was established from eight separate calibration experiments. In this way, manipulation of infected amoebae was kept to a minimum.
Encystation procedure.
The encystation procedure described by Sykes and Band (31) was used: A. polyphaga were grown in PYG for 3 days to 2 × 106 ml−1, scraped with glass beads, and centrifuged for 10 min at 1,000 × g. The pellet was washed with a low-salt solution (50 mM NaCl, 4.6 mM MgSO4, 0.36 mM CaCl2) and resuspended to the original volume in high-salt solution (250 mM NaCl, 4.6 mM MgSO4, 0.36 mM CaCl2). The suspension was incubated for 40 h at 25°C, and the cysts were aliquoted, pelleted as described above, and washed with the high-salt solution. They were stored as a dry pellet either at 4°C or at room temperature.
Growth of S. negevensis in A. polyphaga.
A. polyphaga was grown in 5 ml of PYG in a supine 25-cm2 flask until the posterior wall of the flask was almost covered with organisms. This resulted in a total density of about 1.5 × 106 amoebae ml−1. Growth medium was gently removed without disturbing the layer of amoebae, purified S. negevensis grown in Vero cells was added at a multiplicity of infection (MOI) of 1.0 IFU per amoeba in 1 ml of PYG, and the flask was incubated at 25°C for 2 h. Amoebae were gently displaced with glass beads (1 mm in diameter) and diluted to 30 ml, and the suspension was equally divided among three new flasks (time zero). For the growth curve, samples were taken at various times postinfection and frozen at −70°C in the presence of 50% fetal calf serum until assayed by titration on Vero cells.
Titration on Vero cells of IFU of S. negevensis from amoebae.
Prior to titration, samples of frozen infected amoebae were diluted in RPMI infection medium containing 1 μg of cycloheximide ml−1, which prevents A. polyphaga from feeding on Vero cells. Titrations were carried out in triplicate in 96-well microtiter plates, and 3 days later, the number of IFU was determined after staining of the wells in our standard PIPA (19).
Light microscopic detection of S. negevensis-infected A. polyphaga
An immunoperoxidase assay (IPA) for detection of A. polyphaga infected with S. negevensis in 25-cm2 flasks was developed based on a similar assay used for cell cultures infected with S. negevensis (19). After the standard fixation in 95% ethanol for 10 min, additional treatment with a solution containing 90% methanol, 5% H2O, and 5% H2O2 (30%) for 10 min was necessary in order to neutralize the endogenous peroxidase activity of the amoebae. The remainder of the procedure was identical to that for peroxidase-based detection of infected cell cultures.
EM of infected amoebae.
Cultures of infected amoebae were pelleted, fixed, embedded in aralyte, and stained for electron microscopy (EM) as described by Biberfeld (4).
RESULTS
Infection of A. polyphaga trophozoites with S. negevensis
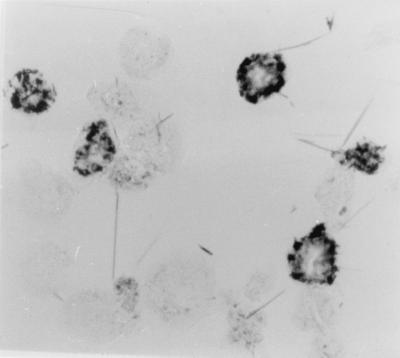
A. polyphaga trophozoites could be infected with S. negevensis in several ways: the amoebae could be added to infected Vero cell cultures, purified S. negevensis particles grown in Vero cells could be incubated with A. polyphaga in PYG medium, or persistently infected amoebae could be diluted in PYG in the presence of uninfected amoebae. The IPA was developed to stain S. negevensis-infected A. polyphaga, as shown in Fig. 1. Infected amoebae displayed one or more vesicles stained blue with antibodies specific for S. negevensis. Uninfected amoebae were not stained so long as endogenous peroxidase activity was neutralized after fixation, as described in Materials and Methods. The IPA was used for simple monitoring of the presence of S. negevensis in amoebal trophozoites.
