Summary
Background
Despite widespread adoption of potent acid suppression treatment with proton pump inhibitors (PPI) for reflux‐like symptoms, persistent symptoms are commonly reported in primary care and community studies.
Aims
This multidisciplinary review critically evaluates how the management of reflux‐like symptoms could better reflect their multifactorial pathophysiology.
Methods
A panel of experts (from general practice, gastroenterology and gastropsychology) attended a series of workshops to review current management and propose a framework for the provision of more individualised care.
Results
It was agreed that the perceptual (as well as the physiological) causes of reflux‐like symptoms should be considered at the start of management, not as a last resort when all else has failed. A short course of PPI is a pragmatic approach to address reflux‐like symptoms, but equally important is counselling about the gut‐brain axis and provision of symptom‐specific behavioural interventions for those who show signs of somatisation, hypervigilance or co‐existing disorders of gut‐brain interaction. Other low‐harm interventions such as lifestyle and dietary advice, should also be better integrated into care at an early stage. Multidisciplinary care management programmes (including dietary, weight loss, exercise and behavioural intervention) should be developed to promote greater self‐management and take advantage of the general shift toward the use of remotely accessed health care resources.
Conclusions
Management of reflux‐like symptoms should be adapted to reflect the advances in knowledge about the multifactorial aetiology of these symptoms, addressing both acid‐related and behavioural components early in management. The time has come to treat the patient, not the “disease”.

1. INTRODUCTION
Oesophageal symptoms are extremely common but the response to treatments targeting gastro‐oesophageal reflux disease (GERD) can be highly variable. 1 , 2 This challenge has, in part, been fostered by the Montreal definition of GORD which provided a rationale for diagnosis and treatment based on the presence of reflux‐like symptoms (heartburn, regurgitation, chest pain) or oesophageal injury, assumed to be the result of refluxing gastric contents. 3 However, equating non‐specific oesophageal symptoms (often with minimal contribution of underlying acidic gastro‐oesophageal reflux 4 ) with reflux oesophagitis is a gross oversimplification. Although proton pump inhibitors (PPIs) have an excellent safety profile and have revolutionised the treatment of oesophagitis, 5 the physiological determinants of reflux‐like symptoms and reflux oesophagitis only partly overlap, and PPI efficacy is less impressive for symptomatic syndromes (40%–60%). 2 This is exemplified by the significant proportion of patients with refractory symptoms that emerged after the widespread adoption of PPI therapy as first‐line treatment. In fact, up to half of the PPI‐treated patients with reflux‐like symptoms in primary care and community‐based studies report persistent symptoms. 6 , 7
While subsequent consensus has acknowledged that non‐reflux factors (e.g., altered perception, visceral hypersensitivity) contribute to refractory symptoms, 8 no clear clinical strategy has emerged to adequately address this in primary care, and escalation or modification of PPI therapy remains the primary strategy to address refractory symptoms. 2 , 9 This raises two major concerns about the modern‐day acid‐targeted approach to management; unnecessarily high levels of chronic exposure to potent acid inhibitors 10 and poor outcomes in patients with persistently unresolved symptoms. 6 , 7 Clearly, a clinical pathway that better addresses the aetiology of symptoms is needed to achieve the desired symptomatic improvement for patients.
2. TREATING THE PATIENT, NOT THE DISEASE
To critically evaluate how the management of reflux‐like symptoms could better reflect the multifactorial pathophysiology, a panel of experts from the fields of general practice, gastroenterology and gastropsychology were assembled. A series of three workshops were held to review current management strategy and treatment options and propose a framework for the provision of a more personalised approach to care. This is especially relevant in the COVID‐19 era. Although viral protein has not been detected directly in the oesophageal epithelium (as it has in the lower gastrointestinal [GI] tract), 11 data from Euromonitor International’s Health and Nutrition Survey (2021) show upper GI symptoms, have ticked upward during the pandemic, together with stress, anxiety and weight gain. 12 This underscores the importance of a holistic approach whereby symptoms are considered in the context of the patient’s overall health, lifestyle and psychological wellbeing. Moreover, the unprecedented strain on health services operating under the shadow of the pandemic, emphasises the need to address the problems of reflux mismanagement (overprescribing with poor outcomes) through better allocation of stretched health care resources.
3. THE STATUS QUO OF REFLUX MANAGEMENT: THE “LADDER” APPROACH
The Rome IV consensus on functional GI diseases (more recently termed disorders of gut‐brain interaction) has categorised symptomatic patients without endoscopic oesophagitis into three subsets: true non‐erosive reflux disease (NERD), reflux hypersensitivity and functional heartburn. 8 , 13 However, the Rome construct relies on physiological testing with prolonged oesophageal pH or pH‐impedance‐metry to identify these subsets. 8 Such testing is not feasible in most clinical settings and is usually employed only as a last resort in specialist centres after the one‐dimensional “ladder” approach of escalating acid inhibition (Figure 1) has failed. Given the prevalence of reflux‐like symptoms in the community, management should not rely on testing that is not readily available in primary care, the setting most frequently attended by these patients.
FIGURE 1.
