Abstract
Background
There is no registry data on morbidity and mortality of high‐risk cutaneous squamous cell carcinoma (cSCC) in Australia.
Aim
To examine the clinicopathological features, mortality and morbidity in high‐risk cSCC patients in Western Australia (WA).
Methods
A retrospective cohort study was conducted through hospital record review on cSCC patients discussed at multidisciplinary meetings at the two largest WA hospitals between March 2015 and December 2016.
Results
Of 141 patients, 129 were evaluable, with median follow up of 43.9 (range 3.0–53.2) months. Patients were predominantly older males (84%) with significant comorbidities (Charlson Comorbidity Index (CCI) ≥5; 76%) and history of previous nonmelanoma skin cancer (57%) with advanced disease (57% stage IV without distant metastasis; American Joint Committee on Cancer, 7th edition). Pathological high‐risk features were common including nodal extracapsular extension (47%) and cranial nerve involvement (16%). Clinical morbidity was significant with a median of 2 (range 0–13) excisions and 2 (range 0–21) cSCC‐related hospitalisations for any cSCC event following the index case discussion. Recurrences of the primary index lesion occurred in 60% of patients and 20% had ≥2 recurrences. Median overall survival for patients with nonmetastatic disease was 39.8 (range 25.9–53.7) months and 16.1 (range 0.2–32.0) months for metastatic disease. CCI ≥5, advanced nodal stage and ≥2 recurrences were significantly associated with mortality on multivariable analyses (P < 0.05). Nodal extracapsular extension and any recurrences were identified as significant risk factors for disease‐specific mortality on multivariable analyses (P < 0.05).
Conclusion
High‐risk cSCC patients have significant health needs represented by high‐baseline comorbidities, multiplicity of cSCC events and the number of healthcare‐associated interventions. There is an unmet need for robust cancer data collection.
Keywords: cutaneous squamous cell carcinoma, Australia, high risk, outcome
Introduction
Cutaneous squamous cell carcinoma (cSCC) is a malignancy that arises from the epidermal keratinocytes and is the second most prevalent cancer worldwide with increasing incidence rates. 1 , 2 Australia has the highest incidence of cSCC worldwide with rates up to 387 per 100 000 person‐years and projections estimate that there will be over 200 000 new cases annually by 2024. 2 , 3 Up to 80% of cSCC occur in the sun‐exposed head and neck region. 4 Most cases are considered low risk and respond well to surgical intervention with 5‐year cure rates greater than 95% and approximately 2% risk of disease‐specific death for all patients. 5 , 6 The definition of high‐risk clinicopathological features include tumour size >2 cm, depth of invasion >6 mm, scalp, ear or lip as the primary site, certain histological findings (poorly differentiated, perineural invasion, lymphovascular invasion) and immunosuppression. 7 However, consensus definition of high‐risk disease and optimal management of cSCC are not established. 8 , 9
Significant disparity exists regarding the absence of high‐quality prospective data on its epidemiology, natural history, burden of disease and patient outcomes as most cancer registries do not mandate data collection on keratinocyte cancers. 2 In Australia, geographical variation in incidence of cSCC related to higher ultraviolet radiation exposure is known to occur in particular regions including Queensland but limited comprehensive data is available from other states. 10 , 11 Collectively, the absence of uniform cancer data collection and country‐specific data represents a significant area of need to define the morbidity of cSCC on patients and the healthcare system to provide a robust evidence base to guide accurate identification of high‐risk patients and selection of patients for optimal treatment approaches. Immunotherapy use has redefined the management landscape of advanced and metastatic cSCC with approximately 50% response rate, excellent tolerability and durable disease control. 12 , 13 , 14 , 15 There are several clinical trials investigating the use of these novel therapies in earlier clinical settings (e.g. NCT04154943, NCT03916627, NCT03969004 and NCT03833167) highlighting the urgent unmet need to comprehensively define the current epidemiology and morbidity of cSCC to understand how such treatments have and will revolutionise patient outcomes.
Therefore, the present study aims primarily to report the clinicopathological features, mortality and disease‐related morbidity for patients with complex or high‐risk cSCC of the head and neck region discussed at the two largest tertiary hospitals' multidisciplinary meetings (MDM) in Perth, Western Australia (WA).
