Abstract
Neutrophil granulocytes are a central component of the innate immune system. In recent years, they have gained considerable attention due to newly discovered biological effector functions and their involvement in various pathological conditions. They have been shown to trigger mechanisms that can either promote or inhibit the development of autoimmunity, thrombosis, and cancer. One mechanism for their modulatory effect is the release of extracellular vesicles (EVs), that trigger appropriate signaling pathways in immune cells and other target cells. In addition, activated neutrophils can release bactericidal DNA fibers decorated with proteins from neutrophil granules (neutrophil extracellular traps, NETs). While NETs are very effective in limiting pathogens, they can also cause severe damage if released in excess or cleared inefficiently. Since NETs and EVs share a variety of neutrophil molecules and initially act in the same microenvironment, differential biochemical and functional analysis is particularly challenging. This review focuses on the biochemical and functional parallels and the extent to which the overlapping spectrum of effector molecules has an impact on biological and pathological effects.
Keywords: extracellular vesicles, neutrophil extracellular traps, neutrophil granulocytes, cancer, autoimmune disease, thrombosis
1. Neutrophil Extracellular Traps
Upon activation, neutrophil granulocytes can release decondensed chromatin, which forms a scaffold of extracellular fibers decorated with granule proteins. Originally, this structure was thought to function as a trap, killing invading microorganisms, and was given the designation neutrophil extracellular trap (NET) [1]. Although numerous studies have demonstrated that comparable structures may also be produced by eosinophils, mast cells, and macrophages, neutrophil-derived extracellular traps have been the most intensively studied [2,3,4].
NET formation is triggered by contact with a variety of pro-inflammatory stimuli. These include molecules collectively referred to as “pathogen-related molecular patterns” (PAMPs) derived from bacterial, fungal, viral, or parasitic pathogens, e.g., lipopolysaccharides (LPS) or N-formylated chemotactic oligopeptides (e.g., N-formyl-methionyl-leucyl-phenylalanine (fMLP)). Intracellular proteins and extracellular matrix proteins released by stressed or dying cells represent another group of pro-inflammatory signaling molecules (summarized as “damage-related molecular patterns” (DAMPs)). Neutrophils carry specific pattern recognition receptors (PRRs) on their cell membrane that bind PAMPs or DAMPs and trigger a signal transduction cascade, leading to the formation of NETs. PRRs involved in neutrophil NETosis include the families of Toll-like receptors (TLRs), nucleotide-binding oligomerization domain (NOD)-like receptors, and C-type lectin receptors, with each receptor triggered by a distinct set of stimuli [5].
In the canonical pathway of NET generation, contact of PRRs with compounds derived from pathogenic microorganisms, immune complexes, cytokines, or mitogens, including phorbol-12-myristate-13-acetate (PMA), initiates mitogen-activated protein kinases (MAPKs)-dependent signaling pathways that induce the generation of reactive oxygen species (ROS). The subsequently activated peptidyl-arginine-deiminase 4 (PAD-4) and the granule enzymes neutrophil elastase (NE) and myeloperoxidase (MPO) induce chromatin de-condensation by modifying histone proteins [6]. Citrullination by PAD enzymes and enzymatic degradation of histones destabilizes the chromatin structure. As a result, chromatin undergoes a process of entropic swelling that leads to rupture of the nuclear and cell membrane and release of decondensed chromatin [7]. Since the physical swelling of chromatin results in the lysis of a netting cell (a cell releasing NETs) within 2 to 4 h after stimulation, this type of NETosis has been termed “suicidal” NETosis. It represents a distinct cell death pathway, different from classical pathways such as apoptosis, and is characterized by NADPH-oxidase (NOX)-mediated ROS generation [8].
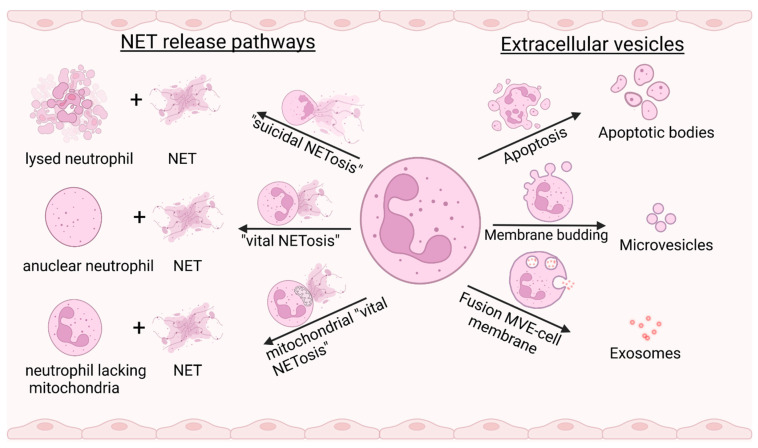
Another non-lytic mode of NET generation is characterized by a rapid cellular response to an external stimulus that occurs within minutes. Nuclear DNA (nDNA)-containing vesicles are formed by budding of a decondensing nucleus and are then transported to the cell membrane [9]. Remarkably, in this process, termed “vital” NETosis, neutrophils become anuclear cytoplasts that are still motile and can sense chemotactic gradients [10]. A third mode of NET generation, which is ROS-dependent and “non-suicidal”, has been described by Yousefi et al. [11]. When granulocyte/macrophage-colony stimulating factor (GM-CSF)-primed neutrophils are stimulated with LPS or complement factor 5a, they release NETs exclusively derived from mitochondrial DNA (mtDNA, Figure 1).
Figure 1.
Mechanisms of NET formation (left) and EV classes released by neutrophils (right). In “suicidal NETosis”, chromatin is released from the lysing neutrophil after chromatin de-condensation and subsequent rupture of the nuclear and cell membrane. “Vital NETosis” is characterized by the fusion of vesicles containing nuclear DNA with the plasma membrane, finally resulting in an anuclear cytoplast. In a second form of “vital NETosis”, neutrophils release mitochondrial DNA by an unknown NOX-dependent mechanism. Extracellular vesicles comprise a group of heterogeneous membranous vesicles of varying size and morphology. Apoptotic bodies represent subcellular fragments after the disassembly of a dying cell. The smaller microvesicles bud from the cell membrane and contain cytoplasmic material. The smallest EVs are referred to as exosomes and are released from the lumen of multivesicular endosomes (MVE), fusing with the cell membrane. Created with BioRender.com.
At this point, it should be emphasized that there is still an ongoing debate in the scientific community concerning key aspects of NETs arising from conflicting data. Currently, there is no consensus on the origin of NET-DNA and the exact sequence of events leading to NET formation and cell death [12]. However, an important role of NETs in the areas described in the following sections is supported by a large body of evidence published by various research groups.
The comparability of different studies on NET biology and pathophysiology is often limited because widely accepted standardized procedures for neutrophil stimulation, NET isolation, and characterization have not been established. Recently, a detailed proteomic analysis of NETs revealed a stimulus-dependent pattern of protein composition and post-transcriptional modifications [13]. NETs may even be identified by a differential proteomic signature that appears to be associated with particular diseases [14]. Neutrophil heterogeneity and inter-individual variations, including varying predisposition for NETotic activity, pose a challenge for the identification of specific, functionally relevant differences between NETs that have been generated in different pathways and are composed of DNA from diverse subcellular origins (nuclear, mitochondrial, or both) [15,16]. Pieterse et al. could demonstrate that NETs generated by a NOX-dependent mechanism have a reduced capacity to activate endothelial cells compared to NOX-independent NETs [17]. However, it is unclear whether N-terminally truncated core histones found in NOX-dependent NETs have a significant effect in vivo. Considering the differences of mtDNA and nDNA in size and structure, it is reasonable to assume that NETs derived from either source might differ in their structural composition and thus also in their function [18]. However, further studies are required to clarify to what extent the structural differences are functionally involved in the development of diseases and how they can be integrated into diagnostic procedures.
2. Extracellular Vesicles
Classically, remote intercellular communication is achieved by the release of soluble molecules into the extracellular space and subsequent (receptor-mediated) uptake by recipient cells. In contrast to this simple mode of delivering effector molecules, extracellular vesicles (EVs) serve as carriers for multiple cytosolic proteins, nucleic acids, and lipids protected by a lipid bilayer, as well as transmembrane and membrane-associated proteins that contribute to cellular targeting [19]. The release of EVs represents a fundamental mechanism of cell-to-cell communication in eukaryotic and prokaryotic organisms, including Archaea [20]. Originally considered membrane debris with no biological function, EVs are today recognized as crucial mediators of intercellular communication in health and disease [21].
The term extracellular vesicle describes a group of membrane-enveloped vesicles of varying size and genesis that are shed by a variety of cells, including endothelial cells, blood cells, and cancer cells, often as a result of activation or cell death. Three major classes of EVs are distinguished [22]: (1) exosomes (30–150 nm in size) released by the fusion of multivesicular endosomes with the cell membrane, (2) microvesicles (100–1000 nm) formed by budding of the cell membrane, and (3) apoptotic bodies (50–5000 nm) released by dying cells [23] (Figure 1). EVs have recently gained increasing attention in the scientific literature because they appear to play an important role in inflammation, thrombosis, and cancer. Since EVs are found in circulating blood and in various types of extracellular fluids, they are ideal candidates for new diagnostic and therapeutic approaches [24].
Communication between immune cells and other cells involved in immune reactions is a prerequisite to orchestrate an appropriate response to infection and the resolution of inflammation and its sequelae. Neutrophil granulocytes, representing the first line of defense against infectious agents, are able to phagocytose, directly kill, and digest microbial pathogens through the action of various enzymes and proteins stored in their granules [25]. In addition, they release EVs either spontaneously or in response to various priming or activating stimuli. These stimuli include bacteria and fungi, endogenous inflammatory mediators (cytokines and chemokines, complement components), and pharmacologic agents (ionophores, phorbolesters) [26]. The stimulation of EV release, though, is not inevitably linked to the activation of neutrophils. While LPS-induced EV release appears to be related to neutrophil degranulation, GM-CSF-induced EV release is not [27]. In fact, many single-receptor stimulants do not enhance EV release from neutrophils. Activation of PRRs by fMLP or LPS or engagement of Fc-receptors by immunoglobulins alone does not lead to enhanced EV release, but additional co-stimulation of complement receptors induces a marked increase of EV shedding [28].
Depending on the stimulus and the physiologic state, neutrophils are able to release EVs with even opposing functional properties [29]. By targeting different cell types with specific pro- or anti-inflammatory molecules, neutrophils modulate immune responses of both the innate and adaptive immune system. For example, azurophilic granule enzymes, Annexin A1, and the membrane phospholipid phosphatidylserine (PS) can modulate dendritic cell and T-cell activity [30].
Recently, microparticles with an elongated shape (termed elongated neutrophil-derived structures, ENDS) released by rolling neutrophils in the vessel lumen have been reported [31]. Mass spectrometric analysis revealed an EV-like proteome, although classical EV markers such as tetraspanins CD9, CD63, or CD81 could not be detected. Unlike EVs, ENDS do not appear to contain DNA. Plasma levels of ENDS are greatly increased in septic patients and may be involved in the pathology of sepsis through the release of a pro-inflammatory DAMP protein complex consisting of S100A8-S100A9. However, current data are insufficient to define the role of ENDS in inflammatory processes in vivo.
3. Common Players: NETs and Neutrophil-Derived EVs
Neutrophils undergo a complex stepwise activation process during their recruitment to sites of injury or infection, while receiving inflammatory signals from their environment [32]. The idea that EVs reflect the activation state of the cell is supported by the fact that EVs from stimulated neutrophils have been shown to exhibit a wide range of functional diversity and target numerous different cell types [33,34,35]. Unlike NETs, which are released upon cell activation, EVs are produced by both resting and activated neutrophils. EVs from resting or apoptotic neutrophils preferentially have an anti-inflammatory effect, possibly contributing to the resolution of inflammation and preventing autoimmunity [26]. Whether an activated cell releases pro- or anti-inflammatory EVs depends largely on the type and amount of stimulus. However, the degree of activation does not seem to be the sole decisive factor in determining the EV type. Neutrophils infected with Mycobacterium tuberculosis produce EVs that exert a much stronger activating effect on macrophages than EVs from neutrophils activated with the highly potent NETosis inducer PMA, as evidenced by the induction of pro-inflammatory cytokines and the expression of costimulatory molecules by monocyte-derived macrophages [36].
A dichotomous mode of immune modulation observed with EVs has also been described for NETs: Despite their well-documented pro-inflammatory activity, NETs tend to form inflammation-resolving aggregates at a high cellular density in neutrophil-rich inflammatory foci [37,38]. NET aggregation protects NET-associated NE from inhibition by its physiologic inhibitor α1-antitrypsin (α1-AT). The preserved proteolytic activity exerts an anti-inflammatory effect by degrading pro-inflammatory cytokines and chemokines.
Since both NETs and EVs are released simultaneously upon activation, the question arises of whether they act as antagonistic, synergistic, or independent mediators. Given their common cellular origin, it is not surprising that both share a broad spectrum of molecules involved in the regulation of immune responses in a narrow or broader sense (Table 1). Nonetheless, due to quantitative differences in effector molecule load, different accessibility to interacting molecules, differing cellular and molecular targeting, and clearing routes, considerably different and even opposing functions of the same molecule derived from either NETs or EVs can be anticipated. However, published data suggest that NETs and EVs do not act completely independently of each other in these aspects either: Wang et al. were able to prove a histone-PS-mediated binding of EVs to NETs from murine neutrophils stimulated with PMA or streptococcal M1 protein [39]. By inhibiting EV formation with caspase and calpain inhibitors, they demonstrated that EVs bound to NETs contribute substantially to neutrophil attraction and prothrombotic activity of NETs [39,40]. It is interesting to note that, at least under static conditions, heterologous EV-NET complexes can also be formed. 4T1 breast cancer cell-derived EVs can bind to NETs and appear to synergistically augment the pro-coagulative and prothrombotic capacity of NETs by delivering tissue factor (TF) [41,42,43,44].
Table 1.
