Abstract
A new reliable, fast, and simple method for the detection of aflatoxigenic Aspergillus strains, consisting of the addition of a cyclodextrin (a methylated β-cyclodextrin derivative) to common media used for testing mycotoxin production ability, was developed. We propose the use of this compound as an additive for fungal culture media to enhance the natural fluorescence of aflatoxins. The production of aflatoxins coincided with the presence of a bright blue or blue-green fluorescent area surrounding colonies when observed under long-wavelength (365-nm) UV light after 3 days of incubation at 28°C. The presence of aflatoxins was confirmed by extracting the medium with chloroform and examining the extracts by high-pressure liquid chromatography with fluorescence detection.
Aflatoxins are mycotoxins with highly toxic and carcinogenic properties produced by some strains of Aspergillus flavus, Aspergillus parasiticus, and Aspergillus nomius. These fungi are frequently found in foodstuffs and animal feeds. However, not all strains are able to produce aflatoxins, and this has encouraged the use of screening for their aflatoxin production abilities. The methodology commonly used for this survey involves the culture of strains in a suitable liquid or solid medium and their later extraction and analysis for the presence of aflatoxins by chromatographic techniques. Yeast extract-sucrose (YES) medium (3) and natural media with wheat, rice, or peanut (9) have been used for this purpose. Testing large numbers of isolates on a variety of substrates with this procedure is tedious and time-consuming. For this reason, several screening methods for direct visual determination of aflatoxin production have been developed. These methods use more or less complicated culture media containing additives to enhance the production of aflatoxins in order to achieve direct visual determination of a bright blue or blue-green fluorescent area surrounding colonies under UV radiation. Thus, a complex agar medium containing sucrose, various salts, and an aqueous extract of aflatoxin-free peanuts (5); a modified Czapek agar medium containing corn steep liquor, named aflatoxin-producing ability (APA) medium (9); media containing coconut, named coconut agar medium (4, 13), coconut extract agar (11, 12), and coconut cream agar (6); the synthetic liquid medium of Adye and Mateles (1); and a silica gel medium (15) are currently in use.
The natural fluorescence of aflatoxins arises from their oxygenated pentaheterocyclic structure. The cyclodextrins (cyd) are molecules formed by the action of the enzyme cyd-transglycolase on dextrans and have different sizes [they contain from six to eight units of glucose in an α(1–4) configuration, according to which they are called α-, β-, or γ-cyd]. They are available commercially, and their physical and chemical properties have been described in the literature (14). These oligomers are able to include a large number of organic and inorganic species in their cavities, and in this work, excitation of the natural fluorescence of aflatoxins has been accomplished using a derivative of these molecules (2, 8, 16, 17).
Experimental results obtained in previous work (2, 16, 17) revealed that β-cyd and its methylated derivatives have excellent cavities for exciting the fluorescence responses of aflatoxins B1 and G1 through the formation of inclusion complexes. In this work, we have exploited this property for the direct visual measurement of aflatoxin production by A. flavus and A. parasiticus strains. The enhancement phenomenon was optimized for representative aflatoxigenic or nonaflatoxigenic Aspergillus strains directly in a culture medium traditionally used for fungal isolation from foods (Sabouraud dextrose agar [SAB]) and in a culture medium used for mycotoxin production (YES).
In the present paper we propose the use of a methylated β-cyd derivative, the β-W7M 1.8-cyd (Mβ-cyd), as an additive to enhance the natural fluorescence of aflatoxins in culture media. We report a series of experiments that led us to the development of a rapid and simple method for the detection of aflatoxin-producing strains using common fungal culture media (7a).
MATERIALS AND METHODS
Strains used.
The representative strains of A. flavus, A. parasiticus, Aspergillus ochraceus, and Aspergillus versicolor used in our experiments are listed in Table 1 and were supplied by the Spanish Type Culture Collection (Burjassot, Valencia, Spain). The method was also evaluated with 24 additional A. flavus group strains belonged to the culture collection of our laboratory. Twelve of these strains were isolated from foods (18), and the rest were isolated from feed. Of these 24 strains, 20 are nonaflatoxigenic.
TABLE 1.
