Abstract
The insulin receptor (IR) is a transmembrane protein that is activated by ligands in insulin signaling pathways. The IR has been considered as a novel therapeutic target for clinical intervention, considering the overexpression of its protein and A-isoform in multiple cancers, Alzheimer’s disease, and Type 2 diabetes mellitus in humans. Meanwhile, it may also serve as a potential target in pest management due to its multiple physiological influences in insects. In this review, we provide an overview of the structural and molecular biology of the IR, functions of IRs in humans and insects, physiological and nonpeptide small molecule modulators of the IR, and the regulating mechanisms of the IR. Xenobiotic compounds and the corresponding insecticidal chemicals functioning on the IR are also discussed. This review is expected to provide useful information for a better understanding of human IR-related diseases, as well as to facilitate the development of novel small-molecule activators and inhibitors of the IR for use as medicines or pesticides.
Keywords: insulin receptor, function, agonists, antagonists, mechanism, medicine, pesticide
1. Introduction
The insulin receptor (IR) is a transmembrane protein and part of the tyrosine kinase receptors (RTK). It exists as covalently bound receptor dimers at the cell surface [1]. The IR plays essential roles in metabolism, cell growth, and development by transmitting the binding of extracellular ligands into several intracellular signaling cascades [2,3,4]. Previous studies have demonstrated that ligands and the insulin signaling IR are highly conserved among human beings and insects [5,6,7].
In human beings, the function of the IR has been studied for many years, and it has been found to play a crucial role in multiple chronic diseases, including Alzheimer’s disease (AD) [8], Type 2 diabetes mellitus (T2DM) [9,10], and various cancers [2,11,12,13], as well as neurodegenerative disorders [14] and metabolic syndromes [15]. For T2DM, the destruction and dysfunction of pancreatic β-cells are common occurrences, and insulin injection is the only choice for glycemic control [16]. The dramatic increase in T2DM over the globe has led to increasing requirements for insulin. Moreover, insulin injection may require more than one shot each day, is hazardous and inconvenient, causes tissue irritation, abscesses, discomfort, etc., and local allergic reactions, lipoatrophy, lipohypertrophy, etc., are common complications of subcutaneous injections [17,18]. Because of the multiple problems associated with insulin injection, orally active insulin-mimetic compounds would be an ideal substitute [19]. For cancer, IR makes an attractive anticancer target owing to its overexpression in a variety of cancers, especially prostate and breast cancers [20]. Therefore, regulators of the IR, such as β-site amyloid precursor protein cleaving enzyme 1 (BACE1), have been regarded as potential therapeutic target [20,21]. Similarly, IR modulators such as ceritinib and anti-idiotypic antibody AK98 (an off-target IR inhibitor) have been suggested as promising drugs for the treatment of brain tumors and breast cancer, respectively [22,23].
In insects, current evidence points to the roles of the IR in regulating development, reproduction, lifespan, caste differentiation, and wing polyphenism [24,25,26]. To our knowledge, neonicotinoid insecticides (e.g., imidacloprid) are selective agonists of the nicotinic acetylcholine receptor (nAChR) that have been widely used to control various insects [27]. Likewise, ryanodine and diamides are commercial insecticides that are antagonists or activators of insect ryanodine receptors (RyRs) [28]. It may be deduced that modulators of the IR that selectively activate or inhibit the IR may be of considerable value in providing promising drugs for the control of human disease, or as insecticides for the control of insects [29]. In this regard, medicines specifically targeting the IR are diverse. However, IR-targeting insecticides are still lacking. Owing to the persistent use of traditional synthetic insecticides, insect resistance has become increasingly serious. Therefore, there is a growing need for new insecticides with new mechanisms of action. Thus IR-targeting insecticides represent an opportunity in the research and development of insecticides.
In this review, we summarize recent studies focused on the structure, conformation, and modulators of the IR in order to provide insight into the mechanisms of its activation and regulation, which is indispensable for understanding the functions of the IR in human and insect physiological processes, including development, differentiation, metabolism, and aging. This information may facilitate better understanding of IR regulation, ligand specificity, crosstalk, and signaling of IR homologues. We also aim to present the available information that may be useful for the discovery of novel medicines and insecticides.
2. Biology Studies of the IR
2.1. Molecular Structure of the IR
Biochemically, the IR is encoded by a single gene. The coding region of the IR gene has 22 exons and 21 introns [30]. The alternative splicing of exon 11 encodes a 12-amino-acid sequence at the C-terminus of the α-subunit of the IR gene during transcription, resulting in the formation of the isoforms IR-A and IR-B [31]. IR-B is a mature isoform due to the fact that it includes the 12-amino-acid sequence, while the fetal isoform IR-A does not [10,32]. Both isoforms are expressed in most of the cells associated with energy homeostasis, such as adipocytes, hepatocytes, myocytes, and placenta vascular endothelium; however, they present different functional features [10,33]. Several in vitro and in vivo studies have confirmed that the expression and response of the two isoforms are different in breast cancer and T2DM [11]. IR-B possesses important metabolic functions and is the dominant isoform [2]. Conversely, the less-differentiated isoform IR-A is principally expressed in cancer cells [32]. Activation of IR-A promotes the growth of the cancer cells [34].
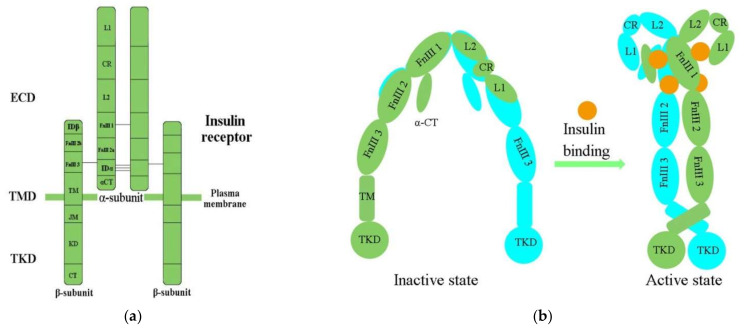
IR structural studies have previously been described in detail [35,36,37,38] (Table 1). The IR is a glycosylated, disulfide-linked (αβ)2 transmembrane homodimer consisting of two repeated ectodomains (ECD), a single transmembrane helix, and two intracellular cytoplasmic domain that includes a tyrosine kinase domain (TKD) (Figure 1a) [38,39]. The α-subunit constitutes most of the IR-ECD, while the β-subunit is necessary for the IR-ECD, the transmembrane domain (TMD), and the intracellular TKD [38].
Table 1.
Summary of the available structures of IR.
| Classification | Structure of IR | References |
|---|---|---|
| Domain layout | an (αβ)2 disulfide-linked homodimer | [35] |
| cDNA sequenced | α chain lies on the N-terminal of the β chain | |
| 3D structure of human apo IR ectodomain | intracellular unphosphorylated from TKD (2.1 Å resolution, PDB 1IRK) | |
| receptor’s isolated L1-CR-L2 module (2.32 Å resolution, PDB 2HR7) | ||
| intact receptor ectodomain in apo form (3.8 Å resolution, PDB 2DTG) | ||
| CryoEM structures of IR | insulin holoreceptor (full-length receptor inclusive of transmembrane and cytoplasmic elements) | [42] |
| isolated receptor ectodomain | [41,43] | |
| an ectodomain construct (leucine-zippered receptor ectodomain) | [44] |
Figure 1.
(a) The architectural domain of the IR (αβ) 2 homodimer. Black lines indicate the intersubunit disulfide bonds; (b) Inactive and active states of the IR; L1, L2, leucine-rich repeat domains 1, 2; CR, cysteine-rich domain; FnIII-1, 2, 3, fibronectin type-III domains 1, 2, 3; αCT, α C-terminal regions; TM, transmembrane; JM, juxtamembrane; KD, kinase domain; CT, C-terminal tail; ECD, ectodomain; TMD, transmembrane domain; TKD, tyrosine kinase domain.
Determination of the three-dimensional (3D) crystal structure of the insulin-free IR-ECD through crystallography has revealed that the IR-ECD dimer roughly displays an inverted “U”- or “V”-shaped architecture [37,40]. Specifically, L1 and CR together with L2 form one leg, while the linearly arranged FnIII domains form the other leg [37]. However, the modular organization of the ECD, with high intrinsic flexibility and its complex ligand-binding properties, poses a challenge for structural studies of the IR. Furthermore, single-particle cryo-electron microscopy (cryo-EM) has revealed that the IR-ECD dimer converts the overall architecture from an autoinhibited inverted “V” shape into a “T”-shaped conformation, which was stabilized after binding insulin molecules to the N-terminal domains (Figure 1b) [39,41,42]. The L1, CR, and L2 domains of both IR promoters constitute the “T” horizontal part, while the FnIII-1, -2, and -3 domains of the IR dimer constitute the vertical piece of the “T” [42].
Previous biochemical and mutagenesis models of insulin binding have identified two distinct binding sites on both the IR and insulin, termed site 1 (S1) and site 2 (S2) [36]. The L1 subdomain and the α-CT helix residue have been confirmed to represent IR S1 site (IR-S1) [39,45,46]. Evidence has indicated that IR-S1 is indispensable for insulin binding, and minor modifications of it were sufficient to change the IR’s specificity for insulin [47]. Scapin (2018) has defined the full S2 binding site [39], and Gutmann (2020) first observed the connection of insulin with discrete IR-S2 [43]. Studies have demonstrated that optimal IR activation requires multiple insulin molecules bound to S1 and S2 [48,49]. A similar result has also been presented in a study of the cryo-EM structure of the IR–insulin complex at 3.2 Å resolution [42]. The binding of insulin to S1 of apo-IR could release the autoinhibited conformation, which was an essential step for IR activation, while binding to S2 was important for the IR to adopt the active T-shape [50,51,52]. Cryo-EM analysis of the insulin–IR complex has revealed that insulin binds independently to the site of S2 between the FnIII-1 and FnIII-2 domains [43]. Another study has shown that the fibronectin domain is folded inwards, in a pincer-like fashion, which brings domains FnIII-3 and FnIII-3′ into contact [44].
The cryo-EM structure of the full-length human IR–insulin complex (human HEK293F cells) in the active state at an overall resolution of 3.2 Å unexpectedly revealed that a maximum of four insulin molecules can bind to the “T”-shaped IR dimer at four distinct sites [42]. Furthermore, at least one insulin molecule bound to two S2s and a maximum of four insulin molecules at four sites are required to form the “T”-shaped dimer [39,42,52]. Insulin 1 mainly binds to the primary site formed by the L1 domain, and α-CT then makes contact with a loop of the FnIII-1 domain from the IR promoter that donates α-CT [42]. During IR activation, a tripartite interface between insulin 1 and site 1 stabilizes the active IR dimer. Insulin 2 binds to a novel binding site on the FnIII-1 domain, located on the backside of the β sheet [42].
However, there is still a lack of detailed analysis of which site is connected first and how the first and second insulin binding results in different phosphorylation status of the IR [52]. The reported findings have emphasized the importance of the conformational changes of the IR-ECD and IR–insulin complex in the insulin/insulin-like growth factor signaling (IIS) pathway. Hence, the precise mechanism of how insulin binds to the IR at first remains elusive, and further research is still needed.
2.2. Activation of the IR
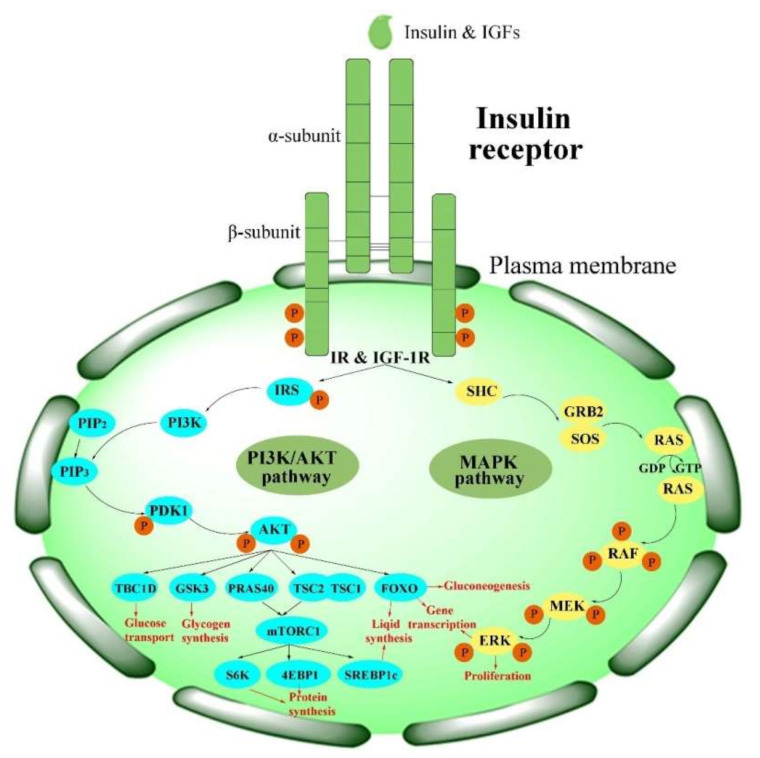
Physiologically, the function of the IR is activated in the insulin/IGF-1-like signal (IIS) pathway by the ligand [2]. The IIS pathway is commonly known as a significant nutrient-dependent endocrine pathway and regulates numerous physiological processes, such as metabolism, growth and development, and so on [6]. In the IIS pathway, the IR regulates two primary cell-signaling cascades (Figure 2) [53]: the phosphatidylinositol-3-kinase (PI3K)/AKT signaling pathway and the mitogen-activated protein kinase (MAPK) pathway (extracellular-signal regulated kinase signaling pathway (ERK)) [53,54,55,56]. The PI3K/AKT pathway is primarily responsible for controlling metabolic processes such as glucose transportation and the synthesis of lipids, proteins, and glycogen. In contrast, the MAPK pathway is primarily related to the mitogenic effects of insulin and is mainly responsible for cell growth and proliferation [56,57].
