Abstract
Swine enteric viruses are a major cause of piglet diarrhea, causing a devastating impact on the pork industry. To further understand the molecular epidemiology and evolutionary diversity of swine enteric viruses, we carried out a molecular epidemiological investigation of swine enteric viruses (PEDV, PDCoV, PoRVA, and TGEV) on 7107 samples collected from pig farms in south-central China. The results demonstrated that PEDV is the predominant pathogen causing piglet diarrhea, and its infection occurs mainly in relatively cold winter and spring in Hunan and Hubei provinces. The positive rate of PEDV showed an abnormal increase from 2020 to 2021, and that of PoRVA and PDCoV exhibited gradual increases from 2018 to 2021. PEDV-PoRVA and PEDV-PDCoV were the dominant co-infection modes. A genetic evolution analysis based on the PEDV S1 gene and ORF3 gene revealed that the PEDV GII-a is currently epidemic genotype, and the ORF3 gene of DY2020 belongs to a different clade relative to other GII-a strains isolated in this study. Overall, our results indicated that the variant PEDV GII-a is the main pathogen of piglet diarrhea with a trend of outbreak. G9 is the dominant PoRVA genotype and has the possibility of outbreak as well. It is therefore critical to strengthen the surveillance of PEDV and PoRVA, and to provide technical reserves for the prevention and control of piglet diarrhea.
Keywords: swine enteric viruses, porcine epidemic diarrhea virus, epidemic and evolutionary characteristics, south-central China
1. Introduction
Swine diarrhea is a pathological symptom caused by multi-factor disorders, including pathogenic microorganisms, feeding environment, and the immune level. Viral diarrhea is a prevalent form of piglet diarrhea in farms, which is mainly manifested as anorexia, vomiting, watery diarrhea, and then body failure and even death [1]. At present, the most common viral pathogens causing piglet diarrhea include porcine epidemic diarrhea virus (PEDV), porcine Group A rotarvirus (PoRVA), porcine deltacoronavirus (PDCoV), transmissible gastroenteritis virus (TGEV), etc. [2]. Both PEDV and TGEV have caused the pandemic of swine diarrhea [3,4].
PEDV can cause acute contact infectious diseases characterized by watery diarrhea and dehydration. Pigs of all ages are susceptible to PEDV, particularly suckling piglets within 7 days of age, which tend to have the most severe symptom with an infection fatality rate up to 90–100% [5]. The clinical symptoms caused by TGEV are similar to those caused by PEDV. TGEV mainly infects piglets within 10 days of age, and the infection rate has been maintained at a relatively low level in recent years [6,7]. PoRVA causes diarrhea, vomiting, and other symptoms, and mainly infects piglets within 60 days of age [8]. PoRVA is distributed worldwide, and its prevalence has shown a steady upward trend in these years, which has aroused the attention of many countries. Moreover, the PoRVA is also a main cause of severe diarrhea in human infants and other young animals through zoonotic transmission [9,10,11]. It has been reported that rotavirus infection kills between 454,000 and 705,000 children under the age of 5 each year in developing countries [12].
After 2010, large-scale outbreaks of porcine epidemic diarrhea (PED) have frequently occurred worldwide. Etiological studies have demonstrated that the PEDV epidemic strains that cause this epidemic have greater genetic variations than the PEDV vaccine strain CV777. The homology of the S gene between the PEDV epidemic strains and CV777 is only 91% to 94% [5,13]. With the commercialization of the PEDV-TGEV inactivated vaccine, PEDV-TGEV-PoRVA (G5 type) live vaccine, and some other antiviral products [14,15,16,17,18], the PEDV epidemic has been better controlled. However, mutation still frequently occurs in the PEDV gene. Therefore, it is highly necessary to monitor the PED epidemic trend and PEDV gene mutation for the timely prevention and control of the epidemic [14,18]. In this study, we conducted an epidemiological investigation and genetic evolution analysis of swine enteric viruses in major pig-producing provinces in China, aiming to clarify the genetic variation trend of PEDV and PoRVA epidemic strains in recent years. The findings are expected to provide technical and material support for the prevention and control of PEDV and PoRVA epidemics.
2. Materials and Methods
2.1. Collection and Pre-Treatment of Clinical Samples
A total of 7107 intestinal and fecal samples were collected from diseased piglets during the outbreak of diarrhea on immunized farms in Henan, Hubei, Jiangsu, Shandong, Guangdong, and Hunan, Jiangxi, and Sichuan provinces of China from 2018 to 2021. The specific source information of the samples is presented in Table 1. The samples were frozen and thawed three times to release the virus after homogenization with phosphate-buffered saline (PBS, Gibco, Thermo Fisher Scientific, Waltham, MA, USA). After centrifugation at 15,000 rpm for 10 min, the supernatants were collected for the extraction of viral nucleic acid and the isolation of the virus.
Table 1.
The information of the samples collected from each province from 2018 to 2021.
| Year | Number of Samples | |||||||
|---|---|---|---|---|---|---|---|---|
| Henan | Hubei | Jiangsu | Shandong | Guangdong | Hunan | Jiangxi | Sichuan | |
| 2018 | 241 | 242 | 201 | 220 | 231 | 257 | 211 | 298 |
| 2019 | 225 | 217 | 177 | 211 | 203 | 221 | 197 | 271 |
| 2020 | 317 | 266 | 232 | 259 | 254 | 286 | 237 | 322 |
| 2021 | 188 | 147 | 141 | 167 | 153 | 171 | 148 | 196 |
2.2. Reverse Transcription PCR (RT-PCR)
The viral RNA was extracted by using MolPure® Viral DNA/RNA Kit (Yeasen, Shanghai, China) following the manufacturer’s instructions. The quantity and quality of the extracted RNA were measured by using a Nanodrop spectrophotometer (Thermo, Waltham, MA, USA). Viral RNA was then transcribed into cDNA by utilizing reverse transcription using the HiScript II 1st Strand cDNA Synthesis Kit (Vazyme, Nanjing, China). The primers targeting the PEDV M gene, PDCoV M gene, PoRVA VP7 gene, and TGEV N gene were designed and shown in Table 2. The cDNA was screened by RT-PCR using the 2 × Taq Master Mix (Dye Plus) (Vazyme, Nanjing, China) on SimpliAmpTM Thermal Cycler (Thermo Fisher Scientific, Waltham, MA, USA).
Table 2.
Primers for swine enteric virus gene detection.
| Primer Name | Sequence | Product Length | Target Gene |
|---|---|---|---|
| PDCoV-P1 | CCAGCAACCACTCGTGTTA | 620 bp | M gene |
| PDCoV-P2 | GTCCTTAGTTGGTTTGGTGGGT | ||
| PEDV-P2 | GTCTAACGGTTCTATTCCCG | 460 bp | M gene |
| PEDV-P3 | ATAGCCCTCTACAAGCAATG | ||
| TGEV-P5 | TTACAAACTCGCTATCGCATGG | 528 bp | N gene |
| TGEV-P6 | TCTTGTCACATCACCTTTACCTGC | ||
| PORVA-P7 | CCCCGGTATTGAATATACCACAGT | 333 bp | VP7 gene |
| PORVA-P8 | TTTCTGTTGGCCACCCTTTAGT |
2.3. Sanger Sequencing
For amplification of the PEDV S gene and ORF3 gene, the primers against the S1, S2, and ORF3 genes were designed by using Primer 5.0. The sequences of primers are shown in Table 3. Then, the cDNA of 96 clinical samples and five isolated PEDV strains was used to amplify the PEDV S1, S2, and ORF3 genome by using 2 × Phanta Max Master Mix (Dye Plus) (Vazyme, Nanjing, China). The PCR products were observed and acquired under ultraviolet after electrophoresis in 0.01–0.03% YeaRed (Yeasen, Shanghai, China) stained agarose gel. Subsequently, the PCR products were extracted by E.Z.N.A.® Gel Extraction Kit (OmegaBio-tek, Inc., Norcross, GA, USA) and sent to Tsingke Biotechnology Co., Ltd. (Beijing, China) for sanger sequencing.
Table 3.
