Abstract
Several moderately halophilic gram-positive, spore-forming bacteria have been isolated by conventional enrichment cultures from damaged medieval wall paintings and building materials. Enrichment and isolation were monitored by denaturing gradient gel electrophoresis and fluorescent in situ hybridization. 16S ribosomal DNA analysis showed that the bacteria are most closely related to Halobacillus litoralis. DNA-DNA reassociation experiments identified the isolates as a population of hitherto unknown Halobacillus species.
Recently, a number of investigations based on molecular techniques (9, 22, 24, 25) as well as on cultivation techniques (10, 16, 17, 27) have demonstrated that objects of art and building materials may be habitats for extremely salt tolerant and moderately halophilic bacteria. In fact, this group of microorganisms has been detected by molecular means on the mural paintings from the 14th century located in the Catherine chapel of the castle of Herberstein (Styria, Austria) (24, 25), the subject of this study. The aim of this work was the selective isolation of halophilic bacteria from wall paintings and building materials.
Two samples were collected from different areas delimited in squares of around 20 cm2 from the wall paintings of the chapel of the castle. Sample H1 was taken below the chancel's east wall window, where a brownish biofilm was observed; sample H6 was taken from the chancel's north wall from a zone of the painting with an intense rosy discoloration. An additional sample (m7) was taken on the outside of the chapel from a lime wall also showing an intense rosy discoloration. All samples were collected with a sterile scalpel by scraping off surface material and plaster to a depth of 1 to 3 mm. Small amounts of samples were used immediately for cultivation.
Enrichment cultures were set up in 300-ml Erlenmeyer flasks containing 30 ml of M2 medium (20% [wt/vol] NaCl) (34) and maintenance medium (MM) medium (10% [wt/vol] NaCl) (31). To avoid fungal growth, media were supplemented with 50 μg of cycloheximide (Sigma) ml−1. All enrichments were incubated aerobically at room temperature (22 ± 3°C) and at 37oC in a water bath using magnetic stirring. Aliquots of the same enrichment cultures were used for denaturing gradient gel electrophoresis (DGGE) analysis, whole-cell hybridization, and isolation of bacteria by plating as described below.
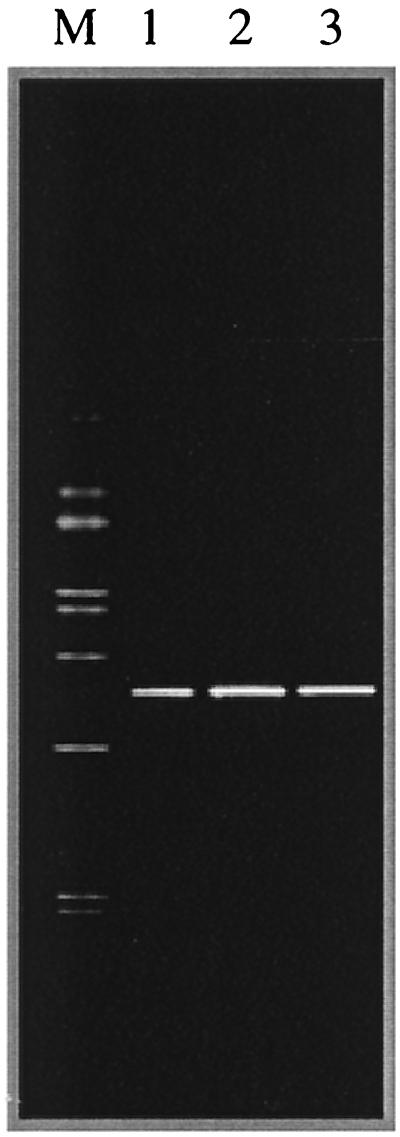
Genomic DNA from enrichment cultures was extracted according to the protocol described by Ausubel et al. (1). Amplification of 16S ribosomal DNA (rDNA) using specific archaeal primers (22) did not give any positive results, indicating that no Archaea were present in the enrichment cultures. However, 16S rDNA amplification using specific eubacterial primers (29) gave positive results. For DGGE analysis, 200-bp fragments of the 16S rDNA were amplified using the forward primer 341f GC (19) and the reverse primer 518r (19). PCR and DGGE were performed as described by Schabereiter-Gurtner et al. (29) and Muyzer et al. (19), respectively. DGGE profiles revealed only one band, demonstrating the low microbial diversity present in the enrichments (Fig. 1).
