Abstract
RNA viromes of nine commonly encountered Ochlerotatus mosquito species collected around Finland in 2015 and 2017 were studied using next-generation sequencing. Mosquito homogenates were sequenced from 91 pools comprising 16–60 morphologically identified adult females of Oc. cantans, Oc. caspius, Oc. communis, Oc. diantaeus, Oc. excrucians, Oc. hexodontus, Oc. intrudens, Oc. pullatus and Oc. punctor/punctodes. In total 514 viral Reverse dependent RNA polymerase (RdRp) sequences of 159 virus species were recovered, belonging to 25 families or equivalent rank, as follows: Aliusviridae, Aspiviridae, Botybirnavirus, Chrysoviridae, Chuviridae, Endornaviridae, Flaviviridae, Iflaviridae, Negevirus, Partitiviridae, Permutotetraviridae, Phasmaviridae, Phenuiviridae, Picornaviridae, Qinviridae, Quenyavirus, Rhabdoviridae, Sedoreoviridae, Solemoviridae, Spinareoviridae, Togaviridae, Totiviridae, Virgaviridae, Xinmoviridae and Yueviridae. Of these, 147 are tentatively novel viruses. One sequence of Sindbis virus, which causes Pogosta disease in humans, was detected from Oc. communis from Pohjois-Karjala. This study greatly increases the number of mosquito-associated viruses known from Finland and presents the northern-most mosquito-associated viruses in Europe to date.
Keywords: Aedini, mosquito virome, Ochlerotatus, NGS, Finland, RNA virus, Sindbis virus
1. Introduction
Mosquitoes (Diptera, Culicidae) are vectors of a variety of medically significant pathogens worldwide. The known endemic mosquito-borne viral pathogens in Finland are Sindbis virus (Togaviridae: Alphavirus) [1,2], Inkoo virus [3,4] and Chatanga virus (Peribunyaviridae: Orthobunyavirus) [5,6]. Three insect-associated flaviviruses are present in the southern half of the country: Lammi virus [7], Hanko virus [8] and Ilomantsi virus [9]. Of these, Lammi and Ilomantsi viruses represent a separate flavivirus group genetically associated with vector-borne flaviviruses. Most recently, a novel Negevirus was isolated from mosquitoes collected in eastern Finland [10].
Forty-three species of mosquitoes are recorded from Finland, which belong to Aedes, Aedimorphus, Culex, Culiseta, Dahliana and Ochlerotatus [11]. Some species have rarely been encountered during recent or historical collections, but most have been reported as human-biting either in Finland or in neighbouring countries [11,12]. Species of the genus Ochlerotatus are most numerous, with 23 recorded from across Finland, but distributions vary according to species-specific life strategies. Halophilic species, including Oc. caspius and Oc. dorsalis, are usually restricted to costal locations, while other species, including Oc. communis and Oc. punctor, are widely distributed across the entire country. In Lapland, the dominant human-biting species include Oc. communis, Oc. excrucians, Oc. hexodontus, Oc. impiger, Oc. nigripes, Oc. pullatus and Oc. punctor/punctodes. Further south, the most commonly encountered human-biting species include Oc. cantans, Oc. caspius, Oc. communis, Oc. diantaeus, Oc. intrudens, Oc. pullatus and Oc. punctor/punctodes. Mosquitoes, generally, are abundant in summer months between June and August, but only Ochlerotatus species have been associated with the known mosquito-borne pathogens in Finland, although vector species associations are not yet confirmed. Sindbis virus has been isolated from mosquitoes twice: once from a pool of unidentified specimens at least containing Ochlerotatus [1] and again, from a pool of 13 specimens morphologically identified as species of Ochlerotatus [2]. Californian serogroup orthobunyaviruses Inkoo virus and Chatanga virus have also been isolated from Ochlerotatus species. Inkoo virus was first identified from Oc. communis and/or Oc. punctor/punctodes from Inkoo in southern Finland [4], while Chatanga virus was originally isolated in eastern Finland from pooled unidentified specimens, likely including specimens of Ochlerotatus or Aedes [5].
More broadly, Ochlerotatus is a widely distributed genus, with 199 species located in tropical, subtropical and temperate regions, and is known to include several species which are naturally infected with arboviruses [13]. In Europe, four native species of Ochlerotatus, Oc. caspius, Oc. communis, Oc. dorsalis and Oc. excrucians, are classed as being of particular interest for targeted surveillance due to their vector potential for a series of listed pathogens by the European Centre for Disease Prevention and Control [14]. Although other known vector species are present in Finland, e.g., Culex pipiens, it is of interest to first pursue the study of identified Ochlerotatus species in order to ascertain their potential virus associations, particularly when they have been implicated as vectors for all three endemic mosquito-borne viruses in Finland and are regularly attracted to humans. As such, females of nine commonly encountered species, Oc. cantans, Oc. caspius, Oc. communis, Oc. diantaeus, Oc. excrucians, Oc. hexodontus, Oc. intrudens, Oc. pullatus and Oc. punctor/punctodes were chosen from suitable specimens that were collected for a nation-wide distribution study [11] for inclusion in NGS studies to analyse their RNA viromes. From 91 pools of identified adult female Ochlerotatus mosquitoes that were collected from a variety of habitats around Finland in summer 2015 and 2017, 514 unique sequences of RNA-dependent RNA polymerase (RdRp) > 1000 nt, belonging to 159 viruses, were recovered. Of these, 147 potentially novel viruses were identified as well as sequences belonging to 12 established viruses, including Sindbis virus. Final decisions on the taxonomic placement and species’ status of these viruses will be determined by the ICTV.
2. Materials and Methods
2.1. Mosquito Collection and Identification
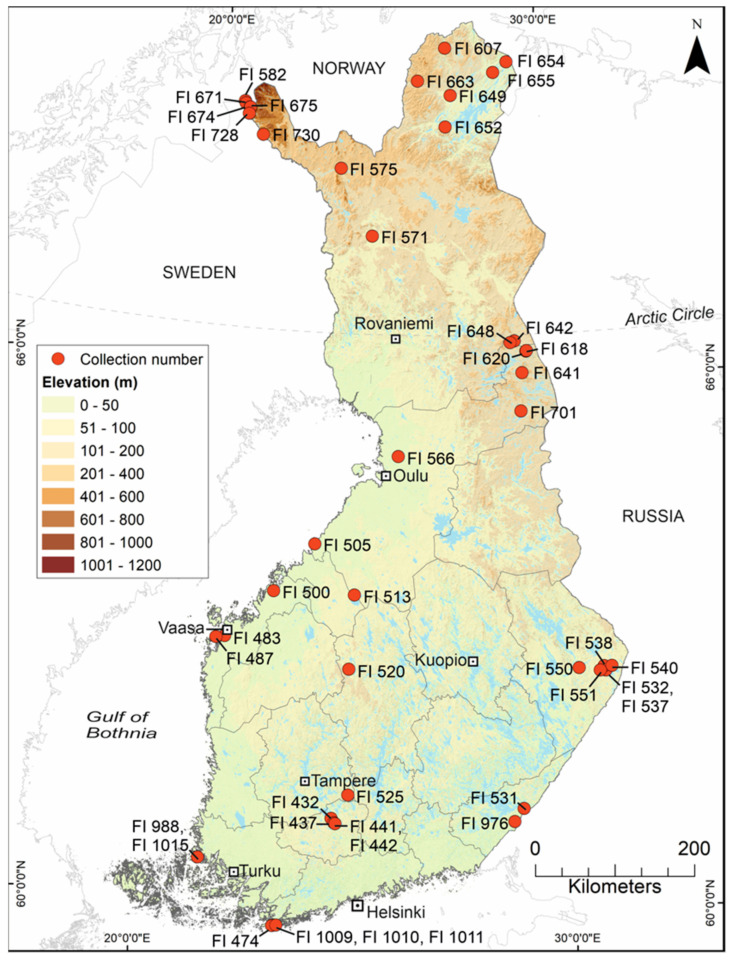
As part of a larger study, 52,466 mosquitoes were collected from around Finland between 2012 and 2018, using a variety of collection methods and from a multitude of different habitats [11]. The primary goal of that study was to collect distribution data for each of the native species, and the secondary aim was to collect specimens that were suitable for other studies. Each of the 1031 collections were numbered with a unique running code prefixed with “FI” (Figure 1, Table 1). Specimens were stored and processed in several ways, such that they could be used in one or more distribution, morphology, genetics or virus studies. Multiple factors, including access to dry ice, RNAlater or specialist freezers, time available for processing, whether the specimens were rare or common, and whether they were alive when reaching field stations, affected their designation for virus or other studies. In total, 18,394 specimens were not suitable for virus studies; 15,096 specimens were stored in RNA stabilisation solutions, including RNAlater; and 18,976 specimens were deep frozen at −70 °C or colder. Deep-frozen specimens were processed along a cold chain of initially −20 °C, −70 °C or on dry ice, and transported in dry ice to storage at −80 °C prior to the study. Mosquitoes were identified over dry ice using morphological keys [15,16] and then either (i) pooled by species, or (ii) stored individually in 1.2 mL collection microtubes (QIAGEN, Venlo, The Netherlands). From the 18,976 deep-frozen specimens, 14,092 were collected as adults, of which 13,927 were females, and 11,835 were adult female Ochlerotatus. A subset of 2333 of these deep-frozen adult female specimens was chosen for inclusion in this study (see below). Notes were made if any specimens were visibly engorged with blood, or if they had ectoparasites (Acarid mites).
Figure 1.
Locations of collections in Finland from which mosquitoes were pooled. Owing to the large numbers of mosquito pools from certain locations, the collection site number is given and not the pool number. Collections were made from a variety of unstandardised habitats while attempting to collect distribution data for all of Finland’s species. See Table 1 for the pool numbers, mosquito species and collection dates, and Table A1 for the viruses found at each location.
Table 1.
Details of the 91 mosquito pools included in this study by collection site (see Figure 1). Pools shaded grey were made up of specimens from more than one collection. Where several collections were combined, the “number of specimens from a collection/total number of specimens in the pool” are given.
| Collection No. | Latitude (N) | Longitude (E) | Location-Pool No. | No. of Specimens |
Collection Date | Mosquito Species |
|---|---|---|---|---|---|---|
| FI 432 | 61.0766 | 24.3912 | FIN/KH-2018/029 | 30 | 27 May 2015 | Oc. pullatus |
| FIN/KH-2018/047 | 20 | 27 May 2015 | Oc. punctor/punctodes | |||
| FI 437 | 61.0285 | 24.4596 | FIN/KH-2018/048 | 20 | 02 June 2015 | Oc. communis |
| FI 441 | 61.0201 | 24.4877 | FIN/KH-2018/038 | 13/20 | 02 June 2015 | Oc. intrudens |
| FIN/KH-2018/049 | 24 | 02 June 2015 | Oc. communis | |||
| FI 442 | 61.0223 | 24.4912 | FIN/KH-2018/038 | 7/20 | 02 June 2015 | Oc. intrudens |
| FI 474 | 59.8372 | 23.1595 | FIN/U-2018/050 | 20 | 14 June 2015 | Oc. communis |
| FI 483 | 63.0630 | 21.5680 | FIN/Po-2018/022 | 24 | 16 June 2015 | Oc. communis |
| FI 487 | 63.0410 | 21.3539 | FIN/Po-2018/009 | 27 | 16 June 2015 | Oc. excrucians |
| FI 500 | 63.6071 | 22.7055 | FIN/Po-2018/031 | 20 | 17 June 2015 | Oc. communis |
| FI 505 | 64.1637 | 23.6876 | FIN/PP-2018/010 | 60 | 17 June 2015 | Oc. communis |
| FI 513 | 63.6039 | 24.7534 | FIN/KP-2018/032 | 25 | 18 June 2015 | Oc. communis |
| FIN/KP-2018/033 | 16 | 18 June 2015 | Oc. diantaeus | |||
| FIN/KP-2018/034 | 20 | 18 June 2015 | Oc. intrudens | |||
| FI 520 | 62.7665 | 24.6814 | FIN/KS-2018/035 | 24 | 18 June 2015 | Oc. communis |
| FI 525 | 61.3473 | 24.7655 | FIN/Pi-2018/051 | 20 | 19 June 2015 | Oc. communis |
| FIN/Pi-2018/052 | 20 | 19 June 2015 | Oc. communis | |||
| FIN/Pi-2018/053 | 20 | 19 June 2015 | Oc. communis | |||
| FIN/Pi-2018/054 | 20 | 19 June 2015 | Oc. communis | |||
| FIN/Pi-2018/055 | 21 | 19 June 2015 | Oc. communis | |||
| FI 531 | 61.2013 | 28.9019 | FIN/EK-2018/056 | 22 | 25 June 2015 | Oc. communis |
| FI 532 | 62.7189 | 31.0050 | FIN/PK-2018/041 | 9/24 | 25 June 2015 | Oc. hexodontus |
| FIN/PK-2018/057 | 20 | 25 June 2015 | Oc. intrudens | |||
| FIN/PK-2018/058 | 20 | 25 June 2015 | Oc. diantaeus | |||
| FIN/PK-2018/059 | 20 | 25 June 2015 | Oc. communis | |||
| FIN/PK-2018/060 | 20 | 25 June 2015 | Oc. communis | |||
| FIN/PK-2018/061 | 20 | 25 June 2015 | Oc. intrudens | |||
| FI 537 | 62.7189 | 31.0050 | FIN/PK-2018/011 | 60 | 26 June 2015 | Oc. punctor/punctodes |
| FIN/PK-2018/041 | 15/24 | 26 June 2015 | Oc. hexodontus | |||
| FIN/PK-2018/042 | 20 | 26 June 2015 | Oc. cantans | |||
| FIN/PK-2018/062 | 20 | 26 June 2015 | Oc. communis | |||
| FIN/PK-2018/063 | 20 | 26 June 2015 | Oc. diantaeus | |||
| FIN/PK-2018/064 | 20 | 26 June 2015 | Oc. diantaeus | |||
| FIN/PK-2018/065 | 20 | 26 June 2015 | Oc. intrudens | |||
| FIN/PK-2018/066 | 20 | 26 June 2015 | Oc. intrudens | |||
| FIN/PK-2018/067 | 20 | 26 June 2015 | Oc. punctor/punctodes | |||
| FIN/PK-2018/068 | 20 | 26 June 2015 | Oc. intrudens | |||
| FIN/PK-2018/069 | 20 | 26 June 2015 | Oc. intrudens | |||
| FIN/PK-2018/070 | 20 | 26 June 2015 | Oc. communis | |||
| FIN/PK-2018/071 | 18 | 26 June 2015 | Oc. punctor/punctodes | |||
| FI 538 | 62.7700 | 30.9733 | FIN/PK-2018/072 | 20 | 26 June 2015 | Oc. intrudens |
| FIN/PK-2018/073 | 20 | 26 June 2015 | Oc. intrudens | |||
| FI 540 | 62.7666 | 31.1629 | FIN/PK-2018/021 | 24 | 26 June 2015 | Oc. communis |
| FI 550 | 62.7650 | 30.3541 | FIN/PK-2018/036 | 20 | 27 June 2015 | Oc. communis |
| FIN/PK-2018/074 | 20 | 27 June 2015 | Oc. communis | |||
| FIN/PK-2018/075 | 20 | 27 June 2015 | Oc. intrudens | |||
| FIN/PK-2018/076 | 20 | 27 June 2015 | Oc. communis | |||
| FIN/PK-2018/077 | 20 | 27 June 2015 | Oc. communis | |||
| FIN/PK-2018/078 | 20 | 27 June 2015 | Oc. communis | |||
| FIN/PK-2018/079 | 20 | 27 June 2015 | Oc. communis | |||
| FI 551 | 62.7241 | 30.8721 | FIN/PK-2018/080 | 21 | 27 June 2015 | Oc. intrudens |
| FI 566 | 65.1798 | 25.8002 | FIN/PP-2018/020 | 16 | 03 July 2015 | Oc. diantaeus |
| FI 571 | 67.6588 | 24.9049 | FIN/L-2018/008 | 48 | 03 July 2015 | Oc. intrudens |
| FI 575 | 68.4076 | 23.8850 | FIN/L-2018/005 | 32/48 | 04 July 2015 | Oc. communis |
| FIN/L-2018/027 | 8/24 | 04 July 2015 | Oc. communis | |||
| FI 582 | 69.0870 | 20.7600 | FIN/L-2018/005 | 8/48 | 02 July 2015 | Oc. communis |
| FI 607 | 69.7904 | 27.0549 | FIN/L-2018/001 | 48 | 07 July 2015 | Oc. hexodontus |
| FIN/L-2018/006 | 48 | 07 July 2015 | Oc. communis | |||
| FI 618 | 66.3588 | 29.3260 | FIN/PP-2018/015 | 40/57 | 09 July 2015 | Oc. punctor/punctodes |
| FIN/PP-2018/28 | 20 | 09 July 2015 | Oc. intrudens | |||
| FI 620 | 66.3639 | 29.3429 | FIN/PP-2018/015 | 17/57 | 09 July 2015 | Oc. punctor/punctodes |
| FIN/PP-2018/016 | 60 | 09 July 2015 | Oc. communis | |||
| FI 641 | 66.1148 | 29.1976 | FIN/PP-2018/082 | 20 | 18 July 2015 | Oc. communis |
| FIN/PP-2018/083 | 17 | 18 July 2015 | Oc. communis | |||
| FI 642 | 66.4756 | 29.0116 | FIN/L-2018/024 | 10/24 | 19 July 2015 | Oc. communis |
| FI 648 | 66.4597 | 28.8963 | FIN/L-2018/024 | 14/24 | 19 July 2015 | Oc. communis |
| FI 649 | 69.2558 | 27.2301 | FIN/L-2018/007 | 40/48 | 22 July 2015 | Oc. excrucians |
| FIN/L-2018/084 | 24 | 22 July 2015 | Oc. excrucians | |||
| FIN/L-2018/085 | 20 | 22 July 2015 | Oc. hexodontus | |||
| FIN/L-2018/086 | 20 | 22 July 2015 | Oc. hexodontus | |||
| FI 652 | 68.9008 | 27.0658 | FIN/L-2018/023 | 8/16 | 22 July 2015 | Oc. pullatus |
| FI 654 | 69.6249 | 29.0415 | FIN/L-2018/019 | 4/16 | 23 July 2015 | Oc. diantaeus |
| FIN/L-2018/007 | 1/48 | 23 July 2015 | Oc. excrucians | |||
| FI 655 | 69.5095 | 28.5965 | FIN/L-2018/019 | 12/16 | 23 July 2015 | Oc. diantaeus |
| FIN/L-2018/007 | 7/48 | 23 July 2015 | Oc. excrucians | |||
| FI 663 | 69.4178 | 26.1809 | FIN/L-2018/088 | 21 | 24 July 2015 | Oc. communis |
| FI 671 | 69.0617 | 20.7936 | FIN/L-2018/002 | 48 | 26 July 2015 | Oc. hexodontus |
| FIN/L-2018/003 | 48 | 26 July 2015 | Oc. punctor/punctodes | |||
| FIN/L-2018/026 | 24 | 26 July 2015 | Oc. punctor/punctodes | |||
| FI 674 | 69.0205 | 20.9304 | FIN/L-2018/089 | 20 | 28 July 2015 | Oc. hexodontus |
| FIN/L-2018/090 | 20 | 28 July 2015 | Oc. hexodontus | |||
| FI 675 | 69.0227 | 20.9380 | FIN/L-2018/030 | 22 | 28 July 2015 | Oc. hexodontus |
| FI 701 | 65.6855 | 29.1345 | FIN/PP-2018/004 | 48 | 23 August 2015 | Oc. punctor/punctodes |
| FI 728 | 68.9490 | 20.9210 | FIN/L-2018/005 | 8/48 | 02 July 2015 | Oc. communis |
| FIN/L-2018/023 | 8/16 | 02 July 2015 | Oc. pullatus | |||
| FI 730 | 68.7270 | 21.4220 | FIN/L-2018/027 | 16/24 | 03 July 2015 | Oc. communis? |
| FI 976 | 61.0569 | 28.6785 | FIN/EK-2018/040 | 20 | 04 July 2017 | Oc. communis |
| 61.0569 | 28.6785 | FIN/EK-2018/091 | 20 | 04 July 2017 | Oc. communis | |
| FI 988 | 60.5481 | 21.3696 | FIN/VS-2018/017 | 60 | 11 July 2017 | Oc. caspius |
| FI 1009 | 59.8439 | 23.2466 | FIN/U-2018/092 | 20 | 22 August 2017 | Oc. caspius |
| FIN/U-2018/093 | 17 | 22 August 2017 | Oc. punctor/punctodes | |||
| FI 1010 | 59.8439 | 23.2466 | FIN/U-2018/018 | 60 | 22–23 August 2017 | Oc. caspius |
| FIN/U-2018/039 | 25 | 22–23 August 2017 | Oc. punctor/punctodes | |||
| FIN/U-2018/094 | 20 | 22–23 August 2017 | Oc. caspius | |||
| FIN/U-2018/095 | 20 | 22–23 August 2017 | Oc. caspius | |||
| FI 1011 | 59.8439 | 23.2466 | FIN/U-2018/044 | 20 | 23–24 August 2017 | Oc. caspius |
| FIN/U-2018/045 | 21 | 23–24 August 2017 | Oc. punctor/punctodes | |||
| FIN/U-2018/096 | 20 | 23–24 August 2017 | Oc. caspius | |||
| FIN/U-2018/097 | 19 | 23–24 August 2017 | Oc. caspius | |||
| FI 1015 | 60.5481 | 21.3696 | FIN/VS-2018/098 | 20 | 24 August 2017 | Oc. caspius |
| FIN/VS-2018/099 | 20 | 24 August 2017 | Oc. caspius | |||
| FIN/VS-2018/100 | 26 | 24 August 2017 | Oc. caspius |
2.2. Pooling and Homogenisation
Pools were constructed using identifiable females of commonly encountered human-biting Ochlerotatus, by species, collection location and collection date (Figure 1, Table 1). Rare species with fewer than 16 specimens were not considered; neither were specimens which were found in low numbers over several collection sites over several years such that location or temporal data would not be confused in the results. Since these species are difficult to identify when scales are denuded, 2176 specimens were immediately excluded from the potential specimens as they were either unidentified or the identification was not confirmed. To suit the available resources, 2333 females belonging to nine species, which were collected in May–August 2015 and July–August 2017, met these criteria, and were divided into 91 pools, as follows: Oc. cantans (n = 1), Oc. caspius (n = 11), Oc. communis (n = 35), Oc. diantaeus (n = 6), Oc. excrucians (n = 3), Oc. hexodontus (n = 8), Oc. intrudens (n = 14), Oc. pullatus (n = 2) and Oc. punctor/punctodes (n = 11) (Table 1).
Pools varied in size, from 16–60 whole individuals, with most later pools comprising 20 specimens. Females that were noticeably blood fed or gravid, or which had one or more ectoparasites were maintained in individual tubes for homogenisation. Pools were assigned a running number corresponding to the date when they were processed, from FIN/L-2018/001 to FIN/VS-2018/100 (Table 1). Most pools comprised mosquitoes from a single collection site, but several contained specimens from up to three locations. In these few cases, specimens were pooled from the same region and within a few days of being collected.
For the purpose of interpreting the collection locations when reading the phylogenetic trees, an additional code was added after “FIN” to represent the 11 (of 19) regions of Finland from which collections were made, as follows: EK, Etelä-Karjala; KH, Kanta-Häme; Kl, Kymenlaakso; KP, Keski-Pohjanmaa; KS, Keski-Suomi; L, Lappi/Lapland; PK, Pohjois-Karjala; Pi, Pirkanmaa; Po, Pohjanmaa; PP, Pohjois-Pohjanmaa; U, Uusimaa; and VS, Varsinais-Suomi.
Individually stored specimens were homogenised in microtubes with 100 µL of Dulbecco’s phosphate-buffered saline (PBS) + 0.2% bovine serum albumin (BSA), sterile sand and a 3 mm tungsten carbide bead (QIAGEN, Venlo, The Netherlands). After homogenisation, the tubes were centrifuged at full speed for 5 min at 5 °C. Subsequently, 50 µL of supernatant from each specimen was then combined in a “super pool”. For pre-pooled mosquitoes, 1.8 mL of Dulbecco’s PBS + 0.2% BSA was added to each 2 mL tube, with a 5 mm tungsten carbide bead. These were homogenised using the QIAGEN TissueLyser II for 2 min at full speed, then centrifuged at 5 °C for 5 min. From each of the 91 pooled mosquito homogenates, aliquots were taken for next-generation sequencing (NGS).
2.3. Illumina MiSeq Sequencing
Prior to sequencing, the mosquito homogenates were treated following an established protocol [17] with minor modifications. Specifically, they were each filtered through a 0.8 µm polyethersulfone (PES) filter and treated with micrococcal nuclease (New England Biolabs, Ipswich, MA, USA) and benzonase (Millipore, Merck KGaA, Darmstadt, Germany). RNA was then extracted using TRIzol (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. The RNA samples were treated with DNase I and purified with Agencourt RNA Clean XP magnetic beads (Beckman Life Industries). Ribosomal RNA was removed using a NEBNext rRNA depletion kit according to the manufacturer’s protocol, followed by amplification using a whole transcriptome amplification WTA2 kit (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany). The sequencing libraries were prepared using a Nextera XT kit (Illumina, San Diego, CA, USA) and sequenced using the Illumina Miseq platform and v2 reagent kit with 150 bp paired-end reads.
2.4. NGS Data Analysis
Sequence reads from the initial homogenates (Figure S1, Table S1) were analysed in Lazypipe v.1.2, an automated bioinformatics pipeline [18]. Preassembly quality control was first performed on the FASTQ reads using Trimmomatic v.0.39 [19] to remove and trim low quality reads, bases and Illumina adapters. MEGAHIT v.1.2.8 [20] was used to perform de novo assembly with the initial quality-controlled reads. Gene-like regions were detected using MetaGeneAnnotator [21] and translated to amino acids with BioPerl [22]. The amino acid sequences were then queried against the UniProtKB database using SANSparallel [23] and assigned NCBI taxonomy IDs. Any sequences that were unclassified according to NCBI Taxonomy were not possible to identify following the steps, above, so were manually identified using BLASTx. Any contigs longer than 1000 nt, with the highest similarity to viral RNA-dependent RNA polymerases (RdRps), were selected for phylogenetic analyses.
Analyses were performed on amino acid sequences, which were derived by analysing each contig with getorf [24] to identify open reading frames (ORFs) and converting them into an amino acid format. These were aligned with MAFFT v. 7.490 [25] and the resulting alignments trimmed with trimal v.1.2 [26]. Finally, maximum likelihood (ML) trees were constructed with IQ-TREE2 v.2 [27], which employs the ModelFinder algorithm [28] to determine the optimal protein substitution model, and the UFBoot2 algorithm [29] to compute 1000 bootstraps. The final trees were visualised in R v.4.1.2 using the GGTREE package v.3.0.4 [30].
The novel viruses discovered in this study (Table S2) were named according to the nearest town or municipality to the, or one of the site(s) from which the mosquitoes were collected, but with diacritical marks removed as they were not supported in GGTREE. If more than one virus variant or species was found from the same pool an additional, final, running number was appended to the end. Representative virus sequences for each virus family were downloaded from those available in GenBank, compared to newly generated sequences, and included in the ML trees.
3. Results
3.1. RNA Viromes Obtained Directly from Mosquito Homogenates
3.1.1. Positive-Sense ssRNA Virus Sequences
Positive-sense ssRNA viruses belonging to eight established viral families were detected during this study; Endornaviridae, Flaviviridae, Iflaviridae, Permutotetraviridae, Picornaviridae, Solemoviridae, Togaviridae and Virgaviridae. Sequences which belong to two proposed taxa, Negevirus and Quenyavirus were also recovered. The +ssRNA viruses are listed below, with all variant names and associated mosquito species in Table 2.
Table 2.
+ssRNA viruses sequenced from Finnish mosquitoes. Previously described viruses are shaded grey. Where more than one virus was sequenced from a pool, an additional code was appended to the pool number.
