Abstract
Seabird species world‐wide are integral to both marine and terrestrial environments, connecting the two systems by transporting vast quantities of marine‐derived nutrients and pollutants to terrestrial breeding, roosting and nesting grounds via the deposition of guano and other allochthonous inputs (e.g. eggs, feathers).
We conducted a systematic review and meta‐analysis and provide insight into what types of nutrients and pollutants seabirds are transporting, the influence these subsidies are having on recipient environments, with a particular focus on soil, and what may happen if seabird populations decline.
The addition of guano to colony soils increased nutrient levels compared to control soils for all seabirds studied, with cascading positive effects observed across a range of habitats. Deposited guano sometimes led to negative impacts, such as guanotrophication, or guano‐induced eutrophication, which was often observed where there was an excess of guano or in areas with high seabird densities.
While the literature describing nutrients transported by seabirds is extensive, literature regarding pollutant transfer is comparatively limited, with a focus on toxic and bioaccumulative metals. Research on persistent organic pollutants and plastics transported by seabirds is likely to increase in coming years.
Studies were limited geographically, with hotspots of research activity in a few locations, but data were lacking from large regions around the world. Studies were also limited to seabird species listed as Least Concern on the IUCN Red List. As seabird populations are impacted by multiple threats and steep declines have been observed for many species world‐wide, gaps in the literature are particularly concerning. The loss of seabirds will impact nutrient cycling at localized levels and potentially on a global scale as well, yet it is unknown what may truly happen to areas that rely on seabirds if these populations disappear.
Keywords: enrichment, guano, marine‐derived, mobile link, vector
This is the first systematic literature review and meta‐analysis on the topic of seabirds as vectors for nutrients and pollutants to terrestrial ecosystems, with 181 publications evaluated. Results indicate the importance of seabirds for localized nutrient cycling, while also signalling the potential negative consequences of seabird‐mediated pollutant deposition.
1. INTRODUCTION
Abiotic and biotic vectors are responsible for the transport of subsidies across ecosystem boundaries, leading to the replenishment of vital resources in recipient communities (Payne & Moore, 2006) and can be profoundly significant by altering ecosystem‐level processes and trophic dynamics (Earl & Zollner, 2017). Abiotic vectors, such as wind (e.g. transport of dust from the Sahara Desert to the Atlantic Ocean; Kaufman et al., 2005), gravity (e.g. fallout of marine snow from surface waters to the deep sea; Kiko et al., 2017) and ocean currents (e.g. dispersal of mangrove seedlings from one island to another; Hodel et al., 2018) move materials from one system to another, usually unidirectional, and are determined by the force of the factor (Payne & Moore, 2006). In comparison, biotic vectors, such as mobile organisms, are often less restricted in their movement and can therefore transport subsidies in a multitude of directions and against those common gradients found with abiotic vectors.
Migratory animals are well‐known biotic vectors, connecting ecological communities all across the globe by transporting significant quantities of spatial subsidies, with the potential to influence community structure, diversity and ecosystem function at resident habitats (Bauer & Hoye, 2014). However, the effects of vectors can be highly variable. Migrants can transport parasites and pathogens (e.g. highly pathogenic avian influenza virus (H5Nx); Wille et al., 2019) as well as propagules (e.g. Old World fruit bats Cynopterus sphinx consume fruits and deposit the seeds through their excreta; Shilton et al., 1999), but perhaps the most common transport effects are nutrients, toxicants and energy. Migrants act as transport mechanisms for these subsidies by feeding in one ecosystem, moving to another system, and then offloading the materials via a range of outputs (e.g. defecation; Bauer & Hoye, 2014; Blais et al., 2005). Vectors can be incredibly beneficial—and the ecological effects can be much more pronounced—when the transport of subsidies is from a nutrient‐rich ecosystem to an oligotrophic system (Savage, 2019).
In the marine environment, transfer of spatial subsidies to terrestrial ecosystems has been documented for Pacific salmon (Oncorhynchus spp.; Payne & Moore, 2006), sea lions (e.g. Zalophus wollebaecki; Fariña et al., 2003) and numerous seabird species (Kolb et al., 2015). Seabirds feed on marine prey (e.g. fish, squid, zooplankton) and transport nitrogen (N) and phosphorous (P) from their marine feeding grounds to their roosting and breeding habitats on land (Daher et al., 2019; Dominguez et al., 2017; Sanchez‐Pinero & Polis, 2000). Here, seabirds congregate in dense colonies and deposit these marine‐derived nutrients (MDN) in huge quantities primarily through guano, but also through other allochthonous inputs of feathers, eggs, boluses (regurgitated pellets) and carcasses (Adame et al., 2015; Duffy, 1994; Szpak, Longstaffe, et al., 2012). For these reasons, seabirds are one of the most significant vectors for the transportation of these vital nutrients (Marmen et al., 2017).
Humans have been aware of the many benefits of seabird guano and have exploited it for the purposes of fertilizing crops as early as the 16th century (Cushman, 2013). The most well‐known areas of guano mining were along the Pacific Margin in Peru and northern Chile where thick deposits of guano (>50 m in extreme cases) were mined extensively from islands (Lucassen et al., 2017; Szpak, Longstaffe, et al., 2012). The trade of guano peaked at 20 million tons/year during the mid‐19th century before declining as guano ran low, prices soared and chemical fertilizers became widely available (Mathew, 1970; Szpak, Longstaffe, et al., 2012). When left to accumulate naturally in the wild, the benefits of guano as a fertilizer can include significantly increased soil N and P, and can also impact soil pH, moisture and salinity (Wait et al., 2005). These changes in soil characteristics leads to myriad positive changes for terrestrial ecosystems and have been widely documented (e.g. Magnússon et al., 2014; Mosbech et al., 2018; Sanchez‐Pinero & Polis, 2000). The positive impacts provided by seabird guano can include enhanced primary and secondary productivity, increased invertebrate abundance and diversity, and changes to ecological communities (Buelow et al., 2018). Referred to as ornithogenic soils, the effects of nutrient enrichment derived from birds can be exceptionally distinct compared to areas without seabirds and can last for centuries (Mizutani et al., 1991).
On islands, the effect of seabirds on their environment can be more pronounced compared to mainland systems. Islands often receive few external nutrient subsidies besides what the birds transport, thus seabirds can play an integral role in the functioning and resilience of islands (Buelow et al., 2018), and the communities that live within and around colonies (Sanchez‐Pinero & Polis, 2000). For example, when a new volcanic island, Surtsey, formed off the coast of Iceland in 1963, the establishment of a gull colony which improved soil nutrient status was the main agent of ecosystem development (Magnússon et al., 2014). Seabirds can therefore facilitate the colonization and regeneration of marginal and nutrient‐limited habitats, such as barren, polar, post‐glacial or volcanic habitats (Şekercioğlu, 2006).
While the positive benefits of seabirds to a terrestrial system are plentiful, high seabird abundance in an area can also contribute to negative consequences (Wootton, 1991). Guano adds valuable nutrients to a system, but excessive deposition can alter plant and invertebrate communities, kill vegetation or cause dead zones (Gillham, 1961; Martín‐Vélez et al., 2019; Saifutdinov & Korobushkin, 2020). This ‘guanotrophication’, or seabird‐induced eutrophication, occurs when the level of nutrients added to a system exceeds what is required (Signa et al., 2015). As apex predators, many seabirds also exhibit high contaminant loads due to biomagnification and bioaccumulation along the marine food chain (Shoji et al., 2019). Chemicals, including persistent organic pollutants (POPs) and trace elements, can pass through individuals via feathers, eggs and guano (Furness & Camphuysen, 1997; Otero et al., 2018). Like the transport of nutrients, many recipient habitats do not already contain these pollutants prior to the arrival of seabirds, and thus the birds introduce pollutants in, at times, substantial quantities (Michelutti et al., 2009; Santamans et al., 2017). In addition to POPs and trace elements, many seabird species consume plastic debris and deposit these pollutants within their colonies via several means. Micro‐plastics (<5 mm in diameter; Barnes et al., 2009) can be excreted with guano; however, it is unknown whether plastics can be digested or broken up to small enough pieces to be passed via guano, or whether it is only micro‐ and nano‐sized particles (ingested at that size) that can be passed (see Bourdages et al., 2021; Gil‐Delgado et al., 2017; Provencher et al., 2018; Reynolds & Ryan, 2018). While deposition of plastics via guano is a relatively new topic, it has been known for some time that seabirds can deposit plastics in colonies through other means, such as regurgitation or the death of birds with high loads of ingested plastics (Buxton et al., 2013; Grant et al., 2021; Nel & Nel, 1999).
Here we critically review the literature regarding seabirds as vectors for nutrients and pollutants from marine to terrestrial ecosystems. While our review captured literature encompassing multiple pathways of nutrients and pollutants as provided by seabirds (i.e. guano, feathers, eggshells, regurgitations including boluses/pellets, carrion/carcasses), we focused on guano as this is the most significant and common pathway. We highlight global patterns and areas of high research activity and provide recommendations for consistent data collection and reporting in the future studies. We assess the various nutrients and pollutants associated with guano and determine if differences lie between seabird orders. Finally, we conduct a meta‐analysis on the impact of guano deposition on soils. We did this using published studies that compare common nutrients in colony soils and control soils. We hypothesize that colony soils will be enriched in nutrients compared to control soils because of the deposition of guano in colonies.
2. MATERIALS AND METHODS
2.1. Literature searching
We conducted a systematic review of the available literature on the online platform Web of Science and using the Core Collection database, containing the following Citation Indexes: Science Citation Index Expanded: 1945–2021; Social Sciences Citation Index: 1956–2021; Arts & Humanities Citation Index: 1975–2021; Conference Proceedings Citation Index—Science: 1990–2021; Conference Proceedings Citation Index—Social Science & Humanities: 1990–2021; and Emerging Sources Citation Index: 2015–2021. We also conducted an identical search on the online database Scopus. We followed established methods outlined in Haddaway et al. (2018) with the aim to capture all published literature that is listed on Web of Science and Scopus on the topic of seabirds as vectors for nutrients and pollutants to terrestrial environments. ‘Terrestrial’, in the case of this review, is defined as any land that seabirds nest, roost, breed or loaf in and therefore is a site where guano is deposited persistently in space and time. This also includes intertidal regions. Papers that studied terrestrial habitats and freshwater habitats (e.g. stream, lake, wetlands) simultaneously were also included. Purely marine‐based studies are not included in this review.
The search was conducted on 26 October 2021 and included key terms synonymous with the four broad topics, namely seabird, vector, nutrient/pollutant and guano (Table 1, see Supporting Information for full search string), and captured published literature up until 26 October 2021. We included all relevant peer‐reviewed original research papers. Review articles, book chapters and grey literature were excluded.
TABLE 1.
List of all search terms applied when using the ISI Web of Science and Scopus databases. Each topic includes a list of all synonyms relevant to that topic and each term (within each topic) was connected by the Boolean operator ‘OR’. A total of 265 papers were returned by this search in Web of Science and 316 from Scopus. An asterisk represents a string of any characters and is used when the word could have different endings, for example, transport* could be transporting or transportation. A question mark represents a single character and is used when a letter within the word could change, for example, fertili?* could be fertilization or fertilization. Please see Supporting Information for the entire search string with all syntax included
|
Topic 1: Seabird |
AND |
Topic 2: Vector |
AND |
Topic 3: Nutrient/pollutant |
AND |
Topic 4: Guano |
NOT |
TOPIC 5: Poultry |
|---|---|---|---|---|---|---|---|---|
| Seabird | Transport* | Contamin* | Guano | Poultry | ||||
| ‘Marine bird’ | Marine‐derived | Toxin | Faeces | |||||
| Avian | Route | Organic | Feces | |||||
| Vector | Inorganic | Dropping* | ||||||
| Enrich* | Metal | Excre* | ||||||
| Fertili?* | ‘Trace element’ | Manur* | ||||||
| Path | ‘Persistent organic pollutant’* | |||||||
| Translocat* | Plastic | |||||||
| Biotransport* | Debris | |||||||
| Deposit* | Nutrient | |||||||
| Engineer | Pollut* | |||||||
In addition, we performed backward literature searching on all papers included in the full review to gain more articles relevant to this topic that were not picked up through searching Web of Science or Scopus. For this, the reference list of each paper was combed through and any paper with a relevant title was extracted and added to the results (Figure 1).
FIGURE 1.
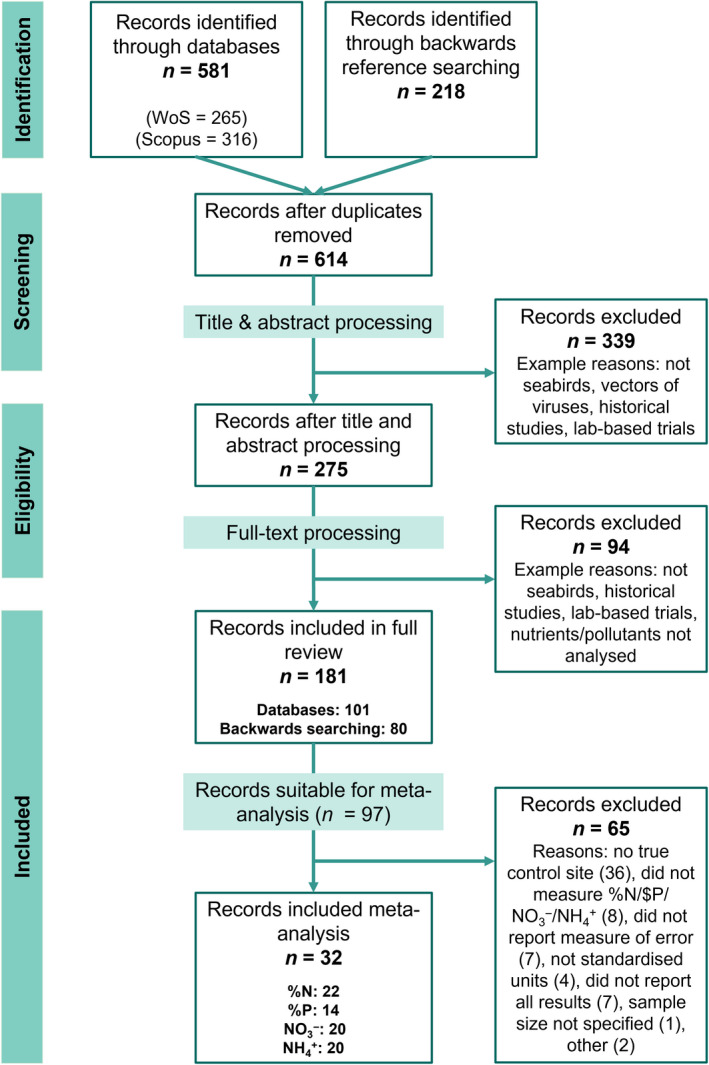
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA; Page et al., 2021) flow diagram for study selection. Diagram depicts the number of studies retained and discarded at each step. The total number of records included in the meta‐analysis was 32; however, many publications studied multiple nutrients simultaneously and thus the sum of records for nutrient types does not equal 32
2.2. Literature screening
The searches returned a combined total of 581 papers, with 265 from Web of Science and 316 from Scopus. Once duplicate records were removed, there were 396 unique papers from Web of Science and Scopus. Backwards literature searching produced a further 218 papers (Figure 1). Each paper underwent a preliminary filtering stage where titles and abstracts were read and if the research was not directly related to the role of seabirds as vectors to terrestrial ecosystems, then it was removed. The remaining papers then went through full‐text processing to remove any more irrelevant articles (Figure 1; Figure S1). For papers to be included in the final dataset, they had to demonstrate that the focal seabirds influence their terrestrial environment. This meant that papers had to do two things: (a) measure seabird influence (i.e. analyse guano, measure presence/absence) and (b) measure the impact of seabirds on recipient environment (e.g. soil, vegetation, invertebrates). Papers that studied historical trends in pollutants in sediments, seabirds as vectors of viruses, transport of seabird‐derived nutrients back to marine environments, atmospheric emissions (above seabird colonies) or laboratory‐based fertilization studies were not included (Figure S1).
We used the International Ornithological Congress World Bird List v11.2 (Gill & Donsker, 2021) for classification of species and for defining ‘seabirds’. Papers were included only if the focal species were in the following orders: Charadriiformes (gulls, terns, auks, shorebirds, skuas), Phaethontiformes (tropicbirds), Sphenisciformes (penguins), Procellariiformes (petrels, storm‐petrels, diving petrels, shearwaters, albatrosses) and Suliformes (boobies, gannets, cormorants, frigatebirds, anhingas).
The total number of peer‐reviewed articles after the completion of title and abstract processing and full‐text screening was 181, with 101 from database searching (Web of Science and Scopus) and 80 from backwards literature searching. All studies used in the systematic review and meta‐analysis are listed in the Data Sources section. M.L.G. and J.L.L. performed the literature screening.
2.2.1. Screening for meta‐analysis
To demonstrate how powerful guano can be, we ran meta‐analyses by comparing nutrient concentrations in colony soils (where guano is deposited) to control soils (where guano is not deposited). For the meta‐analysis, we decided to use studies that compared nutrients in soils because this was the most common abiotic factor that was sampled across all studies. Thus, this decision was made post hoc (i.e. after the studies were collected for review). The nutrients included within the meta‐analyses were ammonium (NH4 +; μg/g), nitrate (NO3 ‐; μg/g), total nitrogen (%N) and total phosphorous (%P). All nutrients were measured in dry weight. For papers to be included, they had to:
Include a control site(s) along with a site with seabirds (i.e. a seabird colony). The control site(s) must be a strictly defined area without seabirds present. Many papers included a control site that was along a gradient of seabird influence, but as these are not strict control sites, they were excluded from analysis.
Measure one or more of the above nutrients in soils in both colony and control soils.
Report mean nutrient/pollutant concentrations in colony and control soils, standard deviations (SD) and sample sizes (n), or allow for them to be calculated. For studies that only reported these values within plots, we used GetData Graph Digitizer v.2.26 to extract the relevant data.
After following these conditions, our list of papers that could be included within the meta‐analysis was reduced to 32 for nutrients in soils (see Figure 1 for more details, Figure S2). However, not all these papers analysed contain all the chosen nutrients (e.g. a paper may have analysed total nitrogen only). As such, for each of the different nutrients, there were 14 to 22 papers.
2.3. Data extraction
From the 181 papers, we then extracted data and information on the following: location (island or mainland, coordinates) and habitat of the study (as defined by the IUCN habitat classification scheme; The International Union for Conservation of Nature, 2012), study duration (single or multi‐year studies as well as the number of years), study site information (number of sites, whether was there a control/s site, that is, a nearby site without the presence of seabirds to compare against), seabird focal species, what metrics were used to measure seabirds' influence (presence/absence or guano) and if guano was used we included the analysis type (nutrient content, elemental analysis, guano cover, isotope analysis or from literature), what the seabirds were documented to be transporting (nutrients, inorganic pollutants, organic pollutants or physical pollutants) and what abiotic and biotic factors were sampled to determine the effect of seabirds. Due to the diversity of biotic and abiotic factors sampled, most were put into broad categories: terrestrial vegetation (e.g. grass, trees and moss), vertebrates (e.g. fish and reptiles), terrestrial invertebrates (e.g. insects and tardigrades), benthic/aquatic vegetation (e.g. macroalgae, seagrass), intertidal organisms (e.g. molluscs and bivalves) and plankton (e.g. zooplankton and phytoplankton). Soil, water, detritus/leaf litter and sediment were retained in their own categories. The overall effect of the focal species on the given area (as stated in the results or conclusion of the paper, for example, soil properties improved, species abundance increased) was also recorded. As guano was the focus of this study, we extracted information on trace elements analysed within guano and the respective concentrations of those elements, as well as nutrient concentrations in guano.
2.3.1. Data extraction for meta‐analysis
For each of the 32 papers suitable to be included in the meta‐analysis, we extracted the following data: mean ± SD concentration of nutrient/s (NH4 +, NO3 −, %N and %P) in seabird soil and in control soil, as well as the number of soil samples for each group (in addition to data extracted for each paper in Section 2.3). For this subset of papers, we also recorded the latitudinal zone the study was undertaken in (polar = >60°N or S; temperate = 30–60°N or S; and tropical = <30°N or S). If a paper measured nutrient concentrations in soils of more than one seabird species, then this was recorded for each species separately.
