Abstract
Individuals with social anxiety disorder (SAD) report less habitual reappraisal and more frequent suppression compared to healthy controls (HC). However, it is unclear whether a neurophysiological index of emotional reactivity, the late positive potential (LPP), is aberrant in SAD or whether self-reported reappraisal or suppression relates to the LPP during on-line emotion reactivity and reappraisal.
Participants with SAD (n = 51) and HC (n = 31) completed an Emotion Regulation Task. Emotion reactivity and regulation were measured via LPP when viewing negative images (‘Look Negative’) and when using a cognitive strategy to reduce negative affect (‘Reappraise Negative’). Participants also completed a self-report measure of habitual reappraisal and suppression.
SAD participants displayed heightened LPP for ‘Look Negative’ compared to HC. However, LPP for online reappraisal was comparable between groups. Self-reported suppression predicted the LPP during ‘Look Negative’ in HC, and there was a trend-level relationship in SAD. LPP findings suggest targeted reappraisal approaches may benefit individuals with SAD.
Keywords: Late positive potential, Social anxiety disorder, Emotion processing, Emotion regulation, EEG
1. Introduction
Social anxiety disorder (SAD) is characterized by excessive reactivity to negative stimuli. Behavioral studies consistently show evidence of an automatic negative processing bias in SAD; for example, SAD is associated with faster response times relative to healthy controls (HC) on probe detection or Stroop tasks comprising threat stimuli (see Amir & Bomyea, 2010; Bögels & Mansell, 2004 for reviews). Moreover, extensive neuroimaging research in SAD has consistently shown excessive reactivity in regions key to the emotion generative process (e.g., amygdala; see Brühl, Delsignore, Komossa, & Weidt, 2014 for a meta-analysis).
In addition to excessive reactivity to negative stimuli, individuals with SAD have difficulty managing emotions. Well-studied emotion regulation strategies include reappraisal and suppression. Reappraisal is considered to be an adaptive strategy that entails altering the way one thinks about a situation to modify the emotional response that would otherwise occur, whereas suppression, a maladaptive strategy, involves avoiding the expression of emotions (Gross, 2002). Several studies have found individuals with SAD report they rely on reappraisal less frequently (Blalock, Kashdan, & Farmer, 2016; D’Avanzato, Joormann, Siemer, & Gotlib, 2013; Jazaieri, Goldin, & Gross, 2017; Kivity & Huppert, 2018a) and suppression more frequently (e.g., D’Avanzato et al., 2013; Dryman & Heimberg, 2018; Jazaieri et al., 2017; Kivity & Huppert, 2018a; Spokas, Luterek, & Heimberg, 2009; Turk, Heimberg, Luterek, Mennin, & Fresco, 2005) than HC. Additionally, literature suggests SAD is characterized by ineffective use of reappraisal (see Dryman & Heimberg, 2018 for a review). Furthermore, neuroimaging studies show that individuals with SAD (relative to HC) show attenuated brain activation in regions associated with cognitive control (e.g., dorsolateral prefrontal cortex) during online reappraisal (e.g., Goldin, Manber, Shabnam, Canlie, & Gross, 2009).
Intriguingly, few studies have taken a neurophysiological approach towards understanding the processing and regulation of negative information in SAD. Event-related potentials (ERPs) use an electroencephalography (EEG) signal that is time-locked to specific events, have excellent temporal resolution, and can be used as an objective measure of emotion processing and regulation (Hajcak, MacNamara, & Olvet, 2010). Specifically, the late positive potential (LPP) is a reliable (Auerbach et al., 2016; Huffmeijer, Bakermans-Kranenburg, Alink, & van IJzendoorn, 2014; Kujawa, Klein, & Proudfit, 2013) ERP elicited by emotional stimuli which is typically analyzed using several time windows following stimulus onset to examine changes in emotion processing over time (Cuthbert, Schupp, Bradley, Birbaumer, & Lang, 2000; Hajcak et al., 2010; Schupp et al., 2000). In unselected samples, the LPP amplitude is positively correlated with self-reported affective arousal (Cuthbert et al., 2000) and is, therefore, considered an index of emotional intensity (Hajcak et al., 2010). Furthermore, the LPP has been shown to decrease as a result of reappraising negative images compared to viewing negative images (Hajcak & Nieuwenhuis, 2006; Moser, Most, & Simons, 2010; Parvaz, MacNamara, Goldstein, & Hajcak, 2012). Additionally, the degree of the LPP reduction during reappraisal was associated with reductions in self-reported ratings of emotional intensity (Hajcak & Nieuwenhuis, 2006). Altogether, the LPP can also be considered an index of online reappraisal facility.