Aflatoxin production by the Aspergillus species used
| Species | Strain | APA response | HPLC of YES extracts for aflatoxins |
|---|---|---|---|
| A. parasiticus | NRRL 2999 | + | + |
| A. parasiticus | NRRL 3145 | + | + |
| A. flavus | NRRL 6540 | + | + |
| A. flavus group | 1316 | + | + |
| A. flavus group | 876 | + | + |
| A. flavus group | 42 | + | + |
| A. flavus group | 19 | + | + |
| A. flavus group | 893 | − | − |
| A. flavus | NRRL 6538 | − | − |
| A. flavus | NRRL 6541 | − | − |
| A. flavus | NRRL A-3537 | − | − |
| A. ochraceus | NRRL 3174a | − | − |
| A. versicolor | NRRL 3499b | − | − |
| A. versicolor | NRRL 573b | − | − |
Ochratoxin A-producing strain.
Sterigmatocystin-producing strain.
Media.
The culture media used were Czapek agar, SAB, YES agar, and APA medium. All were supplied by Difco (Detroit, Mich.). cyd added to the media tested for aflatoxin detection were β-cyd and a methylated β-cyd derivative, pharmaceutical-grade Mβ-cyd from Wacker (Munich, Germany).
Cultivation and observation of fluorescence.
All strains had been tested previously for aflatoxin production in APA medium (incubation was for 10 days at 28°C). These strains were also cultivated in YES medium under the same conditions used for APA medium, and chloroform extracts (7) of all them were obtained and analyzed by high-pressure liquid chromatography (HPLC) with fluorescence detection (2) to confirm the presence of aflatoxins.
After their aflatoxin production abilities were proven, all strains were cultivated in Czapek agar at 25°C for 7 days in darkness. After this time, an inoculum with a loopful of spores of each strain was placed at the center of each dish containing the media tested. We assayed the following experimental parameters: two kinds of cyd (β-cyd and a methylated β-cyd derivative) at two different concentrations (0.3 and 2%), three incubation temperatures (25, 28, and 30°C), and different incubation times (from 1 to 10 days). The presence or absence of fluorescence in the agar surrounding the colonies assayed was determined under UV radiation (365 nm) and expressed as positive or negative. All experiments were replicated three times.
Extraction and quantitation of aflatoxin.
To confirm the correlation between fluorescence and aflatoxin production, all colonies, whether or not they showed fluorescence, were extracted according to the method described by El-Banna et al. (7) with some modifications. The colonies and the surrounding agar were weighed and introduced into stomacher bags. Extraction was carried out using 20 ml of chloroform (twice with 10 ml each), and homogenization for 3 min in a stomacher. The chloroform phase was filtered through Whatman no. 3 filter paper and concentrated to dryness under a nitrogen steam. The residue was redissolved in 200 μl of mobile phase, and volumes of 50 μl of extract were injected into the HPLC system. The HPLC method used in this study was a modification of the method of Cepeda et al. (2). The apparatus and conditions used were as follows: column, Tracer Inertsil ODS2, 5-μm particle size (150 mm by 4.6 mm [inner diameter]) from Teknokroma (Barcelona, Spain); mobile phase, methanol-water (45:55, vol/vol); and flow rate, 0.6 ml/min. The pump was a PU-l580 from Jasco (Tokyo, Japan), and the injector was a Rheodyne (Cotati, Calif.) model 7125 equipped with a 50-μl loop. The fluorescence detector, an RF-535 (Shimadzu, Kyoto, Japan) was set up with a λex of 365 nm and a λem of 418 nm.
For quantitation, standard curves of aflatoxins B1, B2, G1, and G2 were made. A commercial mixture of aflatoxins (Sigma-Aldrich) was used for this purpose. The results obtained were expressed as nanograms of aflatoxin per gram of agar.
RESULTS AND DISCUSSION
First, we assayed the aflatoxin production abilities of all strains used in this experiment by cultivating them in APA medium at 28°C for 10 days (Table 1). Strains NRRL 6540, NRRL 2999, NRRL 3145, 1316, 876, 42, and 19 (the last four are from our laboratory collection) showed fluorescence in this medium after 10 days of incubation. These results were in concordance with those obtained from the chloroform extracts from all strains cultivated in YES medium under the same conditions used for APA medium with analysis by the chromatographic procedure (Table 1). Results with nonaflatoxigenic strains were as expected (they did not show fluorescence in any of the experiments), and only data from a representative negative strain (strain 893) belonging to our collection are shown.
