Abstract
Clinically unsuspected venous thromboembolism (VTE) in children is defined as a VTE diagnosed via imaging test performed for surveillance (i.e., with an intent to identify clinically silent VTEs) or incidentally found (most often via imaging performed for evaluation of regional pathology unrelated to VTE) in the absence of any VTE‐associated signs or symptoms. Our understanding of the clinical significance of these events in children is limited by a paucity of data on the epidemiology and outcomes of this complication. There is an urgent need for further research in this area to inform optimal management. Recognizing this knowledge gap, this Task Force has previously published a systematic review of the literature in this topic. We now provide guidance recommendations for standardization of definitions and identify future research needs on clinically unsuspected VTE in children. These recommendations will serve to enhance the quantity and quality of evidence on the topic and facilitate the design and execution of cooperative observational studies, and interventional trials of risk‐stratified management approaches aimed at preventing and optimizing long‐term outcomes of clinically unsuspected VTE in children.
Keywords: asymptomatic venous thromboembolism, pediatric, unsuspected venous thromboembolism
Essentials.
There are limited data on the epidemiology and outcomes of pediatric clinically unsuspected venous thromboembolism (VTE).
This communication provides recommendations for standardized definitions and future research needs.
Clinically unsuspected VTE is defined as a VTE diagnosed via imaging performed for surveillance, or incidentally found, in the absence of any signs/symptoms.
These recommendations will enhance the quantity and quality of evidence in this area.
1. BACKGROUND
Clinically unsuspected venous thromboembolism (VTE) in children is defined as a VTE diagnosed via imaging test performed for VTE surveillance (i.e., with an intent to identify clinically silent VTEs) or incidentally found (most often via imaging performed for evaluation of a regional pathology unrelated to VTE) in the absence of any VTE‐associated signs or symptoms. The classification of clinically unsuspected VTE can be inherently problematic in pediatrics, particularly in young or critically ill children, who may not be able to verbalize symptoms associated with VTE and for whom clinical signs may be subtle. 1 , 2 , 3 Given a generally low index of suspicion for VTE in children than adults, the importance of clinically unsuspected VTE in children is likely underestimated.
To date, there is a paucity of data from high‐quality studies on the epidemiology and outcomes of clinically unsuspected VTE in children, leading to variability in screening, diagnosis, and management practices for this complication. Thus, there is an urgent need for further research in this area to inform optimal management.
Recognizing this knowledge gap, the Pediatric and Neonatal Thrombosis and Hemostasis Subcommittee of the International Society of Thrombosis and Haemostasis (ISTH) Scientific and Standardization Committee (SSC) assembled a Task Force in 2017 whose objectives were to assess current gaps in knowledge, make recommendations for standardization of definitions, and identify future research needs on clinically unsuspected VTE in children. Regarding the first objectives, a systematic review of the literature was recently conducted and published. 2 The key findings are summarized here.
The Working Group preferred the term “clinically unsuspected VTE” over “asymptomatic VTE” because the literature reflects variable and inconsistent use of the terms “asymptomatic VTE” and “incidentally” diagnosed VTE. It also identified two main types of imaging protocols (surveillance vs. incidental) in which VTE events were diagnosed. Surveillance imaging was performed with an intent to identify clinically silent VTEs, whereas incidental detection occurred when imaging was performed for evaluation of regional pathology unrelated to VTE.
>90% of clinically unsuspected VTEs were central venous catheter (CVC)‐related, most of them diagnosed within 2–7 days following CVC placement in surveillance studies.
The pooled frequency of clinically unsuspected VTE in children varied by: (1) study design – randomized control trials 17.8% (95% confidence interval [CI] 10.2–26.9) compared with retrospective studies 13.9% (95% CI 7.1–22.4); (2) clinical setting – surveillance studies 20.1% (95% CI 15–25.8) compared with incidental VTE studies 3.5% (95% CI 0.7–7.8); and (3) presence of a CVC – 20.8% (95% CI 15.2–26.9) in CVC surveillance studies compared with 7.6% (95% CI 1.1–18) in non‐CVC surveillance studies.
