Summary
Background and Aims
To assess whether corticosteroids improve prognosis in patients with AS‐AIH, and to identify factors at therapy initiation and during therapy predictive of the response to corticosteroids.
Methods
This was a retrospective cohort study including all patients with AS‐AIH admitted to 13 tertiary centres from January 2002 to January 2019. The composite primary outcome was death or liver transplantation within 90 days of admission. Kaplan–Meier and Cox regression methods were used for data analysis.
Results
Of 242 consecutive patients enrolled (mean age [SD] 49.7 [16.8] years), 203 received corticosteroids. Overall 90‐day transplant‐free survival was 61.6% (95% confidence interval [CI] 55.4–67.7). Corticosteroids reduced the risk of a poor outcome (adjusted hazard ratio [HR] 0.25; 95% CI 0.2–0.4), but this treatment failed in 30.5%. An internally validated nomogram composed of older age, MELD, encephalopathy and ascites at the initiation of corticosteroids accurately predicted the response (C‐index 0.82; [95% CI 0.8–0.9]). In responders, MELD significantly improved from days 3 to 14 but remained unchanged in non‐responders. MELD on day 7 with a cut‐off of 25 (sensitivity 62.5%[95% CI: 47.0–75.8]; specificity 95.2% [95% CI: 89.9–97.8]) was the best univariate predictor of the response. Prolonging corticosteroids did not increase the overall infection risk (adjusted HR 0.75; 95% CI 0.3–2.1).
Conclusion
Older patients with high MELD, encephalopathy or ascites at steroid therapy initiation and during treatment are unlikely to show a favourable response and so prolonged therapy in these patients, especially if they are transplantation candidates, should be avoided.
Abstract
Corticosteroids in acute‐severe autoimmune hepatits.

1. INTRODUCTION
Autoimmune hepatitis (AIH) is an immune‐mediated necro‐inflammatory liver disease affecting all ages, genders and ethnic groups. 1 , 2 Clinically, the spectrum of presentation at diagnosis is broad, ranging from mild subclinical disease to an acute disease course in 20%, even leading to fulminant liver failure. 3 , 4 Acute severe‐AIH (AS‐AIH) has been defined as acute onset of jaundice (<30 days) and an international normalised ratio (INR) 1.5 in patients without known chronic liver disease. 4 , 5 AS‐AIH has been scarcely characterised and its actual prevalence might have been underestimated owing to the difficulty of achieving an accurate diagnosis. 6 , 7 , 8 In 50%–60% of patients, AS‐AIH progress to acute liver failure (ALF), which has a dismal prognosis with death rates as high as 20% and a need for liver transplantation in 20%. 5 , 9 , 10 , 11 The risk of this progression is unknown, and there is much controversy over the factors that can modify outcomes, including treatments. Current guidelines recommend a trial with corticosteroids with subsequent reference to emergency liver transplantation in case of failure to improve within the first 7–14 days. 2 , 5 This approach means that liver transplantation could be unnecessarily delayed and therapy prolonged in patients unlikely to respond to corticosteroids. Further, immunosuppression could increase the risk of infection and compromise prognosis and liver transplantation. 12 , 13 , 14 A recent multicentric French study has shown the accuracy of the response to 3‐days of corticosteroids to predict liver transplantation or death in patients with AS‐AIH and has provided a new score (SURFASA score) to identify non‐responders. 15 We propose that a model able to identify factors that can be assessed as soon as possible to predict the response to corticosteroids will be crucial for decision‐making.
The aims of our study were, therefore: (i) to assess whether corticosteroids improve prognosis and (ii) to identify factors at presentation and early during therapy that could serve to predict the individual response to this therapy.
2. PATIENTS AND METHODS
2.1. Study design and patients
This retrospective cohort study was conducted at 13 Spanish tertiary centres from January 2002 to January 2019. All consecutive adults diagnosed with AS‐AIH were included. AS‐AIH was defined as (i) acute hepatic dysfunction (<3 months) as confirmed by an INR 1.5 at the time of presentation; (ii) no known history of chronic liver disease, and (iii) fulfilling simplified International Auto‐Immune Hepatitis Group (IAHG) criteria for a diagnosis of “probable” or “definite” AIH. 8 Patients with an INR 1.5 and hepatic encephalopathy were subclassified as having ALF‐AIH. 5 A favourable corticosteroid response was defined as the absence of death or liver transplantation within 90 days of admission. 16 The study protocol adhered to the principles of the Declaration of Helsinki and was approved by the Ethics Committees for Clinical Research of all the participating centres (Institutional review board code: HRC 070/18). The study was performed according to the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement. 17 The need for written informed consent was waived due to the retrospective nature of the study.
2.2. Study outcomes, procedures and variables
Our primary outcome was a composite of death or liver transplantation within 90 days of hospital admission. 15 Secondary outcomes included histological markers of severity and the risk of infection during the first 90 days. A dedicated database was created and demographic, clinical and laboratory information was retrospectively obtained from paper and electronic records from the in‐hospital, emergency department and primary healthcare databases at each centre. Patients were screened to rule out other causes of ALF, including Wilson’s disease. We considered the results of viral serology with immunoglobulin G and M (IgG and IgM) for Hepatitis A, B and E, Herpes simplex, Epstein–Barr virus and Cytomegalovirus (CMV), as well as the determination of hepatitis C RNA. All potentially hepatotoxic drugs administered within the previous 3 months were evaluated to rule out drug‐induced liver injury. Abdominal ultrasound was performed in all patients. Ascites was considered present from grade 1 (i.e., only detectable by ultrasound examination). 18 Serum IgG and specific AIH‐related antibodies were measured in all patients. Laboratory parameters of serum creatinine, total bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), INR and platelet count were recorded at admission, at initiation and at days 3, 7 and 14 of corticosteroid therapy. Clinically relevant events (ascites and hepatic encephalopathy) were assessed at each of the established time points, and the model for end‐stage liver disease (MELD) score was calculated. 19 Diagnosis of infection was based on clinical, radiological and laboratory findings, as detailed in supporting information. Finally, histological information indicative of AIH (portal lymphoplasmacytic infiltration, interface hepatitis, central venulitis, hepatocyte rosettes and emperipolesis) was obtained from liver biopsies and explants. 2 Liver fibrosis was graded 0–4 following the METAVIR score; grades 3 and 4 were taken to denote severe fibrosis. 20 Necrosis was classified as absent, mild–moderate and severe (bridging necrosis or massive/submassive necrosis). The decision to initiate corticosteroids, dosing and route of administration was at the discretion of the physician in charge.
