Abstract
Background: The poor quality of care received by mothers and neonates in many limited-resource countries represents a main determinant of newborn mortality. Small and sick hospitalized newborns are the highest-risk population, and they should be one of the prime beneficiaries of quality-of-care interventions. This study aimed to evaluate the impact on neonatal mortality of quality improvement interventions which were implemented at Tosamaganga Council Designated Hospital, Iringa, Tanzania, between 2016 and 2020. Methods: A retrospective comparison between pre- and post-intervention periods was performed using the chi-square test and Fisher’s exact test. Effect sizes were reported as odds ratios with 95% confidence intervals. Results: The analysis included 5742 neonates admitted to the Special Care Unit (2952 in the pre-intervention period and 2790 in the post-intervention period). A decrease in mortality among infants with birth weight between 1500 and 2499 g (overall: odds ratio 0.49, 95% confidence interval 0.27–0.87; inborn: odds ratio 0.50, 95% confidence interval 0.27–0.93) was found. The analysis of cause-specific mortality showed a decrease in mortality for asphyxia (odds ratio 0.33, 95% confidence interval 0.12–0.87) among inborn infants with birth weight between 1500 and 2499 g. Conclusions: A quality improvement intervention was associated with decreased mortality among infants with birth weight between 1500 and 2499 g. Further efforts are needed to improve prognosis in very-low-birth-weight infants.
Keywords: neonatal mortality, low birth weight infants, quality improvement, B.A.B.I.E.S. Matrix tool
1. Introduction
Poor neonatal outcomes represent a significant global health burden [1], with a large proportion of neonatal deaths occurring in low-resource settings [2]. Over the years, under-five-year-old mortality (U5M) declined more significantly than neonatal mortality did, resulting in a larger contribution of neonatal deaths to the U5M rate [3]. The poor quality of care received by mothers and babies in many limited-resource countries contributes to the high levels of newborn mortality [4]. Quality improvement (QI) is defined as “better patient experience and outcomes achieved through changing provider behavior and organization through using a systematic change methods and strategies” [5] Recent evidence shows that quality improvement initiatives can reduce the burden of mortality and morbidity for hospitalized newborns in developing countries [6,7,8].
The aim of the present study was to evaluate the impact on neonatal mortality of quality improvement interventions implemented at Tosamaganga Council Designated Hospital, Iringa, Tanzania, between 2017 and 2020.
2. Materials and Methods
2.1. Study Design
This retrospective study compared “pre-intervention” (1 January–31 December 2016) and “post-intervention” (1 January–31 December 2020) mortality data of newborns admitted to the neonatal Special Care Unit (SCU) of Tosamaganga Hospital, Iringa, Tanzania. The periods were separated by a 3-year time span (2017–2019) which was needed to implement the quality improvement bundle.
2.2. Setting
Tosamaganga Hospital is a District Designated Hospital in the District of Iringa (Tanzania) and is the referral hospital for major obstetric emergencies for around 260,000 people living in the area. Every year, around 3000 deliveries and 500 admissions to the SCU occur at Tosamaganga Hospital. The SCU offers basic intensive care such as intravenous therapies, phototherapy and oxygen supplementation without non-invasive respiratory support and mechanical ventilation. Since January 2019, all babies discharged from the SCU are offered regular follow-up visits (to monitor clinical wellbeing, growth and neurological development) at the neonatal follow-up clinic during their first year of life.
2.3. Patients
All newborns admitted to the SCU of Tosamaganga Hospital (Iringa, Tanzania) during the study periods were included.
2.4. Interventions
During 2017–2019, a structured quality improvement process was implemented by Doctors with Africa CUAMM, an Italian nongovernmental organization operating in the field of healthcare in developing countries [9]. The interventions focused on improving infrastructure, equipment, training and use of clinical protocols, with a specific target on low-birth-weight infants (LBW, <2500 g) and pathologic newborns. Table 1 summarizes the area of interventions, timing and actions which were implemented during the process.
