Abstract
A degradative bacterium, M6, was isolated and presumptively identified as Plesiomonas sp. strain M6 was able to hydrolyze methyl parathion to p-nitrophenol. A novel organophosphate hydrolase gene designated mpd was selected from its genomic library prepared by shotgun cloning. The nucleotide sequence of the mpd gene was determined. The gene could be effectively expressed in Esherichia coli.
Organophosphate pesticides are a group of highly toxic agricultural chemicals widely used in plant protection. Up to the present, organophosphate pesticides, such as parathion, methyl parathion, and methamidophos, are still extensively used worldwide despite their high toxicity. The microbial degradation of parathion has been intensively researched. Two parathion-degrading bacteria, Pseudomonas diminuta GM and Flavobacterium sp. strain ATCC 27551 (16, 22) have been isolated in the Philippines and the United States. Both of them possess parathion hydrolase activity, and the hydrolase from Flavobacterium sp. strain ATCC 27551 was purified (14). The opd genes encoding parathion hydrolases from P. diminuta GM and Flavobacterium sp. strain ATCC 27551 were cloned and sequenced (9, 11, 14, 21). The two genes show high homology (17). Another three bacteria that were able to degrade parathion which showed no homology on the protein level to the those found in Pseudomonas and Flavobacterium parathion hydrolases were isolated (15). Studies on the degradation of methyl parathion were also made. The pathway that P. putida uses to degrade methyl parathion was studied by Rani and Lalithakumari (19). They found that P. putida could hydrolyze methyl parathion and use p-nitrophenol as sole carbon source. However, no further research on the nucleic acid level was reported. Chaudhry and colleagues isolated a methyl parathion-degrading bacterium, a Pseudomonas sp. (3), which possessed DNA homologous to the parathion hydrolase gene from Flavobacterium sp. strain ATCC 27551, as revealed by Southern blot analysis of its total DNA against a PstI fragment containing the opd gene (3). Methyl parathion and parathion have similar chemical structures. So, it seems reasonable that the enzymes degrading these chemicals may have homology. Previous research indeed showed that some methyl parathion degrading-bacteria possess DNA homologous to the opd gene (3). Here, we report a novel gene from Plesiomonas sp. strain M6 that is absolutely different from opd genes encoding parathion hydrolases (Table 1).
TABLE 1.
Bacteria and plasmids used in the study
| Strains or plasmids | Relevant phenotype | Source or reference |
|---|---|---|
| E. coli DH5α | recA1 endA1 hsdR17 D lacU169(φ80lacZΔM15) | 20 |
| E. coli BL21 | hsdS gal(λimm434 cIts b2 red Dam Sam/λ) | 20 |
| M6M | M6 strain deficient in methyl parathion hydrolase | This work |
| pUC19 | Ampr | 20 |
| pET-29 | Ampr | Novagen |
| pMT1 | Ampr, pUC19 vector with 2.2-kb foreign fragment | This work |
| pMT2 | Ampr, pUC19 vector with 4.5-kb foreign fragment | This work |
| pETM | Ampr, pET-29 vector with 1.0-kb foreign fragment | This work |
A methyl parathion-degrading bacterial strain, M6, was isolated. M6 hydrolyzed methyl parathion to dimethyl phosphorothinate and p-nitrophenol but could not degrade p-nitrophenol further. However, it was able to use benzoic acid and phenylacetic acid as sole carbon and energy source. M6 is a rod-shaped gram-negative bacterium with polar flagella. It oxidized and fermented glucose to produce acid, but no gas formed. It could not use starch and molecular nitrogen as carbon and nitrogen sources. Catalase and oxidase reactions of M6 were positive. M6 was sensitive to vibriostatic agent O/129. It was presumptively identified as a Plesiomonas sp. based on its biochemical characteristics.