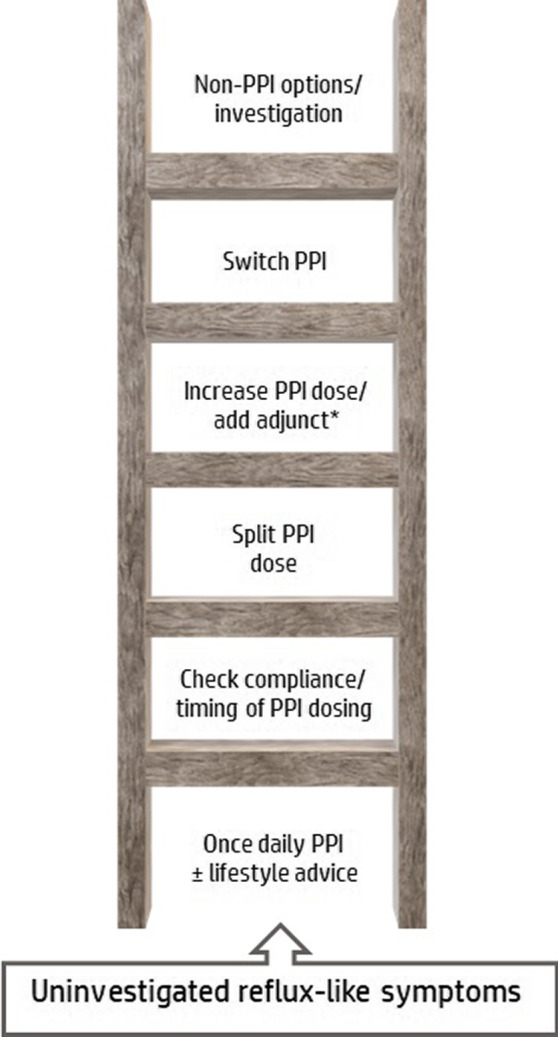
The “ladder” approach to reflux management: Multiple steps are taken to optimise and/or escalate acid suppression before strategies to address potential non‐acidic causes are considered. *For example, H2RAs, alginates, mucosal protectants
When patients first present, over‐the‐counter remedies have generally already been tried unsuccessfully. The next “rung” is empirical PPI treatment and lifestyle measures, followed by interventions to improve compliance and PPI regimen, that is, timing of drug dosing and dose splitting (half‐dose before breakfast and half‐dose before dinner to better control nocturnal acid reflux). This is followed by the escalation of PPI dose and/or switching PPI. 14 There is little rationale for switching (almost all PPIs are similarly effective at equiactive antisecretory doses) unless the switch is toward a more effective antisecretory compound (e.g. esomeprazole, 15 , 16 rabeprazole 17 , 18 ) or formulation (e.g. modified release‐dexlansoprazole 19 or immediate release‐omeprazole 20 ). Modified release formulations may be helpful if nocturnal symptoms or dosing relative to a meal are a problem, and if PPIs are to be dosed intermittently, immediate‐release formulations may be preferable. When acid suppression optimisation fails, patients with persistent symptoms may be referred to a gastroenterologist for further investigation, and treatment subsequently modified with some combination of prokinetics, alginates, mucosal protectants, H2RAs, baclofen, neuromodulators, behavioural intervention, lifestyle modification, alternative non‐reflux treatments or (rarely) surgery. 14 , 21 , 22 Patients may also try natural remedies that are frequently cited by health websites to reduce heartburn, such as apple cider vinegar, turmeric, honey, aloe vera and liquorice.
4. REVIEW OF MANAGEMENT
During the first of three workshops, the multidisciplinary group of experts shared their experience and perceptions of reflux‐like symptom management from the perspective of their speciality. Each member chose an area of management for greater review and presented their findings back to the group at a second meeting. It was noted that there is little high‐level evidence to support the range of therapeutic options for the treatment of reflux‐like symptoms. This data gap is in stark contrast to the multitude of randomised controlled trials (RCTs) demonstrating the efficacy of PPIs in the treatment of oesophagitis. 5 Consequently, the expert panel devised a list of patient categories frequently encountered in clinical practice falling under the umbrella of “PPI refractory” and, based on their own clinical experience and the discussions held, each expert scored their perception of the likely benefit (0%–100%) of each therapeutic option for each patient category. After a further round of discussion during the third and final meeting, the scoring of each therapeutic option was repeated. The polling was then tabulated, and the consolidated opinion is shown in Figure 2. As expected, PPI benefit was considered to diminish significantly beyond manifestations associated with abnormal acid exposure (oesophagitis and NERD), emphasising that long‐term use is unwarranted in patients who respond poorly. 5 It was striking that the perceived benefit of low‐risk interventions such as lifestyle modification, behavioural intervention and alginate‐antacid treatment cut across all symptomatic patient categories, raising the question of how these interventions could be better integrated into care. The similarity of profile across the non‐erosive categories also casts doubt on the value of this subcategorization in clinical practice as it is unlikely to have a significant impact on treatment strategy.
FIGURE 2.
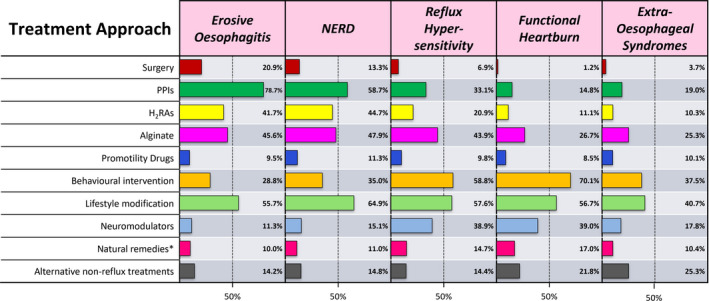
Expert perception of likely treatment benefit (percentage of responsive patients) across reflux‐like symptom categories. *Non‐pharmacological products frequently cited by health websites as beneficial for heartburn, for example., apple cider vinegar, turmeric, honey, aloe vera, liquorice.
5. PERSONALISED MANAGEMENT
5.1. Acknowledging the gut–brain connection
The ladder approach to treatment prioritises suppression of acid, but gut function and central perception are inextricably linked through the gut‐brain axis, 8 and can be influenced by a wide range of environmental and psychosocial factors that also need to be taken into account at the clinical encounter (Figure 3). Any event that dysregulates the gut‐brain pathway (e.g., life stresses, inadequate sleep, infection, mucosal injury) can trigger symptoms through alterations in neural processing and central perception. 23 We know from irritable bowel syndrome (IBS) that visceral hypersensitivity can arise after inflammation caused by an acute infection 24 and persistent symptoms eventually lead to symptom‐specific anxiety and hypervigilance, whereby increased awareness of symptoms, and the settings in which they occur leads to enhanced perception of what should otherwise be benign physiological sensations. 25 , 26 This can occur across the reflux‐like symptom spectrum, irrespective of acid exposure and is more predictive of symptom severity than acid exposure itself. 27 Symptom‐specific anxiety and hypervigilance also manifest in patients with a poor response to medication who may worry about an ominous diagnosis, reinforced by avoidance behaviour—the patient falsely attributes symptom‐free periods to their compulsive attempts to avoid perceived triggers, perpetuating a cycle of hypervigilance, pain and anxiety. 25 , 26
FIGURE 3.
