Abstract
Monoclonal gammopathy of renal significance (MGRS) is a recognized clinical entity. Literature regarding treatment and its outcomes in MGRS is sparse due to the rarity and misdiagnosis of MGRS. We retrospectively analyzed 280 adults with an MGRS diagnosis from 2003 to 2020 across 19 clinical centers from 12 countries. All cases required renal biopsy for the pathological diagnosis of MGRS. Amyloidosis‐related to MGRS (MGRS‐A) was present in 180 patients; nonamyloidosis MGRS (MGRS‐NA), including a broad spectrum of renal pathologies, was diagnosed in 100 patients. The median overall survival in the studied cohort was 121.0 months (95% CI: 105.0–121.0). Patients with MGRS‐A had a shorter overall survival than patients with MGRS‐NA (HR = 0.41, 95%CI: 0.25–0.69; p = 0.0007). Both hematologic and renal responses were associated with longer survival. Achievement of ≥VGPR was generally predictive of a renal response (OR = 8.03 95%CI: 4.04–115.96; p < 0.0001), one‐fourth of patients with ≥VGPR were renal nonresponders. In MGRS‐A, factors associated with poor prognosis included elevated levels of creatinine, beta‐2‐microglobulin, and hemodialysis at diagnosis. In MGRS‐NA, only age >65 years was associated with increased risk of death. Treatments provided similar hematologic response rates in both types of MGRS. Autologous stem cell transplantation led to better response than other treatments. This multicenter and international effort is currently the largest report on MGRS.
1. INTRODUCTION
Monoclonal gammopathy of undetermined significance (MGUS) is defined by a serum or urine monoclonal protein of less than 3 g/dL and 500 mg/24 h, respectively, and by less than 10% monoclonal plasma cells in the bone marrow. 1 Currently, no treatment is indicated for MGUS outside of a clinical trial. The cumulative risk of progression to multiple myeloma (MM) has been widely reported and is about 1% per year in 20 years. 2 Smoldering multiple myeloma (SMM) is characterized by 10%–60% monoclonal plasma cells in the bone marrow and/or ≥3 g/dL of monoclonal component in the serum or urine of ≥500 mg/24 h. In SMM, there is no organ damage, and treatment is indicated only in the context of a clinical trial. 3 , 4 Three new criteria have been introduced to predict increased risk of progression from SMM to symptomatic MM within two years from diagnosis: bone marrow plasma cells ≥60%, free light chain (FLC) ratio > 100 (or if κ/λ ratio is used, ≥100 or ≤0.01), and ≥1 bone lesion detected by magnetic resonance imaging (MRI)—now defined as SLiM‐CRAB. 3 However, both MGUS and SMM may be harmful in the absence of the usual SLiM‐CRAB criteria. A newer term, monoclonal gammopathy of clinical significance (MGCS) indicates organ compromise secondary to the production of a clonal paraprotein(s), which damages the kidney, eye, heart, liver, or neurological compartment. 5 , 6 , 7 , 8 , 9 In addition to MGUS and SMM, this may also be a manifestation of small B‐cell clones arising from lymphoproliferative diseases, that is, chronic lymphocytic leukemia (CLL) or non‐Hodgkin lymphoma (NHL).
In MGRS, a kidney damage is not due to light cast nephropathy. 10 , 11 , 12 , 13 , 14 The mechanisms for organ damage are related to the physicochemical properties of the monoclonal immunoglobulin. A recent consensus statement for the diagnosis and treatment of MGRS was published by the International Kidney and Monoclonal Research Group (IKMG). 12 MGRS is commonly underdiagnosed due to the rarity and lack of familiarity. The clonal deposition of monoclonal immunoglobulin does not respond to immunosuppression as in other nephropathies, tends to progress or relapse after kidney transplant, leading to end‐stage renal disease. 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 For all these reasons, the literature lacks the true incidence and optimal treatment of MGRS. The objective of this multi‐institutional study was to evaluate prognostic indicators and treatment outcomes in MGRS. Considering the high incidence of AL amyloidosis within MGRS, we compared outcomes of patients with amyloidosis and nonamyloidosis MGRS.
