Summary
Background
Vedolizumab registration trials were the first to include elderly patients with moderate‐to‐severe ulcerative colitis (UC) or Crohn’s disease (CD), but few real‐life data have been reported in this population.
Aims
We investigated the effectiveness and safety of vedolizumab in matched cohorts of elderly and nonelderly UC and CD patients.
Methods
The Long‐term Italian Vedolizumab Effectiveness (LIVE) study is a retrospective‐prospective study including UC and CD patients who started vedolizumab from April 2016 to June 2017. Elderly patients (≥65 years) were matched clinically 1:2 to nonelderly patients (18–64 years); the 2 groups were followed until drug discontinuation or June 2019.
Results
The study included 198 elderly (108 UC, 90 CD) and 396 matched nonelderly patients (205 UC, 191 CD). Nonelderly UC patients had a significantly higher persistence on vedolizumab compared to elderly patients (67.6% vs. 51.4%, p = 0.02). No significant difference in effectiveness was observed between elderly and nonelderly CD patients (59.4% vs. 52.4%, p = 0.32). Age ≥65 years was associated with lower persistence in UC; for CD, previous exposure to anti‐TNF‐α agents, Charlson comorbidity index >2 and moderate‐to‐severe clinical activity at baseline were associated with lower persistence. There were recorded 130 adverse events, with comparable rates between the two groups. A Charlson comorbidity index >2 was associated with an increased risk of adverse events.
Conclusion
Vedolizumab can be considered a safe option in elderly IBD patients. Its effectiveness in elderly UC patients may be reduced, while no age‐dependent effect on effectiveness was observed in CD.
Keywords: biologics (IBD), Crohn’s disease, immunosuppression, ulcerative colitis

1. INTRODUCTION
Elderly people (age ≥65 years old) 1 represent a significant percentage of patients with inflammatory bowel disease (IBD), accounting for up to 30% of all cases. 2 Elderly IBD patients may be distinguished according to the age when they received the diagnosis (≥65 years vs. <65 years), and elderly‐onset IBD has been shown to have unique features and specific challenges. 3 , 4 Multiple reports showed that elderly IBD patients have a risk of IBD‐related surgery comparable 5 , 6 to or even greater 7 , 8 than younger patients. This might be partially explained by the underuse of immunosuppressive and biological drugs (mainly anti‐tumour necrosis factor [TNF] α), owing to safety concern. 6 , 7 , 9
Thiopurines are commonly avoided in the elderly, due to the increased risk of severe leukopenia or medullary aplasia, opportunistic infections, lymphoma and non‐melanoma skin cancer. 10 , 11 , 12 Caution is also recommended with the use of anti‐TNF‐α drugs, especially when combined with immunomodulators or prednisone, due to an increased risk of opportunistic and severe infections and mortality. 13 , 14 , 15 However, the stratification of risk with immunosuppressive therapy should not solely be based on patients' chronological age; it should also include the assessment of comorbidities and the more comprehensive notion of “frailty”, which is defined as the “state in which the ability of older people to cope with everyday or acute stressors is compromised”. 16 Recently, a large cohort study of IBD patients found that frailty in the 2 years before treatment with thiopurines or anti‐TNF‐α agents predicted opportunistic infections during the treatment, even after adjusting for age, comorbidities and concomitant medications. 17
Patients with moderate‐to‐severely active Crohn’s disease (CD) or ulcerative colitis (UC) who have failed or are intolerant to conventional therapy or anti‐TNF‐α agents are eligible for vedolizumab, which has been approved by both the US Food and Drug Administration 18 and the European Medicines Agency. 19 Vedolizumab is a fully humanised, monoclonal IgG1k antibody that binds α4β7 integrin expressed on leukocytes and selectively blocks their trafficking to the gut mucosa. Approval was based on results of the GEMINI programme, which were the first clinical trials with biologics that included IBD patients older than 65‐years‐old, up to 80 years of age; however, elderly patients accounted for only 4% and 2% of the enrolled UC and CD populations, respectively. 20 , 21 A post hoc analysis of both trials found no significant differences in terms of efficacy and safety when patients were stratified by age into 3 groups. 22 On the basis of this experience, although limited, and considering the drug’s gut‐selective mechanism of action (supposedly associated with a lower risk of infection), many physicians decide in favour of vedolizumab as the first‐line biological therapy for the real‐life management of elderly IBD patients. 23
To date, data on effectiveness and safety of vedolizumab for the treatment of elderly IBD patients are scarce. 24 , 25 , 26 Most of these studies had small sample sizes and did not include matched comparison groups. Therefore, the aim of our study was to compare the effectiveness and safety of vedolizumab between elderly IBD patients (i.e., starting vedolizumab when 65 years old or over) and a matched group of nonelderly IBD patients.
2. PATIENTS AND METHODS
2.1. Study design
The Long‐term Italian Vedolizumab Effectiveness (LIVE) study was an observational, retrospective‐prospective study conducted at 47 Italian IBD centres affiliated with the Italian Group for the Study of Inflammatory Bowel Disease (IG‐IBD). The study protocol was approved on 4 June 2018 by the Ethics Committee of the coordinating centre (Fondazione Policlinico Universitario A. Gemelli IRCCS, Università Cattolica del Sacro Cuore, Rome, Italy). Patients included in the study provided written informed consent for their clinical data to be used for research purposes.
The study considered consecutive adults with UC or CD diagnosed according to guidelines of the European Crohn’s and Colitis Organisation (ECCO) 27 , 28 who started vedolizumab therapy from April 2016 to June 2017 and had a baseline endoscopy examination within 3 months before vedolizumab initiation. Clinical data from vedolizumab initiation to inclusion in the study were retrospectively extracted from the medical records. After enrolment, ended in December 2018, all patients were prospectively followed‐up until drug discontinuation or June 2019.
Each patient ≥65 years old at vedolizumab starting was included in the elderly cohort and matched with two nonelderly patients (18–64 years old). Younger patients were matched to the elderly patients for sex, type of disease (UC or CD), site and duration of disease (range ± 1 year). Matching patients for previous anti‐TNF‐α exposure was also attempted, but, unfortunately, the model did not perfectly fit for this variable.
2.2. Treatment and clinical observation
Patients received vedolizumab as standard induction and maintenance treatment (300 mg at weeks 0, 2 and 6, and then every 8 weeks [Q8W] thereafter). Some patients received an additional infusion at week 10, according to clinicians' judgement; during maintenance, patients could have the interval between infusions shortened to every 4 weeks (Q4W).
The following baseline characteristics were recorded at enrolment: age, sex, weight, current smoking habit (yes or no), duration of disease, disease type (UC or CD), disease phenotype according to the Montreal Classification, 29 , 30 history of CD‐related surgery, previous and concomitant therapies and extra‐intestinal manifestations. Data on comorbidities were used to calculate the Charlson comorbidity index (CCI), 31 excluding age. Disease activity was assessed using the Partial Mayo Score (PMS) 30 for UC and the Harvey–Bradshaw Index (HBI) 30 for CD. Endoscopic activity was assessed using the endoscopic Mayo score (MS) 30 for UC and the Simple Endoscopic Score for Crohn’s Disease (SES‐CD). 30 Accordingly, disease and endoscopic activity were expressed as quiescent, mild, moderate or severe. 30 No baseline missing data were allowed.
Follow‐up clinical assessments were performed at week 14 and at months 6, 12, 18 and 24. At each visit, disease activity, levels of serum C‐reactive protein (CRP), vedolizumab optimization (from Q8W to Q4W) and new courses of steroids were recorded. Endoscopic activity was also recorded, although the timing of endoscopy during follow‐up was up to each physician’s judgement. Patients’ observation was carried out for 24 months, or until treatment discontinuation or loss to follow‐up.
2.3. Primary and secondary outcome measures
The primary outcomes were vedolizumab persistence and safety in the elderly group compared to the nonelderly group. Persistence was defined as maintenance of vedolizumab therapy, due to sustained clinical benefit, throughout the follow‐up. For each patient, one or two reasons for discontinuation could be indicated. Reasons for discontinuation were categorised as: primary failure, that is, withdrawal within week 14 due to lack of effectiveness; secondary failure, that is withdrawal after week 14 despite an initial benefit; adverse events; and others. All adverse events that occurred during the study were recorded. They were categorised as serious adverse events in case of death, hospitalisation or need for emergency medical care or surgery to prevent permanent disability.
Secondary outcomes were assessed for CD and UC patients separately. These outcomes included: (1) clinical responses at 14 weeks and 6 months; (2) clinical remission at each time point, (3) steroid‐free clinical remission (SFCR) at each time point, (4) biochemical remission at each time point, and (5) endoscopic remission (for patients with follow‐up endoscopic data). Clinical response was defined as a reduction of at least 3 points of HBI, for CD and at least 2 points of PMS, for UC. Clinical remission was defined as HBI ≤4 or PMS ≤2 for CD and UC patients, respectively, and SFCR as clinical remission without concomitant steroid use for at least 3 months. Biochemical remission was defined as the normalisation of CRP (≤5 mg/L) combined with SFCR. Endoscopic remission was classified as an SES‐CD ≤2 or an endoscopic MS ≤1, for CD and UC, respectively.
