Abstract
Interleukin‐22 (IL‐22) is a cytokine mainly produced by T cells and innate lymphoid cells (ILC). IL‐22 primarily targets non‐hematopoietic cells such as epithelial cells and fibroblasts. In the skin, IL‐22 promotes the proliferation of keratinocytes and dermal fibroblasts. IL‐22 furthermore regulates innate immune responses as it induces the production of antimicrobial proteins and neutrophil‐attracting chemokines. IL‐22 plays an important role in wound healing and in the protection against skin infections. However, IL‐22 can also contribute to the pathogenesis of several inflammatory skin diseases such as psoriasis, atopic dermatitis and allergic contact dermatitis. In this review, current information regarding the structure, function and regulation of IL‐22 is discussed with a special focus on the role of IL‐22 in the skin and in skin diseases.
Keywords: Cytokine, interleukin‐22, IL‐22 receptor, skin, inflammatory skin diseases, immunology
INTRODUCTION
Interleukin‐22 (IL‐22) belongs to the IL‐20 subfamily of the large IL‐10 family that, in addition to IL‐22, includes IL‐10, IL‐19, IL‐20, IL‐24, IL‐26, IL‐28A, IL‐28B and IL‐29 [1]. IL‐20 subfamily cytokines primarily act on epithelial cells and help maintain tissue integrity and restore homeostasis of epithelial layers during wound healing activities [1]. Additionally, IL‐22 and other IL‐20 subfamily cytokines induce the production of antimicrobial peptides that protect these cells from various pathogens [2]. In 2000, the il22 gene was identified in mouse T cells and was originally named IL‐10‐related T cell‐derived inducible factor (IL‐TIF) due to the homology with IL‐10 [3]. IL‐22 is mainly produced by T cells and ILC [4, 5]. In the skin, the IL‐22 receptor (IL‐22R) is expressed by keratinocytes and dermal fibroblasts [6]. Thus, IL‐22 facilitates the communication between leukocytes and skin cells, thereby enhancing tissue repair processes and defence mechanisms in the skin.
CELLULAR SOURCES AND REGULATION OF IL‐22 PRODUCTION
Several types of immune cells can produce IL‐22. The most well‐described cells to produce IL‐22 are CD4+ T cells, but other cells, such as ILC type 3 (ILC3), natural killer (NK) cells, CD8+ T cells, γδ+ T cells and dendritic cells (DC), also have the ability to produce IL‐22. IL‐22 production is dependent on stimulation of the cells by various cytokines and the activation of specific transcription factors, which are listed in Table 1 and described in the following sections.
Table 1.
Factors involved in promoting or inhibiting IL‐22 in various immune cells
| Cell type | Species | Promoting factors | Inhibiting factors | Transcription factors involved | References |
|---|---|---|---|---|---|
|
CD4+ αβ T cell |
Human |
IL‐1β IL‐6 IL‐23 TNF AhR agonist TGFβR inhibitor |
AhR antagonist RORγt inhibitor Vitamin D |
AhR RORγt VDR |
[4] |
| Human |
IL‐1β IL‐23 IL‐6 AhR agonist |
AhR siRNA RORγt siRNA |
AhR RORγt |
[14] | |
| Human |
TNF IL‐6 |
[13] | |||
| ILC3 | Mice |
IL‐1β IL‐23 RA |
RORγt RAR |
[15] | |
| Human | IL‐23 |
AhR RORγt |
[16] | ||
| NK cells | Human | IL‐23 |
AhR RORγt |
[17] | |
| Human |
IL‐1β IL‐23 |
AhR RORγt |
[20] | ||
| γδ+ T cells | Mice |
IL‐1β IL‐18 IL‐23 RA |
RAR inhibitor |
RORγt RAR |
[15] |
| Mice | AhR antagonist | AhR | [56] |
Early studies found that IL‐22 was secreted by mouse T lymphocytes in response to IL‐9 [3]. Later, it was found that Th17 cells, in addition to producing its signature cytokine IL‐17, also produced significant amounts of IL‐22 [7]. Despite IL‐17 and IL‐22 were reported to be co‐secreted from Th17 cells, these cytokines were regulated differently. The cytokines IL‐6, IL‐1β and TGFβ are essential for the development of IL‐17‐producing Th17 cells. Furthermore, the addition of IL‐23 led to the concomitant production of IL‐22 from these cells [8]. Another study found that TGFβ induces IL‐17 production whilst inhibiting IL‐22 production in Th17 cells [9]. This differential regulation of IL‐17 and IL‐22 was substantiated by investigating the transcription factors that regulate IL‐17 and IL‐22 production in Th17 cells. A central regulator of IL‐22 production was the aryl hydrocarbon receptor (AhR), and IL‐22 production was less dependent on the retinoic acid receptor (RAR)‐related orphan receptor gamma (RORγt) [10]. In contrast IL‐17 production was highly dependent on RORγt [11]. In 2009, a sub‐population of memory CD4+ T cells was defined as Th22 cells that produced high amounts of IL‐22 with little or no IL‐17 and IFNγ [12, 13]. This cellular subset expressed among others the skin‐homing chemokine receptors (CCR)4, and CCR10, indicating that Th22 cells and IL‐22 could play a central role for skin homeostasis and inflammation [12, 13, 14].
