Summary
Heat waves occurring during droughts can have a devastating impact on yield, especially if they happen during the flowering and seed set stages of the crop cycle. Global warming and climate change are driving an alarming increase in the frequency and intensity of combined drought and heat stress episodes, critically threatening global food security.
Because high temperature is detrimental to reproductive processes, essential for plant yield, we measured the inner temperature, transpiration, sepal stomatal aperture, hormone concentrations and transcriptomic response of closed soybean flowers developing on plants subjected to a combination of drought and heat stress.
Here, we report that, during a combination of drought and heat stress, soybean plants prioritize transpiration through flowers over transpiration through leaves by opening their flower stomata, while keeping their leaf stomata closed. This acclimation strategy, termed ‘differential transpiration’, lowers flower inner temperature by about 2–3°C, protecting reproductive processes at the expense of vegetative tissues.
Manipulating stomatal regulation, stomatal size and/or stomatal density of flowers could serve as a viable strategy to enhance the yield of different crops and mitigate some of the current and future impacts of global warming and climate change on agriculture.
Keywords: climate change, drought, heat stress, soybean, stomata, stress combination, transpiration, yield
Introduction
The unyielding increase in atmospheric and oceanic temperatures, termed ‘global warming’, is causing drastic changes in our climate, termed ‘climate change’ (Lobell et al., 2011; Steg, 2018; Bailey‐Serres et al., 2019; Alizadeh et al., 2020; Overpeck & Udall, 2020; von der Gathen et al., 2021; Zandalinas et al., 2021; Zhai et al., 2021). As a result, large areas of our planet are increasingly exposed to floods or extended droughts combined with extreme temperatures (Mazdiyasni & AghaKouchak, 2015; Alizadeh et al., 2020; Overpeck & Udall, 2020; Rivero et al., 2022; Zandalinas et al., 2021). Historically, extended droughts combined with heat waves have been the cause of catastrophic reductions in agricultural productivity estimated at billions of dollars per episode (e.g. the drought and heat wave combination events that occurred during the summers of 1980 and 1988 in the US resulted in losses to agriculture estimated at $33 and 44 billion, respectively; https://www.ncdc.noaa.gov/billions/; Mittler, 2006; Lobell et al., 2011; Rivero et al., 2022). Because global warming and climate change are increasing the frequency and intensity of drought and heat stress combination events worldwide, more studies are needed to understand how crops and other plants respond to this type of stress combination (Mazdiyasni & AghaKouchak, 2015; Alizadeh et al., 2020; Rivero et al., 2022; Zandalinas et al., 2021; Zhai et al., 2021). Agricultural experience, as well as multiple studies conducted with different crops, revealed that the effects of drought and heat stress combination on yield of many major grain crops is most severe when the stress combination occurs during the reproductive stage of plant growth (Rollins et al., 2013; Mahrookashani et al., 2017; Lawas et al., 2018; Liu et al., 2020; Cohen et al., 2021b; Rivero et al., 2022; Sinha et al., 2021).
Plant reproduction, that is, the developmental process of flower organs (including stamens and stigma), the maturation of pollen and egg cells, pollen shedding, interactions with stigma, germination, growth and eventually fertilization, as well as embryo development and seed filling, are all highly sensitive to elevated temperatures (Santiago & Sharkey, 2019; Djanaguiraman et al., 2020; Chaturvedi et al., 2021; Sze et al., 2021). It was recently proposed that the tightly synchronized nature of the developmental programs involved in these processes, as well as their reliance on certain stress‐related programs (e.g. the stress‐associated dehydration program of pollen grains), reactive oxygen species (ROS) and hormone signaling, under nonstress conditions, makes them more sensitive to stress (Sinha et al., 2021; Sze et al., 2021). Stresses such as drought or heat, or their combinations, may therefore disrupt these tightly synchronized processes by triggering the activation of stress programs and/or the accumulation of different hormones, ROS or other signals, out of sync with the proper developmental process, leading to the production of immature or malnourished pollen, egg cell programmed cell death and other disruptive processes that decrease yield (Martin et al., 2013; Lassig et al., 2014; Daneva et al., 2016; Zhang et al., 2020; Sinha et al., 2021; Sze et al., 2021).
Many important grain crops such as wheat (Triticum aestivum), rice (Oryza sativa) and soybean (Glycine max) are self‐pollinating and do not require vectors such as insects or wind for cross‐pollination (Liu et al., 2006). In many legumes and important grass species, self‐pollination occurs even before the flower opens (i.e. the pollen is transferred to the stigma of the same flower within the closed flower – termed cleistogamy or pseudocleistogamy; Campbell et al., 1983; Takahashi et al., 2001). Although, under controlled nonstressed conditions, cleistogamy/pseudocleistogamy protects many aspects of the reproductive process from external factors such as low humidity, UV radiation, pathogens and/or other potential stressors, under conditions of drought, or drought combined with heat stress, when transpiration is suppressed, the internal temperature of the flower could rise to high values that would inhibit reproduction (Lawas et al., 2018; Wei et al., 2018; Sinha et al., 2021).
Transpiration in plants is primarily controlled by changes in stomatal aperture and the water vapor pressure differential between the plant and the atmosphere (Will et al., 2013; Lawson & Matthews, 2020). When stomata are open, transpiration can occur at a higher rate and cool the plant. This was demonstrated for leaves of different plants subjected to heat stress (Zhou et al., 2015; Balfagón et al., 2019; Zandalinas et al., 2020a). By contrast, during drought, stomata are closed to prevent water loss and plant temperature increases as a result of lack of transpiration. Interestingly, during a combination of drought and heat stress, stomata on leaves of many different plant species are closed and leaf temperature is higher than that of plants subjected to heat alone (Rizhsky et al., 2002, 2004; Carmo‐Silva et al., 2012; Zandalinas et al., 2016; Cohen et al., 2021a). Because the temperature of reproductive processes (occurring within the flowers of cleistogamous plants) plays such a critical role in the overall yield of many crops, we studied how a combination of drought and heat stress (which has a devastating impact on yield; Mittler, 2006; Lobell et al., 2011; Rollins et al., 2013; Mahrookashani et al., 2017; Lawas et al., 2018; Liu et al., 2020; Cohen et al., 2021b; Rivero et al., 2022; Sinha et al., 2021), would impact flower stomatal aperture, transpiration and inner temperature in two different plants: soybean and tobacco (Nicotiana tabacum). Here, we report that during a combination of drought and heat stress, plants prioritize transpiration through flowers over transpiration through leaves by opening their flower stomata, while keeping their leaf stomata closed. This strategy, termed ‘differential transpiration’, lowers flower temperature by about 2–3°C, and represents a newly discovered acclimation mechanism of plants to different abiotic stresses that result in higher inner flower temperature (e.g. combinations of drought, pathogen infection, mechanical injury, high CO2 or air pollution, such as ozone, that cause stomatal closure with heat stress). Manipulating stomatal regulation, stomata size and/or stomata number (i.e. stomatal density) of flowers could therefore serve as a viable strategy to enhance the yield of different crops in the face of our uncertain current and future climates.
Materials and Methods
Plant material and stress treatments
Soybean seeds (Glycine max cv Magellan; United States Department of Agriculture‐Germplasm Resources Information network germplasm collection; https://npgsweb.ars‐grin.gov/) were inoculated with Bradyrhizobium japonicum inoculum (N‐DURE, Verdesian Life Sciences, Cary, NC, USA) and germinated in Promix HP (Premier Tech Horticulture, Quakertown, PA, USA) under short‐day growth conditions (12 h : 12 h, 28°C : 24°C, light : dark, 500 μmol photons m−2 s−1, with the temperature linearly increased from 24 to 28°C between 06:00 and 08:00 h and linearly decreased to 24°C between 16:00 and 20.00 h), for 1 wk in a growth chamber (BDR16; Conviron, Winnipeg, MB, Canada). After 1 wk, seedlings from trays were transplanted into pots containing 1 kg of Promix HP soaked in 1 l of water fertilizer (Zack’s Classic Blossom Booster 10‐30‐20; JR Peters Inc., Allentown, PA, USA) mix (Cohen et al., 2021a). Plants were then grown for the next 16–18 d (until the start of the first open flower, R1 developmental stage; Fehr et al., 1971) under the same 12 h : 12 h, 28°C : 24°C, light : dark conditions, but with the light intensity increased to 1000 μmol photons m−2 s−1. Plants were fertilized twice a week (Cohen et al., 2021a). At R1 plants were randomly divided into four BDR16 growth chambers placed side by side in the same room. One chamber was kept as control (CT), one as heat stress (HS), one as water deficit (WD), and one as WD + HS (Cohen et al., 2021a). The chambers were not randomized between experiments but were all purchased at the same time and were identical. In addition, the relative humidity was maintained at about 60–65% in all chambers, regardless of the treatment, and all internal conditions were continuously monitored. In the WD and WD + HS treatments, plants were supplied with 30% of the water available for transpiration (determined by weighing pots daily as described in Cohen et al., 2021a), while plants in the CT and HS treatments were well watered. The application of water deficit under these conditions mimicked ‘terminal drought’ conditions that negatively impact yield but do not kill the plant (Cohen et al., 2021a). Plants in the HS and WD + HS treatments were further subjected to HS by ramping the temperature from 28 to 38°C between 06:00 and 08:00 h and decreasing it to 28°C between 16:00 and 20:00 h. All measurements were conducted 10 d following the start of the stress treatments using new flowers and leaves that developed under the stress conditions (R2 stage). The temperature regime, overall temperatures, and water deficit conditions we used for our HS, WD and WD + HS treatments are comparable to field conditions in the US Midwest (Bellaloui et al., 2015; Cohen et al., 2021a,b). It should, however, be noted that the light intensity under field conditions during midday when cloud cover is at its minimum is almost double that we used in our chambers (i.e. c. 2000 μmol photons m−2 s−1).
Temperature, gas exchange and water potential
Flower temperature was measured with a microthermocouple sensor (Physitemp Instruments LLC, Clifton, NJ, USA) by inserting the hypodermal needle microprobe (Physitemp Instruments LLC, Clifton, NJ, USA) 0.75–1 mm into soybean flowers (stages II and III, from R2 plants; Supporting Information Fig. S1) and 1.5–2 mm into closed tobacco flowers at 1 d before opening. Data were recorded using a Multi‐Channel Thermocouple Temperature Data Logger (TCTemp X‐Series; ThermoWorks LogMaster, American Fork, UT, USA) between 11:30 and 12:30 h. Stomatal conductance, transpiration, leaf temperature and photosynthesis were recorded using a Li‐Cor Portable Photosynthesis System (LI‐6800; Li‐Cor, Lincoln, NE, USA) between 12:00 and 13:00 h as previously described (Cohen et al., 2021a). Leaf temperature was also recorded using an infrared camera (FLIR C2; FLIR Systems AB, Wilsonville, OR, USA) as previously described (Zandalinas et al., 2020a). Water potential of leaf discs (8 mm) and flowers (cut longitudinally into half) from plants was measured using Dewpoint Potentiometer (WP4C; Meter Group Inc., Pullman, WA, USA) as described previously (Cohen et al., 2021a). Water potential (leaf and flowers) was measured from five to six plants (from each treatment) between 12:00 and 16:00 h.
Measurements of stomatal aperture and stomatal density
The adaxial and abaxial surfaces of fully expanded leaves and the outer surfaces of fully expanded sepals from soybean and tobacco plants were pasted onto microscope slides, between 11:00 and 12:00 h, with a medical adhesive (Hollister Adapt 7730, Libertyville, IL, USA), as described previously (Devireddy et al., 2020; Zandalinas et al., 2020a). Stomatal aperture measurements (as a ratio of stomatal pore width to stomatal pore length) were performed for soybean and tobacco using an EVOS XL microscope (Invitrogen by Thermo Fisher Scientific, Waltham, MA, USA), as described previously (Devireddy et al., 2020; Zandalinas et al., 2020a). Both width and length of stomatal aperture were measured using ImageJ (https://imagej.nih.gov/ij). The number of stomata, epidermal and pavement cells per microscopic field of view were counted using ImageJ to calculate stomatal density and stomatal index. These were calculated as described in Ceulemans et al. (1995). Stomatal pore index was calculated as described in Wang et al. (2019). Flowers at different stages were also fixed in 4% paraformaldehyde, mounted in paraffin, sectioned and stained at the Histochemistry Diagnostic Laboratory at the University of Missouri, Columbia.
Yield measurements
Yield and reproductive traits were measured as described in Cohen et al. (2021a), except that plants were scored while still growing inside the chambers, and not following recovery under glasshouse conditions. The number of flowers and pods were counted from 15 different plants per treatment, for both soybean and tobacco. Seeds from each plant (15 different plants) and seeds from individual flowers (five flowers per plants from 15 different plants per treatment) were pooled and weighed for both soybean and tobacco. Plant height was also measured at the time of yield sampling (Fig. S2).