| Virus Family/ Taxon | Virus Name | Pool/Variant No. | Associated Mosquito Species |
GenBank Accession |
|---|---|---|---|---|
| Endornaviridae | Hallsjon virus | FIN/U-2018/93 | Oc. punctor/punctodes | ON955055 |
| Endornaviridae | Tvarminne alphaendornavirus | FIN/U-2018/93 | Oc. punctor/punctodes | ON955056 |
| Flaviviridae | Hameenlinna flavivirus | FIN/KH-2018/38 | Oc. intrudens | ON955057 |
| Flaviviridae | Kilpisjarvi flavivirus | FIN/L-2018/90 | Oc. hexodontus | ON949931 |
| Flaviviridae | Lestijarvi flavi-like virus | FIN/KP-2018/33 | Oc. diantaeus | ON955060 |
| Flaviviridae | Hanko virus | FIN/U-2018/94 FIN/U-2018/95 FIN/U-2018/96FIN/U-2018/97 |
Oc. caspius
Oc. caspius Oc. caspius Oc. caspius |
ON949927 ON949928 ON949929 ON949930 |
| Flaviviridae | Inari jingmenvirus | FIN/L-2018/30FIN/L-2018/86 | Oc. hexodontusOc. hexodontus |
ON955058 ON955059 |
| Iflaviridae | Enontekio iflavirus | FIN/L-2018/02-1 FIN/L-2018/02-2 FIN/L-2018/89 |
Oc. hexodontusOc. hexodontus
Oc. hexodontus |
ON955061 ON955062 ON949932 |
| Iflaviridae | Hanko iflavirus 1 | FIN/PK-2018/11 FIN/L-2018/24 FIN/L-2018/27 FIN/PP-2018/28 FIN/U-2018/50 FIN/PK-2018/66 FIN/PK-2018/80 |
Oc. punctor/punctodes
Oc. communis Oc. communis Oc. intrudens Oc. communis Oc. intrudens Oc. intrudens |
ON949934 ON955063 ON949933 ON949936 ON949937 ON949935 ON955064 |
| Iflaviridae | Hanko iflavirus 2 | FIN/U-2018/94 FIN/U-2018/97 |
Oc. caspius
Oc. caspius |
ON955065 ON949938 |
| Iflaviridae | Mekrijarvi iflavirus | FIN/PK-2018/69 | Oc. intrudens | ON949939 |
| Iflaviridae | Pedersore iflavirus | FIN/Po-2018/31 FIN/KP-2018/33 FIN/U-2018/92 FIN/U-2018/94 |
Oc. communis
Oc. diantaeus Oc. caspius Oc. caspius |
ON949941 ON949940 ON949942 ON955066 |
| Negevirus | Cordoba virus | FIN/L-2018/02 FIN/PP-2018/04-1 FIN/PP-2018/04-2 FIN/PP-2018/04-3 FIN/L-2018/06 FIN/PP-2018/16-1 FIN/PP-2018/16-2 FIN/PP-2018/82-1 FIN/PP-2018/82-2 FIN/PP-2018/82-3 FIN/PP-2018/82-4 |
Oc. hexodontus
Oc. punctor/punctodes Oc. punctor/punctodes Oc. punctor/punctodes Oc. communis Oc. communis Oc. communis Oc. communis Oc. communis Oc. communis Oc. communis |
ON955067 ON955069 ON955070 ON955071 ON955068 ON955072 ON955073 ON955074 ON955075 ON955076 ON955077 |
| Negevirus | Dezidougou virus | FIN/PP-2018/82 | Oc. communis | ON949943 |
| Negevirus | Kustavi negevirus | FIN/VS-2018/100 | Oc. caspius | ON949944 |
| Negevirus | Mekrijärvi negevirus | FIN/PK-2018/41-1 FIN/PK-2018/41-2 FIN/PK-2018/68 FIN/PK-2018/69 |
Oc. hexodontus
Oc. hexodontus Oc. intrudens Oc. intrudens |
ON955078 ON955079 ON955080 ON955081 |
| Negevirus | Utsjoki negevirus 1 | FIN/L-2018/02-1 | Oc. hexodontus | ON955082 |
| FIN/L-2018/02-2 | Oc. hexodontus | ON955083 | ||
| FIN/L-2018/02-3 | Oc. hexodontus | ON955084 | ||
| FIN/L-2018/03-1 | Oc. punctor/punctodes | ON955085 | ||
| FIN/L-2018/03-2 | Oc. punctor/punctodes | ON955086 | ||
| FIN/PP-2018/04-1 | Oc. punctor/punctodes | ON955088 | ||
| Negevirus | Utsjoki negevirus 1 | FIN/PP-2018/04-2 FIN/PP-2018/04-3 FIN/PP-2018/04-4 FIN/U-2018/06 FIN/PP-2018/16 FIN/PP-2018/82 FIN/L-2018/84 FIN/L-2018/85 FIN/L-2018/90 |
Oc. punctor/punctodes
Oc. punctor/punctodes Oc. punctor/punctodes Oc. communis Oc. communis Oc. communis Oc. excrucians Oc. hexodontus Oc. hexodontus |
ON955089 ON955090 ON955091 ON949945 ON955092 ON949948 ON955087 ON949946 ON949947 |
| Negevirus | Utsjoki negevirus 2 | FIN/L-2018/02-1 FIN/L-2018/02-2 FIN/L-2018/02-3 FIN/PP-2018/04-1 FIN/PP-2018/04-2 FIN/L-2018/06 FIN/L-2018/85 |
Oc. hexodontus
Oc. hexodontus Oc. hexodontus Oc. punctor/punctodes Oc. punctor/punctodes Oc. communis Oc. hexodontus |
ON955093 ON955094 ON955095 ON955098 ON955099 ON955096 ON955097 |
| Negevirus | Utsjoki negevirus 3 | FIN/L-2018/02 FIN/L-2018/06 |
Oc. hexodontus
Oc. communis |
ON955100 ON955101 |
| Permutotetraviridae | Inari permutotetravirus | FIN/PP-2018/04 FIN/L-2018/07-1 FIN/L-2018/07-2 FIN/L-2018/85 FIN/L-2018/86 FIN/L-2018/89 |
Oc. punctor/punctodes
Oc. excrucians Oc. excrucians Oc. hexodontus Oc. hexodontus Oc. hexodontus |
ON955107 ON955102 ON955103 ON955104 ON955105 ON955106 |
| Picornaviridae | Hanko picorna-like virus | FIN/U-2018/92-1 FIN/U-2018/92-2 |
Oc. caspius
Oc. caspius |
ON955108 ON955109 |
| Picornaviridae | Jotan virus | FIN/VS-2018/99-1 FIN/VS-2018/99-2 FIN/VS-2018/99-3 |
Oc. caspius
Oc. caspius Oc. caspius |
ON955110 ON955111 ON955112 |
| Quenyavirus | Enontekio quenyavirus | FIN/L-2018/90 FIN/U-2018/93 |
Oc. hexodontus
Oc. punctor/punctodes |
ON955113 ON955114 |
| Solemoviridae | Enontekio sobemovirus | FIN/L-2018/02 FIN/L-2018/26 FIN/L-2018/89 |
Oc. hexodontus
Oc. punctor/punctodes Oc. hexodontus |
ON955115 ON955116 ON955117 |
| Solemoviridae | Evros sobemo-like virus | FIN/VS-2018/17 FIN/U-2018/18 FIN/U-2018/92 FIN/U-2018/94 FIN/U-2018/95 FIN/U-2018/98 |
Oc. caspius
Oc. caspius Oc. caspius Oc. caspius Oc. caspius Oc. caspius |
ON955122 ON955118 ON955119 ON955120 ON955121 ON955123 |
| Solemoviridae | Hanko sobemovirus | FIN/U-2018/96 | Oc. caspius | ON955124 |
| Solemoviridae | Ilomantsi sobemovirus | FIN/L-2018/07 FIN/PK-2018/42 |
Oc. excrucians
Oc. cantans |
ON955125 ON955126 |
| Solemoviridae | Joensuu sobemovirus | FIN/L-2018/19 FIN/PK-2018/75 FIN/PP-2018/82 |
Oc. diantaeus
Oc. intrudens Oc. communis |
ON955127 ON955128 ON955129 |
| Togaviridae | Sindbis virus | FIN/PK-2018/62 | Oc. communis | ON955130 |
| Virgaviridae | Enontekio virga-like virus 1 | FIN/L-2018/90 | Oc. hexodontus | ON955131 |
| Virgaviridae | Enontekio virga-like virus 2 | FIN/L-2018/90 | Oc. hexodontus | ON955132 |
| Virgaviridae | Pedersore virga-like virus | FIN/Po-2018/31 FIN/EK-2018/40 FIN/L-2018/88 FIN/L-2018/90 FIN/U-2018/93 |
Oc. communis
Oc. communis Oc. communis Oc. hexodontus Oc. punctor/punctodes |
ON955136 ON955133 ON955134 ON955135 ON955137 |
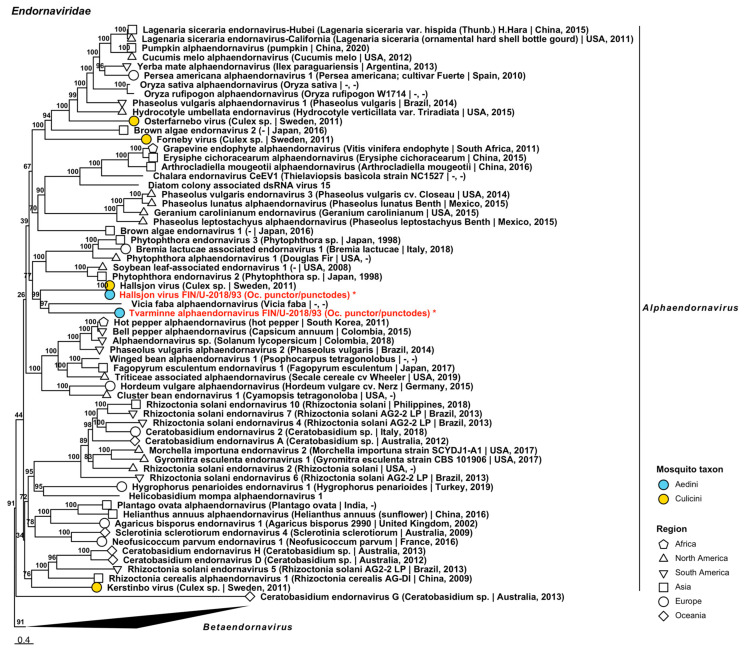
Two species belonging to Alphaendornavirus in Endornaviridae, a family of viruses known to infect plants, fungi and oomycetes, were recovered from one pool of Oc. punctor/punctodes (Figure 2, Table 2). The first was a strain of Hallsjon virus (GenBank accession: QGA70950.1; amino acid identity: 99.77%) and the second was a novel virus, named “Tvarminne alphaendornavirus”, that was distantly similar to Vicia faba alphaendornavirus (GenBank accession: YP_438201.1; amino acid identity: 49.12%). Complete genomes were sequenced for both virus species (GenBank accessions ON955055 and ON955056).
Figure 2.
Maximum likelihood tree of Endornaviridae. Tentative novel viruses are displayed in red and the mosquito species from which they were derived are in parentheses. Sequences from GenBank are black and display the following information after the virus or species name: “(sampled organism(s)|collection country, collection year)”. Tip colours represent the tribe of mosquito from which viruses were obtained. Tip shape represents the continent or region from which the specimens were collected. Trees were constructed from amino acid sequences of virus polymerases >1000 nt, aligned with MAFFT and computed with IQ-TREE2 using ModelFinder and 1000 bootstraps. Asterisks denote that the complete genome was recovered.
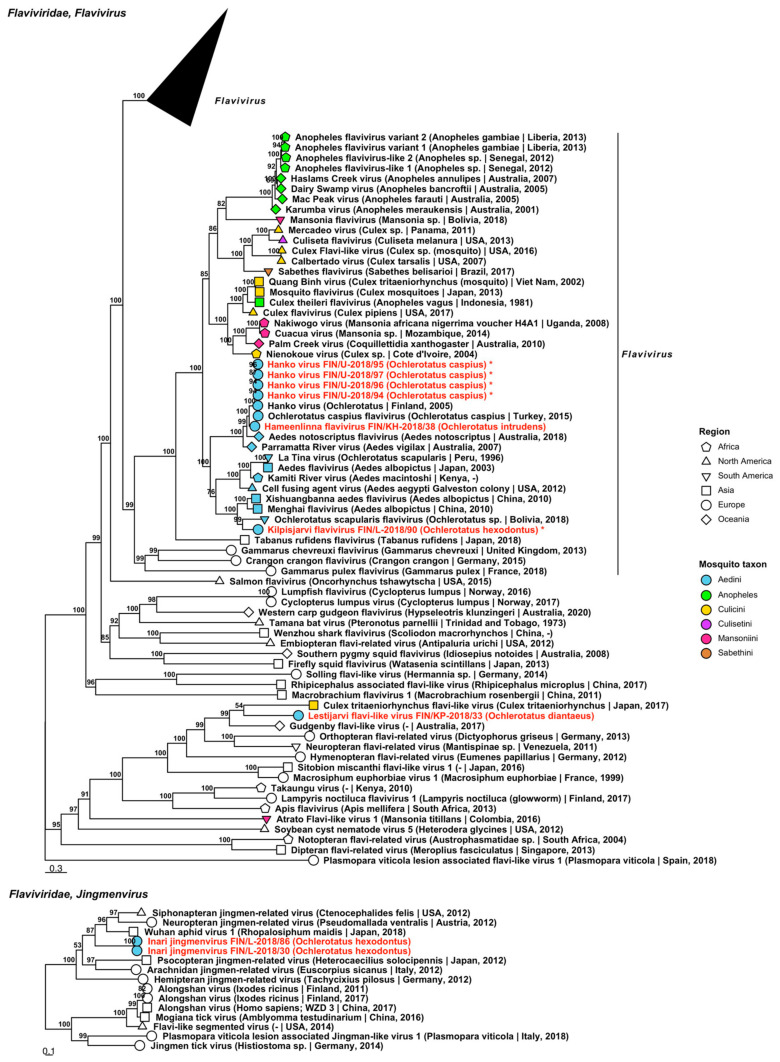
Five species belonging to two genera of Flaviviridae were sequenced from nine mosquito pools, four of which are tentative novel viruses (Figure 3, Table 2). Three viruses grouped within genus Flavivirus, one with flavivirus-like viruses and one within genus Jingmenvirus. Two of the four novel species were named “Hameenlinna flavivirus” and “Kilpisjarvi flavivirus” and these fell within the insect-specific group of flaviviruses. Hameenlinna flavivirus was most similar to another insect-specific flavivirus species that was first detected in Finland, Hanko virus (GenBank accession: YP_009259489.1; amino acid identity: 79.87%). Kilpisjarvi flavivirus was most similar to Xishuangbanna aedes flavivirus (GenBank accession: YP_009350102.1; amino acid identity: 61.88%) although it clustered with Ochlerotatus scapularis flavivirus (GenBank accession: BCI56825.1; amino acid identity: 61.37%) in the phylogenetic tree. The full genome of Kilpisjarvi flavivirus was sequenced (GenBank accessions ON949931). A novel flavivirus-like species, “Lestijarvi flavi-like virus”, was most similar to Hymenopteran flavi-related virus (GenBank accession: QTJ63659.1; amino acid identity: 47.75%), although in the phylogenetic tree it clustered with Gudgenby flavi-like virus (GenBank accession: QTJ63659.1; amino acid identity: 47.3%). Hanko virus, a species which was first described in 2012, was also present in four pools of mosquitoes collected near to the virus’ type locality, which had an average amino acid identity of >99% (Figure 3, Table 2). The full genome of Hanko virus was sequenced from these variants (GenBank accession ON949927–ON949930). One novel member of the genus Jingmenvirus was detected, with two variants provisionally named “Inari jingmenvirus”. This species was not closely related to any species, although it weakly resembled Wuhan aphid virus 1 (GenBank accession: BBV14756.1; amino acid identity: 48.82%), which was derived from aphids from Japan.
Figure 3.
Maximum likelihood trees of Flaviviridae. Tentative novel viruses are displayed in red and the mosquito species from which they were derived are in parentheses. Sequences from GenBank are black and display the following information after the virus or species name: “(sampled organism(s)|collection country, collection year)”. Tip colours represent the tribe (Culicinae) or genus (Anophelinae) of mosquito from which viruses were obtained. Tip shape represents the continent or region from which the specimens were collected. Trees were constructed from amino acid sequences of virus polymerases >1000 nt, aligned with MAFFT and computed with IQ-TREE2 using. Asterisks denote that the complete genome was recovered.
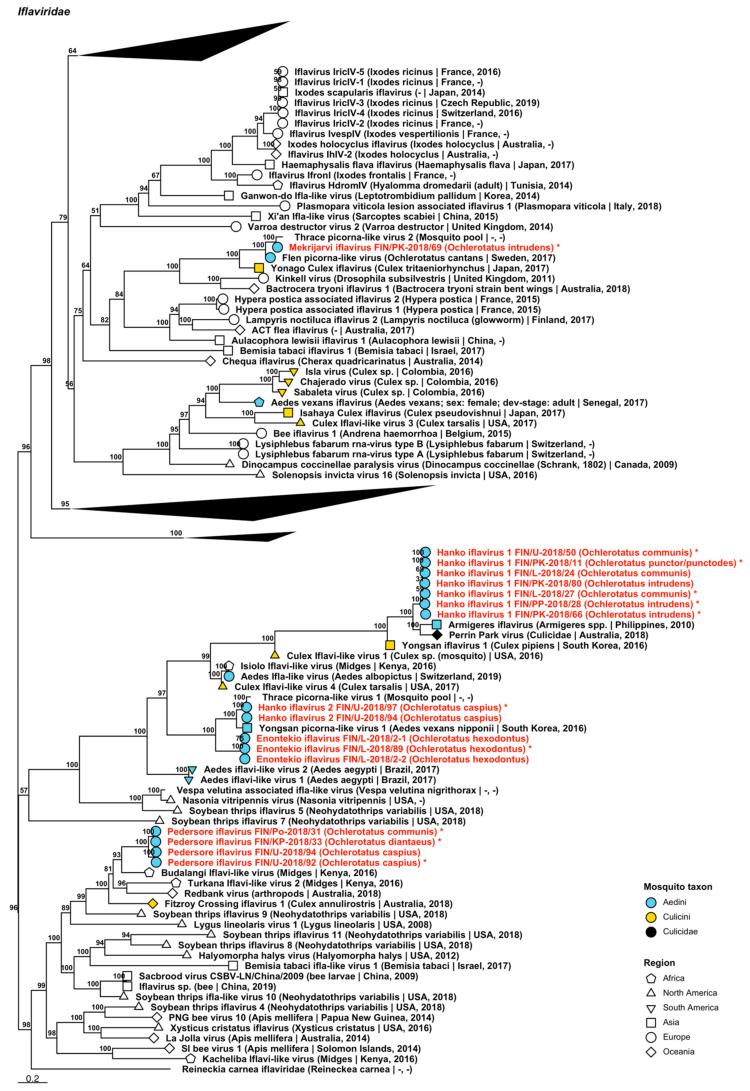
Seventeen variants of sequences representing five tentative novel viruses which grouped within Iflaviridae were sequenced from 15 pools comprised of Oc. caspius, Oc. communis, Oc. diantaeus, Oc. hexodontus, Oc. intrudens and Oc. punctor/punctodes (Figure 4, Table 2). These were named “Enontekio iflavirus”, “Hanko iflavirus 1 and 2”, “Mekrijarvi iflavirus” and “Pedersore iflavirus”. Enontekio iflavirus sequences were most similar to both Culex iflavi-like virus 4 and Yongsan picorna-like virus 1 (GenBank accessions: AXQ04788.1 and AXV43880.1; amino acid identities of 50.2% and 54.28–59.58%, respectively). Hanko iflavirus 1 sequences were most closely related to Perrin Park virus (GenBank accession: QIJ25864.1; amino acid identity: 68.36%) and Armigeres iflavirus (GenBank accession: YP_009448183.1; amino acid identity: 69.56–79.19%) were similar to Yongsan picorna-like virus 1 (GenBank accession: AXV43880.1; amino acid identity: 77.46–81.43%). Mekrijarvi iflavirus resembles most Thrace picorna-like virus 2 (GenBank accession: QRD99887.1; amino acid identity: 89.87%). Lastly, Pedersore iflavirus sequences were most similar to Redbank virus (GenBank accession: QIJ25857.1; amino acid identity: 50.09%), Budalangi iflavi-like virus (GenBank accession: UCW41643.1; amino acid identity: 54.95%) and Fitzroy Crossing iflavirus 1 (GenBank accession: QLJ83497.1; amino acid identity: 49.1%), although these clustered close to Budalangi iflavi-like virus (GenBank accession: UCW41643.1; amino acid identity: 54.95–55.33%).
Figure 4.
Maximum likelihood tree of Iflaviridae. Tentative novel virus species are displayed in red and the mosquito species from which they were derived are in parentheses. Sequences from GenBank are black and display the following information after the virus or species name: “(sampled organism(s)|collection country, collection year)”. Tip colours represent the tribe of mosquito from which viruses were obtained. Tip shape represents the continent or region from which the specimens were collected. Trees were constructed from amino acid sequences of virus polymerases >1000 nt, aligned with MAFFT and computed with IQ-TREE2 using ModelFinder and 1000 bootstraps. Asterisks denote that the complete genome was recovered.
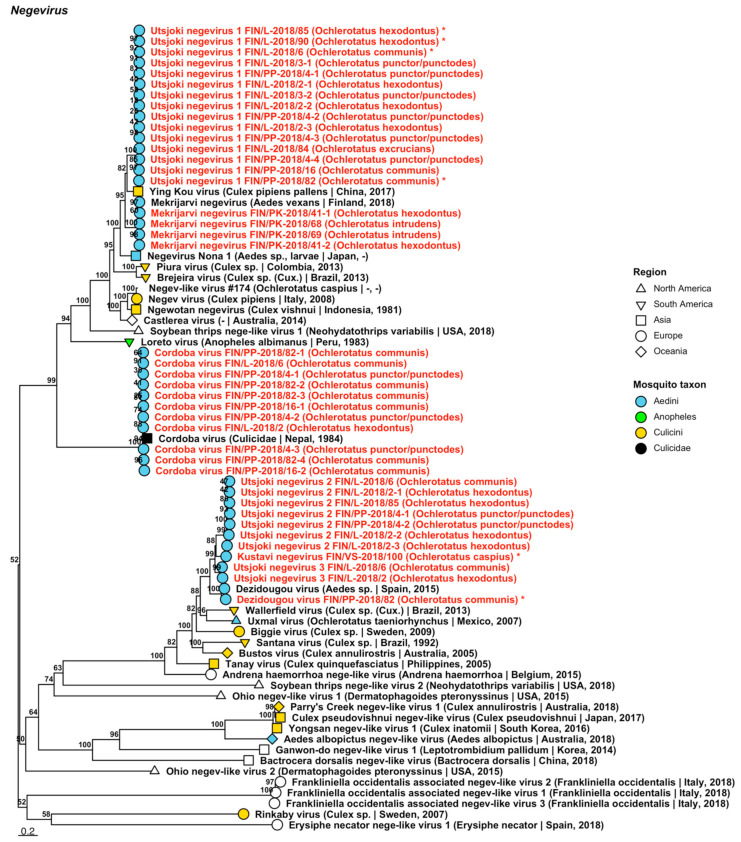
Forty-one strains of seven viruses belonging to the proposed taxon Negevirus were sequenced from 13 mosquito pools. While not yet formally recognised by the ICTV, Negeviruses have been recorded from mosquitoes and sandflies, among other arthropod species. Four of the viruses, “Kustavi negevirus” and “Utsjoki negevirus 1 to 3” were novel; while three, Cordoba virus, Dezidougou virus and Mekrijärvi negevirus (Figure 5, Table 2) have previously been described. Kustavi negevirus is most similar to Dezidougou virus (GenBank accession: AFI24669.1; amino acid identity: 72.41%); Utsjoki negevirus 1 to Ying Kou virus (amino acid identity: 74.11–87.5%) and Mekrijärvi negevirus (amino acid identity: 72.33–78.99%); and Utsjoki negeviruses 2 and 3 are most closely related to Dezidougou virus (protein identities of 62.63–78.81% and 81.93–82.15%). The newly sequenced strains of Cordoba virus and Dezidougou virus shared a high similarity to previously described strains of the same virus species (GenBank accessions: AQM55308.1 and AQM55309.1; amino acid identity: 90.99–95.52%; and QIN93579.1; amino acid identity: 90.12%, respectively). Newly generated Mekrijärvi negevirus sequences were nearly identical to the proposed type of virus species (amino acid identity: 99.37–100%). Full genomes were assembled for Kustavi negevirus, Dezidougou virus and Utsjoki negevirus 1 (GenBank accession ON949944, ON949943 and ON949945–ON949948, respectively).
Figure 5.
Maximum likelihood tree of Negevirus. Tentative novel viruses are displayed in red and the mosquito species from which they were derived are in parentheses. Sequences from GenBank are black and display the following information after the virus or species name: “(sampled organism(s) | collection country, collection year)”. Tip colours represent the tribe (Culicinae) or genus (Anophelinae) of mosquito from which viruses were obtained. Tip shape represents the continent or region from which the specimens were collected. Trees were constructed from amino acid sequences of virus polymerases >1000 nt, aligned with MAFFT and computed with IQ-TREE2 using ModelFinder and 1000 bootstraps. Asterisks denote that the complete genome was recovered.
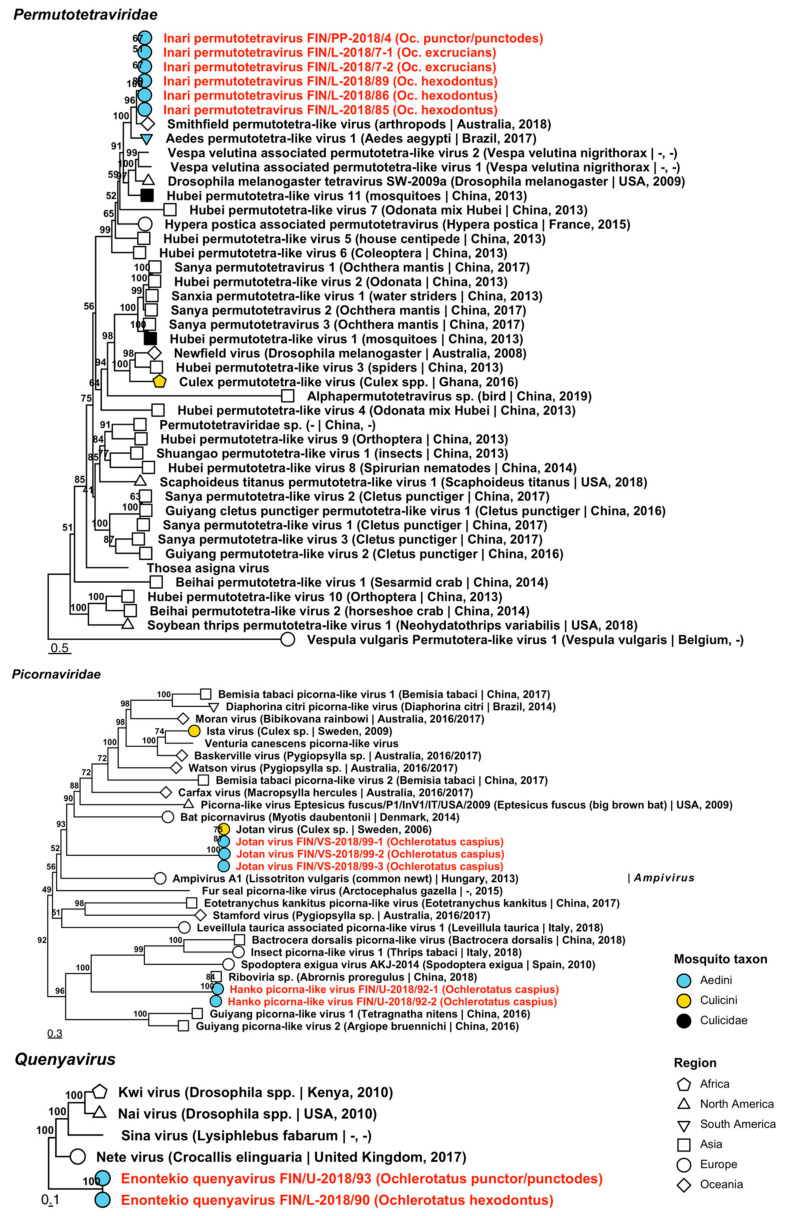
Six variants of one novel species belonging to Permutotetraviridae, a family associated with arthropods, were sequenced from five mosquito pools (Figure 6, Table 2). Named “Inari permutotetravirus”, its amino acid identity was most similar to Smithfield permutotetra-like virus (GenBank accession: QIJ25871.1/QIJ25875.1; amino acid identity: 42.72–66.32%), which were both sequenced from unspecified arthropods collected from Queensland, Australia.
Figure 6.
Maximum likelihood trees of Permutotetraviridae, Picornaviridae and Quenyavirus. Tentative novel viruses are displayed in red and the mosquito species from which they were derived are in parentheses. Sequences from GenBank are black and display the following information after the virus or species name: “(sampled organism(s)|collection country, collection year)”. Tip colours represent the tribe of mosquito from which viruses were obtained. Tip shape represents the continent or region from which the specimens were collected. Trees were constructed from amino acid sequences of virus polymerases >1000 nt, aligned with MAFFT and computed with IQ-TREE2 using ModelFinder and 1000 bootstraps.
Five variants of two species of Picornaviridae, a family of viruses that infect a broad range of vertebrates, were sequenced from two pools of Oc. caspius (Figure 6, Table 2). The first species was a previously described but as yet unnamed RNA virus, tentatively named here as “Hanko picorna-like viruses”. The previously described virus was obtained from an anal swab taken from a passerine bird in a Chinese zoo and was nearly identical to the Finnish variant (GenBank accession: QKN89015.1; amino acid identity: 97.15–99.47%). The second species, Jotan virus, shared high amino acid identity with its previously described counterpart from Culex mosquitoes in Sweden (GenBank accession: QGA70904.1; amino acid identity: 98.25–98.8%).
One virus sequence grouped with the proposed insect-specific taxon Quenyavirus, and was named “Enontekio quenyavirus”, despite being found in specimens collected from northern Lapland and from Uusimaa in the far south of Finland (Figure 6, Table 2). Based on amino acid identity, it is relatively distant from its closest relative, Nete virus (GenBank accession: QIQ61196.1; amino acid identity: 39.71–39.77%) which was sequenced from the moth, Crocallis elinguaria, from the UK.
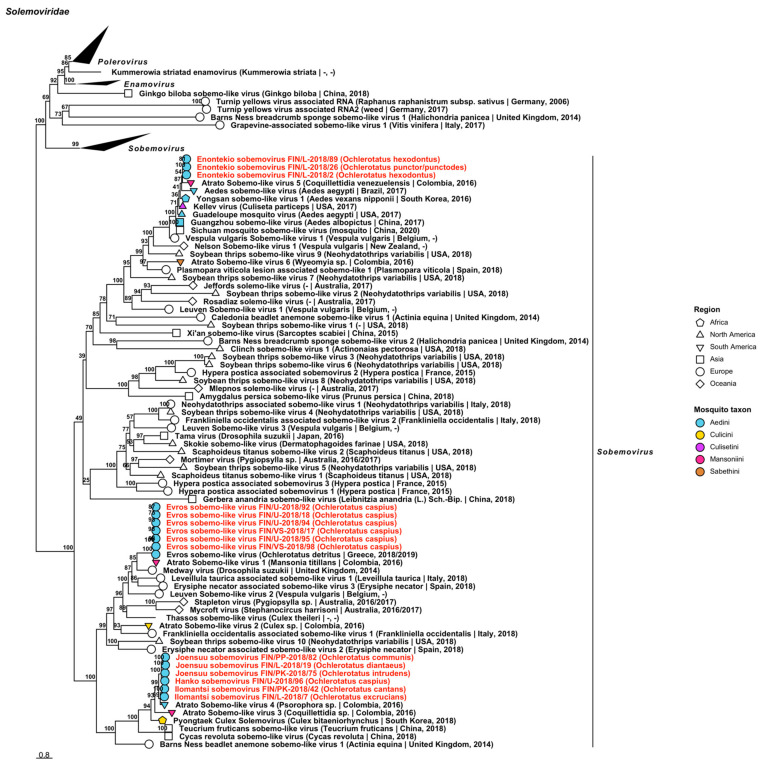
Fifteen variants belonging to the plant-specific Solemoviridae were sequenced, which corresponded to one established virus, Evros sobemo-like virus, and four novel species (Figure 7, Table 2). The novel viruses, “Enontekio sobemovirus”, “Hanko sobemovirus”, “Ilomantsi sobemovirus” and “Joensuu sobemovirus”, clustered with other viruses in Sobemovirus based on our phylogenetic analysis. Enontekio sobemovirus was closely related to Guadeloupe mosquito virus (GenBank accession: QRW42396.1; amino acid identity: 82.86%) and Kellev virus (GenBank accession: QRW41864.1; amino acid identity: 85.91–86.14%). Based on protein similarity, however, it clustered with Atrato Sobemo-like virus 5 (GenBank accession: QHA33869.1; amino acid identity: 80.8–82.31%). The other novel viruses, Hanko sobemovirus (amino acid identity: 83.46%), Ilomantsi sobemovirus (amino acid identity: 84.69–86.06%) and Joensuu sobemovirus (amino acid identity: 83.7–86.07%), in turn, were most similar with Atrato sobemo-like virus 4 (GenBank accession: QHA33876.1). Six sequences (Table 2) shared a high protein similarity with Evros sobemo-like virus (GenBank accession: QRD99867.1/QRD99868.1; amino acid identity: 97.6–98.86%).
Figure 7.
Maximum likelihood tree of Solemoviridae. Tentative novel viruses are displayed in red and the mosquito species from which they were derived are in parentheses. Sequences from GenBank are black and display the following information after the virus or species name: “(sampled organism(s)|collection country, collection year)”. Tip colours represent the tribe of mosquito from which viruses were obtained. Tip shape represents the continent or region from which the specimens were collected. Trees were constructed from amino acid sequences of virus polymerases >1000 nt, aligned with MAFFT and computed with IQ-TREE2 using ModelFinder and 1000 bootstraps.
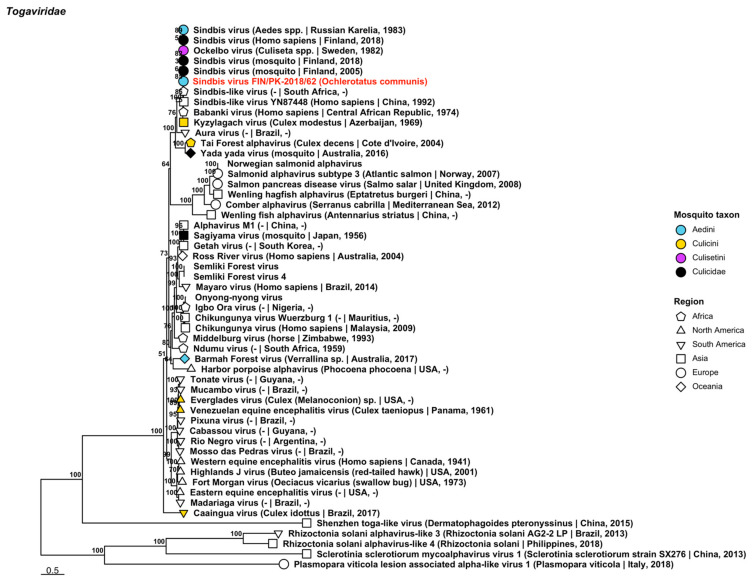
One variant of Sindbis virus (Togaviridae) was sequenced from a pool of Oc. communis collected on 26 June 2015 in Mekrijärvi, Pohjois-Karjala (Figure 8, Table 2). It was closely related to another Finnish mosquito-derived strain (GenBank accession: AFL65801.1; amino acid identity: 99.76%). This new variant is of note as it is the first mosquito species in Finland that has been definitively linked with Sindbis virus, which causes human disease outbreaks in the country.
Figure 8.
Maximum likelihood tree of Togaviridae. The novel strain of Sindbis virus is displayed in red and was derived from Oc. communis. Sequences from GenBank are black and display the following information after the virus or species name: “(sampled organism(s)|collection country, collection year)”. Tip colours represent the tribe of mosquito from which viruses were obtained. Tip shape represents the continent or region from which the specimens were collected. Trees were constructed from amino acid sequences of virus polymerases >1000 nt, aligned with MAFFT and computed with IQ-TREE2 using ModelFinder and 1000 bootstraps.