2. PATIENTS AND METHODS
This was a retrospective, international, multicenter study to survey the landscape of MGRS treatment and its outcomes in collaborating centers in Brazil, Chile, Czech Republic, Germany, France, Italy, Israel, Poland, Romania, Russia, Spain, and the United States. The individual institutional review boards approved the study. Personal data were deidentified to ensure compliance with relevant data privacy regulations. Data collection was based on standardized study forms and aggregation of information by the study coordinator. The diagnostic criteria was consistent with the consensus statement from the IKMG. 12 All patients included in the analysis required local hematopathological confirmation by renal biopsy for one of the following pathological diagnoses: AL amyloidosis, monoclonal immunoglobulin deposition disease (MIDD), proliferative glomerulonephritis (GN) with monoclonal immunoglobulin deposition disease (PGNMID), monoclonal fibrillary GN, immunotactoid GN, cryoglobulinemic GN, light chain proximal tubulopathy (LCPT), crystal‐storing histiocytosis, C3 glomerulopathy with monoclonal gammopathy, and thrombotic microangiopathy. 22 , 23 , 24 , 25 , 26 , 27 Light‐chain cast nephropathy was excluded since it was considered a myeloma‐defining event. 6 The analyzed parameters included: age at diagnosis, gender, heavy and light chain isotype, serum‐free light‐chain κ and λ, the percentage of clonal plasma cells in bone marrow, the presence of B‐cell lymphoproliferative disease, fluorescence in situ hybridization (FISH) for cytogenetic abnormalities [t(14;16), t(4;14), TP53 and/or del 17p] or as per local guidelines 28 hemoglobin level, serum concentrations of calcium, albumin, beta‐2‐microglobulin, LDH, creatinine, estimated glomerular filtration rate (eGFR), hemodialysis required at diagnosis and the duration of hemodialysis, frontline treatments, responses (hematologic and renal response), progression‐free survival (PFS), and OS. Treatment outcomes were classified by the Uniform International Myeloma Working Group (IMWG) criteria. 29 Hematologic response was defined as complete response (CR) if normalization of FLC was obtained when available. Otherwise, a disappearance of monoclonal protein at electrophoresis and with serum or urinary immunofixation or disappearance of the plasmacellular clone. Defining very good partial response (VGPR) and partial response (PR) required 90% or 50% monoclonal protein reduction, respectively.
Renal response was assessed as a reduction of >30% of 24 h proteinuria (in the absence of renal progression defined by progressive decrease of >25% of eGFR). 30
Treatments were divided into proteasome inhibitor–based (bortezomib, carfilzomib or ixazomib, i.e., PI), immunomodulatory (IMiD) drug–based (thalidomide, lenalidomide, pomalidomide, i.e., IMiD), monoclonal antibody–based (daratumumab, rituximab, i.e., MoA), corticosteroids–prednisone, dexamethasone), chemotherapy‐based (chemotherapy alone), and autologous stem cell transplantation (ASCT).
2.1. Statistical analysis
The chi‐square test and the Mann–Whitney U‐test were used to compare categorical and continuous variables, respectively. For the survival analysis, the Kaplan–Meier method was used to generate survival curves, which were then compared using the log‐rank test. The Cox proportional‐hazard regression method was used to fit univariate and multivariate survival models, the results of which are reported as hazard ratios (HRs) with 95% confidence intervals (95% CIs). Variables with >50% of missing data were not included in the survival analyses. All reported p‐values are two‐sided and were considered significant if less than 0.05. All analyses were performed with RStudio Version 1.4.1106, and figures were prepared using MedCalc version 20.014 (MedCalc Software Ltd. Ostend, Belgium). Variables included in the univariate and multivariate models were age, gender, serum concentrations of creatine, albumin, beta‐2microglobulin, LDH, FLC κ/λ, and hemodialysis dependence. The hematologic response was analyzed as CR and VGPR, PR and stable disease (SD), and progressive diseases (PD).
All analyses were carried out in groups of patients with amyloidosis‐related MGRS (MGRS‐A) related and non‐amyloidosis‐related MGRS (MGRS‐NA).
3. RESULTS
3.1. Patients
From 331 patients initially reported, 51 were excluded from analysis due to the lack of clear MGRS diagnosis, that is, unconfirmed by renal biopsy or having symptomatic MM. From January 2003 to June 2020, 280 patients were diagnosed with MGRS. Only 9% of patients (n = 26) were diagnosed before 2010. Patient characteristics are reported in Table 1. Two‐thirds of patients had MGRS‐A (64%). Over half of patients with MGRS‐NA had MIDD. More patients in the MGRS‐NA group had IgG disease and serum‐free light chain κ compared with patients in the MGRS‐A group (Table 1). Renal impairment was more severe in patients with MGRS‐NA than in the group with MGRS‐A (Table 1). MGRS‐associated clonal diagnoses were MGUS (n = 214, 76.4%), SMM (n = 55, 19.6%) and non‐Hodgkin lymphoma (n = 9, 5.0%, including 6 patients with Waldenström's macroglobulinemia, 2 with lymphoplasmacytic lymphoma and 1 with marginal zone lymphoma). FISH was available for 76/280 patients, and t(11;14) was present in 13 patients, gain 1q21 in 5 patients, del17p in 1 patient.