Figure 2.
Activation of the IR in insulin signaling pathways. PI3K/AKT pathways: phosphatidylinositol-3-kinase signaling pathways; MAPK pathway: mitogen-activated protein kinase pathway.
The major upstream factors of the IIS pathway are various insulin-like peptides (ILPs). Based on primary structure and receptor binding preferences, these ILPs can be subdivided into insulin, insulin-related growth factors (IGFs, including IGF-I and IGF-II) in mammals, and ILPs in insects [58]. Insulin is a peptide hormone secreted by pancreas β-cells and is one of the most conserved molecules in animals [59,60]. IGFs are peptides that have a homology of 40–80% with insulin. In humans, both insulin and IGFs can bind to the IR on the cell surface and functionally mediate cellular proliferation and differentiation, lipid metabolism, glucose homeostasis, and DNA synthesis [60,61]. Meanwhile, in insects, ILPs are the most general growth-promotion signaling factors [62,63,64,65], and evidence has suggested that ILPs are homologues of human insulin [6,66]. Insulin is the major regulatory factor in humans, but various ILPs have been identified in different insect species, ranging from one—in the Nevada dampwood termite, Zootermopsis nevadensis (Hagen)—to more than 40—in the silkworm, Bombyx mori L. [60].
ILPs first phosphorylate the IR and then activate IR signaling. The tyrosine-phosphorylated IR, in return, recruits and phosphorylates other intracellular adaptor proteins, such as IR substrate (IRS) proteins and several other substrates, including Src homology 2 domain-containing (SHC), Grb2-associated binder (GAB), APS (SHB2), and Cbl, at several tyrosine residues [56,67]. There are six isoforms (IRS1–6) in the IRS family [68]; among these, IRS1 and IRS2 are the main isoforms [68,69]. These proteins mediate the association with the Src homology 2 (SH2) domains and lead to initiation of the PI3K/AKT pathway, as well as activation of the downstream phosphoinositide-dependent kinase (PDK1) and protein kinase B (PKB, also called AKT) [4]. Phosphorylation of the IR triggers the activation of cellular signaling pathways, which play different roles in human beings and insects.
3. Functions of the IR
3.1. The Functions of the IR in Human Beings
In humans, the IR plays a crucial role in whole-body nutrient homeostasis and in various diseases, such as AD [8], T2DM [4,9,10], obesity [70], atherosclerosis [31], multiple cancers [11,12,13], and cardiovascular disease [71], as well as neurodegenerative disorders [14], metabolic syndrome [15] and polycystic ovary syndrome [72]. Thus, it is necessary to understand the cellular expression and the functions of the IR in order to propose new treatment concepts and to develop novel drugs.
The IR mediates whole-body nutrient homeostasis and is expressed ubiquitously through the classic insulin-responsive targets in the liver, muscle, and adipose tissue [3]. Recent work has demonstrated that the IR is distributed in both dendritic shafts and spines in living hippocampal brain neurons [73]. Knockout of the IR resulted in many impaired target organs. Hepatic deletion of the IR led to hyperglycemia, disorders in fatty acid metabolism, and an increase in the expression of fatty acid oxidation enzymes [74]. In mucosal epithelial cells, the IR interacts with the voltage-dependent anion channel-1 (VDAC1) in mitochondria. Knockdown of the IR gene triggered robust mitochondrial fragmentation and altered polarization [75], while knockout of the β-cell IR gene led to impaired insulin secretion [76]. Additionally, missense mutations of the IR may cause severe inherited insulin resistance syndromes [77].
The IR is a cell-surface receptor translocating to the nucleus, and is associated strongly with RNA polymerase II in the chromatin [78]. In the cell, host cell factor-1 (HCF-1) acts as a transcriptional coregulator functionally mediating the binding of the IR to specific sites located in the gene promoters [79]. HCF-1 mediates the association between the IR and DNA. HCF-1 binds to DNA indirectly through DNA sequence-specific transcription factors, and then forms a complex with the IR and Thanatos-associated protein domain-containing protein 11 (THAP11) in the chromatin. Knockdown of HCF-1 can inhibit the binding ability of the IR to the promotors [79]. Another study indicated that the mRNA and protein levels of the IR were obviously reduced in the subcutaneous and visceral adipose tissue of women with gestational diabetes mellitus (GDMs) [80]. The decrease in IR mRNA was accompanied by a decrease in methylation levels of the IR promoter [80]. This phenomenon has also been observed in the hypothalamus [81]. The methylation degree of the IR nuclear factor I (IRNF-I) binding site within the IR promoter was dramatically inversely correlated with the gene level of the IR. These findings have opened a new avenue for further studies on the functions and mechanisms of the IR. More studies focusing on demonstrating whether epigenetic modifications in the IR sequence impact IR expression of the IR are needed [82].
3.2. The Functions of the IR in Insects
Studies of the IR in human beings have raised interest in the functions of the IR in insects and the consequent possibility for the development of new IR-targeting insecticides with high efficiency and low toxicity.
In insects, multiple functions of the IR have been revealed [83]. The IR is well-known to be implicated—either directly or by crosstalk with other major hormones such as juvenile hormone (JH) and ecdysteroids (especially 20-hydroxyecdysone, 20E)—in post-embryonic development [84,85], nutrition-based phenotypic plasticity and body size control [86,87], reproduction and diapause [55], and circadian rhythmicity and behaviors [88,89,90]. The IR is also indispensable in insect photoperiodism, lifespan, and aging due to its relation to metabolism and growth [25,91,92,93]. Overall, studies have indicated that the IR is indispensable in insect growth [94], development and reproduction [95,96], polymorphism [24], lifespan [97], and oviposition [98]. Therefore, the IR represents an important target for the management of pests and parasites.
3.2.1. Gene Organization of the IR in Insects
The first invertebrate IR was purified from the extracts of adult Drosophila melanogaster based on its binding to radiolabeled insulin [99]. Different from the IR in human beings, the IR presents various types in insects (named InR). For example, only one type of InR has been reported in insects such as the fruit fly Drosophila melanogaster and the silkworm Bombyx mori, while evidence has also indicated that two types of InRs (InR1 and InR2) exist in other insects, such as the honeybee Apis mellifera and the brown planthopper Nilaparvata lugens. Interestingly, three InRs have been discovered in blattodeans, termites, and the firebug, Pyrrhocoris apterus [92,100]. In termites, InR1 and InR3 levels remained stable, while InR2 was differentially expressed between workers and reproductive females [100]. This indicates that the InR2 level is closely related to caste. However, in German cockroaches, there was no difference in the expression of InR2 between larvae and adult females [100].
The IR gene of Drosophila (DInR) has been used as a model for the study of the IR. The DInR is secreted from neurosecretory cells in the brain [29,60] and other tissues, such as the midgut, fat body, and imaginal discs [6]. The DInR exhibits high levels in the central nervous systems of larvae and adult fruit flies. It is necessary for the formation of aversive olfactory learning, associative long-term memory, and intermediate-term memory in aged flies [101,102]. Unlike previously published data, recent evidence has indicated that the DInR in the mushroom body Kenyon cells suppress the formation of anesthesia-resistant memory and stimulated the formation of a longer-lasting memory in larval Drosophila [90]. The DInR mediates and encodes eight insulin-like peptides (DILPs 1–8) [103,104,105]. Among these DILPs, DILPs 2, 3, and 5 are expressed in insulin-producing cells (IPCs), then released into the hemolymph [104]. DILP 6 is structurally and functionally similar to IGF, while DILP 8 is a homolog of relaxin. The overexpression of any of DILPs 1–8 led to an increase in larval size [6]. The interaction of DILPs with the IR is highly conserved through the complex and versatile IIS pathway.
3.2.2. The Effect of Silencing or Knockdown of IR Genes in Insects
The silencing of IR genes produces a variety of phenotypes in insects. In the incomplete metamorphosis brown citrus aphid, Aphis citricidus (Kirkaldy), two IR genes were observed, AcInR1 and AcInR [106]. AcInR1 increased during the transition from nymph to adult in alate aphids, while AcInR2 had the highest expression level in second instar nymphs. The silencing of AcInR1 or AcInR2 by RNAi resulted in 73% or 60%, respectively, of aphids having problems in the transition from nymph to a normal adult. The co-silencing of AcInR1 and AcInR2 genes led to 87% of aphids having problems in the transition from nymph to normal adult and 62% dead nymphs. Therefore, AcInR1 and AcInR2 are essential for a successful nymph–adult transition in alate aphids. In the complete metamorphosis insect red flour beetle, Tribolium castaneum Herbst, two IR genes have also been observed, TcInR1 and TcInR2. TcInR1 presented a high level in the late adult stage and the early pupal stage, whereas TcInR2 was strongly expressed during the late larval stage. The silencing of TcInR1 led to a death rate of 100%, while the silencing of TcInR2 caused a death rate of 42% in larval and parental T. castaneum [95]. For the legume pod borer Maruca vitrata (Fabricius), InR-silenced insects were inhibited at the larval–pupal stage and then died [107].
The silencing of IR genes might relate to the different development stages in insects. However, knockdown of the corresponding IR genes led to various results in different insects. In T. castaneum, the knockdown of TcInRs reduced food consumption and decreased larval weight and size [94]. In the rhinoceros beetle Trypoxylus dichotomus L., knockdown of the IR gene caused a dramatic reduction in the length of the adult horn, with a slight reduction in wing and genital size [86]. In female mosquitoes, such as Aedes aegypti (Linnaeus) [108], Culex quinquefasciatus Say [109], Culex pipiens Linnaeus [110], and Anopheles gambiae Giles [111], knockdown of the IR gene led to smaller ovaries and marked reductions in oviposition. In the female blood-feeding bug Rhodnius prolixus Stål, knockdown of RhoprInR also decreased the formation of eggs [112]. The impairment of IR genes also resulted in negative impacts on the fecundity, immune response, and blood digestion of A. aegypti [108,112].
3.2.3. Polyphenism Is Adjusted by the IR in Insects
Polyphenism is a form of developmental plasticity, and is a successful strategy adopted by organisms to adapt to changing environments [113]. Examples in insects are caste differentiation in eusocial insects, wing polyphenism in planthoppers and aphids, sexual dimorphism in the brown planthopper and fruit fly, and seasonal polyphenism of butterflies [114]. Evidence indicates that insect polyphenism is connected to the multiplicity of the IRs.
Caste differentiation: Social insects, such as bees and ants, are ideal models for investigating caste differentiation mechanisms due to the intraspecific variations in their sexual dimorphism [115]. In the honeybee Apis mellifera and the fire ant Solenopsis invicta, the IR has been shown to regulate caste differentiation [116,117,118]. In critical stages of caste development of the honeybee, the IR genes AmInR-1 and AmInR-2 are primarily expressed in the second instar of queen larvae and sharply declined in the third and fourth instars of queen larvae, while little change is observed in worker bees [117]. AmInR-2 had the highest level in the queen larvae after hatching [116,119]. Similarly, in fire ants, the two SiInRs were differently expressed in the early development of the queen and worker larvae ants [120]. The expression of SiInR-1 in eggs was the highest except for the fourth instar larvae of workers, virgin queens, and males, while the expression of SiInR-2 in eggs was significantly higher than that in other stages [118]. Expression of both of the corresponding receptors was significantly higher in virgin queens and males compared to that in adult workers.
Caste differentiation in cockroaches and termites has been related to InR2 and InR1, while InR3 had no impact [100]. Target of rapamycin (TOR), epidermal growth factor receptor (Egfr), juvenile hormone (JH), and vitellogenin (Vg) are also involved in insect caste determination [120]. In queen predetermined larvae, the knockdown of genes of the IRs, TOR, and Egfr resulted in more workers [121,122].