To our knowledge, only one study has examined LPP to negative stimuli in adults with SAD. Kivity and Huppert (2018a) found no group differences between SAD and HC in either online emotion reactivity or regulation (i.e., reappraisal, suppression) in response to shame and rejection related stimuli, and they found no difference in the LPP amplitude by condition (i.e., between reappraising and viewing negative images). Since the LPP is closely linked to emotional arousal (Cuthbert et al., 2000), the authors hypothesize the shame and rejection stimuli may affect the valence of an individual’s emotional response, but not the arousal level. Additionally, a meta-analysis found that although individuals high in social anxiety self-report less frequent and self-report less effective use of reappraisal compared to individuals low in social anxiety, there was a trend toward larger reappraisal-related reductions in emotional arousal in individuals high in social anxiety compared to those low in social anxiety during a lab-based emotion regulation task, but again there were no group differences in the LPP (Kivity & Huppert, 2018b). In contrast, one study found that youth with anxiety disorders displayed enhanced LPP to threatening faces relative to HC (Kujawa, MacNamara, Fitzgerald, Monk, & Phan, 2015), and the effect was driven by SAD, signifying an exaggerated emotional response in socially anxious youth. Moreover, evidence suggests individuals low in social anxiety are better able attend to and prepare for upcoming emotion regulation tasks compared to individuals high in social anxiety (Yuan, Zhou, & Hu, 2014). Thus, limited studies have revealed inconsistent findings; further research is needed to clarify the effect of SAD on the LPP during emotion reactivity and regulation. Moreover, to our knowledge, no study has examined the relationship between self-reported habitual emotion regulation and the LPP during emotion reactivity or regulation in patients with SAD. However, one study found that habitual reappraisal was correlated with decreased reappraisal-related LPP amplitude in a college sample (Moser, Hartwig, Moran, Jendrusina, & Kross, 2014), which has clinical implications.
The current study aims to extend the literature by 1) replicating previous findings regarding group differences in self-reported habitual reappraisal and suppression, 2) investigating group differences in the LPP in response to negative images in individuals with SAD and HC during emotion reactivity and reappraisal, and 3) examining associations between self-reported reappraisal and suppression tendencies and the LPP during emotion reactivity and online reappraisal. Based on literature and theory, we hypothesized the SAD group would self-report lower reappraisal frequency and higher suppression frequency than HC. We also hypothesized the SAD group would display greater LPP amplitude in response to negative images (i.e., greater emotion reactivity) in comparison to the HC group, but that compared to the SAD group, the HC group would show lower LPP during reappraisal. We hypothesized greater habitual reappraisal would correlate with lower LPP amplitude during online reappraisal. We had no hypothesis regarding the relationship between habitual suppression and the LPP during emotion reactivity and reappraisal, given the absence of previous studies on this topic.
2. Methods
2.1. Participants
Fifty-seven individuals with SAD were identified through local community advertisements and referrals from an outpatient psychiatric clinic based on presenting complaint, prior to initiating treatment. Thirty-four HCs were recruited through community advertisements. Participants completed a consent form approved by the local Institutional Review Board. All participants met with a master’s-level clinician who performed the Structured Clinical Interview for DSM-IV (First, Spitzer, Gibbon, & Williams, 1995) and the Liebowitz Social Anxiety Scale (Liebowitz, 1987). Primary and any comorbid diagnoses were based on consensus among the PI, co-investigators, and clinical staff (e.g., psychiatrist, social worker). Participants were required to be between the ages of 18 and 65, free of major active medical or neurological problems as confirmed by a Board-Certified physician, and not currently receiving treatment (e.g., pharmacotherapy, psychotherapy). Comorbid disorders were permitted for patients, provided SAD was the primary diagnosis (see Table 1 for comorbidity data).
Table 1.
Patient Comorbidity.
| N | % | |
|---|---|---|
| Generalized Anxiety Disorder | 16 | 31 |
| Major Depressive Disorder | 15 | 29 |
| Persistent Depressive Disorder | 5 | 10 |
| Specific Phobia | 5 | 10 |
| Panic Disorder | 4 | 8 |
| Post-Traumatic Stress Disorder | 3 | 6 |
| Acute Adjustment Disorder | 1 | 2 |
| Attention Deficity/Hyperactivity Disorder | 1 | 2 |
| Alcohol abuse | 1 | 2 |
| Eating disorder | 2 | 4 |
Exclusion criteria for all participants included recent substance abuse/dependence (within 6 months of the study), history of major psychiatric illness (e.g., bipolar, psychotic disorder), and current cognitive dysfunction (e.g., traumatic brain injury, pervasive developmental disorder). None of the participants tested positive for alcohol or illegal substances. All participants were compensated for their time, and all procedures complied with the Helsinki Declaration. The Emotion Regulation Questionnaire (Gross & John, 2003) assessed subjective habitual use of reappraisal (ERQ-R) and suppression (ERQ-S). Higher scores on the ERQ indicate more frequent use of the emotion regulation strategy. Table 2 details participants’ demographic and clinical characteristics.
Table 2.
