Abstract
Large structural chromosomal deletions and duplications, referred to as copy number variants (CNVs), play a role in the pathogenesis of neurodevelopmental disorders (NDDs) through effects on gene dosage. This review focuses on our current understanding of genomic disorders that arise from large structural chromosome rearrangements in patients with NDDs, as well as difficulties in overlap of clinical presentation and molecular diagnosis. We discuss the implications of epigenetics, specifically DNA methylation (DNAm), in NDDs and genomic disorders, and consider the implications and clinical impact of copy number and genomic DNAm testing in patients with suspected genetic NDDs. We summarize evidence of global methylation episignatures in CNV-associated disorders that can be used in the diagnostic pathway and may provide insights into the molecular pathogenesis of genomic disorders. Finally, we discuss the potential for combining CNV and DNAm assessment into a single diagnostic assay.
Keywords: DNA methylation, episignature, epigenetics, copy number variant, neurodevelopmental disorder, genomic disorder
1. Introduction
Neurodevelopmental disorders (NDDs) are a class of neurological and neuropsychiatric conditions that manifest in childhood during the developmental phase and persist throughout life [1]. They affect development of the central nervous system and can lead to brain dysfunction, resulting in limitations or impairment in cognition, motor performance, vision, hearing, speech and behavior [2]. NDDs include, but are not limited to, autistic spectrum disorder (ASD), intellectual disability (ID), attention deficit hyperactivity disorder (ADHD) and epilepsy, all of which show high rates of comorbidity and phenotypic overlap [3]. They are estimated to affect approximately 3% of children worldwide [4,5] and therefore, collectively represent a significant impact to families and health care systems.
NDDs present with a broad range of genetic and phenotypic heterogeneity, and clinical presentations are often non-specific. Genetics plays an important role in the etiology of hereditary NDDs. Genetic mutations associated with NDDs vary in size from single nucleotide variants (SNVs) to whole chromosome aneuploidies [6]. Due to the phenotypic overlap exhibited, in addition to targeted gene sequencing approaches, genetic testing often involves global genomic screening including chromosomal microarray analysis (CMA), exome or whole genome sequencing (WES, WGS), or classically G-banded chromosome karyotyping (karyotyping). CMA, considered the first-tier diagnostic test for patients with NDDs, has been used clinically for nearly two decades [7,8] to detect structural imbalances involving deletion or duplication of genetic material, collectively termed copy number variants (CNVs). Whilst some of the first CMAs included bacterial artificial chromosome (BAC) clones in an array-based comparative genomic hybridization (aCGH) with genome-wide coverage at approximately 1 Mb intervals [9,10], more recent platforms involving oligonucleotide or high-resolution single nucleotide polymorphism (SNP) arrays may reach a resolution of a few hundred base pairs [11,12]. Therefore, genomic imbalances beyond the resolution of karyotyping (minimum detection sizes 3–7 Mb) [13,14] are now routinely detected. SNP arrays offer the highest resolution of commercially available microarrays and are designed to determine genotype, structural imbalances, genomic aneuploidy, and loss of heterozygosity [15].
Microarray platforms can be customized to increase coverage and resolution in clinically relevant regions and regions associated with well-defined genomic syndromes. In addition to probe-coverage enriched regions, clinical use microarray platforms have probes equally spaced across the rest of the genome, termed “backbone” coverage. The combination of high probe densities and optimized targeted design is aimed at reducing the rate of ambiguous findings termed variants of uncertain significance (VUSs), since, at this time, there is a limited understanding of the impact of CNVs outside of protein coding regions.
2. The Role of CNVs in Genomic Disorders
Structural variants, defined as “alterations that involve segments of DNA larger than 1 kb” [13], include CNVs linked to phenotypic variation and disease susceptibility [16]. CNVs can contain millions of nucleotides, multiple genes and regulatory elements. Tuzun et al. suggested that individuals carry on average 250 CNVs [17]. As such, compared with SNVs, CNVs are reported to be responsible for more than ten times the total heritable sequence differences observed in the general population [18]. CNVs are also described as polymorphisms in association with several non-pathological conditions, e.g., those involved in variation in olfactory perception [19]. The presence of CNVs in non-pathological conditions can present challenges in the interpretation and classification of variants, especially in the absence of functional studies.
Genomic disorders are a group of genetic conditions caused by CNVs affecting dosage sensitive genes or genes critical for normal development or maintenance and/or their regulatory elements [20]. Recurrent disorders, those with common start and stop breakpoints, include CNVs that are similar in size and gene content, and typically present with similar phenotypes, e.g., deletions and duplications of 17p11.2 (Smith–Magenis and Potocki–Lupski syndrome), 7q11.23 (Williams syndrome), 15q11.2 (Prader–Willi/Angelman syndrome) and 17q21.31 (Koolen–de Vries syndrome) [6,21,22]. In contrast, non-recurrent disorders show variability in size and gene content (typically there is a common region of overlap). Phenotypes in these patients vary substantially, e.g., deletions of 22q13.3 in Phelan–McDermid syndrome (PHMDS) or deletions of 9q34.3 in Kleefstra syndrome [6].
Segmental duplications (also known as low copy repeats; LCRs) are blocks of DNA ranging from 1–400 kb that occur throughout the genome and typically share a high level (>90%) of sequence identity [23,24]. Many structural rearrangements, including CNVs, are mediated by LCRs through non-allelic homologous recombination (NAHR) [25]. These LCRs are highly prone to rearrangements which can result in genomic imbalances, including those associated with the common CNV-related disorders.
A recent study assessed the prevalence and inheritance of CNVs associated with NDDs and estimated recurrent CNVs present in approximately 1 in 200 live births [26]. These results indicate that while individual CNVs may be rare, collectively they contribute significantly to NDDs. The most common CNVs observed in NDDs are those associated with genomic disorders [6], and a recent study estimated deletions of the 16p11.2 proximal region, 17q12, and 1q21.1 regions, and duplications of 15q11.2, 22q11.2, and the 16p11.2 distal region as the most common [26]. These findings vary from previous studies where duplications of 2q13 and deletions of 22q11.2, 15q11.2 and 1p36 were among the most common [21]. These differences likely reflect increased resolution of microarray platforms, as well as a decrease in ascertainment bias.
Considerable research to date has focused on genes within the CNV regions of genomic disorders and how dosage sensitivity may be responsible for the observed phenotypes. While this research has identified some causative or candidate genes for specific Mendelian disorders and phenotypes, the genetic contribution for the majority of the observed clinical phenotypes in CNV disorders are not well defined [21]. One example of a disorder where a contributory gene has been identified is the 5q35 deletion. 5q35 deletion is predominantly mediated by NAHR and is associated with Sotos syndrome 1, where haploinsufficiency of the nuclear receptor-binding set domain protein (NSD1) gene contained within this region is shown, on its own, to be causative for Sotos [27]. This is similar to findings in Smith–Magenis syndrome, where deletions of 17p11.2 are responsible for 90% of causative variants, while approximately 5% are the result of point mutations in the retinoic acid-induced 1 (RAI1) gene contained within this region [22,23,24,25,26,27,28]. This is in contrast to disorders such as 16p11.2 and 22q11.2 deletion and duplication syndromes where no single candidate gene has been identified.
Overall, CNVs may contribute to the clinical features observed in genomic disorders through dosage sensitivity, via haploinsufficiency (as described for Sotos and Smith–Magenis), triplosensitivity (e.g., 22q11.2 duplication syndrome) or imprinting effects (Prader-Willi and Angelman syndromes), or through disruption of gene expression via positional effects, including disruption of transcriptional regulatory elements and changes in the chromatin structure.
3. Clinical Identification of CNVs in Patients with NDDs
CMA screening for CNVs in patients with NDDs has an estimated diagnostic yield of approximately 15–20% [21,22,23,24,25,26,27,28,29], which is a significant increase from karyotyping (3%) [7]. Newer molecular techniques such as WES or WGS in patients with developmental delay (DD) or ID have a reported diagnostic yield of approximately 25–36% [30,31,32,33]. Although these technological advancements have improved diagnostic capabilities in these disorders, half to two thirds of patients with suspected genetic conditions remain without a diagnosis [32,33,34].
This ‘diagnostic odyssey’, the time from initial consultation to diagnosis, often involves multiple clinical evaluations and laboratory tests spanning years [35,36], resulting in significant social and economic burden on both families and health care systems. In addition to CMA as the first-tier screen [7], in males with DD it is often accompanied by assessment for Fragile-X syndrome (FRX). FRX is an X-linked dominant condition and the most common inherited cause of ID. FRX results from abnormal expansion of the CGG trinucleotide repeat (>200 repeats) located in the promoter of the fragile X messenger ribonucleoprotein 1 (FMR1) gene, resulting in promotor DNA hypermethylation and gene silencing [37]. FRX can also be the result of deletions of Xq27.3 containing the FMR1 gene [38]. Reflexive genetic testing, whereby the results of previous tests are used to guide further investigations, include DNA methylation (DNAm) analysis in individuals with CNVs at common imprinting loci, e.g., 15q11.1 and 11p15.5 regions. The average time to diagnosis in patients referred for genetic testing is estimated at 1–8 years [7,35,36,37,38,39], and the cost to healthcare is often difficult to estimate or missing from research. Recent studies describe the substantial positive medical and psychosocial outcome of receiving a genetic diagnosis [40,41]. Therefore, development of novel diagnostic technologies or testing strategies to shorten the diagnostic odyssey or increase the diagnostic yield represent an ongoing priority in NDD research [42].
Many recent studies have demonstrated disruption of genomic DNAm as a functional consequence of genetic defects in patients with NDDs [43,44,45,46]. There is emerging evidence of similar DNAm disruptions as epigenetic biomarkers for CNV disorders and their associated clinical phenotypes.