Major substances shared by NETs and EVs/ENDs upon stimulation with various priming or activating agents.
| Neutrophil Stimulus Used in Study | Substance Detected with Both NETs and EVs/ENDs | References | |
|---|---|---|---|
| EVs/ENDs | NETs | ||
| Granule proteins/peptides | |||
| A. fumigatus conidia | PMA | Azurocidin | [14,45,46,47] |
| A. fumigatus conidia | A23187 | Cathelicidin antimicrobial peptide | [14,47] |
| fMLP | MSU, PMA, IL-8, LPS | Cathepsin G | [8,33,45,46,48] |
| A. fumigatus conidia | A23187 | Cysteine-rich secretory protein 3 | [14,47] |
| A. fumigatus conidia | TNF-α | Defensins | [47,49] |
| fMLP, Ionomycin | PMA, IL-8, LPS | Neutrophil Elastase | [1,45,46,50,51] |
| fMLP, Ionomycin | MSU, PMA, TNF-α, IL-8, LPS | Lactoferrin | [1,33,45,46,48,49,51] |
| A. fumigatus conidia | A23187 | Lipocalin | [14,47] |
| A. fumigatus conidia | PMA | Lysozyme C | [46,47] |
| fMLP, Ionomycin | A23187 | Matrix metallopeptidases | [14,50,51] |
| fMLP, PMA, Ionomycin | MSU, PMA, TNF-α, IL-8, LPS | Myeloperoxidase | [1,8,33,45,46,48,49,51,52] |
| fMLP | PMA | Proteinase-3 | [45,50] |
| DAMPs | |||
| Not cell type or stimulus-specific | PMA, IL-8, LPS | DNA | [1,53] |
| fMLP | MSU, PMA, TNF-α | Histones | [14,33,45,46,48,49] |
| PMA | PMA | HMGB-1 | [40,54] |
| fMLP | PMA, IL-8, amyloid fibrils, Leishmania promastigotes | microRNA | [55,56] |
| fMLP | PMA, TNF-α, | S100 family proteins | [31,33,45,46,49] |
| PMA | |||
| Cytoskeleton proteins | |||
| fMLP | MSU, TNF-α | Myosin-9 | [33,46,48,49] |
| fMLP | MSU, PMA | Actins | [33,46,48] |
| fMLP | A23187, MSU, PMA | α-Enolase | [14,31,48] |
| fMLP, pneumolysin | MSU, PMA | Annexins | [14,33,48,57,58] |
| fMLP | Rheumatoid Factor | Catalase | [33,59] |
| fMLP | PMA, TNF-α plus ANCA | Complement components | [33,60,61] |
| fMLP, LPS | A23187 | Cytokines/Chemokines | [14,35,62,63] |
| fMLP | PMA | Gelsolin | [33,46] |
| fMLP | Plasma from stroke patients | Phosphatidylserine | [64,65] |
| Autoimmune vasculitis | Autoimmune vasculitis; deep vein thrombosis; viral infection | Tissue Factor | [66,67,68] |
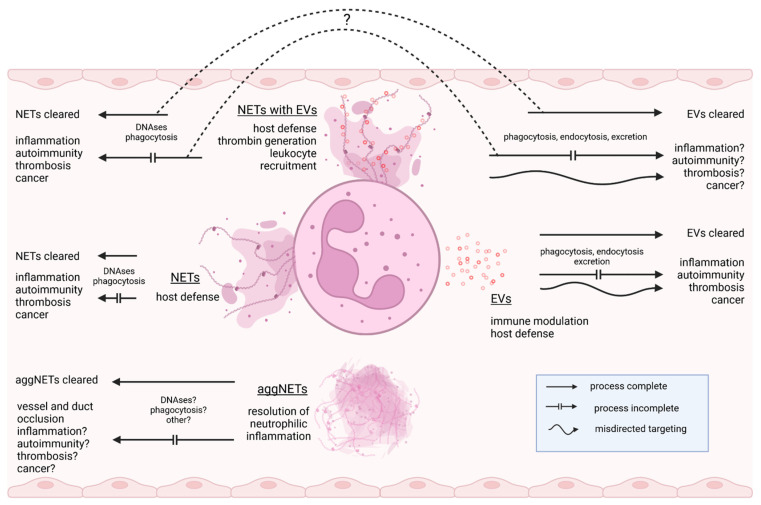
The biological significance of these “EV-NET hybrids” remains elusive. In view of the sparse data available on this subject, it seems expedient to intensify research into their function and clearance pathways. In mice, EVs are cleared from the circulation within a day and then accumulate primarily in the liver [69,70]. The half-life of biotin/luciferase double-labeled EVs in blood has been estimated to be approximately 3 h [71]. In contrast, NETs may persist for at least several days in the vasculature [72]. DNase1 and DNase1-like 3 play a pivotal role in the clearing process by degrading the NET-DNA backbone, followed by phagocytic uptake by macrophages and dendritic cells [73,74]. Consequently, DNase1 and DNase1-like 3 may release NET-associated EVs into the circulation as complexes with DNA fragments, thus affecting their cellular targeting and clearance (Figure 2). It cannot be excluded that the binding of EVs to NETs even influences the NET architecture. Detailed analysis by atomic force microscopy revealed that NET proteins are crucial for the structural integrity of NETs [75]. It can be speculated that EVs influence regular NET structure through proteins exposed on their membrane, comparable to NET-proteins crosslinked by polyamines [76]. The remarkable overlap in the protein equipment of NETs and EVs and the resulting potential functional overlaps have not yet been satisfactorily addressed in the scientific literature. Considering the enormous pathogenic potential of NETs and EVs, it is evident how vitally important it is to study these compounds in a more differentiated way at the biochemical and functional levels.
Figure 2.
Dysregulated expression or impaired clearance of EVs (by phago-/endocytosis, excretion) and NETs (by DNase-digestion, phagocytosis) may trigger various pathologies. Binding of EVs to NETs may hypothetically lead to a reciprocal influence on EV and NET clearance (dashed line). Created with BioRender.com.
4. “Impact beyond Shelf Life”: NETs and Neutrophil-Derived EVs in Disease
Molecules common to NETs and EVs include enzymes with pleiotropic functions such as NE, a major granule serine protease that acts as a pro- and anti-inflammatory effector molecule and is involved in the process of NETosis itself. NE drives chromatin de-condensation by proteolytic cleavage of nuclear proteins during NETosis and during an equivalent process in macrophages [38,77,78,79,80]. The proteolytic activity of NE mediates basic bactericidal and immune modulatory effects, but also contributes to the pathology of various diseases, including autoimmunity, cancer, cardiovascular, and pulmonary diseases (Table 1 and Table 2) [81,82]. Dysregulated release or impaired clearance of NETs and EVs may play a key role by triggering etiopathological mechanisms [83]. The delivery of NET proteases, including the three major azurophilic serine proteases: NE, Cathepsin G, and Proteinase-3 (PR-3), by EVs or NETs in the context of chronic inflammatory lung disease is well-documented [84,85]. Excess NE contributes to disease pathogenesis by activating airway remodeling, interfering with elements of the innate immune system, and triggering pro-inflammatory signaling [80]. In the context of COVID-19 infection, NE contributes to bronchial epithelial cell disruption by diminishing mucociliary transport. Additionally, NE and MPO degrade heparan sulfate, an important structural component of lung parenchyma. In this way, NET/EV-associated proteases directly contribute to acute lung injury in SARS-CoV-2 disease [86].
Table 2.
Overlapping and opposing effects of neutrophil-derived extracellular vesicles and NETs in health and disease. NETs and extracellular vesicles trigger a large variety of secondary effector mechanisms that are not entirely included in this table.
| Biological Context | Neutrophil EVs | NETs | References |
|---|---|---|---|
| General | May act either as a pro-inflammatory or anti-inflammatory mediator depending on target cells and activation context | May act either as a pro-inflammatory or anti-inflammatory mediator depending on activation context | [87,88] |
| Complement | Activate complement | Activate complement | [60,89] |
| Erythrocytes | Bind erythrocytes in the presence of complement | Bind erythrocytes | [89,90] |
| Monocytes/Macrophages | May induce a pro- or anti-inflammatory response in monocytes/macrophages depending on stimulus | May induce a pro- or anti-inflammatory response in monocytes/macrophages | [26,72,91,92] |
| Neutrophils | May induce a pro- or anti-inflammatory response in neutrophils depending on stimulus | Pro-inflammatory, and anti-inflammatory in aggregated form | [26,93,94,95,96] |
| Blood platelets | Activate blood platelets via αMβ2-mediated binding | Activate blood platelets by histones | [97,98] |
| Endothelial cells | May induce a pro- or anti-inflammatory response in endothelial cells and may promote or reduce para-endothelial permeability depending on stimulus | Activate endothelial cells by Interleukin-1α and Cathepsin G and promote endothelial permeability | [26,99,100] |
| T-cells | May induce a pro- or anti-inflammatory response in T-cells | May induce a pro- or anti-inflammatory response in T-cells | [35,101,102] |
| Infection | Antibacterial by: -bacteria aggregation on surface -granule proteins |
Antibacterial by: -bacteria entrapment -granule proteins -antimicrobial peptides -histones, DNA |
[33,50,103,104] |
| No direct evidence for antiviral activity | Antiviral by: -virus entrapment -granule proteins -antimicrobial peptides -histones, DNA |
[105] | |
| Antifungal by granule proteins |
Antifungal by calprotectin |
[47,106] | |
| No direct evidence for antiparasitic activity | Antiparasitic by: -entrapment -killing |
[107] | |
| Non-autoimmune cardiovascular disease | Promote thrombosis by exposing tissue factor, platelet activating factor, and possibly phosphatidylserine | Promote thrombosis by exposing von Willebrand factor, histones, tissue factor, and phosphatidylserine | [98,103,108,109,110] |
| Promote atherosclerosis by delivering microRNA (miR-155) | Promote atherosclerosis by macrophage activation possibly via granule enzymes |
[56,111] | |
| Cancer | Anti-tumorigenic by inducing apoptosis of cancer cells or pro-tumorigenic | Pro-tumorigenic, influencing growth, progression, and spreading of cancer by various mechanisms | [112,113,114] |
| No direct evidence for cancer-associated pro- or anti-thrombotic effect | May promote cancer-associated thrombosis | [115] | |
| Autoimmunity | |||
| ANCA-associated vasculitis | Promote thrombosis by exposing tissue factor; contains autoantigen; may trigger vasculitis |
Promote thrombosis by exposing tissue factor; contains autoantigen; may trigger vasculitis |
[66,116,117,118] |
| Psoriasis | May trigger inflammation | May trigger autoimmunity and inflammation by bound pro-inflammatory IL-17 |
[119,120] |
| Systemic lupus erythematosus (SLE) | No evidence for direct involvement in pathogenesis | Contain autoantigen and may contribute to pathogenesis | [121,122,123] |
| Rheumatoid arthritis (RA) | Protective effect on cartilage | Contain autoantigen and may contribute to pathogenesis of RA; damage cartilage by NE | [49,124,125] |
| Pulmonary disease | Contribute to disease pathology | Contribute to disease pathology | [85,126,127] |
4.1. Autoimmune Disease
The pathogenic potential of proteases shared by NETs and EVs is not limited to a role as effector molecules based on catalytic and direct biochemical functions. Like DNA and histones, they can serve as autoantigens in various autoimmune diseases. Antibodies against MPO and PR-3 are a diagnostic hallmark of anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitides (AAVs) [128]. Netting neutrophils surrounding small vessels are thought to contribute to vessel inflammation in AAV in two ways: (1) NETs as a direct actor by damaging endothelial cells and (2) NETs as autoantigens with high immunogenicity that triggers ANCA production. ANCAs in turn can stimulate the release of neutrophil EVs and induce NETosis of primed neutrophils [117,129]. In addition to excessive NET formation, reduced DNAse1 activity and elevated NET levels in the serum of AAV patients indicate that incomplete NET clearance may be involved in disease pathogenesis [129,130]. Neutrophil-derived PR-3- and MPO-expressing EVs may promote small-vessel vasculitis by triggering an inflammatory cascade and directly damaging endothelial cells through transfer of microRNAs (miRNAs) [117,118]. It is unclear to what extent neutrophil-derived EVs may augment inflammation in AAVs by presenting NET autoantigens as in other autoimmune disorders: Patients with systemic lupus erythematosus (SLE) develop autoantibodies to various NET constituents, which together can form immune complexes, causing nephritis. Delayed removal of NETs due to impaired DNAse1-mediated digestion of the DNA backbone likely promotes the presentation of self-antigens, which initiates the process of SLE [121]. EVs are an additional source of autoantigen in SLE [131]. They have been shown to form non-classical EV-containing immune complexes that are related to disease activity [132]. However, a prominent SLE-associated EV population bearing a neutrophil protein signature has not yet been reported [133,134]. Citrullinated vimentin and citrullinated fibrinogen are major autoantigens in rheumatoid arthritis (RA) [135]. Again, NETosis appears to play a pivotal role in the loss of self-tolerance [136]. Equivalent to SLE, pro-inflammatory complexes of autoantigen-presenting EVs and autoantibodies may be involved in pathogenesis [137]. Potentially immunogenic (or tolerogenic) EVs from various cellular origins carrying the respective autoantigen are found in a variety of further autoimmune diseases [138,139,140]. Pauci-immune glomerulonephritis typically associated with AAVs argues against disease-relevant autoantigen-specific EV-antibody complexes in AAV. In contrast, they might be supported by an electron microscopic analysis of renal biopsies from AAV patients, which revealed weak immune complex deposits in the majority of cases examined [141]. It is unclear whether reported cases of ANCA-negative pauci-immune glomerulonephritis in RA and SLE are associated with EV-antibody complexes or whether they are etiologically different forms of SLE [142,143,144].