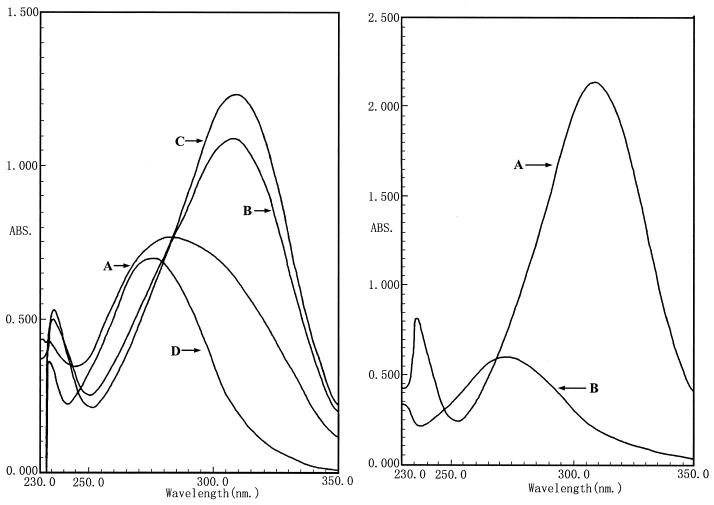
UV scanning was used to measure the hydrolysis of methyl parathion. The scanning results are shown in Fig. 1a. The results showed that M6 had strong methyl parathion-degrading ability. When 200 mg of methyl parathion/ml was added to the liquid medium, it was decomposed into p-nitrophenol in less than 15 min (Fig. 1). The maximum absorption peak of methyl parathion was recorded at 273 nm. A new absorption peak at 310 nm appeared, which was caused by the degradation end product, p-nitrophenol (with maximum absorption at 310 nm). The methyl parathion hydrolase of M6 was an endoenzyme, since no catalytic activity was observed in the supernatant (Fig. 1).
FIG. 1.
(Left) Determination of degradation of methyl parathion by strain M6 at different times by UV scanning. Curves: A, methyl parathion treated with M6 for 5 min; B, treated for 10 min; C, treated for 15 min; D, methyl parathion control. (Right) Localization of the methyl parathion hydrolase enzyme in the cell. Curves: A, methyl parathion treated with the M6 cells; B, methyl parathion treated with supernatant of the culture media. No hydrolase activity was measured in the supernatant.
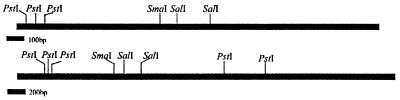
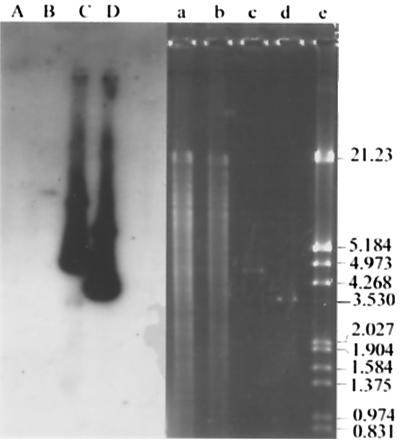
To clone the methyl parathion hydrolase gene, a gene library was constructed from genomic DNA of M6 by the shotgun technique. M6 genomic DNA was extracted by the method of high-salt-concentration precipitation (12). Sau3AI was employed to obtain randomly digested chromosomal fragments. The 2- to 4-kb fragments were recovered by a glassmilk purification kit (Biostar, Canada) and ligated into pUC19 digested with BamHI and treated with calf intestinal alkaline phosphatase (Promega). The ligation product was transformed into E. coli DH5α cells. The bacteria were then plated on Luria-Bertani medium containing 100 mg of methyl parathion/ml as indicator and incubated at 37°C for no more than 12 h. Two positives that produced yellow transparent halos were selected from the gene library. The recombinant plasmids were subsequently designated as pMT1 and pMT2. According to the physical maps of the two positive recombinant plasmids, there were 2.2- and 4.5-kb inserts, respectively, and the 2.2-kb fragment was included in the 4.5-kb fragment (Fig. 2). The cloned methyl parathion hydrolase gene was named mpd. To verify the origination of the inserts, a 1.4-kb SmaI fragment from pMT1 was used as probe to hybridize against pMT1, pMT2, HindIII-digested M6 chromosomal DNA, and HindIII-digested M6M chromosomal DNA. The probe was labeled by [α-32P]dCTP with a Takara random primer DNA labeling kit, version 2 (Takara). The activity of the labeled probe was nearly 109 dpm/μg of DNA. Hybridization result showed that the probe gave strong signals for pMT1 and pMT2 DNA, and a weak band also appeared on the track of M6 chromosomal (Fig. 3, lanes B, C, and D). The result verified that the inserts originated from strain M6 chromosomal DNA. The probe did not give a signal against M6M total DNA (Fig. 3, lane A). The results indicated that the hydrolase gene in M6M was deleted by unknown reasons.
FIG. 2.
Restriction endonuclease maps of the cloned fragments containing the mpd gene from pMT1 (above) and pMT2 (below).