Primers for PEDV S gene and ORF3 gene sequencing.
| Primer Name | Sequence | Product Length | Purpose |
|---|---|---|---|
| S1-F | ATGAAGTCTTTAACGTACTTCTGG | 2208 bp | PEDV gene sequencing |
| S1-R | TAGAAGAAACCAGGCAACTCC | ||
| S2-F | ATGCATTCTAATGATGGCTCTAAT | 1953 bp | |
| S2-R | CTGCACGTGGACCTTTTCAAAAAC | ||
| ORF3-F | ATGTTTCTTGGACTTTTTCAGTACA | 675 bp | |
| ORF3-R | ACTAATTGTAGCATACTCGTCTAG |
2.4. Phylogenetic and Evolution Analysis
A total of 213 PEDV S gene sequences, 208 PEDV ORF3 gene sequences, 59 VP7 gene sequences of human RVA strains, and 61 VP7 gene sequences of PoRVA strains uploaded after 2010 in China were downloaded from GenBank. In addition, the PEDV S1 gene of 96 clinical samples were sequenced. The genomic sequences of each gene were aligned by using MAFFT v7.4.02 [19], respectively. The phylogenetic tree was generated by the neighbor-joining method in MEGAX software with a p-distance model, using 1000 bootstrap replicates [20]. Potential recombination events in the complete genomes of PEDV DY2020 strains isolated in this study and other PEDV strains were assessed by the Recombination Detection Program v4.39 (RDP4), which included nine detection algorithms (RDP, GENECONV, Bootscan, Maxchi, Chimaera, SiSscan, PhylPro, LARD, and 3Seq) [21].
2.5. Cell Culture and Virus Isolation
Vero-E6 cell lines (Purchased from ATCC, Manassas, VA, USA) were cultured in Dulbecco’s modified Eagle medium (DMEM, Gibco, Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS, Natocor, Cordoba, ARG), and antibiotics (100 units/mL of penicillin, 100 μg/mL of streptomycin, and 0.25 μg/mL of Fungizone®, Thermo Fisher Scientific, Waltham, MA, USA), at 37 °C in a humidified incubator containing 5% CO2.
For PEDV isolation, 90%-confluent Vero cell monolayers were washed with PBS twice. Next, 200 μL of inoculum per well for a 6-well plate was added. After incubation at 37 °C for 2 h, 2 mL of PEDV growth medium (DMEM supplemented with antibiotics, and 10 μg/mL of trypsin (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) was added with the removal of the inoculum. After 48–72 h, the plate was frozen at −80 °C and thawed twice. The cells and supernatants were mixed by pipetting and stored at −80 °C. These samples were used as seed stocks for the next passage. For serial passaging, the culture scale was gradually increased, until finally T75 flasks were used for propagation and serial passage of PEDV strains. Virus RNA was extracted every 5 passages for RT-PCR detection of the virus nucleic acids.
2.6. Viral Growth Kinetics
The growth kinetics of isolated PEDV strains was determined by using a one-step growth curve and the 50% tissue culture infective dose (TCID50) assay. Monolayers of Vero cells were inoculated with isolated PEDV strains at a volume ratio of 1:10. After 2 h, the monolayers were washed twice with PBS, and 2 mL of DMEM containing 2% FBS was added. The culture supernatant was collected at 0, 2, 6, 12, 18, 24, 30, and 36 h post-inoculation and was then used to calculate the TCID50 of the virus after being frozen at −80 °C and thawing twice.
For TCID50 determination of PEDV strains, Vero cell monolayers in 96-well plates were inoculated with the culture supernatants of each strain serially diluted in DMEM at 37 °C for 1.5 h. Then, the infected cells were cultured in DMEM supplemented with 10% heat-inactivated FBS at 37 °C with 5% CO2 for 48 h. The cytopathic effect (CPE) on Vero cells was determined to calculate the TCID50 based on the Reed–Muench formula [21].
2.7. Swine Enteric Virus Investigation
The positive rate of four swine enteric viruses from 2018 to 2021 was used to calculate the co-infection rate of each virus in the positive samples. According to the climate in south-central China, the 12 months of the year were divided into four seasons, namely spring (March to March), summer (June to August), autumn (September to November), and winter (December to February), and statistical analysis was performed on the positive rate of PEDV in different seasons to determine the seasonality of PEDV infection. By referring to the conserved sequences of the PEDV reference strains, Primer 5.0 was used to design primers for the main virulence genes of PEDV (S gene and ORF3 gene) and PoRVA (VP4, VP6, and VP7 genes).
2.8. Statistical Analysis
All the data were analyzed using GraphPad Prism 8.0.1 software (GraphPad Software Inc., La Jolla, CA, USA), and presented as means ± standard error of the mean (SEM). The student’s t-test was used for difference analysis between groups, and p < 0.05 was considered a significant difference. All experiments were repeated more than thrice [22].
3. Results
3.1. Positive Rate of Swine Enteric Viruses from 2018 to 2021
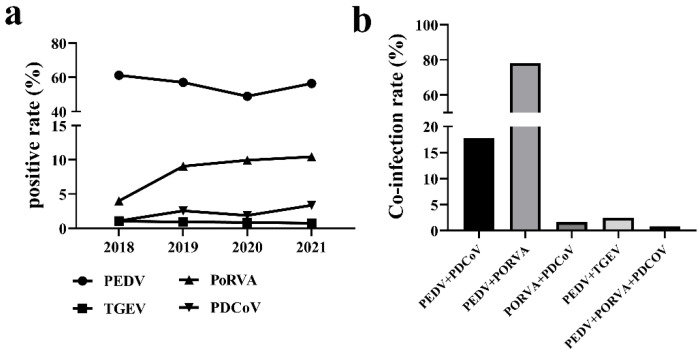
RT-PCR assay was performed to detect PEDV, PDCoV, PoRVA, and TGEV in the intestinal and fecal samples collected from 2018 to 2021. The detection rate of each virus was statistically analyzed and represented by a line graph. The results are shown in Table 4 and Figure 1a. PEDV was found to be the dominant cause of diarrhea in piglets, whose average positive rate was 56.09% during the investigation period. The positive rate of PEDV showed a sustained decreasing trend from 2018 to 2020. Surprisingly, it increased and reached 56.44% (740/1311) in 2021. PoRVA was the second major cause of diarrhea in piglets, whose positive rate increased gradually, reaching 10.45% (137/1311) in 2021. TGEV and PDCoV were the minor causes of diarrhea and remained at low levels, but the positive rate of PDCoV showed a slight increase in 2021.
Table 4.
Positive rates of swine enteric virus from 2018 to 2021.
| Year | Positive Rate (%) and (Number of Positive Samples/Number of Samples) | |||
|---|---|---|---|---|
| PEDV | TGEV | PoRVA | PDCoV | |
| 2018 | 61.02 (1160/1901) |
1.05 (20/1901) |
4.00 (76/1901) |
1.10 (21/1901) |
| 2019 | 57.03 (982/1722) |
0.93 (16/1722) |
9.06 (156/1722) |
2.56 (44/1722) |
| 2020 | 50.81 (1104/2173) |
0.87 (19/2173) |
9.94 (216/2173) |
1.89 (41/2173) |
| 2021 | 56.44 (740/1311) |
0.76 (10/1311) |
10.45 (137/1311) |
3.36 (44/1311) |
Figure 1.
Positive rate of swine enteric viruses from 2018 to 2021. (a) Variation trend of the positive rate of diarrhea virus from 2018 to 2021. The X-axis represents the year, and the Y-axis represents the positive rate of PEDV, TGEV, PoRVA, and PDCoV. (b) Co-infection rate of swine enteric viruses in samples collected from 2018 to 2021. The X-axis represents the pattern of co-infection, and the Y-axis represents the co-infection rate.
The information of all PCR positive samples was used to determine the co-infection of PEDV, PoRVA, TGEV, and PDCoV. As shown in Figure 1b, a total of 125 samples were infected by multiple enteric viruses. About 77.6% (97/125) of PEDV positive samples were found to be co-infected by PoRVA, followed by 17.6% (22/125) by PDCoV and 2.4% (3/125) by TGEV. The positive rate of PoRVA and PDCoV co-infection was 1.6% (2/125). The co-infection of PEDV, PoRVA, and PDCoV was extremely rare, accounting for only 0.8% (1/125).