Subsequently, studies have tried to identify which cytokines and transcription factors that regulate the differentiation of Th22 cells in vitro. One study found that IL‐6 and TNF were important for IL‐22 production [12]. Another study reported that IL‐1β plus IL‐23 constituted the optimal cocktail for Th22 differentiation and that AhR and RORγt were the key transcription factors for IL‐22 secretion [14]. In accordance, a recent study demonstrated that inhibition of AhR and RORγt signalling reduced IL‐22 production in Th22 cells [4]. This study also found that the presence of IL‐6, TNFα, IL‐1β, IL‐23, AhR agonist (FICZ) and TGFβ receptor (TGFβR) inhibitor (galunisertib) constituted the optimal condition for the differentiation of Th22 cells in vitro.
In addition to CD4+ T cells, IL‐22 can also be produced by γδ+ T cells [15], ILC3 [15, 16] and NK cells [17]. IL‐22‐producing ILC3 residing in the skin and gut epithelium underscores that IL‐22 likely plays a role in homeostasis of these tissues [5]. Interestingly, as IL‐22‐producing T cells, ILC3 expresses RORγt and AhR that modulate the production of IL‐17 and IL‐22 [18, 19]. Accordingly, IL‐23 enhanced the development of IL‐22‐producing ILC3 [19]. In line with this, NK cells expressing IL‐22 (NK22) express both AhR and RORγt [17], and IL‐22 production from NK22 cells increased in the presence of IL‐1β and IL‐23 [20].
TGFβ and signalling from the TGFβR affects IL‐22 production. TGFβ is known for its involvement in CD4+ T cell differentiation, where it affects differentiation in a concentration‐dependent manner. Whereas high amounts of TGFβ are required for the generation of regulatory T cells (Treg), lower amounts of TGFβ enhance Th17 cell differentiation [9]. In vitro studies of Th17 cells have suggested that TGFβ inhibits IL‐22 production [8]. This is in line with studies demonstrating that inhibition of TGFβR signalling enhanced the production of IL‐22 in both mouse [21] and human CD4+ T cells [4, 13]. However, another study found that TGFβR signalling enhanced IL‐22 production in Th17 cells [22]. Thus, further studies are required to fully understand the role of TGFβ and TGFβR signalling in IL‐22 production.
AhR is involved in IL‐22 regulation. AhR is a cytoplasmic receptor that is activated by exogenous and endogenous molecules [23]. Several studies have reported that AhR signalling plays a key role in the differentiation and function of CD4+ T cells [9, 10, 12, 14]. It has recently been reported that AhR controls IL‐21‐induced IL‐22 production in murine CD4+ T cells in a STAT3‐dependent manner [24]. In addition, AhR activity promoted the development of Th17 cells and induces IL‐22 production from these cells [9]. In line with this, several studies have identified AhR as a key transcription factor controlling IL‐22 production in Th22 cells and γδ+ T cells [4, 12, 13, 14].
Vitamin D3 is well‐known for its immunomodulatory actions, and studies have elucidated the modulating role of vitamin D3 on T cell differentiation [25]. Recent studies have explored the effect of vitamin D3 on IL‐22‐producing cells such as Th17 and Th22 cells. It was found that vitamin D3 increased the secretion of TNF, IL‐6, IL‐1β and IL‐23 from dendritic cells (DC) leading to augmented IL‐22 secretion from CD4+ T cells [26]. In line with this, other studies found that vitamin D3 enhances plasmacytoid’s (pDC) ability to promote Th22 differentiation [12]. In contrast, another study demonstrated that the vitamin D3 analogue, calcipotriol, inhibited IL‐22 production in human Th17 cells [27]. This is in good agreement with a recent study, which found that vitamin D3 inhibited IL‐22 expression and secretion. This study identified a repressive vitamin D‐responsive element (VDRE) in the il22 promotor allowing for a direct inhibition of IL‐22 production by the active form of vitamin D3 [4]. Consequently, vitamin D3 may be considered as a potential therapeutic to modulate IL‐22‐driven disorders. The vitamin A metabolite retinoic acid (RA) also regulates IL‐22 production. However, in contrast to vitamin D, RA upregulates transcription of the il22 gene [15].
Toll‐like receptor (TLR) signalling has also been reported to induce IL‐22 production. This can either be due to a direct, IL‐23‐independent effect of TLR4 and TLR9 ligands on bone‐marrow‐derived DC [28] or an indirect, IL‐23‐dependent effect of TLR5 and TLR7/8 ligands on lamina propria DC [29] and cells in the skin [30], respectively. TLR7/8 ligands also indirectly increase IL‐22 production in the skin by stimulating IL‐36 production in the keratinocytes [31].