Abscisic acid application and sealing of stomata
Abscisic acid (ABA; Sigma‐Aldrich, St Louis, MO, USA) was dissolved in absolute ethanol, diluted to different concentrations in water (0, 50, 30, 15, and 7.5 µM) and sprayed on flowers of soybean plants (R2) growing under the different stress conditions, as previously described (Zandalinas et al., 2016). Control flowers were sprayed with water that contained the appropriate ethanol concentrations that matched the dilution factor (Zandalinas et al., 2016). Plants were then returned to the chambers and stomatal aperture was measured 60 min after ABA application. To seal stomata, petroleum jelly (Vaseline; Sigma‐Aldrich) was gently applied to flowers of plants growing under the different stress conditions using Q‐tips. Plants were then returned to the chambers and flower temperature was recorded as described above 120 min after petroleum jelly application.
Hormone measurements
Hormone extraction and quantification were performed as previously described (Zandalinas et al., 2016; Balfagón et al., 2019). Chromatographic separation was conducted on a reverse‐phase C18 column (Gravity, 50 × 2.1 mm, 1.8 µm particle size; Macherey‐Nagel GmbH, Dueren, Germany) using a MeOH : H2O (both supplemented with 0.1% acetic acid) gradient at a flow rate of 300 µl min−1. Hormones were quantified with a TQS triple quadrupole mass spectrometer (Micromass, Manchester, UK) connected online to the output of the column through an orthogonal Z‐spray electrospray ion source. All data were acquired and processed using Mass Lynx v.4.1 software.
RNA isolation and RT‐qPCR
Soybean flowers (stages II and III, from R2 plants; Fig. S1) were collected from plants between 11:30 and 12:30 h and immediately frozen in liquid nitrogen. For each biological repeat, 30–40 different flowers, and 15–20 different leaves, at the same developmental stage were pooled from eight to 10 different plants, and RNA was isolated using RNAeasy Plant Mini Kit (Qiagen). RNA was converted to cDNA using PrimeScript RT Master Mix (Takara, Shiga, Japan). Real‐time reverse transcription polymerase chain reaction (RT‐qPCR) was performed with gene‐specific primers (Table S1) using EF1α as internal reference using the CFX Connect Real‐Time PCR Detection System (Bio‐Rad), as previously described (Zandalinas & Mittler, 2021).
RNA sequencing and data analysis
RNA libraries for sequencing were prepared using standard Illumina protocols and RNA sequencing was performed by Novogene Co. Ltd (Sacramento, CA, USA; https://en.novogene.com/) using NovaSeq 6000 PE150. Read quality control was performed using Trim Galore v.0.6.4 (https://www.bioinformatics.babraham.ac.uk/projects/trim_galore/) and FastQC v.0.11.9 (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/). The RNA‐seq reads were aligned to the reference genome for soybean Glycine max v.2.1 (downloaded from ftp://ftp.ensemblgenomes.org/pub/plants/release‐51/fasta/glycine_max/dna/), using Hisat2 short read aligner (Kim et al., 2019). Intermediate file processing of sam to sorted bam conversion was carried out using SAMtools v.1.9 (Danecek et al., 2021). Transcript abundance expressed as fragments per kilobase million (FPKM) was generated using the Cufflinks tool from the Tuxedo suite (Trapnell et al., 2012) guided by genome annotation files downloaded from the same source. Differential gene expression analysis was performed using Cuffdiff tool (Trapnell et al., 2013), also from the same Tuxedo suite. Differentially expressed transcripts were defined as those that had a fold‐change with an adjusted P < 0.05 (negative binomial Wald test followed by Benjamini–Hochberg correction). Functional annotation and quantification of overrepresented gene ontology (GO) terms (P < 0.05) and KEGG pathway enrichment (P < 0.05) were conducted using g:profiler (Raudvere et al., 2019). Venn diagrams were created in Venny 2.1 (BioinfoGP, CNB‐CSIC). Venn diagram overlaps were subjected to hypergeometric testing using the R package phyper (Zandalinas et al., 2020a). Heat maps were generated in Morpheus (https://software.broadinstitute.org/morpheus).
Statistical analysis
All experiments were repeated with three biological repeats, each with 15 plants as technical repeats. Results are shown as box‐and‐whisker plots with borders corresponding to the 25th and 75th percentiles of the data. Statistical analysis was performed using one‐way ANOVA followed by Tukey’s post hoc test (P < 0.05) in GraphPad. Different letters denote statistical significance at P < 0.05. Data collection for yield, stomatal aperture and stomatal index measurements was undertaken blind.
Data availability
The analyzed transcript abundance and differentially expressed transcripts can be accessed interactively via the Differential Expression tool in SoyKB; https://soykb.org/DiffExp/diffExp.php; Joshi et al., 2012, 2014), a comprehensive all‐inclusive web resource for soybean. It provides a set of visualization and analytical tools such as differential expression analysis and gene card pages and provides data in the form of tabs for Gene lists, Venn diagram, Volcano plot, Function Analysis, Pathway Analysis and Gene modules.
Results
Leaf and flower temperature of plants subjected to a combination of water deficit and heat stress
To induce conditions of WD, HS and a combination of WD and HS (WD + HS), we grew soybean plants (Glycine max cv Magellan) in controlled growth chambers. When plants began to flower (R1 stage) we induced conditions of WD, HS and WD + HS (Cohen et al., 2021a) and maintained these conditions for 10 d before starting to analyze and sample leaves and flowers. Using this design, we made sure that the new leaves and flowers we studied (R2 stage) developed under the different stress conditions. As shown in Fig. 1(a), as well as reported previously for different plant species (Rizhsky et al., 2002, 2004; Carmo‐Silva et al., 2012; Zandalinas et al., 2016; Cohen et al., 2021a), compared with plants subjected to CT or WD conditions, leaf temperature of plants subjected to WD + HS was higher by about 3–5°C. To determine whether flowers of plants subjected to WD + HS exhibit a similar higher temperature (compared with flowers of plants subjected to HS or WD), we measured the internal temperature of flowers using a thermocouple thermometer probe (Fig. 1b). For this analysis we used soybean flowers at stages II and III (unopened flowers undergoing self‐pollination; Fig. S1) from plants grown under WD, HS, WD + HS or CT conditions (Fig. 1b). As shown in Fig. 1(c), the inner flower temperature of flowers that developed under WD + HS combination conditions was higher by about 3–4°C than that of flowers grown under CT or WD conditions. The leaf and inner flower temperature of plants subjected to WD + HS was also significantly higher than that of plants subjected to HS (Fig. 1a,b). Water potential (Ψ; psi, measured in MPa) is typically low in tissues subjected to WD, HS or WD + HS, potentially indicating water loss and tissue dehydration (Sattar et al., 2020; Cohen et al., 2021a). In addition to increased temperature (Fig. 1a,c), the water potential of leaves (Fig. 1d) and flowers (Fig. 1e) from plants subjected to WD + HS was lower by about 0.5–1 MPa compared with that of leaves and flowers grown under CT, HS or WD conditions. Taken together, the results presented in Fig. 1 demonstrate that flowers of plants subjected to WD + HS have a high internal temperature that is accompanied by low water potential.
Fig. 1.
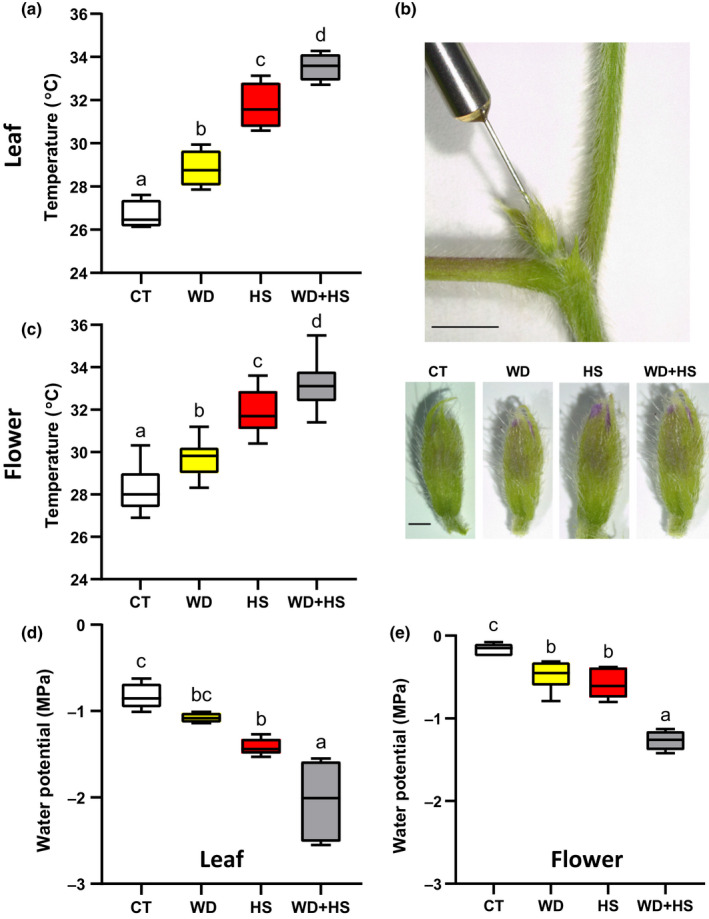
Leaf and flower temperature of soybean (Glycine max) plants subjected to a combination of water deficit (WD) and heat stress (HS). (a) Leaf temperature of soybean plants subjected to control (CT), HS, WD or WD + HS conditions. (b) Representative image of the experimental setup used to measure soybean inner flower temperature with a thermocouple thermometer probe (upper panel; bar, 5 mm) and representative images of closed (stages II and III; Supporting Information Fig. S1) soybean flowers developing under the different stress treatments (lower panel; bar, 1 mm). (c) Inner flower temperature of soybean flowers from plants subjected to CT, WD, HS or WD + HS. (d) Water potential (Ψ; psi, measured in MPa) of soybean leaves subjected to CT, WD, HS or WD + HS. (e) Water potential of soybean flowers from plants subjected to CT, WD, HS or WD + HS. All experiments were conducted with three biological repeats, each with 15 plants as technical repeats. Results are shown as box‐and‐whisker plots with borders corresponding to the 25th and 75th percentiles of the data. Different letters denote significance at P < 0.05 (ANOVA followed by a Tukey's post hoc test).
Stomatal aperture and transpiration of flowers and leaves from plants subjected to a combination of WD and HS
Stomatal aperture, stomatal conductance and transpiration are key physiological parameters that determine plant temperature and water potential (Nilson & Assmann, 2007; Lawson & Matthews, 2020; Hsu et al., 2021). We therefore measured these parameters in leaves and flowers of plants subjected to WD + HS. In agreement with our previous findings obtained with soybean, tobacco and Arabidopsis (Rizhsky et al., 2002, 2004; Zandalinas et al., 2016; Cohen et al., 2021a), leaf stomatal aperture, stomatal conductance and transpiration remained high in plants subjected to HS, significantly decreased in plants subjected to WD, and significantly decreased to similar values in plants subjected to WD + HS (Fig. 2a–c). These findings suggest that, in contrast to HS, leaves subjected to WD + HS could not be cooled via transpiration (Rizhsky et al., 2002, 2004; Mittler, 2006; Carmo‐Silva et al., 2012; Zandalinas et al., 2016; Cohen et al., 2021a), and experience higher temperatures (Fig. 1a). In contrast to leaves, flower (sepal) stomatal aperture and whole‐flower stomatal conductance and transpiration were significantly higher in plants subjected to WD + HS or HS than in those under CT or WD conditions (Fig. 2d,e). This finding suggests that during a combination of WD + HS, stomata of flowers (sepals) respond differently than stomata of leaves and remain open, enabling cooling via transpiration. Interestingly, the inner temperature of flowers subjected to WD + HS was high (Fig. 1c), despite the ongoing transpiration (Fig. 2f). This observation could be explained by differences in the thickness of flowers and leaves. While soybean flower buds have a diameter of about 1.5–2 mm (Fig. 1b), soybean leaves are much thinner (c. 0.12–0.15 mm) and can be cooled by transpiration much more easily.
Fig. 2.