Effect of addition of cyd to the culture media.
The effects of the addition of two different cyd to the culture media at different concentrations on rapid visualization of aflatoxin production are shown in Table 2. When these cyd were used as additives for culture media, they did not negatively affect the fungal growth, and in fact an increase in the diameters of the colonies was observed when strains cultivated under the same conditions in media with and without cyd were compared. This experiment was carried out at 28°C.
TABLE 2.
Comparison of rapid visualization of aflatoxin production results obtained with the addition of cyd to basal culture media at different concentrations on the fifth day of incubation at 28°C
| Species | Strain | Resulta with:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| SAB
|
YES
|
||||||||
| Mβ-cyd at:
|
β-cyd at:
|
Mβ-cyd at:
|
β-cyd at:
|
||||||
| 0.3% | 2% | 0.3% | 2% | 0.3% | 2% | 0.3% | 2% | ||
| A. parasiticus | NRRL 2999 | ++ | ++ | + | + | ++ | ++ | + | + |
| A. parasiticus | NRRL 3145 | ++ | ++ | + | + | ++ | ++ | + | + |
| A. flavus | NRRL 6540 | ++ | ++ | + | + | ++ | ++ | + | + |
| A. flavus group | 1316 | ++ | ++ | + | + | ++ | ++ | + | + |
| A. flavus group | 876 | ++ | ++ | + | + | ++ | ++ | + | + |
| A. flavus group | 42 | ++ | ++ | + | + | ++ | ++ | + | + |
| A. flavus group | 19 | ++ | ++ | + | + | ++ | ++ | + | + |
| A. ochraceus | NRRL 3174 | ± | ± | − | − | − | − | − | − |
++, clear fluorescence; +, weak fluorescence; − no fluorescence; ± fluorescence not clear.
In regard to concentration of cyd used, there was no difference between the subjective intensity of fluorescence observed for all strains assayed in media using 0.3 and 2% cyd. However, in assays carried out with strains NRRL 2999 and NRRL 3145 that were aimed at reducing the additive concentration (0.05 and 0.2% of each cyd) (data not shown), we have found that media with 0.3 and 2% Mβ-cyd produced fluorescence as early as the second day of incubation, while those containing 0.05 and 0.2% did not show fluorescence.
Consistent with earlier work (2), it was also observed that a minimum concentration of cyd was necessary for the formation of inclusion complexes that would enhance the fluorescence signals of aflatoxins; however, an increase in the concentration above this value (0.3% in this case) did not improve the response, since the subjective intensities of fluorescence observed were identical using 0.3 and 2% cyd. Thus, in media containing 2% cyd, results were not improved, and these culture media would be more expensive.
All nonaflatoxigenic strains were negative in both media, except for NRRL 3174, an A. ochraceus ochratoxin A-producing strain, which did not show a clear response in SAB with Mβ-cyd added. The fluorescence exhibited for this strain was observed at the fifth day of incubation, and the blue-violet color of this fluorescence was significantly different from that for the aflatoxigenic Aspergillus strains tested in this work. The aflatoxigenic NRRL 2999 and 1316 (belonging to our collection) strains gave the same positive results with both cyd. However, the response of the rest of aflatoxigenic strains was positive but markedly weak in media containing β-cyd in comparison with the results obtained with the same media containing Mβ-cyd. Since this may lead to reporting of false-negative results with the use of β-cyd, we recommend only the use of Mβ-cyd as an adequate additive for detection of aflatoxigenic strains in culture media. In our previous work (2, 17) using spectroscopic and chromatographic techniques, we observed that a methylated β-cyd derivative was the most suitable compound for enhancing the fluorescence emission of aflatoxins.