4.2. Cancer
Distinct functional plasticity of neutrophils, which is evident in the immunological context, is also reflected in their multifaceted effects on carcinogenesis and the spread of cancer [145]. In the tumor microenvironment, transforming growth factor-β (TGF-β), interferon-β (IFN-β), and granulocyte-colony-stimulating factor (G-CSF) polarize neutrophils towards a pro- or anti-tumorigenic phenotype. Polarized neutrophils modulate tumor growth, progression, and metastasis through a variety of mechanisms. The basis for neutrophil diversity is already laid during neutrophil development in the bone marrow, where neutrophil progenitor cells arise from a presumably heterogeneous pool of granulocyte-monocyte progenitor cells [113]. Granulopoesis and release from the bone marrow can be affected by tumors and tumor-associated cells that release cytokines and chemokines. Consequently, neutrophils of different developmental stages can be found in the circulation [146]. It has been reported that immature circulating neutrophils have a higher global bioenergetic capacity, which facilitates sustained NETosis [147]. NETs are known to play a vital role in fostering pro-tumorigenic neutrophil activity. They can promote tumor growth by either directly inducing cancer proliferation or by shielding tumors from cytotoxic immune cells [148,149,150]. In addition to the inflammatory triggers discussed in the previous sections, NETosis can also be induced by factors released from tumor cells. G-CSF released from 4T1 cells has been found to induce NET formation, resulting in cancer cell invasion in vitro [151]. Furthermore, Cathepsin C released from breast cancer cells can induce NETosis by activating a zymogenic form of NE on the neutrophil cell membrane. Cathepsin C-induced NETs, in turn, have been shown to degrade the metastasis-suppressing protein thrombospondin-1 (TSP-1), promoting metastasis [152]. Neutrophil-triggered degradation of TSP-1 was also shown to be a pro-metastatic mechanism in a mouse model in which tobacco smoke-induced neutrophil NETs awakened dormant breast cancer cells. In this experimental setting, NET-associated NE and matrix metalloproteinase-9 sequentially cleaved laminin, and the processed laminin induced integrin-mediated proliferation of dormant cancer cells [153]. Interestingly, the DNA-scaffold provided by NETs appears to support the proteolytic laminin cleavage by binding to laminin. NET-DNA can therefore be considered a “carrier” for proteolytic neutrophil enzymes. However, DNA itself has been shown to possess pro-metastatic potential as well: NET-DNA exerts chemotactic activity towards cancer cells and is able to capture them from the circulation. Stimulation of the DNA-binding receptor CCDC25 on cancer cells by NET-DNA activates the integrin-linked kinase β-parvin pathway, resulting in increased cell motility [154,155].
While NETs are primarily attributed with pro-tumorigenic activity, EVs originating from anti-tumorigenic neutrophils (termed N1 neutrophils) and pro-tumorigenic neutrophils (termed N2 neutrophils) are reflecting the current cell status [26,146,156]. The anti-tumor activity of EVs is mediated by the release of pro-inflammatory cytokines (IL-1β, IL-2, IL-4), molecules that modulate cell motility and adhesion, such as integrins, granule enzymes, and others [35,63,126,157]. Granule proteins shared by NETs and neutrophil-derived EVs are also an important component of the neutrophil’s pro-carcinogenic arsenal. NE can induce cancer proliferation, migration, and invasion. Although not mandatory, the presence of NE detected in an endosomal compartment in lung tumor cells is consistent with the assumption that NE could be transmitted by EVs [158,159]. An imbalance of the inhibitor and protease apparently favors cancer cell development, growth, and migration [160,161]. NET-DNA might be a good candidate in this context as a participant in the process of dysregulation of serine protease activity: DNA can reversibly inhibit serine protease activity, protecting it from irreversible inhibition by serpins [38,78,162]. Ultimately, irregular release or impaired clearance of NETs and EVs may have an influence on the fine-tuned protease–anti-protease balance.
Another common major granule enzyme, MPO, was discovered in the circulation of ANCA-associated vasculitis patients as a complex with DNA, presumably derived from NETs [116]. Like NE, MPO also participates in the process of NETosis by facilitating chromatin de-condensation [163]. In this way, MPO is likely to exert an indirect pro-tumorigenic effect. However, MPO itself functions as a modulator of cancer development in multiple steps as well [164]. The role of MPO and NETs in autoimmunity and their involvement in cancer development leads to the hypothesis that there may be a mechanistic link between ANCA and cancer: NETosis-inducing ANCA could initiate a self-perpetuating or reinforcing cycle of NET formation, which in turn triggers autoantibody production and furthermore promotes tumorigenesis [165].
4.3. Thrombosis
Remarkably, patients with ANCA-associated autoimmune disease and tumor patients share an increased risk of thrombotic events [115,166]. AAV increases the risk of arterial thrombotic events (ATE) and venous thromboembolism (VTE, including deep vein thrombosis and pulmonary embolism) by at least 2–3-fold [166,167]. Cancer increases the risk of VTE 4- to 7-fold and of ATE approximately 2-fold in the short term [168,169,170]. Although ATE and VTE are classified into two etiologically separate groups, common risk factors for both are well-documented [171,172]. TF has been identified as an important mediator of ANCA-associated thrombosis, which is released packaged in EVs and in NET-bound form by ANCA-activated neutrophils [166]. Vascular injury caused by ANCA-activated neutrophils may lead to additional exposure of TF from adventitial cells that surround blood vessels. By forming a complex with FVII/FVIIa, TF can finally aberrantly trigger blood coagulation, leading to an increased risk of thrombosis [172]. While NET formation appears to be associated with disease activity and thrombosis, TF+ EVs increase the risk of VTE regardless of disease activity [173,174]. Autoantibodies not only contribute to excessive NETosis but may also interfere with appropriate NET elimination. Patients with antiphospholipid syndrome (APS), a disorder associated with elevated titers of antiphospholipid and NET autoantibodies, are at increased risk of arterial, venous, and microvascular thrombosis [175]. Antiphospholipid antibodies can activate neutrophils and induce the release of NETs, accelerating thrombosis [176,177]. Anti-NET antibodies may further increase the risk of thrombotic events in APS by binding to NETs, thereby interfering with their proper degradation and clearance [178,179]. In addition to anti-NET antibodies, DNase inhibition may potentially interfere with NET clearance in autoimmune diseases, as suggested by Hakkim et al. for SLE [121].
Cancer cells can predispose patients to thrombosis in at least two ways: (1) Tumor cells directly secrete prothrombotic factors that can initiate blood coagulation by creating a scaffold for blood clotting factors on EVs (through PS), by activating the extrinsic pathway of coagulation (by TF), and by modulating platelet activity (by podoplanin, Adenosine diphosphate (ADP), and thrombin). In addition, they are able to inhibit fibrinolysis by plasminogen activation inhibitor-1 (PAI-1) [115]. (2) Tumor cells can stimulate neutrophils to release NETs [43,180].
NETs can also be directly triggered by specific viral pathogens such as SARS-CoV-2 [181]. NET-induced thrombosis appears to be a key feature of COVID-19 and has been discussed as a major cause of mortality [86].
The biologic function of the prothrombotic effect of NETs can be interpreted in terms of their role as an element of intravascular innate immunity. In the concept of immunothrombosis, the formation of microvascular thrombi helps fight infection and prevent pathogen dissemination and tissue invasion [98]. Neutrophils, and NETs in particular, play a central role in immunothrombosis, involving structural NET components but also proteins found both on NETs and in neutrophil-derived vesicles. NETs can trigger the extrinsic pathway through NET-bound TF and the intrinsic pathway through activation of FXII by the negative surface charge of the DNA backbone [182]. Both pathways finally trigger the common pathway, which results in the formation of thrombin and fibrin deposits. In addition, platelets are bound and activated by histones mediated by von Willebrand factor (vWF). MPO and serine proteases, including NE, have been shown to indirectly promote blood clotting by inhibiting anticoagulants such as tissue factor pathway inhibitor (TFPI) and thrombomodulin [183].
Injury- and infection-related inflammatory processes that result in thrombus formation are also driven by EVs secreted by neutrophils. Like NETs, they can directly activate the extrinsic and intrinsic pathways of blood coagulation: TF and polyphosphates expressed by EVs mediate the activation of FVII and FXII, respectively [184]. PS, an anionic phospholipid shared by NETs and neutrophil-derived EVs, provides a scaffold for the assembly of coagulation complexes on cell membranes. Electrostatic interactions of PS with positively charged residues of clotting proteins facilitate their binding to the lipid bilayer. In addition, PS+ EVs are able to induce platelet activation and aggregation [185]. The putative relevance of neutrophil-derived PS+ EVs in cardiovascular disease has been demonstrated in non-valvular atrial fibrillation [186]. Thrombosis can additionally be induced by endothelial cell damage caused by the cytotoxic effect of neutrophil EVs carrying MPO [184]. The deleterious effect is probably exerted by enzymatic catalysis of hypochlorous acid (HOCl) generation on the surface of EVs [187]. Likewise, the enzymatic activity of MPO has been demonstrated to contribute to the cytotoxic effects of NETs [188].
5. NET- and Neutrophil EV-Based Diagnostics
The important role of NETs and EVs in inflammation and the pathophysiology of autoimmunity, cancer, and thrombosis suggests their use in the development of new diagnostic methods (Table 3). In recent years, procedural approaches to diagnostics based on circulating neutrophil EVs have significantly improved. Initial achievements in the identification of prognostically and diagnostically potentially useful EVs have been published for cardiovascular and pulmonary diseases, among others [189,190,191,192]. However, neutrophil EV-based diagnostics is still in its fledgling stage. Knowledge of molecular EV signatures, including conditional ones that correlate with a biological and pathophysiological context, is sparse. However, it is desirable to exploit the information provided by EVs on the status and environmental cues to which the releasing neutrophils have been exposed. In addition, technical problems such as the limited availability of antibodies and staining techniques with sufficient sensitivity and specificity for newly discovered EV markers compatible with multiplex technologies hamper the development of clinically applicable diagnostics. The first steps towards establishing such methods for detection and characterization have already been taken, e.g., by identifying suitable marker combinations using flow cytometry [193].
Table 3.
Examples of the potential prognostic and diagnostic use of neutrophil-derived EVs and NETs.
| Disease Setting |
Study Material | Analyte: NET or Neutrophil EV (Used Markers) | Method | Significance | Refs. |
|---|---|---|---|---|---|
| Infection | |||||
| Sepsis | Blood | EV (CD15) | Microbead-based isolation + NTA | Level disease-associated + prognostic potential | [194] |
| Sepsis | Blood | NET formation ex vivo (DNA) | Stimulation of heterologous neutrophils by patient plasma + immunofluorescence microscopic quantification of released DNA | Level disease-associated + prognostic potential | [195] |
| COVID-19 | Blood | EV (PS *, CD15, CD66b) | FC | Level and TF activity associated with thrombotic risk | [196,197] |
| COVID-19 | Blood | NET (MPO-DNA, citrullinated histone, histone H3, cfDNA, NE) | ELISA | Level disease-associated + prognostic potential | [198,199] |
| Cardiovascular | |||||
| Infective endocarditis |
Blood | EV (PS *, CD66b) | FC | Level for differential diagnosis and risk assessment | [189] |
| Infective endocarditis |
Blood | NET (MPO-DNA) | ELISA | Level disease-associated | [200] |
| Unstable plaque in carotid stenosis | Blood | EV (CD11b, CD66b) | FC | Level related to unstable plaque | [190] |
| Familial hypercholesterolemia | Blood | EV (PS *, CD11b, CD66b) | FC | Combined with EVs from different origins: level correlates with coronary calcification and atherosclerotic plaque | [201] |
| Coronary artery disease |
Blood | NET (dsDNA, nucleosomes, MPO-DNA) | DNA-dye, ELISA | Level correlates with coronary calcification and atherosclerotic plaque | [202] |
| Lung | |||||
| COPD | BALF | EV (CD11b, CD66b) | FC | Level disease-associated | [192] |
| COPD | Sputum | NET (MPO-DNA, Elastase-DNA, Histone-elastase) | ELISA | Level disease-associated | [203] |
| ARDS | BALF | EV (CD11b, CD66b) | FC | Level disease-associated | [204] |
| ARDS | BALF, blood | NET (MPO-DNA) | ELISA | Level disease-associated | [205] |
| Cancer | |||||
| Non-small cell lung cancer | Blood | EV (PS *, CD66b) | FC | Level associated with disease progression | [206] |
| Various cancers including lung cancer | Blood | NET (citrullinated histone) | ELISA | Level disease-associated + prognostic potential | [207] |
* PS detected by Annexin V-staining.
Markers of NETosis, on the other hand, may be detected in various, already better-established ways. The concept of liquid biopsy is based on current molecular biology methods such as droplet-based digital PCR and next-generation sequencing technologies. It is used to characterize cell-free DNA (cfDNA) released into the blood and other body fluids by various cells during apoptosis, necrosis, EV-shedding, and as a result of NETosis [208]. Due to the different cellular origins and different cellular processes, it is not feasible to use the release kinetics or levels of cfDNA as NET-related markers. Rather, NET-specific modifications such as citrullinated histones or NET-associated proteins such as MPO can be used as additional markers. Unfortunately, currently, no commercial kit reliably passes the technical difficulties of a clinically validated detection method of DNA-NET-protein complexes [209]. Recently published data even suggest that the widely performed measurement of MPO-DNA complexes by ELISA for the differential determination of NETs is itself questionable, especially in human plasma [210]. The technical challenges of producing reliable measurement results and the lack of standardized methods allowing cross-study analyses also impede the correlation of NET-related data with pathological parameters. Complicating matters further is the conditionally opposing function of NETs, which can act as both pro-inflammatory and anti-inflammatory mediators. Indeed, there are conflicting data, for example, on whether NET levels or NET-degrading activity are correlated with disease activity in autoimmune diseases [130,174,211]. A similar situation is present in cancer diagnostics: although there is some proof of the diagnostic and prognostic relevance of circulating NETs, there is still a lack of solid evidence supported by an adequate amount of clinical data [212].
A combination of NET markers that may be tailored to the experimental or clinical objective could probably improve the quality of study results. Citrullinated histones appear to be a good option as they are directly involved in NETosis. Citrullinated histone H3 in combination with DNA and other NETosis-related markers has been shown to be a suitable analyte for prognosis in cancer, sepsis, or cardiovascular conditions [213,214]. In this context, the formulation and continuous refinement of a guideline based on consensus, comparable to the MISEV guidelines for EVs, would be a valuable aid for the further development of NET-based diagnostics [215].