In addition to the factors that regulate IL‐22 production in immune cells described above, a cell‐extrinsic factor that regulates IL‐22 function exists. This is the IL‐22‐binding protein (IL‐22BP), which strongly binds IL‐22 and thereby controls the bioavailability and activity of IL‐22 [32]. IL‐22BP has high homology with the IL‐22R1 chain of the IL‐22R; however, IL‐22BP binds IL‐22 with much higher affinity compared with the IL‐22R1 subunit [32], explaining its sequestering effect on IL‐22. IL‐22BP is secreted by both immune cells and epithelial cells [33]. During homeostasis, IL‐22BP is highly expressed in immature DC and keratinocytes, and IL‐22BP is thought to neutralize the effects of IL‐22. In DC, IL‐22BP expression is controlled by the inflammasome and RA [33, 34]. During acute inflammation and tissue damage, DC become activated and the activation of the inflammasome results in the inhibition of IL‐22BP secretion [34]. Thus, the bioavailability of IL‐22 increases, allowing IL‐22 to mediate tissue repair at the epithelium. Furthermore, it has been reported that deletion of the IL‐22BP gene results in exacerbated and uncontrolled skin inflammation [35].
In summary, CD4+ and CD8+ T cells, γδ+ T cells, NK cells and ILC3 represent sources of IL‐22. Various cytokines are involved in IL‐22 regulation. These include IL‐1β, IL‐6, IL‐7, IL‐18, IL‐21, IL‐23, TGFβ and TNF. Furthermore, the transcription factors AhR, RORγt and STAT3 play essential roles in regulating il22 transcription. Furthermore, vitamin D3 and vitamin A also modulate IL‐22 expression and lastly IL‐22BP controls the bioavailability of IL‐22 (Fig. 1).
Fig. 1.
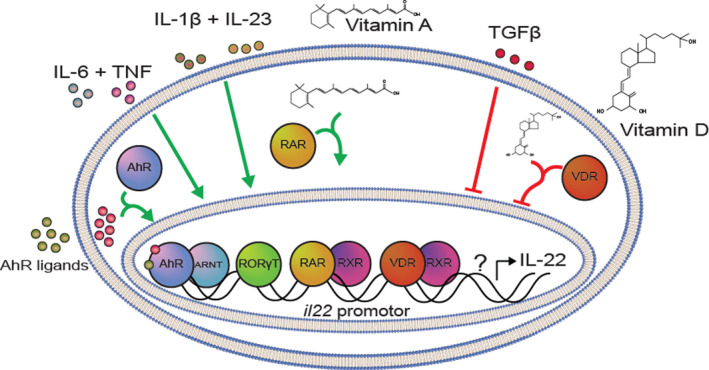
Proposed model for IL‐22 gene regulation. Various cytokines and other factors affect il22 expression and IL‐22 secretion. Some of these factors upregulate IL‐22 levels, whereas others are seen to downregulate IL‐22. The cytokines IL‐6, TNF, IL‐1β and IL‐23 have all been shown to increase IL‐22 levels. This is possibly due to their actions in upregulating the activity of the transcription factor, RORγt, that can directly bind to the promoter region of the il22 gene and mediate transcription. Furthermore, the activity of the transcription factor, AhR, has also been found to regulate IL‐22 expression and production. Thus, ligands that bind and activate AhR (such as the AhR agonist, FICZ), lead to increased IL‐22, whereas inhibition of AhR activity (such as with the AhR antagonist, CH‐223191) leads to decreased IL‐22 levels. Additionally, vitamin A has been shown to augment IL‐22 production. Upon binding to the RAR, the complex translocates to the nucleus, where it together with RXR forms a complex that can upregulate expression of the il22 gene. Other factors inhibit IL‐22 levels. Signalling by TGFβ through the TGFβ receptor has been found to decrease IL‐22 levels. Furthermore, it has recently been shown that vitamin D3 exerts an inhibitory effect on IL‐22. This is due to the presence of a negative vitamin D‐response element (VDRE) in the promotor‐region of the il22 gene. Upon interaction between vitamin D3 and VDR, this complex translocates to the nucleus, where it together with RXR forms a heterodimer that can downregulate expression of the il22 gene and consequently IL‐22 secretion is inhibited. Furthermore, there is most likely also other regulatory factors of IL‐22, which is indicated by the question mark in the figure. AhR, aryl hydrocarbon receptor; ARNT, AhR nuclear translocator; RAR, retinoic acid receptor; RORγt, RAR‐related orphan receptor γ; RXR, retinoid X receptor; TGFβ, transforming growth factor β; TNF, tumor necrosis factor; VDR, vitamin D receptor.
STRUCTURE EXPRESSION AND FUNCTION OF THE IL‐22 RECEPTOR
The IL‐22R is a heterodimer consisting of the IL‐22R1 and the IL‐10R2 chains [36] (Fig. 2). IL‐22 binds with high affinity to the extracellular part of the IL‐22R1 chain [37]. It is believed that IL‐22 binding to IL‐22R1 leads to a conformational change that enhances binding of the IL‐22‐IL‐22R1 complex to the IL‐10R2 chain [37]. Whereas the IL‐10R2 chain is ubiquitously expressed, the IL‐22R1 chain is mainly expressed by epithelial cells and fibroblasts located in the skin, intestine, lung, liver, kidney and pancreas [6].
Fig. 2.