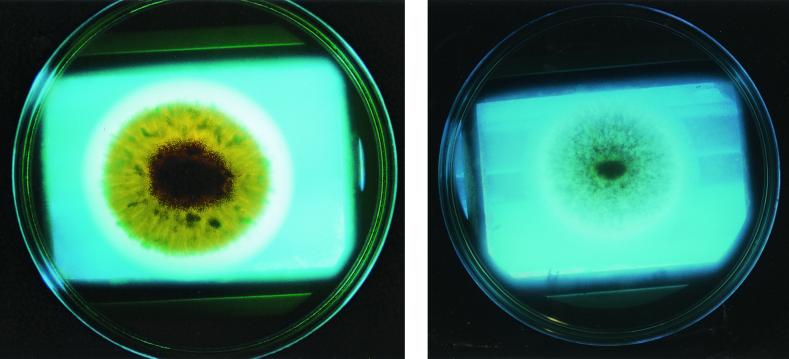
Regarding comparison of the basal culture media, addition of cyd was always effective for SAB and YES medium. With addition of 0.3% Mβ-cyd, either of these media could be used to determine the aflatoxigenic ability of Aspergillus strains. YES medium has already been used as a suitable medium for mycotoxin production (3, 7, 13), and the addition of that cyd only would increase the fluorescence due to the presence of aflatoxins (Fig. 1).
FIG. 1.
Colonies of aflatoxigenic NRRL 2999 (left) and nonaflatoxigenic NRRL 6538 (right) strains observed under UV light. Strains were cultivated in YES medium supplemented with Mβ-cyd and photographed on the third day of incubation at 28°C.
Effect of incubation temperature.
The effect of temperature on the fluorescent response was assayed with all aflatoxigenic and nonaflatoxigenic strains grown on the basal media supplemented with 0.3% Mβ-cyd. At 28 and 30°C, no significant differences in the subjective intensity of fluorescence were observed, while at 25°C the fluorescent responses were weak. However, as the sporulation and size of the colonies were greatest at 30°C, we decided to use 28°C as the incubation temperature.
Time of incubation.
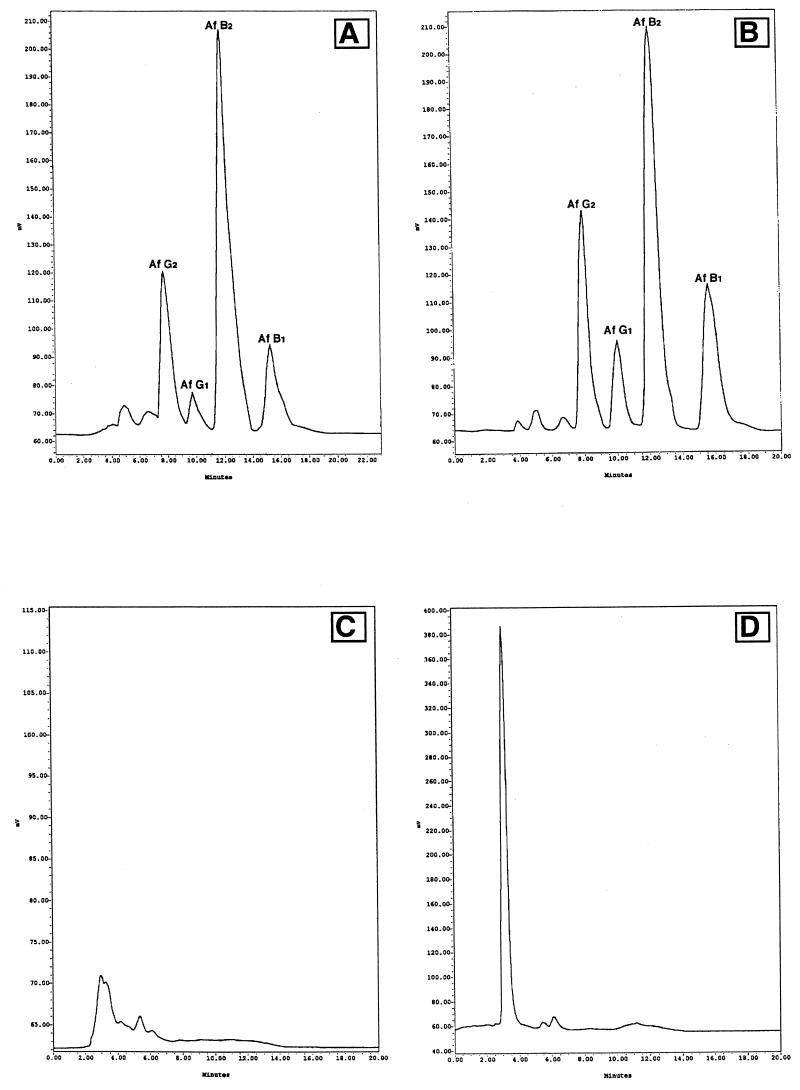
Using SAB and YES agar supplemented with 0.3% Mβ-cyd at 28°C, we looked for the minimum incubation time necessary to detect a clear fluorescent signal of aflatoxigenic strains. Each day for 10 days, a petri dish of both media assayed (with and without cyd) was examined under UV radiation (365 nm), and the chloroform extract of this agar was analyzed by HPLC. For all aflatoxigenic strains tested, the response was clearly conclusive when fluorescence was visualized after 3 days of incubation (only 2 days for the stronger aflatoxin-producing strains NRRL 2999 and NRRL 3145). Aflatoxins were detected in all chloroform extracts from strains showing fluorescence at the third day by an HPLC method with a detection limit of lower than 5 ppb for each aflatoxin. Strain NRRL 3174, an A. ochraceus ochratoxin A-producing strain, did not show fluorescence until the fifth day of incubation, and the aflatoxin HPLC analyses for this strain at the third and fifth days (data not shown) were negative. Like this mycotoxin, other substances produced by Aspergillus strains, i.e., deoxyhydroxy-aspergillic acid (19), flavocol (19), and asperopterin A or B (10), can exhibit blue fluorescence under UV light, but according to our experiments, only the aflatoxigenic strains give fluorescence at the third day of incubation in SAB and YES agar supplemented with Mβ-cyd (Fig. 2). Therefore, we can conclude that the presence of the fluorescence surrounding colonies in both media proposed by us is indicative of aflatoxin production ability. However, a chromatographic determination is recommended to confirm the presence of aflatoxin. The intensity of fluorescence increased with time; the maximum intensity was observed at 3 to 4 days, and after additional incubation (day 5 or 6), cultures became difficult to evaluate because mycelial growth