Summary
Humoral immunity is essential for protection against pathogens, emphasized by the prevention of 2–3 million deaths worldwide annually by childhood immunizations. Long-term protective immunity is dependent on the continual production of neutralizing antibodies by the subset of long-lived plasma cells (LLPC). LLPC are not intrinsically long-lived, but require interaction with LLPC niche stromal cells for survival. However, it remains unclear which and how these interactions sustain LLPC survival and long-term humoral immunity. We now have found that the immunosuppressive enzyme indoleamine 2,3- dioxygenase 1 (IDO1) is required to sustain antibody responses and LLPC survival. Activation of IDO1 occurs upon engagement of CD80/CD86 on the niche dendritic cells by CD28 on LLPC. Kynurenine, the product of IDO1 catabolism, activates the aryl hydrocarbon receptor in LLPC, reinforcing CD28 expression and survival signaling. These findings expand the immune function of IDO1 and uncovers a novel pathway for sustaining LLPC survival and humoral immunity.
Keywords: Durable humoral immunity, plasma cell survival, long lived plasma cells, IDO1, PC niche
eTOC blurb
Sustained protective antibody titers following infection/vaccination are produced by long-lived plasma cells (LLPC). What keeps LLPC alive remains largely unknown. Lightman et al have found unexpected involvement of the immunosuppressive enzyme indoleamine 2,3-dioxygenase 1, produced by dendritic cells in the LLPC nice, in sustaining LLPC survival and long-term antibody titers.
Graphical Abstract
Introduction
Epidemic infectious diseases are a major threat to human health, and protective immunity by vaccination has markedly reduced disease morbidity and mortality. In this decade alone, childhood immunizations have prevented ~10 million deaths (The World Health Organization, 2019, Dec 5, Roush et al., 2007). In contrast to children, generating durable vaccine responses in adults remains a significant challenge (Goodwin et al., 2006), as is waning immunity following natural infection. This is particularly important as natural infection by SARS-CoV-2 confers variable immunity (Dan et al., 2021), and the durability of current COVID-19 vaccines remains to be determined.
The efficacy of most preventive vaccines is through induction of protective antibodies (Ab) produced by terminally differentiated B-lineage plasma cells (PC) (Amanna et al., 2007). Sustained protective Ab titers (particularly without antigen re-exposure) require continuous Ab production by the long-lived PC (LLPC) subset (Mankarious et al., 1988). LLPC arise in germinal center reactions, and reside within a limited number of specific niches primarily in the bone marrow (BM) (Phan et al., 2006), but also in mucosa and gut-associate lymphoid tissue (Manz et al., 1997, Lemke et al., 2016, Cassese et al., 2001). LLPC are distinct from short-lived PC (SLPC), which reside primarily in secondary lymphoid organs (Moser et al., 2006, Sze et al., 2000). The hallmark of LLPC is their longevity (Amanna et al., 2007), allowing for lifelong maintenance of Ab titers without replenishment from reactivated B cells (DiLillo et al., 2007, Hammarlund et al., 2017, Manz et al., 1997, Slifka et al., 1995). However, the estimated LLPC lifespan elicited by different pathogens/vaccines has high variability (Amanna et al., 2007) for unclear reasons. Furthermore, LLPC are critically dependent on interactions with the niche for survival (Nguyen et al., 2018, Minges Wols et al., 2002, Zehentmeier et al., 2014, Tellier and Kallies, 2014). This is underscored in the poor immunization responses in the elderly, attributed to a reduced number of LLPC niches rather than the inability to generate LLPC (Han et al., 2003, Pritz et al., 2015).
Despite its importance, the LLPC niche remains largely uncharacterized. Identified components include eosinophils, megakaryocytes, basophils, T regulatory cells (Treg), mesenchymal, and dendritic cells (DC); extracellular matrix and soluble factors (APRIL, BAFF, IL-6) (Lightman et al., 2019, Garcia De Vinuesa et al., 1999, Glatman Zaretsky et al., 2017, Pioli, 2019). Functionally, the niche must shelter LLPC in a BM microenvironment that is highly proliferative, dynamic, metabolically, and immunologically active. LLPC themselves are not quiescent, with high metabolic demands due to Ab production (Lam et al., 2016, Lam and Bhattacharya, 2018). Additionally, LLPC express a neoantigen (the Ab idiotype) that could be targeted by cytotoxic T cells. How the LLPC niche creates a supportive, protective, and immune-privileged space that supports LLPC survival and function for a lifetime remains undefined.
We now find that the immunosuppressive tryptophan-catabolizing enzyme indoleamine 2,3-dioxygenase 1 (IDO1) (Mellor and Munn, 2004) is a previously unrecognized but essential LLPC niche component, induced upon LLPC engagement to niche DC. In contrast to IDOI’s well characterized suppression of T cell immunity (Ball et al., 2014, Munn et al., 1998, Mellor et al., 2001), we have found that IDO1 enhances durable humoral immunity by sustaining LLPC survival.
Results
IDO1 loss diminishes BM PC numbers and long-lived Ab titers
We have shown that the prototypic T cell costimulatory receptor CD28 is expressed on PC and is essential for LLPC (but not SLPC) survival and maintenance of long-term Ab responses (Rozanski et al., 2011, Rozanski et al., 2015, Utley et al., 2020). CD28 also plays a significant pro-survival role in human multiple myeloma (MM), the malignant counterpart of LLPC (Murray et al., 2014, Nair et al., 2011). PC CD28 is activated upon binding to CD80 and CD86 on DC (Rozanski et al., 2011), and in MM this engagement induces the DC to express active IDO1 (Nair et al., 2011, Koorella et al., 2014) – similar to T cell-DC interactions (Mellor et al., 2001). Given the closely shared biology between LLPC and MM (Boise et al., 2014), this suggested that IDO1 is also involved in LLPC biology.
IDO1’s role in humoral immunity is largely uncharacterized, with prior studies identifying its role in activation and regulation of early B cell responses to both T-independent and T-dependent antigens (Shinde et al., 2015, Matino et al., 2015, Merlo et al., 2016). To further assess this, we measured circulating immunoglobulin (Ig) levels in unvaccinated Ido1−/− (global knockout (KO)) mice vs. wild-type (WT) controls. Given that IDO1 suppresses helper T cell activation (Hwu et al., 2000), it was surprising that IDO1 loss did not enhance T-dependent B cell responses – rather, total IgG and IgA levels were significantly lower in Ido1−/− mice vs. WT, with equivalent IgM levels (Fig. 1A).
Figure 1. IDO1 loss diminishes BM PC numbers and long-lived Ab titers.
A) ELISA of circulating Ig levels from serum of unvaccinated mice, (mean ± SD) n= 16, statistical analysis by Student’s t test. This data is representative of 3 individual experiments. B) Total number of cells in each B cell subset in BM and SP of Ido1−/− (IDO KO) compared to WT mice as determined by flow cytometry, (mean ± SD) n = 10, statistical analysis by Student’s t test. C) Total number of PC in BM (left panel) and SP (right panel) of Ido1−/− mice compared to WT, (mean ± SD) n = 16, statistical analysis by Student’s t test. Data for B and C are representative of 2 individual experiments. D) Vaccination strategy of Ido1−/− and WT mice. Mice were subcutaneously injected with 100μg NP-Ova and Complete Freund’s Adjuvant (CFA) at a 1:1 ratio at day 0 and boosted with 100μg NP-Ova and Incomplete Freund’s Adjuvant (IFA) at a 1:1 ratio 1 week later, serum was serially collected at day 0, week 1, week 3 and then every 3 weeks until endpoint. E) ELISA of total circulating IgG1 (left panel) and NP-specific IgG1 (right panel) from serial serum samples of WT or Ido1−/− mice vaccinated with NP-OVA, n = 18, statistical analysis by Student’s t test. This data is representative of 3 individual experiments. F) ELISpot of NP-specific antigen secreting cells in BM of WT and Ido1−/− mice at Wk 12 post immunization, (mean ± SD), statistical analysis by Student’s t test. This data is aggregated from 2 individual experiments. G) ELISpot of NP-specific antibody secreting cells in BM and SP of WT and Ido1−/− mice 21 weeks post vaccination, (mean ± SD) n= 16, statistical analysis by Student’s t test. Data for BM is aggregated from 4 experiments and SP is aggregated from 2 experiments. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, ns = not significant. See also sFig. 1–3
Loss of B cells may explain diminished Ig levels in the Ido1−/− mice, but we found equivalent numbers in the major B cell subsets in BM and spleen (SP) as WT (Fig. 1B, sFig. 1A–B). There were also no significant differences in CD3+ and CD3+CD4+T cell numbers in BM or SP (sFig. 1C–D, 2A–B), but a small decrease in the CD3+CD8+ subset in the SP of Ido1−/−mice (sFig. 1C–D, sFig. 2C). The Ido1−/− mice had a small increase in macrophages in the BM vs. WT, but no differences in SP (sFig. 1G, 2D). Most striking was a significant decrease in the number of BM PC in the Ido1−/− vs. WT mice, which was not seen for SP PC (Fig. 1C, sFig. 1E–F, 2E–F). The field has classically used anatomic localization of PC to the BM to define LLPC, and PC from secondary lymphoid organs such as the spleen as a surrogate to define SLPC. In our work we have maintained this definition with the understanding that both PC subsets are more complex. This differential loss of BM PC vs. SP PC suggests that IDO1 loss is primarily affecting LLPC.
Loss of BM PC in unvaccinated conditions could be due to defects in B→PC differentiation, PC trafficking or survival once in the BM niche. To assess this and further define LLPC in the BM, Ido1−/− and WT mice were vaccinated with the T-dependent antigen 4-hydroxy-3-nitrophenylacetyl (NP)-ovalbumin and followed for NP-specific IgG1 Ab responses over 21 weeks (Fig. 1D). Compared to Freund’s (Fig. 1D), adjuvanting with alum (sFig. 3A) failed to produce a durable long-term anti-NP response in either WT or Ido1−/− mice (sFig. 3B–C), consistent with its lower potency as a vaccine adjuvant (Bemark et al., 2011). Previous studies have shown that B→PC differentiation and PC trafficking to the BM is complete by Wk 9 post-vaccination (Weisel et al., 2016). Total serum IgG1 levels were equivalent between WT and Ido1−/− mice at all timepoints post-vaccination (Fig. 1E, left). There were no differences in NP-specific IgG1 titers through Wk 12 (Fig. 1E, right), indicating equivalent B cell activation and PC generation (Weisel et al., 2016). However, NP-specific titers began to drop after Wk 15 in the Ido1−/− mice and were significantly lower at Wk 21 vs. WT (Fig. 1E, right). To more clearly define the source of NP-specific Ab at the later timepoints, vaccinated mice were irradiated at Wk 15 - as sub-lethal radiation depletes SLPC and memory B cells, but not LLPC (Slifka et al., 1998). This had no effect on total IgG1 (sFig. 3D) or anti-NP titers at Wk 18 and 21 (sFig. 3E), indicating antibody production predominantly by LLPC. To correlate Ab titer to cellular responses, NP-specific IgG Ab secreting cell (ASC) numbers were quantified at Wk 12 and 21. Equivalent numbers of NP-specific ASC were present in the BM of WT vs. Ido1−/− mice at Wk 12 (Fig. 1F), consistent with the anti-NP titers. This contrasts with fewer total BM PC in unvaccinated Ido1−/− vs. WT mice (Fig. 1C) and suggests there are equivalent numbers of LLPC niches to accommodate the vaccine-generated BM PC. Mirroring the anti-NP titers, by Wk 21 there was a significant decrease in the number of BM NP-specific IgG ASC in the Ido1−/− vs. WT mice (Fig. 1G, left), with no difference in SP NP-specific ASC numbers (Fig. 1G, right).