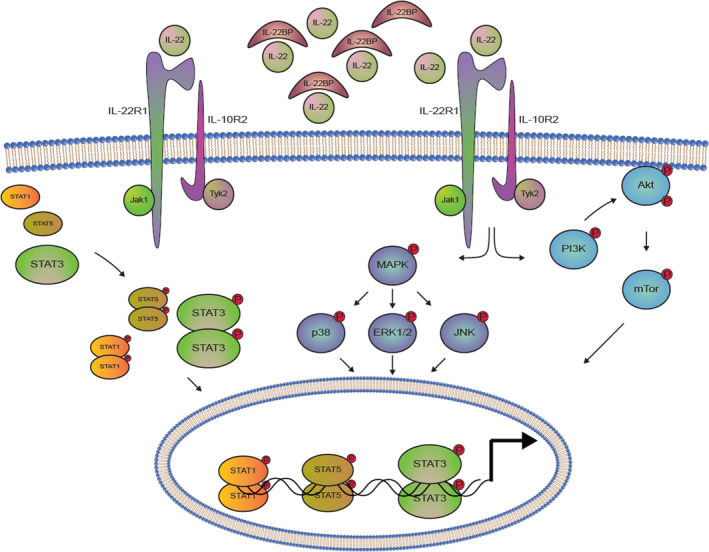
IL‐22R and its intracellular signalling. The heterodimeric IL‐22R consists of the IL‐22R1 and the IL‐10R2. When IL‐22 is not sequestered by the soluble protein, IL‐22BP, it is able to bind the IL‐22 receptor. Upon this binding, intracellular signalling is initiated, which starts with the activation of the receptor‐associated JAKs and TYKs. Activation of these kinases mediates the phosphorylation of various STAT molecules, with STAT3 phosphorylation being the most pronounced. This phosphorylation allows STAT3 to form homodimers, which can translocate to the nucleus and regulate the transcription of STAT3‐responsive genes. Furthermore, STAT1 and STAT5 molecules are also activated. IL‐22R signalling is also seen to activate the MAPK pathways involving ERK1/2, JNK and p38, as well as the PI3K‐Akt‐mTOR pathway. Akt, protein kinase B; ERK, extracellular signal‐regulated kinase; IL‐10R, IL‐10 receptor; IL‐22BP, IL‐22‐binding protein; IL‐22R, IL‐22 receptor; JAK, Janus kinases; JNK, c‐Jun N‐terminal kinase; MAPK, mitogen‐activated protein kinase; mTOR, mammalian target of rapamycin; p38, p38 mitogen‐activated protein kinase; PI3K, phosphoinositide 3‐kinase; STAT, signal transducer of activated T cells; TYK, tyrosine kinases.
Binding of IL‐22 to the IL‐22R activates several intracellular signalling pathways most importantly the JAK/STAT pathway [38] (Fig. 2). Thus, IL‐22 binding to the IL‐22R results in the activation of JAK1 and TYK2 that phosphorylate specific tyrosine residues in the cytoplasmic tail of the IL‐22R chains. The phosphorylated IL‐22R chains attract STAT3 molecules, which are then phosphorylated by the activated JAK1. STAT3 phosphorylation induces the formation of STAT3 homodimers that translocate to the nucleus where they regulate expression of STAT3‐responsive genes. In addition to STAT3, STAT1 and STAT5 play a role in IL‐22R signalling, although to a lesser extent than STAT3 [38]. Other signalling pathways than the JAK/STAT pathway are also activated by IL‐22. These include the phosphoinositide 3‐kinase (PI3K)‐AKT‐mammalian target of rapamycin (mTOR) pathway and the mitogen‐activated protein kinase (MAPK) pathways (ERK1/2, Jun N‐terminal kinase (JNK) and p38 kinase [38] (Fig. 2).
IL‐22 IN SKIN HOMEOSTASIS, WOUND HEALING AND INFLAMMATION
The skin is a highly specialized organ, and the keratinocytes play vital roles for a functional skin barrier and the defence against various environmental microbes and chemicals. IL‐22 affects keratinocytes’ functions as it inhibits the production of several proteins involved in terminal keratinocyte differentiation such as keratin 1 and 10 (KT1/10), involucrin, profilaggrin, loricrin and desmocollin [39, 40, 41]. Thus, IL‐22 affects keratinocyte proliferation, migration and maturation [42]. IL‐22 has also been shown to aid wound healing processes [41, 43]. A study found that IL‐22 was upregulated upon wounding in a mouse model, and as a result, keratinocyte differentiation was inhibited [41]. Furthermore, lack of IL‐22 in mice led to major defects in wound healing processes [43]. In this study, IL‐22‐/‐ mice exerted severely impaired wound healing, but this could to some extent be rescued by exogenous addition of recombinant IL‐22. Moreover, studies have found that IL‐22 induces the expression of anti‐apoptotic genes (e.g. Bcl‐2 and Bcl‐xl) and matrix metalloproteinases (e.g. MMP1/3) that enhance cell proliferation, remodelling of the epidermis and tissue repair mechanisms [39, 41].
In addition to affecting the keratinocytes during wound healing, IL‐22 enhances the antimicrobial responses of the keratinocytes through the induction of antimicrobial peptides such as β‐defensins 2/3, SA1007, 1008, 1009 and lipocalin‐2 [39]. Furthermore, IL‐22 stimulates keratinocytes to release several leukocyte‐attracting chemokines such as chemokine (C‐X‐C motif) ligand (CXCL) 1, CXCL2, CXCL5 and CXCL8 that contributes to the recruitment of leukocytes to the sites of infection [40].