almost reached the margin of the petri dish and so the agar surrounding colonies in which fluorescence must be observed was very reduced. In other media currently in use for the screening of aflatoxin-producing strains (4–6, 9, 11, 15), from 2 to 10 days are necessary to reach conclusive results. The use of a methylated β-cyclodextrin derivative such as Mβ-cyd as an additive for SAB and YES media allows the utilization of a general culture medium for fungal total counts in foods and feeds (SAB) to test the aflatoxigenic ability of Aspergillus strains without the need to use more or less complex media that are exclusive for such purposes. As a basal medium, it is possible to use one that is suitable for the production of mycotoxins in general (YES) and which also allows aflatoxin detection.
FIG. 2.
HPLC fluorescence detection chromatograms of chloroform extracts from strains NRRL 2999 (A and B), NRRL 3174 (C), and NRRL 6538 (D) obtained on the third day of incubation at 28°C. Strains were cultivated in SAB supplemented with Mβ-cyd (A) or in YES medium supplemented with Mβ-cyd (B to D).
ACKNOWLEDGMENTS
This study was supported by the Spanish Government Office for Education and Science as part of research project CICYT-Ali-96/1163. Judith Jaimez Ordaz is supported by a scholarship from CONACYT, Mexico. The method described above belongs to a licensing patent (P9900776).
We thank Encarnación González for technical collaboration.
REFERENCES
- 1.Adye J, Mateles R I. Incorporation of labelled compounds into aflatoxins. Biochim Biophys Acta. 1964;86:418–420. doi: 10.1016/0304-4165(64)90077-7. [DOI] [PubMed] [Google Scholar]
- 2.Cepeda A, Franco C M, Fente C A, Vázquez B I, Rodríguez J L, Prognon P, Mahuzier G. Post-column excitation of aflatoxins using cyclodextrins in liquid chromatography for food analysis. J Chromatogr A. 1996;721:69–74. doi: 10.1016/0021-9673(95)00566-8. [DOI] [PubMed] [Google Scholar]
- 3.Davis N D, Diener U L, Eldridge D W. Production of aflatoxins B1 and G1 by Aspergillus flavus in a semisynthetic medium. Appl Microbiol. 1966;14:378–380. doi: 10.1128/am.14.3.378-380.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis N D, Iyer S K, Diener U L. Improved method of screening for aflatoxin with a coconut agar medium. Appl Environ Microbiol. 1987;53:1593–1595. doi: 10.1128/aem.53.7.1593-1595.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Vogel P, Van Rhee R, Koelensmid W. A rapid screening test for aflatoxin-synthesizing Aspergilli of the flavus-orizae group. J Appl Bacteriol. 1965;28:213–220. doi: 10.1111/j.1365-2672.1965.tb02145.x. [DOI] [PubMed] [Google Scholar]
- 6.Dyer S K, McCammon S. Detection of toxigenic isolates of Aspergillus flavus and related species on coconut cream agar. J Appl Bacteriol. 1994;76:75–78. doi: 10.1111/j.1365-2672.1994.tb04418.x. [DOI] [PubMed] [Google Scholar]
- 7.El-Banna A A, Pitt J I, Leistner L. Production of mycotoxins by Penicillium species. Syst Appl Microbiol. 1987;10:42–46. [Google Scholar]
- 7a.Fente, C. A., J. Jaimez Ordaz, B. I. Vazquez, C. M. Franco, and A. Cepeda. April 1999. Patent P9900776.
