Abstract
Streptomyces cattleya is unusual in that it produces fluoroacetate and 4-fluorothreonine as secondary metabolites. We now report the isolation of an NAD+-dependent fluoroacetaldehyde dehydrogenase from S. cattleya that mediates the oxidation of fluoroacetaldehyde to fluoroacetate. This is the first enzyme to be identified that is directly involved in fluorometabolite biosynthesis. Production of the enzyme begins in late exponential growth and continues into the stationary phase. Measurement of kinetic parameters shows that the enzyme has a high affinity for fluoroacetaldehyde and glycoaldehyde, but not acetaldehyde.
Although the existence of naturally produced organofluorine compounds has been known for over 50 years, particularly fluoroacetate in plants (5), the mechanism by which the C-F bond occurs has not yet been elucidated. The actinomycete Streptomyces cattleya biosynthesizes fluoroacetate and 4-fluorothreonine as secondary metabolites (7, 8) and is a convenient system in which to study biological fluorination. It is our objective to identify the biochemical steps associated with fluorometabolite production by isolating the enzymes on the biosynthetic pathway to these rare compounds.
By determining the incorporation of a stable isotopic label from various precursors into the fluorometabolites, we have attempted to shed light on the biosynthetic pathway and the identity of the carbon substrate involved in the fluorination event (4). The precursors studied showed very similar incorporation into fluoroacetate and C-3 and C-4 of 4-fluorothreonine, in terms of both magnitude and regiochemistry, indicating that a single fluorinating enzyme is present in S. cattleya. Most recently (6), fluoroacetaldehyde has been identified as the common fluorinated precursor of fluoroacetate and 4-fluorothreonine (Fig. 1). Fluoroacetaldehyde is clearly an unlikely metabolic intermediate, but isotopic labeling studies have confirmed its role in 4-fluorothreonine biosynthesis. Furthermore, fluoroacetaldehyde is efficiently converted to fluoroacetate in resting cell cultures of S. cattleya and in cell-free extracts when NAD+ is present.
FIG. 1.
Biotransformation of fluoroacetaldehyde to fluoroacetate and 4-fluorothreonine in S. cattleya.
We now report the isolation and characterization of the enzyme responsible for the oxidation of fluoroacetaldehyde in S. cattleya. Aldehyde dehydrogenases have been studied in a variety of organisms, and although there are reports of the oxidation of chloroacetaldehyde by these enzymes (3, 11), none has been shown to utilize fluoroacetaldehyde as a substrate.
Effect of culture age on fluoroacetaldehyde dehydrogenase activity.
Batch cultures of S. cattleya NRRL 8057 were grown in 500-ml conical flasks containing 90 ml of medium of the composition described by Reid et al. (7). Cells were harvested after periods of growth ranging from 2 to 6 days, and the resting cultures were incubated with 2 mM fluoroacetaldehyde, for 2 h. 19F nuclear magnetic resonance (19F-NMR) analysis of the supernatants demonstrated that fluoroacetaldehyde oxidation was only observed with cells that had been grown in batch culture for 5 or 6 days. Cell extracts from cultures of various ages were prepared by disrupting cells suspended in 100 mM potassium phosphate (pH 6.5) containing 1 mM dithiothreitol and 1 mM EDTA and cooled on ice with a French pressure cell. Cell debris was removed by centrifugation (48,000 × g for 20 min at 4°C).
Fluoroacetaldehyde dehydrogenase activity was assayed at 25°C by monitoring the increase in absorbance at 340 nm when enzyme (0.1 to 0.25 ml) was incubated with NAD+ (1 mM) and fluoroacetaldehyde (0.25 mM) in 200 mM Tris-HCl buffer (pH 9), in a final volume of 1 ml. For comparative purposes, the activity of an enzyme involved in primary metabolism, malate dehydrogenase, was also assayed by monitoring the decrease in absorbance at 340 nm when the cell extract (0.01 ml) was mixed with oxaloacetic acid (1 mM) and NADH (0.1 mM) (Worthington Enzyme Manual; Worthington Biochemical Corporation, Freehold, N.J.). Control experiments were conducted in the absence of fluoroacetaldehyde or oxaloacetic acid.
The results (Fig. 2) indicate that no fluoroacetaldehyde dehydrogenase activity was detected until day 4, just before the growth maximum and activity peaked at day 7, in the stationary phase. In contrast, malate dehydrogenase was detected in the lag phase, and its activity peaked at day 4. These observations are consistent with fluoroacetaldehyde dehydrogenase as an enzyme of secondary metabolism, and its expression coincides with the start of fluorometabolite biosynthesis (7).