6. Conclusions
The high concordance of protein equipment of NETs and EVs can be explained by their common cellular origin. Accordingly, a good scientific study design and future EV- and NET-based diagnostics should be characterized by a careful selection of NET and EV markers, especially if biological effects of NETs and EVs are to be distinguished. For example, tetraspannins characteristic of EVs (CD9, CD63, CD81) may be included as negative NET markers. To date, there is little data on the extent to which previous contact of EVs with NETs affects their protein equipment and which can be identified by standard immunological detection methods. It is possible that EVs, especially under pathological conditions, carry NET-DNA fragments to which NET-DNA-associated protein is bound. The principal ability of DNA to bind onto the EV surface has been demonstrated with cell lines from T-cell leukemia and pancreatic cancer [216]. As shown by Leal et al., even heterologous EV-NET complexes can form, at least under static conditions [44]. This is both a challenge and an opportunity for future research. The technical methods to distinguish DNA packaged during EV generation and passively released DNA (NETs, apoptotic bodies) are not yet sufficiently established [217]. The challenge is compounded by the large stimulus- and biological context-dependent variability of NETs and EVs. However, suitable techniques could turn (heterologous) EV-DNA complexes into a proper complement to NET- and EV-based diagnostics, especially in the fields of autoimmunity and cancer. Considering the outstanding role of phagocytes in modulating cancer development and autoimmunity, remnants of the contact between neutrophil EVs/NETs and cancer cells as well as other immune cells could help identify pathologic mechanisms and differentiate stages of disease progression, and substantially support therapeutic measures.
Acknowledgments
The author would like to thank S. Holdenrieder for his review and thoughtful comments on the manuscript.
Abbreviations
| α1-AT | α1-Antitrypsin |
| ADP | Adenosine Diphosphate |
| ANCA | Anti-Neutrophil Cytoplasmic Antibody |
| APS | Antiphospholipid Syndrome |
| ARDS | Acute Respiratory Distress Syndrome |
| ATE | Arterial Thrombotic Events |
| AVV | ANCA-associated Vasculitis |
| BALF | Bronchoalveolar Fluid |
| cfDNA | Cell-free DNA |
| COPD | Chronic Obstructive Pulmonary Disease |
| DAMP | Damage-related Molecular Pattern |
| ENDS | Elongated Neutrophil-derived Structures |
| EV | Extracellular Vesicle |
| FC | Flow Cytometry |
| fMLP | N-Formyl-Methionyl-Leucyl-Phenylalanine |
| G-CSF | Granulocyte-Colony-Stimulating Factor |
| GM-CSF | Granulocyte/Macrophage-Colony-Stimulating Factor |
| HOCl | Hypochlorous Acid |
| IL-x | Interleukin-x |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-Activated Protein Kinase |
| MPO | Myeloperoxidase |
| miRNA | MicroRNA |
| mtDNA | Mitochondrial DNA |
| MVE | Multivesicular Endosome |
| nDNA | Nuclear DNA |
| NE | Neutrophil Elastase |
| NET | Neutrophil Extracellular Trap |
| NOD | Nucleotide-binding Oligomerization Domain |
| NOX | NADPH-Oxidase |
| NTA | Nanoparticle Tracking Analysis |
| PAD-4 | Peptidyl-Arginine-Deiminase 4 |
| PAI-1 | Plasminogen Activation Inhibitor-1 |
| PAMP | Pathogen-related Molecular Pattern |
| PMA | Phorbol-12-Myristate-13-Acetate |
| PR-3 | Proteinase-3 |
| PRR | Pattern Recognition Receptor |
| PS | Phosphatidylserine |
| RA | Rheumatoid Arthritis |
| ROS | Reactive Oxygen Species |
| SLE | Systemic Lupus Erythematosus |
| TF | Tissue Factor |
| TFPI | Tissue Factor Pathway Inhibitor |
| TGF-β | Transforming Growth Factor-β |
| TLR | Toll-like Receptor |
| TSP-1 | Thrombospondin-1 |
| VTE | Venous Thromboembolism |
| vWF | von Willebrand Factor |
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D.S., Weinrauch Y., Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 2.Yousefi S., Gold J.A., Andina N., Lee J.J., Kelly A.M., Kozlowski E., Schmid I., Straumann A., Reichenbach J., Gleich G.J., et al. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat. Med. 2008;14:949–953. doi: 10.1038/nm.1855. [DOI] [PubMed] [Google Scholar]
- 3.von Kockritz-Blickwede M., Goldmann O., Thulin P., Heinemann K., Norrby-Teglund A., Rohde M., Medina E. Phagocytosis-independent antimicrobial activity of mast cells by means of extracellular trap formation. Blood. 2008;111:3070–3080. doi: 10.1182/blood-2007-07-104018. [DOI] [PubMed] [Google Scholar]
- 4.Wong K.W., Jacobs W.R., Jr. Mycobacterium tuberculosis exploits human interferon gamma to stimulate macrophage extracellular trap formation and necrosis. J. Infect. Dis. 2013;208:109–119. doi: 10.1093/infdis/jit097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takeuchi O., Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 6.Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018;18:134–147. doi: 10.1038/nri.2017.105. [DOI] [PubMed] [Google Scholar]
- 7.Neubert E., Meyer D., Rocca F., Gunay G., Kwaczala-Tessmann A., Grandke J., Senger-Sander S., Geisler C., Egner A., Schon M.P., et al. Chromatin swelling drives neutrophil extracellular trap release. Nat. Commun. 2018;9:3767. doi: 10.1038/s41467-018-06263-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuchs T.A., Abed U., Goosmann C., Hurwitz R., Schulze I., Wahn V., Weinrauch Y., Brinkmann V., Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pilsczek F.H., Salina D., Poon K.K., Fahey C., Yipp B.G., Sibley C.D., Robbins S.M., Green F.H., Surette M.G., Sugai M., et al. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J. Immunol. 2010;185:7413–7425. doi: 10.4049/jimmunol.1000675. [DOI] [PubMed] [Google Scholar]
- 10.Yipp B.G., Petri B., Salina D., Jenne C.N., Scott B.N., Zbytnuik L.D., Pittman K., Asaduzzaman M., Wu K., Meijndert H.C., et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat. Med. 2012;18:1386–1393. doi: 10.1038/nm.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yousefi S., Mihalache C., Kozlowski E., Schmid I., Simon H.U. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 2009;16:1438–1444. doi: 10.1038/cdd.2009.96. [DOI] [PubMed] [Google Scholar]
- 12.Boeltz S., Amini P., Anders H.J., Andrade F., Bilyy R., Chatfield S., Cichon I., Clancy D.M., Desai J., Dumych T., et al. To NET or not to NET: Current opinions and state of the science regarding the formation of neutrophil extracellular traps. Cell Death Differ. 2019;26:395–408. doi: 10.1038/s41418-018-0261-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petretto A., Bruschi M., Pratesi F., Croia C., Candiano G., Ghiggeri G., Migliorini P. Neutrophil extracellular traps (NET) induced by different stimuli: A comparative proteomic analysis. PLoS ONE. 2019;14:e0218946. doi: 10.1371/journal.pone.0218946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chapman E.A., Lyon M., Simpson D., Mason D., Beynon R.J., Moots R.J., Wright H.L. Caught in a Trap? Proteomic Analysis of Neutrophil Extracellular Traps in Rheumatoid Arthritis and Systemic Lupus Erythematosus. Front. Immunol. 2019;10:423. doi: 10.3389/fimmu.2019.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffmann J.H., Schaekel K., Gaiser M.R., Enk A.H., Hadaschik E.N. Interindividual variation of NETosis in healthy donors: Introduction and application of a refined method for extracellular trap quantification. Exp. Dermatol. 2016;25:895–900. doi: 10.1111/exd.13125. [DOI] [PubMed] [Google Scholar]
- 16.Deniset J.F., Kubes P. Neutrophil heterogeneity: Bona fide subsets or polarization states? J. Leukoc. Biol. 2018;103:829–838. doi: 10.1002/JLB.3RI0917-361R. [DOI] [PubMed] [Google Scholar]
- 17.Pieterse E., Rother N., Yanginlar C., Gerretsen J., Boeltz S., Munoz L.E., Herrmann M., Pickkers P., Hilbrands L.B., van der Vlag J. Cleaved N-terminal histone tails distinguish between NADPH oxidase (NOX)-dependent and NOX-independent pathways of neutrophil extracellular trap formation. Ann. Rheum. Dis. 2018;77:1790–1798. doi: 10.1136/annrheumdis-2018-213223. [DOI] [PubMed] [Google Scholar]
- 18.Lood C., Blanco L.P., Purmalek M.M., Carmona-Rivera C., De Ravin S.S., Smith C.K., Malech H.L., Ledbetter J.A., Elkon K.B., Kaplan M.J. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat. Med. 2016;22:146–153. doi: 10.1038/nm.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoshino A., Costa-Silva B., Shen T.L., Rodrigues G., Hashimoto A., Tesic Mark M., Molina H., Kohsaka S., Di Giannatale A., Ceder S., et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gill S., Catchpole R., Forterre P. Extracellular membrane vesicles in the three domains of life and beyond. FEMS Microbiol. Rev. 2019;43:273–303. doi: 10.1093/femsre/fuy042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lo Cicero A., Stahl P.D., Raposo G. Extracellular vesicles shuffling intercellular messages: For good or for bad. Curr. Opin. Cell Biol. 2015;35:69–77. doi: 10.1016/j.ceb.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 22.Morel O., Jesel L., Freyssinet J.M., Toti F. Cellular mechanisms underlying the formation of circulating microparticles. Arterioscler. Thromb. Vasc. Biol. 2011;31:15–26. doi: 10.1161/ATVBAHA.109.200956. [DOI] [PubMed] [Google Scholar]
- 23.Kalra H., Drummen G.P., Mathivanan S. Focus on Extracellular Vesicles: Introducing the Next Small Big Thing. Int. J. Mol. Sci. 2016;17:170. doi: 10.3390/ijms17020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huda M.N., Nafiujjaman M., Deaguero I.G., Okonkwo J., Hill M.L., Kim T., Nurunnabi M. Potential Use of Exosomes as Diagnostic Biomarkers and in Targeted Drug Delivery: Progress in Clinical and Preclinical Applications. ACS Biomater. Sci. Eng. 2021;7:2106–2149. doi: 10.1021/acsbiomaterials.1c00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amulic B., Cazalet C., Hayes G.L., Metzler K.D., Zychlinsky A. Neutrophil function: From mechanisms to disease. Annu. Rev. Immunol. 2012;30:459–489. doi: 10.1146/annurev-immunol-020711-074942. [DOI] [PubMed] [Google Scholar]
- 26.Kolonics F., Szeifert V., Timar C.I., Ligeti E., Lorincz A.M. The Functional Heterogeneity of Neutrophil-Derived Extracellular Vesicles Reflects the Status of the Parent Cell. Cells. 2020;9:2718. doi: 10.3390/cells9122718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mol S., Hafkamp F.M.J., Varela L., Simkhada N., Taanman-Kueter E.W., Tas S.W., Wauben M.H.M., Groot Kormelink T., de Jong E.C. Efficient Neutrophil Activation Requires Two Simultaneous Activating Stimuli. Int. J. Mol. Sci. 2021;22:106. doi: 10.3390/ijms221810106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lorincz A.M., Bartos B., Szombath D., Szeifert V., Timar C.I., Turiak L., Drahos L., Kittel A., Veres D.S., Kolonics F., et al. Role of Mac-1 integrin in generation of extracellular vesicles with antibacterial capacity from neutrophilic granulocytes. J. Extracell. Vesicles. 2020;9:1698889. doi: 10.1080/20013078.2019.1698889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kolonics F., Kajdacsi E., Farkas V.J., Veres D.S., Khamari D., Kittel A., Merchant M.L., McLeish K.R., Lorincz A.M., Ligeti E. Neutrophils produce proinflammatory or anti-inflammatory extracellular vesicles depending on the environmental conditions. J. Leukoc. Biol. 2021;109:793–806. doi: 10.1002/JLB.3A0320-210R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kormelink T.G., Mol S., de Jong E.C., Wauben M.H.M. The role of extracellular vesicles when innate meets adaptive. Semin. Immunopathol. 2018;40:439–452. doi: 10.1007/s00281-018-0681-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marki A., Buscher K., Lorenzini C., Meyer M., Saigusa R., Fan Z., Yeh Y.T., Hartmann N., Dan J.M., Kiosses W.B., et al. Elongated neutrophil-derived structures are blood-borne microparticles formed by rolling neutrophils during sepsis. J. Exp. Med. 2021;218:e20200551. doi: 10.1084/jem.20200551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zarbock A., Ley K. Neutrophil adhesion and activation under flow. Microcirculation. 2009;16:31–42. doi: 10.1080/10739680802350104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dalli J., Montero-Melendez T., Norling L.V., Yin X., Hinds C., Haskard D., Mayr M., Perretti M. Heterogeneity in neutrophil microparticles reveals distinct proteome and functional properties. Mol. Cell Proteom. 2013;12:2205–2219. doi: 10.1074/mcp.M113.028589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lorincz A.M., Schutte M., Timar C.I., Veres D.S., Kittel A., McLeish K.R., Merchant M.L., Ligeti E. Functionally and morphologically distinct populations of extracellular vesicles produced by human neutrophilic granulocytes. J. Leukoc. Biol. 2015;98:583–589. doi: 10.1189/jlb.3VMA1014-514R. [DOI] [PubMed] [Google Scholar]
- 35.Lim K., Hyun Y.M., Lambert-Emo K., Capece T., Bae S., Miller R., Topham D.J., Kim M. Neutrophil trails guide influenza-specific CD8(+) T cells in the airways. Science. 2015;349:aaa4352. doi: 10.1126/science.aaa4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alvarez-Jimenez V.D., Leyva-Paredes K., Garcia-Martinez M., Vazquez-Flores L., Garcia-Paredes V.G., Campillo-Navarro M., Romo-Cruz I., Rosales-Garcia V.H., Castaneda-Casimiro J., Gonzalez-Pozos S., et al. Extracellular Vesicles Released from Mycobacterium tuberculosis-Infected Neutrophils Promote Macrophage Autophagy and Decrease Intracellular Mycobacterial Survival. Front. Immunol. 2018;9:272. doi: 10.3389/fimmu.2018.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Castanheira F.V.S., Kubes P. Neutrophils and NETs in modulating acute and chronic inflammation. Blood. 2019;133:2178–2185. doi: 10.1182/blood-2018-11-844530. [DOI] [PubMed] [Google Scholar]
