Abstract
Aim
To assess the efficacy, safety and tolerability of cotadutide in patients with type 2 diabetes mellitus and chronic kidney disease.
Materials and Methods
In this phase 2a study (NCT03550378), patients with body mass index 25‐45 kg/m2, estimated glomerular filtration rate 30‐59 ml/min/1.73 m2 and type 2 diabetes [glycated haemoglobin 6.5‐10.5% (48‐91 mmol/mol)] controlled with insulin and/or oral therapy combination, were randomized 1:1 to once‐daily subcutaneous cotadutide (50‐300 μg) or placebo for 32 days. The primary endpoint was plasma glucose concentration assessed using a mixed‐meal tolerance test.
Results
Participants receiving cotadutide (n = 21) had significant reductions in the mixed‐meal tolerance test area under the glucose concentration‐time curve (–26.71% vs. +3.68%, p < .001), more time in target glucose range on continuous glucose monitoring (+14.79% vs. –21.23%, p = .001) and significant reductions in absolute bodyweight (–3.41 kg vs. –0.13 kg, p < .001) versus placebo (n = 20). In patients with baseline micro‐ or macroalbuminuria (n = 18), urinary albumin‐to‐creatinine ratios decreased by 51% at day 32 with cotadutide versus placebo (p = .0504). No statistically significant difference was observed in mean change in estimated glomerular filtration rate between treatments. Mild/moderate adverse events occurred in 71.4% of participants receiving cotadutide and 35.0% receiving placebo.
Conclusions
We established the efficacy of cotadutide in this patient population, with significantly improved postprandial glucose control and reduced bodyweight versus placebo. Reductions in urinary albumin‐to‐creatinine ratios suggest potential benefits of cotadutide on kidney function, supporting further evaluation in larger, longer‐term clinical trials.
1. INTRODUCTION
Type 2 diabetes mellitus is the leading cause of chronic kidney disease (CKD), 1 , 2 which affects up to 40% of patients with type 2 diabetes. The development of CKD in patients with type 2 diabetes is multifactorial, with hyperglycaemia, hypertension and obesity each playing significant roles. 3 Type 2 diabetes with CKD increases the risk of major adverse cardiovascular events and all‐cause mortality, 4 placing a high burden on health care services, as well as individuals and their carers. 1 CKD leads to progressive decline in renal function, with approximately one‐third of patients with type 2 diabetes developing microalbuminuria within 15 years of initial diagnosis. 5 Furthermore, the UK Prospective Diabetes Study showed that from the time of diagnosis of type 2 diabetes, 2.0% of patients progress to microalbuminuria, 2.8% to macroalbuminuria and 2.3% to end‐stage kidney disease per year. 6
Data‐driven cluster analyses of adult‐onset type 2 diabetes have established a subgroup of patients with a two to three times greater risk of developing diabetic kidney disease compared with other subgroups. Phenotypic characteristics included severe insulin resistance and a greater prevalence of non‐alcoholic fatty liver disease (NAFLD) compared with other subgroups. 7 This, and other recent evidence linking incidence and severity of NAFLD and CKD, 8 suggests some shared pathogenetic mechanisms underlying liver and kidney injury, and potentially shared therapeutic targets 9 , 10 , 11 ; therapies effective at improving insulin sensitivity and NAFLD may also be beneficial in CKD. However, no treatments that simultaneously address liver and kidney dysfunction, and the underlying metabolic syndrome, are currently available.
Treatments for type 2 diabetes and CKD are frequently limited by restrictions on use in patients with renal impairment, therefore a large unmet need exists for this group. Controlling blood glucose, bodyweight and blood pressure are the cornerstones of disease management. 12 More recently, sodium‐glucose cotransporter‐2 inhibitors, established treatments for type 2 diabetes, have shown benefit beyond glucose lowering in delaying progression of renal disease in individuals with, 13 and without, type 2 diabetes. 14 This has prompted changes in guidelines to direct treatment with this class of drugs to patients with type 2 diabetes and CKD. 15 Similarly, glucagon‐like peptide‐1 receptor agonists (GLP‐1 RAs), approved treatments for type 2 diabetes and obesity, have been shown to delay progression to macroalbuminuria in patients with type 2 diabetes, including in subsets of patients with estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2. 2 , 16 , 17 However, the effect of GLP‐1 RAs on progression to end‐stage kidney disease has not been fully investigated in patients with CKD. 18 , 19 A large phase 3 trial (NCT03819153) of the GLP‐1 RA semaglutide is underway to evaluate whether it can delay progression of renal disease in type 2 diabetes.
Cotadutide (MEDI0382) is a balanced GLP‐1 and glucagon receptor dual agonist, with a ratio of approximately 5:1 GLP‐1 to glucagon activity. This balance of agonistic activity at each receptor is designed to leverage benefits of dual agonism on liver health and bodyweight loss, and to offset glucagon receptor‐driven increases in hepatic glucose production. 20 Indeed, cotadutide showed robust glucose‐lowering and bodyweight loss effects in patients with type 2 diabetes. 21 Cotadutide is under development for the treatment of non‐alcoholic steatohepatitis and type 2 diabetes with CKD. Beneficial liver‐specific effects of cotadutide have been attributed to glucagon receptor signaling. 22 , 23 Cotadutide has been shown to promote a reduction in liver glycogen after short‐term treatment, providing suggestive evidence of target engagement of the glucagon receptor in the liver. 24 Larger improvements in lipid and liver parameters have been shown with cotadutide versus liraglutide. 21 Both the liver and kidney have high levels of glucagon receptor expression, and glucagon acts directly and indirectly to influence GFR. 25 The aim of this study was to investigate the efficacy, safety and tolerability of cotadutide in patients with type 2 diabetes and CKD, including those treated with insulin therapy.