FIG. 1.
Enrichments of wall painting sample H6 monitored by DGGE analysis using eubacterium-specific primers. Lanes: 1, enrichment from sample H6 on M2 medium incubated at 30°C; 2, enrichment from sample H6 on M2 medium incubated at 37°C; 3, H. litoralis DSM 10405T; M, marker (to allow gel-to-gel comparison of different samples). The marker containing 16S rDNA PCR products derived from 10 different bacterial species was designed as previously described by Schabereiter-Gurtner et al. (29).
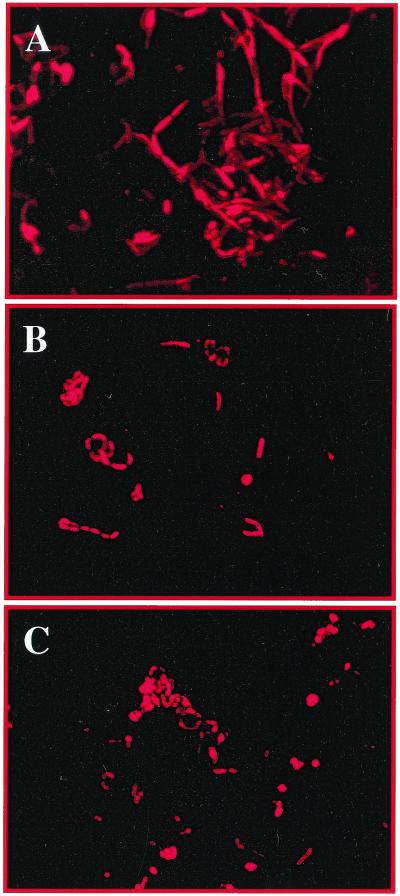
Enrichment samples were fixed and hybridized with the eubacterial probe EUB338 (32) as described before (23). All samples showed a high green autofluorescence probably due to organic-inorganic autofluorescent particles or to cellular interference, as has been reported by other authors (20). However, this problem was overcome by hybridizing bacteria with the probe EUB338 labeled with Cy5 (EUB338-Cy5), which fluoresces in the far red range. No signal was detected without the use of the specific dye, indicating no false results due to autofluorescence using this fluorochrome (data not shown). Figure 2 shows the whole-cell hybridization experiment performed with enrichment cultures of sample H6 in M2 and MM media. A change in cellular morphology of the enrichment cultures was observed at different salt concentrations. In both enrichment media long filaments giving specific hybridization with the eubacterial probe EUB338 were observed. However, the average length of the filaments was greater in 20% NaCl (Fig. 2A) than in 10% NaCl (Fig. 2B and C). Cell discrimination directly on the original sample, H6, using the eubacterial probe EUB338-Cy5 was attempted. However, it was not possible to discriminate the cells in situ due to the high red autofluorescence of the material. Additionally, it could be possible that due to a slow growth activity of the cells in the sample material their ribosomal contents are too low to allow detection using fluorescent in situ hybridization (FISH).
FIG. 2.
Enrichments of wall painting sample H6 monitored by FISH analysis using the specific eubacterium probe EUB338 labeled with the Cy5 reactive fluorescent dye (32). Samples were examined with an epifluorescence microscope (Axioplan; Zeiss) equipped with a 100-W mercury lamp and filter sets for visualization of the fluorescent dyes fluorescein isothiocyanate (green), CY3 (red), and CY5 (far red). For capturing digitized images a slow-scan charge-coupled device camera was used. (A and B) Monitoring of the bacterial growth on M2 (20% NaCl) (A) and MM (10% NaCl) (B) media, using the probe EUB338-Cy5. (C) Halobacillus strain K3-1 isolated from sample H6 growing in MM (10% NaCl) medium, detected by in situ hybridization using the probe EUB338-Cy5.
DGGE and FISH analyses are known to be useful tools for monitoring the microbial composition in enrichment cultures as well as for isolation of pure cultures from cocultures (12, 15, 28, 33).
