Abstract
The aim of this study was to develop a multiplex bead assay using a Brucella rLPS antigen, a Brucella suis smooth antigen, and a Yersinia enterocolitica O:9 antigen that not only discriminates Brucella-infected from Brucella-uninfected pigs and wild boar, but also overcomes the cross reactivity with Y. enterocolitica O:9. Sera from 126 domestic pigs were tested: 29 pigs were Brucella infected, 80 were non-infected and 17 were confirmed to be false positive serological reactors (FPSR). Sera from 49 wild boar were tested: 18 were positive and 31 were negative. Using the rLPS antigen, 26/29 Brucella-infected domestic pigs and 15/18 seropositive wild boar were positive, while 75/80 non-Brucella infected domestic pigs, all FPSR, and all seronegative wild boar were negative. Using the smooth B. suis 1330 antigen, all Brucella-infected domestic pigs, 9/17 FPSR and all seropositive wild boar were positive, while all non-infected pigs and 30/31 seronegative wild boar were negative. The ratio of the readouts from the smooth B. suis antigen and Y. enterocolitica O:9 antigen enabled discriminating all Brucella infected individuals from the FPSR domestic pigs. These results demonstrate the potential of this assay for use in the surveillance of brucellosis, overcoming the cross-reactivity with Y. enterocolitica.
Keywords: multiplex bead assay, Brucella suis, wild boar, domestic pigs, cross-reactivity, rLPS antigen, smooth Brucella suis 1330 antigen, Yersinia enterocolitica O:9 antigen
1. Introduction
Porcine brucellosis is a major concern and is widespread throughout the world, especially in the Mediterranean, Balkans, South America and South East Asia. The primary etiologic agent is the bacterium Brucella suis, and the disease is a cause of severe economic losses in livestock production and may threaten public health [1]. The Eurasian wild boar (Sus scrofa) is widely distributed in the Palearctic and is a wildlife reservoir host for B. suis in many regions. The apparent prevalence of B. suis (based on serology) has been estimated to range from 25–46% in areas of high wild boar density in Spain where epidemiological links with Brucella infection in domestic pigs are suspected [2,3].
Serological methods used for diagnosis of porcine brucellosis include indirect, blocking and competitive enzyme-linked immunosorbent assays (ELISA) based on smooth lipopolysaccharide antigens (sLPS), the Rose Bengal Test (RBT), the complement fixation test (CFT) and the fluorescence polarization assay [1,4]. The B. abortus antigens seem to be suitable for testing swine sera, at least in RBT and CFT, as they can identify antibodies against the three biovars (1,2,3) of B. suis, which infect pigs [1]. A drawback of these serological tests is the lack of reliability for individual diagnosis, because, although they may have acceptable sensitivity, they frequently lack specificity [1]. A major reason for this is infection by Yersinia enterocolitica O:9, which has antigenic determinants (sLPS O-chains) closely related to those of Brucella spp. [5,6,7,8,9]. The structure and the biological properties of the rough Brucella LPS make it a suitable antigen for the serodiagnosis of porcine brucellosis [10]. Specifically, it lacks O-chain and only the lipid A and the core antigens remain. This rLPS structure differs between Brucella and Y. enterocolitica O:9 [11,12,13]. The omission of the cross-reactive O-chain means that rLPS has the potential to be a more specific antigen when applied to samples that are false-positive in assays employing the O-chain [14]. In wild boar, the sensitivity of a multi-species Brucella sLPS iELISA was estimated at being 100% and its specificity was adequate. However, its cross-reactivity with Y. enterocolitica O:9 was not assessed [2].
The above reasons prompted us to use an rLPS-rich antigen, extracted from a rough strain of Brucella, in order to try to enhance the specificity (Sp) of serology. Additionally, a whole cell B. suis biovar 1 (strain 1330) smooth antigen was used to maximize sensitivity (Se) because it is a homologous antigen for infected domestic pigs and wild boar. Being a whole-cell preparation, it contains the most possible immunogenic epitopes, and has a high O-chain content, which includes a low frequency of the OPS M epitope that is not possessed by Y. enterocolitica O:9 [1,15]. The combination of these two antigens in the same multiplex bead assay could enhance the diagnostic accuracy. Finally, a whole cell Y. enterocolitica O:9 antigen was used to detect cross-reacting antibodies and/or antibodies produced after natural exposure to Y. enterocolitica that may be present in Brucella-seropositive domestic pigs and wild boar.
The purpose of the present study was to develop a multiplex bead assay using Brucella rLPS, a whole cell B. suis 1330 smooth, and a whole cell Y. enterocolitica O:9 antigen, which not only discriminates Brucella-seropositive from Brucella-seronegative domestic pigs and wild boar but also overcomes the cross reactivity between B. suis and Y. enterocolitica O:9.
2. Materials and Methods
2.1. Domestic Pig Sera
One-hundred and twenty-six domestic pig sera were used for test development. Group A contained 29 sera from animals that were culture-positive for B. suis biovar 2 (25/29, obtained in Spain), or biovar 1 (4/29, obtained in South America). Group B contained 80 randomly selected sera collected from herds within Great Britain, which is officially brucellosis-free. Group C contained 17 sera from herds within Great Britain that were FPSR (false-positive serological reactors) during routine testing by either RBT (n = 10), cELISA (n = 10), SAT (n = 8) or their combination.