Wing polyphenism: Wing polyphenism is an evolutionarily successful feature that enables insects to adapt to environmental changes [123]. This phenomenon is commonly observed in a wide range of wing polymorphic insects, such as the brown planthopper Nilaparvata lugens (Stal) and the pea aphid Acyrthosiphon pisum (Harris) [106,123]. The interactions between the IIS pathway, ecdysone, and JH signaling are involved in wing dimorphism [124]. The brown planthopper has been well-studied due to its incomplete metamorphosis leading to two wing morphs: long wing (LW) and short wing (SW) [124,125,126,127,128]. The InRs (InR1 and InR2) play antagonistic roles to determine long versus short wing development by regulating forkhead transcription factor subgroup O (FoxO) activity [123,125]. Naturally, the activation of InR1 induces the formation of the long wing through the PI3K–AKT–FoxO signaling cascade, while a high level of InR2 in wing buds leads to short wings [127]. The decapentaplegic (dpp) gene can respond to the switch genes (NlInR1 and NlInR2) and participates in wing morph development through a dose-dependent response [126]. Unlike the brown planthopper, three InRs have been found in the firebug Pyrrhocoris apterus (Linnaeus). Two gene clusters are involved in the wing polyphenism of the firebug. It has been observed that a pattern of Cluster I and II of the InRs impacts wing development, in contrast to that postulated in planthoppers, suggesting independent establishment of IIS in the control of wing development [92].
Sexual dimorphism: Recent research has shown that knockdown of the sex determination gene Transformer-2 in adult N. lugens led to long wing female offspring [129]. This suggests the existence of crosstalk between sex differentiation and wing dimorphism during embryonic stages. For the synthesis of JH, the corpus allatum (ca) is the key gland, while 3-hydroxy-3-methylglutargyl CoA reductase (HMGCR) is also an important enzyme. The InR in the ca and the enzyme HMGCR have been implicated in the control of sexual dimorphism in Drosophila [130,131]. The knockdown of the InR gene in the ca oppressed the gene encoding of HMGCR, resulting in interruption of sexual differentiation and the emergence of dwarf flies [131].
In brief, the InR gene plays important roles in regulating caste differentiation, wing polyphenism, and sexual dimorphism in insects, and thus is significant for future studies on the inhibition, knockdown, or modulation of the InR in insects.
4. Pharmacological and Physiological Modulators of IR Activation
The IR has recently made its mark as an attractive therapeutic target for a variety of cancers and diabetes in humans due to its overexpression in various cancers [13,32]. Thus, agonists and antagonists of the IR that selectively activate or inhibit the IIS pathway may have considerable value in providing promising drugs for human diseases [4,132]. However, in contrast to the diverse regulators of insulin, only a handful of modulators have been discovered for the IR [133]. In the following, we provide a summary of existing IR modulators in order to provide an enriched yet challenging prospect for novel therapeutic approaches.
4.1. Insulin and Its Analogs
As the upstream regulator of the IR, insulin has been studied for more than 100 years since first being reported in 1921. Insulin has an autocrine/paracrine regulatory role on its own signaling system. Binding of insulin to the activated IR leads to negative cooperativity and pleiotropic effects on the IIS pathway [134]. Insulin analogs can be classified as short- or long-acting based on their pharmacokinetics and general principle of protein folding and assembly [32] (Table 2).
Table 2.
Pharmacological and physiological modulators of IR activation.
| Classification | Modulators | IR Modulation Mechanism | Model Organisms or Cells | Side Effects | References |
|---|---|---|---|---|---|
| Insulin and insulin analogs | insulin and IGFs, |
ligand-induced internalization and degradation of the IR | human | tissue irritation, abscesses, allergic edema, weight gain, risk of congestive heart failure | [3] |
| lispro, | [32,135] | ||||
| aspart, | |||||
| glulisine, | |||||
| aspb10, | |||||
| detemir, | |||||
| largine, | |||||
| degludec, | |||||
| ILPs | ligand | insects | - | [103] | |
| Insulin-mimetic peptides | S371, S446 | disrupts the primary insulin binding site of the IR | mice | - | [138] |
| - | |||||
| S519(agonist) | - | ||||
| S597 (partial agonist) |
receptor activation | IR-transfected L6 myoblasts | - | [138,177] | |
| S661 | antagonist of the IR | rat adipocytes | - | [140] | |
| S961(agonist/ antagonist) |
↓IR, blocks expression of the IR without insulin | breast cancer cells | - | [141,178] | |
| Antibodies | XMetA (partial agonist) | ↑IR autophosphorylation (EC50:1.3 nmol/L); ↑Akt phosphorylation (EC50: 1.1 nmol/L) |
CHO-hINSR cells (in vitro); diabetic mice (in vivo) | - | [144,145] |
| XmetS (agonist) | ↑binding affinity with IR; ↑IR autophosphorylation (insulin-dependent); ↑Akt phosphorylation |
MCF-7 human breast cancer (in vitro); mouse models of insulin-resistant diabetes (in vivo) | - | [146] | |
| XmetD (X358) (antagonist) |
↓autophosphorylation of IR (interacte with IR); ↓phosphorylation of Akt and Erk |
adult male CHO-hINSR cells; L6 muscle cells; COLO-205 human colon cancer cells; hyperinsulinemic hypoglycemia mice | - | [147] | |
| healthy adult | insulin resistance (3 d wherein X358-imparted) | [148] | |||
| IRAB-A (agonist/sensitizer) | ↓off-rate of insulin from the IR (stabilizes insulin binding) | diet-induced obese C57 mice | - | [132] | |
| IRAB-B (antagonist) | ↓IR phosphorylation (binds to IR) | C57BL/6N mice | - | [149] | |
| AK98 (antagonist) | competes with insulin (bind to IR) ↓IR expression levels | tumor cell (MCF-7) | - | [23] | |
| Aptamers | IR-A48 (partial agonist) (IR Tyr1150), | ↑IR autophosphorylation (allosteric binds and activates the IR, but not IGF-1R) | HEK293 and 3T3-L1 cells; Rat-1 cells overexpressing human IR (Rat-1/hIR) | - | [152] |
| IR-A43 (sensitizer) | binds to the allosteric site of IR; ↑insulin bind to IR |
- | - | [153] | |
| IR-A62 (agonist and activator) | ↑insulin binding and Y1150; monophosphorylation of the IR (low concentrations); ↓insulin binding and IR phosphorylation (high concentrations) |
C57BL/6 mice; Rat-1 cells overexpressing human IR (Rat-1/hIR); 3T3-L1 and MCF-7 breast cancer cells |
- | [154] | |
| GL56 (inhibitor) | specifically recognizes the IR; ↓IR phosphorylation; ↓phosphorylation of AKT, ERK1/2 and IRS1 |
U87MG; glioblastoma cancer cells |
- | [155] | |
| Proteins | GRB10/14, | ↓activity of the IR as a pseudosubstrate of the IR-TK | mice | - | [157] |
| SOCS1/3, | mice | - | [3] | ||
| GRP78 (IGF-1R) | ↑IGF-1R phosphorylation and activation | hepatoma cells | - | [158] | |
| SH2B1 | ↑IR and IRS1 phosphorylation;↑Akt and Erk activation | CHO–IR, 3T3L1, NIH3T3, and HEK293 cells; mice | - | [157] | |
| SORLA | ↑IR surface expression (redirects internalized IR from endosomes to PM) | mouse with loss of function/tissue -specific over- expression of SORLA; obese human subjects |
- | [70] | |
| Cav-2α | ↑IRS-1 recruitment and association with IR (a substrate of IR tyrosine kinase) | Hirc-B cells, HEK293T cells, 3T3L1 preadipocytes or adipocytes | - | [161] | |
| Cav-2β | desensitization of the IR; ↑IR-TK inactivation via dephospho-rylation by PTP1B and internalization via dynamin- 2-dependent endocytosis |
HEK293T cells, 3T3-L1 preadipocytes (ATCC, CL-173) |
- | [162] | |
| ApoE | interacts with the IR, interfering with insulin binding; ↓insulin–IR interaction and impairs IR trafficking |
human ApoE -targeted replacement mice | - | [164,165] | |
| Others | Glypican-4 | interacts with the IR, causing ↑IR signaling |
visceral and subcutaneous adipose tissue/3T3-L1 preadipocytes | - | [168] |
| mcIRBP-9 | ↑IR kinase activity; ↑phosphorylation of IR; ↑translocation of GLUT4; ↑uptake of glucose |
3T3-L1 preadipocytes; type 1 diabetic mice; type 2 diabetic mice (db/db mice) | - | [169] | |
| Visfatin | binds to the IR site | clonal mouse pancreatic β-cell; β-TC6 cell line (BTC) cells |
- | [170] | |
| SMPDL3b | interferes with the IR isoforms binding to caveolin1 in the PM | podocytes in DKD | - | [171] | |
| PTP1B | dephosphorylates the IR, causing deactivation |
mice | novel therapeutic strategy for T2DM | [172] | |
| PKCε | phosphorylates the IR, blocking IR autophosphorylation |
InsrT1150A mice | improves NAFLD diagnostic screening for the early identification of patients at risk for T2D | [174] | |
| Aroclor 1254 | inhibits the expression of the IR | male C57BL/6 mice/skeletal muscle & liver | _ | [175] | |
| Subetta | increases IR β-subunit phosphorylation | human preadipocytes | _ | [176] | |
| BACE1 | cleaves the IR ECD and decreases the amount of mature IR | mouse models of diabetes (db/db) and impaired glucose tolerance (HFD mice) | _ | [21] |
ILPs: insulin-like peptides; GRB: growth factor receptor-bound protein; SOCS: suppressor of cytokine signaling; SH2B1: SH2 domain-containing adaptor protein; SORLA protein, sorting-related receptor with type A repeats; Cav-2α: caveolin-2α; Cav-2β: caveolin-2β; ApoE: apolipoprotein E; mcIRBP-9: 9-amino-acid-residue peptide; SMPDL3b, sphingomyelin phosphodiesterase acid-like 3b; PTP1B: protein-tyrosine phosphatase 1B; PKCε: protein kinase Cε; NAFLD: nonalcoholic fatty liver disease; BACE1, β-site amyloid precursor protein cleaving enzyme 1; PM, plasma membrane; Hirc-B, human IR-overexpressed rat 1 fibroblast cells; CNS: central nervous system; DKD, diabetic kidney disease; CHO, Chinese hamster ovary; InsrT1150A mice, C57BL/6J mice harboring a threonine-to-alanine mutation at the homologous residue Thr1150.
Short-acting insulin analogs, such as insulin lispro, aspart, glulisine, and AspB10, have a faster onset and shorter duration of action, while long-acting analogs, such as glargine, detemir, and degludec, have a more stable insulin action profile with longer duration than human insulin [135]. Both can interact with the IR and the insulin-like growth factor I receptor (IGF-1R), with varying binding affinities and dissociation rates [32]. Therefore, more and more speculations have been raised that insulin analogs could be used for the treatment of T2DM [136,137].
4.2. Insulin-Mimetic Peptides
Many insulin-mimetic peptides, such as Site 1 insulin-mimetic peptide S371, Site 2 peptide S446, single-chain Site 2–Site 1 optimized peptide S519, Site 2–Site 1 combination peptide S597 and S661, and Site 1–2 peptide S961, have been obtained to selectively function as agonists or antagonists to modulate the IR [138,139] (Table 2). S371 is part of the potent receptor S519, which exhibits agonist activity and competes with the αCT for binding to the L1 domain of the IR. S597 is a further optimized agonist peptide of S519, binding to the IR to adjust its phosphorylation. Furthermore, S661 and S961 are antagonists of the IR [140,141]. Interestingly, S961 is a special example, showing both agonist and antagonist effects on the human IR [140,142].
4.3. Antibodies
Antibodies are agonists of the IR. Many antibodies (non-insulin ligands) have been reported to modulate the activity and function of the IR [4] (Table 2). The first generation of IR autoantibodies was distinguished in the control of some human diseases [143]. They displayed confirmed selectivity, pathophysiological sensitivity, and more excellent safety than the traditional regulating agents [133].
XMetA is a fully human IgG2a monoclonal antibody that is a direct agonist of the IR [144,145]. XMetS is another allosteric IR agonist antibody that has little effect on the IR without the presence of insulin [146]. The combined use of insulin and XMetS modulated the insulin binding affinity and IR autophosphorylation more strongly and positively than insulin and IgG by nearly 18-fold and 14-fold, respectively [145]. In addition to these positive antibodies, many antibodies act as antagonists. XMetD (also known as X358 or XOMA 358) is a negative modulator [147]. XMetD reduced the insulin binding affinity about 3-fold and decreased the sensitivity of insulin-stimulated IR autophosphorylation about 40-fold, and was the first monoclonal antibody to be advanced into human clinical studies [147,148].