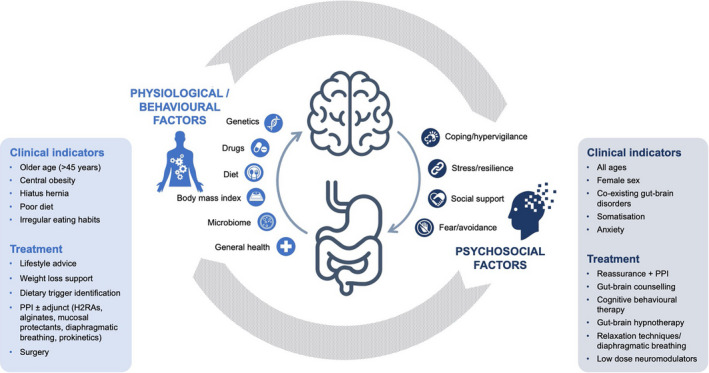
Tailoring treatment and support for reflux‐like symptoms according to the suspected aetiology
5.2. Patient–Clinician communication
Reflux‐like symptoms often form part of a symptom cluster and patients with overlapping symptoms may experience reduced quality of life and greater healthcare resource utilisation versus those with reflux‐like symptoms alone. 28 A comprehensive clinical history will help determine whether the predominant cause of reflux‐like symptoms is likely to be altered perception or altered physiology, an essential distinction to optimise individualised management (Figure 3). 29 Manifestations related to acid reflux are more common in older patients (more than 45 years of age), those with central obesity, hiatus hernia and known lifestyle and dietary risk factors, such as high‐fat diet and irregular eating habits. 29 On the other hand, functional symptoms are more common in younger patients and women, 30 , 31 and frequently co‐present with symptoms suggestive of gut‐brain interaction disorder (postprandial fullness, nausea, early satiation, epigastric burning, IBS), 31 , 32 , 33 somatisation (e.g. fibromyalgia, headache, back pain, chronic fatigue, dizziness), 8 , 34 , 35 and anxiety. 8 , 31 Reassurance and expectation‐setting are particularly important for these patients. 36 Explaining the processes of symptom generation in patient‐friendly language will not only help direct self‐care and treatment adherence but can itself be considered an intervention. As first suggested in the 1950s by the psychoanalyst Michael Balint, “By far the most frequently used drug in general practice is the doctor himself”. 37 He emphasised that a diagnosis based on physical signs and symptoms was insufficient; a deeper understanding of the patient as a unique human being was required. Such a patient‐centred approach is achieved through clinicians “dosing” themselves, maintaining an attentive frame of mind, and listening closely to what is said (and not said) to build an overall picture of the patient. 37 Primary practice, where a deeper understanding of the individual patient may have been fostered over time, is an ideal environment for the implementation of a holistic, individualised approach.
Symptoms that persist despite treatment are more challenging to treat, as they become less associated with visceral triggers (food and stress) and more centrally mediated (increased sensitivity to or failure to inhibit pain signals). To avoid this, there is a need to abandon the treatment ladder and address the psychological and lifestyle aspects up‐front. A care management programme with input from dietitians and behavioural therapists would help support self‐care, while delivering the effective therapeutic intervention. 24 Acid inhibition with PPIs is a pragmatic medical approach, but other interventions (lifestyle advice, alginate‐antacid, mucosal protectants and behavioural therapy) should also be considered ahead of, or in combination with, a PPI. The patient should understand that PPI treatment changes the characteristics of reflux but does not resolve the predisposing factors (obesity, hiatus hernia, diet, stress, anxiety) or address the underlying pathophysiology (lower oesophageal sphincter incompetence, transient lower oesophageal relaxations, gastro‐oesophageal dysmotility) so other approaches are required to address these risks.
5.3. Lifestyle advice
As with other chronic lifestyle disorders, nutritional advice and lifestyle tips should be standard first‐line approaches to management. Supporting data from controlled clinical studies are limited but recommendations included in the latest evidence‐based guidelines include weight loss, smoking cessation, elevating the head of the bed, avoiding dietary triggers (high‐fat, spicy and acidic foods) and not eating close to bedtime. 38 However, evidence suggests that lifestyle advice may not be routinely implemented in general practice. 39 , 40 , 41 Clinicians often incorrectly assume that patients are aware of what constitutes a healthy diet and lifestyle. Perhaps this is because initiating and monitoring behaviour change, especially weight loss, requires motivational interviewing skills, regular contact and encouragement beyond what is feasible in the context of a brief consultation. Nonetheless, simple dietary and lifestyle advice may suffice for patients with reflux‐like symptoms. A recent meta‐analysis found a clear relationship between reflux‐like symptoms and irregular eating habits, including late‐night snacking, skipping breakfast, eating quickly or eating beyond fullness. 42 The strongest risk was associated with “less than 3‐hour interval between dinner and bed” (OR 7.45, 95% CI 3.38–16.4) and “high‐fat diet” (OR 7.568, 95% CI 4.557–8.908), whereas vegetarian diet had the strongest negative relationship (OR 0.34, 95% CI 0.211–0.545). 42 Also, a recent study found that a simple strategy helping GORD patients identify and eliminate dietary triggers significantly reduced symptoms and the need for pharmacological treatment. 43
Lifestyle advice is relevant for all patients, even those on antisecretory therapy. In a study of overweight patients receiving PPI for reflux symptoms, weight loss was associated with PPI discontinuation (>54%) or dose‐reduction (32%), which was not the case for those who maintained their original body mass index. 44 Lifestyle interventions for weight loss, smoking cessation, exercise, etc. are more likely to be successful when there is continuity of care and regular contact. In the context of a busy primary care practice, regular but brief consultations with a general practitioner or nurse practitioner can help monitor a patient’s motivational progress. This can also be augmented through remote/electronic tools or existing programmes. Euromonitor International data suggest that the public have adapted to using online resources and e‐consultations for health advice during the pandemic 12 which may positively impact patient engagement with remote management.