2.2. Wild Boar Sera
Sera from 49 Eurasian wild boar from Spain were also tested: group A—Brucella seropositive, (n = 18)—and group B—Brucella seronegative (n = 31). The discrimination between the seropositive and seronegative wild boars was determined by an indirect ELISA using the sLPS antigen [2].
2.3. Multiplex Bead Assay
2.3.1. Antigen Preparation and Coupling
The antigens used for assay development: (a) rLPS-rich phenol/chloroform/petroleum ether extract from B. abortus RB51 (hereafter referred as rLPS) [14]; (b) whole cells of the smooth B. suis strain 1330 grown on serum dextrose agar at 37 °C and heat-killed [16]; (c) whole cells of the smooth Y. enterocolitica O:9 (strain 234/02) grown on nutrient agar at 27 °C and heat-killed. Ten micrograms of each antigen were bound to 2.5 × 106 Pro Magnetic carboxylated beads according to the manufacturer’s instructions (Bioplex Pro Magnetic COOH Magnetic Beads Amine Coupling Kit, BioRad, Hercules, CA, USA).
2.3.2. Multiplex Bead Assay Protocol
The Bio-Rad Bio-Plex multi-analyte bead suspension array system, which is based on Luminex’s xMAP technology, was used for the assay. The bead reporter fluorescence, expressed as MFI (median fluorescence intensity), was determined with a Bio-Plex 200 (Bio-Rad) instrument that was initially calibrated and set to count 100 beads from each of three bead sets, with the Double Discriminator (DD) gate values set at 7500–25,000. A one-step protocol and normalization method for MFI values were used, as described previously [17]. Fifty microliters of master mix, containing approximately 3500 coupled beads of each type (the rLPS antigen, the smooth B.suis 1330 antigen and the smooth Y. enterocolitica O:9 antigen), biotinylated protein AG (secondary antibody) at a 1:500 dilution and streptavidin–phycoerythrin (2 μg/mL) in dilution buffer, were added to each well of a flat-bottom 96-well plate. Diluted serum (50 μL) was mixed with the master mix and the plate was incubated for 2 h at room temperature, with shaking at 600 rpm. The beads were washed twice with 100 μL Wash buffer (0.1 M PBS and 0.05% Tween 20) using the Bioplex pro Wash Station (BioRad) and finally resuspended in 100 μL of dilution buffer. The bead reporter fluorescence, expressed as MFI, was determined using Bioplex 200 (BioRad) instrument. The machine was calibrated and set to count 100 beads from each of three bead sets. Serum from a known seropositive Brucella-infected domestic pig was included as a positive control on each plate for normalization of test sera MFI values.
2.4. Receiver Operating Characteristic (ROC) Curve Analysis
ROC analysis was used for (i) evaluating overall test performance and (ii) determining cut-points that optimize the diagnostic accuracy of the test. For (i) the trapezoidal rule [18] was used to calculate the area under the ROC curve (AUC) and the corresponding confidence intervals [19]. For (ii) two different criteria were used for cut-point selection: (a) the simultaneous optimization of the Se and Sp and the overall minimization of false results, which corresponds to the maximization of the Youden’s index J = Se + Sp − 1 [20] and (b) the minimization of the quantity min (1 − Se)2 + (1 − Sp)2 that corresponds to the cut-point closest to the upper left corner of the AUC plot [21].
ROC analysis was performed to compare the following combinations: (I) group A and B for the rLPS antigen in domestic pigs; (II) group A and C for the rLPS antigen in domestic pigs; (III) group A and B for the B. suis 1330 smooth antigen in domestic pigs; (IV) group A and C for the B. suis 1330 smooth antigen in domestic pigs; (V) group A and C for the ratio of the readout from the smooth B. suis 1330/the readout from the smooth Y. enterocolitica O:9 in domestic pigs; (VI) group A and B for the rLPS antigen in wild boars; (VII) group A and B for the B. suis 1330 smooth antigen in wild boars.
All analyses were carried out in R [22], using the pROC package [23].