In summary, IL‐22 is a cytokine that plays an essential role in skin homeostasis, wound healing and inflammation. In line with this, dysregulation of IL‐22 and IL‐22‐producing immune cells is associated with several inflammatory skin disorders including psoriasis, atopic dermatitis (AD) and allergic contact dermatitis (ACD).
IL‐22 IN SKIN DISEASES
Psoriasis
Psoriasis is an inflammatory skin disease that affects approximately 2% of the Caucasian population with clinical symptoms such as red, itchy, dry, rough and scaly skin. Signature histological features of psoriatic skin include thickening of the epidermis (acanthosis), hyperproliferation of keratinocytes (hyperkeratosis) and infiltration of immune cells in the dermis and epidermis [44]. Psoriasis is caused by a dysregulation of immune cells and cytokine secretion in the skin [45]. Specifically, Th17 cell activation and IL‐17 are believed to be the main drivers of the pathogenesis of the disease [44]. In addition, it has been reported that IL‐22‐producing cells and dysregulated IL‐22 levels are involved in the pathogenesis of psoriasis [39]. Thus, elevated IL‐22 expression levels in the skin and peripheral blood from patients suffering from psoriasis compared with healthy subjects have been observed [39]. Furthermore, specific genetic variants of IL‐22 lead to altered skin barrier functions and this has been associated with the early onset of psoriasis and correlated with disease severity [46]. In addition to this, an impaired production of IL‐22BP has been linked to the aggravation of skin inflammation in patients suffering from psoriasis [35]. These observations strongly indicate that IL‐22 takes part in the pathogenesis of psoriasis in humans. This is supported by numerous studies that have demonstrated a central role of IL‐22 in different mouse models of psoriasis [30, 40, 47, 48].
Atopic dermatitis
AD is a heterogeneous inflammatory disorder of the skin that affects approximately 3% of the population worldwide. AD signature symptoms include dry, itchy skin with red to brownish‐grey patches [49]. Immunologically, AD is characterized by the dominance of skin‐homing Th2 cells that produce IL‐4 and IL‐13. These Th2 cells are believed to drive the onset and pathogenesis of the disease. Additionally, high levels of IL‐22 are found in skin and blood of patients suffering from acute and chronic moderate‐to‐severe AD [50]. The elevated IL‐22 concentrations are correlated with epidermal hyperplasia, acanthosis and skin barrier defects [50]. In patients suffering from AD, IL‐22‐producing T cells have been identified as the major sources of IL‐22, and even higher frequencies of IL‐22‐producing T cells are found in patients suffering from severe AD than in patients suffering from psoriasis [49, 51]. Therefore, given the pathogenic role of IL‐22 in the development of AD, neutralizing‐IL‐22 treatments are being studied. The first double‐blinded clinical trial using IL‐22 blocking monoclonal antibodies (fezakinumab) improved the clinical symptoms in adults suffering from severe, chronic AD [52]. Subsequently, another study explored the molecular effects of fezakinumab in the skin from these patients compared with placebo. An improvement of epidermal inflammation and molecular skin changes were most robustly observed in patients that had high IL‐22 background levels. This underscores that patients suffering from AD could advantageously be stratified towards different and more precise medical treatment options [53].
Allergic contact dermatitis
ACD is a common inflammatory T cell‐mediated skin disease affecting about 10% of the adult population [54]. ACD can be highly disabling, and it is characterized by an intensely itching erythema, oedema and often vesicles at sites where the allergens contact the skin [54]. High IL‐22 serum levels have been observed in patients suffering from ACD to nickel [55], and a considerable infiltration of IL‐22‐secreting T cells is found in the skin after re‐exposure to nickel [56, 57]. Likewise, the expression of IL‐20 subfamily cytokines, including IL‐19, ‐20, ‐22 and 24, is increased in affected skin from para‐phenylenediamine (PPD) allergic patients compared with unaffected skin [58]. Interestingly, studies in mice did not support the implication of IL‐22 but rather IL‐24 in PPD‐induced ACD [58]. In contrast, other studies in mice supported a pathogenic role of IL‐22 in ACD. Thus, the inflammatory response in oxazolone‐induced ACD was increased in mice lacking the IL‐22BP [59] and prostaglandin E2 promoted oxazolone‐induced ACD by facilitating IL‐22 production from T cells [60]. These studies suggest that IL‐22 can be involved in the pathogenesis of ACD; however, further studies are required to establish the exact role of IL‐22 in ACD in humans.
FINAL REMARKS
IL‐22 is a cytokine that mediates communication between the immune system and tissue barriers, and it plays a central role in skin homeostasis and inflammation but also in the pathogenesis of various skin disorders. Thus, increased IL‐22 levels have been found and linked to several inflammatory skin diseases including psoriasis, AD and ACD. Blocking of IL‐22 is being considered and studied as potential therapy for these diseases. However, the exact mechanisms behind the regulation of IL‐22 and the exact role of IL‐22 in the onset and development of psoriasis, AD and ACD remain to be determined. Increased knowledge on the regulation and function of IL‐22 will provide a better basis for the development of potential IL‐22 therapies against inflammatory skin diseases.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
The authors thank the "T cell Biology and Skin Inflammation Group" at the University of Copenhagen. We would also like to extend our special thanks to Carsten Geisler for valuable discussions and guidance regarding the content of this review.