2.4. Statistical analyses
Absolute numbers and percentages were used for qualitative variables. For quantitative variables, Shapiro–Wilk test for normality was performed, and mean (with standard deviation [SD]) or median (with range) were used, as appropriate.
Baseline characteristics were analysed with descriptive statistics and compared between the two groups using the chi‐square test (dichotomous variables) or t‐test (continuous variables). Vedolizumab persistency was analysed using Kaplan–Meier survival curves, which were compared with the log‐rank test. The proportions of patients in clinical remission, SFCR, biochemical remission and endoscopic remission at each time point were compared using Pearson’s chi‐squared test.
Receiver operating characteristic (ROC) curve analysis was used to establish a CCI cut‐off that predicted the outcomes, choosing the threshold corresponding to the minimum distance between the ROC curve and the point with a specificity and a sensitivity of 1.
Non‐responder imputation was applied to patients lost to follow‐up. Intention‐to‐treat analysis was performed for clinical remission, SFCR and biochemical remission. For endoscopic remission, due to the low number of available endoscopies, per‐protocol analysis was performed.
Univariate and Cox multivariate logistic regression was used to identify baseline predictors of vedolizumab persistence, adverse events and 12‐ and 24‐month SFCR in UC and CD patients separately. Confounding factors were selected according to the literature. 10 , 11 , 13 , 14 , 20 , 21 , 22 , 24 , 26 , 32 Variables with p ≤ 0.250 at univariate analysis were included in the multivariate analyses. Odds ratios (OR) were calculated with 95% confidence intervals (CI). A p < 0.05 indicated statistical significance. Statistical analyses were performed using SPSS 24.0 for Windows.
3. RESULTS
Between April 2016 and June 1, 2017,111 patients began vedolizumab treatment at the participating centres: 198 patients ≥65 years old (elderly group) were matched to 396 others <65 years old (nonelderly group). The characteristics of the two groups are summarised in Table 1 . Besides the difference in age, the groups were similar for most characteristics, reflecting the fact that they were matched for sex and main disease features. The prevalence of perianal disease (p = 0.018), and of previous exposures to immunosuppressants (p < 0.001) and anti‐TNF‐α agents (p < 0.001) was higher among nonelderly patients. Conversely, elderly patients more frequently had a history of cancer (15.7% vs. 9.8%, p = 0.038).
TABLE 1.
Baseline characteristics of IBD patients, by study group
| Characteristics | Elderly | Nonelderly | p value |
|---|---|---|---|
| Age, years, median (range) | 70.3 (65.2–90.0) | 46.8 (19.0–64.9) | <0.001 |
| Female sex, n (%) | 67 (33.8) | 117 (29.5) | ns |
| Weight, kg, mean (SD) | 67.6 (13.1) | 69.7 (13.6) | ns |
| Current smoker, n (%) | 63 (31.8) | 134 (33.8) | ns |
| Disease duration, years, median (range) | 8.3 (0.2–48.3) | 10.5 (0–59.7) | ns |
| Perianal disease, n (%) | 14 (7.1) | 54 (13.6) | 0.018 |
| Extra‐intestinal manifestations, n (%) | 22 (11.1) | 91 (22.9) | ns |
| History of cancer, n (%) | 31 (15.7) | 39 (9.8) | 0.038 |
| Previous immunosuppressive therapy, n (%) | 93 (46.9) | 257 (64.9) | <0.001 |
| Previous exposure to anti‐TNF‐α agents, n (%) | 87 (43.9) | 255 (64.4) | <0.001 |
| Concomitant therapies, n (%) | |||
| 5ASA | 87 (43.9) | 152 (38.4) | ns |
| 5ASA + IMM | 3 (1.5) | 18 (4.5) | ns |
| IMM | 5 (2.5) | 20 (5.1) | ns |
| Steroids | 102 (51.5) | 172 (43.4) | ns |
| CRP (mg/dl), mean (SD) | 13.4 (17.9) | 10.9 (21.0) | ns |
| CCI, mean (SD) | 3.31 (1.37) | 3.29 (1.39) | ns |
| Comorbidities, n (%) | 100 (50.5) | 131 (33.1) | <0.001 |
| CD | 90 (45.5) | 191 (48.2) | ns |
| Age, years, median (range) | 70.4 (65.3–85.9) | 45.1 (19.3–64.7) | <0.001 |
| Female sex, n (%) | 39 (43.3) | 50 (26.2) | 0.004 |
| Weight, kg, mean (SD) | 63.0 (12.8) | 67.6 (13.8) | ns |
| Current smoker, n (%) | 32 (35.6) | 81 (42.2) | ns |
| Disease duration, years, median (range) | 9.2 (0.3–48.3) | 11.3 (0.0–59.7) | ns |
| Localization | |||
| L1 | 39 (43.3) | 91 (47.6) | ns |
| L2 | 9 (10.0) | 26 (13.6) | ns |
| L3 | 40 (44.4) | 71 (37.2) | ns |
| L4 | 2 (2.2) | 3 (1.6) | ns |
| Behaviour | |||
| B1 | 26 (28.9) | 56 (29.3) | ns |
| B2 | 51 (56.7) | 94 (49.2) | ns |
| B3 | 13 (14.4) | 41 (21.5) | ns |
| Disease activity (HBI), n (%) | |||
| <5 | 12 (13.3) | 21 (11.0) | ns |
| 5–7 | 24 (26.7) | 47 (24.6) | ns |
| 8–16 | 52 (57.8) | 115 (60.2) | ns |
| >16 | 2 (2.2) | 8 (4.2) | ns |
| Endoscopic activity (SES‐CD), n (%) | |||
| 0–2 | 3 (3.3) | 8 (4.2) | ns |
| 3–6 | 13 (14.4) | 31 (16.2) | ns |
| 7–15 | 57 (63.3) | 108 (56.5) | ns |
| >15 | 17 (18.9) | 44 (23.0) | ns |
| Perianal disease, n (%) | 13 (14.4) | 46 (24.1) | ns |
| Extra‐intestinal manifestations, n (%) | 14 (15.6) | 52 (27.2) | ns |
| History of cancer, n (%) | 15 (16.7) | 27 (14.1) | ns |
| Previous immunosuppressive therapy, n (%) | 44 (48.9) | 134 (70.2) | 0.001 |
| Previous exposure to anti‐TNF‐α agents, n (%) | 43 (47.8) | 137 (71.7) | <0.001 |
| Concomitant therapies, n (%) | |||
| 5ASA | 21 (23.3) | 37 (19.4) | ns |
| 5ASA + IMM | 0 (0.0) | 3 (1.6) | ns |
| IMM | 2 (2.2) | 12 (6.3) | ns |
| Steroids | 69 (36.1) | 43 (47.8) | ns |
| Previous CD surgery, n (%) | 50 (25.3) | 118 (29.8) | ns |
| CRP (mg/dl), mean (SD) | 12.8 (16.9) | 12.1 (16.6) | ns |
| CCI, mean (SD) | 4.0 (0.99) | 2.4 (1.35) | ns |
| Comorbidities, n (%) | 45 (50.0) | 67 (35.1) | 0.017 |
| UC | 108 (54.5) | 205 (51.8) | ns |
| Age, years, median (range) | 70.2 (65.2–90.0) | 48.0 (19.0–64.9) | <0.001 |
| Female sex, n (%) | 28 (25.9) | 67 (32.7) | ns |
| Weight, kg, mean (SD) | 71.0 (12.4) | 71.9 (13.2) | ns |
| Current smoker, n (%) | 31 (28.7) | 53 (25.9) | ns |
| Disease duration, years, median (range) | 7.6 (0.2–40.5) | 9.4 (0.1–40.7) | ns |
| Extension | |||
| E1 | 3 (2.8) | 12 (5.9) | ns |
| E2 | 50 (46.3) | 103 (50.2) | ns |
| E3 | 55 (50.9) | 90 (43.9) | ns |
| Disease activity (PMS), n (%) | |||
| 0–2 | 5 (4.6) | 5 (2.4) | ns |
| 2–4 | 19 (17.6) | 44 (21.5) | ns |
| 5–7 | 58 (53.7) | 119 (58.0) | ns |
| >7 | 26 (24.1) | 37 (18.0) | ns |
| Endoscopic activity (endoscopic MS), n (%) | |||
| 0 | 2 (1.9) | 2 (1.0) | ns |
| 1 | 5 (4.6) | 15 (7.3) | ns |
| 2 | 46 (42.6) | 105 (51.2) | ns |
| 3 | 55 (50.9) | 83 (40.5) | ns |
| Perianal disease, n (%) | 1 (0.9) | 8 (3.9) | ns |
| Extra‐intestinal manifestations, n (%) | 8 (7.4) | 39 (19.0) | ns |
| History of cancer, n (%) | 16 (14.8) | 12 (5.9) | 0.008 |
| Previous immunosuppressive therapy, n (%) | 49 (45.4) | 123 (60.0) | 0.013 |
| Previous exposure to anti‐TNF‐α agents, n (%) | 44 (40.7) | 118 (57.6) | 0.005 |
| Concomitant therapies, n (%) | |||
| 5ASA | 66 (61.1) | 115 (56.1) | ns |
| 5ASA + IMM | 3 (2.8) | 15 (7.3) | ns |
| IMM | 3 (2.8) | 8 (3.9) | ns |
| Steroids | 59 (54.6) | 103 (50.2) | ns |
| CRP (mg/dl), mean (SD) | 13.5 (18.0) | 11.0 (21.1) | ns |
| CCI, mean (SD) | 3.88 (1.13) | 3.34 (1.35) | ns |
| Comorbidities, n (%) | 55 (50.9) | 64 (31.2) | 0.001 |
Abbreviations: ASA, acetylsalicylic acid; CCI, Charlson comorbidity index; CD, Crohn’s disease; CRP, C‐reactive protein; HBI, Harvey–Bradshaw Index; IMM, Immunosuppressants; MS, Mayo score; ns, not significant; PMS, partial Mayo score; SES‐CD; Simple Endoscopic Score for Crohn’s Disease; TNF, tumour necrosis factor; UC, ulcerative colitis. Bold values indicates p value 0.53.