3. Results
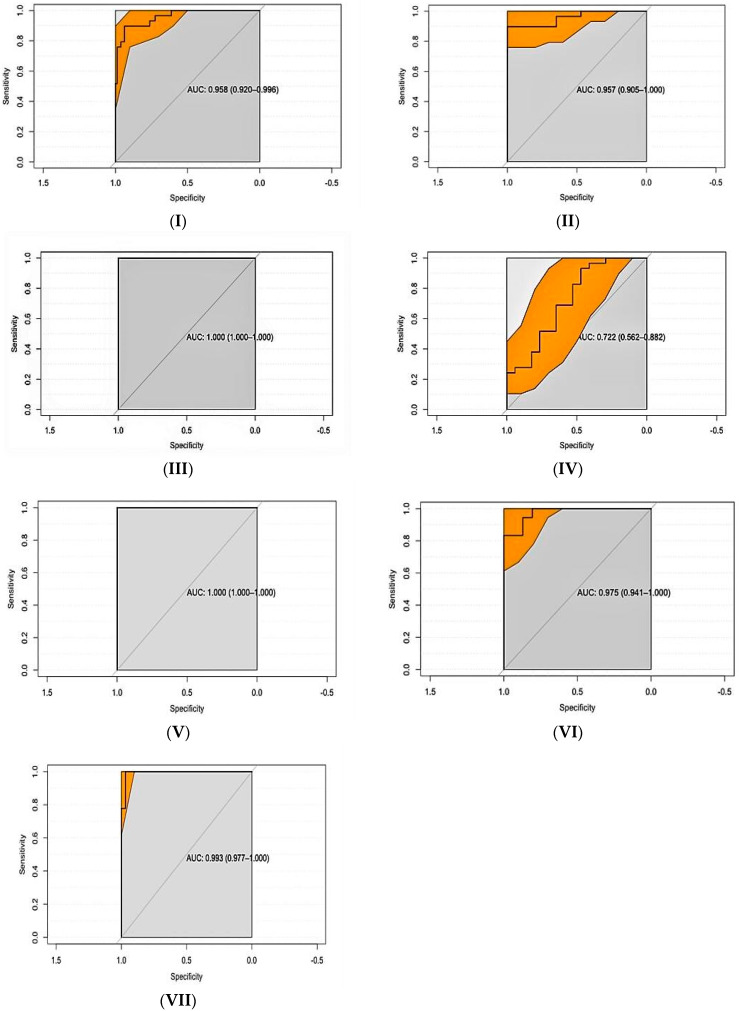
The distribution of the normalized values for each species are shown in Figure 1. ROC curves are shown in Figure 2. The overall discriminatory power for all combinations was high as indicated by the AUCs, which were, in all instances, higher than 0.95 except for the smooth antigen between groups A and C in domestic pigs which was 0.722 (Table 1). Se and Sp combinations at the selected cut-offs were also high for all combinations with the exception of the B. suis 1330 smooth antigen between groups A and C in domestic pigs.
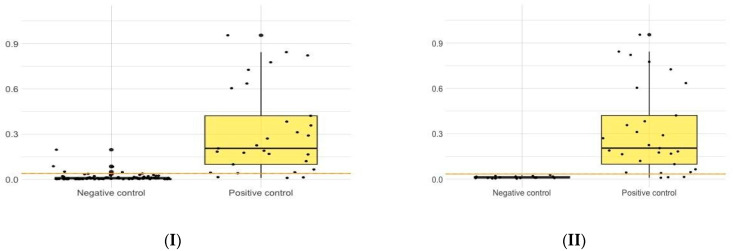
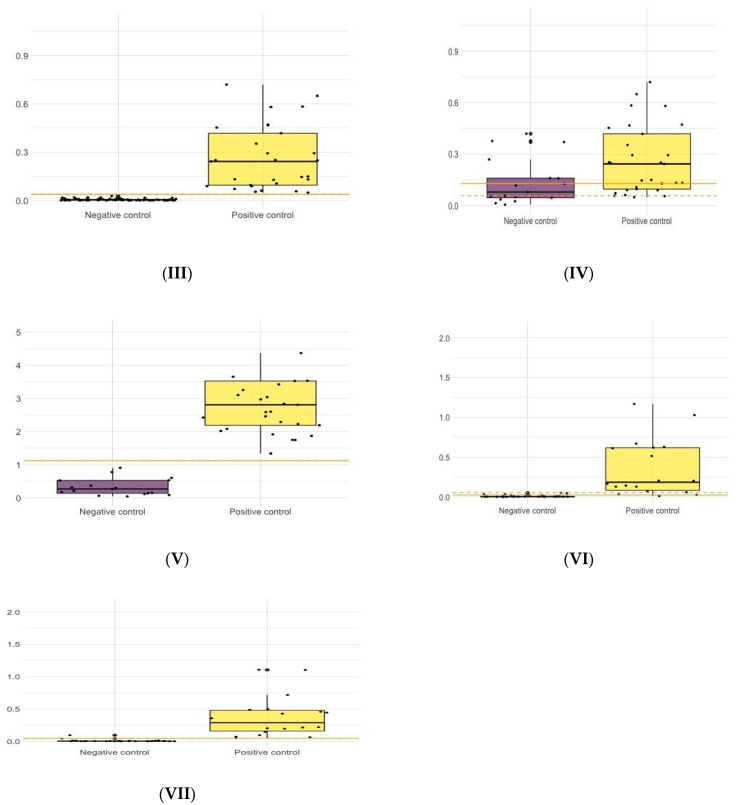
Figure 1.
Distribution of the test values for the following combinations: group A and B for the rLPS antigen in domestic pigs (I); group A and C for the rLPS antigen in domestic pigs (II); group A and B for the smooth B. suis 1330 antigen in domestic pigs (III); group A and C for the smooth B. suis 1330 antigen in domestic pigs (IV); group A and C for the ratio of smooth B. suis 1330/the smooth Y. enterocolitica O:9 in domestic pigs (V); group A and B for the rLPS antigen in wild boars (VI); group A and B for the smooth B. suis 1330 antigen in wild boars (VII). Dots correspond to actual values. Boxes represent interquartile ranges while the solid black line at the approximate center of each box is the median; the arms of each box extend to cover the central 95% of the distribution of the normalized values.