3.2. Seasonal Characteristics and Distribution of PEDV
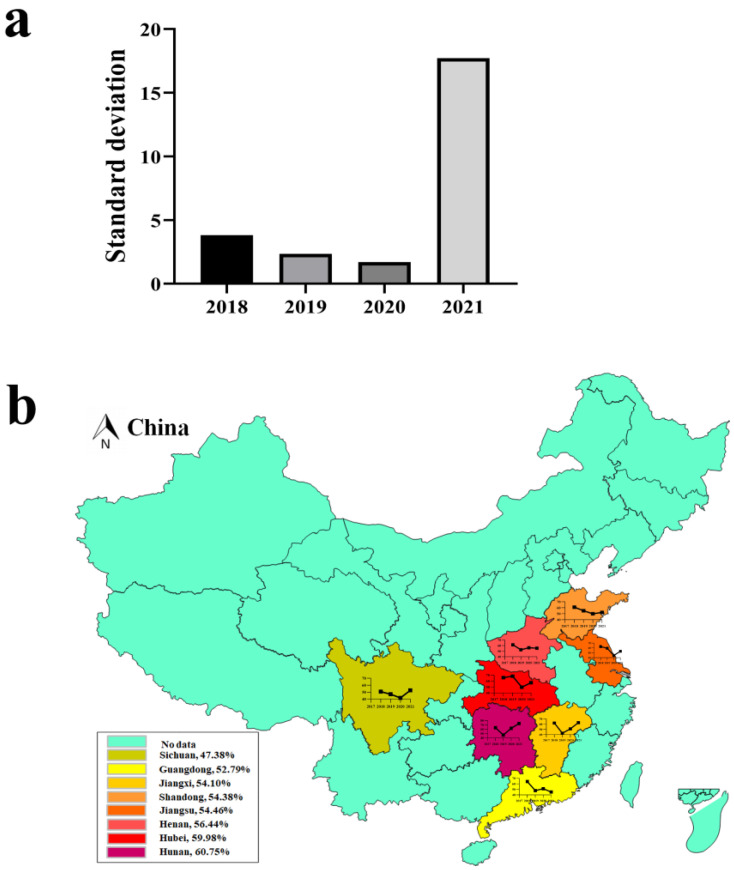
According to the climatic conditions of south-central China, the 12 months were divided into four seasons, namely spring (March–May), summer (June–August), autumn (September–November), and winter (December–February). The information of all PCR positive samples was used to calculate the positive rate of PEDV for each reason. As shown in Table 5, the positive rate of PEDV reached the highest in spring and winter, which was 71.79% (313/436) and 58.76% (191/325), respectively, while it was relatively low in the other two seasons, with the lowest level of 36.88% (97/263). The standard deviation of the PEDV positive rate in the four seasons from 2018 to 2021 was calculated. As shown in Figure 2a, the standard deviation from 2018 to 2021 was 3.81, 2.35, 1.70, and 17.72, respectively. The seasonality for the prevalence of PEDV tended to decrease from 2018 to 2020, and high positive rates may be present in any season in south-central China, however, the standard deviation in winter and spring in 2021 increased significantly, which may be due to the PEDV pandemic.
Table 5.
Positive rates of swine enteric virus from 2018 to 2021.
| Year | Positive Rate (%) and (Number of Positive Samples/Number of Samples) | |||
|---|---|---|---|---|
| Spring | Summer | Autumn | Winter | |
| 2018 | 63.14 (322/510) |
55.56 (210/378) |
59.34 (244/411) |
63.79 (384/602) |
| 2019 | 57.54 (271/471) |
54.76 (190/347) |
54.86 (209/381) |
59.66 (312/523) |
| 2020 | 52.87 (276/522) |
49.28 (239/485) |
49.60 (249/502) |
51.20 (340/664) |
| 2021 | 71.79 (313/436) |
36.88 (97/263) |
48.43 (139/287) |
58.76 (191/325) |
Figure 2.
Seasonal characteristics and distribution of PEDV. (a) The standard deviation of PEDV in samples collected in different seasons from 2018 to 2021. The X-axis represents the year, and the Y-axis represents the standard deviation of the PEDV positive rate in the four seasons from 2018 to 2021. (b) Distribution of PEDV detection rate in eight major pork-producing provinces of China from 2018 to 2021. The yellow area indicates a low positive rate from 2018 to 2021; the red area indicates the highly endemic areas; a darker color indicates a higher positive rate. Percentages represent the overall positive rate from 2018 to 2021. The histogram indicates the positive rate in each province, and the point indicates the positive rate of PEDV in that year (Note: the map does not represent the true borders of administrative regions of China).
We identified the incidence of PEDV infection in major pork-producing provinces in China from 2018 to 2021. As shown in Figure 2b, the incidence of PEDV infection varied in different provinces of China. Cases occurred in south-central China provinces, like Henan, Hubei, Jiangsu, Shandong, Guangdong, Hunan, Jiangxi, and Sichuan, with the overall incidence of 56.44% (548/971), 59.98% (523/872), 54.46% (409/751), 54.38% (466/857), 52.79% (444/841), 60.75% (568/935), 54.10% (429/793), and 47.38% (515/1087). In 2018, 2019, 2020, and 2021, the provinces with the highest PEDV positive rate were Hubei (65.70%), Hubei (68.20%), Hunan (61.89%), and Hunan (73.10%), respectively. These results revealed that the provinces of central China, such as Hunan and Hubei, are the most severely affected areas by PED, while Sichuan, the largest province of pig production in China, has the lowest detection rate of PEDV.
3.3. Phylogenetic and Recombination Analysis of PEDV
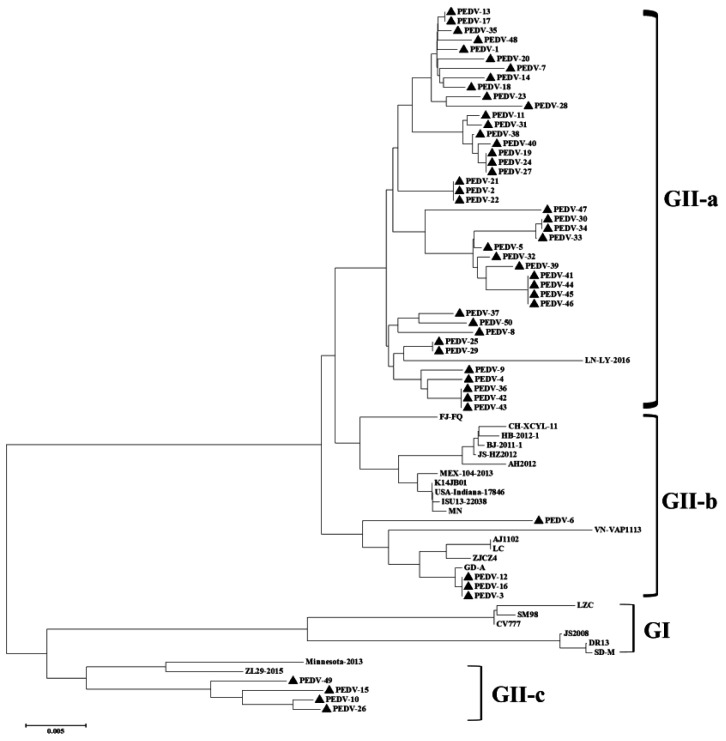
The S gene is the main virulence gene of PEDV, whose mutation leads to significant variations in PEDV virulence. A genetic evolution analysis was performed on the reference strains within each gene subgroup of PEDV, and the S1 gene sequences of 50 clinical samples were determined in this study. As shown in Figure 3, the S1 genes in this study all belonged to the new variant strain of PEDV, with 43 falling into the GII-a subgroup, four belonging to the GII-b subgroup, and three belonging to the GII-c subgroup.
Figure 3.
Phylogenetic analysis based on the PEDV S1 genes sequenced in this study. The phylogenetic tree was generated by the neighbor-joining method in MEGAX software with a p-distance model, using 1000 bootstrap replicates. The triangles represent the strains isolated in this study, and those without triangles represent the reference strains.
The S genes of the five viruses isolated in this study were compared with the reference sequences. As shown in Figure 4a, the phylogenetic tree constructed based on the S gene genome revealed that the PEDV strains in China could be mainly divided into two major groups (GI group and GII group). The GI group mainly included classic PEDV strains and recombinant strains, while most of the PEDV epidemic strains in China emerging in recent years belong to the GII group (variant strains). The strains of the GII group can be divided into two subgroups, GII-a and GII-b. In recent years, the strains of the GII-a subgroup have increased rapidly and tend to become a dominant subgroup. The five isolates isolated in this study were in different branches from the classic strain CV777, and all belonged to the GII-a subgroup, among which DY2020, HB2020, and HB2021 were located in the same branch.