Risk factors identified for clinically unsuspected VTE have included increased CVC duration, 3 , 4 , 5 , 6 , 7 younger age, 3 , 8 , 9 premature birth and low birth weight, and a diagnosis of cancer. 3 , 5 , 10 , 11
Outcomes of clinically unsuspected VTE were favorable, with pooled frequencies of 0% for VTE‐related mortality and 0.4% for pulmonary embolism (PE) despite variability in length of follow‐up and imaging modalities used. Although the frequency of signs/symptoms compatible with postthrombotic syndrome was 26% (95% CI 21.5–31), symptoms were typically not associated with functional limitations. Additionally, the occurrence of clinically unsuspected VTE was not associated with the need for CVC replacement, nor with increased duration of mechanical ventilation, intensive care unit stay, or hospital stay. 3 , 4
2. RATIONALE
The key limitations of the aforementioned systematic review stem from the studies themselves and include the following: paucity of high‐quality studies; high degree of heterogeneity among studies and underlying populations; variability in definition of clinically unsuspected VTE; infrequent reporting of outcomes by symptomatic VTE versus asymptomatic VTE subgroups, and, among patients with clinically unsuspected VTE, infrequent reporting of outcomes by anatomic site and CVC‐relatedness. Yet, it is likely that the rates of acute and long‐term complications associated with clinically unsuspected VTE vary by VTE type (CVC‐related vs. non‐CVC), VTE anatomic location (extremity deep venous thrombosis [DVT], PE, cerebral sinus venous thrombosis [CSVT]), patient's age, and underlying comorbidities, indicating the need to adapt specific outcome measures of relevance to each specific subgroup of interest. 12
Accordingly, in our Task Force efforts, we sought to address each of these limitations in our recommendations for standardized definitions and future research.
3. METHODS
The recommendations made in this guidance statement are based on the findings of the previously mentioned systematic review of the literature and expert consensus reached during a series of virtual meetings convened by the members of the Clinically Unsuspected VTE Task Force of the Pediatric and Neonatal Subcommittee of the ISTH SSC. The Task Force comprised all authors listed on this guidance statement and was cochaired by S. Sharathkumar and N.A. Goldenberg.
4. RECOMMENDATIONS
4.1. Recommendation 1
Clinical assessment of clinically unsuspected VTE in children. For all newly diagnosed VTEs by surveillance‐type or episodic‐type imaging, we recommend that clinicians evaluate for signs/symptoms relevant to the respective anatomic site and/or organ system of involvement (see Figure 1 for workflow) within the prior 2 weeks or longer based on chronicity (i.e., chronic headache in patients with CSVT). Examples of signs/symptoms by anatomic site are provided in Table 1. If any signs/symptoms of VTE are observed on this assessment even if imaging had not originally been performed based on those signs or symptoms, then the VTE should be classified as “symptomatic.”
FIGURE 1.