Hinke (2018) recently reported a novel allosteric agonist IR monoclonal antibody, IRAB-A, which exhibited sensitizing effects on IR and Akt phosphorylation based on cell assays [132]. Further, another novel antagonist antibody, IRAB-B, has been found, which can specifically bind to the IR with nanomolar affinity, and thus can induce rapid and continuous insulin resistance [149].
Interestingly, a new method (anti-idiotypic antibody strategy) by hybridoma technology for the development of IR antagonists has led to the anti-idiotypic antibody AK98. AK98 has exhibited good antagonistic activity against the IR in a tumor cell model. In particular, AK98 presented a dose-dependent effect in the inhibition of IR-mediated signaling pathways [23].
4.4. Allosteric Aptamers
Another type of selective allosteric activator of the IR is aptamers. Aptamers are single-strand oligonucleotides (DNA or RNA) from random oligonucleotide libraries [150]. They can specifically and strongly bind to the target with high affinity due to their unique 3D structures [151]. Studies have reported on various aptamers (Table 2), such as IR-A48 [152], IR-A43 [153], IR-A62 [154], and GL56 [155]. IR-A48 is an agonistic aptamer that binds to and then activates the IR [152]. IR-A43 is a sensitizing aptamer that effectively binds to the IR and enhances insulin sensitivity [153,156]. IR-A62 acts as a biased agonist that binds to the extracellular domain of the IR and preferentially induces Y1150 monophosphorylation of IR [154].
In contrast to IR-A48, IR-A43, and IR-A62, GL56 is a nuclease-resistant RNA aptamer that specifically recognizes the IR and acts as a neutralizing ligand to inhibit IR activity and IR-dependent signaling [155]. GL56 provides a novel avenue for future research on the clinical therapy of IR-dependent cancers.
4.5. Proteins
4.5.1. Adaptor Proteins and Regulatory Proteins
Some cytoplasmic adaptor proteins (Table 2), such as growth factor receptor-bound protein 10 (GRB10), growth factor receptor-bound protein 14 (GRB14), and suppressors of cytokine signaling proteins (SOCS; in particular, SOCS1 and SOCS3), can inhibit IR activity by acting as pseudosubstrates of IR kinase [157]. Further information can be found in the paper by Haeusler (2018) [3].
New proteins have been reported to regulate the IR, such as SH2 domain-containing adaptor protein (SH2B1). SH2B1 can directly bind to the IR and insulin receptor substrate (IRS), and enhances the catalytic activity of the IR and suppresses the dephosphorylation of the IR as an endogenous insulin sensitizer [157]. GRP78 (78 kDa glucose-regulated protein) is a multi-functional chaperone that promotes the phosphorylation and activation of IGF-1R. Thus, GRP78 inhibitors could inhibit IGF-1R signaling in hepatoma cells [158]. Additionally, the expression of the IR in target cells is also influenced by factors such as the sorting-related receptor with type A repeats (SORLA) protein, which acts as a sorting factor for the IR, enhancing its surface expression [70].
4.5.2. Membrane Proteins
Caveolins are lipid-raft-associated integral membrane proteins [159]. Proteins in the caveolin family are encoded by three genes and consist of six known caveolin subtypes: caveolin-1α, caveolin-1β, caveolin-2α, caveolin-2β, caveolin-2γ, and caveolin-3 [160]. Of the six known caveolins, caveolin-2α (Cav-2α) is a positive regulator of insulin signaling [161], while caveolin-2β (Cav-2β) is a negative regulator [162]. Cav-2α gathers the IR and initiates the IRS-1 signaling system [161,163]. Cav-2β desensitizes the IR through the dephosphorylation of protein-tyrosine phosphatase 1B (PTP1B), followed by IR endocytosis and lysosomal degradation, thus resulting in insulin resistance [162,163].
Apolipoprotein E (ApoE) is a glycoprotein consisting of 299 amino acids. ApoE has three isoforms; namely, E2, E3, and E4 [164]. Among the ApoE genes, the ε4 allele (apoE4) is the most substantial genetic risk factor for AD, compared with the ε2 allele (apoE2) and ε3 allele (apoE3). In primary neurons, ApoE4 specifically binds to the IR and reduces its transportation by trapping it in the endosomes [165]; hence, its binding negatively affects the IIS pathway and insulin-evoked mitochondrial respiration and glycolysis due to disruption of the interaction between insulin and the IR in an isoform-dependent manner [166,167].
4.6. Other Pharmacological and Physiological Modulators
In addition to the IR regulators mentioned above, some biomolecules, such as proteoglycan, peptides, enzymes, and some drug mixtures, also regulate the IR. In detail, glypican-4 (Gpc4) is a member of the glycosylphosphatidylinositol (GPI)-anchored heparan sulfate proteoglycan family and binds to the IR and potentiates the IIS pathway. Gpc4 also promotes adipocyte differentiation. Impairment of Gpc4 led to a reduction in IR activation and suppression of adipocyte differentiation in vitro [168].
The 9-amino-acid-residue peptide (mcIRBP-9) is a peptide from the bitter gourd Momordica charantia L. that targets the IR and enhances the activity of IR kinase [169]. Another peptide is visfatin, which binds to the IR at a site distinct from that of insulin and causes hypoglycemia by reducing glucose release from liver cells and stimulating glucose utilization in adipocytes and myocytes [170].
Sphingomyelin phosphodiesterase acid-like 3b (SMPDL3b) is a lipid-raft enzyme that impairs IR-B-dependent insulin signaling by interfering with IR binding to Cav-1 in the plasma membrane (PM) [171]. Protein tyrosine phosphatase 1B (PTP1B) and protein kinase Cε (PKCε) are also negatively involved in regulation of the IR. PTP1B dephosphorylates the IR, leading to its deactivation. Studies in PTP1B knockout mice have revealed improved insulin sensitivity and IR phosphorylation in muscle and liver [172,173]. PKCε impairs IR autophosphorylation by phosphorylating its substrate, Thr1160, in the functionally critical IR kinase activation loop [174].
Aroclor 1254 is a commercial polychlorinated biphenyl (PCB) mixture that inhibits the IR signaling pathway, including the IR, IRS, PI3K-AKT, and PKB, in skeletal muscle and the liver [175]. Subetta is a drug that releases active forms of antibodies to the IR β-subunit and endothelial nitric oxide synthase. Subetta increased phosphorylation of the β-subunit of IR with or without insulin in vitro [176].
4.7. Mechanism of the Pharmacological and Physiological Modulators of IR Activation
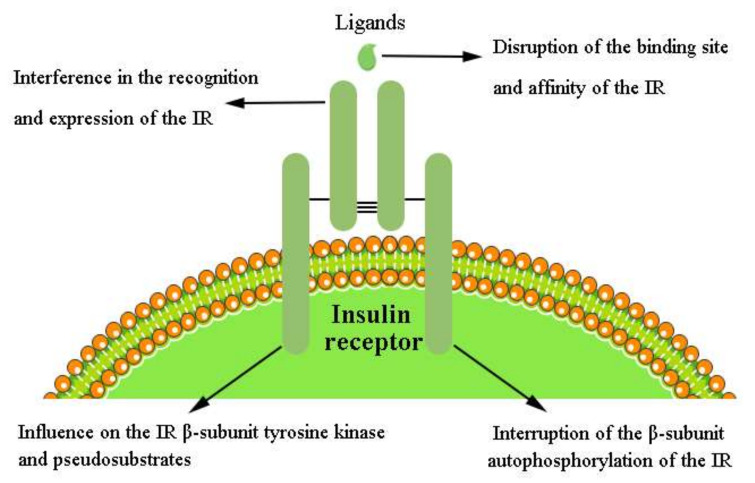
In conclusion, insulin and insulin analogs, insulin-mimetic peptides, monoclonal antibodies, aptamers, proteins, proteoglycan, peptides, enzymes, and some drug mixtures are the main pharmacological and physiological modulators of the IR (Table 2). In vitro evidence has indicated that the IR is regulated through diverse and complex mechanisms. Herein, we classify these mechanisms into four main types [68] (Figure 3):
Figure 3.
Modulation mechanism of pharmacological and physiological modulators of IR activation.
4.7.1. Disruption of the Binding Site and Affinity of the IR
The IR is a heavily glycosylated membrane-spanning protein. Therefore, the insulin binding site and affinity are crucial for modulation of the IR [146]. Disruption of the primary binding site of the IR is a prominent positive modulation [177]. In contrast, the primary negative modulation of IR is through interference with the interaction of insulin and the IR (e.g., ApoE).
4.7.2. Interference in the Recognition and Expression of the IR in the IIS Pathway
Normally, insulin binds to the IR and, in turn, activates the insulin-induced (IIS) pathway. Therefore, the ligand itself is undoubtedly the crucial negative regulator of its signaling [179]. Adjustment of the recognition and expression of the IR by modulators could positively regulate IR signaling. On the contrary, insulin, picropodophyllin (a specific IGF-IR inhibitor that completely abolishes insulin- and IGF-II-induced migration in IRB), Cav-2β, GL56, and Aroclor 1254 are negative regulators of the IR that inhibit IR-dependent signaling.
4.7.3. Interruption of β-Subunit Autophosphorylation of the IR
After insulin binding, the IR undergoes conformational changes, resulting in autophosphorylation of tyrosine residues in the intracellular β-subunits of IR (IR-β) to activate the intrinsic tyrosine kinase in IR-β [180]. The activated IR-β triggers phosphorylation of downstream signaling molecules. IR autophosphorylation has been regarded as a hallmark of IR activation and plays a determinative role in the modulation of receptor-associated kinase activity towards exogenous substrates [181]. Recent work has demonstrated that IR autophosphorylation is a stepwise process, where symmetric mono-phosphorylation of the Tyr1150 residue (m-pY1150) is a key characteristic of biased agonists to the IR [50]. The m-pY1150 is necessary for the juxtamembrane (JM) domain to release the kinase and Tyr residues for phosphorylation [50]. Additionally, m-pY1150 determines the substrate specificity of the IR kinase, and the induction of m-pY1150 is an essential step for full IR activation. Hence, stimulation of m-pY1150 induction and IR autophosphorylation activation are favorable regulatory factors for the IIS pathway. However, dephosphorylation and inhibition of autophosphorylation are negative regulatory factors for the IR; the effect of protein tyrosine phosphatase 1B (PTP1B) on the IR is an example of this.
4.7.4. Influence on the IR β-Subunit Tyrosine Kinase and Pseudosubstrates
Activators of IR–tyrosine kinase (IR-TK) could enhance the activity of the kinase after autophosphorylation of the β-subunit of the IR. The adaptor proteins (GRB and SOCS) are inhibitors of the IR and contain two different domains: one being a peptide region binding to the IR substrate-binding cleft, and the other being the SH2 domain that binds to and occupies the IR phosphotyrosine site. These protect the IR phosphotyrosines from phosphatases, thus enhancing IR phosphorylation. These GRBs and SOCS act as pseudosubstrates of IR kinase and inhibit IRS binding [3].