2.4. Meta‐analysis
2.4.1. Effect size calculations
We calculated the log response ratio (LRR, also known as Log(Ratio of Means)) as our effect size since nutrient concentrations are continuous positive variables. LRR is a common effect size calculated in ecological meta‐analyses because it quantifies the proportionate change between the groups (Hedges et al., 1999). It is calculated by:
where C refers to the concentration of the nutrient in question. When LRR is greater than zero, the concentration of the nutrient is greater in colony soils than in control soils, and when LRR is less than zero, the concentration of the nutrient is higher in control soils than in colony soils. Variance is calculated as:
where SD and N represent the standard deviation and sample size of each group from each study.
2.4.2. Statistical analysis
We ran all statistical analyses using the Metafor package (v 2.4‐0; Viechtbauer, 2010) in r version 4.0.2. For each nutrient, we calculated a summary LRR and 95% confidence intervals (CI95) via a random effects model by using the LRR and variances derived from each individual study. We used a random effects model to account for variation within each study as well as between all the studies, as there was considerable variation in study methodologies. Random effects models are commonly used in ecological meta‐analyses because they allow the estimated effect sizes to vary due to differences observed from sampling error and from true ecological differences between the studies (Anderson et al., 2015). For each nutrient, we calculated the Q‐score and I 2 index of heterogeneity to determine the amount of unexplained between‐study variation and we measured this with a restricted maximum likelihood estimator. When Q‐scores and I 2 were significant (p < 0.05), we conducted subgroup analyses using meta‐regressions with the following moderators: island vs. mainland, geographic location of sites (polar, temperate and tropical) and seabird order, to determine if the variation in effect sizes could be explained. For each moderator group, we hypothesize the following:
Island versus mainland: Island ecosystems are isolated from mainland systems, thus are often dependent on subsidies vectored by mobile organisms, such as seabirds (Buelow et al., 2018). As such, we hypothesize that studies on island environments will be more enriched than studies on mainland environments.
Geographic location: studies in nutrient‐limited environments, such as within polar regions, will be more enriched than studies in regions not limited by nutrients.
Seabird order: Enrichment of soils in seabird colonies will vary with seabird order and with nutrients.
Further partitioning (e.g. down to seabird family or genus) was not possible due to our limited sample sizes. We considered the overall effect size and the effect size for each study to be significant if the 95% CI did not overlap zero.
3. RESULTS
3.1. Overall trends in publications
A total of 181 papers were critically reviewed and were published over 53 years (1968–2021). Overall, there was an increase in the number of papers published in recent years, with more than half of the reviewed literature published within the last 15 years (64.1%, n = 116) with 2015 (n = 16) and 2013 (n = 11) having the most publications. The proportion of single‐ and multi‐year studies were similar (44.2% and 41.4% respectively; Table 2); however, 26 studies (14.4%) did not report the number of years of sampling. For multi‐year studies, most were 2 years in duration (22.1%, n = 40), and the two longest studies were 8 years.
TABLE 2.
Proportion of publications (n = 181) included in this review with the variables and reported metrics extracted. Habitat types are based on the IUCN Habitat Classification Scheme which includes 16 broad habitat types at level 1, and a further 119 types listed at level 2 (The International Union for Conservation of Nature, 2012)
| Variable | Reported metrics | % of publications |
|---|---|---|
| Location | Island | 76.8 |
| Mainland | 23.2 | |
| Ecosystem | Terrestrial | 91.2 |
| Intertidal | 3.9 | |
| Both | 5.0 | |
| Habitat a | Grassland | |
| Tundra | 18.2 | |
| Subantarctic | 5.0 | |
| Temperate | 3.9 | |
| Subtropical/Tropical Dry | 0.6 | |
| Forest | ||
| Temperate | 13.8 | |
| Boreal | 5.5 | |
| Subtropical/Tropical Dry | 3.9 | |
| Subtropical/Tropical Mangrove | 1.7 | |
| Subtropical/Tropical Moist Lowland | 0.6 | |
| Subantarctic | 0.6 | |
| Desert | ||
| Cold | 17.1 | |
| Hot | 3.3 | |
| Shrubland | ||
| Mediterranean | 7.7 | |
| Subantarctic | 0.6 | |
| Boreal | 0.6 | |
| Subtropical/Tropical Dry | 0.6 | |
| Temperate | 0.6 | |
| Marine Intertidal | ||
| Tidepools | 3.3 | |
| Rocky Shoreline | 1.1 | |
| Mangrove (Submerged Roots) | 0.6 | |
| Wetlands | ||
| Permanent Freshwater Lakes (> 8 ha) | 1.1 | |
| Permanent Freshwater Pools (< 8 ha) | 1.1 | |
| Tundra Pools | 0.6 | |
| Bogs, Marshes, Swamps, Fens, Peatlands | 0.6 | |
| Marine Coastal/Supratidal | ||
| Sea Cliffs/Rocky Offshore Islands | 1.1 | |
| Coastal Brackish/Saline Lakes/Pools | 0.6 | |
| Artificial Aquatic | ||
| Ponds | 0.6 | |
| Irrigated Land | 0.6 | |
| Savanna (Dry) | 0.6 | |
| Multiple | 4.4 | |
| Control site | Yes | 75.1 |
| No | 22.1 | |
| Not mentioned | 2.8 | |
| Number of sampling sites | 1 | 7.7 |
| 2–4 | 38.1 | |
| 5–7 | 17.7 | |
| 8–10 | 11.0 | |
| 11–15 | 6.1 | |
| 16–20 | 8.8 | |
| >21 (maximum 64) | 7.7 | |
| Not mentioned | 2.8 | |
| Multi‐year study | Yes | 41.4 |
| No | 44.2 | |
| Not mentioned | 14.4 | |
| Study duration (years) | <1 | 44.2 |
| 2 | 22.1 | |
| 3 | 12.7 | |
| >4 (maximum 8) | 6.6 | |
| Not mentioned | 14.4 | |
| Number of focal species | 1 | 56.9 |
| 2 | 16.0 | |
| 3 | 8.3 | |
| >4 (maximum 29) | 15.5 | |
| Not mentioned | 3.3 | |
| Was guano measured? | Yes | 47.0 |
| No | 53.0 | |
| Measure of guano? (n = 85 studies) b | Nutrient content | 32.4 |
| Elemental analysis | 21.6 | |
| Stable isotope analysis | 18.0 | |
| Defecation rate | 12.6 | |
| Guano cover (%) | 5.4 | |
| Pre‐determined from literature | 5.4 | |
| Plastic load | 2.7 | |
| Presence of guano | 1.8 | |
| Other measures of seabird influence | Presence /Absence of seabirds | 81.8 |
| Feathers | 7.7 | |
| Eggs/shells | 4.4 | |
| Carrion/carcasses | 3.3 | |
| Boluses/pellets | 2.2 | |
| Seabirds are vectors of c | Nutrients | 84.5 |
| Inorganic pollutants | 24.3 | |
| Organic pollutants | 5.5 | |
| Physical pollutants | 1.7 | |
| Overall effect of seabirds | Positive | 49.2 |
| Negative | 19.9 | |
| Mixed | 22.7 | |
| Neutral | 8.3 | |
| Factors sampled c | Soil | 64.1 |
| Terrestrial vegetation | 44.2 | |
| Water | 19.3 | |
| Invertebrates | 17.1 | |
| Sediment | 13.3 | |
| Detritus/leaf litter | 9.9 | |
| Vertebrates | 9.9 | |
| Benthic/aquatic vegetation | 9.9 | |
| Intertidal organisms | 5.5 | |
| Plankton | 4.4 | |
| Other | 14.9 |
For definitions of habitat classes, readers are directed to https://www.iucnredlist.org/resources/habitat‐classification‐scheme
20 studies measured guano using two different analyses, and three studies used three different analyses
The variables ‘Seabirds are vectors of’ and ‘Factors sampled’ do not sum to 100% as many publications studied nutrients and pollutants simultaneously or sampled multiple factors
3.2. Distribution of publications
From the 181 publications, a total of 1,418 sites were examined from 30 different countries (Figure 2). Antarctica was the most represented (22.1%, n = 40 publications), followed by Canada (10.5%, n = 19) and Norway (9.9%, n = 18). Six publications spanned multiple countries while all others included only one country. Research was more heavily focused in the Northern Hemisphere, with 104 studies (57.5%). Research spanned both terrestrial (91.2%, n = 165 publications) and intertidal (3.9%) environments (purely oceanic studies were not included in this review), and a small handful of studies incorporated both (5.0%). A diversity of habitats was covered, including grassland (27.6%, n = 50 publications; Table 2), forest (26.0%, n = 47) and desert (cold: 17.1%, n = 31; hot: 3.3%, n = 6), and several papers studied multiple environments (4.4%, n = 8). Islands (76.8%, n = 139 publications) were studied more frequently than mainland habitats (23.2%, n = 42).
FIGURE 2.
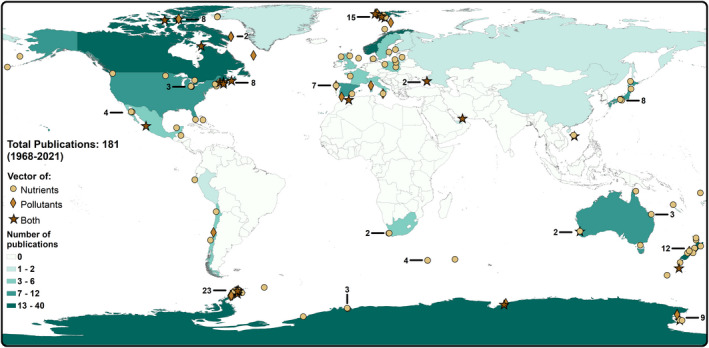
Most publications on seabirds as vectors of nutrients and pollutants were completed in the Northern Hemisphere, with a particular focus on regions in the Arctic. However, the country with the most studies was Antarctica (n = 40). Unique markers are used for publications examining nutrients (yellow circle), pollutants (tan diamond) or a combination of both (brown star; one marker per publication). Countries are shaded teal depending on the number of studies published. Several locations were studied frequently (e.g. King George Island, Antarctica) and for these areas a number is included to indicate how many studies took place in that region
3.3. Focal seabird species
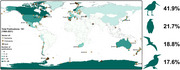
A total of 86 seabird species from 12 families and four orders were covered in the literature, with Charadriiformes accounting for 41.9% (families: Alcidae, Chionidae, Haematopodidae, Laridae and Stercorariidae), followed by Sphenisciformes (21.7%; Spheniscidae), Procellariiformes (18.8%; Diomedeidae, Hydrobatidae and Procellariidae) and Suliformes (17.6%; Fregatidae, Phalacrocoracidae and Sulidae). Adelie penguins Pygoscelis adeliae was the species studied the most (7.1%, n = 23 publications), followed by great cormorants Phalacrocorax carbo (6.8%, n = 22) and gentoo penguins Pygoscelis papua (6.5%, n = 21). Of the 181 papers reviewed, 56.9% (n = 103; Table 2) had a single focal species, while 39.8% included multiple species (n = 72, range = 2–29). A handful of publications did not report the seabird species concerned (8.8%, n = 16) or only reported a portion of the seabird species included within the study (3.9%, n = 7). Research was predominately done on species classified as Least Concern by the International Union for Conservation of Nature's Red List of Threatened Species (IUCN Red List; n = 267 occurrences/65 species), while research on species listed as Endangered on the IUCN Red List was minimal in comparison (n = 15 occurrences/6 species—Procellaria westlandica, Megadyptes antipodes, Phalacrocorax capensis, Phoebetria fusca, Spheniscus demersus and Morus capensis) and there were no studies on species listed as Critically Endangered.
3.4. Metrics used to measure seabird influence
Publications were highly variable in terms of what tools and metrics were used to measure the influence of seabirds on a given system. Guano was examined in 85 publications (47.0%), of which 35 analysed samples for nutrient content (N, P or both), 23 quantified non‐essential trace elements and 20 included stable isotope analysis (Table 2). When guano was not collected and analysed, proxy measures were used instead. The presence and absence of seabirds was commonly used in publications as a simple measure of guano (i.e. the presence of seabirds indicate that guano is being deposited, while the absence of seabirds infers that there is no guano deposition; 81.8%, n = 148), and this was often reported in conjunction with the analysis of guano (30.4%, n = 55). A small portion of publications measured other allochthonous inputs by seabirds, such as feathers (7.7%, n = 14) and eggshells (4.4%, n = 8). Forty publications (22.1%) did not include a control site (i.e. a location without seabirds to compare against).
A diversity of biotic and abiotic samples was measured to determine the effect of seabirds and the flow of subsidies in recipient environments. The most common was soil (64.1%, n = 116 publications), followed by vegetation (44.2%, n = 80) and followed by water (19.3%, n = 35). Most publications measured multiple factors (57.5%, n = 104 publications, range = 2–7 factors), while 33.7% (n = 61) sampled only one factor and 8.8% (n = 16) did not sample any (i.e. only measured guano).
3.5. Seabirds as vectors
Nutrients were the only subsidies recorded in the early literature (1968–1990; Figure 3) and accounted for 84.5% (n = 153) of all studies considered in this review. In contrast, the first publication to consider seabirds as vectors of pollutants was in 1991 (inorganic pollutants; Godzik, 1991). Since that time, there has been an increase in the number of publications investigating the role of seabirds as vectors of pollutants; however, there has been a similar increase in the number of publications focusing on nutrients (43.8% of nutrient papers were published in the past decade; Figure 3) and scientific publishing in general. Overall, inorganic pollutants transported by seabirds were documented more in the literature (24.3%, n = 44) than organic pollutants (5.5%, n = 10). Several papers measured nutrients and pollutants simultaneously (nutrients and inorganic pollutants: n = 27; nutrients and organic pollutants: n = 5). Three publications measured physical pollutants (i.e. plastic particles) in guano.
FIGURE 3.
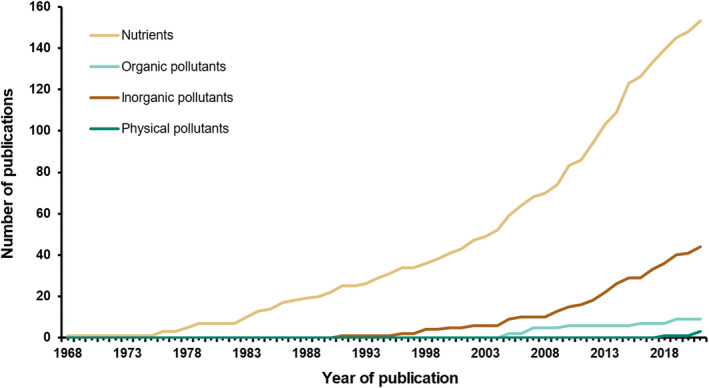
Growth in publications reporting on the different subsidies (nutrients, inorganic, organic or physical pollutants) transported by seabirds to land. Of the 181 publications reviewed, the majority explored nutrients. Inorganic pollutants were first reported in 1998, while organic pollutants were first reported in 2005. Physical pollutants (i.e. plastics) were not reported until 2018. A number of papers reported multiple subsidies (e.g. nutrients and inorganic pollutants), thus lines do not sum to 181
A total of 89 publications (49.2%; Table 2) concluded that seabirds had a positive influence on the terrestrial environment, while 36 (19.9%) returned a detrimental result (see Section 4.4 below). Some publications returned mixed results (22.7%, n = 41), showing both negative and positive effects, while a small portion did not detect a significant change or reported neutral results (8.3%, n = 15).
3.5.1. Contents of guano
There were 35 papers that analysed the nutrient content of guano. All but two of these papers analysed the total percentage of N or P (or both) in guano. Seabirds from the order Suliformes had higher levels of P than all other orders, with a M ± SD of 15.08 ± 1.07%P (range = 14.32–15.83%P; Table 3), whereas Procellariiformes typically exhibited guano with a higher N content (19.13 ± 3.46, range = 15.19–21.70%N).
TABLE 3.
Mean ± SD (range; n) of total nitrogen (%N) and total phosphorous (%P) analysed in guano, separated by seabird order
| Order | Nutrient | |
|---|---|---|
| Nitrogen | Phosphorous | |
| Charadriiformes |
7.07 ± 4.47 (1.39–15.20; 9) |
1.54 ± 0.80 (0.47–2.76; 9) |
| Procellariiformes |
19.13 ± 3.46 (15.19–21.70; 3) |
1.16 ± 0.31 (0.90–1.50; 3) |
| Sphenisciformes |
13.04 ± 6.03 (0.35–20.85; 10) |
3.13 ± 2.80 (0.16–10.28; 10) |
| Suliformes |
9.89 ± 9.35 (3.28–16.50; 2) |
15.08 ± 1.07 (14.32–15.83; 2) |
| Overall |
11.30 ± 6.52 (0.35–21.70; 24) |
3.36 ± 4.22 (0.16–15.83; 24) |
Across the 23 papers that analysed guano for trace elements, there were a total of 30 different elements analysed. The most common elements analysed were zinc and cadmium, examined in 17 papers, followed by copper (n = 16) and lead (n = 15). The concentrations of these elements, plus the next three most analysed (arsenic, manganese and nickel; n = 13, 10 and 10 respectively), were extracted from the relevant papers. Guano was high in zinc across all seabird orders, with a M ± SD concentration of 261.03 ± 235.25 μg/g (range = 4.07–1,200.00 μg/g; Table 4). Copper concentrations were high as well (84.57 ± 100.13, 1.24–350.00 μg/g), followed by manganese (47.25 ± 75.49, 0.82–300.00 μg/g). Across seabird orders, there were some differences; for example, guano from Charadriiformes was high in arsenic, cadmium and lead compared to all other orders (6.48 ± 4.35, 11.40 ± 25.44 and 15.19 ± 14.21 respectively), whereas Sphenisciformes exhibited concentrations of copper and manganese that were much higher than all other orders (177.50 ± 106.63 and 87.47 ± 142.21 respectively; Table 4).
TABLE 4.
Mean ± SD (range; n) of the seven most analysed metals in guano, separated by seabird order. Concentrations are reported in μg/g dry weight. For those where only the mean is reported, n = 1
| Metal | |||||||
|---|---|---|---|---|---|---|---|
| Order | Arsenic | Cadmium | Copper | Manganese | Nickel | Lead | Zinc |
| Charadriiformes |
6.84 ± 4.35 (1.72–13.07; 8) |
11.40 ± 25.44 (0.33–74.16; 9) |
31.88 ± 19.68 (6.25–60.00; 8) |
52.09 ± 48.42 (19.00–147.81; 7) |
9.04 ± 6.07 (0.50–18.45; 6) |
15.10 ± 14.21 (2.04–40.00; 9) |
173.78 ± 114.43 (64.83–412.47; 10) |
| Procellariiformes |
3.22 ± 1.50 (0.97–4.05; 4) |
7.61 ± 1.20 (5.86–8.40; 4) |
11.83 ± 7.07 (7.75–20.00; 3) |
14.57 ± 10.40 (4.30–25.51; 5) |
0.53 ± 0.04 (0.50–0.55; 2) |
0.09 ± 0.01 (0.09–0.09; 2) |
373.70 ± 463.01 (140.00–1,200.00; 5) |
| Sphenisciformes |
1.69 ± 1.58 (0.40–3.81; 6) |
7.88 ± 13.06 (1.67–37.14; 7) |
177.50 ± 106.63 (1.24–350.00; 7) |
87.47 ± 142.21 (0.82–300.00; 4) |
10.23 |
1.76 ± 1.87 (0.41–5.39; 6) |
240.36 ± 140.74 (4.07–510.00; 8) |
| Suliformes | 6.45 |
12.14 ± 8.2 (6.34–17.93, 2) |
21.10 | 20.79 | 20.00 | 1.60 |
390.11 ± 144.77 (232.94–518.00; 3) |
| Overall |
4.29 ± 3.67 (0.40–13.07; 19) |
9.58 ± 16.88 (0.33–74.16; 22) |
84.57 ± 100.13 (1.24–350.00; 19) |
47.25 ± 75.49 (0.82–300.00; 17) |
8.50 ± 7.17 (0.50–20.00; 10) |
7.83 ± 11.82 (0.09–40.00; 18) |
261.03 ± 235.25 (4.07–1,200.00; 26) |
3.5.2. Meta‐analysis
The concentrations of all nutrients were significantly higher in seabird colony soils than in control soils (%N: LRR = 1.15, p < 0.0001; %P: LRR = 2.11, p < 0.0001; NO3 −: LRR = 2.18, p < 0.0001; and NH4 +: LRR = 2.81, p < 0.0001; Figure 4, Figures [Link], [Link]). Ammonium levels in colony soils showed the biggest difference and were on average 41.1 times higher than control soils, followed by nitrate concentrations (5.3× higher), total phosphorous (4.3×) and total nitrogen (2.1×). Q‐scores and I 2 were significant for all models (all p < 0.001; Table S1), suggesting that the heterogeneity in effect sizes between studies was higher than what would be expected by sampling error alone. Subgroup analyses indicated that the impact of guano deposition on island systems was significantly greater for all nutrients in comparison to deposition on mainland systems, while differences in geographic zones or seabird orders was less pronounced and more variable across nutrient types (Figure 4). Studies conducted in polar regions demonstrated significantly higher soil nutrient values across nutrient types, whereas studies in temperate zones showed significant results for all but N. Studies undertaken in tropical zones were minimal thus firm conclusions cannot be drawn. Differences in soil nutrient concentrations due to deposition by different seabird orders were highly variable, with studies examining seabirds from the order Sphenisciformes displaying significantly high levels of ammonium in colony soils (LRR = 4.27, p < 0.0001; Figure 4). Overall, the addition of seabird guano to colony soils increases nutrient concentrations compared to control soils.