Figure 4.
The phylogenetic analysis based on the S genes and ORF3 genes of the PEDV strains isolated in this study. (a) The phylogenetic analysis of the S gene. PEDV was divided into 5 categories: GII-a (Blue), GII-b (Green), GII-c (light pink), GI-a (light red), and GI-b (Yellow). The strains isolated in this study are highlighted in red color. (b). The phylogenetic analysis of the ORF3 gene. PEDV was divided into two categories: A group (Blue) and B group (Green). The strains isolated in this study are highlighted in red color. The phylogenetic trees were generated by the neighbor-joining method in MEGAX software with a p-distance model, using 1000 bootstrap replicates.
ORF3 gene is an important virulence gene of PEDV. We compared the ORF3 gene sequenced in this study with the ORF3 gene sequences of PEDV strains isolated from multiple regions in China and constructed a phylogenetic tree. As shown in Figure 4b, the PEDV strains were clustered into two groups based on the ORF3 gene, namely the A group and B group. The A group mainly included part of members in the GI group and GII-a subgroup, while the B group mainly comprised part of members in the GI group and GII-b subgroup. The ORF3 gene of the HB2018, HB2019, HB2020, and HB2021 strains was in the same branch as the GII-a strain, and that of the DY2020 strain was in the same branch as the GII-b subgroup strain, indicating that there is gene recombination between strains in the GII-a subgroup and GII-b subgroup.
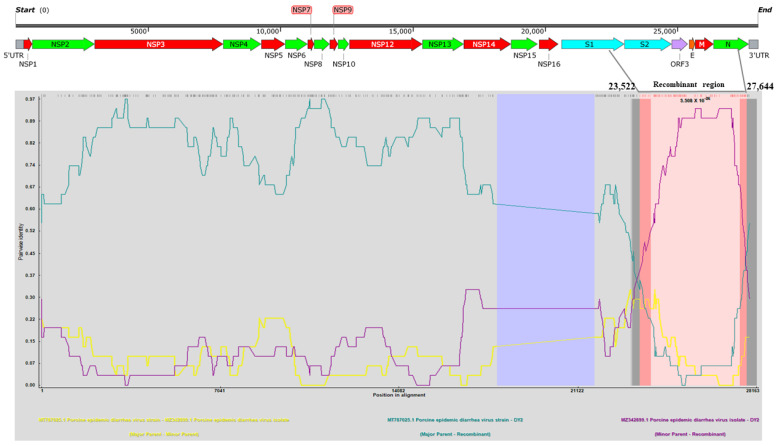
A complete genome recombination analysis was performed between the DY2020 strains and 125 reference strains by using RDP4 software. As shown in Figure 5, the PEDV DY2020 strain might arise from the recombination of MT787025.1 (major parent, GII-a) and MZ342899.1 (minor parent, GII-a), which was supported by six detection algorithms (RDP, p-values ≤ 1.121 × 10−27; GENECONV, p-values ≤ 2.441 × 10−25; Maxchi p-values ≤ 1.125 × 10−13; Chimaera, p-values ≤ 5.111 × 10−15; SiSscan, p-values ≤ 2.409 × 10−16; 3Seq, p-values ≤ 1.374 × 10−35). Further analysis indicated that the position 23,522–27,644 of the complete genome of the DY2020 strain was the predicted genetic recombination fragment, and the identified putative breakpoints were located in the S gene, ORF3 gene, and the genes encoding envelope protein, membrane protein, and part of nucleocapsid proteins of the PEDV DY2020 strain.
Figure 5.
The recombination analysis of the complete genome of PEDV DY2020 strains isolated in this study. The results were described using the RDP method, which was supported by ≥6 programs to further characterize the potential recombination events. The pink box indicates the regions for the occurrence of recombination events. The Y-axis represents the pairwise identity between the recombinant and its putative parents. The X-axis represents the position in alignment with a 30 nt sliding window. The comparison of the recombinant-major parent, recombinant-minor parent, and major-minor parent was indicated by cyan, purple, and yellow lines, respectively.
3.4. Isolation and Titer Analysis of PEDV
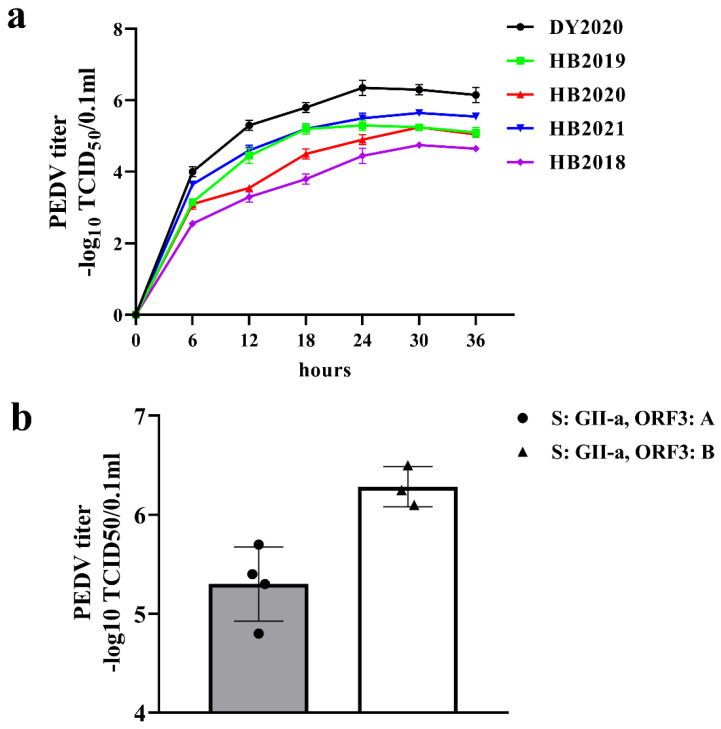
The growth kinetics of the five isolated PEDV strains was evaluated by measuring TCID50 at different infection time points. As shown in Figure 6a, the PEDV titers showed significant time-dependent increases and the DY2020 strain had the fastest growth in the first 12 h after infection. The titers of PEDV DY2020 strains were significantly higher than those of the other four PEDV strains at each infection stage, reaching a peak value of 106.2 TCID50/0.1 mL at 24 h post-infection. The phylogenetic analysis based on the S gene of PEDV strains isolated in this study revealed that the DY2020 strain and the other four strains belonged to the GII-a subgroup. However, based on ORF1gene, the other 4 strains belonged to the A group, and the DY2020 strain belonged to the B group, which was similar to the case of HM2017 and CH/HLJ/18 strains [23,24]. The growth characteristics of HM2017 and CH/HLJ/18 strains were similar to those of DY2020 isolates (Figure 6b).
Figure 6.
The growth kinetics of isolated PEDV strains and titer analysis. (a) Growth curves of isolated PEDV strains in Vero cells. (b) The relationship between PEDV strain titer and ORF3 gene clustering. The dots represent HB2018, HB2019, HB2020, and HB2021 strains, and the triangles represent DY2020, HM2017, and CH/HLJ/18 strains [23,24].