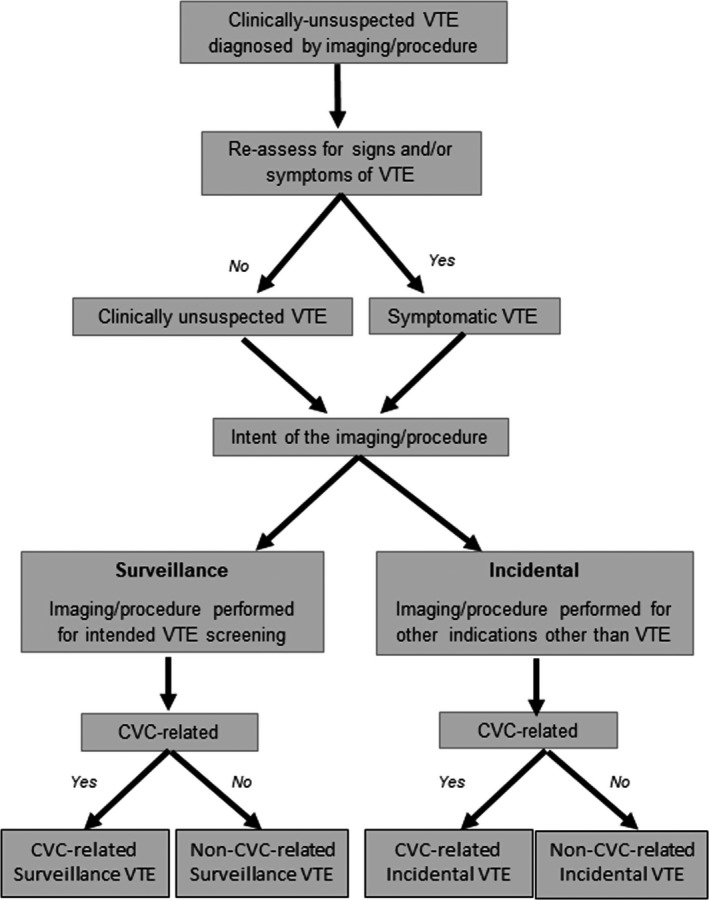
Flow diagram of proposed definition of clinically unsuspected venous thromboembolism in the clinical setting. This is primarily a recommended clinical workflow for the diagnosis of clinically unsuspected VTE. In the research setting, we recommend that investigators evaluate VTE signs and symptoms on a prospective ongoing basis. CVC, central venous catheter; VTE, venous thromboembolism
TABLE 1.
Most common VTE‐associated signs and symptoms in children
| Anatomic location | Clinical presentation |
|---|---|
| Extremity | |
| Upper |
Acute: unilateral extremity pain, swelling, edema, erythema, warmth, chylothorax (subclavian vein) Chronic: signs and symptoms of chronic venous insufficiency including unilateral limb edema, pain or sensation of heaviness, cramping, venous stasis dermatitis, and skin ulceration |
| Lower | |
| Non‐extremity | |
| Cerebral sinus venous system |
Acute: headache, altered mental status, emesis, seizures, papilledema, cranial nerve palsy, intracranial hemorrhage or infarction Chronic: onset of symptoms ≥1 month, chronic recurrent headaches, visual deficits, tinnitus, signs of increased ICP (i.e., elevated pressure on lumbar puncture) |
| Neck | Ipsilateral upper extremity swelling, pain, cervical edema, dilation of superficial collateral venous circulation |
| Pulmonary arteries |
Acute: chest pain, cough, dyspnea, hemoptysis, persistent tachypnea, right‐sided heart failure. Large proximal: hypoxemia, cyanosis, hypotension Chronic: syncopal episodes, progressive dyspnea, exercise intolerance, fatigue, peripheral edema |
| Intra‐abdominal | Renal vein: flank pain, hypertension, hematuria, thrombocytopenia, hydronephrosis, acute kidney injury |
|
Portal vein, acute: abdominal pain, fever, nausea, emesis, diarrhea, ascites, splenomegaly Portal vein, chronic: portal hypertension, esophageal varices, upper gastrointestinal bleed, hepatosplenomegaly, thrombocytopenia Mesenteric vein, acute: positive fecal occult blood, melena, hematochezia, and hematemesis Mesenteric vein, chronic: chronic abdominal pain, malabsorption, portal hypertension | |
| Caval system |
SVC syndrome: swelling, discoloration of head, neck and upper chest, dilation of superficial collateral venous circulation, and headache IVC: nonspecific back/abdominal pain, leg heaviness, pain, swelling, and cramping |
| CVC‐related |
Catheter malfunction (difficulty infusing and/or drawing back), use of tPA Ipsilateral extremity swelling, thrombocytopenia (particularly in premature and critically ill patients) |
This list is not meant to be exhaustive but a comprehensive list of the most commonly VTE‐associated symptoms in children.
Abbreviations: CVC, central venous catheter; ICP, increased intracranial pressure; IVC, inferior vena cava; SVC, superior vena cava; tPA, tissue plasminogen activator.