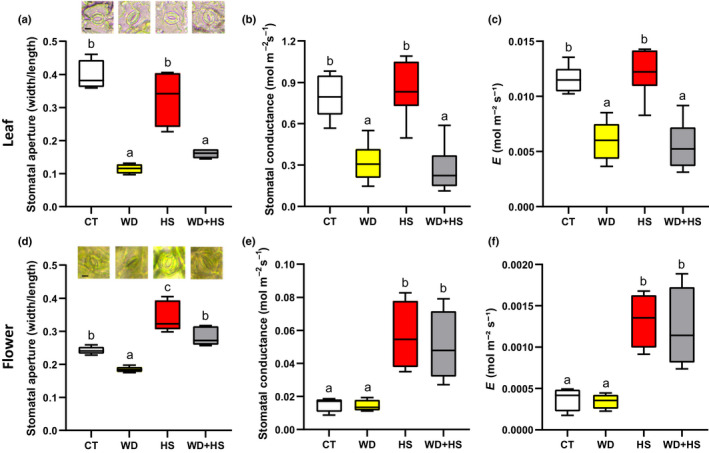
Stomatal aperture and transpiration of flowers and leaves from soybean (Glycine max) plants subjected to a combination of water deficit (WD) and heat stress (HS). (a–c) Stomatal aperture (a), stomatal conductance (b), and transpiration (E) (c) of soybean leaves from plants subjected to control (CT), HS, WD or WD + HS conditions. (d–f) Stomatal aperture (d), stomatal conductance (e), and transpiration (f) of soybean flowers from plants subjected to CT, HS, WD or WD + HS. All experiments were conducted with three biological repeats, each with 15 plants as technical repeats. Twenty microscopic fields from all parts of sepals or from the middle section of leaves (between the main veins) were measured for each plant. Results are shown as box‐and‐whisker plots with borders corresponding to the 25th and 75th percentiles of the data. Different letters denote significance at P < 0.05 (ANOVA followed by a Tukey's post hoc test). Representative images of stomata are shown in (a) and (d). Bar, 10 μm.
Stomatal density of leaves and flowers developed under WD + HS conditions
Plants display a high degree of plasticity when grown under diverse environmental conditions (Chater et al., 2014; Zhu, 2016; Caine et al., 2019; Sakoda et al., 2019; Lloyd & Lister, 2021; Markham & Greenham, 2021). Among the different phenotypes plants can display in response to different growth conditions is a change in the density (number per area) of stomata appearing on the surface of newly developing leaves (Chater et al., 2014; Caine et al., 2019; Sakoda et al., 2019). The differential responses of stomata from sepals and leaves during WD + HS, as well as the lower rates of transpiration measured from whole flowers compared with leaves (Fig. 2), prompted us to examine whether the number of stomata forming on these organs (i.e. stomatal density) during their development under the stress conditions applied in our study would also be different. As shown in Fig. 3(a,b), the stomatal density and index of leaves developed under WD + HS was significantly higher than that of leaves grown under CT conditions. Because stomata on leaves were closed under conditions of WD + HS (Fig. 2a), the stomatal pore index of leaves from plants grown under WD + HS was statistically similar to that of leaves grown under WD conditions (Fig. 3c). By contrast, while the stomatal density and index of sepals from plants subjected to HS or WD + HS was significantly higher than that of plants grown under CT or WD (Fig. 3d,e), because stomata on sepals of plants subjected to WD + HS were open (Fig. 2d), the stomatal pore index of sepals developing under HS or WD + HS was also significantly higher than that of flowers from plants grown under CT or WD conditions (Fig. 3f). The results presented in Figs 2 and 3 suggest that while the developmental responses of leaves and flowers (sepals) to WD + HS (i.e. increase in stomatal density and index; Figs 3a,b,d,e) are similar, the physiological responses of these two different organs (i.e. opening or closing of stomatal aperture; Figs 2, 3b,d) are different. It should also be noted that the expression pattern of three genes involved in the control of stomatal development on leaves (i.e. STOMAGEN, a positive regulator that is upregulated, and Erecta‐like1 (ERL1) and ARF5/MP, negative regulators that are downregulated; Sugano et al., 2010; Zhang et al., 2014; Qi et al., 2020) corresponded with the higher stomatal index and density of flowers from plants subjected to HS and WD + HS compared with CT or WD (Figs 3, S3).
Fig. 3.
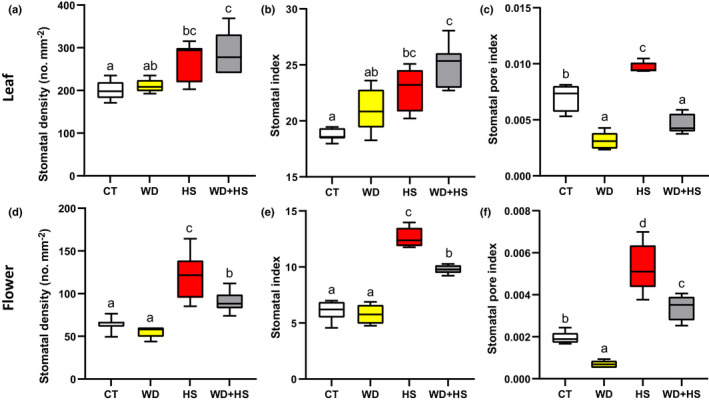
Stomatal density, index and pore index of soybean (Glycine max) leaves and flowers developed under conditions of water deficit (WD) and heat stress (HS) combination. (a–c) Stomatal density (a), stomatal index (b), and stomatal pore index (c) of leaves from plants subjected to control (CT), HS, WD or WD + HS. (d–f) As (a–c) but for sepals from plants subjected to CT, HS, WD or WD + HS. All experiments were conducted with three biological repeats, each with 10 plants as technical repeats. Twenty microscopic fields from all parts of sepals or from the middle section of leaves (between the main veins) were measured for each plant. Results are shown as box‐and‐whisker plots with borders corresponding to the 25th and 75th percentiles of the data. Different letters denote significance at P < 0.05 (ANOVA followed by a Tukey's post hoc test).
External application of ABA to flowers results in stomatal closure, and sealing of stomata results in elevated flower temperature under WD + HS conditions
Stomatal aperture, conductance and overall transpiration are regulated in plants by various signals (Nilson & Assmann, 2007; Buckley, 2019; Hsu et al., 2021). Among these, ABA is well known to play a key role in triggering stomatal closure (Nilson & Assmann, 2007; Lozano‐Juste & Cutler, 2016; Buckley, 2019; Zhang et al., 2022; Hsu et al., 2021). Because stomata of flowers from plants subjected to the WD + HS combination were open, while stomata of leaves from the same plants were closed (Fig. 2), we tested whether external application of ABA would cause stomatal closure in flowers (sepals) from plants subjected to the stress combination. As shown in Fig. 4(a), application of ABA (50 μM) to flowers grown under CT, HS or WD + HS conditions resulted in stomatal closure. By contrast, application of ABA to flowers from plants grown under WD conditions did not change stomatal aperture, as these stomata were already closed. The results presented in Fig. 4(a) suggest that stomata of flowers subjected to WD + HS can respond to external ABA application.
Fig. 4.
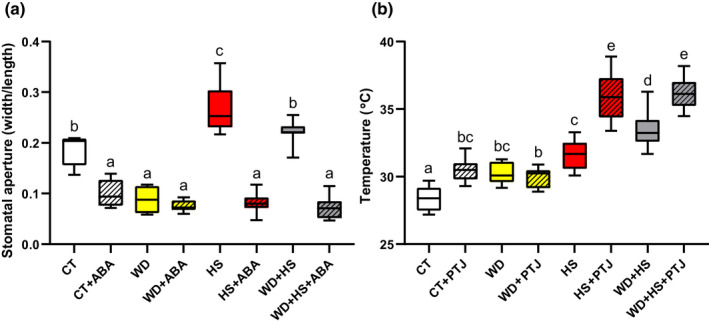
External application of abscisic acid (ABA) to soybean (Glycine max) flowers results in stomatal closure, and sealing of stomata results in elevated flower temperature under conditions of water deficit (WD) and heat stress (HS) combination. (a) Stomatal aperture of sepals from plants subjected to control (CT), HS, WD or WD + HS conditions at 60 min following application of 0 or 50 μM ABA. (b) Inner flower temperature of flowers from plants subjected to CT, WD, HS or WD + HS, coated or uncoated with a thin layer of petroleum jelly (PTJ) for 120 min. All experiments were conducted with three biological repeats, each with 10 plants as technical repeats. Results are shown as box‐and‐whisker plots with borders corresponding to the 25th and 75th percentiles of the data. Different letters denote significance at P < 0.05 (ANOVA followed by a Tukey's post hoc test).
A possible reason why flowers would keep their sepal stomata open, maintaining transpiration under WD + HS conditions (Fig. 2), is that this process helps to lower the inner flower temperature. This could be highly important for protecting the reproductive processes occurring within the flowers of pseudocleistogamous plants such as soybean. To test whether reducing flower transpiration, by sealing stomatal apertures will cause an increase in inner flower temperature under WD + HS conditions, we used a thin layer of petroleum jelly to cover flowers (stages II and III) of plants (R2 stage) grown under CT, WD, HS and WD + HS conditions, and measured their inner flower temperature. As shown in Fig. 4(b), sealing stomatal pores with a thin petroleum jelly layer caused a significant increase of 2–3°C in inner flower temperature of flowers grown under CT, HS or WD + HS conditions. By contrast, the inner flower temperature of flowers from plants subjected to WD did not increase, as the stomata of these flowers were closed. These findings demonstrate that the opening of stomata on sepals of flowers from plants subjected to HS or WD + HS plays an important role in modulating the internal temperature of flowers, potentially mitigating some of the high temperature‐derived negative consequences for plant fertilization in cleistogamous/pseudocleistogamous plants (Rollins et al., 2013; Mahrookashani et al., 2017; Lawas et al., 2018; Xie et al., 2018; Cohen et al., 2021a,b; Sinha et al., 2021).
RNA‐seq analysis of flower buds subjected to WD + HS
To obtain a better understanding of the different processes occurring within flowers under WD + HS conditions and to compare them with the processes that occur in leaves (Cohen et al., 2021a), we conducted an RNA‐seq analysis of whole flowers (R2, stages II and III; Fig. 1b) collected from plants grown under CT, WD, HS, or WD + HS conditions (Datasets S1–S6). Because WD, HS and WD + HS conditions are likely to affect global processes in all tissues and cell types found in flowers, we did not dissect the flower buds into different tissues. This also allowed us to compare the RNA‐seq data obtained in the current study with a previous RNA‐seq analysis of whole leaves (that also contain multiple tissues and cell types subjected to the same conditions, reanalyzed using the same pipeline as described here; Datasets S7–S12), performed in the same growth chambers on plants from the same seed batch, under the same growth conditions (Cohen et al., 2021a). As shown in Fig. 5(a), RT‐qPCR analysis conducted on RNA samples before RNA‐seq analysis revealed that flowers from plants subjected to the different treatments responded differently. Transcripts encoding cytosolic ascorbate peroxidase 1 (APX1), a key ROS metabolizing and signaling enzyme (Davletova et al., 2005; Koussevitzky et al., 2008), significantly accumulated, for example, in response to HS, while transcripts encoding the key transcriptional regulator dehydration responsive element binding (DREB; Agarwal et al., 2017) DREB‐1H significantly accumulated during WD, and transcripts encoding DREB‐1B significantly accumulated during HS and WD + HS.
Fig. 5.
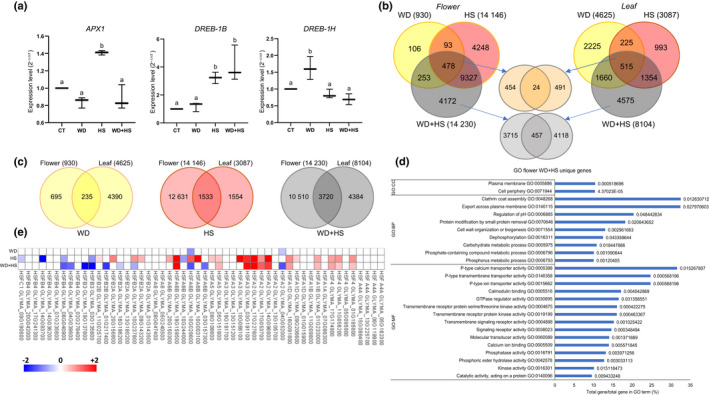
RNA‐seq analysis of soybean (Glycine max) flowers subjected to a combination of water deficit and heat stress. (a) Quantitative reverse transcription polymerase chain reaction (RT‐qPCR) analysis of ascorbate peroxidase 1 (APX1), and dehydration‐responsive element binding (DREB) 1B and 1H in flowers from plants subjected to control (CT), heat stress (HS), water deficit (WD) or WD + HS. Different letters denote significance at P < 0.05 (ANOVA followed by a Tukey's post hoc test). (b) Venn diagrams showing the overlap between transcripts with significantly altered expression (up‐ or downregulated) in flowers (left) and leaves (right) in response to HS, WD or WD + HS. Overlap between transcripts common to all stresses in leaves and flowers is shown in the upper middle and overlap between transcripts unique to WD + HS in flowers and leaves is shown in the lower middle. (c) Venn diagrams showing the overlap between transcripts with significantly altered expression (up‐ or downregulated) in flower and leaves in response to HS, WD or WD + HS. (d) Gene ontology (GO) enrichment analysis of transcripts unique to WD + HS in flowers (4177). (e) Heat map showing the expression pattern of all heat shock transcription factors (HSFs) in flowers subjected to HS, WD or WD + HS. Analysis was performed in three biological repeats. For each biological repeat 30–40 different flowers, and 15–20 different leaves, at the same developmental stage were pooled from eight to 10 different plants. All transcripts shown are significant at P < 0.05 (negative binomial Wald test followed by Benjamini–Hochberg correction). RT‐qPCR results are shown as box‐and‐whisker plots with borders corresponding to the 25th and 75th percentiles of the data. APX, ascorbate peroxidase.