FIG. 3.
Southern blot analysis of chromosomal DNA from M6 and M6M and recombinant plasmids pMT1 and pMT2 by using a 1.4-kb SmaI fragment as probe. Lanes A to D are the blots while restriction digests are shown in lanes a to d. Lanes: a and b, HindIII-digested M6M and M6 chromosomal DNA; c and d, recombinants plasmids pMT2 and pMT1; e, the marker, HindIII-digested lambda DNA, with molecular sizes of bands given in kilobases on the right.
The nucleotide sequence of the 2.2 kb of pMT1 was determined by primer extension. The sequencing primers were R-primer (5′-GAGCGGATAACAATTTCACACAGG-3′), NJS215P1 (5′-GCCACTGCCCAGTCACTT-3′), NJS215P3-02 (5′-AATCGGCTTCTTTCTGGTCC-3′) and NJS215P4 (5′-CGGTTCTTCAGGGGCATGGTGA-3′). Sequence was determined at Takara Biotechnology (Dalian) Co. Ltd. The complete length of the fragment was 2,191 bp.
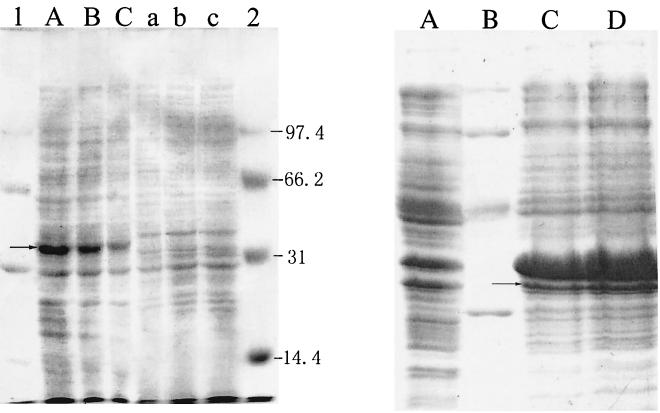
The mpd gene could be expressed in E. coli DH5α without induction of IPTG (isopropyl-β-d-thiogalactopyranoside) as shown in the process of gene cloning. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (9) analysis of the E. coli DH5α cells harboring mpd(pMT1) showed that a new protein band at about 35 kDa appeared compared with the control of harboring pUC19 vector (Fig. 4). The methyl parathion hydrolase activities of the crude cell-free extracts of E. coli DH5α, M6, and E. coli DH5α containing pMT1 were measured. Total protein of the crude extracts was detected by the Bradford method (1). The activity was expressed by the formation of p-nitrophenol in 1 min per microgram of protein. Catalytic activities (micromoles/microgram of protein/minute) (μmol/μg.protein/min) of the crude cell-free extracts were as follows: E. coli DH5α, 0; M6, 2.6 × 10−5; E. coli DH5α containing pMT1, 1.4 × 10−5; and E. coli BL21 containing pETM, 1.5 × 10−4.
FIG. 4.
(Left) SDS-PAGE gel displaying new protein band in E. coli DH5α cells containing pMT1. Lanes A to C, samples of E. coli DH5α containing plasmid pMT1; lanes a to c, samples of E. coli DH5α containing vector plasmid pUC19. Lanes A, B, and C were loaded with 20, 10, and 5 μl of loading sample, respectively. Lanes a, b, and c were treated the same as lanes A, B, and C. Lanes 1 and 2 were the protein molecular markers with molecular sizes of bands given in kilodaltons on the right. (Right) Expression of the amplified mpd gene in E. coli BL21. Lane A, 10 μl of sample of the control, E. coli BL21; lane B, protein molecular marker with the sizes 97.4, 66.2, 42.7, and 31.0 kDa (top to bottom); lanes C and D, samples of E. coli BL21 harboring pETM after inducing with IPTG for 4 h. Lanes C and D were loaded with 10- and 20-μl samples, respectively. Arrows showed the new protein bands expressed in the recombinant E. coli strains.