Participant Characteristics.
| HC (n = 31) | SAD (n = 51) | |
|---|---|---|
|
| ||
| % | % | |
| Female | 70.97 | 74.51 |
| Race/Ethnicity | ||
| Caucasian | 45.16 | 54.90 |
| African-American | 9.68 | 1.96 |
| Asian/Pacific Islander | 35.48 | 23.53 |
| Hispanic/Latino | 25.81 | 35.29 |
| American Indian or Alaskan Native | 0.00 | 1.96 |
| Other/Unknown | 9.68 | 17.65 |
| M (SD) | M (SD) | |
| Age | 24.81 (7.20) | 25.20 (6.19) |
| Education | 15.73 (2.45) | 15.49 (2.40) |
| Hamilton Anxiety Rating Scale | 1.06 (1.65) | 12.51 (6.84)** |
| Hamilton Depression Rating Scale | 0.74 (1.21) | 8.22 (4.77)** |
| Liebowitz Social Anxiety Scale | 15.06 (11.80) | 78.76 (17.21)** |
| Emotion Regulation Questionnaire-Reappraisal | 34.39 (6.51) | 23.51 (6.50)** |
| Emotion Regulation Questionnaire-Suppression | 13.23 (4.73) | 15.75 (5.96)* |
p < 0.05.
p < 0.001.
2.2. Emotion regulation task
Participants completed a validated Emotion Regulation Task (ERT) (e.g., Fitzgerald et al., 2016; Ochsner, Bunge, Gross, & Gabrieli, 2002; Parvaz et al., 2012) during continuous EEG recording. Task stimuli comprised 50 negative and 50 neutral images from the International Affective Picture System (IAPS; Lang, Bradley, & Cuthbert, 1997). Participants were asked to look at negative images (‘Look Negative’), use a cognitive strategy to reduce negative affect in the context of negative images (‘Reappraise Negative’), or look at neutral pictures (i.e., ‘Look Neutral’). Participants completed eight practice trials with images that were not used in the task to confirm understanding of instructions (Fitzgerald et al., 2016; Parvaz et al., 2012).
Each participant completed four blocks of 25 trials each: two blocks of neutral images (‘Look Neutral’), and two blocks of negative images (during which participants were instructed to either ‘Reappraise’ or ‘Look’) for a total of 50 ‘Look Neutral’ trials, 25 trials, and 25 ‘Look Negative’ trials. Participants received auditory instructions 1000 ms after the image appeared. The picture remained on the screen for 6000 ms after instruction onset; thus, total trial duration was 7000 ms. A fixation cross appeared in the center of the screen for 1000 ms after picture offset. Block order was pseudorandomized. Picture order was pseudorandomized across the two blocks of each block type, and the order of ‘Look Negative’ and Reappraise trials was pseudorandomized across the two negative blocks.
2.2.1. Electroencephalographic recording
EEG recordings were continuously collected during the task using an elastic cap and the ActiveTwo BioSemi system (BioSemi, Amsterdam, The Netherlands). Thirty-four electrode sites were used: the standard 32 channel montage, FCz, and Iz, based on the international 10/20 system. Also, two electrodes were used for the left and right mastoids to serve as the reference, and four electrodes were placed on the participant’s face to record electroculogram generated by eye blinks and eye movements. All channels were digitized at 1024 Hz with 24 bits of resolution. No online filter was used.
2.2.2. Offline electroencephalographic data processing
Brain Vision Analyzer 2 software (Brain Products, Gilching, Germany) was used for offline data processing and data reduction. Electrodes were re-referenced to the average of the left and right mastoids. Data were band-pass filtered from 0.01 to 30 Hz and split into segments beginning 200 ms prior to picture onset and ending 7000 ms after picture onset, resulting in 7200 ms segments. Eye blink and ocular corrections were made following the Miller, Gratton, and Yee, (1988) method. Artifact analysis was used to identify a voltage step of more than 50μV between sample points, a voltage difference of 300μV within a segment, and a maximum voltage difference of less than 0.50μV within 100 ms intervals. Trials were inspected visually for any remaining artifacts, and data from individual channels containing artifacts were rejected on a trial-by-trial basis. Two HC and 6 SAD participants were excluded from the analyses for having fewer than 8 usable trials (Moran, Jendrusina, & Moser, 2013) in at least one electrode, resulting in a final sample of 31 HC and 51 SAD participants. For these participants, the average electrophysiological activity for each condition was calculated while using a 200 ms pre-stimulus baseline correction to account for noise.
2.2.3. Late positive potential
An electrode pooling was created (P3, P4, P7, P8, PZ, PO3, PO4) based on visual inspection of where the electrocortical activity was maximal and prior work with the LPP (Foti & Hajcak, 2008; Hajcak & Nieuwenhuis, 2006; Parvaz et al., 2012). Following an established method (Dunning & Hajcak, 2009; Fitzgerald et al., 2016; Hajcak et al., 2010; Parvaz et al., 2012), the pre-instruction LPP was calculated using the mean amplitude from 400 to 1000 ms post-picture onset. The post-instruction LPP was calculated using the mean amplitude in three time windows: early (1500–3000 ms post-picture onset), middle (3000–5000 ms post-picture onset), and late (5000–7000 ms post-picture onset) in order to comprehensively evaluate the time course of emotion processing and regulation.
2.3. Analytic approach
2.3.1. Self-reported reappraisal and suppression
Two independent-samples t-tests were conducted to determine if the total scores on the ERQ-R and ERQ-S differed by group.
2.3.2. Manipulation Check
A repeated-measures ANOVA was conducted on the pre-instruction LPP in each condition (‘Look Neutral’, ‘Look Negative’, ‘Reappraise Negative’), collapsing across patients and HC. The main effect was followed up with paired-samples t-tests to determine which conditions drove the effect. A 2 (Group: HC, SAD) x 3 (Time: Early, Middle, Late) mixed ANOVA on the LPP in the ‘Look Neutral’ condition is included in the Supplementary Materials.