To confirm that the effect of the genetic ablation of IDO1 on LLPC was not due to an undetected distortion in cell development/differentiation, the effect of pharmacological inhibition of IDO1 was assessed. WT mice were vaccinated in the same manner and monitored over the course of 18 weeks (Fig. 2A). From week 15 to 18 mice were given the IDO1 inhibitor epacadostat (Yue et al., 2017). Total serum IgG 1 levels were equivalent in both groups at all time points post-vaccination (Fig. 2B, top), however NP-specific titers dropped significantly after initiation of epacadostat treatment compared to control (Fig. 2B, bottom). A significant decrease in NP-specific ASC in the BM of the epacadostat treated group compared to control was also observed at Wk 18 (Fig. 2C), but not in total IgG ASCs. This supports the observations seen in the IDO1 knockout mice.
Figure 2. Chemical Inhibition of IDO1 diminishes long-lived Ab titers.
A) Experimental design. C57BL/6 WT mice were vaccinated with NP-OVA + CFA at Day 0 and boosted at Wk 1 with NP-OVA + IFA. At Wk 15 experimental group was treated with Epacadostat (100 mg/kg/mouse) via oral gavage for 3 weeks. Serial samples taken pre-vaccination, Wk 1, Wk 3, Wk 6, Wk 9, Wk 12, Wk 15 (post-treatment), and Wk 18. B) ELISA of total circulating IgG1 (top panel) and NP-specific IgG1 (bottom panel) from serial serum samples of WT mice vaccinated with NP-OVA and treated with Epacadostat or vehicle control, n = 10, statistical analysis by Student’s t test. This data is representative of 2 individual experiments. C) ELISpot of total IgG and NP-specific IgG antigen secreting cells in BM of Vehicle and Epacadostat treated mice at Wk 18, n = 10, (mean ± SD), statistical analysis by Student’s t test. This data is aggregated from experiments. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, ns = not significant.
PC-DC interaction through CD28:CD80/CD86 induces active IDO1
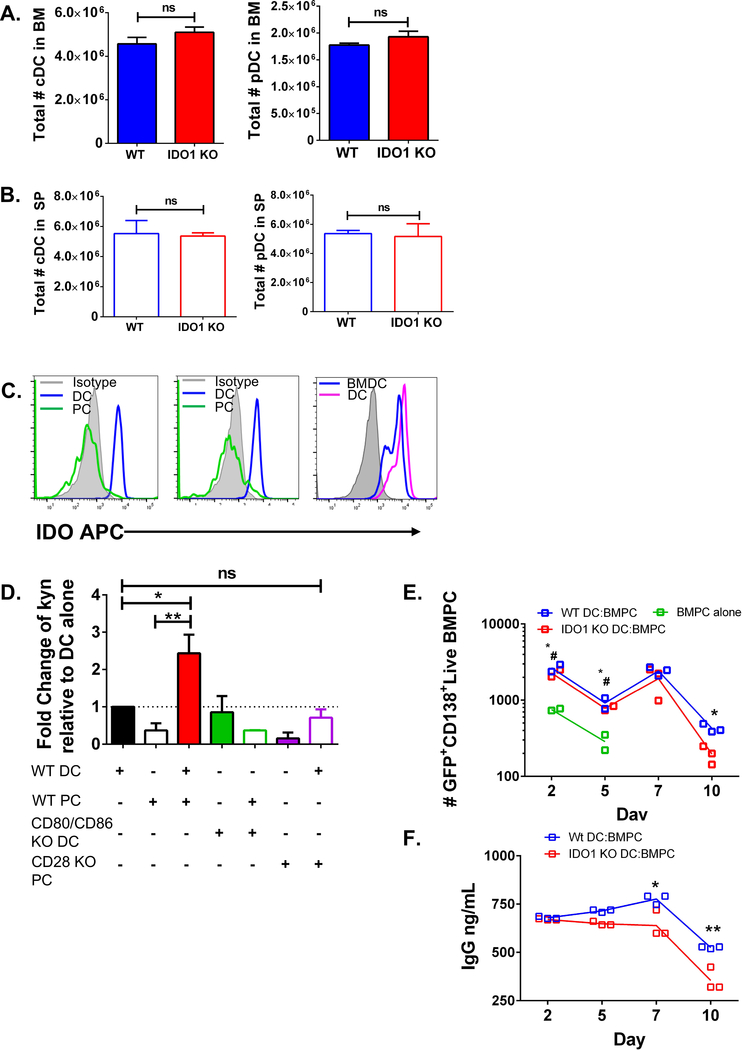
The effect of IDO1 on LLPC survival in the BM niche raises the question of where the IDO1 is coming from. Given human MM cells can induce IDO1 from DC in vitro (Nair et al., 2011, Koorella et al., 2014), we first assessed the number of classical DC (cDC) and plasmacytoid DC (pDC) in Ido1−/− and WT mice in BM and SP, and found no differences (Fig. 3A–B, sFig. 1H). IDO1 protein was expressed in WT CD11c+ DC purified directly from the BM and in in vitro BM-derived DC (BMDC) (Fig. 3C, right panel). In contrast, IDO1 was not expressed in BM PC, cultured alone or with BMDC (Fig. 3C, left and middle panels).
Figure 3. PC-DC interaction through CD28:CD80/CD86 induces active IDO1.
A) Quantification of total cDC (left panel) and pDC (right panel) in the BM and SP of Ido1−/− (IDO KO) and WT mice, (mean ± SD) n = 10, statistical analysis by Student’s t test. This data is representative of 2 individual experiments. B) Quantification of SP cDC (left panel) and pDC (right panel) from WT and Ido1−/− (mean ± SD) n= 10, statistical analysis by Student’s t test. Data are representative of 2 individual experiments. C) Representative flow plot of IDO1 protein expression in PC or BMDC cultured alone in vitro at 24 hrs (left), representative histogram of IDO1 expression in PC and BMDC after co-culture together at 24hr (middle panel) and of BMDC and purified CD11c+ DC alone (right panel) in culture at 24hr compared to isotype control, representative of 3 individual experiments. C) IDO activity as measured by amount of kynurenine in supernatant normalized to DC alone condition, (mean ± SD), statistical analysis by one-way ANOVA. Data is aggregated from 3 individual experiments. D) Number of live GFP+CD138+ PC (purified from UBC-GFP mouse BM) from co-culture with WT BMDC or Ido1−/− BMDC measured over 10 days, statistical analysis by Student’s t test. Data is aggregated from 3 individual experiments. *# = PC alone compared with WT DC:BMPC, * = WT DC:BM PC compared with Ido1−/− DC:BM PC. E) ELISA of IgG in the supernatant of PC co-cultured with WT BMDC or Ido1−/− BMDC measured over 10 days, statistical analysis by one-way ANOVA. This data is representativa of 3 individual experiments. *p<0.05, **p<0.01, ***p<0.001, ns = not significant. See also sFig. 4 & 5.
IDO1 activity was significantly increased when WT BMDC were co-cultured with purified WT BM PC vs. BMDC or PC alone (Fig. 3D, sFig. 4A), as measured by the level of the tryptophan catabolite kynurenine (kyn) (Taylor and Feng, 1991)), which was completely lost when BMDC lacked both CD80 and CD86 (Fig. 3D, sFig. 4B) or BM PC lacked CD28 (Fig. 3D). To assess IDOI’s role in BMDC-supported BM PC survival, WT BM PC were co-cultured with BMDC generated from WT or Ido1−/− mice (sFig. 4B and 5A). BM PC cultured alone rapidly died as measured by number of viable cells (Fig. 3E) and IgG production (sFig. 5B). Co-culture with WT or Ido1−/− BMDC increased BM PC survival (Fig. 3E, sFig. 5C), but WT DC were significantly better at sustaining PC survival and function (IgG production, Fig. 3F) at later time points. These data demonstrate that active IDO1 is directly induced in DC by BM PC through a CD28-CD80/CD86 interaction, and this supports LLPC survival and function in vitro.
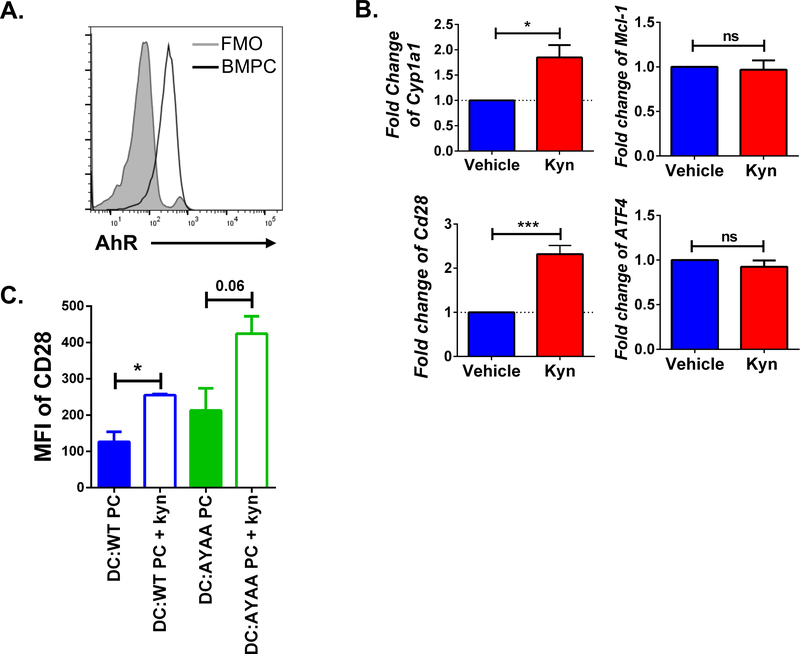
Kynurenine activates AhR in LLPC
The above data suggest a pro-survival role for IDOI-produced kyn. Kyn is a ligand for the aryl-hydrocarbon receptor (AhR) (Mezrich et al., 2010), which regulates survival and proliferation in a wide range of cell types, and modulates immune responses (Vogel et al., 2013, Piper et al., 2019, Bianchi-Smiraglia et al., 2018). AhR mRNA expression and an AhR-response gene signature have been reported in PC (Sherr and Monti, 2013, Valor et al., 2017), but its biological role in normal PC is unknown. We found that BM PC express AhR protein (Fig. 4A), and that kyn treatment activated AhR as measured by upregulated expression of the AhR-target gene Cyp1a1 (Nebert et al., 1993) (Fig. 4B, top left). Kyn did not increase the expression of Mcl-1, Atf4, or Blimp-1 (which play central roles in PC survival (Peperzak et al., 2013, Zhu et al., 2019)) (Fig. 4B, right and sFig. 6A), but increased CD28 expression (Fig. 4B, bottom left). CD28 protein expression was similarly upregulated in BM PC co-cultured with DC, and in the CD28-responsive PC cell line J558 (Rozanski et al., 2015) when treated with kyn (Fig. 4C, sFig. 6B).
