Abstract
Natural compounds have diverse structures and are present in different forms of life. Metabolites such as tannins, anthocyanins, and alkaloids, among others, serve as a defense mechanism in live organisms and are undoubtedly compounds of interest for the food, cosmetic, and pharmaceutical industries. Plants, bacteria, and insects represent sources of biomolecules with diverse activities, which are in many cases poorly studied. To use these molecules for different applications, it is essential to know their structure, concentrations, and biological activity potential. In vitro techniques that evaluate the biological activity of the molecules of interest have been developed since the 1950s. Currently, different methodologies have emerged to overcome some of the limitations of these traditional techniques, mainly via reductions in time and costs. These emerging technologies continue to appear due to the urgent need to expand the analysis capacity of a growing number of reported biomolecules. This review presents an updated summary of the conventional and relevant methods to evaluate the natural compounds’ biological activity in vitro.
Keywords: natural product, bioactive compounds, antimicrobial, antioxidant
1. Introduction
According to the World Health Organization (WHO), 80% of the global population uses medicinal-plant-based medicine to alleviate or cure diseases [1]. In addition, although estimates vary depending on what is considered a natural-product-derived drug, it is safe to say that up to 50% of currently marketed drugs owe their origins to natural products [2]. New molecules from natural resources with potential bioactivity are reported every day; however, only a few of these molecules are evaluated for their suitability for use as drugs [3]. Identifying bioactive compounds (hits or leads) is the initial step for drug discovery. Therefore, it is necessary to select suitable bioassays to evaluate both the activity against the disease and the potency. For this purpose, target-based screening is mainly used to identify compounds that modulate the activity of a target involved in a disease. This screening involves different in vitro biological assays designed to measure primary activities, selectivity, cellular toxicity, and physiologically relevant activity. The initial phases of a target-based screening cascade typically employ a range of in vitro assays, especially high-throughput screening (HTS); however, the study is more expensive and time-consuming [4,5]. In the first instance, the assays can be selected considering that structurally similar compounds have similar biological activity; however, this cannot always be carried out, especially when working with natural compounds or natural extracts.
Extracts from various plants are commonly used in traditional medicine, either alone or in combination, but in many cases only a few of them are evaluated for their biological activity. However, due to inadequate fractionation processes or the degradation of active compounds during separation, it is not always easy to identify the molecules responsible for the activity of these extracts. Since obtaining an isolated compound very often requires infrastructure and specialized personnel and is expensive, it is challenging to offer pharmacological alternatives of this nature to people with low incomes. In addition, since medicinal herbs can be grown locally at a reasonable cost, natural extracts remain an option for treating some diseases in populations with limited resources or living in remote areas [6].
An important advantage of using crude extracts vs. isolated molecules is the presence of molecules in the extract that can interact synergistically with the bioactive compound, potentiating its beneficial effect [7]. However, despite their potential as accessible treatment options and as sources of bioactive molecules for drug discovery, it must be highlighted that similar to other pharmacological alternatives, natural extracts can also present adverse effects that should be considered and evaluated. Having many assays on hand to discard or confirm activities is critical when looking for bioactive compounds because the aim while looking for therapeutic agents is to identify an acceptable technique that can screen the source material for bioactivity. Within this context, the current review presents some of the most common in vitro assays currently used for the identification of the pharmacological activity of bioactive compounds or natural extracts and that allow the preliminary identification of their biological potential and possible targets. Additionally, each section highlights the advantages and disadvantages of these methods, guiding the reader to the selection of the best assays based on the type of sample and available resources. Finally, alternatives or future trends to these approaches are also included in each section.
2. Cytotoxicity Activity in Cultured Mammalian Cells
In the early stages of drug development, extensive toxicity screening is essential [8,9]. Animal studies involve high costs and are often restricted by differential responses due to the physiological differences between species and limitations in test feasibility. Alternatively, in vitro cytotoxicity assays are advantageous in preclinical studies due to their eligibility, cost-effectiveness, and reproducibility. Natural compounds have become particularly relevant in identifying safer and more effective treatments [10].
To evaluate the cytotoxicity in mammalian cells, it is critical to select the proper cell line for each particular experiment, considering the relevant species, specific organs, and chosen route of administration. Multiple cell lines are available for in vitro testing, including immortalized cell lines, primary cultures, and stem cells. Each cell line has requirements that need to be defined before the experiment to ensure a proper understanding of the potential mechanisms of toxicity [11,12].
A wide range of in vitro assays are currently available for cytotoxicity testing [13,14]. While direct cytotoxicity assays focus on detecting a loss of membrane integrity associated with cell death, cell viability assays are developed to measure the activity related to cellular maintenance and survival. Additionally, other assays allow the direct and indirect quantification of changes in the population at specific phases of the cell cycle and provide information on the mechanism of cell death [15]. Different approaches are employed in parallel to better understand the cytotoxicity mechanisms, such as the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay and the comet assay for the analysis of DNA fragmentation [16]; the determination of the activation of apoptosis-related caspases [17,18]; or the detection of the relative level of telomerase activity (TRAP, telomerase repeat amplification protocol) [19,20]. Determining the cytotoxicity against tumor cells; detecting the rate and regulation of cell migration; and analyzing anchorage-independent proliferation, chemotaxis, and invasion are essential factors usually evaluated using colony-forming assays in soft agar and scratch assays using dual-chamber systems [21,22,23].
In short, each assay has its limitations. Although the 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assay has been generally accepted as the gold standard in cytotoxicity testing, this method is not always the most pertinent due to interference with the test compounds, particularly with natural extracts [24,25]. Therefore, before selecting an appropriate methodology, different assays should be compared and more than one should be used when possible. The cost, reliability, timing, user-friendliness, and equipment requirements should also be considered [26]. Table 1 presents the advantages and limitations of these assays.
Table 1.
Most common methods for the determination of cytotoxicity in cultured mammalian cells.
| Method | Principle | Detection | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|
| Cell Viability and Proliferation | |||||
| Dye exclusion | Detection of plasma membrane integrity | Trypan blue colorimetric/H + M |
|
|
[27,28,29,30] |
| Crystal violet colorimetric/M, PR |
|
|
[13,31] | ||
| Metabolic activity | Detection of mitochondrial dehydrogenase and oxidoreductase activity | Tetrazolium salts (MTT, XTT, MTS, WST) colorimetric/PR |
|
|
[27,31,32,33,34] |
| Resazurin fluorescent/PR |
|
|
[35,36,37] | ||
| Energy metabolism | Correlation between a bioluminescent reaction and the ability to synthesize ATP | Luciferase and luciferin luminescent/PR |
|
|
[14,27] |
| Enzyme release-based | Determination of plasma membrane integrity (LDH release) | Lactate + tetrazolium salts/fluorescent probe colorimetric, fluorescent/PR |
|
|
[27,32,38] |
| Colony formation | Determination of clonogenic growth | Low-adherence plates/M |
|
|
[38,39,40,41] |
| Cell Cycle and Apoptosis | |||||
| Cell cycle arrest | Distribution of cell population in each cell cycle phase | PI, 7-AAD fluorescent/FC |
|
|
[42,43] |
| Apoptosis/ necrosis |
Detection of membrane integrity | PI/7-AAD, annexin V fluorescent/F |
|
|
[44,45,46] |
H: Hemocytometer, M: microscopy; PR: plate reader; FC: flow cytometry; LI: light imager; T: thermocycler; GE: gel electrophoresis; PI: propidium iodide; 7-AAD: 7–aminoactinomycin D; MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide); XTT: (2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-carboxanilide-2H-tetrazolium); MTS: 3-(4,5-dimethylthiazol-2-yl)-5(3-carboxymethonyphenol)-2-(4-sulfophenyl)-2H-tetrazolium; WST: water-soluble tetrazolium salts; EC: electric conductivity.
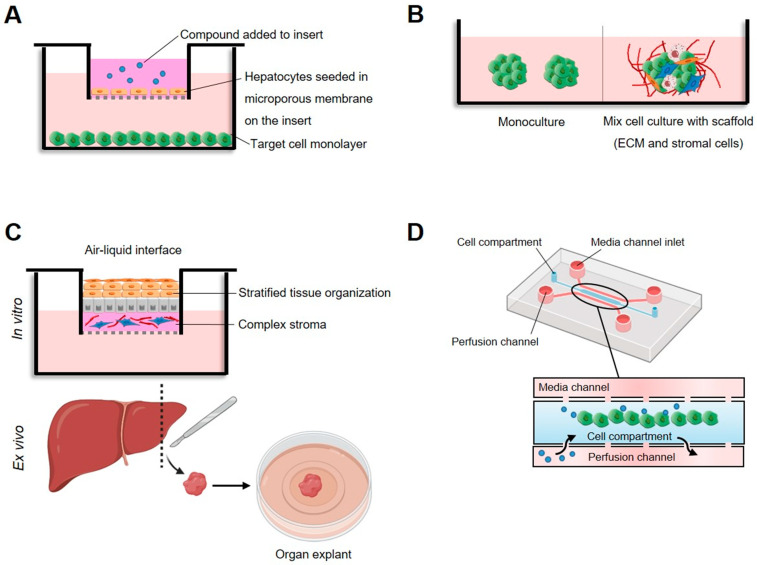
Although two-dimensional mammalian monocultures stemming from specific cell types are widely used based on their reproducible and rapid growth, high productivity levels, ease of data interpretation, and value, the artificial nature of the culture environment presents limitations in drug safety and efficacy evaluation [47,48]. In this sense, even though improved versions of the classical reagents are being developed, the field’s current focus is shifting towards co-culture systems, human organoids, and other sophisticated three-dimensional culture models that collect more physiologically relevant data and represent methods that could better connect traditional cell culture and in vivo models [49,50,51] (Figure 1).
Figure 1.
High-tech in vitro models to assess cytotoxicity in cultured mammalian cells. (A) Dual chamber, test compound, and metabolites diffuse through the microporous barrier toward target cells. (B) Three-dimensional cellular models based on multicellular spheroids or organoids consisting of target cells or the co-cultivation of several types of cells on extracellular matrix (ECM). (C) Organotypic cultures, whereby cells, organ slices, or whole organs are cultured on a tissue culture insert that is either submerged in medium or maintained at an air–liquid interface to ensure sufficient oxygen supply. (D) Microfluidic system based on a mixture of cells and matrix collected in the central channel and medium flowing from the lateral channels that keeps particles in homogenous suspension.
From the array of cell cultures available for in vitro testing that offer diverse degrees of intricacy and similarity to the in vivo setting, organotypic cultures are tissue slices that maintain cell interactions and the extracellular matrix composition of the original tissue and tissue function [52,53,54,55]. However, this system lacks intercommunication with the circulatory and immune systems and is inadequate for medium- to high-throughput analysis [11]. Three-dimensional spheroids and organoids self-organize into organ-specific structures that accurately replicate paracrine and direct intercellular interactions [56,57,58]. While spheroids are usually made from cell lines and offer lower complexity, organoids are derived from the stem cells of different origins and resemble the original tissue in the structure, histologically and genetically [59,60]. Tumor organoids are particularly relevant, since these systems provide suitable platforms to recapitulate the complex tumor microenvironment and its heterogeneity, allowing the study of chemical and metabolic gradients and mechanisms of resistance [49,61].
The evidence indicates that microfluidic devices are gaining traction in the area of cytotoxicity assays [62,63]. Compared to static conditions, microfluidic systems can reproduce the specific flow, temperature, pressure, and chemical gradients of the in vivo systems [64,65,66]. Thus, they can reconstruct the continuous renewal of nutrients, gasses and toxic wastes, migration, and microcirculation. These systems also support longer culture times and drug treatments that are more pharmacologically significant [67].
3. Antihyperglycemic Activity
Diabetes is a global health disease affecting 422 million people worldwide [68]. This disease is characterized by elevated blood glucose levels, which if untreated leads to severe multi-organ failure and 1.5 million deaths each year. Antihyperglycemic agents are a heterogeneous group of molecules obtained via chemical synthesis or isolation from natural sources that lower the glucose concentration in the blood or prevent its increase [69].
The methods used to evaluate the in vitro antidiabetic properties of natural compounds are summarized in Table 2 and can be classified into two major groups: (i) assays based on the inhibition of isolated enzymes involved in the regulation of blood glucose levels and (ii) assays used to measure major cellular processes that directly alter glucose levels, mainly glucose uptake and insulin secretion [70]. The first group includes enzymes that catalyze the breakdown of poly- and oligosaccharides such as α-amylase and α-glucosidase, respectively (Table 2). The inhibition of the mentioned enzymes and others with a similar role in carbohydrate digestion is considered antidiabetic. The reduction in the glucose concentration available to absorb in the intestine prevents a further increase in blood glucose [71]. The α-amylase and α-glucosidase inhibition assays are reactions of commercially available enzymes and substrates in the optimal conditions (buffer, pH, cofactors), allowing the detection of the reaction product(s) [72].
Table 2.
Antihyperglycemic activity.
| Method Name | Assay Type | Description | Detection (Output) | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|---|
| α-amylase inhibition | Isolated enzymes | Measurement of the ability of novel molecules to inhibit the activity of α-amylase | Colorimetric and fluorometric assays |
|
|
[89] |
| α-glucosidase inhibition | Isolated enzymes | Measurement of the ability of novel molecules to inhibit the activity of α-glucosidase | Colorimetric, fluorometric and bioluminescent assays |
|
|
[89,90] |
| Dipeptidyl peptidase IV inhibition |
Isolated enzymes | Measurement of the ability of molecules to inhibit the activity of dipeptidyl peptidase IV | Colorimetric, fluorometric, immunoassay |
|
|
[89,90] |
| Tyrosine phosphatase 1B inhibition | Isolated enzymes | Measurement of the ability of molecules to inhibit the activity of tyrosine phosphatase 1B | Colorimetric, fluorometric |
|
|
[74,77] |
| Glucose uptake | Cell-based | Measurement of the ability of molecules to modify glucose uptake into cells | Colorimetric, fluorometric, bioluminescent assays |
|
|
[91,92] |
| Insulin secretion | Cell-based | Measurement of the ability of molecules to modulate insulin secretion | Bioluminescent assay, immuno-/radioimmunoassay |
|
|
[87,88] |
The most common substrate used to measure α-amylase is starch. The method is based on the reaction of starch with dinitrosalicylic acid (DNS), which reacts with reducing sugars, producing 3-amino-5-nitrosalicylic acid, which is measured spectrophotometrically at 540 nm. Most α-glucosidase assays rely on the spectrophotometric detection of p-nitrophenol liberated after the hydrolysis of p-nitrophenyl-α-d-glucopyranoside (pNPG), which can be measured at 400 nm. Dipeptidyl peptidase IV (DPP4) and tyrosine phosphatase 1B (TP1B) are involved in the indirect regulation of glucose levels by modulating insulin secretion (DPP4) [73] and signaling (TP1B) [74], respectively (Table 2). DPP4 is a serine exopeptidase that cleaves different peptides, including GLP-1, a major regulator of insulin secretion in response to glucose [75]. Thus, inhibiting DPP-4 in vivo increases the availability of GLP-1 and insulin secretion, reducing blood glucose [76]. TP1B negatively regulates insulin and leptin signaling by dephosphorylating the insulin receptor (IR) and its downstream signaling components [77,78]. The inhibition of TP1B releases insulin signaling from TP1B-mediated dephosphorylation and allows insulin downstream signaling [72]. The second group includes assays designed to measure the potential inhibitory effects of different molecules on relevant cellular processes controlling blood glucose levels, such as glucose uptake and insulin secretion (Table 2). The glucose uptake assay is based on the internalization of a labeled glucose analog that cannot be fully utilized because of its modification. It accumulates inside the cells, facilitating its detection. The output generated by the accumulation of labeled analogs is proportional to the glucose uptake and can be detected and quantified using standard equipment such as fluorescence or bioluminescence readers [79,80] or fluorescence-activated cell sorting (FACS) [81]. These assays can be performed in mammalian cell lines, given the importance of measuring the physiologically relevant effects. Some studies report using yeast cells as an alternative to mammalian cell lines [82]. For example, a recent study described a label-free method to measure glucose uptake in yeast cells using pHluorin, a genetically encoded pH-sensitive green fluorescent protein [83]. In general, insulin secretion assays are performed using ß-cells isolated from pancreatic or islet cell cultures. The cells are stimulated by glucose and incubated with the compound or plant extract to measure the insulin secretion modulation effect [84]. After being released from cells, insulin can be measured via radioimmunoassay [85,86] or ELISA. Recently, a luminescent alternative to detect insulin was described by Hager et al. and Kalwat et al. [87,88].