Venn diagrams depicting the overlap between transcripts responding to the different treatments in flowers and leaves revealed that, in contrast to leaves, flowers accumulated many more transcripts in response to HS (14 146) or WD + HS (14 230), but fewer transcripts in response to WD (930) (Fig. 5b; Datasets S13–S41). Interestingly, the numbers of transcripts with a common response to all treatments in flowers (478) and leaves (515) were very similar, suggesting that these transcripts represent a core set of WD, HS and WD + HS response transcripts. However, the overlap between these core sets of leaf and flower transcripts was low (24; Fig. 5b), demonstrating that even when it comes to the most common transcripts, the response of flower and leaf tissues to stress is different. A relatively low overlap (457) was also found between transcripts specific for a combination of WD + HS in flowers (4172) and leaves (4575) (Fig. 5b), further suggesting that the response of flowers to this stress combination is different from that of leaves. A comparison between the overall transcriptomics responses of flowers and leaves to the individual WD, HS and WD + HS treatments (930, 14 146 and 14 230 in flowers, and 4625, 3087 and 8104 in leaves, respectively) also revealed that these two tissues responded differently (overlap of 235, 1533 and 3720, respectively) (Fig. 5c). Although some overlap was found between flowers and leaves, in general there were many more flower‐specific transcripts that respond to HS and WD + HS (12 613 and 10 510, respectively), and many more leaf‐specific transcripts that responded to WD (4390). Overall, the results presented in Fig. 5(b,c) demonstrate that the response of soybean flowers to WD + HS is very different from that of leaves.
Gene ontology annotation analysis of transcripts with a unique response to WD + HS in flowers (4172; Fig. 5d) revealed that this group of transcripts is enriched in calcium signaling, kinase and protease activity, clathrin‐associated vesicle transport and other types of membrane transport mechanisms and pumps. Because different transcription factor (TF) families, such as heat shock transcription factors (HSFs), MYBs and AP2‐EREBP, play a critical role in plant acclimation to stress combination (Zandalinas et al., 2020b), we compared the pattern of their expression between leaves and flowers of soybean plants subjected to CT, WD, HS and WD + HS treatments (Datasets S42–S44). The pattern of expression of many of these TF families was different between flowers subjected to CT, WD, HS or WD + HS treatments (Datasets S42–S44). The pattern of expression of HSFs was for example different between flowers subjected to WD + HS, HS, or WD (Fig. 5e). This finding suggests that, compared with leaves, different types of heat and other stress responses might be activated in flowers when WD and HS are combined (i.e. WD + HS).
Enhanced abundance of transcripts encoding the ABA degradation enzyme ABA 8′‐hydroxylase in flowers from plants subjected to HS or WD + HS
A deeper analyses of our RNA‐seq data revealed that the abundance of several transcripts encoding the key ABA biosynthetic enzymes zeaxanthin epoxidase (ABA1) and 9‐cis‐epoxycarotenoid dioxygenase (NCED) was significantly elevated in flowers from plants subjected to WD or WD + HS, while the abundance of several other key ABA biosynthetic enzymes encoding xanthoxin dehydrogenase (ABA2) and aldehyde oxidase (AAO) was significantly elevated in flowers from plants subjected to HS or WD + HS (Fig. 6a). By contrast, the abundance of several transcripts encoding the key ABA degradation enzyme ABA 8′‐hydroxylase (CYP707A) was specifically and significantly elevated in flowers subjected to HS or WD + HS (Fig. 6a), while the expression level of the suppressor that downregulates CYP707A (short vegetative phase; SVP; Wang et al., 2018) was significantly suppressed (Fig. 6a). These findings, coupled with the stomatal closure response to ABA application of sepals from flowers subjected to CT, HS or WD + HS (Fig. 4a), suggest that enhanced degradation of ABA in flowers from plants subjected to HS or WD + HS could keep ABA concentrations suppressed, and therefore stomata open under HS and WD + HS conditions (Fig. 2d).
Fig. 6.
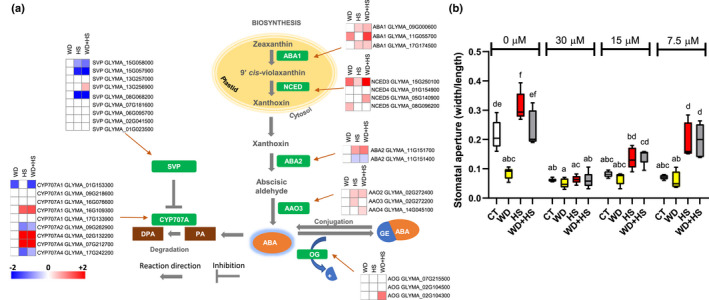
Enhanced expression of transcripts encoding the abscisic acid (ABA) degradation enzyme ABA 8′‐hydroxylase in flowers from soybean (Glycine max) plants subjected to heat stress (HS) or water deficit (WD) combined with HS, and higher resistance of these flowers to external ABA application. (a) Heat maps and a pathway showing the expression of transcripts involved in ABA biosynthesis and degradation in whole flowers from plants grown under control (CT), WD, HS or WD + HS conditions. All transcripts shown are significant at P < 0.05 (negative binomial Wald test followed by Benjamini–Hochberg correction). (b) Stomatal aperture of sepals from plants subjected to CT, HS, WD or WD + HS at 60 min following application of 30, 15, 7.5 or 0 µM ABA. All experiments were conducted with three biological repeats, each with 10 plants as technical repeats. Twenty microscopic fields from all parts of sepals were measured for each plant. Results are shown as box‐and‐whisker plots with borders corresponding to the 25th and 75th percentiles of the data. Different letters denote significance at P < 0.05 (ANOVA followed by a Tukey's post hoc test).
To test whether the rate of ABA degradation is enhanced in flowers from plants subjected to HS or WD + HS, we sprayed flowers from plants grown under CT, WD, HS and WD + HS conditions with different concentrations of ABA and measured stomatal aperture (like Fig. 4a, but with lower concentrations of ABA; Fig. 6b). While higher concentrations of ABA (50 or 30 μM) caused a complete stomatal closure in flowers grown under CT, HS and WD + HS (Figs 4a, 6b), lower concentrations of ABA (i.e. 7.5, and to a lesser extent 15 μM) failed to cause a complete or significant stomatal closure in flowers from plants grown under HS or WD + HS conditions (yet caused a complete stomatal closure in flowers from CT plants; Fig. 6b). In agreement with Fig. 4(a), stomata on flowers from plants grown under WD conditions were closed and did not respond to any of the ABA concentrations applied (Fig. 6b). The findings presented in Fig. 6 suggest that the rate of ABA degradation is enhanced in flowers from plants subjected to HS or WD + HS.
Suppressed accumulation of ABA and JA in flowers from plants subjected to HS or a combination of WD + HS
The results presented in Figs 4 and 6 suggest that the rate of ABA degradation is enhanced in flowers from plants subjected to HS or WD + HS. To determine the concentrations of ABA and its degradation product dihydrophaseic acid (DPA) directly, as well as the concentrations of other hormones potentially involved in stomatal aperture regulation, we measured the concentrations of ABA, DPA, jasmonic acid (JA), JA‐isoleucine (JA‐Ile), salicylic acid (SA) and auxin (IAA) in flowers and leaves from plants subjected to CT, WD, HS and WD + HS conditions (Figs 7, S4). As previously reported for soybean, the overall concentrations of ABA were higher in flowers than in leaves (Yarrow et al., 1988; Liu et al., 2003; Wong et al., 2009). In agreement with our findings that transcripts encoding the ABA biosynthetic enzymes ABA1 and NCED are significantly elevated in flowers in response to WD or WD + HS (Fig. 6a), the concentration of ABA in flowers from plants subjected to WD or WD + HS was significantly higher than that of plants subjected to CT or HS (Fig. 7a). By contrast, and in agreement with our findings that transcripts encoding the ABA degradation enzyme CYP707A are significantly and specifically elevated in flowers in response to HS or WD + HS (Fig. 6a), and that flowers of plants grown under HS or WD + HS conditions could potentially have a higher degradation rate of ABA (Fig. 6b), the concentration of DPA, a product of ABA degradation by CYP707A, was significantly elevated only in flowers from plants subjected to HS or WD + HS (Fig. 7b). These findings support our RNA‐seq analysis (Fig. 6a) and ABA application study (Fig. 6b) and demonstrate that an enhanced process of ABA degradation probably occurs in flowers from plants subjected to HS or WD + HS. Interestingly, compared with flowers from plants subjected to CT or WD stress, flowers from plants subjected to HS or WD + HS contained significantly lower concentrations of JA and the active form of JA, JA‐Ile (Fig. 7c,d). Because both ABA and JA can induce stomatal closure during stress in plants (Nilson & Assmann, 2007; Zandalinas et al., 2016; Zhu, 2016; Hsu et al., 2021; Markham & Greenham, 2021), our findings that flowers from plants subjected to HS or WD + HS contained significantly lower concentrations of JA (Fig. 7c) and JA‐Ile (Fig. 7d), as well as actively degrading ABA (Figs 6, 7b), provide a hormone‐based mechanistic understanding of the opening of stomata on flowers during HS and WD + HS (Fig. 2). In contrast to flowers (Fig. 7a–d), the concentrations of ABA, JA and JA‐Ile in leaves subjected WD + HS were not suppressed (Fig. 7e–g), and stomata on leaves of flowers subjected to WD + HS were closed (Fig. 2). Interestingly, compared with leaves from CT or WD stress, the concentration of IAA was significantly higher in leaves subjected to HS or WD + HS (Fig. S4). In addition, compared with flowers from plants subjected to WD or WD + HS, the concentration of SA was higher in flowers subjected to HS (Fig. S4; but not significantly higher than CT flowers). Further studies are needed to determine the roles of SA and IAA in plant responses to WD + HS.
Fig. 7.
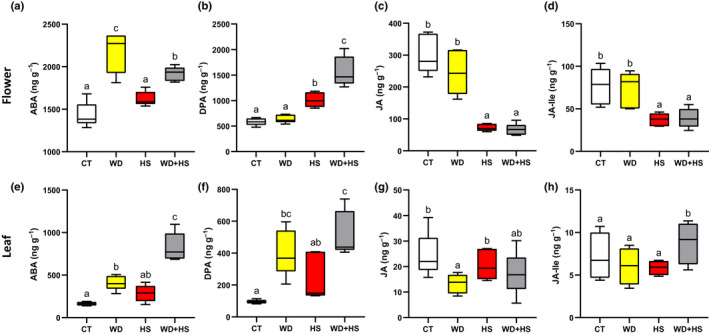
Suppressed accumulation of abscisic acid (ABA) and jasmonic acid (JA) in soybean (Glycine max) flowers from plants subjected to heat stress (HS) or a combination of water deficit (WD) and HS. Concentrations of ABA (a), the ABA degradation product dihydrophaseic acid (DPA) (b), JA (c), and JA‐isoleucine (JA‐Ile) (d) in flowers from plants subjected to control (CT), water deficit (WD), heat stress (HS) or WD + HS. (e–h) As (a–d), but for leaves. Experiments were conducted with three biological repeats, each with 12 plants. For each biological repeat, 30–40 different flowers and 15–20 different leaves at the same developmental stage were pooled in five technical repeats. Results are shown as box‐and‐whisker plots with borders corresponding to the 25th and 75th percentiles of the data. Different letters denote significance at P < 0.05 (ANOVA followed by a Tukey’s post hoc test).
The effect of WD + HS on flower and leaf stomatal aperture, transpiration and temperature in tobacco
To determine whether stomata of sepals and leaves belonging to a different plant species respond in a similar manner to soybean (Fig. 2), we studied the response of Nicotiana tabacum (cv SR1, petite Havana) plants to WD, HS and WD + HS. As shown in Fig. 8(a), and in agreement with our previous analysis of tobacco plants subjected to a combination of WD + HS (Rizhsky et al., 2002), the leaf temperature of plants subjected to a combination of WD + HS was significantly higher than that of plants subjected to WD or HS. This increase was accompanied by closure of stomata and suppressed transpiration (Fig. 8b,c). In contrast to leaves, and similar to soybean (Fig. 2), stomata on sepals of tobacco plants subjected to WD + HS were open, allowing transpiration to occur (Fig. 8e). Although stomata were open and transpiration occurred (Fig. 8f), the inner flower temperature of tobacco plants subjected to WD + HS (measured for unopened flowers) was significantly higher than that of flowers subjected to HS or WD (Fig. 8d; similar to our findings with soybean (Fig. 2)). As in soybean, it is possible that, owing to differences in tissue thickness between leaves and flowers, keeping transpiration ongoing in flowers is not sufficient to reduce the inner temperature of flowers more extensively.