Figure 4. Kynurenine upregulates CD28 in LLPC.
A) Representative histogram of AhR protein expression in PC from the BM of a WT mouse compared to fluorescence minus one (FMO), representative of 4 individual experiments. B) Fold change of Cyp1a1, Mcl-1, Atf4, and Cd28 mRNA expression from purified BM PC cultured in media with or without 100 μM kynurenine, (mean ± SD) n = 3, statistical analysis by Student’s t test. This data is representative of 3 individual experiments. C) MFI of CD28 on purified BM PC from WT and CD28-AYAA KI mice in co-culture with BMDC with or without kyn (100 μM) (mean ± SD), statistical analysis by Student’s t test. Data is aggregated from 2 individual experiments. *p<0.05, **p<0.01, ***p<0.001, ns = not significant. See also sFig. 6.
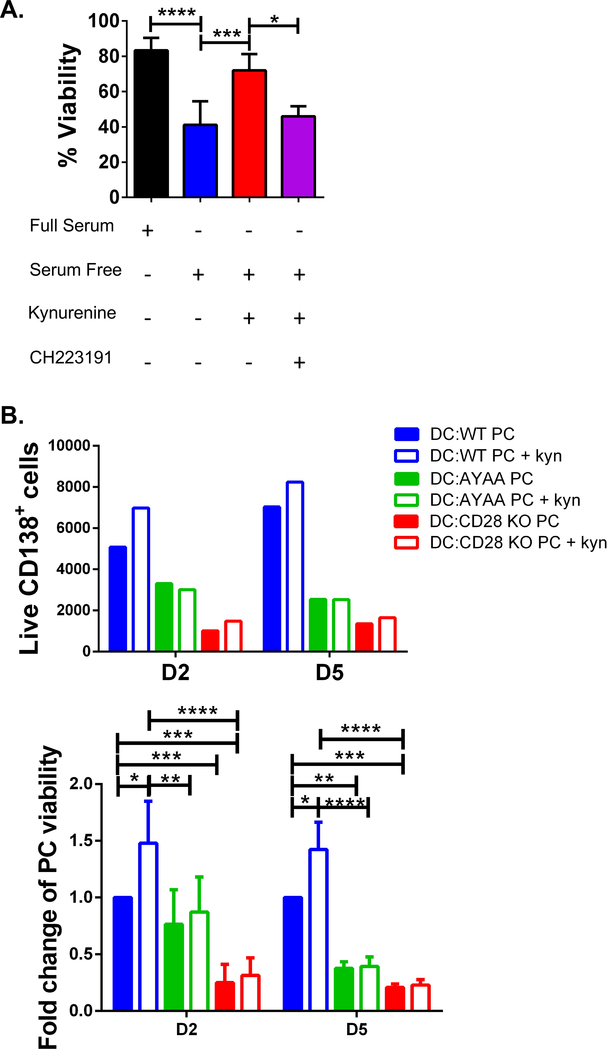
Since purified BM PC rapidly die when cultured alone, the direct pro-survival effect of kyn-induced AhR activation was first assessed in J558. Kyn treatment significantly increased J558 viability following serum starvation, which was blocked by the AhR inhibitor CH223191 (Fig. 5A). We next examined whether kyn had a pro-survival effect on BM PC co-cultured with DC. BM PC from WT, CD28-AYAA knock-in (KI), or CD28−/− (global KO) mice were co-cultured with BMDC ± kyn. CD28-AYAA KI mice have a mutation in the receptor tail that abrogates downstream signaling to Vav (Boomer and Green, 2010), which is required for LLPC survival and long-lived Ab titers (Rozanski et al., 2015). BMDC co-culture supported WT PC but not CD28−/− PC survival (Fig. 5B, consistent with (Rozanski et al., 2011)). Kyn addition significantly increased the survival of WT PC, but not CD28-AYAA or CD28−/− PC. Given the CD28 AYAA PC still express surface CD28 that can bind to DC CD80/CD86 and induce IDO1, this indicates that signaling-competent CD28 is necessary for kyn’s pro-survival effect. This was also seen in kyn-treated J558 (no DC co-culture) when constitutive CD28 signaling was blocked (sFig. 6C–D). Altogether these findings suggest IDO1-generated kyn mediates its pro-LLPC survival effects via AhR-induced upregulation of CD28 expression.
Figure 5. Kynurenine activates AhR and CD28 supporting survival of LLPC.
A) Viability of J558 cell line as determined by trypan blue exclusion in complete media, serum free media (SF), SF + kynurenine (100 μM), or SF + kynurenine (100 μM) + CH 223191 (20 μM), (mean ± SD) n =3, statistical analysis by one-way ANOVA. This data is aggregated from 2 individual experiments. E) (Above panel) Number of live CD138+ BM PC (determined by Live Dead Fixable Dye) from WT, CD28-AYAA KI and CD28−/− (CD28 KO) mice in co-culture with WT BMDC with or without kyn (100 μM), representative plot of 3 individual experiments. (Bottom panel) Fold change of viability of live BM PC compared to the WT PC:BMDC condition, n = 3, statistical analysis by Student’s t test. This is aggregated data from 3 individual experiments. *p<0.05, **p<0.01, ***p<0.001, ns = not significant. See also sFig. 6.
DC are necessary to maintain BM LLPC and long-lived Ab responses in vivo
An in vivo requirement for DC to support LLPC survival (suggested by above and (Garcia De Vinuesa et al., 1999, Glatman Zaretsky et al., 2017, Koorella et al., 2014, Rozanski et al., 2011)) was examined by using CD11c-diptheria toxin receptor (DTR) transgenic mice (Jung et al., 2002) – allowing for depletion of CD11c+ DC in vivo by diphtheria toxin (DT) administration and assessment of the effect on established Ab titers. DTR+ and DTR− littermates were immunized with NP-ovalbumin, boosted once at Wk 1, and given DT for 1 week starting at Wk 15 when antibody titers had plateaued and the NP-specific LLPC were established in the BM (Fig. 6A). DT administration caused a significant decrease in the number of CD11c+ cells in the BM of DTR+ mice vs. DTR− littermates as determined by serial BM aspirates (Fig. 6B). While NP-specific IgG1 titers were equivalent at Wk 15, there was a significant decrease post DT administration (Wk 18) in the DTR+ vs. DTR− controls (Fig. 6C), with no significant differences in the total IgG 1 levels (Fig. 6D). Consistent with this, while the percentage of PC in the BM pre-DT was equivalent as determined by serial BM aspirates (Fig. 6E), DT administration caused a significant decrease in the NP-specific IgG ASC in the BM of DTR+ vs. DTR− mice (Fig. 6F), but not in SP NP-specific ASC (Fig. 6G). Altogether these data directly demonstrate a requirement for DC in supporting BM PC survival in vivo.
Figure 6. DC are necessary to maintain BM LLPC and long-lived Ab responses in vivo.
A) Immunization and diphtheria toxin (DT) administration schedule. B) Percent of CD11c+ cells in DTR+ and DTR− post DT treatment, (mean ± SD) n = 6, statistical analysis by Student’s t test. This data is representative of 3 individual experiments. C) ELISA of NP-specific IgG1 from serum of DTR+ and DTR− mice vaccinated with NP-OVA, (mean ± SD) n = 14, statistical analysis by Students t-test. Data are aggregated from 2 individual experiments. D) ELISA of total circulating IgG1 from serum of DTR+ and DTR− mice vaccinated with NP-OVA (mean ± SD), n = 14, statistical analysis by Student’s t test. Data is aggregated from 2 individual experiments. E) Percent of B220−CD138+ and IgG+ of CD138+ PC from BM aspirate of DTR+ and DTR− mice pre-DT treatment at Wk 15, (mean ± SD) n =14, statistical analysis by Student’s t test. Data is aggregated from 2 individual experiments. F & G) ELISpot of NP-specific antigen secreting cells in BM and SP of DTR+ and DTR− mice 18 weeks post vaccination, (mean ± SD) n = 20, statistical analysis by Student’s t test. Data for F and G are aggregated data of 2 individual experiments. *p<0.05, **p<0.01, ***p<0.001, ns = not significant.
LLPC survival is dependent on DC production of IDO1
Our findings demonstrate that PC-DC interactions upregulate DC IDO1 activity in vitro, and separately that DC and IDO1 are required for LLPC survival in vivo. This suggests that DC are the primary producers of the IDO1 that supports LLPC survival. To interrogate this, we generated BM chimeric mice utilizing BM from Ido1−/− or WT mice mixed with CD11c-DTR BM (which express IDO1), resulting in WT/CD11c-DTR+ or Ido1−/−/CD11c-DTR+ chimeras. After BM reconstitution these chimeras were vaccinated with NP-Ova (Fig. 7A). In the latter chimera the IDO+ CD11c-DTR DC can be depleted with DT after a long-lived Ab response is established, leaving only Ido1−/− DC to support LLPC survival. Chimerism by the percentage of DTR+ genomic DNA ranged from 37%−75% DTR+ in both groups (sFig 7A). Pre-DT serial BM aspirates show the number of BM CD11c+ cells were equivalent (Fig. 7B), and the Ido1−/−/CD11c-DTR+ group had both IDO+ and IDO− DC (sFig. 7B). DT administration at Wk 15 equivalently depleted CD11c+ DC in both chimeras (sFig. 7C), and IDO1 was decreased only in the Ido1−/−/CD11c-DTR+ group (Fig. 7C–D). While NP-specific titers were equivalent pre-DT, they were significantly lower in the Ido1−/−/CD11c-DTR+ chimeras following DT administration at Wk 18 vs. WT/CD11c-DTR+ (Fig. 7E). Similarly, while NP-specific BM PC by BM aspirates were equivalent pre-DT (Fig. 7F and sFig. 7D), the Ido1−/−/CD11c-DTR+ group had significantly fewer BM NP-specific IgG ASC vs. WT/CD11c-DTR+ post-DT administration at Wk 18 (Fig. 7G). Altogether these data reveal a previously unrecognized but essential role for IDO1 production by BM niche DC in maintaining LLPC populations and persistent Ab titers.
Figure 7. LLPC survival is dependent on DC production of IDO1.