2.3.3. Effect of group, condition, and time on the LPP
Our primary contrast of interest was ‘Look Negative’ vs. ‘Reappraise Negative’ to evaluate reactivity relative to reappraisal of threat. Therefore, a 2 (Group: SAD, HC) x 2 (Condition: ‘Look Negative’, ‘Reappraise Negative’) x 3 (Time: Early, Middle, Late) mixed ANOVA, with repeated measures on the last two factors, was conducted to determine the effects of group, condition, and time on the LPP.
2.3.4. Regression
For significant LPP-related group differences, we conducted separate regressions in each group to examine the effect of self-reported reappraisal and suppression (i.e., ERQ-R, ERQ-S) on the LPP in order to understand what is driving any significant differences found in the ANOVA. Bivariate correlations of ERQ-R and ERQ-S and the LPP in each Group, Time Window, and Condition are presented in the Supplementary Materials, as well.
2.3.5. Group comparison of difference waves (‘Reappraise negative’ – ‘look negative’)
We performed exploratory independent samples t-tests to compare the mean difference wave for Reappraise Negative (vs. Look Negative) at each post-instruction time window in the HC and SAD groups.
All analyses were performed in the Statistical Package for the Social Sciences Statistics (Version 24.0); all analyses were two-tailed with an alpha level of 0.05.
3. Results
See Table 3 and Fig. 1 for the mean LPP amplitude by group, condition. Scalp distributions by Group, Condition, and Time are included in the Supplementary Materials.
Table 3.
Late Positive Potential Amplitude by Condition, Group, and Time.
| HC | SAD | |
|---|---|---|
| M (SD) | M (SD) | |
| 400-1000 ms (Pre-Instruction) | ||
| ‘Look Neutral’ | 5.84 (4.62) | 4.55 (3.32) |
| ‘Look Negative’ | 9.48 (5.45) | 7.9 (4.53) |
| ‘Reappraise Negative’ | 8.69 (4.86) | 7.86 (5.21) |
| 1500-3000 ms (Early) | ||
| ‘Look Neutral’ | 3.89 (5.32) | 3.22 (4.6) |
| ‘Look Negative’ | 7.58 (7.51) | 7.42 (5.25) |
| ‘Reappraise Negative’ | 6.81 (6.32) | 5.75 (6.75) |
| 3000-5000 ms (Middle) | ||
| ‘Look Neutral’ | 2.76 (5.64) | 2.82 (4.63) |
| ‘Look Negative’ | 4.7 (7.45) | 6.8 (6.07) |
| ‘Reappraise Negative’ | 5.13 (6.02) | 3.61 (7.08) |
| 5000-7000 ms (Late) | ||
| ‘Look Neutral’ | 1.6 (6.16) | 1.36 (4.71) |
| ‘Look Negative’ | 2.08 (7.42) | 5.18 (6.38) |
| ‘Reappraise Negative’ | 3.02 (6.44) | 1.75 (5.31) |
Fig. 1.
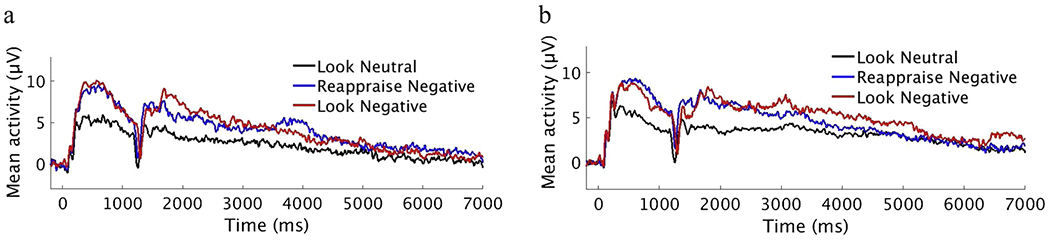
Mean Waveform by Condition in HC (a) and SAD (b).
3.1. Self-reported reappraisal and suppression
The independent-samples t-tests revealed significant group differences in ERQ-R, t(80) = 7.35, p < 0.001, d = 1.67 and ERQ-S, t(80) = −2.00, p = 0.049, d = 0.47.
3.2. Manipulation check
The repeated-measures ANOVA on the pre-instruction LPP revealed a significant effect of condition, F(2, 162) = 37.24, p < 0.001, ηp2 = 0.36. Follow-up paired-samples t-tests revealed a larger LPP in ‘Look Negative’ (M = 8.50, SD = 4.93) than ‘Look Neutral’ (M = 5.03, SD = 3.89), t(81) = −8.06, p < 0.001, d = 1.04 and a larger LPP in ‘Reappraise Negative’ (M = 8.17, SD = 5.07) than ‘Look Neutral’, t(81) = −6.79, p < 0.001, d = 0.89. As expected, there was no difference in the pre-instruction LPP between ‘Look Negative’ and ‘Reappraise Negative’, t(81) = 0.75, p = 0.46, d = 0.08.