FIGURE 4.
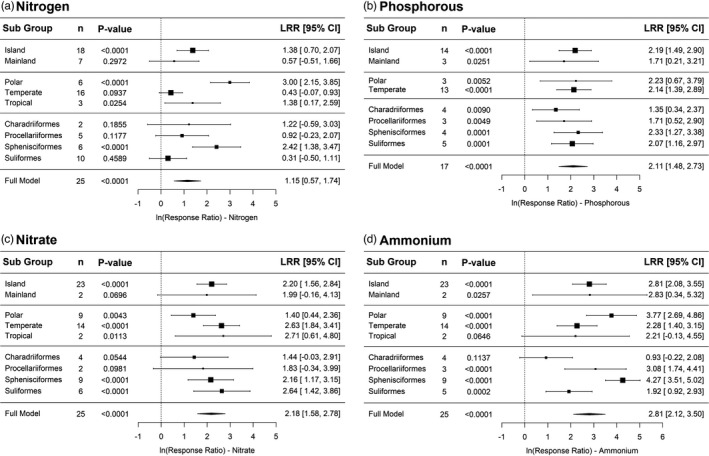
Forest plots showing the effect of guano deposition on nutrient concentrations ((a) nitrogen, (b) phosphorous, (c) nitrate and (d) ammonium) in soils. Effect sizes >0 show that nutrient concentrations were greater in seabird soils compared to control soils not impacted by guano deposition. The mean effect size (LRR, log risk ratio) and 95% confidence intervals are shown for the overall result (full model) and for each subgroup analysis: island vs mainland study sites, location of study sites (polar = >60°N or S; temperate = 30–60°N or S; and tropical = <30°N or S) and seabird order (Charadriiformes, Procellariiformes, Sphenisciformes and Suliformes). Confidence intervals that overlap zero are not significant. N and P were measured as percentage dry weight, while NO3 ‐ and NH4 + were measured in μg/g dry weight. The subgroup ‘Tropical’ for phosphorous is not included as n = 1. There were three publications that studied multiple seabird species, thus the n for the full model for each nutrient type does not equal the corresponding n in Figure 1. Four studies included in all plots did not clarify the seabird species, thus the sum of n for the subgroup seabird order does not equal the corresponding n for the full model
4. DISCUSSION
Seabirds have a vast array of ecological functions both on land and at sea. They have been relied upon as ocean sentinels for decades, providing information on fish stocks (Montevecchi, 1993; Piatt et al., 2007) and trends in chemical and physical pollutants at sea (Burger & Gochfeld, 2004; O'Hanlon et al., 2019). On land, we acknowledge seabirds as ecosystem engineers (Bancroft et al., 2005), capable of changing the physical and chemical conditions in their breeding and roosting grounds. While seabirds as physical engineers, such as burrowing, has been well described (Bancroft et al., 2004; McKechnie, 2006), data on the mechanisms surrounding seabirds as chemical engineers are comparatively limited. For example, only in the past 53 years have data on seabirds as vectors of nutrients or pollutants become available (Figure 3), with nearly 50% of the literature published in the last decade. This influx can be attributed to our growing knowledge of the important ecological services provided by seabirds (Mosbech et al., 2018; Şekercioğlu, 2006). The growth of literature on seabirds as vectors for nutrients has demonstrated their capacity to transport subsidies and alter environments in beneficial ways, but with the increasing awareness of ocean pollution, the birds' abilities to act as vectors for harmful subsidies has also been featured (Bourdages et al., 2021; Grant et al., 2021). This trend is mirrored in other fields globally where there has been a steady increase in pollutant research interest over time in both terrestrial and marine environments.
4.1. Geographic distribution of studies
Many studies were undertaken in nutrient‐limited environments, such as the Arctic and Antarctic, where external nutrient subsidies are considered integral for maintaining local production as resident sources of subsidies are often very minimal (Adame et al., 2015). For example, a large proportion of studies were undertaken in high latitude regions (>60 N/S; 40.9%, n = 74 publications), where terrestrial areas are characterized by cold temperatures and low nutrients (Maron et al., 2006); however, in places where seabirds congregate, there are hotspots of biological productivity and diversity (Brimble et al., 2009; Griffiths et al., 2010; Pereira et al., 2013), and this pattern was observed through our meta‐regressions with colony soils in polar regions being significantly more enriched in nutrients compared to control soils (Figure 4). Similar observations were recorded in the Gulf of Mexico, which is known for its relatively productive waters juxtaposed against hyper‐arid deserts, where the presence of seabirds increased production in comparison to areas without seabirds (Polis & Hurd, 1996). In general, the dynamics of many components (e.g. vegetation, consumers, soil properties) in terrestrial regions across the globe are highly dependent on nutrient subsidies transported by seabirds (Sanchez‐Pinero & Polis, 2000).
Similar to the presence of colonial seabirds in nutrient‐limited environments, seabirds are integral to many islands, as these ecosystems are isolated from external nutrient inputs and are often reliant on seabirds and other mobile vectors for the continued replenishment of vital subsidies (Buelow et al., 2018). Overall, island environments were studied more intensively than mainland environments, with 76.8% of publications based on islands. Most studies reported that islands with seabirds typically exhibited increased nutrient concentration in vegetation (Adame et al., 2015; Richardson et al., 2019) and a higher abundance of invertebrates (Markwell & Daugherty, 2002; Sanchez‐Pinero & Polis, 2000; Zawierucha et al., 2016). In comparison, islands without seabirds (e.g. control sites) often reported lower soil N and P (Rajakaruna et al., 2009; De la Peña‐Lastra et al., 2020; Figure 4), and hence did not see those same benefits as islands with seabirds present. Across island ecosystems, as well as nutrient‐limited environments, the effects of MDN from seabirds are substantial and promote the maintenance of these ecological communities (Polis & Hurd, 1996).
While there were hotspots of research activity in a handful of locations around the world, such as Svalbard, the Gulf of Mexico and King George Island (Antarctica), there are some obvious gaps. There were few studies in Africa (all were based in South Africa), with few data available from south‐east and west Asia, as well as the east coast of South America (Figure 2). This is worrying considering that seabird populations around the world are in decline (Croxall et al., 2012; Dias et al., 2019) or have disappeared in some cases (Feare, 1978), which will have implications for recipient habitats as the transfer of MDN will be reduced. It is important to have a wide representation from a range of ecosystems and habitat types, and not just studies from nutrient‐limited environments.
4.2. Focal seabird species
Most seabirds were listed as Least Concern (77.2%) on the IUCN Red List, with very few data available for globally threatened species (Vulnerable, Endangered or Critically Endangered). While it is important to study all species, regardless of population status, it is concerning that very few studies have examined globally threatened species, given the significant influence seabirds have on their terrestrial environments (Rodrigues et al., 2021). Interestingly, seabirds that were historically important guano‐producing birds (whose guano was mined for fertilizer) in South America were only studied once (Peruvian Booby, Sula variegata; Lucassen et al., 2017), despite two of the species (Guanay cormorant Phalacrocorax bougainvillii and the Peruvian brown pelican Pelecanus thagus) being listed as Near Threatened on the Red List. The guano produced by these species is evidently laden with nutrients (Szpak, Millaire, et al., 2012), thus a decline in their populations would lead to changes to their habitats, yet as these species have not been studied, we do not know what these changes would be.
Substantial, cascading and often detrimental changes may occur in areas where there once were thriving seabird colonies due to the reduction in MDN transported by the birds (Duda et al., 2020; Maron et al., 2006). The loss or major reduction in seabird colonies could result in the alteration in important ecosystem functions (Şekercioğlu et al., 2004). We could expect to see changes to invertebrate communities and vegetation assemblages, as observed on islands where predators have decimated seabird populations. For example, in the Aleutian Archipelago introduced Arctic foxes Vulpes lagopus preyed on seabirds resulting in a reduction in MDN and declining soil fertility, which transformed the habitats on multiple islands from grassland to a dwarf shrub‐dominated landscape (Croll et al., 2005). Furthermore, the loss of seabird populations may not just impact nutrient cycling at localized levels, but potentially on a global scale as well (Riddick et al., 2018) as this could lead to a reduction in global N emissions.
4.3. Metrics used to measure seabird influence
A range of methods were used to measure the influence of seabirds, ranging from simple methods such as the presence/absence of seabirds or percentage guano cover, to more quantitative, analytical methods (e.g. nutrient concentrations in guano). The presence/absence of seabirds is an easy, low‐cost way to test the impact of seabirds in each area, with sites where seabirds are absent functioning as controls. Many studies benefitted from access to islands with and without seabirds (e.g. Markwell & Daugherty, 2002; Powell et al., 1991) while others used a gradient of seabird abundance (e.g. sites were chosen with increasing distance from a colony; Signa et al., 2013; Zmudczynska‐Skarbek et al., 2013; Ziołek & Melke, 2014). While there is still merit in these qualitative studies, it can potentially be difficult to identify the exact cause of the observed effects, and definitively say that it was the presence of seabirds that resulted in the observed changes (Kolb et al., 2015), as the effects of seabirds can last long after they have left the area (Mizutani et al., 1991). This is particularly important as the nutrient concentration in guano varies considerably among seabird orders (Table 3) and by trophic level and foraging behaviours, as has been observed between coastal, oceanic and predatory seabirds (Wing et al., 2014) as well as between planktivorous and piscivorous seabirds (Zwolicki et al., 2013, 2016). While our review was limited by the number of studies examining the nutrient content of guano, and thus we only separated guano by order (Table 3), there is some discussion that guano nutrient content is different within seabird species, particularly those foraging in different locations and feeding on a variety of prey types. Several studies (5.4%) based their analyses on nutrient or pollutant levels in guano from previous literature. While there is some benefit in this (e.g. low cost), the results of this study demonstrate the highly variable nature of nutrients and pollutants in guano (Tables 3 and 4), thus authors should attempt to use values from the same species (or family) to ensure accuracy.
Stable isotope analyses were a popular method for tracing MDN in recipient terrestrial environments in many of the studies (Table 2). Stable isotopes of a variety of elements (C, N, O, H and S) have been used extensively in seabird ecology for inferring foraging locations and providing insights into diet (Bond & Jones, 2009; Callaham et al., 2012; Hoenig et al., 2022) and their application as tracers of nutrient flows is well established (Hebert et al., 2006; Michener & Schell, 1994). They can easily trace seabird‐derived nutrients in coastal and island food webs because of the distinction in marine δ 15N and δ 13C values compared to terrestrial N and C isotopes (Harding et al., 2004). The δ 15N values of consumers are enriched by 3–5‰ with each trophic transfer along the marine food web (Post, 2002), thus seabirds—being mostly tertiary consumers—generally have high δ 15N values (Lucassen et al., 2017). As guano contains considerable amounts of marine‐derived N, stable isotope analyses can be applied to materials (e.g. soil, vegetation, invertebrates; Hawke & Clark, 2010) from within and outside seabird colonies to trace the flow of nutrients from the guano, and have been used successfully to determine the presence of seabirds in areas where colonies are no longer present (Kameda et al., 2006).
4.4. Detrimental impacts of seabirds
While most literature focused on the transport of MDN and the substantial positive benefits derived from the addition of seabirds in terrestrial environments, there were some negative impacts associated with seabirds as well. The deposition of excessive amounts of guano can cause guanotrophication, or seabird‐induced eutrophication, as has been observed with a range of seabirds, including cormorants and gulls (Kolb et al., 2012; Otero et al., 2015). An excess in nutrients can indirectly lead to decreases in abundance and diversity of faunal groups (Signa et al., 2015), or directly lead to the destruction of vegetation through poisoning (Molina‐Montenegro et al., 2013), but the effects of guanotrophication are most often detected in waterbodies adjacent to seabird colonies (e.g. coastal lakes, rockpools; McColl & Burger, 1976; Portnoy, 1990; Martín‐Vélez et al., 2019).
Guanotrophication is not the only negative consequence derived from guano deposition. Guano can contain high concentrations of inorganic pollutants (i.e. heavy metals) and POPs which can bioaccumulate in flora and fauna in recipient environments (Godzik, 1991; Otero et al., 2018). The literature regarding seabirds as vectors for such pollutants commonly focused on metals with very few studies exploring POPs (Figure 3). Several metals, particularly cadmium, copper and lead, were consistently analysed in guano samples (e.g. Celis et al., 2014; Espejo et al., 2014; Otero, 1998). These metals are often the focus of other environmental pollutant studies as they are well known for their toxicity, ability to bioaccumulate in organisms and their persistence and longevity in the environment (Šerić Jelaska et al., 2014). Many studies demonstrated the impact of pollutant laden guano on recipient environments by measuring metal concentrations in soils, vegetation and invertebrates, with results showing higher contaminant levels in samples from colonies compared to samples from control sites (Headley, 1996; Santamans et al., 2017; Shoji et al., 2019). Furthermore, of the few papers that did explore POP transport by seabirds, the majority of papers established a similar pattern, with higher concentrations of POPs in soils and organisms within colonies in comparison to areas without seabirds (Choy et al., 2010; Evenset et al., 2004; Foster et al., 2011; Roosens et al., 2007). The transport of POPs by seabirds is 30 times more efficient than atmospheric transport, with potential risks to human health (Evenset et al., 2007).
While this review only returned three papers that examined microplastics in guano (<5 mm diameter; Provencher et al., 2018; Bourdages et al., 2021; Hamilton et al., 2021), a small number of papers (not included in this review) have also demonstrated that it is not just microplastics that can be transported to seabird colonies via guano, with meso‐ (5–20 mm) and macro‐plastics (>20 mm; Barnes et al., 2009) being deposited through boluses and carcasses (Buxton et al., 2013; Grant et al., 2021), or collected from sea or shorelines and used as nesting materials (Grant et al., 2018; Van De Crommenacker et al., 2021). These papers indicate that the incidence of plastics in seabird colonies is likely widespread and increasing. The literature surrounding the movement of plastics by seabirds to their colonies has increased in recent years, like the increase in pollutant papers detected in this review (Figure 3). It is unknown whether these plastics negatively impact the terrestrial systems they are deposited in; however, research in other fields (e.g. agriculture) suggest that plastics in soils can significantly affect temperature and N cycling (Seeley et al., 2020; Lavers et al., 2021) and microbial activity (de Souza Machado et al., 2018). As the deposition of guano increases the N content of soils (Figure 4a,c,d), the potential for concurrent positive and negative effects, if guano contains microplastics, is plausible, or may lead to a reduction in the incorporation of guano‐derived N. Research in this field is much needed, as plastic pollution is predicted to increase in coming years and, subsequently, the incidence of plastics in colonies is also likely to increase.
4.5. Future directions
Many papers do not report the most basic information, including the year(s) sampled, exact location studied or the seabird species involved (Table 2). It is critical that all published studies contain sufficient information so that the work may be repeated, included in meta‐analyses, and results compared (Cassey & Blackburn, 2006). Furthermore, papers often did not report variance, presenting only mean values, or did not include detailed information on samples sizes. This made it challenging to conduct the meta‐analysis, as papers that did not include these measures had to be excluded, drastically reducing the number of papers that could be synthesized (Figure 1). Soil types vary considerably (WRB, 2015) and play a significant role in the flux of nutrients and pollutants (Tian et al., 2017), yet this information was rarely presented across papers, particularly in those exploring the effects of guano addition in colony soils. The integration of soil science with pollution research in the context of ornithogenic transport would be a useful avenue to explore in the future studies. In addition to these recommendations of reporting basic but necessary information, we recommend focusing research efforts on declining seabird species (e.g. those listed as Critically Endangered or Endangered on the IUCN Red List), the transport of pollutants by seabirds, particularly POPs, as this has been minimally studied over the years (Figure 3), as well as longer studies (i.e. >2 years) to measure annual trends.
5. CONCLUSIONS
While we have highlighted the role of seabirds as vectors of both beneficial nutrients and potentially detrimental pollutants, seabirds remain integral to the continued success of many environments all around the world. In addition to providing nutrients, seabirds can regulate habitats through seed dispersal, physical ecosystem engineering, and assist with carcass and waste disposal. More broadly, birds (both terrestrial and marine) are well known to provide a highly diverse range of ecological functions and services, through regulation and supportive measures (Şekercioğlu, 2006). The potential decline and loss of these keystone species and the critical services they provide could be devasting for habitats globally (Bauer & Hoye, 2014; Ellis, 2005; Şekercioğlu et al., 2004). Considering that many bird groups are currently in decline, with the biggest declines seen in seabirds, it is of utmost importance to continue to study avian groups, so that we can anticipate and mitigate potential consequences.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS' CONTRIBUTIONS
All authors conceived the ideas and designed the methodology; M.L.G. and J.L.L. collected the data; M.L.G. analysed the data and led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Supporting information
Figure S1.The inclusion and exclusion criteria used for the systematic review during the title and abstract processing and full‐text screening. The number of papers at the beginning of the process was 614 and after title and abstraction processing was reduced to 275. These papers then went through full‐text screening and a final count of 181 papers were included in the full review.
Figure S2.From the 181 papers that were included in the full review, these were screened for the meta‐analysis using the above decision tree.
Figure S3.Forest plot for individual studies comparing nitrogen concentrations (%N dry weight) in seabird colony soils and control soils. Effect sizes (log response ratio, LRR) and 95% confidence intervals are displayed. When confidence intervals do not overlap zero (dotted vertical line) and are positive, results can be considered significant, that is, colony soils were demonstrated to be more enriched than control soils. One paper is listed more than once (Smith, 1978) as this paper measured nitrogen concentrations in soils for four different seabird species.
Figure S4.Forest plot for individual studies comparing phosphorous concentrations (%P dry weight) in seabird colony soils and control soils. Effect sizes (log response ratio, LRR) and 95% confidence intervals are displayed. When confidence intervals do not overlap zero (dotted vertical line) and are positive, results can be considered significant, that is, colony soils were demonstrated to be more enriched than control soils.
Figure S5.Forest plot for individual studies comparing nitrate concentrations (μg/g dry weight) in seabird colony soils and control soils. Effect sizes (log response ratio, LRR) and 95% confidence intervals are displayed. When confidence intervals do not overlap zero (dotted vertical line) and are positive, results can be considered significant, that is, colony soils were demonstrated to be more enriched than control soils. Two papers are listed more than once (Smith, 1978; Ramirez‐Fernandez, 2019) as these papers measured nitrate concentrations in soils for four and three different seabird species respectively.
Figure S6.Forest plot for individual studies comparing ammonium concentrations (μg/g dry weight) in seabird colony soils and control soils. Effect sizes (log response ratio, LRR) and 95% confidence intervals are displayed. When confidence intervals do not overlap zero (dotted vertical line) and are positive, results can be considered significant, that is, colony soils were demonstrated to be more enriched than control soils. Two papers are listed more than once (Smith, 1978; Ramirez‐Fernandez, 2019) as these papers measured ammonium concentrations in soils for four and three different seabird species respectively.
Table S1.Heterogeneity (Q‐score and I 2) of effect sizes for full models and for subgroup analyses of studies comparing nutrient concentration of nitrogen, phosphorous, nitrate and ammonium in soils between seabird colonies and control sites.