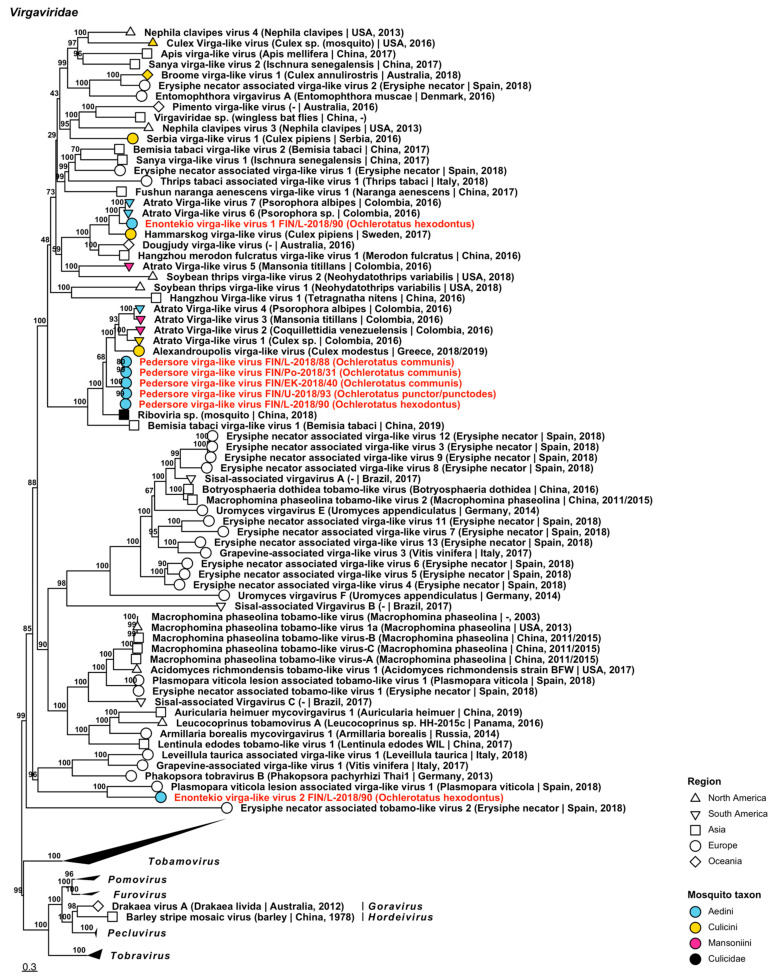
Seven variants of viruses that were closely related to plant-specific viruses in Virgaviridae were recovered, belonging to three viruses (Figure 9, Table 2). They did not, however, cluster with established virgavirus genera in the ML tree, and as such were all named virga-like viruses “Enontekio virga-like virus 1 and 2” and “Pedersore virga-like virus”. The closest matches for these three novel viruses were as follows: Enontekio virga-like virus 1 was closest to mosquito-derived Atrato virga-like virus 6 (GenBank accession: QHA33758.1; amino acid identity: 62.86%) from Columbia; Enontekio virga-like virus 2 was distantly similar to the plant pathogen Plasmopara viticola lesion associated virga-like virus 1 (GenBank accession: QHD64722.1; amino acid identity: 34.46%) from Spain; and Pedersore virga-like virus was similar to an unnamed RNA virus which was sequenced from mosquitoes in China (GenBank accession: QTW97796.1; amino acid identity: 63.74–65.16%) as well as Atrato virga-like virus 3 (GenBank accession: QHA33742.1; amino acid identity: 48.74–56.47%), a mosquito-derived virus from Columbia.
Figure 9.
Maximum likelihood tree of Virgaviridae. Tentative novel viruses are displayed in red and the mosquito species from which they were derived are in parentheses. Sequences from GenBank are black and display the following information after the virus or species name: “(sampled organism(s)|collection country, collection year)”. Tip colours represent the tribe of mosquito from which viruses were obtained. Tip shape represents the continent or region from which the specimens were collected. Trees were constructed from amino acid sequences of virus polymerases >1000 nt, aligned with MAFFT and computed with IQ-TREE2 using ModelFinder and 1000 bootstraps.
3.1.2. Negative-Sense ssRNA Virus Sequences
Negative-sense ssRNA viruses belonging to nine virus families, Aliusviridae, Aspiviridae, Chuviridae, Phasmaviridae, Phenuiviridae, Qinviridae, Rhabdoviridae, Xinmoviridae and Yueviridae were recovered during this study. The −ssRNA viruses are listed below, with all tentative variant names and associated mosquito species in Table 3 and Table 4.
Table 3.
Novel −ssRNA viruses sequenced from Finnish mosquitoes, part 1. Where more than one virus was sequenced from a pool, an additional code was appended to the pool number.
| Virus Family/ Taxon |
Virus Name | Pool/Variant No. | Associated Mosquito Species |
GenBank Accession |
|---|---|---|---|---|
| Aliusviridae | Lestijarvi obscuruvirus | FIN/KP-2018/32 | Oc. communis | ON955144 |
| Aspiviridae | Kilpisjarvi aspivirus | FIN/L-2018/90 | Oc. hexodontus | ON955145 |
| Chuviridae | Hattula chuvirus | FIN/L-2018/01-1 FIN/L-2018/01-2 FIN/L-2018/02 FIN/PP-2018/10-1 FIN/PP-2018/10-2 FIN/PP-2018/28-1 FIN/PP-2018/28-2 FIN/KH-2018/29 FIN/KP-2018/32 FIN/KS-2018/35 FIN/EK-2018/40 FIN/PK-2018/74 |
Oc. hexodontus
Oc. hexodontus Oc. hexodontus Oc. communis Oc. communis Oc. intrudens Oc. intrudens Oc. pullatus Oc. communis Oc. communis Oc. communis Oc. communis |
ON955150 ON955151 ON955152 ON955154 ON955155 ON955156 ON955157 ON955147 ON955148 ON955149 ON955146 ON955153 |
| Chuviridae | Kustavi chuvirus 1 | FIN/VS-2018/17 | Oc. caspius | ON955158 |
| Chuviridae | Kustavi chuvirus 2 | FIN/VS-2018/17 | Oc. caspius | ON955159 |
| Phasmaviridae | Hameenlinna orthophasmavirus 1 | FIN/EK-2018/40 FIN/KH-2018/48 FIN/Pi-2018/51 FIN/Pi-2018/52 |
Oc. communis
Oc. communis Oc. communis Oc. communis |
ON955160 ON955161 ON955162 ON955163 |
| Phasmaviridae | Hameenlinna orthophasmavirus 2 | FIN/EK-2018/40 FIN/KH-2018/48 |
Oc. communis
Oc. communis |
ON955164 ON955165 |
| Phasmaviridae | Kuusamo orthophasmavirus 1 | FIN/PP-2018/83 | Oc. communis | ON955166 |
| Phasmaviridae | Kuusamo orthophasmavirus 2 | FIN/PP-2018/83 | Oc. communis | ON955167 |
| Phasmaviridae | Kuusamo orthophasmavirus 3 | FIN/PP-2018/83 | Oc. communis | ON955168 |
| Phasmaviridae | Kuusamo orthophasmavirus 4 | FIN/EK-2018/40 FIN/PP-2018/83 |
Oc. communis
Oc. communis |
ON955169 ON955170 |
| Phasmaviridae | Lestijarvi orthophasmavirus 1 | FIN/KP-2018/34 | Oc. intrudens | ON955171 |
| Phasmaviridae | Lestijarvi orthophasmavirus 2 | FIN/KP-2018/34 | Oc. intrudens | ON955172 |
Table 4.
−ssRNA viruses sequenced from Finnish mosquitoes, part 2. Previously described viruses are shaded grey.
| Virus Family/ Taxon | Virus Name | Pool/Variant No. | Associated Mosquito Species |
GenBank Accession |
|---|---|---|---|---|
| Phenuiviridae | Hameenlinna phasivirus | FIN/KP-2018/34-1 FIN/KP-2018/34-2 FIN/KP-2018/34-3 FIN/KH-2018/38-1 FIN/KH-2018/38-2 FIN/KH-2018/38-3 FIN/PK-2018/57-1 FIN/PK-2018/57-2 FIN/PK-2018/57-3 FIN/PK-2018/58-1 FIN/PK-2018/58-2 FIN/PK-2018/61 FIN/PK-2018/65-1 FIN/PK-2018/65-2 FIN/PK-2018/66-1 FIN/PK-2018/66-2 FIN/PK-2018/68 FIN/PK-2018/69-1 FIN/PK-2018/69-2 FIN/PK-2018/69-3 FIN/PK-2018/73-1 FIN/PK-2018/73-2 FIN/PK-2018/73-3 FIN/PK-2018/73-4 FIN/PK-2018/74-1 FIN/PK-2018/74-2 FIN/PK-2018/74-3 FIN/PK-2018/74-4 FIN/PK-2018/75-1 FIN/PK-2018/75-2 FIN/PK-2018/77-1 FIN/PK-2018/77-2 FIN/PK-2018/80-1 FIN/PK-2018/80-2 FIN/PK-2018/80-3 |
Oc. intrudens
Oc. intrudens Oc. intrudens Oc. intrudens Oc. intrudens Oc. intrudens Oc. intrudens Oc. intrudens Oc. intrudens Oc. diantaeus Oc. diantaeus Oc. intrudens Oc. intrudens Oc. intrudens Oc. intrudens Oc. intrudens Oc. intrudens Oc. intrudens Oc. intrudens Oc. intrudens Oc. intrudens Oc. intrudens Oc. intrudens Oc. intrudens Oc. communis Oc. communis Oc. communis Oc. communis Oc. intrudens Oc. intrudens Oc. communis Oc. communis Oc. intrudens Oc. intrudens Oc. intrudens |
ON955181 ON955182 ON955183 ON955178 ON955179 ON955180 ON955184 ON955185 ON955186 ON955187 ON955188 ON955189 ON955190 ON955191 ON955192 ON955193 ON955138 ON955194 ON955195 ON955196 ON955197 ON955198 ON955199 ON955200 ON955201 ON955202 ON955203 ON955204 ON955205 ON955206 ON955207 ON955208 ON955209 ON955210 ON955211 |
| Phenuiviridae | Enontekio phenui-like virus 1 | FIN/L-2018/90 | Oc. hexodontus | ON955173 |
| Phenuiviridae | Enontekio phenui-like virus 2 | FIN/EK-2018/91 | Oc. communis | ON955174 |
| Phenuiviridae | Enontekio phenui-like virus 3 | FIN/L-2018/90 | Oc. hexodontus | ON955175 |
| Phenuiviridae | Enontekio phenui-like virus 4 | FIN/U-2018/93 | Oc. punctor/punctodes | ON955176 |
| Phenuiviridae | Enontekio phenui-like virus 5 | FIN/L-2018/90 | Oc. hexodontus | ON955177 |
| Phenuiviridae | Hanko phenui-like virus 1 | FIN/U-2018/96 | Oc. caspius | ON955212 |
| Phenuiviridae | Hanko phenui-like virus 2 | FIN/U-2018/93 | Oc. punctor/punctodes | ON955213 |
| Phenuiviridae | Hanko phenui-like virus 3 | FIN/U-2018/93 | Oc. punctor/punctodes | ON955214 |
| Phenuiviridae | Ilomantsi phenui-like virus | FIN/PK-2018/62 | Oc. communis | ON955215 |
| Phenuiviridae | Kalajoki phenui-like virus 1 | FIN/PP-2018/10 FIN/PK-2018/21 FIN/KP-2018/34-1 FIN/KP-2018/34-2 FIN/PK-2018/62 FIN/PK-2018/70 |
Oc. communis
Oc. communis Oc. intrudens Oc. intrudens Oc. communis Oc. communis |
ON955221 ON955218 ON955216 ON955217 ON955219 ON955220 |
| Phenuiviridae | Kalajoki phenui-like virus 2 | FIN/PP-2018/10 FIN/KH-2018/48 FIN/Pi-2018/51 FIN/PK-2018/59 |
Oc. communis
Oc. communis Oc. communis Oc. communis |
ON955225 ON955222 ON955223 ON955224 |
| Phenuiviridae | Palkane phenui-like viruses 1 | FIN/Pi-2018/55 | Oc. communis | ON955226 |
| Phenuiviridae | Palkane phenui-like viruses 2 | FIN/Pi-2018/52 FIN/Pi-2018/53 FIN/EK-2018/91 |
Oc. communis
Oc. communis Oc. communis |
ON955228 ON955229 ON955227 |
| Qinviridae | Ilomantsi qinvirus | FIN/PK-2018/62 | Oc. communis | ON955230 |
| Qinviridae | Kalajoki qinvirus | FIN/PP-2018/10 FIN/Pi-2018/54 FIN/PK-2018/60 |
Oc. communis
Oc. communis Oc. communis |
ON955233 ON955231 ON955232 |
| Qinviridae | Palkane qinvirus | FIN/Pi-2018/54 FIN/PK-2018/60-1 FIN/PK-2018/60-2 |
Oc. communis
Oc. communis Oc. communis |
ON955234 ON955235 ON955236 |
| Rhabdoviridae | Enontekio merhavirus | FIN/L-2018/90 | Oc. hexodontus | ON955141 |
| Rhabdoviridae | Enontekio ohlsrhavirus | FIN/L-2018/30-1 FIN/L-2018/30-2 FIN/L-2018/30-3 FIN/L-2018/89 |
Oc. hexodontus
Oc. hexodontus Oc. hexodontus Oc. hexodontus |
ON955237 ON955238 ON955239 ON955240 |
| Rhabdoviridae | Enontekio rhabdovirus | FIN/L-2018/03 | Oc. punctor/punctodes | ON955241 |
| Rhabdoviridae | Hattula rhabdovirus | FIN/KH-2018/29 FIN/KS-2018/35-1 FIN/KS-2018/35-2 FIN/PK-2018/59-1 FIN/PK-2018/59-2 FIN/PK-2018/62 FIN/PK-2018/76-1 FIN/PK-2018/76-2 FIN/L-2018/86-1 FIN/L-2018/86-2 |
Oc. pullatus
Oc. communis Oc. communis Oc. communis Oc. communis Oc. communis Oc. communis Oc. communis Oc. hexodontus Oc. hexodontus |
ON955242 ON955243 ON955244 ON955247 ON955248 ON955142 ON955249 ON955250 ON955245 ON955246 |
| Rhabdoviridae | Inari rhabdovirus | FIN/L-2018/84 | Oc. excrucians | ON955143 |
| Rhabdoviridae | Joutseno rhabdovirus 1 | FIN/EK-2018/91 | Oc. communis | ON955251 |
| Rhabdoviridae | Joutseno rhabdovirus 2 | FIN/EK-2018/91 | Oc. communis | ON955252 |
| Rhabdoviridae | Ohlsdorf virus | FIN/L-2018/07 FIN/L-2018/84 |
Oc. excrucians
Oc. excrucians |
ON955253 ON955254 |
| Xinmoviridae | Enontekio anphevirus 1 | FIN/L-2018/90 | Oc. hexodontus | ON955255 |
| Xinmoviridae | Enontekio anphevirus 2 | FIN/L-2018/90 | Oc. hexodontus | ON955256 |
| Xinmoviridae | Hanko anphevirus | FIN/U-2018/96 | Oc. caspius | ON955257 |
| Xinmoviridae | Joensuu anphevirus | FIN/PK-2018/74 FIN/PP-2018/82 FIN/PP-2018/83-1 FIN/PP-2018/83-2 FIN/U-2018/93-1 FIN/U-2018/93-2 |
Oc. communis
Oc. communis Oc. communis Oc. communis Oc. punctor/punctodes Oc. punctor/punctodes |
ON955258 ON955259 ON955260 ON955261 ON955262 ON955263 |
| Yueviridae | Enontekio yuevirus | FIN/L-2018/90 | Oc. caspius | ON955264 |
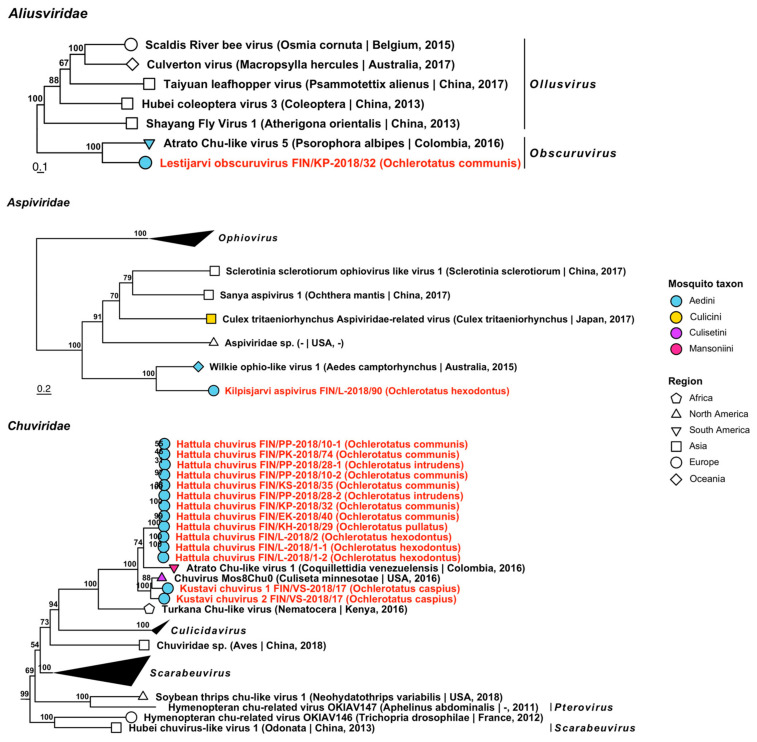
Aliusviridae is comprised of two genera, Ollusvirus and Obscuruvirus, and its member species have previously been from insects. One novel virus belonging to Obscuruvirus was sequenced from a pool of Oc. communis, which was tentatively named “Lestijarvi obscuruvirus” (Figure 10, Table 3). It was most similar to Atrato chu-like virus 5 (GenBank accession: QHA33675.1; amino acid identity: 41.87%), which was sequenced from Psorophora ciliata, an aedine mosquito from Columbia.
Figure 10.
Maximum likelihood trees of Aliusviridae, Aspiviridae and Chuviridae. Tentative novel viruses are displayed in red and the mosquito species from which they were derived are in parentheses. Sequences from GenBank are black and display the following information after the virus or species name: “(sampled organism(s)|collection country, collection year)”. Tip colours represent the tribe of mosquito from which viruses were obtained. Tip shape represents the continent or region from which the specimens were collected. Trees were constructed from amino acid sequences of virus polymerases >1000 nt, aligned with MAFFT and computed with IQ-TREE2 using ModelFinder and 1000 bootstraps.
3.1.3. Negative-Sense ssRNA Virus Sequences
Negative-sense ssRNA viruses belonging to nine virus families, Aliusviridae, Aspiviridae, Chuviridae, Phasmaviridae, Phenuiviridae, Qinviridae, Rhabdoviridae, Xinmoviridae and Yueviridae were recovered during this study. The −ssRNA viruses are listed below, with all tentative variant names and associated mosquito species in Table 3 and Table 4.
Aliusviridae is comprised of two genera, Ollusvirus and Obscuruvirus, and its member species have previously been from insects. One novel virus belonging to Obscuruvirus was sequenced from a pool of Oc. communis, which was tentatively named “Lestijarvi obscuruvirus” (Figure 10, Table 3). It was most similar to Atrato chu-like virus 5 (GenBank accession: QHA33675.1; amino acid identity: 41.87%), which was sequenced from Psorophora ciliata, an aedine mosquito from Columbia.
Similarly, one virus grouped with Aspiviridae, a plant pathogenic family of viruses, and was tentatively named “Kilpisjarvi aspivirus” (Figure 10, Table 3). Its closest match was Wilkie ophio-like virus 1 (GenBank accession: ASA47457.1; amino acid identity: 50.45%), which was derived from a mosquito from Western Australia.
Thirteen variants from ten mosquito pools belonging to Chuviridae (arthropod-associated) were sequenced and grouped into three tentative novel species: “Hattula chuvirus” and “Kustavi chuvirus 1 and 2” (Figure 10, Table 3). By comparing amino acid identities, Hattula chuvirus is most similar to Atrato chu-like virus 1, which was detected in Coquillettidia venezuelensis from Colombia (GenBank accession: QHA33913.1, QHA33917.1; amino acid identity: 69.29–70.66%); and to Chuvirus Mos8Chu0 which was detected in Culiseta minnesotae from the USA (GenBank accession: API61887.1; amino acid identity: 51.79–63.21%). Kustavi chuviruses 1 and 2 were also most similar to Chuvirus Mos8Chu0 (amino acid identities: 82.24% and 79.7%, respectively); thus, all of the three novel species were most closely related to mosquito-derived chuviruses from the Americas.
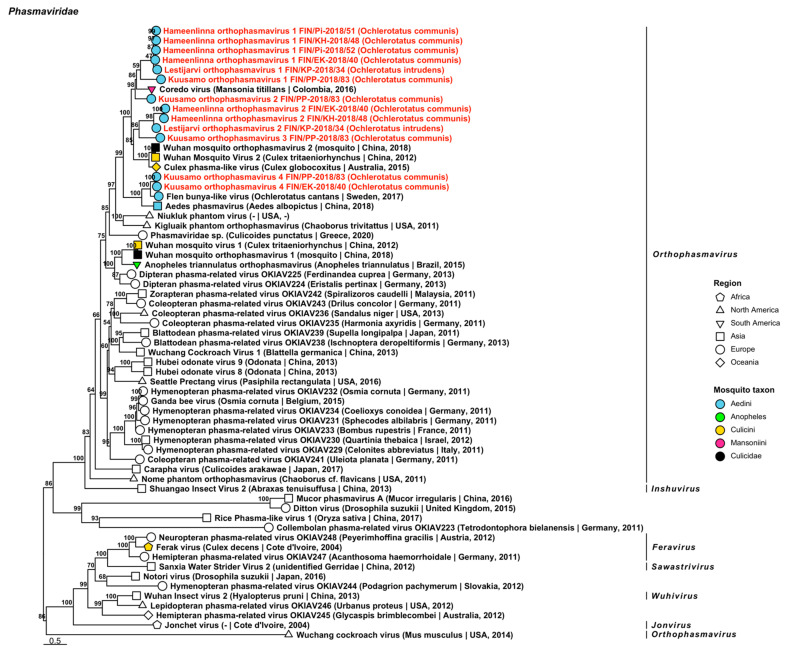
Eight novel viruses closely related to species in genus Orthophasmavirus from family Phasmaviridae were identified from six mosquito pools comprised of Oc. communis or Oc. intrudens (Figure 11, Table 3). These include the tentatively named “Hameenlinna orthophasmavirus 1 and 2”, “Kuusamo orthophasmavirus 1 to 4” and “Lestijarvi orthophasmavirus 1 and 2”. Hameenlinna orthophasmavirus 1 is most similar to Coredo virus (GenBank accession: QHA33845.1; amino acid identity: 59.25–61.89%), a mosquito-derived virus from Mansoniini mosquitoes in Colombia. Hameenlinna orthophasmavirus 2 had a weak similarity to both Wuhan mosquito orthophasmavirus 2 (GenBank accession: QTW97787.1; amino acid identity: 36.14%) and Culex phasma-like virus (officially Culex orthophasmavirus) (GenBank accession: YP_010085109.1; amino acid identity: 39.08%), mosquito-derived viruses from China and Australia, respectively. Kuusamo orthophasmavirus 1 had a low similarity to its closest matching virus, Coredo virus (amino acid identity: 41%) and Kuusamo orthophasmavirus 2 has a slightly higher similarity to Coredo virus (amino acid identity: 67.6%). Kuusamo orthophasmavirus 3 was most similar to Culex phasma-like virus (GenBank accession: ASA47365.1; amino acid identity: 45.95%) from Australia, and Kuusamo orthophasmavirus 4 to Flen bunya-like virus (GenBank accession: QGA87322.1; amino acid identity: 62.26–71.76%) from Oc. cantans that were collected in Sweden. Lastly, Lestijarvi orthophasmavirus 1 was similar to Coredo virus (amino acid identity: 64.1%) and Lestijarvi orthophasmavirus 2 to Culex phasma-like virus (GenBank accession: QHA33850.1; amino acid identity: 40.92%), the latter of which was derived from Columbian Culex.
Figure 11.
Maximum likelihood tree of Phasmaviridae. Tentative novel viruses are displayed in red and the mosquito species from which they were derived are in parentheses. Sequences from GenBank are black and display the following information after the virus or species name: “(sampled organism(s)|collection country, collection year)”. Tip colours represent the tribe (Culicinae) or genus (Anophelinae) of mosquito from which viruses were obtained. Tip shape represents the continent or region from which the specimens were collected. Trees were constructed from amino acid sequences of virus polymerases >1000 nt, aligned with MAFFT and computed with IQ-TREE2 using ModelFinder and 1000 bootstraps.
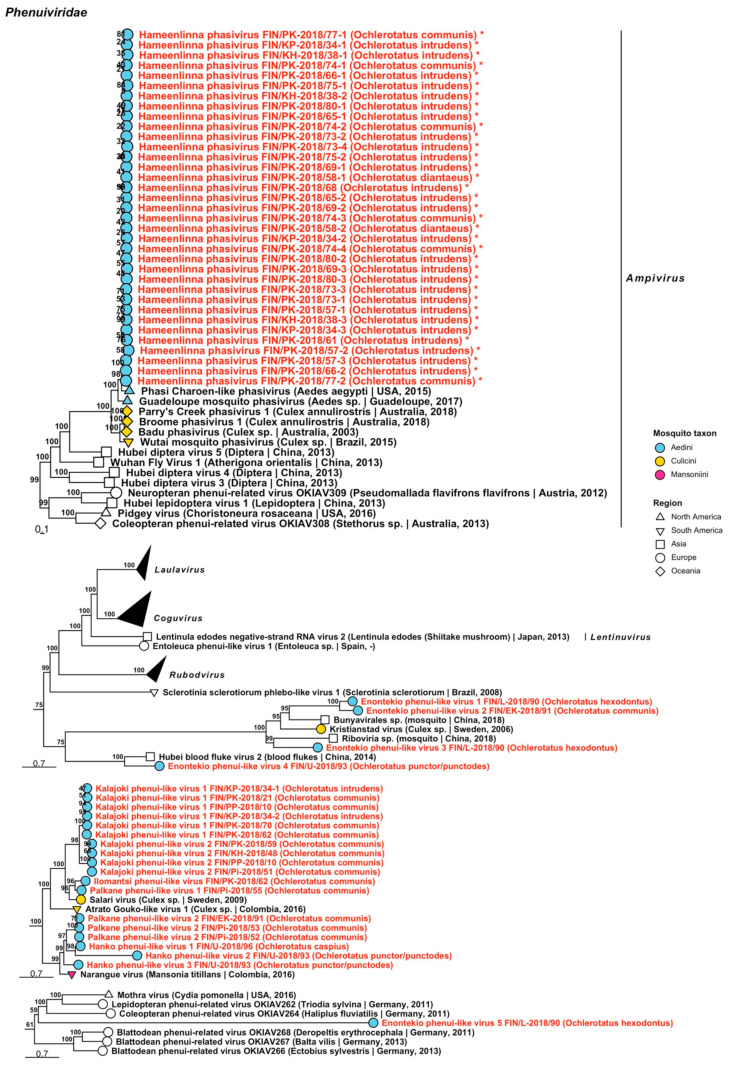
Family Phenuiviridae mainly includes arthropod-specific and vector-borne viruses that primarily infect mammals. We detected one sequence representing a novel virus belonging to genus Phasivirus and 13 phenui-like viruses (Figure 12, Table 4). These were tentatively named “Hameenlinna phasivirus”, “Enontekio phenui-like virus 1 to 5”, “Hanko phenui-like viruses 1 to 3”, “Ilomantsi phenui-like virus”, “Kalajoki phenui-like viruses 1 and 2” and “Palkane phenui-like virus 1 and 2”. The complete genome of Hameenlinna phasivirus was sequenced (GenBank accession ON955138) and was most similar to Phasi Charoen-like phasivirus (GenBank accession: QEM39210.1, QHT65014.1, QKV44090.1, QKV44092.1, QKV44096.1, QKV44098.1, QKV44099.1, QKV44101.1, QKV44103.1, QKV44109.1, QPF16713.1, YP_009505332.1; amino acid identity: 62.78–87.14%). The closest matching viruses by amino acid identity for the putative novel phenui-like viruses were as follows: Enontekio phenui-like virus 1 had a low similarity to an unnamed bunyavirus that was sequenced from a Chinese mosquito (GenBank accession: QTW97784.1; amino acid identity: 34.56%); Enontekio phenui-like virus 2 to Kristianstad virus, a virus described from Sweden that was sequenced from a Culex mosquito [31] (GenBank accession: QGA70932.1; amino acid identity: 34.27%) despite clustering together with Enontekio phenui-like virus 1 and the unnamed bunyavirus sequence (amino acid identity: 35.63%); Enontekio phenui-like virus 3 and Enontekio phenui-like virus 5 to an unnamed RNA virus (GenBank accession: QTW97783.1; amino acid identities: 35.6% and 37.5%); and Enontekio phenui-like virus 4 to Hubei blood fluke virus 2 (GenBank accession: APG79250.1; amino acid identity: 54.2%). Curiously, a phylogenetic analysis suggested that Enontekio phenui-like virus 5 was highly divergent compared to other phenui-related viruses. Hanko phenui-like viruses 1 to 3 were distantly similar to Narangue virus (officially Narangue mobuvirus) (GenBank accession: QHA33858.1; protein identities: 51.77%, 39.16% and 65.15%, correspondingly. Ilomantsi phenui-like virus and Kalajoki phenui-like viruses 1 and 2 matched partially with Salari virus (GenBank accession: QGA70945.1; amino acid identities: 60.64%, 39.15–49.47% and 37.5–53.78%). Lastly, Palkane phenui-like virus 1 also matched closely to Salari virus (amino acid identity: 69.63%), while Palkane phenui-like virus 2 (FIN/Pi-2018/52, FIN/Pi-2018/53 and FIN/EK-2018/91) shared the highest amino acid identity with Narangue virus (GenBank accession: QHA33858.1; amino acid identity: 62.44–68.81%).
Figure 12.
Maximum likelihood subtrees of Phenuiviridae. Tentative novel viruses are displayed in red and the mosquito species from which they were derived are in parentheses. Sequences from GenBank are black and display the following information after the virus or species name: “(sampled organism(s)|collection country, collection year)”. Tip colours represent the tribe of mosquito from which viruses were obtained. Tip shape represents the continent or region from which the specimens were collected. Trees were constructed from amino acid sequences of virus polymerases >1000 nt, aligned with MAFFT and computed with IQ-TREE2 using ModelFinder and 1000 bootstraps. Asterisks denote that the complete genome was recovered.
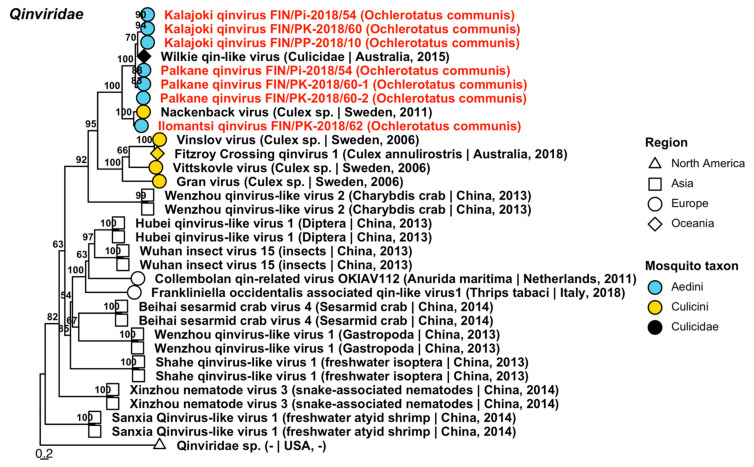
Three novel variants of Qinviridae were detected from pools of Oc. communis (Figure 13, Table 4), which were provisionally named “Ilomantsi qinvirus”, “Kalajoki qinvirus” and “Palkane qinvirus”. The first one was distantly similar to Nackenback virus (GenBank accession: QGA70919.1; amino acid identity: 63.3%), which was detected in Sweden from a Culex mosquito, while the two others were distantly similar to Wilkie qin-like viruses (GenBank accessions: ASA47357.1 and ASA47455.1; amino acid identities: 54.5–58.2% and 56.61–75.3%).
Figure 13.
Maximum likelihood tree of Qinviridae. Tentative novel viruses are displayed in red and the mosquito species from which they were derived are in parentheses. Sequences from GenBank are black and display the following information after the virus or species name: “(sampled organism(s)|collection country, collection year)”. Tip colours represent the tribe of mosquito from which viruses were obtained. Tip shape represents the continent or region from which the specimens were collected. Trees were constructed from amino acid sequences of virus polymerases >1000 nt, aligned with MAFFT and computed with IQ-TREE2 using ModelFinder and 1000 bootstraps.