FIG. 1.
Determination of percentage of acanthamoebae infected with S. negevensis by IPA. Infected amoebae stain dark blue with hyperimmune rabbit serum, peroxidase-conjugated swine anti-rabbit antibodies, and benzidine substrate. Stained and unstained amoebae are easily distinguished. Magnification, ×400.
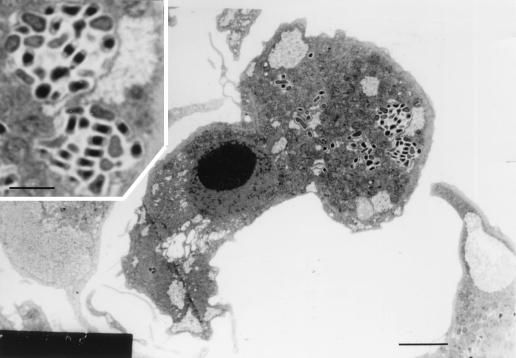
To determine the morphology of S. negevensis in infected amoebae, S. negevensis-infected trophozoites of A. polyphaga were fixed and prepared for EM. Thin sections displayed inclusions containing one or more EB and RB particles of S. negevensis (Fig. 2). The inset in Fig. 2 shows two of the inclusions at higher magnification. The EB are somewhat elongated, with condensed chromatin, and the RB are rather pleomorphic. Such inclusions were observed in A. polyphaga axenic cultures even weeks and months after original infection, demonstrating the ability of S. negevensis to exist as endocytobionts of the amoebae.
FIG. 2.
Transmission electron micrograph of a thin section of an A. polyphaga trophozoite infected with S. negevensis. Inclusion vesicles containing numerous or single RB and EB are seen. Bar = 1 μm. (Inset) Enlarged portion of the figure showing two inclusions containing somewhat elongated, condensed EB and rather pleomorphic RB. Bar = 0.3 μm.
The developmental cycle of S. negevensis in A. polyphaga
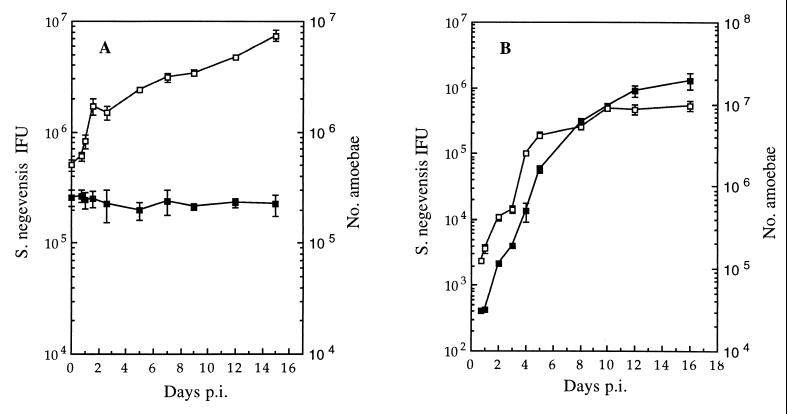
A. polyphaga amoebae were infected at an MOI of 1 with purified S. negevensis grown in Vero cells. The increase in the number of amoebae as well as the number of infectious S. negevensis particles (IFU, assayed on Vero cells) was monitored over time. Figure 3A shows survival of S. negevensis for 15 days without significant proliferation. The rather dense population of A. polyphaga continued to grow. When a sample from a 15-day flask from the experiment depicted in Fig. 3A was diluted 80-fold in fresh PYG, the increase in the number of amoebae as well as S. negevensis infectivity was monitored as a function of time, and the results shown in Fig. 3B were obtained. Exponential growth of both amoebae and the S. negevensis organisms infecting them was observed.
FIG. 3.