5.4. PPI and adjunct therapies
For patients who gain symptomatic benefit, the lowest effective dose or a step‐down approach should be used. 45 PPI overuse and misuse are frequent in the community and deprescribing (reducing to the lowest effective dose, stopping or using intermittently) should be attempted to reduce the risk of rare side effects, medication burden and the unnecessary costs of long‐term prescribing when PPI may no longer be providing benefit. 45 , 46 Furthermore, clinicians are increasingly encountering patients who are opposed to long‐term PPI use, even when they have responded well to it. Research into PPI deprescribing (although limited) suggests that most patients can be successfully stepped down or stopped without symptom recurrence requiring reinstitution of PPI. 46 , 47 , 48 Evidence‐based practice guidelines, including a decision‐support algorithm, have been developed to help clinicians decide when and how to reduce or stop PPI therapy, according to the patient’s individual circumstances and preferences. 46 , 49 Alginate‐antacids have been shown to be an effective rescue therapy during deprescribing 50 , 51 and are a recommended option for the self‐management of patients stepping‐off PPI therapy. 52 , 53 They act to neutralise and displace the source of postprandial acid reflux (the acid pocket) 54 and have bioadhesive properties, allowing them to coat the oesophageal lining thereby improving its resistance to symptom‐provoking reflux constituents. 55 , 56 Another mucoadhesive formulation, based on hyaluronic acid‐chondroitin sulphate, has also been shown to improve symptoms in patients partially responding to PPIs. 57
A number of small studies suggest that increasing lower oesophageal sphincter (LOS) pressure with diaphragmatic breathing exercises may also be a useful adjunct to pharmacological therapy.Implementation of a standardised diaphragmatic breathing protocol led to significant improvements in acid exposure, reflux‐like symptoms, quality of life and a reduction in PPI usage.
There is rationale for using prokinetic medications to increase lower oesophageal sphincter pressure and augment peristalsis, but evidence of efficacy in controlled clinical trials, either as monotherapy or combined with PPIs, is disappointing and the risk of adverse events may be increased. 61 , 62
In many countries prokinetics are not generally available.
5.5. Gut–brain behavioural therapies and neuromodulators
Increased understanding of functional oesophageal disorders has accumulated over several decades, resulting in a recent resurgence in the field of psycho‐gastroenterology, which was largely set aside with the introduction of PPIs. Symptom‐specific psychotherapies can target visceral hypersensitivity and centrally mediated hyperalgesia (increased sensitivity to pain signals), as well as avoidance behaviours, hypervigilance and pain catastrophizing. 24 , 36 Applying these therapies depends on first gaining insight into the patient symptom experience because this often aligns poorly with the clinician’s initial assessment. Interpretation of symptoms as harmful or threatening can negatively impact health‐related quality of life (HRQOL) just as much as symptom severity and frequency. 63 Tools, including the Patient Health Questionnaire 12 (PHQ‐12) 64 and the Oesophageal Hypervigilance and Anxiety Scale (EHAS), 63 are easily administered during a typical consultation to identify somatisation and hypervigilance, respectively. The PHQ‐12 is a shortened form of the PHQ‐15 (excluding three GI‐specific questions) which can detect generalised hypersensitivity by assessing associated symptoms such as backache, limb pain, chest pain, palpitations, breathlessness, sexual dysfunction, lethargy and headache. The short form of EHAS is a reliable and validated tool, which can help understand the patient’s personal experience of symptoms. 63 EHAS score has been shown to correlate with symptom severity and psychological stress in GORD patients, but not with acid reflux burden or mucosal integrity. 65 However, elevation in EHAS does not necessarily indicate the need for psychological services; it may be that clarifying misunderstandings or simple relaxation strategies will be sufficient. 63 Diaphragmatic breathing has demonstrated benefits for reducing reflux events but is also a useful relaxation technique shown to reduce anxiety. 59 In some countries, social prescribing has become a routine element of primary care practice to address the link between psychological well‐being and physical health. Social prescribers help patients find relevant support services in the local community, anything from gardening or sports clubs to address social isolation, to financial advice services or assisted navigation of welfare entitlements. 66
When further behavioural intervention is required, key barriers are lack of access to professionals experienced in gut‐brain psychotherapies and/or resistance from the patient to acknowledge the psychological aspect of their condition. Clinician training and discussion aids for explaining the gut‐brain connection will help improve communication and patient acceptance, whereas forging networks with local specialists who can provide gut‐brain psychotherapy in person or remotely and/or the use of commercialised digital GI‐specific behavioural therapy packages has the potential to improve accessibility. 24 Gut‐directed hypnosis may help patients with somatisation, visceral hypersensitivity and attentional bias. For example, a study to investigate oesophageal‐directed hypnotherapy in patients with PPI‐refractory heartburn demonstrated consistent significant improvements in symptom severity, visceral anxiety and emotional quality of life over seven sessions. 26 Cognitive behavioural therapy may be more appropriate for pain catastrophising and fear of symptoms, as it can help teach patients new ways of thinking and reduce stress induced by their symptoms. 36 These approaches are usually implemented after PPI therapy has failed and investigation points to a functional diagnosis, a strategy which may itself compound fear and anxiety. It should also be noted that disordered perception can occur in patients with oesophagitis. A recent large‐scale study showed that symptomatic erosive oesophagitis was associated with psychological factors (state anxiety, depression), whereas asymptomatic erosive oesophagitis was not. 67 This suggests that behavioural intervention may be relevant for a proportion of patients of all GERD phenotypes.