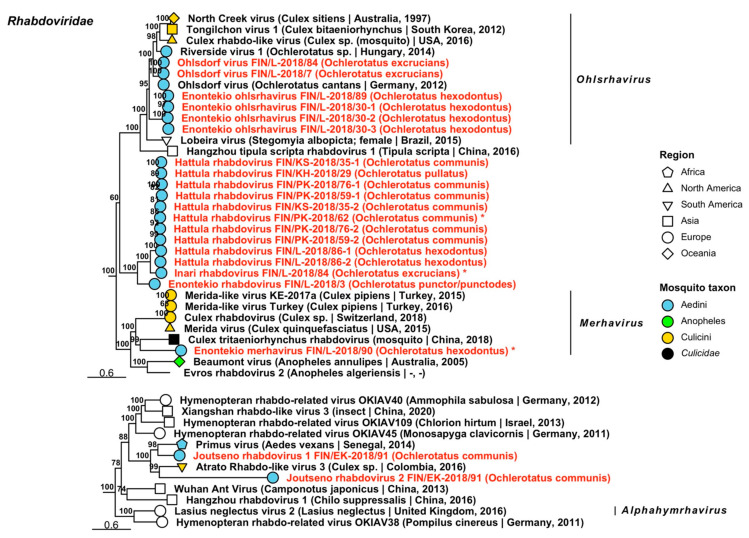
Twenty-one variants of Rhabdoviridae, viruses which infect vertebrates, invertebrates and plants, were sequenced from 13 mosquito pools and grouped into eight viruses (Figure 14, Table 4). Seven of these were novel tentative rhabdoviruses and one an established species. Of the tentative novel viruses, two fell within established genera, “Enontekio merhavirus” (Merhavirus) and “Enontekio ohlsrhavirus” (Ohlsrhavirus), while the remaining species, “Enontekio rhabdovirus”, “Hattula rhabdovirus”, “Inari rhabdovirus”, “Joutseno rhabdovirus 1” and “Joutseno rhabdovirus 2” did not. Two variants of Ohlsdorf virus (officially Ohlsdorf ohlsrhavirus) were also sequenced, which were nearly identical to the originally described virus from Oc. cantans mosquitoes from Germany [32] (GenBank accessions: YP_010086786.1; amino acid identity: 97.87–98.31%). Enontekio merhavirus had a low similarity to Culex tritaeniorhynchus rhabdovirus (officially Tritaeniorhynchus merhavirus) (GenBank accession: BBQ05111.1; amino acid identity: 42.06%), while Enontekio ohlsrhavirus had a moderate similarity to both Ohlsdorf virus (GenBank accessions: ATG83565.1, ATG83567.1 and YP_010086786.1; amino acid identity: 55.01–66.93%) and Riverside virus 1 (Riverside ohlsrhavirus), described from Ochlerotatus sp. mosquitoes from Hungary [33] (GenBank accession: AMJ52368.1; amino acid identity: 75.39%). Enontekio rhabdovirus shared a low amino acid identity with Culex rhabdovirus detected from Culex sp. mosquitoes in California, USA [34] (GenBank accession: AXQ04764.1; amino acid identity: 41.06%), Hattula rhabdovirus to Culex rhabdo-like virus (officially Culex ohlsrhavirus) (GenBank accessions: ASA47473.1; amino acid identity: 63.04%), Merida virus (officially Merida merhavirus) (Culex pipiens/torrentium, Sweden) (GenBank accessions: QGA70896.1 and YP_009552115.1; amino acid identity: 31.2–36.41%), Ohlsdorf virus (GenBank accession: ATG83563.1, ATG83566.1 and YP_010086786.1; amino acid identity: 38.4–45.43%) and Perinet vesiculovirus detected in Madagascar (GenBank accession: YP_009094388.1; amino acid identity: 45.78–46.12%); Inari rhabdovirus to Ohlsdorf virus (GenBank accession: ATG83565.1; amino acid identity: 40.96%); and both Joutseno rhabdovirus 1 and 2 to Primus virus, detected from Aedes vexans in Senegal (GenBank accession: QIS62334.1; amino acid identities: 70.55% and 48.48%, respectively). Complete genomes were sequenced for Enontekio merhavirus, Hattula rhabdovirus and Inari rhabdovirus (GenBank accessions ON955141, ON955142 and ON955143, respectively).
Figure 14.
Maximum likelihood subtrees of Rhabdoviridae. Tentative novel viruses are displayed in red and the mosquito species from which they were derived are in parentheses. Sequences from GenBank are black and display the following information after the virus or species name: “(sampled organism(s)|collection country, collection year)”. Tip colours represent the tribe (Culicinae) or genus (Anophelinae) of mosquito from which viruses were obtained. Tip shape represents the continent or region from which the specimens were collected. Trees were constructed from amino acid sequences of virus polymerases >1000 nt, aligned with MAFFT and computed with IQ-TREE2 using ModelFinder and 1000 bootstraps. Asterisks denote that the complete genome was recovered.
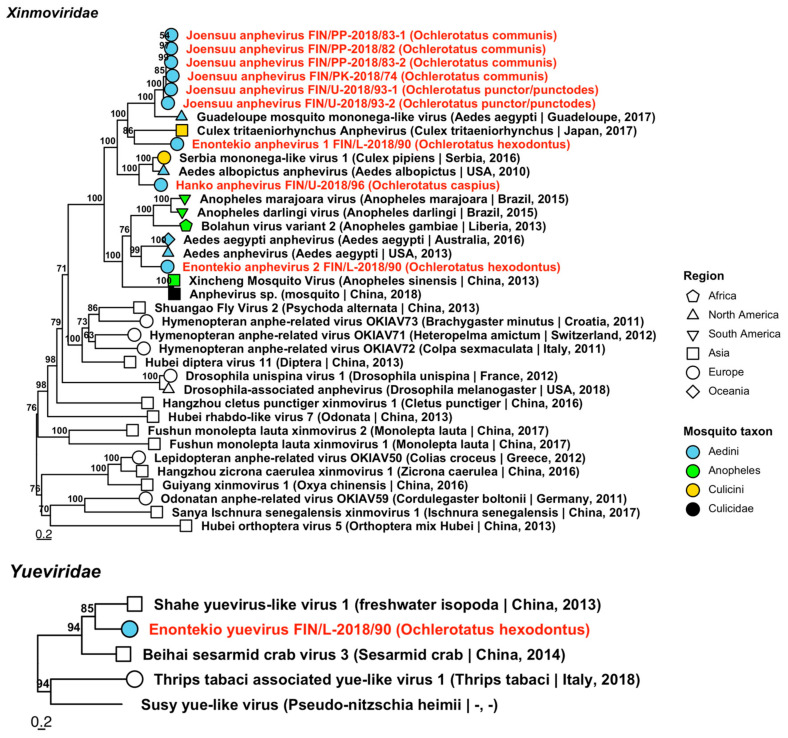
Xinmoviridae includes member species that have been isolated from insects. Nine sequences from four mosquito pools grouped into four novel species, which were tentatively named “Enontekio anphevirus 1 and 2”, “Hanko anphevirus” and “Joensuu anphevirus” (Figure 15, Table 4). The closest sequences available on GenBank for each of these novel species were as follows: Enontekio anphevirus 1 had a medium protein similarity with Culex tritaeniorhynchus anphevirus (GenBank accession: BBQ04822.1; amino acid identity: 53.53%), which was sequenced from Japanese Culex mosquitoes; Enontekio anphevirus 2 with Aedes anphevirus (GenBank accession: AWW13453.1; amino acid identity: 60.48%), from a colony of aedine mosquitoes from Thailand; Hanko anphevirus with Serbia mononega-like virus 1 (GenBank accession: QNS17450.1; amino acid identity: 57.88%) from Serbian specimens of Culex pipiens; and Joensuu anphevirus with Guadeloupe mosquito mononega-like virus (GenBank accession: QEM39171.1; amino acid identity: 49.73–70.95%) in aedine mosquitoes from Guadeloupe. The variant sequences were detected in pools of Oc. caspius, Oc. communis, Oc. hexodontus and Oc. punctor/punctodes from across Finland.
Figure 15.
Maximum likelihood trees of Xinmoviridae and Yueviridae. Tentative novel viruses are displayed in red and the mosquito species from which they were derived are in parentheses. Sequences from GenBank are black and display the following information after the virus or species name: “(sampled organism(s)|collection country, collection year)”. Tip colours represent the tribe (Culicinae) or genus (Anophelinae) of mosquito from which viruses were obtained. Tip shape represents the continent or region from which the specimens were collected. Trees were constructed from amino acid sequences of virus polymerases >1000 nt, aligned with MAFFT and computed with IQ-TREE2 using ModelFinder and 1000 bootstraps.
Yueviridae is another recently validated virus family and includes viruses that have been detected from arthropods and marine diatoms. Among our specimens, we isolated one virus sequence from Oc. hexodontus, which we named “Enontekio yuevirus” (Figure 15, Table 4). It was very distantly similar to Shahe yuevirus-like virus 1 (officially Shahe yuyuevirus) (GenBank accession: YP_009337854.1; amino acid identity: 38.47%), which was sequenced from freshwater isopoda from China.
Finally, while analysing other sequence data that were generated during this study, a fragmentary genome of Inkoo virus (Family Peribunyavirus) was identified. The sequences comprised four contigs of 301 to 630 nucleotides which mapped to the M glycoprotein segment, with >99% nucleotide identity to Russian mosquito-derived strain LEIV-15248Iv (GenBank accession; KT288270). While of a different (polymerase) gene than was included in this study, they are still of interest, as Inkoo virus is pathogenic to humans. The sequences were derived from a pool of 60 Oc. punctor/punctodes (FIN/PK-2018/11), which were collected in late June 2015.
3.1.4. Double-Stranded RNA Virus Sequences
Double-stranded RNA viruses belonging to five established viral families Chrysoviridae, Partitiviridae, Sedoreoviridae, Spinareoviridae and Totiviridae and one proposed family Botybirnaviridae were recovered during the analyses. The dsRNA viruses sequenced in this study are listed, below, with all variant names and associated mosquito species listed in Table 5, Table 6 and Table 7.
Table 5.
dsRNA viruses sequenced from Finnish mosquitoes, part 1. Previously described viruses are shaded grey.
| Virus Family/ Taxon |
Virus Name | Pool/Strain No. | Associated Mosquito Species |
GenBank Accession |
|---|---|---|---|---|
| Botybirnavirus | Palkane botybirna-like virus | FIN/Pi-2018/51-1 FIN/Pi-2018/51-2 FIN/Pi-2018/52 FIN/Pi-2018/53 FIN/Pi-2018/54 FIN/Pi-2018/55 FIN/PK-2018/68 FIN/PK-2018/70 |
Oc. communis
Oc. communis Oc. communis Oc. communis Oc. communis Oc. communis Oc. intrudens Oc. communis |
OP019912 OP019913 OP019914 OP019915 OP019916 OP019917 OP019918 OP019919 |
| Chrysoviridae | Enontekio alphachrysovirus | FIN/L-2018/03 | Oc. punctor/punctodes | OP019837–OP019840 |
| Chrysoviridae | Hanko alphachrysovirus | FIN/U-2018/97 | Oc. caspius | OP019841–OP019844 |
| Chrysoviridae | Lestijarvi alphachrysovirus | FIN/PP-2018/28 FIN/KP-2018/32 FIN/L-2018/88 |
Oc. intrudens
Oc. communis Oc. communis |
OP019911, OP019846–OP019848 OP019910 OP019845 |
Table 6.
dsRNA viruses sequenced from Finnish mosquitoes, part 2, Partitiviridae.
| Virus Family/ Taxon |
Virus Name | Pool/Strain No. | Associated Mosquito Species |
GenBank Accession |
|---|---|---|---|---|
| Partitiviridae | Enontekio alphapartitivirus 1 | FIN/L-2018/90 | Oc. hexodontus | OP019920 |
| Partitiviridae | Enontekio alphapartitivirus 2 | FIN/L-2018/90 | Oc. hexodontus | OP019921 |
| Partitiviridae | Hanko alphapartitivirus 1 | FIN/U-2018/94 FIN/U-2018/95 FIN/U-2018/96 |
Oc. caspius
Oc. caspius Oc. caspius |
OP019929 OP019930 OP019931 |
| Partitiviridae | Hanko alphapartitivirus 2 | FIN/U-2018/96 | Oc. caspius | OP019932 |
| Partitiviridae | Hanko alphapartitivirus 3 | FIN/U-2018/93 | Oc. punctor/punctodes | OP019933 |
| Partitiviridae | Kalajoki alphapartitivirus | FIN/PP-2018/10 FIN/Pi-2018/52 FIN/PK-2018/68 |
Oc. communis
Oc. communis Oc. intrudens |
OP019958 OP019956 OP019957 |
| Partitiviridae | Kuusamo alphapartitivirus | FIN/PP-2018/83 FIN/L-2018/90-1 FIN/L-2018/90-2 |
Oc. communis
Oc. hexodontus Oc. hexodontus |
OP019963 OP019961 OP019962 |
| Partitiviridae | Palkane alphapartitivirus 1 | FIN/Pi-2018/51 FIN/Pi-2018/53 FIN/Pi-2018/55 |
Oc. communis
Oc. communis Oc. communis |
OP019967 OP019968 OP019969 |
| Partitiviridae | Palkane alphapartitivirus 2 | FIN/Pi-2018/53 | Oc. communis | OP019970 |
| Partitiviridae | Enontekio betapartitivirus 1 | FIN/L-2018/90 | Oc. hexodontus | OP019922 |
| Partitiviridae | Enontekio betapartitivirus 2 | FIN/L-2018/90 | Oc. hexodontus | OP019923 |
| Partitiviridae | Kalajoki betapartitivirus | FIN/PP-2018/10 FIN/Pi-2018/51 |
Oc. communis
Oc. communis |
OP019960 OP019959 |
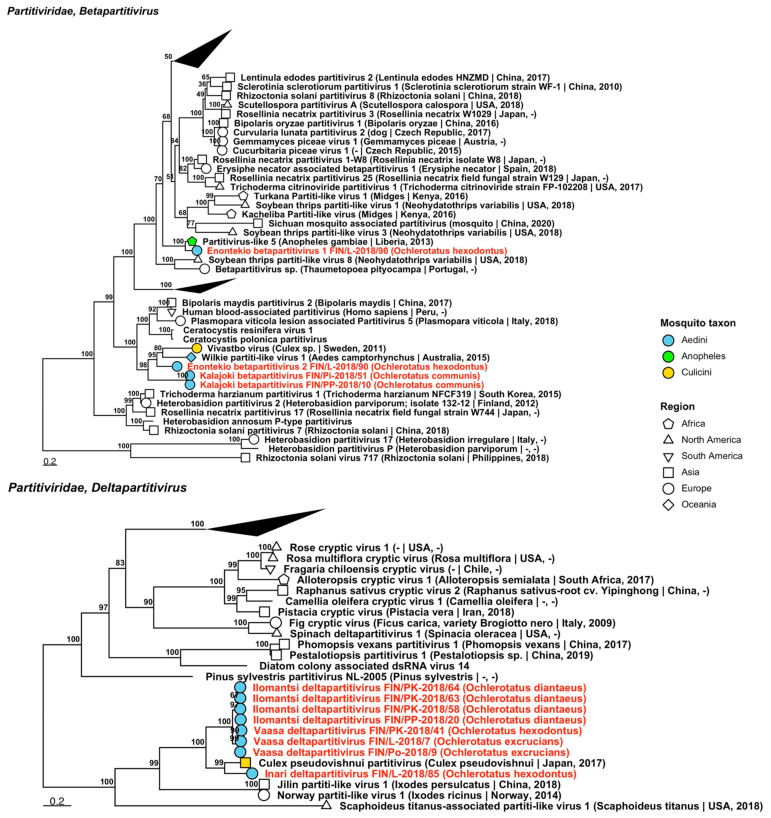
| Partitiviridae | Ilomantsi deltapartitivirus | FIN/PP-2018/20 FIN/PK-2018/58 FIN/PK-2018/63 FIN/PK-2018/64 |
Oc. diantaeus
Oc. diantaeus Oc. diantaeus Oc. diantaeus |
OP019944 OP019941 OP019942 OP019943 |
| Partitiviridae | Inari deltapartitivirus | FIN/L-2018/85 | Oc. hexodontus | OP019955 |
| Partitiviridae | Vaasa deltapartitivirus | FIN/L-2018/07 FIN/Po-2018/09 FIN/PK-2018/41 |
Oc. excrucians
Oc. excrucians Oc. hexodontus |
OP019971 OP019972 OP019973 |
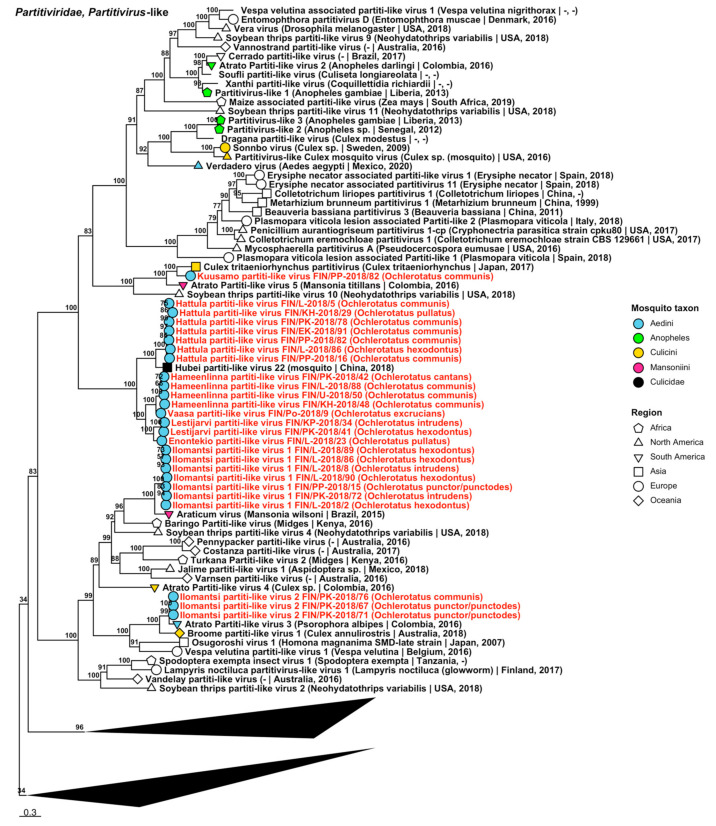
| Partitiviridae | Enontekio partiti-like virus | FIN/L-2018/23 | Oc. pullatus | OP019924 |
| Partitiviridae | Hattula partiti-like virus | FIN/L-2018/05 FIN/PP-2018/16 FIN/KH-2018/29 FIN/PK-2018/78 FIN/PP-2018/82 FIN/L-2018/86 FIN/EK-2018/91 |
Oc. communis
Oc. communis Oc. pullatus Oc. communis Oc. communis Oc. hexodontus Oc. communis |
OP019936 OP019939 OP019935 OP019938 OP019940 OP019937 OP019934 |
| Partitiviridae | Hameenlinna partiti-like virus | FIN/PK-2018/42 FIN/KH-2018/48 FIN/U-2018/50 FIN/L-2018/88 |
Oc. cantans
Oc. communis Oc. communis Oc. communis |
OP019927 OP019925 OP019928 OP019926 |
| Partitiviridae | Ilomantsi partiti-like virus 1 | FIN/L-2018/02 FIN/L-2018/08 FIN/PP-2018/15 FIN/PK-2018/72 FIN/L-2018/86 FIN/L-2018/89 FIN/L-2018/90 |
Oc. hexodontus
Oc. intrudens Oc. punctor/punctodes Oc. intrudens Oc. hexodontus Oc. hexodontus Oc. hexodontus |
OP019945 OP019946 OP019951 OP019950 OP019947 OP019948 OP019949 |
| Partitiviridae | Ilomantsi partiti-like virus 2 | FIN/PK-2018/67 FIN/PK-2018/71 FIN/PK-2018/76 |
Oc. punctor/punctodes
Oc. punctor/punctodes Oc. communis |
OP019952 OP019953 OP019954 |
| Partitiviridae | Kuusamo partiti-like virus | FIN/PP-2018/82 | Oc. communis | OP019964 |
| Partitiviridae | Lestijarvi partiti-like virus | FIN/KP-2018/34 FIN/PK-2018/41 |
Oc. intrudens
Oc. hexodontus |
OP019965 OP019966 |
| Partitiviridae | Vaasa partiti-like virus | FIN/Po-2018/09 | Oc. excrucians | OP019974 |
Table 7.
dsRNA viruses sequenced from Finnish mosquitoes, part 3. Previously described viruses are shaded grey.
| Virus Family/ Taxon | Virus Name | Pool/Strain No. | Associated Mosquito Species | GenBank Accession |
|---|---|---|---|---|
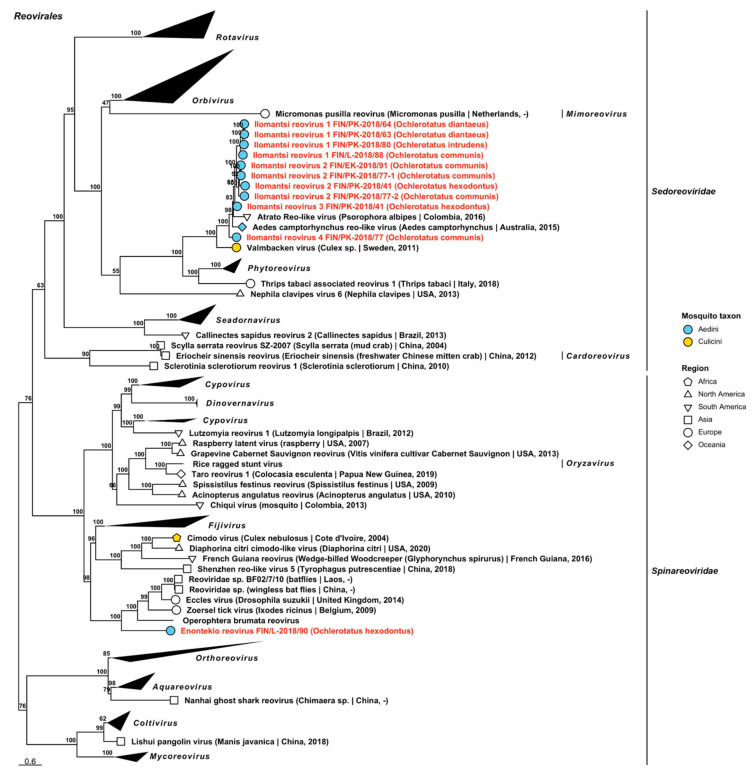
| Sedoreoviridae | Ilomantsi reovirus 1 | FIN/PK-2018/63 FIN/PK-2018/64 FIN/PK-2018/80 FIN/L-2018/88 |
Oc. diantaeus
Oc. diantaeus Oc. intrudens Oc. communis |
OP019977 OP019978 OP019979 OP019976 |
| Sedoreoviridae | Ilomantsi reovirus 2 | FIN/PK-2018/41 FIN/PK-2018/77-1 FIN/PK-2018/77-2 FIN/EK-2018/91 |
Oc. hexodontus
Oc. communis Oc. communis Oc. communis |
OP019981 OP019982 OP019983 OP019980 |
| Sedoreoviridae | Ilomantsi reovirus 3 | FIN/PK-2018/41 | Oc. hexodontus | OP019984 |
| Sedoreoviridae | Ilomantsi reovirus 4 | FIN/PK-2018/77 | Oc. communis | OP019985 |
| Spinareoviridae | Enontekio reovirus | FIN/L-2018/90 | Oc. hexodontus | OP019975 |
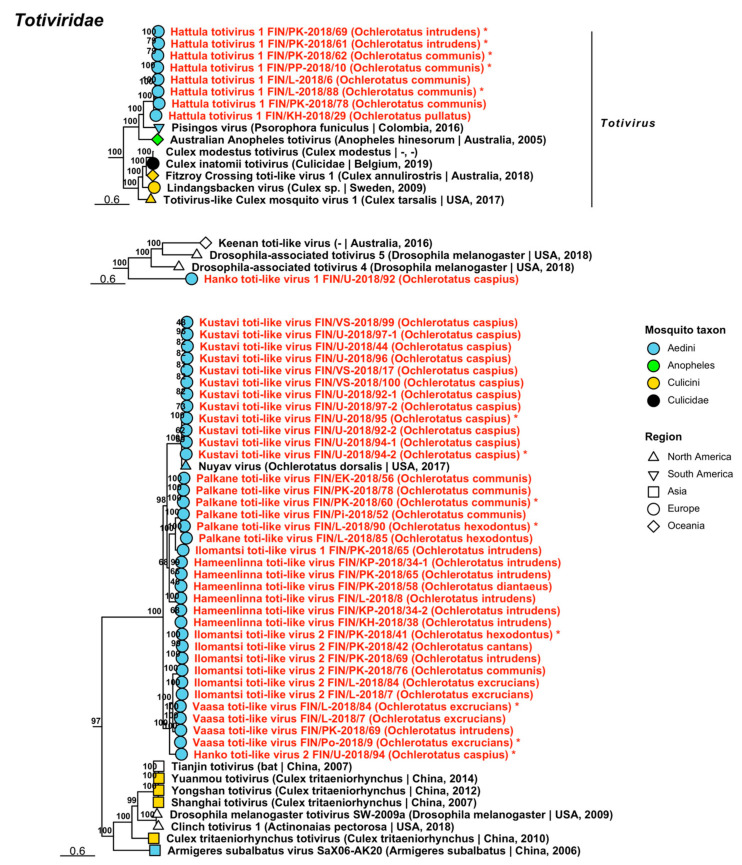
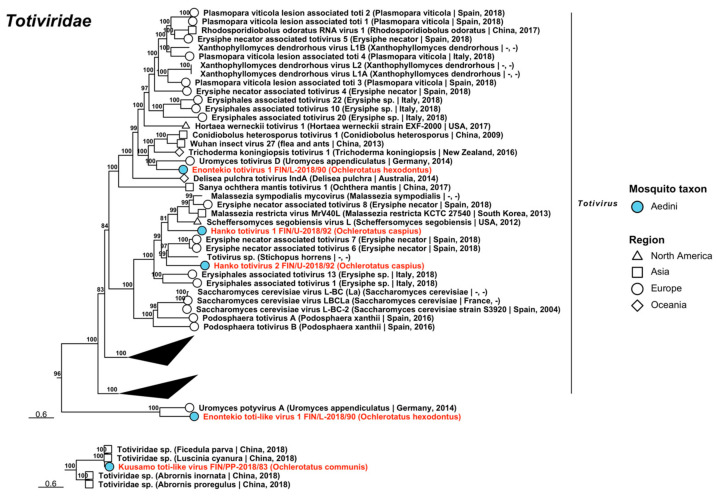
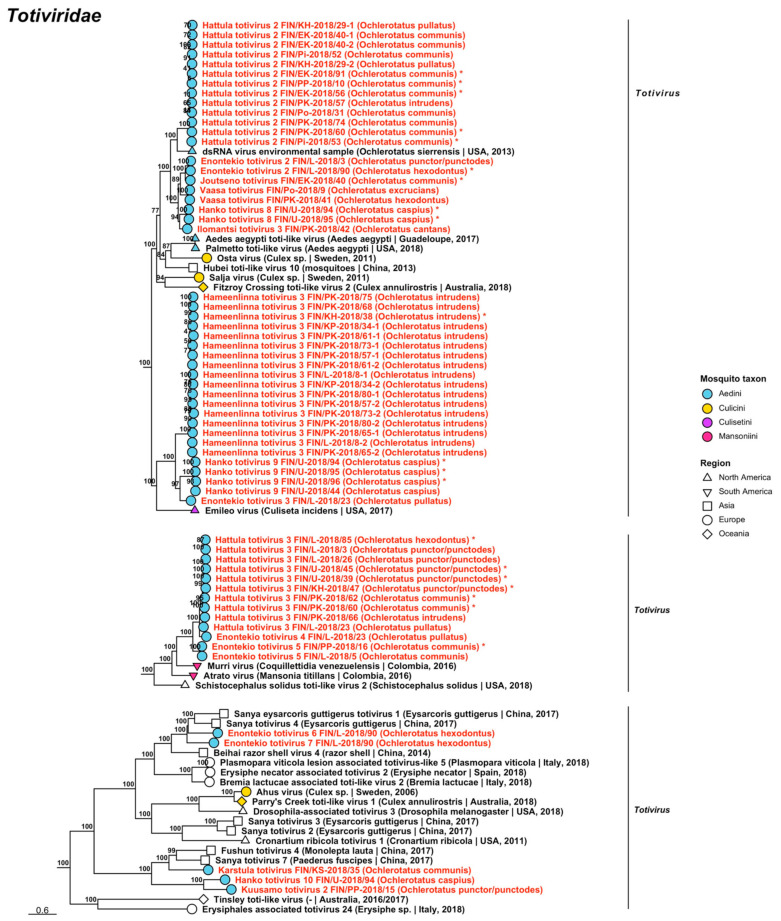
| Totiviridae | Hanko toti-like virus 1 | FIN/U-2018/92 | Oc. caspius | OP020048 |
| Totiviridae | Hanko toti-like virus 2 | FIN/U-2018/94 | Oc. caspius | OP019860 |
| Totiviridae | Hanko toti-like virus 3 | FIN/U-2018/93 | Oc. punctor/punctodes | OP020049 |
| Totiviridae | Enontekio toti-like virus 1 | FIN/L-2018/90 | Oc. hexodontus | OP019986 |
| Totiviridae | Enontekio toti-like virus 2 | FIN/L-2018/90 | Oc. hexodontus | OP019987 |
| Totiviridae | Enontekio toti-like virus 3 | FIN/L-2018/90 | Oc. hexodontus | OP019988 |
| Totiviridae | Enontekio toti-like virus 4 | FIN/L-2018/90 | Oc. hexodontus | OP019849 |
| Totiviridae | Enontekio totivirus 1 | FIN/L-2018/90 | Oc. hexodontus | OP019989 |
| Totiviridae | Enontekio totivirus 2 | FIN/L-2018/03 FIN/L-2018/90 |
Oc. punctor/punctodes
Oc. hexodontus |
OP019990 OP019850 |
| Totiviridae | Enontekio totivirus 3 | FIN/L-2018/23 | Oc. pullatus | OP019991 |
| Totiviridae | Enontekio totivirus 4 | FIN/L-2018/23 | Oc. pullatus | OP019992 |
| Totiviridae | Enontekio totivirus 5 | FIN/L-2018/05 FIN/PP-2018/16 |
Oc. communis
Oc. communis |
OP019993 OP019851 |
| Totiviridae | Enontekio totivirus 6 | FIN/L-2018/90 | Oc. hexodontus | OP019994 |
| Totiviridae | Enontekio totivirus 7 | FIN/L-2018/90 | Oc. hexodontus | OP019995 |
| Totiviridae | Hameenlinna toti-like virus | FIN/L-2018/08 FIN/KP-2018/34-1 FIN/KP-2018/34-2 FIN/KH-2018/38 FIN/PK-2018/58 FIN/PK-2018/65 |
Oc. intrudens
Oc. intrudens Oc. intrudens Oc. intrudens Oc. diantaeus Oc. intrudens |
OP019999 OP019997 OP019998 OP019996 OP020000 OP020001 |
| Totiviridae | Hameenlinna totivirus 1 | FIN/L-2018/05 FIN/PP-2018/10 FIN/PP-2018/16 FIN/PK-2018/21 FIN/Po-2018/31 FIN/PK-2018/36 FIN/EK-2018/40 FIN/KH-2018/48 FIN/KH-2018/49 FIN/U-2018/50 FIN/Pi-2018/51 FIN/Pi-2018/52-1 FIN/Pi-2018/52-2 FIN/Pi-2018/53 FIN/Pi-2018/54-1 FIN/Pi-2018/54-2 FIN/Pi-2018/55 FIN/EK-2018/56 FIN/PK-2018/59 FIN/PK-2018/60 FIN/PK-2018/68 FIN/PK-2018/69 FIN/PK-2018/70 FIN/PK-2018/74 FIN/PK-2018/76 FIN/PK-2018/78 FIN/PK-2018/79 FIN/PP-2018/82 FIN/PP-2018/83 FIN/L-2018/85-1 FIN/L-2018/85-2 FIN/L-2018/88 FIN/EK-2018/91 |
Oc. communis
Oc. communis Oc. communis Oc. communis Oc. communis Oc. communis Oc. communis Oc. communis Oc. communis Oc. communis Oc. communis Oc. communis Oc. communis Oc. communis Oc. communis Oc. communis Oc. communis Oc. communis Oc. communis Oc. communis Oc. intrudens Oc. intrudens Oc. communis Oc. communis Oc. communis Oc. communis Oc. communis Oc. communis Oc. communis Oc. hexodontus Oc. hexodontus Oc. communis Oc. communis |
OP020006 OP020025 OP020026 OP020015 OP019856 OP020016 OP020002 OP020004 OP020005 OP020027 OP019854 OP020009 OP020010 OP020011 OP020012 OP020013 OP020014 OP020003 OP020017 OP020018 OP020019 OP020020 OP020021 OP020022 OP019855 OP020023 OP020024 OP019857 OP019858 OP020007 OP020008 OP019853 OP019852 |
| Totiviridae | Hameenlinna totivirus 2 | FIN/L-2018/08 FIN/KH-2018/38 FIN/PK-2018/75 FIN/PK-2018/80 |
Oc. intrudens
Oc. intrudens Oc. intrudens Oc. intrudens |
OP020029 OP020028 OP020030 OP020031 |
| Totiviridae | Hameenlinna totivirus 3 | FIN/L-2018/08-1 FIN/L-2018/08-2 FIN/KP-2018/34-1 FIN/KP-2018/34-2 FIN/KH-2018/38 FIN/PK-2018/57-1 FIN/PK-2018/57-2 FIN/PK-2018/61-1 FIN/PK-2018/61-2 FIN/PK-2018/65-1 FIN/PK-2018/65-2 FIN/PK-2018/68 FIN/PK-2018/73-1 FIN/PK-2018/73-2 FIN/PK-2018/75 FIN/PK-2018/80-1 FIN/PK-2018/80-2 |
Oc. intrudens
Oc. intrudens Oc. intrudens Oc. intrudens Oc. intrudens Oc. intrudens Oc. intrudens Oc. intrudens Oc. intrudens Oc. intrudens Oc. intrudens Oc. intrudens Oc. intrudens Oc. intrudens Oc. intrudens Oc. intrudens Oc. intrudens |
OP020034 OP020035 OP020032 OP020033 OP019859 OP020036 OP020037 OP020038 OP020039 OP020040 OP020041 OP020042 OP020043 OP020044 OP020045 OP020046 OP020047 |
| Totiviridae | Hanko toti-like virus 1 | FIN/U-2018/92 | Oc. caspius | OP020048 |
| Totiviridae | Hanko toti-like virus 2 | FIN/U-2018/94 | Oc. caspius | OP019860 |
| Totiviridae | Hanko toti-like virus 3 | FIN/U-2018/93 | Oc. punctor/punctodes | OP020049 |
| Totiviridae | Hanko totivirus 1 | FIN/U-2018/92 | Oc. caspius | OP020050 |
| Totiviridae | Hanko totivirus 2 | FIN/U-2018/92 | Oc. caspius | OP020052 |
| Totiviridae | Hanko totivirus 3 | FIN/U-2018/18 FIN/U-2018/44 FIN/U-2018/92 FIN/U-2018/94 FIN/U-2018/95 FIN/U-2018/96 FIN/U-2018/97 FIN/VS-2018/99 FIN/VS-2018/100 |
Oc. caspius
Oc. caspius Oc. caspius Oc. caspius Oc. caspius Oc. caspius Oc. caspius Oc. caspius Oc. caspius |
OP020053 OP019861 OP019902 OP019909 OP019903 OP019904 OP019862 OP019905 OP020054 |
| Totiviridae | Hanko totivirus 4 | FIN/U-2018/18 FIN/U-2018/94 FIN/VS-2018/100 |
Oc. caspius
Oc. caspius Oc. caspius |
OP020055 OP020056 OP020057 |
| Totiviridae | Hanko totivirus 5 | FIN/U-2018/18 FIN/U-2018/44 FIN/U-2018/92-1 FIN/U-2018/92-2 FIN/U-2018/92-3 FIN/U-2018/94 FIN/U-2018/95 FIN/U-2018/96 FIN/U-2018/97 FIN/VS-2018/99 FIN/VS-2018/100 |
Oc. caspius
Oc. caspius Oc. caspius Oc. caspius Oc. caspius Oc. caspius Oc. caspius Oc. caspius Oc. caspius Oc. caspius Oc. caspius |
OP020058 OP020059 OP020060 OP020061 OP020062 OP020063 OP019906 OP020064 OP019863 OP020066 OP020065 |
| Totiviridae | Hanko totivirus 6 | FIN/U-2018/45 | Oc. punctor/punctodes | OP020067 |
| Totiviridae | Hanko totivirus 7 | FIN/U-2018/45 | Oc. punctor/punctodes | OP020068 |
| Totiviridae | Hanko totivirus 8 | FIN/U-2018/94 FIN/U-2018/95 |
Oc. caspius
Oc. caspius |
OP019864 OP019865 |
| Totiviridae | Hanko totivirus 9 | FIN/U-2018/44 FIN/U-2018/94 FIN/U-2018/95 FIN/U-2018/96 |
Oc. caspius
Oc. caspius Oc. caspius Oc. caspius |
OP020069 OP019866 OP019867 OP019900 |
| Totiviridae | Hanko totivirus 10 | FIN/U-2018/94 | Oc. caspius | OP020051 |
| Totiviridae | Hattula totivirus 1 | FIN/L-2018/06 FIN/PP-2018/10 FIN/KH-2018/29 FIN/PK-2018/61 FIN/PK-2018/62 FIN/PK-2018/69 FIN/PK-2018/78 FIN/L-2018/88 |
Oc. communis
Oc. communis Oc. pullatus Oc. intrudens Oc. communis Oc. intrudens Oc. communis Oc. communis |
OP020071 OP019871 OP020070 OP019868 OP019869 OP019870 OP020072 OP019901 |
| Totiviridae | Hattula totivirus 2 | FIN/PP-2018/10 FIN/KH-2018/29-1 FIN/KH-2018/29-2 FIN/Po-2018/31 FIN/EK-2018/40-1 FIN/EK-2018/40-2 FIN/Pi-2018/52 FIN/Pi-2018/53 FIN/EK-2018/56 FIN/PK-2018/57 FIN/PK-2018/60 FIN/PK-2018/74 FIN/EK-2018/91 |
Oc. communis
Oc. pullatus Oc. pullatus Oc. communis Oc. communis Oc. communis Oc. communis Oc. communis Oc. communis Oc. intrudens Oc. communis Oc. communis Oc. communis |
OP019876 OP020075 OP020076 OP020080 OP020073 OP020074 OP020077 OP019874 OP019872 OP020078 OP019875 OP020079 OP019873 |
| Totiviridae | Hattula totivirus 3 | FIN/L-2018/03 FIN/L-2018/23 FIN/L-2018/26 FIN/U-2018/39 FIN/U-2018/45 FIN/KH-2018/47 FIN/PK-2018/60 FIN/PK-2018/62 FIN/PK-2018/66 FIN/L-2018/85 |
Oc. punctor/punctodes
Oc. pullatus Oc. punctor/punctodes Oc. punctor/punctodes Oc. punctor/punctodes Oc. punctor/punctodes Oc. communis Oc. communis Oc. intrudens Oc. hexodontus |
OP020083 OP020081 OP020082 OP019881 OP019882 OP019877 OP019879 OP019880 OP020084 OP019878 |
| Totiviridae | Ilomantsi toti-like virus 1 | FIN/PK-2018/65 | Oc. intrudens | OP020085 |
| Totiviridae | Ilomantsi toti-like virus 2 | FIN/L-2018/07 FIN/PK-2018/41 FIN/PK-2018/42 FIN/PK-2018/69 FIN/PK-2018/76 FIN/L-2018/84 |
Oc. excrucians
Oc. hexodontus Oc. cantans Oc. intrudens Oc. communis Oc. excrucians |
OP020086 OP019883 OP020088 OP020089 OP020090 OP020087 |
| Totiviridae | Ilomantsi toti-like virus 3 | FIN/PK-2018/58 | Oc. diantaeus | OP020091 |
| Totiviridae | Ilomantsi totivirus 1 | FIN/PK-2018/58 FIN/PP-2018/82 |
Oc. diantaeus
Oc. communis |
OP020092 OP019884 |
| Totiviridae | Ilomantsi totivirus 2 | FIN/PK-2018/42-1 FIN/PK-2018/42-2 FIN/PK-2018/76 |
Oc. cantans
Oc. cantans Oc. communis |
OP020093 OP020094 OP020095 |
| Totiviridae | Ilomantsi totivirus 3 | FIN/PK-2018/42 | Oc. cantans | OP020096 |
| Totiviridae | Inari toti-like virus | FIN/L-2018/84 FIN/U-2018/93 |
Oc. excrucians
Oc. punctor/punctodes |
OP020097 OP020098 |
| Totiviridae | Inari totivirus 1 | FIN/L-2018/07-1 FIN/L-2018/07-2 FIN/L-2018/84-1 FIN/L-2018/84-2 |
Oc. excrucians
Oc. excrucians Oc. excrucians Oc. excrucians |
OP020099 OP020100 OP019885 OP020101 |
| Totiviridae | Inari totivirus 2 | FIN/L-2018/19 FIN/L-2018/85 FIN/L-2018/88 |
Oc. diantaeus
Oc. hexodontus Oc. communis |
OP019886 OP019887 OP019888 |
| Totiviridae | Joutseno totivirus | FIN/EK-2018/40 | Oc. communis | OP019889 |
| Totiviridae | Karstula totivirus | FIN/KS-2018/35 | Oc. communis | OP020102 |
| Totiviridae | Kustavi toti-like virus | FIN/VS-2018/17 FIN/U-2018/44 FIN/U-2018/92-1 FIN/U-2018/92-2 FIN/U-2018/94-1 FIN/U-2018/94-2 FIN/U-2018/95 FIN/U-2018/96 FIN/U-2018/97-1 FIN/U-2018/97-2 FIN/VS-2018/99 FIN/VS-2018/100 |
Oc. caspius
Oc. caspius Oc. caspius Oc. caspius Oc. caspius Oc. caspius Oc. caspius Oc. caspius Oc. caspius Oc. caspius Oc. caspius Oc. caspius |
OP020111 OP020103 OP020104 OP020105 OP020106 OP019890 OP019891 OP020107 OP020108 OP020109 OP020112 OP020110 |
| Totiviridae | Kuusamo toti-like virus | FIN/PP-2018/83 | Oc. communis | OP020113 |
| Totiviridae | Kuusamo totivirus 1 | FIN/PP-2018/15 | Oc. punctor/punctodes | OP020114 |
| Totiviridae | Kuusamo totivirus 2 | FIN/PP-2018/15 | Oc. punctor/punctodes | OP020115 |
| Totiviridae | Lestijarvi totivirus | FIN/L-2018/19 FIN/KP-2018/33 FIN/PK-2018/58 FIN/PK-2018/63 FIN/PK-2018/64 FIN/PP-2018/82 |
Oc. diantaeus
Oc. diantaeus Oc. diantaeus Oc. diantaeus Oc. diantaeus Oc. communis |
OP019892 OP020116 OP019893 OP020117 OP020118 OP019894 |
| Totiviridae | Palkane toti-like virus | FIN/Pi-2018/52 FIN/EK-2018/56 FIN/PK-2018/60 FIN/PK-2018/78 FIN/L-2018/85 FIN/L-2018/90 |
Oc. communis