Despite continuous improvements in measuring glucose and glucose-associated processes, a relevant challenge is to fully understand the physiological and pathological roles of blood glucose levels and their impact on health conditions and disease. In vitro methods are critical for discovering natural compounds with antidiabetic activity. Although diabetes and other health issues associated with high blood glucose levels can be treated using available antidiabetics, given the vast chemical diversity of natural products with unknown but potentially beneficial effects, the evaluation of the antidiabetic activity of molecules obtained from natural sources is a very relevant research topic. Some parameters that are actively being improved to allow the efficient prospection of potential candidates for developing novel antidiabetic drugs from natural sources are: (i) increasing the sensitivity and robustness of the assays; (ii) reducing the laborious steps needed for the preparation of samples and cell extracts; and (iii) increasing the throughput of the assays.
4. Anti-Inflammatory Activity
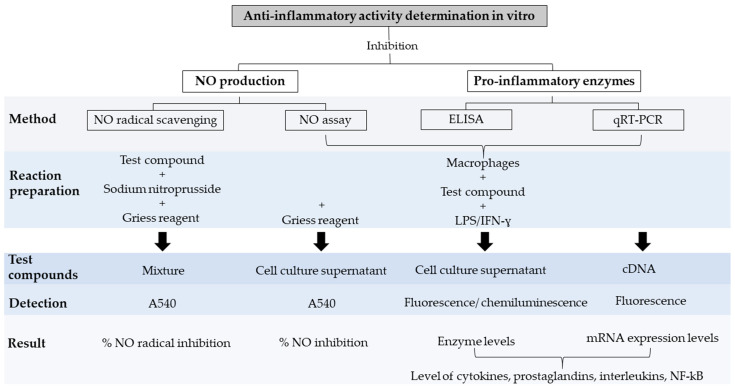
Inflammation is a protective response of a given organism’s immune system against harmful external agents, such as pathogens, toxins, or irritants [93]. This mechanism aids in the recovery from infections, disease, and tissue damage, favoring the healing process [94]. Inflammatory responses include the activation of macrophages by pro-inflammatory mediators such as lipopolysaccharide (LPS), interleukin-1β (IL-1β), interferon-γ (IFN-γ), and the nuclear factor kappa B (NF-κB), which trigger the cyclooxygenase (COX) and lipoxygenase (LOX) pathways and the production of nitric oxide (NO), tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6) primarily [95,96,97]. All of these pro-inflammatory mediators are studied to identify the anti-inflammatory properties of natural molecules in vitro. In terms of the anti-inflammatory treatments, non-steroidal drugs have been shown to inhibit the production of arachidonic acid and to lower prostaglandin levels, contributing to reducing pain and inflammation [98]. However, their use is associated with multiple side effects, including stomach ulcers, indigestion, headaches, allergic reactions, and increased cardiovascular conditions [98]. Fortunately, the incredible variety of phytoconstituents present in plants such as flavonoids, alkaloids, saponins, coumarins, anthraquinones, saccharides, glucosinolates, tannins, phenolic acids, and nitrile glycosides are excellent sources for drug development due to their vast biological activities [99]. Specifically, flavonoids [100,101], anthocyanins [102], and some polyphenols [103] have shown anti-inflammatory properties. Similarly, secondary metabolites, such as atranorin from lichens [104] and sulfated polysaccharides from the brown alga Sargassum cristaefolium [105], inhibit the inflammatory process [104]. These few examples highlight the incredible potential of natural extracts for developing anti-inflammatory pharmaceuticals. For a complete review of natural active molecules with anti-inflammatory potential, refer to Wang and Zeng [106]. A simple way of measuring inflammation is through the overproduction of nitric oxide (NO), which is associated with tissue toxicity, several inflammatory conditions, and carcinomas [58]. Easy, cheap, and rapid quantification of nitric oxide levels directly from a given compound or from a cell culture subjected to an inflammatory stimulus is possible through the Griess assay (Figure 2) [95,107,108,109]. Thus, a compound inducing low NO levels has anti-inflammatory potential. However, this method has drawbacks, including its variable sensitivity, low detection levels, and the interference of some compounds with the Griess reaction assay [110,111]. Alternatives to the Griess assay for NO detection are covered by Bryan and Grisham [112].
Figure 2.
Strategies employed to assess anti-inflammatory activity in vitro. Nitric oxide (NO), lipopolysaccharide (LPS), interferon-gamma (IFN-γ), nuclear factor kappa B (NF-κB).
Other approaches, such as the enzyme-linked immunosorbent assay (ELISA) and qRT-PCR, have been extensively employed to determine the decreases in pro-inflammatory enzyme levels and their changes in expression, respectively (Figure 2) [94]. The advantages of ELISA are its simplicity, high specificity, and sensitivity. However, on the other hand, it is a time-consuming process and involves high costs associated with the antibodies [113]. In the case of qRT-PCR, the advantages include its high sensitivity and relatively high throughput. In contrast, its disadvantages are associated with its increased complexity, variable reproducibility, and the high cost of the equipment and reagents [114]. Additionally, qRT-PCR measures the mRNA levels but does not provide information about the overall enzyme levels. Alternative methods to measure inflammation include the nuclear factor kappa B (NF-κB) luciferase assay [108]; Western blotting assay on COX-2 and inducible nitric oxide synthase (iNOS) expression [115]; immunofluorescence staining of NF-κB p65 and iNOS [96]; ferrous oxidation−xylenol orange (FOX) assay for the determination of lipid hydroperoxides [116]; and protein denaturation inhibitory activity, heat-induced hemolysis, and lipoxygenase inhibitory activity assays [117]. However, these are less common during the early stages of anti-inflammatory activity identification.
It is essential to identify the best method for an investigation based on its advantages and limitations. Currently, researchers employ a combination of methods (e.g., NO inhibition assay and pro-inflammatory enzyme quantification) to obtain more information about the potential anti-inflammatory properties of different compounds. Figure 2 details the most common strategies used to establish the anti-inflammatory potential of natural molecules in vitro.
5. Analgesic Activity
Pain is a symptom of many diseases that require analgesic treatment [118]. An analgesic is a drug used to relieve pain without loss of consciousness [119]. Analgesics can act on the central or peripheral nervous system [120]. There are two main groups of drugs used to reduce pain: (i) non-steroidal anti-inflammatory analgesics, which alleviate pain by reducing local inflammatory responses; (ii) opiate analgesics, which are highly effective because they reduce but do not block the perception of the central nervous system and even induce sedation, transforming pain into a non-bothersome sensation [121,122]. There are several types of opioid receptors: µ-receptors, which when stimulated, the typical effects of narcotic analgesics are produced; κ-receptors, which are responsible for spinal analgesia [123], and when the drug acts on these receptors, it does not have addictive effects but causes unpleasant hallucinations; δ-receptors, which are responsible for cardiovascular manifestations [118,124].
Radioligand binding assays are used to assess the affinity and selectivity of the new molecules for specific receptors to evaluate the potential analgesic activity of new compounds. The three experimental types of radioligand binding assays are saturation, competitive, and kinetic assays [125]. The competitive assay is the most widely used, which studies equilibrium binding at a fixed radioligand concentration and with different concentrations of an unlabeled competitor [126]. In Table 3, the in vitro methods for analgesic activity are described.
Table 3.
Methods used for the determination of the analgesic activity of molecules based on the displacement of radioligands.
| Method Name | Assay Type | Description | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|
| Displacement of radioligands at receptors | 3H-N binding assay | Radiolabeled ligand: 3H-N Type of receptor: opiate Tissue culture: rat brain |
|
|
[118,119] |
| 3H-DHM binding assay | Radiolabeled ligand: 3H-DHM Type of receptor: µ-opiate Tissue culture: rat brain |
[124] | |||
| 3H-B binding assay | Radiolabeled ligand: 3H-B Type of receptor: κ-opiate Tissue culture: guinea pig cerebellum |
[119,131] | |||
| 3H-NCNH2 binding assay | Radiolabeled ligand: 3H-NCNH2 assay Type of receptor: nociceptin Tissue culture: rat brain |
[131] | |||
| 35S-GT binding assay | Radiolabeled ligand: 35S-GT Type of receptor: cannabinoid Tissue culture: human brain |
[131,132] | |||
| 3H-R binding assay | Radiolabeled ligand: 3H-R Type of receptor: vanilloid Tissue culture: rat brain |
[133,134] |
3H-N: 3H-naloxone; 3H-DHM: 3H-dihydromorphine; 3H-B: 3H-bremazocine; 3H-NCNH2: 3H-nociceptin amide; 3H-R: 3H-resiniferatoxin; 35S-GT: 35S-guanosine-5′-O-(3-thio) triphosphate.
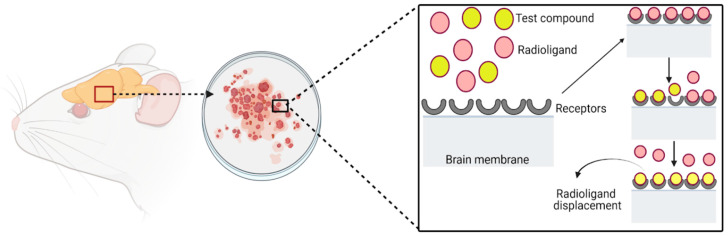
There is a good correlation between the in vivo pharmacological potency of opiate agonists and antagonists and their ability to displace radiolabeled compounds such as naloxone, a substance that binds to opiate receptors [127]. The correlation mentioned above can be exploited to evaluate new compounds. As shown in Figure 3, these experiments are performed on animal brain membranes, which have a high density of the receptors of interest; in the assay, different concentrations of the test compound are evaluated against a fixed concentration of specific radioligands [128,129,130]. These assays help identify compounds that recognize the same binding site as known radiolabeled ligands. The results can be quantified and expressed as a percentage displacement of the radioactive compound and as IC50 value [119].
Figure 3.
Sites in the rat brain tissue, where both opiate antagonists and agonists compete for the same receptors, opiate potencies, and antagonists in displacing 3H-naloxone binding parallel to their pharmacological potencies.
Enzyme assays are also used to determine analgesic activity (Table 4). The inhibition of enkephalinases has shown antinociceptive properties [126]. The determination of the inhibition is carried out via fluorescence, for which the dansyl-d-Ala-Gly-Phe(pNO2)-Gly (DAGNPG) molecule is used as a selective enzymatic substrate and the enkephalinase breaks the Gly-Phe(pNO2) DAGNPG peptide bond, which causes an increase in fluorescence [121].
Table 4.
Enzyme inhibition method for the determination of the analgesic activity of molecules.
| Method Type | Detection (Output) | Description | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|
| Enzyme inhibition | Fluorometric detection using fluorogenic peptide DANGPG |
|
|
|
[131,135,136] |
DANGPG: dansyl-D-Ala-Gly-Phe(pNO2)-Gly; ANF: atrial natriuretic factor.
Some of the current widely used analgesic treatments such as the non-steroidal anti-inflammatory drugs (NSAID) (e.g., acetylsalicylic acid) are associated with certain adverse effects, including gastrointestinal irritation and bleeding, fluid retention, and prolonged bleeding times [125]. Similarly, opioids, the current “gold standard” medication for nociceptive pain, also cause dose-limiting side effects including constipation, potentially lethal respiratory arrest, and drowsiness and are associated with the development of tolerance, dependence, and addiction [137]. Medicinal plant extracts offer a diverse source of new molecules with analgesic potential; however, it is essential to evaluate their adverse effects. Ideally, these natural compounds must be highly selective for a target that is specifically expressed in the nociceptive system, minimizing the risks of side effects [128]. Morphine, steroids, salicin, nicotine, artemisinin, atropine, pilocarpine, capsaicin, quinine, scopolamine, and captopril are a few examples of naturally derived molecules with analgesic properties [135]. Moreover, other plant-derived molecules such as monoterpenes, sesquiterpenes, and phenylpropanoids [136] and some venom peptides from animals such as spiders [138,139] have shown promising analgesic potential as well.
Overall, the assays presented above allow the screening of the analgesic activity of compounds in new extracts or natural products and encourage the isolation of the main active molecules present to relate them to their biological responses. Furthermore, knowing the chemical structure of the isolated molecules would allow an understanding of the possible biomolecular targets and their mechanisms of action to mitigate pain. Future research will need to focus on developing and improving multiple radio- and fluorescent ligands for studies with other types of specific binding, such as NaV channel isoforms, the σ-opioid receptor, the ε-opioid receptor, and the bradykinin B1 receptor, among others.
6. Anticoagulant Activity
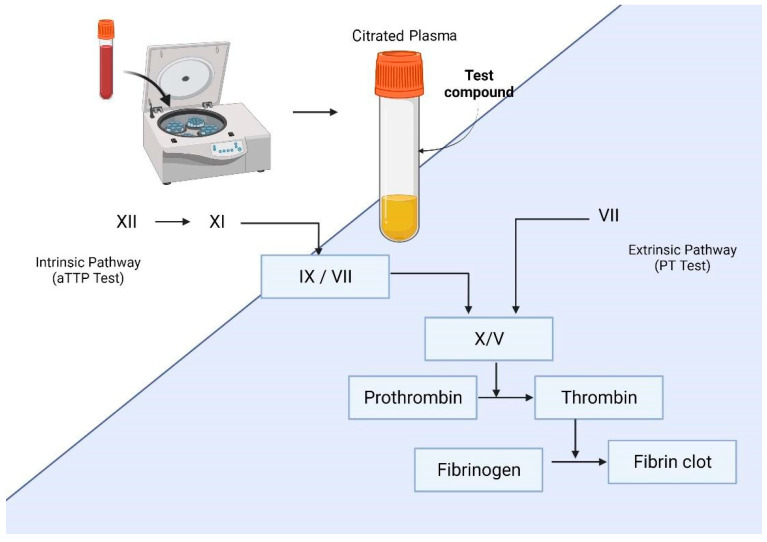
The coagulation process is usually described as a “waterfall” or “cascade”, referring to the steps involved in the development of a blood clot following injury by activating a cascade of proteins known as clotting factors. The coagulation cascade involves a series of enzymatic reactions, where each of the participating molecules in the coagulation process is referred to as a "factor." These coagulation factors are commonly identified by using a designated Roman numeral chosen according to the order in which they were discovered and with an “a” lowercase to indicate the active form [140]. In these reactions, a zymogen (an inactive enzyme precursor) and its glycoprotein cofactor are activated to become active components that then catalyze the subsequent reaction in the cascade. An active enzyme cleaves a portion of the next inactive protein in the cascade (activating it), ending in the formation of cross-linked fibrin [141], as shown in Figure 4.