For the isolation of detected microorganisms, 100-μl aliquots of enrichment cultures were plated onto solid versions of M2 and MM medium as well as on M2A medium (6). All media were incubated aerobically at room temperature and 37°C. After 3 weeks, four brick red-pigmented colonies (K3, K5, U5, and U6) were isolated from enrichment cultures of sample H6. Two different colony types were observed: small, circular colonies with an intense coloration in the center and larger colonies which were less intensely pigmented. The two colony types were separated, and pure cultures were designated K3-1, K3-2, K5-1, K5-2, U5-1, U5-2, U6-1, and U6-2. In addition, we isolated from samples H1 and m7 two yellow-to-brown-pigmented bacterial strains, I3 and I7, respectively. DGGE analysis was performed with all isolates, and the migrations of the DGGE bands were compared. The positions of the DGGE bands were identical for all isolates to the positions of DGGE bands obtained from the same enrichment cultures shown in Fig. 1 (data not shown). These results indicate that the isolated strains are phylogenetically related despite their different colony morphologies.
All isolated strains were able to grow in M2A or MM medium in the presence of 5 to 20% (wt/vol) NaCl. Optimum growth occurred in media containing 5 to 10% NaCl; no growth occurred in the absence of NaCl. In the standard bacteriological tests (30) all of the strains were catalase and oxidase positive and did not grow anaerobically, as tested by using an atmospheric generation system (Anaerogen; Oxoid). Cells were gram positive and rod shaped, and in stationary cultures single endospores were observed in the cells by phase-contrast microscopy (Leitz Diaplan). One strain of the eight red isolates (strain K3-1) was selected from the H6 enrichment culture, as well as strains I3 and I7 from H1 and m7 enrichment cultures, respectively, for further phylogenetic investigations.
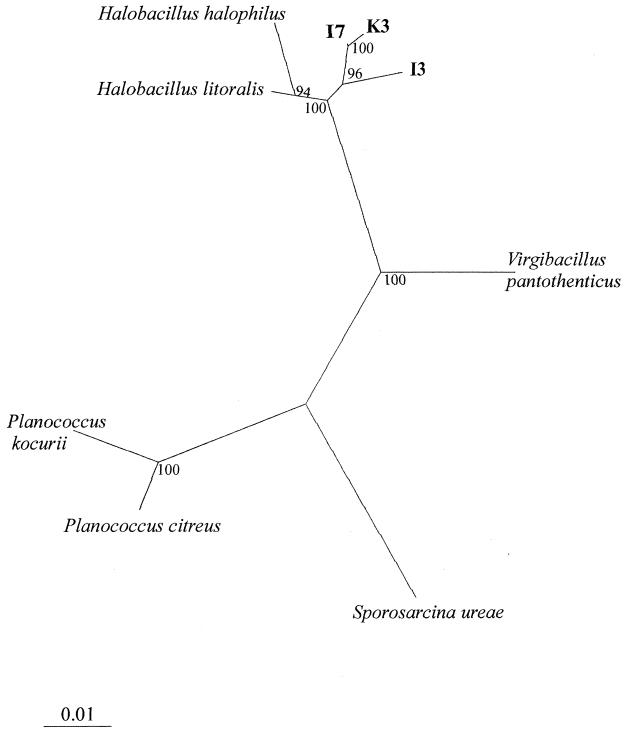
For sequence analysis, 16S rDNA fragments were amplified according to the method of Lane (18). Individual 16S rDNA fragments amplified from isolates K3-1, I3, and I7 (1,425 bp, 1,326 bp, and 1,322 bp, respectively) were purified and sequenced as previously described (29). Sequences were compared with sequences of known microorganisms using the EMBL nucleotide sequence database. The FASTA search option (21) for the EMBL database was used to search for close evolutionary relatives. Comparison of 16S rDNA sequences showed that the new strains are most closely related to the genus Halobacillus (97.1 to 98.4% similarity). To verify their phylogenetic positions, evolutionary distances were calculated and a phylogenetic tree was constructed (Fig. 3). The sequence alignment was corrected manually and end gaps on the 5′ and 3′ ends, as well as positions of ambiguous base pairs, were omitted from the analysis. Therefore, a total of 1,298 bases were compared. Pairwise evolutionary similarities and distances (14) were computed by using the DNADIST program in the phylogeny inference package PHYLIP (version 3.57c, University of Washington, Seattle). The phylogenetic tree was constructed by using the neighbor-joining method of Saitou and Nei (26). To determine the statistical significance of branching patterns, a bootstrap analysis (1,000 replicates) was performed using the SEQBOOT program; subsequently a consensus tree was produced by using the CONSENSE program.