- 8.Franco C M, Fente C A, Vázquez B I, Cepeda A, Mahuzier G, Prognon P. Interaction between cyclodextrins and aflatoxins Q1, M1 and P1: fluorescence and chromatographic studies. J Chromatogr A. 1998;815:21–29. doi: 10.1016/s0021-9673(98)00509-3. [DOI] [PubMed] [Google Scholar]
- 9.Hara S, Fennell D L, Hesseltine C W. Aflatoxin producing strains of Aspergillus flavus detected by fluorescence of agar medium under ultraviolet light. Appl Microbiol. 1974;27:1118–1123. doi: 10.1128/am.27.6.1118-1123.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaneko Y, Sanada M. Studies on the fluorescent substances produced by Aspergillus fungi. VII. Purification and isolation of asperopterin B and chemical properties of asperopterin B and A. J Ferment Technol. 1969;47:8–19. [Google Scholar]
- 11.Lemke P A, Davis N D, Iyer S K, Creech G W. Direct visual detection of aflatoxin synthesis by minicolonies of Aspergillus species. Appl Environ Microbiol. 1989;55:1808–1810. doi: 10.1128/aem.55.7.1808-1810.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lemke P A, Davis N D, Iyer S K, Creech G W, Diener U L. Fluorimetric analysis of iodinated aflatoxin in minicultures of Aspergillus parasiticus. J Ind Microbiol. 1988;3:119–125. [Google Scholar]
- 13.Lin M T, Dianese J C. A coconut-agar medium for rapid detection of aflatoxin production by Aspergillus spp. Phytopathology. 1976;66:1466–1469. [Google Scholar]
- 14.Sozsef S. Cyclodextrin technology. Dordrecht, The Netherlands: De Kluver; 1988. [Google Scholar]
- 15.Torrey G S, Marth E H. Silica gel medium to detect molds that produce aflatoxin. Appl Environ Microbiol. 1976;32:376–380. doi: 10.1128/aem.32.3.376-380.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vázquez B I, Fente C A, Franco C M, Cepeda A, Mahuzier G, Prognon P. Preliminary study on fluorimetry detection of aflatoxins Q1, P1 and B1 using heptakis-di-o-methyl-β-cyclodextrin as post-column HPLC reagent. Anal Commun. 1999;36:5–7. [Google Scholar]
- 17.Vázquez M L, Cepeda A, Prognon P, Mahuzier G, Blais J. Cyclodextrins as modifiers of the luminescence characteristics of aflatoxins. Anal Chim Acta. 1991;255:343–350. [Google Scholar]
- 18.Vázquez-Belda B, Fente-Sampayo C A, Quinto-Fernández E, Franco-Abuin C, Rodríguez-Otero J L, Cepeda-Sáez A. Incidence of toxigenic molds in farm-level cheesemaking units from Arzúa (La Coruña, Spain) Food Sci Technol Int. 1995;1:91–95. [Google Scholar]
- 19.Yokotsuka T, Sasaki M, Kikuchi I, Asao Y, Nobuhara A. Studies on the compounds produced by moulds. 1. Fluorescent compounds produced by Japanese industrial moulds. Nippon Nogei Kagaku Zasshi. 1967;41:32–38. [Google Scholar]