Mean CCI values (excluding age) were 3.31 and 3.29 in the elderly versus nonelderly group, respectively. ROC curve analysis identified a cut‐off of ≤2 to discriminate between elderly and nonelderly patients (area under the curve, 0.593; 95% CI, 0.542–0.644). Overall, 231 patients (38.8%) had at least one comorbidity and 78 (13.1%) had two or more, with a higher incidence among elderly patients (50.5% vs. 33.1%, p < 0.0001). The most common comorbidities were hypertension (64 patients, 27.7%), a history of cardiovascular disease (42 patients, 18.1%) and concomitant immune‐mediated diseases (26 patients, 11.2%).
In terms of treatment schedule, 84 of the 594 patients (14.1%) required the adjunctive infusion at week 10 (27 [13.6%] elderly patients and 57 [14.4%] nonelderly patients, p > 0.05). Furthermore, 188 patients (31.6%) received dose escalation to Q4W (62 [31.3%] elderly patients and 126 [31.8%] nonelderly patients, p > 0.05).
3.1. Cumulative vedolizumab persistence
Overall, 248 patients (41.8%; 95 elderly and 153 nonelderly) discontinued vedolizumab after a median time of 44.5 weeks (range, 1–117 weeks). Treatment ineffectiveness (on intestinal, extraintestinal and/or perianal disease) led to vedolizumab withdrawal in 198 cases (83.4%): 37 patients (18.6%) stopped the treatment within the first 14 weeks (primary failure), while the remaining 161 (64.9%) stopped after 14 weeks (secondary failure). Among all non‐responders, 11 patients (4.4%) withdrew for lack of effectiveness on concomitant arthritis (4 of them also had active intestinal disease), while 7 patients (2.8%) withdrew for lack of effectiveness on perianal disease (2 of them also had active intestinal disease); the remaining non‐responders (180, 72.6%) experience only lack of effectiveness on intestinal disease. The rate of withdrawal among elderly patients (47.9%) was significantly higher than among nonelderly patients (38.6%; p = 0.029). Cumulative vedolizumab persistence was analysed in UC and CD patients separately (Figure 1). For UC, nonelderly patients confirmed a significantly higher rate of cumulative persistence on vedolizumab therapy than elderly patients (67.6% vs. 51.4%, log‐rank test, p = 0.02) (Figure 1A). On the contrary, no significant differences emerged for CD patients between the two groups (59.4% vs. 52.4%, log‐rank test, p = 0.32) (Figure 1B). Table 2 summarises the causes of withdrawal in elderly vs nonelderly patients: for each patient, one or two reasons for discontinuation could be specified, leading to a total of 258 causes of withdrawal for 248 patients who suspended vedolizumab. Of note, a significantly higher rate of suspension due to adverse events was observed in elderly patients (18.2% in elderly vs 8.9% in nonelderly patients, p = 0.03); when stratified by disease, a significant difference was observed only in CD patients (20.8% in elderly vs 8.4% in nonelderly patients, p = 0.04).
FIGURE 1.
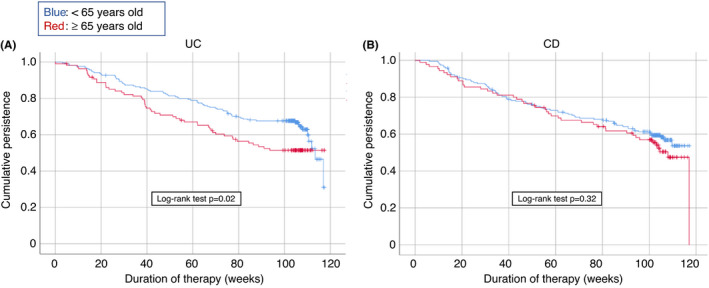
Kaplan–Meier survival curves for the persistency (total number of discontinued patients and number of patients lost to follow‐up) of vedolizumab therapy in elderly versus nonelderly patients with inflammatory bowel disease. (A) Patients with ulcerative colitis. (B) Patients with Crohn’s disease
TABLE 2.
Reasons for vedolizumab discontinuation
| Reason for discontinuation | Elderly | Nonelderly | p value |
|---|---|---|---|
| Ineffectiveness on intestinal disease, n (%) | 71 (71.7) | 118 (75.2) | ns |
| Adverse events, n (%) | 18 (18.2) | 14 (8.8) | 0.026 |
| Ineffectiveness on EIMs, n (%) | 3 (3.0) | 8 (5.0) | ns |
| Ineffectiveness on perianal disease, n (%) | 2 (2.0) | 5 (3.2) | ns |
| Remission, n (%) | 1 (1.0) | 0 (0.0) | ns |
| Other, n (%) | 4 (4.0) | 14 (8.8) | ns |
| CD | |||
| Ineffectiveness on intestinal disease, n (%) | 30 (62.5) | 56 (67.5) | ns |
| Adverse events, n (%) | 10 (20.8) | 7 (8.4) | 0.026 |
| Ineffectiveness on EIMs, n (%) | 3 (6.3) | 6 (7.2) | ns |
| Ineffectiveness on perianal disease, n (%) | 2 (4.2) | 5 (6.0) | ns |
| Remission, n (%) | 1 (2.1) | 0 (0.0) | ns |
| Other, n (%) | 2 (4.2) | 9 (10.8) | ns |
| UC | |||
| Ineffectiveness on intestinal disease, n (%) | 41 (80.5) | 62 (81.6) | ns |
| Adverse events, n (%) | 8 (15.7) | 7 (9.2) | ns |
| Ineffectiveness on EIMs, n (%) | 0 (0.0) | 2 (2.6) | ns |
| Ineffectiveness on perianal disease, n (%) | 0 (0.0) | 0 (0.0) | ns |
| Remission, n (%) | 0 (0.0) | 0 (0.0) | ns |
| Other, n (%) | 2 (3.9) | 5 (6.6) | ns |
Note: For 10 patients, two reasons for discontinuation were specified: 4 elderly CD (2 ineffectiveness on intestinal disease + ineffectiveness on EIMs, 1 ineffectiveness on intestinal disease + adverse event, 1 ineffectiveness on intestinal disease + ineffectiveness on perianal disease), 5 nonelderly CD (1 ineffectiveness on intestinal disease + ineffectiveness on EIMs, 1 ineffectiveness on intestinal disease + adverse event, 1 ineffectiveness on intestinal disease + ineffectiveness on perianal disease, 1 ineffectiveness on EIMs + adverse event, 1 adverse event + other), 1 nonelderly UC (ineffectiveness on intestinal disease + ineffectiveness on EIMs).
Age was the only independent factor associated with persistence in UC (OR = 1.43; 95% CI, 1.00–2‐05, p = 0.047). In CD, multivariate analysis identified being naive to anti‐TNF‐α agents (OR = 1.92; 95% CI, 1.24–2.96, p = 0.003), concomitant steroid therapy (OR = 1.61; 95% CI, 1.12–2.33 p = 0.011), and quiescent‐mild clinical activity (OR = 1.77; 95% CI, 1.18–2.66, p = 0.006) as independent predictors of persistence on vedolizumab therapy (Table 3).
TABLE 3.