4. The Role of Epigenetics in NDDs and Subsequent Episignature Mapping
Epigenetics refers to mitotically heritable gene regulatory mechanisms without changes in the DNA sequence [47,48]. Epigenetic regulation of gene expression occurs at the level of chromatin and typically involves processes that modify chromatin or histones, the proteins around which DNA is wrapped, or covalent modifications in the associated DNA molecule [49]. DNAm is the most extensively studied epigenetic modification and refers to the mechanism of addition or removal of a methyl group to cytosine nucleotides [50]. Most cytosines subject to DNAm are adjacent to guanine residues and referred to as CpG dinucleotides (CpGs) [50]. High density clusters of CpGs, often associated with gene promoters, are referred to as CpG islands [50]. Unmethylated (hypomethylated) CpGs and CpG islands are generally associated with open, transcriptionally accessible chromatin, while DNA hypermethylation correlates with compact, transcriptionally repressive chromatin [51]. The majority of CpGs in the human genome are methylated except for those contained within CpG islands [52]. Therefore, in addition to affecting chromatin states and stability, disruptions in DNAm patterns can alter gene expression [51].
An increasing number of chromatin and epigenetic regulatory genes are becoming implicated in a variety of NDDs. Mutations in these genes result in DNAm episignatures, whole genome methylation changes, which are routinely detectable in the peripheral blood of patients affected by these disorders [43]. An episignature is defined as a recurring epigenetic pattern associated with a common genetic or environmental etiology in a disorder-specific patient population. Episignatures are highly sensitive and specific biomarkers that can be used to help resolve ambiguous clinical and genetic findings, and for screening patients with suspected genetic conditions [45]. Episignatures have the potential to provide insight into functional effects of certain mutations and genomic alterations on widespread DNAm and their contribution to the pathophysiology of genetic disorders [46].
Histone modifications refer to the chemical modification of histone tails by processes including methylation, acetylation, phosphorylation and ubiquitination. Histone tails are loosely structured protein segments that can mediate interaction between nucleosomes, and their modifications can result in either condensed or more relaxed chromatin, ultimately exhibiting an effect on gene transcription, as well as accessibility of DNA to other chromatin remodeling factors, including those involved in DNA methylation. Our group and others have identified unique episignatures in multiple NDDs that are the consequence of mutations in genes associated with histone modification [44], including, e.g., Kabuki syndrome caused by mutations in the lysine-specific methyltransferase 2D gene (KMT2D) [53]. We have mapped episignatures in several other histone modifying genes including lysine-specific methyltransferase 2B (KMT2B), set domain-containing protein 2 (SETD2), creb-binding protein (CREBBP), lysine acetyltransferase 6A (KAT6A) and lysine demethylase 4B (KDM4B) [43]. In addition, unique episignatures have also been reported in genes associated with the removal of histone methylation marks, the so-called “eraser” genes, such as the histone lysine demethylase 5C gene (KDM5C) in Claes–Jensen syndrome [54]. Histone modifications work in concert with DNAm to affect chromatin remodeling and gene expression.
The DNAm reaction is catalyzed by enzymes known as DNA methyltransferases (DNMT), which are responsible for mediating the transfer of the methyl group from S-adenosylmethionine (SAM) to cytosine residues. Robust episignatures have been reported in NDDs caused by mutations in the DNA methyltransferase genes DNMT1, DNMT3A and DNMT3B [44,55]. These genes are involved in the establishment and maintenance of DNAm during DNA replication and are termed “writers” since they are responsible for the addition of the methyl group to cytosines. Unique episignatures in two disorders are associated with mutations in DNMT1, hereditary sensory neuropathy with dementia and hearing loss (HSNDHL), and autosomal dominant cerebellar ataxia, deafness and narcolepsy (ADCADN) ([56,57]), while loss of function mutations in DNMT3A result in an episignature in Tatton–Brown–Rahman syndrome (TBRS) [44,58]. Mutations in DNMT3B, which cause immunodeficiency, centromere instability and facial anomalies (ICF) syndrome, also result in genomic defects in DNAm [59]. We recently demonstrated a genome-wide DNA hypermethylation episignature in a DNA demethylation gene Tet methylcytosine dioxygenase 3 (TET3), an “eraser” gene that opposes the writer function of DNMT1 [60]. Mutations in the highly conserved catalytic domain of TET3 cause Beck–Fahrner syndrome (BEFAHRS). Inheritance patterns of BEFAHRS vary and include autosomal dominant or recessive forms [61]. Through episignature mapping, we were able to differentiate between affected individuals with mono- and bi-allelic mutations [60].
To date, chromatin remodeling genes comprise the largest group of epigenetic modifier genes with mapped episignatures, e.g., truncating mutations in the SNF2-related CBP activator protein gene (SRCAP) result in an episignature specific for Floating-Harbor syndrome [62]. Schenkel et al. reported a unique methylation profile associated with mutations in the ATRX chromatin remodeler gene ATRX in alpha-thalassemia X-linked intellectual disability syndrome [63]. In addition, our group previously described a shared DNAm episignature in Coffrin–Siris and Nicolaides–Baraitser syndromes (NCBRS) [64], which are two phenotypically similar NDDs associated with mutations in subunits of the BAF chromatin remodeling complex (commonly referred to as BAFopathies). This study described a shared BAFopathies episignature and supported the findings from previous studies suggesting that these conditions represent a disease spectrum rather than two distinct disorders [65]. Furthermore, this study indicates that methylation analysis may uncover or provide further support for the “relatedness” of genes and their disorders. In a subsequent study by our group, we described a new syndrome involving the BAF complex and the SWI/SNF-related matrix-associated, actin-dependent regulator of the chromatin gene (SMARCA2)—a gene reported in multiple individuals with NCBRS. This new syndrome was identified based on unique methylation patterns observed in individuals with intragenic variants located in the helicase domain of the SMARCA2 gene compared to individuals with pathogenic variants located outside the helicase domain [66]. In support of these findings, clinical features of patients with SMARCA2 helicase domain mutations exhibited a common phenotype distinct from NCBRS. Similarly, functional studies in yeast supported a different molecular mechanism underlying these two disorders [66]. Therefore, by analyzing variants from multiple regions within a gene, we were able to identify two unique episignatures and uncover functional data to explain the phenotypic differences seen between patients harboring variants in the same gene, resulting in the discovery of a new syndrome.
Interestingly, two distinct domain-specific episignatures have also been described in Helsmoortel-van der Aa syndrome associated with dominant negative truncating mutations in the activity-dependent neuroprotector homeobox gene (ADNP), which has chromatin regulatory functions [67]. These signatures were partially opposing, with mutations in the N- and C-terminus resulting in a predominantly hypomethylated signature; in contrast, mutations centered on the nuclear localization sequence resulted in a predominant hypermethylation signature. A subsequent study confirmed phenotypic differences between patients that correlated with the two episignatures [68].
Genes whose primary function is not associated with epigenetic and chromatin regulatory mechanisms, such as ubiquitin-conjugating enzyme E2 A (UBE2A) and spermine synthase (SMS) in X-linked syndromic forms of mental retardation—Nascimento and Snyder–Robinson types, respectively [44]—have also shown evidence of unique episignatures. The UBE2A gene at Xq24 encodes the RAD6 ubiquitin-conjugating enzyme and has been recently shown to be involved in histone modifications that control gene expression [69,70]. The SMS gene at Xp22.11 encodes for an enzyme involved in polyamine synthesis and recycling and is directly related to decarboxylated SAM. Previous studies have suggested that alterations in this polyamine synthesis could result in an excess of SAM and may lead to aberrant DNAm status [71].
Taken together, these studies show that DNAm episignatures can be detected in genes with various functions and provide strong evidence for the clinical utility of episignatures as diagnostic biomarkers in NDDs [43,44,72], while also enabling broader understanding of the clinical associations and biological roles of DNAm in genetic disorders.
5. Current Episignature Detection in NDDs
We recently described an approach to episignature mapping and development of a clinical EpiSign classifier in 65 genetic syndromes [43] involving bisulfite converted peripheral blood samples analyzed using methylation microarrays. Blood presents itself as the ideal tissue type for episignature development as it is a common clinical sample type and is easily accessible. Since episignatures represent a fundamental defect in NDDs caused by genetic variation in the germline, DNAm changes will be present in all subsequent tissues. This microarray technology enables a genome-wide, cost-effective, standardized, scalable and high throughput assessment of DNAm patterns, amenable to clinical validation in a diagnostic laboratory setting. This technology enables simultaneous assessment of up to 850,000 CpGs across the genome. By applying a custom bioinformatic pipeline to the methylation data obtained from these arrays, we are able to identify sensitive and disorder-specific episignatures. Using unsupervised machine learning models (MLMs), the sensitivity of an episignature can be assessed. Construction of multiclass supervised MLMs to compare a patient’s DNAm data against controls and samples from other clinically validated episignatures at the same time, through the use of an expansive tissue-specific database, should be applied to confirm the specificity of a signature [44]. These methods rely on the ability to perform concurrent assessment of multiple disorders and controls, and highlights the importance of development of large-scale reference databases. To use episignatures in different tissues, a reference database would be required to establish the unique DNAm changes in the particular tissue that arise in development during differentiation and determine how this may impact the specific episignature. Our group has focused on episignatures in blood and has not assessed peripheral blood episignatures in other tissue types. Use of these supervised and unsupervised MLMs is also important when we consider the scalability of testing, as the list of episignatures continues to expand, requiring these algorithms to be capable of handling large amounts of data and computations in a cost-effective and timely fashion.
The ability to detect episignatures is highly contingent upon the intensity (effect size) and extent (number of differentially methylated CpGs) of the observed DNAm changes [44]. Some disorders, such as Sotos or TBRS, are associated with robust changes to the extent of involvement of tens of thousands of CpGs. In contrast, disorders such as the BAFopathies only exhibit a few hundred differentially methylated CpGs [64]. In light of this, sample size can play a role in the ability to detect episignatures in disorders associated with mild or moderate DNAm changes.
When analyzing methylation effects, it is important to consider confounding biological factors such as age, sex and blood cell composition, which are known to be associated with changes in methylation patterns in healthy individuals [73,74]. Methods should be in place to account for such factors when trying to decipher which observed methylation changes contribute to the underlying NDD.
To identify regions containing methylation changes, referred to as differentially methylated regions (DMRs), a ‘bump hunting’ approach [75] can be used, which typically considers regions containing 3–5 CpGs with greater than 10% methylation change between case samples and controls, and gaps of no more than 500 bp between neighboring CpGs [53]. DMRs can be useful in determining significant downstream effects of gene disruption and pathogenesis of disorders such as up- and down-regulated gene expression.