TABLE 1.
Patient's characteristics
| MGRS‐A | MGRS‐NA | p‐value | |
|---|---|---|---|
| Number of patients | 180 | 100 | |
| Median age (range), years | 61 (28–87) | 60 (25–87) | 0.2790 |
| Male sex, n (%) | 90 (50.0%) | 51 (49.0%) | 0.8728 |
| Monoclonal component | |||
| Heavy chains, n (%) | <0.0001 | ||
| IgA κ/ λ | 1/9 (5.8%) | 3/3 (6.2%) | |
| IgG κ/ λ | 10/41 (29.7%) | 39/16 (56.7%) | |
| IgM κ/ λ | 4/8 (7.0%) | 5/1 (6.2%) | |
| Free light chains | |||
| κ | 24 (14.0%) | 23 (23.7%) | |
| λ | 75 (43.6%%) | 7 (7.2%) | |
| Type of MGRS | |||
| MIDD | Not applicable | 53 (53%) | ‐ |
| PGNMID | 14 (14%) | ||
| LCPT | 11 (11%) | ||
| Monoclonal fibrillary GN | 4 (4%) | ||
| Immunotactoid GN | 4 (4%) | ||
| C3 glomerulopathy with monoclonal gammopathy | 7 (7%) | ||
| Other | 5 (5%) | ||
| Cryoglobulinemic GN | 2 (2%) | ||
| Laboratory parameters, median (range) | |||
| Bone marrow involvement (% PCs), median* (range) | 5.7% (0–50) | 7.5% (0–55) | 0.1386 |
| Monoclonal component (mg/dL), median* (range) | 5.0 (0–3000) | 57 (0–2460) | 0.0763 |
| FLC κ, median (range) (mg/dL), median* (range) | 21.1 (0–2152) | 71.4 (1.4–6680) | <0.0001 |
| FLC λ, (mg/dL), median* (range) | 21.8 (0–4260) | 68.6 (0–1815) | <0.0001 |
| FLC κ/λ, median* (range) | 0.4 (0–309) | 3.5 (0–1040) | < 0.0001 |
| Albumin (≥3.5 mg/dL), n (%) | 153 (93.3%) | 85 (92.4%) | 0.7871 |
| B‐2‐microglobulin (≥5.5 mg/L), n (%) | 26 (34.2%) | 34 (54.8%) | 0.0055 |
| LDH ≥300 U/L, n (%) | 46 (36.8%) | 22 (26.5%) | 0.1221 |
| Creatinine ≥177 mg/dL, n (%) | 51 (32.3%) | 58 (58.0%) | <0.0001 |
| eGFR <60 ml/min/1.73m2, n (%) | 97 (54.5%) | 72 (88.8%) | <0.0001 |
| 24 h urine protein (g) | 6.2 (0.1–14 850) | 3.4 (0–7000) | 0.0001 |
| Dialysis, n (%) | 33 (18.3%) | 26 (26.0%) | 0.0004 |
| Treatment, n (%) | |||
| Untreated | 25 (13.9%) | 12 (12.0%) | 0.0614 |
| 1 line | 106 (58.9%) | 65 (65.0%) | |
| 2 lines | 35 (19.4%) | 17 (17.0%) | |
| 3 lines | 14 (7.8%) | 6 (6.0%) | |
Abbreviations: eGFR, estimated glomerular filtration rate; FLC, free light chains; GN, glomerulonephritis; LCPT, light chain proximal tubulopathy; LDH, lactate dehydrogenase; MGRS‐A, amyloidoid‐associated monoclonal gammaglobulinemia of renal significance; MGRS‐NA, non‐amyloidosis‐associated gammaglobulinemia of renal significance; MIDD, monoclonal immunoglobulin deposition disease; PGNMID, proliferative glomerulonephritis with monoclonal immunoglobulin deposition disease monoclonal fibrillary glomerulonephritis.
Values based on the nonmissing data.
Typically, untreated patients refused treatment or were referred and lost to follow‐up (20/37, 51.3%) or died within two years from the diagnosis (9/37, 24.3%). Causes of death included multiorgan failures (n = 3), disease progression (n = 3), sepsis (n = 2), and gastrointestinal bleeding (n = 1). Response and survival analyses were performed only among patients who received treatment.