Figure 2.
Plot of the Receiver Operating Characteristic (ROC) curve analysis for the following combinations: group A and B for the rLPS antigen in domestic pigs (I); group A and C for the rLPS antigen in domestic pigs (II); group A and B for the smooth B. suis 1330 antigen in domestic pigs (III); group A and C for the smooth B. suis 1330 antigen in domestic pigs (IV); group A and C for the ratio of smooth B. suis 1330/the smooth Y. enterocolitica O:9 in domestic pigs (V); group A and B for the rLPS antigen in wild boars (VI); group A and B for the smooth B. suis 1330 antigen in wild boars (VII).
Table 1.
Estimated AUCs, Se, Sp and associated 95% Confidence Intervals (CIs) at selected cut-offs that maximize the Youden’s J statistic (1) or correspond to the point closest to upper left corner of the AUC plot (2). Results are for the following combinations: group A and B for the rLPS antigen in domestic pigs (I); group A and C for the rLPS antigen in domestic pigs (II); group A and B for the B. suis 1330 smooth antigen in domestic pigs (III); group A and C for the B. suis 1330 smooth antigen in domestic pigs (IV); group A and C for the ratio of smooth B. suis 1330/the smooth Y. enterocolitica O:9 in domestic pigs (V); group A and B for the rLPS antigen in wild boars (VI); group A and B for the B. suis 1330 smooth antigen in wild boars (VII).
| Combination | AUC (CIs) | Cut-Off (1) | Cut-Off (2) | ||||
|---|---|---|---|---|---|---|---|
| Se (CIs) | Sp (CIs) | Se (CIs) | Sp (CIs) | ||||
| I | 0.958 (0.920; 0.996) | 0.039 | 0.90 (0.79; 1.00) | 0.94 (0.88; 0.98) | 0.039 | 0.90 (0.79; 1.00) | 0.94 (0.88; 0.98) |
| II | 0.957 (0.905; 1.000) | 0.034 | 0.89 (0.76; 1.00) | 1.00 (1.00; 1.00) | 0.034 | 0.89 (0.76; 1.00) | 1.00 (1.00; 1.00) |
| III | 1.000 (1.000; 1.000) | 0.039 | 1.00 (1.00; 1.00) | 1.00 (1.00; 1.00) | 0.039 | 1.00 (1.00; 1.00) | 1.00 (1.00; 1.00) |
| IV | 0.722 (0.562; 0.882) | 0.058 | 0.93 (0.83; 1.00) | 0.47 (0.23; 0.71) | 0.128 | 0.69 (0.52; 0.83) | 0.65 (0.41; 0.88) |
| V | 1.000 (1.000; 1.000) | 1.123 | 1.00 (1.00; 1.00) | 1.00 (1.00; 1.00) | 1.123 | 1.00 (1.00; 1.00) | 1.00 (1.00; 1.00) |
| VI | 0.975 (0.941; 1.000) | 0.056 | 0.83 (0.67; 1.00) | 1.00 (1.00; 1.00) | 0.026 | 0.94 (0.83; 1.00) | 0.87 (0.74; 0.97) |
| VII | 0.993 (0.977; 1.000) | 0.047 | 1.00 (1.00; 1.00) | 0.97 (0.90; 1.00) | 0.047 | 1.00 (1.00; 1.00) | 0.97 (0.90; 1.00) |
The selected cut-offs that maximize the Youden’s J statistic showed that 26/29 group A domestic pigs (23/25 Brucella infected by biovar 2 and 3/4 infected by biovar 1) were positive using the rough B. abortus RB51 antigen, while 75/80 group B domestic pigs and all (17/17) group C (FPSR) domestic pigs were negative. The same antigen detected 15/18 of the seropositive wild boar and was negative for all (31/31) seronegative wild boar. The smooth B. suis 1330 antigen detected in all (29/29) group A domestic pigs was negative in all (80/80) group B animals, but was positive in just over half (9/17) of the group C (FPSR) samples. In wild boars, the same antigen was detected in all (18/18) group A seropositive samples and was negative in 30/31 group B seronegative animals. Finally, the ratio of the smooth B. suis 1330 and the smooth Y. enterocolitica O:9 normalized MFI values discriminated with 100% sensitivity and 100% specificity between group A and group C (FPSR) domestic pigs.
4. Discussion
This study shows that a multiplex bead assay could be a useful serodiagnostic tool for porcine brucellosis due to the high Se and Sp (whole cell B. suis 1330 smooth antigen) and the ability to identify cross-reactions due to Y. enterocolitica O:9 (rLPS antigen; smooth B. suis 1330/smooth Y. enterocolitica O:9 normalized MFI values ratio).