Figure 4.
The activation of coagulation factors in vitro for clot formation by adding the test compound as a possible therapeutic agent.
When new compounds with anticoagulant potential are evaluated, their ability to prevent the action of coagulation factors is determined, thereby blocking the cascade almost from its inception [135,142]. Each component must function correctly and sufficiently in the process to ensure clot formation. If one or more coagulation factors are deficient, or if one or more are not working correctly, the clot may not form and bleeding may not be controlled [140].
Anticoagulants make it difficult for the blood to coagulate, thereby preventing the formation of clots or their growth and favoring their disappearance [141]. When an injury occurs to a blood vessel or tissue within the body and bleeding occurs, the body initiates a process of clot formation at the site of the damage to help stop the bleeding; this mechanism is known as hemostasis [141]. During this process, platelets stick together and become activated at the injury site. In parallel, the coagulation cascade is initiated, and coagulation factors including fibrinogen are also activated [143]. Fibrinogen is converted by thrombin into insoluble fibrin strands that cross each other, forming a fibrin network that adheres to the main focus of the lesion. Thus, a stable clot prevents further blood loss with platelets and remains in place until the wound heals [140].
Heparin is a sulfated polysaccharide commonly used as an anticoagulant. It acts by inactivating thrombin and activating factor X (factor Xa) through an antithrombin (AT)-dependent mechanism [144]. Despite being one of the most common anticoagulants, heparin has some drawbacks, such as the risk of contamination with oversulfated chondroitin sulfate, which can cause death due to its severe hypotensive effects [145]. In addition, adverse reactions to heparin include hemorrhage, heparin-induced thrombocytopenia, osteoporosis, general hypersensitivity reactions, and elevations of aminotransferase levels [146]. Notably, the number of molecules with anticoagulant activity isolated from natural sources has increased in recent years [139]. Some prominent examples include the anticoagulant active fraction (AAFCC) purified from the leaves of C. colebrookianum [147], the ethanolic extract of Mikania laevigata [142], the water-soluble polysaccharide fraction (GSP-3) from Gentiana scabra bunge roots [148], the active components obtained from Safflower [135], and the sulphated polysaccharide isolated from Globularia alypum L. [149].
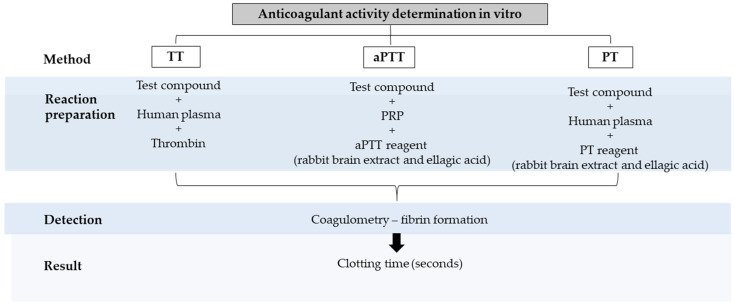
Coagulation tests such as prothrombin time (PT) and activated partial thromboplastin (aPTT) methods are used in vitro to assess the influence of compounds on blood plasma coagulation [150]. PT, aPTT, and thrombin time (TT) tests can inhibit blood coagulation through the intrinsic, extrinsic, and common pathways of the thrombin cascade and allow an assessment of coagulation, respectively [151]. The intrinsic and extrinsic pathways are two separate pathways that lead to the formation of a blood clot. The main difference between the intrinsic and extrinsic pathways in blood coagulation is that the former pathway is activated after exposure to endothelial collagen, which is only exposed when endothelial damage occurs. In contrast, the second pathway is activated through tissue factor release by endothelial cells after external damage [140,141]. As shown in Figure 5, these three tests are mainly used to discover drugs with anticoagulant properties. First, the test compound is incubated with human-platelet-rich plasma using a specific reagent for each type of test. Then, fibrin formation is evaluated through clots, and the test result is determined through an analyzer and expressed in coagulation time (seconds) [142]. To perform anticoagulant activity tests, platelet-rich plasma (PRP) must be obtained from healthy people because contamination by anticoagulant agents and liver diseases can result in false-positive results with the compounds of interest. For anticoagulant evaluation, the parallel use of intrinsic and extrinsic pathway tests (aPTT, PT) is recommended to determine the anticoagulant properties of the compound of interest [150]. The aPTT and PT assays are the most used in vitro tests nowadays due to their low costs and rapidity.
Figure 5.
Strategies employed to assess analgesic activity in vitro. TT: thrombin time; aPTT: activated partial thromboplastin; PT: prothrombin time; PRP: platelet-rich plasma.
7. Antihypertensive Activity
Hypertension or high blood pressure is a complex, multifactorial disease that contributes to morbidity and mortality in industrialized countries [152]. Although numerous preventive and therapeutic pharmacological interventions exist, as of 2021, approximately 700 million people worldwide still suffer from poorly controlled hypertension [153]. Therefore, there is growing interest in finding novel natural or synthetic molecules that are helpful in preventing and treating hypertension.
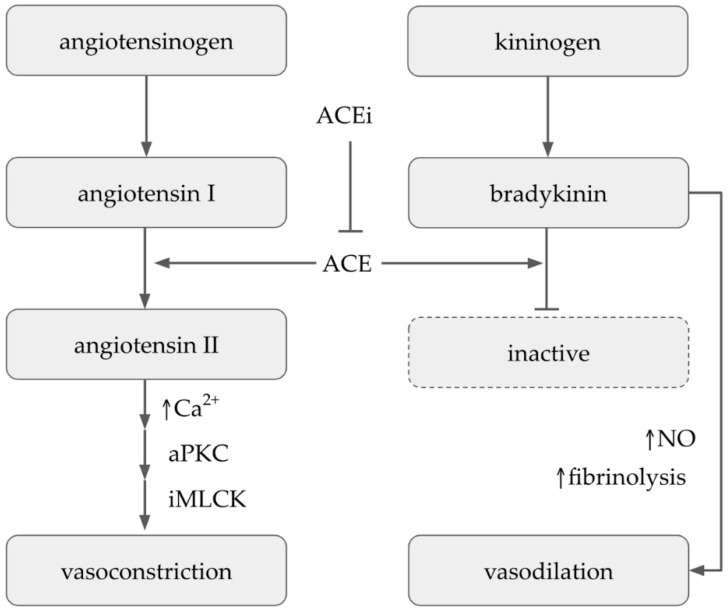
Angiotensin-converting enzyme (ACE) is one of the primary regulators of blood pressure. It acts via two central mechanisms: (i) converting the decapeptide angiotensin I (angI) into the potent vasoconstrictor (hypertensive) octapeptide angiotensin II (angII) and (ii) catalyzing the degradation of the antihypertensive peptide bradykinin. Angiotensin II increases blood pressure by stimulating a GPCR-activated pathway [154], resulting in the release of Ca2+, activation of protein kinase C (PKC), and subsequent vasoconstriction via the inactivation of the myosin-light-chain kinase (MLCK) (Figure 6).
Figure 6.
The inhibition of angiotensin-converting enzyme. Calcium (Ca2+), angiotensin-converting enzyme (ACE), angiotensin-converting enzyme inhibitors (ACEi), atypical protein kinase C (aPKC), myosin-light-chain kinase (MLCK), nitric oxide (NO).
ACE is a dicarboxypeptidyl peptidase, which cleaves off the two terminal amino acids of angI to form angII. Unlike other peptidases, ACE is not specific to a single substrate. Instead, it cleaves several natural peptides such as bradykinin, substance P, and tetrapeptide N-acetyl-Ser-Asp-Lys-Pro (Ac-SDKP) [155] and several synthetic substrates. Therefore, the ability of molecules to inhibit the production of angII by ACE is an effective strategy for measuring the antihypertensive activity of isolated plant extracts in vitro (Figure 6).
Most of the methods for measuring ACE activity in vitro rely on the incubation of a reaction mix containing purified ACE and synthetic substrates such as furanacryloyl (FA-PGG) [156], 3-hydroxybutyryl-Gly-Gly-Gly (3HB-GGG) [157], hippuryl-histidyl-leucine (HHL) [158], dansyltriglycyne [159,160], or benzoyl-[l1-14C] glycyl-l-histidyl-l-leucine. Each technique uses the same principle, only differing in the output (colorimetric, fluorometric, radioactive, etc.). The ACE activity or inhibition is determined by adding the substrate and the potential inhibitor compound and comparing the activity to a sample without the inhibitor molecule or extract. One of the advantages of using ACE activity tests to evaluate the activity of potential antihypertensive agents is that due to the central role of ACE in controlling blood pressure in vivo, finding novel ACE inhibitors can directly impact the development of antihypertensive agents. The assay has been used since the 1960s and different substrates and outputs have been described; it can be adapted to evaluate the activity of a vast, diverse array of isolated molecules or mixtures.
Many of the original ACE tests require several intermediate reactions and laborious sample preparation steps (purification, extraction, etc.) to finally read the test result, making the assay hard to automate or increase its throughput. Improvements have been made in the last decades, and the current methods are more straightforward, cheaper, and faster, allowing the development of some high-throughput ACE assays [161,162]. Despite these efforts, given the enormous impact of hypertension as a global health concern, there is still room for further improvements in assay parameters, such as by developing simpler readouts and high-performance assays or reducing the overall cost and enhancing the robustness of ACE activity tests.
8. Antioxidant Activity
The antioxidant activity can be described as the property of a given compound that in low concentrations can inhibit or decrease the oxidation of its substrate [163]. An antioxidant acts on free radicals that negatively affect biological systems by neutralizing them through electron donation or by breaking down processes [164]. This inhibition or neutralization can be measured in vitro through chemical (e.g., ABTS, DPPH, FRAP, ORAC, CUPRAC) and biochemical methods (e.g., oxidation of low-density lipoprotein (LDL) assay, thiobarbituric acid reactive substances (TBARS) assay). Nevertheless, the first ones are preferred over the second ones due to their simplicity, speed, and low costs. Therefore, this section is focused on the most frequently used chemical-based methods to evaluate the antioxidant activity of natural molecules in vitro (Table 5). For a complete review of the antioxidant methods, please refer to [164,165,166,167]. Several metabolites and phytoconstituents have shown antioxidant properties because of their scavenging ability; these include phenolic compounds, flavonoids [168,169], anthocyanins [170], and polysaccharides [171]. Many of these molecules are commonly present in natural sources, making the study of the antioxidant properties a must in almost every study about the biological activities. Chemical-based assays fall into two categories: hydrogen atom transfer (HAT) reaction-based and single-electron transfer (ET) reaction-based assays. However, some combine the two mechanisms (i.e., ABTS, DPPH). Notably, several methods (i.e., ABTS, DPPH, FRAP) have limited biological relevance because they measure the inhibition of radicals that do not exist in biological systems (e.g., DPPH•, ABTS•+) or the reducing capacity based upon one specific ion (Fe3+) [165]. The exception to this is the oxygen radical absorbance capacity assay (ORAC), which monitors the inhibition of the biologically relevant peroxyl radical (ROO•), and chemiluminescence, which can detect the quenching capacity of different oxygen and reactive nitrogen species [172]. The advantages and disadvantages of the chemical methods are described in Table 5 and should be considered when assessing the antioxidant activity and selecting the best method for a given sample. Overall, the selection of a specific antioxidant method is mainly based on its simplicity, cost, and type of sample. Nevertheless, currently the coupling of many chemical assays to high-throughput or automated systems (i.e., HPLC) facilitates the analysis and saves time [173,174], which makes the parallel use of more than one method possible. It is recommended that the antioxidant activity of natural products be evaluated using assays of biological relevance or complementary assays (e.g., chemical and biochemical) to eliminate possible over- or underestimations due to the absence of a biological system and its components [175]. However, the use of methods of biological irrelevance could provide insights into the antioxidant properties and could guide the research towards more appropriate quantification methods, including in vivo analyses. The future research is focused on developing electrochemical sensors and biosensors, which could be employed for the rapid quantification of complex samples in small amounts and could provide additional information about the kinetics and mechanisms involved in the antioxidant activity [176].
Table 5.
Common chemical methods for determining the antioxidant activity of natural molecules.
| Method Name | Description | Detection Method | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|
| Radical/ROS-based scavenging assays | |||||
| 2,2′-azinobis (3-ethylbenzo-thiazoline 6- sulphonate) (ABTS)/Trolox equivalent antioxidant capacity (TEAC) test |
|
Spectrophotometry (A734) |
|
|
[167,177,178,179] |
| N, N-diphenyl-N’-picrylhydrazyl (DPPH) free radical |
|
|
|
|
[174,177] |
| Oxygen radical absorbance capacity (ORAC) |
|
Fluorometry |
|
|
[175,180,181,182] |
| Chemiluminescence |
|
Fluorometry |
|
|
[178,179,180,183,184] |
| Non-radical redox-potential-based assays | |||||
| Ferric reducing antioxidant power (FRAP) |
|
|
|
|
[173,178,179,185,186,187] |
| Cupric reducing antioxidant capacity (CUPRAC) |
|
Spectrophotometry (A450) |
|
|
[164,166,188,189,190] |
HAT (hydrogen atom transfer), ET (single-electron transfer), EPR (electron paramagnetic resonance), HPLC (high-performance liquid chromatography), TPTZ (tripyridyltriazine).
9. Antimicrobial Activity
The usefulness of plant extracts for antimicrobial therapy has been promising since ancient times [191]. However, over the last few years, about 250,000 higher plant species have been described, 17% of which have been evaluated according to biological aspects and only 10% which been assessed for antimicrobial activity and several classes of secondary metabolites (alkaloids, flavonoids, saponins, etc.) [192].
Antimicrobial resistance (AMR) is a significant threat to human health worldwide. In 2019, an estimated 4.95 million deaths were associated with AMR, of which 1.95 million were deaths associated with infections caused by fungi, mainly Candida, Aspergillus, and Cryptococcus [193]. Among the bacteria, the leading pathogens for fatalities related to antibiotic resistance are Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Streptococcus pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa. The synergistic effect of antibiotics with plant extracts against resistant bacteria may lead to new options for treating infectious diseases when the antibiotic is no longer effective during treatment [194].
The Clinical Guidelines of the European Committee on Antimicrobial Susceptibility Testing (CLSI) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) [195,196] seek to standardize testing for clinical purposes; however, when it comes to natural extracts, these guidelines are also used since there are no standards of their own [197,198,199]. Therefore, it is essential to consider the limitations that this entails. For example, natural extracts may not be hydrophilic, and there is no database of minimum inhibitory concentrations for mixtures of molecules to compare results. This lack of standardization regarding the techniques used to determine antimicrobial activity does not always allow the results to be compared between investigations focused on natural products [200].