FIG. 3.
Unrooted phylogenetic tree showing the relationship of isolates K3-1, I3, and I7 to related genera based on a comparison of 16S rDNA sequences. 16S rDNA sequences used in this study for phylogenetic analysis are as follows: isolate K3-1, AJ291752; isolate I3, AJ291753; isolate I7, AJ291754; Sporosarcina ureae DSM 2281T, AF202057; Halobacillus litoralis SL-4T, X94558; Halobacillus halophilus NCIMB 2269T, X62174; Virgibacillus pantothenticus IAM 11061T, D16275; Planococcus citreus NCIMB 1493T, X62172; and Planococcus kocurii NCIMB 629T, X62173. The tree was derived from a distance matrix based on a selection of 16S rRNA sequences of moderately halophilic and nonhalophilic gram-positive bacteria with low DNA G+C contents. Scale bar, 1% estimated sequence divergence.
The high sequence similarity clearly indicates that the isolates are closely related phylogenetically but does not allow single-species differentiation (8). Therefore, a DNA-DNA hybridization experiment was performed by the Identification Service of the Deutsche Sammlung von Mikroorganismen und Zellkulturen, Braunschweig, Germany. Genomic DNA from strains K3-1, I3, I7, and Halobacillus litoralis DSM 10405T was isolated by hydroxyapatite chromatography using the procedure of Cashion et al. (3). DNA-DNA hybridizations were determined spectrophotometrically, as described by De Ley et al. (5) with modifications (7, 11), using a Gilford System model 2600 spectrophotometer equipped with a Gilford model 2527-R thermoprogrammer and plotter. Renaturation rates were computed with the TRANSFER.BAS program (13). The levels of DNA relatedness were 23.5% between K3-1 and DSM 10405T, 23.7% between I3 and DSMT, and 25.0% between I7 and DSM 10405T. The DNA homology levels between the wall paintings and building material isolates were 42.7% between K3-1 and I3, 29.7% between I3 and I7, and 83.4% between K3-1 and I7. The level of DNA relatedness found between strains K3-1 and I7 clearly suggests that the two organisms are highly related genotypically. DNA reassociation values obtained with Halobacillus litoralis DSM10405T were otherwise clearly below the usual standard criterion of approximately 70%, which supports the idea that the new isolates represent two distinct taxa (species) within the genus Halobacillus.
Halobacillus species have been previously isolated from hypersaline sediments, Antarctic sea ice, and fermented foods (2, 4, 31). To our knowledge this is the first report on the isolation of Halobacillus species from historic buildings and monuments.
Acknowledgments
This work is part of the European Union-funded project “Novel molecular tools for the analysis of unknown microbial communities of mural paintings and their implementation into the conservation/restoration practise” (EU-ENV4-CT98-0705). The work of G.P. was supported by a European Community Marie Curie Training Grant (Contract BIO4-CT98-5057).
We thank Søren Molin for permission to use the facilities at the Technical University of Denmark (Lyngby).