Predictors of persistence with vedolizumab at baseline, by disease type
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Ulcerative colitis | ||||
| Age (<65 vs. ≥65 years) | 1.55 (0.96–2.48) | 0.045 | 1.43 (1.00–2.05) | 0.047 |
| Sex (male vs. female) | 0.67 (0.40–1.10) | 0.074 | 0.71 (0.47–1.06) | 0.102 |
| Naive to anti‐TNF‐α agents | 1.22 (0.77–1.92) | 0.224 | ‐ | |
| Concomitant steroids (no vs. yes) | 1.29 (0.82–2.03) | 0.161 | 0.83 (0.58–1.19) | 0.321 |
| CCI ≤2 | 1.30 (0.35–4.85) | 0.478 | ‐ | |
| Disease duration <10 years | 0.93 (0.59–1.46) | 0.422 | ‐ | |
| Clinical activity (quiescent‐mild vs. moderate–severe) | 1.39 (0.80–2.41) | 0.145 | 1.19 (0.76–1.88) | 0.432 |
| Endoscopic activity (quiescent‐mild–moderate vs. severe) | 1.34 (0.85–2.11) | 0.125 | 1.19 (0.82–1.71) | 0.348 |
| Disease extent (E1‐E2 vs. E3) | 1.03 (0.65–1.62) | 0.488 | ‐ | |
| Crohn’s disease | ||||
| Age (<65 vs. ≥65 years) | 1.39 (0.84–2.29) | 0.204 | 1.15 (0.77–1.73) | 0.492 |
| Sex (male vs. female) | 1.25 (0.76–2.07) | 0.385 | 1.30 (0.87–1.94) | 0.196 |
| Naive to anti‐TNF‐α agents | 1.76 (1.07–2.92) | 0.026 | 1.92 (1.24–2.96) | 0.003 |
| Concomitant steroids (no vs. yes) | 1.66 (1.02–2.67) | 0.040 | 1.61 (1.12–2.33) | 0.011 |
| CCI ≤2 | 1.88 (0.92–3.85) | 0.080 | 1.63 (0.98–2.68) | 0.059 |
| Disease duration <10 years | 0.71 (0.44–1.14) | 0.156 | 0.75 (0.52–1.08) | 0.118 |
| Clinical activity (quiescent‐mild vs. moderate–severe) | 2.18 (1.31–3.61) | 0.002 | 1.77 (1.18–2.66) | 0.006 |
| Endoscopic activity (quiescent‐mild–moderate vs. severe) | 0.88 (0.50–1.57) | 0.665 | ‐ | ‐ |
| Disease extent (E1‐E2 vs. E3) | 0.74 (0.36–1.54) | 0.424 | ‐ | ‐ |
Abbreviations: CCI, Charlson comorbidity index; CI, confidence interval; OR, odds ratio; TNF, tumour necrosis factor.
During follow‐up, surgical procedures related to disease activity were required in 26 elderly patients and in 38 nonelderly patients (13.2% vs 9.6%, p > 0.05). Surgical procedures were, however, more frequent among patients with CD than UC [38 of 281 (13.5%) vs. 26 of 313 (8.3%), p = 0.028].
3.2. Safety
Overall, 130 adverse events were reported by 107 patients, leading to drug discontinuation in 32 cases (29.9%). The incidence rates of adverse events were comparable between the two groups (0.10 per patient‐year in the nonelderly group vs. 0.13 in the elderly group, p > 0.05 s). One elderly patient (a 72‐year‐old man) died after the first infusion of vedolizumab because of a worsening of congestive heart failure. Among the adverse events recorded (Table 4), the most frequent were infections (50, 38.5%). Sixteen patients (12.3%) received a diagnosis of cancer or dysplasia during follow‐up: six patients were diagnosed with gastrointestinal cancer (two colorectal, two colon polypoid dysplasia, one small bowel and one non‐polypoid colonic dysplasia), four with urogenital cancer (three prostate and one seminoma), three with skin (two melanoma and one Bowen’s disease) and one each with lung cancer, cerebellar neurinoma and recurrence of tongue dysplasia. It should be noted that elderly patients (both CD and UC) of our cohort presented with an increased risk of receiving a diagnosis of cancer during vedolizumab therapy, compared to nonelderly ones (OR = 4.62, 95% CI 1.56–12.13, p = 0.002). Adverse events leading to vedolizumab discontinuation are included in Table S1.
TABLE 4.
Adverse events reported during vedolizumab treatment
| Adverse event | Whole cohort | Elderly cohort | Nonelderly cohort | OR (95% CI), p | |||
|---|---|---|---|---|---|---|---|
| Occurrence, n (%) | Patients, n | Elderly cohort – Occurrence, n (%) | Elderly cohort – Patients, n | Nonelderly cohort – Occurrence, n (%) | Nonelderly cohort – Patients, n | ||
| Infections, n (%) | 50 (38.5) | 41 | 16 (32.7) | 15 | 34 (42.0) | 26 | 1.21 (0.64–2.3), 0.568 |
| Upper respiratory tract | 17 | 14 | 5 | 4 | 12 | 10 | |
| Lower respiratory tract | 15 | 11 | 5 | 5 | 10 | 6 | |
| Gastrointestinal tract | 7 | 5 | 2 | 2 | 5 | 3 | |
| Skin and mucosa infections | 2 | 2 | 0 | 0 | 2 | 2 | |
| Urinary tract | 2 | 2 | 1 | 1 | 1 | 1 | |
| Others | 7 | 7 | 3 | 3 | 4 | 4 | |
| Cancer or dysplasia, n (%) | 16 (12.3) | 16 | 11 (22.4) | 11 | 5 (6.2) | 5 | 4.62 (1.56–12.13), 0.002 |
| Arthralgia or arthritis, n (%) | 21 (16.2) | 21 | 5 (10.2) | 5 | 16 (19.8) | 16 | 0.66 (0.26–1.80), 0.417 |
| Skin reaction, n (%) | 8 (6.15) | 8 | 2 (4.1) | 2 | 6 (7.4) | 6 | 0.70 (0.14–2.91), 0.663 |
| Cholestatic hepatitis, n (%) | 6 (4.6) | 6 | 4 (8.2) | 4 | 2 (2.5) | 2 | 4.2 (0.97–22.22), 0.074 |
| Infusion reactions, n (%) | 3 (2.3) | 3 | 0 (0.0) | 0 | 3 (3.7) | 3 | 0.00 (0.00–2.45), 0.233 |
| Other, n (%) | 26 (20.0) | 21 | 11 (22.4) | 8 | 15 (18.5) | 13 | 1.12 (0.49–2.61), 0.799 |
| CD | |||||||
| Infections, n (%) | 31 (36.5) | 26 | 11 (32.4) | 10 | 20 (39.2) | 16 | 1.49 (0.65–3.3), 0.353 |
| Upper respiratory tract | 9 | 7 | 3 | 2 | 6 | 5 | |
| Lower respiratory tract | 9 | 8 | 4 | 4 | 5 | 4 | |
| Gastrointestinal tract | 5 | 3 | 1 | 1 | 4 | 2 | |
| Skin and mucosa infections | 2 | 2 | 0 | 0 | 2 | 2 | |
| Urinary tract | 1 | 1 | 0 | 0 | 1 | 1 | |
| Others | 5 | 5 | 3 | 3 | 2 | 2 | |
| Cancer or dysplasia, n (%) | 7 (8.2) | 7 | 5 (14.7) | 5 | 2 (3.9) | 2 | 5.95 (1.22–30.29), 0.018 |
| Arthralgia or arthritis, n (%) | 17 (20.0) | 17 | 5 (14.7) | 5 | 12 (23.5) | 12 | 0.99 (0.37–2.93), 0.989 |
| Skin reaction, n (%) | 5 (5.9) | 5 | 2 (5.9) | 2 | 3 (5.9) | 3 | 1.59 (0.28–7.91), 0.615 |
| Cholestatic hepatitis, n (%) | 3 (3.5) | 3 | 1 (2.9) | 1 | 2 (3.9) | 2 | 1.19 (0.08–10.37), 0.888 |
| Infusion reactions, n (%) | 2 (2.4) | 2 | 0 (0.0) | 0 | 2 (3.9) | 2 |
0.00 (0.00–5.22), 0.360 |
| Other, n (%) | 20 (23.5) | 15 | 10 (29.4) | 7 | 10 (19.6) | 8 | 2.08 (0.78–6.10), 0.166 |
| UC | |||||||
| Infections, n (%) | 19 (42.2) | 15 | 5 (33.3) | 5 | 14 (46.7) | 10 | 0.96 (0.36–2.65), 0.938 |
| Upper respiratory tract | 8 | 7 | 2 | 2 | 6 | 5 | |
| Lower respiratory tract | 6 | 3 | 1 | 1 | 5 | 2 | |
| Gastrointestinal tract | 2 | 2 | 1 | 1 | 1 | 1 | |
| Skin and mucosa infections | 0 | 0 | 0 | 0 | 0 | 0 | |
| Urinary tract | 1 | 1 | 1 | 1 | 0 | 0 | |
| Others | 2 | 2 | 0 | 0 | 2 | 2 | |
| Cancer or dysplasia, n (%) | 9 (20.0) | 9 | 6 (40.0) | 6 | 3 (10.0) | 3 | 3.83 (0.99–14.16), 0.046 |
| Arthralgia or arthritis, n (%) | 4 (8.9) | 4 | 0 (0.0) | 0 | 4 (13.3) | 4 | 0.00 (0.00–1.97), 0.150 |
| Skin reaction, n (%) | 3 (6.7) | 3 | 0 (0.0) | 0 | 3 (10.0) | 3 | 0.00 (0.00–2.24), 0.212 |
| Cholestatic hepatitis, n (%) | 3 (6.7) | 3 | 3 (20.0) | 3 | 0 (0.0) | 0 | ∞ (1.63‐∞), 0.018 a |
| Infusion reactions, n (%) | 1 (2.2) | 1 | 0 (0.0) | 0 | 1 (3.3) | 1 | 0.00 (0.00 to 17.33), 0.470 |
| Other, n (%) | 6 (13.3) | 6 | 1 (6.7) | 1 | 5 (16.7) | 5 | 0.38 (0.03–2.85), 0.367 |
Notes: Table summarising AEs. For each cohort considered (whole cohort, elderly and nonelderly), it is reported the number of AEs occurred (left column, expressed as percentage of the total number of AEs in the reference cohort) and the number of patients experiencing an AE (right column). Odds ratio is expressed as the odds of elderly patients experiencing an AE during observation compared to nonelderly patients; statistical significance is set at p < 0.05.