If, in contrast, no signs/symptoms are observed on assessment at the time of radiologic discovery of the VTE, a classification of “clinically unsuspected” VTE is appropriate. The Task Force recommends that CVC malfunction, defined as the inability to either draw or infuse, or requirement of tissue plasminogen activator at least once for catheter patency, be considered a clinical sign associated with CVC‐related DVT.
4.2. Recommendation 2
Standardization of clinically unsuspected VTE definition, classification, and recommended nomenclature for data reporting in children. We recommend that clinically unsuspected VTE be defined, for both clinical use and for future research work, as a VTE (PE, nonextremity including intracardiac and intra‐abdominal DVT, extremity DVT, and CSVT) radiologically confirmed by imaging performed either as surveillance for risk of VTE, or for non‐VTE‐related clinical issues, in the absence of any VTE‐associated signs and symptoms. A list of the most common VTE‐associated signs and symptoms in pediatrics can be seen in Table 1. We further recommend that clinically unsuspected VTE be categorized by intent for imaging and etiology, with each of the following features as applicable (Figure 1): detected via surveillance imaging with an intent to screen for VTE versus incidental detection because of imaging performed for evaluation of regional pathology unrelated to VTE; and CVC‐related versus non‐CVC‐related. Thus, the suggested nomenclature includes CVC‐related surveillance or incidental VTE and non‐CVC‐related surveillance or incidental VTEs (Figure 1). In addition, we recommend that the following clinical data domains be reported as part of the nomenclature used to classify asymptomatic VTE in future research work: (1) patient‐related elements; (2) treatment‐related elements; (3) thrombus‐related characteristics; and (4) imaging modality‐related characteristics (Table 2).
TABLE 2.
Recommended clinical data domains for the classification of asymptomatic VTE in children
| Clinical domains | |
|---|---|
| Patient‐related elements | Age group, inpatient setting (PICU vs. NICU vs. CVICU vs. wards), underlying medical conditions (i.e., cancer, congenital heart disease, TPN dependence) |
| Treatment‐related elements | Use of anticoagulation or antiplatelet therapy as primary or secondary prophylaxis |
| Thrombus‐related elements | Occlusive vs. nonocclusive, vessel territories affected, CVC‐related vs. non‐CVC‐related |
| Imaging modality‐related elements | Type of imaging modality, type of study (i.e., screening, surveillance) |
Abbreviations: CVC, central venous catheter; CVICU, cardiovascular intensive care unit; NICU, neonatal intensive care unit; PICU, pediatric intensive care unit; TPN, total parenteral nutrition.
4.3. Recommendation 3
Follow‐up of pediatric patients with clinically unsuspected VTE and standardization of outcome definitions. Given the risks of postthrombotic sequelae (including postthrombotic syndrome among those with DVT in the venous systems draining the limbs), we recommend that children with clinically unsuspected VTE be followed by clinicians with expertise in the management of pediatric thrombosis for at least 1 year after diagnosis of the asymptomatic VTE event. We further recommend that outcomes assessed during follow‐up visits, and reported in research studies, adhere to outcome definitions previously recommended by the Pediatric and Neonatal Thrombosis and Hemostasis Subcommittee of the ISTH SSC, 13 and that research studies reporting these VTE outcomes do so separately for clinically unsuspected and symptomatic VTE. Beyond those outcomes previously outlined, 13 additional outcomes of clinically unsuspected VTE should be monitored in accordance with the anatomic location and/or organ system affected by asymptomatic VTE. For example, outcomes in patients with clinically unsuspected CSVT should include neurological (including visual) impairments, whereas outcomes of clinically unsuspected PE should include cardiac and pulmonary structural, and functional abnormalities such as dyspnea and exercise intolerance, and the development of chronic thromboembolic pulmonary hypertension. For DVTs involving various organs like kidneys, heart, or liver, the organ function should be followed in collaboration with respective specialty depending on severity of organ involvement. Validated pediatric general Quality of Life and VTE‐specific Quality of Life measures 14 , 15 are also recommended as outcomes for inclusion in future clinical/translational research studies. 16