3.5. Phylogenetic Analysis of PoRVA
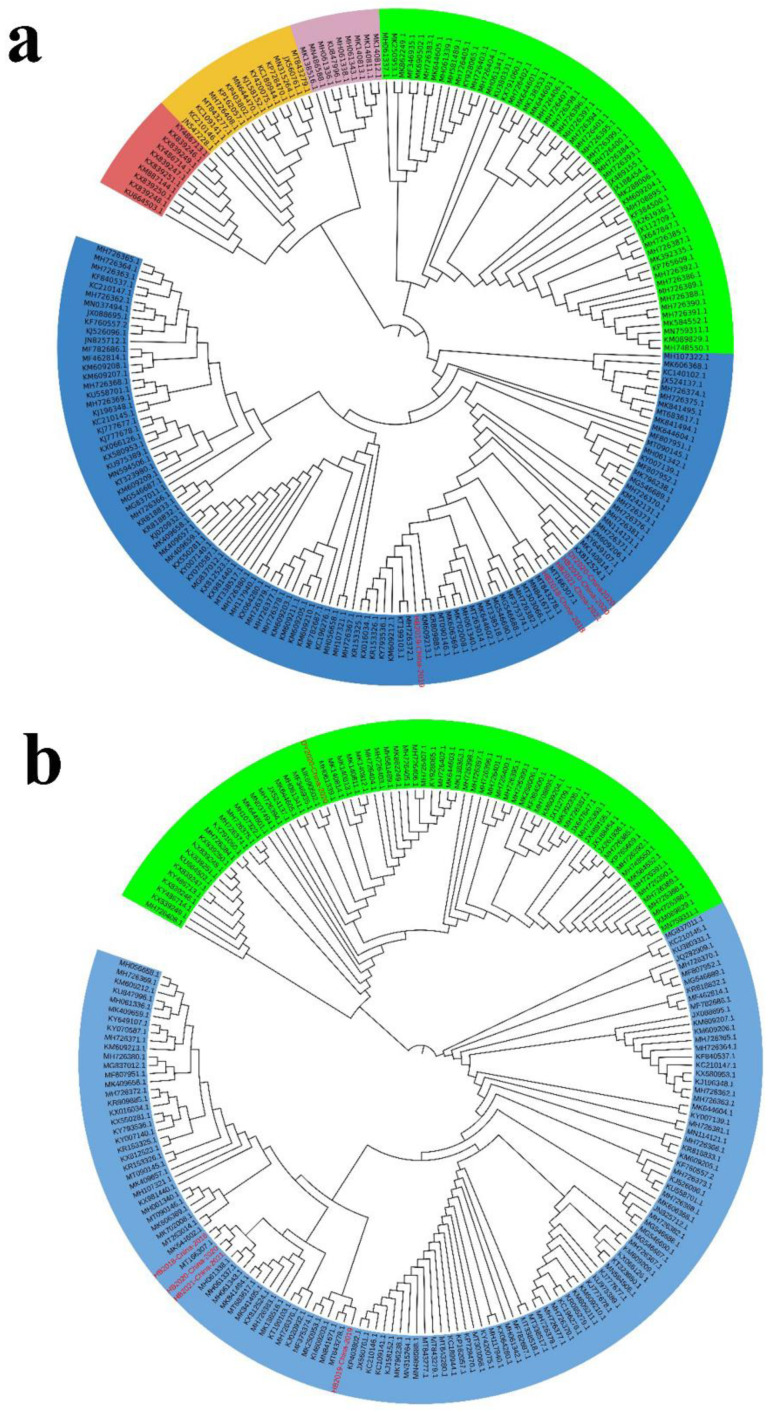
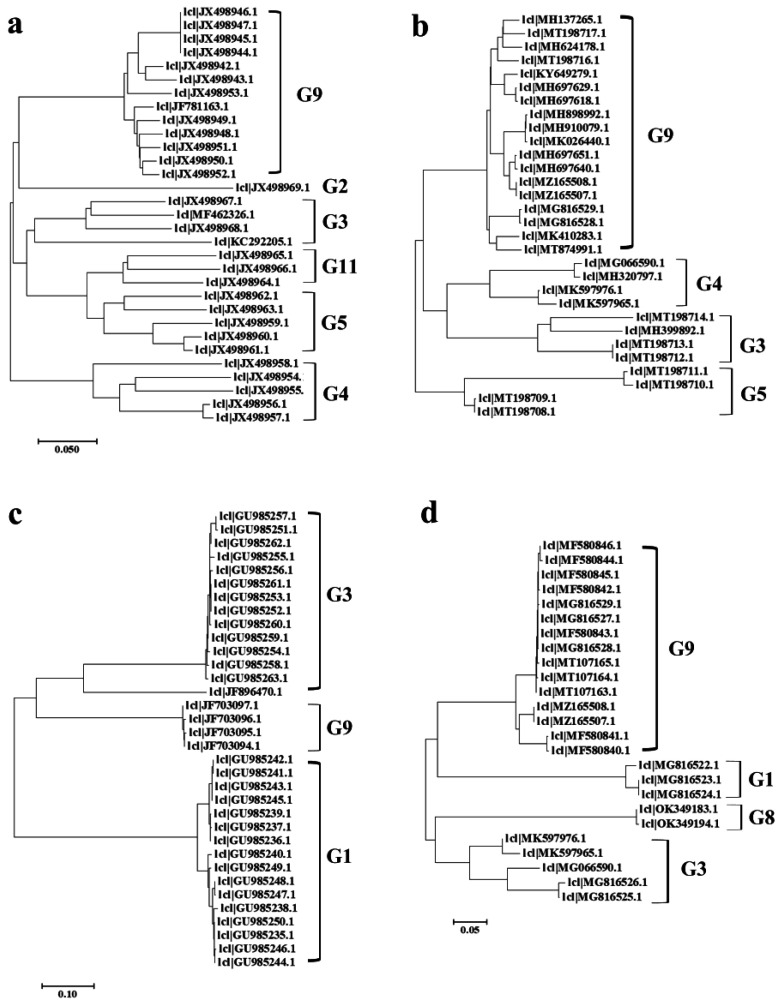
The G genotype of PoRVA is determined by its VP7 gene [11]. VP7 nucleotide with homology higher than 80% can be identified as the same genotype (G type). The phylogenetic analysis based on VP7 genes of porcine PoRVA strains demonstrated that G9 is always the most common G genotype of PoRVA in China (Figure 7a,b). The phylogenetic analysis based on VP7 gene of human rotaviruses strains showed that before 2017, human rotaviruses in China were mainly G1 and G3 types, while the G9 type only accounted for a small proportion. However, after 2018, the G9 type strain became the dominant genotype of human rotavirus (Figure 7c,d).
Figure 7.
Phylogenetic analysis based on the VP7 genes of the PoRVA strains and human rotavirus strains. (a,b) Phylogenetic analysis based on the VP7 genes of the PoRVA strains before (a) and after (b) 2018. (c,d) Phylogenetic analysis based on VP7 genes of human rotaviruses strains before (c) and after (d) 2018.
4. Discussion
Piglet viral diarrhea is an important cause of piglet death, leading to serious production reduction and huge economic loss in the pork industry. PEDV, PoRVA, PDCoV, and TGEV are the most common pathogens causing piglet diarrhea [25,26]. In this study, a total of 7107 diarrhea-related samples collected from pig farms in south-central China from 2018 to 2021 were examined to identify the major diarrhea pathogens. As a result, PEDV was identified as the most important cause of diarrhea, and its detection rate decreased from 2018 to 2020, which may be due to the wide use of the PEDV vaccine (GII-b) in China [18,27,28,29]. In addition, in 2018, the outbreak of African swine fever (ASF) in pigs was first reported in China, and was transmitted rapidly through the legal trade of live pigs, which led to the development of ASFV monitoring and control plans and the upgrading of the biosecurity system of pig farms [30,31,32]. These may be important reasons for the decline in the PED positive rate after 2018, and also provide some useful data for pig farms and the government to develop a bio-safety system for more efficient prevention and control of infectious diseases. In 2019, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first reported in Wuhan city of China, causing the coronavirus disease 2019 (COVID-19). As a result, strict nationwide prevention and control of COVID were implemented in China, which significantly restricted the flow of pedestrian, logistics, and animal products [33,34]. Due to the impact of COVID-19, the number of samples collected each year was inconsistent between 2018 and 2021, with the smallest number in 2021. However, our results still showed that the detection rate of PEDV increased sharply in 2021, indicating the potential risk of another outbreak and therefore the need to develop new PEDV vaccine strains. In addition, the detection rate of TGEV has been at a low level without a trend of large-scale epidemics, while the detection rate of PoRVA and PDCoV have had a rising tendency in recent years. The detection results of co-infection revealed that PEDV and PoRVA co-infection is currently the most important form of co-infection rather than the previous PEDV and TGEV co-infection [35,36], indicating that the monitoring and prevention of PoRVA should be strengthened. The seasonality for the prevalence of PEDV has been gradually decreasing [37,38], and many provinces showed high incidence throughout the year, suggesting that the prevention and control of the epidemic should be strengthened in the summer and autumn when PEDV was low in the past. Furthermore, we found that Hunan and Hubei were the provinces with the most severe PEDV epidemics in south-central China, which is consistent with the study of He et al. [39]. In addition, our results revealed that the GII-a subgroup has been increasing rapidly and has become dominant in recent years. The PEDV epidemic has been circulating many times and there is a trend of re-emergence [40,41,42]. Therefore, more attention should be paid to the PEDV GII-a subgroup [43]. The epidemiological investigation and research on the latest PEDV variants will provide theoretical guidance for the prevention and control of PED and provide vaccine reserves for preventing the outbreak of PED epidemics.