2.3. Statistical analysis
Quantitative variables are expressed as the mean and standard deviation (SD) or the median and interquartile range (first quartile‐third quartile) when skewed. Normality was tested through distributional graphs and the Shapiro–Wilk’s test. Frequency counts and percentages were used for categorical data. Continuous variables were compared using parametric (t‐test), and non‐parametric tests (Mann–Whitney U test) when appropriate. Chi‐squared and Fisher’s exact tests were used for categorical data. Cumulative incidences were calculated by the Kaplan–Meier method. To assess the impact of corticosteroids on 90‐day transplant‐free survival, we selected confounders based on prior knowledge instead of a data‐driven approach. Thus, we adjusted for age, liver function (MELD score), sex and the presence of ascites and hepatic encephalopathy. We tested first‐order interactions via a global likelihood ratio test. In the second stage, predictors of a poor outcome and lack of response to corticosteroids (death or liver transplantation within the 90 first days) were assessed by univariate and multivariate survival analysis with Cox’s regression. To minimise the risk of a type I error, we only considered variables that were readily available, reproducible and had a plausible pathophysiological link to the outcome of interest. Variables found to be significant (p < 0.05) were considered in the final multivariate models. Finally, a nomogram model was developed and internally validated via bootstrapping with 200 resamples. The predictive performance of our nomogram was assessed through Harrell’s concordance index (C‐index) and calibration plots. Additional information is detailed in supporting information.
Based on previous data and expert opinion, we performed the following exploratory analyses: (i) the value of dynamic changes in liver function to predict response to corticosteroids; (ii) the usefulness of MELD score as a single parameter and the accuracy of new multivariate predictive models relying on data obtained at days 3 and 7 of treatment; (iii) the benefits of adding histological findings in predicting the response to corticosteroids and (iv) the impact of corticosteroids on the risk of infection. These analyses included the development of new predictive models, which were constructed using the same approach as described above. For the histological analysis, we only included those samples obtained before the initiation of corticosteroids. For the infection risk analysis, patients diagnosed with infection at admission were excluded. Additionally, we assessed the performance in our population of the SURFASA score developed by De Martin et al. based on data from a multicenter French cohort of patients with AS‐AIH: −6.80 + 1.92 × (D0‐INR) + 1.94× (∆%3‐INR) + 1.64 × (∆%3‐bilirubin). 15
All tests were two‐tailed. Significance was set at p < 0.05. Data were analysed at the promoting institution (Hospital Universitario Ramón y Cajal, Madrid, Spain) using STATA software 14.1 (StataCorp.).
3. RESULTS
3.1. Patient baseline characteristics
Of 253 patients with probable or definitive AS‐AIH identified, 242 fulfilled all the selection criteria and presented 90‐day follow‐up data (Figure 1, study flowchart). The mean age of the 242 participants was 49.6 (16.8) years, 180 (74.4%) were female, 199 (82.2%) tested positive for ANA, smooth muscle antibodies (SMA), or LKM‐1 and 156 (64.5%) showed increased serum IgG levels. The diagnosis of AIH was considered probable or definitive in 130 (53.7%) and 112 (46.3%) patients, respectively.
FIGURE 1.
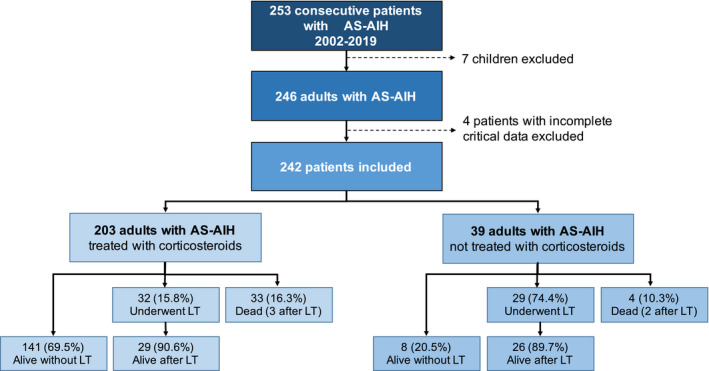
Patient recruitment flowchart. AS‐AIH, acute severe autoimmune hepatitis; LT, Liver transplantation
On admission, all participants had impaired liver function with mean bilirubin of 14.5 (8.7) mg/dl, INR of 2.1 (0.8) and a MELD score of 24 (5). Ascites was present in 22 (21.5%), and 33 (13.6%) debuted with hepatic encephalopathy, which was graded III or IV in only 6 (2.5%). In consequence, 33 (13.6%) fulfilled the criteria for ALF on admission. Additional clinical and analytical baseline characteristics are provided in Table 1.
TABLE 1.
Clinical, histological and analytical characteristics at baseline
| Variable | All (n = 242) | Treated (n = 203) | Untreated (n = 39) | p value |
|---|---|---|---|---|
| Age, years | 49.6 (16.8) | 50.4 (16.9) | 45.0 (15.6) | 0.07 |
| Female sex | 180 (74.4%) | 147 (72.4%) | 33 (84.6%) | 0.16 |
| Arterial hypertension | 42 (17.4%) | 36 (17.7%) | 61 (15.4%) | 0.82 |
| Diabetes Mellitus | 14 (5.8%) | 12 (5.9%) | 2 (5.1%) | 1.0 |
| Extrahepatic autoimmune disease | 47 (19.4%) | 42 (20.7%) | 5 (12.8%) | 0.38 |
| Alcohol consumption (>20–30 g/day) | 11 (4.5%) | 11 (5.4%) | 0 (0%) | 0.22 |
| Laboratory | ||||
| Total bilirubin (mg/dl) | 14.5 (8.7) | 13.7 (7.6) | 18.9 (12.1) | 0.01 |
| Creatinine (mg/dl) | 0.82 (0.45) | 0.81 (0.41) | 0.89 (0.60) | 0.28 |
| AST (IU/L) | 1373 (878) | 1377 (879) | 1359 (1073) | 0.92 |
| ALT (IU/L) | 1309 (950) | 1300 (928) | 1358 (1073) | 0.75 |
| Albumin (g/L) | 2.9 (0.6) | 2.9 (0.6) | 2.9 (0.5) | 0.72 |
| Platelets × 103/mm3 | 193 (83) | 189 (81) | 218 (89) | 0.07 |
| INR | 2.1 (0.8) | 2.0 (0.6) | 2.6 (1.3) | <0.01 |
| MELD score | 24 (5) | 23 (5) | 27 (7) | <0.01 |
| Clinical presentation | ||||
| Ascites (any grade) | 52 (21.5%) | 40 (19.7%) | 12 (30.8%) | 0.14 |
| Hepatic encephalopathy | ||||
| Absent | 209 (86.4%) | 180 (88.7%) | 29 (74.4%) | 0.04 |
| Grade 1–2 | 27 (11.2%) | 19 (9.4%) | 8 (20.5%) | |
| Grade 3–4 | 6 (2.5%) | 4 (2.0%) | 2 (5.1%) | |
| Immunological features | ||||
| Seropositive | 199 (82.2%) | 167 (82.3%) | 32 (82.1%) | 0.56 |
| ANA | 176 (72.7%) | 152 (74.9%) | 24 (61.5%) | 0.12 |
| LKM‐1 | 12 (72.7%) | 8 (3.9%) | 4 (10.3%) | 0.11 |
| SMA | 88 (36.4%) | 75 (36.9%) | 13 (33.3%) | 0.72 |
| IgG concentration | 2381 (1491) | 2430 (1501) | 2123 (832) | 0.22 |
| IgG elevated (>1600 mg/dl) | 156 (64.5%) | 127 (62.6%) | 29 (74.4%) | 0.11 |
| Histological features | ||||
| Number of patients | 155 | 134 | 21 | |
| Interface hepatitis | 109 (70.3%) | 99 (73.9%) | 10 (47.6%) | 0.02 |
| Portal inflammation (lymphoplasmacytic) | 84 (54.2%) | 77 (57.5%) | 7 (33.3%) | 0.06 |
| Emperipolesis | 32 (20.6%) | 27 (20.1%) | 5 (23.8%) | 0.17 |
| Hepatocyte rosetting | 55 (35.5%) | 50 (37.3%) | 5 (23.8%) | 0.45 |
| Centrilobular venulitis | 108 (69.7%) | 40 (29.9%) | 7 (33.3%) | 0.36 |
| Severe necrosis | 85 (54.8%) | 68 (50.7%) | 17 (81.0%) | <0.01 |
| Severe fibrosis (grade 3–4) | 35 (22.5%) | 32 (23.8%) | 3 (14.3%) | 0.23 |
Note: Numbers represent absolute values or means with rates or standard deviations in parenthesis, respectively.