4.4. Recommendation 4
Additional priorities for future clinical/translational research. To best characterize the incidence, natural history, and outcomes of clinically unsuspected VTE in children, and reduce heterogeneity within studies, we recommend that specific subpopulations are distinguished within future studies and addressed by devoted studies of those subpopulations, including (but not limited to) premature infants, critically ill children, children with cancer, and neonates and older children with congenital and acquired cardiac disease. Future research efforts should also report outcomes by VTE type (i.e., clinically unsuspected vs. symptomatic), and for patients with clinically unsuspected VTE, by treatment status (i.e., treatment vs. no‐treatment) to allow outcome comparisons between those children with clinically unsuspected VTE who receive therapy and those who do not, and permit a better understanding of the role of anticoagulation therapy in the prevention of adverse long‐term outcomes in this population. 1 Although retrospective studies designed to address some of the previously mentioned knowledge gaps may be useful as an initial step in these subpopulations of interest, prospective studies are preferred to capture high‐quality data using the standardized definitions and classifications recommended here. Generating high‐quality evidence on clinically unsuspected VTE in these pediatric subpopulations of interest will require active research collaboration among pediatric centers on national and international levels. It is hoped that the International Pediatric Thrombosis Network, recently established via the Subcommittee, will serve as a platform for multicenter collaboration and data collection in future prospective observational studies of clinically unsuspected VTE. 17
5. CONCLUSIONS
These recommendations will serve to enhance the quantity and quality of evidence on pediatric clinically unsuspected VTE and facilitate the design and execution of cooperative prospective observational studies that will, in turn, lead to interventional trials of risk‐stratified management approaches aimed at preventing, and optimizing long‐term outcomes of, clinically unsuspected VTE in children. The enhanced understanding of clinically unsuspected VTE gained through such research will also require that these recommendations be periodically updated in the future.
CONFLICT OF INTEREST
M.B. receives consulting fees from Janssen Pharmaceuticals; N. A. G. receives consulting fees from Bayer, Boerhinger Ingelheim, and Anthos for advisory board activities, from Novartis for data and safety monitoring board activities, from Daiichi Sankyo for steering committee activities, from the university‐affiliated Academic Research Organization CPC Clinical Research for data and safety monitoring board activities for clinical trials sponsored by Bristol Myers Squibb and Pfizer, and he also receives research and salary support from the U.S. National Institutes of Health (NIH), via a U01 award; E. V. S. F. receives funding from the American Heart Association, NIH and Grifols for research support, and consulting fees from Boehringer Ingelheim for advisory board activities; A. S., M. R., L. R. B., K. K., and S. J. declare no potential conflicts of interest related to this work.
AUTHOR CONTRIBUTION
M. B., N. A. G., and A. S. contributed to the concept and design, and writing of drafts and final version of the manuscript. K. K., M. R., S. J., L. R. B., and E. V. S. F. contributed to the concept and design, critical revision of the intellectual content, and final approval of the version to be published.
INFORMED CONSENT
No informed consent was required for this manuscript.