Neuromodulators, including tricyclic antidepressants (TCAs) and selective serotonin reuptake inhibitors (SSRIs), are further treatment options for modulating pain threshold and oesophageal sensations. 68 , 69 , 70 , 71 It is important to explain clearly to the patient that these medications are being prescribed to modulate the gut‐brain axis and not to treat anxiety and depression. They should also be prepared for the fact that it can take up to 6 weeks for the medication to take effect68 and that they may experience some side effects in the first 7–10 days. To limit side effects, treatment is initiated at very low dose (5–10 mg) 72 , 73 and then titrated after 10 days according to the patient’s symptomatic response. In the experience of the authors, it is generally useful to consult with the patient about 2 months after starting treatment to assess tolerability and adjust the dose, and to reinforce the expected benefits of the medication.
6. CONCLUSION
Three key conclusions were drawn from the critical evaluation of current management of reflux‐like symptoms: (1) GERD is an inappropriate and misleading diagnosis when applied to non‐specific oesophageal symptoms. Reflux‐like symptoms have a complex multifactorial pathophysiology involving both perceptual and physiological disturbances and require a holistic, personalised management strategy; (2) The current therapeutic “ladder” strategy of addressing symptoms with the one‐dimensional approach of escalating acid inhibition is oversimplified with respect to symptoms and has resulted in PPIs being overused and overdosed, ignoring the importance of low‐harm interventions such as lifestyle advice and behavioural therapy; (3) The perceptual causes of reflux‐like symptoms (hypervigilance, visceral hypersensitivity and altered central processing) and lifestyle components (obesity, poor eating habits) should be identified and addressed early in management, either prior to or in combination with pharmacological intervention. Continuity of care and support is required through multi‐disciplinary referral networks and/or online tools for nutritional management, weight loss, exercise programmes and symptom‐specific behavioural therapies.
AUTHOR CONTRIBUTIONS
Pali Hungin: Conceptualization (equal); supervision (lead); writing – original draft (equal). Carmelo Scarpignato: Writing – review and editing (supporting). Laurie Keefer: Writing – review and editing (equal). Maura Corsetti: Writing – review and editing (supporting). Foteini Anastasiou: Writing – review and editing (supporting). Jean Muris: Writing – review and editing (supporting). Juan Mendive: Writing – review and editing (supporting). Peter Kahrilas: Conceptualization (equal); writing – original draft (equal); writing – review and editing (supporting).
AUTHORSHIP
Guarantor of the article: A. Pali S. Hungin.
ACKNOWLEDGEMENTS
Declaration of personal interests: All authors attended the online workshops organised and funded by Reckitt Benckiser Healthcare Ltd. Pali Hungin has served as a consultant for Sandoz and Chair of Rome IV Primary care committee. Carmelo Scarpignato has served as a consultant for Alfasigma, Dompé Farmaceutici, Shionogi and Biofarma Group. Jean Muris and Maura Corsetti have no further disclosures to declare. Juan Mendive has participated in educational activities funded by Reckitt Benckiser Healthcare Ltd. Foteini Anastasiou is a member of the Programme for NASH in Primary Health Care at the University of Crete, funded by Gilead. Laurie Keefer has served as a consultant for Abbvie and Eli Lilly, is cofounder and owns stocks and shares in Trellus Health and is on the Board of Directors for the Rome Foundation. Peter JK has served as a consultant for Ironwood, Reckitt and Johnson & Johnson.
Hungin AP, Scarpignato C, Keefer L, Corsetti M, Anastasiou F, Muris JW, et al. Review article: rethinking the “ladder” approach to reflux‐like symptom management in the era of PPI “resistance” ‐ a multidisciplinary perspective. Aliment Pharmacol Ther. 2022;55:1492–1500. 10.1111/apt.16930
Funding informationThe series of workshops on which this article is based were organised and funded by Reckitt Benckiser Healthcare Ltd. Editorial assistance was provided by Lisa O’Rourke PhD of Lumanity, UK, and funded by Reckitt Benckiser Healthcare Ltd.
The Handling Editor for this article was Dr Mike Burkitt, and this uncommissioned review was accepted for publication after full peer‐review.
REFERENCES
- 1. Nirwan JS, Hasan SS, Babar Z‐U‐D, Conway BR, Ghori MU. Global prevalence and risk factors of gastro‐oesophageal reflux disease (GORD). Systematic Review with Meta‐analysis Scientific reports. 2020;10(1):5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Katzka DA, Pandolfino JE, Kahrilas PJ. Phenotypes of gastroesophageal reflux disease: where Rome, Lyon, and Montreal meet. Clin Gastroenterol Hepatol. 2020;18(4):767–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vakil N, van Zanten SV, Kahrilas P, et al. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence‐based consensus. Am J Gastroenterol. 2006;101(8):1900–20; quiz 1943. [DOI] [PubMed] [Google Scholar]
- 4. Savarino E, Zentilin P, Savarino V. NERD: an umbrella term including heterogeneous subpopulations. Nat Rev Gastroenterol Hepatol. 2013;10(6):371–80. [DOI] [PubMed] [Google Scholar]
- 5. Scarpignato C, Gatta L, Zullo A, et al. Effective and safe proton pump inhibitor therapy in acid‐related diseases—a position paper addressing benefits and potential harms of acid suppression. BMC Med. 2016;14(1):179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. El‐Serag H, Becher A, Jones R. Systematic review: persistent reflux symptoms on proton pump inhibitor therapy in primary care and community studies. Aliment Pharmacol Ther. 2010;32(6):720–37. [DOI] [PubMed] [Google Scholar]
- 7. Delshad SD, Almario CV, Chey WD, Spiegel BMR. Prevalence of gastroesophageal reflux disease and proton pump inhibitor‐refractory symptoms. Gastroenterology. 2020;158(5):1250–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aziz Q, Fass R, Gyawali CP, Miwa H, Pandolfino JE, Zerbib F. Functional esophageal disorders. Gastroenterology. 2016;150:1368–79. 10.1053/j.gastro.2016.02.012 [DOI] [PubMed] [Google Scholar]
- 9. Hungin APS, Molloy‐Bland M, Scarpignato C. Revisiting Montreal: new insights into symptoms and their causes, and implications for the future of GERD. Am J Gastroenterol. 2019;114(3):414–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heidelbaugh JJ, Kim AH, Chang R, Walker PC. Overutilization of proton‐pump inhibitors: what the clinician needs to know. Therap Adv Gastroenterol. 2012;5(4):219–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hunt RH, East JE, Lanas A, Malfertheiner P, Satsangi J, Scarpignato C, et al. COVID‐19 and gastrointestinal disease: implications for the gastroenterologist. Dig Dis. 2021;39(2):119–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Euromonitor International . Consumer Health: Changes in consumer behaviour during Covid‐19 https://go.euromonitor.com/webinar‐health‐and‐wellness‐21‐05‐04‐changes‐in‐consumer‐health.html (accessed November 2021).