3.2. Treatment and response
The majority of patients received treatment (87%). Frontline treatments and the corresponding hematologic responses are summarized in Table S1. The overall response rate (ORR) was 56% and 72% in the MGRS‐A and MGRS‐NA groups, respectively. The most common first‐line treatment was PI‐based followed by conventional chemotherapy. Overall, 16% of patients received ASCT as part of the first‐line treatment. There were no differences in hematologic responses regardless of the induction regimens and/or ASCT in patients with different types of MGRS. There was no difference in the rate of ≥VGPR between patients with MGRS‐A (37%, 45/122) and MGRS‐NA (43%, 34/78), p = 0.3449. Around 30% of patients received the second line of treatment, and <10% also the third line. The rate of ≥VGPR in the subsequent lines of treatment was increasing (Table S2).
Both hematologic and renal responses were available in 74% of treated patients (182/243). Among patients with ≥VGPR, 73.8% (31/42) and 76.9% (20/26) achieved renal response in the MGRS‐A and MGRS‐NA groups, respectively. A renal response was less common than hematologic. Overall, in patients with ≥VGPR, 25% did not achieve a renal response. Among patients with kidney function evaluated, the renal response was confirmed in 39% of patients with MGRS‐A (50/129) and 60% of patients with MGRS‐NA (39/65), p = 0.0052. In the MGRS‐A group, among patients with both hematologic and renal response evaluated, the renal response was present in 73.8% (31/42) and 19.0% (12/63) of patients with ≥VGPR and PR or SD, respectively (p < 0.0001). In the MGRS‐NA group, the renal response was present in 76.9% (20/26) and 47.0% (16/34) of patients with ≥VGPR and PR or SD, respectively (p = 0.0202). There was no difference in the renal response between patients with ≥VGPR in both groups (p = 0.7759); however, patients with PR or SD in the MGRS‐NA group were more likely renal responders than in the MGRS‐A group (p = 0.0038).
In general, achievement of ≥VGPR increased the likelihood for a renal response (OR = 8.03 95%CI: 4.04–115.96; p < 0.0001).
3.3. Survival analysis and prognostic factors of survival
Survival analysis was performed only among patients who received treatment. After a median follow‐up of 30 months (range 1–192 months), the median OS was 121.0 months (95%CI: 105.0–121.0) (Figure 1A, B), Patients with MGRS‐NA were at a significantly lower risk of death than patients with MGRS‐A (HR = 0.41, 95% CI: 0.25–0.69; p = 0.0007). This effect was observed during the first 120 months, and the curves converged since that time, with 50% of patients in both groups remaining alive. There were more deaths in the MGRS‐A (31%, 51/154) compared to the MGRS‐NA group (14%, 12/86); p = 0.0130. The most common causes of death in the MGRS‐A group included disease progression (29%), infection (20%), and heart failure (16%); in 20% of cases, a cause of death was not recorded. In the MGRS‐NA group, disease progression (42%) and infections (25%) were the most common causes of death. In contrast to the MGRS‐A group, only one case of death in the MGRS‐NA group was related to a cardiovascular event. Thirty‐seven patients either refused treatment or were referred and lost to follow‐up (20/37, 51%) or died within two years from the diagnosis (9/37, 24%). Causes of death included multiorgan failure (n = 3), disease progression (n = 3), sepsis (n = 2), and gastrointestinal bleeding (n = 1).
FIGURE 1.
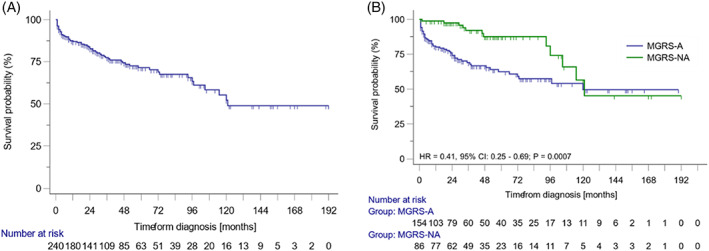
Overall survival of all patients and amyloidosis‐associated monoclonal gammopathy of renal significance (MGRS‐A) and non‐amyloidosis‐associated gammopathy of renal significance (MGRS‐NA). Overall survival of all patients with monoclonal gammopathy of renal significance (A) and overall survival of patients with MGRS‐A, and MGRS‐NA (B) [Color figure can be viewed at wileyonlinelibrary.com]
Achievement of ≥VGPR after the first‐line of treatment was associated with improved survival in the MGRS‐A group (HR = 0.17, 95% CI: 0.08–0.33; p < 0.0001) and in the MGRS‐NA group (HR = 0.27, 95% CI: 0.08–0.87; p = 0.0295) (Figure 2). The renal response was associated with better survival both in the MGRS‐A (HR = 0.23, 95%CI: 0.19–0.43; p < 0.0001) and MGRS‐NA group (HR = 0.15, 95%CI: 0.03–0.80; p = 0.0260) (Figure 3). In a univariate analysis, elevations of beta‐2‐microglobulin and creatinine, as well as hemodialysis dependence, were associated with increased risk of death in the MGRS‐A group (Table S3). None of these factors were significant in the multivariate analysis (beta‐2‐microglobulin HR = 1.66, 95% CI: 0.52–5.28, p = 0.556; creatinine HR = 1.41, 95% CI: 0.45–4.43; p = 0.556; hemodialysis HR = 1.87, 95% CI: 0.72–4.85, p = 0.201). For the MGRS‐NA, patients >65 years old had an increased risk of death compared to the younger group.