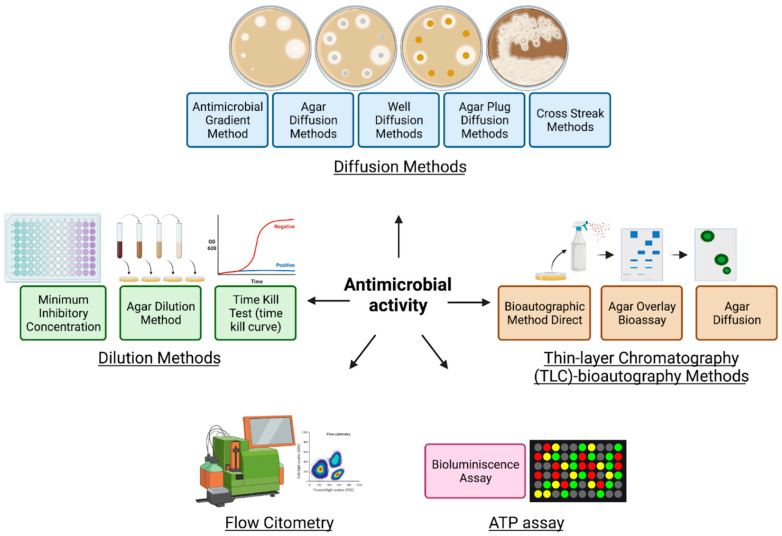
Although new technologies have emerged to assess antimicrobial activity, traditional technologies are the most widely used for bacteria and fungi. For example, in vitro antimicrobial evaluation methods emerged in the 1960s and responded to rapid, simple tests that evaluate various plant extracts or pure compounds at a low cost [201]. These methods involve the diffusion of the potential antimicrobial compound through solid or semi-solid culture media to inhibit the growth of sensitive microorganisms (Figure 7) [197]. The reference methods are disk diffusion and broth or agar dilution assays; however, in the last decades, new evaluation methods have emerged to overcome some of the disadvantages of traditional methods, such as the response time, low sensitivity, and reproducibility [202,203]. An overview of the commonly used susceptibility testing methods is shown in Table 6.
Figure 7.
Summary of the most relevant antimicrobial activity methods.
Table 6.
Traditional and emerging methodologies used to assess the antimicrobial activity of natural products.
| Method Name | Description | Advantages | Disadvantages | Ref. | |
|---|---|---|---|---|---|
| Diffusion methods | Agar diffusion method | The antimicrobial agent diffuses from disks or strips into the solid culture medium that has been seeded with a pure culture |
|
|
[198,199,204] |
| Well diffusion method | Diffusion of a liquid antimicrobial agent placed in a well punched into the solid culture medium that has been seeded with a pure culture | ||||
| Agar plug diffusion method | The first bacterium or fungus is grown on agar plates, here it will secrete molecules that diffuse in the agar which then is cut and placed on another agar plate inoculated with a different microorganism |
|
|
[199,200,201] | |
| Antimicrobial gradient method (Etest) | Based on creating a concentration gradient of the antimicrobial agent tested in the agar medium where it is exposed to the selected microorganism |
|
|
[205,206] | |
| Cross streak method | The first microbial strain is streaked in the center of the agar plate and incubated then in the same plate is seeded the second microorganism by a single streak perpendicular to the central streak |
|
|
[207,208] | |
| Thin-layer chromatography (TLC)–bioautography methods | Bioautographic method direct | The antimicrobial activity is assessed directly onto the TLC plates where the extracts are separated by chromatography across a TLC plate, then the microorganisms are also applied by spray-identifying the localization of the fraction with antimicrobial potential |
|
|
[209,210] |
| Agar overlay bioassay | The TLC plate is covered with agar seeded with the test microbe and the antimicrobial compounds are diffused onto the agar medium |
|
|
[211,212,213] | |
| Agar diffusion | The antimicrobial agent is transferred from a TLC to an agar plate previously inoculated with the test microorganism |
|
|
[212,214] | |
| Dilution methods | Broth dilution method Minimum inhibitory concentration (MIC) determination |
Uses tubes or microdilution plates to measures the lowest concentration of antimicrobial agent that completely inhibits the growth of the bacteria/fungi |
|
|
[215,216] |
| Agar dilution method | Similarly, to the procedure used in the disk diffusion method, a desired concentration of the antimicrobial agent is placed into an agar medium |
|
|
[217,218,219] | |
| Time-kill test (time-kill curve) | Based on a time/concentration-dependent analysis of antimicrobial effects. Several tubes containing varying concentrations of the antimicrobial agent are seeded with the bacteria/fungi and the percentage of dead cells is determined along with the assay |
|
|
[220,221] | |
| ATP bioluminescence assay | Bioluminescence ATP based | Based on the capacity to measure adenosine triphosphate (ATP) produced by bacteria or fungi |
|
|
[222,223,224] |
| Flow cytofluorometric method | Flow cytofluorometric | Based on the capacity of damaged cells to emit a positive signal that is detected by flow cytometry analysis. The microorganism is exposed to antimicrobial agents and then stained with the intercalating agent propidium iodide. |
|
|
[225,226,227] |
| Biofilm inhibition | Microtiter plate biofilm production assay | Based on the potential of the extracts to prevent initial cell attachment to microtiter plates. Biofilm formation/inhibition is observed through (OD) of the crystal violet present in the destaining solution measured at 595 nm |
|
|
[228,229] |
| Quorum sensing inhibition | Violacein quantification | The inhibitory activity is measured by quantifying violacein production in a microplate reader using a spectrophotometer at 585 nm where the microorganism and extracts are cultured at different concentrations. |
|
|
[230,231] |
ATP: adenosine triphosphate; OD: optical density.
The research into natural compounds has shown significant progress in discovering new molecules with antimicrobial activity. The primary natural compounds with valuable antimicrobial activity are medicinal plants and microorganisms. However, given the great diversity of compounds with antimicrobial potential, it is necessary to have adequate tools to facilitate screening, reduce costs, and obtain rapid, quality results [232].
According to the CLSI and EUCAST guidelines, disk diffusion assays are considered the gold standard for evaluating antimicrobial activity. However, in the case of natural extracts, there is no adequate method in all cases, so the decision must consider, among other aspects, the extract’s polarity and complexity, the available equipment, and the possibility of automation [233]. The emergent methodologies to assess the antimicrobial activity of natural extracts include isothermal microcalorimetry (IMC) and mass spectrometry. However, in both cases, these methodologies are expensive and require specialized equipment and training, which limits their use broadly [234,235,236].
In addition, the emergence of new antimicrobial resistance mechanisms requires that the performance of susceptible devices be constantly reassessed and updated periodically, along with new automated instruments that can provide faster results, save money, and reduce the labor requirements [205,237]. Hence, further improvements in the currently used and novel antimicrobial susceptibility test (AST) methods and instruments are mandatory to speed up the determination of antimicrobial efficacy in clinical and research microbiology laboratories in the foreseeable future.
10. Conclusions
The high structural and physicochemical diversity of natural molecules produced by plants, microorganisms, and insects makes them a virtually inexhaustible source with potential for the discovery and development of novel bioactive compounds with applications in the pharmaceutical, food, and biotechnological industries, to name only a few [238,239]. Additionally, the selectivity, specificity, and potency are common features of many natural compounds as the result of their ongoing evolutionary specialization over millions of years. Given the immense variety of biological molecules and the high array of promising activities and evaluation methods, it is crucial to establish the most relevant tests for the initial screening stage [234].
The first step in the biological evaluation of natural molecules commonly involves evaluating the compound in a range of in vitro biochemical and pharmacological assays. These experiments aim to establish a solid grounding regarding the compound’s properties and to understand its mechanism of action. This information is then used to select the most suitable compounds and evaluate their activity in more complex in vitro and in vivo assays [240]. The selection of the methods to be used must be made on a case-by-case basis, considering their advantages and limitations. Additionally, the use of complementary assays during the evaluation of biological activities to avoid underestimating their potential activity and to aid with identifying the pharmacological mechanisms is highly recommended. The future trends in assessing the biological activity of natural molecules are focused on establishing simplified and automated protocols that enable high-throughput screening [235], facilitating subsequent analyses, shortening the evaluation time, and allowing the parallel use of multiple methods [236]. Moreover, different systems are being developed to support more physiologically relevant data collection and enable real-time monitoring.
It is also important, after the initial identification of the biological potential from an extract or a natural source, to determine the specific molecule responsible for the given activity. Thus, this initial screening can be further complemented by identifying specific metabolites or active molecules present in the sample through approaches such as fractionation, NMR, HPLC-MS/MS, high-resolution Fourier transform mass spectrometry (FTMS), and photo-diode arrays. Notably, the data generated from MS and NMR are large and complex. Therefore, several computational approaches and multivariate analysis techniques such as the cluster analysis and partial least squares regression (PLS) methods, as well as the principal component analysis (PCA) method, are employed to identify and discriminate the metabolites present in each sample [241]. Another approach is to depict the interactions between metabolites and biological pathways as networks. Here, the metabolite is placed in a node and linked to a metabolic interaction. KEGG, Human Recon 1, and EHMN are some of the existing metabolic databases focused on metabolic pathways [242]. Moreover, high-throughput data can be used to identify pathways through Bayesian-based networks, correlation-based measures, and Gaussian graphical models (GGMs) [243,244]. Overall, complementary strategies should be employed to extract as much information as possible from an experiment and to help develop novel therapeutics.
Finally, as discussed throughout this review, it is worth mentioning that identifying natural products with biological activities is crucial for drug discovery. However, it is also important to recall that regardless of whether the compounds are obtained from natural sources or chemically synthesized, they are not exempt from potentially causing adverse reactions. Therefore, their adverse effects must be evaluated with the same rigor required when evaluating their biological activity.
Acknowledgments
We thank Federico Zertuche for his technical support. Figure 3 and Figure 7 were created with BioRender.com.
Author Contributions
Conceptualization C.B.-O., S.E.C.-P. and L.P.G., writing—original draft preparation, C.B.-O., S.E.C.-P., R.G.-P., J.H.-M., A.M.-R., C.R.-P., J.Z.-M. and B.A.-A., writing—review and editing C.B.-O., L.P.G., S.E.C.-P. and R.G.-P., supervision, L.P.G.; project administration L.P.G. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All tables were created by the authors. All sources of information were adequately referenced. There was no need to obtain copyright permission.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Malaquias G., Santos Cerqueira G., Pinheiro Ferreira P.M., Landim Pacheco A.C., de Castro e Souza J.M., do Socorro Meireles de Deus M., Peron A.P. Utilização na medicina popular, potencial terapêutico e toxicidade em nível celular das plantas Rosmarinus officinalis L., Salvia officinalis L. e Mentha piperita L. (Família Lamiaceae) Rev. Intertox Toxicol. Risco Ambient. Soc. 2015;7:50–68. doi: 10.22280/revintervol7ed3.183. [DOI] [Google Scholar]
- 2.Kingston D.G.I. Modern natural products drug discovery and its relevance to biodiversity conservation. J. Nat. Prod. 2011;74:496–511. doi: 10.1021/np100550t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fabricant D.S., Farnsworth N.R. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 2001;109((Suppl. S1)):69–75. doi: 10.1289/ehp.01109s169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Batool M., Ahmad B., Choi S. A Structure-Based Drug Discovery Paradigm. Int. J. Mol. Sci. 2019;20:2783. doi: 10.3390/ijms20112783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin Y.C., Kofron J.L., Traphagen L.M. Do structurally similar molecules have similar biological activity? J. Med. Chem. 2002;45:4350–4358. doi: 10.1021/jm020155c. [DOI] [PubMed] [Google Scholar]
- 6.Rasoanaivo P., Wright C.W., Willcox M.L., Gilbert B. Whole plant extracts versus single compounds for the treatment of malaria: Synergy and positive interactions. Malar. J. 2011;10((Suppl. S1)):S4. doi: 10.1186/1475-2875-10-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilbert B., Alves L. Synergy in plant medicines. Curr. Med. Chem. 2003;10:13–20. doi: 10.2174/0929867033368583. [DOI] [PubMed] [Google Scholar]
- 8.Vinken M., Blaauboer B.J. In vitro testing of basal cytotoxicity: Establishment of an adverse outcome pathway from chemical insult to cell death. Toxicol. Vitr. 2017;39:104–110. doi: 10.1016/j.tiv.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Nunzio M., Valli V., Tomás-Cobos L., Tomás-Chisbert T., Murgui-Bosch L., Danesi F., Bordoni A. Is cytotoxicity a determinant of the different in vitro and in vivo effects of bioactives? BMC Complement. Altern. Med. 2017;17:453. doi: 10.1186/s12906-017-1962-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ling T., Lang W.H., Maier J., Quintana Centurion M., Rivas F. Cytostatic and Cytotoxic Natural Products against Cancer Cell Models. Molecules. 2019;24:2012. doi: 10.3390/molecules24102012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sachana M., Hargreaves A.J. Veterinary Toxicology. 3rd ed. Academic Press; Cambridge, MA, USA: 2018. Chapter 9—Toxicological testing: In vivo and in vitro models; pp. 145–161. [Google Scholar]
- 12.Jablonská E., Kubásek J., Vojtěch D., Ruml T., Lipov J. Test conditions can significantly affect the results of in vitro cytotoxicity testing of degradable metallic biomaterials. Sci. Rep. 2021;11:6628. doi: 10.1038/s41598-021-85019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamiloglu S., Sari G., Ozdal T., Capanoglu E. Guidelines for cell viability assays. Food Front. 2020;1:332–349. doi: 10.1002/fft2.44. [DOI] [Google Scholar]