Abbreviations: CD, Crohn’s disease; CI, Confidence Interval; OR, Odds Ratio; UC, ulcerative colitis.
Infinity value due to 0 as denominator in the OR, unlikely to be clinically meaningful.
On multivariate analysis (Table S2), Crohn’s disease was associated with a significantly higher risk of developing any adverse event (OR = 2.34; 95% CI, 1.51–3.62, p < 0.0001). Conversely, a CCI ≤2 was protective from developing any adverse event (OR = 0.44, 95% CI, 0.26–0.77, p = 0.004).
3.3. Secondary outcomes – UC patients
Clinical response was observed in 70 elderly (64.8%) and 148 nonelderly (72.2%) patients at week 14, and in 71 (65.7%) and 141 (68.8%) patients at 6 months, respectively (p > 0.05 for both comparisons between groups). Nonelderly patients had a numerically higher rate of clinical remission at every time point, reaching a maximum of 48.3% at 12 months, with significant differences at 12, 18 and 24 months (Figure 2A). Similarly, they had higher rates of SFCR, with significant differences at 12 and 24 months (Figure 2B), and of biochemical remission, with significant differences almost at every timepoint (Figure 2C). Follow‐up endoscopy was performed at least once in 231 patients (80 elderly patients and 151 nonelderly). The median times from vedolizumab initiation to the last endoscopic evaluation were 15 months (range 3–27) for elderly and 18 months (range, 2–27) for nonelderly patients. Between 6 and 12 months, endoscopic remission was recorded in 6 elderly patients (16.7%) and 12 nonelderly patients (23.5%) (p = 0.4; Figure 2D). During the subsequent year, 11 (28.2%) and 38 (42.2%) patients, respectively, achieved endoscopic remission (p = 0.1).
FIGURE 2.
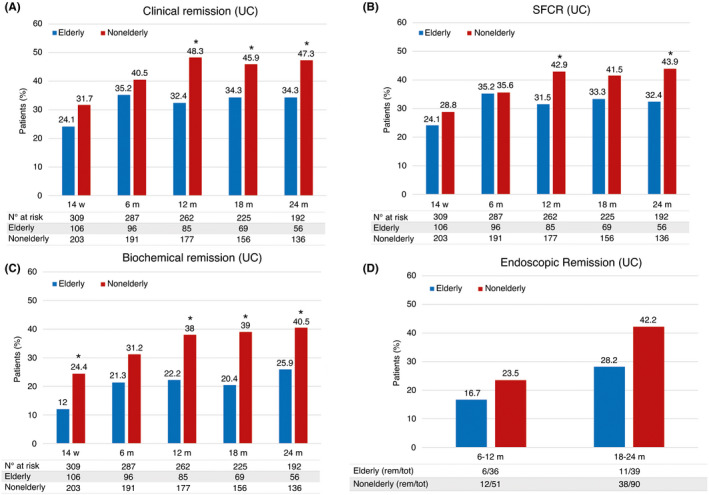
Secondary outcomes in UC patients, by study group and time of assessment. (A) Clinical remission. (B) Steroid‐free clinical remission. (C) Biochemical remission. (D) Endoscopic remission. *p < 0.05
On multivariate analysis, age < 65 years (OR = 1.72; 95% CI, 1.03–2.89, p = 0.038), being naive to anti‐TNF‐α agents (OR = 1.63; 95% CI, 0.99–2.67, p = 0.05) and no concomitant steroid therapy (OR = 1.86; 95% CI, 1.16–2.98, p = 0.01) were associated with SFCR at 24 months (Table S3).
3.4. Secondary outcomes – CD patients
Clinical response was recorded in 47 elderly (52.2%) and 99 nonelderly (51.8%) patients at week 14, and in 48 (53.3%) and 101 (52.9%) patients at 6 months, respectively (p > 0.05 for both comparisons between groups). No significant differences in clinical remission and SFCR were observed between the two groups. (Figure 3A,B). The rates of biochemical remission were lower for elderly patients at 18 months (20.0% vs. 30.4%, p = 0.068) and 24 months (21.1% vs. 30.9%, p = 0.088) without reaching significance (Figure 3C). Follow‐up endoscopy was performed at least once in 158 patients (49 elderly and 109 nonelderly). The median times from vedolizumab initiation to the last endoscopic evaluation were 18 months (range, 3–26) for elderly and 17.5 months (range, 3–27) for nonelderly patients. Between 6 and 12 months, endoscopic remission was recorded in 6 elderly (31.6%) and 5 nonelderly (13.5%) patients (p = 0.1, Figure 3D). During the second year, 7 (24.1%) and 29 (32.8%) patients, respectively, achieved endoscopic remission (p = 0.16).
FIGURE 3.
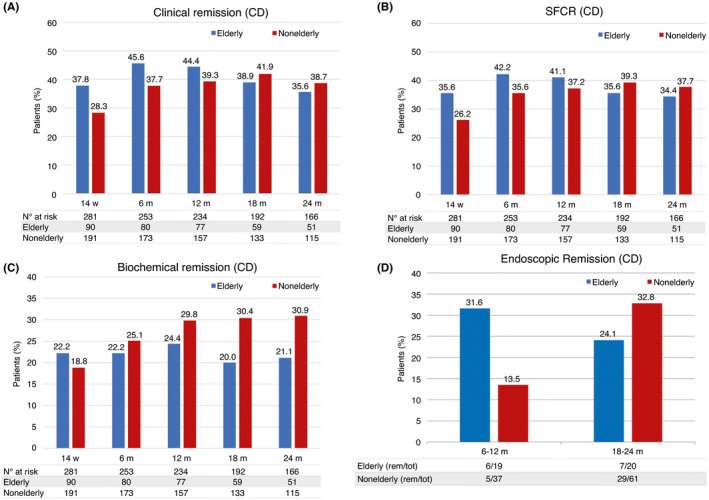
Secondary outcomes in CD patients, by study group and time of assessment. (A) Clinical remission. (B) Steroid‐free clinical remission. (C) Biochemical remission. (D) Endoscopic remission. *p < 0.05
On multivariate analysis, absence of previous exposure to anti‐TNF‐α agents (OR = 2.85; 95% CI, 1.61–5.05, p < 0.001), concomitant steroid therapy (OR = 1.80; 95% CI, 1.04–3.10, p = 0.034) and mild clinical activity at baseline (OR = 2.60; 95% CI, 1.52–4.42, p < 0.001) were associated with a higher likelihood of SFCR (Table S4).
4. DISCUSSION
The management of elderly patients with IBD is a growing challenge due to the progressive ageing of the population 33 and the high frequency of comorbidities in older people. Physicians must be proficient in managing several aspects of ageing, including frailty and multiple comorbidities (e.g. cardiovascular diseases and cancer). The safety of IBD treatments is the biggest concern, especially when immunosuppressants therapies are used to achieve and maintain disease control. Vedolizumab is the first biological therapy tested, in phase 3 trials, in IBD patients older than 65 years, even though they accounted for, at most, 4% of the study cohort. 20 , 21 Few real‐life data support the effectiveness of vedolizumab therapy in the elderly, and there are conflicting results about safety. 24 , 25 , 26 , 32
Our study is the largest, to our knowledge, to have assessed the effectiveness and safety of vedolizumab in elderly compared to matched nonelderly patients. Our elderly group included mainly patients who had an apparently milder course, as suggested by less frequent prior use of immunosuppressant drugs or anti‐TNF‐α agents (compared to the nonelderly group of patients). Nevertheless, elderly patients had a significantly higher rate of vedolizumab discontinuation. This difference was attributable to UC patients, especially after the first year. Importantly, the causes of withdrawal were equally distributed between elderly and nonelderly UC patients, thus excluding that decreased persistence in the population of elderly UC patients was attributable to a reduced safety of vedolizumab in this population. Furthermore, nonelderly UC patients outperformed elderly UC patients in terms of SFCR and biochemical remission throughout follow‐up. No significant difference was found in the rates of endoscopic remission between the two groups, which may be due to the small number of endoscopic assessments available. As expected, age >65 years was associated with a lower persistence with vedolizumab and a lower likelihood of achieving SFCR at 24 months in UC. In CD patients, age did not seem to affect vedolizumab effectiveness, as no significant differences in terms of persistence and SFCR were observed between the elderly and nonelderly groups. Our findings in UC patients partially contradict the observations of a recent large retrospective study from the Veterans Affairs Healthcare System (VAHS), where Khan et al. compared the effectiveness of vedolizumab between two not‐matched groups of patients (i.e., <60 years old vs. ≥60 years old at the time of starting vedolizumab), showing similar rates of steroid‐free clinical remission at 6 and 12 months, 1‐year IBD hospitalisation and surgery. 34 Such differences might be attributable to the absence of matching, the different age threshold for defining elderly patients or the choice to restrict the effectiveness analysis only to patients who were assuming steroids at baseline.