Lopez DV, Kongsbak‐Wismann M. Role of IL‐22 in homeostasis and diseases of the skin. APMIS. 2022; 130: 314–322.
References
- 1. Rutz S, Wang X, Ouyang W. The IL‐20 subfamily of cytokines‐from host defence to tissue homeostasis. Nat Rev Immunol. 2014;14:783–95. [DOI] [PubMed] [Google Scholar]
- 2. Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, et al. Interleukin‐22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–9. [DOI] [PubMed] [Google Scholar]
- 3. Dumoutier L, Louahed J, Renauld J‐C. Cloning and characterization of IL‐10‐related T cell‐derived inducible factor (IL‐TIF), a novel cytokine structurally related to IL‐10 and inducible by IL‐9. J Immunol. 2000;164:1814–9. [DOI] [PubMed] [Google Scholar]
- 4. Lopez DV, Al‐Jaberi FAH, Damas ND, Weinert BT, Pus U, Torres‐Rusillo S, et al. Vitamin D inhibits IL‐22 production through a repressive vitamin D response element in the il22 promoter. Front Immunol. 2021;12:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sawa S, Cherrier M, Lochner M, Satoh‐Takayama N, Fehling HJ, Langa F, et al. Lineage relationship analysis of RORγt+ innate lymphoid cells. Science. 2010;330:665–9. [DOI] [PubMed] [Google Scholar]
- 6. Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL‐22 increases the innate immunity of tissues. Immunity. 2004;21:241–54. [DOI] [PubMed] [Google Scholar]
- 7. Liang SC, Tan X‐Y, Luxenberg DP, Karim R, Dunussi‐Joannopoulos K, Collins M, et al. Interleukin (IL)‐22 and IL‐17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frazer LC, Scurlock AM, Zurenski MA, Riley MM, Mintus M, Pociask DA, et al. IL‐23 induces IL‐22 and IL‐17 production in response to Chlamydia muridarum genital tract infection, but the absence of these cytokines does not influence disease pathogenesis. Am J Reprod Immunol. 2013;70:472–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rutz S, Noubade R, Eidenschenk C, Ota N, Zeng W, Zheng Y, et al. Transcription factor c‐Maf mediates the TGF‐beta‐dependent suppression of IL‐22 production in TH17 cells. Nat Immunol. 2011;12:1238–47. [DOI] [PubMed] [Google Scholar]
- 10. Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld J‐C, et al. The aryl hydrocarbon receptor links TH17‐cell‐mediated autoimmunity to environmental toxins. Nature. 2008;453:106–10. [DOI] [PubMed] [Google Scholar]
- 11. Ivanov II, Mckenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, et al. The orphan nuclear receptor RORgt directs the differentiation program of proinflammatory IL‐17+ T helper cells. Cell. 2006;126:1121–33. [DOI] [PubMed] [Google Scholar]
- 12. Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin‐homing memory T cells. Nat Immunol. 2009;10:857–63. [DOI] [PubMed] [Google Scholar]
- 13. Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest. 2009;119:3573–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from TH‐17, TH1 and TH2 cells. Nat Immunol. 2009;10:864–72. [DOI] [PubMed] [Google Scholar]
- 15. Mielke LA, Jones SA, Raverdeau M, Higgs R, Stefanska A, Groom JR, et al. Retinoic acid expression associates with enhanced IL‐22 production by γδ T cells and innate lymphoid cells and attenuation of intestinal inflammation. J Exp Med. 2013;210:1117–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Takatori H, Kanno Y, Watford WT, Tato CM, Weiss G, Ivanov II, et al. Lymphoid tissue inducer‐like cells are an innate source of IL‐17 and IL‐22. J Exp Med. 2009;206:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JKM, et al. A human natural killer cell subset provides an innate source of IL‐22 for mucosal immunity. Nature. 2009;457:722–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gladiator A, Wangler N, Trautwein‐Weidner K, LeibundGut‐Landmann S. Cutting edge: IL‐17–secreting innate lymphoid cells are essential for host defense against fungal infection. J Immunol. 2013;190:521–5. [DOI] [PubMed] [Google Scholar]
- 19. Lee JS, Cella M, McDonald KG, Garlanda C, Kennedy GD, Nukaya M, et al. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat Immunol. 2012;13:144–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tang Q, Ahn YO, Southern P, Blazar BR, Miller JS, Verneris MR. Development of IL‐22‐producing NK lineage cells from umbilical cord blood hematopoietic stem cells in the absence of secondary lymphoid tissue. Blood. 2011;117:4052–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Plank MW, Kaiko GE, Maltby S, Weaver J, Tay HL, Shen W, et al. Th22 cells form a distinct Th lineage from Th17 cells in vitro with unique transcriptional properties and Tbet‐dependent Th1 plasticity. J Immunol. 2017;198:2182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Perez LG, Kempski JM, McGee H, Pelzcar P, Agalioti T, Giannou A, et al. TGF‐β signaling in Th17 cells promotes IL‐22 production and colitis‐associated colon cancer. Nat Commun. 2020;11:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rothhammer V, Quintana FJ. The aryl hydrocarbon receptor: an environmental sensor integrating immune responses in health and disease. Nat Rev Immunol. 2019;19:184–97. [DOI] [PubMed] [Google Scholar]