Growth curves of S. negevensis in A. polyphaga and of the amoebae themselves in the same cultures. (A) Infection of a relatively high-density amoebal culture at an MOI of 1, with S. negevensis purified from infected Vero cell cultures. (B) Results after dilution of the 15-day culture shown in panel A into fresh PYG medium (see text). Solid symbols, IFU of S. negevensis; open symbols, number of amoebae in the culture at the given time point. Bars indicate the standard deviation of the mean for amoebal counts and S. negevensis titers determined in triplicate. p.i., postinfection.
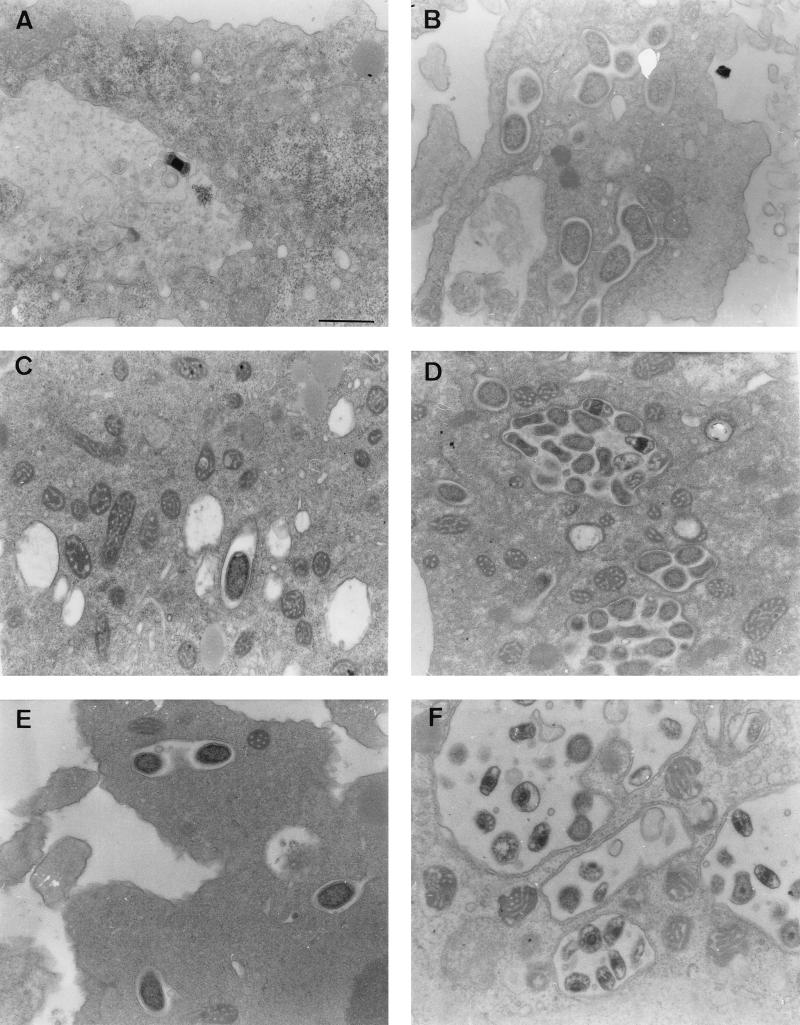
EM studies were carried out to characterize morphological features of the two types of infection represented by the growth curves of S. negevensis in Fig. 3. In the nonproliferative state (Fig. 3A), only a small proportion (<1%) of amoebae were seen to be infected. S. negevensis particles, mainly EB, were seen inside inclusions (usually containing only single particles), almost no dividing RB were seen, and mitochondria were not seen in the proximity of the inclusions (Fig. 4 A, C, and E). In contrast, in the “proliferative” mode (Fig. 3B), EB and dividing RB could easily be seen, even at 24 h postinfection (Fig. 4B). Later in infection, many S. negevensis inclusions were seen containing both EB and dividing RB particles (Fig. 4D and F). The inclusions were closely surrounded by a large number of host mitochondria.