The complex bioinformatic pipeline required to identify episignatures, and to overcome previously mentioned confounding variables, relies heavily on a large-scale tissue-specific reference DNAm database, as well as bioinformatic and clinical genetic expertise [45]. Broadening utility of episignature assessment in the clinical setting involve screening of patients with suspected NDDs, as well as a functional assay for reclassification of VUSs.
6. The Use of Episignatures in the Diagnosis of NDDs
EpiSign is a clinical genome-wide DNAm test that has been available since 2019 that can detect over 60 disorders in more than 80 genes associated with Mendelian disorders through assessment of peripheral blood DNA [43]. A list of the current disorders detectable by EpiSign version 3 are listed in Table 1.
Table 1.
EpiSign v3 assay gene content.
| Syndrome | Episignature Abbreviation | Underlying Gene(s) or Region | OMIM |
|---|---|---|---|
| Alpha-thalassemia mental retardation syndrome | ATRX | ATRX | 301040 |
| Angelman syndrome | Angelman | UBE3A | 105830 |
| Arboleda–Tham syndrome | ARTHS | KAT6A | 616268 |
| Autism, susceptibility to, 18 | AUTS18 | CHD8 | 615032 |
| Beck–Fahrner syndrome | BEFAHRS | TET3 | 618798 |
| Beckwith–Wiedemann syndrome | BWS | Chr11p15 (ICR1, KCNQ1OT1, CDKN1C) | 130650 |
| Blepharophimosis intellectual disability SMARCA2 syndrome | BIS | SMARCA2 | 619293 |
| Börjeson–Forssman–Lehmann syndrome | BFLS | PHF6 | 301900 |
| Cerebellar ataxia, deafness, and narcolepsy, autosomal dominant | ADCADN | DNMT1 | 604121 |
| CHARGE syndrome | CHARGE | CHD7 | 214800 |
| Chr16p11.2 deletion syndrome | Chr16p11.2del | Chr16p11.2 deletion | 611913 |
| Coffin–Siris syndrome-1, 2 (CSS1,2) | CSS_c.6200 | ARID1A; ARID1B | 135900; 614607 |
| Coffin–Siris 1–4 (CSS1–4) and Nicolaides–Baraitser syndrome (NCBRS) | BAFopathy | ARID1B; ARID1A; SMARCB1; SMARCA4; SMARCA2 | 135900; 614607; 614608; 614609; 601358 |
| Coffin–Siris syndrome-4 (CSS4) | CSS_c.2656 | SMARCA4 | 614609 |
| Coffin–Siris syndrome-9 (CSS9) | CSS9 | SOX11 | 615866 |
| Cohen–Gibson syndrome; Weaver syndrome | PRC2 | EED; EZH2 | 617561; 277590 |
| Cornelia de Lange syndromes 1–4 | CdLS | NIPBL; SMC1A; SMC3; RAD21 | 122470; 300590; 610759; 614701 |
| Down syndrome | Down | Chr21 trisomy | 190685 |
| Dystonia-28, childhood onset | DYT28 | KMT2B | 617284 |
| Epileptic encephalopathy, childhood onset | EEOC | CHD2 | 615369 |
| Floating-Harbour syndrome | FLHS | SRCAP | 136140 |
| Fragile X syndrome | FXS | FMR1 | 300624 |
| Gabriele de Vries syndrome | GADEVS | YY1 | 617557 |
| Genitopatellar syndrome (see also Ohdo syndrome, SBBYSS variant) | GTPTS | KAT6B | 606170 |
| Helsmoortel–Van der Aa syndrome (ADNP syndrome (Central)) | HVDAS_C | ADNP | 615873 |
| Helsmoortel–Van der Aa syndrome (ADNP syndrome (Terminal)) | HVDAS_T | ADNP | 615873 |
| Hunter–McAlpine craniosynostosis syndrome | HMA | Chr5q35-qter duplication | 601379 |
| Immunodeficiency, centromeric instability, facial anomalies syndrome 1 (ICF1) | ICF_1 | DNMT3B | 242860 |
| Immunodeficiency, centromeric instability, facial anomalies syndrome 2,3,4 (ICF2,3,4) | ICF_2_3_4 | ZBTB24; CDCA7; HELLS | 614069; 616910; 616911 |
| Intellectual developmental disorder-65 | KDM4B | KDM4B | 619320 |
| Intellectual developmental disorder with seizures and language delay | IDDSELD | SETD1B | 619000 |
| Intellectual developmental disorder, X-linked 93 | MRX93 | BRWD3 | 300659 |
| Intellectual developmental disorder, X-linked 97 | MRX97 | ZNF711 | 300803 |
| Intellectual developmental disorder, X-linked, Snyder–Robinson type | MRXSSR | SMS | 309583 |
| Intellectual developmental disorder, X-linked, syndromic, Armfield type | MRXSA | FAM50A | 300261 |
| Intellectual developmental disorder, X-linked, syndromic, Claes-Jensen type | MRXSCJ | KDM5C | 300534 |
| Intellectual developmental disorder, X-linked syndromic, Nascimento-type | MRXSN | UBE2A | 300860 |
| Kabuki syndromes 1, 2 | Kabuki | KMT2D; KDM6A | 147920; 300867 |
| Kagami–Ogatta syndrome | KOS | Chr14q32 | 608149 |
| KDM2B-related syndrome | KDM2B | KDM2B | unofficial |
| Kleefstra syndrome 1 | Kleefstra | EHMT1 | 610253 |
| Koolen de Vries syndrome | KDVS | KANSL1 | 610443 |
| Luscan–Lumish syndrome | LLS | SETD2 | 616831 |
| Menke–Hennekam syndrome-1, 2 | MKHK_ID4 | CREBBP; EP300 | 618332; 618333 |
| Mental retardation, autosomal dominant 23 | MRD23 | SETD5 | 615761 |
| Mental retardation, autosomal dominant 51 | MRD51 | KMT5B | 617788 |
| Mental retardation, FRA12A type | DIP2B | DIP2B | 136630 |
| Myopathy, lactic acidosis, and sideroblastic anemia-2 | MLASA2 | YARS2 | 613561 |
| Ohdo syndrome, SBBYSS variant | SBBYSS | KAT6B | 603736 |
| Phelan–McDermid syndrome | PHMDS | Chr22q13.3 deletion | 606232 |
| Prader–Willi syndrome | PWS | Chr15q11 (SNRPN, NDN) | 176270 |
| Rahman syndrome | RMNS | HIST1H1E | 617537 |
| Renpenning syndrome | RENS1 | PQBP1 | 309500 |
| Rubinstein–Taybi syndrome 1 | RSTS1 | CREBBP | 180849 |
| Rubinstein–Taybi syndrome-1, 2 | RSTS | CREBBP; EP300 | 180849; 613684 |
| Rubinstein–Taybi syndrome-2 | RSTS2 | EP300 | 613684 |
| Silver–Russell syndrome 1 | SRS1 | Chr11p15.5 | 180860 |
| Silver–Russell syndrome 2 | SRS2 | Chr7p11.2 | 618905 |
| Sotos syndrome 1 | Sotos | NSD1 | 117550 |
| Tatton–Brown–Rahman syndrome | TBRS | DNMT3A | 615879 |
| Temple syndrome | Temple | Chr14q32 | 616222 |
| Velocardiofacial syndrome | VCFS | Chr22q11.2 deletion | 192430 |
| Wiedemann–Steiner syndrome | WDSTS | KMT2A | 605130 |
| Williams–Beuren deletion syndrome (Chr7q11.23 deletion syndrome) | Williams | Chr7q11.23 deletion | 194050 |
| Williams–Beuren duplication syndrome (Chr7q11.23 duplication syndrome) | Dup7 | Chr7q11.23 duplication | 609757 |
| Wolf–Hirschhorn syndrome | WHS | Chr4p16.13 deletion | 194190 |
In parallel with screening for episignatures, EpiSign also permits concurrent detection of FRX in males [37] and common imprinting disorders [76], thereby consolidating tests and reducing the need for additional reflexive testing [45]. In addition, its clinical utility in the assessment and reclassification of VUSs in genes with existing episignatures was recently reported in multiple studies [45,72,77,78]. EpiSign is the first and currently the only genome-wide DNAm clinical test offered for screening individuals with NDDs, and can be used as part of the diagnostic work up or for reclassification of VUSs. The reference EpiSign Knowledge Database (EKD) [44] utilized by the EpiSign assay contains thousands of peripheral blood DNAm profiles from both reference controls and NDD.
The cost of completing a methylation array is comparable to the cost of most CMAs, and the results are highly reproducible. The assay uses peripheral blood, similar to current CMA platforms, allowing streamlined adaption in the clinical setting given the overlap in equipment and laboratory techniques.
The ability to detect episignatures in patients with NDDs has been shown to increase the diagnostic yield, helping to resolve the diagnostic odyssey. A recent study assessing the clinical impact of EpiSign for patients with rare Mendelian disorders demonstrated a 27.6% diagnostic yield among patients with previous ambiguous/inconclusive genetic findings including genetic VUSs [45]. As episignature discovery expands and more disorders are added to the test repertoire, the diagnostic yield is likely to increase significantly, ultimately benefiting patients, their families and the related health care systems. This expansion in episignatures, however, may bring with it challenges, as it is likely to uncover syndromes with overlapping episignatures which may require the implementation of novel computational methods in order to classify these disorders.
7. Episignature Development in CNV-Associated Genomic Disorders Provides Insight into Pathological Mechanism
Changes in DNAm profiles, or episignatures, in patients with large CNV defects associated with genomic disorders have not been systematically studied, and it is plausible that large CNVs, much like gene specific variants, may exhibit unique diagnostic methylation signatures in patients with NDDs.