- 24. Yeste A, Mascanfroni ID, Nadeau M, Burns EJ, Tukpah A‐M, Santiago A, et al. IL‐21 induces IL‐22 production in CD4+ T cells. Nat Commun. 2014;5:3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Al‐Jaberi FAH, Kongsbak‐Wismann M, Aguayo‐Orozco A, Krogh N, Buus TB, Lopez DV, et al. Impaired vitamin D signaling in T cells from a family with hereditary vitamin D resistant rickets. Front Immunol. 2021;12:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sommer A, Fabri M. Vitamin D regulates cytokine patterns secreted by dendritic cells to promote differentiation of IL‐22‐Producing T cells. PLoS One. 2015;10:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lovato P, Norsgaard H, Tokura Y, Røpke MA. Calcipotriol and betamethasone dipropionate exert additive inhibitory effects on the cytokine expression of inflammatory dendritic cell‐Th17 cell axis in psoriasis. J Dermatol Sci. 2016;81:153–64. [DOI] [PubMed] [Google Scholar]
- 28. Fumagalli S, Torri A, Papagna A, Citterio S, Mainoldi F, Foti M. IL‐22 is rapidly induced by pathogen recognition receptors stimulation in bone‐marrow‐derived dendritic cells in the absence of IL‐23. Sci Rep. 2016;6:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kinnebrew M, Buffie C, Diehl G, Zenewicz L, Leiner I, Hohl T, et al. Interleukin 23 production by intestinal CD103 +CD11b + dendritic cells in response to bacterial flagellin enhances mucosal innate immune defense. Immunity. 2012;36:276–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Van Belle AB, de Heusch M, Lemaire MM, Hendrickx E, Warnier G, Dunussi‐Joannopoulos K, et al. IL‐22 is required for imiquimod‐induced psoriasiform skin inflammation in mice. J Immunol. 2012;188:462–9. [DOI] [PubMed] [Google Scholar]
- 31. Goldstein JD, Bassoy EY, Caruso A, Palomo J, Rodriguez E, Lemeille S, et al. IL‐36 signaling in keratinocytes controls early IL‐23 production in psoriasis‐like dermatitis. Life Sci Alliance. 2020;3:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kotenko SV, Izotova LS, Mirochnitchenko OV, Esterova E, Dickensheets H, Donnelly RP, et al. Identification, cloning, and characterization of a novel soluble receptor that binds IL‐22 and neutralizes its activity. J Immunol. 2001;166:7096–103. [DOI] [PubMed] [Google Scholar]
- 33. Martin JC, Bériou G, Heslan M, Chauvin C, Utriainen L, Aumeunier A, et al. Interleukin‐22 binding protein (IL‐22BP) is constitutively expressed by a subset of conventional dendritic cells and is strongly induced by retinoic acid. Mucosal Immunol. 2014;7:101–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huber S, Gagliani N, Zenewicz LA, Huber FJ, Bosurgi L, Hu BO, et al. IL‐22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature. 2012;491:259–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Martin JC, Wolk K, Bériou G, Abidi A, Witte‐Händel E, Louvet C, et al. Limited presence of IL‐22 binding protein, a natural IL‐22 inhibitor, strengthens psoriatic skin inflammation. J Immunol. 2017;198:3671–9. [DOI] [PubMed] [Google Scholar]
- 36. Xie M‐H, Aggarwal S, Ho W‐H, Foster J, Zhang Z, Stinson J, et al. Interleukin (IL)‐22, a novel human cytokine that signals through the interferon receptor‐related proteins CRF2‐4 and IL‐22R. J Biol Chem. 2000;275:31335–9. [DOI] [PubMed] [Google Scholar]
- 37. Logsdon NJ, Jones BC, Josephson K, Cook J, Walter MR. Soluble receptor complexes. J Interf Cytokine Res. 2002;22:1099–112. [DOI] [PubMed] [Google Scholar]
- 38. Lejeune D, Dumoutier L, Constantinescu S, Kruijer W, Schuringa JJ, Renauld JC. Interleukin‐22 (IL‐22) activates the JAK/STAT, ERK, JNK, and p38 MAP kinase pathways in a rat hepatoma cell line: Pathways that are shared with and distinct from IL‐10. J Biol Chem. 2002;277:33676–82. [DOI] [PubMed] [Google Scholar]
- 39. Wolk K, Witte E, Wallace E, Döcke W‐D, Kunz S, Asadullah K, et al. IL‐22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol. 2006;36:1309–23. [DOI] [PubMed] [Google Scholar]
- 40. Wolk K, Haugen HS, Xu W, Witte E, Waggie K, Anderson M, et al. IL‐22 and IL‐20 are key mediators of the epidermal alterations in psoriasis while IL‐17 and IFN‐γ are not. J Mol Med. 2009;87:523–36. [DOI] [PubMed] [Google Scholar]