Table 1.
Summary of the quality improvement process which was implemented in 2017–2019 at Tosamaganga Hospital (Iringa, Tanzania).
| Area of Intervention | Year | Action |
|---|---|---|
| Infrastructures | January 2017 | A Neonatal ward was constructed near the Maternity Ward, divided into three areas: Neonatal Intensive Care Unit (one room), Neonatal Sub-intensive Care Unit (one room) and Kangaroo Mother Care Unit (two rooms) |
| Equipment | January 2017 | Four oxygen concentrators (increased over the years up to 10), two phototherapy machines, four infusion pumps and a syringe pump, a capillary hemoglobin dosing machine and an electric aspirator were purchased. The staff received training on their use. |
| Protocols | 2017 and 2019 | Operational protocols were updated and presented to the staff in dedicated training sessions. Laminated copies of the most commonly used protocols were displayed for quick consultation even by on-call staff during night shifts and holidays. A further update of the ward guidelines was carried out in 2019, in light of the publication of the first edition of the national neonatal guidelines. |
| Procedures | January 2017 | New procedures were introduced: antenatal administration of dexamethasone for lung maturity and magnesium sulfate for neuroprotection, positioning of an umbilical venous catheter in newborns weighing <1200 g, administration of paracetamol in newborns with suspected patent ductus arteriosus, administration of hydrocortisone in newborns with oxygen dependence and suspected bronchopulmonary dysplasia. |
| Staff | 2017 | A dedicated nursing team was created, consisting of 5 nurses (increased over the years up to 8). From February 2017, a Tanzanian doctor started working in Neonatology. |
| Training activity | 2017–2019 | Over years, the Neonatal Unit and Maternity Ward staff were periodically trained on partogram use and interpretation, management of a complicated pregnancy (gestational hypertension, gestational diabetes, prolonged rupture of the membranes); management of labor and delivery (1st, 2nd, 3rd stage), prolonged rupture of the membranes, complicated labor and the most common maternal peripartum complications, neonatal resuscitation, management of common neonatal severe conditions (sepsis, jaundice, asphyxia, prematurity, respiratory distress syndrome), essential newborn care and care of low-birth-weight and very-low-birth-weight infants. |
2.5. Outcome Measures
The main indicators were derived from the World Health Organization (WHO) B.A.B.I.E.S. Matrix tool (Birth weight group, Age at death, Boxes for an Intervention Evaluation System) [10], which is described in paragraph 2.7. The primary outcome measures included deaths/live births before discharge <1499 g (B.A.B.I.E.S. Matrix Cell 3), deaths/live births before discharge 1500–2499 g (B.A.B.I.E.S. Matrix Cell 7), and deaths/live births before discharge ≥2500 g (B.A.B.I.E.S. Matrix Cell 11). The secondary outcome measures included the mortality rates from asphyxia, infection and prematurity, according to the main causes of death (as defined by the tool for cells 3, 7 and 11).
2.6. Data Collection
All data were retrospectively and anonymously collected from hospital charts by a researcher who was not involved in clinical activity. The researcher was not masked to the intervention period. Retrieved data did not contain any information that might be used to identify individual patients.
2.7. Definitions
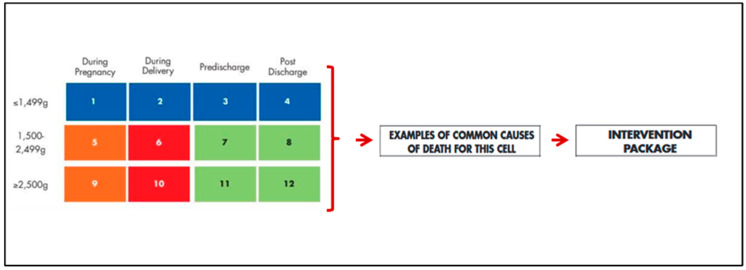
The B.A.B.I.E.S. Matrix tool works by segregating, organizing, analyzing and transforming data regarding fetal and neonatal deaths. Through stratification of data by weight and by moment of death, the tool guides identifications of the main problems related to pregnancy, labor, delivery and postnatal management, suggesting the causes and the appropriate interventions needed to reduce neonatal mortality (Figure 1). The stratification works on three birth weight categories (<1499, 1500–2499 and ≥2500 g) and on four time categories (during pregnancy: from 28 weeks of gestational age to the beginning of labor; during labor: from the beginning of labor to delivery time; pre-discharge: from delivery time to discharge time; post-discharge: from discharge to 28 days of life).