FIG. 2.
Effect of culture age on fluoroacetaldehyde dehydrogenase (102) (●) and malate dehydrogenase (▴) activity. Error bars indicate standard deviation. The growth of the organism was monitored by measuring the optical density (OD) at 600 nm (×) of 0.2 ml of the batch culture in 3.8 ml of H2O.
Cell extract containing fluoroacetaldehyde dehydrogenase activity was applied to an anion-exchange column (DEAE-Sepharose Fast Flow and AKTA Prime) and eluted with a stepwise gradient of KCl (0 to 0.5 M) in 100 mM phosphate (pH 6.5) with 1 mM dithiothreitol and 1 mM EDTA. Activity eluted as a single peak, indicating that only one fluoroacetaldehyde dehydrogenase was present in the extract.
Purification of fluoroacetaldehyde dehydrogenase.
Solid (NH4)2SO4 was added to cell extract to 40% saturation, and after stirring for 20 min, the solution was centrifuged and the pellet was discarded. The supernatant was adjusted to 55% saturation with solid (NH4)2SO4, stirred for 20 min, and centrifuged, and the supernatant was discarded. The pellet was resuspended in 100 mM potassium phosphate with 1 mM dithiothreitol and 1 mM EDTA (5 ml), and the protein was eluted from a Hi-Trap desalting column using an AKTA Prime system (Pharmacia). Desalted protein (4 ml) was applied to an anion-exchange column (Pharmacia DEAE-Sepharose Fast Flow), and fluoroacetaldehyde dehydrogenase activity eluted with 0.25 M KCl. Fractions containing the highest activity were pooled and applied to a 5′-AMP–agarose column (1-ml bed volume), and the column was washed first with buffer and then with buffer containing 2 mM NAD+. The (NH4)2SO4 precipitation and the anion exchange were conducted at 4°C, and the affinity chromatography step was performed at room temperature. Protein content was determined using the Coomassie blue binding method (1). Using this procedure, the enzyme was purified 63-fold (Table 1; Fig. 3), but the overall yield was quite low. In the last step, the enzyme was purified almost 20-fold, but much of the activity did not bind to the column; hence, the yield was quite poor.
TABLE 1.
Purification of fluoroacetaldehyde dehydrogenase from S. cattleya
| Purification step | Vol (ml) | Total activity (U)a | Yield (%) | Protein (mg/ml) | Sp act (U/mg) |
|---|---|---|---|---|---|
| Cell extract | 28 | 1.44 | 100 | 3.73 | 0.014 |
| Desalted (NH4)2SO4 fraction | 9 | 0.75 | 52 | 2.61 | 0.032 |
| Anion-exchange | 10 | 0.35 | 24 | 0.71 | 0.049 |
| 5′-AMP–agarose | 4.5 | 0.04 | 3 | 0.01 | 0.889 |
One unit is defined as the amount of enzyme required to reduce 1 μmol of NAD+ min−1.
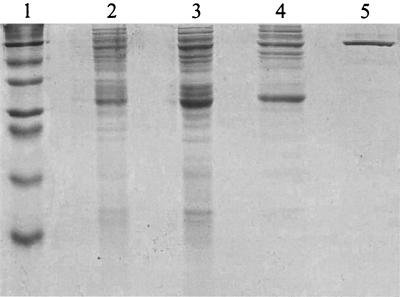
FIG. 3.
SDS-PAGE of fractions containing fluoroacetaldehyde dehydrogenase during purification. Lanes: 1, marker proteins (bovine albumin [66 kDa], egg albumin [44 kDa], glyceraldehyde-3-phosphate dehydrogenase [36 kDa], carbonic anhydrase [29 kDa], trypsinogen [24 kDa], trypsin inhibitor [20 kDa], and α-lactalbumin [14.2 kDa]); 2, crude extract; 3, (NH4)2SO4 fractionation; 4, anion exchange; 5, affinity chromatography.
Because of this, the enzyme from the anion-exchange step was used for characterization, unless otherwise stated. Only one aldehyde dehydrogenase was present in the anion-exchange fraction, since the relative rates of oxidation of fluoroacetaldehyde and other aldehydes (see below) were the same with this fraction as with the completely pure enzyme.
Biochemical properties of the enzyme.