A) Experimental design and chimerism strategy, immunization, and DT treatment schedule. B and D) Quantification of percent of CD11c+ cells and IDO1 expression of CD11c+ cells from serial BM aspirates of Ido1−/− (IDO KO)/DTR+ and WT/DTR+, (mean ± SD) n= 20, statistical analysis by Student’s t test. Data is aggregated from 3 experiments. C) Representative histograms of IDO1 expression in CD11c+ cells from BM aspirates of Ido1−/−/DTR+ and WT/DTR+ at Wk 18 post-DT treatment, representative of 3 individual experiments. E) NP-specific IgG1 Elisa of serum from Ido1−/−/DTR+ and WT/DTR+ mice, n= 8, statistical analysis by Student’s t test. This data is aggregated from 2 individual experiments. F) Percent of B220−CD138+IgG+NP+ cells from BM aspirates of Ido1−/−/DTR+ and WT/DTR+ chimeras at Wk 15 pre-DT administration, (mean ± SD) n= 10, statistical analysis by Student’s t test. Data is representative of 2 individual experiments. G) ELISpot of NP-specific ASC in BM of Ido1−/−/DTR+ and WT/DTR+ mice post-DT administration (Wk 18) (mean ± SD) n= 20, statistical analysis by Student’s t test. This data is aggregated from 3 individual experiments. *p<0.05, **p<0.01, ***p<0.001, ns = not significant. See also sFig. 7.
Discussion
Neutralizing Ab are essential for protective immunity against a wide range of infectious diseases (VanBlargan et al., 2016, Amanna et al., 2007), but the lack of persistence of Ab titers remains a major challenge for vaccination in adults (The World Health Organization, 2019, Dec 5). Furthermore, understanding how to establish a persistent protective response is critical in light of the COVID-19 pandemic. While it was initially reported that serum antibodies rapidly decreased after infection with SARS-Cov-2 (Ibarrondo et al., 2020), new findings have determined that LLPC can be present in the BM after infection (Turner et al., 2021). In 15 out of 19 volunteers infected with mild SARS-Cov-2, 7–11 months later SARS-Cov-2 specific BMPC were detected. However, 4 of the volunteers did not have detectable SARS-Cov-2 specific BMPC. Additionally, the authors state that of the S-binding BMPC detected, it is unclear what fraction of these BMPC encode for neutralizing antibodies and to what level, which is a critical component to long term protection after infection or vaccination. While sustained Ab production by LLPC is a major component of long-term humoral immunity (Amanna et al., 2007), why the longevity of different LLPC is so varied remains unclear. It is known that LLPC are critically dependent on both intrinsic factors (e.g. signaling from BAFF and CD28) and extrinsic factors in the niche (e.g. stromal cell interactions and cytokines like IL-6), however the interplay of these factors are largely unknown (Moser et al., 2006, Lightman et al., 2019).
We and others have shown that CD28 on PC modulates PC survival and Ab titers (Rozanski et al., 2011, Rozanski et al., 2015, Utley et al., 2020) (Njau et al., 2012), with our work demonstrating that CD28 delivers an essential pro-survival signal for LLPC. In vitro, LLPC CD28 binding to CD80/CD86 expressed on DC sustains LLPC survival (Rozanski et al., 2011). Interaction with DC is also pro-survival for human myeloma cells (Nair et al., 2011, Koorella et al., 2014, Murray et al., 2014), and induces DC production of active IDO1 through CD28-CD80/CD86 engagement (Koorella et al., 2014). This suggested a previously unrecognized role for IDO1 in the interaction between normal LLPC and the niche. The regulation of humoral immunity by IDO enzymes is largely unexplored, with only a few studies examining B cell activation and initial PC formation (Shinde et al., 2015, Merlo et al., 2014, Merlo et al., 2016, Matino et al., 2015). Previous studies largely focus in on IDO1 mediating T cell suppression and IDO1 and IDO2 in mediating B cell activation and initial responses (Merlo et al., 2020). We found in contrast to IDO1’s well-characterized suppression of T cell responses and the effect on SLPC (Mellor et al., 2001, Merlo et al., 2020), that unvaccinated Ido1−/− mice had significantly less total IgG and IgA (but not IgM) and BM PC numbers vs. WT controls. This was not due to clear abnormalities in the B, T or myeloid cell compartments, and Ido1−/− mice could generate an initial NP-specific Ab response following vaccination equivalent to WT controls. However, the absence of IDO1 compromised LLPC survival within the BM and the ability to sustain long-term Ab titers. In contrast, IDO1 loss did not affect IgM-secreting PC, suggesting they occupy a different niche (Reynolds et al., 2015).
The induction of IDO1 by BM PC engagement of DC (via CD28:CD80/CD86) suggests that the occupancy of the niche by LLPC is what brings IDO1 into play. Interestingly, induction of IDO1 activity did not require exogenous pro-inflammatory cytokines (IFNγ, IFNα, or TGFβ) (sFig. 4C) as has been reported in settings of immune/T cell activation (Jurgens et al., 2009, Mellor and Munn, 2008). Whether this IDO1 induction is truly independent of IFN is not known, as a role for endogenously produced IFN cannot be ruled out in our experiments. We have also previously reported that CD80/CD86 induction of IDO1 required co-signaling through Notch1 (Koorella et al., 2014), suggesting the potential for additional interactions to modulate DC IDO1 induction that may differ between T cells and PC. Altogether these findings identify the importance of IDO1 activity of DC origin upon interaction with PC in the direct maintenance of LLPC survival. Production of IDO1 by the LLPC niche may also suppress proliferation of adjacent hematopoietic progenitors, “carving out” space in the BM.
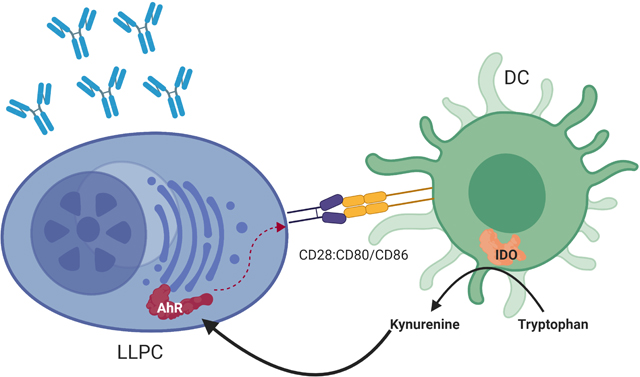
Activation of IDO1 results in low levels of Trp leading to activation of the GCN2 stress-response kinase pathway in T cells (Munn et al., 2005), however upregulation of this pathway as reflected in Atf4 upregulation (Kilberg et al., 2009) was not observed in PC (sFig.6E). Therefore, the direct pro-survival effect of IDO1 on BM PC in DC co-cultures indicates involvement of some product of IDO1 activity, and we found that the Trp catabolite kyn activated a pro-survival signal through AhR. AhR has multifaceted roles in immune responses, including Treg activation (Mezrich et al., 2010, Vogel et al., 2013) and regulation of B cell activation and maturation (Vaidyanathan et al., 2017, Piper et al., 2019). Hyperactivation of AhR prevents B→PC differentiation in vitro by regulating Blimp-1 and PAX5 expression (Sherr and Monti, 2013). Counterintuitively however, PC have the highest expression of AhR mRNA within the B lineage (Sherr and Monti, 2013), and AhR response elements are enriched in the regulatory regions associated with the transcriptional signature in early PC (Valor et al., 2017). Little is known about AhR function in normal PC biology, although we have recently reported it has a pro-survival role in MM (Bianchi-Smiraglia et al., 2018). We found that kyn-mediated AhR activation upregulated CD28 expression on PC, and that kyn-AhR enhancement of BM PC survival was lost if CD28 was unable to signal. This uncovers a self-reinforcing loop where PC CD28 engages DC CD80/CD86 and induces active IDO1, which generates kyn that activates PC AhR, which upregulates PC CD28 expression. This sustains both CD28’s direct pro-LLPC survival signal and ability to engage niche DC through CD80/CD86, in addition to modulating the surrounding microenvironment.
Previous in vitro studies have demonstrated DC support BM PC survival (Rozanski et al., 2011, Garcia De Vinuesa et al., 1999), but in vivo relevance is unknown. We found that DC are required to maintain established Ab titers and support the SP SLPC and BM LLPC survival in vivo. Furthermore, it was not just the presence of DC that was necessary, but also their ability to express IDO1. It is likely that other LLPC niche cells also regulate IDO1 activity. For example, CTLA4+ Tregs have been directly visualized as components of the niche (Glatman Zaretsky et al., 2017), and can engage DC CD80/CD86 to induce IDO1 (Mellor and Munn, 2004). This interaction and the induction of IDO1 promotes bone marrow homeostasis by limiting excessive osteoclast formation and bone reabsorption (Bozec et al., 2014). IDO1-produced kyn also activates and regulates Treg function (Mezrich et al., 2010) and also has the ability to tolerize DC through AhR autocrine signaling, reducing their ability to trigger a proinflammatory response (Li et al., 2016, Quintana et al., 2010). These adjacent signals mediated by AhR could potentially represent other self-reinforcing pathways that facilitates LLPC survival by indirectly providing a stable, protected, and less inflammatory niche.
Altogether our work demonstrates that DC-produced IDO1 is an essential component in the niche that supports LLPC survival and thus sustained humoral responses. We believe that IDO1 is both a key modulator of the BM niche and a central component of a self-reinforcing loop that maintains the pro-survival interactions between LLPC and this niche. IDO’s involvement in sustaining long-lived antibody responses also raises the possibility that the physiological counter-regulatory immunosuppressive responses to initial inflammation/immune activation have an unappreciated role in sustaining durable immune responses. These insights may lead to additional understanding of the basis for antibody titer persistence, as well as novel therapeutic approaches to enhance protective humoral immunity or suppress pathologic antibody responses.
STAR Methods
Resources Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Kelvin P. Lee (kplee@iu.edu).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
Section 1: Data
All data reported in this paper will be shared by the lead contact upon request.
Section 2: Code
This paper does not report original code.
Section 3:
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Experimental Model and Subject Details
Mice
Procedures were approved by the Institutional Animal Care and Use Committee at Roswell Park Comprehensive Cancer Center. Retired female C567BL6/Ncr mice (6–8 month old), and female and male 6–8 week old C57BL6/Ncr mice were purchased from Charles River and were housed in pathogen-free facilities. 6–8 week female and male C57BL/6J mice were purchased from Jackson Laboratories for chimeric experiments and housed in pathogen-free facilities. Female and male breeding pairs of B6.129-Ido1tm1Alm/J mice (Ido1−/−), B6.FVB-1700016L21RjkTg(Itgax-DTR/EGFP)57Lan/j mice (CD11c-DTR), and B6.129S2-Cd28tm1Mak/J (CD28−/−) mice were purchased from Jackson Laboratories. Female and male breeding pairs of CD28-AYAA knock in (KI) mice were derived as previously reported (Boomer and Green, 2010). Upon receipt all animals were house and bred in the Division of Laboratory Animal Resources (RPCI, Buffalo, NY) in a pathogen-free barrier facility. Age matched 6–8 week old femurs and tibias from B6.129S4-Cd80tm1Shr Cd86tm2Shr/J (CD80/CD86−/−) crossed with C57BL/6 Tg(TcraTcrb)1100Mjb/J mice were kindly provided by Drs. Stephen Schoenberger and Joey Lee (La Jolla Institute for Allergy and Immunology). Age matched 6–8 week old femurs and tibias from C57BL/6Tg(UBC-GFP)30Scha/J were generously provided by Dr. Michael Nemeth and Jennifer Peresie (RPCCC).