Figure 1.
World Health Organization B.A.B.I.E.S. Matrix tool, modified from Joy Lawn et al. [10].
To identify the cause-specific mortality, the criteria reported by Mmbaga et al. [11] were used: (a) Birth asphyxia: birth asphyxia with weight >1000 g or gestational age >27 weeks; birth asphyxia and prematurity with gestational age ≥33 weeks and birth weight ≥2500 g or birth weight ≥1800 g if gestational age is unknown; neonatal encephalopathy with 5-min Apgar lower than 7; (b) Prematurity: prematurity; prematurity and asphyxia with gestational age <33 weeks and birth weight <2500 g or birth weight <1800 g if gestational age is unknown; respiratory distress syndrome in preterm; necrotizing enterocolitis; birth asphyxia gestational age <27 weeks or birth weight <1000 g; infection with gestational age <33 weeks; (c) Infection: neonatal infection; sepsis/septicemia; meningitis; pneumonia; impetigo neonatorum.
2.8. Statistical Analysis
Categorical data were summarized as frequencies and percentages. Comparisons between pre- and post-intervention periods were performed using the chi-square test and Fisher’s exact test. Effect sizes were reported as odds ratios with 95% confidence intervals for each outcome measure. All tests were 2-sided, and a p-value less than 0.05 was considered statistically significant. Adjustment for multiple testing was not performed due to the exploratory purpose of the study. Statistical analysis was performed using R 4.1 (R Foundation for Statistical Computing, Vienna, Austria) [12].
3. Results
The analysis included 2952 neonates admitted to the SCU in the pre-intervention period and 2790 neonates admitted to the SCU in the post-intervention period. The characteristics of deliveries and neonates are reported in Table 2. The baseline characteristics were clinically comparable between the two periods, with small changes in cesarean sections (36.4% vs. 30.7%, p < 0.0001), inborn neonates (97.9% vs. 96.6%, p < 0.0001) and LBW neonates (10.4% vs. 13.7%; p < 0.0001).
Table 2.
Baseline characteristics of neonates admitted to the SCU in the pre- vs. post-intervention periods.
| Pre-Intervention | Post-Intervention Period: | p-Value | |
|---|---|---|---|
| Period: | |||
| Deliveries | N = 2901 | N = 2732 | - |
| Mode of delivery: | <0.0001 | ||
| Caesarean section | 1056/2901 (36.4%) | 840/2732 (30.7%) | |
| Vaginal delivery | 1845/2901 (63.6%) | 1892/2732 (69.3%) | |
| Twin deliveries | 50/2901 (1.7%) | 55/2732 (2.0%) | 0.48 |
| Neonates | N = 2952 | N = 2790 | |
| Inborn neonates | 2890/2952 (97.9%) | 2675/2790 (95.6%) | <0.0001 |
| Males | 1472/2952 (49.9%) | 1370/2790 (49.1%) | 0.58 |
| Birth weight: | <0.0001 | ||
| ≤1499 g | 24/2952 (0.8%) | 63/2790 (2.3%) | |
| 1500–2499 g | 282/2952 (9.6%) | 318/2790 (11.4%) | |
| ≥2500 g | 2646/2952 (89.6%) | 2409/2790 (86.3%) | |
| 5-min Apgar score < 7 (only inborn) | 121/2890 (4.2%) | 109/2675 (4.1%) | 0.89 |
Data were summarized as n/N (%).