2. MATERIALS AND METHODS
2.1. Study design
This was a randomized, double‐blind, placebo‐controlled, phase 2a study to evaluate the efficacy, safety and tolerability of 50‐300 μg cotadutide in patients with type 2 diabetes and CKD. The primary objective was to assess the effect of cotadutide on postprandial glucose control, measured by a standardized mixed‐meal tolerance test (MMTT). Secondary objectives were to characterize the safety profile of cotadutide, assess the effects of cotadutide on additional measures of glycaemic control and bodyweight, and characterize the pharmacokinetics (PK) profile and immunogenicity of cotadutide. The exploratory objectives were to evaluate the effect of cotadutide on renal biomarkers and albuminuria.
2.2. Ethics
This study is registered on ClinicalTrials.gov (NCT03550378) and was conducted in accordance with the principles of the Declaration of Helsinki, the International Council for Harmonization Guidance for Good Clinical Practice and with any applicable laws and conditions required by relevant regulatory authorities. The study protocol and informed consent documents were approved by the relevant Independent Ethics Committee. All participants provided written informed consent before study entry.
2.3. Patients
Eligible participants were aged 18‐84 years, overweight or obese (body mass index 25‐45 kg/m2) with CKD (eGFR 30‐59 ml/min/1.73 m2) and diagnosed with type 2 diabetes with glucose control managed with any insulin and/or oral therapy combination (except GLP‐1 RAs) where no significant dose changes (>50%) had occurred within 3 months before screening. Participants had glycated haemoglobin (HbA1c) of 6.5‐10.5% (48‐91 mmol/mol) and stage 3 CKD; 40% of patients were required to have eGFR 30‐44 ml/min/1.73 m2 and 40% were required to have eGFR 45‐59 ml/min/1.73 m2, to ensure a balance between the stage 3A and 3B CKD subgroups. At least 50% of participants should have been taking insulin at a total daily dose of ≥20 units, to enable the study of patients on established, high‐dose insulin therapy. Patients with renal transplant, acute/subacute renal function deterioration, or significant hepatic disease were excluded. See https://clinicaltrials.gov/ct2/show/NCT03550378 for full eligibility criteria.
2.4. Study design and interventions
Participants were randomized 1:1 to receive subcutaneous cotadutide or placebo in the morning. Randomization was stratified by insulin dose (≥20 vs. <20 units per day). Participants in the cotadutide group received 50 μg once daily for 4 days,100 μg for 7 days, 200 μg for 7 days and then 300 μg for 14 days. Patients in the placebo group received once‐daily subcutaneous injections for 32 days (Figure 1).
FIGURE 1.
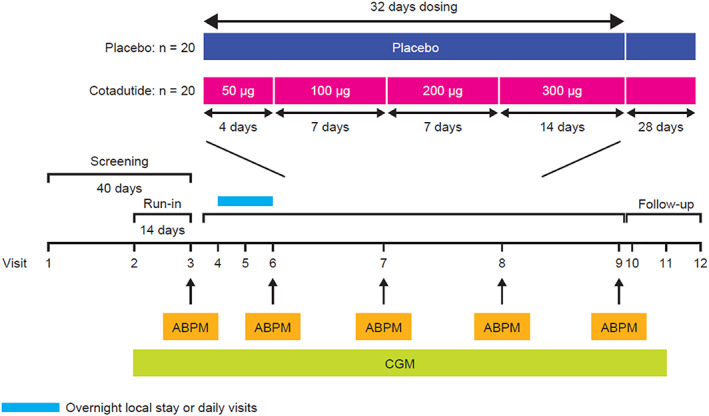
Study design. Abbreviations: ABPM, ambulatory blood pressure monitoring; CGM, continuous glucose monitoring; n, number of subjects
A standardized MMTT [Ensure Plus (Abbott Nutrition, Maidenhead, UK) nutritional supplement containing fat, carbohydrate and protein] was used to assess change in plasma glucose area under the curve (AUC) from baseline to day 32 of treatment. Further details are provided in the Appendix S1.
2.5. Endpoints
The primary efficacy endpoint was the percentage change in MMTT plasma glucose AUC from baseline to day 32. Secondary safety and tolerability endpoints included incidence of adverse events (AEs) and serious AEs (SAEs), changes from baseline in vital signs, blood pressure, electrocardiogram, 24‐h pulse rate, bioimpedance spectroscopy to evaluate fluid balance and clinical laboratory test results. Secondary efficacy endpoints included change from baseline in HbA1c, fasting plasma glucose, percentage of time spent within target interstitial glucose range (70‐180 mg/dl or 3.9‐10 mmol/L) over a 7‐day period at baseline to the final week of treatment and percentage and absolute change in bodyweight. PK parameters and titre of anti‐drug antibody (ADA) were additional secondary endpoints. Exploratory endpoints included change from baseline in mean UACR and eGFR, and percentage change and change in total daily insulin dose.
Post hoc analyses included changes in plasma and urinary renal biomarkers, and inflammatory biomarkers (Table S1) from baseline to day 32. Lithium clearance was calculated as a surrogate of proximal tubule sodium excretion using plasma and urine creatinine and lithium levels.
2.6. Statistical analyses
The sample size was chosen to provide >85% power to detect 18.1% difference between treatment arms for the primary efficacy endpoint, with a two‐sided significance level of 0.1, assuming a standard deviation (SD) of 20%.
All efficacy analyses were performed on the intent‐to‐treat population, unless otherwise specified. Safety analyses were performed on the as‐treated population.
Continuous endpoints were analysed using an analysis of covariance model, with treatment as a fixed factor and baseline as a covariate. Change in percentage of time spent within target interstitial glucose range was analysed using a Wilcoxon rank‐sum test. Percentage change and change in total daily insulin dose was analysed using a two‐sample t‐test (unequal variance with Satterthwaite approximation). Descriptive statistics were generated for analysis for clinical laboratory parameters, vital signs, electrocardiogram, PK and immunogenicity. Change in UACR was analysed using a t‐test with unequal variance.