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley and Sons, Inc.; 1991. [Google Scholar]
- 2.Bowman J P, McCammon S A, Brown M V, Nichols D S, McMeekin T A. Diversity and association of psychrophilic bacteria in Antarctic sea ice. Appl Environ Microbiol. 1997;63:3068–3078. doi: 10.1128/aem.63.8.3068-3078.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cashion P, Hodler-Franklin M A, McCully J, Franklin M. A rapid method for base ratio determination of bacterial DNA. Anal Biochem. 1977;81:461–466. doi: 10.1016/0003-2697(77)90720-5. [DOI] [PubMed] [Google Scholar]
- 4.Chaiyanan S, Chaiyanan S, Maugel T, Huq A, Robb F T, Colwell R R. Polyphasic taxonomy of a novel Halobacillus, Halobacillus thailandesis sp. nov. isolated from fish sauce. Syst Appl Microbiol. 1999;22:360–365. doi: 10.1016/S0723-2020(99)80043-5. [DOI] [PubMed] [Google Scholar]
- 5.De Ley J, Cattoir H, Reynaerts A. The quantitative measurement of DNA hybridization from renaturation rates. Eur J Biochem. 1970;12:133–142. doi: 10.1111/j.1432-1033.1970.tb00830.x. [DOI] [PubMed] [Google Scholar]
- 6.Denner E B M, McGenity T J, Busse H-J, Grant W D, Wanner G, Stan-Lotter H. Halococcus salifodine sp. nov., an archaeal isolate from an Austrian salt mine. Int J Syst Bacteriol. 1994;44:774–780. [Google Scholar]
- 7.Escara J F, Hutton J R. Thermal stability and renaturation of DNA in dimethyl sulfoxide solutions: acceleration of renaturation rate. Biopolymers. 1980;19:1315–1327. doi: 10.1002/bip.1980.360190708. [DOI] [PubMed] [Google Scholar]
- 8.Fox G C, Wisotzkey J D, Jurtshuk P., Jr How close is close: 16S rRNA sequence identity may not be sufficient to guarantee species identity. Int J Syst Bacteriol. 1992;42:166–170. doi: 10.1099/00207713-42-1-166. [DOI] [PubMed] [Google Scholar]
- 9.Gurtner C, Heyrman J, Piñar G, Lubitz W, Swings J, Rölleke S. Comparative analyses of the bacterial diversity on two different biodeteriorated wall paintings by DGGE and 16S rDNA sequence analysis. Int Biodeterior Biodegradation. 2000;46:229–239. [Google Scholar]
- 10.Heyrman J, Mergaert J, Denys R, Swings J. The use of fatty acid methyl ester analysis (FAME) for the identification of heterotrophic bacteria present on three mural paintings showing severe damage by microorganisms. FEMS Microbiol Lett. 1999;181:55–62. doi: 10.1111/j.1574-6968.1999.tb08826.x. [DOI] [PubMed] [Google Scholar]
- 11.Huss V A R, Festl H, Schleifer K H. Studies on the spectrometric determination of DNA hybridization from renaturation rates. Syst Appl Microbiol. 1983;4:184–192. doi: 10.1016/S0723-2020(83)80048-4. [DOI] [PubMed] [Google Scholar]
- 12.Jackson C R, Roden E E, Churchill P F. Changes in bacterial species composition in enrichment cultures with various dilutions of inoculum as monitored by denaturing gradient gel electrophoresis. Appl Environ Microbiol. 1998;64:5046–5048. doi: 10.1128/aem.64.12.5046-5048.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jahnke K-D. Basic computer program for evaluation of spectroscopic DNA renaturation data from Gilford System 2600 spectrometer on a PC/XT/AT type personal computer. J Microbiol Methods. 1992;15:61–73. [Google Scholar]
- 14.Jukes T H, Cantor C R. Evolution of protein molecules. In: Munro H N, editor. Mammalian protein metabolism. New York, N.Y: Academic Press, Inc.; 1969. pp. 21–132. [Google Scholar]
- 15.Kane M D, Poulsen L K, Stahl D A. Monitoring the enrichment and isolation of sulfate-reducing bacteria using oligonucleotide hybridization probes designed from environmentally derived 16S rRNA sequences. Appl Environ Microbiol. 1993;59:682–686. doi: 10.1128/aem.59.3.682-686.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krumbein W E, Schostak V, Petersen K. On novel halophilic and extremely halotolerant bacteria from environments near the North Sea coast and the Steinhuder Meer. Kiel Meeresforsch Sonderh. 1991;8:173–177. [Google Scholar]
- 17.Laiz L, Recio D, Hermosin B, Saiz-Jimenez C. Microbial communities in salt efflorescences. In: Ciferri O, Tiano P, Mastromei G, editors. On microbes and art. Proceedings of an International Conference on Microbiology and Conservation (ICMC). New York, N.Y: Kluwer Academic/Plenum Publishers; 2000. pp. 77–88. [Google Scholar]