5. Nonpeptide Small Molecule Modulators of the IR
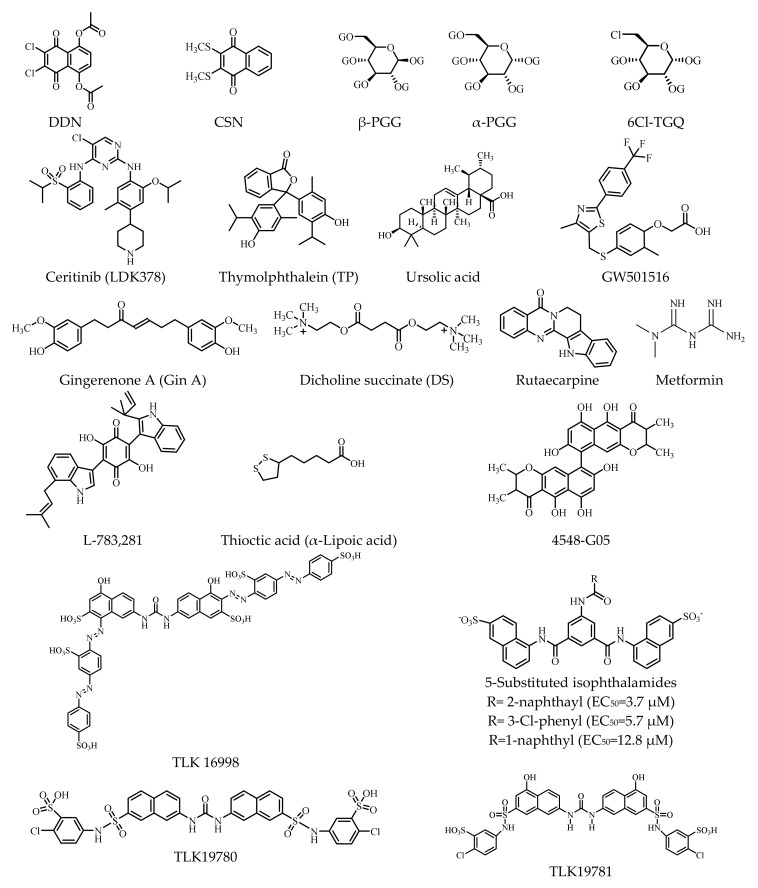
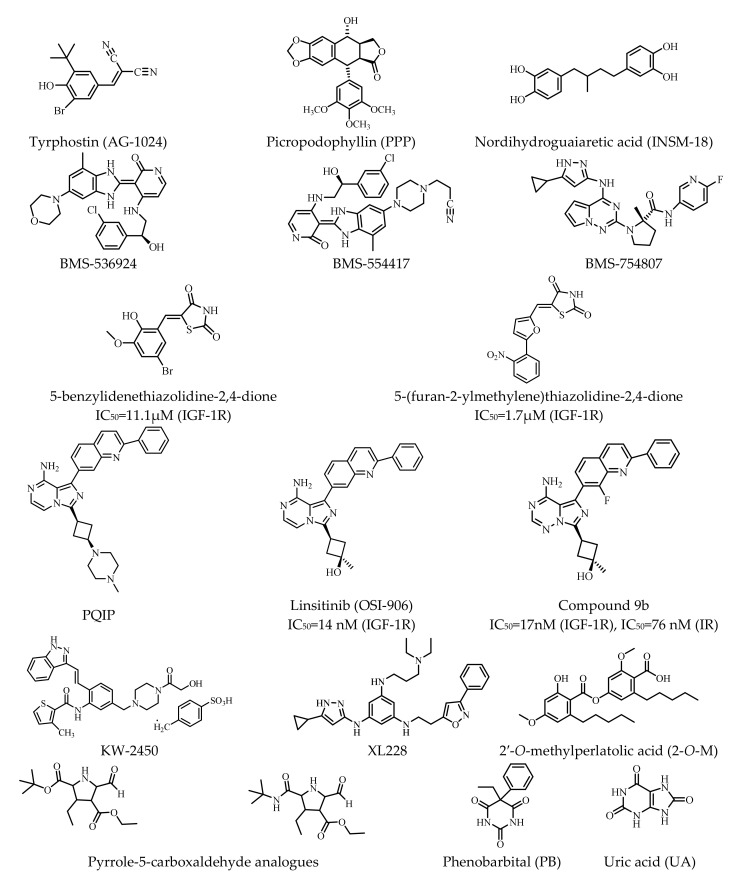
Small molecules with molecular weights below 500 Da have recently received more attention due to their high potential for specificity based on the physiological abnormalities of human diseases [182]. Therefore, they are considered promising in the development of new drugs. Basically, they can be classified into three groups: (1) small-molecule inducers of IR autophosphorylation, (2) modulators of the tyrosine kinase domain of the IR β-subunit, and (3) regulators of the IR and IGF-1R (Table 3). The structures of these small molecules are presented in Figure 4.
Table 3.
Small-molecule positive modulators of the IR.
| Group | Compound | Pharmacological Activity | Experimental Model |
References |
|---|---|---|---|---|
| 1 | Thymolphthalein (TP) weak agonist |
displaces insulin from IR, binds to the IR; ↑auto- and substrate-based phosphorylation of IR |
isolated primary mouse adipocytes | [183] |
| 1 | Dicholine succinate (DS) (sensitizers) |
↑IR-mediated signaling | mice | [184,185] |
| 1 | GW501516 | ↑expression of the IR (1.3-fold than insulin); ↓TNF-α (tumor necrosis factor α)-induced IR expression |
differentiated 3T3-L1 adipocytes | [186] |
| 1 | DDN (activator) | ↑phosphorylation of Akt and ERK (bind to IR-TKD); ↓blood glucose |
male C57BL/6J, C57BL/KsJ db/db mice; female C57BL/KsJ ob/ob mice | [187] |
| 1 | CSN (activator) | ↑IR phosphorylation (time-dependent manner) |
||
| 1 | Ceritinib (LDK378) (off-target inhibitor) |
↓IGF-1R (IC50: 8 nm) phosphorylation and downstream effector AKT; ↓IR (IC50: 7 nm) phosphorylation |
human primary cell culture PhKh1 of a pediatric HGNET-BCOR patient (P1) | [188,189,190] |
| 1 | Penta-O-galloyl-D-glucose (PGG) | ↑phosphorylation of the IR and Akt (α-PGG/β-PGG isoform) | 3T3-L1 adipocytes | [191,192] |
| 1 | 6Cl-TGQ | ↑IR (without activating IGF-1R); ↑glucose uptake |
3T3-L1 adipocytes | [193] |
| 1 | Adenosine | ↑phosphorylation and activation of IR (interacted with IR-α) | HepG2 liver cells; insulin-resistant T2D Leprdb/db mice | [194] |
| 1 | Gingerenone A (Gin A) | ↑tyrosine phosphorylation of the IR; ↑translocation of GLUT4; ↑insulin-stimulated glucose uptake |
murine 3T3-L1 adipocytes; rat L6 myotubes | [195] |
| 1 | Ursolic acid | ↑autophosphorylation of the β-subunit of the IR; ↑glucose uptake (dose-dependent manner) |
3T3-L1 adipocytes | [196,197] |
| 1 | Metformin | ↑autophosphorylation of the human IR (activator of AMP-activated protein kinase (AMPK)) lactic acidosis | CHO cells expressing the human IR | [198,199] |
| 1 | Rutaecarpine (activator) | ↑autophosphorylation of the human IR (bind to IR-ECD) | [200] | |
| 2 | L-783,281 (insulin mimetic) (activator) |
↑phosphorylation of the IR β subunit & IRS-1; ↑PI 3-kinase activity (13); ↑phosphorylation Akt kinase; ↑glucose uptake |
Chinese hamster ovary cells (overexpress the human IR) (CHO.IR) | [201] |
| 2 | Thioctic acid (α-lipoic acid) (activator) | ↑activation of the IR (bind to IR-TKD) | mice primary hepatocytes | [202] |
| 2 | 4548-G05 (insulin mimetics) (activator) | ↑phosphorylations of IR, IRS-1, Akt | C2C12 myotubes [197] | [203] |
| 2 | TLK16998 (sensitizer) | ↑IR autophosphorylation (activates IR-TKD β-subunit); ↑IRS-1 phosphorylation;↑PI3-kinase recruitment;↑GLUT4 translocation; ↑glucose uptake |
3T3-L1 adipocytes | [204] |
| 2 | TLK19780 (activator) | ↑the amount of autophosphorylated IR; | HTC-IR cells | [205] |
| 2 | TLK19781 | ↑phosphorylation of the IR-TKD; ↑GLUT4 translocation;↑glucose transport |
3T3-L1 fibroblasts | [206,207] |
| 2 | 5-substituted isophthalamides (sensitizer) | IR sensitizer, inactive without insulin | 3T3-L1 adipocytes | [208] |
| 2 | Tyrphostin (AG-1024) | ↓autophosphorylation IGF-1R (IC50:0.4 μM)/IR (IC50:0.1 μM) | NIH-3T3 fibroblasts | [20] |
| 2 | Picropodophyllin (PPP) | ↓IGF-1R autophosphorylation at the substrate level | mice | [209] |
| 2 | Nordihydroguaiaretic acid (INSM-18) | ↓activation of the IGF-1R (inhibitor); ↓phosphorylation of the Akt/PKB serine kinase |
MCF-7 human breast cancer cells | [210] |
| 3 | 5-benzylidenethiazolidine-2,4-dione | ↓IGF-IR and IR kinase activity | MCF-7 human breast cancer cell line | [211] |
| 3 | 5-(furan-2-ylmethylene) thiazolidine-2,4-dione | ↓IGF-IR and IR kinase activity | ||
| 3 | PQIP | ↓autophosphorylation of the IGF-1R (IC50: 19 nmol/L) | 3T3/huIGF1R fibrosarcoma cells | [212] |
| 3 | linsitinib (OSI-906) | ↓IR/IGF-1R kinase; ↓autophosphorylation of IR and IGF-1R (human) |
3T3/huIGF-1R fibrosarcoma cells; GEO human colorectal cancer cells | [213] |
| 3 | Compound 9b | ↓phosphorylation of IR and IGF-1R; ↓pAkt |
GEO human colorectal tumor cell line/tumor xenograft models | [214] |
| 3 | BMS-536924 | ↓phosphorylation of the IR and IGF-1R tyrosine kinase; ↓Akt and MAPK phosphorylation |
Sal tumor model | [215] |
| 3 | BMS-554417 | ↓IGF-IR and IR kinase activity and proliferation | carcinoma cell lines (Colo205 and OV202) | [216] |
| 3 | BMS-754807 | ↓phosphorylation of IGF-1R and IR | postmenopausal, estrogen-dependent breast cancer | [217] |
| 3 | KW-2450 | ↓tyrosine kinase of IR (IC50: 5.64 nM)/IGF-1R (IC50: 7.39 nM) | HT-29/GFP colon cancer xenograft model | [218,219] |
| 3 | XL228 | ↓tyrosine kinase of IGF-1R and other protein kinases | patients with advanced malignancies | [220] |
| 3 | pyrrole-5-carboxaldehyde analogues | ↓tyrosine kinase of IR and IGF-1R; ↓autophosphorylation of IR and IGF-1R |
human embryonic kidney cells (HEK-293) | [221] |
| 3 | Phenobarbital (PB) (antagonist) |
↓dephosphorylate-activated IR; ↓dephosphorylation of phosphorylated Akt/FOXO1 |
primary hepatocytes and HepG2 cells; mouse | [222] |
| 3 | Uric acid (UA) | induced ENPP1 binding to IR α-subunit; ↓autophosphorylation of IR and IRS; ↓glucose transport |
human umbilical vein endothelial cell | [223,224,225] |
| 3 | 2′-O-methylperlatolic acid (sensitizer) | ↑insulin signaling pathway (binds to IR-ECD); ↑cellular glucose uptake |
Hepa and C2C12 myotubes | [226] |
GLUT4, glucose transporter 4; HGNET-BCOR, high-grade neuroepithelial tumor with BCOR alteration; ENPP1, ectonucleotide pyrophosphatase/phosphodiesterase 1.
Figure 4.
Structure of small-molecule positive modulators of the IR.
5.1. Small Molecule Inducers of IR Autophosphorylation
Once insulin binds to the α-subunit of the IR, autophosphorylation of tyrosine residues of the β-subunit occurs. Hence, compounds—such as thymolphthalein (TP, an agonist), dicholine succinate (DS, a mitochondrial complex II substrate), and GW501516 (2-methyl-4-((4-methyl-2-(4-trifluoromethylphenyl)-1,3-thiazol-5-yl)-methylsulfanyl)phenoxy-acetic acid, a PPAR β/δ agonist), as well as some other naphthoquinone derivatives and natural chemicals from plants—that stimulate the phosphorylation of the IR present one pattern of modulation [183,184,185,186,187] (Table 3).
Presently, the naphthoquinone derivatives include DDN (5,8-diacetyloxy-2,3-dichloro-1,4-naphthoquinone), CSN (2,3-bismethylsulfanyl-1,4-naphthoquinone), and ceritinib (LDK378, 5-chloro-N2-(2-isopropoxy-5-methyl-4-(piperidin-4-yl)phenyl)-N4-(2-(isopropylsulfonyl) phenyl) pyrimidine-2,4-diamine) [188,189,190], while the natural chemicals from plants consist of tannin chemicals such as α-PGG (α-penta-galloyl-glucose), β-PGG (β-penta-galloyl-glucose), and 6Cl-TGQ (6-chloro-6-deoxy-1,2,3,4-tetra-O-galloyl-α-D-glucopyranose) [191,192,193].
Additionally, adenosine (5′-Se-methyl-5′-seleno-,2′,3′-diacetate) [non-peptidyl compound 43 (NPC43)] and a plant-derived polyphenol chemical, gingerenone A (Gin A), have also been reported as inducers of IR autophosphorylation [194,195]. In addition to these tannin derivatives and polyphenols, polycyclic natural products such as ursolic acid can also enhance the autophosphorylation of the β-subunit of the IR and subsequently affect the PI3K pathway downstream [196,197]. Rutaecarpine is extremely significant and is regarded as an analog of metformin (dimethyl biguanide, the first-line drug for the treatment of T2DM) [198,199,200] (Table 3).
5.2. Modulators of the Tyrosine Kinase Domain of the IR β-Subunit
Modulators in this group can be further classified into two subgroups, namely, activators or sensitizers of IR tyrosine kinase and inhibitors of the IR tyrosine kinase.