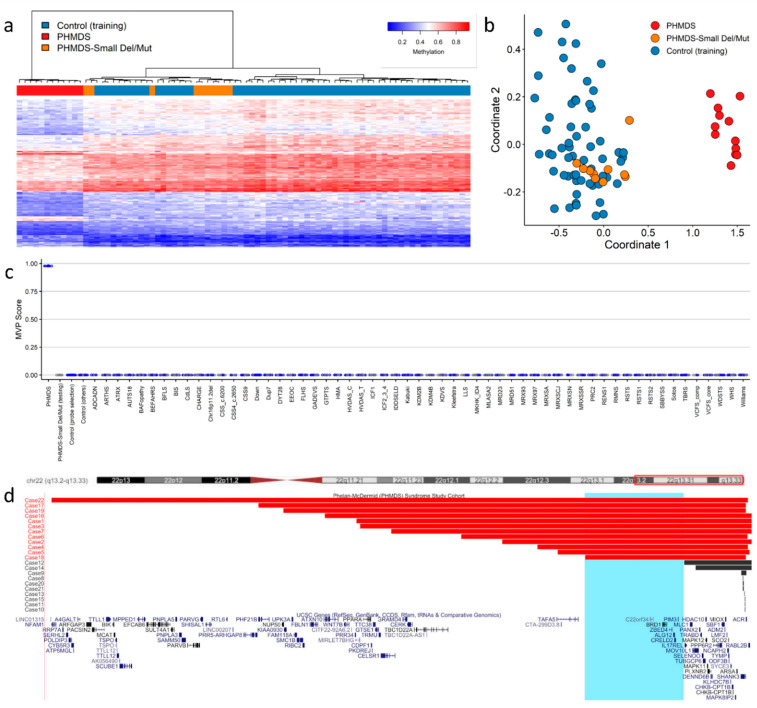
Our group recently published findings describing episignature discovery in patients with PHMDS [46], highlighting the novel insights DNA methylation analysis can contribute to the pathogenesis of CNV disorders. PHMDS is a genomic disorder associated with deletions of chromosome 22, involving partial or whole-gene disruption of the SH3 and multiple ankyrin repeat domains 3 gene (SHANK3). Intragenic variants in SHANK3 alone are responsible for a broad range of the phenotypic features observed in PHMDS [79]. However, this gene does not explain the entire phenotype in many patients, particularly speech and motor deficits, as well as renal abnormalities. The phenotypic variability and potential involvement of additional genes within the region has been previously assessed by multiple groups [80,81]. We demonstrated an episignature in patients with large deletions that was not observed in those with small deletions or SHANK3 gene level variants (Figure 1a–c). The minimal region of difference between these two deletion types, large versus small, included the bromodomain-containing protein 1 gene (BRD1), a gene involved in epigenetic mechanisms and a likely candidate gene for the methylation signature observed in these patients (Figure 1d). BRD1 is a component of a histone acetyltransferase complex that interacts with chromatin remodeling proteins and, before now, there was limited genotype–phenotype association reported in this gene. In addition, metabolic studies confirmed that these patients also exhibited very different metabolic profiles [46], further providing functional evidence for disease pathogenesis, as well as indicating targets for future therapies.
Figure 1.
Phelan−McDermid syndrome (PHMDS) episignature demonstrating the critical BRD1 region: (a) Euclidean hierarchical clustering (heatmap); each column represents a single PHMDS case or control, each row represents one of the CpG probes selected for the episignature. This heatmap shows clear separation between large deletion (2−6 Mb in size) PHMDS cases (red) from controls (blue). Smaller deletions (0.01−1 Mb) and intragenic SHANK3 gene variants (Small Del/Mut) (orange) are shown to segregate with controls. (b) Multidimensional scaling (MDS) plot shows segregation of large deletion PHMDS cases from both controls and Small Del/Mut cases. (c) Support vector machine (SVM) classifier model. Model was trained using the selected probes for the PHMDS episignature, 75% of controls and 75% of other neurodevelopmental disorder samples (blue). The remaining 25% of controls and 25% of other disorder samples were used for testing (grey). Plot shows the large deletion PHMDS cases with a methylation variant pathogenicity (MVP) score close to 1 compared with all other samples, showing the specificity of the classifier and episignature. (d) PHMDS deletions illustrating the critical region of interest associated with DNA methylation episignature. The horizontal red bars represent large deletion PHMDS cases associated with the presence of a distinct episignature. The horizontal black bars represent Small Del/Mut cases that do not have a distinct DNA methylation episignature. Highlighted in light blue is the common critical region of interest (Chr22:49,228,863−50,429,645) of deletions associated with the episignature. The common region of interest contains the candidate BRD1 gene. Cytogenetic bands and known genes are presented in this figure using the UCSC genome browser [82] 2009 (GRCh37/hg19) genome build. Figure adapted with permission from Schenkel et al. [46].
8. Defined Episignatures in Other CNV-Associated Genomic Disorders Provide Rationale to Further Expand Episignature Discovery
Symmetrical dose-dependent DNAm profiles have been reported in individuals with deletion of the 7q11.23 region (Williams syndrome; WS) or duplication of the same region (7q11.23 duplication syndrome) [83], highlighting the importance of DNAm in the pathogenesis of these disorders. This region contains a number of genes associated with epigenetic mechanisms, and a study by Aref-Eshghi et al. later showed that these methylation changes resulted in unique episignatures that could differentiate WS and 7q11.23 duplication syndrome from 40 other NDDs and congenital anomaly disorders [44]. In the same study, Aref-Eshghi et al. demonstrated another example of symmetrical DNAm pattern, this time when comparing Hunter–McAlpine syndrome (HMS) and Sotos syndrome. A distinct hypermethylation episignature is observed in HMS patients with duplications involving the 5q35 region containing the NSD1 gene, a direct contrast to the robust hypomethylation episignature seen in patients with Sotos syndrome, which is the result of loss of function variants in the same NSD1 gene [44].
A DNAm signature was reported in a cohort with the genomic disorder 16p11.2 deletion syndrome (16p11.2DS) [84]—a disorder associated with a variable phenotype that includes increased susceptibility to ASD. Several genes within this region play a role in histone or chromatin function; however, to date, no single candidate gene has been identified to be causative of this disorder or its resultant episignature. Moreover, 16p11.2DS shows reduced penetrance and variable expressivity, and although most deletions are de novo, many are inherited from apparently unaffected parents. These so-called “susceptibility CNVs” present challenges for clinicians in counselling families [41]. Due to the presence of a cluster of LCRs in this region that mediate CNVs through NAHR, there is a reciprocal duplication disorder (16p11.2 duplication syndrome) with similar diagnostic challenges. Studying methylation changes in patients with these susceptibility CNVs and their carrier parents could potentially unlock novel insights into the role of aberrant DNAm in reduced penetrance CNV disorders.
Our group recently described an aberrant DNAm pattern in patients with deletions of 12q24.31 encompassing the known histone modifier gene SET domain-containing protein 1B (SETD1B), and demonstrated that patients who harbored point mutations within SETD1B shared the same methylation episignature [78]. This study highlights that larger CNVs may exhibit the same methylation affects as gene specific variants within these regions.
The most common genomic disorder is a 22q11.2 deletion syndrome and is the result of a 1.5–3 Mb deletion also mediated by NAHR at a cluster of LCRs. Clinical manifestations of this disorder include DiGeorge and Velocardiofacial syndromes, and, to date, the phenotype–genotype relationship has not been fully elucidated. Through analysis of a cohort of individuals with 22q11.2 deletions, we identified an episignature that can differentiate 22q11.2 deletion syndrome from other NDDs on the clinical EpiSign test, including those considered in the differential diagnosis of this syndrome [85]. Among other findings, assessment of DMRs showed overlap with loci for orofacial clefting, a key phenotypic feature of this disorder. Through further analysis of atypical deletions and gene level variants, it may be possible to determine the gene, or genes, that play a role in the aberrant DNAm pattern observed, as well as insight into the mechanisms contributing to this disorder.
Only a few of the most prevalent genomic disorders have a candidate gene considered responsible for the entire phenotypic spectrum. Interestingly, where these candidate genes have been identified, they are predominantly involved in epigenetic regulation including chromatin remodeling or histone modification, e.g., CREBBP in Rubinstein–Taybi syndrome [86] and NSD1 in Sotos syndrome [87] (Table 2). Variants in most of these genes have already been assessed for genome-wide DNAm changes, and have been shown to exhibit unique and specific episignatures [43]. Overall, the majority of CNV disorders do not have a known or suspected candidate gene of interest. However, almost all of these regions contain one or more genes with epigenetic function (Table 2), e.g., chromodomain helicase DNA-binding protein 1-like (CHD1L) gene in 1q21.1 deletions and duplications, a gene that has a role in chromatin remodeling following DNA damage [88].
Table 2.
Common CNV disorders including the candidate genes involved in the clinical phenotype (where applicable), and genes contained within with reported epigenetic machinery roles.