FIGURE 2.
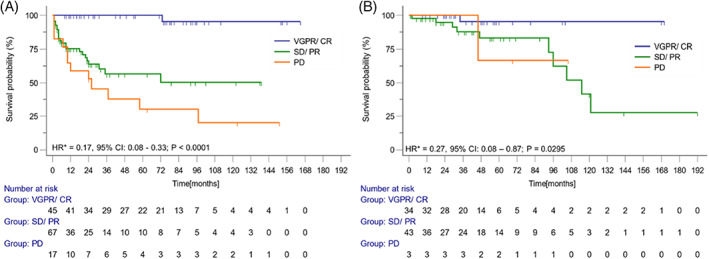
Overall survival and hematological response. Overall survival in the groups with amyloidosis‐associated monoclonal gammopathy of renal significance (A) and non‐amyloidosis‐associated gammopathy of renal significance (B) depending on hematological response.* ‐ Hazard ratio for comparison between survival of patients with ≥VGPR versus ≤PR or PD [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 3.
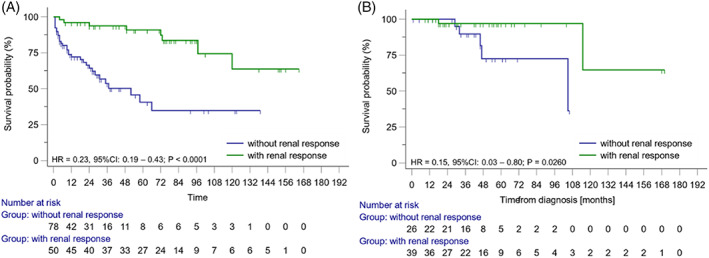
Overall survival and renal response. Overall survival in the groups with amyloidosis‐associated monoclonal gammopathy of renal significance (A) and non‐amyloidosis‐associated gammopathy of renal significance (B) depending on renal response [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
This is the largest report of biopsy‐proven MGRS. 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 The results comprise the real clinical practice of MGRS management, mainly in the past decade. Of note, many of these patients did not meet the criteria for myeloma, so only a subset received PIs or IMiDs as would be utilized in myeloma.
Patients with MGRS are heterogeneous. Different types of paraprotein‐associated renal involvement include all elements of the nephron. 12 , 31 , 32 Primary differentiation between the most common MGRS‐A and MGRS‐NA revealed that these entities differed in disease burden and survival. Renal disease was more severe in patients with MGRS‐NA than with MGRS‐A, with a higher proportion of patients with elevated creatinine, low eGFR, and more frequently requiring hemodialysis in MGRS‐NA than in MGRS‐A. Despite similar bone marrow plasma cell percentages in MGRS‐NA and MGRS‐A, M‐protein levels were higher in MGRS‐NA. In addition, κ light chain paraprotein was more frequently produced in MGRS‐NA than in MGRS‐A, whereas FLC κ/λ was lower in MGRS‐A than in MGRS‐NA. 27
Despite a lower clonal and renal burden, patients with MGRS‐A had shorter OS than patients with MGRS‐NA. Univariate analysis revealed that elevated creatinine level, requiring hemodialysis at diagnosis, and elevated beta‐2‐microglobulin were associated with a higher risk of death in the MGRS‐A group. None of these was an independent prognostic factor in multivariate analysis. However, there were no differences between groups in the hematologic response; patients with MGRS‐A had the renal response less likely than in the MGRS‐NA group. The second most frequently involved organ in AL amyloidosis is the heart. Cardiac involvement is the key driver of disease prognosis and mortality. 33 , 34 , 35 , 36 , 37 We observed more deaths due to heart failure in the MGRS‐A group than in the MGRS‐NA group. These findings are similar to AL amyloidosis, in which mortality rates depend on cardiac dysfunction rather than the renal function. 35 The above indirect evidence and the literature review support the importance of cardiac‐related mortality in MGRS‐A. Based on the study results, we cannot definitively explain excess mortality observed in MGRS‐A. However, in MGRS‐A 8/51 (16%), deaths were related to cardiac failure, while in MGRS‐NA only 1/12 (1%) death was related to cardiac failure, and we can assume that cardiac involvement was a possible prognosticator. The contribution of other factors can not be excluded. For example, the dosing and timing of dexamethasone use in AL amyloidosis with cardiac involvement have been associated with early mortality. 37
Despite confirmation of the diagnosis with a renal biopsy, approximately 10% of the patient were not treated. In the case of MGRS, no treatment or delayed treatment are factors associated with a poor prognosis. 16 , 17