ACKNOWLEDGEMENTS
The authors acknowledge the traditional owners of the lands where this research was completed. They thank the numerous researchers whose papers have been included in this review and for focusing their efforts on this important and growing topic and everyone from Adrift Lab for being so supportive throughout this process. They extend their special thanks to H. Charlton‐Howard, K. Montanaro, E, Styles and A. Teo for reading papers. They also thank two anonymous reviewers, the associate editor and the editor for providing their thoughts. Open access publishing facilitated by University of Tasmania, as part of the Wiley ‐ University of Tasmania agreement via the Council of Australian University Librarians. [Correction added on 14‐May‐22, after first online publication: CAUL funding statement has been added.]
Grant, M. L. , Bond, A. L. & Lavers, J. L. (2022). The influence of seabirds on their breeding, roosting and nesting grounds: A systematic review and meta‐analysis. Journal of Animal Ecology, 91, 1266–1289. 10.1111/1365-2656.13699
Handling Editor Antica Culina
DATA AVAILABILITY STATEMENT
The datasets supporting this article and relevant R code are freely available from the Institute for Marine and Antarctic Studies (IMAS) Data Portal: https://doi.org/10.25959/ENWE‐GB98 (Grant et al., 2022).
References
REFERENCES
- Adame, M. F. , Fry, B. , Gamboa, J. N. , & Herrera‐Silveira, J. A. (2015). Nutrient subsidies delivered by seabirds to mangrove islands. Marine Ecology Progress Series, 525, 15–24. 10.3354/meps11197 [DOI] [Google Scholar]
- Anderson, L. G. , Rocliffe, S. , Haddaway, N. R. , & Dunn, A. M. (2015). The role of tourism and recreation in the spread of non‐native species: A systematic review and meta‐analysis. PLoS ONE, 10, e0140833. 10.1371/journal.pone.0140833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft, W. J. , Garkaklis, M. J. , & Roberts, J. D. (2005). Burrow building in seabird colonies: A soil‐forming process in island ecosystems. Pedobiologia, 49, 149–165. 10.1016/j.pedobi.2004.10.002 [DOI] [Google Scholar]
- Bancroft, W. J. , Hill, D. , & Roberts, J. D. (2004). A new method for calculating volume of excavated burrows: The geomorphic impact of Wedge‐Tailed Shearwater burrows on Rottnest Island. Functional Ecology, 18, 752–759. 10.1111/j.0269-8463.2004.00898.x [DOI] [Google Scholar]
- Barnes, D. K. A. , Galgani, F. , Thompson, R. C. , & Barlaz, M. (2009). Accumulation and fragmentation of plastic debris in global environments. Philosophical Transactions of the Royal Society B, 364, 1985–1998. 10.1098/rstb.2008.0205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer, S. , & Hoye, B. (2014). Migratory animals couple biodiversity and ecosystem functioning worldwide. Science, 344, 1242552. 10.1126/science.1242552 [DOI] [PubMed] [Google Scholar]
- Blais, J. M. , Kimpe, L. E. , McMahon, D. , Keatley, B. E. , Mallory, M. L. , Douglas, M. S. , & Smol, J. P. (2005). Arctic seabirds transport marine‐derived contaminants. Science, 309, 445. 10.1126/science.1112658 [DOI] [PubMed] [Google Scholar]
- Bond, A. L. , & Jones, I. L. (2009). A practical introduction to stable‐isotope analysis for seabird biologists: Approaches, cautions and caveats. Marine Ornithology, 37, 183–188. [Google Scholar]
- Bourdages, M. P. T. , Provencher, J. F. , Baak, J. E. , Mallory, M. L. , & Vermaire, J. C. (2021). Breeding seabirds as vectors of microplastics from sea to land: Evidence from colonies in Arctic Canada. Science of the Total Environment, 764, 142808. 10.1016/j.scitotenv.2020.142808 [DOI] [PubMed] [Google Scholar]
- Brimble, S. K. , Blais, J. M. , Kimpe, L. E. , Mallory, M. L. , Keatley, B. E. , Douglas, M. S. V. , & Smol, J. P. (2009). Bioenrichment of trace elements in a series of ponds near a Northern Fulmar (Fulmarus glacialis) colony at Cape Vera, Devon Island. Canadian Journal of Fisheries and Aquatic Sciences, 66, 949–958. 10.1139/f09-053 [DOI] [Google Scholar]
- Buelow, C. A. , Baker, R. , Reside, A. E. , & Sheaves, M. (2018). Nutrient subsidy indicators predict the presence of an avian mobile‐link species. Ecological Indicators, 89, 507–515. 10.1016/j.ecolind.2018.02.029 [DOI] [Google Scholar]
- Burger, J. , & Gochfeld, M. (2004). Marine birds as sentinels of environmental pollution. EcoHealth, 1, 263–274. 10.1007/s10393-004-0096-4 [DOI] [Google Scholar]
- Buxton, R. T. , Currey, C. A. , Lyver, P. O. B. , & Jones, C. J. (2013). Incidence of plastic fragments among burrow‐nesting seabird colonies on offshore islands in northern New Zealand. Marine Pollution Bulletin, 74, 420–424. 10.1016/j.marpolbul.2013.07.011 [DOI] [PubMed] [Google Scholar]
- Callaham, M. A. , Butt, K. R. , & Lowe, C. N. (2012). Stable isotope evidence for marine‐derived avian inputs of nitrogen into soil, vegetation, and earthworms on the isle of Rum, Scotland, UK. European Journal of Soil Biology, 52, 78–83. 10.1016/j.ejsobi.2012.07.004 [DOI] [Google Scholar]
- Cassey, P. , & Blackburn, T. M. (2006). Reproducibility and repeatability in ecology. Bioscience, 56, 958–959. 10.1641/0006-3568(2006)56[958,RARIE]2.0.CO;2 [DOI] [Google Scholar]
- Celis, J. E. , Espejo, W. , González‐Acuña, D. , Jara, S. , & Barra, R. (2014). Assessment of trace metals and porphyrins in excreta of Humboldt penguins (Spheniscus humboldti) in different locations of the northern coast of Chile. Environmental Monitoring and Assessment, 186, 1815–1824. 10.1007/s10661-013-3495-6 [DOI] [PubMed] [Google Scholar]
- Choy, E. S. , Gauthier, M. , Mallory, M. L. , Smol, J. P. , Douglas, M. S. V. , Lean, D. , & Blais, J. M. (2010). An isotopic investigation of mercury accumulation in terrestrial food webs adjacent to an Arctic seabird colony. Science of the Total Environment, 408, 1858–1867. 10.1016/j.scitotenv.2010.01.014 [DOI] [PubMed] [Google Scholar]
- Croll, D. A. , Maron, J. L. , Estes, J. A. , Danner, E. M. , & Byrd, G. V. (2005). Introduced predators transform subarctic islands from grassland to tundra. Science, 307, 1959–1961. 10.1126/science.1108485 [DOI] [PubMed] [Google Scholar]
- Croxall, J. P. , Butchart, S. H. M. , Lascelles, B. , Stattersfield, A. J. , Sullivan, B. , Symes, A. , & Taylor, P. (2012). Seabird conservation status, threats and priority actions: A global assessment. Bird Conservation International, 22, 1–34. 10.1017/s0959270912000020 [DOI] [Google Scholar]
- Cushman, G. T. (2013). Guano and the opening of the Pacific world: A global ecological history. Cambridge University Press. [Google Scholar]
- Daher, M. , Schaefer, C. E. G. R. , Thomazini, A. , de Lima Neto, E. , Souza, C. D. , & do Vale Lopes, D. (2019). Ornithogenic soils on basalts from maritime Antarctica. CATENA, 173, 367–374. 10.1016/j.catena.2018.10.028 [DOI] [Google Scholar]
- De la Peña‐Lastra, S. , Affre, L. , & Otero, X. L. (2020). Soil nutrient dynamics in colonies of the yellow‐legged seagull (Larus michahellis) in different biogeographical zones. Geoderma, 361, 114109. 10.1016/j.geoderma.2019.114109 [DOI] [Google Scholar]
- de Souza Machado, A. A. , Lau, C. W. , Till, J. , Kloas, W. , Lehmann, A. , Becker, R. , & Rillig, M. C. (2018). Impacts of microplastics on the soil biophysical environment. Environmental Science & Technology, 52, 9656–9665. 10.1021/acs.est.8b02212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias, M. P. , Martin, R. , Pearmain, E. J. , Burfield, I. J. , Small, C. , Phillips, R. A. , Yates, O. , Lascelles, B. , Borboroglu, P. G. , & Croxall, J. P. (2019). Threats to seabirds: A global assessment. Biological Conservation, 237, 525–537. 10.1016/j.biocon.2019.06.033 [DOI] [Google Scholar]
- Dominguez, M. T. , Gutierrez, E. , Gonzalez‐Dominguez, B. , Roman, M. , Avila, J. M. , Ramo, C. , Gonzalez, J. M. , & Garcia, L. V. (2017). Impacts of protected colonial birds on soil microbial communities: When protection leads to degradation. Soil Biology & Biochemistry, 105, 59–70. 10.1016/j.soilbio.2016.11.007 [DOI] [Google Scholar]
- Duda, M. P. , Glew, J. R. , Michelutti, N. , Robertson, G. J. , Montevecchi, W. A. , Kissinger, J. A. , Eickmeyer, D. C. , Blais, J. M. , & Smol, J. P. (2020). Long‐term changes in terrestrial vegetation linked to shifts in a colonial seabird population. Ecosystems, 23, 1643–1656. 10.1007/s10021-020-00494-8 [DOI] [Google Scholar]
- Duffy, D. C. (1994). The guano islands of Peru: The once and future management of a renewable resource. Birdlife Conservation Series, 1, 68–76. [Google Scholar]
- Earl, J. E. , & Zollner, P. A. (2017). Advancing research on animal‐transported subsidies by integrating animal movement and ecosystem modelling. Journal of Animal Ecology, 86, 987–997. 10.1111/1365-2656.12711 [DOI] [PubMed] [Google Scholar]
- Ellis, J. C. (2005). Marine birds on land: A review of plant biomass, species richness, and community composition in seabird colonies. Plant Ecology, 181, 227–241. 10.1007/s11258-005-7147-y [DOI] [Google Scholar]
- Espejo, W. , Celis, J. E. , González‐Acuña, D. , Jara, S. , & Barra, R. (2014). Concentration of trace metals in excrements of two species of penguins from different locations of the Antarctic Peninsula. Polar Biology, 37, 675–683. 10.1007/s00300-014-1468-z [DOI] [Google Scholar]
- Evenset, A. , Carroll, J. , Christensen, G. N. , Kallenborn, R. , Gregor, D. , & Gabrielsen, G. W. (2007). Seabird guano is an efficient conveyer of persistent organic pollutants (POPs) to Arctic lake ecosystems. Environmental Science & Technology, 41, 1173–1179. 10.1021/es0621142 [DOI] [PubMed] [Google Scholar]
- Evenset, A. , Christensen, G. N. , Skotvold, T. , Fjeld, E. , Schlabach, M. , Wartena, E. , & Gregor, D. (2004). A comparison of organic contaminants in two high Arctic lake ecosystems, Bjornoya (Bear Island), Norway. Science of the Total Environment, 318, 125–141. 10.1016/s0048-9697(03)00365-6 [DOI] [PubMed] [Google Scholar]
- Fariña, J. , Salazar, S. , Wallem, K. , Witman, J. , & Ellis, J. (2003). Nutrient exchanges between marine and terrestrial ecosystems: The case of the Galapagos sea lion Zalophus wollebaecki . Journal of Animal Ecology, 72, 873–887. 10.1046/j.1365-2656.2003.00760.x [DOI] [Google Scholar]
- Feare, C. J. (1978). The decline of Booby (Sulidae) populations in the western Indian Ocean. Biological Conservation, 14, 295–305. 10.1016/0006-3207(78)90046-0 [DOI] [Google Scholar]
- Foster, K. L. , Kimpe, L. E. , Brimble, S. K. , Liu, H. J. , Mallory, M. L. , Smol, J. P. , Macdonald, R. W. , & Blais, J. M. (2011). Effects of seabird vectors on the fate, partitioning, and signatures of contaminants in a high Arctic ecosystem. Environmental Science & Technology, 45, 10053–10060. 10.1021/es202754h [DOI] [PubMed] [Google Scholar]
- Furness, R. W. , & Camphuysen, C. J. (1997). Seabirds as monitors of the marine environment. ICES Journal of Marine Science, 54, 726–737. 10.1006/jmsc.1997.0243 [DOI] [Google Scholar]
- Gil‐Delgado, J. A. , Guijarro, D. , Gosálvez, R. U. , López‐Iborra, G. M. , Ponz, A. , & Velasco, A. (2017). Presence of plastic particles in waterbirds faeces collected in Spanish lakes. Environmental Pollution, 220, 732–736. 10.1016/j.envpol.2016.09.054 [DOI] [PubMed] [Google Scholar]
- Gill, F. , & Donsker, D. (2021). IOC World Bird List (v11.2). 10.14344/IOC.ML.11.2 [DOI]
- Gillham, M. E. (1961). Alteration of the breeding habitat by sea‐birds and seals in Western Australia. Journal of Ecology, 49, 289–300. 10.2307/2257263 [DOI] [Google Scholar]
- Godzik, B. (1991). Heavy metals and macroelements in the tundra of southern Spitsbergen: The effect of Little Auk Alle alle (L.) colonies. Polar Research, 9, 121–131. 10.1111/j.1751-8369.1991.tb00608.x [DOI] [Google Scholar]
- Grant, M. L. , Lavers, J. L. , & Bond, A. L. (2022). Data from: The influence of seabirds on their breeding, roosting, and nesting grounds: A systematic review and meta‐analysis. Institute for Marine and Antarctic Studies, University of Tasmania, IMAS Data Portal . 10.25959/ENWE-GB98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant, M. L. , Lavers, J. L. , Hutton, I. , & Bond, A. L. (2021). Seabird breeding islands as sinks for marine plastic debris. Environmental Pollution, 276, 116734. 10.1016/j.envpol.2021.116734 [DOI] [PubMed] [Google Scholar]
- Grant, M. L. , Lavers, J. L. , Stuckenbrock, S. , Sharp, P. B. , & Bond, A. L. (2018). The use of anthropogenic marine debris as a nesting material by Brown Boobies (Sula leucogaster). Marine Pollution Bulletin, 137, 96–103. 10.1016/j.marpolbul.2018.10.016 [DOI] [PubMed] [Google Scholar]
- Griffiths, K. , Michelutti, N. , Blais, J. M. , Kimpe, L. E. , & Smol, J. P. (2010). Comparing nitrogen isotopic signals between bulk sediments and invertebrate remains in High Arctic seabird‐influenced ponds. Journal of Paleolimnology, 44, 405–412. 10.1007/s10933-009-9354-3 [DOI] [Google Scholar]
- Haddaway, N. R. , Macura, B. , Whaley, P. , & Pullin, A. S. (2018). ROSES RepOrting standards for Systematic Evidence Syntheses: Pro forma, flow‐diagram and descriptive summary of the plan and conduct of environmental systematic reviews and systematic maps. Environmental Evidence, 7, 7. 10.1186/s13750-018-0121-7 [DOI] [Google Scholar]
- Hamilton, B. M. , Bourdages, M. P. T. , Geoffroy, C. , Vermaire, J. C. , Mallory, M. L. , Rochman, C. M. , & Provencher, J. F. (2021). Microplastics around an Arctic seabird colony: Particle community composition varies across environmental matrices. Science of the Total Environment, 773, 145536. 10.1016/j.scitotenv.2021.145536 [DOI] [PubMed] [Google Scholar]
- Harding, J. S. , Hawke, D. J. , Holdaway, R. N. , & Winterbourn, M. J. (2004). Incorporation of marine‐derived nutrients from petrel breeding colonies into stream food webs. Freshwater Biology, 49, 576–586. 10.1111/j.1365-2427.2004.01210.x [DOI] [Google Scholar]
- Hawke, D. J. , & Clark, J. M. (2010). Isotopic signatures (13C/12C, 15N/14N) of Blue Penguin burrow soil invertebrates: Carbon sources and trophic relationships. New Zealand Journal of Zoology, 37, 313–321. 10.1080/03014223.2010.519036 [DOI] [Google Scholar]
- Headley, A. D. (1996). Heavy metal concentrations in peat profiles from the high Arctic. Science of the Total Environment, 177, 105–111. 10.1016/0048-9697(95)04867-7 [DOI] [Google Scholar]
- Hebert, C. E. , Arts, M. T. , & Weseloh, D. V. C. (2006). Ecological tracers can quantify food web structure and change. Environmental Science & Technology, 40, 5618–5623. 10.1021/es0520619 [DOI] [PubMed] [Google Scholar]
- Hedges, L. V. , Gurevitch, J. , & Curtis, P. S. (1999). The meta‐analysis of response ratios in experimental ecology. Ecology, 80, 1150–1156. 10.1890/0012-9658(1999)080[1150:TMAORR]2.0.CO;2 [DOI] [Google Scholar]
- Hodel, R. G. , Knowles, L. L. , McDaniel, S. F. , Payton, A. C. , Dunaway, J. F. , Soltis, P. S. , & Soltis, D. E. (2018). Terrestrial species adapted to sea dispersal: Differences in propagule dispersal of two Caribbean mangroves. Molecular Ecology, 27, 4612–4626. 10.1111/mec.14894 [DOI] [PubMed] [Google Scholar]
- Hoenig, B. D. , Snider, A. M. , Forsman, A. M. , Hobson, K. A. , Latta, S. C. , Miller, E. T. , Polito, M. J. , Powell, L. L. , Rogers, S. L. , Sherry, T. W. , Toews, D. P. L. , Welch, A. J. , Taylor, S. S. , & Porter, B. A. (2022). Current methods and future directions in avian diet analysis. Ornithology, 139, 1–28. 10.1093/ornithology/ukab077 [DOI] [Google Scholar]
- Kameda, K. , Koba, K. , Hobara, S. , Osono, T. , & Terai, M. (2006). Pattern of natural 15N abundance in lakeside forest ecosystem affected by cormorant‐derived nitrogen. Hydrobiologia, 567, 69–86. 10.1007/s10750-006-0052-0 [DOI] [Google Scholar]
- Kaufman, Y. J. , Koren, I. , Remer, L. A. , Tanré, D. , Ginoux, P. , & Fan, S. (2005). Dust transport and deposition observed from the Terra‐Moderate Resolution Imaging Spectroradiometer (MODIS) spacecraft over the Atlantic Ocean. Journal of Geophysical Research: Atmospheres, 110, D10S12. 10.1029/2003jd004436 [DOI] [Google Scholar]
- Kiko, R. , Biastoch, A. , Brandt, P. , Cravatte, S. , Hauss, H. , Hummels, R. , Kriest, I. , Marin, F. , McDonnell, A. M. P. , Oschlies, A. , Picheral, M. , Schwarzkopf, F. U. , Thurnherr, A. M. , & Stemmann, L. (2017). Biological and physical influences on marine snowfall at the equator. Nature Geoscience, 10, 852–858. 10.1038/Ngeo3042 [DOI] [Google Scholar]
- Kolb, G. S. , Jerling, L. , Essenberg, C. , Palmborg, C. , & Hambäck, P. A. (2012). The impact of nesting cormorants on plant and arthropod diversity. Ecography, 35, 726–740. 10.1111/j.1600-0587.2011.06808.x [DOI] [Google Scholar]