3.3. Effect of group, condition, and time on the LPP
The mixed ANOVA revealed a main effect of Time, F(1.37, 109.48) = 37.49, p < 0.001, ηp2 = 0.32, and a Group x Condition x Time interaction, F(1.64, 130.89) = 3.58, p = 0.039, ηp2 = 0.04. The main effect of Condition, F(1, 80) = 2.54, p = 0.115, ηp2 = 0.03, the Time x Condition interaction, F(1.64, 109.48) = 0.03, p = 0.97, ηp2 < 0.001, and the Group x Condition interaction, F(1, 80) = 3.42, p = 0.068, ηp2 = 0.04 were not significant.
To follow up the main effect of time, we conducted paired samples t-tests collapsing across condition (i.e., ‘Look Negative’, ‘Reappraise Negative’), revealing the mean LPP was larger in the early (M = 6.82, SD = 5.50) than the middle (M = 5.09, SD = 5.08) time window, t(81) = 5.39, p < 0.001, d = 0.58, and the LPP was larger in the middle than the late (M = 3.12, SD = 4.68) time window, t(81) = 6.56, p < 0.001, d = 0.59.
To follow up the three-way interaction, we conducted separate 2 (Group: SAD, HC) x 2 (Condition: ‘Look Negative’, ‘Reappraise Negative’) repeated measures ANOVAs in each time window. The Group x Condition interaction was not significant in the early, F(2, 80) = 0.37 p = 0.543, ηp2 = 0.01 or middle, F(2, 80) = 3.47, p = 0.066, ηp2 = 0.04) time window. The late time window (5000–7000 ms) revealed a Group x Condition interaction, F(1, 80) = 5.23, p = 0.025, ηp2 = 0.06. Follow-up independent samples t-tests revealed a group difference in the LPP during ‘Look Negative’ in the late time window, t(80) = 2.01, p = 0.048, such that the SAD group (M = 5.18, SD = 6.38) displayed a larger LPP than the HC group (M = 2.08, SD = 7.42), d = 0.45. There was no group difference in the LPP during ‘Reappraise Negative’, t(80) = 0.97, p = 0.334, d = 0.22. The 3-way interaction was also examined via separate Condition x Time ANOVAs in each Group; results are presented in the Supplementary Materials.
3.4. Self-reported habitual emotion regulation and the LPP during ‘look negative’ in the late time window
Regression analyses revealed that for the HC group, ERQ-S significantly predicted the LPP during ‘Look Negative’ in the late time window, β = 0.49, p = 0.007, but ERQ-R did not, β = 0.01, p = 0.940. Self-reported regulation tendencies explained a significant proportion of the variance in the LPP during ‘Look Negative’ in the late time window in HCs, R2 = 0.24, F(2, 28) = 4.40, p = 0.022. In the SAD group, the regression analysis revealed a trend toward ERQ-S predicting the LPP during ‘Look Negative’ in the late time window, β = 0.27, p = 0.055, but there was no such trend for ERQ-R, β = −0.10, p = 0.461. Self-reported emotion regulation tendencies did not explain a significant proportion of the variance in the LPP during ‘Look Negative’ in the late time window in the SAD group, R2 = 0.08, F(2, 48) = 2.07, p = 0.137. The correlations between mean post-instruction LPP and the ERQ across all conditions are presented in the Supplementary Materials.
3.5. Group comparison of difference waves (‘Reappraise negative’ – ‘look negative’)
Independent samples t-tests revealed a greater reduction of the LPP from ‘Look Negative’ to ‘Reappraise Negative’ in the SAD group (M = −3.43, SD = 8.20) than the HC group (M = 0.95, SD = 8.74) in the late time window only, t(80) = 2.29, p = 0.025, d = 0.52. Groups did not differ on the mean difference wave in the early, t(80) = 0.61, p = 0.543, d = 0.43 or middle, t(80) = 1.86, p = 0.066, d = 0.13 time window.
4. Discussion
In the current study, we examined self-reported habitual emotion regulation tendencies (i.e., reappraisal, suppression) together with the LPP during an emotion regulation task in patients with SAD and HCs. As predicted, SAD patients reported less frequent reappraisal use and more frequent suppression use compared to HCs. The SAD group displayed heightened LPP during emotion processing (i.e., ‘Look Negative’) in the late time window compared to HCs. There was no group difference in LPP during ‘Reappraise Negative’. Additionally, self-reported frequency of suppression, but not reappraisal, predicted the LPP during ‘Look Negative’ in the late time window for the HC group, but there was only a trend toward suppression frequency predicting the LPP in the SAD group. Finally, the SAD group showed a greater decrease in the LPP from ‘Look Negative’ to ‘Reappraise Negative’ than the HC group.
The findings on self-reported habitual reappraisal (i.e., ERQ-R) and suppression (i.e., ERQ-S) are consistent with the literature. We demonstrated that HC participants self-report greater use of reappraisal and less use of suppression compared to individuals with SAD. These results contribute to a growing body of literature implicating less habitual use of adaptive and more frequent use of maladaptive emotion regulation strategies in SAD in real-life settings (e.g., Blalock et al., 2016; D’Avanzato et al., 2013; Jazaieri et al., 2017; Kivity & Huppert, 2018a; Spokas et al., 2009; Turk et al., 2005). Given the negative processing biases present in SAD (e.g., Amir & Bomyea, 2010), emotion regulation strategies may play an especially important role in the etiology and maintenance of the disorder. Emotion dysregulation could serve as a potential target for treatments aimed at reducing social anxiety symptoms.