Fig. 8.
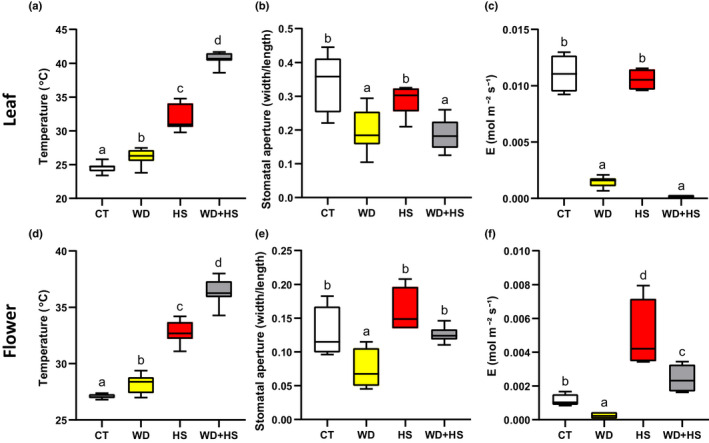
The effect of water deficit (WD) and heat stress (HS) combination on flower and leaf stomatal aperture, transpiration and temperature in tobacco (Nicotiana tabacum). (a) Leaf temperature of tobacco plants subjected to control (CT), HS, WD or WD + HS. (b) Stomatal aperture of tobacco leaves subjected to CT, WD, HS or WD + HS. (c) Transpiration (E) of tobacco leaves subjected to CT, WD, HS or WD + HS. (d–f) As (a–c), but for tobacco flowers from plants subjected to CT, WD, HS or WD + HS. All experiments were conducted with three biological repeats, each with 10 plants as technical repeats. Twenty microscopic fields from all parts of sepals or from the middle section of leaves (between the main veins) were measured for each plant. Results are shown as box‐and‐whisker plots with borders corresponding to the 25th and 75th percentiles of the data. Different letters denote significance at P < 0.05 (ANOVA followed by a Tukey's post hoc test).
Yield of soybean and tobacco subjected to WD + HS
Our findings that stomata of sepals are open during HS and WD + HS, and that this process limits increases in internal flower temperature (Figs 2, 4), suggest that the opening of stomata on sepals could curb the extent of yield losses that may otherwise be caused by WD + HS. Because the temperatures of flowers from plants grown under conditions of HS and WD + HS were comparable (albeit higher in plants subjected to WD + HS; Figs 1, 4, 8), we hypothesized that yield penalty in plants subjected to WD + HS will be comparable to that of plants subjected to HS alone. To test this hypothesis, we grew soybean and tobacco plants under CT, WD, HS and WD + HS conditions and scored them for number of flowers, number of pods and seed weight per plant and flower. In contrast to our previous analysis of soybean yield under these conditions (Cohen et al., 2021a), plants were scored for the different parameters while in the chambers, and not following a recovery period in the glasshouse. As shown in Fig. 9(a), soybean and tobacco plants subjected to HS produced significantly more flowers compared with plants subjected to CT, WD or WD + HS. The number of pods and seeds produced by plants and flowers subjected to HS was, however, significantly lower than that of plant and flowers subjected to CT or WD conditions, suggesting that most of these flowers could not produce pods and seeds (Fig. 9b–d). Interestingly, the numbers of pods and seeds produced per plant in soybean plants subjected to HS or WD + HS were comparable (Fig. 9b,c), while the number of seeds produced per flower was significantly higher in plants subjected to WD + HS vs HS (Fig. 9d). These findings suggest that the differential transpiration response of soybean plants (Figs 2, 4) could help to protect flowers during WD + HS. By contrast, HS and WD + HS had a much more severe impact on pod and seed production per plant or flower in tobacco, with WD + HS being the more severe of the two (Figs 9b–d). Our findings suggest that, at least in soybean, which uses pseudocleistogamy for plant reproduction (Takahashi et al., 2001; Khan et al., 2008; Benitez et al., 2010), the differential transpiration of sepals during a combination WD + HS could keep flower temperature under control and help to prevent excessive yield losses.
Fig. 9.
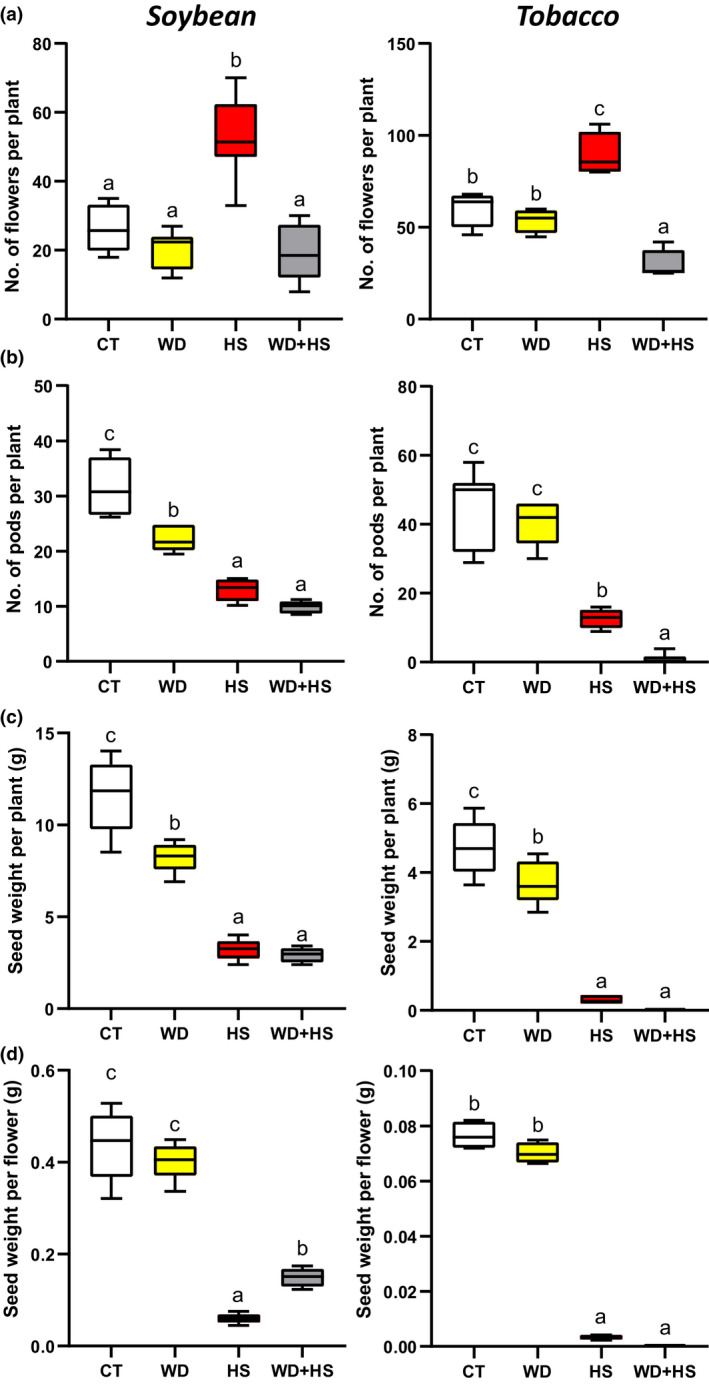
Yield of soybean (Glycine max) and tobacco (Nicotiana tabacum) plants subjected to a combination of water deficit (WD) and heat stress (HS). (a–d) Number of flowers per plant (a), number of pods per plant (b), total seed weight per plant (c), and total seed weight per flower (d) of soybean (left bar graphs) and tobacco (right bar graphs) plants subjected to control (CT), HS, WD or WD + HS. All experiments were conducted with three biological repeats, each with 10 plants as technical repeats. Results are shown as box‐and‐whisker plots with borders corresponding to the 25th and 75th percentiles of the data. Different letters denote significance at P < 0.05 (ANOVA followed by a Tukey's post hoc test).
Discussion
Heat waves occurring during periods of drought can inflict heavy losses to agricultural production, especially if they occur during the reproductive growth phase of crops (Mittler, 2006; Rollins et al., 2013; Mazdiyasni & AghaKouchak, 2015; Mahrookashani et al., 2017; Lawas et al., 2018; Liu et al., 2020; Cohen et al., 2021b; Rivero et al., 2022; Sinha et al., 2021). Because water is needed to cool the plant via transpiration, we reasoned that when WD is combined with HS it would limit the ability of plants to cool their flowers and cause a severe heat‐induced reduction in yield. Here, we show that WD + HS conditions, which were found to reduce yield in many different crops (Mittler, 2006; Rollins et al., 2013; Mahrookashani et al., 2017; Lawas et al., 2018; Liu et al., 2020; Rivero et al., 2022; Sinha et al., 2021), are indeed accompanied by higher inner flower temperatures (Figs 1, 8). Higher leaf temperatures were previously reported for plants subjected to WD + HS and linked to the closure of stomata on leaves during stress combination (Rizhsky et al., 2002, 2004; Carmo‐Silva et al., 2012; Zandalinas et al., 2016; Cohen et al., 2021a). We therefore expected stomata of flowers from plants subjected to WD + HS to be closed as well. Surprisingly, however, they were open (Figs 2, 8). Moreover, transpiration rates of flowers from plants subjected to WD + HS were as high as those of flowers subjected to HS alone (Figs 2, 8). In contrast to flowers, stomata on leaves of plants subjected to WD + HS were closed (Figs 2, 8). Our results therefore reveal that during a combination of WD + HS annual plants prioritize transpiration through flowers over transpiration through leaves by opening their sepal stomata, while keeping their leaf stomata closed (Fig. 10). This ‘differential transpiration’ strategy lowers flower internal temperature (Fig. 4) and enables some reproductive processes to occur (Fig. 9). Under WD + HS conditions the plant might therefore attempt to protect reproductive processes, at the expense of vegetative organs. This acclimation strategy could also prove effective in other scenarios that may result in higher inner flower temperatures (e.g. combinations of pathogen infection, mechanical wounding, high CO2 or air pollution, such as ozone, that cause stomal closure with heat stress; Melotto et al., 2006; Vahisalu et al., 2010; Raven, 2014; Deger et al., 2015; Chen et al., 2017; Zhang et al., 2018; Devireddy et al., 2020).
Fig. 10.
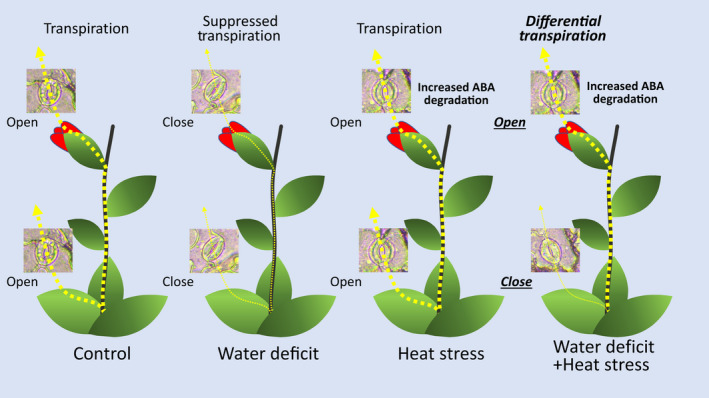
A model depicting ‘differential transpiration’ during a combination of water deficit (WD) and heat stress (HS). Control plants (left) conduct transpiration through open stomata on their leaves and flowers. In response to WD, plants (second from left) close their stomata on leaves and flowers and suppress transpiration. During HS, plants (third from left) keep their stomata on leaves and flowers open and maintain transpiration. By contrast, during a combination of WD and HS, plants (right) keep their stomata on flowers open, while closing their stomata on leaves. The opening of stomata on flowers from plants subjected to HS or a combination of WD + HS is proposed to result from an enhanced rate of abscisic acid (ABA) degradation that specifically occurs in flowers from plants grown under these conditions. The strategy of differential transpiration allows plants subjected to the stress combination to cool their flowers and limit heat‐induced negative impacts on yield.
Enhanced transpiration of flowers grown under WD + HS conditions could cause flowers to undergo dehydration as a result of limited water resources. Indeed, flowers of plants subjected to WD + HS had a lower water potential (Ψ) compared with flowers of plants subjected to HS alone (Fig. 2). This observation suggests that the strategy of differential transpiration under WD + HS conditions (Fig. 10) has its limits, and once flowers will reach a dehydration point of no return, reproductive processes will be further, and perhaps irreversibly, damaged. Another point to consider is the intensity of transpiration occurring from plants and whether it is sufficient to cool flowers by 2–3°C (Figs 2, 4). It is possible that other factors, as well as the cocooning effect of reproductive tissues within flowers, are playing a role in this process. The challenges faced by flowers of plants subjected to WD + HS, and the findings that they may be subjected to a combination of heat and dehydration stress (Fig. 2), is also reflected in our RNA‐seq analysis. Flowers from plants subjected to WD + HS displayed a unique transcriptomics response that was different from that of flowers from plants subjected to HS or WD (Fig. 5a,b). Interestingly, the overall transcriptomics response of flowers to WD, HS or WD + HS was different from that of leaves (with the highest degree of similarity observed between flowers and leaves subjected to a combination of WD + HS; Fig. 5c). This observation might reflect the many different reproductive processes that occur in developing flowers compared with leaves, but could also suggest that flowers are subjected to different types or degrees of stress compared with leaves under WD + HS conditions. Further studies are, of course, needed to address these intriguing possibilities. In future studies it would also be important to test whether intermittent HS or WD, or even short episodes of WD + HS, occurring during the vegetative growth stage of soybean plants, can prime the transcriptomic responses of flowers to these different stresses and their combination during the reproductive growth phase.