- Kolb, G. S. , Palmborg, C. , Taylor, A. R. , Baath, E. , & Hamback, P. A. (2015). Effects of nesting cormorants (Phalacrocorax carbo) on soil chemistry, microbial communities and soil fauna. Ecosystems, 18, 643–657. 10.1007/s10021-015-9853-1 [DOI] [Google Scholar]
- Lavers, J. L. , Rivers‐Auty, J. , & Bond, A. L. (2021). Plastic debris increases circadian temperature extremes in beach sediments. Journal of Hazardous Materials, 416, 126140. 10.1016/j.jhazmat.2021.126140 [DOI] [PubMed] [Google Scholar]
- Lucassen, F. , Pritzkow, W. , Rosner, M. , Sepulveda, F. , Vasquez, P. , Wilke, H. , & Kasemann, S. A. (2017). The stable isotope composition of nitrogen and carbon and elemental contents in modern and fossil seabird guano from Northern Chile—Marine sources and diagenetic effects. PLoS ONE, 12, e0179440. 10.1371/journal.pone.0179440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnússon, B. , Magnússon, S. H. , Ólafsson, E. , & Sigurdsson, B. D. (2014). Plant colonization, succession and ecosystem development on Surtsey with reference to neighbouring islands. Biogeosciences (Online), 11, 5521–5537. 10.5194/bg-11-5521-2014 [DOI] [Google Scholar]
- Markwell, T. J. , & Daugherty, C. H. (2002). Invertebrate and lizard abundance is greater on seabird‐inhabited islands than on seabird‐free islands in the Marlborough Sounds, New Zealand. Ecoscience, 9, 293–299. 10.1080/11956860.2002.11682715 [DOI] [Google Scholar]
- Marmen, M. B. , Kenchington, E. , Ardyna, M. , & Archambault, P. (2017). Influence of seabird colonies and other environmental variables on benthic community structure, Lancaster Sound Region, Canadian Arctic. Journal of Marine Systems, 167, 105–117. 10.1016/j.jmarsys.2016.11.021 [DOI] [Google Scholar]
- Maron, J. L. , Estes, J. A. , Croll, D. A. , Danner, E. M. , Elmendorf, S. C. , & Buckelew, S. L. (2006). An introduced predator alters Aleutian Island plant communities by thwarting nutrient subsidies. Ecological Monographs, 76, 3–24. 10.1890/05-0496 [DOI] [Google Scholar]
- Martín‐Vélez, V. , Sánchez, M. I. , Shamoun‐Baranes, J. , Thaxter, C. B. , Stienen, E. W. M. , Camphuysen, K. C. J. , & Green, A. J. (2019). Quantifying nutrient inputs by gulls to a fluctuating lake, aided by movement ecology methods. Freshwater Biology, 64, 1821–1832. 10.1111/fwb.13374 [DOI] [Google Scholar]
- Mathew, W. M. (1970). Peru and the British Guano Market, 1840–1870. The Economic History Review, 23, 112–128. 10.2307/2594566 [DOI] [Google Scholar]
- McColl, J. G. , & Burger, J. (1976). Chemical inputs by a colony of Franklin's Gulls nesting in cattails. The American Midland Naturalist, 96, 270–280. 10.2307/2424068 [DOI] [Google Scholar]
- McKechnie, S. (2006). Biopedturbation by an island ecosystem engineer: Burrowing volumes and litter deposition by Sooty Shearwaters (Puffinus griseus). New Zealand Journal of Zoology, 33, 259–265. 10.1080/03014223.2006.9518455 [DOI] [Google Scholar]
- Michelutti, N. , Keatley, B. E. , Brimble, S. , Blais, J. M. , Liu, H. , Douglas, M. S. , Mallory, M. L. , Macdonald, R. W. , & Smol, J. P. (2009). Seabird‐driven shifts in Arctic pond ecosystems. Proceedings: Biological Sciences, 276, 591–596. 10.1098/rspb.2008.1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michener, R. , & Schell, D. (1994). Stable isotopes as tracers in marine aquatic foodwebs. In Lajtha K. & Michener R. (Eds.), Stable isotopes in ecology and environmental science (pp. 138–157). Blackwell Scientific Publications. [Google Scholar]
- Mizutani, H. , Kabaya, Y. , Moors, P. J. , Speir, T. W. , & Lyon, G. L. (1991). Nitrogen isotope ratios identify deserted seabird colonies. The Auk, 108, 960–964. 10.1093/auk/108.4.960 [DOI] [Google Scholar]
- Molina‐Montenegro, M. A. , Torres‐Diaz, C. , Gallardo‐Cerda, J. , Leppe, M. , & Gianoli, E. (2013). Seabirds modify El Nino effects on tree growth in a southern Pacific island. Ecology, 94, 2415–2425. 10.1890/12-1054.1 [DOI] [PubMed] [Google Scholar]
- Montevecchi, W. A. (1993). Birds as indicators of change in marine prey stocks. In Furness R. W. & Greenwood D. J. (Eds.), Birds as monitors of environmental change (pp. 261–271). Chapman & Hall. [Google Scholar]
- Mosbech, A. , Johansen, K. L. , Davidson, T. A. , Appelt, M. , Gronnow, B. , Cuyler, C. , Lyngs, P. , & Flora, J. (2018). On the crucial importance of a small bird: The ecosystem services of the little auk (Alle alle) population in Northwest Greenland in a long‐term perspective. Ambio, 47, 226–243. 10.1007/s13280-018-1035-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nel, D. C. , & Nel, J. L. (1999). Marine debris and fishing gear associated with seabirds at sub‐Antarctic Marion Island, 1996/97 and 1997/98: In relation to longline fishing activity. CCAMLR Science, 6, 85–96. [Google Scholar]
- O'Hanlon, N. J. , Bond, A. L. , Lavers, J. L. , Masden, E. A. , & James, N. A. (2019). Monitoring nest incorporation of anthropogenic debris by Northern Gannets across their range. Environmental Pollution, 255, 113152. 10.1016/j.envpol.2019.113152 [DOI] [PubMed] [Google Scholar]
- Otero, X. L. (1998). Effects of nesting yellow‐legged gulls (Larus cachinnans Pallas) on the heavy metal content of soils in the Cies Islands (Galicia, North‐west Spain). Marine Pollution Bulletin, 36, 267–272. 10.1016/S0025-326X(98)80010-6 [DOI] [Google Scholar]
- Otero, X. L. , de la Pena‐Lastra, S. , Romero, D. , Nobrega, G. N. , Ferreira, T. O. , & Perez‐Alberti, A. (2018). Trace elements in biomaterials and soils from a yellow‐legged gull (Larus michahellis) colony in the Atlantic Islands of Galicia National Park (NW Spain). Marine Pollution Bulletin, 133, 144–149. 10.1016/j.marpolbul.2018.05.027 [DOI] [PubMed] [Google Scholar]
- Otero, X. L. , Tejada, O. , Martín‐Pastor, M. , De La Peña, S. , Ferreira, T. O. , & Pérez‐Alberti, A. (2015). Phosphorus in seagull colonies and the effect on the habitats. The case of yellow‐legged gulls (Larus michahellis) in the Atlantic Islands National Park (Galicia‐NW Spain). Science of the Total Environment, 532, 383–397. 10.1016/j.scitotenv.2015.06.013 [DOI] [PubMed] [Google Scholar]
- Page, M. J. , McKenzie, J. E. , Bossuyt, P. M. , Boutron, I. , Hoffmann, T. C. , Mulrow, C. D. , Shamseer, L. , Tetzlaff, J. M. , Akl, E. A. , Brennan, S. E. , Chou, R. , Glanville, J. , Grimshaw, J. M. , Hróbjartsson, A. , Lalu, M. M. , Li, T. , Loder, E. W. , Mayo‐Wilson, E. , McDonald, S. , … Moher, D. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Systematic Reviews, 10, 89. 10.1186/s13643-021-01626-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne, L. X. , & Moore, J. W. (2006). Mobile scavengers create hotspots of freshwater productivity. Oikos, 115, 69–80. 10.1111/j.2006.0030-1299.14899.x [DOI] [Google Scholar]
- Pereira, T. T. C. , Schaefer, C. E. G. R. , Ker, J. C. , Almeida, C. C. , Almeida, I. C. C. , & Pereira, A. B. (2013). Genesis, mineralogy and ecological significance of ornithogenic soils from a semi‐desert polar landscape at Hope Bay, Antarctic Peninsula. Geoderma, 209–210, 98–109. 10.1016/j.geoderma.2013.06.012 [DOI] [Google Scholar]
- Piatt, J. F. , Harding, A. M. A. , Shultz, M. , Speckman, S. G. , van Pelt, T. I. , Drew, G. S. , & Kettle, A. B. (2007). Seabirds as indicators of marine food supplies: Cairns revisited. Marine Ecology Progress Series, 352, 221–234. 10.3354/meps07078 [DOI] [Google Scholar]
- Polis, G. A. , & Hurd, S. D. (1996). Linking marine and terrestrial food webs: Allochthonous input from the ocean supports high secondary productivity on small islands and coastal land communities. The American Naturalist, 147, 396–423. 10.1086/285858 [DOI] [Google Scholar]
- Portnoy, J. W. (1990). Gull contributions of phosphorus and nitrogen to a Cape Cod kettle pond. Hydrobiologia, 202, 61–69. 10.1007/BF00027092 [DOI] [Google Scholar]
- Post, D. M. (2002). Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology, 83, 703–718. 10.1890/0012-9658(2002)083[0703,Usitet]2.0.Co;2 [DOI] [Google Scholar]
- Powell, G. V. N. , Fourqurean, J. W. , Kenworthy, W. J. , & Zieman, J. C. (1991). Bird colonies cause seagrass enrichment in a subtropical estuary: Obsevational and experimental evidence. Estuarine, Coastal and Shelf Science, 32, 567–579. 10.1016/0272-7714(91)90075-m [DOI] [Google Scholar]
- Provencher, J. F. , Vermaire, J. C. , Avery‐Gomm, S. , Braune, B. M. , & Mallory, M. L. (2018). Garbage in guano? Microplastic debris found in faecal precursors of seabirds known to ingest plastics. Science of the Total Environment, 644, 1477–1484. 10.1016/j.scitotenv.2018.07.101 [DOI] [PubMed] [Google Scholar]
- Rajakaruna, N. , Pope, N. , Perez‐Orozco, J. , & Harris, T. B. (2009). Ornithocoprophilous plants of Mount Desert Rock, a remote bird‐nesting island in the Gulf of Maine, U.S.A. Rhodora, 111, 417–447. 10.3119/08-12.1 [DOI] [Google Scholar]
- Reynolds, C. , & Ryan, P. G. (2018). Micro‐plastic ingestion by waterbirds from contaminated wetlands in South Africa. Marine Pollution Bulletin, 126, 330–333. 10.1016/j.marpolbul.2017.11.021 [DOI] [PubMed] [Google Scholar]
- Richardson, K. M. , Iverson, J. B. , & Kurle, C. M. (2019). Marine subsidies likely cause gigantism of iguanas in the Bahamas. Oecologia, 189, 1005–1015. 10.1007/s00442-019-04366-4 [DOI] [PubMed] [Google Scholar]
- Riddick, S. N. , Dragosits, U. , Blackall, T. D. , Tomlinson, S. J. , Daunt, F. , Wanless, S. , Hallsworth, S. , Braban, C. F. , Tang, Y. S. , & Sutton, M. A. (2018). Global assessment of the effect of climate change on ammonia emissions from seabirds. Atmospheric Environment, 184, 212–223. 10.1016/j.atmosenv.2018.04.038 [DOI] [Google Scholar]
- Rodrigues, W. F. , Oliveira, F. S. D. , Schaefer, C. E. G. R. , Leite, M. G. P. , & Pavinato, P. S. (2021). Phosphatization under birds' activity: Ornithogenesis at different scales on Antarctic Soilscapes. Geoderma, 391, 114950. 10.1016/j.geoderma.2021.114950 [DOI] [Google Scholar]
- Roosens, L. , Van Den Brink, N. , Riddle, M. , Blust, R. , Neels, H. , & Covaci, A. (2007). Penguin colonies as secondary sources of contamination with persistent organic pollutants. Journal of Environmental Monitoring, 9, 822–825. 10.1039/B708103K [DOI] [PubMed] [Google Scholar]
- Saifutdinov, R. A. , & Korobushkin, D. I. (2020). Impact of overwintering cormorants (Phalacrocorax carbo) on springtail (Hexapoda: Collembola) communities of the Azov and Black Sea coastal forests. Turkish Journal of Zoology, 44, 303–311. 10.3906/zoo-2003-24 [DOI] [Google Scholar]
- Sanchez‐Pinero, F. , & Polis, G. A. (2000). Bottom‐up dynamics of allochthonous input: Direct and indirect effects of seabirds on islands. Ecology, 81, 3117–3132. 10.2307/177405 [DOI] [Google Scholar]
- Santamans, A. C. , Boluda, R. , Picazo, A. , Gil, C. , Ramos‐Miras, J. , Tejedo, P. , Pertierra, L. R. , Benayas, J. , & Camacho, A. (2017). Soil features in rookeries of Antarctic penguins reveal sea to land biotransport of chemical pollutants. PLoS ONE, 12, e0181901. 10.1371/journal.pone.0181901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage, C. (2019). Seabird nutrients are assimilated by corals and enhance coral growth rates. Scientific Reports, 9, 4248. 10.1038/s41598-019-41030-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley, M. E. , Song, B. , Passie, R. , & Hale, R. C. (2020). Microplastics affect sedimentary microbial communities and nitrogen cycling. Nature Communications, 11, 2372. 10.1038/s41467-020-16235-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Şekercioğlu, Ç. H. (2006). Increasing awareness of avian ecological function. Trends in Ecology & Evolution, 21, 464–471. 10.1016/j.tree.2006.05.007 [DOI] [PubMed] [Google Scholar]
- Şekercioğlu, Ç. H. , Daily, G. C. , & Ehrlich, P. R. (2004). Ecosystem consequences of bird declines. Proceedings of the National Academy of Sciences of the United States of America, 101, 18042. 10.1073/pnas.0408049101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šerić Jelaska, L. , Jurasović, J. , Brown, D. S. , Vaughan, I. P. , & Symondson, W. O. C. (2014). Molecular field analysis of trophic relationships in soil‐dwelling invertebrates to identify mercury, lead and cadmium transmission through forest ecosystems. Molecular Ecology, 23, 3755–3766. 10.1111/mec.12566 [DOI] [PubMed] [Google Scholar]
- Shilton, L. A. , Altringham, J. D. , Compton, S. G. , & Whittaker, R. J. (1999). Old World fruit bats can be long‐distance seed dispersers through extended retention of viable seeds in the gut. Proceedings of the Royal Society of London. Series B: Biological Sciences, 266, 219–223. 10.1098/rspb.1999.0625 [DOI] [Google Scholar]
- Shoji, A. , Elliott, K. H. , Aris‐Brosou, S. , Mizukawa, H. , Nakayama, S. M. M. , Ikenaka, Y. , Ishizuka, M. , Kuwae, T. , Watanabe, K. , Escoruela Gonzalez, J. , & Watanuki, Y. (2019). Biotransport of metallic trace elements from marine to terrestrial ecosystems by seabirds. Environmental Toxicology and Chemistry, 38, 106–114. 10.1002/etc.4286 [DOI] [PubMed] [Google Scholar]
- Signa, G. , Mazzola, A. , Costa, V. , & Vizzini, S. (2015). Bottom‐up control of macrobenthic communities in a guanotrophic coastal system. PLoS ONE, 10, e0117544. 10.1371/journal.pone.0117544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signa, G. , Mazzola, A. , Tramati, C. D. , & Vizzini, S. (2013). Gull‐derived trace elements trigger small‐scale contamination in a remote Mediterranean nature reserve. Marine Pollution Bulletin, 74, 237–243. 10.1016/j.marpolbul.2013.06.051 [DOI] [PubMed] [Google Scholar]
- Szpak, P. , Longstaffe, F. J. , Millaire, J. F. , & White, C. D. (2012). Stable isotope biogeochemistry of seabird guano fertilization: Results from growth chamber studies with maize (Zea mays). PLoS ONE, 7, e33741. 10.1371/journal.pone.0033741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpak, P. , Millaire, J.‐F. , White, C. D. , & Longstaffe, F. J. (2012). Influence of seabird guano and camelid dung fertilization on the nitrogen isotopic composition of field‐grown maize (Zea mays). Journal of Archaeological Science, 39, 3721–3740. 10.1016/j.jas.2012.06.035 [DOI] [Google Scholar]
- The International Union for Conservation of Nature . (2012). Data from: Habitat Classification Scheme. IUCN. [Google Scholar]
- Tian, L. , Zhao, L. , Wu, X. , Fang, H. , Zhao, Y. , Yue, G. , Liu, G. , & Chen, H. (2017). Vertical patterns and controls of soil nutrients in alpine grassland: Implications for nutrient uptake. Science of the Total Environment, 607–608, 855–864. 10.1016/j.scitotenv.2017.07.080 [DOI] [PubMed] [Google Scholar]
- Van De Crommenacker, J. , Soares, J. H. , Larose, C. S. , & Feare, C. J. (2021). Importation of plastic fragments into a seabird colony: Accident or design, threat or benign? Bird Conservation International, 1–14. 10.1017/S0959270921000538 [DOI] [Google Scholar]
- Viechtbauer, W. (2010). Conducting meta‐analyses in R with the metafor package. Journal of Statistical Software, 36, 1–48. 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- Wait, D. A. , Aubrey, D. P. , & Anderson, W. B. (2005). Seabird guano influences on desert islands: Soil chemistry and herbaceous species richness and productivity. Journal of Arid Environments, 60, 681–695. 10.1016/j.jaridenv.2004.07.001 [DOI] [Google Scholar]
- Wille, M. , Lisovski, S. , Risely, A. , Ferenczi, M. , Roshier, D. , Wong, F. Y. K. , Breed, A. C. , Klaassen, M. , & Hurt, A. C. (2019). Serologic evidence of exposure to highly pathogenic avian influenza H5 viruses in migratory shorebirds, Australia. Emerging Infectious Diseases, 25, 1903–1910. 10.3201/eid2510.190699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing, S. R. , Jack, L. , Shatova, O. , Leichter, J. J. , Barr, D. , Frew, R. D. , & Gault‐Ringold, M. (2014). Seabirds and marine mammals redistribute bioavailable iron in the Southern Ocean. Marine Ecology Progress Series, 510, 1–13. 10.3354/meps10923 [DOI] [Google Scholar]
- Wootton, J. T. (1991). Direct and indirect effects of nutrients on intertidal community structure: Variable consequences of seabird guano. Journal of Experimental Marine Biology and Ecology, 151, 139–153. 10.1016/0022-0981(91)90121-c [DOI] [Google Scholar]
- WRB . (2015). Data from: World Reference Base for Soil Resources 2014, update 2015 International soil classification system for naming soils and creating legends for soil maps.