To date, nine molecules have been reported as activators or sensitizers of the IR by acting on the IR tyrosine kinase (Table 3). These molecules are L-783281 (2,5-dihydroxy-6-(1-methylindol-3-yl)-3phenyl-1,4-benzoquinone), thioctic acid (α-lipoic acid), the chaetochromin derivatives (4548-G05 ([9,9′-bi-4H-Naphtho [2,3-b]pyran]-4,4′-dione,2,2′,3,3′-tetrahydri-5,5′,5,6′,8,8′-hexahydroxy-2,2′,3,3′-tetramethyl), TLK16998, TLK19780, and TLK19781, and three 5-substituted isophthalamides [201,202,203,204,205,206,207,208] (Figure 3). At present, tyrphostin (AG-1024), picropodophyllin (PPP), and nordihydroguaiaretic acid (INSM 18) are known to be inhibitors of the IR tyrosine kinase [209,210].
5.3. Regulators of the IR and IGF-1R
Regulators in this group consist of thiazolidinediones, pyrimidines, and others (Table 3).
Thiazolidinediones or thiazolidine-2,4-dione (TZDs) have recently emerged as potent antidiabetic agents, and several TZDs have been approved by the FDA for treating type 2 diabetes. Two TZDs, 5-benzylidenethiazolidine-2,4-dione and 5-(furan-2-ylmethylene) thiazolidine-2,4-dione, have been reported as selective IGF-1R inhibitors. Both of them can inhibit the IGF-1R kinase domain in vitro, with IC50 < 15.0 μM. This finding uncovered the potential of adding TZD derivatives to the portfolio of IGF-1R inhibitors [211].
The pyrimidine derivative PQIP (cis-3-[3-(4-methyl-piperazin-l-yl)-cyclobutyl]-1-(2-phenyl-quinoline-7-yl)-imidazo[1,5-α]pyrazin-8-ylamine) is an IGF-1R kinase inhibitor that potently inhibits the autophosphorylation of IGF-1R (IC50 = 19 nM) with 14-fold cellular selectivity relative to human IR [212]. Furthermore, linsitinib (OSI-906, 3-[8-amino-1-(2-phenylquinolin-7-yl) imidazo[1,5-a]pyrazin-3-yl]-1-methylcyclobutan-1-ol), a derivative of PQIP, is known to be an orally bioavailable, ATP-competitive, imidazopyrazine-based inhibitor [21,213,214].
Other chemicals such as BMS-536924 ((S)-4-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]-3-(4-methyl-6- morpholin-4-yl-1H-benzoim-idazol-2-yl)-1H-pyridin-2-one), BMS-554417 (3-[4-[(2Z)-2-[4-[[(2S)-2-(3-chlorophenyl)-2-hydroxyethyl] amino]-2-oxopyridin-3-ylidene]-7-methyl-1,3-dihydrobenzimidazol-5-yl]piperazin-1-yl]propanenitrile), and BMS-754807 ((2S)-1-[4-[(5-cyclopropyl-1H-pyrazol-3-yl)amino] pyrrolo-[2,1-f][1,2,4]triazin-2-yl]-N-(6-fluoropyridin-3-yl)-2-methylpyrrolidine-2-carboxamide) are inhibitors of the IR tyrosine kinase [215,216,217]. BMS-536924 and BMS-554417 belong to the pyridinones and play crucial roles in inhibiting IGF-1R and IR kinase activity [217].
Other heterocyclic compounds include the indazole derivative KW-2450 (2-thiophenecarboxamide, N-[5-[4-(2-hydroxyacetyl)-1-piperazinyl]methyl]-2-[(1E)-2-(1H-indazol-3-yl)ethenyl]phenyl]-3-methyl-4-methylbenzenesulfonate), the pyrazol derivative XL228 (4-N-(5-cyclopropyl-1H-pyrazol-3-yl)-6-(4-methylpiperazin-1-yl)-2-N-[(3-propan-2-yl-1,2-oxazol-5-yl)methyl]pyrimidine-2,4-diamine), and two pyrrole-5-carboxaldehyde analogues (2-tert-butyl-4-ethyl-3-ethyl-5-formyl-1H-pyrrole- 2,4-dicarboxylate and ethyl-5-[(tert-butylamino)carbonyl]-4-ethyl-2-formyl-1H-pyrrole-3- carboxylate) [218,219,220,221].
Additionally, three other chemicals, phenobarbital (5-Ethyl-5-phenylbarbituric acid), uric acid (7,9-dihydro-1H-purine-2,6,8(3H)-trione), and 2′-O-methylperlatolic acid (2-O-M, 4-(2-hydroxy-4-methoxy-6-pentylbenzoyl)oxy-2-methoxy-6-pentylbenzoic acid) are also effective inhibitors of the IR (Table 3). Structurally, phenobarbital and uric acid consist of a trione bond and a dione bond, respectively (Figure 3). The trione chemical phenobarbital (PB) is an IR antagonist. PB and insulin crosstalk regulated glucose through the IR in HepG2 cells [222]. The trioxopurine chemical uric acid (UA) is a final product of purine metabolism [223,224,225]. 2-O-M is a polyphenolic compound that binds to the extracellular domain of the IR. Combination treatment with 2-O-M and insulin resulted in significant activation of the insulin signaling pathway in vitro [226].
6. Molecular Mechanisms of Modulators Targeting the IR
6.1. Gene Expression of the IR Changed by DNA Methylation
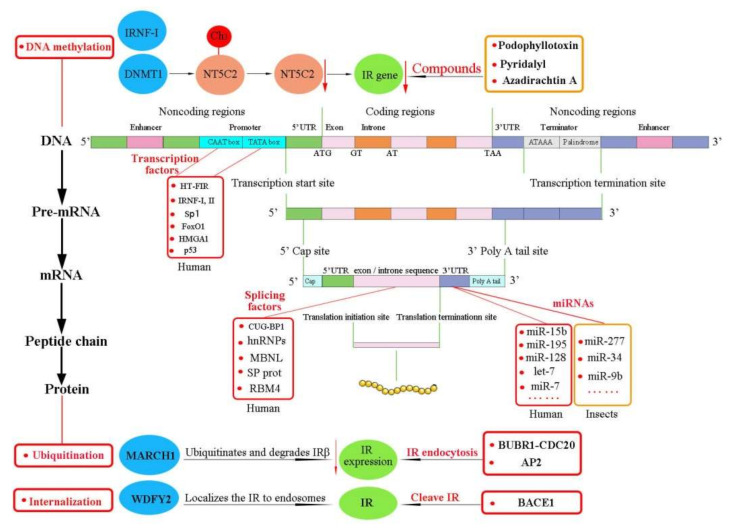
DNA methylation is a conserved epigenetic modification that can lead to gene silencing and regulated gene expression and the resulting cellular functions [227]. Therefore, it is indispensable for embryonic development, transcriptional regulation, and genome stability [228]. The pattern of DNA methylation in the genome is achieved by DNA methyltransferases such as DNMT1, DNMT3A, and DNMT3B [229,230,231]. DNMT1 affects IR gene expression through the transfection of 5′-nucleotidase, cytosolic II (NT5C2), an enzyme that dephosphorylates noncyclic nucleoside monophosphates into nucleoside and inorganic phosphate [232,233] (Figure 5). DNMT1 epigenetically regulates NT5C2 and the IR. Overexpression of DNMT1 stimulated DNA hypermethylation of NT5C2, leading to the silencing of the NT5C2 gene and the inhibition of IR gene expression [32,232]. Knockdown of NT5C2 induced the overexpression of DNMT1 and inhibition of the IR [232]. In contrast, overexpression of NT5C2 downregulated the expression of DNMT1 and upregulated the activation of the IR in RIN-m5F cells. In brief, NT5C2 epigenetically regulates the IR, thus potentially providing a novel therapeutic strategy. Analysis of DNA methylation might afford new diagnostic and therapeutic approaches for patients.
Figure 5.
Summary of the principal IR regulators acting at the gene level (DNA methylation, xenobiotic compounds, and transcription factors at promoter), mRNA level (miRNAs at 3′UTR), and protein level (ubiquitination, endocytosis, internalization, and cleavage). DNMT1, DNA methyltransferase 1; IRNF-I, IR nuclear factor I; NT5C2, transfection 5′-nucleotidase, cytosolic II.
IR nuclear factor I (IRNF-I) also positively regulates IR promoter activity, and thus controls expression of the IR gene [32]. The methylation degree of the IRNF-I binding site within the InR promoter-pancreatic and duodenal homeobox 1 (PDX1) has been shown to be inversely and significantly correlated with InR gene expression [81]. In high-fat diet (HFD)-overfed adult offspring of the outbred Wistar rats, hypothalamic InR mRNA suppression was relevant to DNA hypermethylation of the InR promoter. Hypothalamic InR expression and DNA promoter methylation might lead to insulin resistance and T2DM. Interestingly, insulin resistance was more pronounced in male offspring [81]. These epigenetic phenomena were not associated with InR expression and DNA methylation alteration in female offspring, while males were predisposed. Chronic high-fat (HF) feeding decreased the insulin content and the IR level in the hippocampus [234]. New findings regarding sex-specific expression and alteration of the IR with respect to food intake and bodyweight should be considered in future studies on developmental nutritional programming.
6.2. Gene Expression of the IR Regulated by Xenobiotic Compounds
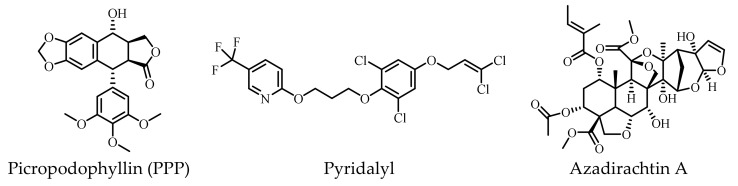
To date, podophyllotoxin and two commercial pesticides (pyridalyl and azadirachtin A) have been found to affect IR genes (Figure 6).
Figure 6.
Structures of xenobiotic compounds regulating IR genes.
Podophyllotoxin is a plant-derived cyclolignan [235]. The medicinal and pesticidal activities of podophyllotoxin and its derivatives have recently become hot topics [236]. Podophyllotoxin has presented anti-cancer activity [237,238,239], and one of its derivatives—etoposide—is an essential anti-cancer drug included in the WHO Model List of Essential Medicines and currently in clinical use [240]. Etoposide can inhibit topoisomerase-II and promote DNA damage and apoptosis in cancer cells [241]. Interestingly, podophyllotoxin is structurally related to the IGF-1R inhibitor picropodophyllin mentioned above. Numerous studies have also reported that podophyllotoxin and its analogs possess insecticidal activity against the oriental armyworm moth Mythimna separata Walker, the moth Athetis dissimilis Hampson, and other agricultural pests [235,242,243,244,245]. RNA-seq has suggested that the podophyllotoxin derivative Compound 2a (5R,5aR,8aR,9R)-8-oxo-9-(3,4,5-trimethoxyphenyl)-5,5a,6,8,8a,9–hexahydrofuro [3’,4’:6,7] naphtha [2,3-d] [1,3] dioxol-5-yl 3-nitrobenzoate might target the IR and markedly repress the wing-development-related genes of the IR in the oriental armyworm moth [29,246]. These findings emphasize the importance of podophyllotoxin and its derivatives and provide possible guidance for further design and structural modification for the development of novel drugs and insecticidal agents acting through regulation of the IR (Figure 5).
Pyridalyl is an insecticide that has shown significant toxicity against several lepidopterous and thysanopterous pests on cotton and vegetables [247]. Oral exposure to pyridalyl resulted in upregulation of the IR (Figure 5), cytochrome P450, antioxidant enzymes, and other enzymes involved in cell death in the olive fruit fly Bactrocera oleae Rossi (Diptera) [248]. Activities of some enzymatic and nonenzymatic components were also changed after oral exposure to pyridalyl. Biochemical experiments have found that adult flies fed a pyridalyl-added protein hydrolysate diet presented significant mortality, with an LC50 value of 0.517 μg/mL. The fecundity of treated females showed no significant differences after 7 days, while the mortality of the laid eggs was obviously higher than those of controls. Activation of the IR, cytochrome P450, cell death (via apoptosis), and oxidative stress were the main alterations observed in pyridalyl-treated olive fruit flies [249]. These results are also consistent with earlier findings [250]. In a cultured cell line (Spodoptera litura (Fabricius), SL-1 cell), azadirachtin A, a well-known botanical insecticide, also enhanced the IR expression level. Azadirachtin A blocked the combination of insulin and IR, leading to a change of phosphorylation of the InR, thus affecting phosphorylation in the downstream signaling pathway [251].