Oc. communis Oc. communis Oc. communis Oc. hexodontus Oc. hexodontus |
OP020121 OP020119 OP019896 OP020122 OP020120 OP019895 |
| Totiviridae | Palkane totivirus | FIN/EK-2018/40 FIN/Pi-2018/54-1 FIN/Pi-2018/54-2 FIN/Pi-2018/55 FIN/EK-2018/91 |
Oc. communis
Oc. communis Oc. communis Oc. communis Oc. communis |
OP019907 OP020123 OP020124 OP020125 OP019908 |
| Totiviridae | Utsjoki toti-like virus | FIN/L-2018/88 | Oc. communis | OP019897 |
| Totiviridae | Vaasa toti-like virus | FIN/L-2018/07 FIN/Po-2018/09 FIN/PK-2018/69 FIN/L-2018/84 |
Oc. excrucians
Oc. excrucians Oc. intrudens Oc. excrucians |
OP020126 OP019899 OP020127 OP019898 |
| Totiviridae | Vaasa totivirus | FIN/Po-2018/09 FIN/PK-2018/41 |
Oc. excrucians
Oc. hexodontus |
OP020129 OP020128 |
Botybirnavirus is a recently proposed virus taxon, whose species have been isolated from plants and phytopathogenic fungi. One novel virus was sequenced and tentatively named “Palkane botybirna-like virus”, which had a low resemblance to Bremia lactucae-associated dsRNA virus 1 (GenBank accession: QIP68006.1; amino acid identity: 40.17–44.61%.). Eight variants were found in six pools of Oc. communis and one of Oc. intrudens (Figure 16, Table 5).
Figure 16.
Maximum likelihood trees of Botybirnavirus and Chrysoviridae. Tentative novel viruses are displayed in red and the mosquito species from which they were derived are in parentheses. Sequences from GenBank are black and display the following information after the virus or species name: “(sampled organism(s)|collection country, collection year)”. Tip colours represent the tribe (Culicinae) or genus (Anophelinae) of mosquito from which viruses were obtained. Tip shape represents the continent or region from which the specimens were collected. Trees were constructed from amino acid sequences of virus polymerases >1000 nt, aligned with MAFFT and computed with IQ-TREE2 using ModelFinder and 1000 bootstraps. Asterisks denote that the complete genome was recovered.
Five variants of three novel Chrysoviridae viruses, which mainly infect fungi as well as plants and insects, were sequenced from pools of Oc. caspius, Oc. communis, Oc. intrudens and Oc. punctor/punctodes (Figure 16, Table 5). All species belonged to Alphachrysovirus and were provisionally named “Enontekio alphachrysovirus”, “Hanko alphachrysovirus” and “Lestijarvi alphachrysovirus”. These viruses had a moderate similarity to Keturi virus (GenBank accession: QRW42852.1; amino acid identities: 73.68%, 77.62% and 72.98–74.71%, respectively).
Fifty-five strains grouped into 23 novel species belonging to Partitiviridae, viruses traditionally associated with fungi, plants and protozoa, but recently associated also with arthropods [35,36,37] (Table 6). Eight of these species were partiti-like viruses and did not fall within an established genus, but the remaining fifteen belonged to three established genera: nine in Alphapartitivirus (Figure 17), three in Betapartitivirus and three in Deltapartitivirus (Figure 18). The novel alphapartitiviruses were named “Enontekio alphapartitivirus 1 to 2”, “Hanko alphapartitivirus 1 to 3”, “Kalajoki alphapartitivirus”, “Kuusamo alphapartitivirus” and “Palkane alphapartitivirus 1 and 2”. Enontekio alphapartitivirus 1 was most similar to Hubei partiti-like virus 27 (GenBank accession: APG78241.1; amino acid identity: 63.67%), while Enontekio alphapartitivirus 2, Hanko alphapartitivirus 1, Kuusamo alphapartitivirus and Palkane alphapartitiviruses 1 and 2 were most similar to Wilkie partiti-like virus 2 (GenBank accessions: ASA47308.1 and YP_009388578.1; amino acid identities: 42.66%, 59.29–61.27%, 59.89–62.01%, 56.58–61.03% and 60.8%, respectively). Hanko alphapartitivirus 2 shared a high amino acid identity with Erysiphe necator-associated partitivirus 5 (GenBank accession: QJW70310.1; amino acid identity: 84.66%) and Hanko alphapartitivirus 3 had a slightly lower amino acid identity to Gaeumannomyces tritici partitivirus 1 (GenBank accession: AZT88602.1; amino acid identity: 71.76%). Lastly, Kalajoki alphapartitivirus sequences had moderate similarity to soybean-leaf-associated partitivirus 1 (GenBank accession: ALM62245.1; amino acid identity: 56.23–61.67%). The novel betapartitiviruses detected included the tentatively named “Enontekio betapartitivirus 1”, “Enontekio betapartitivirus 2” and “Kalajoki betapartitivirus”. The closest matching virus to Enontekio betapartitivirus 1 was Partitivirus-like 5 (GenBank accession: AOR51392.1; amino acid identity: 74.96%), the closest to Enontekio betapartitivirus 2 was Wilkie partiti-like virus 1 (GenBank accession: ASA47307.1; amino acid identity: 59.36%) and the closest to Kalajoki betapartitivirus was Vivastbo virus (GenBank accession: QGA70914.1; amino acid identity: 46.63–46.89%). The novel deltapartitiviruses, which were provisionally named “Ilomantsi deltapartitivirus”, “Inari deltapartitivirus” and “Vaasa deltapartitivirus”, were all moderately similar to Culex pseudovishnui partitivirus based on amino acid identity (GenBank accession: BBQ05103.1; amino acid identities: 67.13–67.33%, 76.75% and 66.4–66.6%, respectively).
Figure 17.
Maximum likelihood tree of Alphapartitivirus (Partitiviridae). Tentative novel viruses are displayed in red, with the mosquito species from which they were derived in parentheses. Sequences from GenBank are black and display the following information after the virus or species name: “(sampled organism(s)|collection country, collection year)”. Tip colours represent the tribe of mosquito from which viruses were obtained. Tip shape represents the continent or region from which the specimens were collected. Trees were constructed from amino acid sequences of virus polymerases >1000 nt, aligned with MAFFT and computed with IQ-TREE2 using ModelFinder and 1000 bootstraps.
Figure 18.
Maximum likelihood trees of Betapartitivirus and Deltapartitivirus (Partitiviridae). Tentative novel viruses are displayed in red and the mosquito species from which they were derived are in parentheses. Sequences from GenBank are black and display the following information after the virus or species name: “(sampled organism(s)|collection country, collection year)”. Tip colours represent the tribe (Culicinae) or genus (Anophelinae) of mosquito from which viruses were obtained. Tip shape represents the continent or region from which the specimens were collected. Trees were constructed from amino acid sequences of virus polymerases >1000 nt, aligned with MAFFT and computed with IQ-TREE2 using ModelFinder and 1000 bootstraps.
Finally, the eight partiti-like viruses included the tentatively named “Enontekio partiti-like virus”, “Hameenlinna partiti-like virus”, “Hattula partiti-like virus”, “Ilomantsi partiti-like virus 1”, “Ilomantsi partiti-like virus 2”, “Kuusamo partiti-like virus”, “Lestijarvi partiti-like virus” and “Vaasa partiti-like virus” (Figure 19, Table 6). Of these viruses, Ilomantsi partiti-like virus 1 shared the highest amino acid identity with Araticum virus detected from Mansonia wilsoni mosquitoes from Brazil (GenBank accession: ASV45859.1; amino acid identity: 78.17–78.49%); Ilomantsi partiti-like virus 2 with Atrato partiti-like virus 3 (GenBank accession: QHA33899.1; amino acid identity: 88.14%); Kuusamo partiti-like virus with Culex tritaeniorhynchus partitivirus from Japan [38] (GenBank accession: BBQ05106.1; amino acid identity: 72.03%); and Enontekio partiti-like virus, Hameenlinna partiti-like virus, Hattula partiti-like virus, Lestijarvi partiti-like virus and Vaasa partiti-like virus with different strains of Hubei partiti-like virus 22 (GenBank accessions: APG78217.1, BBQ05104.1 and BBQ05105.1; amino acid identities: 64.58%, 60–62.12%, 76.11–78.57%, 64.04–64.12% and 61.54%).
Figure 19.
Maximum likelihood tree of partiti-like viruses (Partitiviridae). Tentative novel viruses are displayed in red and the mosquito species from which they were derived are in parentheses. Sequences from GenBank are black and display the following information after the virus or species name: “(sampled organism(s)|collection country, collection year)”. Tip colours represent the tribe (Culicinae) or genus (Anophelinae) of mosquito from which viruses were obtained. Tip shape represents the continent or region from which the specimens were collected. Trees were constructed from amino acid sequences of virus polymerases >1000 nt, aligned with MAFFT and computed with IQ-TREE2 using ModelFinder and 1000 bootstraps.
Five novel reoviruses belonging to Reovirales, a diverse order of viruses that infect organisms from several phyla, were sequenced (Figure 20, Table 7). Four novel viruses belonging to the family Sedoreoviridae were tentatively named “Ilomantsi reovirus 1”, “Ilomantsi reovirus 2”, “Ilomantsi reovirus 3” and “Ilomantsi reovirus 4”, while one novel virus belonging to Spinareoviridae was named “Enontekio reovirus”. According to the phylogenetic analyses, none of these five viruses clustered within established genera. Enontekio reovirus was distantly similar to Operophtera brumata reovirus (GenBank accession: YP_392501.1; amino acid identity: 29.59%), while Ilomantsi reoviruses 1–4 were moderately similar to Aedes camptorhynchus reo-like virus (GenBank accession: YP_009389547.1; amino acid identities: 64.96–66.33%, 67.77–70.69%, 74.94% and 64.98%, respectively). However, a phylogenetic analysis suggested that Atrato reo-like virus (GenBank accession: QHA33824.1) was more related to Ilomantsi reoviruses 1–3, while Ilomantsi reovirus 4 clustered near the root of the Ilomantsi reovirus clade.
Figure 20.
Maximum likelihood tree of Reovirales. Tentative novel viruses are displayed in red and the mosquito species from which they were derived are in parentheses. Sequences from GenBank are black and display the following information after the virus or species name: “(sampled organism(s)|collection country, collection year)”. Tip colours represent the tribe of mosquito from which viruses were obtained. Tip shape represents the continent or region from which the specimens were collected. Trees were constructed from amino acid sequences of virus polymerases >1000 nt, aligned with MAFFT and computed with IQ-TREE2 using ModelFinder and 1000 bootstraps.
The most viral sequences in this study grouped within Totiviridae, which includes viruses of fungi and protozoans, among others. From 205 sequences, 52 viruses were identified, of which 50 were novel and two were strains of previously described, albeit unnamed, viruses (Figure 21, Figure 22, Figure 23, Figure 24 and Figure 25, Table 7). Virus strains were found in all nine mosquito species and from across the country. The novel viruses included 33 provisionally named viruses which clustered with member species of Totivirus. These included “Enontekio totivirus 1 to 7”, “Hameenlinna totivirus 1 to 3”, “Hanko totivirus 1 to 10”, “Hattula totivirus 1 to 3”, “Ilomantsi totivirus 1 to 3”, “Inari totivirus 1 and 2”, “Joutseno totivirus”, “Karstula totivirus”, “Kuusamo totivirus 1 and 2”, “Lestijarvi totivirus”, “Palkane totivirus” and “Vaasa totivirus”. Protein blast results suggested that the closest matching virus by relatively low amino acid identity values for Enontekio totivirus 1 was Wuhan insect virus 27 (GenBank accession: YP_009342434.1; amino acid identity: 55.87%). Similarly, Enontekio totivirus 2, Hanko totiviruses 8 and 9, Hattula totivirus 2, Ilomantsi totivirus 3, Joutseno totivirus and Vaasa totivirus had a low to moderate amino acid identity to an unnamed dsRNA from an environmental sample (GenBank accession: AJT39583.1; amino acid identities: 61–61.1%, 61.4–61.49%, 46.27–47.07%, 63.78–69.55%, 51.72%, 61.18% and 50–65.23%). Hameenlinna totivirus 3 sequences had a low amino acid identity with multiple previously established viruses including the aforementioned unnamed virus (amino acid identity: 36.36–54.53%), Aedes aegypti toti-like virus (GenBank accession: QEM39133.1; amino acid identity: 39.66–47.3%), Emileo virus (GenBank accession: QRW41692.1; amino acid identity: 44.42%) and Hubei toti-like virus 10 (GenBank accession: YP_009336493.1, amino acid identity: 53.33%). Enontekio totivirus 3 was also similar to Hubei toti-like virus 10 (amino acid identity: 56.29%).
Figure 21.
Maximum likelihood subtrees of Totiviridae. Tentative novel viruses are displayed in red and the mosquito species from which they were derived are in parentheses. Sequences from GenBank are black and display the following information after the virus or species name: “(sampled organism(s)|collection country, collection year)”. Tip colours represent the tribe (Culicinae) or genus (Anophelinae) of mosquito from which viruses were obtained. Tip shape represents the continent or region from which the specimens were collected. Trees were constructed from amino acid sequences of virus polymerases >1000 nt, aligned with MAFFT and computed with IQ-TREE2 using ModelFinder and 1000 bootstraps. Asterisks denote that the complete genome was recovered.
Figure 22.
Maximum likelihood subtrees of Totiviridae. Tentative novel viruses are displayed in red and the mosquito species from which they were derived are in parentheses. Sequences from GenBank are black and display the following information after the virus or species name: “(sampled organism(s)|collection country, collection year)”. Tip colours represent the tribe of mosquito from which viruses were obtained. Tip shape represents the continent or region from which the specimens were collected. Trees were constructed from amino acid sequences of virus polymerases >1000 nt, aligned with MAFFT and computed with IQ-TREE2 using ModelFinder and 1000 bootstraps.
Figure 23.
Maximum likelihood subtree of Totiviridae. Tentative novel viruses are displayed in red and the mosquito species from which they were derived are in parentheses. Sequences from GenBank are black and display the following information after the virus or species name: “(sampled organism(s)|collection country, collection year)”. Tip colours represent the tribe of mosquito from which viruses were obtained. Tip shape represents the continent or region from which the specimens were collected. Trees were constructed from amino acid sequences of virus polymerases >1000 nt, aligned with MAFFT and computed with IQ-TREE2 using ModelFinder and 1000 bootstraps. Asterisks denote that the complete genome was recovered.
Figure 24.
Maximum likelihood subtrees of Totiviridae. Tentative novel viruses are displayed in red and the mosquito species from which they were derived are in parentheses. Sequences from GenBank are black and display the following information after the virus or species name: “(sampled organism(s)|collection country, collection year)”. Tip colours represent the tribe of mosquito from which viruses were obtained. Tip shape represents the continent or region from which the specimens were collected. Trees were constructed from amino acid sequences of virus polymerases >1000 nt, aligned with MAFFT and computed with IQ-TREE2 using ModelFinder and 1000 bootstraps. Asterisks denote that the complete genome was recovered.
Figure 25.
Maximum likelihood subtree of Totiviridae. Tentative novel viruses are displayed in red and the mosquito species from which they were derived are in parentheses. Sequences from GenBank are black and display the following information after the virus or species name: “(sampled organism(s)|collection country, collection year)”. Tip colours represent the tribe of mosquito from which viruses were obtained. Tip shape represents the continent or region from which the specimens were collected. Trees were constructed from amino acid sequences of virus polymerases >1000 nt, aligned with MAFFT and computed with IQ-TREE2 using ModelFinder and 1000 bootstraps. Asterisks denote that the complete genome was recovered.
Enontekio totiviruses 4 and 5 as well as Hattula totivirus 3 were most similar to Murri virus (GenBank accession: QHA33714.1; amino acid identities: 57.2%, 57.8% and 57.48–71.43%, correspondingly). Hattula totivirus 3 also shared amino acid identity with Atrato virus (GenBank accession: QHA33710.1; amino acid identity: 57.46–62.73%), although based on our phylogenetic tree, the virus in general was more related to Murri virus. Enontekio totiviruses 6 and 7 had the most similarity with Beihai razor shell virus 4 (GenBank accession: YP_009333409.1; amino acid identities: 41.12% and 46.59%). Thirteen viruses shared a moderate amino acid identity with an unnamed uncultured virus (GenBank accession: AGW51771.1). These were Hameenlinna totiviruses 1 and 2 (amino acid identities: 48.73–65.96% and 62.42–70.73%), Hanko totiviruses 5, 7 and 10 (amino acid identities: 66.05–75.56%, 70.14% and 49.36%), Ilomantsi totiviruses 1 and 2 (amino acid identities: 54.61–64.58% and 59.68–72.16%), Inari totivirus 1 (amino acid identity: 48.98–57.46%), Karstula totivirus (amino acid identity: 41.18%), Kuusamo totiviruses 1 and 2 (amino acid identities: 73.75% and 45.61%), Lestijarvi totivirus (amino acid identity: 39.84–42.51%) and Palkane totivirus (amino acid identity: 60.16–61.96%). Despite sharing the highest amino acid identity with the uncultured virus, Karstula totivirus, Hanko totivirus 10 and Kuusamo totivirus 2 clustered with Fushun totivirus 4 and Sanya totivirus 7 (GenBank accessions: UHM27684.1 and UHM27502.1; these viruses did not appear in the BLASTx results). Hattula totivirus 1 was similar to Pisingos virus (GenBank accession: QHA33716.1; amino acid identity: 68.82–71.3%), Hanko totivirus 2 to Erysiphe necator associated totivirus 7 (GenBank accession: QJW70337.1; amino acid identity: 51.07%) and Hanko totivirus 6 to Aedes alboannulatus toti-like virus 1 (GenBank accession: YP_009388609.1; amino acid identity: 51.62%). Other detected totiviruses that shared similar amino acid identities to Aedes alboannulatus toti-like virus 1 were strains of the aforementioned Hameenlinna totivirus 1 (amino acid identity: 62.6%) and Inari totivirus 1 (amino acid identity: 62.2%). Several strains of our novel totiviruses were moderately similar to Aedes camptorhynchus toti-like virus 1 (GenBank accession: YP_009388611.1), Inari totivirus 2 (amino acid identity: 52.88–53.19%), Hameenlinna totivirus 1 (amino acid identity: 60.98%) and Lestijarvi totivirus (amino acid identity: 53.3%). Lastly, Hanko totivirus 1 shared a low amino acid identity with Malassezia sympodialis mycovirus (GenBank accession: QNJ34610.1; amino acid identity: 35.14%).
Two totivirus sequences were Finnish strains of a previously described “uncultured virus” from France (GenBank accession: AGW51771.1), which have nearly identical protein identities. In the absence of a name, these viruses were therefore tentatively named “Hanko totivirus 3” and “Hanko totivirus 4” (amino acid identities: 96.76–97.35% and 94–94.5%). Complete genomes were detected for 16 aforementioned totiviruses, as follows: Enontekio totiviruses 2 and 5, Hameenlinna totiviruses 1 and 3, Hanko totiviruses 3, 5, 8 and 9, Hattula totiviruses 1 to 3, Ilomantsi totivirus 1, Inari totiviruses 1 and 2, Joutseno totivirus and Lestijarvi totivirus.
Seventeen novel toti-like viruses were recovered, which did not cluster within any established genera. As such, they were provisionally named “Enontekio toti-like virus 1 to 4”, “Hameenlinna toti-like virus”, “Hanko toti-like virus 1 to 3”, “Ilomantsi toti-like virus 1 to 3”, “Inari toti-like virus”, “Kustavi toti-like virus”, “Kuusamo toti-like virus”, “Palkane toti-like virus”, “Utsjoki toti-like virus” and “Vaasa toti-like virus”. Based on amino acid identity, Enontekio toti-like virus 1 was distantly similar to Uromyces totivirus D (GenBank accession: QED43018.1; amino acid identity: 43.84%), yet clustered with Uromyces potyvirus A (GenBank accession: QED42911.1). Enontekio toti-like viruses 2 and 3 had a low similarity to diatom-colony-associated dsRNA virus 7 (GenBank accession: YP_009553338.1; amino acid identities: 46.85% and 52.13%), while Enontekio toti-like virus 4 to diatom-colony-associated dsRNA virus 11 (GenBank accession: YP_009552795.1; amino acid identity: 35.11%). Seven of the viruses matched Nuyav virus (GenBank accession: QRW41699.1). These included Hameenlinna toti-like virus (amino acid identity: 68.09–73.6%), Hanko toti-like virus 2 (amino acid identity: 68.98%), Ilomantsi toti-like viruses 1 and 2 (amino acid identities: 76.01% and 66.82–71.27%), Kustavi toti-like virus (amino acid identity: 88.62–91.38), Palkane toti-like virus (amino acid identity: 72.59–76.81%) and Vaasa toti-like virus (amino acid identity: 68.53–69.73%). Ilomantsi toti-like virus 3 were distantly similar to an unnamed RNA virus (GenBank accession: QTW97791.1; amino acid identity: 37.9%). Hanko toti-like virus 1 was distantly related to Keenan toti-like virus (GenBank accession: QIJ70132.1; amino acid identity: 39.55%) and according to the phylogenetic analysis (Figure 21) to two Drosophila-associated totiviruses (GenBank accession: UFT26914.1 and UFT26909.1). Both Hanko toti-like virus 3 and Inari toti-like virus were related to Umbelopsis ramanniana virus 2 (GenBank accession: VFI65724.1; protein identities: 55.56% and 52.29%). One strain of the Inari toti-like virus also shared a low amino acid identity with Thelebolus microsporus totivirus 1 (GenBank accession: AZT88643.1; amino acid identity: 49.27%). However, our phylogenetic analysis suggested that both viruses were more related to soybean-thrips-associated totivirus 1 (GenBank accession: QQP18682.1). Lastly, Kuusamo toti-like virus had a high protein similarity to an unnamed totivirus (GenBank accession: QJI53453.1; amino acid identity: 86.43%), while Utsjoki toti-like virus had a low similarity to Wuhan insect virus 28 (GenBank accession: YP_009342430.1; amino acid identity: 34.55%). Complete genomes were sequenced for seven of our novel toti-like viruses. These were Enontekio toti-like virus 4, Hanko toti-like virus 2, Ilomantsi toti-like virus 2, Kustavi toti-like virus, Palkane toti-like virus, Utsjoki toti-like virus and Vaasa toti-like virus (GenBank accessions OP019849, OP019860, OP019883, OP019890, OP019895, OP019897 and OP019898, respectively).