- 18.Lane D J. 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M, editors. Nucleic acid techniques in bacterial systematics. Chichester, United Kingdom: John Wiley & Sons; 1991. pp. 115–175. [Google Scholar]
- 19.Muyzer G, de Waal E C, Uitterlinden A G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol. 1993;59:695–700. doi: 10.1128/aem.59.3.695-700.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muyzer G, Ramsing N B. Molecular methods to study the organization of microbial communities. Water Sci Technol. 1995;32:1–9. [Google Scholar]
- 21.Pearson W R. Rapid and sensitive sequence comparison with FAST and FASTA. Methods Enzymol. 1994;183:63–98. doi: 10.1016/0076-6879(90)83007-v. [DOI] [PubMed] [Google Scholar]
- 22.Piñar G, Gurtner C, Lubitz W, Rölleke S. Identification of Archaea in objects of art by DGGE analysis and shotgun cloning. Methods Enzymol. 2001;336:356–366. doi: 10.1016/s0076-6879(01)36601-6. [DOI] [PubMed] [Google Scholar]
- 23.Poulsen L K, Ballard G, Stahl D A. Use of rRNA fluorescence in situ hybridization for measuring the activity of single cells in young and established biofilms. Appl Environ Microbiol. 1993;59:1354–1360. doi: 10.1128/aem.59.5.1354-1360.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rölleke S, Muyzer G, Wawer C, Wanner G, Lubitz W. Identification of bacteria in a biodegraded wall painting by denaturing gradient gel electrophoresis of PCR-amplified gene fragments coding for 16S rRNA. Appl Environ Microbiol. 1996;62:2059–2065. doi: 10.1128/aem.62.6.2059-2065.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rölleke S, Witte A, Wanner G, Lubitz W. Medieval wall painting—a habitat for archaea: identification of archaea by denaturing gradient gel electrophoresis (DGGE) of PCR-amplified gene fragments coding 16S rRNA in a medieval wall painting. Int Biodeterior Biodegradation. 1998;41:85–92. [Google Scholar]
- 26.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 27.Saiz-Jimenez C, Laiz L. Occurrence of halotolerant/halophilic bacterial communities in deteriorated monuments. Int Biodeterior Biodegradation. 2000;46:319–326. [Google Scholar]
- 28.Santegoeds C M, Nold S C, Ward D M. Denaturing gradient gel electrophoresis used to monitor the enrichment culture of aerobic chemoorganotrophic bacteria from a hot spring cyanobacterial mat. Appl Environ Microbiol. 1996;62:3922–3928. doi: 10.1128/aem.62.11.3922-3928.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schabereiter-Gurtner C, Piñar G, Lubitz W, Rölleke S. An advanced molecular strategy to identify bacterial communities on art objects. J Microbiol Methods. 2001;45:77–87. doi: 10.1016/s0167-7012(01)00227-5. [DOI] [PubMed] [Google Scholar]
- 30.Smibert R M, Krieg N R. Phenotypic characterization. In: Gerhardt P, Murray R G E, Woods W A, Krieg N R, editors. Methods for general and molecular bacteriology. Washington, D.C.: American Society for Microbiology; 1994. pp. 607–654. [Google Scholar]
- 31.Spring S, Ludwig W, Marquez M C, Ventosa A, Schleifer K-H. Halobacillus gen. nov., with descriptions of Halobacillus litoralis sp. nov. and Halobacillus trueperi sp. nov., and transfer of Sporosarcina halophila to Halobacillus halophilus comb. nov. Int J Syst Bacteriol. 1996;46:492–496. [Google Scholar]
- 32.Stahl D A, Devereux R, Amann R I, Flesher B, Lin C, Stromley J. Ribosomal RNA based studies of natural microbial diversity and ecology. In: Hattori T, Ishida Y, Maruyama Y, Morita R, Uchida A, editors. Recent advances in microbial ecology. Tokyo, Japan: Japan Scientific Societies Press; 1989. pp. 669–673. [Google Scholar]
- 33.Teske A, Sigalevich P, Cohen Y, Muyzer G. Molecular identification of bacteria from a coculture by denaturing gradient gel electrophoresis of 16S ribosomal DNA fragments as a tool for isolation in pure cultures. Appl Environ Microbiol. 1996;62:4210–4215. doi: 10.1128/aem.62.11.4210-4215.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tomlinson G A, Hochstein L I. Halobacterium saccharovorum sp. nov., a carbohydrate-metabolizing, extremely halophilic bacterium. Can J Microbiol. 1976;22:587–591. doi: 10.1139/m76-087. [DOI] [PubMed] [Google Scholar]