- 14.Riss T., Niles A., Moravec R., Karassina N., Vidugiriene J. Cytotoxicity assays: In vitro methods to measure dead cells. In: Sittampalam G.S., Coussens N.P., Brimacombe K., Grossman A., Arkin M., Auld D., Austin C., Baell J., Bejcek B., Caaveiro J.M.M., et al., editors. Assay Guidance Manual. Eli Lilly & Company and the National Center for Advancing Translational Sciences; Bethesda, MD, USA: 2004. [PubMed] [Google Scholar]
- 15.Gordon J.L., Brown M.A., Reynolds M.M. Cell-Based Methods for Determination of Efficacy for Candidate Therapeutics in the Clinical Management of Cancer. Diseases. 2018;6:85. doi: 10.3390/diseases6040085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.King T.C. Elsevier’s Integrated Pathology. Elsevier; Amsterdam, The Netherlands: 2007. Cell injury, cellular responses to injury, and cell death; pp. 1–20. [Google Scholar]
- 17.McStay G.P., Green D.R. Measuring apoptosis: Caspase inhibitors and activity assays. Cold Spring Harb. Protoc. 2014;2014:799–806. doi: 10.1101/pdb.top070359. [DOI] [PubMed] [Google Scholar]
- 18.Elmore S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heller-Uszynska K., Kilian A. Microarray TRAP—A high-throughput assay to quantitate telomerase activity. Biochem. Biophys. Res. Commun. 2004;323:465–472. doi: 10.1016/j.bbrc.2004.08.109. [DOI] [PubMed] [Google Scholar]
- 20.Menyhárt O., Harami-Papp H., Sukumar S., Schäfer R., Magnani L., de Barrios O., Győrffy B. Guidelines for the selection of functional assays to evaluate the hallmarks of cancer. Biochim. Biophys. Acta. 2016;1866:300–319. doi: 10.1016/j.bbcan.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 21.Borowicz S., Van Scoyk M., Avasarala S., Karuppusamy Rathinam M.K., Tauler J., Bikkavilli R.K., Winn R.A. The soft agar colony formation assay. J. Vis. Exp. 2014;92:e51998. doi: 10.3791/51998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hulkower K.I., Herber R.L. Cell migration and invasion assays as tools for drug discovery. Pharmaceutics. 2011;3:107–124. doi: 10.3390/pharmaceutics3010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grada A., Otero-Vinas M., Prieto-Castrillo F., Obagi Z., Falanga V. Research techniques made simple: Analysis of collective cell migration using the wound healing assay. J. Investig. Dermatol. 2017;137:e11–e16. doi: 10.1016/j.jid.2016.11.020. [DOI] [PubMed] [Google Scholar]
- 24.van Tonder A., Joubert A.M., Cromarty A.D. Limitations of the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay when compared to three commonly used cell enumeration assays. BMC Res. Notes. 2015;8:47. doi: 10.1186/s13104-015-1000-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akter S., Addepalli R., Netzel M.E., Tinggi U., Fletcher M.T., Sultanbawa Y., Osborne S.A. Antioxidant-Rich Extracts of Terminalia ferdinandiana Interfere with Estimation of Cell Viability. Antioxidants. 2019;8:191. doi: 10.3390/antiox8060191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niles A.L., Moravec R.A., Riss T.L. Update on in vitro cytotoxicity assays for drug development. Expert Opin. Drug Discov. 2008;3:655–669. doi: 10.1517/17460441.3.6.655. [DOI] [PubMed] [Google Scholar]
- 27.Aslantürk Ö.S. In vitro cytotoxicity and cell viability assays: Principles, advantages, and disadvantages. In: Larramendy M.L., Soloneski S., editors. Genotoxicity—A Predictable Risk to Our Actual World. InTech; London, UK: 2018. [Google Scholar]
- 28.Jain A.K., Singh D., Dubey K., Maurya R., Mittal S., Pandey A.K. In Vitro Toxicology. Elsevier; Amsterdam, The Netherlands: 2018. Models and methods for in vitro toxicity; pp. 45–65. [Google Scholar]
- 29.Strober W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2015;111:A3.B.1-3. doi: 10.1002/0471142735.ima03bs111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lebeau P.F., Chen J., Byun J.H., Platko K., Austin R.C. The trypan blue cellular debris assay: A novel low-cost method for the rapid quantification of cell death. MethodsX. 2019;6:1174–1180. doi: 10.1016/j.mex.2019.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Śliwka L., Wiktorska K., Suchocki P., Milczarek M., Mielczarek S., Lubelska K., Cierpiał T., Łyżwa P., Kiełbasiński P., Jaromin A., et al. The comparison of MTT and CVS assays for the assessment of anticancer agent interactions. PLoS ONE. 2016;11:e0155772. doi: 10.1371/journal.pone.0155772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bopp S.K., Lettieri T. Comparison of four different colorimetric and fluorometric cytotoxicity assays in a zebrafish liver cell line. BMC Pharmacol. 2008;8:8. doi: 10.1186/1471-2210-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barile F.A. Vitro Methods in Pharmaceutical Research. Elsevier; Amsterdam, The Netherlands: 1997. Continuous cell lines as a model for drug toxicity assessment; pp. 33–54. [Google Scholar]
- 34.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 35.Vega-Avila E., Pugsley M.K. An overview of colorimetric assay methods used to assess survival or proliferation of mammalian cells. Proc. West. Pharmacol. Soc. 2011;54:10–14. [PubMed] [Google Scholar]
- 36.Walzl A., Unger C., Kramer N., Unterleuthner D., Scherzer M., Hengstschläger M., Schwanzer-Pfeiffer D., Dolznig H. The Resazurin Reduction Assay Can Distinguish Cytotoxic from Cytostatic Compounds in Spheroid Screening Assays. J. Biomol. Screen. 2014;19:1047–1059. doi: 10.1177/1087057114532352. [DOI] [PubMed] [Google Scholar]
- 37.Präbst K., Engelhardt H., Ringgeler S., Hübner H. Basic colorimetric proliferation assays: MTT, WST, and resazurin. Methods Mol. Biol. 2017;1601:1–17. doi: 10.1007/978-1-4939-6960-9_1. [DOI] [PubMed] [Google Scholar]
- 38.Jakštys B., Ruzgys P., Tamošiūnas M., Šatkauskas S. Different cell viability assays reveal inconsistent results after bleomycin electrotransfer in vitro. J. Membr. Biol. 2015;248:857–863. doi: 10.1007/s00232-015-9813-x. [DOI] [PubMed] [Google Scholar]
- 39.Gutiérrez L., Stepien G., Gutiérrez L., Pérez-Hernández M., Pardo J., Pardo J., Grazú V., de la Fuente J.M. Comprehensive Medicinal Chemistry III. Elsevier; Amsterdam, The Netherlands: 2017. Nanotechnology in drug discovery and development; pp. 264–295. [Google Scholar]
- 40.Weisenthal L.M., Lippman M.E. Clonogenic and nonclonogenic in vitro chemosensitivity assays. Cancer Treat. Rep. 1985;69:615–632. [PubMed] [Google Scholar]
- 41.Weisenthal L.M., Dill P.L., Kurnick N.B., Lippman M.E. Comparison of dye exclusion assays with a clonogenic assay in the determination of drug-induced cytotoxicity. Cancer Res. 1983;43:258–264. [PubMed] [Google Scholar]
- 42.Juan-García A., Taroncher M., Font G., Ruiz M.-J. Micronucleus induction and cell cycle alterations produced by deoxynivalenol and its acetylated derivatives in individual and combined exposure on HepG2 cells. Food Chem. Toxicol. 2018;118:719–725. doi: 10.1016/j.fct.2018.06.024. [DOI] [PubMed] [Google Scholar]
- 43.Wu H., Chen L., Zhu F., Han X., Sun L., Chen K. The cytotoxicity effect of resveratrol: Cell cycle arrest and induced apoptosis of breast cancer 4T1 cells. Toxins. 2019;11:731. doi: 10.3390/toxins11120731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wlodkowic D., Telford W., Skommer J., Darzynkiewicz Z. Apoptosis and beyond: Cytometry in studies of programmed cell death. Methods Cell Biol. 2011;103:55–98. doi: 10.1016/B978-0-12-385493-3.00004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rieger A.M., Nelson K.L., Konowalchuk J.D., Barreda D.R. Modified annexin V/propidium iodide apoptosis assay for accurate assessment of cell death. J. Vis. Exp. 2011;50:2597. doi: 10.3791/2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khazaei S., Esa N.M., Ramachandran V., Hamid R.A., Pandurangan A.K., Etemad A., Ismail P. In vitro Antiproliferative and Apoptosis Inducing Effect of Allium atroviolaceum Bulb Extract on Breast, Cervical, and Liver Cancer Cells. Front. Pharmacol. 2017;8:5. doi: 10.3389/fphar.2017.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ballav S., Jaywant Deshmukh A., Siddiqui S., Aich J., Basu S. Cell Culture—Advanced Technology and Applications in Medical and Life Sciences. IntechOpen; London, UK: 2021. Two-Dimensional and Three-Dimensional Cell Culture and Their Applications. Biochemistry. [Google Scholar]
- 48.Kapałczyńska M., Kolenda T., Przybyła W., Zajączkowska M., Teresiak A., Filas V., Ibbs M., Bliźniak R., Łuczewski Ł., Lamperska K. 2D and 3D cell cultures—A comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018;14:910–919. doi: 10.5114/aoms.2016.63743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jensen C., Teng Y. Is it time to start transitioning from 2D to 3D cell culture? Front. Mol. Biosci. 2020;7:33. doi: 10.3389/fmolb.2020.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoarau-Véchot J., Rafii A., Touboul C., Pasquier J. Halfway between 2D and Animal Models: Are 3D Cultures the Ideal Tool to Study Cancer-Microenvironment Interactions? Int. J. Mol. Sci. 2018;19:181. doi: 10.3390/ijms19010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berrouet C., Dorilas N., Rejniak K.A., Tuncer N. Comparison of Drug Inhibitory Effects (IC50) in Monolayer and Spheroid Cultures. Bull. Math. Biol. 2020;82:68. doi: 10.1007/s11538-020-00746-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martin S.Z., Wagner D.C., Hörner N., Horst D., Lang H., Tagscherer K.E., Roth W. Ex vivo tissue slice culture system to measure drug-response rates of hepatic metastatic colorectal cancer. BMC Cancer. 2019;19:1030. doi: 10.1186/s12885-019-6270-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roelants C., Pillet C., Franquet Q., Sarrazin C., Peilleron N., Giacosa S., Guyon L., Fontanell A., Fiard G., Long J.-A., et al. Ex-Vivo Treatment of Tumor Tissue Slices as a Predictive Preclinical Method to Evaluate Targeted Therapies for Patients with Renal Carcinoma. Cancers. 2020;12:232. doi: 10.3390/cancers12010232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koerfer J., Kallendrusch S., Merz F., Wittekind C., Kubick C., Kassahun W.T., Schumacher G., Moebius C., Gaßler N., Schopow N., et al. Organotypic slice cultures of human gastric and esophagogastric junction cancer. Cancer Med. 2016;5:1444–1453. doi: 10.1002/cam4.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Temblador A., Topalis D., van den Oord J., Andrei G., Snoeck R. Organotypic Epithelial Raft Cultures as a Three-Dimensional In Vitro Model of Merkel Cell Carcinoma. Cancers. 2022;14:1091. doi: 10.3390/cancers14041091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin R.-Z., Lin R.-Z., Chang H.-Y. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol. J. 2008;3:1172–1184. doi: 10.1002/biot.200700228. [DOI] [PubMed] [Google Scholar]
- 57.Cui X., Hartanto Y., Zhang H. Advances in multicellular spheroids formation. J. R. Soc. Interface. 2017;14:20160877. doi: 10.1098/rsif.2016.0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shankaran A., Prasad K., Chaudhari S., Brand A., Satyamoorthy K. Advances in development and application of human organoids. 3 Biotech. 2021;11:257. doi: 10.1007/s13205-021-02815-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Caipa Garcia A.L., Arlt V.M., Phillips D.H. Organoids for toxicology and genetic toxicology: Applications with drugs and prospects for environmental carcinogenesis. Mutagenesis. 2022;37:143–154. doi: 10.1093/mutage/geab023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fey S.J., Wrzesinski K. Determination of drug toxicity using 3D spheroids constructed from an immortal human hepatocyte cell line. Toxicol. Sci. 2012;127:403–411. doi: 10.1093/toxsci/kfs122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weeber F., Ooft S.N., Dijkstra K.K., Voest E.E. Tumor Organoids as a Pre-clinical Cancer Model for Drug Discovery. Cell Chem. Biol. 2017;24:1092–1100. doi: 10.1016/j.chembiol.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 62.Kitaeva K.V., Rutland C.S., Rizvanov A.A., Solovyeva V.V. Cell Culture Based in vitro Test Systems for Anticancer Drug Screening. Front. Bioeng. Biotechnol. 2020;8:322. doi: 10.3389/fbioe.2020.00322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garcia-Hernando M., Benito-Lopez F., Basabe-Desmonts L. Advances in microtechnology for improved cytotoxicity assessment. Front. Mater. 2020;7:582030. doi: 10.3389/fmats.2020.582030. [DOI] [Google Scholar]
- 64.Mi S., Du Z., Xu Y., Wu Z., Qian X., Zhang M., Sun W. Microfluidic co-culture system for cancer migratory analysis and anti-metastatic drugs screening. Sci. Rep. 2016;6:35544. doi: 10.1038/srep35544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bhatia S.N., Ingber D.E. Microfluidic organs-on-chips. Nat. Biotechnol. 2014;32:760–772. doi: 10.1038/nbt.2989. [DOI] [PubMed] [Google Scholar]
- 66.Bhise N.S., Ribas J., Manoharan V., Zhang Y.S., Polini A., Massa S., Dokmeci M.R., Khademhosseini A. Organ-on-a-chip platforms for studying drug delivery systems. J. Control. Release. 2014;190:82–93. doi: 10.1016/j.jconrel.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lubamba B., Jensen T., McClelland R. Rapid Detection of Direct Compound Toxicity and Trailing Detection of Indirect Cell Metabolite Toxicity in a 96-Well Fluidic Culture Device for Cell-Based Screening Environments: Tactics in Six Sigma Quality Control Charts. Appl. Sci. 2022;12:2786. doi: 10.3390/app12062786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roglic G., World Health Organization . Global Report on Diabetes. World Health Organization; Geneva, Switzerland: 2016. [Google Scholar]
- 69.Reyes B.A.S., Dufourt E.C., Ross J., Warner M.J., Tanquilut N.C., Leung A.B. Studies in Natural Products Chemistry. Volume 55. Elsevier; Amsterdam, The Netherlands: 2017. Selected phyto and marine bioactive compounds: Alternatives for the treatment of type 2 diabetes; pp. 111–143. [Google Scholar]
- 70.Thete M., Dilip A. Recent advances and methods for in-vitro evaluation of antidiabetic activity: A review. Int. J. Eng. Appl. Sci. Technol. 2020;4:194–198. [Google Scholar]
- 71.Gromova L.V., Fetissov S.O., Gruzdkov A.A. Mechanisms of glucose absorption in the small intestine in health and metabolic diseases and their role in appetite regulation. Nutrients. 2021;13:2474. doi: 10.3390/nu13072474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xue B., Kim Y.-B., Lee A., Toschi E., Bonner-Weir S., Kahn C.R., Neel B.G., Kahn B.B. Protein-tyrosine phosphatase 1B deficiency reduces insulin resistance and the diabetic phenotype in mice with polygenic insulin resistance. J. Biol. Chem. 2007;282:23829–23840. doi: 10.1074/jbc.M609680200. [DOI] [PubMed] [Google Scholar]