In this study, five PEDV variants were isolated from diarrhea samples using a Vero-E6 cell line, and primers were designed to sequence their main virulence genes (S gene and ORF3 gene) [18]. According to the genetic evolution analysis of the S gene, these five PEDV strains belong to the GII-a subgroup, which is closely related to the PEDV variant strains isolated in China in recent years, and possesses relative low homology to the commercial vaccine strain CV777 [44,45,46,47,48,49]. According to the genetic evolution analysis of the ORF3 gene, other four PEDV strains, except for DY2020 isolated in this study, are in the same branch as the strains of the GII-a subgroup, and these four strains form a small branch independently with the PEDV variant strains isolated in China in recent years, which may be caused by mutations at multiple loci. In addition, the ORF3 gene of one PEDV variant strain was in the same branch as that of the GII-b subgroup, indicating that the strains of the GII-a subgroup and the GII-b subgroup of PEDV might have undergone recombination in the natural environment. In addition, the recombinant DY2020 strain exhibited earlier and more obvious CPE on Vero cells, suggesting that mutation in the ORF3 protein may have altered its infectivity [23,24], which needs further verification. A comparison of the nucleotide and amino acid homology between the five strains and the commercial vaccine strains revealed that they have rather low homology. In recent years, the re-emergence of PEDV may be caused by these genetic variants [18]. Further research on the variant strains will help to understand the mechanisms for the re-emergence of PEDV and help the prevention of PED.
The gene sequences of PEDV epidemic strains at present are significantly different from those of previous classic strains and vaccine strains, and the nucleotide sequences of the S gene and ORF3 gene are also significantly different from those of PEDV epidemic strains and the classic strains CV777 reported previously [18]. Since 2010, large-scale PED outbreaks have frequently occurred in China due to the variation of PEDV. In addition, PEDV tends to have continuous mutations, posing new challenges to the prevention and control of PED. It is therefore necessary to strengthen the epidemiological study of PEDV mutant strains and pay close attention to the genetic variation of the pathogens. There are great differences between PEDV and existing commercial vaccine strains, and more PEDV mutant strains are emerging [43,50]. Therefore, China is in urgent need for vaccine strains corresponding to the epidemic strains, and research on the characteristics of epidemic strains will provide technical reserves for the control of PED epidemics.
The prevalent PoRVA G genotypes mainly include G9, G11, G2, G3, G4, and G5, among which G5 and G9 are the main genotypes in China [51]. The wide application of commercial vaccines, such as the TGEV-PEDV-PoRVA (G5) triple live vaccine, has effectively controlled the epidemic of the PoRVA G5 genotype in China. However, at the same time, we found that more G9-type PoRVAs were isolated from piglet diarrhea samples in China and other regions of Asia, which also demonstrates that G9-type PoRVA has been increasing in China and other Asian countries and has become the most predominant G type [52,53]. The G5 strains of PoRVA may be gradually reduced under the selection pressure of PoRVA (G5) vaccines, while the G9 strains slowly emerged through the results of epidemiological investigations. The typical sequences of the VP7 gene of human rotavirus strains (59) and pig PoRVA strains (61) from 2010 to 2021 were downloaded from GenBank. The evolutionary analysis revealed that rotavirus G1 and G3 strains were dominant before 2017, while after 2018 there was a surge of human G9 strain. The G9 strain, meanwhile, has been present and dominant in swine and dogs [51,54]. Previous studies have revealed that the G3 and G9 strains of RAV cause diarrhea in human infants and young children, and the recombination of RVA in humans and animals causes the generation of new PoRVA genotypes [55,56,57,58,59]. Therefore, we hypothesize that the wide application of related vaccines may largely reduce the prevalence of human G1 and G3 and pig G5 genotypes, while the rotavirus G9 genotype will eventually become the dominant genotype, which will undoubtedly increase the pressure on the prevention and control of porcine rotavirus. In addition, the G9 genotype also has the risk of infecting infants and young children, and thus calls for more attention [51,57,60,61,62]. By analyzing the prevalence of PoRVA and human rotavirus in China and studying the genetic evolution relationship of its genotypes, this study facilitates a better understanding of the trend of the G9 subtype and provides some important implications for the control of the rotavirus epidemic.
Acknowledgments
We thank the Shaobo Xiao research group from Huazhong Agricultural University for providing suggestions on the data analysis.
Author Contributions
Conceptualization, C.L., H.L. and D.Z.; Methodology, C.L., H.L. and D.Z.; software, C.L., H.L. and C.G.; validation, C.L., H.L., C.G., K.Y., W.L., Z.L., F.Y., T.G., S.W., H.S. and P.W.; investigation, C.L., H.L., C.G., K.Y., W.L., Z.L., F.Y., T.G., S.W., H.S. and P.W.; Visualization, C.L., H.L. and C.G.; Writing—Original Draft Preparation, C.L. and D.Z.; Writing—Review & Editing, C.L. and D.Z.; Supervision, Y.T. and D.Z.; Project Administration, Y.T. and D.Z.; Funding Acquisition, Y.T. and D.Z. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was supported by The Central Public-interest Scientific Institution Basal Research Fund (National Data Center of Animal Health), Technical Innovation Project of Hubei Province (2021ABA005), Key Laboratory of Prevention and Control Agents for Animal Bacteriosis (Ministry of Agriculture) (grant number: KLPCAAB-YTP-1801), and Hubei Province Innovation Center of Agricultural Sciences and Technology (2019–620–000-001-017). The funders had no role in the study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pan Z., Lu J., Wang N., He W.-T., Zhang L., Zhao W., Su S. Development of a TaqMan-probe-based multiplex real-time PCR for the simultaneous detection of emerging and reemerging swine coronaviruses. Virulence. 2020;11:707–718. doi: 10.1080/21505594.2020.1771980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhai S.-L., Wei W.-K., Li X.-P., Wen X.-H., Zhou X., Zhang H., Lv D.-H., Li F., Wang D. Occurrence and sequence analysis of porcine deltacoronaviruses in southern China. Virol. J. 2016;13:136. doi: 10.1186/s12985-016-0591-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li W., Li H., Liu Y., Pan Y., Deng F., Song Y., Tang X., He Q. New variants of porcine epidemic diarrhea virus, China, 2011. Emerg. Infect. Dis. 2012;18:1350–1353. doi: 10.3201/eid1803.120002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng S., Wu H., Chen Z. Evolution of Transmissible Gastroenteritis Virus (TGEV): A Codon Usage Perspective. Int. J. Mol. Sci. 2020;21:7898. doi: 10.3390/ijms21217898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun R.-Q., Cai R.-J., Chen Y.-Q., Liang P.-S., Chen D.-K., Song C.-X. Outbreak of porcine epidemic diarrhea in suckling piglets, China. Emerg. Infect. Dis. 2012;18:161–163. doi: 10.3201/eid1801.111259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwegmann-Wessels C., Herrler G. Transmissible gastroenteritis virus infection: A vanishing specter. Dtsch. Tierarztl. Wochenschr. 2006;113:157–159. [PubMed] [Google Scholar]
- 7.Gao D.-M., Yu H.-Y., Zhou W., Xia B.-B., Li H.-Z., Wang M.-L., Zhao J. Inhibitory effects of recombinant porcine interferon-α on porcine transmissible gastroenteritis virus infections in TGEV-seronegative piglets. Vet. Microbiol. 2021;252:108930. doi: 10.1016/j.vetmic.2020.108930. [DOI] [PubMed] [Google Scholar]