6.3. IR Expression Regulated Posttranscriptionally via MicroRNAs
MicroRNAs (miRNAs) are evolutionarily conserved small noncoding RNAs that can inhibit translation or promote mRNA degradation [252].
A growing number of studies have shown that miRNAs are critical components of posttranscriptional gene expression regulation and are critical in biological processes for humans and insects [253,254,255]. Different miRNAs have been reported to participate in human IR modulation [11,57] (Figure 4); for example, Let-7 miRNA family members regulated the IR expression in human HEK293T cells and pancreatic ductal adenocarcinoma (PDAC) [256,257]. Let-7 family members also targeted and downregulated the IR/IGF pathway in PDAC; miR-7 is a brain-abundant miRNA that targets the IR, IRS-2, and insulin-degrading enzyme (IDE), and thus affects insulin signaling through posttranscriptional regulation of the IRS-2, IR, and IDE pathways in AD patients [258]. Consequently, miRNAs might thus provide an effective target for the development of IR/IGF pathway-specific treatment strategies.
Further, miRNAs are known to regulate development, metabolism, reproduction, and many physiological syntheses in insects. In mosquitoes, miR-277 targets ILP7 and ILP8 and acts as a monitor to control ILP7 and ILP8 mRNA levels [259]. Additionally, miRNAs have functions during wing development [260]; miR-34 mediates the crosstalk between JH, 20E, and IIS pathways by targeting two binding sites in the 3′UTR of NlIR1, and formed a positive autoregulatory loop to control wing morphs in N. lugens [120]. Another study reported that miR-9b played a key role in regulating wing dimorphism in the brown citrus aphid Aphis citricidus (Kirkaldy) [260]. Likewise, miR-9b-ABCG4-insulin signaling has been shown to be involved in the transition from the fourth instar winged nymph to the winged adult. Inhibition of Aci-miR-9b increased the proportion of winged offspring under normal conditions, while overexpression of aci-miR-9b resulted in a decline in the proportion of winged offspring under crowded conditions and resulted in malformed wings in adults [260]. These results enrich our knowledge of miRNA as an important factor that could help insects adapt to changing environments. Such information might stimulate the development of methods for controlling migratory insect pests and the corresponding viral diseases transmitted by these insects.
6.4. Regulation of Expression of the IR Protein
6.4.1. Ubiquitination of the IR
Dynamic modulation and posttranslational modification of proteins are multistep enzymatic biological processes that occur in response to physiological cues [261]. Ubiquitination is one of the most common forms of posttranslational dynamic modification of proteins in cells [262]. Thousands of proteins are targeted for ubiquitination at some time during their life. Ubiquitination regulates extensive complex physiological processes, including protein degradation and interactions, endocytosis, and cell-cycle progression and differentiation, as well as the activation or inactivation of substrates [261]. Dysregulation of ubiquitination contributes to various diseases [263], and both IR and IRS proteins are regulated by ubiquitination [264,265,266,267] (Figure 5).
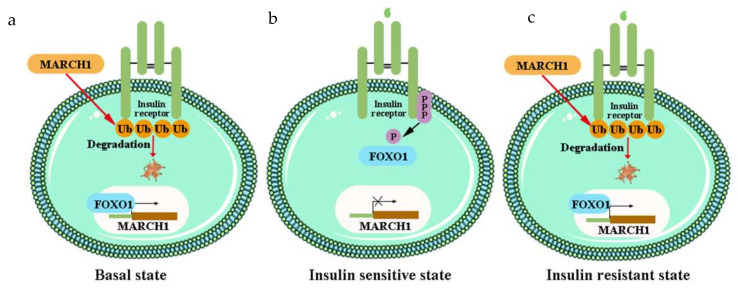
The negative regulator E3 ubiquitin ligase MARCH 1 impairs cellular insulin action by degrading the cell surface IR. MARCH 1 ubiquitinates the IR to decrease the level of cell surface IR in the basal state, rather than after insulin stimulation [267]. This is different to other IR ubiquitin ligases. MARCH 1 also controls IR tyrosine phosphorylation, regulation of PTP1B, variation in clathrin-mediated IR endocytosis, and alteration of IR compartmentation into lipid rafts and caveolae [267]. MARCH 1 is conducive to the pathophysiology of T2DM, and thus could provide a novel therapeutic approach (Figure 7) [32,267].
Figure 7.
MARCH 1 regulates the IR: (a) MARCH 1 ubiquitinates and degrades the β-subunit of the IR, thereby decreasing IR surface expression; (b) IR activation inhibits FOXO, resulting in transcriptional repression of MARCH 1 and an increase in surface IR levels; (c) Insulin fails to inhibit FOXO, leading to enhanced MARCH 1 expression, reduced surface IR levels, and an impaired IIS pathway. Ub, ubiquitin. FOXO, forkhead transcription factor subgroup O.
6.4.2. Endocytosis of the IR
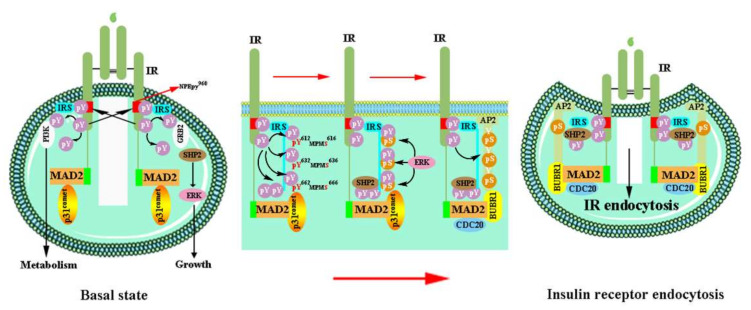
Protein ubiquitination acts as a signal for sorting, trafficking, and membrane protein removal by endocytosis [261]. Active IR is internalized by dynamin-mediated endocytosis [268]. Therefore, IR endocytosis is a crucial factor that regulates the insulin signaling intensity and duration (Figure 5). The IRS module and MAD2 protein collaborate to trigger and regulate activated IR endocytosis [56] (Figure 8). The IRS module is activated by the SHP2-MAPK pathway [56,269]. The phosphotyrosine-binding domain of the IRS is directly bound to the phosphorylated NPEpy960 motif in the IR-JM domain, interacts with assembly polypeptide 2 (AP2), and then triggers IR endocytosis. Src homology phosphatase 2 (SHP2) binds to the C-terminal phosphotyrosine sites on the IRS1 and dephosphorylates pY612/pY632/pY662 of the doubly phosphorylated IRS1 (pY/pS) to facilitate the IRS1–AP2 interaction.
Figure 8.
Activated IR endocytosis, regulated by the IRS and MAD2. The IRS proteins directly bind to the IR-JM domain and interact with AP2; p31comet suppresses the interaction of BUBR1-CDC20-AP2 and IR-bound MAD2, thus interfering with IR endocytosis in the basal state. The connection of BUBR1-CDC20-AP2 and IR-MAD2 causes IR endocytosis. SHP2, Src homology phosphatase 2; ERK, extracellular-signal-regulated kinase; AP2, assembly polypeptide 2; Y612/Y632/Y662, IR tyrosine phosphorylates the YXXΦ motifs on the IRS1. S616/S636/S666, activate ERK phosphorylates on the IRS1.
SHP2 promotes IR endocytosis directly by removing IRS tyrosine phosphorylation and indirectly by activating the MAPK pathway [56]. In the MAD2-dependent module, MAD2 binds to the C-terminal MAD2-interacting motifs (MIMs) of IR then interacts with BUBR1-CDC20 and provides another binding site for AP2 [270]. The successful connection of BUBR1-CDC20-AP2 and IR-MAD2 leads to IR endocytosis in cells. In the basal state, p31comet disrupts IR endocytosis by inhibiting the interaction between BUBR1-CDC20-AP2 and the IR-bound MAD2 [271]. Persistent hyperinsulinemia also accelerates IR endocytosis and impairs the functional IR level at the PM. SHP2 inhibition provides a hopeful means to interfere with the endocytosis feedback loop, prolonged insulin signaling at the PM, and potentiated insulin sensitivity [271]. These findings support that the feedback regulation of IR endocytosis may contribute to diabetes therapy in human patients.
6.4.3. Endosome Localization and Cleavage of the IR
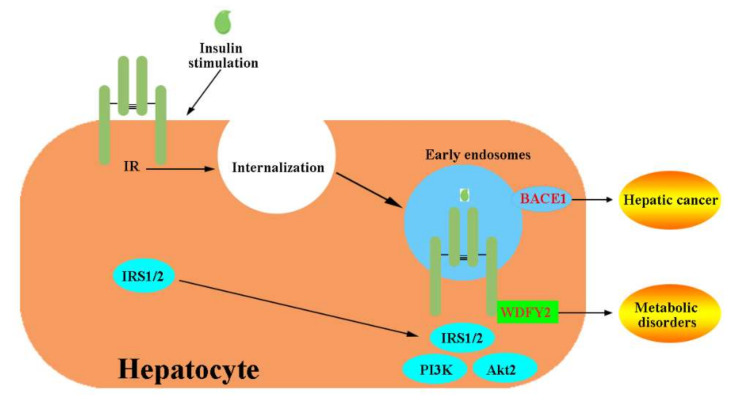
The liver is the largest parenchymal organ, regulating detoxification in the body and playing a fundamental role in coordinating systemic metabolic homeostasis [15,272]. In hepatocytes, IR ligand binding results in IR-TK autophosphorylation and internalization to generate intracellular signaling endosomes, which plays an important role in activating the hepatic insulin-evoked PI3K-AKT pathway [273] (Figure 5). The IR continues to signal at endosomal compartment Akt. Akt isoform 2 (Akt2), the most abundant Akt isoform in tissues, is also recruited to the endosomes and exhibits higher specific enzymatic activity than PM. The endosome-located protein WD Repeat and FYVE domain containing 2 (WDFY2) is highly expressed in the liver [274,275] and has been reported to act as an adaptor-like protein for the regulation of protein phosphorylation and endocytosis [276]. WDFY2 interacts with the IR through its WD1-4 domain and localizes the IR to endosomes after insulin stimulation, ensuring that downstream IRS1/2 is recruited [275] (Figure 9). Disturbing the IR-WDFY2 interaction ultimately impairs downstream PI3K-AKT signaling. Therefore, increasing WDFY2 liver expression might provide a new treatment concept for metabolic disorders.
Figure 9.
Model for the role of WDFY2 and BACE1 in IR internalization into endosomes in hepatocytes. After insulin stimulation, the IR internalizes into endosomes. WDFY2 interacts with the IR to localize it to endosomes such that downstream IRS1/2 and AKT2 can be recruited to the endosomal IR. BACE1, located at the early endosomes, cleaves the IR-ECD and decreases the number of mature IRs. WDFY2, WD Repeat and FYVE domain containing 2; BACE1, β-site amyloid precursor protein cleaving enzyme 1.
In the liver, β-site amyloid precursor protein cleaving enzyme 1 (BACE1), a therapeutic target of AD [277], regulates the number of IRs and insulin signaling in a glucose concentration-dependent manner [21]. BACE1 is located in the trans-Golgi network (TGN), PM, and early endosomes [278] (Figure 9). BACE1 can cleave IR-ECD and decrease the number of mature IRs [21]. BACE1 inhibition recovered functional IR and enhanced insulin signaling in diabetes patients. A soluble truncated IR (IRsol, especially the IR α-subunit) has been shown to be elevated in the plasma of T2DM patients compared to control groups [279,280]. Consequently, the use of BACE1 inhibitors may potentially assist in hepatic cancer management, and the cleaved IR might serve as a novel biomarker for hepatic cancer diagnosis and management.
7. Conclusions and Future Perspectives
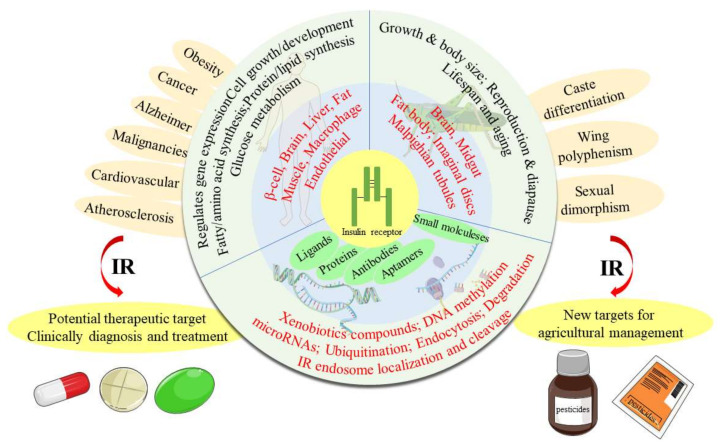
Briefly, the contents and the conclusions of this paper are summarized in the following diagram (Figure 10).
Figure 10.
Summary of the contents and the conclusions.