p < 0.05 are indicated in bold.
Abbreviations: ANA, antinuclear antibodies; AST, aspartate aminotransferase; ALT, alanine aminotransferase; INR, International normalised ratio; LKM‐1, liver kidney microsomal type 1; MELD, model for end‐stage liver disease; SMA, smooth muscle antibodies.
Two hundred and three patients (83.9%) received corticosteroids, with an initial mean dose of predniso(lo)ne of 58 (12) mg per day, mostly administered intravenously (59.1%). Most patients (79.8%) were treated with the standard dose of predniso(lo)ne of 1.0 mg/kg day, 18.2% with a dose of 0.5 mg/kg day, and only four patients received high doses (1.5 mg/kg.day) of methylprednisolone. The median time from admission to initiation of corticosteroids was 4 (2–8) days, and 90 (44.3%) patients were treated within the first 3 days of admission (early treatment). The 39 (16.1%) untreated patients had worse liver function on admission, as shown by higher bilirubin levels, INR, MELD scores and prevalence of hepatic encephalopathy. Laboratory, demographic, clinical and histological characteristics of treated and untreated patients are summarised in Table 1.
3.2. Transplant‐free survival and mortality
Of 72 patients with AS‐AIH (29.8%) listed for emergency liver transplantation, 7 died on the waiting list, and 4 were delisted due to significant improvement. Thus, 61 patients (25.2%) were eventually transplanted. The median time from admission to wait listing was 8 (3–14) days, and 10 of 61 (16.4%) underwent liver transplantation within 3 days (median time from admission to transplantation 11 [5–26] days). Thirty‐seven patients (15.3%) died within 90 days of admission, including 5 in the early postoperative period. The median time from admission to death was 29 (15–39) days. The overall 90‐day transplant‐free survival was 61.6% (95% CI 55.4–67.7%). In the subset of patients fulfilling ALF criteria on admission, this rate decreased to 21.1% (95% CI 5.5–35.9%).
3.3. Effect of corticosteroid therapy on outcomes
Ninety‐day transplant‐free survival in patients treated with corticosteroids was significantly higher than in untreated patients (69.5% [95% CI 63.1–75.8] vs 20.5% [95% CI 7.2–33.8], p < 0.01) (Figure S1). As shown in Table 2, the benefit of corticosteroids was independent of sex, age, liver function (MELD score at baseline), and presence of hepatic encephalopathy on admission (HR 0.25 [95% CI 0.2–0.4], p < 0.01). We observed remission of hepatic encephalopathy in response to corticosteroids in 7 of 23 (30.4%) treated patients fulfilling ALF criteria on admission, yet this effect was not noted in any untreated patient. In contrast, the beneficial effect of corticosteroids on outcome was not observed in patients with any grade of ascites on admission (adjusted HR 0.61 [95% CI 0.3–1.3]; p = 0.22), which indicates a different effect of steroids depending on ascites presence (first‐order interaction p < 0.01).
TABLE 2.
Effect of corticosteroids on 90 days transplant‐free survival based on baseline variables (determined at admission)
| Variable | Hazard ratio (95% CI); p value |
|---|---|
| Age, years | 1.00 (0.9–1.0); 0.84 |
| Female sex | 0.64 (0.4–1.0); 0.05 |
| MELD score | 1.11 (1.1–1.2); <0.01 |
| Hepatic encephalopathy (any grade) | 2.18 (1.3–3.6); <0.01 |
| Corticosteroid therapy | 0.25 (0.2–0.4); <0.01 a /0.61 (0.3–1.3); 0.22b |
| High volume transplant centrec | 1.41 (0.8–2.4); 0.21 |
| Ascites | 0.56 (0.25–1.7); 0.16 |
Note: Significant interaction between corticosteroids and ascites: aHazard ratio for patients without ascites; bHazard ratio for patients with ascites. cHigh volume transplant centre = 15 liver transplants due to AS‐AIH during the study period.
p < 0.05 are indicated in bold.
Abbreviation: MELD, model for end‐stage liver disease.
3.4. Response to corticosteroid therapy
Treatment failed in 62 (30.5% [95% CI 24.2–36.9]) out of the 203 patients receiving corticosteroids: 32 patients (15.8%) were transplanted, and 33 (16.3%) died, 3 of them after transplantation. In the univariate analysis, older age, male sex, worse liver function and the presence of ascites and hepatic encephalopathy recorded upon initiation of corticosteroids increased the risk of treatment failure. In Cox’s regression model, the factors emerging as predictors of a poor response were: older age (HR 1.02 [95% CI 1.0–1.1]; p < 0.01), MELD score (HR 1.2 [95% CI 1.1–1.3]; p < 0.01), the presence of hepatic encephalopathy (HR 2.3 [95% CI 1.3–4.2]; p < 0.01) and ascites (HR 2.1 [95% CI 1.2–3.8]; p < 0.01) at initiation of corticosteroids. In contrast, the early administration (<3 days after admission) and dose or route of corticosteroids had no impact on the primary outcome (Table 3). The mean dose of corticosteroids was similar in responders and non‐responders (57[13] vs 60[11] mg/day), and the intravenous route was even more frequently used in non‐responders (75 vs 54%). Neither the dose (p = 0.14) nor the administration route (p = 0.23) was selected as predictors of response during the multivariate modelling as detailed in Table S1. A predictive nomogram based on the final model is provided in Figure 2A. The model showed good accuracy in estimating the 90‐day risk of death or liver transplantation, with a C‐index of 0.82 (95% CI 0.77–0.87) (Figure 3A). Calibration at days 7 and 14 of treatment, and days 30 and 90 post‐admission was good as shown in (Figure 3B–E).
TABLE 3.