- 73.Deacon C.F. Physiology and Pharmacology of DPP-4 in Glucose Homeostasis and the Treatment of Type 2 Diabetes. Front. Endocrinol. 2019;10:80. doi: 10.3389/fendo.2019.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Prabhakar P.K., Sivakumar P.M. Protein Tyrosine Phosphatase 1B Inhibitors: A Novel Therapeutic Strategy for the Management of type 2 Diabetes Mellitus. Curr. Pharm. Des. 2019;25:2526–2539. doi: 10.2174/1381612825666190716102901. [DOI] [PubMed] [Google Scholar]
- 75.Mulvihill E.E., Drucker D.J. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocr. Rev. 2014;35:992–1019. doi: 10.1210/er.2014-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Omar B., Ahrén B. Pleiotropic mechanisms for the glucose-lowering action of DPP-4 inhibitors. Diabetes. 2014;63:2196–2202. doi: 10.2337/db14-0052. [DOI] [PubMed] [Google Scholar]
- 77.Yip S.-C., Saha S., Chernoff J. PTP1B: A double agent in metabolism and oncogenesis. Trends Biochem. Sci. 2010;35:442–449. doi: 10.1016/j.tibs.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Elchebly M., Payette P., Michaliszyn E., Cromlish W., Collins S., Loy A.L., Normandin D., Cheng A., Himms-Hagen J., Chan C.C., et al. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science. 1999;283:1544–1548. doi: 10.1126/science.283.5407.1544. [DOI] [PubMed] [Google Scholar]
- 79.Csepregi R., Temesfői V., Sali N., Poór M., Needs P.W., Kroon P.A., Kőszegi T. A One-Step Extraction and Luminescence Assay for Quantifying Glucose and ATP Levels in Cultured HepG2 Cells. Int. J. Mol. Sci. 2018;19:2670. doi: 10.3390/ijms19092670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Blodgett A.B., Kothinti R.K., Kamyshko I., Petering D.H., Kumar S., Tabatabai N.M. A fluorescence method for measurement of glucose transport in kidney cells. Diabetes Technol. Ther. 2011;13:743–751. doi: 10.1089/dia.2011.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dong S., Alahari S. FACS-based Glucose Uptake Assay of Mouse Embryonic Fibroblasts and Breast Cancer Cells Using 2-NBDG Probe. Bio Protoc. 2018;8:e2816. doi: 10.21769/BioProtoc.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cirillo V.P. Mechanism of glucose transport across the yeast cell membrane. J. Bacteriol. 1962;84:485–491. doi: 10.1128/jb.84.3.485-491.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schmidl S., Iancu C.V., Reifenrath M., Choe J.-Y., Oreb M. A label-free real-time method for measuring glucose uptake kinetics in yeast. FEMS Yeast Res. 2021;21:foaa069. doi: 10.1093/femsyr/foaa069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee J., Noh S., Lim S., Kim B. Plant extracts for type 2 diabetes: From traditional medicine to modern drug discovery. Antioxidants. 2021;10:81. doi: 10.3390/antiox10010081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schmidt S., Jakab M., Jav S., Streif D., Pitschmann A., Zehl M., Purevsuren S., Glasl S., Ritter M. Extracts from Leonurus sibiricus L. increase insulin secretion and proliferation of rat INS-1E insulinoma cells. J. Ethnopharmacol. 2013;150:85–94. doi: 10.1016/j.jep.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 86.Ansari P., Flatt P.R., Harriott P., Abdel-Wahab Y.H.A. Insulin secretory and antidiabetic actions of Heritiera fomes bark together with isolation of active phytomolecules. PLoS ONE. 2022;17:e0264632. doi: 10.1371/journal.pone.0264632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hager R., Pitsch J., Kerbl-Knapp J., Neuhauser C., Ollinger N., Iken M., Ranner J., Mittermeier-Kleßinger V., Dawid C., Lanzerstorfer P., et al. A High-Content Screen for the Identification of Plant Extracts with Insulin Secretion-Modulating Activity. Pharmaceuticals. 2021;14:809. doi: 10.3390/ph14080809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kalwat M.A., Wichaidit C., Nava Garcia A.Y., McCoy M.K., McGlynn K., Hwang I.H., MacMillan J.B., Posner B.A., Cobb M.H. Insulin promoter-driven Gaussia luciferase-based insulin secretion biosensor assay for discovery of β-cell glucose-sensing pathways. ACS Sens. 2016;1:1208–1212. doi: 10.1021/acssensors.6b00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bhatia A., Singh B., Arora R., Arora S. In vitro evaluation of the α-glucosidase inhibitory potential of methanolic extracts of traditionally used antidiabetic plants. BMC Complement. Altern. Med. 2019;19:74. doi: 10.1186/s12906-019-2482-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mechchate H., Es-Safi I., Louba A., Alqahtani A.S., Nasr F.A., Noman O.M., Farooq M., Alharbi M.S., Alqahtani A., Bari A., et al. In Vitro Alpha-Amylase and Alpha-Glucosidase Inhibitory Activity and In Vivo Antidiabetic Activity of Withania frutescens L. Foliar Extract. Molecules. 2021;26:293. doi: 10.3390/molecules26020293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yamamoto N., Ueda-Wakagi M., Sato T., Kawasaki K., Sawada K., Kawabata K., Akagawa M., Ashida H. Measurement of glucose uptake in cultured cells. Curr. Protoc. Pharmacol. 2015;71:12.14.1–12.14.26. doi: 10.1002/0471141755.ph1214s71. [DOI] [PubMed] [Google Scholar]
- 92.Maric T., Mikhaylov G., Khodakivskyi P., Bazhin A., Sinisi R., Bonhoure N., Yevtodiyenko A., Jones A., Muhunthan V., Abdelhady G., et al. Bioluminescent-based imaging and quantification of glucose uptake in vivo. Nat. Methods. 2019;16:526–532. doi: 10.1038/s41592-019-0421-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen L., Deng H., Cui H., Fang J., Zuo Z., Deng J., Li Y., Wang X., Zhao L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018;9:7204–7218. doi: 10.18632/oncotarget.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shehata I.A., El-harshany E., Abdallah H.M., Esmat A., Abdel-sattar E.A. Anti-inflammatory activity of Kleinia odora. Eur. J. Integr. Med. 2018;23:64–69. doi: 10.1016/j.eujim.2018.10.005. [DOI] [Google Scholar]
- 95.Ondua M., Njoya E.M., Abdalla M.A., McGaw L.J. Anti-inflammatory and antioxidant properties of leaf extracts of eleven South African medicinal plants used traditionally to treat inflammation. J. Ethnopharmacol. 2019;234:27–35. doi: 10.1016/j.jep.2018.12.030. [DOI] [PubMed] [Google Scholar]
- 96.Chan P.-M., Tan Y.-S., Chua K.-H., Sabaratnam V., Kuppusamy U.R. Attenuation of Inflammatory Mediators (TNF-α and Nitric Oxide) and Up-Regulation of IL-10 by Wild and Domesticated Basidiocarps of Amauroderma rugosum (Blume & T. Nees) Torrend in LPS-Stimulated RAW264.7 Cells. PLoS ONE. 2015;10:e0139593. doi: 10.1371/journal.pone.0139593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Abdulkhaleq L.A., Assi M.A., Abdullah R., Zamri-Saad M., Taufiq-Yap Y.H., Hezmee M.N.M. The crucial roles of inflammatory mediators in inflammation: A review. Vet. World. 2018;11:627–635. doi: 10.14202/vetworld.2018.627-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Oguntibeju O.O. Medicinal plants with anti-inflammatory activities from selected countries and regions of Africa. J. Inflamm. Res. 2018;11:307–317. doi: 10.2147/JIR.S167789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Alamgir A.N.M. Therapeutic Use of Medicinal Plants and Their Extracts: Volume 2. Volume 74. Springer; Cham, Switzerland: 2018. Phytoconstituents—Active and inert constituents, metabolic pathways, chemistry and application of phytoconstituents, primary metabolic products, and bioactive compounds of primary metabolic origin; pp. 25–164. Progress in Drug Research. [Google Scholar]
- 100.Ożarowski M., Karpiński T.M. Extracts and Flavonoids of Passiflora Species as Promising Anti-inflammatory and Antioxidant Substances. Curr. Pharm. Des. 2021;27:2582–2604. doi: 10.2174/1381612826666200526150113. [DOI] [PubMed] [Google Scholar]
- 101.Xu Y.-B., Chen G.-L., Guo M.-Q. Antioxidant and Anti-Inflammatory Activities of the Crude Extracts of Moringa oleifera from Kenya and Their Correlations with Flavonoids. Antioxidants. 2019;8:296. doi: 10.3390/antiox8080296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Blando F., Calabriso N., Berland H., Maiorano G., Gerardi C., Carluccio M.A., Andersen Ø.M. Radical Scavenging and Anti-Inflammatory Activities of Representative Anthocyanin Groupings from Pigment-Rich Fruits and Vegetables. Int. J. Mol. Sci. 2018;19:169. doi: 10.3390/ijms19010169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hewlings S.J., Kalman D.S. Curcumin: A review of its effects on human health. Foods. 2017;6:92. doi: 10.3390/foods6100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Studzińska-Sroka E., Dubino A. Lichens as a source of chemical compounds with anti-inflammatory activity. Herba Pol. 2018;64:56–64. doi: 10.2478/hepo-2018-0005. [DOI] [Google Scholar]
- 105.Wu G.-J., Shiu S.-M., Hsieh M.-C., Tsai G.-J. Anti-inflammatory activity of a sulfated polysaccharide from the brown alga Sargassum cristaefolium. Food Hydrocoll. 2016;53:16–23. doi: 10.1016/j.foodhyd.2015.01.019. [DOI] [Google Scholar]
- 106.Wang Y.-H., Zeng K.-W. Natural products as a crucial source of anti-inflammatory drugs: Recent trends and advancements—ProQuest. TMR. 2019;4:257–268. doi: 10.53388/TMR20190831133. [DOI] [Google Scholar]
- 107.Chan P.-M., Kanagasabapathy G., Tan Y.-S., Sabaratnam V., Kuppusamy U.R. Amauroderma rugosum (Blume & T. Nees) Torrend: Nutritional Composition and Antioxidant and Potential Anti-Inflammatory Properties. Evid. Based Complement. Alternat. Med. 2013;2013:304713. doi: 10.1155/2013/304713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Benevides Bahiense J., Marques F.M., Figueira M.M., Vargas T.S., Kondratyuk T.P., Endringer D.C., Scherer R., Fronza M. Potential anti-inflammatory, antioxidant and antimicrobial activities of Sambucus australis. Pharm. Biol. 2017;55:991–997. doi: 10.1080/13880209.2017.1285324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rao U.M. In vitro nitric oxide scavenging and anti inflammatory activities of different solvent extracts of various parts of Musa paradisiaca. Malays. J. Anal. Sci. 2016;20:1191–1202. doi: 10.17576/mjas-2016-2005-26. [DOI] [Google Scholar]
- 110.Hunter R.A., Storm W.L., Coneski P.N., Schoenfisch M.H. Inaccuracies of nitric oxide measurement methods in biological media. Anal. Chem. 2013;85:1957–1963. doi: 10.1021/ac303787p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sun J., Zhang X., Broderick M., Fein H. Measurement of nitric oxide production in biological systems by using griess reaction assay. Sensors. 2003;3:276–284. doi: 10.3390/s30800276. [DOI] [Google Scholar]
- 112.Bryan N.S., Grisham M.B. Methods to detect nitric oxide and its metabolites in biological samples. Free Radic. Biol. Med. 2007;43:645–657. doi: 10.1016/j.freeradbiomed.2007.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Crowther J.R. The ELISA Guidebook. Volume 516. Humana Press; Totowa, NJ, USA: 2009. Methods in Molecular Biology. [Google Scholar]
- 114.Deepak S., Kottapalli K., Rakwal R., Oros G., Rangappa K., Iwahashi H., Masuo Y., Agrawal G. Real-Time PCR: Revolutionizing Detection and Expression Analysis of Genes. Curr. Genom. 2007;8:234–251. doi: 10.2174/138920207781386960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhao H., Wang Q.-L., Hou S.-B., Chen G. Chemical constituents from the rhizomes of Polygonatum sibiricum Red. and anti-inflammatory activity in RAW264.7 macrophage cells. Nat. Prod. Res. 2019;33:2359–2362. doi: 10.1080/14786419.2018.1440220. [DOI] [PubMed] [Google Scholar]
- 116.del Carmen Pinto M., Tejeda A., Duque A.L., Macías P. Determination of lipoxygenase activity in plant extracts using a modified ferrous oxidation-xylenol orange assay. J. Agric. Food Chem. 2007;55:5956–5959. doi: 10.1021/jf070537x. [DOI] [PubMed] [Google Scholar]
- 117.Gunathilake K.D.P.P., Ranaweera K.K.D.S., Rupasinghe H.P.V. Influence of Boiling, Steaming and Frying of Selected Leafy Vegetables on the In Vitro Anti-inflammation Associated Biological Activities. Plants. 2018;7:22. doi: 10.3390/plants7010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Marzouk B., Marzouk Z., Haloui E., Fenina N., Bouraoui A., Aouni M. Screening of analgesic and anti-inflammatory activities of Citrullus colocynthis from southern Tunisia. J. Ethnopharmacol. 2010;128:15–19. doi: 10.1016/j.jep.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 119.Auld D.S., Farmen M.W., Kahl S.D., Kriauciunas A., McKnight K.L., Montrose C., Weidner J.R. Receptor Binding Assays for HTS and Drug Discovery. In: Sittampalam G.S., Coussens N.P., Nelson H., Arkin M., Auld D., Austin C., Bejcek B., Glicksman M., Inglese J., Iversen P.W., et al., editors. Assay Guidance Manual. Eli Lilly & Company and the National Center for Advancing Translational Sciences; Bethesda, MD, USA: 2004. [PubMed] [Google Scholar]
- 120.Koyyalagunta D. Pain Management. Elsevier; Amsterdam, The Netherlands: 2007. Opioid Analgesics; pp. 939–964. [Google Scholar]
- 121.Vardanyan R.S., Hruby V.J. Synthesis of Essential Drugs. Elsevier; Amsterdam, The Netherlands: 2006. Analgesics; pp. 19–55. [Google Scholar]
- 122.Sharma A. Dehydroacetic Acid and Its Derivatives. Elsevier; Amsterdam, The Netherlands: 2017. Various biological activities of DHA derivatives; pp. 81–118. [Google Scholar]
- 123.Sullivan K.R., Cammarano W.B., Wiener-Kronish J.P. Cardiac Intensive Care. Elsevier; Amsterdam, The Netherlands: 2010. Analgesics, tranquilizers, and sedatives; pp. 504–515. [Google Scholar]
- 124.Eguchi K., Makimura M., Murakoshi Y. Advances in Endogenous and Exogenous Opioids. Elsevier; Amsterdam, The Netherlands: 1981. Properties of opiate receptor binding in an opiate tolerant state; pp. 428–430. [Google Scholar]
- 125.Yunes R.A., Filho V.C., Ferreira J., Calixto J.B. Bioactive Natural Products (Part K) Volume 30. Studies in natural products chemistry; Elsevier; Amsterdam, The Netherlands: 2005. The use of natural products as sources of new analgesic drugs; pp. 191–212. [Google Scholar]
- 126.Pert C.B., Snyder S.H. Properties of opiate-receptor binding in rat brain. Proc. Natl. Acad. Sci. USA. 1973;70:2243–2247. doi: 10.1073/pnas.70.8.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vogel H.G., Vogel W.H., Schölkens B.A., Sandow J., Müller G., Vogel W.F. Analgesic, anti-inflammatory, and anti-pyretic activity1. In: Vogel H.G., Vogel W.H., Schölkens B.A., Sandow J., Müller G., Vogel W.F., editors. Drug Discovery and Evaluation. Springer; Berlin/Heidelberg, Germany: 2002. pp. 670–773. [Google Scholar]