3.2. Viruses by Mosquito Species
Variable numbers of pools, ranging from 1 to 35, were prepared for each mosquito species included in this study, with pooled material obtained from multiple collection locations. This made direct comparison of some results between species less meaningful, but each species was associated with multiple viruses.
Ochlerotatus cantans, which was the least represented species in the study with only one pool of 20 specimens collected in late June 2015 in Ilomantsi, PK, was found to have six viral sequences. These represented five novel species, and clustered within Solemoviridae, Partitiviridae and Totiviridae (Table 8).
Table 8.
Virus families detected by NGS, their host/vector associations and the (number of virus species/novel virus variants) by mosquito species. Brackets next to mosquito species names denotes the number of pools studied. Where a single digit is given, no novel viruses were detected for the given virus family/mosquito.
| Virus Family | No. Virus Variants | No. of Viruses | No. Novel Viruses | Host Associations | Oc. cantans (1) | Oc. caspius (11) | Oc. communis (35) | Oc. diantaeus (6) | Oc. excrucians (3) | Oc. hexodontus (8) | Oc. intrudens (14) | Oc. pullatus (2) | Oc. punctor/punctodes (11) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| + ssRNA |
Endornaviridae | 2 | 2 | 1 | Plants, fungi and oomycetes; host-specific [39]. | - | - | - | - | - | - | - | - | 2/1 | No. of virus species per mosquito species/no. of novel virus species |
| Flaviviridae | 9 | 5 | 4 | Arthropod-borne; mammalian hosts [40] mosquitoes [7,8,9,41,42,43]. | - | 1 | - | 1/1 | - | 2/2 | 1/1 | - | - | ||
| Iflavirus | 17 | 5 | 5 | Arthropoda [44], mosquitoes, inc. Culex sp. [45]. | - | 2/2 | 2/2 | 1/1 | - | 1/1 | 2/2 | - | 1/1 | ||
| Negevirus | 41 | 7 | 4 | Phlebotomine sandflies and mosquitoes [10,46]. | - | 1/1 | 5/3 | - | 1/1 | 5/3 | 1 | - | 3/2 | ||
| Permutotetraviridae | 6 | 1 | 1 | Insecta: Setothosea asigna [47], Euprosterna elaeasa [48]. Fungus: Botrytis cinerea [49]. Mosquitoes [42]. | - | - | - | - | 1/1 | 1/1 | - | - | 1/1 | ||
| Picornaviridae | 5 | 2 | 0 | Vertebrates (six of the seven classes) [50], Culex mosquitoes [51] and fleas [52]. | - | 2 | - | - | - | - | - | - | - | ||
| Quenyavirus | 2 | 1 | 1 | Insecta: Crocallis elinguaria, Drosophila sp. and Lysiphlebus fabarum [53]. | - | - | - | - | - | 1/1 | - | - | 1/1 | ||
| Solemoviridae | 15 | 5 | 4 | Plants (monocotyledons and dicotyledons) [54]. | 1/1 | 2/1 | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | - | 1/1 | ||
| Togaviridae | 1 | 1 | 0 | Humans and nonhuman primates, mosquitoes, amphibians, arthropods, birds, equids, pigs, reptiles, rodents, salmonids and sea mammals; most are mosquito-borne [55]. Mosquitoes [43]. |
- | - | 1 | - | - | - | - | - | - | ||
| Virgaviridae | 7 | 3 | 3 | Plants, plasmodiophorids, nematodes and pollen [56]. | - | - | 1/1 | - | - | 3/3 | - | - | 1/1 | ||
| − ssRNA |
Aliusviridae | 1 | 1 | 1 | Mosquitoes, Coleoptera, Hymenoptera, leafhopper [57] fleas [52] | - | - | 1/1 | - | - | - | - | - | - | |
| Aspiviridae | 1 | 1 | 1 | Plants and plant-infecting fungi [58]. | - | - | - | - | - | 1/1 | - | - | - | ||
| Chuviridae | 14 | 3 | 3 | Mosquitoes [59,60], earwigs, Odonata, ticks, cockroaches, snakes, fish [61]. | - | 2/2 | 1/1 | - | - | 1/1 | 1/1 | 1/1 | - | ||
| Phasmaviridae | 13 | 8 | 8 | Mosquitoes [43], Hymenoptera, Hemiptera, Coleoptera [59]. | - | - | 6/6 | - | - | - | 2/2 | - | - | ||
| Phenuiviridae | 58 | 14 | 14 | Mosquitoes [43,62], fleas [52], ticks, Coleoptera, phlebotomine sandflies, plants, humans [61]. | - | 1/1 | 7/7 | 1/1 | - | 3/3 | 2/2 | - | 3/3 | ||
| Qinviridae | 7 | 3 | 3 | Insects [63,64], marine diatoms [57]. | - | - | 3/3 | - | - | - | - | - | - | ||
| Rhabdoviridae | 21 | 8 | 7 | Vertebrates, invertebrates and plants [61]. Insect vectors infect vertebrates [65], mosquitoes [32]. | - | - | 3/3 | - | 2/1 | 3/3 | - | 1/1 | 1/1 | ||
| Xinmoviridae | 9 | 4 | 4 | Mosquitoes [66,67], Odonata & Hymenoptera [68]. | - | 1/1 | 1/1 | - | - | 2/2 | - | - | 1/1 | ||
| Yueviridae | 1 | 1 | 1 | Invertebrates (freshwater isopoda/sesarmid crab) [35]. | - | - | - | - | - | 1/1 | - | - | - | ||
|
dsRNA |
Botybirnavirus | 8 | 1 | 1 | Phytopathogenic fungi [69]. | - | - | 1/1 | - | - | - | 1/1 | - | - | |
| Chrysoviridae | 5 | 3 | 3 | Fungi and plants; possibly insects [70]. | - | 1/1 | 1/1 | - | - | - | 1/1 | - | 1/1 | ||
| Partitiviridae | 55 | 23 | 23 | Plants, fungi, protozoa [71], mosquitoes [60]. | 1/1 | 2/2 | 9/9 | 1/1 | 2/2 | 10/10 | 3/3 | 2/2 | 3/3 | ||
| Sedoreoviridae | 10 | 4 | 4 | Pathogenic viruses; arthropods [72,73,74], mammals, inc. humans [75,76], plants [77]. | - | - | 3/3 | 1/1 | - | 2/2 | 1/1 | - | - | ||
| Spinareoviridae | 1 | 1 | 0 | Pathogenic viruses; mosquitoes [78], plants [79], fish [79], reptiles, birds and mammals [80]. | - | - | - | - | - | 1/1 | - | - | - | ||
| Totiviridae | 205 | 52 | 50 | Fungi [81], protozoa [82], mosquitoes [60], fleas [52]. | 3/3 | 11/9 | 16/16 | 5/5 | 5/5 | 14/14 | 10/10 | 5/5 | 8/8 | ||
| Totals | 514 | 159 | 147 | 5 | 26 | 62 | 11 | 12 | 52 | 26 | 9 | 27 | |||
Ochlerotatus caspius was represented with 11 mosquito pools comprised of 305 specimens collected from the southern, coastal regions of Uusimaa and Varsinais-Suomi in July and August 2017. In total, 76 viral sequences grouped into 26 virus species, and of these, 20 represented new virus species within Chrysoviridae, Chuviridae, Iflaviridae, Negevirus, Partitiviridae, Phenuiviridae, Solemoviridae, Totiviridae and Xinmoviridae. The seven previously described viruses fell within Flaviviridae, Picornaviridae, Solemoviridae and Totiviridae (Table 8). It was found to be virus-positive for Hanko virus in Uusimaa (FI 1010 and FI 1011), but not in Varsinais-Suomi (FI 988 and FI 1015) (see Figure 1).
Ochlerotatus communis was overrepresented in this study since it is one of the most common human-biting mosquitoes in Finland and is active across the summer months. As such, 35 pools were constructed, comprised of 866 specimens that were collected from around the country in May to August of 2015 and 2017. Inevitably, it also had the most unique viral sequences, with 179 that grouped into 62 species, of which 58 were novel. The three established viruses were Cordoba and Dezidougou viruses (Negevirus) and Sindbis virus (Alphavirus, Togaviridae). This is the first confirmed mosquito species to be associated with Sindbis virus in Finland. The single strain was found in Mekrijärvi, Pohjois-Karjala, an area where the only other Finnish mosquito-borne Sindbis virus strains have been recovered. The remaining 58 novel species belong to Aliusviridae, Botybirnavirus, Chrysoviridae, Chuviridae, Iflaviridae, Negevirus, Partitiviridae, Phasmaviridae, Phenuiviridae, Qinviridae, Sedoreoviridae, Rhabdoviridae, Solemoviridae, Totiviridae, Virgaviridae and Xinmoviridae (Table 8).
Ochlerotatus diantaeus was represented by six pools, comprised of 108 specimens, which were collected from northern, eastern and central Finland in June and July 2015. From these, 20 virus sequences were assembled, which grouped into 11 novel viruses in Flaviviridae, Iflaviridae, Partitiviridae, Phenuiviridae, Sedoreoviridae, Solemoviridae and Totiviridae (Table 8).
Ochlerotatus excrucians was represented by 99 specimens divided into three (unequal) pools, which were collected from northern and western Finland in June and July 2015. Twenty sequences were assembled, which grouped into 12 virus species, 11 of which were novel. Two strains of the previously described Ohlsdorf virus (Rhabdoviridae) were found from Inari in Lapland. The other 11 species grouped within Negevirus, Partitiviridae, Permutotetraviridae, Solemoviridae and Totiviridae (Table 8).
Ochlerotatus hexodontus, despite being represented by only eight pools comprised of 222 specimens, had the second most viruses of the nine mosquito species analysed herein. Most collections were made in Lapland in July 2015, with only one being from Ilomantsi, PK, eastern Finland in June 2015. In all, 78 virus sequences were assembled, which grouped into 52 species, of which 50 were novel. Two Negeviruses, Cordoba virus and Mekrijärvi negevirus, have previously been described. Novel viruses all belonged to Aspiviridae, Chuviridae, Flaviviridae, Iflaviridae, Negevirus, Partitiviridae, Permutotetraviridae, Phenuiviridae, Quenyavirus, Rhabdoviridae, Sedoreoviridae, Solemoviridae, Spinareoviridae, Totiviridae, Virgaviridae, Xinmoviridae and Yueviridae (Table 8).
Ochlerotatus intrudens was also well represented, with 14 pools assembled from 309 specimens, which were collected in June and July 2015, from around the country, but in particular from eastern Finland. Eighty-three virus sequences were assembled, which grouped into 26 species, of which 25 were novel: Botybirnavirus, Chrysoviridae, Chuviridae, Flaviviridae, Iflaviridae, Negevirus, Partitiviridae, Phasmaviridae, Phenuiviridae, Sedoreoviridae, Solemoviridae and Totiviridae. Two strains of the previously described Mekrijärvi negevirus were also sequenced (Table 8).
Ochlerotatus pullatus was the second least represented species in this study, with 46 specimens divided into two pools: one from Lapland and the other from Hattula, KH. Both were collected in 2017, in May and July. Ten virus sequences were detected, which grouped into nine novel species, which belong to Chuviridae, Partitiviridae, Rhabdoviridae and Totiviridae (Table 8).
Ochlerotatus punctor/punctodes was also represented by 11 pools, comprised of 358 specimens that were collected around Finland between May to August in 2015 and 2017. Forty-one strains were sequenced, which grouped into 27 species, of which 25 were novel. The novels species belong to Chrysoviridae, Endornaviridae, Iflaviridae, Negevirus, Partitiviridae, Permutotetraviridae, Phenuiviridae, Quenyavirus, Rhabdoviridae, Solemoviridae, Totiviridae, Virgaviridae and Xinmoviridae. The two established species were Hallsjon virus (Endornaviridae) and Cordoba virus (Negevirus) (Table 8). Short M glycoprotein sequences from Inkoo virus were also recovered in addition to the RdRp sequences used to assess species diversity.
4. Discussion
This is the first in-depth study of the viromes of mosquitoes from Finland. The aim was to investigate RNA viromes of identified Ochlerotatus mosquitoes, thereby ascertaining both the diversity of associated viruses and the potential vector associations of these mosquito species. RNA sequences were generated from nine identified species of female Ochlerotatus (2333 specimens), which were divided into 91 species-specific pools. Viral sequences were present in all mosquito pools, but only 90 contained sequences of RdRp greater than 1000 nucleotides and were included in further analyses. In total, 514 viral RNA sequences were identified that grouped into 159 species, 147 of which were likely to be novel. Strains for 12 viruses which had previously been described were sequenced, although only nine had been named when published: Hallsjon virus (Endornaviridae), Hanko virus (Flaviviridae), Cordoba virus, Dezidougou virus and Mekrijärvi negevirus (Negevirus), Jotan virus (Picornaviridae), Ohlsdorf virus (Ohlsrhavirus, Rhabdoviridae), Evros sobemo-like virus (Solemoviridae) and Sindbis virus (Togaviridae). The remaining three unnamed viruses were given suggested names in this study, to correspond with where the Finnish sequences originated: Hanko picorna-like virus (Picornaviridae), Hanko totivirus 3 and Hanko totivirus 4 (Totiviridae). Only two of these previously described viruses are currently recognised by the ICTV: Sindbis virus and Ohlsdorf virus. Three viruses, which had previously been detected using virus cell culture with Finnish mosquitoes, were sequenced and linked to named mosquitoes as follows: Hanko virus with Oc. caspius, Inkoo virus with Oc. punctor/punctodes and Sindbis virus with Oc. communis. These results affirm the high degree of viral diversity found in mosquitoes from Finland, despite only nine of the forty-three endemic mosquito species [11] being included in this study.
4.1. Classification and Interpretation of the Viruses Detected in this Study
Constructing phylogenetic trees of RNA viruses using RNA-dependent RNA polymerase sequences is a common practice to infer evolutionary relationships and classify newly detected viruses. This is because RdRp is a core viral protein which has conserved sequence motifs that make it a preferable gene to utilise in phylogenetic analyses. The nucleotide sequences of RNA viruses change constantly due to the high mutation rate in RNA viruses, but in contrast, amino acid sequences remain relatively stable and conserved [83]. Phylogenetic trees made from RdRps, therefore, tend to be more accurate compared to those made using other core proteins [84,85,86].
The putative viruses sequenced in this study were assigned as novel based on several criteria: (1) novel virus RNA dependent RNA polymerases submitted to NCBI BLASTx had to have an amino acid identity value lower than 90% compared to the most similar virus; (2) phylogenetic analyses were run to ascertain their evolutionary relationship with previously described viruses and their likely classification within virus families; (3) associated GenBank records from closely related taxa were examined and compared with potentially novel viruses. These included the country of sampling, collection date and the organism from which the virus was isolated, to infer their novelty. Finally, (4) the criteria set by ICTV, including sequence lengths, amino acid identity and clustering in phylogenetic trees were also considered. Additionally, we computed supplementary pairwise distances from the protein alignments to ascertain the novelty of the detected viruses (Table S3). Certain viruses named in this study were on the borderline of being novel, since they came close to, but above, the 90% amino acid identity threshold with the closest described viruses. Such cases were noted where relevant. These viruses might indeed turn out to be Finnish strains of established viruses, but confirmation would require additional research including more sequence information on the related viral genetic diversity, especially from other geographical regions. All of the virus names proposed in this study are working names, as the final decision on their nomenclature and classification will be made by the ICTV.
Most (147) of the 159 viruses reported in this study were designated as novel since they had low similarities in RdRp amino acid identity with the most similar existing viruses (average 65.88%). The lowest amino acid identity was seen with Enontekio reovirus, which was only 29.6% similar to Operophtera brumata reovirus (Spinareoviridae), but low values were encountered many times throughout the analysis. This highlights the issues presented by “viral dark matter”, i.e., the lack of available sequences in databases to which viral sequences can be aligned [87], as well as the capacity of Lazypipe, the virus discovery and annotation pipeline established in our laboratory [18], to unravel viral sequences that are only remotely related to previously known viruses. Palkane botybirna-like virus (described in Section 3.1.3), shared a low average amino acid identity with another unclassified botybirna-like virus, Bremia lactucae associated dsRNA virus 1. Whether these two viruses are distant relatives, are ancestral viruses to the taxon, whether they can be classified as botybirnaviruses or whether they constitute a novel group of viruses remains undetermined. This study falls short of suggesting new virus genera, but it is likely that many of these sequences will form new genera in future revisions of the affected virus families.
Many more new viruses could have been named from the sequences obtained from this study but were excluded as their contigs fell below the 1000-nucleotide minimum length requirement that was set for any sequences to be considered for analysis. These discarded sequences formed approximately 75.5% of the total viral sequence data generated. In particular, short sequences of the pathogenic species Inkoo virus and Chatanga virus (Peribunyaviridae) were affected by these strict parameters, since short contigs containing polymerase, glycoprotein and nucleocapsid sequences were recovered.
A common pattern observed in the phylogenetic trees was that novel viruses clustered with available sequences of mosquito-derived viruses, inferring that these might be more mosquito-specific than insect-specific. Moreover, many novel viruses obtained during this study clustered with viruses that were sequenced from other mosquitoes, many of which belonged to Aedini, a cosmopolitan tribe of Culicidae with 1263 extant species and which includes 35% of all valid mosquito species [13]. One explanation could be that these viruses share a common ancestry [85]. Viral sequences that grouped within Iflaviridae, Aliusviridae and especially in Flaviviridae (Flavivirus) clustered near to or with insect-specific and Aedini-associated viruses. Several of the novel viruses which grouped within Picornaviridae, Chuviridae and Chrysoviridae were the first mosquito-associated viruses detected which belonged to tribe Aedini. These findings could be indicative of broader mosquito association ranges among these RNA virus families. Among the virus families which infect plants and fungi, e.g., Alphapartitivirus, the discovery of these novel Finnish viruses would suggest that Ochlerotatus mosquitoes (and most likely mosquitoes in general) act in some capacity as vectors for these viruses, whether by mechanical transmission or otherwise.
The proportion of totivirus sequences detected in all Ochlerotatus pools was very high in this study (Figures S2 and S3), despite them being viruses traditionally more associated with fungi and protozoa (https://ictv.global/taxonomy/ (accessed on 20 May 2022)). GenBank records show that totiviruses have been found in arthropods, plants, mammals and fish, thus indicate that these viruses might have a wider host range than is currently recognised by the ICTV (https://ictv.global/taxonomy/ (accessed on 20 May 2022)). Another factor to potentially explain the high prevalence of totiviruses could be that they are part of the core virome of Ochlerotatus species [88]. Either way, this study highlights the need for an expert group to subject Totiviridae to a critical review, since at present only 28 species belonging to five genera are currently officially recognised by the ICTV (https://ictv.global/taxonomy/ (accessed on 20 May 2022)), but in this study alone, 52 novel viruses were proposed. Similarly, partitiviruses were the next most represented species in this study, with 52 strains belonging to 23 viruses.
In recent years, most of the novel mosquito-borne viruses have been detected and reported from temperate and equatorial regions, since that is where most of the known mosquito-borne diseases are distributed [89]. The number of viromic studies from northern latitudes are increasing [31,32,90,91], but the uneven distribution of global research effort emphasises the importance of investigating mosquito viromes of these regions for more accurate information about the virosphere.
4.2. Reflections on the Methods and Their Impact upon Interpreting the Results
Since the lab work for this study was completed, a viromics study of Swedish mosquitoes was published in which a rinse step was added prior to homogenisation to remove surface contaminants from their specimens [90]. On reflection, this additional step would have been very beneficial to exclude any viruses which may have been mechanically transmitted to mosquitoes, or which were associated with bacteria/protozoa on the mosquito’s integument. Many of the viruses that were sequenced during this study, e.g., Chrysoviridae, Endornaviridae, Solemoviridae, Totiviridae and Virgaviridae are more traditionally associated with protozoa, plants or fungi than mosquitoes (see Table 8) [39,54,56,70,81,82]. Species of Virgaviridae even use pollen grains to disperse and infect new hosts [56]. The downside of viromics is not knowing the association of the novel viruses that are recovered, e.g., whether the mosquito happened to be covered in pollen grains which were in turn covered in viruses; whether the viruses were present in undigested gut contents; whether they infected the mosquito; or whether the mosquito is a vector for that virus, and so on.
Mosquitoes also have many interactions with other organisms in the environment. Some species are known to feed on honeydew, a sugar-rich excrement that some insects including ants (Hymenoptera) and aphids (Hemiptera) excrete after feeding on plants [92,93]. It would be interesting to determine, since some species actively seek out honeydew [93], if such interactions affect virus transmission between insects, particularly since so many plant-associated viruses were recovered in this study. In addition, three of the females that were included in the study, one Oc. excrucians (FIN/L-2018/007) and two Oc. punctor/punctodes (FIN/PP-2018/015 and FIN/L-2018/026) were noted to have parasitic or phoretic mites attached to them (nine mites, one and one mite, respectively). If truly phoretic, then the mites may just have been temporarily attached to the mosquito for dispersal, so they may not have been so relevant for interspecies transmission. If, however, they were parasitic, then the transfer of viruses between mites and mosquitoes is not out of the realm of possibility [94]. More work is required in the future to elucidate these relationships.
Taking these points into consideration, a further laboratory step would have also increased our understanding of which viruses may be vectored by the mosquitoes included in these analyses. Honey-baited nucleic acid cards, such as FTA® Elute Cards (Whatman, Maidstone, UK), have been used in several studies in recent years in order to collect mosquito saliva, preserve any viral RNA, and ultimately sequenced to determine which viruses/virus species are present [95,96,97]. By first collecting mosquitoes, and then allowing them time to feed upon such cards either singularly, or in small groups, it would certainly be possible to refine results from metagenomic studies such as this one to see which viruses were common to the nucleic acid cards/saliva and mosquitoes, and which were only present in the mosquitoes, thereby determining which viruses have higher or lower likelihoods of being pathogenic. This could be then tested further using virus cell culture methods to isolate possible viruses on vertebrate or mosquito cells.
When mosquitoes were collected for this study, a note was made whenever a female was noticeably blood fed or gravid, but not if they had distended abdomens which looked as though they had recently fed upon plant juices. All but three of the females were not visibly blood-fed with only one female from pools FIN/L-2018/07 and FIN/L-2018/27 being confirmed as such, and one female which looked like it had possibly blood-fed several days earlier, in pool FIN/L-2018/88. Pool FIN/L-2018/07 contained seven viruses, one described from Oc. cantans in Germany, Ohlsdorf virus [32] (Rhabdoviridae), and six novel viruses belonging to Partitiviridae (1), Permutotetraviridae (1), Solemoviridae (1) and Totiviridae (3). Pool FIN/L-2018/27 only contained a single virus, Hanko iflavirus 1 (Iflaviridae). Pool FIN/L-2018/88 contained eight novel viruses, which belonged to Chrysoviridae (1), Partitiviridae (1), Sedoreoviridae (1), Totiviridae (4) and Virgaviridae (1). Competition between different viruses within mosquitoes might inhibit the replication or transmission of other viruses, resulting in the over representation of more competitive viruses [98,99,100]. Defective viral genomes have also been observed to inhibit replication or transmission of other viruses in mosquitoes [98,99], or in the case of identical or closely related viruses, the virus which manages to infect a host cell first might inhibit the replication of another via a process named “superinfection exclusion” [100].
Viromes of other mosquitoes which are native to Finland would also be of interest to study further in the future. This study only included nine of 43 (21%) currently recognised endemic species [11], and 38% of the pools were Oc. communis, creating a heavy bias to one species. Additional topics that would be of interest to explore further include the geographic and seasonal variations in the virome, as well as differences between males and females and at different developmental stages. Seasonal variation has been observed in Aedes (Stegomyia) albopictus [101] and Culex mosquitoes [102], though the core virome remains similar across different life stages in Ae. albopictus [103]. The sole focus on female mosquitoes might also limit virus discovery, akin to a study done with Ae. albopictus mosquitoes, in which Aedes iflavi-like virus genomes were only detected in a pool of male mosquitoes [104]. The authors do however note that the explanation for this is uncertain and that there might be other causal factors, such as the location of mosquito sampling [104].
4.3. Geographical Distribution of Viruses in Finland
This study has significantly increased the number of locations from which virus-positive mosquitoes have been collected in Finland. Prior studies have detected Hanko and Inkoo viruses from Uusimaa [3,4,8], Lammi virus from Kainuu, Pohjois-Karjala and Päijät-Häme [7,9], Chatanga virus from Kainuu (same location as for Lammi virus) and Pohjois-Karjala [5], Ilomantsi virus from Pohjois-Karjala [9], Sindbis virus from Pohjois-Karjala [1,2] and finally Mekrijärvi negevirus from Pohjois-Karjala [10]. In all, these viruses were found in only 4 of the 19 regions, and from only seven approximate locations, since six publications all included specimens from around Mekrijärvi in Pohjois-Karjala. The previously most northern mosquito-associated viruses in Finland were found in mosquitoes from around Sotkamo in Kainuu, approximately N64°08′, E28°23′ [5,9].
In contrast, this study included specimens which were collected from 49 collection efforts at 43 sites (min 1 km separation) in 11 regions and extended the sampling locations of virus-positive mosquitoes to the entire country (see Table A1 for a list of the proposed novel viruses by collection location, which can be compared with Figure 1). Moreover, the most northerly record of mosquito-positive viruses in Europe is now from collection FI 607 from Utsjoki in Lapland at N69°47′, E27°03′, where six viruses (Hattula chuvirus, Cordoba virus, Utsjoki negeviruses 1–3 and Hattula totivirus 1) were sequenced from pools FIN/L-2018/01 and FIN/L-2018/06. This overtakes the previous northernmost European record of a mosquito-associated bunyavirus from Masi, Norway, which was located at N69°26′, E23°39′ in the 1970s [105]. Two other collections which contribute to this study, FI 654 and FI 655 from Inari, Lapland, were also made further north than the Norwegian study (pools FIN/L-2018/07 and FIN/L-2018/19).
Hanko virus, an insect-specific virus which was first described from Finland [8], was sequenced in this study from mosquitoes that were collected near to the type locality in Hanko, Uusimaa. The four virus-positive pools all comprised Oc. caspius, which were collected in late August 2017. This is the first instance where a named mosquito species is confirmed to be associated with the virus. With future analyses, it will be interesting to see if Hanko virus is restricted to Oc. caspius, a halophilic/coastal mosquito species [16], or if it is also associated with mosquito species with larger distributions in Finland. Other specimens of Oc. caspius were included in the analysis, from Kustavi in Varsinais-Suomi from collections made in July and August 2017 (collection numbers FI 988 and FI 1015 in Figure 1), but the virus was not found therein.
A disproportionate number of pools were comprised of specimens which were collected from around Mekrijärvi, or in the municipality of Ilomantsi, Pohjois-Karjala. This was in part because the material in this study was all snap-frozen, identified and stored at −70 °C immediately following identification, to permit virus cell culture experiments. Such specialist facilities are located at a few field stations around Finland, which also explains why many collections were also made around the municipalities of Enontekiö and Utskoki in Lapland and in Hanko, Uusimaa. There were other factors, however, which influenced the decision to include material from eastern Finland. Prior to this study, Pohjois-Karjala was the only region where Sindbis virus [1,2], and one of only two locations from which Chatanga virus, has been found in Finnish mosquitoes [5], and vector species had not been confirmed. However, Sindbis virus has been detected in other parts of Finland in recent years [2]. Chatanga virus was not confirmed within the parameters of the study, but Sindbis virus was, as already mentioned, sequenced from a pool of Oc. communis mosquitoes. This sampling strategy did provide the first record for Inkoo virus in Oc. punctor/punctodes mosquito outside of Uusimaa, so from that perspective, it was very interesting, particularly as seroprevalence to California serogroup viruses is high amongst the Finnish population [106], but virus-positive mosquitoes have rarely been encountered. Since Ilomantsi, Hanko and Enontekiö had the majority of mosquito pools, they also had the most unique virus detections. The most widespread virus families in turn were Totiviridae and Partitiviridae. Totiviruses were detected in all sampled regions, which supports them being part of the core virome of Ochlerotatus mosquitoes. Similarly, partitiviruses were detected in all regions with the exception of Keski-Suomi (Central Finland) and Varsinais-Suomi (Southwest Finland). This, however, is very likely explained by sampling bias, since only one mosquito pool included specimens from Keski-Suomi and four pools from Varsinais-Suomi.
4.4. Brief Comparison with Other Virome Studies
Metagenomics studies published in recent years have identified diverse viromes in mosquitoes from around the world [31,34,37,43,45,66,90,107,108,109,110,111,112].These viromes appear to differ between species and can include anywhere from tens to hundreds of different virus species in a given sample, which often comprises several individuals of a species [31,34,37,43,45,66,90,107,108,109,110,111,112]. The nearest comparable study to Finland is a single-year, two-location study of 953 specimens of six mosquito species in Sweden [90]. It examined the viromes of Coquillettidia richiardii, Oc. communis, Oc. annulipes, Oc. cantans, Culex pipiens and Cx. torrentium, all species which are common to both Sweden and Finland, and two of which were common to both studies. They found viruses which belonged to multiple families, but ultimately there were none that were common to both studies [90]. They did, however, find viruses belonging to several families/orders which are yet to be detected in Finnish mosquitoes, including Nodaviridae, Orthomyxoviridae, Tombusviridae and Articulavirales [90]. Another Swedish study focused on comparing the viromes of Culex pipiens and Cx. torrentium collected from two locations over several years. They found 40 viruses (28 novel viruses) belonging to 14 families/orders: Bunyavirales, Endornaviridae, Luteoviridae, Mogonegavirales, Negevirus, Nidovirales, Orthomyxoviridae, Partitiviridae, Picornaviridae, Qinviridae, Reoviridae, Togaviridae (wrongly attributed to “Alphaviridae”, an invalid family) Totiviridae and Virgaviridae [31]. Sindbis virus, Hallsjon virus and Jotan virus were common to both this and the Swedish study [31], but viruses from Luteoviridae and Orthomyxoviridae were not sequenced in this study.
It is also of interest to compare these findings with those of other virus studies from Finland, to determine if other distant host taxa share any close virus associations and therefore explore the potential origins or pathogenicity of novel viruses. A study of glow-worms (Coleoptera: Lampyridae) amplified targeted RNA sequences from adults collected in central and southern Finland [113]. They recovered 11 novel viruses belonging to Flaviviridae, Iflaviviridae, Tymoviridae, Bunyavirales, Rhabdoviridae, Partitiviridae, Totiviridae and Metaviridae. Lampyris noctiluca flavivirus 1 grouped within the same clade as Lestijarvi flavi-like virus in the Flavivirus tree (Figure 2), in a branch separate from all other flavivirus sequences that were recovered in this study. Similarly, Lampyris noctiluca iflavirus 2 grouped in the same clade with Mekrijarvi iflavirus (Figure 3), in a branch away from all of the other iflaviruses. The glow-worm totivirus and rhabdovirus sequences also featured within the trees generated for this study, but not as closely as for the two named viruses. Since glow-worms are not haematophagous, have predatory larvae, do not feed as adults and are nonsocial, their associated viruses have limited sources [113]. The origins, host associations and pathogenicity of the novel viruses in this study are still to be determined.
4.5. Viruses Which Have Pathogenic Associations in Vertebrates
Two of the viruses which were sequenced in this study, Inkoo virus (Peribunyaviridae: Orthobunyavirus) and Sindbis virus (Togaviridae: Alphavirus) have known disease associations in Finland, and, although infrequent, can cause severe enough symptoms for patients to require hospitalisation [6,106,114]. These viruses have been detected in mosquitoes in previous Finnish studies, but it is worth mentioning that two mosquito species have now been implicated as being at the very least hosts for these viruses, if not vectors, Oc. punctor/punctodes and Oc. communis, respectively. The first isolations of Inkoo virus in the 1960s did include mixed pools containing Oc. communis and Oc. punctor/punctodes but now Oc. punctor/punctodes is confirmed as being virus positive. While most of the detected viral diversity has not been, and likely will not be, associated with pathogenic traits, it is nevertheless notable that without targeted sampling to capture outbreaks spatially or temporally, we have been able to detect sequences of the two previously well-established mosquito-borne pathogenic viruses in Finland.
Reovirales (until recently Reoviridae) is an order comprised of two families, Sedoreoviridae and Spinareoviridae (formerly Sedoreovirinae and Spinareovirinae), each of which has pathogenic virus species among their members. It is for this reason that the five novel Reovirales viruses in this study are of particular interest for future examination, to determine if hitherto unrecognised pathogenic mosquito-borne viruses are present in Finland. The proposed Sedoreoviridae viruses (Ilomantsi reovirus 1 to 4) all group with other viruses which were sequenced from mosquitoes, and are related to Phytoreovirus, which includes plant-pathogenic viruses based on a phylogenetic analysis. Valmbacken virus (see Figure 20), which is at the root of the novel virus cluster, is likely a mosquito-associated virus [31], indicating that the novel viruses potentially could have such associations. The single tentative Spinareoviridae virus, Enontekio reovirus, is distantly related to Fijivirus, which includes plant-infecting viruses that may spread via an insect vector. The sequences which group together in Figure 20 are all derived from insects.