- 38.Hahn J., Schauer C., Czegley C., Kling L., Petru L., Schmid B., Weidner D., Reinwald C., Biermann M.H.C., Blunder S., et al. Aggregated neutrophil extracellular traps resolve inflammation by proteolysis of cytokines and chemokines and protection from antiproteases. FASEB J. 2019;33:1401–1414. doi: 10.1096/fj.201800752R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y., Luo L., Braun O.O., Westman J., Madhi R., Herwald H., Morgelin M., Thorlacius H. Neutrophil extracellular trap-microparticle complexes enhance thrombin generation via the intrinsic pathway of coagulation in mice. Sci. Rep. 2018;8:4020. doi: 10.1038/s41598-018-22156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y., Du F., Hawez A., Morgelin M., Thorlacius H. Neutrophil extracellular trap-microparticle complexes trigger neutrophil recruitment via high-mobility group protein 1 (HMGB1)-toll-like receptors(TLR2)/TLR4 signalling. Br. J. Pharmacol. 2019;176:3350–3363. doi: 10.1111/bph.14765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brill A., Fuchs T.A., Savchenko A.S., Thomas G.M., Martinod K., De Meyer S.F., Bhandari A.A., Wagner D.D. Neutrophil extracellular traps promote deep vein thrombosis in mice. J. Thromb. Haemost. 2012;10:136–144. doi: 10.1111/j.1538-7836.2011.04544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Massberg S., Grahl L., von Bruehl M.L., Manukyan D., Pfeiler S., Goosmann C., Brinkmann V., Lorenz M., Bidzhekov K., Khandagale A.B., et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat. Med. 2010;16:887–896. doi: 10.1038/nm.2184. [DOI] [PubMed] [Google Scholar]
- 43.Demers M., Krause D.S., Schatzberg D., Martinod K., Voorhees J.R., Fuchs T.A., Scadden D.T., Wagner D.D. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc. Natl. Acad. Sci. USA. 2012;109:13076–13081. doi: 10.1073/pnas.1200419109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leal A.C., Mizurini D.M., Gomes T., Rochael N.C., Saraiva E.M., Dias M.S., Werneck C.C., Sielski M.S., Vicente C.P., Monteiro R.Q. Tumor-Derived Exosomes Induce the Formation of Neutrophil Extracellular Traps: Implications for the Establishment of Cancer-Associated Thrombosis. Sci. Rep. 2017;7:6438. doi: 10.1038/s41598-017-06893-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Urban C.F., Ermert D., Schmid M., Abu-Abed U., Goosmann C., Nacken W., Brinkmann V., Jungblut P.R., Zychlinsky A. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009;5:e1000639. doi: 10.1371/journal.ppat.1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O’Donoghue A.J., Jin Y., Knudsen G.M., Perera N.C., Jenne D.E., Murphy J.E., Craik C.S., Hermiston T.W. Global substrate profiling of proteases in human neutrophil extracellular traps reveals consensus motif predominantly contributed by elastase. PLoS ONE. 2013;8:e75141. doi: 10.1371/journal.pone.0075141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shopova I.A., Belyaev I., Dasari P., Jahreis S., Stroe M.C., Cseresnyes Z., Zimmermann A.K., Medyukhina A., Svensson C.M., Kruger T., et al. Human Neutrophils Produce Antifungal Extracellular Vesicles against Aspergillus fumigatus. mBio. 2020;11:e00596-20. doi: 10.1128/mBio.00596-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chatfield S.M., Grebe K., Whitehead L.W., Rogers K.L., Nebl T., Murphy J.M., Wicks I.P. Monosodium Urate Crystals Generate Nuclease-Resistant Neutrophil Extracellular Traps via a Distinct Molecular Pathway. J. Immunol. 2018;200:1802–1816. doi: 10.4049/jimmunol.1701382. [DOI] [PubMed] [Google Scholar]
- 49.Khandpur R., Carmona-Rivera C., Vivekanandan-Giri A., Gizinski A., Yalavarthi S., Knight J.S., Friday S., Li S., Patel R.M., Subramanian V., et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci. Transl. Med. 2013;5:178ra140. doi: 10.1126/scitranslmed.3005580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gasser O., Hess C., Miot S., Deon C., Sanchez J.C., Schifferli J.A. Characterisation and properties of ectosomes released by human polymorphonuclear neutrophils. Exp. Cell Res. 2003;285:243–257. doi: 10.1016/S0014-4827(03)00055-7. [DOI] [PubMed] [Google Scholar]
- 51.Thiam H.R., Wong S.L., Qiu R., Kittisopikul M., Vahabikashi A., Goldman A.E., Goldman R.D., Wagner D.D., Waterman C.M. NETosis proceeds by cytoskeleton and endomembrane disassembly and PAD4-mediated chromatin decondensation and nuclear envelope rupture. Proc. Natl. Acad. Sci. USA. 2020;117:7326–7337. doi: 10.1073/pnas.1909546117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Slater T.W., Finkielsztein A., Mascarenhas L.A., Mehl L.C., Butin-Israeli V., Sumagin R. Neutrophil Microparticles Deliver Active Myeloperoxidase to Injured Mucosa To Inhibit Epithelial Wound Healing. J. Immunol. 2017;198:2886–2897. doi: 10.4049/jimmunol.1601810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elzanowska J., Semira C., Costa-Silva B. DNA in extracellular vesicles: Biological and clinical aspects. Mol. Oncol. 2021;15:1701–1714. doi: 10.1002/1878-0261.12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tohme S., Yazdani H.O., Al-Khafaji A.B., Chidi A.P., Loughran P., Mowen K., Wang Y., Simmons R.L., Huang H., Tsung A. Neutrophil Extracellular Traps Promote the Development and Progression of Liver Metastases after Surgical Stress. Cancer Res. 2016;76:1367–1380. doi: 10.1158/0008-5472.CAN-15-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Linhares-Lacerda L., Temerozo J.R., Ribeiro-Alves M., Azevedo E.P., Mojoli A., Nascimento M.T.C., Silva-Oliveira G., Savino W., Foguel D., Bou-Habib D.C., et al. Neutrophil extracellular trap-enriched supernatants carry microRNAs able to modulate TNF-alpha production by macrophages. Sci. Rep. 2020;10:2715. doi: 10.1038/s41598-020-59486-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gomez I., Ward B., Souilhol C., Recarti C., Ariaans M., Johnston J., Burnett A., Mahmoud M., Luong L.A., West L., et al. Neutrophil microvesicles drive atherosclerosis by delivering miR-155 to atheroprone endothelium. Nat. Commun. 2020;11:214. doi: 10.1038/s41467-019-14043-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dalli J., Norling L.V., Renshaw D., Cooper D., Leung K.Y., Perretti M. Annexin 1 mediates the rapid anti-inflammatory effects of neutrophil-derived microparticles. Blood. 2008;112:2512–2519. doi: 10.1182/blood-2008-02-140533. [DOI] [PubMed] [Google Scholar]
- 58.Letsiou E., Teixeira Alves L.G., Felten M., Mitchell T.J., Muller-Redetzky H.C., Dudek S.M., Witzenrath M. Neutrophil-Derived Extracellular Vesicles Activate Platelets after Pneumolysin Exposure. Cells. 2021;10:3581. doi: 10.3390/cells10123581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carmona-Rivera C., Carlucci P.M., Moore E., Lingampalli N., Uchtenhagen H., James E., Liu Y., Bicker K.L., Wahamaa H., Hoffmann V., et al. Synovial fibroblast-neutrophil interactions promote pathogenic adaptive immunity in rheumatoid arthritis. Sci. Immunol. 2017;2:eaag3358. doi: 10.1126/sciimmunol.aag3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yuen J., Pluthero F.G., Douda D.N., Riedl M., Cherry A., Ulanova M., Kahr W.H., Palaniyar N., Licht C. NETosing Neutrophils Activate Complement both on Their Own NETs and Bacteria via Alternative and Non-alternative Pathways. Front. Immunol. 2016;7:137. doi: 10.3389/fimmu.2016.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang H., Wang C., Zhao M.H., Chen M. Neutrophil extracellular traps can activate alternative complement pathways. Clin. Exp. Immunol. 2015;181:518–527. doi: 10.1111/cei.12654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ye C., Li H., Bao M., Zhuo R., Jiang G., Wang W. Alveolar macrophage—Derived exosomes modulate severity and outcome of acute lung injury. Aging. 2020;12:6120–6128. doi: 10.18632/aging.103010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gao K., Jin J., Huang C., Li J., Luo H., Li L., Huang Y., Jiang Y. Exosomes Derived from Septic Mouse Serum Modulate Immune Responses via Exosome-Associated Cytokines. Front. Immunol. 2019;10:1560. doi: 10.3389/fimmu.2019.01560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou P., Li T., Jin J., Liu Y., Li B., Sun Q., Tian J., Zhao H., Liu Z., Ma S., et al. Interactions between neutrophil extracellular traps and activated platelets enhance procoagulant activity in acute stroke patients with ICA occlusion. EBioMedicine. 2020;53:102671. doi: 10.1016/j.ebiom.2020.102671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eken C., Martin P.J., Sadallah S., Treves S., Schaller M., Schifferli J.A. Ectosomes released by polymorphonuclear neutrophils induce a MerTK-dependent anti-inflammatory pathway in macrophages. J. Biol. Chem. 2010;285:39914–39921. doi: 10.1074/jbc.M110.126748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kambas K., Chrysanthopoulou A., Vassilopoulos D., Apostolidou E., Skendros P., Girod A., Arelaki S., Froudarakis M., Nakopoulou L., Giatromanolaki A., et al. Tissue factor expression in neutrophil extracellular traps and neutrophil derived microparticles in antineutrophil cytoplasmic antibody associated vasculitis may promote thromboinflammation and the thrombophilic state associated with the disease. Ann. Rheum. Dis. 2014;73:1854–1863. doi: 10.1136/annrheumdis-2013-203430. [DOI] [PubMed] [Google Scholar]
- 67.von Bruhl M.L., Stark K., Steinhart A., Chandraratne S., Konrad I., Lorenz M., Khandoga A., Tirniceriu A., Coletti R., Kollnberger M., et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J. Exp. Med. 2012;209:819–835. doi: 10.1084/jem.20112322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Skendros P., Mitsios A., Chrysanthopoulou A., Mastellos D.C., Metallidis S., Rafailidis P., Ntinopoulou M., Sertaridou E., Tsironidou V., Tsigalou C., et al. Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J. Clin. Investig. 2020;130:6151–6157. doi: 10.1172/JCI141374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peinado H., Aleckovic M., Lavotshkin S., Matei I., Costa-Silva B., Moreno-Bueno G., Hergueta-Redondo M., Williams C., Garcia-Santos G., Ghajar C., et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ohno S., Takanashi M., Sudo K., Ueda S., Ishikawa A., Matsuyama N., Fujita K., Mizutani T., Ohgi T., Ochiya T., et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol. Ther. 2013;21:185–191. doi: 10.1038/mt.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lai C.P., Mardini O., Ericsson M., Prabhakar S., Maguire C., Chen J.W., Tannous B.A., Breakefield X.O. Dynamic biodistribution of extracellular vesicles In Vivo using a multimodal imaging reporter. ACS Nano. 2014;8:483–494. doi: 10.1021/nn404945r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Santocki M., Kolaczkowska E. On Neutrophil Extracellular Trap (NET) Removal: What We Know Thus Far and Why So Little. Cells. 2020;9:2079. doi: 10.3390/cells9092079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jimenez-Alcazar M., Rangaswamy C., Panda R., Bitterling J., Simsek Y.J., Long A.T., Bilyy R., Krenn V., Renne C., Renne T., et al. Host DNases prevent vascular occlusion by neutrophil extracellular traps. Science. 2017;358:1202–1206. doi: 10.1126/science.aam8897. [DOI] [PubMed] [Google Scholar]
- 74.Singhal A., Kumar S. Neutrophil and remnant clearance in immunity and inflammation. Immunology. 2022;165:22–43. doi: 10.1111/imm.13423. [DOI] [PubMed] [Google Scholar]
- 75.Pires R.H., Felix S.B., Delcea M. The architecture of neutrophil extracellular traps investigated by atomic force microscopy. Nanoscale. 2016;8:14193–14202. doi: 10.1039/C6NR03416K. [DOI] [PubMed] [Google Scholar]
- 76.Csomos K., Kristof E., Jakob B., Csomos I., Kovacs G., Rotem O., Hodrea J., Bagoly Z., Muszbek L., Balajthy Z., et al. Protein cross-linking by chlorinated polyamines and transglutamylation stabilizes neutrophil extracellular traps. Cell Death Dis. 2016;7:e2332. doi: 10.1038/cddis.2016.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kummarapurugu A.B., Zheng S., Ma J., Ghosh S., Hawkridge A., Voynow J.A. Neutrophil Elastase Triggers the Release of Macrophage Extracellular Traps: Relevance to Cystic Fibrosis. Am. J. Respir. Cell Mol. Biol. 2022;66:76–85. doi: 10.1165/rcmb.2020-0410OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Papayannopoulos V., Metzler K.D., Hakkim A., Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 2010;191:677–691. doi: 10.1083/jcb.201006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Farley K., Stolley J.M., Zhao P., Cooley J., Remold-O’Donnell E. A serpinB1 regulatory mechanism is essential for restricting neutrophil extracellular trap generation. J. Immunol. 2012;189:4574–4581. doi: 10.4049/jimmunol.1201167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Voynow J.A., Shinbashi M. Neutrophil Elastase and Chronic Lung Disease. Biomolecules. 2021;11:1065. doi: 10.3390/biom11081065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Belaaouaj A., Kim K.S., Shapiro S.D. Degradation of outer membrane protein A in Escherichia coli killing by neutrophil elastase. Science. 2000;289:1185–1188. doi: 10.1126/science.289.5482.1185. [DOI] [PubMed] [Google Scholar]