The most commonly used serological tests are generally designed to measure antibodies against a single antigen preparation, whereas the one-step multiplex bead assay is capable of detecting antibody responses to a range of different antigens at the same time. This offers significant benefits over other tests, including reduced reagent costs [24]. The potential advantages of multiplex bead assays over conventional serologic tests provide a strong impetus for their routine use in both research and clinical laboratories. In our study the beads were conjugated on three separate occasions, one conjugation per antigen and bead type, and were easily mixed and efficiently combined.
The frequency of the false-positive reactions in group B domestic pigs and wild boar was low (<10%), despite the fact that multiplexed assays are usually characterized by a lower Sp when compared to conventional serological tests due to the simultaneous presence of multiple ligands [25]. One possible explanation could be the use of protein AG instead of a species/isotype-specific secondary antibody. The results of this study show that the multiplex bead assay using rLPS and smooth B. suis 1330 antigens effectively distinguishes between sera from group A and group B in both domestic pigs and wild boar. Based on the calculated AUC values, Se and Sp of the latter antigen seems to be better than rLPS for this purpose.
In a recent study, the Se and Sp of a conventional iELISA, in which the sLPS antigen was used, were 0.66 and 0.97, respectively, leading to the conclusion that this assay is not sensitive enough for the diagnosis of brucellosis in domestic pigs [26]. Furthermore, in another study the use of sLPS in an iELISA showed a DSn of 95.07% and a DSp of 99.75% but only 24.77 with FPSR samples, results similar to ours [27]. In another study, the use of sLPS antigen in the iELISA resulted in 0.94 Se and 1.00 Sp [14], confirming the results of several previous publications that also support the diagnostic accuracy of sLPS in discriminating Brucella-infected from non-infected domestic pigs [28,29]. Our results indicate that a multiplex bead assay using the whole smooth B. suis 1330 antigen may be better than conventional serologic tests at discriminating between sera from Brucella infected and non-infected non-FPSR domestic pigs (Se: 1.00, Sp: 1.00).
The use of rLPS had a satisfactory diagnostic performance in the multiplex bead assay. The good distinction between group A and group B samples from domestic pigs was also found in a recent study where the same antigen was used in an iELISA, resulting in a Se of 0.91 and a Sp of 0.99 [14]. The results of the multiplex bead assay clearly show that in domestic pigs, the rLPS antigen can discriminate group A (Brucella infected animals) from group C (non-Brucella infected FPSR) sera. This attribute of the rLPS rich antigen was anticipated, as the structure of the core sugars within the rLPS is very different between Brucella spp. [30] and Y. enterocolitica O:9 [13] and the absence of the O-chain in this antigen [31] avoids cross-reactivity with antibodies against Y. enterocolitica O:9, as has been previously shown using an iELISA method [14]. Other studies have shown Brucella rLPS antigen to be less effective at serodiagnosis [27]. However, in this case, the antigen was pure and so differs from the less pure preparation used in this study, within which the co-extractants may behave as excipients and enhance the efficacy of the rLPS antigen.
The application of serological tests in wildlife is usually carried out for screening purposes or surveillance. Wild boar are indigenous in many countries and may contribute to the transmission of B. suis to livestock and hamper the success of eradication programs [32]. Based on our results, the best antigen for screening wild boar populations with the multiplex bead assay is the smooth B. suis 1330 (Se: 1.00, Sp: 0.97). Furthermore, given the high seroprevalence (up to 63%) against Brucella spp. in European wild boar populations [33], the concomitant use of rLPS may improve the combined specificity, considering that this antigen gave a negative result in only one group B serum sample, with a positive normalized MFI value for the smooth B. suis 1330 antigen. Further studies with larger sample sizes are obviously needed to confirm this hypothesis.
According to a previous study [34], the cut-off should be selected by taking into consideration the epidemiologic situation in each area. For example, for countries which are brucellosis-free, by taking into account the low prevalence of the disease and the serious consequences of a false-positive diagnosis, it may be advisable to choose a cut-off at the lower part of the ROC curve in order to maximize the Sp. On the other hand, a maximum Se would be appropriate for countries where the disease occurs at high prevalence. Therefore, the Se and Sp of the multiplex bead assay may change depending on the criteria used for cut-off selection.
The poor ability of the smooth B. suis 1330 antigen to differentiate between group A and group C domestic pigs was also expected based on previous studies in cattle [35,36] and pigs [8,9], as the antigen did differentiate between most of the samples, but not nearly so well as the rLPS antigen, However, the concomitant use of the smooth Y. enterocolitica O:9 antigen in the multiplex bead assay and the calculation of the ratio between the smooth B. suis 1330 and the smooth Y. enterocolitica O:9 normalized MFI values fully overcame this drawback, permitting the clear differentiation between groups A and C. This would most likely be due to the binding of antibodies on each antigen that are not shared but are distinct to each antigen types. Furthermore, the use of the rLPS antigen in a multiplex bead assay may be helpful in cases of dual infections of Brucella spp. and Y. enterocolitica O:9, given that the OPS is so similar between the B. suis biovar 2 and the Y. enterocolitica O:9 [15].