3. RESULTS
3.1. Patients
Of 101 patients screened across seven sites in the UK and Germany between 29 June 2018 and 4 February 2019, 41 patients were enrolled. Overall, 21 and 20 patients were randomized to receive cotadutide and placebo, respectively. Twenty‐eight participants were on ≥20 units per day of insulin (14 per treatment arm) with or without additional oral treatment, two were receiving <20 units per day and oral treatment (one per treatment arm) and 11 oral treatment only (five in the placebo group and six in the cotadutide group) (Table 1).
TABLE 1.
Patient demographics and baseline characteristics
| Cotadutide (n = 21) | Placebo (n = 20) | Total (N = 41) | |
|---|---|---|---|
| Age, years ± SD | 71.1 ± 7.4 | 70.9 ± 4.7 | 71.0 ± 6.1 |
| Sex, n (%) | |||
| Female | 9 (42.9) | 11 (55.0) | 20 (48.8) |
| Male | 12 (57.1) | 9 (45.0) | 21 (51.2) |
| Weight, kg; mean ± SD | 94.7 ± 17.6 | 91.6 ± 15.8 | 93.2 ± 16.6 |
| Height, cm; mean ± SD | 170.5 ± 9.8 | 167.0 ± 10.0 | 168.8 ± 10.0 |
| BMI, kg/m2; mean ± SD | 32.4 ± 4.1 | 32.9 ± 5.5 | 32.7 ± 4.8 |
| eGFR, ml/min/1.73 m2; mean ± SD | 44.7 ± 8.7 | 47.6 ± 8.8 | 46.1 ± 8.8 |
| HbA1c | |||
| %, mean ± SD | 7.85 ± 0.7 | 7.88 ± 1.3 | 7.87 ± 1.0 |
| mmol/mol, mean ± SD | 62 ± 7.7 | 63 ± 14.2 | 64 ± 10.9 |
| Fasting plasma glucose | |||
| mg/dl, mean ± SD | 166.6 ± 26.9 | 177.9 ± 50.7 | 172.1 ± 40.2 |
| mmol/L, mean ± SD | 9.3 ± 1.5 | 9.9 ± 2.8 | 9.6 ± 2.2 |
| Duration of type 2 diabetes, years; mean ± SD | 16.3 ± 8.5 | 15.9 ± 7.2 | 16.1 ± 7.8 |
| Insulin dose, n (%) | |||
| ≥20 units per day | 14 (66.7) | 14 (70.0) | 28 (68.3) |
| <20 units per day | 7 (33.3) | 6 (30.0) | 13 (31.7) |
| Other concomitant medications, n (%) | |||
| Metformin | 11 (52.4) | 9 (45.0) | 20 (48.8) |
| Sulphonylureas/glitinides | 6 (28.6) | 5 (25.0) | 11 (26.8) |
| SGLT2i | 4 (19.0) | 2 (10.0) | 6 (14.6) |
| DPP4i | 2 (9.5) | 3 (15.0) | 5 (12.2) |
| ACEi/A2RB | 16 (76.2) | 16 (80.0) | 32 (78.0) |
Abbreviations: A2RB, angiotensin II receptor blocker; ACEi, angiotensin‐converting enzyme inhibitor; BMI, body mass index; DPP4i, dipeptidyl‐peptidase 4 inhibitor; eGFR, estimated glomerular filtration rate; HbA1c, glycated haemoglobin; SD, standard deviation; SGLT2i, sodium‐glucose cotransporter 2 inhibitor.
Four participants (9.8%) did not complete treatment; three discontinued treatment because of an AE (one in the placebo group and two in the cotadutide group) and one did not complete the study because of death (cotadutide group). All participants completed the study, except for the participant who died. Further details are provided in the Appendix S1.
3.2. Efficacy
3.2.1. Glycaemic endpoints
The primary objective of the study was met, with a significant reduction in the least‐squares (LS) mean MMTT plasma glucose AUC0−4h from baseline to day 32 in the cotadutide group versus placebo (–26.71% vs. +3.68%, p < .001; Figure 2A). A larger but non‐significant reduction in LS mean fasting plasma glucose from baseline to day 32 was observed in the cotadutide group versus placebo [–19.55 mg/dl (1.09 mmol/L) vs. +0.60 mg/dl (0.03 mmol/L), p = .089]. There was a significant decline in LS mean 7‐day continuous glucose monitoring average blood glucose concentrations from baseline to the last week of treatment (day 32) in the cotadutide group versus placebo [–41.37 mg/dl (90% CI: –58.06, –24.67) or 2.3 mmol/L vs. +6.52 mg/dl (90% CI: –12.00, 25.02) or +0.4 mmol/L, p = .003; Figure 2B].
FIGURE 2.