Methods
This is a retrospective cohort study that examined clinicopathological and outcome data for patients with cSCC that were discussed at head and neck cancer/skin cancer MDM at the two major tertiary cancer centres in WA between 1 March 2015 and 31 December 2016. Patients deemed to have complexities regarding the management of cSCC are referred to this weekly meeting for review and discussion of treatment options by their specialists. We defined the need for discussion at MDM as a means to identify patients with complex or high‐risk disease and thus those potentially requiring significant health resource utilisation. The meeting is attended by staff from surgical teams (Otolaryngology, Neurosurgery and Plastic Surgery), Medical Oncology, Radiation Oncology, Pathology, Radiology and cancer nurse coordinators. Records of these meetings were screened to identify patients with cSCC and subsequently demographic, clinical, pathological and health resource utilisation data were extracted from hospitals' cancer database. Patients with SCC in situ (Bowen disease), premalignant lesions (actinic keratosis or actinic cheilitis), metastatic squamous cell carcinoma of unknown primary, basal cell carcinoma, age <18 years or enrolment into a clinical trial during the study period were excluded. Clinicopathological information obtained included sex, age, smoking status, comorbidities, immunosuppression (defined as the presence of concurrent haematological malignancy, previous solid organ transplant, regular immunosuppressive medications or immunodeficiency syndrome), anatomical site of origin of cSCC, stage of disease, pathological features (tumour size, depth of invasion, differentiation, perineural invasion, lymphovascular invasion, extracapsular extension), and treatment modalities. The Charlson Comorbidity Index (CCI) was used to categorise patient comorbidities. The CCI is a validated tool that predicts 10‐year mortality in patients with higher CCI scores predictive of higher mortality and greater resource utilisation. 16 , 17 To assess disease‐related morbidity, hospitalisations (overall and those related to cSCC events), cSCC recurrence including number of recurrences, subsequent primary cSCC and survival outcome data were collected. Recurrence was defined as the first instance of histopathologically confirmed relapse (local, nodal or distant) of the index lesion discussed at the MDM in the absence of another culprit cSCC lesion. Patients were staged using the seventh edition of American Joint Committee on Cancer staging manual, which was the current version at the time of clinical discussion. 8 The censor date for data collection was 10 August 2019. Ethics approval was obtained by Sir Charles Gairdner Group Human Research Ethics Committee (reference number 13938) and Fiona Stanley Hospital Human Research Ethics Committee (reference number 0000000144).
Descriptive statistics, including frequency distributions for categorical data and means and standard deviations or medians and interquartile ranges for continuous data, were used to summarise clinicopathological data. Survival outcomes including median progression‐free survival (PFS), disease‐specific survival (DSS) and overall survival (OS) were examined using Kaplan–Meier survival probabilities. Logistic regression analyses were conducted to examine associations between clinicopathological features and patient survival outcomes including mortality, disease‐specific death, recurrence and subsequent primary cSCC. Significance was set at 0.05. Stata 16 (StataCorp, College Station, TX, USA) was used for data analysis.
Results
One hundred and forty‐one patients with cSCC were evaluated with a median follow up of 43.9 (range 3.0–53.2) months over the study period. Twelve cases were excluded (six had squamous cell carcinoma of non‐cutaneous primary, four were on an immunotherapy clinical trial during the study period with metastatic cSCC disease and one each had basal cell carcinoma and squamous cell carcinoma in situ).
Patient characteristics
Of 129 patients, the majority (84%; n = 108) were male and the mean age was 72 (range 30–110) years. The majority of patients had significant comorbidities with 76% (n = 98) of patients having a CCI score of 5 or more (higher scores are associated with higher mortality), of which 31% (n = 40) had a score greater than 7. 17 The majority (57%; n = 73) of patients had a history of managed non‐melanoma skin cancer. Regarding predisposition to cSCC, 21% (n = 27) of patients were immunosuppressed with a history of haematological malignancy, solid organ transplant or iatrogenic immunosuppression (Table 1).
Table 1.