| Syndrome | Chromosome Region | Candidate Gene | Genes in Region with Epigenetic Function |
|---|---|---|---|
| 1p36 Deletion/Duplication | 1p36 | - | ICMT, CHD5, TP73, PMRD16, SKI, NOC2L |
| 1q21.1 Deletion/Duplication | 1q21.1 | - | CHD1L |
| 1q43q44 Deletion | 1q43q44 | - | HNRNPU, DESI2, ZBTB18, AKT3 |
| 2q11.2 Deletion/Duplication | 2q11.2 | - | KANSL3, ARID5A |
| 2q13 Deletion/Duplication | 2q13 | - | MIR4435-2HG |
| 2q37 Deletion | 2q37 | - | HDAC4, D2HGDH, ING5, HDLBP, PASK |
| 3q29 Deletion/Duplication | 3q29 | - | PAK2, RNF168 |
| 4p16.3 Deletion (Wolf–Hirschhorn)/4p16.3 Duplication | 4p16.3 | NSD2 | NSD2, CTBP1, SLBP, CTBP1, PCGF3 |
| 5p15 Deletion (Cri du Chat)/5p15 Duplication | 5p15 | - | ATPSCKMT, MTRR, NSUN2, LPCAT1, BRD9 |
| 5q35 Deletion (Sotos)/5q35 Duplication (Hunter–McAlpine) | 5q35 | NSD1 | NSD1, UIMC1 |
| 7q11.23 Deletion (Williams–Beuren)/7q11.23 Duplication | 7q11.23 | - | METTL27, BUD23, BCL7B, BAZ1B |
| 8p23.1 Deletion/Duplication | 8p23.1 | - | TNKS |
| 9q34 Deletion (Kleefstra)/9q34 Duplication | 9q34 | EHMT1 | EHMT1 |
| 10q22.3q23.2 Deletion/Duplication | 10q22.3q23.2 | - | WAPL, DYDC1, MAT1A |
| 11p11.2 Deletion (Potocki–Shaffer)/11p11.2 Duplication | 11p11.2 | - | PHF21A, CD82, ALKBH3 |
| 11q13.2q13.4 Deletion | 11q13.2q13.4 | - | KMT5B |
| 15q11.2 Deletion (non-imprinting region) | 15q11.2 | - | - |
| 15q11q13 Deletion (Prader–Willi/Angelman)/15q11q13 Duplication | 15q11q13 | - | HERC2 |
| 15q13.3 Deletion/Duplication | 15q13.3 | - | OTUD7A, KLF13 |
| 15q24 (BP0-BP1) Deletion/Duplication | 15q24 | - | - |
| 15q24 (BP2-BP3) Deletion | 15q24 | - | SIN3A, COMMD4 |
| 15q25.2 Deletion | 15q25.2 | - | HDGFL3, BNC1 |
| 16p13.3 Deletion (Rubinstein–Taybi)/16p13.3 Duplication | 16p13.3 | CREBBP | CREBBP |
| 16p13.11 Deletion/16p13.11 Duplication | 16p13.11 | - | NDE1 |
| 16p11.2 Distal Deletion/Duplication | 16p11.2 | - | SH2B1 |
| 16p11.2 Deletion/Duplication | 16p11.2 | - | PPP4C, HIRIP3, PAGR1, INO80E |
| 17p13.3 Deletion (Miller–Dieker)/17p13.3 Duplication | 17p13.3 | - | HIC1, SMYD4, MYO1C |
| 17p11.2 Deletion (Smith–Magenis)/17p11.2 Duplication (Potocki–Lupski) | 17p11.2 | RAI1 | ALKBH5, RAI1, PEMT |
| 17q11.2 Deletion/Duplication | 17q11.2 | - | SUZ12 |
| 17q12 Deletion/Duplication | 17q12 | - | HNF1B, TADA2A, AATF, PIGW |
| 17q21.31 Deletion (Koolen–de Vries)/17q21.31 Duplication | 17q21.31 | KANSL1 | KANSL1 |
| 22q11.2 Tetrasomy/Triplication (Cat eye syndrome) | 22q11.2 | - | CECR2, ADA2 |
| 22q11.2 Deletion (DiGeorge/Velocardiofacial)/22q11.2 Duplication | 22q11.2 | - | THAP7, TRMT2A, COMT, HIRA |
| 22q11.2 recurrent region distal type I (D-E/F) Deletion/Duplication | 22q11.2 | - | TOP3B, PPM1F |
| 22q13.3 Deletion (Phelan–McDermid) | 22q13.3 | SHANK3 | BRD1 |
| Xp11.22 Duplication (MRX17) | Xp11.22 | - | HUWE1, HSD17B10, SMC1A |
Taken together, the evidence suggests that CNV-associated genomic disorders may exhibit aberrant DNAm as the result of genes affected in their underlying deletions and duplications, especially when those regions include genes with epigenetic regulatory roles. CNV-associated genomic disorders are therefore strong candidates for episignature discovery. Investigating these syndromes further, including atypical CNVs and gene level variants within the same regions for possible sub-signatures, may uncover novel insights into the pathogenesis of these disorders. These studies may also identify new candidate genes responsible for some of the phenotypic presentation—should sub-signatures be uncovered for specific deleted or duplicated regions—and potentially unlock novel targets for more personalized treatment approaches.
9. Combined Detection of CNVs and DNA Methylation Episignatures in a Single Assay
Recent studies have shown it is possible to detect CNVs by applying computational methods to data obtained from DNAm arrays, such as the Illumina 450K and EPIC Bead Chip arrays [89,90,91]. Many of these pipelines are publicly available in Bioconductor, e.g., ChAMP [91,92], CopyNumber450k [93] and EpiCopy [89] (https://bioconductor.org/packages/, accessed on 19 May 2022). The ability to integrate the detection of genetic and epigenetic findings can provide a more complete view of underlying pathogenic mechanisms.
We applied a similar computational approach using the DNAcopy package (Bioconductor.org) to our PHMDS cohort, and confirmed we could detect breakpoint coordinates similar to those obtained from conventional clinical CMA at the time of original diagnosis [46]; these findings are in line with previous studies [89,90,91,92,93].
Combining these detection methods is not without challenges, most notably in coverage of the genome, as CpG sites are not uniformly distributed throughout the genome and therefore methylation arrays lack the “backbone coverage” observed in high-density SNP arrays. However, it is plausible that, with modifications, a combined array could be developed containing a combination of copy number and CpG targeted probes to produce a clinically targeted array enabling accurate episignature and CNV analysis on a single platform. This has the potential to impact healthcare resource utilization by reducing concurrent testing in NDD patients, and decreasing the need for reflexive testing for disorders such as those associated with imprinting. There would continue to be limitations in the ability to detect low level mosaicism, as seen with existing CNV platforms; however, studies have shown the ability to detect mosaicism from methylation arrays in Kabuki syndrome 1 [94], imprinting disorders [76] and FRX [37].
Additional benefits of a combined testing platform include those to the patient; a combined array would permit screening for more disorders in a single assay, thereby potentially increasing diagnostic yield over that of the current first-tier clinical test (chromosome microarray), and shortening the time spent in the diagnostic odyssey. This approach could concurrently reduce the burden on clinical services and genetic counselling by providing results for CMA, FRX, imprinting and methylation in a single report, leading to a reduction in requisitions and clinic visits. A combined platform would also benefit oncology studies, where limitations in tumor sample availability can often impact research and diagnosis; this would permit the detection of CNVs and methylation status from the same volume of tissue as traditional testing.
10. Conclusions
The identification of episignatures in genomic disorders associated with CNVs could facilitate the expansion of screening capabilities for patients with NDDs, improving the diagnostic yield of clinical testing. This work may also provide novel insights into the pathogenesis of genomic disorders and provide targets for future therapies. The ability to combine CNV and episignature detection into a single assay would reduce the overall cost of testing, increase the number of disorders being screened for, and contribute to the reduction in alternative reflexive and concurrent genetic tests ordered. The benefit to patients and families in reducing wait times and increasing screened disorders, as well as reducing clinician and laboratory burden via fewer clinic visits and fewer genetic tests ordered, would have significant impacts on healthcare resource utilization and the costs associated with the diagnosis of NDDs.
Acknowledgments
The authors would like to thank Haley McConkey for administrative support.
Abbreviations
aCGH, array-based comparative genomic hybridization; ADHD, attention deficit hyperactivity disorder; ASD, autism spectrum disorder; CMA, chromosomal microarray analysis; CNV, copy number variant; DD, developmental delay; DNAm, DNA methylation; DNMT, DNA methyltransferases; FRX, fragile X syndrome; HMS, Hunter–McAlpine syndrome; ID, intellectual disability; LCR, low copy repeats; MLM, machine learning models; NAHR, non-allelic homologous recombination; NCBRS, Nicolaides–Baraitser syndromes; NDD, neurodevelopmental disorder; PHMDS, Phelan–McDermid syndrome; SAM, S-adenosylmethionine; SNV, single nucleotide variants; TBRS, Tatton–Brown–Rahman syndrome; VUS, variant of uncertain significance; WES, whole exome sequencing; WGS, whole genome sequencing; WS, Williams syndrome.