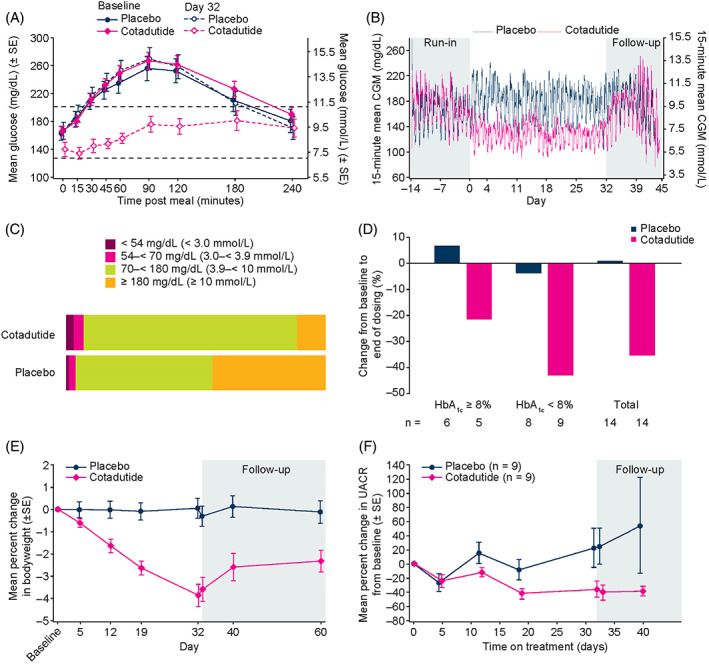
Primary and secondary endpoints. (A) Percentage change in MMTT plasma glucose AUC4h from baseline to day 32. (B) 15‐minute mean CGM over time. (C) Proportion of time spent in target interstitial glucose range across 32 days of dosing. (D) Change from baseline to end of dosing in daily insulin dose in participants receiving insulin ≥20 units per day, according to baseline HbA1c (n = 14 in each treatment arm). (E) Percentage change in bodyweight from baseline to day 32 and over 38 days follow‐up. (F) Mean percentage change in UACR over time in subgroup of patients with baseline micro‐ or macroalbuminuria (n = 18). Abbreviations: AUC, area under the curve; CGM, continuous glucose monitoring; HbA1c, glycated haemoglobin; MMTT, mixed‐meal tolerance test; SE, standard error; UACR, urinary albumin‐to‐creatinine ratio
Participants receiving cotadutide had a significantly greater LS mean increase from baseline to day 32 in the percentage of time spent within target range (70‐180 mg/dl or 3.9‐10 mmol/L) over a 7‐day period versus placebo (+14.79% vs. –21.23%, p = .001; Figure 2C). Cotadutide was associated with a progressively increasing mean percentage of time spent within the target range over a 7‐day period (baseline: 59.02% vs. 57.58% for placebo; 100 μg: 75.82% vs. 48.57%; 200 μg: 78.69% vs. 54.99%; 300 μg: 78.18% vs. 40.66%). Over the entire dosing period, participants receiving cotadutide spent significantly less time in hyperglycaemia (glucose >180 mg/dl or 10 mmol/L) versus placebo (10.50% vs. 37.39%, p = .001; Figure 2C).
A significant reduction in LS mean HbA1c from baseline to day 32 was observed in the cotadutide group versus placebo (–0.65% vs. +0.01%, p < .001).
For participants on insulin ≥20 units per day, median reductions in total daily insulin dose in the cotadutide group were significantly larger than they were for the placebo group [–37.0% (–100, 33%) vs. 0.0% (–35, 40%), p = .012; Figure 2D]. The median absolute change in dose from baseline to day 32 was –16.0 units per day (range: –194, 20 units per day) in the cotadutide group and 0.0 units per day (range: –46, 24 units per day) in the placebo group, but the difference was not significant (p = .073).
3.2.2. Bodyweight endpoints
Cotadutide was associated with a significant LS mean reduction in absolute and percentage bodyweight from baseline to day 33 versus placebo [cotadutide, –3.41 kg (90% CI: –4.2, –2.62), –3.69% (90% CI: –4.55, –2.83); placebo, –0.13 kg (90% CI: –0.9, 0.64), –0.21% (90% CI: –1.05, 0.62) p < .001] (Figure 2E).
3.2.3. Renal and cardiovascular endpoints
Mean UACR was lower in the cotadutide group than in the placebo group from day 19 to 32 and for 8 days into follow‐up. There was a reduction in LS mean UACR from baseline to day 33 in the cotadutide group versus placebo (–7.65% vs. 5.43%) but the difference was not significant (Table S2). In a subgroup of patients with baseline micro‐ or macroalbuminuria (n = 18), UACR was non‐significantly reduced by 51% (90% CI: 27.7%, 88.4%) at day 32 with cotadutide versus placebo (p = .0504; Figure 2F). No significant correlation was found between change in HbA1c and percentage change in UACR in this subgroup (p = .604, R = 0.04), supporting that this effect was independent of glucose control.
No statistically significant difference was observed in the LS mean change in eGFR between treatment arms [cotadutide, 1.17 ml/min/1.73 m2 (90% CI: –1.29, 3.62); placebo, –1.10 ml/min/1.73 m2 (90% CI: –3.43, 1.23), p = .268]. Similarly, there were no clinically meaningful differences between treatment arms in terms of changes in body water volume or intracellular or extracellular fluid volume.
In post hoc analyses, cotadutide was associated with a larger reduction in N‐terminal pro‐brain natriuretic peptide (n = 17; –79.73 ng/L) from baseline to day 32 than placebo (n = 19; –9.42 ng/L; p = .04; Table S2). Increases in LS mean supine blood pressure from baseline to day 32 were observed, but the changes were not clinically significant and were greater in the placebo group (systolic, cotadutide vs. placebo, 1.44 mmHg vs. 8.69 mmHg, p = .144; diastolic, 0.57 vs. 3.40 mmHg, p = .421). Increases from baseline to day 32 in LS mean heart rate were 14.13 and 3.14 beats per minute in the cotadutide and placebo groups, respectively (p < .001).
3.2.4. Post hoc analyses of renal biomarkers
Serum ammonia was reduced from baseline to day 32 in the cotadutide group (n = 17; –29.53 mmol/L) versus placebo (n = 19; +1.88 mmol/L; p = .155; Table S2). There were also small reductions in urinary fractional lithium excretion (FELi; day 1, mean ± SD 13.82% ± 12.46%; day 32, 11.82% ± 5.48%) and lithium clearance (day 1, mean ± SD 6.40 ± 5.01 ml/min; day 32, 5.85 ± 2.51 ml/min) in the cotadutide group that were not observed in the placebo group (Table S3).
There were no significant changes in other plasma or urinary inflammatory (pH, interleukin‐18, monocyte chemoattractant protein, tumour necrosis factor receptor 1/2) or kidney injury (blood urea nitrogen, uric acid, renin, aldosterone, kidney‐injury marker‐1) biomarkers in either treatment arm from day 1 to day 32 (Table S2).