Next, SAD patients displayed heightened LPP to negative images during ‘Look Negative’ in the late time window compared to HCs, suggesting aberrant sustained emotional processing of negative images in the SAD group. Thus, patients did not experience the same decrease in emotional reactivity as HCs did when experiencing their emotions naturally. Though findings are inconsistent with the Kivity and Huppert (2018a) study, further study is warranted given methodological differences. In their study, the authors propose that the shame and rejection stimuli affected the valence of the individual’s emotional response, but not the arousal level (Kivity & Huppert, 2018a). On the other hand, our study used images of general negative content (Lang et al., 1997), which may have elicited a different response than the images used by Kivity and Huppert (2018a). Moreover, our study used a larger sample and fewer conditions than the Kivity and Huppert (2018a) study, thereby potentially increasing power to detect an effect. Furthermore, the stimulus presentation time was shorter in Kivity and Huppert (2018a) than in the present study. It is possible that group differences would have emerged with a longer presentation time. Our findings suggest elevated sustained LPP to negative, arousing images may serve as a neurophysiological marker of SAD and may signify aberrant extended elaborative processing of negative information in SAD, but further research is needed to replicate our findings.
Our finding that self-reported suppression frequency (ERQ-S) was associated with heightened LPP during ‘Look Negative’ in the late time window in the HC group indicates that habitual suppression relates to increased extended emotional reactivity and processing. It is possible that those who typically suppress their emotions are actually suppressing their emotions during the task, even when instructed to view the images and experience their emotions naturally. Future research should explore this possibility by asking participants to report the strategy they used following task completion or by including an online suppression condition, as in Kivity and Huppert (2018a). There was no relationship between self-reported reappraisal frequency (ERQ-R) and the LPP. Thus, habitual reappraisal tendencies may not predict the LPP when participants are viewing negative IAPS images.
In contrast to our hypothesis, there was no group difference in the LPP during online reappraisal. Consistent with other studies, all participants practiced reappraisal strategies before completing the ERT to ensure understanding of instructions. Also, analogous to other studies (e.g., Fitzgerald, Kinney, Phan, & Klumpp, 2018, 2016; Klumpp et al., 2017; Parvaz et al., 2012), all participants were provided examples of cognitive approaches to reframe negative images, which may have contributed to the null results. Moreover, the combined findings that individuals with SAD reported less frequent reappraisal than HCs, but were equally able to employ reappraisal when instructed to are consistent with previous research suggesting self-reported reappraisal frequency may not track reappraisal success (Ford, Karnilowicz, & Mauss, 2017). Furthermore, the task comprised images of general negative content, rather than images related to social interaction. Future studies should examine whether individuals with SAD are as effective at reappraising stimuli more closely related to social evaluative fears (e.g., negative facial expressions) as HCs. However, findings suggest targeted reappraisal approaches may benefit individuals with SAD. Further research is needed to determine how this may translate to clinical settings.
Moreover, exploratory analyses suggest individuals with SAD were better at decreasing their LPP during reappraisal than HCs. This result may represent a floor effect in the HC group. Additionally, literature suggests individuals with SAD may be especially susceptible to demand characteristics. Fear of negative evaluation is a cardinal feature of SAD (Schneier, 2006; Wittchen & Fehm, 2001); thus, it is possible that the SAD patients were more engaged in the reappraisal task, relative to the HC group. However, results of the present study suggest that individuals with SAD are able to Reappraise negative images in a controlled lab task after receiving reappraisal instruction.
The present study is not without important limitations. First, given the comorbidity in SAD, it is unclear whether the findings would generalize to individuals without such comorbidity. Also, the interrater reliability was not collected for clinician-administered measures. Additionally, our study did not include a measure of confidence in one’s ability to successfully employ reappraisal, which has been shown to be lower in individuals with SAD than in HCs (Werner, Goldin, Ball, Heimberg, & Gross, 2011). Moreover, the ERT used IAPS images (Lang et al., 1997). It is possible that idiographic images may have shown a stronger relationship between the frequency of reappraisal and the LPP during reappraise because those who use reappraisal likely do so in personally relevant situations. Therefore, personally relevant images may better reflect the participants’ real-world reappraisal ability. Additionally, subjective ratings of emotional reactivity were not collected during the ERT. Thus, it is unclear how closely the LPP correlates with the participants’ subjective emotional experience in the present sample. Even so, several studies have found the LPP relates to subjective emotional valence and arousal during lab-based ERTs (Cuthbert et al., 2000; Hajcak et al., 2010). Despite these limitations, the present study extends previous findings concerning self-reported emotion regulation and represents advancement in characterizing the neurophysiology of emotion processing and regulation in SAD. Evidence that patients with SAD are successful in online reappraisal when instructed has important clinical implications.