- 82.Tralau T., Meyer-Hoffert U., Schroder J.M., Wiedow O. Human leukocyte elastase and cathepsin G are specific inhibitors of C5a-dependent neutrophil enzyme release and chemotaxis. Exp. Dermatol. 2004;13:316–325. doi: 10.1111/j.0906-6705.2004.00145.x. [DOI] [PubMed] [Google Scholar]
- 83.Masuda S., Nonokawa M., Futamata E., Nishibata Y., Iwasaki S., Tsuji T., Hatanaka Y., Nakazawa D., Tanaka S., Tomaru U., et al. Formation and Disordered Degradation of Neutrophil Extracellular Traps in Necrotizing Lesions of Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis. Am. J. Pathol. 2019;189:839–846. doi: 10.1016/j.ajpath.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 84.Korkmaz B., Horwitz M.S., Jenne D.E., Gauthier F. Neutrophil elastase, proteinase 3, and cathepsin G as therapeutic targets in human diseases. Pharmacol. Rev. 2010;62:726–759. doi: 10.1124/pr.110.002733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Genschmer K.R., Russell D.W., Lal C., Szul T., Bratcher P.E., Noerager B.D., Abdul Roda M., Xu X., Rezonzew G., Viera L., et al. Activated PMN Exosomes: Pathogenic Entities Causing Matrix Destruction and Disease in the Lung. Cell. 2019;176:113–126.e115. doi: 10.1016/j.cell.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Szturmowicz M., Demkow U. Neutrophil Extracellular Traps (NETs) in Severe SARS-CoV-2 Lung Disease. Int. J. Mol. Sci. 2021;22:8854. doi: 10.3390/ijms22168854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hong C.W. Extracellular Vesicles of Neutrophils. Immune Netw. 2018;18:e43. doi: 10.4110/in.2018.18.e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Neubert E., Meyer D., Kruss S., Erpenbeck L. The power from within—Understanding the driving forces of neutrophil extracellular trap formation. J. Cell Sci. 2020;133:jcs241075. doi: 10.1242/jcs.241075. [DOI] [PubMed] [Google Scholar]
- 89.Gasser O., Schifferli J.A. Microparticles released by human neutrophils adhere to erythrocytes in the presence of complement. Exp. Cell Res. 2005;307:381–387. doi: 10.1016/j.yexcr.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 90.Fuchs T.A., Brill A., Duerschmied D., Schatzberg D., Monestier M., Myers D.D., Jr., Wrobleski S.K., Wakefield T.W., Hartwig J.H., Wagner D.D. Extracellular DNA traps promote thrombosis. Proc. Natl. Acad. Sci. USA. 2010;107:15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Guimaraes-Costa A.B., Rochael N.C., Oliveira F., Echevarria-Lima J., Saraiva E.M. Neutrophil Extracellular Traps Reprogram IL-4/GM-CSF-Induced Monocyte Differentiation to Anti-inflammatory Macrophages. Front. Immunol. 2017;8:523. doi: 10.3389/fimmu.2017.00523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Barrientos L., Bignon A., Gueguen C., de Chaisemartin L., Gorges R., Sandre C., Mascarell L., Balabanian K., Kerdine-Romer S., Pallardy M., et al. Neutrophil extracellular traps downregulate lipopolysaccharide-induced activation of monocyte-derived dendritic cells. J. Immunol. 2014;193:5689–5698. doi: 10.4049/jimmunol.1400586. [DOI] [PubMed] [Google Scholar]
- 93.Domer D., Walther T., Moller S., Behnen M., Laskay T. Neutrophil Extracellular Traps Activate Proinflammatory Functions of Human Neutrophils. Front. Immunol. 2021;12:636954. doi: 10.3389/fimmu.2021.636954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schauer C., Janko C., Munoz L.E., Zhao Y., Kienhofer D., Frey B., Lell M., Manger B., Rech J., Naschberger E., et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat. Med. 2014;20:511–517. doi: 10.1038/nm.3547. [DOI] [PubMed] [Google Scholar]
- 95.Walters N., Nguyen L.T.H., Zhang J., Shankaran A., Reategui E. Extracellular vesicles as mediators of In Vitro neutrophil swarming on a large-scale microparticle array. Lab Chip. 2019;19:2874–2884. doi: 10.1039/C9LC00483A. [DOI] [PubMed] [Google Scholar]
- 96.Majumdar R., Tameh A.T., Arya S.B., Parent C.A. Exosomes mediate LTB4 release during neutrophil chemotaxis. PLoS Biol. 2021;19:e3001271. doi: 10.1371/journal.pbio.3001271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pluskota E., Woody N.M., Szpak D., Ballantyne C.M., Soloviev D.A., Simon D.I., Plow E.F. Expression, activation, and function of integrin alphaMbeta2 (Mac-1) on neutrophil-derived microparticles. Blood. 2008;112:2327–2335. doi: 10.1182/blood-2007-12-127183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Engelmann B., Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat. Rev. Immunol. 2013;13:34–45. doi: 10.1038/nri3345. [DOI] [PubMed] [Google Scholar]
- 99.Ma Y., Yang X., Chatterjee V., Meegan J.E., Beard R.S., Jr., Yuan S.Y. Role of Neutrophil Extracellular Traps and Vesicles in Regulating Vascular Endothelial Permeability. Front. Immunol. 2019;10:1037. doi: 10.3389/fimmu.2019.01037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Folco E.J., Mawson T.L., Vromman A., Bernardes-Souza B., Franck G., Persson O., Nakamura M., Newton G., Luscinskas F.W., Libby P. Neutrophil Extracellular Traps Induce Endothelial Cell Activation and Tissue Factor Production through Interleukin-1alpha and Cathepsin G. Arterioscler. Thromb. Vasc. Biol. 2018;38:1901–1912. doi: 10.1161/ATVBAHA.118.311150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Minns D., Smith K.J., Findlay E.G. Orchestration of Adaptive T Cell Responses by Neutrophil Granule Contents. Mediat. Inflamm. 2019;2019:8968943. doi: 10.1155/2019/8968943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shen G., Krienke S., Schiller P., Niessen A., Neu S., Eckstein V., Schiller M., Lorenz H.M., Tykocinski L.O. Microvesicles released by apoptotic human neutrophils suppress proliferation and IL-2/IL-2 receptor expression of resting T helper cells. Eur. J. Immunol. 2017;47:900–910. doi: 10.1002/eji.201546203. [DOI] [PubMed] [Google Scholar]
- 103.Timar C.I., Lorincz A.M., Csepanyi-Komi R., Valyi-Nagy A., Nagy G., Buzas E.I., Ivanyi Z., Kittel A., Powell D.W., McLeish K.R., et al. Antibacterial effect of microvesicles released from human neutrophilic granulocytes. Blood. 2013;121:510–518. doi: 10.1182/blood-2012-05-431114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kaplan M.J., Radic M. Neutrophil extracellular traps: Double-edged swords of innate immunity. J. Immunol. 2012;189:2689–2695. doi: 10.4049/jimmunol.1201719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schonrich G., Raftery M.J. Neutrophil Extracellular Traps Go Viral. Front. Immunol. 2016;7:366. doi: 10.3389/fimmu.2016.00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Urban C.F., Nett J.E. Neutrophil extracellular traps in fungal infection. Semin. Cell Dev. Biol. 2019;89:47–57. doi: 10.1016/j.semcdb.2018.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Diaz-Godinez C., Carrero J.C. The state of art of neutrophil extracellular traps in protozoan and helminthic infections. BioSci. Rep. 2019;39:BSR20180916. doi: 10.1042/BSR20180916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Biro E., Sturk-Maquelin K.N., Vogel G.M., Meuleman D.G., Smit M.J., Hack C.E., Sturk A., Nieuwland R. Human cell-derived microparticles promote thrombus formation in vivo in a tissue factor-dependent manner. J. Thromb. Haemost. 2003;1:2561–2568. doi: 10.1046/j.1538-7836.2003.00456.x. [DOI] [PubMed] [Google Scholar]
- 109.Thakur M., Evans B., Schindewolf M., Baumgartner I., Doring Y. Neutrophil Extracellular Traps Affecting Cardiovascular Health in Infectious and Inflammatory Diseases. Cells. 2021;10:1689. doi: 10.3390/cells10071689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Watanabe J., Marathe G.K., Neilsen P.O., Weyrich A.S., Harrison K.A., Murphy R.C., Zimmerman G.A., McIntyre T.M. Endotoxins stimulate neutrophil adhesion followed by synthesis and release of platelet-activating factor in microparticles. J. Biol. Chem. 2003;278:33161–33168. doi: 10.1074/jbc.M305321200. [DOI] [PubMed] [Google Scholar]
- 111.Warnatsch A., Ioannou M., Wang Q., Papayannopoulos V. Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science. 2015;349:316–320. doi: 10.1126/science.aaa8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang J., Ji C., Zhang H., Shi H., Mao F., Qian H., Xu W., Wang D., Pan J., Fang X., et al. Engineered neutrophil-derived exosome-like vesicles for targeted cancer therapy. Sci. Adv. 2022;8:eabj8207. doi: 10.1126/sciadv.abj8207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hedrick C.C., Malanchi I. Neutrophils in cancer: Heterogeneous and multifaceted. Nat. Rev. Immunol. 2022;22:173–187. doi: 10.1038/s41577-021-00571-6. [DOI] [PubMed] [Google Scholar]
- 114.Rubenich D.S., Omizzollo N., Szczepanski M.J., Reichert T.E., Whiteside T.L., Ludwig N., Braganhol E. Small extracellular vesicle-mediated bidirectional crosstalk between neutrophils and tumor cells. Cytokine Growth Factor Rev. 2021;61:16–26. doi: 10.1016/j.cytogfr.2021.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Razak N.B.A., Jones G., Bhandari M., Berndt M.C., Metharom P. Cancer-Associated Thrombosis: An Overview of Mechanisms, Risk Factors, and Treatment. Cancers. 2018;10:380. doi: 10.3390/cancers10100380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kessenbrock K., Krumbholz M., Schonermarck U., Back W., Gross W.L., Werb Z., Grone H.J., Brinkmann V., Jenne D.E. Netting neutrophils in autoimmune small-vessel vasculitis. Nat. Med. 2009;15:623–625. doi: 10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hong Y., Eleftheriou D., Hussain A.A., Price-Kuehne F.E., Savage C.O., Jayne D., Little M.A., Salama A.D., Klein N.J., Brogan P.A. Anti-neutrophil cytoplasmic antibodies stimulate release of neutrophil microparticles. J. Am. Soc. Nephrol. 2012;23:49–62. doi: 10.1681/ASN.2011030298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Glemain A., Neel M., Neel A., Andre-Gregoire G., Gavard J., Martinet B., Le Bloas R., Riquin K., Hamidou M., Fakhouri F., et al. Neutrophil-derived extracellular vesicles induce endothelial inflammation and damage through the transfer of miRNAs. J. Autoimmun. 2022;129:102826. doi: 10.1016/j.jaut.2022.102826. [DOI] [PubMed] [Google Scholar]
- 119.Lin A.M., Rubin C.J., Khandpur R., Wang J.Y., Riblett M., Yalavarthi S., Villanueva E.C., Shah P., Kaplan M.J., Bruce A.T. Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J. Immunol. 2011;187:490–500. doi: 10.4049/jimmunol.1100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shao S., Fang H., Zhang J., Jiang M., Xue K., Ma J., Zhang J., Lei J., Zhang Y., Li B., et al. Neutrophil exosomes enhance the skin autoinflammation in generalized pustular psoriasis via activating keratinocytes. FASEB J. 2019;33:6813–6828. doi: 10.1096/fj.201802090RR. [DOI] [PubMed] [Google Scholar]
- 121.Hakkim A., Furnrohr B.G., Amann K., Laube B., Abed U.A., Brinkmann V., Herrmann M., Voll R.E., Zychlinsky A. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc. Natl. Acad. Sci. USA. 2010;107:9813–9818. doi: 10.1073/pnas.0909927107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Salemme R., Peralta L.N., Meka S.H., Pushpanathan N., Alexander J.J. The Role of NETosis in Systemic Lupus Erythematosus. J. Cell Immunol. 2019;1:33–42. doi: 10.33696/immunology.1.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tsai C.Y., Li K.J., Hsieh S.C., Liao H.T., Yu C.L. What’s wrong with neutrophils in lupus? Clin. Exp. Rheumatol. 2019;37:684–693. [PubMed] [Google Scholar]
- 124.Headland S.E., Jones H.R., Norling L.V., Kim A., Souza P.R., Corsiero E., Gil C.D., Nerviani A., Dell’Accio F., Pitzalis C., et al. Neutrophil-derived microvesicles enter cartilage and protect the joint in inflammatory arthritis. Sci. Transl. Med. 2015;7:315ra190. doi: 10.1126/scitranslmed.aac5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Carmona-Rivera C., Carlucci P.M., Goel R.R., James E., Brooks S.R., Rims C., Hoffmann V., Fox D.A., Buckner J.H., Kaplan M.J. Neutrophil extracellular traps mediate articular cartilage damage and enhance cartilage component immunogenicity in rheumatoid arthritis. JCI Insight. 2020;5:e139388. doi: 10.1172/jci.insight.139388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vargas A., Roux-Dalvai F., Droit A., Lavoie J.P. Neutrophil-Derived Exosomes: A New Mechanism Contributing to Airway Smooth Muscle Remodeling. Am. J. Respir. Cell Mol. Biol. 2016;55:450–461. doi: 10.1165/rcmb.2016-0033OC. [DOI] [PubMed] [Google Scholar]
- 127.Twaddell S.H., Baines K.J., Grainge C., Gibson P.G. The Emerging Role of Neutrophil Extracellular Traps in Respiratory Disease. Chest. 2019;156:774–782. doi: 10.1016/j.chest.2019.06.012. [DOI] [PubMed] [Google Scholar]
- 128.Kitching A.R., Anders H.J., Basu N., Brouwer E., Gordon J., Jayne D.R., Kullman J., Lyons P.A., Merkel P.A., Savage C.O.S., et al. ANCA-associated vasculitis. Nat. Rev. Dis. Primers. 2020;6:71. doi: 10.1038/s41572-020-0204-y. [DOI] [PubMed] [Google Scholar]