- 8.Jones T., Pritchard G., Paton D. Transmissible gastroenteritis of pigs. Vet. Rec. 1997;141:427–428. [PubMed] [Google Scholar]
- 9.Martella V., Bányai K., Matthijnssens J., Buonavoglia C., Ciarlet M. Zoonotic aspects of rotaviruses. Vet. Microbiol. 2010;140:246–255. doi: 10.1016/j.vetmic.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 10.Midgley S., Böttiger B., Jensen T.G., Friis-Møller A., Person L.K., Nielsen L., Barzinci S., Fischer T.K. Human group A rotavirus infections in children in Denmark: Detection of reassortant G9 strains and zoonotic P[14] strains. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2014;27:114–120. doi: 10.1016/j.meegid.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Midgley S.E., Bányai K., Buesa J., Halaihel N., Hjulsager C.K., Jakab F., Kaplon J., Larsen L.E., Monini M., Poljšak-Prijatelj M., et al. Diversity and zoonotic potential of rotaviruses in swine and cattle across Europe. Vet. Microbiol. 2012;156:238–245. doi: 10.1016/j.vetmic.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 12.Atii D.J., Ojeh C.K., Durojaiye O.A. Detection of rotavirus antigen in diarrhoeic and non-diarrhoeic piglets in Nigeria. Rev. Elev. Med. Vet. Pays Trop. 1990;42:494–496. [PubMed] [Google Scholar]
- 13.Zhou Y., Wu Y., Zhu J., Tong W., Yu H., Jiang Y., Tong G. Complete genome sequence of a virulent porcine epidemic diarrhea virus strain. J. Virol. 2012;86:13862. doi: 10.1128/JVI.02635-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerdts V., Zakhartchouk A. Vaccines for porcine epidemic diarrhea virus and other swine coronaviruses. Vet. Microbiol. 2017;206:45–51. doi: 10.1016/j.vetmic.2016.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pascual-Iglesias A., Sanchez C.M., Penzes Z., Sola I., Enjuanes L., Zuñiga S. Recombinant Chimeric Transmissible Gastroenteritis Virus (TGEV)—Porcine Epidemic Diarrhea Virus (PEDV) Virus Provides Protection against Virulent PEDV. Viruses. 2019;11:682. doi: 10.3390/v11080682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo T., Gao C., Hao J., Lu X., Xie K., Wang X., Li J., Zhou H., Cui W., Shan Z., et al. Strategy of Developing Oral Vaccine Candidates Against Co-infection of Porcine Diarrhea Viruses Based on a Lactobacillus Delivery System. Front. Microbiol. 2022;13:872550. doi: 10.3389/fmicb.2022.872550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meng F., Ren Y., Suo S., Sun X., Li X., Li P., Yang W., Li G., Li L., Schwegmann-Wessels C., et al. Evaluation on the efficacy and immunogenicity of recombinant DNA plasmids expressing spike genes from porcine transmissible gastroenteritis virus and porcine epidemic diarrhea virus. PLoS ONE. 2013;8:e57468. doi: 10.1371/journal.pone.0057468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang D., Fang L., Xiao S. Porcine epidemic diarrhea in China. Virus Res. 2016;226:7–13. doi: 10.1016/j.virusres.2016.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katoh K., Standley D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin D.P., Murrell B., Golden M., Khoosal A., Muhire B. RDP4: Detection and analysis of recombination patterns in virus genomes. Virus Evol. 2015;1:vev003. doi: 10.1093/ve/vev003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li C., Sun Y., Jiang C., Cao H., Zeng W., Zhang X., Li Z., He Q. Porcine circovirus type 2 infection activates NF-κB pathway and cellular inflammatory responses through circPDCD4/miR-21/PDCD4 axis in porcine kidney 15 cell. Virus Res. 2021;298:198385. doi: 10.1016/j.virusres.2021.198385. [DOI] [PubMed] [Google Scholar]
- 23.Wang X.N., Wang L., Zheng D.Z., Chen S., Shi W., Qiao X.Y., Jiang Y.P., Tang L.J., Xu Y.G., Li Y.J. Oral immunization with a Lactobacillus casei-based anti-porcine epidemic diarrhoea virus (PEDV) vaccine expressing microfold cell-targeting peptide Co1 fused with the COE antigen of PEDV. J. Appl. Microbiol. 2018;124:368–378. doi: 10.1111/jam.13652. [DOI] [PubMed] [Google Scholar]
- 24.Yang D., Su M., Li C., Zhang B., Qi S., Sun D., Yin B. Isolation and characterization of a variant subgroup GII-a porcine epidemic diarrhea virus strain in China. Microb. Pathog. 2020;140:103922. doi: 10.1016/j.micpath.2019.103922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jung K., Saif L.J., Wang Q. Porcine epidemic diarrhea virus (PEDV): An update on etiology, transmission, pathogenesis, and prevention and control. Virus Res. 2020;286:198045. doi: 10.1016/j.virusres.2020.198045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vlasova A.N., Amimo J.O., Saif L.J. Porcine Rotaviruses: Epidemiology, Immune Responses and Control Strategies. Viruses. 2017;9:48. doi: 10.3390/v9030048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu X., Zhang Q., Zhang L., Zhou P., Yang J., Fang Y., Dong Z., Zhao D., Li W., Feng J., et al. A newly isolated Chinese virulent genotype GIIb porcine epidemic diarrhea virus strain: Biological characteristics, pathogenicity and immune protective effects as an inactivated vaccine candidate. Virus Res. 2019;259:18–27. doi: 10.1016/j.virusres.2018.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y., Wang G., Wang J., Man K., Yang Q. Cell attenuated porcine epidemic diarrhea virus strain Zhejiang08 provides effective immune protection attributed to dendritic cell stimulation. Vaccine. 2017;35:7033–7041. doi: 10.1016/j.vaccine.2017.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bi J., Zeng S., Xiao S., Chen H., Fang L. Complete genome sequence of porcine epidemic diarrhea virus strain AJ1102 isolated from a suckling piglet with acute diarrhea in China. J. Virol. 2012;86:10910–10911. doi: 10.1128/JVI.01919-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao X., Liu T., Liu Y., Xiao J., Wang H. Transmission of African swine fever in China Through Legal Trade of Live Pigs. Transbound. Emerg. Dis. 2021;68:355–360. doi: 10.1111/tbed.13681. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y., Zhang X., Qi W., Yang Y., Liu Z., An T., Wu X., Chen J. Prevention and Control Strategies of African Swine Fever and Progress on Pig Farm Repopulation in China. Viruses. 2021;13:2552. doi: 10.3390/v13122552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ge S., Li J., Fan X., Liu F., Li L., Wang Q., Ren W., Bao J., Liu C., Wang H., et al. Molecular Characterization of African Swine Fever Virus, China, 2018. Emerg. Infect. Dis. 2018;24:2131–2133. doi: 10.3201/eid2411.181274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Du Z., Wang L., Cauchemez S., Xu X., Wang X., Cowling B.J., Meyers L.A. Risk for Transportation of Coronavirus Disease from Wuhan to Other Cities in China. Emerg. Infect. Dis. 2020;26:1049–1052. doi: 10.3201/eid2605.200146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flahault A. Has China faced only a herald wave of SARS-CoV-2? Lancet. 2020;395:947. doi: 10.1016/S0140-6736(20)30521-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Z.-L., Zhu L., Ma J.-Y., Zhou Q.-F., Song Y.-H., Sun B.-L., Chen R.-A., Xie Q.-M., Bee Y.-Z. Molecular characterization and phylogenetic analysis of porcine epidemic diarrhea virus (PEDV) field strains in south China. Virus Genes. 2012;45:181–185. doi: 10.1007/s11262-012-0735-8. [DOI] [PubMed] [Google Scholar]
- 36.Luo L., Chen J., Li X., Qiao D., Wang Z., Wu X., Du Q., Tong D., Huang Y. Establishment of method for dual simultaneous detection of PEDV and TGEV by combination of magnetic micro-particles and nanoparticles. J. Infect. Chemother. Off. J. Jpn. Soc. Chemother. 2020;26:523–526. doi: 10.1016/j.jiac.2020.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kong F., Xu Y., Ran W., Yin B., Feng L., Sun D. Cold Exposure-Induced Up-Regulation of Hsp70 Positively Regulates PEDV mRNA Synthesis and Protein Expression In Vitro. Pathogens. 2020;9:246. doi: 10.3390/pathogens9040246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hsueh F.-C., Lin C.-N., Chiou H.-Y., Chia M.-Y., Chiou M.-T., Haga T., Kao C.-F., Chang Y.-C., Chang C.-Y., Jeng C.-R., et al. Updated phylogenetic analysis of the spike gene and identification of a novel recombinant porcine epidemic diarrhoea virus strain in Taiwan. Transbound. Emerg. Dis. 2020;67:417–430. doi: 10.1111/tbed.13365. [DOI] [PubMed] [Google Scholar]