Patient and disease characteristics
| Characteristic | Number of patients (n = 129), n (%) |
|---|---|
| Age, mean (range) (years) | 72 (30–101) |
| Sex | |
| Male | 108 (84) |
| Female | 21 (16) |
| Smoking | |
| Current | 19 (15) |
| Former | 33 (26) |
| Never | 48 (37) |
| Unknown | 29 (22) |
| Charlson Comorbidity Index score | |
| <5 | 31 (24) |
| 5–7 | 58 (45) |
| >7 | 40 (31) |
| Other malignancy | |
| None | 41 (32) |
| Prior/managed cutaneous | 73 (57) |
| Current or prior haematological | 12 (9) |
| Current or prior solid organ | 11 (9) |
| Immunosuppression | |
| No | 102 (79) |
| Yes (haematological/transplant/iatrogenic) | 27 (21) |
| Depth of invasion >6 mm | |
| No | 17 (13) |
| Yes | 51 (40) |
| Unknown | 61 (47) |
| Poorly differentiated histology | |
| No | 74 (58) |
| Yes | 52 (40) |
| Unknown | 3 (2) |
| Perineural invasion | |
| No | 82 (64) |
| Yes | 40 (31) |
| Unknown | 7 (5) |
| Lymphovascular invasion | |
| No | 97 (75) |
| Yes | 23 (18) |
| Unknown | 9 (7) |
| Nodal extracapsular extension | |
| No | 25 (19) |
| Yes | 60 (47) |
| Unknown | 44 (34) |
| Cranial nerve involvement (pathological) | |
| No | 106 (82) |
| Yes | 21 (16) |
| Unknown | 2 (2) |
| Tumour stage (AJCC 7th edition) | |
| I | 8 (6) |
| II | 20 (16) |
| III | 21 (16) |
| IV | 80 (62) |
| Stage IV | |
| M0 | 122 (95) |
| M1 | 7 (5) |
AJCC, American Joint Committee on Cancer; M0, patients without distant metastasis; M1, patients with distant metastasis; SD, standard deviation.
Disease characteristics
Fifty‐seven percent (n = 73) of patients discussed at the MDM had advanced cSCC (Stage IV disease without distant metastasis) and 5% (n = 7) of patients had distant metastatic disease. Pathological information obtained from surgery was reflective of the high proportion of patients with locally advanced disease with high‐risk features such as tumour greater than 2 cm (36%; n = 47), poor differentiation status (40%; n = 52), cranial nerve involvement (16%; n = 21), depth of invasion >6 mm (40%; n = 51), perineural invasion (31%; n = 40), lymphovascular space invasion (18%; n = 23) and nodal extracapsular extension (47%; n = 60). However, information on tumour depth of invasion and nodal extracapsular extension was not present in 47% (n = 61) and 34% (n = 44) of specimens, respectively, indicating a need to standardise pathological reporting to identify patients with high‐risk disease (Table 1).
Treatment consisted of surgery in 88% (n = 114) of patients, 67% (n = 86) of patients received adjuvant radiotherapy and 7% (n = 9) received adjuvant chemoradiotherapy. No patients included in the study received immunotherapy due to the study exclusion criteria aimed to define the contemporaneous burden of disease without immunotherapy use.
In our cohort, clinical morbidity was high as demonstrated by the number of hospitalisations, number of cSCC recurrences, number of second primary cSCC and the number of resultant excisions of lesions (Table 2, Fig. S1). The total median number of any hospitalisations postdiscussion at MDM was 3 (range 0–21) and the median number of hospitalisations related to the management or associated complications of the index lesion or any subsequent primary cSCC was 2 (range 0–21). Subsequent to the discussion on the management of the index lesion at the MDM, most (60%; n = 78) patients had at least one recurrence of the primary index lesion with 20% (n = 26) having two or more recurrences. Approximately 18% (n = 23) of patients had a second primary cSCC lesion postdiscussion at the MDM. The median number of excisions of the index lesion and any subsequent primary cSCC lesions was 2 (range 0–13).
Table 2.
Clinical morbidity associated with cutaneous squamous cell carcinoma (cSCC)
| Variable | Number of patients (n = 129), n (%) |
|---|---|
| Number of recurrences of the index primary cSCC lesions | |
| 0 | 51 (40) |
| 1 | 52 (40) |
| 2 | 20 (15) |
| >2 | 6 (5) |
| Number of other subsequent primary cSCC lesions | |
| 0 | 106 (82) |
| 1 | 15 (12) |
| ≥2 | 8 (6) |
| Number of hospitalisations | |
| Median: total (range) | 3 (0–21) |
| Median: related to cSCC (range) | 2 (0–21) |
Survival outcomes
Sixty‐nine (54%) patients died during the study duration, of which 44 of 69 (64%) deaths were due to cSCC. Of note, the remaining patients died due to relevant other medical comorbidities including other cancers and pneumonia. The median OS and PFS of patients in the cohort excluding those with distant metastatic disease were 39.8 and 14.9 months respectively (Fig. 1). The median OS and PFS in those with distant metastatic disease were 16.1 and 9.9 months respectively (Fig. 1). The median DSS was not reached in the patient cohort without distant metastatic disease and was 16.1 months in those with distant metastatic disease.