5. Conclusions
This study, by using high throughput next-generation sequencing methods and an unbiased virus discovery pipeline, has vastly increased the knowledge of viruses associated with mosquitoes in Northern Europe and has confirmed the number of known mosquito-associated viruses and virus families in Finland from seven and four, to 159 and 25, respectively. Such a large increase in knowledge of the diversity of mosquito-associated viruses is certainly interesting and begins to enlighten the “viral dark matter”, but inevitably brings with it new questions and challenges. It also highlights the pressing need for additional study to bring relevance to the names and sequences presented herein, as well as to investigate arthropod viromes of northern regions more thoroughly. It is evident from the points we have raised that the floodgates have opened, and the real work of elucidating the relationships between mosquitoes, viruses, the environment and host species must now begin.
Acknowledgments
We thank Joni Uusitalo and Lynne Edward for assisting in mosquito collections, and Mira Utriainen, Johanna Martikainen and Viktor Olander for technical assistance. We would also like to thank Ilya Plyusnin for his contribution to Lazypipe.
Supplementary Materials
The following interactive, figures are available online at https://www.mdpi.com/article/10.3390/v14071489/s1; Figure S1: Viral read distribution per pool, Figure S2a: The number of virus contigs per family per mosquito species, Figure S2b: The number of viruses per family per mosquito species, Figure S3: The number of contigs from each virus family by mosquito species, Table S1: Summary of numbers and lengths of reads per sequenced pool, Table S2: Summary of detected viruses and supplementary information about them, Table S3: Pairwise distances of detected viruses computed from protein alignments with seqinR (v4.2-16).
Appendix A
Table A1.
All virus species that were sequenced in this study are displayed by the collection number from which they were obtained. Cross-reference this table with Figure 1 to visualise where each virus was present. Collections FI 1009, FI 1010 and FI 1011 were kept separate in the analyses but were made at the same location over two days and so are displayed together to avoid repetition. Region names are abbreviated, as per the codes in methods Section 2.2.
| Collection No. | Region, Municipality Date |
Genome Type |
Virus Family/ Taxon |
Proposed or Established Name |
|---|---|---|---|---|
| FI 432 | KH, Hattula | –ssRNA | Chuviridae | Hattula chuvirus |
| 27 May 2015 | −ssRNA | Rhabdoviridae | Hattula rhabdovirus | |
| dsRNA | Partitiviridae | Hattula partiti-like virus | ||
| dsRNA | Totiviridae | Hattula totivirus 1 | ||
| dsRNA | Totiviridae | Hattula totivirus 2 | ||
| dsRNA | Totiviridae | Hattula totivirus 3 | ||
| FI 437 | KH, Hämeenlinna | −ssRNA | Phasmaviridae | Hameenlinna orthophasmavirus 1 |
| 2 June 2015 | −ssRNA | Phasmaviridae | Hameenlinna orthophasmavirus 2 | |
| −ssRNA | Phenuiviridae | Kalajoki phenui-like virus 2 | ||
| dsRNA | Partitiviridae | Hameenlinna partiti-like virus | ||
| dsRNA | Totiviridae | Hameenlinna totivirus 1 | ||
| FI 441, FI 442 | KH, Hämeenlinna | +ssRNA | Flaviviridae | Hameenlinna flavivirus |
| 2 June 2015 | −ssRNA | Phenuiviridae | Hameenlinna phasivirus | |
| dsRNA | Totiviridae | Hameenlinna toti-like virus | ||
| dsRNA | Totiviridae | Hameenlinna totivirus 1 | ||
| dsRNA | Totiviridae | Hameenlinna totivirus 2 | ||
| dsRNA | Totiviridae | Hameenlinna totivirus 3 | ||
| FI 474 | Po, Vaasa | +ssRNA | Iflaviridae | Hanko iflavirus 1 |
| 14 June 2015 | dsRNA | Partitiviridae | Hameenlinna partiti-like virus | |
| dsRNA | Totiviridae | Hameenlinna totivirus 1 | ||
| FI 487 | Po, Vaasa | dsRNA | Partitiviridae | Vaasa deltapartitivirus |
| 16 June 2015 | dsRNA | Partitiviridae | Vaasa partiti-like virus | |
| dsRNA | Totiviridae | Vaasa toti-like virus | ||
| dsRNA | Totiviridae | Vaasa totivirus | ||
| FI 500 | Po, Pedersöre | +ssRNA | Iflaviridae | Pedersore iflavirus |
| 17 June 2015 | dsRNA | Totiviridae | Hameenlinna totivirus 1 | |
| dsRNA | Totiviridae | Hattula totivirus 2 | ||
| +ssRNA | Virgaviridae | Pedersore virga-like virus | ||
| FI 505 | PP, Kalajoki | −ssRNA | Chuviridae | Hattula chuvirus |
| 17 June 2015 | −ssRNA | Phenuiviridae | Kalajoki phenui-like virus 1 | |
| −ssRNA | Phenuiviridae | Kalajoki phenui-like virus 2 | ||
| −ssRNA | Qinviridae | Kalajoki qinvirus | ||
| dsRNA | Partitiviridae | Kalajoki alphapartitivirus | ||
| dsRNA | Partitiviridae | Kalajoki betapartitivirus | ||
| dsRNA | Totiviridae | Hameenlinna totivirus 1 | ||
| dsRNA | Totiviridae | Hattula totivirus 1 | ||
| dsRNA | Totiviridae | Hattula totivirus 2 | ||
| FI 513 | KP, Lestijärvi | +ssRNA | Flaviviridae | Lestijarvi flavi-like virus |
| 18 June 2015 | +ssRNA | Iflaviridae | Pedersore iflavirus | |
| −ssRNA | Aliusviridae | Lestijarvi obscuruvirus | ||
| −ssRNA | Chuviridae | Hattula chuvirus | ||
| −ssRNA | Phasmaviridae | Lestijarvi orthophasmavirus 1 | ||
| −ssRNA | Phasmaviridae | Lestijarvi orthophasmavirus 2 | ||
| dsRNA | Chrysoviridae | Lestijarvi alphachrysovirus | ||
| dsRNA | Partitiviridae | Lestijarvi partiti-like virus | ||
| dsRNA | Totiviridae | Lestijarvi totivirus | ||
| FI 520 | KS, Karstula | −ssRNA | Chuviridae | Hattula chuvirus |
| 18 June 2015 | −ssRNA | Rhabdoviridae | Hattula rhabdovirus | |
| dsRNA | Totiviridae | Karstula totivirus | ||
| FI 525 | Pi, Pälkäne | −ssRNA | Phasmaviridae | Hameenlinna orthophasmavirus 1 |
| 19 June 2015 | −ssRNA | Phenuiviridae | Kalajoki phenui-like virus 2 | |
| −ssRNA | Phenuiviridae | Palkane phenui-like virus 1 | ||
| −ssRNA | Phenuiviridae | Palkane phenui-like virus 2 | ||
| −ssRNA | Qinviridae | Kalajoki qinvirus | ||
| −ssRNA | Qinviridae | Palkane qinvirus | ||
| dsRNA | Botybirnavirus | Palkane botybirna-like virus | ||
| dsRNA | Partitiviridae | Kalajoki alphapartitivirus | ||
| dsRNA | Partitiviridae | Kalajoki betapartitivirus | ||
| dsRNA | Partitiviridae | Palkane alphapartitivirus 1 | ||
| dsRNA | Partitiviridae | Palkane alphapartitivirus 2 | ||
| dsRNA | Totiviridae | Hameenlinna totivirus 1 | ||
| dsRNA | Totiviridae | Hattula totivirus 2 | ||
| dsRNA | Totiviridae | Palkane toti-like virus | ||
| dsRNA | Totiviridae | Palkane totivirus | ||
| FI 531 | EK, Imatra | dsRNA | Totiviridae | Hameenlinna totivirus 1 |
| 23 June 2015 | dsRNA | Totiviridae | Hattula totivirus 2 | |
| dsRNA | Totiviridae | Palkane toti-like virus | ||
| FI 532, FI 537 | VS, Ilomantsi | +ssRNA | Iflaviridae | Hanko iflavirus 1 |
| 25–26 June 2015 | +ssRNA | Iflaviridae | Mekrijarvi iflavirus | |
| +ssRNA | Negevirus | Mekrijarvi negevirus | ||
| +ssRNA | Solemoviridae | Ilomantsi sobemovirus | ||
| +ssRNA | Togaviridae | Sindbis virus | ||
| −ssRNA | Phenuiviridae | Hameenlinna phasivirus | ||
| −ssRNA | Phenuiviridae | Ilomantsi phenui-like virus | ||
| −ssRNA | Phenuiviridae | Kalajoki phenui-like virus 1 | ||
| −ssRNA | Phenuiviridae | Kalajoki phenui-like virus 2 | ||
| −ssRNA | Qinviridae | Kalajoki qinvirus | ||
| −ssRNA | Qinviridae | Palkane qinvirus | ||
| −ssRNA | Qinviridae | Ilomantsi qinvirus | ||
| −ssRNA | Rhabdoviridae | Hattula rhabdovirus | ||
| dsRNA | Botybirnavirus | Palkane botybirna-like virus | ||
| dsRNA | Partitiviridae | Ilomantsi deltapartitivirus | ||
| dsRNA | Partitiviridae | Ilomantsi partiti-like virus 2 | ||
| dsRNA | Partitiviridae | Kalajoki alphapartitivirus | ||
| dsRNA | Partitiviridae | Lestijarvi partiti-like virus | ||
| dsRNA | Partitiviridae | Vaasa deltapartitivirus | ||
| dsRNA | Partitiviridae | Hameenlinna partiti-like virus | ||
| dsRNA | Sedoreoviridae | Ilomantsi reovirus 1 | ||
| dsRNA | Sedoreoviridae | Ilomantsi reovirus 2 | ||
| dsRNA | Sedoreoviridae | Ilomantsi reovirus 3 | ||
| dsRNA | Totiviridae | Hameenlinna toti-like virus | ||
| dsRNA | Totiviridae | Hameenlinna totivirus 1 | ||
| dsRNA | Totiviridae | Hameenlinna totivirus 3 | ||
| dsRNA | Totiviridae | Hattula totivirus 1 | ||
| dsRNA | Totiviridae | Hattula totivirus 2 | ||
| dsRNA | Totiviridae | Hattula totivirus 3 | ||
| dsRNA | Totiviridae | Ilomantsi toti-like virus 1 | ||
| dsRNA | Totiviridae | Ilomantsi toti-like virus 2 | ||
| dsRNA | Totiviridae | Ilomantsi toti-like virus 3 | ||
| dsRNA | Totiviridae | Ilomantsi totivirus 1 | ||
| dsRNA | Totiviridae | Ilomantsi totivirus 2 | ||
| dsRNA | Totiviridae | Ilomantsi totivirus 3 | ||
| dsRNA | Totiviridae | Lestijarvi totivirus | ||
| dsRNA | Totiviridae | Palkane toti-like virus | ||
| dsRNA | Totiviridae | Vaasa toti-like virus | ||
| dsRNA | Totiviridae | Vaasa totivirus | ||
| FI 538 | VS, Ilomantsi | −ssRNA | Phenuiviridae | Hameenlinna phasivirus |
| 26 June 2015 | dsRNA | Partitiviridae | Ilomantsi partiti-like virus 1 | |
| dsRNA | Totiviridae | Hameenlinna totivirus 3 | ||
| FI 540 | VS, Ilomantsi | −ssRNA | Phenuiviridae | Kalajoki phenui-like virus 1 |
| 26 June 2015 | dsRNA | Totiviridae | Hameenlinna totivirus 1 | |
| FI 550 | VS, Joensuu | +ssRNA | Solemoviridae | Joensuu sobemovirus |
| 7 June 2015 | −ssRNA | Chuviridae | Hattula chuvirus | |
| −ssRNA | Phenuiviridae | Hameenlinna phasivirus | ||
| −ssRNA | Rhabdoviridae | Hattula rhabdovirus | ||
| −ssRNA | Xinmoviridae | Joensuu anphevirus | ||
| dsRNA | Partitiviridae | Ilomantsi partiti-like virus 2 | ||
| dsRNA | Partitiviridae | Hattula partiti-like virus | ||
| dsRNA | Sedoreoviridae | Ilomantsi reovirus 2 | ||
| dsRNA | Sedoreoviridae | Ilomantsi reovirus 4 | ||
| dsRNA | Totiviridae | Hameenlinna totivirus 1 | ||
| dsRNA | Totiviridae | Hameenlinna totivirus 2 | ||
| dsRNA | Totiviridae | Hameenlinna totivirus 3 | ||
| dsRNA | Totiviridae | Hattula totivirus 1 | ||
| dsRNA | Totiviridae | Hattula totivirus 2 | ||
| dsRNA | Totiviridae | Ilomantsi toti-like virus 2 | ||
| dsRNA | Totiviridae | Ilomantsi totivirus 2 | ||
| dsRNA | Totiviridae | Palkane toti-like virus | ||
| FI 551 | VS, Ilomantsi | +ssRNA | Iflaviridae | Hanko iflavirus 1 |
| 27 June 2015 | −ssRNA | Phenuiviridae | Hameenlinna phasivirus | |
| dsRNA | Sedoreoviridae | Ilomantsi reovirus 1 | ||
| dsRNA | Totiviridae | Hameenlinna totivirus 2 | ||
| dsRNA | Totiviridae | Hameenlinna totivirus 3 | ||
| FI 566 | PP, Kiiminki 3 July 2015 |
dsRNA | Partitiviridae | Ilomantsi deltapartitivirus |
| FI 571 | L, Kittilä | dsRNA | Partitiviridae | Ilomantsi partiti-like virus 1 |
| 3 July 2015 | dsRNA | Totiviridae | Hameenlinna toti-like virus | |
| dsRNA | Totiviridae | Hameenlinna totivirus 2 | ||
| dsRNA | Totiviridae | Hameenlinna totivirus 3 | ||
| FI 575, FI 730 | L, Enontekiö 3–4 July 2015 |
+ssRNA | Iflaviridae | Hanko iflavirus 1 |
| FI 575, FI 582, FI 728 | L, Enontekiö | dsRNA | Partitiviridae | Hattula partiti-like virus |
| 2 and 4 July 2015 | dsRNA | Totiviridae | Enontekio totivirus 5 | |
| dsRNA | Totiviridae | Hameenlinna totivirus 1 | ||
| FI 607 | L, Utsjoki | +ssRNA | Negevirus | Cordoba virus |
| 7 July 2015 | +ssRNA | Negevirus | Utsjoki negevirus 1 | |
| +ssRNA | Negevirus | Utsjoki negevirus 2 | ||
| +ssRNA | Negevirus | Utsjoki negevirus 3 | ||
| −ssRNA | Chuviridae | Hattula chuvirus | ||
| dsRNA | Totiviridae | Hattula totivirus 1 | ||
| FI 618 | PP, Kuusamo | +ssRNA | Iflaviridae | Hanko iflavirus 1 |
| 9 July 2015 | −ssRNA | Chuviridae | Hattula chuvirus | |
| dsRNA | Chrysoviridae | Lestijarvi alphachrysovirus | ||
| FI 618, FI 620 | PP, Kuusamo | dsRNA | Partitiviridae | Ilomantsi partiti-like virus 1 |
| 9 July 2015 | dsRNA | Totiviridae | Kuusamo totivirus 1 | |
| dsRNA | Totiviridae | Kuusamo totivirus 2 | ||
| FI 620 | PP, Kuusamo | +ssRNA | Negevirus | Cordoba virus |
| 9 July 2015 | +ssRNA | Negevirus | Cordoba virus | |
| +ssRNA | Negevirus | Utsjoki negevirus 1 | ||
| dsRNA | Partitiviridae | Hattula partiti-like virus | ||
| dsRNA | Totiviridae | Enontekio totivirus 5 | ||
| dsRNA | Totiviridae | Hameenlinna totivirus 1 | ||
| FI 641 | PP, Kuusamo | +ssRNA | Negevirus | Cordoba virus |
| 18 July 2015 | +ssRNA | Negevirus | Dezidougou virus | |
| +ssRNA | Negevirus | Utsjoki negevirus 1 | ||
| +ssRNA | Solemoviridae | Joensuu sobemovirus | ||
| −ssRNA | Phasmaviridae | Kuusamo orthophasmavirus 1 | ||
| −ssRNA | Phasmaviridae | Kuusamo orthophasmavirus 2 | ||
| −ssRNA | Phasmaviridae | Kuusamo orthophasmavirus 3 | ||
| −ssRNA | Phasmaviridae | Kuusamo orthophasmavirus 4 | ||
| −ssRNA | Xinmoviridae | Joensuu anphevirus | ||
| dsRNA | Partitiviridae | Kuusamo partiti-like virus | ||
| dsRNA | Partitiviridae | Kuusamo alphapartitivirus | ||
| dsRNA | Partitiviridae | Hattula partiti-like virus | ||
| dsRNA | Totiviridae | Hameenlinna totivirus 1 | ||
| dsRNA | Totiviridae | Ilomantsi totivirus 1 | ||
| dsRNA | Totiviridae | Kuusamo toti-like virus | ||
| dsRNA | Totiviridae | Lestijarvi totivirus | ||
| FI 642, FI 648 | L, Salla 19 July 2015 |
+ssRNA | Iflaviridae | Hanko iflavirus 1 |
| FI 649 | L, Inari | +ssRNA | Flaviviridae | Inari jingmenvirus |
| 22 July 2015 | +ssRNA | Negevirus | Utsjoki negevirus 1 | |
| +ssRNA | Negevirus | Utsjoki negevirus 2 | ||
| +ssRNA | Permutotetraviridae | Inari permutotetravirus | ||
| −ssRNA | Rhabdoviridae | Hattula rhabdovirus | ||
| −ssRNA | Rhabdoviridae | Inari rhabdovirus | ||
| −ssRNA | Rhabdoviridae | Ohlsdorf virus | ||
| dsRNA | Partitiviridae | Ilomantsi partiti-like virus 1 | ||
| dsRNA | Partitiviridae | Hattula partiti-like virus | ||
| dsRNA | Partitiviridae | Inari deltapartitivirus | ||
| dsRNA | Totiviridae | Hameenlinna totivirus 1 | ||
| dsRNA | Totiviridae | Hattula totivirus 3 | ||
| dsRNA | Totiviridae | Ilomantsi toti-like virus 2 | ||
| dsRNA | Totiviridae | Inari toti-like virus | ||
| dsRNA | Totiviridae | Inari totivirus 1 | ||
| dsRNA | Totiviridae | Inari totivirus 2 | ||
| dsRNA | Totiviridae | Palkane toti-like virus | ||
| dsRNA | Totiviridae | Vaasa toti-like virus | ||
| FI 649, FI 654, FI 655 | L, Inari | +ssRNA | Permutotetraviridae | Inari permutotetravirus |
| 22–23 July 2015 | +ssRNA | Solemoviridae | Ilomantsi sobemovirus | |
| −ssRNA | Rhabdoviridae | Ohlsdorf virus | ||
| dsRNA | Partitiviridae | Vaasa deltapartitivirus | ||
| dsRNA | Totiviridae | Ilomantsi toti-like virus 2 | ||
| dsRNA | Totiviridae | Inari totivirus 1 | ||
| dsRNA | Totiviridae | Vaasa toti-like virus | ||
| FI 652, FI 728 | L, Inari/Enontekiö | dsRNA | Partitiviridae | Enontekio partiti-like virus |
| 2 July 2015 | dsRNA | Totiviridae | Enontekio totivirus 3 | |
| dsRNA | Totiviridae | Enontekio totivirus 4 | ||
| dsRNA | Totiviridae | Hattula totivirus 3 | ||
| FI 654, FI 655 | L, Inari | +ssRNA | Solemoviridae | Joensuu sobemovirus |
| 23 July 2015 | dsRNA | Totiviridae | Inari totivirus 2 | |
| dsRNA | Totiviridae | Lestijarvi totivirus | ||
| FI 663 | L, Utsjoki | +ssRNA | Virgaviridae | Pedersore virga-like virus |
| 24 July 2015 | dsRNA | Chrysoviridae | Lestijarvi alphachrysovirus | |
| dsRNA | Partitiviridae | Hameenlinna partiti-like virus | ||
| dsRNA | Sedoreoviridae | Ilomantsi reovirus 1 | ||
| dsRNA | Totiviridae | Hameenlinna totivirus 1 | ||
| dsRNA | Totiviridae | Hattula totivirus 1 | ||
| dsRNA | Totiviridae | Inari totivirus 2 | ||
| dsRNA | Totiviridae | Utsjoki toti-like virus | ||
| FI 671 | L, Enontekiö | +ssRNA | Iflaviridae | Enontekio iflavirus |
| 26 July 2015 | +ssRNA | Negevirus | Cordoba virus | |
| +ssRNA | Negevirus | Utsjoki negevirus 1 | ||
| +ssRNA | Negevirus | Utsjoki negevirus 2 | ||
| +ssRNA | Negevirus | Utsjoki negevirus 3 | ||
| +ssRNA | Solemoviridae | Enontekio sobemovirus | ||
| −ssRNA | Chuviridae | Hattula chuvirus | ||
| −ssRNA | Rhabdoviridae | Enontekio rhabdovirus | ||
| dsRNA | Chrysoviridae | Enontekio alphachrysovirus | ||
| dsRNA | Partitiviridae | Ilomantsi partiti-like virus 1 | ||
| dsRNA | Totiviridae | Enontekio totivirus 2 | ||
| dsRNA | Totiviridae | Hattula totivirus 3 | ||
| dsRNA | Totiviridae | Hattula totivirus 3 | ||
| FI 674 | L, Enontekiö | +ssRNA | Flaviviridae | Kilpisjarvi flavivirus |
| 28 July 2015 | +ssRNA | Iflaviridae | Enontekio iflavirus | |
| +ssRNA | Negevirus | Utsjoki negevirus 1 | ||
| +ssRNA | Permutotetraviridae | Inari permutotetravirus | ||
| +ssRNA | Quenyavirus | Enontekio quenyavirus | ||
| +ssRNA | Solemoviridae | Enontekio sobemovirus | ||
| +ssRNA | Virgaviridae | Enontekio virga-like virus 1 | ||
| +ssRNA | Virgaviridae | Enontekio virga-like virus 2 | ||
| +ssRNA | Virgaviridae | Pedersore virga-like virus | ||
| −ssRNA | Aspiviridae | Kilpisjarvi aspivirus | ||
| −ssRNA | Phenuiviridae | Enontekio phenui-like virus 1 | ||
| −ssRNA | Phenuiviridae | Enontekio phenui-like virus 3 | ||
| −ssRNA | Phenuiviridae | Enontekio phenui-like virus 5 | ||
| −ssRNA | Rhabdoviridae | Enontekio merhavirus | ||
| −ssRNA | Rhabdoviridae | Enontekio ohlsrhavirus | ||
| −ssRNA | Xinmoviridae | Enontekio anphevirus 1 | ||
| −ssRNA | Xinmoviridae | Enontekio anphevirus 2 | ||
| −ssRNA | Yueviridae | Enontekio yuevirus | ||
| dsRNA | Partitiviridae | Enontekio alphapartitivirus 1 | ||
| dsRNA | Partitiviridae | Enontekio alphapartitivirus 2 | ||
| dsRNA | Partitiviridae | Enontekio betapartitivirus 1 | ||
| dsRNA | Partitiviridae | Enontekio betapartitivirus 2 | ||
| dsRNA | Partitiviridae | Ilomantsi partiti-like virus 1 | ||
| dsRNA | Partitiviridae | Kuusamo alphapartitivirus | ||
| dsRNA | Spinareoviridae | Enontekio reovirus | ||
| dsRNA | Totiviridae | Enontekio toti-like virus 1 | ||
| dsRNA | Totiviridae | Enontekio toti-like virus 2 | ||
| dsRNA | Totiviridae | Enontekio toti-like virus 3 | ||
| dsRNA | Totiviridae | Enontekio toti-like virus 4 | ||
| dsRNA | Totiviridae | Enontekio totivirus 1 | ||
| dsRNA | Totiviridae | Enontekio totivirus 2 | ||
| dsRNA | Totiviridae | Enontekio totivirus 6 | ||
| dsRNA | Totiviridae | Enontekio totivirus 7 | ||
| dsRNA | Totiviridae | Palkane toti-like virus | ||
| FI 675 | L, Enontekiö | +ssRNA | Flaviviridae | Inari jingmenvirus |
| 28 July 2015 | −ssRNA | Rhabdoviridae | Enontekio ohlsrhavirus | |
| FI 701 | PP, Kuusamo | +ssRNA | Negevirus | Cordoba virus |
| 23 August 2015 | +ssRNA | Negevirus | Utsjoki negevirus 1 | |
| +ssRNA | Negevirus | Utsjoki negevirus 2 | ||
| +ssRNA | Permutotetraviridae | Inari permutotetravirus | ||
| FI 976 | EK, Joutseno | +ssRNA | Virgaviridae | Pedersore virga-like virus |
| 4 July 2017 | −ssRNA | Chuviridae | Hattula chuvirus | |
| −ssRNA | Phasmaviridae | Hameenlinna orthophasmavirus 1 | ||
| −ssRNA | Phasmaviridae | Hameenlinna orthophasmavirus 2 | ||
| −ssRNA | Phasmaviridae | Kuusamo orthophasmavirus 4 | ||
| −ssRNA | Phenuiviridae | Enontekio phenui-like virus 2 | ||
| −ssRNA | Phenuiviridae | Palkane phenui-like virus 2 | ||
| −ssRNA | Rhabdoviridae | Joutseno rhabdovirus 1 | ||
| −ssRNA | Rhabdoviridae | Joutseno rhabdovirus 2 | ||
| dsRNA | Partitiviridae | Hattula partiti-like virus | ||
| dsRNA | Sedoreoviridae | Ilomantsi reovirus 2 | ||
| dsRNA | Totiviridae | Hameenlinna totivirus 1 | ||
| dsRNA | Totiviridae | Hattula totivirus 2 | ||
| dsRNA | Totiviridae | Joutseno totivirus | ||
| dsRNA | Totiviridae | Palkane totivirus | ||
| FI 988 | VS, Kustavi | +ssRNA | Solemoviridae | Evros sobemo-like virus |
| 11 July 2017 | −ssRNA | Chuviridae | Kustavi chuvirus 1 | |
| −ssRNA | Chuviridae | Kustavi chuvirus 2 | ||
| dsRNA | Totiviridae | Kustavi toti-like virus | ||
| FI 1009, FI 1010, FI 1011 | U, Hanko | +ssRNA | Flaviviridae | Hanko virus |
| 22–23 August 2017 | +ssRNA | Iflaviridae | Hanko iflavirus 2 | |
| +ssRNA | Iflaviridae | Pedersore iflavirus | ||
| +ssRNA | Picornaviridae | Hanko picorna-like virus | ||
| +ssRNA | Quenyavirus | Enontekio quenyavirus | ||
| +ssRNA | Solemoviridae | Evros sobemo-like virus | ||
| +ssRNA | Solemoviridae | Hanko sobemovirus | ||
| +ssRNA | Virgaviridae | Pedersore virga-like virus | ||
| −ssRNA | Phenuiviridae | Enontekio phenui-like virus 4 | ||
| −ssRNA | Phenuiviridae | Hanko phenui-like virus 1 | ||
| −ssRNA | Phenuiviridae | Hanko phenui-like virus 2 | ||
| −ssRNA | Phenuiviridae | Hanko phenui-like virus 3 | ||
| −ssRNA | Xinmoviridae | Hanko anphevirus | ||
| −ssRNA | Xinmoviridae | Joensuu anphevirus | ||
| dsRNA | Chrysoviridae | Hanko alphachrysovirus | ||
| dsRNA | Endornaviridae | Hallsjon virus | ||
| dsRNA | Endornaviridae | Tvarminne alphaendornavirus | ||
| dsRNA | Partitiviridae | Hanko alphapartitivirus 1 | ||
| dsRNA | Partitiviridae | Hanko alphapartitivirus 2 | ||
| dsRNA | Partitiviridae | Hanko alphapartitivirus 3 | ||
| dsRNA | Totiviridae | Hanko toti-like virus 1 | ||
| dsRNA | Totiviridae | Hanko toti-like virus 2 | ||
| dsRNA | Totiviridae | Hanko toti-like virus 3 | ||
| dsRNA | Totiviridae | Hanko totivirus 1 | ||
| dsRNA | Totiviridae | Hanko totivirus 2 | ||
| dsRNA | Totiviridae | Hanko totivirus 3 | ||
| dsRNA | Totiviridae | Hanko totivirus 4 | ||
| dsRNA | Totiviridae | Hanko totivirus 5 | ||
| dsRNA | Totiviridae | Hanko totivirus 6 | ||
| dsRNA | Totiviridae | Hanko totivirus 7 | ||
| dsRNA | Totiviridae | Hanko totivirus 8 | ||
| dsRNA | Totiviridae | Hanko totivirus 9 | ||
| dsRNA | Totiviridae | Hanko totivirus 10 | ||
| dsRNA | Totiviridae | Hattula totivirus 3 | ||
| dsRNA | Totiviridae | Inari toti-like virus | ||
| dsRNA | Totiviridae | Kustavi toti-like virus | ||
| FI 1015 | VS, Kustavi | +ssRNA | Negevirus | Kustavi negevirus |
| 24 August 2017 | +ssRNA | Picornaviridae | Jotan virus | |
| +ssRNA | Solemoviridae | Evros sobemo-like virus | ||
| dsRNA | Totiviridae | Hanko totivirus 3 | ||
| dsRNA | Totiviridae | Hanko totivirus 4 | ||
| dsRNA | Totiviridae | Hanko totivirus 5 | ||
| dsRNA | Totiviridae | Kustavi toti-like virus |
Author Contributions
Conceptualization, E.H., O.V., C.L.C., E.M.K. and T.S.; methodology, E.H., P.T.T.N., C.L.C., E.M.K. and T.S.; field work and mosquito identifications, C.L.C.; formal analysis, P.T.T.N. and T.S.; writing—original draft preparation, P.T.T.N., C.L.C., E.M.K., T.S. and E.H.; writing—review and editing, P.T.T.N., C.L.C., M.T.S., E.M.K., O.V., T.S. and E.H.; visualization, P.T.T.N. and R.U.; funding acquisition, O.V., E.H., C.L.C. and E.M.K. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The produced sequence data is openly available in the NCBI BioProject database with the accession code PRJNA852425 and the virus sequences are publicly available in the NCBI GenBank database (see Table 2,Table 3,Table 4,Table 5,Table 6,Table 7 for accession codes).