FIG. 4.
Time course of S. negevensis nonproliferative infection (left panels) versus productive infection (right panels) of amoebae, as monitored by EM. (A, C, and E) One, 7, and 15 days postinfection, respectively. (B, D, and F) One, 10, and 16 days postinfection, respectively. Bar = 0.5 μm.
Encystation of S. negevensis in A. polyphaga
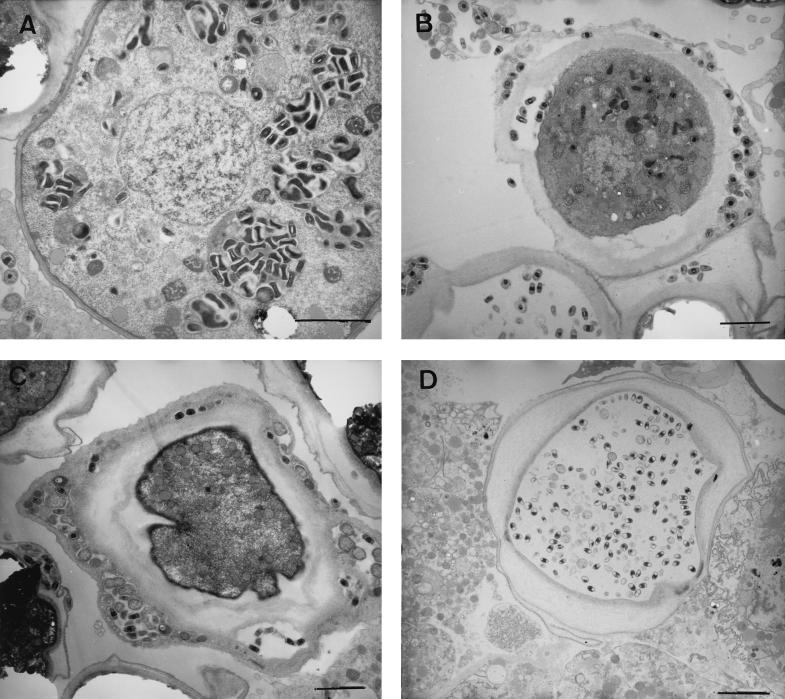
The life cycle of Acanthamoeba spp. comprises two distinct stages: the trophozoite (vegetative, feeding form) and the cyst (dormant form, which develops under adverse conditions). The cysts are double-walled, resilient entities, which can survive exposure to temperatures between −20°C and +42°C (2). The fate of S. negevensis residing in A. polyphaga under conditions in which encystation occurred was examined by EM of thin sections of encysted material. Figure 5A shows a precyst in which a number of S. negevensis inclusions containing both EB and RB particles are seen. In the process of encystation, when the double wall was formed, S. negevensis particles could be seen between the cyst walls or on the inner side of the outer wall (Fig. 5B and C). In Fig. 5B, some bacteria could still be seen residing inside the cyst cytoplasm, while in Fig. 5C, it appears as if the amoeba succeeded in excluding S. negevensis from the central cytoplasm of the cyst. However, another outcome was observed (Fig. 5D) in which only the bacteria seemed to reside inside the double wall of the cyst. In parallel with the EM studies, cysts were prepared and stored dry at room temperature or at 4°C. The ability of S. negevensis-infected cysts and uninfected cysts to convert to trophozoites upon return to optimal growth conditions was observed by microscopy, and the recovery of S. negevensis infectivity was monitored by PIPA (Table 1). After 79 days at 4°C, infectivity of S. negevensis that had been sequestered in amoebal cysts was still greater than 50% of initial infectivity, while purified S. negevensis EB particles did not survive 12 days of exposure to room temperature or 4°C, even when they were suspended in SPG (sucrose-phosphate-glutamic acid medium), a standard storage solution used to preserve the microorganisms at −70°C (data not shown).