CD patients who were naive to anti‐TNF‐α agents were more likely to benefit from vedolizumab therapy, as suggested by multivariate analysis. These findings are in line with results from the US VICTORY Consortium, which found higher rates of clinical and endoscopic effectiveness and lower progression to surgery in CD patients without prior exposure to these drugs. 35 Our data suggest that vedolizumab responders have a less aggressive phenotype of IBD. For example, in our study, vedolizumab responders were less likely to take steroids and generally had milder clinical activity at baseline. In our UC patients, previous exposure to anti‐TNF‐α agents correlated only with a lower rate of SFCR at 24 months. Conversely, no use of steroids at baseline was associated with achieving SFCR at 12 and 24 months. Differences in responses to therapy between nonelderly and elderly IBD patients have already been reported for anti‐TNF‐α agents, with a more favourable profile for younger patients 36 , 37 , 38 in terms of persistence with therapy and short‐term clinical benefit. However, we cannot exclude that these outcomes have been influenced by the higher propensity of physicians to stop biologics earlier in elderly patients in case of initial ineffectiveness or partial response and to reserve these drugs only for more severe cases.
Underlying molecular mechanisms could also influence the effectiveness of vedolizumab. Immune‐ageing is characterised by a tendency towards the unopposed activation of the innate immune system and a reduction in wound healing capabilities, 39 and these features might be—at least, partially—responsible for the observed differences in terms of effectiveness between elderly and nonelderly patients. However, they cannot explain why such differences were only observed in UC patients. Changes in cytokine production and T helper cell differentiation have been extensively documented in ageing people 40 , 41 , 42 , 43 , 44 : while there is no consensus about the specifics of these changes, we might speculate that this immunological phenotype renders elderly UC patients less susceptible to vedolizumab’s mechanism of action.
Regarding safety, no significant difference in the rate of adverse events was observed between elderly and nonelderly patients, supporting the notion that vedolizumab can be considered safe also in the elderly population. Indeed, data from Veterans Affairs Healthcare System (VAHS) seemingly confirm, in a large cohort of elderly IBD patients, a better safety profile of vedolizumab over chronic steroids and comparable to mesalamine in terms of risk of infections and malignancies. 45 It should be noted that, in our study, cancer diagnosis was more frequent in elderly versus nonelderly patients: however, elderly age increases the risk of receiving an oncologic diagnosis per se, 46 and the design of this present research does not allow us to infer whether vedolizumab further contributes to that risk. At multivariate analysis, CD and a CCI score >2 were the only factors associated with an increased risk of adverse events after starting vedolizumab treatment, regardless of the patients' age. This finding confirms the importance of considering comorbidities as well as age when assessing each patient’s risk from immunosuppressive therapies. This finding also confirms the work by Asscher et al., who reported that CCI, but not age, was independently associated with infections and hospitalizations, but not with non‐infectious adverse events. 32 Finally, it is worth mentioning that, while no difference in overall persistence was observed between elderly and nonelderly CD patients, elderly CD patients in our study more frequently discontinued vedolizumab due to adverse events than did nonelderly patients, which might suggest that the tolerance to vedolizumab could be reduced in elderly CD patients.
Our study does have limitations, primarily a potential selection bias in the elderly group that could limit the generalisation of the results. It is possible that the accessibility of biological therapies for elderly patients, regardless of comorbidities, might have been influenced by the expertise of the centre where they were treated. As this was a multicentric study involving 47 centres, it was not possible to guarantee that each patient received standardised management – especially the elderly ones. Differences in the protocols for prescribing steroids and optimising therapy could have influenced the effectiveness outcomes. The inability of matching on anti‐TNF‐α exposure represents another limitation of this study since it has been suggested that previous anti‐TNF‐α treatment impacts the effectiveness of vedolizumab. 47 , 48 , 49 In our cohort, anti‐TNF‐α exposure correlated with lower vedolizumab persistence in CD but, notably, not in UC, where the only independent factor found, on multivariate analysis, to correlate with reduced persistence was indeed age; nevertheless, being naïve to TNF‐α inhibitors was associated with better rates of SFCR in both UC and CD. Collectively, this body of evidence suggests that age affects vedolizumab effectiveness in UC independently from previous anti‐TNF‐α treatment; however, we cannot exclude that unbalanced proportions of patients exposed to anti‐TNF‐α might have contributed to determining some of the differences we observed in elderly versus nonelderly patients.
While CCI, being a relatively simple score, is commonly used in longitudinal studies to adjust for comorbidities, 50 it should be acknowledged that it does not necessarily correlate with performance status and frailty, 51 and that other, more sophisticated scores outperform CCI in predicting mortality.. 52 Furthermore, differences in indication and timing to elective surgery among centres cannot be excluded, depending on the specific accessibility and waitlist of surgical facilities of each centre. Moreover, the results might have been partially influenced by the significant difference in terms of anti‐TNF‐α exposure between the two groups. We did not report data on faecal calprotectin, as the high costs of this test for patients in our country limit its use. Finally, the limited number of endoscopic assessments and the variability in their timing precluded a more reliable analysis of endoscopic outcomes.
In conclusion, these data show that vedolizumab can be considered a valid option for elderly patients. Notably, a higher number of comorbidities (CCI >2), but not age, correlated with an increased risk of adverse events. Vedolizumab effectiveness in elderly UC patients (in terms of persistence, SFCR and biochemical remission) seems to be lower than in nonelderly UC patients, while no such difference was observed in CD. In multivariate analysis, previous exposure to anti‐TNF‐α agents consistently associated with worse outcomes in CD, while it did not have a significant impact in UC (besides SFCR at 24 months).
AUTHORSHIP
Guarantor of the article: Alessandro Armuzzi
Author contributions: Daniela Pugliese, Giuseppe Privitera and Alessandro Armuzzi are responsible for the planning of the study, drafting of the article, statistical analysis and interpretation of data. All other authors performed data collections and critical revision of article for important intellectual content. All authors approved the final version of the manuscript including authorship list.
Supporting information
Tables S1‐S4
ACKNOWLEDGMENTS
Declaration of personal interest: Ennio Sarli provided statistical consulting. Valerie Matarese provided scientific editing.
Daniela Pugliese received speaker fees and/or advisory board from AbbVie, MSD, Takeda and Janssen, Pfizer. Giuseppe Privitera received consultancy fees from Alphasigma and Janssen. Alessandro Armuzzi: consulting and/or advisory board fees from AbbVie, Allergan, Amgen, Arena, Biogen, Bristol‐Myers Squibb, Celgene, Celltrion, Eli‐Lilly, Ferring, Galapagos, Gilead, Janssen, MSD, Mylan, Pfizer, Roche, Samsung Bioepis, Sandoz, Takeda; lecture and/or speaker bureau fees from AbbVie, Amgen, Arena, Biogen, Bristol‐Myers Squibb, Eli‐Lilly, Ferring, Galapagos, Gilead, Janssen, MSD, Mitsubishi‐Tanabe, Novartis, Pfizer, Roche, Sandoz, Samsung Bioepis, Takeda; and research grants from MSD, Pfizer, Takeda and Biogen. The remaining authors declare no competing interests.
Appendix 1.
1.1. IG‐IBD LIVE Study Group
Davide Giuseppe Ribaldone, MD; 24 Giuseppe Biscaglia, MD; 25 Andrea Buda, MD, PhD;26 Giammarco Mocci, MD; 27 Angelo Viscido, MD, PhD;28 Maria Carla Di Paolo, MD; 29 Sara Onali, MD, PhD; 30 Stefano Rodino’, MD; 31 Marina Coletta, MD; 32 Mariabeatrice Principi, MD, PhD; 33 Agnese Miranda, MD; 34 Arnaldo Amato, MD; 35 Cristina Bezzio, MD, PhD; 36 Carlo Petruzzellis, MD; 37 Silvia Mazzuoli, MD; 38 Stefano Festa, MD; 39 Alessandro Sartini, MD; 40 Davide Checchin, MD; 41 Libera Fanigliulo, MD; 42 Sara Gallina, MD; 43 Monica Cesarini, MD, PhD; 44 Giorgia Bodini, MD, PhD; 45 Davide Stradella, MD; 46 Rocco Spagnuolo, MD; 47 Luisa Guidi, MD, PhD; 1,2 Edoardo Savarino, MD, PhD; 11 Barbara Scrivo, MD;10 Pietro Soru, MD;9 Francesco Costa, MD; 8 Walter Fries, MD, PhD; 7 Franco Scaldaferri, MD, PhD; 1 Mariangela Allocca, MD, PhD; Lucienne Pellegrini, MD, PhD;5 Alessandro Massari, MD; 4 Ambrogio Orlando, MD; 3.