3.3. Safety and tolerability
The majority of participants experienced at least one treatment‐emergent AE (TEAE), with the incidence higher in the cotadutide group (95.2%) than in the placebo group (65.0%) (Table 2). Similarly, TEAEs related to the investigational product were more prevalent in the cotadutide group (71.4%) than in the placebo group (35.0%).
TABLE 2.
Summary of safety events
| Cotadutide (n = 21) | Placebo (n = 20) | Overall (N = 41) | |
|---|---|---|---|
| Treatment‐emergent adverse event, n (%) | |||
| Any | 20 (95.2) | 13 (65.0) | 33 (80.5) |
| Any related to cotadutide | 15 (71.4) | 7 (35.0) | 22 (53.7) |
| Serious (no. patients) | 2 (9.5) | 2 (10.0) | 4 (9.8) |
| Serious related to cotadutide | 1 (4.8) | 0 | 1 (2.4) |
| Grade ≥3 severity | |||
| Leading to death (no. patients) | 1 (4.8) | 0 | 1 (2.4) |
| Leading to study discontinuation (no. patients) | 2 (9.5) | 1 (5.0) | 3 (7.3) |
| Occurring in ≥15% patients | |||
| Diarrhoea | 5 (23.8) | 0 | 5 (12.2) |
| Dyspepsia | 5 (23.8) | 1 (5.0) | 6 (14.6) |
| Nausea | 9 (42.9) | 4 (20.0) | 13 (31.7) |
| Vomiting | 6 (28.6) | 1 (5.0) | 7 (17.1) |
| Occurring in <15% patients | |||
| Flatulence | 2 (9.5) | 0 | 2 (4.9) |
| Nasopharyngitis | 3 (14.3) | 2 (10.0) | 5 (12.2) |
| Decreased appetite | 3 (14.3) | 0 | 3 (7.3) |
| Hypoglycaemia a | 3 (14.3) | 1 (5.0) | 4 (9.8) |
| Dizziness | 2 (9.5) | 1 (5.0) | 3 (7.3) |
| Headache | 2 (9.5) | 2 (10.0) | 4 (9.8) |
Clinically significant hypoglycaemia defined as a capillary or venous plasma glucose of <54 mg/dl (3.0 mmol/L).
Two SAEs were recorded in each treatment arm. One participant treated with insulin in the cotadutide group experienced an SAE of diabetic ketoacidosis in association with a serious infection (cholecystitis) and presumed cessation of insulin therapy that resulted in death. The event was deemed related to cotadutide by the investigator because an association could not be ruled out. No clear indication of a relationship to cotadutide was identified by the sponsor. An additional participant in the cotadutide group experienced an SAE of hypertensive crisis secondary to inadequate postoperative analgesia 8 days after their last dose. This was considered not related to cotadutide by the investigator. Two participants in the placebo group experienced SAEs of carotid artery stenosis and syncope; both were assessed as not related to the investigational product.
Most TEAEs were mild or moderate (grade 1 or 2) in severity, with an approximately equal number of participants experiencing a grade ≥3 AE across the two treatment arms. Three participants (one in the placebo group and two in the cotadutide group) experienced a TEAE that led to study discontinuation.
The most common TEAEs in the cotadutide group were gastrointestinal disorders, infections and infestations, and metabolism and nutrition disorders. TEAEs reported in more than 15% of participants in the cotadutide group were nausea (42.9%), vomiting (28.6%), diarrhoea (23.8%) and dyspepsia (23.8%); nausea was the only TEAE reported in more than 15% of participants in the placebo group (20.0%).
There were no recorded AEs of severe hypoglycaemia. Continuous glucose monitoring showed an initial small statistically significant increase in time spent in level 2 (clinically significant) hypoglycaemia (glucose range <54 mg/dl or <3.0 mmol/L). This abated through insulin dose reduction as dosing progressed. Over the entire dosing period, those receiving cotadutide spent numerically more time in hypoglycaemia (glucose range <70 mg/dl or <3.9 mmol/L) than those receiving placebo (6.07% vs. 2.73%, p = .061; Figure 2C) and significantly more time in clinically significant hypoglycaemia (2.01% vs. 0.66%, p = .010).
3.3.1. Clinical chemistry endpoints
There were no clinically meaningful trends or shifts from baseline in serum chemistry in the cotadutide or placebo treatment arms, including no change in eGFR from baseline to day 32 in the cotadutide group. There were also no clinically meaningful changes in urinalysis results (Table S4).
3.4. Pharmacokinetics and immunogenicity
Repeat daily administration of cotadutide 50‐300 μg showed linear increased exposure at Ctrough. Two participants, both in the cotadutide group, were ADA‐positive post‐baseline. No participants had a treatment‐boosted ADA response.