The sequence was analyzed with the software Bioedit (18). There were eight open reading frames (ORFs) that encoded proteins larger than 100 amino acids on the strand and an incomplete large ORF on the reverse. The eight ORFs started from positions 570, 654, 699, 723, 870, 1164, 1191, and 1323 and all ended at TGA (position 1718). Promoter prediction (http://www.fruitfly.org/seq_tools/promoter.html) revealed that there was a promoter-like sequence located at position 651 to 696. It had a typical E. coli promoter structure of TTGCAA-17 nucleotides-TATACT. There was a ribosome binding site (AGGA) 5 nucleotides upstream of the start code at 711 to 716. Considering that the regulation element of the mpd gene could be recognized by E. coli DH5α translation mechanisms and the molecular size of the gene product was about 35 kDa, it was deduced that the region ranging from 657 to 1718 was the complete sequence of the methyl parathion hydrolase gene. Primers 5′-GAATTCATATGCCCCTGAAGAAC-3′ (forward) and 5′-CGGAATTCTCACTTGGGGTTGAC-3′ (reverse) were synthesized on the basis of the nucleotides sequence of the 2.2-kb fragment. They were designed to amplify DNA containing the entire ORF of the mpd gene with an NdeI site at the 5′ end and an EcoRI site at the 3′ end. PCR was carried out with rTaq DNA polymerase (Takara). The reaction mixture contained 0.5 μM concentrations of each forward and reverse primer, 0.2 mM deoxynucleoside triphosphate, 2.0 mM MgSO4, 1× PCR buffer, 50 ng of pMT1 DNA, and 2.5 U of rTaq DNA polymerase per 50 μl. The mixture was subjected to 30 cycles of the following treatment: denaturation for 1 min at 94°C, annealing for 1 min at 52°C, and a final extension for 1 min at 72°C, with a final 5-min 72°C extension step after cycling was complete. The amplified DNA fragment of 1.0 kb was cloned into NdeI-BamHI sites of the vectors pET-29 (Novagen) to yield pETM. pETM was transformed into E. coli BL21 cells (Novagen). Recombinant harboring pETM showed remarkable expression of mpd by SDS-PAGE (Fig. 4). The molecular mass of the enzyme coincided with that of mpd expressed in DH5α cells.
The mpd nucleotide sequence and predicted protein sequence were compared with those in the GenBank database by an online alignment search (http://www.ncbi.nlm.nih.gov/BLAST/). No regions of extensive DNA homology could be detected. The predicted protein had 31% identity in a 307-amino-acid overlap with a hypothetical beta-lactamase SCJ21.1 (CAB52357) from Streptomyces coelicolor and also 27 to 29% identity with beta-lactamase (CAB39710 and CAB73577) from other sources, indicating that the hydrolase might be a member of the lactamase superfamily.
Biodegradation of organophosphate agents was widely studied for its high toxicity to the environment and animals (3, 9, 10, 18, 19, 22). Two organophosphate pesticides hydrolase genes (opd; GenBank accession numbers, M29593 and M20392) from P. diminuta GM and Flavobacterium sp. strain ATCC 27551 have been cloned and sequenced. The two genes shared 86.3% identity on the nucleic acid level at the coding region. Southern blot analysis of a methyl parathion-degrading strain of a Pseudomonas sp. against a α-32P-labeled 1.2-kb probe containing an opd gene from P. diminuta GM showed that its methyl parathion hydrolase gene had homology to the opd gene (3). The restriction map of the mpd gene we cloned was completely different from that of the opd gene. Sequence comparison also revealed that the sequence of the mpd gene shared no intensive homology with the opd genes. A comparison of the coding regions of the three sequences was made (data not shown).
Organophosphorus hydrolases play important roles in the decontamination of organophosphate pesticides and will be useful in the bioremediation of pollution caused by these pesticides (5). Recombination expression of these enzymes can supply enzyme products at low cost for environment protection.
Nucleotide sequence accession number.
The nucleotide sequence of the mpd gene has been assigned the GenBank accession number AF338729.
Acknowledgments
This work was granted by the 863 hi-tech research and development program of Ministry of Science and Technology of The People's Republic of China (project no. SZ0308).
We thank Zhou Junchu for providing experimental conditions and useful suggestion in the work. We are also grateful to Lu Zhaoxin of Nanjing Agricultural University and Gu Xiangyang of Hongkong Baptism University for helpful comments on the manuscript.