- 129.Soderberg D., Segelmark M. Neutrophil Extracellular Traps in ANCA-Associated Vasculitis. Front. Immunol. 2016;7:256. doi: 10.3389/fimmu.2016.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nakazawa D., Shida H., Tomaru U., Yoshida M., Nishio S., Atsumi T., Ishizu A. Enhanced formation and disordered regulation of NETs in myeloperoxidase-ANCA-associated microscopic polyangiitis. J. Am. Soc. Nephrol. 2014;25:990–997. doi: 10.1681/ASN.2013060606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pisetsky D.S., Gauley J., Ullal A.J. Microparticles as a source of extracellular DNA. Immunol. Res. 2011;49:227–234. doi: 10.1007/s12026-010-8184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fortin P.R., Cloutier N., Bissonnette V., Aghdassi E., Eder L., Simonyan D., Laflamme N., Boilard E. Distinct Subtypes of Microparticle-containing Immune Complexes Are Associated with Disease Activity, Damage, and Carotid Intima-media Thickness in Systemic Lupus Erythematosus. J. Rheumatol. 2016;43:2019–2025. doi: 10.3899/jrheum.160050. [DOI] [PubMed] [Google Scholar]
- 133.Ostergaard O., Nielsen C.T., Tanassi J.T., Iversen L.V., Jacobsen S., Heegaard N.H.H. Distinct proteome pathology of circulating microparticles in systemic lupus erythematosus. Clin. Proteom. 2017;14:23. doi: 10.1186/s12014-017-9159-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ostergaard O., Nielsen C.T., Iversen L.V., Tanassi J.T., Knudsen S., Jacobsen S., Heegaard N.H. Unique protein signature of circulating microparticles in systemic lupus erythematosus. Arthritis Rheum. 2013;65:2680–2690. doi: 10.1002/art.38065. [DOI] [PubMed] [Google Scholar]
- 135.Corsiero E., Bombardieri M., Carlotti E., Pratesi F., Robinson W., Migliorini P., Pitzalis C. Single cell cloning and recombinant monoclonal antibodies generation from RA synovial B cells reveal frequent targeting of citrullinated histones of NETs. Ann. Rheum. Dis. 2016;75:1866–1875. doi: 10.1136/annrheumdis-2015-208356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Corsiero E., Pratesi F., Prediletto E., Bombardieri M., Migliorini P. NETosis as Source of Autoantigens in Rheumatoid Arthritis. Front. Immunol. 2016;7:485. doi: 10.3389/fimmu.2016.00485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cloutier N., Tan S., Boudreau L.H., Cramb C., Subbaiah R., Lahey L., Albert A., Shnayder R., Gobezie R., Nigrovic P.A., et al. The exposure of autoantigens by microparticles underlies the formation of potent inflammatory components: The microparticle-associated immune complexes. EMBO Mol. Med. 2013;5:235–249. doi: 10.1002/emmm.201201846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tanaka T., Warner B.M., Odani T., Ji Y., Mo Y.Q., Nakamura H., Jang S.I., Yin H., Michael D.G., Hirata N., et al. LAMP3 induces apoptosis and autoantigen release in Sjogren’s syndrome patients. Sci. Rep. 2020;10:15169. doi: 10.1038/s41598-020-71669-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kapsogeorgou E.K., Abu-Helu R.F., Moutsopoulos H.M., Manoussakis M.N. Salivary gland epithelial cell exosomes: A source of autoantigenic ribonucleoproteins. Arthritis Rheum. 2005;52:1517–1521. doi: 10.1002/art.21005. [DOI] [PubMed] [Google Scholar]
- 140.Hasilo C.P., Negi S., Allaeys I., Cloutier N., Rutman A.K., Gasparrini M., Bonneil E., Thibault P., Boilard E., Paraskevas S. Presence of diabetes autoantigens in extracellular vesicles derived from human islets. Sci. Rep. 2017;7:5000. doi: 10.1038/s41598-017-04977-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Haas M., Eustace J.A. Immune complex deposits in ANCA-associated crescentic glomerulonephritis: A study of 126 cases. Kidney Int. 2004;65:2145–2152. doi: 10.1111/j.1523-1755.2004.00632.x. [DOI] [PubMed] [Google Scholar]
- 142.Van Paassen P., Tervaert J.W., Heeringa P. Mechanisms of vasculitis: How pauci-immune is ANCA-associated renal vasculitis? Nephron. Exp. Nephrol. 2007;105:e10–e16. doi: 10.1159/000096960. [DOI] [PubMed] [Google Scholar]
- 143.Ayaash A., Maan D., Kapetanos A., Bunker M., Wasko M.C., Clark B. Significance of Crescentic Glomeruli in Acute Kidney Injury with Rheumatoid Arthritis. Case Rep. Nephrol. Dial. 2019;9:42–48. doi: 10.1159/000500105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cansu D.U., Temiz G., Acikalin M.F., Korkmaz C. Pauci-immune lupus nephritis: Possibility or co-incidence? Eur. J. Rheumatol. 2017;4:73–75. doi: 10.5152/eurjrheum.2016.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sagiv J.Y., Michaeli J., Assi S., Mishalian I., Kisos H., Levy L., Damti P., Lumbroso D., Polyansky L., Sionov R.V., et al. Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Rep. 2015;10:562–573. doi: 10.1016/j.celrep.2014.12.039. [DOI] [PubMed] [Google Scholar]
- 146.Coffelt S.B., Wellenstein M.D., de Visser K.E. Neutrophils in cancer: Neutral no more. Nat Rev. Cancer. 2016;16:431–446. doi: 10.1038/nrc.2016.52. [DOI] [PubMed] [Google Scholar]
- 147.Hsu B.E., Tabaries S., Johnson R.M., Andrzejewski S., Senecal J., Lehuede C., Annis M.G., Ma E.H., Vols S., Ramsay L., et al. Immature Low-Density Neutrophils Exhibit Metabolic Flexibility that Facilitates Breast Cancer Liver Metastasis. Cell Rep. 2019;27:3902–3915.e3906. doi: 10.1016/j.celrep.2019.05.091. [DOI] [PubMed] [Google Scholar]
- 148.Cristinziano L., Modestino L., Loffredo S., Varricchi G., Braile M., Ferrara A.L., de Paulis A., Antonelli A., Marone G., Galdiero M.R. Anaplastic Thyroid Cancer Cells Induce the Release of Mitochondrial Extracellular DNA Traps by Viable Neutrophils. J. Immunol. 2020;204:1362–1372. doi: 10.4049/jimmunol.1900543. [DOI] [PubMed] [Google Scholar]
- 149.Miller-Ocuin J.L., Liang X., Boone B.A., Doerfler W.R., Singhi A.D., Tang D., Kang R., Lotze M.T., Zeh H.J., 3rd DNA released from neutrophil extracellular traps (NETs) activates pancreatic stellate cells and enhances pancreatic tumor growth. Oncoimmunology. 2019;8:e1605822. doi: 10.1080/2162402X.2019.1605822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Teijeira A., Garasa S., Gato M., Alfaro C., Migueliz I., Cirella A., de Andrea C., Ochoa M.C., Otano I., Etxeberria I., et al. CXCR1 and CXCR2 Chemokine Receptor Agonists Produced by Tumors Induce Neutrophil Extracellular Traps that Interfere with Immune Cytotoxicity. Immunity. 2020;52:856–871.e858. doi: 10.1016/j.immuni.2020.03.001. [DOI] [PubMed] [Google Scholar]
- 151.Park J., Wysocki R.W., Amoozgar Z., Maiorino L., Fein M.R., Jorns J., Schott A.F., Kinugasa-Katayama Y., Lee Y., Won N.H., et al. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci. Transl. Med. 2016;8:361ra138. doi: 10.1126/scitranslmed.aag1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Xiao Y., Cong M., Li J., He D., Wu Q., Tian P., Wang Y., Yang S., Liang C., Liang Y., et al. Cathepsin C promotes breast cancer lung metastasis by modulating neutrophil infiltration and neutrophil extracellular trap formation. Cancer Cell. 2021;39:423–437.e427. doi: 10.1016/j.ccell.2020.12.012. [DOI] [PubMed] [Google Scholar]
- 153.Albrengues J., Shields M.A., Ng D., Park C.G., Ambrico A., Poindexter M.E., Upadhyay P., Uyeminami D.L., Pommier A., Kuttner V., et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science. 2018;361:eaao4227. doi: 10.1126/science.aao4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yang L., Liu Q., Zhang X., Liu X., Zhou B., Chen J., Huang D., Li J., Li H., Chen F., et al. DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25. Nature. 2020;583:133–138. doi: 10.1038/s41586-020-2394-6. [DOI] [PubMed] [Google Scholar]
- 155.Cools-Lartigue J., Spicer J., McDonald B., Gowing S., Chow S., Giannias B., Bourdeau F., Kubes P., Ferri L. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J. Clin. Investig. 2013;123:3446–3458. doi: 10.1172/JCI67484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rayes R.F., Mouhanna J.G., Nicolau I., Bourdeau F., Giannias B., Rousseau S., Quail D., Walsh L., Sangwan V., Bertos N., et al. Primary tumors induce neutrophil extracellular traps with targetable metastasis promoting effects. JCI Insight. 2019;5:e128008. doi: 10.1172/jci.insight.128008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Szatmary A.C., Nossal R., Parent C.A., Majumdar R. Modeling neutrophil migration in dynamic chemoattractant gradients: Assessing the role of exosomes during signal relay. Mol. Biol. Cell. 2017;28:3457–3470. doi: 10.1091/mbc.E17-05-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Houghton A.M., Rzymkiewicz D.M., Ji H., Gregory A.D., Egea E.E., Metz H.E., Stolz D.B., Land S.R., Marconcini L.A., Kliment C.R., et al. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nat. Med. 2010;16:219–223. doi: 10.1038/nm.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lerman I., Garcia-Hernandez M.L., Rangel-Moreno J., Chiriboga L., Pan C., Nastiuk K.L., Krolewski J.J., Sen A., Hammes S.R. Infiltrating Myeloid Cells Exert Protumorigenic Actions via Neutrophil Elastase. Mol. Cancer Res. 2017;15:1138–1152. doi: 10.1158/1541-7786.MCR-17-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chou R.H., Wen H.C., Liang W.G., Lin S.C., Yuan H.W., Wu C.W., Chang W.S. Suppression of the invasion and migration of cancer cells by SERPINB family genes and their derived peptides. Oncol. Rep. 2012;27:238–245. doi: 10.3892/or.2011.1497. [DOI] [PubMed] [Google Scholar]
- 161.Caruso J.A., Akli S., Pageon L., Hunt K.K., Keyomarsi K. The serine protease inhibitor elafin maintains normal growth control by opposing the mitogenic effects of neutrophil elastase. Oncogene. 2015;34:3556–3567. doi: 10.1038/onc.2014.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Belorgey D., Bieth J.G. DNA binds neutrophil elastase and mucus proteinase inhibitor and impairs their functional activity. FEBS Lett. 1995;361:265–268. doi: 10.1016/0014-5793(95)00173-7. [DOI] [PubMed] [Google Scholar]
- 163.Metzler K.D., Fuchs T.A., Nauseef W.M., Reumaux D., Roesler J., Schulze I., Wahn V., Papayannopoulos V., Zychlinsky A. Myeloperoxidase is required for neutrophil extracellular trap formation: Implications for innate immunity. Blood. 2011;117:953–959. doi: 10.1182/blood-2010-06-290171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Valadez-Cosmes P., Raftopoulou S., Mihalic Z.N., Marsche G., Kargl J. Myeloperoxidase: Growing importance in cancer pathogenesis and potential drug target. Pharmacol. Ther. 2021;236:108052. doi: 10.1016/j.pharmthera.2021.108052. [DOI] [PubMed] [Google Scholar]
- 165.Scandolara T.B., Panis C. Neutrophil traps, anti-myeloperoxidase antibodies and cancer: Are they linked? Immunol. Lett. 2020;221:33–38. doi: 10.1016/j.imlet.2020.02.011. [DOI] [PubMed] [Google Scholar]
- 166.Misra D.P., Thomas K.N., Gasparyan A.Y., Zimba O. Mechanisms of thrombosis in ANCA-associated vasculitis. Clin. Rheumatol. 2021;40:4807–4815. doi: 10.1007/s10067-021-05790-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Berti A., Matteson E.L., Crowson C.S., Specks U., Cornec D. Risk of Cardiovascular Disease and Venous Thromboembolism Among Patients with Incident ANCA-Associated Vasculitis: A 20-Year Population-Based Cohort Study. Mayo Clin. Proc. 2018;93:597–606. doi: 10.1016/j.mayocp.2018.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Navi B.B., Reiner A.S., Kamel H., Iadecola C., Okin P.M., Elkind M.S.V., Panageas K.S., DeAngelis L.M. Risk of Arterial Thromboembolism in Patients with Cancer. J. Am. Coll. Cardiol. 2017;70:926–938. doi: 10.1016/j.jacc.2017.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sud R., Khorana A.A. Cancer-associated thrombosis: Risk factors, candidate biomarkers and a risk model. Thromb. Res. 2009;123((Suppl. S4)):S18–S21. doi: 10.1016/S0049-3848(09)70137-9. [DOI] [PubMed] [Google Scholar]
- 170.Blom J.W., Doggen C.J., Osanto S., Rosendaal F.R. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293:715–722. doi: 10.1001/jama.293.6.715. [DOI] [PubMed] [Google Scholar]
- 171.Mi Y., Yan S., Lu Y., Liang Y., Li C. Venous thromboembolism has the same risk factors as atherosclerosis: A PRISMA-compliant systemic review and meta-analysis. Medicine. 2016;95:e4495. doi: 10.1097/MD.0000000000004495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mackman N., Bergmeier W., Stouffer G.A., Weitz J.I. Therapeutic strategies for thrombosis: New targets and approaches. Nat. Rev. Drug Discov. 2020;19:333–352. doi: 10.1038/s41573-020-0061-0. [DOI] [PubMed] [Google Scholar]
- 173.Mendoza C.E., Brant E.J., McDermott M.L., Froment A., Hu Y., Hogan S.L., Jennette J.C., Falk R.J., Nachman P.H., Derebail V.K., et al. Elevated Microparticle Tissue Factor Activity Differentiates Patients with Venous Thromboembolism in Anti-neutrophil Cytoplasmic Autoantibody Vasculitis. Kidney Int. Rep. 2019;4:1617–1629. doi: 10.1016/j.ekir.2019.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Barnado A., Crofford L.J., Oates J.C. At the Bedside: Neutrophil extracellular traps (NETs) as targets for biomarkers and therapies in autoimmune diseases. J. Leukoc. Biol. 2016;99:265–278. doi: 10.1189/jlb.5BT0615-234R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.De Groot P.G., de Laat B. Mechanisms of thrombosis in systemic lupus erythematosus and antiphospholipid syndrome. Best Pract. Res. Clin. Rheumatol. 2017;31:334–341. doi: 10.1016/j.berh.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 176.Yalavarthi S., Gould T.J., Rao A.N., Mazza L.F., Morris A.E., Nunez-Alvarez C., Hernandez-Ramirez D., Bockenstedt P.L., Liaw P.C., Cabral A.R., et al. Release of neutrophil extracellular traps by neutrophils stimulated with antiphospholipid antibodies: A newly identified mechanism of thrombosis in the antiphospholipid syndrome. Arthritis Rheumatol. 2015;67:2990–3003. doi: 10.1002/art.39247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Meng H., Yalavarthi S., Kanthi Y., Mazza L.F., Elfline M.A., Luke C.E., Pinsky D.J., Henke P.K., Knight J.S. In Vivo Role of Neutrophil Extracellular Traps in Antiphospholipid Antibody-Mediated Venous Thrombosis. Arthritis Rheumatol. 2017;69:655–667. doi: 10.1002/art.39938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zuo Y., Yalavarthi S., Gockman K., Madison J.A., Gudjonsson J.E., Kahlenberg J.M., Joseph McCune W., Bockenstedt P.L., Karp D.R., Knight J.S. Anti-Neutrophil Extracellular Trap Antibodies and Impaired Neutrophil Extracellular Trap Degradation in Antiphospholipid Syndrome. Arthritis Rheumatol. 2020;72:2130–2135. doi: 10.1002/art.41460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Leffler J., Stojanovich L., Shoenfeld Y., Bogdanovic G., Hesselstrand R., Blom A.M. Degradation of neutrophil extracellular traps is decreased in patients with antiphospholipid syndrome. Clin. Exp. Rheumatol. 2014;32:66–70. [PubMed] [Google Scholar]