BM-derived dendritic cell (BMDC) generation:
BM from both male and female C57BL/6 WT, Ido1−/−, and CD80/CD86−/− mice were used to generate BMDC. BM cells were taken from the tibia and femurs of age matched mice and put into culture in 6 well plates with 20 ng/mL recombinant murine GM-CSF (Miltenyi mouse recombinant GM-CSF research grade 130–094-043) for 7 days at a concentration of 1 × 106 cells/mL, 2mL per well. On day 3 and 5, 1mL was taken from each well and replenished with fresh media + 20 ng/mL of GM-CSF.
Co-culture experiments
PC were purified from the bone marrow of male and female UBC-GFP mice or C57BL/6 WT mice (and stained with Cell Trace Violet- ThermoFisher) for use in the co-culture experiments to differentiate PC from BMDC by flow cytometry. Co-culture was plated in a 96 well plate at a 1:2 ratio, 2 × 104 PC with or without 4 × 104 BMDC in 0.2 mL of culture medium (10% FBS RPMI) supplemented with 10 ng/mL GM-CSF at 37°C with 5% CO2 per well for 10 days. Supernatant was collected on Day 2, 5, 7, and 10 for IgG analysis by ELISA. At time of supernatant collection, fresh media was given to cells, supplemented with GM-CSF. Cells were harvested from Day 2–10 and analyzed by flow cytometry for viability.
Cell Culture
J558 (TIB-6 ATCC, sex for cell line not disclosed upon purchase from ATCC) and purified BM PC from female C57BL/6 WT BM were cultured in vitro in complete RPMI with or without the addition of L-kynurenine (Sigma Aldrich 2922–83-0) at a concentration of 100 μM per well. BM PC were harvested at 1 hr for RNA extraction and qPCR analysis. J558 were harvested at 24 and 48 hours for flow cytometry and viability experiments.
Co-culture
BM from male and female C57BL/6 WT and CD80/CD86−/− mice were used to generate BMDC. PC were purified from the bone marrow of female C57BL/6 WT or CD28−/− mice. Co-culture was plated in a 96 well plate at a 1:2 ratio, 2 × 104 PC with or without 4 × 104 BMDC in 0.2 mL of culture medium/well supplemented with 100 pM L-tryptophan (Sigma Aldrich 73–22-3) at 37°C with 5% CO2 for 2 hours. IFNγ (Miltenyi mouse IFNγ research grade, 130–105-790) was given at 1000 Units/mL. At 2 hours the supernatant was collected from each well to be used in the IDO activity assay as previously described (Asghar et al., 2015).
BM chimeras
Chimeras were generated at a 1:1 ratio of male and female BM from either C57BL/6 WT and CD11c-DTR+ or Ido1−/− and CD11c-DTR+ BM into lethally irradiated Ido1−/− or C57BL/6 WT hosts. Bone marrow was injected IV at a concentration of 106 cells. BM was allowed to repopulate for 6 weeks in mice before use in immunizations and further experimentation. At 6 weeks mice were bled retro-orbitally to collect whole blood for genomic DNA isolation and chimerism quantification.
Method Details
Antibodies and Flow Cytometry
All antibodies were used at a 1:100 dilution concentration unless otherwise noted. For intracellular staining of IDO1 (eBioscience, clone mIDO-48) and AhR (eBioscience, clone 4MEJJ), cells were surface stained with antibodies mentioned in key resources table and supplemental figures to delineate cell populations. The eBioscience Fixation and Perm Buffer Set (IC fixation buffer and 10x Permeabilization buffer, Cat # 88–8824-00) were used for intracellular staining and used per the manufacturer’s protocol.
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| AhR Monoclonal Antibody (4MEJJ)- PE | eBioscience/ThermoFisher Scientific | 12-5925-82 |
| IDO Monoclonal Antibody (mIDO-48), eFluor 660 | eBioscience/ThermoFisher Scientific | 50-9473-82 |
| Rat IgG2b kappa Isotype Control (eB149/10H5), eFluor 660 | eBioscience/ThermoFisher Scientific | 50-4031-82 |
| APC anti-mouse CD3 | Biolegend | 100236 |
| FITC anti-mouse CD3 | Biolegend | 100203 |
| APC anti-mouse CD43 | Biolegend | 143207 |
| APC/Cyanine 7 anti-mouse/human CD45R/B220 | Biolegend | 103223 |
| Brilliant Violet 421 anti-mouse/human CD45R/B220 | Biolegend | 103240 |
| Brilliant Violet 421 anti-mouse CD24 | Biolegend | 101825 |
| FITC anti-mouse IgM | Biolegend | 406505 |
| PE anti-mouse IgD | Biolegend | 405705 |
| Brilliant Violet 605 anti-mouse CD184 (CXCR4) | Biolegend | 146519 |
| PerCP/Cyanine 5.5 anti-mouse CD93 | Biolegend | 136511 |
| FITC anti-mouse CD19 | Biolegend | 152403 |
| Pacific Blue anti-mouse/human CD11b | Biolegend | 101223 |
| APC/Cynanine 7 anti-mouse CD3 | Biolegend | 100221 |
| PerCP/Cyanine 5.5 anti-mouse CD45R/B220 | Biolegend | 103235 |
| APC anti-mouse CD19 | Biolegend | 152409 |
| PE anti-mouse CD21/CD35 (CR2/CR1) | Biolegend | 123409 |
| PerCP/Cyanine 5.5 anti-mouse CD4 | Biolegend | 100539 |
| APC/Cyanine 7 anti-mouse CD8b.2 | Biolegend | 140421 |
| PE anti-mouse CD4 | Biolegend | 116005 |
| PE anti-mouse CD28 | Biolegend | 102106 |
| PerCP/Cyanine 5.5 anti-mouse CD28 | Biolegend | 102114 |
| APC anti-mouse CD138 (Syndecan-1) | Biolegend | 142506 |
| PE anti-mouse CD138 (Syndecan-1) | Biolegend | 142504 |
| PE anti-mouse F4/80 | Biolegend | 123109 |
| PE anti-mouse CD11c | Biolegend | 117307 |
| Brilliant Violet 785 anti-mouse CD11c | Biolegend | 117335 |
| PE anti-mouse CD80 | Biolegend | 104708 |
| PE anti-mouse CD86 | Biolegend | 105007 |
| PE anti-mouse/human CD11b | Biolegend | 101207 |
| PE anti-mouse I-A/I-E (MHC class II) | Biolegend | 107607 |
| PE anti-mouse CD40 | Biolegend | 124609 |
| APC/Cyanine 7 Goat anti-mouse IgG (minimal x-reactivity) | Biolegend | 405316 |
| APC anti-mouse CD45 | Biolegend | 103111 |
| FITC anti-mouse CD45 | Biolegend | 103107 |
| Pacific Blue anti-mouse CD45 | Biolegend | 103125 |
| APC/Cyanine 7 anti-mouse CD45 | Biolegend | 103115 |
| PE anti-mouse CD45 | Biolegend | 103106 |
| Brilliant Violet 421 anti-mouse CD45 | Biolegend | 103134 |
| Brilliant Violet 785 anti-mouse CD45 | Biolegend | 103149 |
| PerCP/Cyanine 5.5 anti-mouse CD45 | Biolegend | 103131 |
| Live/Dead Fixable Blue Dead Cell Stain Kit, for UV excitation | ThermoFisher Scientific | L23105 |
| Live/Dead Fixable Near-IR Dead Cell Stain Kit | ThermoFisher Scientific | L10119 |
| Cell Trace Violet Cell Proliferation Kit | ThermoFisher Scientific | C34571 |
| CD138+ Plasma Cell Isolation Kit, mouse | Miltenyi Biotec | 130-092-530 |
| Mojosort Mouse CD11c Nanobeads | Biolegend | 480077 |
| Bacterial and virus strains | ||
| Biological samples | ||
| Chemicals, peptides, and recombinant proteins | ||
| NP-PE (phycoerythrin) | Biosearch Technologies | N-5070-1 |
| NP-KLH (Keyhole Limpet Hemocyanin) | Biosearch Technologies | N-5060-5 |
| NP-OVAL (Ovalbumin) | Biosearch Technologies | N-5051-100 |
| Blocker Casein in PBS | ThermoFisher Scientific | 37528 |
| eBioscience Intracellular Fixation & Permeabilization Buffer Set | eBioscience/ThermoFisher Scientific | 88-8824-00 |
| 4-(Dimethylamino)benzaldehyde | Millipore Sigma | 156477 |
| Substrate Reagent Pack | R&D systems | DY999 |
| Diphtheria Toxin from Corynebacterium diphtheriae | Millipore Sigma | D0564 |
| Imject Freund’s Complete Adjuvant (FCA) | ThermoFisher Scientific | 77140 |
| Imject Freund’s Incomplete Adjuvant (FIA) | ThermoFisher Scientific | 77145 |
| Imject Alum Adjuvant | ThermoFisher Scientific | 77161 |
| Epacadostat | Chemietek | CT-EPAC |
| Recombinant Mouse IFN-γ research grade | Miltenyi Biotech | 130-105-790 |
| Recombinant mouse GM-CSF research grade | Miltenyi Biotech | 130-094-043 |
| L-Kynurenine | Millipore Sigma | 2922-83-0 |
| L-Tryptophan | Millipor Sigma | 73-22-3 |
| PowerUp SYBR Green Master Mix | ThermoFisher Scientific/Applied Biosystems | A25779 |
| Critical commercial assays | ||
| Mouse IgG ELISA Quantitation Kit | Bethyl Laboratories Inc. | E90-131 |
| Mouse IgG ELISpot Basic Kit (HRP) | Mabtech | 3825-2H |
| RNeasy Mini Kit | Qiagen | 74106 |
| iScript cDNA Synthesis Kit | BioRad | 1708890 |
| Deposited data | ||
| Experimental models: Cell lines | ||
| J558 | ATCC | TIB-6 |
| Experimental models: Organisms/strains | ||
| C57BL/6J | Jackson Labs | 000664 |
| C57BL/6NCrl | Charles River Labs | 027 |
| B6.129-Ido1tm1Alm/J | Jackson Labs | 005867 |
| B6.FVB-1700016L21 RikTg(Itgax0DTR/EGFP)57Lan/J | Jackson Labs | 004509 |
| B6.129S2-Cd28tm1Mak/J | Jackson Labs | 002666 |
| B6.129X1-Cd28tm1Jmg/Mmjax | Jackson Labs | 32039-JAX |
| B6.129S4-Cd80tm1ShrCd86tm2Shr/J | Jackson Labs | 003610 |
| C57BL/6-Tg(UBC-GFP)30Scha/J | Jackson Labs | 004353 |
| Oligonucleotides | ||
| Murine GAPDH: FWD: ‘CTT TGT CAA GCT CAT TTC CTG G’ REV: ‘TCT TGC TCA GTG TCC TTG C’ |
ThermoFisher Scientific | Custom |
| Murine Cyp1a1: FWD: ‘AGA GCA CTA CAG GAC ATT TGA G’ REV: ‘CCA AAG AGG TCC AAA ACA ATC G’ |
ThermoFisher Scientific | Custom |
| Murine Cd28: FWD: ‘GCC TTA CCT AGA CAA CGA GAG’ REV: ‘CAA GCC ATA ACA AAA CAG GAC TC’ |
ThermoFisher Scientific | Custom |
| Murine Mcl-1: FWD: ‘TTG TAA GGA CGA AAC GGG AC’ REV: ‘TCT AGG TCC TGT ACG TGG AAG’ |
ThermoFisher Scientific | Custom |
| Murine Atf-4: FWD: ‘GGT TCT CCA GCG ACA AGG’ REV: ‘GCA TCG AAG TCA AAC TCT TTC AG’ |
ThermoFisher Scientific | Custom |
| Murine Blimp-1: FWD: ‘ATT AAG CCT ATC CCT GCC AAC’ REV: ‘CTA CTG TAT TGC TTT GGG TTG C’ |
ThermoFisher Scientific | Custom |
| Murine DTR-GFP: FWD: ‘AGT GCT TCA GCC GCT ACC’ REV: ‘GAA GAT GGT GCG CTC CTG’ |
ThermoFisher Scientific | Custom |
| Recombinant DNA | ||
| Software and algorithms | ||
| Prism Graph Pad version 6–8 | Prism | https://www.graphpad.com/scientific-software/prism/ |
| Flow Jo | BD Biosciences | https://www.flowjo.com/solutions/flowjo |
| Other | ||
The J558 cell line was purchased from ATCC and cultured in RPMI 10% FBS culture media. After in vitro culture, these cells were stained at a 1:100 dilution with anti-mouse CD28 PE. Trypan blue exclusion was used to determine viability. To assess viability in BM PC: BMDC co-cultures Live Dead Near IR and Blue Fixable Viability Dyes were used from Thermo Fisher.