4. DISCUSSION
In adults with type 2 diabetes and CKD, cotadutide showed significant improvements in postprandial glucose control. This was also accompanied by significant reductions in bodyweight and HbA1c. The results are consistent with the efficacy of other GLP‐1 RAs. 26 , 27 In patients receiving insulin, reductions in plasma glucose with cotadutide were achieved alongside significant reductions in daily total insulin dose. In these patients, cotadutide led to a small increase in time spent in hypoglycaemia at initiation; however, this was abated through insulin dose reduction. Cotadutide had an acceptable tolerability profile comparable with previously observed tolerability in patients with type 2 diabetes but without CKD, 21 , 28 and to other GLP‐1 RAs. 29 , 30
Patients receiving cotadutide had a 51% reduction in UACR not observed in those receiving placebo, which may suggest cotadutide being beneficial in terms of long‐term renal outcomes in patients with CKD and more pronounced albuminuria. This supports findings from cardiovascular outcome trials of other GLP‐1 RAs in subsets of patients with type 2 diabetes and CKD, in which the placebo‐corrected UACR reduction observed in patients with type 2 diabetes with and without albuminuria ranged 18‐26% across 1‐4 years of treatment. 31 , 32 While direct benchmarking is challenging, the magnitude of UACR reduction with cotadutide observed here suggests that combining GLP‐1 receptor and glucagon receptor agonism has the potential to drive additional benefit in this disease setting compared with GLP‐1 receptor agonism alone. In this small, short‐term substudy, reductions in UACR did not correlate significantly with HbA1c. Changes in glucose control have been shown to influence UACR in some, but not all, studies of GLP‐1 RAs. 33 , 34
GLP‐1 receptors are expressed in the renal vasculature 35 and there is debate as to whether expression is also observed in proximal tubular cells. 36 Glucagon receptors are widely distributed in the kidney, which is second only to the liver in density of glucagon receptor expression. 25 Glucagon receptor expression is significantly upregulated in the kidney and in infiltrating leucocyte B cells in patients with type 2 diabetes and proteinuria, compared with those without proteinuria. 37 Furthermore, elevated glucagon levels have been observed in patients with diabetes and in those with CKD. 25 In addition to its anti‐hyperglycaemic effects, GLP‐1 has been shown in clinical 38 and non‐clinical 35 , 39 studies to promote vasodilation of the renal afferent arteriole via nitric oxide and prostaglandin E, respectively, thereby increasing GFR and natriuresis. Glucagon has also been shown to play a role in vasodilation. In a study of the effect of glucagon and GLP‐1 on vasodilation in isolated rat thoracic aorta, vasodilation by glucagon was mediated by both the glucagon and GLP‐1 receptors, and GLP‐1 vasodilation was partly mediated by the glucagon receptor. 40 Another study in isolated rat heart found that glucagon affected ischaemic vasodilation via nitric oxide and histamine. 41 In the kidney, glucagon plays a key role in sodium, potassium, magnesium and calcium homeostasis, and is associated with renal vasodilation and increases in GFR. 25 , 40 , 42 , 43 Despite the known effects of GLP‐1 and glucagon on natriuresis, there was no evidence for depletion of intra‐ or extracellular fluid volume in patients treated with cotadutide. Analysis of renal biomarkers in this study did not reveal any changes in inflammatory or kidney injury markers over 32 days of dosing; this could potentially indicate that the impact on UACR reduction in the short term is primarily mediated by vascular changes, although these results will need to be confirmed in larger, longer‐term clinical studies.
Lithium clearance as a surrogate for proximal tubular sodium excretion did not show evidence of increased natriuresis, suggesting bodyweight and blood pressure changes were not the result of water loss. Similarly, measures of renin and aldosterone did not provide conclusive insights into changes in the renin‐angiotensin system in patients receiving cotadutide. However, most patients were on a renin‐angiotensin‐aldosterone system inhibitor among other antihypertensives, and sodium content in the diet was not controlled, precluding the ability to draw meaningful conclusions on measures of sodium handling by the kidney following treatment with cotadutide.
Limitations of this study include its short duration, as well as the small number of participants receiving active treatment and of those with albuminuria included in the substudy. Evaluation of the longer‐term benefits and risks of cotadutide in the renal population awaits larger and longer studies. Another limitation is that a greater proportion of participants in the placebo group had eGFR in the 45‐59 ml/min/1.73 m2 range than in the cotadutide group, which had an approximately equal distribution of patients in the eGFR subgroups. In addition, a slightly higher proportion of patients in the cotadutide group (n = 4, 19%) than in the placebo group (n = 2, 10%) were on sodium‐glucose cotransporter‐2 inhibitors. Finally, because cotadutide is a dual agonist of GLP‐1 and glucagon receptors, and in the absence of a monoagonist comparator arm such as liraglutide or semaglutide, it was not possible in this study to separate out their potential synergistic effects, and longer‐term randomized controlled trials are needed to examine the independent effect of glucagon.
In conclusion, cotadutide led to improvements in glycaemic control and weight loss in patients with type 2 diabetes and CKD, and further showed the potential to reduce albuminuria. Based on the growing evidence of the shared pathogenetic mechanisms underlying type 2 diabetes, NAFLD/non‐alcoholic steatohepatitis and CKD, and the enhanced insulin sensitivity, improved glycaemic control, reduction in bodyweight and improved lipid and hepatic biomarkers in obese patients with type 2 diabetes, 21 , 23 , 28 cotadutide may be a potentially beneficial therapy in patients with CKD and type 2 diabetes. Further evaluation in larger, longer‐term clinical studies is warranted.
CONFLICT OF INTEREST
VP, TH, YC, MPe, LH, PA and LJ are employees and shareholders of AstraZeneca. TH has stock or stock options with AstraZeneca, GW Pharma and Jazz Pharma. FG has received payment for lectures, presentations, speaker bureaus, manuscript writing or educational events from Eli Lilly and Novo Nordisk. LR has participated in advisory panels for Novo Nordisk and acted as a consultant for and is a member of the Association of Statutory Health Insurance Physicians. HH is a steering committee member and consultant for clinical trials sponsored by AstraZeneca, has received research grants from AstraZeneca for the present manuscript, is an advisor for AbbVie, Bayer, Boehringer Ingelheim, Chinook, CSL Behring, Dimerix, Eli Lilly, Janssen, Gilead, Merck, MundiPharma, Mitsubishi Tanabe, NovoNordisk, Travere Pharmaceuticals, and has received grants from AbbVie, Boehringer Ingelheim, Janssen, and NovoNordisk. RM has received research support from AstraZeneca, royalties or licenses from Elsevier, lecture fees from Sanofi Aventis and NovoNordisk, has participated on the Advisory Board for Sanofi Aventis, is a non‐executive member of NHS Tayside Health Board, and a panel member of MRC Population and Systems Medicine Board. HS, BW and MPo declare that they have no competing interests. Editorial support was provided by Oxford PharmaGenesis, Oxford, UK, and was funded by AstraZeneca.