Management of MGRS requires monitoring by a hematologist and nephrologist. 7 This remains an unmet medical need since 25% of patients had not assessed hematologic and/or renal response. 8 Treatment of MGRS requires targeting of the underlying plasma cell or lymphoplasmacytic clone. Antimyeloma agents, typically bortezomib‐based regimens, were commonly used in plasmacytic disorders and anti‐CD20 immunotherapy in cases related to B‐cell lymphoproliferative disorders. 38 , 39 Alkylating agents were commonly used in our study population, whereas other antimyeloma drugs like PIs, IMiDs, and monoclonal antibodies were rarely used due to lack of availability in many countries at the analyzed time of the study. The majority of treated patients received only one line of treatment. In our study, different frontline regimens had similar efficacy in both types of MGRS. ASCT, in the frontline treatment, was the only treatment with a higher ORR. 40 , 41 Hematologic response increased chances for renal response and was associated with better survival. However, one‐fourth of patients did not improve renal function despite achieving ≥VGPR. This makes MGRS an entity with a significant medical need.
The study has some important limitations associated with its retrospective nature and limited follow‐up information. A limitation was the lack of information about the involvement of other organs than the kidney.
5. CONCLUSIONS
MGRS is a recognized and heterogeneous entity that requires treatment upon diagnosis. A significant proportion of patients remain untreated or not adequately followed up by hematologists and nephrologists. Typically, MGRS‐NA has a higher clonal and renal burden than MGRS‐A. MGRS‐A was associated with shorter survival, even though the biochemical disease burden was lower than in MGRS‐NA. Although direct evidence is not available, MGRS‐A probably has higher mortality due to cardiac involvement. The exact reason of higher mortality in MGRS‐A needs to be further investigated. Heart failure, contributing to 16% of reasons of death in MGRS‐A, cannot itself explain the difference in survival between two types of MGRS. Treatment and outcomes were similar in different types of MGRS. Whether novel treatments such as anti‐CD38 monoclonal antibodies will change responses and survival requires further investigations.
CONFLICT OF INTEREST
All authors declare that they have no conflicts of interest.
Supporting information
Table S1. First‐line treatment and hematologic response in patients with amyloidosis‐associated and non‐amyloidosis‐associated monoclonal gammopathy of renal significance.
Table S2. Patients with very good partial response of better in the subsequent lines of treatment. Data presented only of treated patients with evaluation of the response.
Table S3. Baseline patients characteristics with univariate analysis.
ACKNOWLEDGMENT
Open Access Funding provided by Universita degli Studi di Siena within the CRUI‐CARE Agreement.
Gozzetti A, Guarnieri A, Zamagni E, et al. Monoclonal gammopathy of renal significance (MGRS): Real‐world data on outcomes and prognostic factors. Am J Hematol. 2022;97(7):877‐884. doi: 10.1002/ajh.26566
Alessandro Gozzetti and Artur Jurczyszyn share first authorship.
[Correction added on May 05, 2022, after first online publication: New affiliations for Serena Rocchi and Michele Cavo were included and affiliations 15 to 23 were renumbered.]
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author [Alessandro Gozzetti].