Supplementary Material
Acknowledgements
This work was supported by the National Institute of Mental HealthK23MH093679 (HK), R01MH112705 (HK), and the Center for Clinical and Translational Research (CCTS)UL1RR029879.
Footnotes
Conflicts of interest
The authors have no conflicts of interest to report
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi: https://doi.org/10.1016/j.biopsycho.2019.01.019.
References
- Amir N, & Bomyea J (2010). Cognitive biases in social anxiety disorderSocial anxiety (second edition). Elsevier; 373–393. 10.1016/B978-0-12-375096-9.00014-6. [DOI] [Google Scholar]
- Auerbach RP, Bondy E, Stanton CH, Webb CA, Shankman SA, & Pizzagalli DA (2016). Self-referential processing in adolescents: Stability of behavioral and ERP markers. Psychophysiology, 53(9), 1398–1406. 10.1111/psyp.12686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blalock DV, Kashdan TB, & Farmer AS (2016). Trait and daily emotion regulation in social anxiety disorder. Cognitive Therapy and Research, 40(3), 416–425. 10.1007/s10608-015-9739-8. [DOI] [Google Scholar]
- Bögels SM, & Mansell W (2004). Attention processes in the maintenance and treatment of social phobia: Hypervigilance, avoidance and self-focused attention. Clinical Psychology Review, 24(7), 827–856. 10.1016/j.cpr.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Brühl AB, Delsignore A, Komossa K, & Weidt S (2014). Neuroimaging in social anxiety disorder—A meta-analytic review resulting in a new neurofunctional model. Neuroscience and Biobehavioral Reviews, 47, 260–280. 10.1016/j.neubiorev.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, & Lang PJ (2000). Brain potentials in affective picture processing: Covariation with autonomic arousal and affective report. Biological Psychology, 52(2), 95–111. 10.1016/S0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- D’Avanzato C, Joormann J, Siemer M, & Gotlib IH (2013). Emotion regulation in depression and anxiety: Examining diagnostic specificity and stability of strategy use. Cognitive Therapy and Research, 37(5), 968–980. 10.1007/s10608-013-9537-0. [DOI] [Google Scholar]
- Dryman MT, & Heimberg RG (2018). Emotion regulation in social anxiety and depression: A systematic review of expressive suppression and cognitive reappraisal. Clinical Psychology Review, 65, 17–42. 10.1016/j.cpr.2018.07.004. [DOI] [PubMed] [Google Scholar]
- Dunning JP, & Hajcak G (2009). See no evil: Directing visual attention within unpleasant images modulates the electrocortical response. Psychophysiology, 46(1), 28–33. 10.1111/j.1469-8986.2008.00723.x. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, & Williams J (1995). Structured clinical interview for DSM-IV Axis I disorders, patient edition (SCID-P), version 2. New York, NY: Biometrics Research. [Google Scholar]
- Fitzgerald JM, Kinney KL, Phan KL, & Klumpp H (2018). Distinct neural engagement during implicit and explicit regulation of negative stimuli. Neuropsychologia. 10.1016/j.neuropsychologia.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald JM, MacNamara A, DiGangi JA, Kennedy AE, Rabinak CA, Patwell R, … Phan KL (2016). An electrocortical investigation of voluntary emotion regulation in combat-related posttraumatic stress disorder. Psychiatry Research Neuroimaging, 249, 113–121. 10.1016/j.pscychresns.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford BQ, Karnilowicz HR, & Mauss IB (2017). Understanding reappraisal as a multicomponent process: The psychological health benefits of attempting to use reappraisal depend on reappraisal success. Emotion. 10.1037/emo0000310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D, & Hajcak G (2008). Deconstructing reappraisal: Descriptions preceding arousing pictures modulate the subsequent neural response. Journal of Cognitive Neuroscience, 20(6), 977–988. [DOI] [PubMed] [Google Scholar]
- Goldin CRD, Manber T, Shabnam H, Canlie T, & Gross J (2009). Neural bases of social anxiety disorder. Archives of General Psychiatry, 66(2), 170–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ (2002). Emotion regulation: Affective, cognitive, and social consequences. Psychophysiology, 39(03), 281–291. [DOI] [PubMed] [Google Scholar]
- Gross JJ, & John OP (2003). Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. Journal of Personality and Social Psychology, 85(2), 348–362. 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Hajcak G, & Nieuwenhuis S (2006). Reappraisal modulates the electrocortical response to unpleasant pictures. Cognitive, Affective & Behavioral Neuroscience, 6(4), 291–297. [DOI] [PubMed] [Google Scholar]
- Hajcak G, MacNamara A, & Olvet DM (2010). Event-related potentials, emotion, and emotion regulation: An integrative review. Developmental Neuropsychology, 35(2), 129–155. 10.1080/87565640903526504. [DOI] [PubMed] [Google Scholar]
- Huffmeijer R, Bakermans-Kranenburg MJ, Alink LRA, & van IJzendoorn MH (2014). Reliability of event-related potentials: The influence of number of trials and electrodes. Physiology & Behavior, 130, 13–22. 10.1016/j.physbeh.2014.03.008. [DOI] [PubMed] [Google Scholar]