All flow samples were run on the BD LSR Fortessa Flow Cytometer. All analysis was done using the FloJo software.
BM aspiration
Bone marrow aspirates were taken from live mice as previously described (Chung et al., 2014). In brief, mice are anesthetized using isoflurane and maintained. Leg is shaved up to abdomen and cleaned with alternating betadine and alcohol swipes. Mice are injected with 0.1 mL sterile 0.9% NaCl with 0.1 mg/kg buprenorphine SC for pain management. A 27G insulin syringe is inserted through the patellar tendon between the condyles of the femur. Gentle extraction of the marrow cavity is done for a total of 5μL of marrow aspirated. For recovery mice are placed under a heat lamp to maintain body temp during recovery.
PC purification
Plasma cells were purified from male and female C57BL/6 WT, CD28−/−, CD28-AYAA-KI, and C57BL/6 UBC-GFP mice using the Miltenyi Biotech MACS CD138+ PC isolation kit. In brief cells from the bone marrow were labeled with Non-PC depletion cocktail and anti-Biotin microbeads per the manufacture’s instruction for non-PC depletion and run through the LD magnetic column. These cells were then labeled with CD138 microbeads and run through the MS magnetic column twice for positive selection of CD138+ cells. The purity of the CD138+ population was 75–97%.
Purification of CD11c+ DC from BM of WT mice:
CD11c+ DC were isolated from female BM of C57BL/6 WT mice to observe IDO1 expression via flow cytometry. The MojoSort Mouse CD11c Nanobeads kits (Biolegend, Cat 480077) was used for positive selection per the manufacturer’s instructions.
IDO Enzymatic Assay
Kynurenine levels were measured spectrophotometrically. 100μL of supernatant samples from primary co-cultures were mixed with 100μL of 30% trichloroacetic acid, vortexed and centrifuged at 10,000 × g for 5 min. 100μL of the supernatant from the mixed samples were added to an equal volume of Ehrlich’s reagent (100mg p-dimethylaminobenzaldehyde and 5mL glacial acetic acid) in a 96 well microtiter plate. OD values were measured at 490nm using the Biotek Synergy H1 hybrid plate reader. A standard curve of kynurenine concentrations 0–100μM was used in determining concentrations of experimental samples.
ELISA
Amount of IgG from the supernatant of co-cultures and antibody titers for murine IgM, IgG, IgA, and IgG1 were determined by ELISA per the manufacturer’s instructions (Bethyl Laboratories, Inc.). All samples were plated in triplicate. NP-IgG1 antibody titers were measured utilizing the IgG1 ELISA from Bethyl Laboratories with minor modification. In short, NUNC 96 well plates were precoated with NP-KLH (Biosearch Technologies) at 10 μg/mL in coating buffer overnight at 4°C. Congruent steps were per manufacturer’s instructions. Casein blocking buffer in PBS (ThermoFisher) and R&D substrate reagents were used instead of recommended blocking and substrate reagents.
ELISpot assay
Total IgG and NP-specific IgG antibody secreting cells (ASC) were quantified by ELISpot per manufacturer’s instructions (Mabtech). In short 96 well plates were pre-coated with 15 μg/mL anti-IgG capture antibody or 10μg/mL NP-KLH in PBS overnight at 4°C. Bone marrow cells were plated at 0.1 × 106 cells/well for IgG and 0.25 × 106 cells/well for NP-IgG in triplicate for each sample. Spleen cells were plated at 0.5 × 105 cells/well for IgG and 0.1 × 106 cells/well for NP-IgG in triplicate for each sample.
Diphtheria treatments
CD11c-DTR and chimeric mice were treated with DT via IP injection at a concentration of 100 ng/mouse. Dosing for DTR+ and DTR− mice was every other day for one week at Wk 15. Chimeric mice were treated every other day consecutively for three weeks starting at Wk 15 and ceasing at Wk 18.
Immunizations
Freund’s immunizations were done by mixing at a 1:1 ratio, 100 μg NP-ovalbumin (Biosearch technologies) in Complete Freund’s adjuvant (ThermoFisher Scientific) on day 0 subcutaneously. On day 7 mice were boosted with a 1:1 ratio of 100 μg NP-ovalbumin in Incomplete Freund’s adjuvant (ThermoFisher Scientific) subcutaneously. Serum was serially taken from mice before vaccination at day 0, at 1 week before boost, at week 3, and every 3 weeks thereafter until endpoint. At endpoint mice were bled and BM and SP were harvested for further analysis.
Alum Immunizations were done by mixing at a 1:1 ratio, 100 μg NP-ovalbumin (Biosearch technologies) with Imject Alum adjuvant (ThermoFisher Scientific Cat: 77161) per the manufacturer’s instructions and injected subcutaneously on day 0. On day 21 mice were boosted in the same fashion as the initial immunization. Serum was serially taken from mice before vaccination at day 0, at 1 week before boost, at week 3, and every 3 weeks thereafter until endpoint. At endpoint mice were bled and BM and SP were harvested for further analysis.
Epacadostat Treatment
Epacadostat (Chemietek, Cat: CT-EPAC) was reconstituted in 0.5% methylcellulose (Millipore Sigma, Cas #: 9004–67-5) with overnight end-over-end mixing. Dosage given to mice was 100mg/kg/mouse via oral gavage 5 days on and 2 days off consecutively for 3 weeks. Vehicle, 0.5% methylcellulose, was administered via oral gavage at the same time as drug treatment.
qPCR
RNA extraction was completed using the RNA RNeasy Mini Kit (Qiagen). Biorad iScript cDNA synthesis kit was used. For qPCR analysis using a 364 well plate, equal loading volumes of GAPDH were used as an endogenous control target using a QuantStudio 6 Flex Real-Time PCR machine (ThermoFisher) with PowerUp SYBR Green Master Mix (ThermoFisher). Experimental samples were plated in quadruplicate wells. The same machine was also utilized to determine percentage of DTR+ genomic DNA in chimeric mice based on a standard curve of DTR+ and WT gDNA.
Quantification and Statistical Analysis
All statistical analysis was done using GraphPad Prism 7. All error bars represent standard deviation. Student’s t test was performed for statistical analysis using a two-tailed, non-equal variances, and 95% CI. For Fig 2C, 2E, Fig 3D, and sFig 10D an ordinary one-way ANOVA test was used. Errors bars are ± standard deviation. All statistical details of experiments can be found in corresponding figure legends.
Supplementary Material
Highlights:
Long-lived plasma cells (LLPC) are essential for durable antibody (Ab) titers
The pro-survival interactions that maintain LLPC populations remain unclear
DC in the LLPC niche are essential for LLPC survival and sustained Ab titers
DC-produced indoleamine 2,3-dioxygenase 1 sustains LLPC survival and Ab titers
Acknowledgments
This research was supported by NIH grants R01CA121044, R01AI100157, P30 CA016056 (K.P.L.), T32 CA085183 (S.M.L), R00CA175189 (S.H.O), and the Roswell Park Alliance Foundation (M.J.N.). CD80/CD86−/− BM generously provided by Dr. Stephen Schoenberger.
Footnotes
Declaration of Interests:
The authors declare no competing interests.