- 13. Drossman DA, Hasler WL. Rome IV‐functional GI disorders: disorders of gut‐brain interaction. Gastroenterology. 2016;150(6):1257–61. [DOI] [PubMed] [Google Scholar]
- 14. Gyawali CP, Fass R. Management of gastroesophageal reflux disease. Gastroenterology. 2018;154(2):302–18. [DOI] [PubMed] [Google Scholar]
- 15. Miner P Jr, Katz PO, Chen Y, Sostek M. Gastric acid control with esomeprazole, lansoprazole, omeprazole, pantoprazole, and rabeprazole: a five‐way crossover study. Am J Gastroenterol. 2003;98(12):2616–20. [DOI] [PubMed] [Google Scholar]
- 16.Li M‐J, Li Q, Sun M, Liu L‐Q. Comparative effectiveness and acceptability of the FDA‐licensed proton pump inhibitors for erosive esophagitis: a PRISMA‐compliant network meta‐analysis. Medicine 2017;96(39):e8120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Graham DY, Tansel A. Interchangeable use of proton pump inhibitors based on relative potency. Clin Gastroenterol Hepatol. 2018;16(6):800–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kirchheiner J, Glatt S, Fuhr U, Klotz U, Meineke I, Seufferlein T, et al. Relative potency of proton‐pump inhibitors‐comparison of effects on intragastric pH. Eur J Clin Pharmacol. 2009;65(1):19–31. [DOI] [PubMed] [Google Scholar]
- 19. Frye JW, Peura DA. Managing gastroesophageal reflux disease – comparative efficacy and outcomes of dexlansoprazole MR. Ther Clin Risk Manag. 2015;11:1649–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Howden CW. Review article: immediate‐release proton‐pump inhibitor therapy‐‐potential advantages. Aliment Pharmacol Ther. 2005;22(Suppl. 3):25–30. [DOI] [PubMed] [Google Scholar]
- 21. Zerbib F, Bredenoord AJ, Fass R, Kahrilas PJ, Roman S, Savarino E, et al. ESNM/ANMS consensus paper: diagnosis and management of refractory gastro‐esophageal reflux disease. Neurogastroenterol Motil. 2021;33(4):e14075. [DOI] [PubMed] [Google Scholar]
- 22. Jobe BA, Richter JE, Hoppo T, Peters JH, Bell R, Dengler WC, et al. Preoperative diagnostic workup before antireflux surgery: an evidence and experience‐based consensus of the esophageal diagnostic advisory panel. J Am Coll Surg. 2013;217(4):586–97. [DOI] [PubMed] [Google Scholar]
- 23. Keefer L, Palsson OS, Pandolfino JE. Best practice update: incorporating Psychogastroenterology into Management of Digestive Disorders. Gastroenterology. 2018;154(5):1249–57. [DOI] [PubMed] [Google Scholar]
- 24. Chey WD, Keefer L, Whelan K, Gibson PR. Behavioral and diet therapies in integrated care for patients with irritable bowel syndrome. Gastroenterology. 2021;160(1):47–62. [DOI] [PubMed] [Google Scholar]
- 25. Kahrilas PJ, Keefer L, Pandolfino JE. Patients with refractory reflux symptoms: what do they have and how should they be managed? Neurogastroenterol Motil. 2015;27(9):1195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Riehl ME, Pandolfino JE, Palsson OS, Keefer L. Feasibility and acceptability of esophageal‐directed hypnotherapy for functional heartburn. Dis Esophagus. 2016;29(5):490–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guadagnoli L, Yadlapati R, Taft T, Pandolfino JE, Tye M, Keefer L. Esophageal hypervigilance is prevalent across gastroesophageal reflux disease presentations. Neurogastroenterol Motil. 2021;33(8):e14081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Klausen KM, Bomme Høgh M, David M, Schaffalitzky de Muckadell OB, Hansen JM. How dyspepsia, gastroesophageal reflux symptoms, and overlapping symptoms affect quality of life, use of health care, and medication – a long‐term population based cohort study. Scand J Gastroenterol. 2021;56(7):753–60. [DOI] [PubMed] [Google Scholar]
- 29. Kahrilas PJ, Savarino E, Anastasiou F, Bredenoord AJ, Corsetti M, Lagergren J, et al. The tapestry of reflux syndromes: translating new insight into clinical practice. Br J Gen Pract. 2021;71(711):470–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hershcovici T, Zimmerman J. Functional heartburn vs. non‐erosive reflux disease: similarities and differences. Aliment Pharmacol Ther. 2008;27(11):1103–9. [DOI] [PubMed] [Google Scholar]
- 31. de Bortoli N, Frazzoni L, Savarino EV, Frazzoni M, Martinucci I, Jania A, et al. Functional heartburn overlaps with irritable bowel syndrome more often than GERD. Am J Gastroenterol. 2016;111(12):1711–7. [DOI] [PubMed] [Google Scholar]
- 32. Surdea Blaga T, Dumitrascu D, Galmiche JP, Bruley des Varannes S. Functional heartburn: clinical characteristics and outcome. Eur J Gastroenterol Hepatol. 2013;25(3):282–90. [DOI] [PubMed] [Google Scholar]
- 33. Ribolsi M, Cicala M, Zentilin P, Neri M, Mauro A, Efthymakis K, et al. Prevalence and clinical characteristics of refractoriness to optimal proton pump inhibitor therapy in non‐erosive reflux disease. Aliment Pharmacol Ther. 2018;48(10):1074–81. [DOI] [PubMed] [Google Scholar]