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the Jane and Aatos Erkko Foundation; the Sigrid Jusélius Foundation; the Academy of Finland; EU Horizon 2020 VEO, grant number 874735; Helsinki University Hospital Funds (TYH2018322 and TYH2021343), the Finnish Cultural Foundation, grant number 00210264; Societas Pro Fauna et Flora Fennica; the Kymenlaakso Regional Fund; and the University of Helsinki Doctoral Programs in Integrated Life Sciences and Interdisciplinary Environmental Sciences. Open access funding provided by University of Helsinki.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sane J., Kurkela S., Putkuri N., Huhtamo E., Vaheri A., Vapalahti O. Complete coding sequence and molecular epidemiological analysis of Sindbis virus isolates from mosquitoes and humans, Finland. J. Gen. Virol. 2012;93:1984–1990. doi: 10.1099/vir.0.042853-0. [DOI] [PubMed] [Google Scholar]
- 2.Korhonen E.M., Suvanto M.T., Uusitalo R., Faolotto G., Smura T., Sane J., Vapalahti O., Huhtamo E. Sindbis virus strains of divergent origin isolated from humans and mosquitoes during a recent outbreak in Finland. Vector Borne Zoonotic Dis. 2020;20:843–849. doi: 10.1089/vbz.2019.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brummer-Korvenkontio M., Saikku P., Korhonen P., Reunala T. The Inkoo virus, a member of the California encephalitis group in Finland. Scand. J. Clin. Lab. Invest. 1969;23((Suppl. 108)):159. [Google Scholar]
- 4.Brummer-Korvenkontio M., Saikku P., Korhonen P., Ulmanen I., Reunala T., Karvonen J. Arboviruses in Finland. IV. Isolation and characterization of Inkoo virus, a Finnish representative of the California group. Am. J. Trop. Med. Hyg. 1973;22:404–413. doi: 10.4269/ajtmh.1973.22.404. [DOI] [PubMed] [Google Scholar]
- 5.Putkuri N., Kurkela S., Levanov L., Huhtamo E., Vaheri A., Sironen T., Vapalahti O. Isolation and characterization of a California encephalitis serogroup orthobunyavirus from Finnish mosquitoes. Infect. Genet. Evol. 2014;22:164–173. doi: 10.1016/j.meegid.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 6.Putkuri N., Kantele A., Levanov L., Kivisto I., Brummer-Korvenkontio M., Vaheri A., Vapalahti O. Acute human Inkoo and Chatanga Virus infections, Finland. Emerg Infect. Dis. 2016;22:810–817. doi: 10.3201/eid2205.151015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huhtamo E., Putkuri N., Kurkela S., Manni T., Vaheri A., Vapalahti O., Uzcátegui N.Y. Characterization of a novel flavivirus from mosquitoes in northern Europe that is related to mosquito-borne flaviviruses of the tropics. J. Virol. 2009;83:9532–9540. doi: 10.1128/JVI.00529-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huhtamo E., Moureau G., Cook S., Julkunen O., Putkuri N., Kurkela S., Uzcátegui N.Y., Harbach R.E., Gould E.A., Vapalahti O., et al. Novel insect-specific flavivirus isolated from northern Europe. Virology. 2012;433:471–478. doi: 10.1016/j.virol.2012.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huhtamo E., Cook S., Moureau G., Uzcátegui N.Y., Sironen T., Kuivanen S., Putkuri N., Kurkela S., Harbach R.E., Firth A.E., et al. Novel flaviviruses from mosquitoes: Mosquito-specific evolutionary lineages within the phylogenetic group of mosquito-borne flaviviruses. Virology. 2014;464–465:320–329. doi: 10.1016/j.virol.2014.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suvanto M.T., Truong Nguyen P., Uusitalo R., Korhonen E.M., Faolotto G., Vapalahti O., Huhtamo E., Smura T. A novel negevirus isolated from Aedes vexans mosquitoes in Finland. Arch. Virol. 2020;165:2989–2992. doi: 10.1007/s00705-020-04810-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Culverwell C.L., Uusitalo R.J., Korhonen E.M., Vapalahti O.P., Huhtamo E., Harbach R.E. The mosquitoes of Finland: Updated distributions and bionomics. Med. Vet. Entomol. 2021;35:1–29. doi: 10.1111/mve.12475. [DOI] [PubMed] [Google Scholar]
- 12.Utrio P. Geographic distribution of mosquitoes (Diptera, Culicidae) in eastern Fennoscandia. Neotrop. Entomol. 1979;59:105–123. [Google Scholar]
- 13.Harbach R.E. Mosquito Taxonomic Inventory. [(accessed on 7 April 2022)]. Available online: http://mosquito-taxonomic-inventory.info.
- 14.ECDC . Guidelines for the Surveillance of Native Mosquitoes in Europe. European Centre for Disease Prevention and Control, Stockholm; Stockholm, Sweden: 2014. [Google Scholar]
- 15.Stojanovich C.J., Scott H.G. Illustrated Key to the Mosquitoes of Fennoscandia: Finland, Sweden, Denmark, Norway. Scott; Portland, OR, USA: 1995. p. 132. [Google Scholar]
- 16.Becker N., Petrić D., Zgomba M., Boase C., Madon M.B., Dahl C., Kaiser A. Mosquitoes and Their Control. 2nd ed. Springer; Berlin/Heidelberg, Germany: 2010. [Google Scholar]
- 17.Conceição-Neto N., Zeller M., Lefrère H., De Bruyn P., Beller L., Deboutte W., Yinda C.K., Lavigne R., Maes P., Ranst M.V., et al. Modular approach to customise sample preparation procedures for viral metagenomics: A reproducible protocol for virome analysis. Sci. Rep. 2015;5:16532. doi: 10.1038/srep16532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plyusnin I., Kant R., Jääskelainen A.J., Sironen T., Holm L., Vapalahti O., Smura T. Novel NGS pipeline for virus discovery from a wide spectrum of hosts and sample types. Virus Evol. 2020;6:veaa091. doi: 10.1093/ve/veaa091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bolger A.M., Lohse M., Usadel B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li D., Luo R., Liu C.-M., Leung C.-M., Ting H.-F., Sadakane K., Yamashita H., Lam T.-W. MEGAHIT v1.0: A fast and scalable metagenome assembler driven by advanced methodologies and community practices. Methods. 2016;102:3–11. doi: 10.1016/j.ymeth.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 21.Noguchi H., Taniguchi T., Itoh T. MetaGeneAnnotator: Detecting species-specific patterns of ribosomal binding site for precise gene prediction in anonymous prokaryotic and phage genomes. DNA Res. 2008;15:387–396. doi: 10.1093/dnares/dsn027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stajich J.E., Block D., Boulez K., Brenner S.E., Chervitz S.A., Dagdigian C., Fuellen G., Gilbert J.G., Korf I., Lapp H., et al. The Bioperl toolkit: Perl modules for the life sciences. Genome Res. 2002;12:1611–1618. doi: 10.1101/gr.361602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Somervuo P., Holm L. SANSparallel: Interactive homology search against Uniprot. Nucleic Acids Res. 2015;43:W24–W29. doi: 10.1093/nar/gkv317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rice P., Longden I., Bleasby A. EMBOSS: The European Molecular Biology Open Software Suite. Trends Genet. 2000;16:276–277. doi: 10.1016/S0168-9525(00)02024-2. [DOI] [PubMed] [Google Scholar]
- 25.Katoh K., Standley D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Capella-Gutiérrez S., Silla-Martínez J.M., Gabaldón T. trimAl: A tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics. 2009;25:1972–1973. doi: 10.1093/bioinformatics/btp348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Minh B.Q., Schmidt H.A., Chernomor O., Schrempf D., Woodhams M.D., von Haeseler A., Lanfear R. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020;37:1530–1534. doi: 10.1093/molbev/msaa015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalyaanamoorthy S., Minh B.Q., Wong T.K.F., von Haeseler A., Jermiin L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods. 2017;14:587–589. doi: 10.1038/nmeth.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoang D.T., Chernomor O., von Haeseler A., Minh B.Q., Vinh L.S. UFBoot2: Improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 2018;35:518–522. doi: 10.1093/molbev/msx281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu G., Smith D.K., Zhu H., Guan Y., Lam T.T.Y. GGTREE: An R package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods Ecol. Evol. 2017;8:28–36. doi: 10.1111/2041-210X.12628. [DOI] [Google Scholar]
- 31.Pettersson J.H., Shi M., Eden J.S., Holmes E.C., Hesson J.C. Meta-transcriptomic comparison of the RNA viromes of the mosquito vectors Culex pipiens and Culex torrentium in Northern Europe. Viruses. 2019;11:1033. doi: 10.3390/v11111033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shahhosseini N., Lühken R., Jöst H., Jansen S., Börstler J., Rieger T., Krüger A., Yadouleton A., de Mendonça Campos R., Cirne-Santos C.C., et al. Detection and characterization of a novel rhabdovirus in Aedes cantans mosquitoes and evidence for a mosquito-associated new genus in the family Rhabdoviridae. Infect. Genet. Evol. 2017;55:260–268. doi: 10.1016/j.meegid.2017.09.026. [DOI] [PubMed] [Google Scholar]
- 33.Reuter G., Boros Á., Pál J., Kapusinszky B., Delwart E., Pankovics P. Detection and genome analysis of a novel (dima)rhabdovirus (Riverside virus) from Ochlerotatus sp. mosquitoes in Central Europe. Infect. Genet. Evol. 2016;39:336–341. doi: 10.1016/j.meegid.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 34.Sadeghi M., Altan E., Deng X., Barker C.M., Fang Y., Coffey L.L., Delwart E. Virome of > 12 thousand Culex mosquitoes from throughout California. Virology. 2018;523:74–88. doi: 10.1016/j.virol.2018.07.029. [DOI] [PubMed] [Google Scholar]
- 35.Shi M., Lin X.-D., Tian J.-H., Chen L.-J., Chen X., Li C.-X., Qin X.-C., Li J., Cao J.-P., Eden J.-S., et al. Redefining the invertebrate RNA virosphere. Nature. 2016;540:539–543. doi: 10.1038/nature20167. [DOI] [PubMed] [Google Scholar]
- 36.Pettersson J.H., Shi M., Bohlin J., Eldholm V., Brynildsrud O.B., Paulsen K.M., Andreassen Å., Holmes E.C. Characterizing the virome of Ixodes ricinus ticks from northern Europe. Sci. Rep. 2017;7:10870. doi: 10.1038/s41598-017-11439-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi M., Neville P., Nicholson J., Eden J.S., Imrie A., Holmes E.C. High-resolution metatranscriptomics reveals the ecological dynamics of mosquito-associated RNA viruses in Western Australia. J. Virol. 2017;91:e00680-17. doi: 10.1128/JVI.00680-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Faizah A.N., Kobayashi D., Isawa H., Amoa-Bosompem M., Murota K., Higa Y., Futami K., Shimada S., Kim K.S., Itokawa K., et al. Deciphering the virome of Culex vishnui subgroup mosquitoes, the major vectors of Japanese Encephalitis, in Japan. Viruses. 2020;12:264. doi: 10.3390/v12030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valverde R.A., Khalifa M.E., Okada R., Fukuhara T., Sabanadzovic S., Ictv Report C. ICTV Virus Taxonomy Profile: Endornaviridae. J. Gen. Virol. 2019;100:1204–1205. doi: 10.1099/jgv.0.001277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simmonds P., Becher P., Bukh J., Gould E.A., Meyers G., Monath T., Muerhoff S., Pletnev A., Rico-Hesse R., Smith D.B., et al. ICTV Virus Taxonomy Profile: Flaviviridae. J. Gen. Virol. 2017;98:2–3. doi: 10.1099/jgv.0.000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akiner M.M., Ozturk M., Baser A.B., Gunay F., Hacioglu S., Brinkmann A., Emanet N., Alten B., Ozkul A., Nitsche A., et al. Arboviral screening of invasive Aedes species in northeastern Turkey: West Nile virus circulation and detection of insect-only viruses. PLoS Negl. Trop. Dis. 2019;13:e0007334. doi: 10.1371/journal.pntd.0007334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Supriyono R.K., Torii S., Shimoda H., Ishijima K., Yonemitsu K., Minami S., Kuroda Y., Tatemoto K., Tran N.T.B., Takano A., et al. Mosquito-borne viruses, insect-specific flaviviruses (family Flaviviridae, genus Flavivirus), Banna virus (family Reoviridae, genus Seadornavirus), Bogor virus (unassigned member of family Permutotetraviridae), and alphamesoniviruses 2 and 3 (family Mesoniviridae, genus Alphamesonivirus) isolated from Indonesian mosquitoes. J. Vet. Med. Sci. 2020;82:1030–1041. doi: 10.1292/jvms.20-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He X., Yin Q., Zhou L., Meng L., Hu W., Li F., Li Y., Han K., Zhang S., Fu S., et al. Metagenomic sequencing reveals viral abundance and diversity in mosquitoes from the Shaanxi-Gansu-Ningxia region, China. PLoS Negl. Trop. Dis. 2021;15:e0009381. doi: 10.1371/journal.pntd.0009381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valles S.M., Chen Y., Firth A.E., Guerin D.M.A., Hashimoto Y., Herrero S., de Miranda J.R., Ryabov E., ICTV Report Consortium ICTV Virus Taxonomy Profile: Iflaviridae. J Gen. Virol. 2017;98:527–528. doi: 10.1099/jgv.0.000757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Atoni E., Wang Y., Karungu S., Waruhiu C., Zohaib A., Obanda V., Agwanda B., Mutua M., Xia H., Yuan Z. Metagenomic virome analysis of Culex mosquitoes from Kenya and China. Viruses. 2018;10:30. doi: 10.3390/v10010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vasilakis N., Forrester N.L., Palacios G., Nasar F., Savji N., Rossi S.L., Guzman H., Wood T.G., Popov V., Gorchakov R., et al. Negevirus: A proposed new taxon of insect-specific viruses with wide geographic distribution. J. Virol. 2013;87:2475–2488. doi: 10.1128/JVI.00776-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pringle F.M., Gordon K.H., Hanzlik T.N., Kalmakoff J., Scotti P.D., Ward V.K. A novel capsid expression strategy for Thosea asigna virus (Tetraviridae) Pt 7J Gen. Virol. 1999;80:1855–1863. doi: 10.1099/0022-1317-80-7-1855. [DOI] [PubMed] [Google Scholar]
- 48.Zeddam J.-L., Gordon K.H.J., Lauber C., Alves C.A.F., Luke B.T., Hanzlik T.N., Ward V.K., Gorbalenya A.E. Euprosterna elaeasa virus genome sequence and evolution of the Tetraviridae family: Emergence of bipartite genomes and conservation of the VPg signal with the dsRNA Birnaviridae family. Virology. 2010;397:145–154. doi: 10.1016/j.virol.2009.10.042. [DOI] [PubMed] [Google Scholar]
- 49.Ruiz-Padilla A., Rodríguez-Romero J., Gómez-Cid I., Pacifico D., Ayllón M.A. Novel mycoviruses discovered in the mycovirome of a necrotrophic fungus. Mbio. 2021;12:e03705-20. doi: 10.1128/mBio.03705-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zell R., Delwart E., Gorbalenya A.E., Hovi T., King A.M.Q., Knowles N.J., Lindberg A.M., Pallansch M.A., Palmenberg A.C., Reuter G., et al. ICTV Virus Taxonomy Profile: Picornaviridae. J Gen. Virol. 2017;98:2421–2422. doi: 10.1099/jgv.0.000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cholleti H., Hayer J., Fafetine J., Berg M., Blomström A.-L. Genetic characterization of a novel picorna-like virus in Culex spp. mosquitoes from Mozambique. Virol. J. 2018;15:71. doi: 10.1186/s12985-018-0981-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harvey E., Rose K., Eden J.-S., Lawrence A., Doggett S.L., Holmes E.C. Identification of diverse arthropod associated viruses in native Australian fleas. Virology. 2019;535:189–199. doi: 10.1016/j.virol.2019.07.010. [DOI] [PubMed] [Google Scholar]
- 53.Obbard D.J., Shi M., Roberts K.E., Longdon B., Dennis A.B. A new lineage of segmented RNA viruses infecting animals. Virus Evol. 2020;6:vez061. doi: 10.1093/ve/vez061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sõmera M., Fargette D., Hébrard E., Sarmiento C., Ictv Report C. ICTV Virus Taxonomy Profile: Solemoviridae 2021. J. Gen. Virol. 2021;102:001707. doi: 10.1099/jgv.0.001707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen R., Mukhopadhyay S., Merits A., Bolling B., Nasar F., Coffey L.L., Powers A., Weaver S.C., Consortium I.R. ICTV Virus Taxonomy Profile: Togaviridae. J. Gen. Virol. 2018;99:761–762. doi: 10.1099/jgv.0.001072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Adams M.J., Adkins S., Bragard C., Gilmer D., Li D., MacFarlane S.A., Wong S.M., Melcher U., Ratti C., Ryu K.H., et al. ICTV Virus Taxonomy Profile: Virgaviridae. J. Gen. Virol. 2017;98:1999–2000. doi: 10.1099/jgv.0.000884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Charon J., Murray S., Holmes E.C. Revealing RNA virus diversity and evolution in unicellular algae transcriptomes. Virus Evol. 2021;7:veab070. doi: 10.1093/ve/veab070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.García M.L., Bó E.D., da Graça J.V., Gago-Zachert S., Hammond J., Moreno P., Natsuaki T., Pallás V., Navarro J.A., Reyes C.A., et al. ICTV Virus Taxonomy Profile: Ophioviridae. J. Gen. Virol. 2017;98:1161–1162. doi: 10.1099/jgv.0.000836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abudurexiti A., Adkins S., Alioto D., Alkhovsky S.V., Avšič-Županc T., Ballinger M.J., Bente D.A., Beer M., Bergeron É., Blair C.D., et al. Taxonomy of the order Bunyavirales: Update 2019. Arch. Virol. 2019;164:1949–1965. doi: 10.1007/s00705-019-04253-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De Lara Pinto A.Z., Santos de Carvalho M., de Melo F.L., Ribeiro A.L.M., Ribeiro B.M., Slhessarenko R.D. Novel viruses in salivary glands of mosquitoes from sylvatic Cerrado, Midwestern Brazil. PLoS ONE. 2017;12:e0187429. doi: 10.1371/journal.pone.0187429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kuhn J.H., Adkins S., Agwanda B.R., Al Kubrusli R., Alkhovsky S.V., Amarasinghe G.K., Avšič-Županc T., Ayllón M.A., Bahl J., Balkema-Buschmann A., et al. 2021 Taxonomic update of phylum Negarnaviricota (Riboviria: Orthornavirae), including the large orders Bunyavirales and Mononegavirales. Arch. Virol. 2021;166:3513–3566. doi: 10.1007/s00705-021-05143-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Silva M., Morais P., Maia C., de Sousa C.B., de Almeida A.P.G., Parreira R. A diverse assemblage of RNA and DNA viruses found in mosquitoes collected in southern Portugal. Virus Res. 2019;274:197769. doi: 10.1016/j.virusres.2019.197769. [DOI] [PubMed] [Google Scholar]
- 63.Wu H., Pang R., Cheng T., Xue L., Zeng H., Lei T., Chen M., Wu S., Ding Y., Zhang J., et al. Abundant and diverse RNA viruses in insects revealed by RNA-Seq analysis: Ecological and evolutionary emplications. mSystems. 2020;5:e00039-20. doi: 10.1128/mSystems.00039-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chiapello M., Bosco L., Ciuffo M., Ottati S., Salem N., Rosa C., Tavella L., Turina M., Pfeiffer J.K. Complexity and local specificity of the virome associated with tospovirus-transmitting Thrips species. J. Virol. 2021;95:e00597-21. doi: 10.1128/JVI.00597-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Walker P.J., Siddell S.G., Lefkowitz E.J., Mushegian A.R., Dempsey D.M., Dutilh B.E., Harrach B., Harrison R.L., Hendrickson R.C., Junglen S., et al. Changes to virus taxonomy and the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses (2019) Arch. Virol. 2019;164:2417–2429. doi: 10.1007/s00705-019-04306-w. [DOI] [PubMed] [Google Scholar]
- 66.Fauver J.R., Grubaugh N.D., Krajacich B.J., Weger-Lucarelli J., Lakin S.M., Fakoli L.S., 3rd, Bolay F.K., Diclaro J.W., 2nd, Dabire K.R., Foy B.D., et al. West African Anopheles gambiae mosquitoes harbor a taxonomically diverse virome including new insect-specific flaviviruses, mononegaviruses, and totiviruses. Virology. 2016;498:288–299. doi: 10.1016/j.virol.2016.07.031. [DOI] [PubMed] [Google Scholar]
- 67.Parry R., Asgari S., Williams B.R.G. Aedes anphevirus: An insect-specific virus distributed worldwide in Aedes aegypti mosquitoes that has complex interplays with Wolbachia and Dengue Virus infection in cells. J. Virol. 2018;92:e00224-18. doi: 10.1128/JVI.00224-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Käfer S., Paraskevopoulou S., Zirkel F., Wieseke N., Donath A., Petersen M., Jones T.C., Liu S., Zhou X., Middendorf M., et al. Re-assessing the diversity of negative strand RNA viruses in insects. PLoS Pathog. 2019;15:e1008224. doi: 10.1371/journal.ppat.1008224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhai L., Yang M., Zhang M., Hong N., Wang G. Characterization of a Botybirnavirus conferring hypovirulence in the phytopathogenic fungus Botryosphaeria dothidea. Viruses. 2019;11:266. doi: 10.3390/v11030266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ghabrial S.A., Caston J.R., Coutts R.H.A., Hillman B.I., Jiang D., Kim D.H., Moriyama H., Ictv Report C. ICTV Virus Taxonomy Profile: Chrysoviridae. J Gen. Virol. 2018;99:19–20. doi: 10.1099/jgv.0.000994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vainio E.J., Chiba S., Ghabrial S.A., Maiss E., Roossinck M., Sabanadzovic S., Suzuki N., Xie J., Nibert M., ICTV Report Consortium ICTV Virus Taxonomy Profile: Partitiviridae. J Gen. Virol. 2018;99:17–18. doi: 10.1099/jgv.0.000985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Duan Y., Yang Z., Bellis G., Xie J., Li L. Full genome sequencing of three Sedoreoviridae viruses isolated from Culicoides spp. (Diptera, Ceratopogonidae) in China. Viruses. 2022;14:971. doi: 10.3390/v14050971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Attoui H., Jaafar F.M., Belhouchet M., Aldrovandi N., Tao S., Chen B., Liang G., Tesh R.B., de Micco P., de Lamballerie X. Yunnan orbivirus, a new orbivirus species isolated from Culex tritaeniorhynchus mosquitoes in China. J. Gen. Virol. 2005;86:3409–3417. doi: 10.1099/vir.0.81258-0. [DOI] [PubMed] [Google Scholar]
- 74.Attoui H., Stirling J.M., Munderloh U.G., Billoir F., Brookes S.M., Burroughs J.N., de Micco P., Mertens P.P.C., de Lamballerie X. Complete sequence characterization of the genome of the St Croix River virus, a new orbivirus isolated from cells of Ixodes scapularis. J. Gen. Virol. 2001;82:795–804. doi: 10.1099/0022-1317-82-4-795. [DOI] [PubMed] [Google Scholar]
- 75.Murphy M.D., Howerth E.W., MacLachlan N.J., Stallknecht D.E. Genetic variation among epizootic hemorrhagic disease viruses in the southeastern United States: 1978–2001. Infect. Genet. Evol. 2005;5:157–165. doi: 10.1016/j.meegid.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 76.Liu H., Li M.-H., Zhai Y.-G., Meng W.-S., Sun X.-H., Cao Y.-X., Fu S.-H., Wang H.-Y., Xu L.-H., Tang Q., et al. Banna virus, China, 1987–2007. Emerg. Infect. Dis. 2010;16:514–517. doi: 10.3201/eid1603.091160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lu G., Zhou Z.H., Baker M.L., Jakana J., Cai D., Wei X., Chen S., Gu X., Chiu W. Structure of double-shelled rice dwarf virus. J. Virol. 1998;72:8541–8549. doi: 10.1128/JVI.72.11.8541-8549.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shapiro A., Green T., Rao S., White S., Carner G., Mertens P.P.C., Becnel J.J. Morphological and molecular characterization of a Cypovirus (Reoviridae) from the mosquito Uranotaenia sapphirina (Diptera: Culicidae) J. Virol. Methods. 2005;79:9430–9438. doi: 10.1128/JVI.79.15.9430-9438.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brcák J., Králík O. Cytopathic effects of oat sterile dwarf virus in enation cells of oat leaves. Acta Virol. 1980;24:346–350. [PubMed] [Google Scholar]
- 80.Day J.M. The diversity of the orthoreoviruses: Molecular taxonomy and phylogentic divides. Infect. Genet. Evol. 2009;9:390–400. doi: 10.1016/j.meegid.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 81.Bruenn J., Madura K., Siegel A., Miner Z., Lee M. Long internal inverted repeat in a yeast viral double-stranded RNA. Nucleic Acids Res. 1985;13:1575–1591. doi: 10.1093/nar/13.5.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Goodman R.P., Ghabrial S.A., Fichorova R.N., Nibert M.L. Trichomonasvirus: A new genus of protozoan viruses in the family Totiviridae. Arch. Virol. 2011;156:171–179. doi: 10.1007/s00705-010-0832-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Koonin E.V., Gorbalenya A.E. Evolution of RNA genomes: Does the high mutation rate necessitate high rate of evolution of viral proteins? J. Mol. Evol. 1989;28:524–527. doi: 10.1007/BF02602932. [DOI] [PubMed] [Google Scholar]
- 84.Koonin E.V., Dolja V.V., Morris T.J. Evolution and taxonomy of positive-strand RNA viruses: Implications of comparative analysis of amino acid sequences. Crit. Rev. Biochem. Mol. Biol. 1993;28:375–430. doi: 10.3109/10409239309078440. [DOI] [PubMed] [Google Scholar]
- 85.Wolf Y.I., Kazlauskas D., Iranzo J., Lucía-Sanz A., Kuhn J.H., Krupovic M., Dolja V.V., Koonin E.V. Origins and evolution of the global RNA virome. Mbio. 2018;9:e02329-18. doi: 10.1128/mBio.02329-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mönttinen H.A.M., Ravantti J.J., Poranen M.M. Structure unveils relationships between RNA virus polymerases. Viruses. 2021;13:313. doi: 10.3390/v13020313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Krishnamurthy S.R., Wang D. Origins and challenges of viral dark matter. Virus Res. 2017;239:136–142. doi: 10.1016/j.virusres.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 88.Coatsworth H., Bozic J., Carrillo J., Buckner E.A., Rivers A.R., Dinglasan R.R., Mathias D.K. Intrinsic variation in the vertically transmitted core virome of the mosquito Aedes aegypti. Mol. Ecol. 2022;31:2545–2561. doi: 10.1111/mec.16412. [DOI] [PubMed] [Google Scholar]
- 89.Atoni E., Zhao L., Karungu S., Obanda V., Agwanda B., Xia H., Yuan Z. The discovery and global distribution of novel mosquito-associated viruses in the last decade (2007–2017) Rev. Med. Virol. 2019;29:e2079. doi: 10.1002/rmv.2079. [DOI] [PubMed] [Google Scholar]
- 90.Öhlund P., Hayer J., Lundén H., Hesson J.C., Blomström A.L. Viromics reveal a number of novel RNA viruses in Swedish mosquitoes. Viruses. 2019;11:1027. doi: 10.3390/v11111027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang L., Rosales Rosas A.L., De Coninck L., Shi C., Bouckaert J., Matthijnssens J., Delang L. Establishment of Culex modestus in Belgium and a glance into the virome of Belgian mosquito species. mSphere. 2021;6:e01229-20. doi: 10.1128/mSphere.01229-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Peach D.A.H., Gries R., Young N., Lakes R., Galloway E., Alamsetti S.K., Ko E., Ly A., Gries G. Attraction of female Aedes aegypti (L.) to aphid honeydew. Insects. 2019;10:43. doi: 10.3390/insects10020043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Peach D.A.H., Gries G. Mosquito phytophagy—Sources exploited, ecological function, and evolutionary transition to haematophagy. Entom. Exp. Appl. 2019;168:120–136. doi: 10.1111/eea.12852. [DOI] [Google Scholar]
- 94.Beaurepaire A.L., Moro A., Mondet F., Le Conte Y., Neumann P., Locke B. Population genetics of ectoparasitic mites suggest arms race with honeybee hosts. Sci. Rep. 2019;9:11355. doi: 10.1038/s41598-019-47801-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wipf N.C., Guidi V., Tonolla M., Ruinelli M., Müller P., Engler O. Evaluation of honey-baited FTA cards in combination with different mosquito traps in an area of low arbovirus prevalence. Parasit Vectors. 2019;12:554. doi: 10.1186/s13071-019-3798-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Birnberg L., Temmam S., Aranda C., Correa-Fiz F., Talavera S., Bigot T., Eloit M., Busquets N. Viromics on honey-baited FTA cards as a new tool for the detection of circulating viruses in mosquitoes. Viruses. 2020;12:274. doi: 10.3390/v12030274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Flies E.J., Toi C., Weinstein P., Doggett S.L., Williams C.R. Converting mosquito surveillance to arbovirus surveillance with honey-baited nucleic acid preservation cards. Vector-Borne Zoonotic Dis. 2015;15:397–403. doi: 10.1089/vbz.2014.1759. [DOI] [PubMed] [Google Scholar]
- 98.Levi L.I., Rezelj V.V., Henrion-Lacritick A., Erazo D., Boussier J., Vallet T., Bernhauerová V., Suzuki Y., Carrau L., Weger-Lucarelli J., et al. Defective viral genomes from chikungunya virus are broad-spectrum antivirals and prevent virus dissemination in mosquitoes. PLoS Pathog. 2021;17:e1009110. doi: 10.1371/journal.ppat.1009110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rezelj V.V., Carrau L., Merwaiss F., Levi L.I., Erazo D., Tran Q.D., Henrion-Lacritick A., Gausson V., Suzuki Y., Shengjuler D., et al. Defective viral genomes as therapeutic interfering particles against flavivirus infection in mammalian and mosquito hosts. Nat. Commun. 2021;12:2290. doi: 10.1038/s41467-021-22341-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Laureti M., Paradkar P.N., Fazakerley J.K., Rodriguez-Andres J. Superinfection exclusion in mosquitoes and its potential as an arbovirus control strategy. Viruses. 2020;12:1259. doi: 10.3390/v12111259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.He W., Chen Y., Zhang X., Peng M., Xu D., He H., Gao Y., Chen J., Zhang J., Li Z., et al. Virome in adult Aedes albopictus captured during different seasons in Guangzhou City, China. Parasites Vectors. 2021;14:415. doi: 10.1186/s13071-021-04922-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Feng Y., Gou Q.Y., Yang W.H., Wu W.C., Wang J., Holmes E.C., Liang G., Shi M. A time-series meta-transcriptomic analysis reveals the seasonal, host, and gender structure of mosquito viromes. Virus Evol. 2022;8:veac006. doi: 10.1093/ve/veac006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shi C., Zhao L., Atoni E., Zeng W., Hu X., Matthijnssens J., Yuan Z., Xia H. Stability of the virome in lab- and field-collected Aedes albopictus mosquitoes across different developmental stages and possible core viruses in the publicly available virome data of Aedes mosquitoes. mSystems. 2020;5:e00640-20. doi: 10.1128/mSystems.00640-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kubacki J., Flacio E., Qi W., Guidi V., Tonolla M., Fraefel C. Viral metagenomic analysis of Aedes albopictus mosquitos from southern Switzerland. Viruses. 2020;12:929. doi: 10.3390/v12090929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Traavik T., Mehl R., Wiger R. California encephalitis group viruses isolated from mosquitoes collected in Southern and Arctic Norway. Acta Path. Microbiol. Scand. Sect. B. 1978;86B:335–342. doi: 10.1111/j.1699-0463.1978.tb00053.x. [DOI] [PubMed] [Google Scholar]
- 106.Putkuri N., Vaheri A., Vapalahti O. Prevalence and protein specificity of human antibodies to Inkoo virus infection. Clin. Vaccine Immunol. 2007;14:1555–1562. doi: 10.1128/CVI.00288-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xia H., Wang Y., Shi C., Atoni E., Zhao L., Yuan Z. Comparative metagenomic profiling of viromes associated with four common mosquito species in China. Virol. Sin. 2018;33:59–66. doi: 10.1007/s12250-018-0015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hameed M., Wahaab A., Shan T., Wang X., Khan S., Di D., Xiqian L., Zhang J.J., Anwar M.N., Nawaz M., et al. A metagenomic analysis of mosquito virome collected from different animal farms at Yunnan-Myanmar border of China. Front. Microbiol. 2020;11:591478. doi: 10.3389/fmicb.2020.591478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Oguzie J.U., Nwangwu U.C., Oluniyi P.E., Olumade T.J., George U.E., Kazeem A., Bankole B.E., Brimmo F.O., Asadu C.C., Chukwuekezie O.C., et al. Metagenomic sequencing characterizes a wide diversity of viruses in field mosquito samples in Nigeria. Sci. Rep. 2022;12:7616. doi: 10.1038/s41598-022-11797-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shi C., Beller L., Deboutte W., Yinda K.C., Delang L., Vega-Rúa A., Failloux A.-B., Matthijnssens J. Stable distinct core eukaryotic viromes in different mosquito species from Guadeloupe, using single mosquito viral metagenomics. Microbiome. 2019;7:121. doi: 10.1186/s40168-019-0734-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hameed M., Liu K., Anwar M.N., Wahaab A., Li C., Di D., Wang X., Khan S., Xu J., Li B., et al. A viral metagenomic analysis reveals rich viral abundance and diversity in mosquitoes from pig farms. Transbound Emerg. Dis. 2020;67:328–343. doi: 10.1111/tbed.13355. [DOI] [PubMed] [Google Scholar]
- 112.Nebbak A., Monteil-Bouchard S., Berenger J.-M., Almeras L., Parola P., Desnues C. Virome diversity among mosquito populations in a sub-urban region of Marseille, France. Viruses. 2021;13:768. doi: 10.3390/v13050768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Viljakainen L., Borshagovski A.-M., Saarenpää S., Kaitala A., Jurvansuu J. Identification and characterisation of common glow-worm RNA viruses. Virus Genes. 2020;56:236–248. doi: 10.1007/s11262-019-01724-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kurkela S., Manni T., Myllynen J., Vaheri A., Vapalahti O. Clinical and laboratory manifestations of Sindbis virus infection: Prospective study, Finland, 2002–2003. J. Infect. Dis. 2005;191:1820–1829. doi: 10.1086/430007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The produced sequence data is openly available in the NCBI BioProject database with the accession code PRJNA852425 and the virus sequences are publicly available in the NCBI GenBank database (see Table 2,Table 3,Table 4,Table 5,Table 6,Table 7 for accession codes).