Inclusion and Diversity
We worked to ensure sex-balance in the selection of non-human subjects. One or more of the authors of this paper self-identifies as an underrepresented ethnic minority in science.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- AMANNA IJ, CARLSON NE & SLIFKA MK 2007. Duration of Humoral Immunity to Common Viral and Vaccine Antigens. New England Journal of Medicine, 357, 1903–1915. [DOI] [PubMed] [Google Scholar]
- ASGHAR K, ASHIQ MT, ZULFIQAR B, MAHROO A, NASIR K & MURAD S 2015. Indoleamine 2,3-dioxygenase expression and activity in patients with hepatitis C virus-induced liver cirrhosis. Exp Ther Med, 9, 901–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALL HJ, JUSOF FF, BAKMIWEWA SM, HUNT NH & YUASA HJ 2014. Tryptophan-catabolizing enzymes - party of three. Front Immunol, 5, 485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEMARK M, BERGQVIST P, STENSSON A, HOLMBERG A, MATTSSON J & LYCKE NY 2011. A unique role of the cholera toxin A1-DD adjuvant for long-term plasma and memory B cell development. J Immunol, 186, 1399–410. [DOI] [PubMed] [Google Scholar]
- BIANCHI-SMIRAGLIA A, BAGATI A, FINK EE, AFFRONTI HC, LIPCHICK BC, MOPARTHY S, LONG MD, ROSARIO SR, LIGHTMAN SM, MOPARTHY K, WOLFF DW, YUN DH, HAN Z, POLECHETTI A, ROLL MV, GITLIN II, LEONOVA KI, ROWSAM AM, KANDEL ES, GUDKOV AV, BERGSAGEL PL, LEE KP, SMIRAGLIA DJ & NIKIFOROV MA 2018. Inhibition of the aryl hydrocarbon receptor/polyamine biosynthesis axis suppresses multiple myeloma. J Clin Invest, 128, 4682–4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOISE LH, KAUFMAN JL, BAHLIS NJ, LONIAL S & LEE KP 2014. The Tao of myeloma. Blood, 124, 1873–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOOMER JS & GREEN JM 2010. An enigmatic tail of CD28 signaling. Cold Spring Harbor perspectives in biology, 2, a002436–a002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOZEC A, ZAISS MM, KAGWIRIA R, VOLL R, RAUH M, CHEN Z, MUELLER-SCHMUCKER S, KROCZEK RA, HEINZERLING L, MOSER M, MELLOR AL, DAVID JP & SCHETT G 2014. T cell costimulation molecules CD80/86 inhibit osteoclast differentiation by inducing the IDO/tryptophan pathway. Sci Transl Med, 6, 235ra60. [DOI] [PubMed] [Google Scholar]
- CASSESE G, LINDENAU S, DE BOER B, ARCE S, HAUSER A, RIEMEKASTEN G, BEREK C, HIEPE F, KRENN V, RADBRUCH A & MANZ RA 2001. Inflamed kidneys of NZB / W mice are a major site for the homeostasis of plasma cells. Eur J Immunol, 31, 2726–32. [DOI] [PubMed] [Google Scholar]
- CHUNG YR, KIM E & ABDEL-WAHAB O 2014. Femoral bone marrow aspiration in live mice. J Vis Exp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAN JM, MATEUS J, KATO Y, HASTIE KM, YU ED, FALITI CE, GRIFONI A, RAMIREZ SI, HAUPT S, FRAZIER A, NAKAO C, RAYAPROLU V, RAWLINGS SA, PETERS B, KRAMMER F, SIMON V, SAPHIRE EO, SMITH DM, WEISKOPF D, SETTE A & CROTTY S 2021. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science (New York, N.Y.), 371, eabf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DILILLO DJ, HAMAGUCHI Y, UEDA Y, YANG K, UCHIDA J, HAAS KM, KELSOE G & TEDDER TF 2007. Maintenance of Long-Lived Plasma Cells and Serological Memory Despite Mature and Memory B Cell Depletion during CD20 Immunotherapy in Mice. The Journal of Immunology, 180, 361–371. [DOI] [PubMed] [Google Scholar]
- GARCIA DE VINUESA C, GULBRANSON-JUDGE A, KHAN M, O’LEARY P, CASCALHO M, WABL M, KLAUS GG, OWEN MJ & MACLENNAN IC 1999. Dendritic cells associated with plasmablast survival. Eur J Immunol, 29, 3712–21. [DOI] [PubMed] [Google Scholar]
- GLATMAN ZARETSKY A, KONRADT C, DEPIS F, WING JB, GOENKA R, ATRIA DG, SILVER JS, CHO S, WOLF AI, QUINN WJ, ENGILES JB, BROWN DC, BEITING D, ERIKSON J, ALLMAN D, CANCRO MP, SAKAGUCHI S, LU LF, BENOIST CO & HUNTER CA 2017. T Regulatory Cells Support Plasma Cell Populations in the Bone Marrow. Cell Rep, 18, 1906–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODWIN K, VIBOUD C & SIMONSEN L 2006. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine, 24, 1159–69. [DOI] [PubMed] [Google Scholar]
- HAMMARLUND E, THOMAS A, AMANNA IJ, HOLDEN LA, SLAYDEN OD, PARK B, GAO L & SLIFKA MK 2017. Plasma cell survival in the absence of B cell memory. Nat Commun, 8, 1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAN S, YANG K, OZEN Z, PENG W, MARINOVA E, KELSOE G & ZHENG B 2003. Enhanced differentiation of splenic plasma cells but diminished long-lived high-affinity bone marrow plasma cells in aged mice. J Immunol, 170, 1267–73. [DOI] [PubMed] [Google Scholar]
- HWU P, DU MX, LAPOINTE R, DO M, TAYLOR MW & YOUNG HA 2000. Indoleamine 2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. J Immunol, 164, 3596–9. [DOI] [PubMed] [Google Scholar]
- IBARRONDO FJ, FULCHER JA, GOODMAN-MEZA D, ELLIOTT J, HOFMANN C, HAUSNER MA, FERBAS KG, TOBIN NH, ALDROVANDI GM & YANG OO 2020. Rapid Decay of Anti-SARS-CoV-2 Antibodies in Persons with Mild Covid-19. N Engl J Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUNG S, UNUTMAZ D, WONG P, SANO G, DE LOS SANTOS K, SPARWASSER T, WU S, VUTHOORI S, KO K, ZAVALA F, PAMER EG, LITTMAN DR & LANG RA 2002. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity, 17, 211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JURGENS B, HAINZ U, FUCHS D, FELZMANN T & HEITGER A 2009. Interferon-gamma-triggered indoleamine 2,3-dioxygenase competence in human monocyte-derived dendritic cells induces regulatory activity in allogeneic T cells. Blood, 114, 3235–43. [DOI] [PubMed] [Google Scholar]
- KILBERG MS, SHAN J & SU N 2009. ATF4-dependent transcription mediates signaling of amino acid limitation. Trends Endocrinol Metab, 20, 436–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOORELLA C, NAIR JR, MURRAY ME, CARLSON LM, WATKINS SK & LEE KP 2014. Novel regulation of CD80/CD86-induced phosphatidylinositol 3-kinase signaling by NOTCH1 protein in interleukin-6 and indoleamine 2,3-dioxygenase production by dendritic cells. J Biol Chem, 289, 7747–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAM WY, BECKER AM, KENNERLY KM, WONG R, CURTIS JD, LLUFRIO EM, MCCOMMIS KS, FAHRMANN J, PIZZATO HA, NUNLEY RM, LEE J, WOLFGANG MJ, PATTI GJ, FINCK BN, PEARCE EL & BHATTACHARYA D 2016. Mitochondrial Pyruvate Import Promotes Long-Term Survival of Antibody-Secreting Plasma Cells. Immunity, 45, 60–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAM WY & BHATTACHARYA D 2018. Metabolic Links between Plasma Cell Survival, Secretion, and Stress. Trends Immunol, 39, 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEMKE A, KRAFT M, ROTH K, RIEDEL R, LAMMERDING D & HAUSER AE 2016. Long-lived plasma cells are generated in mucosal immune responses and contribute to the bone marrow plasma cell pool in mice. Mucosal Immunol, 9, 83–97. [DOI] [PubMed] [Google Scholar]
- LI Q, HARDEN JL, ANDERSON CD & EGILMEZ NK 2016. Tolerogenic Phenotype of IFN-γ-Induced IDO+ Dendritic Cells Is Maintained via an Autocrine IDO− Kynurenine/AhR-IDO Loop. J Immunol, 197, 962–70. [DOI] [PubMed] [Google Scholar]
- LIGHTMAN SM, UTLEY A & LEE KP 2019. Survival of Long-Lived Plasma Cells (LLPC): Piecing Together the Puzzle. Front Immunol, 10, 965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANKARIOUS S, LEE M, FISCHER S, PYUN KH, OCHS HD, OXELIUS VA & WEDGWOOD RJ 1988. The half-lives of IgG subclasses and specific antibodies in patients with primary immunodeficiency who are receiving intravenously administered immunoglobulin. J Lab Clin Med, 112, 634–40. [PubMed] [Google Scholar]
- MANZ RA, THIEL A & RADBRUCH A 1997. Lifetime of plasma cells in the bone marrow. Nature, 388, 133–4. [DOI] [PubMed] [Google Scholar]
- MATINO D, GARGARO M, SANTAGOSTINO E, DI MINNO MN, CASTAMAN G, MORFINI M, ROCINO A, MANCUSO ME, DI MINNO G, COPPOLA A, TALESA VN, VOLPI C, VACCA C, ORABONA C, IANNITTI R, MAZZUCCONI MG, SANTORO C, TOSTI A, CHIAPPALUPI S, SORCI G, TAGARIELLO G, BELVINI D, RADOSSI P, LANDOLFI R, FUCHS D, BOON L, PIRRO M, MARCHESINI E, GROHMANN U, PUCCETTI P, IORIO A & FALLARINO F 2015. IDO1 suppresses inhibitor development in hemophilia A treated with factor VIII. J Clin Invest, 125, 3766–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MELLOR AL & MUNN DH 2004. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol, 4, 762–74. [DOI] [PubMed] [Google Scholar]
- MELLOR AL & MUNN DH 2008. Creating immune privilege: active local suppression that benefits friends, but protects foes. Nat Rev Immunol, 8, 74–80. [DOI] [PubMed] [Google Scholar]
- MELLOR AL, SIVAKUMAR J, CHANDLER P, SMITH K, MOLINA H, MAO D & MUNN DH 2001. Prevention of T cell-driven complement activation and inflammation by tryptophan catabolism during pregnancy. Nat Immunol, 2, 64–8. [DOI] [PubMed] [Google Scholar]
- MERLO LMF, DUHADAWAY JB, GRABLER S, PRENDERGAST GC, MULLER AJ & MANDIK-NAYAK L 2016. IDO2 Modulates T Cell–Dependent Autoimmune Responses through a B Cell–Intrinsic Mechanism. The Journal of Immunology, 196, 4487–4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MERLO LMF, DUHADAWAY JB, MONTGOMERY JD, PENG W-D, MURRAY PJ, PRENDERGAST GC, CATON AJ, MULLER AJ & MANDIK-NAYAK L 2020. Differential Roles of IDO1 and IDO2 in T and B Cell Inflammatory Immune Responses. Frontiers in Immunology, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MERLO LMF, PIGOTT E, DUHADAWAY JB, GRABLER S, METZ R, PRENDERGAST GC & MANDIK-NAYAK L 2014. IDO2 is a critical mediator of autoantibody production and inflammatory pathogenesis in a mouse model of autoimmune arthritis. J Immunol, 192, 2082–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEZRICH JD, FECHNER JH, ZHANG X, JOHNSON BP, BURLINGHAM WJ & BRADFIELD CA 2010. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol, 185, 3190–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MINGES WOLS HA, UNDERHILL GH, KANSAS GS & WITTE PL 2002. The role of bone marrow-derived stromal cells in the maintenance of plasma cell longevity. J Immunol, 169, 4213–21. [DOI] [PubMed] [Google Scholar]