Predictors of response to corticosteroids at initiation and at day 7 of corticosteroids
| Variable | Responders | Non‐responders | Univariate p‐values | Cox’s multivariate regression model Hazard ratio (95% CI); p value |
|---|---|---|---|---|
| At initiation of corticosteroids | N = 141 | N = 62 | ||
| Age, years | 48.0 (16.4) | 55.9 (17.0) | <0.01 | 1.02 (1.0–1.1); <0.01 |
| Female sex | 111 (78.7%) | 36 (58.1%) | <0.01 | |
| Diabetes Mellitus | 8 (5.7%) | 4 (6.5%) | 0.76 | |
| Alcohol consumption (>20–30 g/day) | 6 (4.3%) | 5 (8.1%) | 0.32 | |
| AST | 1401 (751) | 1309 (1087) | 0.55 | |
| ALT | 1366 (913) | 1150 (953) | 0.14 | |
| Total bilirubin (mg/dl) | 14.8 (8.4) | 18.7 (8.6) | <0.01 | |
| Creatinine (mg/dl) | 0.76 (0.33) | 1.02 (0.63) | <0.01 | |
| INR | 1.8 (0.5) | 2.4 (0.9) | <0.01 | |
| MELD score | 22 (4) | 27 (5) | <0.01 | 1.17 (1.1–1.3); <0.01 |
| Ascites (any grade) | 13 (9.2%) | 27 (43.3%) | <0.01 | 2.08 (1.2–3.8); <0.01 |
| Hepatic encephalopathy (any grade) | 7 (5.0%) | 16 (25.8%) | <0.01 | 2.29 (1.3–4.2); <0.01 |
| Early corticosteroids (<3 days) | 60 (42.6%) | 30 (48.4%) | 0.45 | |
| Dose of corticosteroids, mg per day | 57 (13) | 60 (11) | 0.24 | |
| Route of corticosteroids (intravenous) | 76 (53.9%) | 45 (75.0%) | 0.14 | |
| At day 7 of corticosteroids | N = 125 | N = 42 | ||
| Age, years | 47.4 (11.7) | 58.5 (9.4) | <0.01 | |
| Female sex | 101 (80.8%) | 18 (42.9%) | <0.01 | |
| Diabetes Mellitus | 7 (5.6%) | 3 (7.1%) | 0.76 | |
| Alcohol consumption (>20–30 g/day) | 6 (4.8%) | 5 (11.9%) | 0.32 | |
| Total bilirubin (mg/dl) | 8.8 (6.8) | 18.6 (9.6) | <0.01 | |
| Creatinine (mg/dl) | 0.79 (0.77) | 1.13 (1.11) | 0.04 | |
| INR | 1.4 (0.3) | 2.4 (1.2) | <0.01 | |
| MELD score | 17 (4) | 26 (6) | <0.01 | 1.16 (1.1–1.2); <0.01 |
| %ΔMELD | −22 (13) | 1.6 (16) | <0.01 | |
| Ascites (any grade) | 8 (6.4%) | 27 (63.3%) | <0.01 | 3.26 (1.4–7.6); <0.01 |
| Hepatic encephalopathy (any grade) | 4 (3.2%) | 27 (63.4%) | <0.01 | 3.63 (1.7–7.8); 0.01 |
| Early corticosteroids (<3 days) | 58 (46.4%) | 20 (47.6%) | 0.45 | |
| Dose of corticosteroids, mg per day | 57 (13) | 60 (11) | 0.24 | |
| Route of corticosteroids (intravenous) | 73 (58.4%) | 29 (42) | 0.14 |
Note: Numbers represent absolute values or means with rates or standard deviations in parenthesis, respectively.
p < 0.05 are indicated in bold.
Abbreviations: AST, aspartate aminotransferase; ALT, alanine aminotransferase; MELD,: model for end‐stage liver disease; INR, International normalised ratio.
FIGURE 2.
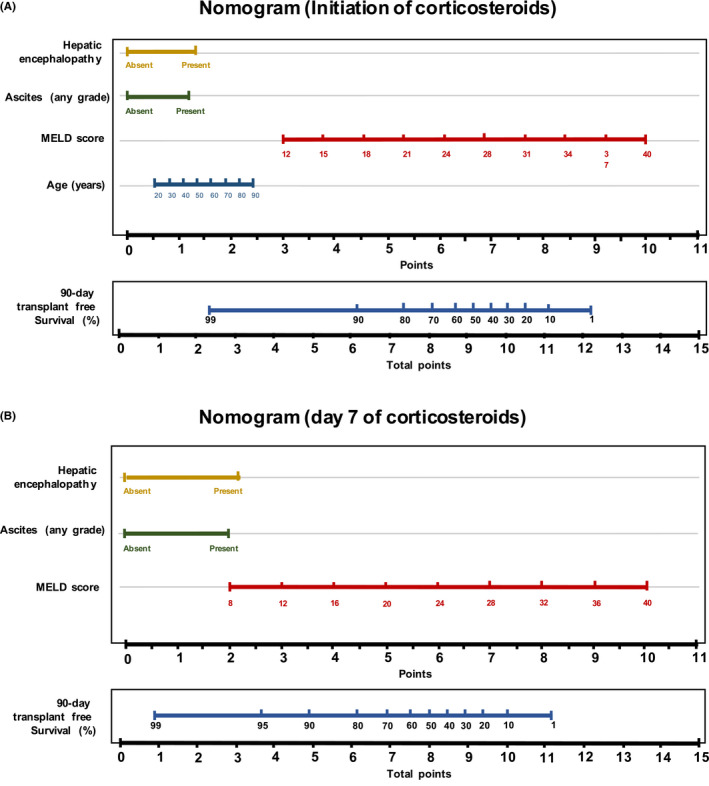
Nomogram to estimate the risk of treatment failure. (A) Nomogram at the initiation of treatment; (B) nomogram at day 7 of treatment. To use the nomogram, we first draw a line from each parameter value to the score axis for the score. The points for all the parameters are then added. Finally, a line from the total score points axis is drawn to the lower line of the nomogram to obtain the predicted 90‐day transplant‐free survival. MELD: Model for end‐stage liver disease
FIGURE 3.