Figure 1.
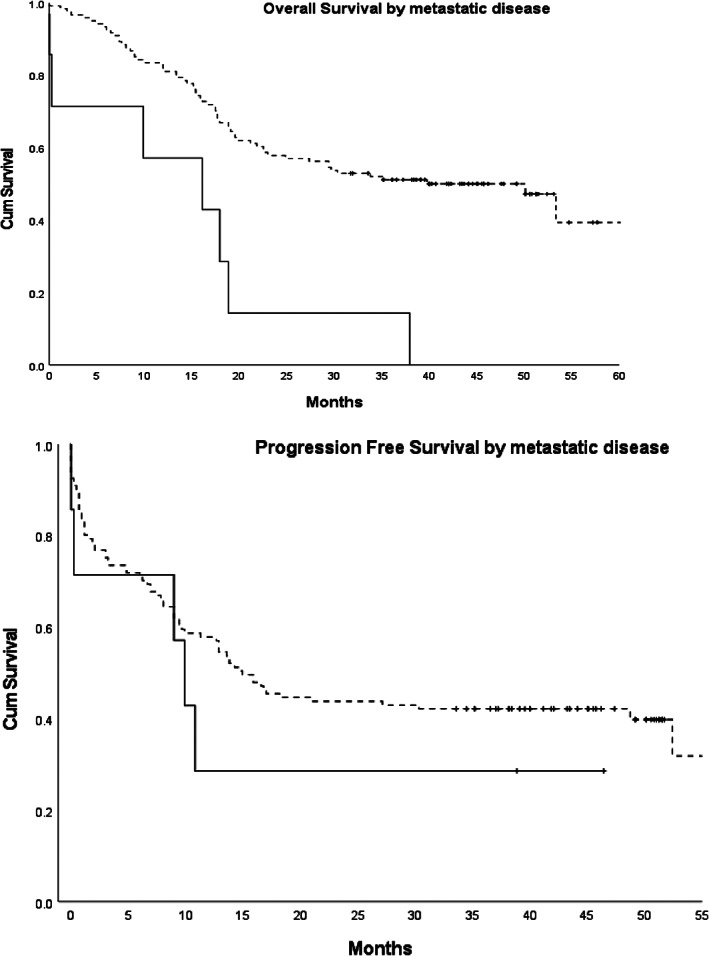
Kaplan–Meier estimates of overall survival and progression‐free survival in patients with metastatic disease (M1, continuous line) and without (M0, dotted line) distant metastatic disease. Censored data are indicated by tick marks. Cum Survival, probability of cumulative survival.
Associations between clinicopathological risk factors and survival outcomes
Clinicopathological risk factors significantly associated with mortality, disease‐specific death, recurrence and subsequent primary cSCC are summarised in Supporting Information Table S1A–D. For the outcome of mortality (Table S1A), logistic regression modelling showed on univariable analyses that advanced age (odds ratio (OR): 1.03; 95% confidence interval (CI): 1.00–1.06; P‐value 0.02), CCI score ≥ 5 (OR: 3.18; 95% CI: 1.35–7.47; P‐value 0.01), immunosuppression (OR: 10.13; 95% CI: 2.87–35.81; P < 0.01), advanced nodal stage (OR: 3.00; 95% CI: 1.17–7.71; P‐value 0.02), perineural invasion (OR: 2.79; 95% CI: 1.26–6.17; P‐value 0.01), lymphovascular invasion (OR: 3.56; 95% CI: 1.29–9.80; P‐value 0.01), nodal extracapsular extension (OR: 6.00; 95% CI: 2.14–16.86; P‐value 0.01) and ≥2 recurrences (OR: 6.00; 95% CI: 1.95–18.45; P‐value 0.01) were significant risk factors for mortality. On multivariable analyses, only CCI score ≥ 5 (OR: 3.03; 95% CI: 1.12–8.15; P‐value 0.03), advanced nodal stage (OR: 5.54; 95% CI: 1.26–24.34; P‐value 0.02) and ≥2 recurrences (OR: 7.66; 95% CI: 2.13–27.60; P‐value 0.01) were significant.