Author Contributions
K.R. and B.S. conceived, performed the literature review and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was funded by the Government of Canada through Genome Canada and the Ontario Genomics Institute (OGI-188).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.López-Rivera J.A., Pérez-Palma E., Symonds J., Lindy A.S., McKnight D.A., Leu C., Zuberi S., Brunklaus A., Møller R., Lal D. A catalogue of new incidence estimates of monogenic neurodevelopmental disorders caused by de novo variants. Brain. 2020;143:1099–1105. doi: 10.1093/brain/awaa051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Institute of Medicine (US) Committee on Nervous System Disorders in Developing Countries . Neurological, Psychiatric, and Developmental Disorders: Meeting the Challenge in the Developing World. National Academies Press; Washington, DC, USA: 2001. [PubMed] [Google Scholar]
- 3.Morris-Rosendahl D.J., Crocq M.-A. Neurodevelopmental disorders—The history and future of a diagnostic concept. Dialogues Clin. Neurosci. 2020;22:65–72. doi: 10.31887/DCNS.2020.22.1/macrocq. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parenti I., Rabaneda L.G., Schoen H., Novarino G. Neurodevelopmental Disorders: From Genetics to Functional Pathways. Trends Neurosci. 2020;43:608–621. doi: 10.1016/j.tins.2020.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Sheridan E., Wright J., Small N., Corry P.C., Oddie S., Whibley C., Petherick E.S., Malik T., Pawson N., McKinney P.A., et al. Risk factors for congenital anomaly in a multiethnic birth cohort: An analysis of the Born in Bradford study. Lancet. 2013;382:1350–1359. doi: 10.1016/S0140-6736(13)61132-0. [DOI] [PubMed] [Google Scholar]
- 6.Pinto D., Delaby E., Merico D., Barbosa M., Merikangas A., Klei L., Thiruvahindrapuram B., Xu X., Ziman R., Wang Z., et al. Convergence of Genes and Cellular Pathways Dysregulated in Autism Spectrum Disorders. Am. J. Hum. Genet. 2014;94:677–694. doi: 10.1016/j.ajhg.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller D.T., Adam M.P., Aradhya S., Biesecker L.G., Brothman A.R., Carter N.P., Church D.M., Crolla J.A., Eichler E.E., Epstein C.J., et al. Consensus Statement: Chromosomal Microarray Is a First-Tier Clinical Diagnostic Test for Individuals with Developmental Disabilities or Congenital Anomalies. Am. J. Hum. Genet. 2010;86:749–764. doi: 10.1016/j.ajhg.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silva M., De Leeuw N., Mann K., Schuring-Blom H., Morgan S., Giardino D., Rack K., Hastings R. European guidelines for constitutional cytogenomic analysis. Eur. J. Hum. Genet. 2019;27:1–16. doi: 10.1038/s41431-018-0244-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaw-Smith C., Redon R., Rickman L., Rio M., Willatt L., Fiegler H., Firth H., Sanlaville D., Winter R., Colleaux L., et al. Microarray based comparative genomic hybridisation (array-CGH) detects submicroscopic chromosomal deletions and duplications in patients with learning disability/mental retardation and dysmorphic features. J. Med. Genet. 2004;41:241–248. doi: 10.1136/jmg.2003.017731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vissers L.E., de Vries B.B., Osoegawa K., Janssen I.M., Feuth T., Choy C.O., Straatman H., van der Vliet W., Huys E.H., van Rijk A., et al. Array-Based Comparative Genomic Hybridization for the Genomewide Detection of Submicroscopic Chromosomal Abnormalities. Am. J. Hum. Genet. 2003;73:1261–1270. doi: 10.1086/379977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haraksingh R.R., Abyzov A., Urban A.E. Comprehensive performance comparison of high-resolution array platforms for genome-wide Copy Number Variation (CNV) analysis in humans. BMC Genom. 2017;18:321. doi: 10.1186/s12864-017-3658-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCarroll S., Kuruvilla F.G., Korn J.M., Cawley S., Nemesh J., Wysoker A., Shapero M.H., Bakker P.I.W.D., Maller J., Kirby A., et al. Integrated detection and population-genetic analysis of SNPs and copy number variation. Nat. Genet. 2008;40:1166–1174. doi: 10.1038/ng.238. [DOI] [PubMed] [Google Scholar]
- 13.Feuk L., Carson A.R., Scherer S. Structural variation in the human genome. Nat. Rev. Genet. 2006;7:85–97. doi: 10.1038/nrg1767. [DOI] [PubMed] [Google Scholar]
- 14.Levy B., Burnside R.D. Are all chromosome microarrays the same? What clinicians need to know. Prenat. Diagn. 2019;39:157–164. doi: 10.1002/pd.5422. [DOI] [PubMed] [Google Scholar]
- 15.D’Amours G., Langlois M., Mathonnet G., Fetni R., Nizard S., Srour M., Tihy F., Phillips M.S., Michaud J.L., Lemyre E. SNP arrays: Comparing diagnostic yields for four platforms in children with developmental delay. BMC Med. Genom. 2014;7:70. doi: 10.1186/s12920-014-0070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y., Haraksingh R., Grubert F., Abyzov A., Gerstein M., Weissman S., Urban A.E. Child Development and Structural Variation in the Human Genome. Child Dev. 2013;84:34–48. doi: 10.1111/cdev.12051. [DOI] [PubMed] [Google Scholar]
- 17.Tuzun E., Sharp A.J., Bailey J.A., Kaul R., Morrison V.A., Pertz L.M., Haugen E., Hayden H.S., Albertson D.G., Pinkel D., et al. Fine-scale structural variation of the human genome. Nat. Genet. 2005;37:727–732. doi: 10.1038/ng1562. [DOI] [PubMed] [Google Scholar]
- 18.Liu J., Zhou Y., Liu S., Song X., Yang X.Z., Fan Y., Chen W., Akdemir Z.C., Yan Z., Zuo Y., et al. The coexistence of copy number variations (CNVs) and single nucleotide polymorphisms (SNPs) at a locus can result in distorted calculations of the significance in associating SNPs to disease. Hum. Genet. 2018;137:553–567. doi: 10.1007/s00439-018-1910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akhtar M.S., Ashino R., Oota H., Ishida H., Niimura Y., Touhara K., Melin A.D., Kawamura S. Genetic variation of olfactory receptor gene family in a Japanese population. Anthr. Sci. 2022:211024. doi: 10.1537/ase.211024. [DOI] [Google Scholar]
- 20.Lee J.A., Lupski J.R. Genomic Rearrangements and Gene Copy-Number Alterations as a Cause of Nervous System Disorders. Neuron. 2006;52:103–121. doi: 10.1016/j.neuron.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 21.Cooper G.M., Coe B.P., Girirajan S., Rosenfeld J.A., Vu T.H., Baker C., Williams C., Stalker H., Hamid R., Hannig V., et al. A Copy Number Variation Morbidity Map of Developmental Delay. Nat. Genet. 2011;43:838–846. doi: 10.1038/ng.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le Gouard N.R., Jacquinet A., Ruaud L., Deleersnyder H., Ageorges F., Gallard J., Lacombe D., Odent S., Mikaty M., Manouvrier-Hanu S., et al. Smith-Magenis syndrome: Clinical and behavioral characteristics in a large retrospective cohort. Clin. Genet. 2021;99:519–528. doi: 10.1111/cge.13906. [DOI] [PubMed] [Google Scholar]
- 23.Eichler E.E. Recent duplication, domain accretion and the dynamic mutation of the human genome. Trends Genet. 2001;17:661–669. doi: 10.1016/S0168-9525(01)02492-1. [DOI] [PubMed] [Google Scholar]
- 24.Sharp A.J., Cheng Z., Eichler E.E. Structural Variation of the Human Genome. Annu. Rev. Genom. Hum. Genet. 2006;7:407–442. doi: 10.1146/annurev.genom.7.080505.115618. [DOI] [PubMed] [Google Scholar]
- 25.Liu P., Lacaria M., Zhang F., Withers M., Hastings P., Lupski J.R. Frequency of Nonallelic Homologous Recombination Is Correlated with Length of Homology: Evidence that Ectopic Synapsis Precedes Ectopic Crossing-Over. Am. J. Hum. Genet. 2011;89:580–588. doi: 10.1016/j.ajhg.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smajlagić D., Lavrichenko K., Berland S., Helgeland Ø., Knudsen G.P., Vaudel M., Haavik J., Knappskog P.M., Njølstad P.R., Houge G., et al. Population prevalence and inheritance pattern of recurrent CNVs associated with neurodevelopmental disorders in 12,252 newborns and their parents. Eur. J. Hum. Genet. 2021;29:205–215. doi: 10.1038/s41431-020-00707-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tatton-Brown K., Douglas J., Coleman K., Baujat G., Chandler K., Clarke A., Collins A., Davies S., Faravelli F., Firth H., et al. Multiple mechanisms are implicated in the generation of 5q35 microdeletions in Sotos syndrome. J. Med. Genet. 2005;42:307–313. doi: 10.1136/jmg.2004.027755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldenberg P. An Update on Common Chromosome Microdeletion and Microduplication Syndromes. Pediatr. Ann. 2018;47:e198–e203. doi: 10.3928/19382359-20180419-01. [DOI] [PubMed] [Google Scholar]
- 29.Bernardini L., Alesi V., Loddo S., Novelli A., Bottillo I., Battaglia A., Digilio M.C., Zampino G., Ertel A., Fortina P., et al. High-resolution SNP arrays in mental retardation diagnostics: How much do we gain? Eur. J. Hum. Genet. 2010;18:178–185. doi: 10.1038/ejhg.2009.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Ligt J., Willemsen M.H., Van Bon B.W., Kleefstra T., Yntema H.G., Kroes T., Vulto-van Silfhout A.T., Koolen D.A., De Vries P., Gilissen C., et al. Diagnostic Exome Sequencing in Persons with Severe Intellectual Disability. N. Engl. J. Med. 2012;367:1921–1929. doi: 10.1056/NEJMoa1206524. [DOI] [PubMed] [Google Scholar]
- 31.Lemke J.R., Riesch E., Scheurenbrand T., Schubach M., Wilhelm C., Steiner I., Hansen J., Courage C., Gallati S., Bürki S., et al. Targeted next generation sequencing as a diagnostic tool in epileptic disorders. Epilepsia. 2012;53:1387–1398. doi: 10.1111/j.1528-1167.2012.03516.x. [DOI] [PubMed] [Google Scholar]
- 32.Schwarze K., Buchanan J., Taylor J.C., Wordsworth S. Are whole-exome and whole-genome sequencing approaches cost-effective? A systematic review of the literature. Genet. Med. 2018;20:1122–1130. doi: 10.1038/gim.2017.247. [DOI] [PubMed] [Google Scholar]