A comparison of neonatal mortality between pre- and post-intervention periods is summarized in Table 3. Overall mortality did not change after the implementation of the interventions (odds ratio 1.05, 95% confidence interval 0.78 to 1.41). However, there was a decrease in overall mortality among infants with birth weight between 1500 and 2499 g (odds ratio 0.49, 95% confidence interval 0.27–0.87) and in inborn neonates of the same birth weight category (odds ratio 0.50, 95% confidence interval 0.27–0.93).
Table 3.
Comparison of mortality of neonates admitted to the SCU in the pre- vs. post-intervention periods.
| Outcome Measure | Pre-Intervention Period: | Post-Intervention Period: | Post vs. Pre Comparison: Odds Ratio (95% Confidence Interval) |
p-Value |
|---|---|---|---|---|
| All neonates | N = 2952 | N = 2790 | - | - |
| Overall mortality | 87/2952 (2.9%) | 92/2790 (3.3%) | 1.05 (0.78 to 1.41) | 0.49 |
| Mortality in BW categories: | ||||
| ≤1499 g | 13/24 (54.2%) | 31/63 (49.2%) | 0.81 (0.32 to 2.10) | 0.86 |
| 1500–2499 g | 34/282 (12.1%) | 20/318 (6.3%) | 0.49 (0.27 to 0.87) | 0.02 |
| ≥2500 g | 40/2646 (1.5%) | 41/2409 (1.7%) | 1.12 (0.72 to 1.75) | 0.67 |
| Mortality for prematurity | 21/2952 (0.7%) | 36/2790 (1.3%) | 1.82 (1.03 to 3.29) | 0.04 |
| Mortality for asphyxia: | ||||
| Overall | 44/2952 (1.5%) | 40/2790 (1.4%) | 0.96 (0.62 to 1.47) | 0.94 |
| ≤1499 g | 0/24 (0.0%) | 0/63 (0.0%) | NA | NA |
| 1500–2499 g | 15/282 (5.3%) | 7/318 (2.2%) | 0.40 (0.16 to 1.00) | 0.07 |
| ≥2500 g | 29/2646 (1.1%) | 33/2409 (1.4%) | 1.25 (0.75 to 2.07) | 0.45 |
| Mortality for infection: | ||||
| Overall | 12/2952 (0.4%) | 6/2790 (0.2%) | 0.52 (0.19 to 1.40) | 0.29 |
| ≤1499 g | 0/24 (0.0%) | 0/63 (0.0%) | NA | NA |
| 1500–2499 g | 5/282 (1.8%) | 2/318 (0.6%) | 0.35 (0.06 to 1.82) | 0.26 |
| ≥2500 g | 7/2646 (0.3%) | 4/2409 (0.2%) | 0.62 (0.18 to 2.14) | 0.55 |
| Inborn neonates | N = 2890 | N = 2675 | - | - |
| Overall mortality | 77/2890 (2.7%) | 73/2675 (2.7%) | 1.02 (0.74 to 1.41) | 0.94 |
| Mortality in BW categories: | ||||
| ≤1499 g | 10/15 (66.7%) | 22/30 (73.3%) | 1.37 (0.35 to 5.27) | 0.90 |
| 1500–2499 g | 29/249 (11.6%) | 18/287 (6.3%) | 0.50 (0.27 to 0.93) | 0.04 |