5. Conclusions
Based on the results of this study, the multiplex bead assay can be considered to be an accurate diagnostic test for brucellosis in domestic pigs and wild boar, if at least two antigens are included. For domestic pigs, the use of the smooth B. suis 1330 antigen along with the Y. enterocolitica O:9 antigen (thus enabling calculation of the ratio between MFI values for the two antigens) seems to be the best combination to discriminate between sera from Brucella-infected and non-Brucella-infected (FPSR and non-FPSR) animals, although the addition of the rLPS would help in the case of dual infection. In wild boar, the smooth B. suis 1330 antigen seems to be more accurate in terms of Se and Sp but the addition of the rLPS may further increase Sp.
Author Contributions
Conceptualization, A.T., J.M., V.S., L.P. and C.B.; methodology, A.T., J.M., S.C., G.V., L.D., V.S., L.P. and C.B.; software, P.K.; validation, A.T., S.C., G.V., C.G., M.B., V.S., L.P. and C.B.; formal analysis, P.K.; investigation, A.T., S.C., G.V., C.G., M.B., M.S., Z.A., D.C.C., V.S., L.P. and C.B.; resources, J.M., S.C., C.G. and M.B.; data curation, A.T., S.C. and L.P.; writing—original draft preparation, A.T.; writing—review and editing, A.T., J.M., S.C., L.D., C.G., M.S., Z.A., D.C.C., L.P. and C.B.; visualization, P.K.; supervision, S.C., V.S., L.P. and C.B.; project administration, L.P. and C.B.; funding acquisition, L.P. and C.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study is part of European Union Seventh Framework Programme (2007–2013), a large collaboration project under grant agreement no. 222633 (Novel Technologies for Surveillance of Emerging and Re-emerging Infections of Wildlife—WildTech). All samples used in this study represent material collected by partners and other organizations for other purposes than this project as specified in deliverable D4.5/5.5 entitled ‘Guidelines for ethical sample collection’ submitted to European Commission (26 February 2010, Dissemination Level: PP, Restricted to other programme participants, including Commission Services). The wild boar serum samples were collected opportunistically (no active capture, killing and sampling of wild animals specifically for this study was performed) from animals hunter harvested by members of Hunting Federations. Thus, special approval was not necessary, and steps to ameliorate suffering were not applicable to this study. Research on animals as defined in the EU Ethics for Researchers document (European Commission, 2007, Ethics for Researchers—Facilitating Research Excellence in FP7, Luxembourg: Office for Official Publications of the European Communities, ISBN 978-92-79-05474-7) is not applicable to this study.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are presented in the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Funding Statement
We thankfully acknowledge the financial support of the European Union Seventh Framework Programme (2007–2013) under grant agreement no. 222633 (WildTech) entitled “Novel Technologies for Surveillance of Emerging and Re-emerging Infections of Wildlife”.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brucellosis. OIE—World Organisation for Animal Health. [(accessed on 22 March 2021)]. Available online: https://www.oie.int/en/animal-health-in-theworld/animal-diseases/brucellosis/
- 2.Muñoz P.M., Boadella M., Arnal M., de Miguel M.J., Revilla M., Martínez D., Vicente J., Acevedo P., Oleaga A., Ruiz-Fons F., et al. Spatial Distribution and Risk Factors of Brucellosis in Iberian Wild Ungulates. BMC Infect. Dis. 2010;10:46. doi: 10.1186/1471-2334-10-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muñoz P.M., Mick V., Sacchini L., Janowicz A., de Miguel M.J., Cherfa M.-A., Nevado C.R., Girault G., Andrés-Barranco S., Jay M., et al. Phylogeography and Epidemiology of Brucella Suis Biovar 2 in Wildlife and Domestic Swine. Vet. Microbiol. 2019;233:68–77. doi: 10.1016/j.vetmic.2019.04.025. [DOI] [PubMed] [Google Scholar]
- 4.Muñoz P.M., Blasco J.M., Engel B., de Miguel M.J., Marín C.M., Dieste L., Mainar-Jaime R.C. Assessment of Performance of Selected Serological Tests for Diagnosing Brucellosis in Pigs. Vet. Immunol. Immunopathol. 2012;146:150–158. doi: 10.1016/j.vetimm.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 5.Meikle P.J., Perry M.B., Cherwonogrodzky J.W., Bundle D.R. Fine Structure of A and M Antigens from Brucella Biovars. Infect. Immun. 1989;57:2820–2828. doi: 10.1128/iai.57.9.2820-2828.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cloeckaert A., Weynants V., Godfroid J., Verger J.-M., Grayon M., Zygmunt M.S. O-Polysaccharide Epitopic Heterogeneity at the Surface of Brucella Spp. Studied by Enzyme-Linked Immunosorbent Assay and Flow Cytometry. Clin. Diagn. Lab. Immunol. 1998;5:862–870. doi: 10.1128/CDLI.5.6.862-870.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kittelberger R., Bundesen P.G., Cloeckaert A., Greiser-Wilke I., Letesson J.-J. Serological Cross-Reactivity between Brucella Abortus and Yersinia Enterocolitica 0:9:: IV. Evaluation of the M- and C-Epitope Antibody Response for the Specific Detection of B. Abortus Infections. Vet. Microbiol. 1998;60:45–57. doi: 10.1016/S0378-1135(97)00202-2. [DOI] [PubMed] [Google Scholar]