- 33.Sikkema-Raddatz B., Johansson L.F., de Boer E.N., Almomani R., Boven L.G., van den Berg M.P., van Spaendonck-Zwarts K.Y., van Tintelen J.P., Sijmons R.H., Jongbloed J.D.H., et al. Targeted Next-Generation Sequencing can Replace Sanger Sequencing in Clinical Diagnostics. Hum. Mutat. 2013;34:1035–1042. doi: 10.1002/humu.22332. [DOI] [PubMed] [Google Scholar]
- 34.Fraiman Y.S., Wojcik M.H. The influence of social determinants of health on the genetic diagnostic odyssey: Who remains undiagnosed, why, and to what effect? Pediatr. Res. 2021;89:295–300. doi: 10.1038/s41390-020-01151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michaels-Igbokwe C., McInnes B., MacDonald K.V., Currie G.R., Omar F., Shewchuk B., Bernier F.P., Marshall D.A. (Un)standardized testing: The diagnostic odyssey of children with rare genetic disorders in Alberta, Canada. Genet. Med. 2021;23:272–279. doi: 10.1038/s41436-020-00975-0. [DOI] [PubMed] [Google Scholar]
- 36.Thevenon J., Duffourd Y., Masurel-Paulet A., Lefebvre M., Feillet F., El Chehadeh-Djebbar S., St-Onge J., Steinmetz A., Huet F., Chouchane M., et al. Diagnostic odyssey in severe neurodevelopmental disorders: Toward clinical whole-exome sequencing as a first-line diagnostic test. Clin. Genet. 2016;89:700–707. doi: 10.1111/cge.12732. [DOI] [PubMed] [Google Scholar]
- 37.Schenkel L.C., Schwartz C., Skinner C., Rodenhiser D.I., Ainsworth P.J., Pare G., Sadikovic B. Clinical Validation of Fragile X Syndrome Screening by DNA Methylation Array. J. Mol. Diagn. 2016;18:834–841. doi: 10.1016/j.jmoldx.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 38.Coffee B., Ikeda M., Budimirovic D.B., Hjelm L.N., Kaufmann W.E., Warren S.T. Mosaic FMR1 Deletion Causes Fragile X Syndrome and Can Lead to Molecular Misdiagnosis. Am. J. Med. Genet. Part A. 2008;146A:1358–1367. doi: 10.1002/ajmg.a.32261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Nimwegen K., Schieving J., Willemsen M., Veltman J., van der Burg S., van der Wilt G., Grutters J. The diagnostic pathway in complex paediatric neurology: A cost analysis. Eur. J. Paediatr. Neurol. 2015;19:233–239. doi: 10.1016/j.ejpn.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 40.Copeland H., Kivuva E., Firth H.V., Wright C.F. Systematic assessment of outcomes following a genetic diagnosis identified through a large-scale research study into developmental disorders. Genet. Med. 2021;23:1058–1064. doi: 10.1038/s41436-021-01110-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kleinendorst L., Heuvel L.M.V.D., Henneman L., Van Haelst M.M. Who ever heard of 16p11.2 deletion syndrome? Parents’ perspectives on a susceptibility copy number variation syndrome. Eur. J. Hum. Genet. 2020;28:1196–1204. doi: 10.1038/s41431-020-0644-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Savatt J.M., Myers S.M. Genetic Testing in Neurodevelopmental Disorders. Front. Pediatr. 2021;9:526779. doi: 10.3389/fped.2021.526779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levy M.A., McConkey H., Kerkhof J., Barat-Houari M., Bargiacchi S., Biamino E., Bralo M.P., Cappuccio G., Ciolfi A., Clarke A., et al. Novel diagnostic DNA methylation episignatures expand and refine the epigenetic landscapes of Mendelian disorders. Hum. Genet. Genom. Adv. 2021;3:100075. doi: 10.1016/j.xhgg.2021.100075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aref-Eshghi E., Kerkhof J., Pedro V.P., Barat-Houari M., Ruiz-Pallares N., Andrau J.-C., Lacombe D., Van-Gils J., Fergelot P., Dubourg C., et al. Evaluation of DNA Methylation Episignatures for Diagnosis and Phenotype Correlations in 42 Mendelian Neurodevelopmental Disorders. Am. J. Hum. Genet. 2020;106:356–370. doi: 10.1016/j.ajhg.2020.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sadikovic B., Levy M.A., Kerkhof J., Aref-Eshghi E., Schenkel L., Stuart A., McConkey H., Henneman P., Venema A., Schwartz C.E., et al. Clinical epigenomics: Genome-wide DNA methylation analysis for the diagnosis of Mendelian disorders. Genet. Med. 2021;23:1065–1074. doi: 10.1038/s41436-020-01096-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schenkel L.C., Aref-Eshghi E., Rooney K., Kerkhof J., Levy M.A., McConkey H., Rogers R.C., Phelan K., Sarasua S.M., Jain L., et al. DNA methylation epi-signature is associated with two molecularly and phenotypically distinct clinical subtypes of Phelan-McDermid syndrome. Clin. Epigenetics. 2021;13:2. doi: 10.1186/s13148-020-00990-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berger S.L., Kouzarides T., Shiekhattar R., Shilatifard A. An operational definition of epigenetics. Genes Dev. 2009;23:781–783. doi: 10.1101/gad.1787609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deans C., Maggert K.A. What Do You Mean, “Epigenetic”? Genetics. 2015;199:887–896. doi: 10.1534/genetics.114.173492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gibney E.R., Nolan C.M. Epigenetics and gene expression. Heredity. 2010;105:4–13. doi: 10.1038/hdy.2010.54. [DOI] [PubMed] [Google Scholar]
- 50.Li E., Zhang Y. DNA Methylation in Mammals. Cold Spring Harb. Perspect. Biol. 2014;6:a019133. doi: 10.1101/cshperspect.a019133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miller J.L., Grant P.A. The Role of DNA Methylation and Histone Modifications in Transcriptional Regulation in Humans. In: Kundu T.K., editor. Epigenetics: Development and Disease. Springer; Dordrecht, The Netherlands: 2013. pp. 289–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jones P.A. Functions of DNA methylation: Islands, start sites, gene bodies and beyond. Nat. Rev. Genet. 2012;13:484–492. doi: 10.1038/nrg3230. [DOI] [PubMed] [Google Scholar]
- 53.Aref-Eshghi E., Schenkel L.C., Lin H., Skinner C., Ainsworth P., Paré G., Rodenhiser D., Schwartz C., Sadikovic B. The defining DNA methylation signature of Kabuki syndrome enables functional assessment of genetic variants of unknown clinical significance. Epigenetics. 2017;12:923–933. doi: 10.1080/15592294.2017.1381807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schenkel L.C., Aref-Eshghi E., Skinner C., Ainsworth P., Lin H., Paré G., Rodenhiser D.I., Schwartz C., Sadikovic B. Peripheral blood epi-signature of Claes-Jensen syndrome enables sensitive and specific identification of patients and healthy carriers with pathogenic mutations in KDM5C. Clin. Epigenetics. 2018;10:21. doi: 10.1186/s13148-018-0453-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kernohan K.D., Schenkel L.C., Huang L., Smith A., Pare G., Ainsworth P., Care4Rare Canada Consortium. Boycott K.M., Warman-Chardon J., Sadikovic B. Identification of a methylation profile for DNMT1-associated autosomal dominant cerebellar ataxia, deafness, and narcolepsy. Clin. Epigenetics. 2016;8:91. doi: 10.1186/s13148-016-0254-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klein C.J., Bird T., Ertekin-Taner N., Lincoln S., Hjorth R., Wu Y., Kwok J., Mer G., Dyck P.J., Nicholson G.A. DNMT1 mutation hot spot causes varied phenotypes of HSAN1 with dementia and hearing loss Background: Mutations in DNA methyltransferase 1 (DNMT1) have been identified in 2 autosomal. Neurology. 2013;80:824–828. doi: 10.1212/WNL.0b013e318284076d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Klein C.J., Botuyan M., Wu Y., Ward C.J., Nicholson G.A., Hammans S., Hojo K., Yamanishi H., Adam R., Wallace D.C., et al. Mutations in DNMT1 cause hereditary sensory neuropathy with dementia and hearing loss. Nat. Genet. 2011;43:595–600. doi: 10.1038/ng.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tatton-Brown K., Childhood Overgrowth Consortium. Seal S., Ruark E., Harmer J., Ramsay E., Duarte S.D.V., Zachariou A., Hanks S., O’Brien E., et al. Mutations in the DNA methyltransferase gene DNMT3A cause an overgrowth syndrome with intellectual disability. Nat. Genet. 2014;46:385–388. doi: 10.1038/ng.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jin B., Tao Q., Peng J., Soo H.M., Wu W., Ying J., Fields C.R., Delmas A.I., Liu X., Qiu J., et al. DNA methyltransferase 3B (DNMT3B) mutations in ICF syndrome lead to altered epigenetic modifications and aberrant expression of genes regulating development, neurogenesis and immune function. Hum. Mol. Genet. 2008;17:690–709. doi: 10.1093/hmg/ddm341. [DOI] [PubMed] [Google Scholar]
- 60.Levy M.A., Beck D.B., Metcalfe K., Douzgou S., Sithambaram S., Cottrell T., Ansar M., Kerkhof J., Mignot C., Nougues M.-C., et al. Deficiency of TET3 leads to a genome-wide DNA hypermethylation episignature in human whole blood. NPJ Genom. Med. 2021;6:92. doi: 10.1038/s41525-021-00256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beck D.B., Petracovici A., He C., Moore H.W., Louie R.J., Ansar M., Douzgou S., Sithambaram S., Cottrell T., Santos-Cortez R.L.P., et al. Delineation of a Human Mendelian Disorder of the DNA Demethylation Machinery: TET3 Deficiency. Am. J. Hum. Genet. 2020;106:234–245. doi: 10.1016/j.ajhg.2019.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hood R.L., Schenkel L.C., Nikkel S.M., Ainsworth P.J., Pare G., Boycott K.M., Bulman D.E., Sadikovic B. The defining DNA methylation signature of Floating-Harbor Syndrome. Sci. Rep. 2016;6:38803. doi: 10.1038/srep38803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schenkel L.C., Kernohan K.D., McBride A., Reina D., Hodge A., Ainsworth P.J., Rodenhiser D.I., Pare G., Bérubé N.G., Skinner C., et al. Identification of epigenetic signature associated with alpha thalassemia/mental retardation X-linked syndrome. Epigenet. Chromatin. 2017;10:10. doi: 10.1186/s13072-017-0118-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aref-Eshghi E., Bend E.G., Hood R.L., Schenkel L.C., Carere D.A., Chakrabarti R., Nagamani S.C.S., Cheung S.W., Campeau P.M., Prasad C., et al. BAFopathies’ DNA methylation epi-signatures demonstrate diagnostic utility and functional continuum of Coffin–Siris and Nicolaides–Baraitser syndromes. Nat. Commun. 2018;9:4885. doi: 10.1038/s41467-018-07193-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wieczorek D., Bögershausen N., Beleggia F., Steiner-Haldenstätt S., Pohl E., Li Y., Milz E., Martin M., Thiele H., Altmüller J., et al. A comprehensive molecular study on Coffin–Siris and Nicolaides–Baraitser syndromes identifies a broad molecular and clinical spectrum converging on altered chromatin remodeling. Hum. Mol. Genet. 2013;22:5121–5135. doi: 10.1093/hmg/ddt366. [DOI] [PubMed] [Google Scholar]