FIG. 5.
Electron micrographs of A. polyphaga infected with S. negevensis, 2 days after encystation, demonstrating several possible interactions between the two organisms. (A) Precyst. (B to D) Double-walled cysts. Bars = 1 μm.
TABLE 1.
Survival of S. negevensis in cysts of A. polyphaga as a function of timea
| Time (days) | Infectivity (% of initial) at:
|
|
|---|---|---|
| Room temp | 4°C | |
| 0 | 2.5 × 106 (100) | 2.5 × 106 (100) |
| 21 | 2.13 × 106 (85) | 2.6 × 106 (100) |
| 48 | 2.23 × 106 (89) | NDb |
| 79 | ND | 1.4 × 106 (56) |
| 148 | 7.75 × 103 (0.3) | 8.6 × 103 (0.3) |
Results of a representative experiment are shown. Other experiments gave similar results, but the longest time point has not yet been repeated.
ND, not determined.
DISCUSSION
S. negevensis was initially isolated as a contaminant of cell cultures, and its natural host or hosts were unknown. However, the finding that its 23S rRNA contains an unspliced group I intron similar to those found in chloroplasts of algae and mitochondria of amoebae (9) stimulated investigation of its ability to infect amoebae. In this study, the ability of S. negevensis to grow in A. polyphaga as well as in cultured cells of human or simian origin was demonstrated for the first time. Infection of A. polyphaga with purified S. negevensis grown in Vero cells resulted in loss of most of the bacterial infectivity; however, in some fraction of the amoebal culture, S. negevensis was able to survive for more than 15 days and eventually multiply (Fig. 3). A similar phenomenon was observed when Acanthamoeba castellani amoebae were infected with Chlamydophila pneumoniae (7), an intracellular microorganism of the Chlamydiaceae family that causes epidemic and endemic respiratory infections in humans and has been associated with chronic and acute cardiovascular disease. Dilution of amoebae persistently infected with S. negevensis and their transfer to favorable nutritional conditions allowed exponential growth of S. negevensis (Fig. 3B). By EM, EB and dividing RB particles could be seen inside very distinct inclusions, which were closely surrounded by a large number of mitochondria (Fig. 4B, D, and F); these images were very similar to those seen in infected HeLa cells (17) and Vero cells (unpublished results). EB were 0.2 to 0.3 μm in diameter and usually contained some white, electrolucent areas in addition to areas of condensed DNA. RB were more pleomorphic than EB, with dimensions of 0.3 to 0.7 μm, and had no areas of condensed DNA.
Encystation of S. negevensis-infected amoebae under unfavorable environmental conditions resulted in competition for survival between S. negevensis and the amoebae. Several possible outcomes were observed: (i) amoebal cysts in which S. negevensis was found between the cyst walls (Fig. 5B and C), as was previously described by Steinert et al. (30) in the case of Mycobacterium avium; (ii) cysts in which both S. negevensis and normal amoebic cytoplasm were seen; and (iii) cysts in which S. negevensis remained alone, protected by the double wall of the cyst (Fig. 5D). Similarly, legionellae have been shown to be able to survive and be transmitted inside amoebal cysts (21, 29; reviewed in reference 15).
Free-living protozoa such as Acanthamoeba are commonly found in water supplies, air, desert dust, and cooling systems (24, 25). These organisms have been associated with amoebic keratitis, especially in wearers of contact lenses (24), and can also produce a rare encephalitic infection in immunocompromised individuals. Many types of bacteria have been found within free-living amoebae, including Legionella spp., Burkholderia pickettii, Vibrio cholerae, Mycobacterium avium, and Listeria monocytogenes (26, 30; reviewed in references 14 and 15). These bacteria are able to survive inside the protozoa as endocytobionts and to take advantage of them as vectors for their dissemination as pathogens.