For the outcome of disease‐specific death (Table S1B), CCI (OR: 2.85; 95% CI: 1.08–7.48; P‐value 0.03), immunosuppression (OR: 11.96; 95% CI: 3.23–44.34; P‐value < 0.01), perineural invasion (OR: 3.81; 95% CI: 1.58–9.16; P‐value 0.01), lymphovascular invasion (OR: 4.32; 95% CI: 1.45–12.83; P‐value 0.01), nodal extracapsular extension (OR: 6.50; 95% CI: 1.88–22.44; P‐value 0.01) and any recurrences (OR: 3.40; 95% CI: 1.43–8.10; P‐value 0.01) were identified as significant on univariable analyses. However, only nodal extracapsular extension (OR: 7.27; 95% CI: 1.98–26.79; P‐value 0.01) and any recurrences (OR: 3.74; 95% CI: 1.20–11.69; P‐value 0.02) were identified as significant multivariable risk factors.
For the outcome of recurrence (Table S1C), perineural invasion (OR: 3.42; 95% CI: 1.40–8.33; P‐value 0.01) was the only significant variable identified on univariable and multivariable analyses. For the outcome of subsequent primary cSCC (Table S1D), immunosuppression due to a history of a transplant (n = 4) was the only significant risk factor identified on univariable and multivariable analyses (OR: 14.63; 95% CI: 1.43–149.75; P‐value 0.02).
Discussion
This retrospective cohort study of 129 patients with high‐risk cSCC discussed at the two largest WA tertiary hospital MDMs demonstrates significant disease morbidity and healthcare resource utilisation. The majority of the patients were older, had advanced disease with multiple comorbidities, high‐risk pathology features and multiple recurrences in which logistic regression analyses correlated these variables significantly with risk of death. Of particular note, more than half (60%) of the cohort had at least one recurrence of the index lesion with 20% of patients having more than two recurrences. Although the median number of any cSCC‐related hospitalisations in our cohort following definitive management was 2, the upper limit of the range was 21 episodes. Similarly, although the median number of excisions for any cSCC lesion post‐MDM discussion was 2, the upper limit of the range was 13 excisions. Furthermore, of the 69 deaths that occurred in the entire cohort, the majority (64%) were due to cSCC. This is considerably higher than the approximately 2–3% disease‐specific mortality reported when disease of all risk categories are included which dilutes the appreciation of the mortality associated with high‐risk disease simply defined by discussion at a tertiary hospital MDM. 5 , 18 , 19 Thus, the selection of the patients discussed at a tertiary hospital MDM was successful in identifying patients with more complex health‐related and cSCC‐related care needs.
The present study population primarily comprised older men (84%) with a mean age of 72 years at diagnosis, which is similar to other reported cohorts in the literature with respect to age and sex, with the mean age at diagnosis in the seventh decade and the majority male patients. 20 , 21 , 22 , 23 The older mean age of our patients was associated with an increased complexity of healthcare needs reflected by the high CCI scores, which is predictive of an increasing risk of death. 17 , 18
Given the grouping of survival data without consideration of disease risk and the ambiguity and lack of consensus regarding risk stratification, the survival of patients with locally advanced and metastatic disease is not clearly defined. It is important to identify patients with high‐risk disease for allocation of optimal treatment including access to clinical trials. While previous publications have reported low cSCC disease‐specific mortality, these cohorts included all patients with cSCC that would underestimate the morbidity and mortality in those with high‐risk disease. 5 , 19 In our cohort, the disease‐specific mortality was high (64%) and median OS in those with distant metastatic disease was 16.1 months. Our cohort of patients had high‐risk and advanced disease as demonstrated by the presence of multiple high‐risk pathology features and advanced stage at the time of MDM discussion, explaining the higher mortality rate. Our findings are similar to other retrospective studies, including a study of 190 patients by Hillen et al., 24 and 82 patients by Cowey et al., 20 which reported the median OS in those with distant metastases was 16.0 and 15.3 months respectively. This affirms the high mortality burden in those with advanced or metastatic disease and the need for robust epidemiological data and better therapeutic interventions to mitigate the unmet medical need.