- Zawierucha, K. , Zmudczynska‐Skarbek, K. , Kaczmarek, A. , & Wojczulanis‐Jakubas, K. (2016). The influence of a seabird colony on abundance and species composition of water bears (Tardigrada) in Hornsund (Spitsbergen, Arctic). Polar Biology, 39, 713–723. 10.1007/s00300-015-1827-4 [DOI] [Google Scholar]
- Ziołek, M. , & Melke, J. (2014). The impact of seabirds on the content of various forms of phosphorus in organic soils of the Bellsund coast, western Spitsbergen. Polar Research, 33, 19986. 10.3402/polar.v33.19986 [DOI] [Google Scholar]
- Zmudczynska‐Skarbek, K. , Barcikowski, M. , Zwolicki, A. , Iliszko, L. , & Stempniewicz, L. (2013). Variability of polar scurvygrass Cochlearia groenlandica individual traits along a seabird influenced gradient across Spitsbergen tundra. Polar Biology, 36, 1659–1669. 10.1007/s00300-013-1385-6 [DOI] [Google Scholar]
- Zwolicki, A. , Zmudczynska‐Skarbek, K. , Matula, J. , Wojtun, B. , & Stempniewicz, L. (2016). Differential responses of Arctic vegetation to nutrient enrichment by plankton‐ and fish‐eating colonial seabirds in Spitsbergen. Frontiers in Plant Science, 7, 1959. 10.3389/fpls.2016.01959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwolicki, A. , Zmudczynska‐Skarbek, K. M. , Iliszko, L. , & Stempniewicz, L. (2013). Guano deposition and nutrient enrichment in the vicinity of planktivorous and piscivorous seabird colonies in Spitsbergen. Polar Biology, 36, 363–372. 10.1007/s00300-012-1265-5 [DOI] [Google Scholar]
DATA SOURCES
- Abakumov, E. (2018). Content of available forms of nitrogen, potassium and phosphorus in ornithogenic and other soils of the Fildes Peninsula (King George Island, Western Antarctica). Biological Communications, 63, 109–116. 10.21638/spbu03.2018.203 [DOI] [Google Scholar]
- Abakumov, E. , Lupachev, A. , Andreev, M. , Wang, W. , & Ji, X. (2021). The influence of Brown and South Polar Skua on the content of plant nutrient in the soils from the Fildes Peninsula (King George Island, West Antarctica). Chemistry and Ecology, 37, 185–199. 10.1080/02757540.2020.1839435 [DOI] [Google Scholar]
- Adame, M. F. , Fry, B. , Gamboa, J. N. , & Herrera‐Silveira, J. A. (2015). Nutrient subsidies delivered by seabirds to mangrove islands. Marine Ecology Progress Series, 525, 15–24. 10.3354/meps11197 [DOI] [Google Scholar]
- Allaway, W. , & Ashford, A. (1984). Nutrient input by seabirds to the forest on a coral island of the Great Barrier Reef. Marine Ecology Progress Series, 19, 297–298. [Google Scholar]
- Anderson, W. B. , & Polis, G. A. (1999). Nutrient fluxes from water to land: Seabirds affect plant nutrient status on Gulf of California islands. Oecologia, 118, 324–332. 10.1007/s004420050733 [DOI] [PubMed] [Google Scholar]
- Balciauskas, L. , Skipityte, R. , Jasiulionis, M. , Balciauskiene, L. , & Remeikis, V. (2018). Immediate increase in isotopic enrichment in small mammals following the expansion of a Great Cormorant colony. Biogeosciences (Online), 15, 3883–3891. 10.5194/bg-15-3883-2018 [DOI] [Google Scholar]
- Balčiauskas, L. , Skipitytė, R. , Jasiulionis, M. , Trakimas, G. , Balčiauskienė, L. , & Remeikis, V. (2016). The impact of Great Cormorants on biogenic pollution of land ecosystems: Stable isotope signatures in small mammals. Science of the Total Environment, 565, 376, 10.1016/j.scitotenv.2016.04.185–383. [DOI] [PubMed] [Google Scholar]
- Ball, B. A. , Tellez, C. R. , & Virginia, R. A. (2015). Penguin activity influences soil biogeochemistry and soil respiration in rookeries on Ross Island, Antarctica. Polar Biology, 38, 1357–1368. 10.1007/s00300-015-1699-7 [DOI] [Google Scholar]
- Bancroft, W. J. , Garkaklis, M. J. , & Roberts, J. D. (2005). Burrow building in seabird colonies: A soil‐forming process in island ecosystems. Pedobiologia, 49, 149–165. 10.1016/j.pedobi.2004.10.002 [DOI] [Google Scholar]
- Bancroft, W. J. , Roberts, J. D. , & Garkaklis, M. J. (2005). Burrowing seabirds drive decreased diversity and structural complexity, and increased productivity in insular‐vegetation communities. Australian Journal of Botany, 53, 231–241. 10.1071/BT04079 [DOI] [Google Scholar]
- Bargagli, R. , Sanchez‐Hernandez, J. C. , Martella, L. , & Monaci, F. (1998). Mercury, cadmium and lead accumulation in Antarctic mosses growing along nutrient and moisture gradients. Polar Biology, 19, 316–322. 10.1007/s003000050252 [DOI] [Google Scholar]
- Blais, J. M. , Kimpe, L. E. , McMahon, D. , Keatley, B. E. , Mallory, M. L. , Douglas, M. S. , & Smol, J. P. (2005). Arctic seabirds transport marine‐derived contaminants. Science, 309, 445. 10.1126/science.1112658 [DOI] [PubMed] [Google Scholar]
- Blakemore, L. , & Gibbs, H. (1968). Effects of gannets on soil at Cape Kidnappers, Hawkes Bay. New Zealand Journal of Science, 11, 54–62. [Google Scholar]
- Bokhorst, S. , Huiskes, A. , Convey, P. , & Aerts, R. (2007). External nutrient inputs into terrestrial ecosystems of the Falkland Islands and the Maritime Antarctic region. Polar Biology, 30, 1315–1321. 10.1007/s00300-007-0292-0 [DOI] [Google Scholar]
- Bosman, A. , & Hockey, P. (1986). Seabird guano as a determinant of rocky intertidal community structure. Marine Ecology Progress Series, 32, 247–257. [Google Scholar]
- Bosman, A. L. , Du Toit, J. T. , Hockey, P. A. R. , & Branch, G. M. (1986). A field experiment demonstrating the influence of seabird guano on intertidal primary production. Estuarine, Coastal and Shelf Science, 23, 283–294. 10.1016/0272-7714(86)90028-4 [DOI] [Google Scholar]
- Bourdages, M. P. T. , Provencher, J. F. , Baak, J. E. , Mallory, M. L. , & Vermaire, J. C. (2021). Breeding seabirds as vectors of microplastics from sea to land: Evidence from colonies in Arctic Canada. Science of the Total Environment, 764, 142808. 10.1016/j.scitotenv.2020.142808 [DOI] [PubMed] [Google Scholar]
- Boutin, C. , Dobbie, T. , Carpenter, D. , & Hebert, C. E. (2011). Effects of Double‐Crested Cormorants (Phalacrocorax auritus Less.) on island vegetation, seedbank, and soil chemistry: Evaluating island restoration potential. Restoration Ecology, 19, 720–727. 10.1111/j.1526-100X.2010.00769.x [DOI] [Google Scholar]
- Breuning‐Madsen, H. , Ehlers, C. B. , & Borggaard, O. K. (2008). The impact of perennial cormorant colonies on soil phosphorus status. Geoderma, 148, 51–54. 10.1016/j.geoderma.2008.09.002 [DOI] [Google Scholar]
- Brimble, S. K. , Blais, J. M. , Kimpe, L. E. , Mallory, M. L. , Keatley, B. E. , Douglas, M. S. V. , & Smol, J. P. (2009). Bioenrichment of trace elements in a series of ponds near a Northern Fulmar (Fulmarus glacialis) colony at Cape Vera, Devon Island. Canadian Journal of Fisheries and Aquatic Sciences, 66, 949–958. 10.1139/f09-053 [DOI] [Google Scholar]
- Brimble, S. K. , Foster, K. L. , Mallory, M. L. , MacDonald, R. W. , Smol, J. P. , & Blais, J. M. (2009). High Arctic ponds receiving biotransported nutrients from a nearby seabird colony are also subject to potentially toxic loadings of arsenic, cadmium, and zinc. Environmental Toxicology and Chemistry, 28, 2426–2433. 10.1897/09-235.1 [DOI] [PubMed] [Google Scholar]
- Bukancinski, D. , Rutkowska, A. , & Bukacinska, M. (1994). The effect of nesting Black‐Headed Gulls (Larus ridibundus L.) on the soil and vegetation of a Vistula River Island, Poland. Annales Botanici Fennici, 31(4), 233–243. [Google Scholar]
- Burger, A. , Lindeboom, H. , & Williams, A. (1978). The mineral and energy contributions of guano of selected species of birds to the Marion Island terrestrial ecosystem. South African Journal of Antarctic Research, 8, 59–70. [Google Scholar]
- Callaham, M. A. , Butt, K. R. , & Lowe, C. N. (2012). Stable isotope evidence for marine‐derived avian inputs of nitrogen into soil, vegetation, and earthworms on the isle of Rum, Scotland, UK. European Journal of Soil Biology, 52, 78–83. 10.1016/j.ejsobi.2012.07.004 [DOI] [Google Scholar]
- Caut, S. , Angulo, E. , Pisanu, B. , Ruffino, L. , Faulquier, L. , Lorvelec, O. , Chapuis, J.‐L. , Pascal, M. , Vidal, E. , & Courchamp, F. (2012). Seabird modulations of isotopic nitrogen on islands. PLoS ONE, 7, e39125. 10.1371/journal.pone.0039125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celis, J. , Jara, S. , González‐Acuña, D. , Barra, R. , & Espejo, W. (2012). A preliminary study of trace metals and porphyrins in excreta of Gentoo penguins (Pygoscelis papua) at two locations of the Antarctic Peninsula. Archivos de Medicina Veterinaria, 44, 311–316. [Google Scholar]
- Celis, J. E. , Barra, R. , Espejo, W. , Gonzalez‐Acuna, D. , & Jara, S. (2015). Trace element concentrations in biotic matrices of Gentoo Penguins (Pygoscelisp papua) and coastal soils from different locations of the Antarctic Peninsula. Water, Air, and Soil Pollution, 226, 2266. 10.1007/s11270-014-2266-5 [DOI] [Google Scholar]
- Celis, J. E. , Espejo, W. , González‐Acuña, D. , Jara, S. , & Barra, R. (2014). Assessment of trace metals and porphyrins in excreta of Humboldt penguins (Spheniscus humboldti) in different locations of the northern coast of Chile. Environmental Monitoring and Assessment, 186, 1815–1824. 10.1007/s10661-013-3495-6 [DOI] [PubMed] [Google Scholar]
- Cheng, W. , Sun, L. , Kimpe, L. E. , Mallory, M. L. , Smol, J. P. , Gallant, L. R. , Li, J. , & Blais, J. M. (2016). Sterols and stanols preserved in pond sediments track seabird biovectors in a high Arctic environment. Environmental Science & Technology, 50, 9351–9360. 10.1021/acs.est.6b02767 [DOI] [PubMed] [Google Scholar]
- Choy, E. S. , Gauthier, M. , Mallory, M. L. , Smol, J. P. , Douglas, M. S. V. , Lean, D. , & Blais, J. M. (2010). An isotopic investigation of mercury accumulation in terrestrial food webs adjacent to an Arctic seabird colony. Science of the Total Environment, 408, 1858–1867. 10.1016/j.scitotenv.2010.01.014 [DOI] [PubMed] [Google Scholar]
- Choy, E. S. , Kimpe, L. E. , Mallory, M. L. , Smol, J. P. , & Blais, J. M. (2010). Contamination of an arctic terrestrial food web with marine‐derived persistent organic pollutants transported by breeding seabirds. Environmental Pollution, 158, 3431–3438. 10.1016/j.envpol.2010.07.014 [DOI] [PubMed] [Google Scholar]
- Cocks, M. , Balfour, D. , & Stock, W. (1998). On the uptake of ornithogenic products by plants on the inland mountains of Dronning Maud Land, Antarctica, using stable isotopes. Polar Biology, 20, 107–111. 10.1007/s003000050283 [DOI] [Google Scholar]
- Cocks, M. P. , Harris, J. M. , Steele, W. K. , & Balfour, D. A. (1999). The influence of ornithogenic products on the nutrient status of soils surrounding nests on nunataks in Dronning Maud Land, Antarctica. Polar Research, 18, 19–26. 10.1111/j.1751-8369.1999.tb00274.x [DOI] [Google Scholar]
- Corsolini, S. , Baroni, D. , Martellini, T. , Pala, N. , & Cincinelli, A. (2019). PBDEs and PCBs in terrestrial ecosystems of the Victoria Land, Antarctica. Chemosphere, 231, 233–239. 10.1016/j.chemosphere.2019.05.126 [DOI] [PubMed] [Google Scholar]
- Daher, M. , Schaefer, C.E.G.R. , Thomazini, A. , de Lima Neto, E. , Souza, C.D. & do Vale Lopes, D. (2019). Ornithogenic soils on basalts from maritime Antarctica. CATENA, 173, 367‐374. 10.1016/j.catena.2018.10.028 [DOI] [Google Scholar]
- De la Peña‐Lastra, S. , Affre, L. , & Otero, X. L. (2020). Soil nutrient dynamics in colonies of the yellow‐legged seagull (Larus michahellis) in different biogeographical zones. Geoderma, 361, 114109. 10.1016/j.geoderma.2019.114109 [DOI] [Google Scholar]
- De La Pena‐Lastra, S. , Perez‐Alberti, A. , & Otero, X. L. (2019). Enrichment of trace elements in colonies of the yellow‐legged gull (Larus michahellis) in the Atlantic Islands National Park (Galicia‐NW Spain). Science of the Total Environment, 648, 1536–1548. 10.1016/j.scitotenv.2018.08.284 [DOI] [PubMed] [Google Scholar]
- De La Peña‐Lastra, S. , Pérez‐Alberti, A. , & Otero, X. L. (2021). Seabird colonies as the main source of nutrients for the coastal ecosystems in the Atlantic Islands of Galicia National Park (NW Spain). Chemosphere, 275, 130077. 10.1016/j.chemosphere.2021.130077 [DOI] [PubMed] [Google Scholar]
- Dowdall, M. , Gwynn, J. P. , Gabrielsen, G. W. , & Lind, B. (2005). Assessment of elevated radionuclide levels in soils associated with an avian colony in a high arctic environment. Soil & Sediment Contamination, 14, 1–11. 10.1080/15320380590891781 [DOI] [Google Scholar]
- Ellis, J. C. , Fariña, J. M. , & Witman, J. D. (2006). Nutrient transfer from sea to land: The case of gulls and cormorants in the Gulf of Maine. Journal of Animal Ecology, 75, 565–574. 10.1111/j.1365-2656.2006.01077.x [DOI] [PubMed] [Google Scholar]
- Erskine, P. D. , Bergstrom, D. M. , Schmidt, S. , Stewart, G. R. , Tweedie, C. E. , & Shaw, J. D. (1998). Subantarctic Macquarie Island—A model ecosystem for studying animal‐derived nitrogen sources using 15N natural abundance. Oecologia, 117, 187–193. 10.1007/s004420050647 [DOI] [PubMed] [Google Scholar]
- Espejo, W. , Celis, J. E. , González‐Acuña, D. , Jara, S. , & Barra, R. (2014). Concentration of trace metals in excrements of two species of penguins from different locations of the Antarctic Peninsula. Polar Biology, 37, 675–683. 10.1007/s00300-014-1468-z [DOI] [Google Scholar]
- Espejo, W. , Celis, J. E. , Sandoval, M. , Gonzalez‐Acuna, D. , Barra, R. , & Capulin, J. (2017). The impact of penguins on the content of trace elements and nutrients in coastal soils of north western Chile and the Antarctic Peninsula area. Water, Air, and Soil Pollution, 228, 116. 10.1007/s11270-017-3303-y [DOI] [Google Scholar]
- Evenset, A. , Carroll, J. , Christensen, G. N. , Kallenborn, R. , Gregor, D. , & Gabrielsen, G. W. (2007). Seabird guano is an efficient conveyer of persistent organic pollutants (POPs) to Arctic lake ecosystems. Environmental Science & Technology, 41, 1173–1179. 10.1021/es0621142 [DOI] [PubMed] [Google Scholar]
- Foster, K. L. , Kimpe, L. E. , Brimble, S. K. , Liu, H. J. , Mallory, M. L. , Smol, J. P. , Macdonald, R. W. , & Blais, J. M. (2011). Effects of seabird vectors on the fate, partitioning, and signatures of contaminants in a high Arctic ecosystem. Environmental Science & Technology, 45, 10053–10060. 10.1021/es202754h [DOI] [PubMed] [Google Scholar]
- Gagnon, K. , Yli‐Rosti, J. , & Jormalainen, V. (2015). Cormorant‐induced shifts in littoral communities. Marine Ecology Progress Series, 541, 15–30. 10.3354/meps11548 [DOI] [Google Scholar]
- García, L. V. , Maranón, T. , Ojeda, F. , Clemente, L. , & Redondo, R. (2002). Seagull influence on soil properties, chenopod shrub distribution, and leaf nutrient status in semi‐arid Mediterranean islands. Oikos, 98, 75–86. 10.1034/j.1600-0706.2002.980108.x [DOI] [Google Scholar]
- Geizer, H. D. , Klapstein, S. J. , Mallory, M. L. , & O'Driscoll, N. J. (2021). Total mercury, methylmercury, phosphate, and sulfate inputs to a bog ecosystem from Herring Gull (Larus smithsoniansus) guano. Ecotoxicology and Environmental Safety, 226, 112845. 10.1016/j.ecoenv.2021.112845 [DOI] [PubMed] [Google Scholar]
- Godzik, B. (1991). Heavy metals and macroelements in the tundra of southern Spitsbergen: The effect of Little Auk Alle alle (L.) colonies. Polar Research, 9, 121–131. 10.1111/j.1751-8369.1991.tb00608.x [DOI] [Google Scholar]
- Gonzalez‐Bergonzoni, I. , Johansen, K. L. , Mosbech, A. , Landkildehus, F. , Jeppesen, E. , & Davidson, T. A. (2017). Small birds, big effects: The Little Auk (Alle alle) transforms high Arctic ecosystems. Proceedings of the Royal Society B: Biological Sciences, 284, 20162572. 10.1098/rspb.2016.2572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths, K. , Michelutti, N. , Blais, J. M. , Kimpe, L. E. , & Smol, J. P. (2010). Comparing nitrogen isotopic signals between bulk sediments and invertebrate remains in High Arctic seabird‐influenced ponds. Journal of Paleolimnology, 44, 405–412. 10.1007/s10933-009-9354-3 [DOI] [Google Scholar]
- Guo, Y. , Wang, N. , Li, G. , Rosas, G. , Zang, J. , Ma, Y. , Liu, J. , Han, W. , & Cao, H. (2018). Direct and indirect effects of penguin feces on microbiomes in Antarctic ornithogenic soils. Frontiers in Microbiology, 9, 552. 10.3389/fmicb.2018.00552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton, B. M. , Bourdages, M. P. T. , Geoffroy, C. , Vermaire, J. C. , Mallory, M. L. , Rochman, C. M. , & Provencher, J. F. (2021). Microplastics around an Arctic seabird colony: Particle community composition varies across environmental matrices. Science of the Total Environment, 773, 145536. 10.1016/j.scitotenv.2021.145536 [DOI] [PubMed] [Google Scholar]
- Harding, J. S. , Hawke, D. J. , Holdaway, R. N. , & Winterbourn, M. J. (2004). Incorporation of marine‐derived nutrients from petrel breeding colonies into stream food webs. Freshwater Biology, 49, 576–586. 10.1111/j.1365-2427.2004.01210.x [DOI] [Google Scholar]
- Hargan, K. E. , Michelutti, N. , Coleman, K. , Grooms, C. , Blais, J. M. , Kimpe, L. E. , Gilchrist, G. , Mallory, M. , & Smol, J. P. (2017). Cliff‐nesting seabirds influence production and sediment chemistry of lakes situated above their colony. Science of the Total Environment, 576, 85–98. 10.1016/j.scitotenv.2016.10.024 [DOI] [PubMed] [Google Scholar]
- Havik, G. , Catenazzi, A. , & Holmgren, M. (2014). Seabird nutrient subsidies benefit non‐nitrogen fixing trees and alter species composition in South American coastal dry forests. PLoS ONE, 9, e86381. 10.1371/journal.pone.0086381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawke, D. , & Holdaway, R. (2005). Avian assimilation and dispersal of carbon and nitrogen brought ashore by breeding Westland Petrels (Procellaria westlandica): A stable isotope study. Journal of Zoology, 266, 419–426. 10.1017/S0952836905007065 [DOI] [Google Scholar]
- Hawke, D. , & Powell, H. (1995). Soil solution chemistry at a Westland Petrel breeding colony, New Zealand: Paleoecological implications. Soil Research, 33, 915–924. 10.1071/SR9950915 [DOI] [Google Scholar]
- Hawke, D. J. (2005). Soil P in a forested seabird colony: Inventories, parent material contributions, and N:P stoichiometry. Australian Journal of Soil Research, 43, 957–962. 10.1071/sr05075 [DOI] [Google Scholar]