| ≥2500 g | 38/2626 (1.4%) | 33/2358 (1.4%) | 0.96 (0.60 to 1.54) | 0.98 |
| Mortality for prematurity | 15/2890 (0.5%) | 26/2675 (1.0%) | 1.88 (0.99 to 3.55) | 0.07 |
| Mortality for asphyxia: | ||||
| Overall | 43/2890 (1.5%) | 33/2675 (1.2%) | 0.82 (0.52 to 1.30) | 0.48 |
| ≤1499 g | 0/15 (0.0%) | 0/30 (0.0%) | NA | NA |
| 1500–2499 g | 15/249 (6.0%) | 6/287 (2.1%) | 0.33 (0.12 to 0.87) | 0.03 |
| ≥2500 g | 28/2626 (1.1%) | 27/2358 (1.1%) | 1.07 (0.63 to 1.82) | 0.90 |
| Mortality for infection: | ||||
| Overall | 10/2890 (0.3%) | 5/2675 (0.2%) | 0.53 (0.18 to 1.57) | 0.38 |
| ≤1499 g | 0/15 (0.0%) | 0/30 (0.0%) | NA | NA |
| 1500–2499 g | 4/269 (1.5%) | 2/287 (0.7%) | 0.46 (0.08 to 2.55) | 0.44 |
| ≥2500 g | 6/2626 (0.2%) | 3/2358 (0.1%) | 0.55 (0.13 to 2.22) | 0.51 |
| Outborn neonates | N = 62 | N = 115 | - | - |
| Overall mortality | 10/62 (16.1%) | 19/115 (16.5%) | 1.02 (0.44 to 2.37) | 0.99 |
| Mortality in BW categories: | ||||
| ≤1499 g | 3/9 (33.3%) | 9/33 (27.3%) | 0.75 (0.15 to 3.65) | 0.69 |
| 1500–2499 g | 5/33 (15.2%) | 2/31 (6.5%) | 0.38 (0.06 to 2.15) | 0.42 |
| ≥2500 g | 2/20 (10.0%) | 8/51 (15.7%) | 1.67 (0.32 to 8.66) | 0.71 |
| Mortality for prematurity | 6/62 (9.7%) | 10/115 (8.7%) | 0.88 (0.30 to 1.57) | 0.99 |
| Mortality for asphyxia: | ||||
| Overall | 1/62 (1.6%) | 7/115 (6.1%) | 3.95 (0.47 to 32.89) | 0.26 |
| ≤1499 g | 0/9 (0.0%) | 0/33 (0.0%) | NA | NA |
| 1500–2499 g | 0/33 (0.0%) | 1/31 (3.2%) | 3.29 (0.12 to 89.97) | 0.48 |
| ≥2500 g | 1/20 (5.0%) | 6/51 (11.8%) | 2.53 (0.28 to 22.49) | 0.66 |
| Mortality for infection: | ||||
| Overall | 2/62 (3.2%) | 1/115 (0.9%) | 0.26 (0.02 to 2.96) | 0.28 |
| ≤1499 g | 0/9 (0.0%) | 0/33 (0.0%) | NA | NA |
| 1500–2499 g | 1/33 (3.0%) | 0/31 (0.0%) | 0.34 (0.01 to 8.76) | 0.99 |
| ≥2500 g | 1/20 (5.0%) | 1/51 (2.0%) | 0.38 (0.02 to 6.38) | 0.48 |
Data were summarized as n/N (%).
The analysis of cause-specific mortality found a decrease in mortality for asphyxia (odds ratio 0.33, 95% confidence interval 0.12–0.87) among infants with birth weight between 1500 and 2499 g and an increase in overall mortality for prematurity (odds ratio 1.82, 95% confidence interval 1.03–3.29). No statistically significant differences in mortality were observed among outborn neonates.