Multiplicity of non‐melanoma skin cancers has previously been reported, which is reflected in our cohort, with the majority (57%) of patients having had a history of prior lesion and 18% developing a second primary cSCC lesion post‐MDM discussion. 25 While the incidence of cSCC is plateauing or falling in Australia, the number of hospitalisations and deaths each year from nonmelanoma skin cancers appear to be increasing from 109 000 hospitalisations and 600 deaths in 2014 to 115 000 hospitalisation and 679 deaths in 2016 highlighting the increasing burden of disease and the need for better therapies. 18 , 26 , 27 Our cohort of patients is not reflective of all patients with cSCC but rather represents those with high‐risk or advanced disease. These patients have complex needs requiring multimodal treatment, long‐term follow up and significant utilisation of healthcare resources as demonstrated by the number of comorbidities, recurrences, excisions and hospitalisations. In at least one‐third of our cohort of patients evaluated, the cause of the death was attributed to cSCC. This calls for public health measures to further reduce the incidence of cSCC, develop better therapeutic interventions to treat advanced disease and routinely capture data on these patients to better guide management.
The economic impact of advanced cSCC is not well understood and to date there are scant published data in this area. The present study would predict for high‐cost utilisation in those with advanced and/or high‐risk disease. The high number of recurrences and hospitalisations highlights the significant cost incurred in investigation and management of cSCC. An analysis of direct costs of unresectable and advanced cSCC from Italy estimated cost to be between €10 281 and €12 882 (A$16 216–20 319; 1€ = A$1.58) per patient per annum. 28 In the Australian context, it is difficult to accurately estimate the cost of advanced cSCC as most of the reported dataset is based on information obtained through Medicare Australia, which does not report episode of care on patients treated in public hospitals and captures patients with more indolent disease.
In 2018, cemiplimab was approved by the United States Food and Drug Administration (FDA) as the first immunotherapy agent for the treatment of metastatic or locally advanced cSCC not amenable to curative treatment. 13 , 29 , 30 , 31 , 32 Recent updated data from the landmark phase II trial in patients with advanced cSCC demonstrated durable significant clinical responses and an increasing number of complete responses. 33 With a median follow up of 15.7 (range 0.6–36.1) months, the objective response rate was reported as 46.1% (95% CI: 38.9–53.4), the number of complete responses had increased from 7% to 16.1%, the durable disease control rate was 61% (95% CI: 53.3–67.6) and median OS was not reached. 33 High tumour mutational burden is associated with durable response to immunotherapies, from which precedence of excellent survival has been demonstrated in other cancers such as advanced melanoma, with cSCC known to have the highest tumour mutational burden of all cancers. 34 , 35 In Australia, immunotherapy is not yet available for reimbursement under the Pharmaceutical Benefits Scheme. Our report excluded four patients who were on immunotherapy clinical trials, but at least seven additional patients with advanced disease in our cohort would have been eligible for cemiplimab based on FDA approved criteria. 36
Limitations
This was a retrospective, observational analysis of patients' electronic medical records and it is limited by the factors common to this study design, including incomplete and missing data. The use of head and neck MDM to identify patients with advanced/high‐risk cSCC is open to selection bias and means that the applicability of our findings is limited to those patients with head and neck disease only. However, the use of MDM discussion to select patients was successful in identifying patients with high‐risk disease and high health resource utilisation.
Conclusion
Advanced cSCC has a high burden of disease and represents an area of unmet clinical need. Its increasing incidence and prevalence calls for further research to better understand its epidemiology and pave the way for more refined management options.
Supporting information
Figure S1. Summary of selected clinical morbidity associated with cutaneous squamous cell carcinoma.
Table S1. (A) Clinicopathological risk factors for mortality on univariable and multivariable analyses. (B) Clinicopathological risk factors for disease‐specific death on univariable and multivariable analyses. (C) Clinicopathological risk factor for recurrence on univariable and multivariable analyses. (D) Clinicopathological risk factor for subsequent primary cSCC on univariable and multivariable analyses.
Funding: None.
Conflict of interest: None.