Our RNA‐seq analysis further revealed that the expression of several transcripts encoding the key ABA degradation enzymes ABA 8′‐hydroxylase is specifically enhanced in flowers from plants subjected to HS or WD + HS (Fig. 6a). Interestingly, stomata of flowers grown under HS or WD + HS closed in response to external application of ABA (Fig. 4a), suggesting that ABA may not accumulate to high concentrations in flowers from plants subjected to these stresses. ABA biosynthesis might therefore occur in flowers from plants subjected to WD, HS or WD + HS, but under conditions of HS and WD + HS, ABA degradation could keep ABA concentrations low and, hence, stomata open (Figs 2, 6, 7). Indeed, the concentrations of the ABA degradation product DPA were specifically and significantly elevated in flowers from plant subjected to HS or WD + HS (Fig. 7b), and lower concentrations of ABA were unable to cause complete stomatal closure in flowers from plants grown under HS or WD + HS conditions (Fig. 6b), supporting this hypothesis. In addition to enhanced ABA degradation (Figs 6b, 7b), the concentrations of JA and JA‐Ile were also specifically and significantly altered (reduced) in flowers from plants subjected to HS or WD + HS (Fig. 7c,d). Because JA‐Ile and ABA are both involved in the regulation of stomatal closure during stress (Nilson & Assmann, 2007; Zandalinas et al., 2016; Zhu, 2016; Zhang et al., 2022; Hsu et al., 2021; Markham & Greenham, 2021), the acclimation strategy of ‘differential transpiration’ during WD + HS (Fig. 10) could be explained by differential accumulation of these two hormones between flowers (enhanced degradation of ABA and reduced concentrations of JA‐Ile; Figs 6, 7b,d) and leaves (enhanced accumulation of ABA and JA‐Ile; Fig. 7e,h). Further studies are, of course, needed to determine how genes involved in the biosynthesis, degradation, and transport of these two hormones are differentially regulated in flowers and leaves in response to WD, HS, WD + HS and other stressful conditions.
For plants that use cleistogamy or pseudocleistogamy for reproduction, cooling of flowers by opening stomata on sepals could be especially important to protect reproductive processes from high temperatures. Although most of the closed soybean flower surface is covered by sepals (Fig. 1b), cooling of a closed flower that has a diameter of c. 1.5–2 mm by transpiration from sepals is much harder than cooling a leaf that has a thickness of c. 0.12–0.15 mm. It is likely that as a result of the thickness of flower buds, cooling by transpiration can only contribute to a reduction of c. 2–3°C in flower temperature during HS or WD + HS conditions (Fig. 4) and this reduction would, of course, depend on the water status of the plant. It therefore seems logical to speculate that the smaller the closed flower, the easier it will be to cool it by transpiration. Moreover, the strategy of differential transpiration, revealed by this work (Fig. 10), may be primarily beneficial for annual plants that need to produce seeds every season, as opposed to perennials that need to protect their vegetative tissues and might abort or skip flowering during entire seasons if conditions are not permissive. Because many important crops, such as soybean, wheat and barley, are annual, use cleistogamy or pseudocleistogamy for reproduction, and have relatively small flowers, the strategy of differential transpiration could play an important economic role in preventing yield penalty under different stress conditions, especially when they occur during the reproductive stage of plant growth. Further studies are needed to dissect the different pathways involved in this response and identify key regulators that control it.
The identification of differential transpiration as a potential mechanism that prevents yield losses under WD + HS conditions highlights new avenues for crop improvements. For example, the density and size of stomata on sepals or other floral organs might be altered (e.g. by manipulating the expression of different stomatal development genes; Fig. S3) to improve transpirational cooling of reproductive tissues. In addition, the pathways and mechanisms controlling stomatal responses of flowers could be manipulated to modulate opening or closing (e.g. by regulating ABA concentrations via ABA degradation; Figs 6, 7), depending on different environmental conditions, protecting flowers from overheating. These manipulations could target the timing of opening or closing as well as the different stimuli and stresses that trigger them.
In summary, our work reveals a novel acclimation strategy of plants that prioritize the transpiration of reproductive tissues over that of vegetative tissues (Fig. 10). This mechanism, termed ‘differential transpiration’, protects flowers of plants from overheating and could be important to minimize yield losses under conditions of stress combination. In addition, it can serve as a new example for plant plasticity in response to abiotic stress.
Author contributions
RS, SIZ and YF performed experiments and analyzed the data. SS and TJ analyzed the transcriptomics data, SZ incorporated the transcriptomics data in SoyKB database, RM, FBF, RS, TJ, SS, AG‐C, SIZ and YF designed experiments, analyzed the data, and/or wrote the manuscript.
Supporting information
Dataset S1 Transcripts significantly upregulated in soybean flowers subjected to water deficit stress (Fig. 5b).
Dataset S2 Transcripts significantly downregulated in soybean flowers subjected to water deficit stress (Fig. 5b).
Dataset S3 Transcripts significantly upregulated in soybean flowers subjected to heat stress (Fig. 5b).
Dataset S4 Transcripts significantly downregulated in soybean flowers subjected to heat stress (Fig. 5b).
Dataset S5 Transcripts significantly upregulated in soybean flowers subjected to combination of water deficit and heat stress (Fig. 5b).
Dataset S6 Transcripts significantly downregulated in soybean flowers subjected to combination of water deficit and heat stress (Fig. 5b).
Dataset S7 Transcripts significantly upregulated in soybean leaves subjected to water deficit (Fig. 5b).
Dataset S8 Transcripts significantly downregulated in soybean leaves subjected to water deficit (Fig. 5b).
Dataset S9 Transcripts significantly upregulated in soybean leaves subjected to heat stress (Fig. 5b).
Dataset S10 Transcripts significantly downregulated in soybean leaves subjected to heat stress (Fig. 5b).
Dataset S11 Transcripts significantly upregulated in soybean leaves subjected to combination of water deficit and heat stress (Fig. 5b).
Dataset S12 Transcripts significantly downregulated in soybean leaves subjected to combination of water deficit and heat stress (Fig. 5b).
Dataset S13 Transcripts exclusively differentially expressed in soybean flowers subjected to water deficit (Fig. 5b).
Dataset S14 Transcripts exclusively differentially expressed in soybean flower subjected to heat stress (Fig. 5b).
Dataset S15 Transcripts exclusively differentially expressed in soybean flower subjected to combination of water deficit and heat stress (Fig. 5b).
Dataset S16 Transcripts commonly expressed in soybean flower subjected to water deficit, and combination of water deficit and heat stress (Fig. 5b).
Dataset S17 Transcripts commonly expressed in soybean flower subjected to water deficit stress and heat stress (Fig. 5b).
Dataset S18 Transcripts commonly expressed in soybean flowers subjected to heat stress, and combination of water deficit and heat stress (Fig. 5b).
Dataset S19 Transcripts commonly expressed in soybean flowers subjected to water deficit, heat stress and combination of water deficit and heat stress (Fig. 5b).
Dataset S20 Transcripts exclusively expressed in soybean leaves subjected to water deficit (Fig. 5b).
Dataset S21 Transcripts exclusively expressed in soybean leaves subjected to heat stress (Fig. 5b).
Dataset S22 Transcripts exclusively expressed in soybean leaves subjected to combination of water deficit and heat stress (Fig. 5b).
Dataset S23 Transcripts commonly expressed in soybean leaves subjected to water deficit stress and heat stress (Fig. 5b).
Dataset S24 Transcripts commonly expressed in soybean leaves subjected to heat stress, and combination of water deficit and heat stress (Fig. 5b).
Dataset S25 Transcripts commonly expressed in soybean leaves subjected to water deficit, and combination of water deficit and heat stress (Fig. 5b).
Dataset S26 Transcripts commonly expressed in soybean leaves subjected to water deficit, heat stress and combination of water deficit and heat stress (Fig. 5b).
Dataset S27 Transcripts exclusive to soybean flowers in response to water deficit, heat stress and combination of water deficit and heat stress compared with leaves (Fig. 5b).
Dataset S28 Transcripts exclusive to soybean leaves in response to water deficit, heat stress and combination of water deficit and heat stress compared with soybean flowers (Fig. 5b).
Dataset S29 Unique transcripts in response to combination of water deficit and heat stress exclusive to soybean flower compared with leaves (Fig. 5b).
Dataset S30 Unique transcripts in response to combination of water deficit and heat stress exclusive to soybean leaves compared with flower (Fig. 5b).
Dataset S31 Transcripts commonly expressed in soybean flowers and leaves when subjected to water deficit, heat stress and combination of water deficit and heat stress (Fig. 5b).
Dataset S32 Unique transcripts in response to combination of water deficit and heat stress common between soybean flower and leaves (Fig. 5b).
Dataset S33 Transcripts exclusively expressed in soybean flowers compared with soybean leaves when subjected to water deficit (Fig. 5c).
Dataset S34 Transcripts exclusively expressed in soybean leaves compared with soybean flowers when subjected to water deficit (Fig. 5c).
Dataset S35 Transcripts commonly expressed in soybean flowers and soybean leaves when subjected to water deficit (Fig. 5c).
Dataset S36 Transcripts exclusively expressed in soybean flowers compared with soybean leaves when subjected to heat stress (Fig. 5c).
Dataset S37 Transcripts exclusively expressed in soybean leaves compared with soybean flowers when subjected to heat stress (Fig. 5c).
Dataset S38 Transcripts commonly expressed in soybean flowers and soybean leaves subjected to heat stress (Fig. 5c).
Dataset S39 Transcripts exclusively expressed in soybean flowers compared with soybean leaves subjected to combination of water deficit and heat stress (Fig. 5c).
Dataset S40 Transcripts exclusively expressed in soybean leaves compared with soybean flowers subjected to combination of water deficit and heat stress (Fig. 5c).
Dataset S41 Transcripts commonly expressed in soybean flowers and soybean leaves subjected to combination of water deficit and heat stress (Fig. 5c).
Dataset S42 Expression of heat shock factor (HSF) transcripts in soybean flowers and leaves subjected to water deficit, heat stress and combination of water deficit and heat stress (Fig. 5e).
Dataset S43 Expression of MYB transcripts in soybean flowers and leaves subjected to water deficit, heat stress and combination of water deficit and heat stress.
Dataset S44 Expression of APETALA 2 (AP2) transcripts in soybean flowers and leaves subjected to water deficit, heat stress and combination of water deficit and heat stress.
Fig. S1 Cross‐section light microscopy analysis of fixed and embedded soybean (Glycine max) flowers from plants grown under controlled growth conditions.
Fig. S2 Height of soybean (Glycine max) plants grown under control (CT), water deficit (WD), heat stress (HS) and WD + HS.
Fig. S3 Expression of transcripts involved in stomatal development in flowers from soybean (Glycine max) plants grown under control (CT), water deficit (WD), heat stress (HS), or WD + HS conditions.
Fig. S4 Accumulation of SA and IAA in flowers from soybean (Glycine max) plants subjected to heat stress or a combination of water deficit and heat stress.
Table S1 List of primers used for RT‐PCR.
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Acknowledgements
This work was supported by funding from the National Science Foundation (IOS‐2110017, IOS‐1353886, MCB‐1936590, IOS‐1932639), the Interdisciplinary Plant Group and the University of Missouri. We thank Professor Robert E. Sharp for valuable comments and discussions. The authors declare there are no competing interests.
Data availability
Data supporting the findings of this work are provided in the main paper and Supporting Information. Raw and processed RNA‐seq data files were deposited in the GEO database (https://www.ncbi.nlm.nih.gov/geo/) (accession no. GSE186317). RNA‐seq data can also be accessed through the SoyKB database (https://soykb.org/DiffExp/diffExp.php).