- 39.He W.-T., Bollen N., Xu Y., Zhao J., Dellicour S., Yan Z., Gong W., Zhang C., Zhang L., Lu M., et al. Phylogeography Reveals Association between Swine Trade and the Spread of Porcine Epidemic Diarrhea Virus in China and across the World. Mol. Biol. Evol. 2022;39:msab364. doi: 10.1093/molbev/msab364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zuo Q., Zhao R., Liu J., Zhao Q., Zhu L., Zhang B., Bi J., Yang G., Liu J., Yin G. Epidemiology and phylogeny of spike gene of porcine epidemic diarrhea virus from Yunnan, China. Virus Res. 2018;249:45–51. doi: 10.1016/j.virusres.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 41.Yu J., Chai X., Cheng Y., Xing G., Liao A., Du L., Wang Y., Lei J., Gu J., Zhou J. Molecular characteristics of the spike gene of porcine epidemic diarrhoea virus strains in Eastern China in 2016. Virus Res. 2018;247:47–54. doi: 10.1016/j.virusres.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 42.Tan L., Li Y., He J., Hu Y., Cai X., Liu W., Liu T., Wang J., Li Z., Yuan X., et al. Epidemic and genetic characterization of porcine epidemic diarrhea virus strains circulating in the regions around Hunan, China, during 2017-2018. Arch. Virol. 2020;165:877–889. doi: 10.1007/s00705-020-04532-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y., Chen Y., Yuan W., Peng Q., Zhang F., Ye Y., Huang D., Ding Z., Lin L., He H., et al. Evaluation of Cross-Protection between G1a- and G2a-Genotype Porcine Epidemic Diarrhea Viruses in Suckling Piglets. Animals. 2020;10:1674. doi: 10.3390/ani10091674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cui J.-T., Qiao H., Hou C.-Y., Zheng H.-H., Li X.-S., Zheng L.-L., Chen H.-Y. Characteristics of the spike and ORF3 genes of porcine epidemic diarrhea virus in Henan and Shanxi provinces of China. Arch. Virol. 2020;165:2323–2333. doi: 10.1007/s00705-020-04744-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang H., Han F., Yan X., Liu L., Shu X., Hu H. Prevalence and phylogenetic analysis of spike gene of porcine epidemic diarrhea virus in Henan province, China in 2015–2019. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2021;88:104709. doi: 10.1016/j.meegid.2021.104709. [DOI] [PubMed] [Google Scholar]
- 46.Wang Z., Li X., Shang Y., Wu J., Dong Z., Cao X., Liu Y., Lan X. Rapid differentiation of PEDV wild-type strains and classical attenuated vaccine strains by fluorescent probe-based reverse transcription recombinase polymerase amplification assay. BMC Vet. Res. 2020;16:208. doi: 10.1186/s12917-020-02424-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tian Y., Yu Z., Cheng K., Liu Y., Huang J., Xin Y., Li Y., Fan S., Wang T., Huang G., et al. Molecular characterization and phylogenetic analysis of new variants of the porcine epidemic diarrhea virus in Gansu, China in 2012. Viruses. 2013;5:1991–2004. doi: 10.3390/v5081991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wen F., Yang J., Li A., Gong Z., Yang L., Cheng Q., Wang C., Zhao M., Yuan S., Chen Y., et al. Genetic characterization and phylogenetic analysis of porcine epidemic diarrhea virus in Guangdong, China, between 2018 and 2019. PLoS ONE. 2021;16:e0253622. doi: 10.1371/journal.pone.0253622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li D., Li Y., Liu Y., Chen Y., Jiao W., Feng H., Wei Q., Wang J., Zhang Y., Zhang G. Isolation and Identification of a Recombinant Porcine Epidemic Diarrhea Virus With a Novel Insertion in S1 Domain. Front. Microbiol. 2021;12:667084. doi: 10.3389/fmicb.2021.667084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu X., Zhang L., Zhang Q., Zhou P., Fang Y., Zhao D., Feng J., Li W., Zhang Y., Wang Y. Evaluation and comparison of immunogenicity and cross-protective efficacy of two inactivated cell culture-derived GIIa- and GIIb-genotype porcine epidemic diarrhea virus vaccines in suckling piglets. Vet. Microbiol. 2019;230:278–282. doi: 10.1016/j.vetmic.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 51.Xue R., Tian Y., Zhang Y., Zhang M., Li Z., Chen S., Liu Q. Diversity of group A rotavirus of porcine rotavirus in Shandong province China. Acta Virol. 2018;62:229–234. doi: 10.4149/av_2018_216. [DOI] [PubMed] [Google Scholar]
- 52.Maneekarn N., Khamrin P. Rotavirus associated gastroenteritis in Thailand. Virusdisease. 2014;25:201–207. doi: 10.1007/s13337-014-0201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mitui M.T., Chandrasena T.N., Chan P.K., Rajindrajith S., Nelson E.A.S., Leung T.F., Nishizono A., Ahmed K. Inaccurate identification of rotavirus genotype G9 as genotype G3 strains due to primer mismatch. Virol. J. 2012;9:144. doi: 10.1186/1743-422X-9-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yan N., Tang C., Kan R., Feng F., Yue H. Genome analysis of a G9P[23] group A rotavirus isolated from a dog with diarrhea in China. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2019;70:67–71. doi: 10.1016/j.meegid.2019.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yan N., Yue H., Wang Y., Zhang B., Tang C. Genomic analysis reveals G3P[13] porcine rotavirus A interspecific transmission to human from pigs in a swine farm with diarrhoea outbreak. J. Gen. Virol. 2021;102:001532. doi: 10.1099/jgv.0.001532. [DOI] [PubMed] [Google Scholar]
- 56.Mao J.-W., Yang Y.-L., Shi C.-C., Chen Z., Li C., Wang Y.-M., Li L.-B., Chen J.-H. Molecular epidemiological characteristics of the virus in 96 children with acute diarrhea in Changdu of Tibet, China. Zhongguo Dang Dai Er Ke Za Zhi. 2022;24:266–272. doi: 10.7499/j.issn.1008-8830.2110012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tian Y., Gao Z., Li W., Liu B., Chen Y., Jia L., Yan H., Wang Q. Group A rotavirus prevalence and genotypes among adult outpatients with diarrhea in Beijing, China, 2011–2018. J. Med. Virol. 2021;93:6191–6199. doi: 10.1002/jmv.27100. [DOI] [PubMed] [Google Scholar]
- 58.Zhao L., Shi X., Meng D., Guo J., Li Y., Liang L., Guo X., Tao R., Zhang X., Gao R., et al. Prevalence and genotype distribution of group A rotavirus circulating in Shanxi Province, China during 2015–2019. BMC Infect. Dis. 2021;21:94. doi: 10.1186/s12879-021-05795-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jing Z., Zhang X., Shi H., Chen J., Shi D., Dong H., Feng L. A G3P[13] porcine group A rotavirus emerging in China is a reassortant and a natural recombinant in the VP4 gene. Transbound. Emerg. Dis. 2018;65:e317–e328. doi: 10.1111/tbed.12756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ahmed S.F., Mansour A.M., Klena J.D., Husain T.S., Hassan K.A., Mohamed F., Steele D. Rotavirus genotypes associated with acute diarrhea in Egyptian infants. Pediatr. Infect. Dis. J. 2014;33((Suppl. S1)):S62–S68. doi: 10.1097/INF.0000000000000052. [DOI] [PubMed] [Google Scholar]
- 61.Sadiq A., Bostan N., Bokhari H., Yinda K.C., Matthijnssens J. Whole Genome Analysis of Selected Human Group A Rotavirus Strains Revealed Evolution of DS-1-Like Single- and Double-Gene Reassortant Rotavirus Strains in Pakistan During 2015–2016. Front. Microbiol. 2019;10:2641. doi: 10.3389/fmicb.2019.02641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Phua K.B., Emmanuel S.C., Goh P., Quak S.H., Lee B.W., Han H.H., Ward R.L., Bernstein D.I., De Vos B., Bock H.L. A rotavirus vaccine for infants: The Asian experience. Ann. Acad. Med. Singap. 2006;35:38–44. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.