- Hawke, D. J. , & Clark, J. M. (2010). Isotopic signatures (13C/12C, 15N/14N) of Blue Penguin burrow soil invertebrates: Carbon sources and trophic relationships. New Zealand Journal of Zoology, 37, 313–321. 10.1080/03014223.2010.519036 [DOI] [Google Scholar]
- Hawke, D. J. , Clark, J. M. , & Vallance, J. R. (2013). Breeding Westland Petrels as providers of detrital carbon and nitrogen for soil arthropods: A stable isotope study. Journal of the Royal Society of New Zealand, 43, 58–65. 10.1080/03036758.2011.616211 [DOI] [Google Scholar]
- Hawke, D. J. , Gamlen‐Greene, R. , Harding, J. S. , & Leishman, D. (2017). Minimal ecosystem uptake of selenium from Westland Petrels, a forest‐breeding seabird. Science of the Total Environment, 574, 148–154. 10.1016/j.scitotenv.2016.08.203 [DOI] [PubMed] [Google Scholar]
- Hawke, D. J. , & Newman, J. (2007). Carbon‐13 and nitrogen‐15 enrichment in coastal forest foliage from nutrient‐poor and seabird‐enriched sites in southern New Zealand. New Zealand Journal of Botany, 45, 309–315. 10.1080/00288250709509719 [DOI] [Google Scholar]
- Hawke, D. J. , & Vallance, J. R. (2015). Microbial carbon concentration in samples of seabird and non‐seabird forest soil: Implications for leaf litter cycling. Pedobiologia, 58, 33–39. 10.1016/j.pedobi.2015.01.002 [DOI] [Google Scholar]
- Hawke, D. J. , & Wu, J. R. (2012). Soil selenium in a forested seabird colony: Distribution, sources, uptake by plants, and comparison with non‐seabird sites. Soil Research, 50, 588–595. 10.1071/sr12137 [DOI] [Google Scholar]
- Headley, A. D. (1996). Heavy metal concentrations in peat profiles from the high Arctic. Science of the Total Environment, 177, 105–111. 10.1016/0048-9697(95)04867-7 [DOI] [Google Scholar]
- Hobara, S. , Koba, K. , Osono, T. , Tokuchi, N. , Ishida, A. , & Kameda, K. (2005). Nitrogen and phosphorus enrichment and balance in forests colonized by cormorants: Implications of the influence of soil adsorption. Plant and Soil, 268, 89–101. 10.1007/s11104-004-0231-6 [DOI] [Google Scholar]
- Hobara, S. , Osono, T. , Koba, K. , Tokuchi, N. , Fujiwara, S. , & Kameda, K. (2001). Forest floor quality and N transformations in a temperate forest affected by avian‐derived N deposition. Water, Air, and Soil Pollution, 130, 679–684. 10.1023/A:1013869115132 [DOI] [Google Scholar]
- Hogg, E. , & Morton, J. (1983). The effects of nesting gulls on the vegetation and soil of islands in the Great Lakes. Canadian Journal of Botany, 61, 3240–3254. 10.1139/b83-361 [DOI] [Google Scholar]
- Huang, T. , Sun, L. , Wang, Y. , Chu, Z. , Qin, X. , & Yang, L. (2014). Transport of nutrients and contaminants from ocean to island by Emperor Penguins from Amanda Bay, East Antarctic. Science of the Total Environment, 468–469, 578–583. 10.1016/j.scitotenv.2013.08.082 [DOI] [PubMed] [Google Scholar]
- Ishida, A. (1996). Changes of soil properties in the colonies of the Common Cormorant, Phalacrocorax carbo . Journal of Forest Research, 1, 31–35. 10.1007/BF02348337 [DOI] [Google Scholar]
- Joly, Y. , Frenot, Y. , & Vernon, P. (1987). Environmental modifications of a subantarctic peat‐bog by the Wandering Albatross (Diomedea exulans): A preliminary study. Polar Biology, 8, 61–72. 10.1007/BF00297166 [DOI] [Google Scholar]
- Juchnowicz‐Bierbasz, M. , & Rakusa‐Suszczewski, S. (2002). Nutrients and cations content in soil solutions from the present and abandoned penguin rookeries (Antarctica, King George Island). Polish Journal of Ecology, 50, 79–91. [Google Scholar]
- Kameda, K. , Koba, K. , Hobara, S. , Osono, T. , & Terai, M. (2006). Pattern of natural 15N abundance in lakeside forest ecosystem affected by cormorant‐derived nitrogen. Hydrobiologia, 567, 69–86. 10.1007/s10750-006-0052-0 [DOI] [Google Scholar]
- Katsumata, S. , Hobara, S. , Osono, T. , & Takeda, H. (2015). Mass, nitrogen content, and decomposition of woody debris in forest stands affected by excreta deposited in nesting colonies of Great Cormorant. Ecological Research, 30, 555–561. 10.1007/s11284-015-1256-4 [DOI] [Google Scholar]
- Kazama, K. , Murano, H. , Tsuzuki, K. , Fujii, H. , Niizuma, Y. , & Mizota, C. (2013). Input of seabird‐derived nitrogen into rice‐paddy fields near a breeding/roosting colony of the Great Cormorant (Phalacrocorax carbo), and its effects on wild grass. Applied Geochemistry, 28, 128–134. 10.1016/j.apgeochem.2012.10.001 [DOI] [Google Scholar]
- Kickbush, J. C. , Mallory, M. L. , Murimboh, J. D. , Rand, J. , Klapstein, S. J. , Loder, A. L. , Hill, N. M. , & O'Driscoll, N. J. (2018). The influence of avian biovectors on mercury speciation in a bog ecosystem. Science of the Total Environment, 637–638, 264–273. 10.1016/j.scitotenv.2018.04.224 [DOI] [PubMed] [Google Scholar]
- Kienel, U. , Plessen, B. , Schettler, G. , Weise, S. , Pinkerneil, S. , Bohnel, H. , Englebrecht, A. C. , & Haug, G. H. (2013). Sensitivity of a hypersaline crater lake to the seasonality of rainfall, evaporation, and guano supply. Fundamental and Applied Limnology, 183, 135–152. 10.1127/1863-9135/2013/0405 [DOI] [Google Scholar]
- Klimaszyk, P. , Brzeg, A. , Rzymski, P. , & Piotrowicz, R. (2015). Black spots for aquatic and terrestrial ecosystems: Impact of a perennial cormorant colony on the environment. Science of the Total Environment, 517, 222–231. 10.1016/j.scitotenv.2015.02.067 [DOI] [PubMed] [Google Scholar]
- Kolb, G. S. , Jerling, L. , Essenberg, C. , Palmborg, C. , & Hambäck, P. A. (2012). The impact of nesting cormorants on plant and arthropod diversity. Ecography, 35, 726–740. 10.1111/j.1600-0587.2011.06808.x [DOI] [Google Scholar]
- Kolb, G. S. , Jerling, L. , & Hambäck, P. A. (2010). The impact of cormorants on plant–arthropod food webs on their nesting islands. Ecosystems, 13, 353–366. 10.1007/s10021-010-9323-8 [DOI] [Google Scholar]
- Kolb, G. S. , Palmborg, C. , & Hamback, P. A. (2013). Ecological stoichiometry and density responses of plant‐arthropod communities on cormorant nesting islands. PLoS ONE, 8, e61772. 10.1371/journal.pone.0061772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb, G. S. , Palmborg, C. , Taylor, A. R. , Baath, E. , & Hamback, P. A. (2015). Effects of nesting cormorants (Phalacrocorax carbo) on soil chemistry, microbial communities and soil fauna. Ecosystems, 18, 643–657. 10.1007/s10021-015-9853-1 [DOI] [Google Scholar]
- Korobushkin, D. , & Saifutdinov, R. (2019). Influence of seabird colonies on soil macrofauna communities at the Black Sea coast forests. Russian Journal of Ecology, 50, 567–573. 10.1134/S1067413619060080 [DOI] [Google Scholar]
- Kristiansen, S. M. , Leinaas, H. P. , Herzke, D. , Hylland, K. , Gabrielsen, G. W. , Harju, M. , & Borga, K. (2019). Seabird‐transported contaminants are reflected in the Arctic tundra, but not in its soil‐dwelling springtails (Collembola). Environmental Science & Technology, 53, 12835–12845. 10.1021/acs.est.9b05316 [DOI] [PubMed] [Google Scholar]
- Ksiksi, T. S. , Muzaffar, S. B. , Gubiani, R. , & Alshihi, R. M. (2015). The impact of nesting Socotra Cormorants on soil chemistry and vegetation in a large colony in the United Arab Emirates. Diversity, 7, 60–73. 10.3390/d7010060 [DOI] [Google Scholar]
- Ligeza, S. , & Smal, H. (2003). Accumulation of nutrients in soils affected by perennial colonies of piscivorous birds with reference to biogeochemical cycles of elements. Chemosphere, 52, 595–602. 10.1016/S0045-6535(03)00241-8 [DOI] [PubMed] [Google Scholar]
- Liu, X. D. , Nie, Y. G. , Sun, L. G. , & Emslie, S. D. (2013). Eco‐environmental implications of elemental and carbon isotope distributions in ornithogenic sediments from the Ross Sea region, Antarctica. Geochimica et Cosmochimica Acta, 117, 99–114. 10.1016/j.gca.2013.04.013 [DOI] [Google Scholar]
- Liu, X. D. , Zhao, S. P. , Sun, L. G. , Yin, X. B. , Xie, Z. Q. , Honghao, L. , & Wang, Y. H. (2006). P and trace metal contents in biomaterials, soils, sediments and plants in colony of Red‐Footed Booby (Sula sula) in the Dongdao Island of South China Sea. Chemosphere, 65, 707–715. 10.1016/j.chemosphere.2006.01.043 [DOI] [PubMed] [Google Scholar]
- Loder, T. C. , Ganning, B. , & Love, J. A. (1996). Ammonia nitrogen dynamics in coastal rockpools affected by gull guano. Journal of Experimental Marine Biology and Ecology, 196, 113–129. 10.1016/0022-0981(95)00126-3 [DOI] [Google Scholar]
- Lucassen, F. , Pritzkow, W. , Rosner, M. , Sepulveda, F. , Vasquez, P. , Wilke, H. , & Kasemann, S. A. (2017). The stable isotope composition of nitrogen and carbon and elemental contents in modern and fossil seabird guano from Northern Chile—Marine sources and diagenetic effects. PLoS ONE, 12, e0179440. 10.1371/journal.pone.0179440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallory, M. L. , Mahon, L. , Tomlik, M. D. , White, C. , Milton, G. R. , & Spooner, I. (2015). Colonial marine birds influence island soil chemistry through biotransport of trace elements. Water, Air, and Soil Pollution, 226, 31. 10.1007/s11270-015-2314-9 [DOI] [Google Scholar]
- Marion, L. , Clergeau, P. , Brient, L. , & Bertru, G. (1994). The importance of avian‐contributed nitrogen (N) and phosphorus (P) to Lake Grand‐Lieu, France. Hydrobiologia, 279, 133–147. 10.1007/bf00027848 [DOI] [Google Scholar]
- Markwell, T. , & Daugherty, C. (2003). Variability in δ15N, δ13C and Kjeldahl nitrogen of soils from islands with and without seabirds in the Marlborough Sounds, New Zealand. New Zealand Journal of Ecology, 27, 25–30. [Google Scholar]
- Markwell, T. J. , & Daugherty, C. H. (2002). Invertebrate and lizard abundance is greater on seabird‐inhabited islands than on seabird‐free islands in the Marlborough Sounds, New Zealand. Ecoscience, 9, 293–299. 10.1080/11956860.2002.11682715 [DOI] [Google Scholar]
- Maron, J. L. , Estes, J. A. , Croll, D. A. , Danner, E. M. , Elmendorf, S. C. , & Buckelew, S. L. (2006). An introduced predator alters Aleutian Island plant communities by thwarting nutrient subsidies. Ecological Monographs, 76, 3–24. 10.1890/05-0496 [DOI] [Google Scholar]
- Martín‐Vélez, V. , Hortas, F. , Taggart, M. A. , Green, A. J. , ÓHanlon, N. J. , & Sánchez, M. I. (2021). Spatial variation and biovectoring of metals in gull faeces. Ecological Indicators, 125, 107534. 10.1016/j.ecolind.2021.107534 [DOI] [Google Scholar]
- McColl, J. G. , & Burger, J. (1976). Chemical inputs by a colony of Franklin's Gulls nesting in cattails. The American Midland Naturalist, 96, 270–280. 10.2307/2424068 [DOI] [Google Scholar]
- Metcheva, R. , Yurukova, L. & Teodorova, S.E. (2011). Biogenic and toxic elements in feathers, eggs, and excreta of Gentoo penguin (Pygoscelis papua ellsworthii) in the Antarctic. Environmental Monitoring and Assessment, 182, 571‐585. 10.1007/s10661‐011‐1898‐9 [DOI] [PubMed] [Google Scholar]
- Methratta, E. T. (2004). Top‐down and bottom‐up factors in tidepool communities. Journal of Experimental Marine Biology and Ecology, 299, 77–96. 10.1016/j.jembe.2003.09.004 [DOI] [Google Scholar]
- Michelutti, N. , Blais, J. M. , Mallory, M. L. , Brash, J. , Thienpont, J. , Kimpe, L. E. , Douglas, M. S. V. , & Smol, J. P. (2010). Trophic position influences the efficacy of seabirds as metal biovectors. Proceedings of the National Academy of Sciences of the United States of America, 107, 10543–10548. 10.1073/pnas.1001333107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizota, C. , & Naikatini, A. (2007). Nitrogen isotope composition of inorganic soil nitrogen and associated vegetation under a sea bird colony on the Hatana islands, Rotuma Group, Fiji. Geochemical Journal, 41, 297–301. 10.2343/geochemj.41.297 [DOI] [Google Scholar]
- Mizutani, H. , Hasegawa, H. , & Wada, E. (1986). High nitrogen isotope ratio for soils of seabird rookeries. Biogeochemistry, 2, 221–247. 10.1007/BF02180160 [DOI] [Google Scholar]
- Mizutani, H. , Kabaya, Y. , & Wada, E. (1985). Ammonia volatilization and high 15N/14N ratio in a penguin rookery in Antarctica. Geochemical Journal, 19, 323–327. 10.2343/geochemj.19.323 [DOI] [Google Scholar]
- Mizutani, H. , & Wada, E. (1988). Nitrogen and carbon isotope ratios in seabird rookeries and their ecological implications. Ecology, 69, 340–349. 10.2307/1940432 [DOI] [Google Scholar]
- Molina‐Montenegro, M. A. , Torres‐Diaz, C. , Gallardo‐Cerda, J. , Leppe, M. , & Gianoli, E. (2013). Seabirds modify El Nino effects on tree growth in a southern Pacific island. Ecology, 94, 2415–2425. 10.1890/12-1054.1 [DOI] [PubMed] [Google Scholar]
- Mosbech, A. , Johansen, K. L. , Davidson, T. A. , Appelt, M. , Gronnow, B. , Cuyler, C. , Lyngs, P. , & Flora, J. (2018). On the crucial importance of a small bird: The ecosystem services of the little auk (Alle alle) population in Northwest Greenland in a long‐term perspective. Ambio, 47, 226–243. 10.1007/s13280-018-1035-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder, C. P. H. , & Keall, S. N. (2001). Burrowing seabirds and reptiles: Impacts on seeds, seedlings and soils in an island forest in New Zealand. Oecologia, 127, 350–360. 10.1007/s004420000600 [DOI] [PubMed] [Google Scholar]
- Nedzarek, A. (2010). Change in N and P concentrations in Antarctic streams as a response to change in penguin populations. Papers on Global Change, 17, 67–80. 10.2478/v10190-010-0006-2 [DOI] [Google Scholar]
- Nie, Y. G. , Liu, X. D. , Wen, T. , Sun, L. G. , & Emslie, S. D. (2014). Environmental implication of nitrogen isotopic composition in ornithogenic sediments from the Ross Sea region, East Antarctica: Delta N‐15 as a new proxy for avian influence. Chemical Geology, 363, 91–100. 10.1016/j.chemgeo.2013.10.031 [DOI] [Google Scholar]
- Odasz, A. M. (1994). Nitrate reductase activity in vegetation below an arctic bird cliff, Svalbard, Norway. Journal of Vegetation Science, 5, 913–920. 10.2307/3236203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki, M. , Oshida, Y. , Malony, R. , & Warham, J. (1993). Effects of Sooty Shearwaters Puffinus griseus on surface soils on Motuara Island, New Zealand. Journal of the Yamashina Institute for Ornithology, 25, 137–143. 10.3312/jyio1952.25.137 [DOI] [Google Scholar]
- Osono, T. , Hobara, S. , Fujiwara, S. , Koba, K. , & Kameda, K. (2002). Abundance, diversity, and species composition of fungal communities in a temperate forest affected by excreta of the Great Cormorant Phalacrocorax carbo . Soil Biology and Biochemistry, 34, 1537–1547. 10.1016/S0038-0717(02)00123-2 [DOI] [Google Scholar]
- Osono, T. , Hobara, S. , Koba, K. , Kameda, K. , & Takeda, H. (2006). Immobilization of avian excreta‐derived nutrients and reduced lignin decomposition in needle and twig litter in a temperate coniferous forest. Soil Biology & Biochemistry, 38, 517–525. 10.1016/j.soilbio.2005.05.022 [DOI] [Google Scholar]
- Otero, X. , & Fernández‐Sanjurjo, M. (1999). Seasonal variation in inorganic nitrogen content of soils from breeding sites of yellow‐legged gulls (Larus cachinnans) in the Cíes Islands Natural Park (NW Iberian Peninsula). Fresenius Environmental Bulletin, 8, 685–692. [Google Scholar]
- Otero, X. , & Fernández‐Sanjurjo, M. (2000). Mercury in faeces and feathers of yellow‐legged gulls (Larus cachinnans) and in soils from their breeding sites (Cies Islands‐NW Spain) in the vicinity of a chlor‐alkali plant. Fresenius Environmental Bulletin, 9, 56–63. [Google Scholar]
- Otero, X. L. (1998). Effects of nesting yellow‐legged gulls (Larus cachinnans Pallas) on the heavy metal content of soils in the Cies Islands (Galicia, North‐west Spain). Marine Pollution Bulletin, 36, 267–272. 10.1016/S0025-326X(98)80010-6 [DOI] [Google Scholar]
- Otero, X. L. , de la Pena‐Lastra, S. , Romero, D. , Nobrega, G. N. , Ferreira, T. O. , & Perez‐Alberti, A. (2018). Trace elements in biomaterials and soils from a yellow‐legged gull (Larus michahellis) colony in the Atlantic Islands of Galicia National Park (NW Spain). Marine Pollution Bulletin, 133, 144–149. 10.1016/j.marpolbul.2018.05.027 [DOI] [PubMed] [Google Scholar]
- Otero, X. L. , Fernández‐Balado, C. , Ferreira, T. O. , Pérez‐Alberti, A. , & Revilla, G. (2021). Soil eutrophication in seabird colonies affects cell wall composition: Implications for the conservation of rare plant species. Marine Pollution Bulletin, 168, 112469. 10.1016/j.marpolbul.2021.112469 [DOI] [PubMed] [Google Scholar]
- Otero, X. L. , Tejada, O. , Martín‐Pastor, M. , De La Peña, S. , Ferreira, T. O. , & Pérez‐Alberti, A. (2015). Phosphorus in seagull colonies and the effect on the habitats. The case of yellow‐legged gulls (Larus michahellis) in the Atlantic Islands National Park (Galicia‐NW Spain). Science of the Total Environment, 532, 383–397. 10.1016/j.scitotenv.2015.06.013 [DOI] [PubMed] [Google Scholar]
- Pereira, T. T. C. , Schaefer, C. E. G. R. , Ker, J. C. , Almeida, C. C. , Almeida, I. C. C. , & Pereira, A. B. (2013). Genesis, mineralogy and ecological significance of ornithogenic soils from a semi‐desert polar landscape at Hope Bay, Antarctic Peninsula. Geoderma, 209–210, 98–109. 10.1016/j.geoderma.2013.06.012 [DOI] [Google Scholar]
- Perfetti‐Bolaño, A. , Moreno, L. , Urrutia, R. , Araneda, A. , & Barra, R. (2018). Influence of Pygoscelis Penguin Colonies on Cu and Pb Concentrations in Soils on the Ardley Peninsula, Maritime Antarctica. Water, Air, and Soil Pollution, 229, 390. 10.1007/s11270-018-4042-4 [DOI] [Google Scholar]
- Pietr, S. J. , Tatur, A. , & Myrcha, A. (1983). Mineralization of penguin excrements in the Admiralty Bay region (King George Island, South Shetland Islands, Antarctica). Polish Polar Research, 4, 97–112. [Google Scholar]
- Polis, G. A. , & Hurd, S. D. (1996). Linking marine and terrestrial food webs: Allochthonous input from the ocean supports high secondary productivity on small islands and coastal land communities. The American Naturalist, 147, 396–423. 10.1086/285858 [DOI] [Google Scholar]
- Portnoy, J. W. (1990). Gull contributions of phosphorus and nitrogen to a Cape Cod kettle pond. Hydrobiologia, 202, 61–69. 10.1007/BF00027092 [DOI] [Google Scholar]
- Powell, G. V. N. , Fourqurean, J. W. , Kenworthy, W. J. , & Zieman, J. C. (1991). Bird colonies cause seagrass enrichment in a subtropical estuary: Obsevational and experimental evidence. Estuarine, Coastal and Shelf Science, 32, 567–579. 10.1016/0272-7714(91)90075-m [DOI] [Google Scholar]