A variety of chlamydia-like microorganisms have recently been detected by PCR in diverse clinical specimens and environmental samples (27). Additional endocytobionts belonging to the order Chlamydiales as determined by amplification of 16S rDNA are being recovered from clinical and environmental isolates of Acanthamoeba spp. and Hartmannella vermiformis (13, 16). The survival of S. negevensis as an endocytobiont in trophozoites of A. polyphaga and in cysts demonstrated in this study may have implications for the mode of transmission of the microorganism. Although we have shown preliminary evidence for the survival of S. negevensis in amoebal cysts for as long as 21 weeks (Table 1), the potential for survival under various types of adverse conditions still needs to be systematically examined. The widespread seropositivity to the organism in different population groups (11, 12) and the apparently early age of acquisition of infection, at least in some population groups (11; M. G. Friedman, A. Galil, D. Greenberg, S. Greenberg, R. Dagan, B. Sarov, and S. Kahane, Abstr. First Cong. Eur. Soc. Emerg. Infect., Budapest, Hungary, poster 15, 1998), support the possibility that amoebae may have a role in the natural transmission of S. negevensis, since, in the past, early acquisition of infection with Helicobacter pylori was associated with transmission in drinking water (22).
It has been suggested that microorganisms capable of surviving and growing inside amoebal hosts may have gained some evolutionary advantage allowing growth and survival within human macrophages, since in some ways, living protozoa mimic professional macrophages (13). The ability of S. negevensis to grow exponentially both in protozoa and in cell culture implies that this microorganism may be useful as a model system for comparison of the physiology of mechanisms of survival in different hosts and has important implications for transmission and pathogenesis of the microorganism.
ACKNOWLEDGMENTS
This work was supported by grant no. 4672/0 from the Office of the Chief Scientist of the Israel Ministry of Health, via the Keren Kayemet LeIsrael, and under grant no. TA-Mou-99-C19–033, U.S.-Israel Cooperative Development Research Program, Economic Growth, U.S. Agency for International Development.
We acknowledge with thanks the gift of Acanthamoeba polyphaga as well as generous advice from R. J. Birtles and the assistance of R. Jeger with some of the EM.
REFERENCES
- 1.Amann R, Springer N, Schönhuber W, Ludwig W, Schmid E N, Müller K-D, Michel R. Obligate intracellular bacterial parasites of acanthamoebae related to Chlamydia spp. Appl Environ Microbiol. 1997;63:115–121. doi: 10.1128/aem.63.1.115-121.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong M. The pathogenesis of human Acanthamoeba infection. Infect Dis Rev. 2000;2:65–73. [Google Scholar]
- 3.Berk S G, Ting R S, Turner G W, Ashburn R J. Production of respirable vesicles containing live Legionella pneumophila cells by two Acanthamoeba spp. Appl Environ Microbiol. 1998;64:279–286. doi: 10.1128/aem.64.1.279-286.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biberfeld P. Cytological studies on blood lymphocytes activated by phytohaemagglutinin in vitro. Acta Pathol Microbiol Scand. 1971;223:7–8. [PubMed] [Google Scholar]
- 5.Birtles R J, Rowbotham T J, Storey C, Marrie T J, Raoult D. Chlamydia-like obligate parasite of free-living amoebae. Lancet. 1997;349:925–930. doi: 10.1016/s0140-6736(05)62701-8. [DOI] [PubMed] [Google Scholar]
- 6.Caldwell H D, Kromhout J, Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981;31:1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Essig, A., M. Heinemann, U. Simnacher, and R. Marre. Infection of Acanthamoeba castellani by Chlamydia pneumoniae. Appl. Eviron. Microbiol. 63:1396–1399. [DOI] [PMC free article] [PubMed]
- 8.Everett K D E, Bush R M, Andersen A A. Emended description of the order Chlamydiales, proposal of Parachlamydiaceae fam. nov. and Simkaniaceae fam. nov., each containing one monotypic genus, revised taxonomy of the family Chlamydiaceae including a new genus and five new species, and standards for the identification of organisms. Int J Syst Bacteriol. 1999;49:415–440. doi: 10.1099/00207713-49-2-415. [DOI] [PubMed] [Google Scholar]
- 9.Everett K D E, Kahane S, Bush R M, Friedman M G. An unspliced group I intron in 23S rRNA links Chlamydiales, chloroplasts, and mitochondria. J Bacteriol. 1999;181:4734–4740. doi: 10.1128/jb.181.16.4734-4740.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fallon R J, Rowbotham T J. Microbiological investigations into an outbreak of Pontiac fever due to Legionella micdadei associated with use of a whirlpool. J Clin Pathol. 1990;43:479–483. doi: 10.1136/jcp.43.6.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman M G, Dvoskin B, Hartley J, Greenberg D, Chiu C-H, Hammerschlag M, Lieberman D, Roblin P, Gelling M, Ben-Dror S, Kahane S. Seropositivity to the novel microorganism Simkania negevensis in Israel, North America, and Great Britain. In: Saikku P, editor. Proceedings of the Fourth Meeting of the European Society for Chlamydia Research. Helsinki, Finland: Universitas Helsingiensis; 2000. p. 234. [Google Scholar]
- 12.Friedman M G, Galil A, Greenberg S, Kahane S. Seroprevalence of IgG antibodies to the Chlamydia-like microorganism “Simkania Z” by ELISA. Epidemiol Infect. 1999;122:117–123. doi: 10.1017/s095026889800185x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fritsche T R, Horn M, Wagner M, Herwig R P, Schliefer K-H, Gautom R K. Phylogenetic diversity among geographically dispersed Chlamydiales endosymbionts recovered from clinical and environmental isolates of Acanthamoeba spp. Appl Environ Microbiol. 2000;66:2613–2619. doi: 10.1128/aem.66.6.2613-2619.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harb O S, Gao L Y, Abu Kwaik Y. From protozoa to mammalian cells: a new paradigm in the life cycle of intracellular bacterial pathogens. Environ Microbiol. 2000;2:251–265. doi: 10.1046/j.1462-2920.2000.00112.x. [DOI] [PubMed] [Google Scholar]
- 15.Harf C. Free-living amoeba: interactions with environmental pathogenic bacteria. Endocytobiosis Cell Res. 1994;10:167–183. [Google Scholar]
- 16.Horn M, Wagner M, Müller K-D, Schmid E N, Fritsche T R, Schliefer K-H, Michel R. Neochlamydia hartmannellae gen. nov., sp. nov. (Parachlamydiaceae), an endoparasite of the amoeba Hartmannella vermiformis. Microbiology. 2000;146:1231–1239. doi: 10.1099/00221287-146-5-1231. [DOI] [PubMed] [Google Scholar]
- 17.Kahane S, Gonen R, Sayada C, Elion J, Friedman M G. Description and partial characterization of a new chlamydia-like microorganism. FEMS Microbiol Lett. 1993;109:329–334. doi: 10.1016/0378-1097(93)90041-y. [DOI] [PubMed] [Google Scholar]
- 18.Kahane S, Metzer E, Friedman M G. Evidence that the novel microorganism “Z” may belong to a new genus in the family Chlamydiaceae. FEMS Microbiol Lett. 1995;126:203–208. doi: 10.1111/j.1574-6968.1995.tb07417.x. [DOI] [PubMed] [Google Scholar]