AUTHOR CONTRIBUTIONS
VP, TH, PA and LJ contributed to the study design. VP, LH, PA and LJ contributed to funding acquisition. HS, FG, BW, MPo, LR and RM contributed to the study conduct/data collection. VP, YC, MPe, LH, PA, LJ and HH contributed to the analysis. VP contributed to writing of the original draft. All authors participated in reviewing and editing the manuscript and approved the final version of the manuscript. VP is the guarantor of this work, and such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14712.
Supporting information
Appendix S1
Table S1
Table S2
Table S3
Table S4
ACKNOWLEDGMENTS
The authors thank the participants and staff involved in the study. Medical writing and editorial support were provided by Katie Willetts PhD and Paul Williams of Oxford PharmaGenesis, which was funded by AstraZeneca.
Parker VER, Hoang T, Schlichthaar H, et al. Efficacy and safety of cotadutide, a dual glucagon‐like peptide‐1 and glucagon receptor agonist, in a randomized phase 2a study of patients with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab. 2022;24(7):1360‐1369. doi: 10.1111/dom.14712
Funding information This study (NCT03550378) was sponsored by AstraZeneca.
DATA AVAILABILITY STATEMENT
Data underlying the findings described in this article may be obtained in accordance with AstraZeneca's data sharing policy described at:https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure
REFERENCES
- 1. Reutens AT. Epidemiology of diabetic kidney disease. Med Clin North Am. 2013;97(1):1‐18. [DOI] [PubMed] [Google Scholar]
- 2. Gorriz JL, Soler MJ, Navarro‐Gonzalez JF, et al. GLP‐1 receptor agonists and diabetic kidney disease: a call of attention to nephrologists. J Clin Med. 2020;9(4):947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kovesdy CP, Furth SL, Zoccali C. Obesity and kidney disease: hidden consequences of the epidemic. Can J Kidney Health Dis. 2017;4:2054358117698669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cherney DZI, Repetto E, Wheeler DC, et al. Impact of cardio‐renal‐metabolic comorbidities on cardiovascular outcomes and mortality in type 2 diabetes mellitus. Am J Nephrol. 2020;51(1):74‐82. [DOI] [PubMed] [Google Scholar]
- 5. Gheith O, Farouk N, Nampoory N, Halim MA, Al‐Otaibi T. Diabetic kidney disease: world wide difference of prevalence and risk factors. J Nephropharmacol. 2016;5(1):49‐56. [PMC free article] [PubMed] [Google Scholar]
- 6. Adler AI, Stevens RJ, Manley SE, et al. Development and progression of nephropathy in type 2 diabetes: the United Kingdom prospective diabetes study (UKPDS 64). Kidney Int. 2003;63(1):225‐232. [DOI] [PubMed] [Google Scholar]
- 7. Ahlqvist E, Storm P, Karajamaki A, et al. Novel subgroups of adult‐onset diabetes and their association with outcomes: a data‐driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018;6(5):361‐369. [DOI] [PubMed] [Google Scholar]
- 8. Musso G, Gambino R, Tabibian JH, et al. Association of non‐alcoholic fatty liver disease with chronic kidney disease: a systematic review and meta‐analysis. PLoS Med. 2014;11(7):e1001680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Musso G, Cassader M, Cohney S, et al. Fatty liver and chronic kidney disease: novel mechanistic insights and therapeutic opportunities. Diabetes Care. 2016;39(10):1830‐1845. [DOI] [PubMed] [Google Scholar]
- 10. Byrne CD, Targher G. NAFLD as a driver of chronic kidney disease. J Hepatol. 2020;72(4):785‐801. [DOI] [PubMed] [Google Scholar]
- 11. Sun DQ, Jin Y, Wang TY, et al. MAFLD and risk of CKD. Metabolism. 2021;115:154433. [DOI] [PubMed] [Google Scholar]
- 12. Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an ADA consensus conference. Diabetes Care. 2014;37(10):2864‐2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295‐2306. [DOI] [PubMed] [Google Scholar]
- 14. Heerspink HJL, Stefansson BV, Correa‐Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436‐1446. [DOI] [PubMed] [Google Scholar]
- 15. Davies MJ, D'Alessio DA, Fradkin J, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of diabetes (EASD). Diabetologia. 2018;61(12):2461‐2498. [DOI] [PubMed] [Google Scholar]
- 16. Mann JFE, Orsted DD, Buse JB. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377(22):2197‐2198. [DOI] [PubMed] [Google Scholar]
- 17. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834‐1844. [DOI] [PubMed] [Google Scholar]
- 18. Armstrong MJ, Hull D, Guo K, et al. Glucagon‐like peptide 1 decreases lipotoxicity in non‐alcoholic steatohepatitis. J Hepatol. 2016;64(2):399‐408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kawanami D, Takashi Y. GLP‐1 receptor agonists in diabetic kidney disease: from clinical outcomes to mechanisms. Front Pharmacol. 2020;11:967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Henderson SJ, Konkar A, Hornigold DC, et al. Robust anti‐obesity and metabolic effects of a dual GLP‐1/glucagon receptor peptide agonist in rodents and non‐human primates. Diabetes Obes Metab. 2016;18(12):1176‐1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ambery P, Parker VE, Stumvoll M, et al. MEDI0382, a GLP‐1 and glucagon receptor dual agonist, in obese or overweight patients with type 2 diabetes: a randomised, controlled, double‐blind, ascending dose and phase 2a study. Lancet. 2018;391(10140):2607‐2618. [DOI] [PubMed] [Google Scholar]