REFERENCES
- 1.Ausubel F M, Kinston R E, Brent R, et al. Short protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1995. [Google Scholar]
- 2.Chatterjee D K, Chakrabarty A M. Genetic homology between independently isolated chlorobenzoate-degradative plasmids. J Bacteriol. 1983;153:681–686. doi: 10.1128/jb.153.1.532-534.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaudhry G R, Ali A N, Wheeler W B. Isolation of a methyl parathion-degrading Pseudomonas sp. that possesses DNA homologous to the opd gene from a Flavobacterium sp. Appl Environ Microbiol. 1988;54:288–293. doi: 10.1128/aem.54.2.288-293.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng T-C, Harvey S P, Chen G L. Cloning and expression of a gene encoding a bacterial enzyme for decontamination of organophosphorus nerve agents and nucleotide sequence of the enzyme. Appl Environ Microbiol. 1996;62:1636–1641. doi: 10.1128/aem.62.5.1636-1641.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho T H, Wild J R, Donnelly K C. Utility of organophosphorus hydrolase for the remediation of mutagenicity of methyl parathion. Environ Toxicol Chem. 2000;19:2022–2028. [Google Scholar]
- 6.Ghosal D, You I S. Nucleotide homology and organization of chlorocatechol oxidation genes of plasmids pJP4 and pAC27. Mol Gen Genet. 1988;211:113–120. doi: 10.1007/BF00338401. [DOI] [PubMed] [Google Scholar]
- 7.Hall T A. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- 8.Harley C B, Reynolds R P. Analysis of E. coli promoter sequences. Nucleic Acids Res. 1987;15:2343–2361. doi: 10.1093/nar/15.5.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harper L L, McDaniel C S, Miller C E, Wild J R. Dissimilar plasmids isolated from Pseudomonas diminuta MG and a Flavobacterium sp. (ATCC 27551) contain identical opd genes. Appl Environ Microbiol. 1988;54:2586–2589. doi: 10.1128/aem.54.10.2586-2589.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayatsu M, Hirano M, Tokuda S. Involvement of two plasmids in fenitrothion degradation by Burkholderia sp. strain NF100. Appl Environ Microbiol. 2000;66:1737–1740. doi: 10.1128/aem.66.4.1737-1740.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McDaniel C S, Harper L L, Wild J R. Cloning and sequencing of a plasmid-borne gene (opd) encoding a phosphotriesterase. J Bacteriol. 1988;170:2306–2311. doi: 10.1128/jb.170.5.2306-2311.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller S A, Dykes D D, Polesky H F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mulbry W W, Kearney P C. Degradation of pesticides by microorganisms and the potential for genetic manipulation. Crop Protect. 1991;10:334–346. [Google Scholar]
- 14.Mulbry W W, Karns J S. Parathion hydrolase specified by the Flavobacterium opd gene: relationship between the gene and protein. J Bacteriol. 1989;171:6740–6746. doi: 10.1128/jb.171.12.6740-6746.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mulbry W W, Karns J S. Purification and characterization of three parathion hydrolases from gram-negative bacterial strains. Appl Environ Microbiol. 1989;55:289–293. doi: 10.1128/aem.55.2.289-293.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mulbry W W, Karns J S, Kearney P C, Nelson J O, McDaniel C S, Wild J R. Identification of a plasmid-borne parathion hydrolase gene from Flavobacterium sp. by Southern hybridization with opd from Pseudomonas diminuta. Appl Environ Microbiol. 1986;51:926–930. doi: 10.1128/aem.51.5.926-930.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mulbry W W, Kearney P C, Nelson J O, et al. Physical comparison of parathion hydrolase plasmids from Pseudomonas diminuta and Flavobacterium sp. Plasmid. 1987;18:173–177. doi: 10.1016/0147-619x(87)90046-1. [DOI] [PubMed] [Google Scholar]
- 18.Munnecke D M, Hsieh D P H. Microbial decontamination of parathion and p-nitrophenol in aqueous media. Appl Microbiol. 1974;28:212–217. doi: 10.1128/am.28.2.212-217.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rani N L, Lalithakumari D. Degradation of methyl parathion by Pseudomonas putida. Can J Microbiol. 1994;40:1000–1006. doi: 10.1139/m94-160. [DOI] [PubMed] [Google Scholar]
- 20.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 21.Serdar C M, Gibson D T. Enzymatic hydrolysis of organophosphates: cloning and expression of a parathion hydrolase gene from Pseudomonas diminuta. Bio/Technology. 1985;3:567–571. [Google Scholar]
- 22.Serdar C M, Gibson D T, Munnecke D M, Lancaster J H. Plasmid involvement in parathion hydrolysis by Pseudomonas diminuta. Appl Environ Microbiol. 1982;44:246–249. doi: 10.1128/aem.44.1.246-249.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]