- Jazaieri H, Goldin PR, & Gross JJ (2017). Treating social anxiety disorder with CBT: Impact on emotion regulation and satisfaction with life. Cognitive Therapy and Research, 41(3), 406–416. 10.1007/s10608-016-9762-4. [DOI] [Google Scholar]
- Kivity Y, & Huppert JD (2018a). Are individuals diagnosed with social anxiety disorder successful in regulating their emotions? A mixed-method investigation using self-report, subjective, and event-related potentials measures. Journal of Affective Disorders. 10.1016/j.jad.2018.02.029. [DOI] [PubMed] [Google Scholar]
- Kivity Y, & Huppert JD (2018b). Emotion regulation in social anxiety: A systematic investigation and meta-analysis using self-report, subjective, and event-related potentials measures. Cognition & Emotion, 1–18. 10.1080/02699931.2018.1446414. [DOI] [PubMed] [Google Scholar]
- Klumpp H, Roberts J, Kennedy AE, Shankman SA, Langenecker SA, Gross JJ, … Phan LK (2017). Emotion regulation related neural predictors of cognitive behavioral therapy response in social anxiety disorder. Progress in Neuro-psychopharmacology & Biological Psychiatry, 75, 106–112. 10.1016/j.pnpbp.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Klein DN, & Proudfit GH (2013). Two-year stability of the late positive potential across middle childhood and adolescence. Biological Psychology, 94(2), 290–296. 10.1016/j.biopsycho.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, MacNamara A, Fitzgerald KD, Monk CS, & Phan KL (2015). Enhanced neural reactivity to threatening faces in anxious youth: Evidence from event-related potentials. Journal of Abnormal Child Psychology, 43(8), 1493–1501. 10.1007/s10802-015-0029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, & Cuthbert BN (1997). International affective picture system (IAPS): Technical manual and affective ratings. NIMH Center for the Study of Emotion and Attention, 39–58. [Google Scholar]
- Liebowitz MR (1987). Social phobia. Modern Problems of Pharmacopsychiatry, 22, 141–173. 10.1159/000414022. [DOI] [PubMed] [Google Scholar]
- Miller GA, Gratton G, & Yee CM (1988). Generalized implementation of an eye movement correction procedure. Psychophysiology, 25(2), 241–243. [Google Scholar]
- Moran TP, Jendrusina AA, & Moser JS (2013). The psychometric properties of the late positive potential during emotion processing and regulation. Brain Research, 1516, 66–75. 10.1016/j.brainres.2013.04.018. [DOI] [PubMed] [Google Scholar]
- Moser JS, Hartwig R, Moran TP, Jendrusina AA, & Kross E (2014). Neural markers of positive reappraisal and their associations with trait reappraisal and worry. Journal of Abnormal Psychology, 123(1), 91–105. 10.1037/a0035817. [DOI] [PubMed] [Google Scholar]
- Moser JS, Most SB, & Simons RF (2010). Increasing negative emotions by reappraisal enhances subsequent cognitive control: A combined behavioral and electrophysiological study. Cognitive, Affective & Behavioral Neuroscience, 10(2), 195–207. 10.3758/CABN.10.2.195. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, & Gabrieli JDE (2002). Rethinking feelings: An fMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience, 14(8), 1215–1229. 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Parvaz MA, MacNamara A, Goldstein RZ, & Hajcak G (2012). Event-related induced frontal alpha as a marker of lateral prefrontal cortex activation during cognitive reappraisal. Cognitive, Affective & Behavioral Neuroscience, 12(4), 730–740. 10.3758/s13415-012-0107-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneier FR (2006). Social anxiety disorder. The New England Journal of Medicine, 355(10), 1029–1036. 10.1056/NEJMcp060145. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Cacioppo JT, Ito T, & Lang PJ (2000). Affective picture processing: The late positive potential is modulated by motivational relevance. Psychophysiology, 37(2), 257–261. [PubMed] [Google Scholar]
- Spokas M, Luterek JA, & Heimberg RG (2009). Social anxiety and emotional suppression: The mediating role of beliefs. Journal of Behavior Therapy and Experimental Psychiatry, 40(2), 283–291. 10.1016/j.jbtep.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Turk CL, Heimberg RG, Luterek JA, Mennin DS, & Fresco DM (2005). Emotion dysregulation in generalized anxiety disorder: A comparison with social anxiety disorder. Cognitive Therapy and Research, 29(1), 89–106. 10.1007/s10608-005-1651-1. [DOI] [Google Scholar]
- Werner KH, Goldin PR, Ball TM, Heimberg RG, & Gross JJ (2011). Assessing emotion regulation in social anxiety disorder: The emotion regulation interview. Journal of Psychopathology and Behavioral Assessment, 33(3), 346–354. 10.1007/s10862-011-9225-x. [DOI] [Google Scholar]
- Wittchen H-U, & Fehm L (2001). Epidemiology, patterns of comorbidity, and associated disabilities of social phobia. Psychiatric Clinics, 24(4), 617–641. [DOI] [PubMed] [Google Scholar]
- Yuan L, Zhou R, & Hu S (2014). Cognitive reappraisal of facial expressions: Electrophysiological evidence of social anxiety. Neuroscience Letters, 577, 45–50. 10.1016/j.neulet.2014.06.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