- 34. Choung RS, Locke GR III, Schleck CD, et al. Multiple functional gastrointestinal disorders linked to gastroesophageal reflux and somatization: a population‐based study. Neurogastroenterol Motil. 2017;29(7):e13041. 10.1111/nmo.13041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim SE, Chang L. Overlap between functional GI disorders and other functional syndromes: what are the underlying mechanisms? Neurogastroenterol Motil. 2012;24(10):895–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Keefer L, Ballou SK, Drossman DA, Ringstrom G, Elsenbruch S, Ljótsson B. A Rome working team report on brain‐gut behavior therapies for disorders of gut‐brain interaction. Gastroenterology. 2022;162(1):300–15. [DOI] [PubMed] [Google Scholar]
- 37. Balint E. A portrait of Michael Balint: the development of his ideas on the use of the drug "doctor". Int J Psychiatry Med. 1974;5(3):211–22. [DOI] [PubMed] [Google Scholar]
- 38. Katz PO, Dunbar KB, Schnoll‐Sussman FH, Greer KB, Yadlapati R, Spechler SJ. ACG clinical guideline for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2022;117(1):27–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kang JY. Lifestyle measures and reflux. Aliment Pharmacol Ther. 2000;8:1103. [DOI] [PubMed] [Google Scholar]
- 40. Nowak M, Büttner P, Raasch B, Daniell K, McCutchan C, Harrison S. Lifestyle changes as a treatment of gastroesophageal reflux disease: a survey of general practitioners in North Queensland, Australia. Ther Clin Risk Manag. 2005;1(3):219–24. [PMC free article] [PubMed] [Google Scholar]
- 41. Reimer C, Bytzer P. Perceptions and beliefs concerning gastroesophageal reflux disease: physicians and patients disagree. Digestion. 2007;76(3–4):229–34. [DOI] [PubMed] [Google Scholar]
- 42. Zhang M, Hou Z‐K, Huang Z‐B, Chen X‐L, Liu F‐B. Dietary and lifestyle factors related to gastroesophageal reflux disease: a systematic review. Ther Clin Risk Manag. 2021;17:305–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tosetti C, Savarino E, Benedetto E, De Bastiani R. Elimination of dietary triggers is successful in treating symptoms of gastroesophageal reflux disease. Dig Dis Sci. 2021;66(5):1565–71. [DOI] [PubMed] [Google Scholar]
- 44. de Bortoli N, Guidi G, Martinucci I, Savarino E, Imam H, Bertani L, et al. Voluntary and controlled weight loss can reduce symptoms and proton pump inhibitor use and dosage in patients with gastroesophageal reflux disease: a comparative study. Dis Esophagus. 2016;29(2):197–204. [DOI] [PubMed] [Google Scholar]
- 45. Scarpignato C, Tolone S. Addressing long‐term PPI safety. Dig Liver Dis. 2020;52(8):853–6. [DOI] [PubMed] [Google Scholar]
- 46. Farrell B, Pottie K, Thompson W, Boghossian T, Pizzola L, Rashid FJ, et al. Deprescribing proton pump inhibitors: evidence‐based clinical practice guideline. Can Fam Physician. 2017;63(5):354–64. [PMC free article] [PubMed] [Google Scholar]
- 47. Inadomi JM, McIntyre L, Bernard L, Fendrick AM. Step‐down from multiple‐ to single‐dose proton pump inhibitors (PPIs): a prospective study of patients with heartburn or acid regurgitation completely relieved with PPIs. Am J Gastroenterol. 2003;98(9):1940–4. [DOI] [PubMed] [Google Scholar]
- 48. Inadomi JM, Jamal R, Murata GH, Hoffman RM, Lavezo LA, Vigil JM, et al. Step‐down management of gastroesophageal reflux disease. Gastroenterology. 2001;121(5):1095–100. [DOI] [PubMed] [Google Scholar]
- 49. Targownik LE, Fisher DA, Saini SD. AGA clinical practice update on De‐prescribing of proton pump inhibitors: expert review. Gastroenterology. 2022;162(4):1334–42. [DOI] [PubMed] [Google Scholar]
- 50. Coyle C, Symonds R, Allan J, Dawson S, Russell S, Smith A, Daff C, Kotze H. Sustained proton pump inhibitor deprescribing among dyspeptic patients in general practice: a return to self‐management through a programme of education and alginate rescue therapy. A prospective interventional study. BJGP Open 2019;3(3)doi: 10.3399/bjgpopen19X101651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Murie J, Allen J, Simmonds R, de Wet C. Glad you brought it up: a patient‐centred programme to reduce proton‐pump inhibitor prescribing in general medical practice. Qual Prim Care. 2012;20(2):141–8. [PubMed] [Google Scholar]
- 52. Leiman DA, Riff BP, Morgan S, Metz DC, Falk GW, French B, et al. Alginate therapy is effective treatment for GERD symptoms: a systematic review and meta‐analysis. Dis Esophagus. 2017;30(5):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. National Institute for Health and Care Excellence . Gastro‐oesophageal reflux disease and dyspepsia in adults. Clinical guideline 184. September 2014. Gastro‐oesophageal reflux disease and dyspepsia in adults: investigation and management (nice.org.uk). [PubMed]
- 54. Kahrilas PJ, McColl K, Fox M, O'Rourke L, Sifrim D, Smout AJPM, et al. The acid pocket: a target for treatment in reflux disease? Am J Gastroenterol. 2013;108(7):1058–64. [DOI] [PubMed] [Google Scholar]
- 55. Sonmez S, Coyle C, Sifrim D, Woodland P. Duration of adhesion of swallowed alginates to distal oesophageal mucosa: implications for topical therapy of oesophageal diseases. Aliment Pharmacol Ther. 2020;52(3):442–8. [DOI] [PubMed] [Google Scholar]