24 Department of Medical Sciences, University of Turin, Turin, Italy; 25 Division of Gastroenterology, IRCCS Ospedale Casa Sollievo della Sofferenza, San Giovanni Rotondo, Puglia, Italy; 26 Department of Gastrointestinal Oncological Surgery, Gastroenterology and Endoscopy Unit, S. Maria del Prato Hospital, Feltre, Italy; 27 SC Gastroenterologia Ospedale Brotzu, Cagliari, Italy; 28 Gastroenterology Unit, Department of Life, Health and Environmental Sciences, University of L’Aquila, L’Aquila, Italy; 29 Department of Gastroenterology and Digestive Endoscopy, S. Giovanni Addolorata Hospital, Rome, Italy; 30 Gastroenterology Unit, University Hospital, AOU Cagliari, Department of Medical Science and Public Health, University of Cagliari, Cagliari, Italy 31 Division of Gastroenterology, “Ciaccio‐Pugliese” Hospital, Catanzaro; 32 Department of Hepatology and Clinical Gastroenterology, ASST Santi Paolo e Carlo‐Ospedale San Polo Universitario Milano, Milan, Italy.33 University of Bari, Gastroenterology, Bari, Italy; 34 Gastroenterology and Endoscopy Unit, University of Campania “L. Vanvitelli” Naples, Italy; 35 Ospedale Valduce, Gastroenterology, Como, Italy; 36 Gastroenterology Unit, Rho Hospital, Rho (MI), ASST Rhodense, Garbagnate Milanese, Lombardia, Italy; 37 Department of Medicine, Gastroenterology and Endoscopy, Fondazione Poliambulanza, Brescia, Italy; 38 Section of Gastroenterology & Artificial Nutrition, Hospital San Nicola Pellegrino, Bari, Italy; 39 S. Filippo Neri Hospital, IBD Unit, Rome, Lazio, Italy; 40 Gastroenterology and Digestive Endoscopy Unit, Forlì‐Cesena, AUSL della Romagna, Italy; 41 U.O.C. Gastroenterologia, Ospedale HUB di Mestre, Venezia, Italy; 42 Gastroenterology Unit, Ospedale Santissima Annunziata, Taranto, Italy; 43 Division of Gastroenterology, “Belcolle” Hospital, Viterbo.; 44Casa di Cura “Madonna della fiducia”, Roma, Italy; 45 Cattedra di Gastroenterologia, Diparti‐mento di Medicina Interna, Universita`di Genova, Genova, Italy; 46 Gastroenterologia, A.O.U. Maggiore della Caritá di Novara, Piemonte, University of Eastern Piedmont Amedeo Avogadro, Italy; 47 Gastroenterology and Digestive Endoscopy Department, University of Catanzaro, Catanzaro, Italy; 48 AOUP, Gastroenterology, Pisa, Italy.
Pugliese D, Privitera G, Crispino F, Mezzina N, Castiglione F, Fiorino G, et al. IG‐IBD LIVE Study Group . Effectiveness and safety of vedolizumab in a matched cohort of elderly and nonelderly patients with inflammatory bowel disease: the IG‐IBD LIVE study. Aliment Pharmacol Ther. 2022;56:95–109. 10.1111/apt.16923
The Handling Editor for this article was Dr Cynthia Seow, and it was accepted for publication after full peer‐review.
Funding informationTakeda gave unconditional financial support to the study.
Contributor Information
Alessandro Armuzzi, Email: alessandro.armuzzi@hunimed.eu.
IG‐IBD LIVE Study Group:
Davide Giuseppe Ribaldone, Giuseppe Biscaglia, Andrea Buda, Giammarco Mocci, Angelo Viscido, Maria Carla Di Paolo, Sara Onali, Stefano Rodino, Marina Coletta, Mariabeatrice Principi, Agnese Miranda, Arnaldo Amato, Cristina Bezzio, Carlo Petruzzellis, Silvia Mazzuoli, Stefano Festa, Alessandro Sartini, Davide Checchin, Libera Fanigliulo, Sara Gallina, Monica Cesarini, Giorgia Bodini, Davide Stradella, Rocco Spagnuolo, Luisa Guidi, Edoardo Savarino, Barbara Scrivo, Pietro Soru, Francesco Costa, Walter Fries, Franco Scaldaferri, Mariangela Allocca, Lucienne Pellegrini, Alessandro Massari, and Ambrogio Orlando
REFERENCES
- 1. World Health Organization . Men, Ageing and Health: Achieving Health Across the Life Span. Geneva: World Health Organization; 2001. [Google Scholar]
- 2. Jeuring SFG, van den Heuvel TRA, Zeegers MP, Hameeteman WH, Romberg‐Camps MJL, Oostenbrug LE, et al. Epidemiology and long‐term outcome of inflammatory bowel disease diagnosed at elderly age—an increasing distinct entity? Inflamm Bowel Dis. 2016;22(6):1425–34. 10.1097/MIB.0000000000000738 [DOI] [PubMed] [Google Scholar]
- 3. Taleban S, Colombel JF, Mohler MJ, Fain MJ. Inflammatory bowel disease and the elderly: a review. J Crohns Colitis. 2015;9(6):507–15. 10.1093/ecco-jcc/jjv059 [DOI] [PubMed] [Google Scholar]
- 4. Rozich JJ, Dulai PS, Fumery M, Sandborn WJ, Singh S. Progression of elderly onset inflammatory bowel diseases: a systematic review and meta‐analysis of population‐based cohort studies. Clin Gastroenterol Hepatol. 2020;18(11):2437–2447.e6. 10.1016/J.CGH.2020.02.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Charpentier C, Salleron J, Savoye G, Fumery M, Merle V, Laberenne JE, et al. Natural history of elderly‐onset inflammatory bowel disease: a population‐based cohort study. Gut. 2014;63(3):423–32. 10.1136/gutjnl-2012-303864 [DOI] [PubMed] [Google Scholar]
- 6. Lakatos PL, David G, Pandur T, Erdelyi Z, Mester G, Balogh M, et al. IBD in the elderly population: results from a population‐based study in Western Hungary, 1977‐2008. J Crohns Colitis. 2011;5(1):5–13. 10.1016/J.CROHNS.2010.08.004 [DOI] [PubMed] [Google Scholar]
- 7. Everhov ÅH, Halfvarson J, Myrelid P, Sachs MC, Nordenvall C, Söderling J, et al. Incidence and treatment of patients diagnosed with inflammatory bowel diseases at 60 years or older in Sweden. Gastroenterology. 2018;154(3):518–528.e15. 10.1053/J.GASTRO.2017.10.034 [DOI] [PubMed] [Google Scholar]
- 8. Ananthakrishnan AN, Shi HY, Tang W, Law CCY, Sung JJY, Chan FKL, et al. Systematic review and meta‐analysis: phenotype and clinical outcomes of older‐onset inflammatory bowel disease. J Crohns Colitis. 2016;10(10):1224–36. 10.1093/ECCO-JCC/JJW054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fries W, Viola A, Manetti N, et al. Disease patterns in late‐onset ulcerative colitis: results from the IG‐IBD “AGED study”. Dig Liver Dis. 2017;49(1):17–23. 10.1016/j.dld.2016.09.006 [DOI] [PubMed] [Google Scholar]
- 10. Kotlyar DS, Lewis JD, Beaugerie L, Tierney A, Brensinger CM, Gisbert JP, et al. Risk of lymphoma in patients with inflammatory bowel disease treated with azathioprine and 6‐mercaptopurine: a meta‐analysis. Clin Gastroenterol Hepatol. 2015;13(5):847–858.e4. 10.1016/j.cgh.2014.05.015 [DOI] [PubMed] [Google Scholar]
- 11. Calafat M, Mañosa M, Cañete F, Ricart E, Iglesias E, Calvo M, et al. Increased risk of thiopurine‐related adverse events in elderly patients with IBD. Aliment Pharmacol Ther. 2019;50(7):780–8. 10.1111/apt.15458 [DOI] [PubMed] [Google Scholar]
- 12. Peyrin‐Biroulet L, Khosrotehrani K, Carrat F, Bouvier AM, Chevaux JB, Simon T, et al. Increased risk for nonmelanoma skin cancers in patients who receive thiopurines for inflammatory bowel disease. Gastroenterology. 2011;141(5):1621–1628.e5. 10.1053/j.gastro.2011.06.050 [DOI] [PubMed] [Google Scholar]
- 13. Lichtenstein GR, Feagan BG, Cohen RD, Salzberg BA, Diamond RH, Price S, et al. Serious infection and mortality in patients with crohn’s disease: more than 5 years of follow‐up in the TREAT registry. Am J Gastroenterol. 2012;107(9):1409–22. 10.1038/ajg.2012.218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cottone M, Kohn A, Daperno M, Armuzzi A, Guidi L, D'Inca R, et al. Advanced age is an independent risk factor for severe infections and mortality in patients given anti‐tumor necrosis factor therapy for inflammatory bowel disease. Clin Gastroenterol Hepatol. 2011;9(1):30–5. 10.1016/j.cgh.2010.09.026 [DOI] [PubMed] [Google Scholar]
- 15. Piovani D, Danese S, Peyrin‐Biroulet L, Nikolopoulos GK, Bonovas S. Systematic review with meta‐analysis: biologics and risk of infection or cancer in elderly patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2020;51(9):820–30. 10.1111/APT.15692 [DOI] [PubMed] [Google Scholar]
- 16. World Health Organization . WHO Clinical Consortium on Healthy Ageing. Report of consortium meeting 1–2 December 2016 in Geneva, Switzerland. Geneva: World Health Organization; 2017. (WHO/FWC/ALC/17.2). [Google Scholar]
- 17. Kochar B, Cai W, Cagan A, Ananthakrishnan AN. Pretreatment frailty is independently associated with increased risk of infections after immunosuppression in patients with inflammatory bowel diseases. Gastroenterology. 2020;158(8):2104–2111.e2. 10.1053/j.gastro.2020.02.032 [DOI] [PubMed] [Google Scholar]
- 18. Vedolizumab ‐ FDA . https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125476s025s030lbl.pdf. Accessed August 26, 2021.