References
- Agarwal PK, Gupta K, Lopato S, Agarwal P. 2017. Dehydration responsive element binding transcription factors and their applications for the engineering of stress tolerance. Journal of Experimental Botany 68: 2135–2148. [DOI] [PubMed] [Google Scholar]
- Alizadeh MR, Adamowski J, Nikoo MR, AghaKouchak A, Dennison P, Sadegh M. 2020. A century of observations reveals increasing likelihood of continental‐scale compound dry‐hot extremes. Science Advances 6: eaaz4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey‐Serres J, Parker JE, Ainsworth EA, Oldroyd GED, Schroeder JI. 2019. Genetic strategies for improving crop yields. Nature 575: 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balfagón D, Sengupta S, Gómez‐Cadenas A, Fritschi FB, Azad R, Mittler R, Zandalinas SI. 2019. Jasmonic acid is required for plant acclimation to a combination of high light and heat stress. Plant Physiology 181: 1668–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellaloui N, Bruns HA, Abbas HK, Mengistu A, Fisher DK, Reddy KN. 2015. Agricultural practices altered soybean seed protein, oil, fatty acids, sugars, and minerals in the Midsouth USA. Frontiers in Plant Science 6: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitez ER, Khan NA, Matsumura H, Abe J, Takahashi R. 2010. Varietal differences and morphology of cleistogamy in soybean. Crop Science 50: 185–190. [Google Scholar]
- Buckley TN. 2019. How do stomata respond to water status? New Phytologist 224: 21–36. [DOI] [PubMed] [Google Scholar]
- Caine RS, Yin X, Sloan J, Harrison EL, Mohammed U, Fulton T, Biswal AK, Dionora J, Chater CC, Coe RA et al. 2019. Rice with reduced stomatal density conserves water and has improved drought tolerance under future climate conditions. New Phytologist 221: 371–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell CS, Quinn JA, Cheplick GP, Bell TJ. 1983. Cleistogamy in Grasses. Annual Review of Ecology and Systematics 14: 411–441. [Google Scholar]
- Carmo‐Silva AE, Gore MA, Andrade‐Sanchez P, French AN, Hunsaker DJ, Salvucci ME. 2012. Decreased CO2 availability and inactivation of Rubisco limit photosynthesis in cotton plants under heat and drought stress in the field. Environmental and Experimental Botany 83: 1–11. [Google Scholar]
- Ceulemans R, Van Praet L, Jiang XN. 1995. Effects of CO2 enrichment, leaf position and clone on stomatal index and epidermal cell density in poplar (Populus). New Phytologist 131: 99–107. [DOI] [PubMed] [Google Scholar]
- Chater CCC, Oliver J, Casson S, Gray JE. 2014. Putting the brakes on: abscisic acid as a central environmental regulator of stomatal development. New Phytologist 202: 376–391. [DOI] [PubMed] [Google Scholar]
- Chaturvedi P, Wiese AJ, Ghatak A, Záveská Drábková L, Weckwerth W, Honys D. 2021. Heat stress response mechanisms in pollen development. New Phytologist 231: 571–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Cao Y, Li H, Kim D, Ahsan N, Thelen J, Stacey G. 2017. Extracellular ATP elicits DORN1‐mediated RBOHD phosphorylation to regulate stomatal aperture. Nature Communications 8: 2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I, Zandalinas SI, Fritschi FB, Sengupta S, Fichman Y, Azad K, Mittler R. 2021a. The impact of water deficit and heat stress combination on the molecular response, physiology, and seed production of soybean. Physiologia Plantarum 172: 41–52. [DOI] [PubMed] [Google Scholar]
- Cohen I, Zandalinas SI, Huck C, Fritschi FB, Mittler R. 2021b. Meta‐analysis of drought and heat stress combination impact on crop yield and yield components. Physiologia Plantarum 171: 66–76. [DOI] [PubMed] [Google Scholar]
- Danecek P, Bonfield JK, Liddle J, Marshall J, Ohan V, Pollard MO, Whitwham A, Keane T, McCarthy SA, Davies RM et al. 2021. Twelve years of SAMtools and BCFtools. Gigascience 10: giab008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneva A, Gao Z, Van Durme M, Nowack MK. 2016. Functions and regulation of programmed cell death in plant development. Annual Review of Cell and Developmental Biology 32: 441–468. [DOI] [PubMed] [Google Scholar]
- Davletova S, Rizhsky L, Liang H, Shengqiang Z, Oliver DJ, Coutu J, Shulaev V, Schlauch K, Mittler R. 2005. Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell 17: 268–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deger AG, Scherzer S, Nuhkat M, Kedzierska J, Kollist H, Brosché M, Unyayar S, Boudsocq M, Hedrich R, Roelfsema MRG. 2015. Guard cell SLAC1‐type anion channels mediate flagellin‐induced stomatal closure. New Phytologist 208: 162–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devireddy AR, Arbogast J, Mittler R. 2020. Coordinated and rapid whole‐plant systemic stomatal responses. New Phytologist 225: 21–25. [DOI] [PubMed] [Google Scholar]
- Djanaguiraman M, Narayanan S, Erdayani E, Prasad PVV. 2020. Effects of high temperature stress during anthesis and grain filling periods on photosynthesis, lipids and grain yield in wheat. BMC Plant Biology 20: 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr WR, Caviness CE, Burmood DT, Pennington JS. 1971. Stage of development descriptions for soybeans, Glycine max (L.) Merrill. Crop Science 11: 929–931. [Google Scholar]
- von der Gathen P, Kivi R, Wohltmann I, Salawitch RJ, Rex M. 2021. Climate change favours large seasonal loss of Arctic ozone. Nature Communications 12: 3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu P‐K, Dubeaux G, Takahashi Y, Schroeder JI. 2021. Signaling mechanisms in abscisic acid‐mediated stomatal closure. The Plant Journal 105: 307–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi T, Fitzpatrick MR, Chen S, Liu Y, Zhang H, Endacott RZ, Gaudiello EC, Stacey G, Nguyen HT, Xu D. 2014. Soybean knowledge base (SoyKB): a web resource for integration of soybean translational genomics and molecular breeding. Nucleic Acids Research 42: D1245–D1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi T, Patil K, Fitzpatrick MR, Franklin LD, Yao Q, Cook JR, Wang Z, Libault M, Brechenmacher L, Valliyodan B et al. 2012. Soybean knowledge base (SoyKB): a web resource for soybean translational genomics. BMC Genomics 13: S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan NA, Githiri SM, Benitez ER, Abe J, Kawasaki S, Hayashi T, Takahashi R. 2008. QTL analysis of cleistogamy in soybean. Theoretical and Applied Genetics 117: 479–487. [DOI] [PubMed] [Google Scholar]
- Kim D, Paggi JM, Park C, Bennett C, Salzberg SL. 2019. Graph‐based genome alignment and genotyping with HISAT2 and HISAT‐genotype. Nature Biotechnology 37: 907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koussevitzky S, Suzuki N, Huntington S, Armijo L, Sha W, Cortes D, Shulaev V, Mittler R. 2008. Ascorbate peroxidase 1 plays a key role in the response of Arabidopsis thaliana to stress combination. Journal of Biological Chemistry 283: 34197–34203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassig R, Gutermuth T, Bey TD, Konrad KR, Romeis T. 2014. Pollen tube NAD(P)H oxidases act as a speed control to dampen growth rate oscillations during polarized cell growth. The Plant Journal 78: 94–106. [DOI] [PubMed] [Google Scholar]
- Lawas LMF, Shi W, Yoshimoto M, Hasegawa T, Hincha DK, Zuther E, Jagadish SVK. 2018. Combined drought and heat stress impact during flowering and grain filling in contrasting rice cultivars grown under field conditions. Field Crops Research 229: 66–77. [Google Scholar]
- Lawson T, Matthews J. 2020. Guard cell metabolism and stomatal function. Annual Review of Plant Biology 71: 273–302. [DOI] [PubMed] [Google Scholar]
- Liu F, Andersen MN, Jensen CR. 2003. Loss of pod set caused by drought stress is associated with water status and ABA content of reproductive structures in soybean. Functional Plant Biology 30: 271–280. [DOI] [PubMed] [Google Scholar]
- Liu KW, Liu ZJ, Huang LQ, Li LQ, Chen LJ, Da TG. 2006. Self‐fertilization strategy in an orchid. Nature 441: 945–946. [DOI] [PubMed] [Google Scholar]
- Liu X, Wang X, Wang X, Gao J, Luo N, Meng Q, Wang P. 2020. Dissecting the critical stage in the response of maize kernel set to individual and combined drought and heat stress around flowering. Environmental and Experimental Botany 179: 104213. [Google Scholar]
- Lloyd JPB, Lister R. 2022. Epigenome plasticity in plants. Nature Reviews Genetics 23: 55–68. [DOI] [PubMed] [Google Scholar]
- Lobell DB, Schlenker W, Costa‐Roberts J. 2011. Climate trends and global crop production since 1980. Science 333: 616–620. [DOI] [PubMed] [Google Scholar]
- Lozano‐Juste J, Cutler SR. 2016. Hormone signalling: ABA has a breakdown. Nature Plants 2: 16137. [DOI] [PubMed] [Google Scholar]
- Mahrookashani A, Siebert S, Hüging H, Ewert F. 2017. Independent and combined effects of high temperature and drought stress around anthesis on wheat. Journal of Agronomy and Crop Science 203: 453–463. [Google Scholar]
- Markham K, Greenham K. 2021. Abiotic stress through time. New Phytologist 231: 40–46. [DOI] [PubMed] [Google Scholar]
- Martin MV, Fernando Fiol D, Sundaresan V, Julián Zabaleta E, Pagnussat GC. 2013. Oiwa, a female gametophytic mutant impaired in a mitochondrial manganese‐superoxide dismutase, reveals crucial roles for reactive oxygen species during embryo sac development and fertilization in Arabidopsis. Plant Cell 25: 1573–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazdiyasni O, AghaKouchak A. 2015. Substantial increase in concurrent droughts and heatwaves in the United States. Proceedings of the National Academy of Sciences, USA 112: 11484–11489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melotto M, Underwood W, Koczan J, Nomura K, He SY. 2006. Plant stomata function in innate immunity against bacterial invasion. Cell 126: 969–980. [DOI] [PubMed] [Google Scholar]
- Mittler R. 2006. Abiotic stress, the field environment and stress combination. Trends in Plant Science 11: 15–19. [DOI] [PubMed] [Google Scholar]
- Nilson SE, Assmann SM. 2007. The control of transpiration. Insights from Arabidopsis. Plant Physiology 143: 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overpeck JT, Udall B. 2020. Climate change and the aridification of North America. Proceedings of the National Academy of Sciences, USA 117: 11856–11858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X, Yoshinari A, Bai P, Maes M, Zeng SM, Torii KU. 2020. The manifold actions of signaling peptides on subcellular dynamics of a receptor specify stomatal cell fate. eLife 9: e58097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudvere U, Kolberg L, Kuzmin I, Arak T, Adler P, Peterson H, Vilo J. 2019. g:Profiler: a web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Research 47: W191–W198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven JA. 2014. Speedy small stomata? Journal of Experimental Botany 65: 1415–1424. [DOI] [PubMed] [Google Scholar]
- Rivero RM, Mittler R, Blumwald E, Zandalinas SI. 2022. Developing climate‐resilient crops: improving plant tolerance to stress combination. The Plant Journal 109: 373–389. [DOI] [PubMed] [Google Scholar]
- Rizhsky L, Liang H, Mittler R. 2002. The combined effect of drought stress and heat shock on gene expression in tobacco. Plant Physiology 130: 1143–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizhsky L, Liang H, Shuman J, Shulaev V, Davletova S, Mittler R. 2004. When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiology 134: 1683–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins JA, Habte E, Templer SE, Colby T, Schmidt J, Von Korff M. 2013. Leaf proteome alterations in the context of physiological and morphological responses to drought and heat stress in barley (Hordeum vulgare L.). Journal of Experimental Botany 64: 3201–3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakoda K, Watanabe T, Sukemura S, Kobayashi S, Nagasaki Y, Tanaka Y, Shiraiwa T. 2019. Genetic diversity in stomatal density among soybeans elucidated using high‐throughput technique based on an algorithm for object detection. Scientific Reports 9: 7610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago JP, Sharkey TD. 2019. Pollen development at high temperature and role of carbon and nitrogen metabolites. Plant, Cell & Environment 42: 2759–2775. [DOI] [PubMed] [Google Scholar]
- Sattar A, Sher A, Ijaz M, Ul‐Allah S, Rizwan MS, Hussain M, Jabran K, Cheema MA. 2020. Terminal drought and heat stress alter physiological and biochemical attributes in flag leaf of bread wheat. PLoS ONE 15: e0232974. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sinha R, Fritschi FB, Zandalinas SI, Mittler R. 2021. The impact of stress combination on reproductive processes in crops. Plant Science 311: 111007. [DOI] [PubMed] [Google Scholar]
- Steg L. 2018. Limiting climate change requires research on climate action. Nature Climate Change 8: 759–761. [Google Scholar]
- Sugano SS, Shimada T, Imai Y, Okawa K, Tamai A, Mori M, Hara‐Nishimura I. 2010. Stomagen positively regulates stomatal density in Arabidopsis. Nature 463: 241–244. [DOI] [PubMed] [Google Scholar]