4. Discussion
Recent evidence suggests that an effective implementation of quality improvement in the care of small and sick newborns is possible in low-resource settings [6,7,8]. The literature indicates limitations in staff, equipment and protocols as the main barriers for such implementation and underlines the opportunity for meso-level and educational interventions [6]. This study reports neonatal mortality outcomes after a quality improvement intervention in a sub-Saharan setting. According to indications drawn from the literature, our quality improvement approach involved the use of meso and micro interventions (such as strengthening the facility’s infrastructure, continuous quality improvement, supervision, feedback, in-service training, distribution of referencing materials to providers, decision support and care coordination) and used mortality as the main outcome measure [6]. The decrease in mortality among infants with birth weight between 1500 and 2499 g supports the effectiveness of the implementation of specifically targeted interventions and indirectly underlines the importance of the B.A.B.I.E.S Matrix as a guiding tool for improving quality of neonatal care. The decrease in mortality for asphyxia among infants with birth weight between 1500 and 2499 g suggested an improvement in stabilization practices immediately after birth for low-birth-weight infants. Of note, we found increased overall mortality due to prematurity, which might likely be due to a bias in the definition of prematurity. As gestational age is rarely available in low-resource settings, a birth weight <1800 g was used to define prematurity [11]. Within this category, there was a larger number of babies with birth weight <1500 g in 2020 vs. that in 2016. Very-low-birth-weight newborns (<1500 g) are extremely fragile, and their mortality varies considerably among high-income (12–15%) and low–middle-income countries (21–43%) [13,14,15]. Unfortunately, reducing mortality in this subgroup of newborns requires massive human and economic resources, and the B.A.B.I.E.S Matrix tool suggests interventions on pre-pregnancy health and high-tech neonatal care that are very difficult to implement in low-resource settings.
The reader should be aware that reducing neonatal mortality may come at the cost of increased post-discharge morbidity in such vulnerable subjects. A follow-up service for high-risk newborns is currently active at the study site, but unfortunately, the high dropout rate makes any assessment difficult.
The present study has some limitations that should be considered by the reader. First, the retrospective design limited both the availability and quality of data. Second, the quality improvement was implemented in a sub-Saharan referral hospital, hence the generalizability of the findings should be limited to similar settings. Third, adjustment for multiple testing was not performed due to the exploratory purpose of the study, hence we suggest caution in the interpretation of the results. Finally, we could not discriminate the specific impact of each component of the bundle.
Future interventions for reducing morbidity and mortality in this setting would focus on applying new strategies such as the use of devices for non-invasive respiratory support and improving good practices of infection prevention, nutritional support and maintenance of normothermia. Hypothermia, hypoglycemia and infections are the main causes of death in the neonatal period, and their prevention is even more important in very-low-birth-weight newborns. To this end, it would be necessary to pay even greater attention to the management of vascular access, parenteral fluids and enteral nutrition. Strengthening Kangaroo mother care and close monitoring of temperature and blood sugar would also be essential.
5. Conclusions
A quality improvement process based on meso and micro interventions was associated with decreased mortality among infants with birth weight between 1500 and 2499 g. Further efforts are needed to improve prognosis in very-low-birth-weight infants.
Acknowledgments
We are very grateful to the nurses and medical staff of the St. John of the Cross, Tosamaganga Council Designated Hospital for the passion they show in their daily work.
Author Contributions
Conceptualization: A.P.; data curation: A.P., M.P., C.Z. and L.B.; formal analysis, F.C. and A.P.; investigation: M.P., C.Z., L.B., D.M.M. and D.E.L.; project administration: A.P., G.A. and G.P.; supervision: F.C., D.T. and G.P.; visualization: A.P. and F.C.; writing—original draft preparation: A.P. and F.C.; writing—review and editing: M.P., C.Z., L.B., G.A., D.M.M., D.E.L., G.P. and D.T. All authors approved the final version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
All methods were performed in accordance with the relevant guidelines and regulations. The study was approved by the Institutional Review Board of Tosamaganga Hospital (protocol number DOIRA/TCDH/VOL.016/5, date 08 September 2021).