- 22. Boland ML, Laker RC, Mather K, et al. Resolution of NASH and hepatic fibrosis by the GLP‐1R/GcgR dual‐agonist Cotadutide via modulating mitochondrial function and lipogenesis. Nat Metab. 2020;2(5):413‐431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nahra R, Wang T, Gadde KM, et al. Effects of Cotadutide on metabolic and hepatic parameters in adults with overweight or obesity and type 2 diabetes: a 54‐week randomized phase 2b study. Diabetes Care. 2021;44(6):1433‐1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Parker V, Robertson D, Hansen L, et al. Cotadutide (MEDI0382), a dual receptor agonist with balanced glucagon‐like peptide‐1 and glucagon activity, modulates hepatic glycogen stores. Presented at: European Association for the Study of Diabetes 55th Annual Meeting; September 18, 2019; Barcelona, Spain. [Google Scholar]
- 25. Bankir L, Bouby N, Blondeau B, Crambert G. Glucagon actions on the kidney revisited: possible role in potassium homeostasis. Am J Physiol Renal Physiol. 2016;311(2):F469‐F486. [DOI] [PubMed] [Google Scholar]
- 26. Nauck MA, Quast DR, Wefers J, Meier JJ. GLP‐1 receptor agonists in the treatment of type 2 diabetes ‐ state‐of‐the‐art. Mol Metab. 2021;46:101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kolterman OG, Kim DD, Shen L, et al. Pharmacokinetics, pharmacodynamics, and safety of exenatide in patients with type 2 diabetes mellitus. Am J Health Syst Pharm. 2005;62(2):173‐181. [DOI] [PubMed] [Google Scholar]
- 28. Parker VER, Robertson D, Wang T, et al. Efficacy, safety, and mechanistic insights of cotadutide, a dual receptor glucagon‐like peptide‐1 and glucagon agonist. J Clin Endocrinol Metab. 2020;105(3):803‐820. [DOI] [PubMed] [Google Scholar]
- 29. Mann JFE, Fonseca VA, Poulter NR, et al. Safety of liraglutide in type 2 diabetes and chronic kidney disease. Clin J Am Soc Nephrol. 2020;15(4):465‐473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sorli C, Harashima SI, Tsoukas GM, et al. Efficacy and safety of once‐weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double‐blind, randomised, placebo‐controlled, parallel‐group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. 2017;5(4):251‐260. [DOI] [PubMed] [Google Scholar]
- 31. Persson F, Bain SC, Mosenzon O, et al. Changes in albuminuria predict cardiovascular and renal outcomes in type 2 diabetes: a post hoc analysis of the LEADER trial. Diabetes Care. 2021;44(4):1020‐1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mann JFE, Hansen T, Idorn T, et al. Effects of once‐weekly subcutaneous semaglutide on kidney function and safety in patients with type 2 diabetes: a post‐hoc analysis of the SUSTAIN 1‐7 randomised controlled trials. Lancet Diabetes Endocrinol. 2020;8(11):880‐893. [DOI] [PubMed] [Google Scholar]
- 33. Elnaem MH, Mansour NO, Nahas AF, Baraka MA, Elkalmi R, Cheema E. Renal outcomes associated with the use of non‐insulin antidiabetic pharmacotherapy: a review of current evidence and recommendations. Int J Gen Med. 2020;13:1395‐1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van Baar MJB, van der Aart AB, Hoogenberg K, Joles JA, Heerspink HJL, van Raalte DH. The incretin pathway as a therapeutic target in diabetic kidney disease: a clinical focus on GLP‐1 receptor agonists. Ther Adv Endocrinol Metab. 2019;10:2042018819865398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jensen EP, Poulsen SS, Kissow H, et al. Activation of GLP‐1 receptors on vascular smooth muscle cells reduces the autoregulatory response in afferent arterioles and increases renal blood flow. Am J Physiol Renal Physiol. 2015;308(8):F867‐F877. [DOI] [PubMed] [Google Scholar]
- 36. Schlatter P, Beglinger C, Drewe J, Gutmann H. Glucagon‐like peptide 1 receptor expression in primary porcine proximal tubular cells. Regul Pept. 2007;141(1‐3):120‐128. [DOI] [PubMed] [Google Scholar]
- 37. Wilson PC, Wu H, Kirita Y, et al. The single‐cell transcriptomic landscape of early human diabetic nephropathy. Proc Natl Acad Sci USA. 2019;116(39):19619‐19625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Muskiet MH, Tonneijck L, Smits MM, et al. Acute renal haemodynamic effects of glucagon‐like peptide‐1 receptor agonist exenatide in healthy overweight men. Diabetes Obes Metab. 2016;18(2):178‐185. [DOI] [PubMed] [Google Scholar]
- 39. Jensen EP, Moller S, Hviid AV, et al. GLP‐1‐induced renal vasodilation in rodents depends exclusively on the known GLP‐1 receptor and is lost in prehypertensive rats. Am J Physiol Renal Physiol. 2020;318(6):F1409‐F1417. [DOI] [PubMed] [Google Scholar]
- 40. Selley E, Kun S, Szijarto IA, Kertesz M, Wittmann I, Molnar GA. Vasodilator effect of glucagon: receptorial crosstalk among glucagon, GLP‐1, and receptor for glucagon and GLP‐1. Horm Metab Res. 2016;48(7):476‐483. [DOI] [PubMed] [Google Scholar]
- 41. Rosic M, Pantovic S, Rosic G, et al. Glucagon effects on ischemic vasodilatation in the isolated rat heart. J Biomed Biotechnol. 2010;2010:231832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Friedlander G, Blanchet‐Benque F, Nitenberg A, Laborie C, Assan R, Amiel C. Glucagon secretion is essential for aminoacid‐induced hyperfiltration in man. Nephrol Dial Transplant. 1990;5(2):110‐117. [DOI] [PubMed] [Google Scholar]
- 43. Farah AE. Glucagon and the circulation. Pharmacol Rev. 1983;35(3):181‐217. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Table S1
Table S2
Table S3
Table S4
Data Availability Statement
Data underlying the findings described in this article may be obtained in accordance with AstraZeneca's data sharing policy described at:https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure