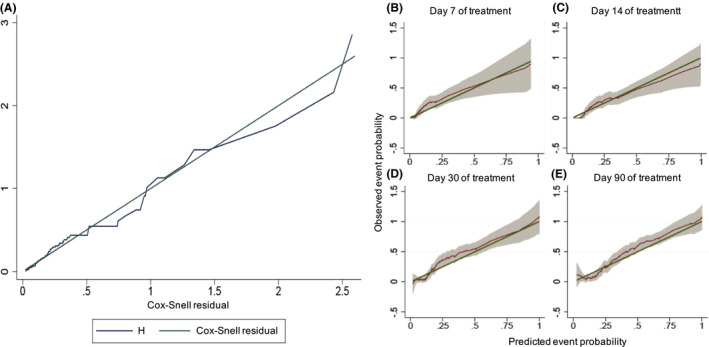
Calibration of a prognostic model. (A) Calibration plots of the nomogram at the initiation of treatment predicting 90‐day transplant‐free survival. (B–E) calibration plots of the nomogram at the initiation of treatment for predicting the corticosteroid therapy response at different time points: (B) 7 days of treatment; (C) 14 days of treatment; (D) 30 days after admission; (E) 90 days after admission. We plotted smoothed pseudovalues with point‐wise 95% confidence intervals against predicted event probabilities. The straight line is the line of identity, denoting perfect calibration
3.5. Changes in liver function during corticosteroids
In a sensitivity analysis, changes in liver function at different time points after the onset of therapy were explored. Figure 4 shows that MELD significantly improved in responders as early as on day 3 of treatment (mean MELD3–2.5 [2.5]), and further on days 7 (meanMELD7–5.0 [3.1]) and 14 of treatment (mean MELD14–7.7 [4.2]). In contrast, mean MELD did not significantly change between initiation of corticosteroids and any of these time points in non‐responders (MELD3 + 0.8 [3.5]MELD7 + 0.4 [4.1]; MELD14 + 0.8 [5.1], respectively). The 90‐day transplant‐free survival rates in patients without MELD improvement at treatment days 3 and 7 were as low as 38.5% and 18.8%, respectively (Figure S2). Interestingly, no statistically significant differences in the time‐dependent AUC at 90‐day transplant‐free survival were found between the absolute value of MELD, %MELD and MELD on days 3 and 7 after treatment onset (Figure S3 and Table S2). In our cohort, the best univariate predictor of response was the absolute value of MELD on day 7 of corticosteroids. The cut‐off of 25 points showed a good specificity and negative predictive value (sensitivity 62.5% [95% CI: 47.0–75.8]; specificity 95.2% [95% CI: 89.9–97.8]; positive predictive value 80.6%; negative predictive value 89.9%).
FIGURE 4.
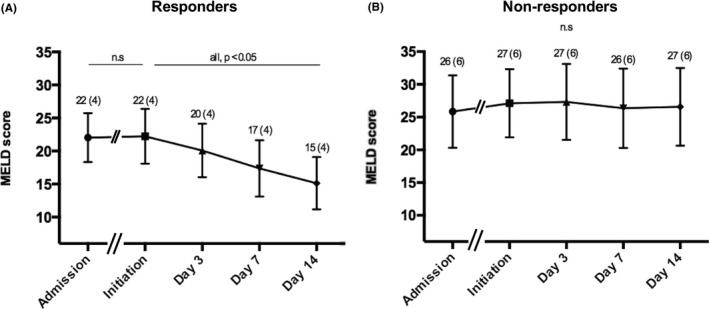
Changes in MELD scores during corticosteroid therapy. MELD, model for end‐stage liver disease. (A) Corticosteroid responders; (B) Corticosteroid non‐responders
Two multivariate predictive models based on information at days 3 and 7 of treatment were developed. One hundred and sixty‐three of the 203 (80.3%) patients on corticosteroids were alive without liver transplantation on day 7 of treatment. In the multivariate analysis, MELD and the presence of hepatic encephalopathy and ascites on day 7 were independently related to treatment failure (Table 3). In consequence, a predictive nomogram based on this model is provided in Figure 3B. This model showed high accuracy (C‐index 0.91 [95% CI: 0.87–0.95]) to predict a poor outcome (Figure S4). In contrast, the C index of the predictive model developed at day 3 of corticosteroids was slightly lower (0.85 [95% CI: 0.81–0.89]) (Table S3). Incorporating MELD on days 3 and 7 did not improve the performance of the models (Table S4).
In an additional analysis designed to examine the performance of the SURFASA score in our cohort, 15 good discrimination capacity to identify non‐responders was observed (AUC 0.85, 95% CI 0.78–0.91), but poor calibration (Hosmer–Lemeshow p < 0.001) (Figure S5).
3.6. Predictive value of histological parameters
One hundred and fifty‐five (64.1%) patients had a liver biopsy prior to the initiation of corticosteroids and were included in this sub‐analysis. The median time from admission to liver biopsy was 3 (1–7) days, and most were performed by the transjugular route (77.4%). Main histological data are provided in Table 1. Histological findings on liver biopsy were critical for the diagnosis of AIH in 103 of the 242 patients (42.3%).
In our series, the only histological feature associated with a poor outcome in the univariate and multivariate analysis was the presence of severe necrosis (HR 5.67 [95% CI 1.6–6.2]; p < 0.01) but not the presence of severe liver fibrosis (HR 1.18 [95% CI 0.6–2.2]; p = 0.61) (Table S5). The inclusion of histological variables did not significantly change the predictive ability of the models (C index: 0.80).
3.7. Risk of systemic infection
Infection was present on admission in three patients and developed during hospitalisation in 40 (Table S6). Thirty‐nine episodes of a bacteriologically proven infection were recorded in 34 patients. Urinary tract infection was the most frequent (9 episodes), followed by primary bacteremia (8 episodes) and spontaneous bacterial peritonitis (7 cases). Five patients developed a viral infection (4 CMV reactivation and 1 pneumonia due to H1N1 influenza virus), and 7 suffered invasive aspergillosis.
The cumulative incidence of infection during hospitalisation was 16.5% (95% CI 11.8–21.2), and the median time from admission was 18 (5–30) days. The incidence of infection was similar in patients treated and not treated with corticosteroids (Figure S6). As many as 12 of the 40 (30.0%) patients with infection had not received corticosteroids. Remarkably, the seven patients with invasive aspergillosis had all received corticosteroids (univariate p = 0.24) for at least 7 days (median time from treatment onset to the diagnosis of aspergillosis 18 [11–34] days). After adjusting by MELD score, age, sex, ascites and hepatic encephalopathy at admission, corticosteroid therapy did not negatively impact the risk of infection (HR 0.75 [95% CI 0.3–2.0]; p = 0.57) (Table S6). Among treated patients, a worse liver function, higher dose of corticosteroids and the presence of ascites and encephalopathy at treatment onset increased the risk of infection in the univariate analysis (Table S7). In Cox’s regression model, only MELD score (HR 1.16 [95% CI 1.1–1.3]; p < 0.01), and ascites (HR 2.12 [95% CI 1.0–4.4]; p = 0.04) at treatment onset emerged as predictors of infection. The model showed moderate accuracy in estimating the infection risk, with a C‐index of 0.74 (95% CI 0.65–0.84).
4. DISCUSSION
In this nationwide retrospective study, we analysed predictive factors associated with a poor response to corticosteroid treatment in the largest series of patients with AS‐AIH examined to date. Our findings revealed that: (i) corticosteroids improved 90‐day transplant‐free survival, but up to one‐third of patients were unresponsive to them, (ii) older patients with poor liver function, ascites or encephalopathy at the initiation of treatment were unlikely to respond, (iii) a lack of improvement in liver function after 7 days of corticosteroids was highly predictive of treatment failure and (iv) prolonging corticosteroids in non‐responders emerged as unlikely to modify the course of events and should be avoided in patients listed for liver transplantation.