- 19. Entyvio | European Medicines Agency . https://www.ema.europa.eu/en/medicines/human/EPAR/entyvio. Accessed August 26, 2021.
- 20. Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369(8):699–710. 10.1056/NEJMoa1215734 [DOI] [PubMed] [Google Scholar]
- 21. Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369(8):711–21. 10.1056/NEJMoa1215739 [DOI] [PubMed] [Google Scholar]
- 22. Yajnik V, Khan N, Dubinsky M, Axler J, James A, Abhyankar B, et al. Efficacy and safety of vedolizumab in ulcerative colitis and Crohn’s disease patients stratified by age. Adv Ther. 2017;34(2):542–59. 10.1007/s12325-016-0467-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chan W, Kariyawasam VC, Kim S, Pudipeddi AV, Paramsothy S, Shim HH, et al. Gastroenterologists' preference and risk perception on the use of immunomodulators and biological therapies in elderly patients with ulcerative colitis: an international survey. Eur J Gastroenterol Hepatol. 2020;32(8):976–83. 10.1097/MEG.0000000000001768 [DOI] [PubMed] [Google Scholar]
- 24. Ibraheim H, Samaan MA, Srinivasan A, et al. Effectiveness and safety of vedolizumab in inflammatory bowel disease patients aged 60 and over: an observational multicenter UKexperience. Ann Gastroenterol. 2020;33(2):170–7. 10.20524/aog.2020.0447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shashi P, Gopalakrishnan D, Parikh MP, Shen B, Kochhar G. Efficacy and safety of vedolizumab in elderly patients with inflammatory bowel disease: a matched case‐control study. Gastroenterol Rep. 2020;8(4):306–11. 10.1093/gastro/goz041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cohen NA, Plevris N, Kopylov U, Grinman A, Ungar B, Yanai H, et al. Vedolizumab is effective and safe in elderly inflammatory bowel disease patients: a binational, multicenter, retrospective cohort study. United Eur Gastroenterol J. 2020;8(9):1076–85. 10.1177/2050640620951400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Harbord M, Eliakim R, Bettenworth D, Karmiris K, Katsanos K, Kopylov U, et al. Third European evidence‐based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis. 2017;11(7):769–84. 10.1093/ecco-jcc/jjx009 [DOI] [PubMed] [Google Scholar]
- 28. Torres J, Bonovas S, Doherty G, Kucharzik T, Gisbert JP, Raine T, et al. ECCO guidelines on therapeutics in Crohn’s disease: medical treatment. J Crohns Colitis. 2020;14(1):4–22. 10.1093/ecco-jcc/jjz180 [DOI] [PubMed] [Google Scholar]
- 29. Maaser C, Sturm A, Vavricka SR, Kucharzik T, Fiorino G, Annese V, et al. ECCO‐ESGAR guideline for diagnostic assessment in IBD part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019;13(2):144–64. 10.1093/ecco-jcc/jjy113 [DOI] [PubMed] [Google Scholar]
- 30. Sturm A, Maaser C, Calabrese E, Annese V, Fiorino G, Kucharzik T, et al. Ecco‐esgar guideline for diagnostic assessment in ibd part 2: Ibd scores and general principles and technical aspects. J Crohns Colitis. 2019;13(3):273–284E. 10.1093/ecco-jcc/jjy114 [DOI] [PubMed] [Google Scholar]
- 31. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 32. Asscher VER, Biemans VBC, Pierik MJ, Dijkstra G, Löwenberg M, van der Marel S, et al. Comorbidity, not patient age, is associated with impaired safety outcomes in vedolizumab‐ and ustekinumab‐treated patients with inflammatory bowel disease‐a prospective multicentre cohort study. Aliment Pharmacol Ther. 2020;52(8):1366–76. 10.1111/apt.16073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.(No Title). https://www.census.gov/content/dam/Census/library/publications/2016/demo/p95‐16‐1.pdf. Accessed April 23, 2021.
- 34. Khan N, Pernes T, Weiss A, Trivedi C, Patel M, Medvedeva E, et al. Efficacy of vedolizumab in a Nationwide cohort of elderly inflammatory bowel disease patients. Inflamm Bowel Dis. 2021;izab163. 10.1093/ibd/izab163 [DOI] [PubMed] [Google Scholar]
- 35. Dulai PS, Singh S, Jiang X, Peerani F, Narula N, Chaudrey K, et al. The real‐world effectiveness and safety of vedolizumab for moderate‐severe Crohn’s disease: results from the US VICTORY consortium. Am J Gastroenterol. 2016;111(8):1147–55. 10.1038/ajg.2016.236 [DOI] [PubMed] [Google Scholar]
- 36. Lobatón T, Ferrante M, Rutgeerts P, Ballet V, Van Assche G, Vermeire S. Efficacy and safety of anti‐TNF therapy in elderly patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2015;42(4):441–51. 10.1111/apt.13294 [DOI] [PubMed] [Google Scholar]
- 37. Desai A, Zator ZA, De Silva P, et al. Older age is associated with higher rate of discontinuation of anti‐TNF therapy in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19(2):309–15. 10.1002/ibd.23026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. De Jong ME, Smits LJT, Van Ruijven B, et al. Increased discontinuation rates of anti‐TNF therapy in elderly inflammatory bowel disease patients. J Crohns Colitis. 2020;14(7):888–95. 10.1093/ECCO-JCC/JJAA012 [DOI] [PubMed] [Google Scholar]
- 39. Weyand CM, Goronzy JJ. Aging of the immune system: Mechanisms and therapeutic targets. Ann Am Thorac Soc. 2016;13(Supp 5):S422–8. 10.1513/AnnalsATS.201602-095AW [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mansfield AS, Nevala WK, Dronca RS, Leontovich AA, Shuster L, Markovic SN. Normal ageing is associated with an increase in Th2 cells, MCP‐1 (CCL1) and RANTES (CCL5), with differences in sCD40L and PDGF‐AA between sexes. Clin Exp Immunol. 2012;170(2):186–93. 10.1111/j.1365-2249.2012.04644.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sakata‐Kaneko S, Wakatsuki Y, Matsunaga Y, Usui T, Kita T. Altered Th1/Th2 commitment in human CD4+ T cells with ageing. Clin Exp Immunol. 2000;120(2):267–73. 10.1046/j.1365-2249.2000.01224.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li M, Yao D, Zeng X, Kasakovski D, Zhang Y, Chen S, et al. Age related human T cell subset evolution and senescence. Immun Ageing. 2019;16(1):24. 10.1186/s12979-019-0165-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Uciechowski P, Kahmann L, Plümäkers B, Malavolta M, Mocchegiani E, Dedoussis G, et al. TH1 and TH2 cell polarization increases with aging and is modulated by zinc supplementation. Exp Gerontol. 2008;43(5):493–8. 10.1016/j.exger.2007.11.006 [DOI] [PubMed] [Google Scholar]
- 44. Lee JS, Lee W‐W, Kim S‐H, Kang SW, Kang I. Age‐associated alteration in naive and memory Th17 cell response in humans (85.8). J Immunol. 2010;184(1 Supplement):84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Khan N, Pernes T, Weiss A, Trivedi C, Patel M, Xie D, et al. Incidence of infections and malignancy among elderly male patients with IBD exposed to vedolizumab, prednisone, and 5‐ASA medications: a Nationwide retrospective cohort study. Adv Ther. 2021;38(5):2586–98. 10.1007/S12325-021-01713-X [DOI] [PubMed] [Google Scholar]
- 46. White MC, Holman DM, Goodman RA, Richardson LC. Cancer risk among older adults: time for cancer prevention to go silver. Gerontologist. 2019;59(Supplement_1):S1–6. 10.1093/GERONT/GNZ038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sands BE, Sandborn WJ, Van Assche G, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease in patients Naïve to or who have failed tumor necrosis factor antagonist therapy. Inflamm Bowel Dis. 2017;23(1):97–106. 10.1097/MIB.0000000000000979 [DOI] [PubMed] [Google Scholar]
- 48. Sands BE, Feagan BG, Rutgeerts P, Colombel JF, Sandborn WJ, Sy R, et al. Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology. 2014;147(3):618–627.e3. 10.1053/J.GASTRO.2014.05.008 [DOI] [PubMed] [Google Scholar]
- 49. Narula N, Peerani F, Meserve J, Kochhar G, Chaudrey K, Hartke J, et al. Vedolizumab for ulcerative colitis: treatment outcomes from the VICTORY consortium. Am J Gastroenterol. 2018;113(9):1345–54. 10.1038/S41395-018-0162-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Moltó A, Dougados M. Comorbidity indices. Clin Exp Rheumatol. 2014;32:S131–4. [PubMed] [Google Scholar]
- 51. Vitzthum LK, Feng CH, Noticewala S, Hines PJ, Nguyen C, Zakeri K, et al. Comparison of comorbidity and frailty indices in patients with head and neck cancer using an online tool. JCO Clin cancer informatics. 2018;2(2):1–9. 10.1200/CCI.18.00082 [DOI] [PubMed] [Google Scholar]
- 52. Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64(7):749–59. 10.1016/J.JCLINEPI.2010.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1‐S4