- MOSER K, TOKOYODA K, RADBRUCH A, MACLENNAN I & MANZ RA 2006. Stromal niches, plasma cell differentiation and survival. Curr Opin Immunol, 18, 265–70. [DOI] [PubMed] [Google Scholar]
- MUNN DH, SHARMA MD, BABAN B, HARDING HP, ZHANG Y, RON D & MELLOR AL 2005. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity, 22, 633–42. [DOI] [PubMed] [Google Scholar]
- MUNN DH, ZHOU M, ATTWOOD JT, BONDAREV I, CONWAY SJ, MARSHALL B, BROWN C & MELLOR AL 1998. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science, 281, 1191–3. [DOI] [PubMed] [Google Scholar]
- MURRAY ME, GAVILE CM, NAIR JR, KOORELLA C, CARLSON LM, BUAC D, UTLEY A, CHESI M, BERGSAGEL PL, BOISE LH & LEE KP 2014. CD28-mediated pro-survival signaling induces chemotherapeutic resistance in multiple myeloma. Blood, 123, 3770–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAIR JR, CARLSON LM, KOORELLA C, ROZANSKI CH, BYRNE GE, BERGSAGEL PL, SHAUGHNESSY JP JR., BOISE LH, CHANAN-KHAN A & LEE KP 2011. CD28 expressed on malignant plasma cells induces a prosurvival and immunosuppressive microenvironment. J Immunol, 187, 1243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEBERT DW, PUGA A & VASILIOU V 1993. Role of the Ah receptor and the dioxin-inducible [Ah] gene battery in toxicity, cancer, and signal transduction. Ann N Y Acad Sci, 685, 624–40. [DOI] [PubMed] [Google Scholar]
- NGUYEN DC, GARIMALLA S, XIAO H, KYU S, ALBIZUA I, GALIPEAU J, CHIANG KY, WALLER EK, WU R, GIBSON G, ROBERSON J, LUND FE, RANDALL TD, SANZ I & LEE FE 2018. Factors of the bone marrow microniche that support human plasma cell survival and immunoglobulin secretion. Nat Commun, 9, 3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NJAU MN, KIM JH, CHAPPELL CP, RAVINDRAN R, THOMAS L, PULENDRAN B & JACOB J 2012. CD28-B7 interaction modulates short- and long-lived plasma cell function. Journal of immunology (Baltimore, Md. : 1950), 189, 2758–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEPERZAK V, VIKSTROM I, WALKER J, GLASER SP, LEPAGE M, COQUERY CM, ERICKSON LD, FAIRFAX K, MACKAY F, STRASSER A, NUTT SL & TARLINTON DM 2013. Mcl-1 is essential for the survival of plasma cells. Nat Immunol, 14, 290–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHAN TG, PAUS D, CHAN TD, TURNER ML, NUTT SL, BASTEN A & BRINK R 2006. High affinity germinal center B cells are actively selected into the plasma cell compartment. J Exp Med, 203, 2419–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIOLI PD 2019. Plasma Cells, the Next Generation: Beyond Antibody Secretion. Frontiers in Immunology, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIPER CJM, ROSSER EC, OLEINIKA K, NISTALA K, KRAUSGRUBER T, RENDEIRO AF, BANOS A, DROZDOV I, VILLA M, THOMSON S, XANTHOU G, BOCK C, STOCKINGER B & MAURI C 2019. Aryl Hydrocarbon Receptor Contributes to the Transcriptional Program of IL-10-Producing Regulatory B Cells. Cell Rep, 29, 1878–1892 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRITZ T, LAIR J, BAN M, KELLER M, WEINBERGER B, KRISMER M & GRUBECK-LOEBENSTEIN B 2015. Plasma cell numbers decrease in bone marrow of old patients. Eur J Immunol, 45, 738–46. [DOI] [PubMed] [Google Scholar]
- QUINTANA FJ, MURUGAIYAN G, FAREZ MF, MITSDOERFFER M, TUKPAH A-M, BURNS EJ & WEINER HL 2010. An endogenous aryl hydrocarbon receptor ligand acts on dendritic cells and T cells to suppress experimental autoimmune encephalomyelitis. Proceedings of the National Academy of Sciences, 107, 20768–20773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS AE, KURAOKA M & KELSOE G 2015. Natural IgM is produced by CD5-plasma cells that occupy a distinct survival niche in bone marrow. J Immunol, 194, 231–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROUSH SW, MURPHY TV, VACCINE-PREVENTABLE DISEASE TABLE WORKING GROUP & THE 2007. Historical Comparisons of Morbidity and Mortality for Vaccine-Preventable Diseases in the United States. JAMA, 298, 2155–2163. [DOI] [PubMed] [Google Scholar]
- ROZANSKI CH, ARENS R, CARLSON LM, NAIR J, BOISE LH, CHANAN-KHAN AA, SCHOENBERGER SP & LEE KP 2011. Sustained antibody responses depend on CD28 function in bone marrow-resident plasma cells. J Exp Med, 208, 1435–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROZANSKI CH, UTLEY A, CARLSON LM, FARREN MR, MURRAY M, RUSSELL LM, NAIR JR, YANG Z, BRADY W, GARRETT-SINHA LA, SCHOENBERGER SP, GREEN JM, BOISE LH & LEE KP 2015. CD28 Promotes Plasma Cell Survival, Sustained Antibody Responses, and BLIMP-1 Upregulation through Its Distal PYAP Proline Motif. J Immunol, 194, 4717–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHERR DH & MONTI S 2013. The role of the aryl hydrocarbon receptor in normal and malignant B cell development. Seminars in Immunopathology, 35, 705–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHINDE R, SHIMODA M, CHAUDHARY K, LIU H, MOHAMED E, BRADLEY J, KANDALA S, LI X, LIU K & MCGAHA TL 2015. B Cell-Intrinsic IDO1 Regulates Humoral Immunity to T Cell-Independent Antigens. J Immunol, 195, 2374–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLIFKA MK, ANTIA R, WHITMIRE JK & AHMED R 1998. Humoral immunity due to long-lived plasma cells. Immunity, 8, 363–72. [DOI] [PubMed] [Google Scholar]
- SLIFKA MK, MATLOUBIAN M & AHMED R 1995. Bone marrow is a major site of long-term antibody production after acute viral infection. J Virol, 69, 1895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZE DMY, TOELLNER K-M, DE VINUESA CG, TAYLOR DR & MACLENNAN ICM 2000. Intrinsic Constraint on Plasmablast Growth and Extrinsic Limits of Plasma Cell Survival. The Journal of Experimental Medicine, 192, 813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR MW & FENG GS 1991. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. Faseb j, 5, 2516–22. [PubMed] [Google Scholar]
- TELLIER J & KALLIES A 2014. Finding a home for plasma cells--a niche to survive. Eur J Immunol, 44, 2243–6. [DOI] [PubMed] [Google Scholar]
- THE WORLD HEALTH ORGANIZATION. 2019, Dec 5. Immunization [Online]. Available: https://www.who.int/en/news-room/fact-sheets/detail/immunization-coverage [Accessed].
- TURNER JS, KIM W, KALAIDINA E, GOSS CW, RAUSEO AM, SCHMITZ AJ, HANSEN L, HAILE A, KLEBERT MK, PUSIC I, O’HALLORAN JA, PRESTI RM & ELLEBEDY AH 2021. SARS-CoV-2 infection induces long-lived bone marrow plasma cells in humans. Nature. [DOI] [PubMed] [Google Scholar]
- UTLEY A, CHAVEL C, LIGHTMAN S, HOLLING GA, COOPER J, PENG P, LIU W, BARWICK BG, GAVILE CM, MAGUIRE O, MURRAY-DUPUIS M, ROZANSKI C, JORDAN MS, KAMBAYASHI T, OLEJNICZAK SH, BOISE LH & LEE KP 2020. CD28 Regulates Metabolic Fitness for Long-Lived Plasma Cell Survival. Cell Rep, 31, 107815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAIDYANATHAN B, CHAUDHRY A, YEWDELL WT, ANGELETTI D, YEN WF, WHEATLEY AK, BRADFIELD CA, MCDERMOTT AB, YEWDELL JW, RUDENSKY AY & CHAUDHURI J 2017. The aryl hydrocarbon receptor controls cell-fate decisions in B cells. J Exp Med, 214, 197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VALOR LM, RODRIGUEZ-BAYONA B, RAMOS-AMAYA AB, BRIEVA JA & CAMPOS-CARO A 2017. The transcriptional profiling of human in vivo-generated plasma cells identifies selective imbalances in monoclonal gammopathies. PLoS One, 12, e0183264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VANBLARGAN LA, GOO L & PIERSON TC 2016. Deconstructing the Antiviral Neutralizing-Antibody Response: Implications for Vaccine Development and Immunity. Microbiology and Molecular Biology Reviews, 80, 989–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL CF, WU D, GOTH SR, BAEK J, LOLLIES A, DOMHARDT R, GRINDEL A & PESSAH IN 2013. Aryl hydrocarbon receptor signaling regulates NF-kappaB RelB activation during dendritic-cell differentiation. Immunol Cell Biol, 91, 568–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISEL FJ, ZUCCARINO-CATANIA GV, CHIKINA M & SHLOMCHIK MJ 2016. A Temporal Switch in the Germinal Center Determines Differential Output of Memory B and Plasma Cells. Immunity, 44, 116–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YUE EW, SPARKS R, POLAM P, MODI D, DOUTY B, WAYLAND B, GLASS B, TAKVORIAN A, GLENN J, ZHU W, BOWER M, LIU X, LEFFET L, WANG Q, BOWMAN KJ, HANSBURY MJ, WEI M, LI Y, WYNN R, BURN TC, KOBLISH HK, FRIDMAN JS, EMM T, SCHERLE PA, METCALF B & COMBS AP 2017. INCB24360 (Epacadostat), a Highly Potent and Selective Indoleamine-2,3-dioxygenase 1 (IDO1) Inhibitor for Immuno-oncology. ACS Med Chem Lett, 8, 486–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZEHENTMEIER S, ROTH K, CSERESNYES Z, SERCAN O, HORN K, NIESNER RA, CHANG HD, RADBRUCH A & HAUSER AE 2014. Static and dynamic components synergize to form a stable survival niche for bone marrow plasma cells. Eur J Immunol, 44, 2306–17. [DOI] [PubMed] [Google Scholar]
- ZHU H, BHATT B, SIVAPRAKASAM S, CAI Y, LIU S, KODEBOYINA SK, PATEL N, SAVAGE NM, SHARMA A, KAUFMAN RJ, LI H & SINGH N 2019. Ufbp1 promotes plasma cell development and ER expansion by modulating distinct branches of UPR. Nat Commun, 10, 1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Section 1: Data
All data reported in this paper will be shared by the lead contact upon request.
Section 2: Code
This paper does not report original code.
Section 3:
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.