Corticosteroids remain the cornerstone treatment of AIH as remission rates approach 60–80%. 15 , 21 , 22 However, their beneficial effects in patients with acute and severe presentations have been a matter of debate. Our results definitively confirm that the usefulness of this treatment extends to this scenario, in which corticosteroids were found to raise transplant‐free survival by 3.4‐fold. The survival rate of non‐responders was as low as those untreated, which highlights a need for accurate scores for early response prediction. As independent predictors of treatment failure, we identified an older age, high MELD, encephalopathy and ascites at treatment initiation. Our results from the largest cohort to date are consistent with the findings of others, confirming that poor liver function and encephalopathy are linked to higher mortality. 15 , 22 , 23 , 24 In fact, patients with ALF showed the worst response to treatment as their transplant‐free survival was as low as 21.1%. However, in up to one‐third, encephalopathy was reversed by treatment. Thus, the isolated presence of encephalopathy should not preclude corticosteroid use, unless other factors are present. Specifically, ascites was found to be a strong modifier of the effect of corticosteroids, as supported by the interaction effect of both variables on transplant‐free survival. Distinctively, our study incorporates a nomogram that can estimate the survival probability of an individual before initiation of therapy with a C‐index of 0.82. The nomogram allows the assessment of the readily available prognostic variables identified in a single time point and as early as possible in the course of the disease, which are criteria of quality of a predictive model. Our nomogram can be used shortly after admission to accurately identify a patient unlikely to respond to treatment and represents a quantitative tool for clinical decision‐making at the bedside. Patients can then be considered for an early referral or diagnostic work‐up for liver transplantation.
Despite the accuracy of our nomogram, patients with AS‐AIH and a low probability of response should receive corticosteroids, as avoiding this treatment was linked to the worst prognosis (transplant‐free survival of 20.5%), and there are no other therapeutic options. Because of this, we also examined whether changes in liver function when under treatment with corticosteroids could improve the predictive capacity of the nomogram. MELD improved as early as day 3 of therapy in responders but remained unchanged on days 3, 7 and 14 in non‐responders. The AUC to identify a non‐response to 7 days of corticosteroids was 0.90 for the absolute value of MELD (MELD7), non‐significantly greater than 0.86 for ∆MELD7, and 0.89 for %∆MELD7. The probability of responding to corticosteroids in patients with MELD7 25 on treatment day 7 was 90% (sensitivity 62.5%, specificity 95.2%), and the risk of dying (or being transplanted) if MELD7 was above 25 was as high as 81%. We, therefore, incorporated MELD, ascites and encephalopathy on treatment day 7 in a new nomogram to very accurately assess the individual response probability (C‐index of 90). Worsening of liver function as assessed by changes in bilirubin, INR, MELD or MELD‐Na on treatment days 3–7 has also been identified in shorter patient series as predictive of a poor prognosis. 25 , 26 Our results are in line with those of a recent large retrospective multicenter French study in 128 patients with AS‐AIH. The ability of a score comprising INR before corticosteroids and changes in INR and bilirubin on treatment day 3 to predict death or transplantation within 3 days of corticosteroids was assessed with an AUC of 0.93. The SURFASA score tested on our cohort showed good accuracy to identify treatment failure at day 3 of treatment, but rather a poor calibration probably reflecting overfitting of the model due to the smaller size of the French cohort. 15 The accuracy of the scores used on days 3 or 7 of treatment in the French study could not be compared because of missing data. We, nevertheless, identified the MELD on day 7 as a powerful bedside tool to identify patients likely to show poor survival. The better performance of the model at day 7 is probably due to the greater prognostic information provided by adding dynamic variables. We cannot completely discard the contribution to this observation of a smaller sample size due to the loss of high‐risk patients that did not reach the day 7 time‐point. However, we consider this reason unlikely as the number of patients that reached day 7 with complete clinical information was quite large, and the performance of the model at day 3 was slightly worse although more patients reached this time point. Inclusion in our model of variables with predictive meaning at a single time point instead of the change in their value from admission (∆values) was intended to facilitate its use for clinicians because incorporating ∆MELD in multivariable models did not improve their prognostic yield. Remarkably, despite the high accuracy of the assessment of liver function on days 3 and especially 7 of therapy to predict a poor outcome, the predictive value of the nomogram including only baseline data was similar.
Our study revealed that a low titre or lack of autoantibodies and IgG normality are not uncommon in AS‐AIH, making liver biopsy necessary to fulfil standard simplified diagnostic criteria, as occurred in 42.3% of the cases in our series. 8 These findings support current EASL clinical practice guidelines that consider liver biopsy a requirement for AIH diagnosis, and that it should be performed before starting treatment. 2 More controversy exists regarding the value of histological findings to predict the response to corticosteroids. Findings such as massive necrosis and central perivenulitis have been related to a worse outcome in most studies. 6 , 27 The predictive value of the fibrosis stage is under discussion. In our study, severe liver necrosis, but not fibrosis stage, was noted to increase the risk of treatment failure. Interestingly, the inclusion of histological variables did not significantly augment the accuracy of the model based on variables obtained before starting corticosteroids.
The dose, route of administration and timing of onset of corticosteroids have also been a matter of debate. 2 , 5 , 16 Most of our patients received the recommended dose of 1 mg/kg day of predniso(lo)ne by the intravenous route. 2 In agreement with the results of De Martin et al., the response to steroids in our series was independent of dose or administration route. 15 However, no firm conclusions can either be drawn in this regard as our cohort was also retrospective in nature and decisions regarding dose and route of corticosteroids were arbitrarily taken by the respective practitioner. Of note, we found no difference in response between “early” (<3 days) or “late” initiation of steroids, indicating sufficient time for an extensive diagnostic work‐up (including the ruling‐out of infection) after admission.
Infection is a concern in patients with severe liver insufficiency undergoing steroid treatment, as an active infection could jeopardise liver transplantation. 13 , 25 , 27 , 28 , 29 , 30 , 31 Corticosteroids alone did not increase the bacterial infection risk. This was supported by the similar rates of infection recorded in patients on and not on steroids, and by the lack of independent association between corticosteroids and infection risk detected in the multivariate analysis. In effect, the infection risk in our study was related to the severity of liver insufficiency, as it was highest in patients with high MELD and ascites, which also emerged as independent factors predictive of a non‐response to steroids. Similarly, bacterial infection risk in patients with severe alcoholic hepatitis is more closely related to a non‐response to corticosteroids than the therapy itself. 12 , 32 However, our results suggest an association between prolonged corticosteroids and systemic fungal infection. Invasive aspergillosis, a condition that compromises transplantation, 13 was detected in seven patients, all of whom had been on corticosteroids for at least 7 days. Physicians should be aware of this risk and avoid extending corticosteroids beyond 7 days in non‐responders, especially in liver transplantation candidates.
We here present the largest published dataset on prognosis and response to therapy in AS‐AIH. Additionally, our complete laboratory data for 3 and 7 days of treatment allowed us to compare the performance accuracy of the models at both time points. To our knowledge, this is the first study to provide a prediction nomogram to anticipate the response to corticosteroids at disease presentation. We should acknowledge several limitations. Firstly, in the final analysis, we included all patients diagnosed with AS‐AIH, even those with severe liver fibrosis and/or cirrhosis detected on liver biopsy, possibly representing a different population. According to current guidelines, patients with acute presentations of AIH are included in the definition of ALF and are considered candidates for emergency liver transplantation, despite the possibility of underlying advanced chronic liver disease. 16 , 18 However, in the sub‐analysis performed to overcome this limitation in patients with complete histological information prior to corticosteroids, the treatment response was independent of the fibrosis stage. Finally, the retrospective nature of this study is an intrinsic limitation that makes it difficult to identify patients warranting a corticosteroids trial and those who should be directly referred for liver transplantation. While we tried to adjust for confounding factors by multivariate regression analysis, residual confounders due to unknown or unmeasured covariates may not be completely ruled out. Consequently, the prognostic models developed need further prospective and external validation.