REFERENCES
- 1. Kyle RA, Therneau TM, Rajkumar SV, et al. Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med. 2006;354(13):1362‐1369. [DOI] [PubMed] [Google Scholar]
- 2. Kyle RA, Larson DR, Therneau TM, et al. Long‐term follow‐up of monoclonal Gammopathy of undetermined significance. N Engl J Med. 2018;378(3):241‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International myeloma working group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538‐e548. [DOI] [PubMed] [Google Scholar]
- 4. Mateos MV, Kumar S, Dimopoulos MA, et al. International myeloma working group risk stratification model for smoldering multiple myeloma (SMM). Blood Cancer J. 2020;10(10):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fermand J‐P, Bridoux F, Dispenzieri A, et al. Monoclonal gammopathy of clinical significance: a novel concept with therapeutic implications. Blood. 2018;132(14):1478‐1485. [DOI] [PubMed] [Google Scholar]
- 6. Dispenzieri A. Monoclonal gammopathies of clinical significance. Hematology Am Soc Hematol Educ Program. 2020;2020:380‐388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bladé J, Cibeira MT. M‐protein–related disorders: MGCS. Blood. 2018;132:1464‐1465. [DOI] [PubMed] [Google Scholar]
- 8. Lomas OC, Mouhieddine TH, Tahri S, Ghobrial IM. Monoclonal Gammopathy of undetermined significance (MGUS)—not so asymptomatic after all. Cancer. 2020;12:1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Merlini G, Stone MJ. Dangerous small B‐cell clones. Blood. 2006;108:2520‐2530. [DOI] [PubMed] [Google Scholar]
- 10. Leung N, Bridoux F, Nasr SH. Monoclonal Gammopathy of renal significance. N Engl J Med. 2021;384(20):1931‐1941. [DOI] [PubMed] [Google Scholar]
- 11. Leung N, Bridoux F, Hutchison CA, et al. Monoclonal gammopathy of renal significance: when MGUS is no longer undetermined or insignificant. Blood. 2012;120(22):4292‐4295. [DOI] [PubMed] [Google Scholar]
- 12. Leung N, Bridoux F, Batuman V, et al. The evaluation of monoclonal gammopathy of renal significance: a consensus report of the international kidney and monoclonal Gammopathy research group. Nat Rev Nephrol. 2019;15(1):45‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Paueksakon P, Revelo MP, Horn RG, Shappell S, Fogo AB. Monoclonal gammopathy: significance and possible causality in renal disease. Am J Kidney Dis. 2003;42(1):87‐95. [DOI] [PubMed] [Google Scholar]
- 14. Batko K, Malyszko J, Jurczyszyn A, et al. The clinical implication of monoclonal gammopathies: monoclonal gammopathy of undetermined significance and of renal significance. Nephrol Dial Transplant. 2019;34(9):1440‐1452. [DOI] [PubMed] [Google Scholar]
- 15. Sethi S, Rajkumar SV, D'Agati VD. The complexity and heterogeneity of monoclonal immunoglobulin‐associated renal diseases. J Am Soc Nephrol. 2018;29:1810‐1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Steiner N, Göbel G, Suchecki P, Prokop W, Neuwirt H, Gunsilius E. Monoclonal gammopathy of renal significance (MGRS) increases the risk for progression to multiple myeloma: an observational study of 2935 MGUS patients. Oncotarget. 2018;9(2):2344‐2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fermand J‐P, Bridoux F, Kyle RA, et al. How I treat monoclonal gammopathy of renal significance (MGRS). Blood. 2013;122(22):3583‐3590. [DOI] [PubMed] [Google Scholar]
- 18. Kourelis TV, Nasr SH, Dispenzieri A, et al. Outcomes of patients with renal monoclonal immunoglobulin deposition disease. Am J Hematol. 2016;91:1123‐1128. [DOI] [PubMed] [Google Scholar]
- 19. Ziogas DC, Kastritis E, Terpos E, et al. Hematologic and renal improvement of monoclonal immunoglobulin deposition disease after treatment with bortezomib‐based regimens. Leuk Lymphoma. 2017;58:1832‐1839. [DOI] [PubMed] [Google Scholar]
- 20. Vignon M, Javaugue V, Alexander MP, et al. Current anti‐myeloma therapies in renal manifestations of monoclonal light chain‐associated Fanconi syndrome: a retrospective series of 49 patients. Leukemia. 2017;31:123‐129. [DOI] [PubMed] [Google Scholar]
- 21. Fervenza FC, Appel GB, Barbour SJ, et al. Rituximab or cyclosporine in the treatment of membranous nephropathy. N Engl J Med. 2019;381:36‐46. [DOI] [PubMed] [Google Scholar]
- 22. Lysenko Kozlovskaya LV, Rameev VV, Androsova TV. Monoclonal gammapathy of renal significance (MGRS) at the current state: terminology, diagnosis and treatment. Ter Arkh. 2020;92(6):15‐22. [DOI] [PubMed] [Google Scholar]
- 23. Bhutani G, Nasr SH, Said SM, et al. Hematologic characteristics of proliferative glomerulonephritides with nonorganized monoclonal immunoglobulin deposits. Mayo Clin Proc. 2015;90(5):587‐596. [DOI] [PubMed] [Google Scholar]