- 19.Kahane S, Greenberg D, Friedman M G, Haikin H, Dagan R. High prevalence of “Simkania Z”, a novel Chlamydia-like bacterium, in infants with acute bronchiolitis. J Infect Dis. 1998;177:1425–1429. doi: 10.1086/517830. [DOI] [PubMed] [Google Scholar]
- 20.Kahane S, Everett K D E, Kimmel N, Friedman M G. Simkania negevensis strain ZT: growth, antigenic and genome characteristics. Int J Syst Bacteriol. 1999;49:815–820. doi: 10.1099/00207713-49-2-815. [DOI] [PubMed] [Google Scholar]
- 21.Kilvington S, Price J. Survival of Legionella pneumophila within cysts of Acanthamoeba polyphaga following chlorine exposure. J Appl Bacteriol. 1990;68:519–525. doi: 10.1111/j.1365-2672.1990.tb02904.x. [DOI] [PubMed] [Google Scholar]
- 22.Klein P D, Graham D Y, Gaillou A, Opekun A R, Smith E O Gastrointestinal Physiology Working Group. Water source as risk factor for Helicobacter pylori infection in Peruvian children. Lancet. 1991;337:1503–1506. doi: 10.1016/0140-6736(91)93196-g. [DOI] [PubMed] [Google Scholar]
- 23.Lieberman D, Kahane S, Lieberman D, Friedman M G. Pneumonia with serological evidence of acute infection with the chlamydia-like microorganism “Z.”. Am J Respir Crit Care Med. 1997;156:578–582. doi: 10.1164/ajrccm.156.2.9608081. [DOI] [PubMed] [Google Scholar]
- 24.Marshall M M, Naumovitz D, Ortega Y, Sterling C R. Waterborne protozoan pathogens. Clin Microbiol Rev. 1997;10:67–85. doi: 10.1128/cmr.10.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mergeryan H. The prevalence of Acanthamoeba in the human environment. Rev Infect Dis. 1991;13:S390–S391. doi: 10.1093/clind/13.supplement_5.s390. [DOI] [PubMed] [Google Scholar]
- 26.Michel R, Hauroder B. Isolation of an Acanthamoeba strain with intracellular Burkholderia pickettii infection. Zentbl Bakteriol. 1997;285:251–257. doi: 10.1016/s0934-8840(97)80116-8. [DOI] [PubMed] [Google Scholar]
- 27.Ossewarde J M, Meijer A. Molecular evidence for the existence of additional members of the order Chlamydiales. Microbiology. 1999;145:411–417. doi: 10.1099/13500872-145-2-411. [DOI] [PubMed] [Google Scholar]
- 28.Rurangirwa F R, Dilbeck P M, Crawford T B, McGuire T C, McElwain T F. Analysis of the 16S rRNA gene of microorganism WSU 86–1044 from an aborted bovine foetus reveals that it is a member of the order Chlamydiales: proposal of Waddliaceae fam. nov., Waddlia chondrophila gen. nov., sp. nov. Int J Syst Bacteriol. 1999;49:577–581. doi: 10.1099/00207713-49-2-577. [DOI] [PubMed] [Google Scholar]
- 29.Skinner A R, Anand C M, Malic A, Kurtz J B. Acanthamoeba and environmental spread of Legionella pneumophila. Lancet. 1983;ii:289–290. doi: 10.1016/s0140-6736(83)90277-5. [DOI] [PubMed] [Google Scholar]
- 30.Steinert M, Birkness K, White E, Fields B, Quinn F. Mycobacterium avium bacilli grow saprozoically in coculture with Acanthamoeba polyphaga and survive within cyst walls. Appl Environ Microbiol. 1998;64:2256–2261. doi: 10.1128/aem.64.6.2256-2261.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sykes D E, Band R N. Polyphenol oxidase production during encystation of Acanthamoeba castellani. J Protozool. 1985;32:512–517. doi: 10.1111/j.1550-7408.1985.tb04052.x. [DOI] [PubMed] [Google Scholar]
- 32.Visvesvara G S. Parasite culture: Acanthamoeba and Naegleria spp. In: Isenberg H, editor. Clinical microbiology procedures handbook. Washington, D.C.: American Society for Microbiology; 1992. pp. 7-9-2-1–7-9-2-8. [Google Scholar]