Our study provides support for current clinical recommendations that are based on expert opinion. Its findings indicate a survival benefit of corticosteroids in AS‐AIH, and a trial of this therapy is warranted in most patients, even those with encephalopathy. Response to therapy should be assessed at treatment onset and after 3 days, or better still 7 days, to early identify patients likely to be non‐responders. Prolonging treatment in non‐responders beyond this time point should be avoided, especially in transplantation candidates, as it is unlikely it will modify the disease course yet may increase the risk of invasive aspergillosis. Importantly, a nomogram including older age, high MELD and presence of encephalopathy and ascites accurately identify newly admitted patients unlikely to respond to steroids thus expediting their referral or work‐up for liver transplantation.
AUTHOR CONTRIBUTIONS
Luis Téllez: Conceptualization (lead); data curation (lead); formal analysis (lead); investigation (lead); methodology (lead); project administration (lead); resources (lead); supervision (lead); validation (lead); visualization (lead); writing – original draft (lead); writing – review and editing (lead). Eugenia Sánchez Rodríguez: Conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); project administration (equal); resources (equal); supervision (equal); validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Enrique Rodríguez de Santiago: Conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); project administration (equal); resources (equal); software (equal); supervision (equal); validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Laura Patricia Llovet: Data curation (equal). Ana Gómez Outomuro: Data curation (equal). Fernando Díaz Fontenla: Data curation (equal). Patricia Álvarez López: Data curation (equal). Maria García‐Eliz: Data curation (equal). Carla Amaral: Data curation (equal). Yolanda Sánchez‐Torrijos: Data curation (equal). José Ignacio Fortea: Data curation (equal). Carlos Ferre Aracil: Data curation (equal). Manuel Rodriguez‐Peralvarez: Data curation (equal). Marta Abadía: Data curation (equal). Judith Gómez Camarero: Data curation (equal). Antonio Olveira: Data curation (equal); validation (equal). José Luis Calleja: Data curation (equal); validation (equal). Javier Crespo: Data curation (equal); validation (equal). Manuel Hernández‐Guerra: Data curation (equal); validation (equal). Marina Carmen Berenguer Hayme: Data curation (equal); validation (equal). Mar Riveiro‐Barciela: Data curation (equal); visualization (equal). Magdalena Salcedo: Data curation (equal); validation (equal). Manuel Rodrıguez: Data curation (equal); visualization (equal). María‐Carlota Londoño: Data curation (equal); validation (equal). Agustin Albillos: Conceptualization (lead); data curation (lead); formal analysis (lead); funding acquisition (lead); investigation (lead); methodology (lead); project administration (lead); resources (lead); supervision (lead); validation (lead); visualization (lead); writing – original draft (equal); writing – review and editing (lead).
Supporting information
DataS 1
ACKNOWLEDGEMENT
Declaration of personal interests: The authors have no conflicts of interest pertaining to this work and all authors have fulfilled the conflict of interest statement.
Téllez L, Sánchez Rodríguez E, Rodríguez de Santiago E, Llovet L, Gómez‐Outomuro A, Díaz‐Fontenla F, et al. Early predictors of corticosteroid response in acute severe autoimmune hepatitis: a nationwide multicenter study. Aliment Pharmacol Ther. 2022;56:131–143. 10.1111/apt.16926
Luis Téllez and Eugenia Sánchez Rodríguez share co‐first authorship.
The Handling Editor for this article was Professor Gideon Hirschfield, and it was accepted for publication after full peer‐review.
Funding information
This study was supported in part by grants from the Spanish Ministry of Science and Innovation, Instituto de Salud Carlos III, number PI20/01302, awarded to Agustín Albillos and number PI 21/01310, awarded to Luis Téllez. CIBEREHD is funded by the Instituto de Salud Carlos III using grants cofinanced by the European Development Regional Fund “A way to achieve Europe” (EDRF). María Carlota Londoño received support from the Plan Nacional de I+D+I co‐funded by ISCIII‐Subdirección General de Evaluación and Fondo Europeo de Desarrollo Regional (FEDER‐"Una manera de Hacer Europa") (PI17/00955). Laura Patricia Llovet received the Resident Award “Clínic‐La Pedrera” granted by the Hospital Clínic de Barcelona, Research, Innovation and Education Department.
DATA AVAILABILITY STATEMENT
Data, analytic methods, and study materials will be available to other researchers upon request to the corresponding author.
REFERENCES
- 1. Manns MP, Czaja AJ, Gorham JD, Krawitt EL, Mieli‐Vergani G, Vergani D, et al. Diagnosis and management of autoimmune hepatitis. Hepatology. 2010;51(6):2193–213. 10.1002/hep.23584 [DOI] [PubMed] [Google Scholar]
- 2. European Association for the Study of the Liver . EASL clinical practice guidelines: autoimmune hepatitis. J Hepatol. 2015;63(4):971–1004. 10.1016/j.jhep.2015.06.030 [DOI] [PubMed] [Google Scholar]
- 3. Lohse AW, Mieli‐Vergani G. Autoimmune hepatitis. J Hepatol. 2011. Jul;55(1):171–82. 10.1016/j.jhep.2010.12.012 [DOI] [PubMed] [Google Scholar]
- 4. Takahashi H, Zeniya M. Acute presentation of autoimmune hepatitis: does it exist? A published work review. Hepatol Res. 2011. Jun;41(6):498–504. 10.1111/j.1872-034X.2011.00808.x [DOI] [PubMed] [Google Scholar]
- 5. Mack CL, Adams D, Assis DN, Kerkar N, Manns MP, Mayo MJ, et al. Diagnosis and Management of Autoimmune Hepatitis in adults and children: 2019 practice guidance and guidelines from the American Association for the Study of Liver Diseases. Hepatology. 2020;72(2):671–722. 10.1002/hep.31065 [DOI] [PubMed] [Google Scholar]
- 6. Stravitz RT, Lefkowitch JH, Fontana RJ, Gershwin ME, Leung PS, Sterling RK, et al. Autoimmune acute liver failure: proposed clinical and histological criteria. Hepatology. 2011;53(2):517–26. 10.1002/hep.24080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alvarez F, Berg PA, Bianchi FB, Bianchi L, Burroughs AK, Cancado EL, et al. International autoimmune hepatitis group report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31(5):929–38. [DOI] [PubMed] [Google Scholar]