A monomer of approximately 55 kDa was observed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Fig. 3), and passage of the native enzyme through a HiLoad 16/60 Superdex 200 gel filtration column (Pharmacia) indicated a molecular weight of approximately 200,000. Thus, the enzyme is probably a tetramer. The pH optimum was found to be 9 after the enzyme was assayed in morpholineethanesulfonic acid (MES), phosphate, Tris, and glycine buffers spanning a pH range of 6 to 10.5. The effect of temperature on the enzyme was investigated by conducting the assay over a range of temperatures from 25 to 55°C. The assay mixture without fluoroacetaldehyde was incubated for 10 min before the reaction was initiated, and the temperature optimum was determined to be 45°C. Enzyme activity was dramatically reduced when iodoacetamide (0.05 mM) or Cu2+ (1 mM) was present in the assay mixture, suggesting that there is an active-site thiol. Addition of EDTA (1 mM) increased enzyme activity, indicating that the enzyme may be sensitive to trace amounts of metal ions. The presence of Mg2+ (1 mM) resulted in an 80% loss of activity, possibly owing to a decreased dissociation rate of NADH from the enzyme (2).
The N-terminal amino acid sequence of the completely purified fluoroacetaldehyde dehydrogenase (determined using an Applied Biosyntems Procise 491 sequencing instrument) was found to be Thr-Val-His-Gln-Ala-Pro-Gly-Ser-Val-Ile-Ser-Leu-Arg-Pro-Pro-Tyr-Asp. A search of the Swall database using the FASTA program revealed homology with the N-terminal sequences (residues 1 to 30) of aldehyde dehydrogenases from Pseudomonas aeruginosa (9) and Deinococcus radiodurans (14), 50 and 52%, respectively. Thus, the fluoroacetaldehyde dehydrogenase in S. cattleya has similar properties to other aldehyde dehydrogenases, indicating that it is a variant of this class of enzyme.
Substrate specificity of fluoroacetaldehyde dehydrogenase.
The kinetic properties of the enzyme were determined by Lineweaver-Burk treatment of the data after the assay was conducted using a range of aldehyde concentrations (0.25 to 0.0125 mM for fluoroacetaldehyde and 1 to 0.05 mM for the others). Of the substrates tested, fluoroacetaldehyde and glycoaldehyde were most efficiently oxidized by the enzyme (Table 2). Acetaldehyde is a relatively poor substrate, with a Km that is 10-fold higher than that for fluoroacetaldehyde, indicating that electronic factors are more important for binding than steric properties. Interestingly, yeast aldehyde dehydrogenase (Sigma) also oxidizes fluoroacetaldehyde, but the Km is almost fourfold higher with this enzyme (0.31 mM). Chloroacetaldehyde also appears to be readily oxidized, but upon extended incubation of this substrate (0.25 mM) with the enzyme, the rate of reaction slowed dramatically, even though only a small amount of substrate was used up. Furthermore, when chloroacetaldehyde (0.25 mM) was incubated with the enzyme for 5 min prior to the addition of NAD+, the rate of oxidation was only 16% of that without preincubation. When this experiment was repeated with fluoroacetaldehyde, there was no difference in the rate of oxidation. Therefore, it is likely that chloroacetaldehyde inactivates the enzyme in a time-dependent fashion, possibly by alkylation of the active-site thiol after nucleophilic attack by the sulfur on the chloromethyl group, liberating chloride. This would not be expected to happen with fluoroacetaldehyde, as fluoride is a relatively poor leaving group.
TABLE 2.
Substrate specificity of fluoroacetaldehyde dehydrogenase from S. cattleya
| Substrate | Relative activity (%)a | Vmax (U/mg)b | Km (μM)b |
|---|---|---|---|
| Fluoroacetaldehyde | 100 | 0.143 | 80 |
| Glycoaldehyde | 150 | 0.206 | 150 |
| Chloroacetaldehyde | 80 | NDc | ND |
| Acetaldehyde | 40 | 0.129 | 810 |
| Propionaldehyde | 28 | ND | ND |
| dl-Glyceraldehyde | 10 | ND | ND |
| Benzaldehyde | 65 | 0.099 | 320 |
Activities expressed relative to the rate obtained with 0.25 mM fluoroacetaldehyde.
Values are the means of duplicate experiments; the variation around the mean was no more than 7%.
ND, not determined.
Secondary metabolic enzymes are derived by duplication and mutation of enzymes from primary metabolic pathways (12); hence, it is possible that the fluoroacetaldehyde dehydrogenase enzyme in S. cattleya evolved from an aldehyde dehydrogenase that utilized glycoaldehyde, or a similar compound, as the natural substrate.
As it is common for the genes coding for enzymes involved in the biosynthesis of secondary metabolites in bacteria to be clustered (13), it may now be conceivable to identify the cluster responsible for fluoroacetate biosynthesis by targeting the genes coding for this dehydrogenase.