References
- 1. Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J Am Acad Dermatol 2013; 68: 957–66. [DOI] [PubMed] [Google Scholar]
- 2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 3. Lomas A, Leonardi‐Bee J, Bath‐Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol 2012; 166: 1069–80. [DOI] [PubMed] [Google Scholar]
- 4. Ramirez CC, Federman DG, Kirsner RS. Skin cancer as an occupational disease: the effect of ultraviolet and other forms of radiation. Int J Dermatol 2005; 44: 95–100. [DOI] [PubMed] [Google Scholar]
- 5. Schmults CD, Karia PS, Carter JB, Han J, Qureshi AA. Factors predictive of recurrence and death from cutaneous squamous cell carcinoma: a 10‐year, single‐institution cohort study. JAMA Dermatol 2013; 149: 541–7. [DOI] [PubMed] [Google Scholar]
- 6. Amoils M, Lee CS, Sunwoo J, Aasi SZ, Hara W, Kim J et al. Node‐positive cutaneous squamous cell carcinoma of the head and neck: survival, high‐risk features, and adjuvant chemoradiotherapy outcomes. Head Neck 2017; 39: 881–5. [DOI] [PubMed] [Google Scholar]
- 7. Martorell‐Calatayud A, Sanmartín Jimenez O, Cruz Mojarrieta J, Guillén Barona C. Cutaneous squamous cell carcinoma: defining the high‐risk variant. Actas Dermosifiliogr 2013; 104: 367–79. [DOI] [PubMed] [Google Scholar]
- 8. Warner CL, Cockerell CJ. The new seventh edition American Joint Committee on Cancer staging of cutaneous non‐melanoma skin cancer: a critical review. Am J Clin Dermatol 2011; 12: 147–54. [DOI] [PubMed] [Google Scholar]
- 9. Karia PS, Jambusaria‐Pahlajani A, Harrington DP, Murphy GF, Qureshi AA, Schmults CD. Evaluation of American Joint Committee on Cancer, International Union against Cancer, and Brigham and Women's Hospital tumor staging for cutaneous squamous cell carcinoma. J Clin Oncol 2014; 32: 327–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Buettner PG, Raasch BA. Incidence rates of skin cancer in Townsville, Australia. Int J Cancer 1998; 78: 587–93. [DOI] [PubMed] [Google Scholar]
- 11. Perera E, Gnaneswaran N, Staines C, Win AK, Sinclair R. Incidence and prevalence of non‐melanoma skin cancer in Australia: a systematic review. Australas J Dermatol 2015; 56: 258–67. [DOI] [PubMed] [Google Scholar]
- 12. Migden MR, Rischin D, Schmults CD, Guminski A, Hauschild A, Lewis KD et al. PD‐1 blockade with cemiplimab in advanced cutaneous squamous‐cell carcinoma. N Engl J Med 2018; 379: 341–51. [DOI] [PubMed] [Google Scholar]
- 13. Rischin D, Migden MR, Lim AM, Schmults CD, Khushalani NI, Hughes BGM et al. Phase 2 study of cemiplimab in patients with metastatic cutaneous squamous cell carcinoma: primary analysis of fixed‐dosing, long‐term outcome of weight‐based dosing. J Immunother Cancer 2020; 8: e000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grob JJ, Gonzalez R, Basset‐Seguin N, Vornicova O, Schachter J, Joshi A et al. Pembrolizumab monotherapy for recurrent or metastatic cutaneous squamous cell carcinoma: a single‐arm phase II trial (KEYNOTE‐629). J Clin Oncol 2020; 38: 2916–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maubec E, Boubaya M, Petrow P, Beylot‐Barry M, Basset‐Seguin N, Deschamps L et al. Phase II study of pembrolizumab as first‐line, single‐drug therapy for patients with unresectable cutaneous squamous cell carcinomas. J Clin Oncol 2020; 38: 3051–61. [DOI] [PubMed] [Google Scholar]
- 16. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–83. [DOI] [PubMed] [Google Scholar]
- 17. Sundararajan V, Henderson T, Perry C, Muggivan A, Quan H, Ghali WA. New ICD‐10 version of the Charlson Comorbidity Index predicted in‐hospital mortality. J Clin Epidemiol 2004; 57: 1288–94. [DOI] [PubMed] [Google Scholar]
- 18. Stang A, Khil L, Kajüter H, Pandeya N, Schmults CD, Ruiz ES et al. Incidence and mortality for cutaneous squamous cell carcinoma: comparison across three continents. J Eur Acad Dermatol Venereol 2019; 33(Suppl 8): 6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eigentler TK, Leiter U, Häfner HM, Garbe C, Röcken M, Breuninger H. Survival of patients with cutaneous squamous cell carcinoma: results of a prospective cohort study. J Invest Dermatol 2017; 137: 2309–15. [DOI] [PubMed] [Google Scholar]