- 128.Wang T.-X., Wu G.-J., Jiang J.-G. Natural Products with Analgesic Effect from Herbs and Nutraceuticals Used in Traditional Chinese Medicines. Curr. Mol. Med. 2020;20:461–483. doi: 10.2174/1566524019666191205111937. [DOI] [PubMed] [Google Scholar]
- 129.Sajid-Ur-Rehman M., Ishtiaq S., Khan M.A., Alshamrani M., Younus M., Shaheen G., Abdullah M., Sarwar G., Khan M.S., Javed F. Phytochemical profiling, in vitro and in vivo anti-inflammatory, analgesic and antipyretic potential of Sesuvium sesuvioides (Fenzl) Verdc. (Aizoaceae) Inflammopharmacology. 2021;29:789–800. doi: 10.1007/s10787-021-00824-9. [DOI] [PubMed] [Google Scholar]
- 130.Alvarez-Perez B., Poras H., Maldonado R. The inhibition of enkephalin catabolism by dual enkephalinase inhibitor: A novel possible therapeutic approach for opioid use disorders. Br. J. Pharmacol. 2021;1:1–15. doi: 10.1111/bph.15656. [DOI] [PubMed] [Google Scholar]
- 131.Vogel H.G., editor. Drug Discovery and Evaluation: Pharmacological Assays. Springer; Berlin/Heidelberg, Germany: 2008. [Google Scholar]
- 132.Harrison C., Traynor J.R. The [35S]GTPγS binding assay: Approaches and applications in pharmacology. Life Sci. 2003;74:489–508. doi: 10.1016/j.lfs.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 133.Finn D. xPharm: The Comprehensive Pharmacology Reference. Elsevier; Amsterdam, The Netherlands: 2007. TRPV1 (VR-1) Vanilloid Receptor; pp. 1–8. [Google Scholar]
- 134.Messeguer A., Planells-Cases R., Ferrer-Montiel A. Physiology and pharmacology of the vanilloid receptor. Curr. Neuropharmacol. 2006;4:1–15. doi: 10.2174/157015906775202995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Thanawala V., Kadam V.J., Ghosh R. Enkephalinase inhibitors: Potential agents for the management of pain. Curr. Drug Targets. 2008;9:887–894. doi: 10.2174/138945008785909356. [DOI] [PubMed] [Google Scholar]
- 136.Benyamin R., Trescot A.M., Datta S., Buenaventura R., Adlaka R., Sehgal N., Glaser S.E., Vallejo R. Opioid complications and side effects. Pain Physician. 2008;11:S105–S120. doi: 10.36076/ppj.2008/11/S105. [DOI] [PubMed] [Google Scholar]
- 137.González-Rodríguez S., Poras H., Menéndez L., Lastra A., Ouimet T., Fournié-Zaluski M.-C., Roques B.P., Baamonde A. Synergistic combinations of the dual enkephalinase inhibitor PL265 given orally with various analgesic compounds acting on different targets, in a murine model of cancer-induced bone pain. Scand. J. Pain. 2017;14:25–38. doi: 10.1016/j.sjpain.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 138.Wang K.-H., Li S.-F., Zhao Y., Li H.-X., Zhang L.-W. In vitro anticoagulant activity and active components of safflower injection. Molecules. 2018;23:170. doi: 10.3390/molecules23010170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.de Sousa D.P. Analgesic-like activity of essential oils constituents. Molecules. 2011;16:2233–2252. doi: 10.3390/molecules16032233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vetter I., Deuis J.R., Mueller A., Israel M.R., Starobova H., Zhang A., Rash L.D., Mobli M. NaV1.7 as a pain target—From gene to pharmacology. Pharmacol. Ther. 2017;172:73–100. doi: 10.1016/j.pharmthera.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 141.Mueller A., Starobova H., Morgan M., Dekan Z., Cheneval O., Schroeder C.I., Alewood P.F., Deuis J.R., Vetter I. Antiallodynic effects of the selective NaV1.7 inhibitor Pn3a in a mouse model of acute postsurgical pain: Evidence for analgesic synergy with opioids and baclofen. Pain. 2019;160:1766–1780. doi: 10.1097/j.pain.0000000000001567. [DOI] [PubMed] [Google Scholar]
- 142.Jin N.Z., Gopinath S.C.B. Potential blood clotting factors and anticoagulants. Biomed. Pharmacother. 2016;84:356–365. doi: 10.1016/j.biopha.2016.09.057. [DOI] [PubMed] [Google Scholar]
- 143.Brinkman H.J.M. Global assays and the management of oral anticoagulation. Thromb. J. 2015;13:9. doi: 10.1186/s12959-015-0037-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Leite P.M., Miranda A.P.N., Amorim J.M., Duarte R.C.F., Bertolucci S.K.V., Carvalho M., das Gracas Carvalho M., Castilho R.O. In Vitro Anticoagulant Activity of Mikania laevigata: Deepening the Study of the Possible Interaction between Guaco and Anticoagulants. J. Cardiovasc. Pharmacol. 2019;74:574–583. doi: 10.1097/FJC.0000000000000745. [DOI] [PubMed] [Google Scholar]
- 145.Syed A.A., Mehta A. Target specific anticoagulant peptides: A review. Int. J. Pept. Res. Ther. 2018;24:1–12. doi: 10.1007/s10989-018-9682-0. [DOI] [Google Scholar]
- 146.Hirsh J., Anand S.S., Halperin J.L., Fuster V. Mechanism of action and pharmacology of unfractionated heparin. Arterioscler. Thromb. Vasc. Biol. 2001;21:1094–1096. doi: 10.1161/hq0701.093686. [DOI] [PubMed] [Google Scholar]
- 147.Liu H., Zhang Z., Linhardt R.J. Lessons learned from the contamination of heparin. Nat. Prod. Rep. 2009;26:313–321. doi: 10.1039/b819896a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Onishi A., St Ange K., Dordick J.S., Linhardt R.J. Heparin and anticoagulation. Front. Biosci. 2016;21:1372–1392. doi: 10.2741/4462. [DOI] [PubMed] [Google Scholar]
- 149.Gogoi D., Ramani S., Bhartari S., Chattopadhyay P., Mukherjee A.K. Characterization of active anticoagulant fraction and a fibrin(ogen)olytic serine protease from leaves of Clerodendrum colebrookianum, a traditional ethno-medicinal plant used to reduce hypertension. J. Ethnopharmacol. 2019;243:112099. doi: 10.1016/j.jep.2019.112099. [DOI] [PubMed] [Google Scholar]
- 150.Cai W., Xu H., Xie L., Sun J., Sun T., Wu X., Fu Q. Purification, characterization and in vitro anticoagulant activity of polysaccharides from Gentiana scabra Bunge roots. Carbohydr. Polym. 2016;140:308–313. doi: 10.1016/j.carbpol.2015.12.054. [DOI] [PubMed] [Google Scholar]
- 151.Ghlissi Z., Krichen F., Kallel R., Amor I.B., Boudawara T., Gargouri J., Zeghal K., Hakim A., Bougatef A., Sahnoun Z. Sulfated polysaccharide isolated from Globularia alypum L.: Structural characterization, in vivo and in vitro anticoagulant activity, and toxicological profile. Int. J. Biol. Macromol. 2019;123:335–342. doi: 10.1016/j.ijbiomac.2018.11.044. [DOI] [PubMed] [Google Scholar]
- 152.Ryu R., Jung U.J., Kim H.-J., Lee W., Bae J.-S., Park Y.B., Choi M.-S. Anticoagulant and Antiplatelet Activities of Artemisia princeps Pampanini and Its Bioactive Components. Prev. Nutr. Food Sci. 2013;18:181–187. doi: 10.3746/pnf.2013.18.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Skalski B., Pawelec S., Jedrejek D., Rolnik A., Pietukhov R., Piwowarczyk R., Stochmal A., Olas B. Antioxidant and anticoagulant effects of phenylpropanoid glycosides isolated from broomrapes (Orobanche caryophyllacea, Phelipanche arenaria, and P. ramosa) Biomed. Pharmacother. 2021;139:111618. doi: 10.1016/j.biopha.2021.111618. [DOI] [PubMed] [Google Scholar]
- 154.Brinks H.L., Eckhart A.D. Regulation of GPCR signaling in hypertension. Biochim. Biophys. Acta. 2010;1802:1268–1275. doi: 10.1016/j.bbadis.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: A pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. 2021;398:957–980. doi: 10.1016/S0140-6736(21)01330-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Herman L.L., Padala S.A., Ahmed I., Bashir K. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2022. Angiotensin converting enzyme inhibitors (ACEI) [PubMed] [Google Scholar]
- 157.Masuyer G., Douglas R.G., Sturrock E.D., Acharya K.R. Structural basis of Ac-SDKP hydrolysis by Angiotensin-I converting enzyme. Sci. Rep. 2015;5:13742. doi: 10.1038/srep13742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Murray B.A., Walsh D.J., FitzGerald R.J. Modification of the furanacryloyl-L-phenylalanylglycylglycine assay for determination of angiotensin-I-converting enzyme inhibitory activity. J. Biochem. Biophys. Methods. 2004;59:127–137. doi: 10.1016/j.jbbm.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 159.Lam L.H., Shimamura T., Sakaguchi K., Noguchi K., Ishiyama M., Fujimura Y., Ukeda H. Assay of angiotensin I-converting enzyme-inhibiting activity based on the detection of 3-hydroxybutyric acid. Anal. Biochem. 2007;364:104–111. doi: 10.1016/j.ab.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 160.Li J., Liu Z., Zhao Y., Zhu X., Yu R., Dong S., Wu H. Novel Natural Angiotensin Converting Enzyme (ACE)-Inhibitory Peptides Derived from Sea Cucumber-Modified Hydrolysates by Adding Exogenous Proline and a Study of Their Structure−Activity Relationship. Mar. Drugs. 2018;16:271. doi: 10.3390/md16080271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Elbl G., Wagner H. A new method for the in vitro screening of inhibitors of angiotensin-converting enzyme (ACE), using the chromophore- and fluorophore-labelled substrate, dansyltriglycine. Planta Med. 1991;57:137–141. doi: 10.1055/s-2006-960050. [DOI] [PubMed] [Google Scholar]
- 162.Baudin B., Bénéteau-Burnat B., Baumann F.C., Giboudeau J. A reliable radiometric assay for the determination of angiotensin I-converting enzyme activity in urine. J. Clin. Chem. Clin. Biochem. 1990;28:857–861. doi: 10.1515/cclm.1990.28.11.857. [DOI] [PubMed] [Google Scholar]
- 163.Jimsheena V.K., Gowda L.R. Colorimetric, high-throughput assay for screening Angiotensin I-converting enzyme inhibitors. Anal. Chem. 2009;81:9388–9394. doi: 10.1021/ac901775h. [DOI] [PubMed] [Google Scholar]
- 164.Schwager S.L., Carmona A.K., Sturrock E.D. A high-throughput fluorimetric assay for angiotensin I-converting enzyme. Nat. Protoc. 2006;1:1961–1964. doi: 10.1038/nprot.2006.305. [DOI] [PubMed] [Google Scholar]
- 165.Francenia Santos-Sánchez N., Salas-Coronado R., Villanueva-Cañongo C., Hernández-Carlos B. Antioxidant compounds and their antioxidant mechanism. In: Shalaby E., editor. Antioxidants. IntechOpen; London, UK: 2019. [Google Scholar]
- 166.Munteanu I.G., Apetrei C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 2021;22:3380. doi: 10.3390/ijms22073380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Florin Danet A. Recent advances in antioxidant capacity assays. In: Waisundara V., editor. Antioxidants—Benefits, Sources, Mechanisms of Action. IntechOpen; London, UK: 2021. [Google Scholar]
- 168.Niu L., Han D. Chemical Analysis of Antioxidant Capacity: Mechanisms and Techniques. De Gruyter; Berlin, Germany: 2020. [Google Scholar]
- 169.Ivanova A., Gerasimova E., Gazizullina E. Study of Antioxidant Properties of Agents from the Perspective of Their Action Mechanisms. Molecules. 2020;25:4251. doi: 10.3390/molecules25184251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Basyal D., Neupane A., Pandey D.P., Pandeya S. Phytochemical Screening and In Vitro Antioxidant and Anti-inflammatory Activities of Aerial Parts of Euphorbia hirta L. J. Nepal Chem. Soc. 2021;42:115–124. doi: 10.3126/jncs.v42i1.35362. [DOI] [Google Scholar]
- 171.Lesjak M., Beara I., Simin N., Pintać D., Majkić T., Bekvalac K., Orčić D., Mimica-Dukić N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods. 2018;40:68–75. doi: 10.1016/j.jff.2017.10.047. [DOI] [Google Scholar]
- 172.Ma Y., Feng Y., Diao T., Zeng W., Zuo Y. Experimental and theoretical study on antioxidant activity of the four anthocyanins. J. Mol. Struct. 2020;1204:127509. doi: 10.1016/j.molstruc.2019.127509. [DOI] [Google Scholar]
- 173.Su Y., Li L. Structural characterization and antioxidant activity of polysaccharide from four auriculariales. Carbohydr. Polym. 2020;229:115407. doi: 10.1016/j.carbpol.2019.115407. [DOI] [PubMed] [Google Scholar]
- 174.Pohl F., Kong Thoo Lin P. The Potential Use of Plant Natural Products and Plant Extracts with Antioxidant Properties for the Prevention/Treatment of Neurodegenerative Diseases: In Vitro, In Vivo and Clinical Trials. Molecules. 2018;23:3283. doi: 10.3390/molecules23123283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hirayama O., Takagi M., Hukumoto K., Katoh S. Evaluation of antioxidant activity by chemiluminescence. Anal. Biochem. 1997;247:237–241. doi: 10.1006/abio.1997.2053. [DOI] [PubMed] [Google Scholar]
- 176.Arslan Burnaz N., Küçük M., Akar Z. An on-line HPLC system for detection of antioxidant compounds in some plant extracts by comparing three different methods. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017;1052:66–72. doi: 10.1016/j.jchromb.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 177.Yan R., Cao Y., Yang B. HPLC-DPPH screening method for evaluation of antioxidant compounds extracted from Semen Oroxyli. Molecules. 2014;19:4409–4417. doi: 10.3390/molecules19044409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Amatatongchai M., Laosing S., Chailapakul O., Nacapricha D. Simple flow injection for screening of total antioxidant capacity by amperometric detection of DPPH radical on carbon nanotube modified-glassy carbon electrode. Talanta. 2012;97:267–272. doi: 10.1016/j.talanta.2012.04.029. [DOI] [PubMed] [Google Scholar]
- 179.Shahidi F., Alasalvar C., Liyana-Pathirana C.M. Antioxidant phytochemicals in hazelnut kernel (Corylus avellana L.) and hazelnut byproducts. J. Agric. Food Chem. 2007;55:1212–1220. doi: 10.1021/jf062472o. [DOI] [PubMed] [Google Scholar]
- 180.Becker E.M., Nissen L.R., Skibsted L.H. Antioxidant evaluation protocols: Food quality or health effects. Eur. Food Res. Technol. 2004;219:561–571. doi: 10.1007/s00217-004-1012-4. [DOI] [Google Scholar]
- 181.Munteanu I.G., Apetrei C. A review on electrochemical sensors and biosensors used in assessing antioxidant activity. Antioxidants. 2022;11:584. doi: 10.3390/antiox11030584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Dong J.-W., Cai L., Xing Y., Yu J., Ding Z.-T. Re-evaluation of ABTS*+ Assay for Total Antioxidant Capacity of Natural Products. Nat. Prod. Commun. 2015;10:2169–2172. doi: 10.1177/1934578X1501001239. [DOI] [PubMed] [Google Scholar]
- 183.Prior R.L., Wu X., Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005;53:4290–4302. doi: 10.1021/jf0502698. [DOI] [PubMed] [Google Scholar]
- 184.Huang D., Ou B., Prior R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005;53:1841–1856. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- 185.Srivastava S., Adholeya A., Conlan X.A., Cahill D.M. Acidic Potassium Permanganate Chemiluminescence for the Determination of Antioxidant Potential in Three Cultivars of Ocimum basilicum. Plant Foods Hum. Nutr. 2016;71:72–80. doi: 10.1007/s11130-016-0527-8. [DOI] [PubMed] [Google Scholar]
- 186.Shahidi F., Zhong Y. Measurement of antioxidant activity. J. Funct. Foods. 2015;18:757–781. doi: 10.1016/j.jff.2015.01.047. [DOI] [Google Scholar]