- 8. Hennes EM, Zeniya M, Czaja AJ, Parés A, Dalekos GN, Krawitt EL, et al. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48(1):169–76. 10.1002/hep.22322 [DOI] [PubMed] [Google Scholar]
- 9. Yeoman AD, Westbrook RH, Zen Y, Bernal W, Al‐Chalabi T, Wendon JA, et al. Prognosis of acute severe autoimmune hepatitis (AS‐AIH): the role of corticosteroids in modifying outcome. J Hepatol. 2014;61(4):876–82. 10.1016/j.jhep.2014.05.021 [DOI] [PubMed] [Google Scholar]
- 10. Moenne‐Loccoz R, Severac F, Baumert TF, Habersetzer F. Usefulness of corticosteroids as first‐line therapy in patients with acute severe autoimmune hepatitis. J Hepatol. 2016;65(2):444–6. 10.1016/j.jhep.2016.03.023 [DOI] [PubMed] [Google Scholar]
- 11. Sonthalia N, Rathi PM, Jain SS, Surude RG, Mohite AR, Pawar SV, et al. Natural history and treatment outcomes of severe autoimmune hepatitis. J Clin Gastroenterol. 2017;51(6):548–56. 10.1097/MCG.0000000000000805 [DOI] [PubMed] [Google Scholar]
- 12. Louvet A, Wartel F, Castel H, Dharancy S, Hollebecque A, Canva‐Delcambre V, et al. Infection in patients with severe alcoholic hepatitis treated with steroids: early response to therapy is the key factor. Gastroenterology. 2009;137(2):541–8. 10.1053/j.gastro.2009.04.062 [DOI] [PubMed] [Google Scholar]
- 13. Fujiwara K, Yasui S, Yonemitsu Y, Arai M, Kanda T, Fukuda Y, et al. Analysis of infectious complications and timing for emergency liver transplantation in autoimmune acute liver failure. J Hepatobiliary Pancreat Sci. 2016;23(4):212–9. 10.1002/jhbp.326 [DOI] [PubMed] [Google Scholar]
- 14. Karkhanis J, Verna EC, Chang MS, Stravitz RT, Schilsky M, Lee WM, et al. Steroid use in acute liver failure. Hepatology. 2014;59(2):612–21. 10.1002/hep.26678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. De Martin E, Coilly A, Chazouillères O, Roux O, Peron JM, Houssel‐Debry P, et al. Early liver transplantation for corticosteroid non‐responders with acute severe autoimmune hepatitis: the SURFASA score. J Hepatol. 2021;74(6):1325–34. 10.1016/j.jhep.2020.12.033 [DOI] [PubMed] [Google Scholar]
- 16. European Association for the Study of the Liver . EASL clinical practical guidelines on the management of acute (fulminant) liver failure. J Hepatol. 2017;66(5):1047–81. 10.1016/j.jhep.2016.12.003 [DOI] [PubMed] [Google Scholar]
- 17. Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Ann Intern Med. 2007;147(8):W163–94. 10.7326/0003-4819-147-8-200,710,160-00010-w1 [DOI] [PubMed] [Google Scholar]
- 18. European Association for the Study of the Liver . EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69(2):406–460. doi: 10.1016/j.jhep.2018.03.024. Epub 2018 Apr 10. Erratum in: J Hepatol. 2018 Nov;69(5):1207. PMID: 29653741. [DOI] [PubMed] [Google Scholar]
- 19. Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31(4):864–71. 10.1053/he.2000.5852 [DOI] [PubMed] [Google Scholar]
- 20. Bedossa P. Presentation of a grid for computer analysis for compilation of histopathologic lesions in chronic viral hepatitis C. cooperative study of the METAVIR group. Ann Pathol. 1993;13(4):260–5. French [PubMed] [Google Scholar]
- 21. Zhang C, Wu SS, Dong XQ, Wu Z, Zhao H, Wang GQ. The efficacy and safety of different doses of glucocorticoid for autoimmune hepatitis: a systematic review and meta‐analysis. Medicine (Baltimore). 2019;98(52):e18313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zachou K, Arvaniti P, Azariadis K, Lygoura V, Gatselis NK, Lyberopoulou A, et al. Prompt initiation of high‐dose i.v. corticosteroids seems to prevent progression to liver failure in patients with original acute severe autoimmune hepatitis. Hepatol Res. 2019;49(1):96–104. 10.1111/hepr.13252 [DOI] [PubMed] [Google Scholar]
- 23. Villamil AG, Casciato P, Eduardo M, Bustos D, Giunta D, Bandi JC, et al. Fulminanant autoimmune hepatitis: clinical presentation, outcome and prognostic factors. Am J Transpl. 2005;5(Suppl. 11):278. [Google Scholar]
- 24. De Martin E, Coilly A, Ichai P, Samuel D, Duclos‐Vallée JC. The role of corticosteroids in acute‐severe autoimmune hepatitis is still highly debatable. J Hepatol. 2015;63(4):1041–2. 10.1016/j.jhep.2015.04.032 [DOI] [PubMed] [Google Scholar]
- 25. Verma S, Maheshwari A, Thuluvath P. Liver failure as initial presentation of autoimmune hepatitis: clinical characteristics, predictors of response to steroid therapy, and outcomes. Hepatology. 2009;49(4):1396–7. 10.1002/hep.22894 [DOI] [PubMed] [Google Scholar]
- 26. Yeoman AD, Westbrook RH, Zen Y, Maninchedda P, Portmann BC, Devlin J, et al. Early predictors of corticosteroid treatment failure in icteric presentations of autoimmune hepatitis. Hepatology. 2011;53(3):926–34. 10.1002/hep.24141 [DOI] [PubMed] [Google Scholar]
- 27. Mendizabal M, Marciano S, Videla MG, Anders M, Zerega A, Balderramo DC, et al. Fulminant presentation of autoimmune hepatitis: clinical features and early predictors of corticosteroid treatment failure. Eur J Gastroenterol Hepatol. 2015;27(6):644–8. 10.1097/MEG.0000000000000353 [DOI] [PubMed] [Google Scholar]
- 28. Rahim MN, Miquel R, Heneghan MA. Approach to the patient with acute severe autoimmune hepatitis. JHEP Rep. 2020;2(6):100149. 10.1016/j.jhepr.2020.100149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rolando N, Harvey F, Brahm J, Philpott‐Howard J, Alexander G, Casewell M, et al. Fungal infection: a common, unrecognised complication of acute liver failure. J Hepatol. 1991;12(1):1–9. 10.1016/0168-8278(91)90900-v [DOI] [PubMed] [Google Scholar]
- 30. Rolando N, Philpott‐Howard J, Williams R. Bacterial and fungal infection in acute liver failure. Semin Liver Dis. 1996;16(4):389–402. 10.1055/s-2007-1,007,252 [DOI] [PubMed] [Google Scholar]
- 31. Vaquero J, Polson J, Chung C, Helenowski I, Schiodt FV, Reisch J, et al. Infection and the progression of hepatic encephalopathy in acute liver failure. Gastroenterology. 2003;125(3):755–64. 10.1016/s0016-5085(03)01051-5 [DOI] [PubMed] [Google Scholar]
- 32. Louvet A, Naveau S, Abdelnour M, Ramond MJ, Diaz E, Fartoux L, et al. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology. 2007;45(6):1348–54. 10.1002/hep.21607 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
DataS 1
Data Availability Statement
Data, analytic methods, and study materials will be available to other researchers upon request to the corresponding author.