- 66.Cappuccio G., Sayou C., Tanno P.L., Tisserant E., Bruel A.L., Kennani S.E., Sá J., Low K.J., Dias C., Havlovicová M., et al. De novo SMARCA2 variants clustered outside the helicase domain cause a new recognizable syndrome with intellectual disability and blepharophimosis distinct from Nicolaides–Baraitser syndrome. Genet. Med. 2020;22:1838–1850. doi: 10.1038/s41436-020-0898-y. [DOI] [PubMed] [Google Scholar]
- 67.Bend E.G., Aref-Eshghi E., Everman D.B., Rogers R.C., Cathey S.S., Prijoles E.J., Lyons M.J., Davis H., Clarkson K., Gripp K.W., et al. Gene domain-specific DNA methylation episignatures highlight distinct molecular entities of ADNP syndrome. Clin. Epigenet. 2019;11:64. doi: 10.1186/s13148-019-0658-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Breen M.S., Garg P., Tang L., Mendonca D., Levy T., Barbosa M., Arnett A.B., Kurtz-Nelson E., Agolini E., Battaglia A., et al. Episignatures Stratifying Helsmoortel-Van Der Aa Syndrome Show Modest Correlation with Phenotype. Am. J. Hum. Genet. 2020;107:555–563. doi: 10.1016/j.ajhg.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tolmacheva E.N., Kashevarova A.A., Nazarenko L.P., Minaycheva L.I., Skryabin N.A., Lopatkina M.E., Nikitina T.V., Sazhenova E.A., Belyaeva E.O., Fonova E.A., et al. Delineation of Clinical Manifestations of the Inherited Xq24 Microdeletion Segregating with sXCI in Mothers: Two Novel Cases with Distinct Phenotypes Ranging from UBE2A Deficiency Syndrome to Recurrent Pregnancy Loss. Cytogenet. Genome Res. 2020;160:245–254. doi: 10.1159/000508050. [DOI] [PubMed] [Google Scholar]
- 70.Wojcik F., Dann G.P., Beh L.Y., Debelouchina G.T., Hofmann R., Muir T.W. Functional crosstalk between histone H2B ubiquitylation and H2A modifications and variants. Nat. Commun. 2018;9:1394. doi: 10.1038/s41467-018-03895-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Selmi C., Feghali-Bostwick C.A., Lleo A., Lombardi S.A., De Santis M., Cavaciocchi F., Zammataro L., Mitchell M.M., LaSalle J.M., Medsger T., et al. X chromosome gene methylation in peripheral lymphocytes from monozygotic twins discordant for scleroderma. Clin. Exp. Immunol. 2012;169:253–262. doi: 10.1111/j.1365-2249.2012.04621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Aref-Eshghi E., Bend E.G., Colaiacovo S., Caudle M., Chakrabarti R., Napier M., Brick L., Brady L., Carere D.A., Levy M.A., et al. Diagnostic Utility of Genome-wide DNA Methylation Testing in Genetically Unsolved Individuals with Suspected Hereditary Conditions. Am. J. Hum. Genet. 2019;104:685–700. doi: 10.1016/j.ajhg.2019.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alisch R.S., Barwick B.G., Chopra P., Myrick L.K., Satten G.A., Conneely K.N., Warren S.T. Age-associated DNA methylation in pediatric populations. Genome Res. 2012;22:623–632. doi: 10.1101/gr.125187.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Houseman E.A., Accomando W.P., Koestler D.C., Christensen B.C., Marsit C.J., Nelson H.H., Wiencke J.K., Kelsey K.T. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinform. 2012;13:86. doi: 10.1186/1471-2105-13-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jaffe A., Murakami P., Lee H., Leek J., Fallin M.D., Feinberg A., Irizarry R.A. Bump hunting to identify differentially methylated regions in epigenetic epidemiology studies. Int. J. Epidemiol. 2012;41:200–209. doi: 10.1093/ije/dyr238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aref-Eshghi E., Schenkel L.C., Lin H., Skinner C., Ainsworth P., Paré G., Siu V., Rodenhiser D., Schwartz C., Sadikovic B. Clinical Validation of a Genome-Wide DNA Methylation Assay for Molecular Diagnosis of Imprinting Disorders. J. Mol. Diagn. 2017;19:848–856. doi: 10.1016/j.jmoldx.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 77.Aref-Eshghi E., Rodenhiser D.I., Schenkel L.C., Lin H., Skinner C., Ainsworth P., Paré G., Hood R.L., Bulman D.E., Kernohan K.D., et al. Genomic DNA Methylation Signatures Enable Concurrent Diagnosis and Clinical Genetic Variant Classification in Neurodevelopmental Syndromes. Am. J. Hum. Genet. 2018;102:156–174. doi: 10.1016/j.ajhg.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Krzyzewska I.M., Maas S.M., Henneman P., Lip K.V.D., Venema A., Baranano K., Chassevent A., Aref-Eshghi E., Van Essen A.J., Fukuda T., et al. A genome-wide DNA methylation signature for SETD1B-related syndrome. Clin. Epigenet. 2019;11:156. doi: 10.1186/s13148-019-0749-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.De Rubeis S., Siper P.M., Durkin A., Weissman J., Muratet F., Halpern D., Trelles M.D.P., Frank Y., Lozano R., Wang A.T., et al. Delineation of the genetic and clinical spectrum of Phelan-McDermid syndrome caused by SHANK3 point mutations. Mol. Autism. 2018;9:31. doi: 10.1186/s13229-018-0205-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sarasua S.M., Dwivedi A., Boccuto L., Chen C.-F., Sharp J.L., Rollins J.D., Collins J.S., Rogers R.C., Phelan K., DuPont B.R. 22q13.2q13.32 genomic regions associated with severity of speech delay, developmental delay, and physical features in Phelan–McDermid syndrome. Genet. Med. 2014;16:318–328. doi: 10.1038/gim.2013.144. [DOI] [PubMed] [Google Scholar]
- 81.Wilson H.L., Crolla J.A., Walker D., Artifoni L., Dallapiccola B., Takano T., Vasudevan P., Huang S., Maloney V., Yobb T., et al. Interstitial 22q13 deletions: Genes other than SHANK3 have major effects on cognitive and language development. Eur. J. Hum. Genet. 2008;16:1301–1310. doi: 10.1038/ejhg.2008.107. [DOI] [PubMed] [Google Scholar]
- 82.Kent W.J., Sugnet C.W., Furey T.S., Roskin K.M., Pringle T.H., Zahler A.M., Haussler D. The Human Genome Browser at UCSC. Genome Res. 2002;12:996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Strong E., Butcher D.T., Singhania R., Mervis C.B., Morris C.A., De Carvalho D., Weksberg R., Osborne L.R. Symmetrical Dose-Dependent DNA-Methylation Profiles in Children with Deletion or Duplication of 7q11.23. Am. J. Hum. Genet. 2015;97:216–227. doi: 10.1016/j.ajhg.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Siu M.T., Butcher D.T., Turinsky A.L., Cytrynbaum C., Stavropoulos D.J., Walker S., Caluseriu O., Carter M., Lou Y., Nicolson R., et al. Functional DNA methylation signatures for autism spectrum disorder genomic risk loci: 16p11.2 deletions and CHD8 variants. Clin. Epigenetics. 2019;11:103. doi: 10.1186/s13148-019-0684-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rooney K., Levy M.A., Haghshenas S., Kerkhof J., Rogaia D., Tedesco M.G., Imperatore V., Mencarelli A., Squeo G.M., Di Venere E., et al. Identification of a DNA Methylation Episignature in the 22q11.2 Deletion Syndrome. Int. J. Mol. Sci. 2021;22:8611. doi: 10.3390/ijms22168611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Van Gils J., Magdinier F., Fergelot P., Lacombe D. Rubinstein-Taybi Syndrome: A Model of Epigenetic Disorder. Genes. 2021;12:968. doi: 10.3390/genes12070968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Choufani S., Cytrynbaum C., Chung B.H.Y., Turinsky A.L., Grafodatskaya D., Chen Y.A., Cohen A.S.A., Dupuis L., Butcher D.T., Siu M.T., et al. NSD1 mutations generate a genome-wide DNA methylation signature. Nat. Commun. 2015;6:10207. doi: 10.1038/ncomms10207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Singh H.R., Nardozza A.P., Möller I.R., Knobloch G., Kistemaker H.A.V., Hassler M., Harrer N., Blessing C., Eustermann S., Kotthoff C., et al. A Poly-ADP-Ribose Trigger Releases the Auto-Inhibition of a Chromatin Remodeling Oncogene. Mol. Cell. 2017;68:860–871. doi: 10.1016/j.molcel.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 89.Cho S., Kim H.-S., Zeiger M.A., Umbricht C.B., Cope L.M. Measuring DNA Copy Number Variation Using High-Density Methylation Microarrays. J. Comput. Biol. 2019;26:295–304. doi: 10.1089/cmb.2018.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Feber A., Guilhamon P., Lechner M., Fenton T., Wilson G.A., Thirlwell C., Morris T.J., Flanagan A.M., Teschendorff A.E., Kelly J.D., et al. Using high-density DNA methylation arrays to profile copy number alterations. Genome Biol. 2014;15:R30. doi: 10.1186/gb-2014-15-2-r30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tian Y., Morris T.J., Webster A.P., Yang Z., Beck S., Feber A., Teschendorff A.E. ChAMP: Updated methylation analysis pipeline for Illumina BeadChips. Bioinformatics. 2017;33:3982–3984. doi: 10.1093/bioinformatics/btx513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Morris T.J., Butcher L.M., Feber A., Teschendorff A.E., Chakravarthy A.R., Wojdacz T.K., Beck S. ChAMP: 450k Chip Analysis Methylation Pipeline. Bioinformatics. 2014;30:428–430. doi: 10.1093/bioinformatics/btt684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Papillon-Cavanagh S., Fortin J.-P., De Jay N. CopyNumber 450k: An R Package for CNV Inference Using Illumina 450k DNA Methylation Assay. 2013. [(accessed on 15 May 2022)]. Available online: http://rdrr.io/github/spapillon/CopyNumber450k/
- 94.Montano C., Britton J.F., Harris J.R., Kerkhof J., Barnes B.T., Lee J.A., Sadikovic B., Sobreira N., Fahrner J.A. Genome-wide DNA methylation profiling confirms a case of low-level mosaic Kabuki syndrome 1. Am. J. Med. Genet. Part A. 2022;188:2217–2225. doi: 10.1002/ajmg.a.62754. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.