- 56. Woodland P, Lee C, Duraisamy Y, et al. Assessment and protection of esophageal mucosal integrity in patients with heartburn without esophagitis. Am J Gastroenterol. 2013;108(4):535–43. [DOI] [PubMed] [Google Scholar]
- 57. Savarino V, Pace F, Scarpignato C. Randomised clinical trial: mucosal protection combined with acid suppression in the treatment of non‐erosive reflux disease—efficacy of Esoxx, a hyaluronic acid‐chondroitin sulphate based bioadhesive formulation. Aliment Pharmacol Ther. 2017;45(5):631–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Eherer AJ, Netolitzky F, Högenauer C, Puschnig G, Hinterleitner TA, Scheidl S, et al. Positive effect of abdominal breathing exercise on gastroesophageal reflux disease: a randomized, controlled study. Am J Gastroenterol. 2012;107(3):372–8. [DOI] [PubMed] [Google Scholar]
- 59. Ong AM, Chua LT, Khor CJ, et al. Diaphragmatic breathing reduces belching and proton pump inhibitor refractory gastroesophageal reflux symptoms. Clin Gastroenterol Hepatol. 2018;16(3):407–16. [DOI] [PubMed] [Google Scholar]
- 60. Halland M, Bharucha AE, Crowell MD, Ravi K, Katzka DA. Effects of diaphragmatic breathing on the pathophysiology and treatment of upright gastroesophageal reflux: a randomized controlled trial. Am J Gastroenterol. 2021;116(1):86–94. [DOI] [PubMed] [Google Scholar]
- 61. Sigterman KE, van Pinxteren B, Bonis PA, Lau J, Numans ME. Short‐term treatment with proton pump inhibitors, H2‐receptor antagonists and prokinetics for gastro‐oesophageal reflux disease‐like symptoms and endoscopy negative reflux disease. Cochrane Database Syst Rev 2013;2013(5):CD002095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ren LH, Chen WX, Qian LJ, Li S, Gu M, Shi RH. Addition of prokinetics to PPI therapy in gastroesophageal reflux disease: a meta‐analysis. World J Gastroenterol. 2014;20(9):2412–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Taft TH, Guadagnoli L, Carlson DA, Kou W, Keefer L, Pandolfino J. Validation of the short‐form esophageal hypervigilance and anxiety scale. Clin Gastroenterol Hepatol. 2021;20:e64–73. 10.1016/j.cgh.2020.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Spiller RC, Humes DJ, Campbell E, Hastings M, Neal KR, Dukes GE, et al. The patient health questionnaire 12 somatic symptom scale as a predictor of symptom severity and consulting behaviour in patients with irritable bowel syndrome and symptomatic diverticular disease. Aliment Pharmacol Ther. 2010;32(6):811–20. [DOI] [PubMed] [Google Scholar]
- 65. Wong MW, Liu TT, Yi CH, Lei WY, Hung JS, Cock C, et al. Oesophageal hypervigilance and visceral anxiety relate to reflux symptom severity and psychological distress but not to acid reflux parameters. Aliment Pharmacol Ther. 2021;54(7):923–30. [DOI] [PubMed] [Google Scholar]
- 66. Roland M, Everington S, Marshall M. Social prescribing—transforming the relationship between physicians and their patients. N Engl J Med. 2020;383(2):97–9. [DOI] [PubMed] [Google Scholar]
- 67. Choi JM, Yang JI, Kang SJ, Han YM, Lee J, Lee C, et al. Association between anxiety and depression and gastroesophageal reflux disease: Results from a large cross‐sectional study. J Neurogastroenterol Motil. 2018;24(4):593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Weijenborg PW, de Schepper HS, Smout AJ, Bredenoord AJ. Effects of antidepressants in patients with functional esophageal disorders or gastroesophageal reflux disease: a systematic review. Clin Gastroenterol Hepatol. 2015;13(2):251–9. [DOI] [PubMed] [Google Scholar]
- 69. Viazis N, Keyoglou A, Kanellopoulos AK, Karamanolis G, Vlachogiannakos J, Triantafyllou K, et al. Selective serotonin reuptake inhibitors for the treatment of hypersensitive esophagus: a randomized, double‐blind, placebo‐controlled study. Am J Gastroenterol. 2012;107(11):1662–7. [DOI] [PubMed] [Google Scholar]
- 70. Viazis N, Katopodi K, Karamanolis G, Denaxas K, Varytimiadis L, Galanopoulos M, et al. Proton pump inhibitor and selective serotonin reuptake inhibitor therapy for the management of noncardiac chest pain. Eur J Gastroenterol Hepatol. 2017;29(9):1054–8. [DOI] [PubMed] [Google Scholar]
- 71. Ostovaneh MR, Saeidi B, Hajifathalian K, Farrokhi‐Khajeh‐Pasha Y, Fotouhi A, Mirbagheri SS, et al. Comparing omeprazole with fluoxetine for treatment of patients with heartburn and normal endoscopy who failed once daily proton pump inhibitors: double‐blind placebo‐controlled trial. Neurogastroenterol Motil. 2014;26(5):670–8. [DOI] [PubMed] [Google Scholar]
- 72. Keefer L, Kahrilas PJ. Low‐dose tricyclics for esophageal hypersensitivity: is it all placebo effect? Am J Gastroenterol. 2016;2:225–7. [DOI] [PubMed] [Google Scholar]
- 73. Dickman R, Maradey‐Romero C, Fass R. The role of pain modulators in esophageal disorders—no pain no gain. Neurogastroenterol Motil. 2014;26(5):603–10. [DOI] [PubMed] [Google Scholar]