Betensky M, Kulkarni K, Rizzi M, et al. Recommendations for standardized definitions, clinical assessment, and future research in pediatric clinically unsuspected venous thromboembolism: Communication from the ISTH SSC subcommittee on pediatric and neonatal thrombosis and hemostasis. J Thromb Haemost. 2022;20:1729–1734. doi: 10.1111/jth.15731
Manuscript Handled by: Joost Meijers
Final decision: Joost Meijers, 11 April 2022
REFERENCES
- 1. Jones S, Monagle P, Newall F. Do asymptomatic clots in children matter? Thromb Res. 2020;189:24‐34. [DOI] [PubMed] [Google Scholar]
- 2. Sharathkumar AA, Biss T, Kulkarni K, et al. Epidemiology and outcomes of clinically unsuspected venous thromboembolism in children: a systematic review. J Thromb Haemost. 2020;18:1100‐1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beck C, Dubois J, Grignon A, Lacroix J, David M. Incidence and risk factors of catheter‐related deep vein thrombosis in a pediatric intensive care unit: a prospective study. J Pediatr. 1998;133:237‐241. [DOI] [PubMed] [Google Scholar]
- 4. Faustino EV, Spinella PC, Li S, et al. Incidence and acute complications of asymptomatic central venous catheter‐related deep venous thrombosis in critically Ill children. J Pediatr. 2013;162:387‐391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim JH, Lee YS, Kim SH, Lee SK, Lim MK, Kim HS. Does umbilical vein catheterization lead to portal venous thrombosis? Prospective US evaluation in 100 neonates. Radiology. 2001;219:645‐650. [DOI] [PubMed] [Google Scholar]
- 6. Menendez JJ, Verdu C, Calderon B, et al. Incidence and risk factors of superficial and deep vein thrombosis associated with peripherally inserted central catheters in children. J Thromb Haemost. 2016;14:2158‐2168. [DOI] [PubMed] [Google Scholar]
- 7. Hanslik A, Thom K, Haumer M, et al. Incidence and diagnosis of thrombosis in children with short‐term central venous lines of the upper venous system. Pediatrics. 2008;122:1284‐1291. [DOI] [PubMed] [Google Scholar]
- 8. Landisch RM, Hanson SJ, Punzalan RC, et al. Efficacy of surveillance ultrasound for venous thromboembolism diagnosis in critically ill children after trauma. J Pediatr Surg. 2018;53:2195‐2201. [DOI] [PubMed] [Google Scholar]
- 9. Anton N, Cox PN, Massicotte MP, et al. Heparin‐bonded central venous catheters do not reduce thrombosis in infants with congenital heart disease: a blinded randomized, controlled trial. Pediatrics. 2009;123:e453‐e458. [DOI] [PubMed] [Google Scholar]
- 10. Haddad H, Lee KS, Higgins A, McMillan D, Price V, El‐Naggar W. Routine surveillance ultrasound for the management of central venous catheters in neonates. J Pediatr. 2014;164:118‐122. [DOI] [PubMed] [Google Scholar]
- 11. Ruud E, Holmstrom H, Hopp E, Wesenberg F. Central line‐associated venous late effects in children without prior history of thrombosis. Acta Paediatr. 1992;2006(95):1060‐1065. [DOI] [PubMed] [Google Scholar]
- 12. Monagle P. Slow progress. How do we shift the paradigm of thinking in pediatric thrombosis and anticoagulation? Thromb Res. 2019;173:186‐190. [DOI] [PubMed] [Google Scholar]
- 13. Mitchell LG, Goldenberg NA, Male C, et al. Definition of clinical efficacy and safety outcomes for clinical trials in deep venous thrombosis and pulmonary embolism in children. J Thromb Haemost. 2011;9(9):1856‐1858. [DOI] [PubMed] [Google Scholar]
- 14. Smith J, Thornhill D, Goldenberg NA, et al. Validation of outcome instruments for pediatric post thrombotic syndrome: Introducing the Peds‐VEINES‐QOL, a new health‐related quality of life instrument. Thromb Haemost. 2021;121(10):1367‐1375. doi: 10.1055/s-0041-1725199 [DOI] [PubMed] [Google Scholar]
- 15. Varni JW, Burwinkle TM, Seid M, Skarr D. The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr. 2003;3(6):329‐341. [DOI] [PubMed] [Google Scholar]
- 16. Bruce AK, Bauman ME, Jones S, Massicotte MP, Monagle P. Recommendations for measuring health‐related quality of life in children on anticoagulation. J Thromb Haemost. 2012;10(12):2596‐2598. [DOI] [PubMed] [Google Scholar]
- 17. van Ommen CH, Albisetti M, Bhatt M, et al. International pediatric thrombosis network to advance pediatric thrombosis research: communication from the ISTH SSC subcommittee on pediatric and neonatal thrombosis and hemostasis. J Thromb Haemost. 2021;19(4):1123‐1129. [DOI] [PMC free article] [PubMed] [Google Scholar]