- 8.Jungersen G., Sørensen V., Giese S.B., Stack J.A., Riber U. Differentiation between Serological Responses to Brucella Suis and Yersinia Enterocolitica Serotype O[Ratio]9 after Natural or Experimental Infection in Pigs. Epidemiol. Infect. 2006;134:347–357. doi: 10.1017/S095026880500511X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nielsen K., Smith P., Yu W., Nicoletti P., Jungersen G., Stack J., Godfroid J. Serological Discrimination by Indirect Enzyme Immunoassay between the Antibody Response to Brucella Sp. and Yersinia Enterocolitica O:9 in Cattle and Pigs. Vet. Immunol. Immunopathol. 2006;109:69–78. doi: 10.1016/j.vetimm.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 10.Salvador-Bescós M., Gil-Ramírez Y., Zúñiga-Ripa A., Martínez-Gómez E., de Miguel M.J., Muñoz P.M., Cloeckaert A., Zygmunt M.S., Moriyón I., Iriarte M., et al. WadD, a New Brucella Lipopolysaccharide Core Glycosyltransferase Identified by Genomic Search and Phenotypic Characterization. Front. Microbiol. 2018;9:2293. doi: 10.3389/fmicb.2018.02293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kubler-Kielb J., Vinogradov E. The Study of the Core Part and Non-Repeating Elements of the O-Antigen of Brucella Lipopolysaccharide. Carbohydr. Res. 2013;366:33–37. doi: 10.1016/j.carres.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barquero-Calvo E., Conde-Alvarez R., Chacón-Díaz C., Quesada-Lobo L., Martirosyan A., Guzmán-Verri C., Iriarte M., Mancek-Keber M., Jerala R., Gorvel J., et al. The Differential Interaction of Brucella and Ochrobactrum with Innate Immunity Reveals Traits Related to the Evolution of Stealthy Pathogens. PLoS ONE. 2009;4:e5893. doi: 10.1371/journal.pone.0005893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Müller-Loennies S., Rund S., Ervelä E., Skurnik M., Holst O. The Structure of the Carbohydrate Backbone of the Core-Lipid A Region of the Lipopolysaccharide from a Clinical Isolate of Yersinia Enterocolitica O:9. Eur. J. Biochem. 1999;261:19–24. doi: 10.1046/j.1432-1327.1999.00243.x. [DOI] [PubMed] [Google Scholar]
- 14.McGiven J.A., Nicola A., Commander N.J., Duncombe L., Taylor A.V., Villari S., Dainty A., Thirlwall R., Bouzelmat N., Perrett L.L., et al. An Evaluation of the Capability of Existing and Novel Serodiagnostic Methods for Porcine Brucellosis to Reduce False Positive Serological Reactions. Vet. Microbiol. 2012;160:378–386. doi: 10.1016/j.vetmic.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Zaccheus M.V., Ali T., Cloeckaert A., Zygmunt M.S., Weintraub A., Iriarte M., Moriyón I., Widmalm G. The Epitopic and Structural Characterization of Brucella Suis Biovar 2 O-Polysaccharide Demonstrates the Existence of a New M-Negative C-Negative Smooth Brucella Serovar. PLoS ONE. 2013;8:e53941. doi: 10.1371/journal.pone.0053941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alton G.G., Jones L.M., Pietz D.E. Laboratory Techniques in Brucellosis. World Health Organization & Food and Agriculture Organization of the United Nations; Geneva, Switzerland: 1975. [PubMed] [Google Scholar]
- 17.Touloudi A., Valiakos G., Cawthraw S., Kostoulas P., Gortázar C., Boadella M., Giannakopoulos A., Birtsas P., Sofia M., Athanasiou L.V., et al. Development of a Multiplex Bead Assay for Simultaneous Serodiagnosis of Antibodies against Mycobacterium Bovis, Brucella Suis, and Trichinella Spiralis in Wild Boar. Microorganisms. 2021;9:904. doi: 10.3390/microorganisms9050904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fawcett T. An Introduction to ROC Analysis. Pattern Recognit. Lett. 2006;27:861–874. doi: 10.1016/j.patrec.2005.10.010. [DOI] [Google Scholar]
- 19.DeLong E.R., DeLong D.M., Clarke-Pearson D.L. Comparing the Areas under Two or More Correlated Receiver Operating Characteristic Curves: A Nonparametric Approach. Biometrics. 1988;44:837–845. doi: 10.2307/2531595. [DOI] [PubMed] [Google Scholar]
- 20.Youden W.J. Index for Rating Diagnostic Tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::AID-CNCR2820030106>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 21.Greiner M., Sohr D., Göbel P. A Modified ROC Analysis for the Selection of Cut-off Values and the Definition of Intermediate Results of Serodiagnostic Tests. J. Immunol. Methods. 1995;185:123–132. doi: 10.1016/0022-1759(95)00121-P. [DOI] [PubMed] [Google Scholar]