- 24. Yadav P, Sathick I, Leung N, et al. Serum free light chain level at diagnosis in myeloma cast nephropathy—a multicentre study. Blood Cancer J. 2020;10:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nasr SH, Valeri AM, Cornell LD, et al. Renal monoclonal immunoglobulin deposition disease: a report of 64 patients from a single institution. Clin J Am Soc Nephrol. 2012;7(2):231‐239. [DOI] [PubMed] [Google Scholar]
- 26. Klomjit N, Leung N, Fervenza F, Sethi S, Zand L. Rate and predictors of finding monoclonal gammopathy of renal significance (MGRS) lesions on kidney biopsy in patients with monoclonal gammopathy. J Am Soc Nephrol. 2020;31:2400‐2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pozzi C, D'Amico M, Fogazzi GB, et al. Light chain deposition disease with renal involvement: clinical characteristics and prognostic factors. Am J Kidney Dis. 2003;42(6):1154‐1163. [DOI] [PubMed] [Google Scholar]
- 28. Gozzetti A, Le Beau MM. Fluorescence in situ hybridization: uses and limitations. Semin Hematol. 2000;37:320‐333. [DOI] [PubMed] [Google Scholar]
- 29. Kumar S, Paiva B, Anderson KC, et al. International myeloma working group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17(8):e328‐e346. [DOI] [PubMed] [Google Scholar]
- 30. Palladini G, Hegenbart U, Milani P, et al. A staging system for renal outcome and early markers of renal response to chemotherapy in AL amyloidosis. Blood. 2014;124(15):2325‐2332. [DOI] [PubMed] [Google Scholar]
- 31. Chauvet S, Frémeaux‐Bacchi V, Petitprez F, et al. Treatment of B‐cell disorder improves renal outcome of patients with monoclonal gammopathy‐associated C3 glomerulopathy. Blood. 2017;129:1437‐1447. [DOI] [PubMed] [Google Scholar]
- 32. Cohen C, Royer B, Javaugue V, et al. Bortezomib produces high hematological response rates with prolonged renal survival in monoclonal immunoglobulin deposition disease. Kidney Int. 2015;88:1135‐1143. [DOI] [PubMed] [Google Scholar]
- 33. Wechalekar AD, Schonland SO, Kastritis E, et al. A European collaborative study of treatment outcomes in 346 patients with cardiac stage III AL amyloidosis. Blood. 2013;121(17):3420‐3427. [DOI] [PubMed] [Google Scholar]
- 34. Dispenzieri A, Gertz MA, Kyle RA, et al. Serum cardiac troponins and N‐terminal pro‐brain natriuretic peptide: a staging system for primary systemic amyloidosis. J Clin Oncol. 2004;22(18):3751‐3757. [DOI] [PubMed] [Google Scholar]
- 35. Lebovic D, Hoffman J, Levine BM, et al. Predictors of survival in patients with systemic Mikhael JR, Schuster SR, Jimenez‐Zepeda VH, et al. cyclophosphamide‐bortezomib‐dexamethasone (CyBorD) produces rapid and complete hematologic response in patients with AL amyloidosis. Blood. 2012;119:4391‐4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Abeykoon JP, Zanwar S, Dispenzieri A, et al. Daratumumab‐based therapy in patients with heavily‐pretreated AL amyloidosis. Leukemia. 2019;33:531‐536. [DOI] [PubMed] [Google Scholar]
- 37. Bézard M, Oghina S, Vitiello D, et al. Dexamethasone is associated with early deaths in light chain amyloidosis patients with severe cardiac involvement. PLoS ONE. 2021;16(9):e0257189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hogan JJ, Mocanu M, Berns JS. The native kidney biopsy: update and evidence for best practice. Clin J Am Soc Nephrol. 2016;11(2):354‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Correia SO, Santos S, Malheiro J, Cabrita A, Martins S, Santos J. Monoclonal gammopathy of renal significance: diagnostic workup. World J Nephrol. 2017;6(2):72‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Telio D, Shepherd J, Forrest D, et al. High‐dose melphalan followed by auto‐SCT has favorable safety and efficacy in selected patients with light chain deposition disease and light and heavy chain deposition disease. Bone Marrow Transplant. 2012;47(3):453‐455. [DOI] [PubMed] [Google Scholar]
- 41. Batalini F, Econimo L, Quillen K, et al. High‐dose Melphalan and stem cell transplantation in patients on dialysis due to immunoglobulin light‐chain amyloidosis and monoclonal immunoglobulin deposition disease. Biol Blood Marrow Transplant. 2018;24(1):127‐132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. First‐line treatment and hematologic response in patients with amyloidosis‐associated and non‐amyloidosis‐associated monoclonal gammopathy of renal significance.
Table S2. Patients with very good partial response of better in the subsequent lines of treatment. Data presented only of treated patients with evaluation of the response.
Table S3. Baseline patients characteristics with univariate analysis.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author [Alessandro Gozzetti].