Informed Consent Statement
Patient consent was waived due to the retrospective nature of the study and the use of anonymized data from hospital records.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Victoria G.C., Requejo J.H., Barros A.J.D., Berman P., Bhutta Z., Boerma T., Chopra M., de Francisco A., Daelmans B., Hazel E., et al. Countdown to 2015 for maternal newborn and child survival. A decade tracking progress maternal, newborn, child survival. Lancet. 2016;387:2049–2059. doi: 10.1016/S0140-6736(15)00519-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawn J.E., Cousens S., Zupan J., Lancet Neonatal Survival Steering Team 4 million neonatal deaths: When? Where? Why? Lancet. 2005;365:891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- 3.Liu L., Oza S., Hogan D., Perin J., Rudan I., Lawn J.E., Cousens S., Mathers C., Black R.E. Global, regional, and national causes of child mortality in 2000–2013, with projections to inform post-2015 priorities: An updated systematic analysis. Lancet. 2015;385:430–440. doi: 10.1016/S0140-6736(14)61698-6. [DOI] [PubMed] [Google Scholar]
- 4.Van den Broek N.R., Graham W.J. Quality of care for maternal and newborn health: The neglected agenda. BJOG. 2009;116:18–21. doi: 10.1111/j.1471-0528.2009.02333.x. [DOI] [PubMed] [Google Scholar]
- 5.Ovretveit J., Appleby J. Does improving quality of care save money? BMJ. 2009;339:b3678. doi: 10.1136/bmj.b3678. [DOI] [PubMed] [Google Scholar]
- 6.Zaka N., Alexander E.C., Manikam L., Norman I., Akhbari M., Moxon S., Ram P.K., Murphy G., English M., Niermeyer S., et al. Quality improvement initiatives for hospitalised small and sick newborns in low- and middle-income countries: A systematic review. Implement. Sci. 2018;13:20. doi: 10.1186/s13012-018-0712-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavicchiolo M.E., Lanzoni P., Wingi M.O., Pizzol D., Daverio M., Da Dalt L., Putoto G., Trevisanuto D. Reduced neonatal mortality in a regional hospital in Mozambique linked to a Quality Improvement intervention. BMC Pregnancy Childbirth. 2016;16:366. doi: 10.1186/s12884-016-1170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ouedraogo P., Villani P.E., Tubaldi L., Bua J., Uxa F., Dell’Anna C., Cavallin F., Thomson M., Plicco C., Chiesi M.P. Impact of a quality improvement intervention on neonatal mortality in a regional hospital in Burkina Faso. J. Matern. Fetal Neonatal Med. 2021;Jan 5:1–6. doi: 10.1080/14767058.2020.1866532. [DOI] [PubMed] [Google Scholar]
- 9.Doctors with Africa CUAMM. [(accessed on 25 June 2022)]. Available online: https://doctorswithafrica.org/
- 10.Lawn J., McCarthy B.J., Ross S.R. HEALTHY NEWBORN: A Reference Manual for Program Managers, PART 2: A Newborn Health Management Information System. The CARE /CDC Health Initiative; Clifton Road Atlanta, GA, USA: 2002. [Google Scholar]
- 11.Mmbaga B.T., Lie R.T., Olomi R., Mahande M.J., Kvåle G., Daltveit A.K. Cause-specific neonatal mortality in a neonatal care unit in Northern Tanzania: A registry based cohort study. BMC Pediatr. 2012;12:116. doi: 10.1186/1471-2431-12-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2021. [(accessed on 25 June 2022)]. Available online: https://www.R-project.org/ [Google Scholar]
- 13.Fanaroff A.A., Stoll B.J., Wright L.L., Carlo W.A., Ehrenkranz R.A., Stark A.R., Bauer C.R., Donovan E.F., Korones S.B., Laptook A.R., et al. Trends in neonatal morbidity and mortality for very low birthweight infants. Am. J. Obstet. Gynecol. 2007;196:147.e1–8. doi: 10.1016/j.ajog.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Bansal A. Comparison of outcome of very-low-birth-weight babies with developed countries: A prospective longitudinal observational study. J. Clin. Neonatol. 2018;7:254. doi: 10.4103/jcn.JCN_76_18. [DOI] [Google Scholar]
- 15.Michaelis I.A., Krägeloh-Mann I., Manyisane N., Mazinu M.C., Jordaan E.R. Prospective cohort study of mortality in very low birthweight infants in a single centre in the Eastern Cape province, South Africa. BMJ Paediatr. Open. 2021;5:e000918. doi: 10.1136/bmjpo-2020-000918. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.