- 41. Boniface K, Bernard F‐X, Garcia M, Gurney AL, Lecron J‐C, Morel F. IL‐22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol. 2005;174:3695–702. [DOI] [PubMed] [Google Scholar]
- 42. Sabat R, Ouyang W, Wolk K. Therapeutic opportunities of the IL‐22‐IL‐22R1 system. Nat Rev Drug Discov. 2014;13:21–38. [DOI] [PubMed] [Google Scholar]
- 43. McGee HM, Schmidt BA, Booth CJ, Yancopoulos GD, Valenzuela DM, Murphy AJ, et al. IL‐22 promotes fibroblast‐mediated wound repair in the skin. J Invest Dermatol. 2013;133:1321–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496–509. [DOI] [PubMed] [Google Scholar]
- 45. Griffiths CEM, Barker JNWN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370:263–71. [DOI] [PubMed] [Google Scholar]
- 46. Nikamo P, Cheuk S, Lysell J, Enerbäck C, Bergh K, Xu Landén N, et al. Genetic variants of the IL22 promoter associate to onset of psoriasis before puberty and increased IL‐22 production in T cells. J Invest Dermatol. 2014;134:1535–41. [DOI] [PubMed] [Google Scholar]
- 47. Ma HL, Liang S, Li J, Napierata L, Brown T, Benoit S, et al. IL‐22 is required for Th17 cell‐mediated pathology in a mouse model of psoriasis‐like skin inflammation. J Clin Invest. 2008;118:597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham‐Anderson J, Wu J, et al. Interleukin‐22, a TH17 cytokine, mediates IL‐23‐induced dermal inflammation and acanthosis. Nature. 2007;445:648–51. [DOI] [PubMed] [Google Scholar]
- 49. Guttman‐Yassky E, Nograles KE, Krueger JG. Contrasting pathogenesis of atopic dermatitis and psoriasis – Part II: immune cell subsets and therapeutic concepts. J Allergy Clin Immunol. 2011;127:1420–32. [DOI] [PubMed] [Google Scholar]
- 50. Gittler JK, Shemer A, Suárez‐Fariñas M, Fuentes‐Duculan J, Gulewicz KJ, Wang CQF, et al. Progressive activation of TH2/TH22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J Allergy Clin Immunol. 2012;130:1344–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nograles KE, Zaba LC, Shemer A, Fuentes‐Duculan J, Cardinale I, Kikuchi T, et al. IL‐22‐producing “T22” T cells account for upregulated IL‐22 in atopic dermatitis despite reduced IL‐17‐producing TH17 T cells. J Allergy Clin Immunol. 2009;123:1244–52.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Guttman‐Yassky E, Brunner PM, Neumann AU, Khattri S, Pavel AB, Malik K, et al. Efficacy and safety of fezakinumab (an IL‐22 monoclonal antibody) in adults with moderate‐to‐severe atopic dermatitis inadequately controlled by conventional treatments: a randomized, double‐blind, phase 2a trial. J Am Acad Dermatol. 2018;78:872–81.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Brunner PM, Pavel AB, Khattri S, Leonard A, Malik K, Rose S, et al. Baseline IL‐22 expression in patients with atopic dermatitis stratifies tissue responses to fezakinumab. J Allergy Clin Immunol. 2019;143:142–54. [DOI] [PubMed] [Google Scholar]
- 54. Martin SF, Rustemeyer T, Thyssen JP. Recent advances in understanding and managing contact dermatitis. F1000 Res. 2018;7:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ricciardi L, Minciullo PL, Saitta S, Trombetta D, Saija A, Gangemi S. Increased serum levels of IL‐22 in patients with nickel contact dermatitis. Contact Dermatitis. 2009;60:57–9. [DOI] [PubMed] [Google Scholar]
- 56. Larsen JM, Bonefeld CM, Poulsen SS, Geisler C, Skov L. IL‐23 and TH17‐mediated inflammation in human allergic contact dermatitis. J Allergy Clin Immunol. 2009;123:486–92.e1. [DOI] [PubMed] [Google Scholar]
- 57. Dyring‐Andersen B, Skov L, Løvendorf MB, Bzorek M, Søndergaard K, Lauritsen JPH, et al. CD4+ T cells producing interleukin (IL)‐17, IL‐22 and interferon‐γ are major effector T cells in nickel allergy. Contact Dermatitis. 2013;68:339–47. [DOI] [PubMed] [Google Scholar]
- 58. Van Belle AB, Cochez PM, de Heusch M, Pointner L, Opsomer R, Raynaud P, et al. IL‐24 contributes to skin inflammation in para‐phenylenediamine‐induced contact hypersensitivity. Sci Rep. 2019;9:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lindahl H, Martini E, Brauner S, Nikamo P, Gallais Serezal I, Guerreiro‐Cacais AO, et al. IL‐22 binding protein regulates murine skin inflammation. Exp Dermatol. 2017;26:444–6. [DOI] [PubMed] [Google Scholar]
- 60. Robb CT, McSorley HJ, Lee J, Aoki T, Yu C, Crittenden S, et al. Prostaglandin E 2 stimulates adaptive IL‐22 production and promotes allergic contact dermatitis. J Allergy Clin Immunol. 2018;141:152–62. [DOI] [PMC free article] [PubMed] [Google Scholar]