- 22.R: The R Project for Statistical Computing. [(accessed on 18 May 2021)]. Available online: https://www.r-project.org/
- 23.Robin X., Turck N., Hainard A., Tiberti N., Lisacek F., Sanchez J.-C., Müller M. PROC: An Open-Source Package for R and S+ to Analyze and Compare ROC Curves. BMC Bioinform. 2011;12:77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raulino R., Thaurignac G., Butel C., Villabona-Arenas C.J., Foe T., Loul S., Ndimbo-Kumugo S.-P., Mbala-Kingebeni P., Makiala-Mandanda S., Ahuka-Mundeke S., et al. Multiplex Detection of Antibodies to Chikungunya, O’nyong-Nyong, Zika, Dengue, West Nile and Usutu Viruses in Diverse Non-Human Primate Species from Cameroon and the Democratic Republic of Congo. PLoS Negl. Trop. Dis. 2021;15:e0009028. doi: 10.1371/journal.pntd.0009028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elshal M.F., McCoy J.P. Multiplex Bead Array Assays: Performance Evaluation and Comparison of Sensitivity to ELISA. Methods. 2006;38:317–323. doi: 10.1016/j.ymeth.2005.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Praud A., Gimenez O., Zanella G., Dufour B., Pozzi N., Antras V., Meyer L., Garin-Bastuji B. Estimation of Sensitivity and Specificity of Five Serological Tests for the Diagnosis of Porcine Brucellosis. Prev. Vet. Med. 2012;104:94–100. doi: 10.1016/j.prevetmed.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 27.Dieste-Pérez L., Blasco J.M., de Miguel M.J., Moriyón I., Muñoz P.M. Diagnostic Performance of Serological Tests for Swine Brucellosis in the Presence of False Positive Serological Reactions. J. Microbiol. Methods. 2015;111:57–63. doi: 10.1016/j.mimet.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Algers B., Blokhuis H.J., Bøtner A., Broom D.M., Costa P., Domingo M., Greiner M., Hartung J., Koenen F., Muller-Graf C., et al. Porcine brucellosis (Brucella suis) scientific opinion of the panel on animal health and welfare. EFSA J. 2009;7:1144. [Google Scholar]
- 29.Paulo P.S., Vigliocco A.M., Ramondino R.F., Marticorena D., Bissi E., Briones G., Gorchs C., Gall D., Nielsen K. Evaluation of Primary Binding Assays for Presumptive Serodiagnosis of Swine Brucellosis in Argentina. Clin. Diagn. Lab. Immunol. 2000;7:828–831. doi: 10.1128/CDLI.7.5.828-831.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Velasco J., Bengoechea J.A., Brandenburg K., Lindner B., Seydel U., González D., Zähringer U., Moreno E., Moriyón I. Brucella Abortus and Its Closest Phylogenetic Relative, Ochrobactrum Spp., Differ in Outer Membrane Permeability and Cationic Peptide Resistance. Infect. Immun. 2000;68:3210–3218. doi: 10.1128/IAI.68.6.3210-3218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cloeckaert A., Zygmunt M.S., Guilloteau L.A. Brucella Abortus Vaccine Strain RB51 Produces Low Levels of M-like O-Antigen. Vaccine. 2002;20:1820–1822. doi: 10.1016/S0264-410X(02)00035-X. [DOI] [PubMed] [Google Scholar]
- 32.Godfroid J., Saegerman C., Wellemans V., Walravens K., Letesson J.-J., Tibor A., Mc Millan A., Spencer S., Sanna M., Bakker D., et al. How to Substantiate Eradication of Bovine Brucellosis When Aspecific Serological Reactions Occur in the Course of Brucellosis Testing. Vet. Microbiol. 2002;90:461–477. doi: 10.1016/S0378-1135(02)00230-4. [DOI] [PubMed] [Google Scholar]
- 33.Grégoire F., Mousset B., Hanrez D., Michaux C., Walravens K., Linden A. A Serological and Bacteriological Survey of Brucellosis in Wild Boar (Sus Scrofa) in Belgium. BMC Vet. Res. 2012;8:80. doi: 10.1186/1746-6148-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greiner M., Pfeiffer D., Smith R.D. Principles and Practical Application of the Receiver-Operating Characteristic Analysis for Diagnostic Tests. Prev. Vet. Med. 2000;45:23–41. doi: 10.1016/S0167-5877(00)00115-X. [DOI] [PubMed] [Google Scholar]
- 35.The Dutch Brucella Abortus Monitoring Programme for Cattle: The Impact of False-Positive Serological Reactions and Comparison of Serological Tests: Veterinary Quarterly: Volume 24, No 1. [(accessed on 18 May 2021)]. Available online: https://www.tandfonline.com/doi/abs/10.1080/01652176.2002.9695123. [DOI] [PubMed]
- 36.Muñoz P.M., Marín C.M., Monreal D., González D., Garin-Bastuji B., Díaz R., Mainar-Jaime R.C., Moriyón I., Blasco J.M. Efficacy of Several Serological Tests and Antigens for Diagnosis of Bovine Brucellosis in the Presence of False-Positive Serological Results Due to Yersinia Enterocolitica O:9. Clin. Diagn. Lab. Immunol. 2005;12:141–151. doi: 10.1128/CDLI.12.1.141-151.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are presented in the manuscript.