- 20. Cowey CL, Robert NJ, Espirito JL, Davies K, Frytak J, Lowy I et al. Clinical outcomes among unresectable, locally advanced, and metastatic cutaneous squamous cell carcinoma patients treated with systemic therapy. Cancer Med 2020; 9: 7381–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brougham ND, Dennett ER, Cameron R, Tan ST. The incidence of metastasis from cutaneous squamous cell carcinoma and the impact of its risk factors. J Surg Oncol 2012; 106: 811–5. [DOI] [PubMed] [Google Scholar]
- 22. Brantsch KD, Meisner C, Schönfisch B, Trilling B, Wehner‐Caroli J, Röcken M et al. Analysis of risk factors determining prognosis of cutaneous squamous‐cell carcinoma: a prospective study. Lancet Oncol 2008; 9: 713–20. [DOI] [PubMed] [Google Scholar]
- 23. Leiter U, Eigentler T, Garbe C. Epidemiology of skin cancer. Adv Exp Med Biol 2014; 810: 120–40. [DOI] [PubMed] [Google Scholar]
- 24. Hillen U, Leiter U, Haase S, Kaufmann R, Becker J, Gutzmer R et al. Advanced cutaneous squamous cell carcinoma: A retrospective analysis of patient profiles and treatment patterns – results of a non‐interventional study of the DeCOG. Eur J Cancer 2018; 96: 34–43. [DOI] [PubMed] [Google Scholar]
- 25. Pandeya N, Olsen CM, Whiteman DC. The incidence and multiplicity rates of keratinocyte cancers in Australia. Med J Aust 2017; 207: 339–43. [DOI] [PubMed] [Google Scholar]
- 26. Australian Institute of Health and Welfare (AIHW) . Cancer in Australia 2019. Cancer series no.119. Cat. no. CAN 123. Canberra: AIHW; 2019.
- 27. Australian Institute of Health and Welfare (AIHW) . Cancer in Australia 2017. Cancer series no.101. Cat. no. CAN 100. Canberra: AIHW; 2017.
- 28. Ronconi G, Piccinni C, Dondi L, Calabria S, Pedrini A, Esposito I et al. Identification of cases and estimate of direct costs of unresectable and advanced cutaneous squamous cell carcinoma: real‐world data from a large Italian database. Br J Dermatol 2020; 183: 172–4. [DOI] [PubMed] [Google Scholar]
- 29. Petersen ET, Ahmed SR, Chen L, Silapunt S, Migden MR. Review of systemic agents in the treatment of advanced cutaneous squamous cell carcinoma. Future Oncol 2019; 15: 3171–84. [DOI] [PubMed] [Google Scholar]
- 30. Migden MR, Khushalani NI, Chang ALS, Lewis KD, Schmults CD, Hernandez‐Aya L et al. Cemiplimab in locally advanced cutaneous squamous cell carcinoma: results from an open‐label, phase 2, single‐arm trial. Lancet Oncol 2020; 21: 294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Keeping S, Xu Y, Chen CI, Cope S, Mojebi A, Kuznik A et al. Comparative efficacy of cemiplimab versus other systemic treatments for advanced cutaneous squamous cell carcinoma. Future Oncol 2021; 17: 611–27. [DOI] [PubMed] [Google Scholar]
- 32. Konidaris G, Paul E, Kuznik A, Keeping S, Chen CI, Sasane M et al. Assessing the value of cemiplimab for adults with advanced cutaneous squamous cell carcinoma: a cost‐effectiveness analysis. Value Health 2021; 24: 377–87. [DOI] [PubMed] [Google Scholar]
- 33. Rischin D, Khushalani NI, Schmults CD, Guminski A, Chang ALS, Lewis KD et al. Integrated analysis of a phase 2 study of cemiplimab in advanced cutaneous squamous cell carcinoma: extended follow‐up of outcomes and quality of life analysis. J Immunother Cancer 2021; 9: e002757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med 2017; 9: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Larkin J, Chiarion‐Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD et al. Five‐year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2019; 381: 1535–46. [DOI] [PubMed] [Google Scholar]
- 36. Markham A, Duggan S. Cemiplimab: first global approval. Drugs 2018; 78: 1841–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Summary of selected clinical morbidity associated with cutaneous squamous cell carcinoma.
Table S1. (A) Clinicopathological risk factors for mortality on univariable and multivariable analyses. (B) Clinicopathological risk factors for disease‐specific death on univariable and multivariable analyses. (C) Clinicopathological risk factor for recurrence on univariable and multivariable analyses. (D) Clinicopathological risk factor for subsequent primary cSCC on univariable and multivariable analyses.