- Sze H, Palanivelu R, Harper JF, Johnson MA. 2021. Holistic insights from pollen omics: co‐opting stress‐responsive genes and ER‐mediated proteostasis for male fertility. Plant Physiology 187: 2361–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi R, Kurosaki H, Yumoto S, Han OK, Abe J. 2001. Genetic and linkage analysis of Cleistogamy in soybean. Journal of Heredity 92: 89–92. [DOI] [PubMed] [Google Scholar]
- Trapnell C, Hendrickson DG, Sauvageau M, Goff L, Rinn JL, Pachter L. 2013. Differential analysis of gene regulation at transcript resolution with RNA‐seq. Nature Biotechnology 31: 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L. 2012. Differential gene and transcript expression analysis of RNA‐seq experiments with TopHat and Cufflinks. Nature Protocols 7: 562–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahisalu T, Puzõrjova I, Brosché M, Valk E, Lepiku M, Moldau H, Pechter P, Wang Y‐S, Lindgren O, Salojärvi J et al. 2010. Ozone‐triggered rapid stomatal response involves the production of reactive oxygen species, and is controlled by SLAC1 and OST1 . The Plant Journal 62: 442–453. [DOI] [PubMed] [Google Scholar]
- Wang MH, Wang JR, Zhang XW, Zhang AP, Sun S, Zhao CM. 2019. Phenotypic plasticity of stomatal and photosynthetic features of four Picea species in two contrasting common gardens. AoB Plants 11: plz034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Wang F, Hong Y, Yao J, Ren Z, Shi H, Zhu JK. 2018. The flowering repressor SVP confers drought resistance in Arabidopsis by regulating abscisic acid catabolism. Molecular Plant 11: 1184–1197. [Google Scholar]
- Wei D, Liu M, Chen H, Zheng Y, Liu Y, Wang X, Yang S, Zhou M, Lin J. 2018. INDUCER OF CBF EXPRESSION 1 is a male fertility regulator impacting anther dehydration in Arabidopsis. PLoS Genetics 14: e1007695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will RE, Wilson SM, Zou CB, Hennessey TC. 2013. Increased vapor pressure deficit due to higher temperature leads to greater transpiration and faster mortality during drought for tree seedlings common to the forest–grassland ecotone. New Phytologist 200: 366–374. [DOI] [PubMed] [Google Scholar]
- Wong CE, Singh MB, Bhalla PL. 2009. Molecular processes underlying the floral transition in the soybean shoot apical meristem. The Plant Journal 57: 832–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Xiong W, Pan J, Ali T, Cui Q, Guan D, Meng J, Mueller ND, Lin E, Davis SJ. 2018. Decreases in global beer supply due to extreme drought and heat. Nature Plants 4: 964–973. [DOI] [PubMed] [Google Scholar]
- Yarrow GL, Brun WA, Brenner ML. 1988. Effect of shading individual soybean reproductive structures on their abscisic acid content, metabolism, and partitioning. Plant Physiology 86: 71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandalinas SI, Balfagón D, Arbona V, Gómez‐Cadenas A, Inupakutika MA, Mittler R. 2016. ABA is required for the accumulation of APX1 and MBF1c during a combination of water deficit and heat stress. Journal of Experimental Botany 67: 5381–5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandalinas SI, Fichman Y, Devireddy AR, Sengupta S, Azad RK, Mittler R. 2020a. Systemic signaling during abiotic stress combination in plants. Proceedings of the National Academy of Sciences, USA 117: 13810–13820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandalinas SI, Fritschi FB, Mittler R. 2020b. Signal transduction networks during stress combination. Journal of Experimental Botany 71: 1734–1741. [DOI] [PubMed] [Google Scholar]
- Zandalinas SI, Fritschi FB, Mittler R. 2021. Global warming, climate change, and environmental pollution: recipe for a multifactorial stress combination disaster. Trends in Plant Science 26: 588–599. [DOI] [PubMed] [Google Scholar]
- Zandalinas SI, Mittler R. 2021. Vascular and non‐vascular transmission of systemic reactive oxygen signals during wounding and heat stress. Plant Physiology 186: 1721–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai P, Pirani A, Connors S, Péan C, Berger S, Caud N, Chen Y, Goldfarb L, Gomis M, Huang M et al., 2021. IPCC 2021: Climate change 2021: the physical science basis. Contribution of working group I to the sixth assessment report of the intergovernmental panel on climate change. Geneva, Switzerland: Intergovernmental Panel on Climate Change. [Google Scholar]
- Zhang H, Zhu J, Gong Z, Zhu J‐K. 2022. Abiotic stress responses in plants. Nature Reviews Genetics 23: 104–119. [DOI] [PubMed] [Google Scholar]
- Zhang J, De‐Oliveira‐Ceciliato P, Takahashi Y, Schulze S, Dubeaux G, Hauser F, Azoulay‐Shemer T, Tõldsepp K, Kollist H, Rappel W‐J et al. 2018. Insights into the molecular mechanisms of CO2‐mediated regulation of stomatal movements. Current Biology 28: R1356–R1363. [DOI] [PubMed] [Google Scholar]
- Zhang JY, He SB, Li L, Yang HQ. 2014. Auxin inhibits stomatal development through MONOPTEROS repression of a mobile peptide gene STOMAGEN in mesophyll. Proceedings of the National Academy of Sciences, USA 111: E3015–E3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang MJ, Zhang XS, Gao XQ. 2020. ROS in the male‐female interactions during pollination: function and regulation. Frontiers in Plant Science 11: 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Yu X, Kjær KH, Rosenqvist E, Ottosen CO, Wu Z. 2015. Screening and validation of tomato genotypes under heat stress using Fv/Fm to reveal the physiological mechanism of heat tolerance. Environmental and Experimental Botany 118: 1–11. [Google Scholar]
- Zhu JK. 2016. Abiotic stress signaling and responses in plants. Cell 167: 313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Dataset S1 Transcripts significantly upregulated in soybean flowers subjected to water deficit stress (Fig. 5b).
Dataset S2 Transcripts significantly downregulated in soybean flowers subjected to water deficit stress (Fig. 5b).
Dataset S3 Transcripts significantly upregulated in soybean flowers subjected to heat stress (Fig. 5b).
Dataset S4 Transcripts significantly downregulated in soybean flowers subjected to heat stress (Fig. 5b).
Dataset S5 Transcripts significantly upregulated in soybean flowers subjected to combination of water deficit and heat stress (Fig. 5b).
Dataset S6 Transcripts significantly downregulated in soybean flowers subjected to combination of water deficit and heat stress (Fig. 5b).
Dataset S7 Transcripts significantly upregulated in soybean leaves subjected to water deficit (Fig. 5b).
Dataset S8 Transcripts significantly downregulated in soybean leaves subjected to water deficit (Fig. 5b).
Dataset S9 Transcripts significantly upregulated in soybean leaves subjected to heat stress (Fig. 5b).
Dataset S10 Transcripts significantly downregulated in soybean leaves subjected to heat stress (Fig. 5b).
Dataset S11 Transcripts significantly upregulated in soybean leaves subjected to combination of water deficit and heat stress (Fig. 5b).
Dataset S12 Transcripts significantly downregulated in soybean leaves subjected to combination of water deficit and heat stress (Fig. 5b).
Dataset S13 Transcripts exclusively differentially expressed in soybean flowers subjected to water deficit (Fig. 5b).
Dataset S14 Transcripts exclusively differentially expressed in soybean flower subjected to heat stress (Fig. 5b).
Dataset S15 Transcripts exclusively differentially expressed in soybean flower subjected to combination of water deficit and heat stress (Fig. 5b).
Dataset S16 Transcripts commonly expressed in soybean flower subjected to water deficit, and combination of water deficit and heat stress (Fig. 5b).
Dataset S17 Transcripts commonly expressed in soybean flower subjected to water deficit stress and heat stress (Fig. 5b).
Dataset S18 Transcripts commonly expressed in soybean flowers subjected to heat stress, and combination of water deficit and heat stress (Fig. 5b).
Dataset S19 Transcripts commonly expressed in soybean flowers subjected to water deficit, heat stress and combination of water deficit and heat stress (Fig. 5b).
Dataset S20 Transcripts exclusively expressed in soybean leaves subjected to water deficit (Fig. 5b).
Dataset S21 Transcripts exclusively expressed in soybean leaves subjected to heat stress (Fig. 5b).
Dataset S22 Transcripts exclusively expressed in soybean leaves subjected to combination of water deficit and heat stress (Fig. 5b).
Dataset S23 Transcripts commonly expressed in soybean leaves subjected to water deficit stress and heat stress (Fig. 5b).
Dataset S24 Transcripts commonly expressed in soybean leaves subjected to heat stress, and combination of water deficit and heat stress (Fig. 5b).
Dataset S25 Transcripts commonly expressed in soybean leaves subjected to water deficit, and combination of water deficit and heat stress (Fig. 5b).
Dataset S26 Transcripts commonly expressed in soybean leaves subjected to water deficit, heat stress and combination of water deficit and heat stress (Fig. 5b).
Dataset S27 Transcripts exclusive to soybean flowers in response to water deficit, heat stress and combination of water deficit and heat stress compared with leaves (Fig. 5b).
Dataset S28 Transcripts exclusive to soybean leaves in response to water deficit, heat stress and combination of water deficit and heat stress compared with soybean flowers (Fig. 5b).
Dataset S29 Unique transcripts in response to combination of water deficit and heat stress exclusive to soybean flower compared with leaves (Fig. 5b).
Dataset S30 Unique transcripts in response to combination of water deficit and heat stress exclusive to soybean leaves compared with flower (Fig. 5b).
Dataset S31 Transcripts commonly expressed in soybean flowers and leaves when subjected to water deficit, heat stress and combination of water deficit and heat stress (Fig. 5b).
Dataset S32 Unique transcripts in response to combination of water deficit and heat stress common between soybean flower and leaves (Fig. 5b).
Dataset S33 Transcripts exclusively expressed in soybean flowers compared with soybean leaves when subjected to water deficit (Fig. 5c).
Dataset S34 Transcripts exclusively expressed in soybean leaves compared with soybean flowers when subjected to water deficit (Fig. 5c).
Dataset S35 Transcripts commonly expressed in soybean flowers and soybean leaves when subjected to water deficit (Fig. 5c).
Dataset S36 Transcripts exclusively expressed in soybean flowers compared with soybean leaves when subjected to heat stress (Fig. 5c).
Dataset S37 Transcripts exclusively expressed in soybean leaves compared with soybean flowers when subjected to heat stress (Fig. 5c).
Dataset S38 Transcripts commonly expressed in soybean flowers and soybean leaves subjected to heat stress (Fig. 5c).
Dataset S39 Transcripts exclusively expressed in soybean flowers compared with soybean leaves subjected to combination of water deficit and heat stress (Fig. 5c).
Dataset S40 Transcripts exclusively expressed in soybean leaves compared with soybean flowers subjected to combination of water deficit and heat stress (Fig. 5c).
Dataset S41 Transcripts commonly expressed in soybean flowers and soybean leaves subjected to combination of water deficit and heat stress (Fig. 5c).
Dataset S42 Expression of heat shock factor (HSF) transcripts in soybean flowers and leaves subjected to water deficit, heat stress and combination of water deficit and heat stress (Fig. 5e).
Dataset S43 Expression of MYB transcripts in soybean flowers and leaves subjected to water deficit, heat stress and combination of water deficit and heat stress.
Dataset S44 Expression of APETALA 2 (AP2) transcripts in soybean flowers and leaves subjected to water deficit, heat stress and combination of water deficit and heat stress.
Fig. S1 Cross‐section light microscopy analysis of fixed and embedded soybean (Glycine max) flowers from plants grown under controlled growth conditions.
Fig. S2 Height of soybean (Glycine max) plants grown under control (CT), water deficit (WD), heat stress (HS) and WD + HS.
Fig. S3 Expression of transcripts involved in stomatal development in flowers from soybean (Glycine max) plants grown under control (CT), water deficit (WD), heat stress (HS), or WD + HS conditions.
Fig. S4 Accumulation of SA and IAA in flowers from soybean (Glycine max) plants subjected to heat stress or a combination of water deficit and heat stress.
Table S1 List of primers used for RT‐PCR.
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Data Availability Statement
The analyzed transcript abundance and differentially expressed transcripts can be accessed interactively via the Differential Expression tool in SoyKB; https://soykb.org/DiffExp/diffExp.php; Joshi et al., 2012, 2014), a comprehensive all‐inclusive web resource for soybean. It provides a set of visualization and analytical tools such as differential expression analysis and gene card pages and provides data in the form of tabs for Gene lists, Venn diagram, Volcano plot, Function Analysis, Pathway Analysis and Gene modules.
Data supporting the findings of this work are provided in the main paper and Supporting Information. Raw and processed RNA‐seq data files were deposited in the GEO database (https://www.ncbi.nlm.nih.gov/geo/) (accession no. GSE186317). RNA‐seq data can also be accessed through the SoyKB database (https://soykb.org/DiffExp/diffExp.php).