- 187.Ashida S., Okazaki S., Tsuzuki W., Suzuki T. Chemiluminescent method for the evaluation of antioxidant activity using lipid hydroperoxide-luminol. Anal. Sci. 1991;7:93–96. doi: 10.2116/analsci.7.93. [DOI] [Google Scholar]
- 188.Bunaciu A.A., Danet A.F., Fleschin Ş., Aboul-Enein H.Y. Recent applications for in vitro antioxidant activity assay. Crit. Rev. Anal. Chem. 2016;46:389–399. doi: 10.1080/10408347.2015.1101369. [DOI] [PubMed] [Google Scholar]
- 189.Antolovich M., Prenzler P.D., Patsalides E., McDonald S., Robards K. Methods for testing antioxidant activity. Analyst. 2002;127:183–198. doi: 10.1039/b009171p. [DOI] [PubMed] [Google Scholar]
- 190.Pascualreguera M., Ortegacarmona I., Molinadiaz A. Spectrophotometric determination of iron with ferrozine by flow-injection analysis. Talanta. 1997;44:1793–1801. doi: 10.1016/S0039-9140(97)00050-7. [DOI] [PubMed] [Google Scholar]
- 191.Baba S.A., Malik S.A. Determination of total phenolic and flavonoid content, antimicrobial and antioxidant activity of a root extract of Arisaema jacquemontii Blume. J. Taibah Univ. Sci. 2015;9:449–454. doi: 10.1016/j.jtusci.2014.11.001. [DOI] [Google Scholar]
- 192.Apak R., Güçlü K., Ozyürek M., Karademir S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004;52:7970–7981. doi: 10.1021/jf048741x. [DOI] [PubMed] [Google Scholar]
- 193.Ribeiro J.P.N., Magalhães L.M., Reis S., Lima J.L.F.C., Segundo M.A. High-throughput total cupric ion reducing antioxidant capacity of biological samples determined using flow injection analysis and microplate-based methods. Anal. Sci. 2011;27:483. doi: 10.2116/analsci.27.483. [DOI] [PubMed] [Google Scholar]
- 194.Özyürek M., Güçlü K., Tütem E., Başkan K.S., Erçağ E., Esin Çelik S., Baki S., Yıldız L., Karaman Ş., Apak R. A comprehensive review of CUPRAC methodology. Anal. Methods. 2011;3:2439. doi: 10.1039/c1ay05320e. [DOI] [Google Scholar]
- 195.Mamedov N. Medicinal plants studies: History, challenges and prospective. Med. Aromat. Plants. 2012;1:1000e133. doi: 10.4172/2167-0412.1000e133. [DOI] [Google Scholar]
- 196.Arastehfar A., Gabaldón T., Garcia-Rubio R., Jenks J.D., Hoenigl M., Salzer H.J.F., Ilkit M., Lass-Flörl C., Perlin D.S. Drug-Resistant Fungi: An Emerging Challenge Threatening Our Limited Antifungal Armamentarium. Antibiotics. 2020;9:877. doi: 10.3390/antibiotics9120877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST) EUCAST definitive document EDef 7.1: Method for the determination of broth dilution MICs of antifungal agents for fermentative yeasts. Clin. Microbiol. Infect. 2008;14:398–405. doi: 10.1111/j.1469-0691.2007.01935.x. [DOI] [PubMed] [Google Scholar]
- 198.Caron F. Antimicrobial susceptibility testing: A four facets tool for the clinician. J. Des Anti-Infect. 2012;14:168–174. doi: 10.1016/j.antinf.2012.10.002. [DOI] [Google Scholar]
- 199.Vaou N., Stavropoulou E., Voidarou C., Tsigalou C., Bezirtzoglou E. Towards advances in medicinal plant antimicrobial activity: A review study on challenges and future perspectives. Microorganisms. 2021;9:41. doi: 10.3390/microorganisms9102041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Aldholmi M., Marchand P., Ourliac-Garnier I., Le Pape P., Ganesan A. A Decade of Antifungal Leads from Natural Products: 2010–2019. Pharmaceuticals. 2019;12:182. doi: 10.3390/ph12040182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ibrahim D., Lee C.C., Sheh-Hong L. Antimicrobial Activity of Endophytic Fungi Isolated from Swietenia macrophylla Leaves. Nat. Prod. Commun. 2014;9:1934578X1400900. doi: 10.1177/1934578X1400900229. [DOI] [PubMed] [Google Scholar]
- 202.Ruban P., Gajalakshmi K. In vitro antibacterial activity of Hibiscus rosa-sinensis flower extract against human pathogens. Asian Pac. J. Trop. Biomed. 2012;2:399–403. doi: 10.1016/S2221-1691(12)60064-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Antimicrobial Resistance Collaborators Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet. 2022;399:629–655. doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Humphries R.M., Ambler J., Mitchell S.L., Castanheira M., Dingle T., Hindler J.A., Koeth L., Sei K. CLSI Methods Development and Standardization Working Group of the Subcommittee on Antimicrobial Susceptibility Testing CLSI methods development and standardization working group best practices for evaluation of antimicrobial susceptibility tests. J. Clin. Microbiol. 2018;56:e01934-17. doi: 10.1128/JCM.01934-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Jorgensen J.H., Ferraro M.J. Antimicrobial susceptibility testing: A review of general principles and contemporary practices. Clin. Infect. Dis. 2009;49:1749–1755. doi: 10.1086/647952. [DOI] [PubMed] [Google Scholar]
- 206.Dannaoui E., Espinel-Ingroff A. Antifungal susceptibly testing by concentration gradient strip etest method for fungal isolates: A review. J. Fungi. 2019;5:108. doi: 10.3390/jof5040108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Songtham S. A Comparison of Two Methods Used for Measuring the Antagonistic Activity of Bacillus Species. Walailak J. Sci. Technol. (WJST) 2011;5:161–171. [Google Scholar]
- 208.Lorelli J.P., Held A.A. Screening of Blastocladialean Fungi for Antibiotic Production by a Modified “Cross-Streak” Test. Mycologia. 1983;75:909. doi: 10.1080/00275514.1983.12023769. [DOI] [Google Scholar]
- 209.Homans A.L., Fuchs A. Direct bioautography on thin-layer chromatograms as a method for detecting fungitoxic substances. J. Chromatogr. A. 1970;51:327–329. doi: 10.1016/S0021-9673(01)96877-3. [DOI] [PubMed] [Google Scholar]
- 210.Suleimana M.M., McGaw L.J., Naidoo V., Eloff J.N. Detection of antimicrobial compounds by bioautography of different extracts of leaves of selected South African tree species. Afr. J. Tradit. Complement. Altern. Med. 2009;7:64–78. doi: 10.4314/ajtcam.v7i1.57269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Dewanjee S., Gangopadhyay M., Bhattacharya N., Khanra R., Dua T.K. Bioautography and its scope in the field of natural product chemistry. J. Pharm. Anal. 2015;5:75–84. doi: 10.1016/j.jpha.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Marston A. Thin-layer chromatography with biological detection in phytochemistry. J. Chromatogr. A. 2011;1218:2676–2683. doi: 10.1016/j.chroma.2010.12.068. [DOI] [PubMed] [Google Scholar]
- 213.Pereira E., Santos A., Reis F., Tavares R.M., Baptista P., Lino-Neto T., Almeida-Aguiar C. A new effective assay to detect antimicrobial activity of filamentous fungi. Microbiol. Res. 2013;168:1–5. doi: 10.1016/j.micres.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 214.Wang M., Zhang Y., Wang R., Wang Z., Yang B., Kuang H. An Evolving Technology That Integrates Classical Methods with Continuous Technological Developments: Thin-Layer Chromatography Bioautography. Molecules. 2021;26:4647. doi: 10.3390/molecules26154647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ericsson H.M., Sherris J.C. Antibiotic sensitivity testing. Report of an international collaborative study. Acta Pathol. Microbiol. Scand. B Microbiol. Immunol. 1971;217:90. [PubMed] [Google Scholar]
- 216.Alexander B.D. Reference Method For Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2017. p. 50. [Google Scholar]
- 217.Wiegand I., Hilpert K., Hancock R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008;3:163–175. doi: 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]
- 218.Marroki A., Bousmaha-Marroki L. Reference Module in Biomedical Sciences. Elsevier; Amsterdam, The Netherlands: 2021. Antibiotic resistance diagnostic methods for pathogenic bacteria. [Google Scholar]
- 219.Therese K.L., Bagyalakshmi R., Madhavan H.N., Deepa P. In-vitro susceptibility testing by agar dilution method to determine the minimum inhibitory concentrations of amphotericin b, fluconazole and ketoconazole against ocular fungal isolates. Indian J. Med. Microbiol. 2006;24:273–279. doi: 10.1016/S0255-0857(21)02288-X. [DOI] [PubMed] [Google Scholar]
- 220.Pfaller M.A., Sheehan D.J., Rex J.H. Determination of fungicidal activities against yeasts and molds: Lessons learned from bactericidal testing and the need for standardization. Clin. Microbiol. Rev. 2004;17:268–280. doi: 10.1128/CMR.17.2.268-280.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Klepser M.E., Ernst E.J., Lewis R.E., Ernst M.E., Pfaller M.A. Influence of test conditions on antifungal time-kill curve results: Proposal for standardized methods. Antimicrob. Agents Chemother. 1998;42:1207–1212. doi: 10.1128/AAC.42.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Vojtek L., Dobes P., Buyukguzel E., Atosuo J., Hyrsl P. Bioluminescent assay for evaluating antimicrobial activity in insect haemolymph. Eur. J. Entomol. 2014;111:335–340. doi: 10.14411/eje.2014.045. [DOI] [Google Scholar]
- 223.Finger S., Wiegand C., Buschmann H.-J., Hipler U.-C. Antibacterial properties of cyclodextrin-antiseptics-complexes determined by microplate laser nephelometry and ATP bioluminescence assay. Int. J. Pharm. 2013;452:188–193. doi: 10.1016/j.ijpharm.2013.04.080. [DOI] [PubMed] [Google Scholar]
- 224.Ke H.-M., Tsai I.J. Understanding and using fungal bioluminescence—Recent progress and future perspectives. Curr. Opin. Green Sustain. Chem. 2021;33:100570. doi: 10.1016/j.cogsc.2021.100570. [DOI] [Google Scholar]
- 225.Green L., Petersen B., Steimel L., Haeber P., Current W. Rapid determination of antifungal activity by flow cytometry. J. Clin. Microbiol. 1994;32:1088–1091. doi: 10.1128/jcm.32.4.1088-1091.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ramani R., Chaturvedi V. Flow cytometry antifungal susceptibility testing of pathogenic yeasts other than Candida albicans and comparison with the NCCLS broth microdilution test. Antimicrob. Agents Chemother. 2000;44:2752–2758. doi: 10.1128/AAC.44.10.2752-2758.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Mani-López E., Cortés-Zavaleta O., López-Malo A. A review of the methods used to determine the target site or the mechanism of action of essential oils and their components against fungi. SN Appl. Sci. 2021;3:44. doi: 10.1007/s42452-020-04102-1. [DOI] [Google Scholar]
- 228.Djordjevic D., Wiedmann M., McLandsborough L.A. Microtiter plate assay for assessment of Listeria monocytogenes biofilm formation. Appl. Environ. Microbiol. 2002;68:2950–2958. doi: 10.1128/AEM.68.6.2950-2958.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Famuyide I.M., Aro A.O., Fasina F.O., Eloff J.N., McGaw L.J. Antibacterial and antibiofilm activity of acetone leaf extracts of nine under-investigated south African Eugenia and Syzygium (Myrtaceae) species and their selectivity indices. BMC Complement. Altern. Med. 2019;19:141. doi: 10.1186/s12906-019-2547-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Singh V.K., Mishra A., Jha B. Anti-quorum Sensing and Anti-biofilm Activity of Delftia tsuruhatensis Extract by Attenuating the Quorum Sensing-Controlled Virulence Factor Production in Pseudomonas aeruginosa. Front. Cell. Infect. Microbiol. 2017;7:337. doi: 10.3389/fcimb.2017.00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Choo J.H., Rukayadi Y., Hwang J.K. Inhibition of bacterial quorum sensing by vanilla extract. Lett. Appl. Microbiol. 2006;42:637–641. doi: 10.1111/j.1472-765X.2006.01928.x. [DOI] [PubMed] [Google Scholar]
- 232.Hayashi M.A., Bizerra F.C., Da Silva P.I. Antimicrobial compounds from natural sources. Front. Microbiol. 2013;4:195. doi: 10.3389/fmicb.2013.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Masota N.E., Vogg G., Ohlsen K., Holzgrabe U. Reproducibility challenges in the search for antibacterial compounds from nature. PLoS ONE. 2021;16:e0255437. doi: 10.1371/journal.pone.0255437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Atanasov A.G., Zotchev S.B., Dirsch V.M., Supuran C.T., International Natural Product Sciences Taskforce Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021;20:200–216. doi: 10.1038/s41573-020-00114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Wilson B.A.P., Thornburg C.C., Henrich C.J., Grkovic T., O’Keefe B.R. Creating and screening natural product libraries. Nat. Prod. Rep. 2020;37:893–918. doi: 10.1039/C9NP00068B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Santana K., do Nascimento L.D., Lima E Lima A., Damasceno V., Nahum C., Braga R.C., Lameira J. Applications of Virtual Screening in Bioprospecting: Facts, Shifts, and Perspectives to Explore the Chemo-Structural Diversity of Natural Products. Front. Chem. 2021;9:662688. doi: 10.3389/fchem.2021.662688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Forry S.P., Madonna M.C., López-Pérez D., Lin N.J., Pasco M.D. Automation of antimicrobial activity screening. AMB Express. 2016;6:20. doi: 10.1186/s13568-016-0191-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Ntie-Kang F., Yong J.N. The chemistry and biological activities of natural products from Northern African plant families: From Aloaceae to Cupressaceae. RSC Adv. 2014;4:61975–61991. doi: 10.1039/C4RA11467A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Zhang Y., Cai P., Cheng G., Zhang Y. A Brief Review of Phenolic Compounds Identified from Plants: Their Extraction, Analysis, and Biological Activity. Nat. Prod. Commun. 2022;17:1934578X2110697. doi: 10.1177/1934578X211069721. [DOI] [Google Scholar]
- 240.Schneider G. Automating drug discovery. Nat. Rev. Drug Discov. 2018;17:97–113. doi: 10.1038/nrd.2017.232. [DOI] [PubMed] [Google Scholar]
- 241.Nyamundanda G., Brennan L., Gormley I.C. Probabilistic principal component analysis for metabolomic data. BMC Bioinform. 2010;11:571. doi: 10.1186/1471-2105-11-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Ren S., Hinzman A.A., Kang E.L., Szczesniak R.D., Lu L.J. Computational and statistical analysis of metabolomics data. Metabolomics. 2015;11:1492–1513. doi: 10.1007/s11306-015-0823-6. [DOI] [Google Scholar]
- 243.Bartel J., Krumsiek J., Theis F.J. Statistical methods for the analysis of high-throughput metabolomics data. Comput. Struct. Biotechnol. J. 2013;4:e201301009. doi: 10.5936/csbj.201301009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Cakır T., Hendriks M.M.W.B., Westerhuis J.A., Smilde A.K. Metabolic network discovery through reverse engineering of metabolome data. Metabolomics. 2009;5:318–329. doi: 10.1007/s11306-009-0156-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All tables were created by the authors. All sources of information were adequately referenced. There was no need to obtain copyright permission.