- Provencher, J. F. , Vermaire, J. C. , Avery‐Gomm, S. , Braune, B. M. , & Mallory, M. L. (2018). Garbage in guano? Microplastic debris found in faecal precursors of seabirds known to ingest plastics. Science of the Total Environment, 644, 1477–1484. 10.1016/j.scitotenv.2018.07.101 [DOI] [PubMed] [Google Scholar]
- Qin, X. Y. , Sun, L. G. , Blais, J. M. , Wang, Y. H. , Huang, T. , Huang, W. , & Xie, Z. Q. (2014). From sea to land: Assessment of the bio‐transport of phosphorus by penguins in Antarctica. Chinese Journal of Oceanology and Limnology, 32, 148–154. 10.1007/s00343-014-3115-5 [DOI] [Google Scholar]
- Rajakaruna, N. , Pope, N. , Perez‐Orozco, J. , & Harris, T. B. (2009). Ornithocoprophilous plants of Mount Desert Rock, a remote bird‐nesting island in the Gulf of Maine, U.S.A. Rhodora, 111, 417–447. 10.3119/08-12.1 [DOI] [Google Scholar]
- Ramírez‐Fernández, L. , Trefault, N. , Carú, M. , & Orlando, J. (2019). Seabird and pinniped shape soil bacterial communities of their settlements in Cape Shirreff, Antarctica. PLoS ONE, 14, e0209887. 10.1371/journal.pone.0209887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin, A. M. , & Wolff, E. W. (2000). Ammonium and potassium in snow around an emperor penguin colony. Antarctic Science, 12, 154–159. 10.1017/S0954102000000201 [DOI] [Google Scholar]
- Richardson, K. M. , Iverson, J. B. , & Kurle, C. M. (2019). Marine subsidies likely cause gigantism of iguanas in the Bahamas. Oecologia, 189, 1005–1015. 10.1007/s00442-019-04366-4 [DOI] [PubMed] [Google Scholar]
- Rodrigues, W. F. , Oliveira, F. S.d. , Schaefer, C. E. G. R. , Leite, M. G. P. , & Pavinato, P. S. (2021). Phosphatization under birds' activity: Ornithogenesis at different scales on Antarctic Soilscapes. Geoderma, 391, 114950. 10.1016/j.geoderma.2021.114950 [DOI] [Google Scholar]
- Roosens, L. , Van Den Brink, N. , Riddle, M. , Blust, R. , Neels, H. , & Covaci, A. (2007). Penguin colonies as secondary sources of contamination with persistent organic pollutants. Journal of Environmental Monitoring, 9, 822–825. 10.1039/B708103K [DOI] [PubMed] [Google Scholar]
- Rush, S. A. , Dobbie, T. , & Fisk, A. T. (2013). Quantification of cormorant litter and nutrient deposition to Great Lakes island ecosystems. Journal of Great Lakes Research, 39, 303–307. 10.1016/j.jglr.2013.03.002 [DOI] [Google Scholar]
- Rush, S. A. , Verkoeyen, S. , Dobbie, T. , Dobbyn, S. , Hebert, C. E. , Gagnon, J. , & Fisk, A. T. (2011). Influence of increasing populations of Double‐crested Cormorants on soil nutrient characteristics of nesting islands in western Lake Erie. Journal of Great Lakes Research, 37, 305–309. 10.1016/j.jglr.2011.03.006 [DOI] [Google Scholar]
- Ryan, P. G. , & Watkins, B. P. (1989). The influence of physical factors and ornithogenic products on plant and arthropod abundance at an Inland Nunatak group in Antarctica. Polar Biology, 10, 151–160. 10.1007/BF00239162 [DOI] [Google Scholar]
- Saifutdinov, R. A. , & Korobushkin, D. I. (2020). Impact of overwintering cormorants (Phalacrocorax carbo) on springtail (Hexapoda: Collembola) communities of the Azov and Black Sea coastal forests. Turkish Journal of Zoology, 44, 303–311. 10.3906/zoo-2003-24 [DOI] [Google Scholar]
- Sale, M. , & Arnould, J. (2013). Inflated population density of island antechinus: A case of allochthonous marine inputs leading to increased food availability? Australian Journal of Zoology, 60, 343–351. 10.1071/ZO12073 [DOI] [Google Scholar]
- Sanchez‐Pinero, F. , & Polis, G. A. (2000). Bottom‐up dynamics of allochthonous input: Direct and indirect effects of seabirds on islands. Ecology, 81, 3117–3132. 10.2307/177405 [DOI] [Google Scholar]
- Santamans, A. C. , Boluda, R. , Picazo, A. , Gil, C. , Ramos‐Miras, J. , Tejedo, P. , Pertierra, L. R. , Benayas, J. , & Camacho, A. (2017). Soil features in rookeries of Antarctic penguins reveal sea to land biotransport of chemical pollutants. PLoS ONE, 12, e0181901. 10.1371/journal.pone.0181901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, S. , Dennison, W. C. , Moss, G. J. , & Stewart, G. R. (2004). Nitrogen ecophysiology of Heron Island, a subtropical coral cay of the Great Barrier Reef, Australia. Functional Plant Biology, 31, 517–528. 10.1071/FP04024 [DOI] [PubMed] [Google Scholar]
- Schmidt, S. , Mackintosh, K. , Gillett, R. , Pudmenzky, A. , Allen, D. E. , Rennenberg, H. , & Mueller, J. F. (2010). Atmospheric concentrations of ammonia and nitrogen dioxide at a tropical coral cay with high seabird density. Journal of Environmental Monitoring, 12, 460–465. 10.1039/b910922f [DOI] [PubMed] [Google Scholar]
- Sebastian‐Gonzalez, E. , Navarro, J. , Sanchez‐Zapata, J. A. , Botella, F. , & Delgado, A. (2012). Water quality and avian inputs as sources of isotopic variability in aquatic macrophytes and macroinvertebrates. Journal of Limnology, 71, 191–199. 10.4081/jlimnol.2012.e20 [DOI] [Google Scholar]
- Shoji, A. , Elliott, K. H. , Aris‐Brosou, S. , Mizukawa, H. , Nakayama, S. M. M. , Ikenaka, Y. , Ishizuka, M. , Kuwae, T. , Watanabe, K. , Escoruela Gonzalez, J. , & Watanuki, Y. (2019). Biotransport of metallic trace elements from marine to terrestrial ecosystems by seabirds. Environmental Toxicology and Chemistry, 38, 106–114. 10.1002/etc.4286 [DOI] [PubMed] [Google Scholar]
- Signa, G. , Mazzola, A. , Costa, V. , & Vizzini, S. (2015). Bottom‐up control of macrobenthic communities in a guanotrophic coastal system. PLoS ONE, 10, e0117544. 10.1371/journal.pone.0117544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signa, G. , Mazzola, A. , Tramati, C. D. , & Vizzini, S. (2013). Gull‐derived trace elements trigger small‐scale contamination in a remote Mediterranean nature reserve. Marine Pollution Bulletin, 74, 237–243. 10.1016/j.marpolbul.2013.06.051 [DOI] [PubMed] [Google Scholar]
- Signa, G. , Mazzola, A. , & Vizzini, S. (2012). Effects of a small seagull colony on trophic status and primary production in a Mediterranean coastal system (Marinello ponds, Italy). Estuarine, Coastal and Shelf Science, 111, 27–34. 10.1016/j.ecss.2012.06.008 [DOI] [Google Scholar]
- Signa, G. , Tramati, C. D. , & Vizzini, S. (2013). Contamination by trace metals and their trophic transfer to the biota in a Mediterranean coastal system affected by gull guano. Marine Ecology Progress Series, 479, 13–24. 10.3354/meps10210 [DOI] [Google Scholar]
- Simas, F. N. , Schaefer, C. E. G. , Melo, V. F. , Albuquerque‐Filho, M. R. , Michel, R. F. , Pereira, V. V. , Gomes, M. R. , & da Costa, L. M. (2007). Ornithogenic cryosols from Maritime Antarctica: Phosphatization as a soil forming process. Geoderma, 138, 191–203. 10.1016/j.geoderma.2006.11.011 [DOI] [Google Scholar]
- Simpson, L. T. , Canty, S. W. J. , Cissell, J. R. , Steinberg, M. K. , Cherry, J. A. , & Feller, I. C. (2021). Bird rookery nutrient over‐enrichment as a potential accelerant of mangrove cay decline in Belize. Oecologia, 197, 771–784. 10.1007/s00442-021-05056-w [DOI] [PubMed] [Google Scholar]
- Smith, J. S. , & Johnson, C. R. (1995). Nutrient inputs from seabirds and humans on a populated coral cay. Marine Ecology Progress Series, 124, 189–200. 10.3354/meps124189 [DOI] [Google Scholar]
- Smith, V. (1976). The effect of burrowing species of procellariidae on the nutrient status of inland tussock grasslands on Marion Island. Journal of South African Biology, 42, 262–272. [Google Scholar]
- Smith, V. R. (1978). Animal‐plant‐soil nutrient relationships on Marion Island (Subantarctic). Oecologia, 32, 239–253. 10.1007/BF00366075 [DOI] [PubMed] [Google Scholar]
- Smith, V. R. (1979). The influence of seabird manuring on the phosphorus status of Marion Island (Subantarctic) soils. Oecologia, 41, 123–126. 10.1007/BF00344842 [DOI] [PubMed] [Google Scholar]
- Sobey, D. G. , & Kenworthy, J. B. (1979). The relationship between Herring Gulls and the vegetation of their breeding colonies. Journal of Ecology, 67, 469–496. 10.2307/2259108 [DOI] [Google Scholar]
- Speir, T. W. , & Cowling, J. C. (1984). Ornithogenic soils of the Cape Bird Adelie Penguin rookeries, Antarctica. Polar Biology, 2, 199–205. 10.1007/BF00263625 [DOI] [Google Scholar]
- Stempniewicz, L. (1990). Biomass of dovekie excreta in the vicinity of a breeding colony. Colonial Waterbirds, 13, 62–66. 10.2307/1521421 [DOI] [Google Scholar]
- Tatur, A. , & Myrcha, A. (1983). Changes in chemical composition of waters running off from the penguin rookeries in the Admiralty Bay region (King George Island, South Shetland Islands, Antarctica). Polish Polar Research, 4(1–4), 113–125. [Google Scholar]
- Tatur, A. , & Myrcha, A. (1984). Ornithogenic soils on King George Island, South Shetland Islands (Maritime Antarctic Zone). Polish Polar Research, 5, 31–60. [Google Scholar]
- Wainright, S. C. , Haney, J. C. , Kerr, C. , Golovkin, A. N. , & Flint, M. V. (1998). Utilization of nitrogen derived from seabird guano by terrestrial and marine plants at St. Paul, Pribilof islands, Bering sea, Alaska. Marine Biology, 131, 63–71. 10.1007/s002270050297 [DOI] [Google Scholar]
- Wait, D. A. , Aubrey, D. P. , & Anderson, W. B. (2005). Seabird guano influences on desert islands: Soil chemistry and herbaceous species richness and productivity. Journal of Arid Environments, 60, 681–695. 10.1016/j.jaridenv.2004.07.001 [DOI] [Google Scholar]
- Wardle, D. A. , Bellingham, P. J. , Bonner, K. I. , & Mulder, C. P. H. (2009). Indirect effects of invasive predators on litter decomposition and nutrient resorption on seabird‐dominated islands. Ecology, 90, 452–464. 10.1890/08-0097.1 [DOI] [PubMed] [Google Scholar]
- Wing, S. R. , Jack, L. , Shatova, O. , Leichter, J. J. , Barr, D. , Frew, R. D. , & Gault‐Ringold, M. (2014). Seabirds and marine mammals redistribute bioavailable iron in the Southern Ocean. Marine Ecology Progress Series, 510, 1–13. 10.3354/meps10923 [DOI] [Google Scholar]
- Wojciechowska, A. , Zwolicki, A. , Barcikowski, A. , & Stempniewicz, L. (2015). The structure of Cochlearia groenlandica population along the bird colony influence gradient (Hornsund, Spitsbergen). Polar Biology, 38, 1919–1930. 10.1007/s00300-015-1755-3 [DOI] [Google Scholar]
- Wootton, J. T. (1991). Direct and indirect effects of nutrients on intertidal community structure: Variable consequences of seabird guano. Journal of Experimental Marine Biology and Ecology, 151, 139–153. 10.1016/0022-0981(91)90121-c [DOI] [Google Scholar]
- Wright, D. G. , van der Wal, R. , Wanless, S. , & Bardgett, R. D. (2010). The influence of seabird nutrient enrichment and grazing on the structure and function of island soil food webs. Soil Biology and Biochemistry, 42, 592–600. 10.1016/j.soilbio.2009.12.008 [DOI] [Google Scholar]
- Wu, L. , Liu, X. , Fang, Y. , Hou, S. , Xu, L. , Wang, X. , & Fu, P. (2018). Nitrogen cycling in the soil–plant system along a series of coral islands affected by seabirds in the South China Sea. Science of the Total Environment, 627, 166–175. 10.1016/j.scitotenv.2018.01.213 [DOI] [PubMed] [Google Scholar]
- Zawierucha, K. , Cytan, J. , Smykla, J. , Wojczulanis‐Jakubas, K. , Kaczmarek, L. , Kosicki, J. Z. , & Michalczyk, L. (2015). Seabird guano boosts body size of water bears (Tardigrada) inhabiting the arctic tundra. Polar Biology, 38, 579–582. 10.1007/s00300-014-1591-x [DOI] [Google Scholar]
- Zhang, H. S. , Wang, Z. P. , Lu, B. , Zhu, C. , Wu, G. H. , & Vetter, W. (2007). Occurrence of organochlorine pollutants in the eggs and dropping‐amended soil of Antarctic large animals and its ecological significance. Science in China Series D‐Earth Sciences, 50, 1086–1096. 10.1007/s11430-007-0021-0 [DOI] [Google Scholar]
- Zhu, R. , Wang, Q. , Ding, W. , Wang, C. , Hou, L. , & Ma, D. (2014). Penguins significantly increased phosphine formation and phosphorus contribution in maritime Antarctic soils. Scientific Reports, 4, 7055. 10.1038/srep07055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziółek, M. , Bartmiński, P. , & Stach, A. (2017). The influence of seabirds on the concentration of selected heavy metals in organic soil on the Bellsund Coast, Western Spitsbergen. Arctic, Antarctic, and Alpine Research, 49, 507–520. 10.1657/AAAR0016-024 [DOI] [Google Scholar]
- Ziołek, M. , & Melke, J. (2014). The impact of seabirds on the content of various forms of phosphorus in organic soils of the Bellsund coast, western Spitsbergen. Polar Research, 33, 19986. 10.3402/polar.v33.19986 [DOI] [Google Scholar]
- Zmudczynska, K. , Olejniczak, I. , Zwolicki, A. , Iliszko, L. , Convey, P. , & Stempniewicz, L. (2012). Influence of allochtonous nutrients delivered by colonial seabirds on soil collembolan communities on Spitsbergen. Polar Biology, 35, 1233–1245. 10.1007/s00300-012-1169-4 [DOI] [Google Scholar]
- Zmudczynska, K. , Zwolicki, A. , Barcikowski, M. , Iliszko, L. , & Stempniewicz, L. (2008). Variability of individual biomass and leaf size of Saxifraga nivalis L. along a transect between seabirds colony and seashore in Hornsund, Spitsbergen. Quest Ecology, 9, 37–44. [Google Scholar]
- Zmudczyńska‐Skarbek, K. , Balazy, P. , & Kuklinski, P. (2015). An assessment of seabird influence on Arctic coastal benthic communities. Journal of Marine Systems, 144, 48–56. 10.1016/j.jmarsys.2014.11.013 [DOI] [Google Scholar]
- Zmudczynska‐Skarbek, K. , Barcikowski, M. , Zwolicki, A. , Iliszko, L. , & Stempniewicz, L. (2013). Variability of polar scurvygrass Cochlearia groenlandica individual traits along a seabird influenced gradient across Spitsbergen tundra. Polar Biology, 36, 1659–1669. 10.1007/s00300-013-1385-6 [DOI] [Google Scholar]
- Zwolicki, A. , Barcikowski, M. , Barcikowski, A. , Cymerski, M. , Stempniewicz, L. , & Convey, P. (2015). Seabird colony effects on soil properties and vegetation zonation patterns on King George Island, Maritime Antarctic. Polar Biology, 38, 1645–1655. 10.1007/s00300-015-1730-z [DOI] [Google Scholar]
- Zwolicki, A. , Zmudczynska‐Skarbek, K. , Matula, J. , Wojtun, B. , & Stempniewicz, L. (2016). Differential responses of Arctic vegetation to nutrient enrichment by plankton‐ and fish‐eating colonial seabirds in Spitsbergen. Frontiers in Plant Science, 7, 1959. 10.3389/fpls.2016.01959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwolicki, A. , Zmudczyńska‐Skarbek, K. , Richard, P. , & Stempniewicz, L. (2016). Importance of marine‐derived nutrients supplied by planktivorous seabirds to high Arctic tundra plant communities. PLoS ONE, 11, e0154950. 10.1371/journal.pone.0154950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwolicki, A. , Zmudczynska‐Skarbek, K. M. , Iliszko, L. , & Stempniewicz, L. (2013). Guano deposition and nutrient enrichment in the vicinity of planktivorous and piscivorous seabird colonies in Spitsbergen. Polar Biology, 36, 363–372. 10.1007/s00300-012-1265-5 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1.The inclusion and exclusion criteria used for the systematic review during the title and abstract processing and full‐text screening. The number of papers at the beginning of the process was 614 and after title and abstraction processing was reduced to 275. These papers then went through full‐text screening and a final count of 181 papers were included in the full review.
Figure S2.From the 181 papers that were included in the full review, these were screened for the meta‐analysis using the above decision tree.
Figure S3.Forest plot for individual studies comparing nitrogen concentrations (%N dry weight) in seabird colony soils and control soils. Effect sizes (log response ratio, LRR) and 95% confidence intervals are displayed. When confidence intervals do not overlap zero (dotted vertical line) and are positive, results can be considered significant, that is, colony soils were demonstrated to be more enriched than control soils. One paper is listed more than once (Smith, 1978) as this paper measured nitrogen concentrations in soils for four different seabird species.
Figure S4.Forest plot for individual studies comparing phosphorous concentrations (%P dry weight) in seabird colony soils and control soils. Effect sizes (log response ratio, LRR) and 95% confidence intervals are displayed. When confidence intervals do not overlap zero (dotted vertical line) and are positive, results can be considered significant, that is, colony soils were demonstrated to be more enriched than control soils.
Figure S5.Forest plot for individual studies comparing nitrate concentrations (μg/g dry weight) in seabird colony soils and control soils. Effect sizes (log response ratio, LRR) and 95% confidence intervals are displayed. When confidence intervals do not overlap zero (dotted vertical line) and are positive, results can be considered significant, that is, colony soils were demonstrated to be more enriched than control soils. Two papers are listed more than once (Smith, 1978; Ramirez‐Fernandez, 2019) as these papers measured nitrate concentrations in soils for four and three different seabird species respectively.
Figure S6.Forest plot for individual studies comparing ammonium concentrations (μg/g dry weight) in seabird colony soils and control soils. Effect sizes (log response ratio, LRR) and 95% confidence intervals are displayed. When confidence intervals do not overlap zero (dotted vertical line) and are positive, results can be considered significant, that is, colony soils were demonstrated to be more enriched than control soils. Two papers are listed more than once (Smith, 1978; Ramirez‐Fernandez, 2019) as these papers measured ammonium concentrations in soils for four and three different seabird species respectively.
Table S1.Heterogeneity (Q‐score and I 2) of effect sizes for full models and for subgroup analyses of studies comparing nutrient concentration of nitrogen, phosphorous, nitrate and ammonium in soils between seabird colonies and control sites.
Data Availability Statement
The datasets supporting this article and relevant R code are freely available from the Institute for Marine and Antarctic Studies (IMAS) Data Portal: https://doi.org/10.25959/ENWE‐GB98 (Grant et al., 2022).