Acknowledgments
This work was supported by the Biotechnological and Biological Sciences Research Council.
We thank David Harper (The Queen's University of Belfast) for helpful comments.
REFERENCES
- 1.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilising the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 2.Dickinson F M, Hart G J. Effects of Mg2+, Ca2+ and Mn2+ on sheep liver cytosolic aldehyde dehydrogenase. Biochem J. 1982;205:343–448. doi: 10.1042/bj2050443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eckfelt J H, Yonetani T. Isozymes of aldehyde dehydrogenase from horse liver. Methods Enzymol. 1982;89:474–479. doi: 10.1016/s0076-6879(82)89081-2. [DOI] [PubMed] [Google Scholar]
- 4.Hamilton J T G, Murphy C D, Amin M R, O'Hagan D, Harper D B. Exploring the biosynthetic origin of fluoroacetate and 4-fluorothreonine in Streptomyces cattleya. J Chem Soc Perkin Trans. 1998;1:759–767. [Google Scholar]
- 5.Harper D B, O'Hagan D. The fluorinated natural products. Nat Prod Rep. 1994;11:123–133. doi: 10.1039/np9941100123. [DOI] [PubMed] [Google Scholar]
- 6.Moss S J, Murphy C D, Hamilton J T G, McRoberts W C, O'Hagan D, Schaffrath C, Harper D B. Fluoroacetaldehyde: a precursor of both fluoroacetate and 4-fluorothreonine in Streptomyces cattleya. Chem Commun. 2000;2000:2281–2282. [Google Scholar]
- 7.Reid K A, Hamilton J T G, Bowden R D, O'Hagan D, Dasardhi L, Amin M R, Harper D B. Biosynthesis of fluorinated secondary metabolites by Streptomyces cattleya. Microbiology. 1995;141:1385–1393. doi: 10.1099/13500872-141-6-1385. [DOI] [PubMed] [Google Scholar]
- 8.Sanada M, Miyano T, Iwadare S, Williamson J M, Arison B H, Smith J L, Douglas A W, Liesch J M, Inamine E. Biosynthesis of fluorothreonine and fluoroacetic acid by the thienamycin producer Streptomyces cattleya. J Antiobiot. 1984;39:259–265. doi: 10.7164/antibiotics.39.259. [DOI] [PubMed] [Google Scholar]
- 9.Schobert M, Görisch H. Cytochrome c550 is an essential component of the quinoprotein ethanol oxidation system in Pseudomonas aeruginosa: cloning and sequencing of the genes encoding cytochrome c550 and an adjacent aldehyde dehydrogenase. Microbiology. 1999;145:471–481. doi: 10.1099/13500872-145-2-471. [DOI] [PubMed] [Google Scholar]
- 10.Takeuchi A, Uritani I. Partial purification and characterisation of aldehyde dehydrogenase from sweet potato roots. Agric Biol Chem. 1981;45:1753–1759. [Google Scholar]
- 11.Van der Ploeg J, Smidt M P, Landa A S, Janssen D B. Identification of chloroacetaldehyde dehydrogenase involved in 1,2-dichloroethane degradation. Appl Environ Microbiol. 1994;60:1599–1605. doi: 10.1128/aem.60.5.1599-1605.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vining L C. Roles of secondary metabolites from microbes. In: Chadwick D J, Whelan J, editors. Secondary metabolites: their function and evolution. Ciba Foundation Symposium. New York, N.Y: John Wiley and Sons; 1992. pp. 184–194. [DOI] [PubMed] [Google Scholar]
- 13.Vining L C. Functions of secondary metabolites. Annu Rev Microbiol. 1990;44:395–427. doi: 10.1146/annurev.mi.44.100190.002143. [DOI] [PubMed] [Google Scholar]
- 14.White O, Eisen J A, Heidelberg J F, Hickey E K, Peterson J D, Dodson R J, Haft D H, Gwinn M L, Nelson W C, Richardson D L, Moffat K S, Qin H Y, Jiang L X, Pamphile W, Crosby M, Shen M, Vamathevan J J, Lam P, McDonald L, Utterback T, Zalewski C, Makarova K S, Aravind L, Daly M J, Minton K W, Fleischmann R D, Ketchum K A, Nelson K E, Salzberg S, Smith H O, Venter J C, Fraser C M. Genome sequence of the radioresistant bacterium Deinococcus radiodurans R1. Science. 1999;286:1571–1577. doi: 10.1126/science.286.5444.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]