- 180.Jung H.S., Gu J., Kim J.E., Nam Y., Song J.W., Kim H.K. Cancer cell-induced neutrophil extracellular traps promote both hypercoagulability and cancer progression. PLoS ONE. 2019;14:e0216055. doi: 10.1371/journal.pone.0216055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Veras F.P., Pontelli M.C., Silva C.M., Toller-Kawahisa J.E., de Lima M., Nascimento D.C., Schneider A.H., Caetite D., Tavares L.A., Paiva I.M., et al. SARS-CoV-2-triggered neutrophil extracellular traps mediate COVID-19 pathology. J. Exp. Med. 2020;217:e20201129. doi: 10.1084/jem.20201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Noubouossie D.F., Whelihan M.F., Yu Y.B., Sparkenbaugh E., Pawlinski R., Monroe D.M., Key N.S. In vitro activation of coagulation by human neutrophil DNA and histone proteins but not neutrophil extracellular traps. Blood. 2017;129:1021–1029. doi: 10.1182/blood-2016-06-722298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kapoor S., Opneja A., Nayak L. The role of neutrophils in thrombosis. Thromb. Res. 2018;170:87–96. doi: 10.1016/j.thromres.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Blanch-Ruiz M.A., Ortega-Luna R., Martinez-Cuesta M.A., Alvarez A. The Neutrophil Secretome as a Crucial Link between Inflammation and Thrombosis. Int. J. Mol. Sci. 2021;22:4170. doi: 10.3390/ijms22084170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Owens A.P., 3rd, Mackman N. Microparticles in hemostasis and thrombosis. Circ. Res. 2011;108:1284–1297. doi: 10.1161/CIRCRESAHA.110.233056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wang L., Bi Y., Yu M., Li T., Tong D., Yang X., Zhang C., Guo L., Wang C., Kou Y., et al. Phosphatidylserine-exposing blood cells and microparticles induce procoagulant activity in non-valvular atrial fibrillation. Int. J. Cardiol. 2018;258:138–143. doi: 10.1016/j.ijcard.2018.01.116. [DOI] [PubMed] [Google Scholar]
- 187.Pitanga T.N., de Aragao Franca L., Rocha V.C., Meirelles T., Borges V.M., Goncalves M.S., Pontes-de-Carvalho L.C., Noronha-Dutra A.A., dos-Santos W.L. Neutrophil-derived microparticles induce myeloperoxidase-mediated damage of vascular endothelial cells. BMC Cell Biol. 2014;15:21. doi: 10.1186/1471-2121-15-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Saffarzadeh M., Juenemann C., Queisser M.A., Lochnit G., Barreto G., Galuska S.P., Lohmeyer J., Preissner K.T. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: A predominant role of histones. PLoS ONE. 2012;7:e32366. doi: 10.1371/journal.pone.0032366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Guimaraes Junior M.H., Ferrari T.C.A., Teixeira-Carvalho A., Moreira M.L., de Souza Santos L.J., Costa-Silva M.F., Coelho R.M.P., Pinto P., Ris T.H., Salles J.T., et al. Cell-derived microvesicles in infective endocarditis: Role in diagnosis and potential for risk stratification at hospital admission. J. Infect. 2019;79:101–107. doi: 10.1016/j.jinf.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 190.Sarlon-Bartoli G., Bennis Y., Lacroix R., Piercecchi-Marti M.D., Bartoli M.A., Arnaud L., Mancini J., Boudes A., Sarlon E., Thevenin B., et al. Plasmatic level of leukocyte-derived microparticles is associated with unstable plaque in asymptomatic patients with high-grade carotid stenosis. J. Am. Coll. Cardiol. 2013;62:1436–1441. doi: 10.1016/j.jacc.2013.03.078. [DOI] [PubMed] [Google Scholar]
- 191.Martinez G.J., Barraclough J.Y., Nakhla S., Kienzle V., Robertson S., Mallat Z., Celermajer D.S., Patel S. Neutrophil-derived microparticles are released into the coronary circulation following percutaneous coronary intervention in acute coronary syndrome patients. BioSci. Rep. 2017;37:BSR20160430. doi: 10.1042/BSR20160430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Soni S., Garner J.L., O’Dea K.P., Koh M., Finney L., Tirlapur N., Srikanthan K., Tenda E.D., Aboelhassan A.M., Singh S., et al. Intra-alveolar neutrophil-derived microvesicles are associated with disease severity in COPD. Am. J. Physiol. Lung Cell Mol. Physiol. 2021;320:L73–L83. doi: 10.1152/ajplung.00099.2020. [DOI] [PubMed] [Google Scholar]
- 193.Bonifay A., Robert S., Champagne B., Petit P.R., Eugene A., Chareyre C., Duchez A.C., Velier M., Fritz S., Vallier L., et al. A new strategy to count and sort neutrophil-derived extracellular vesicles: Validation in infectious disorders. J. Extracell. Vesicles. 2022;11:e12204. doi: 10.1002/jev2.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Chen H.P., Wang X.Y., Pan X.Y., Hu W.W., Cai S.T., Joshi K., Deng L.H., Ma D. Circulating Neutrophil-Derived Microparticles Associated with the Prognosis of Patients with Sepsis. J. Inflamm. Res. 2020;13:1113–1124. doi: 10.2147/JIR.S287256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Abrams S.T., Morton B., Alhamdi Y., Alsabani M., Lane S., Welters I.D., Wang G., Toh C.H. A Novel Assay for Neutrophil Extracellular Trap Formation Independently Predicts Disseminated Intravascular Coagulation and Mortality in Critically Ill Patients. Am. J. Respir. Crit. Care Med. 2019;200:869–880. doi: 10.1164/rccm.201811-2111OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Petito E., Falcinelli E., Paliani U., Cesari E., Vaudo G., Sebastiano M., Cerotto V., Guglielmini G., Gori F., Malvestiti M., et al. Association of Neutrophil Activation, More than Platelet Activation, with Thrombotic Complications in Coronavirus Disease 2019. J. Infect. Dis. 2021;223:933–944. doi: 10.1093/infdis/jiaa756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Guervilly C., Bonifay A., Burtey S., Sabatier F., Cauchois R., Abdili E., Arnaud L., Lano G., Pietri L., Robert T., et al. Dissemination of extreme levels of extracellular vesicles: Tissue factor activity in patients with severe COVID-19. Blood Adv. 2021;5:628–634. doi: 10.1182/bloodadvances.2020003308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zuo Y., Yalavarthi S., Shi H., Gockman K., Zuo M., Madison J.A., Blair C., Weber A., Barnes B.J., Egeblad M., et al. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020;5:e138999. doi: 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Huckriede J., Anderberg S.B., Morales A., de Vries F., Hultstrom M., Bergqvist A., Ortiz-Perez J.T., Sels J.W., Wichapong K., Lipcsey M., et al. Evolution of NETosis markers and DAMPs have prognostic value in critically ill COVID-19 patients. Sci. Rep. 2021;11:15701. doi: 10.1038/s41598-021-95209-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kuo Y.M., Lin Y.C., Lee M.J., Chen J.W., Hsu C.C., Huang T.Y., Chen J.H., Tzeng S.J., Chiu Y.L., Wang S.R., et al. Biomarker of neutrophil extracellular traps is associated with deep-seated infections and predicts mortality and cardiovascular morbidity in commensal streptococcal bacteremia. J. Microbiol. Immunol. Infect. 2022. in press . [DOI] [PubMed]
- 201.Chiva-Blanch G., Padro T., Alonso R., Crespo J., Perez de Isla L., Mata P., Badimon L. Liquid Biopsy of Extracellular Microvesicles Maps Coronary Calcification and Atherosclerotic Plaque in Asymptomatic Patients with Familial Hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 2019;39:945–955. doi: 10.1161/ATVBAHA.118.312414. [DOI] [PubMed] [Google Scholar]
- 202.Borissoff J.I., Joosen I.A., Versteylen M.O., Brill A., Fuchs T.A., Savchenko A.S., Gallant M., Martinod K., Ten Cate H., Hofstra L., et al. Elevated levels of circulating DNA and chromatin are independently associated with severe coronary atherosclerosis and a prothrombotic state. Arterioscler. Thromb. Vasc. Biol. 2013;33:2032–2040. doi: 10.1161/ATVBAHA.113.301627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Dicker A.J., Crichton M.L., Pumphrey E.G., Cassidy A.J., Suarez-Cuartin G., Sibila O., Furrie E., Fong C.J., Ibrahim W., Brady G., et al. Neutrophil extracellular traps are associated with disease severity and microbiota diversity in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2018;141:117–127. doi: 10.1016/j.jaci.2017.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Guervilly C., Lacroix R., Forel J.M., Roch A., Camoin-Jau L., Papazian L., Dignat-George F. High levels of circulating leukocyte microparticles are associated with better outcome in acute respiratory distress syndrome. Crit. Care. 2011;15:R31. doi: 10.1186/cc9978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Bendib I., de Chaisemartin L., Granger V., Schlemmer F., Maitre B., Hue S., Surenaud M., Beldi-Ferchiou A., Carteaux G., Razazi K., et al. Neutrophil Extracellular Traps Are Elevated in Patients with Pneumonia-related Acute Respiratory Distress Syndrome. Anesthesiology. 2019;130:581–591. doi: 10.1097/ALN.0000000000002619. [DOI] [PubMed] [Google Scholar]
- 206.Liu T., Wang J., Li T., Cui P., Hou B., Zhuang C., Wei G., Zhang S., Li H., Hu Y. Predicting disease progression in advanced non-small cell lung cancer with circulating neutrophil-derived and platelet-derived microparticles. BMC Cancer. 2021;21:939. doi: 10.1186/s12885-021-08628-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Rosell A., Aguilera K., Hisada Y., Schmedes C., Mackman N., Wallen H., Lundstrom S., Thalin C. Prognostic value of circulating markers of neutrophil activation, neutrophil extracellular traps, coagulation and fibrinolysis in patients with terminal cancer. Sci. Rep. 2021;11:5074. doi: 10.1038/s41598-021-84476-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Poulet G., Massias J., Taly V. Liquid Biopsy: General Concepts. Acta Cytol. 2019;63:449–455. doi: 10.1159/000499337. [DOI] [PubMed] [Google Scholar]
- 209.Goggs R., Jeffery U., LeVine D.N., Li R.H.L. Neutrophil-Extracellular Traps, Cell-Free DNA, and Immunothrombosis in Companion Animals: A Review. Vet. Pathol. 2020;57:6–23. doi: 10.1177/0300985819861721. [DOI] [PubMed] [Google Scholar]
- 210.Hayden H., Ibrahim N., Klopf J., Zagrapan B., Mauracher L.M., Hell L., Hofbauer T.M., Ondracek A.S., Schoergenhofer C., Jilma B., et al. ELISA detection of MPO-DNA complexes in human plasma is error-prone and yields limited information on neutrophil extracellular traps formed in vivo. PLoS ONE. 2021;16:e0250265. doi: 10.1371/journal.pone.0250265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Wang H., Sha L.L., Ma T.T., Zhang L.X., Chen M., Zhao M.H. Circulating Level of Neutrophil Extracellular Traps Is Not a Useful Biomarker for Assessing Disease Activity in Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. PLoS ONE. 2016;11:e0148197. doi: 10.1371/journal.pone.0148197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ronchetti L., Boubaker N.S., Barba M., Vici P., Gurtner A., Piaggio G. Neutrophil extracellular traps in cancer: Not only catching microbes. J. Exp. Clin. Cancer Res. 2021;40:231. doi: 10.1186/s13046-021-02036-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Mao L., Mostafa R., Ibili E., Fert-Bober J. Role of protein deimination in cardiovascular diseases: Potential new avenues for diagnostic and prognostic biomarkers. Expert Rev. Proteom. 2021;18:1059–1071. doi: 10.1080/14789450.2021.2018303. [DOI] [PubMed] [Google Scholar]
- 214.Mauracher L.M., Posch F., Martinod K., Grilz E., Daullary T., Hell L., Brostjan C., Zielinski C., Ay C., Wagner D.D., et al. Citrullinated histone H3, a biomarker of neutrophil extracellular trap formation, predicts the risk of venous thromboembolism in cancer patients. J. Thromb. Haemost. 2018;16:508–518. doi: 10.1111/jth.13951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Thery C., Witwer K.W., Aikawa E., Alcaraz M.J., Anderson J.D., Andriantsitohaina R., Antoniou A., Arab T., Archer F., Atkin-Smith G.K., et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Nemeth A., Orgovan N., Sodar B.W., Osteikoetxea X., Paloczi K., Szabo-Taylor K.E., Vukman K.V., Kittel A., Turiak L., Wiener Z., et al. Antibiotic-induced release of small extracellular vesicles (exosomes) with surface-associated DNA. Sci. Rep. 2017;7:8202. doi: 10.1038/s41598-017-08392-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ghanam J., Chetty V.K., Barthel L., Reinhardt D., Hoyer P.F., Thakur B.K. DNA in extracellular vesicles: From evolution to its current application in health and disease. Cell BioSci. 2022;12:37. doi: 10.1186/s13578-022-00771-0. [DOI] [PMC free article] [PubMed] [Google Scholar]