Since the discovery of the IR, the structure and signaling pathways of this vital receptor have been systematically studied. Structurally, the IR-ECD converts the overall architecture from an autoinhibited inverted “V” shape into a “T”-shaped conformation after insulin binding, where at least one insulin molecule at two sites and a maximum of four insulin molecules at four sites are required to form the “T”-shaped dimer. Strikingly, insulin binds to the majority of IRs in a symmetric manner.
Functionally, the IR plays essential roles in metabolism, cell growth, development, and numerous physiological processes. Moreover, the IR has been related to the most prevalent diseases, such as T2DM and cancers, and thus has been considered as a novel therapeutic target of IR-related diseases in humans [263]. In-depth analysis of IR regulators, including insulin, insulin analogs, insulin-mimetic peptides, antibodies, aptamers, and proteins, would help develop an understanding of the regulation of cellular IIS pathways, substantially contributing to the development of novel drugs for T2DM and other diseases related to insulin signaling [281]. Therefore, design and synthesis of novel small molecules as oral drugs has been regarded as a source of potential IR activation modulators. Metformin is a good example that has emerged in recent years.
Noteworthy, there exists high conservation in the IIS pathway between mammals and insects [5]. This has led to the possibility for the IR to be used as a potential target for the development of new insecticides in order to control insects. To date, insecticides have been claimed as exactly targeting the IR, even though the IR is known to play roles in caste differentiation, wing polyphenism, and some other important physiological processes. Moreover, IR-silenced insects are blocked at the larval–pupal transformation and then die [107]. It is notable that podophyllotoxin, pyridalyl, and azadirachtin A affect the IR.
In summary, these discoveries are expected to be useful for the development of novel oral drugs and insecticidal agents targeting the IR. DNA methylation, microRNAs, protein ubiquitination and endocytosis, endosome localization, and cleavage are remarkable categories of IR regulation to which significant attention should be paid in the development of novel drugs and insecticides.
Acknowledgments
We appreciate Meirong Yan (College of Ecology and Environment, Hainan Tropical Ocean University) very much for good suggestions in our work.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the Science and Technology Planning Programs of Guangdong Province, China (2017A020208040).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ward C.W., Lawrence M.C. Ligand-induced activation of the insulin receptor: A multi-step process involving structural changes in both the ligand and the receptor. Bioessays. 2009;31:422–434. doi: 10.1002/bies.200800210. [DOI] [PubMed] [Google Scholar]
- 2.Vigneri R., Goldfine I.D., Frittitta L. Insulin, insulin receptors, and cancer. J. Endocrinol. Investig. 2016;39:1365–1376. doi: 10.1007/s40618-016-0508-7. [DOI] [PubMed] [Google Scholar]
- 3.Haeusler R.A., McGraw T.E., Accili D. Biochemical and cellular properties of insulin receptor signalling. Nat. Rev. Mol. Cell Biol. 2018;19:31–44. doi: 10.1038/nrm.2017.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yunn N.O., Kim J., Kim Y., Leibiger I., Berggren P.O., Ryu S.H. Mechanistic understanding of insulin receptor modulation: Implications for the development of anti-diabetic drugs. Pharmacolo. Therapeut. 2018;185:86–98. doi: 10.1016/j.pharmthera.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Brogiolo W., Stocker H., Ikeya T., Rintelen F., Fernandez R., Hafen E. An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr. Biol. 2001;11:213–221. doi: 10.1016/S0960-9822(01)00068-9. [DOI] [PubMed] [Google Scholar]
- 6.Lin X., Smagghe G. Roles of the insulin signaling pathway in insect development and organ growth. Peptides. 2019;122:169923. doi: 10.1016/j.peptides.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Liu C.Y., Zhao W.L., Wang J.X., Zhao X.F. Cyclin-dependent kinase regulatory subunit 1 promotes cell proliferation by insulin regulation. Cell Cycle. 2015;14:3045–3057. doi: 10.1080/15384101.2015.1053664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Craft S., Cholerton B., Baker L.D. Insulin and Alzheimer’s disease: Untangling the web. J. Alzheimer’s Dis. 2013;33((Suppl. S1)):S263–S275. doi: 10.3233/JAD-2012-129042. [DOI] [PubMed] [Google Scholar]
- 9.Czech M.P. Insulin action and resistance in obesity and type 2 diabetes. Nat. Med. 2017;23:804–814. doi: 10.1038/nm.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Westermeier F., Saez T., Arroyo P., Toledo F., Gutierrez J., Sanhueza C., Pardo F., Leiva A., Sobrevia L. Insulin receptor isoforms: An integrated view focused on gestational diabetes mellitus. Diabetes Metab. Res. Rev. 2016;32:350–365. doi: 10.1002/dmrr.2729. [DOI] [PubMed] [Google Scholar]
- 11.Vella V., Milluzzo A., Scalisi N., Vigneri P., Sciacca L. Insulin receptor isoforms in cancer. Int. J. Mol. Sci. 2018;19:3615. doi: 10.3390/ijms19113615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sciacca L., Le Moli R., Vigneri R. Insulin analogs and cancer. Front. Endocrinol. 2012;3:21. doi: 10.3389/fendo.2012.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malaguarnera R., Belfiore A. The insulin receptor: A new target for cancer therapy. Front. Endocrinol. 2011;2:93. doi: 10.3389/fendo.2011.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stoeckel L.E., Arvanitakis Z., Gandy S., Small D., Kahn C.R., Pascual-Leone A., Pawlyk A., Sherwin R., Smith P. Complex mechanisms linking neurocognitive dysfunction to insulin resistance and other metabolic dysfunction. F1000Res. 2016;5:353. doi: 10.12688/f1000research.8300.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Titchenell P.M., Lazar M.A., Birnbaum M.J. Unraveling the regulation of hepatic metabolism by insulin. Trends Endocrinol Metab. 2017;28:497–505. doi: 10.1016/j.tem.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell R.K. Type 2 diabetes: Where we are today: An overview of disease burden, current treatments, and treatment strategies. J. Am. Pharm. Assoc. 2009;49:S3–S9. doi: 10.1331/JAPhA.2009.09077. [DOI] [PubMed] [Google Scholar]
- 17.Ermakova A., Stauffer M.E., Sieradzan R., Taylor S. Characterizing the clinical and economic burden of type 2 diabetes (T2DM) patients on multiple daily injections (MDI) of insulin: A systematic literature review. Value Health. 2018;21:S70. doi: 10.1016/j.jval.2018.04.461. [DOI] [Google Scholar]
- 18.Buyruk B.A., Kebapci N., Yorulmaz G., Alaguney E.S., Akalin A., Efe B. Prevalence and risk factors of lipohypertrophy and lipoatrophy in diabetes patients receiving insulin therapy. Diabetes. 2019;68:59. doi: 10.2337/db19-59-LB. [DOI] [Google Scholar]
- 19.Brietzke S.A. Oral antihyperglycemic treatment options for type 2 diabetes mellitus. Med. Clin. North Am. 2015;99:87–106. doi: 10.1016/j.mcna.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Singh P., Alex J.M., Bast F. Insulin receptor (IR) and insulin-like growth factor receptor 1 (IGF-1R) signaling systems: Novel treatment strategies for cancer. Med. Oncol. 2014;31:805. doi: 10.1007/s12032-013-0805-3. [DOI] [PubMed] [Google Scholar]
- 21.Meakin P.J., Mezzapesa A., Benabou E., Haas M.E., Bonardo B., Grino M., Brunel J.M., Desbois-Mouthon C., Biddinger S.B., Govers R., et al. The beta secretase BACE1 regulates the expression of insulin receptor in the liver. Nat. Commun. 2018;9:1306. doi: 10.1038/s41467-018-03755-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russo A., Paret C., Alt F., Burhenne J., Fresnais M., Wagner W., Glaser M., Bender H., Huprich S., Harter P.N., et al. Ceritinib-induced regression of an insulin-like growth factor-driven neuroepithelial brain tumor. Int. J. Mol. Sci. 2019;20:4267. doi: 10.3390/ijms20174267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wenbin K., Xiaoqin L., Qiuchan D., Xinwen Z., Xiaoqin X., Fangyuan S., Dabao H., Shuangjiu Z. Development of a novel insulin receptor (IR) antagonist that exhibits anti-breast tumor activity. Human Cell. 2020;33:1204–1217. doi: 10.1007/s13577-020-00381-3. [DOI] [PubMed] [Google Scholar]
- 24.Lin X., Lavine L.C. Endocrine regulation of a dispersal polymorphism in winged insects: A short review. ScienceDirect. 2018;25:20–24. doi: 10.1016/j.cois.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Guo S., Wang X., Kang L. Special significance of non-Drosophila insects in aging. Front. Cell Dev. Biol. 2020;8:576571. doi: 10.3389/fcell.2020.576571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alvarez-Rendon J.P., Salceda R., Riesgo-Escovar J.R. Drosophila melanogaster as a model for diabetes type 2 progression. Biomed. Res. Int. 2018;2018:1417528. doi: 10.1155/2018/1417528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Houchat J.N., Cartereau A., Le Mauff A., Taillebois E., Thany S.H. An overview on the effect of neonicotinoid insecticides on mammalian cholinergic functions through the activation of neuronal nicotinic acetylcholine receptors. Int. J. Environ. Res. Public Health. 2020;17:3222. doi: 10.3390/ijerph17093222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun Z., Xu H. Ryanodine receptors for drugs and insecticides: An overview. Mini. Rev. Med. Chem. 2019;19:22–33. doi: 10.2174/1389557518666180330112908. [DOI] [PubMed] [Google Scholar]
- 29.Sun Z., Lv M., Huang W., Li T., Xu H. Development of botanical pesticides: Exploration on the phenotype of vestigial wings of insect pests induced by plant natural products or their derivatives by blocking tyrosine phosphorylation of insulin receptor 1. J. Agric. Food Chem. 2022;70:2117–2126. doi: 10.1021/acs.jafc.1c06341. [DOI] [PubMed] [Google Scholar]
- 30.Belfiore A., Frasca F., Pandini G., Sciacca L., Vigneri R. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr. Rev. 2009;30:586–623. doi: 10.1210/er.2008-0047. [DOI] [PubMed] [Google Scholar]
- 31.Beneit N., Fernandez-Garcia C.E., Martin-Ventura J.L., Perdomo L., Escribano O., Michel J.B., Garcia-Gomez G., Fernandez S., Diaz-Castroverde S., Egido J., et al. Expression of insulin receptor (IR) A and B isoforms, IGF-IR, and IR/IGF-IR hybrid receptors in vascular smooth muscle cells and their role in cell migration in atherosclerosis. Cardiovasc. Diabetol. 2016;15:161. doi: 10.1186/s12933-016-0477-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Belfiore A., Malaguarnera R., Vella V., Lawrence M.C., Sciacca L., Frasca F., Morrione A., Vigneri R. Insulin receptor isoforms in physiology and disease: An updated view. Endocr. Rev. 2017;38:379–431. doi: 10.1210/er.2017-00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang J., Morehouse C., Streicher K., Higgs B.W., Gao J., Czapiga M., Boutrin A., Zhu W., Brohawn P., Chang Y., et al. Altered expression of insulin receptor isoforms in breast cancer. PLoS ONE. 2011;6:e26177. doi: 10.1371/journal.pone.0026177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heidegger I., Kern J., Ofer P., Klocker H., Massoner P. Oncogenic functions of IGF1R and INSR in prostate cancer include enhanced tumor growth, cell migration and angiogenesis. Oncotarget. 2014;5:2723–2735. doi: 10.18632/oncotarget.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lawrence M.C. Understanding insulin and its receptor from their three-dimensional structures. Mol Metab. 2021;52:101255. doi: 10.1016/j.molmet.2021.101255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Meyts P. Insulin/receptor binding: The last piece of the puzzle? What recent progress on the structure of the insulin/receptor complex tells us (or not) about negative cooperativity and activation. Bioessays. 2015;37:389–397. doi: 10.1002/bies.201400190. [DOI] [PubMed] [Google Scholar]
- 37.Croll T.I., Smith B.J., Margetts M.B., Whittaker J., Weiss M.A., Ward C.W., Lawrence M.C. Higher-resolution structure of the human insulin receptor ectodomain: Multi-modal inclusion of the insert domain. Structure. 2016;24:469–476. doi: 10.1016/j.str.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ye L., Maji S., Sanghera N., Gopalasingam P., Gorbunov E., Tarasov S., Epstein O., Klein-Seetharaman J. Structure and dynamics of the insulin receptor: Implications for receptor activation and drug discovery. Drug Discov. Today. 2017;22:1092–1102. doi: 10.1016/j.drudis.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 39.Scapin G., Dandey V.P., Zhang Z., Prosise W., Hruza A., Kelly T., Mayhood T., Strickland C., Potter C.S., Carragher B. Structure of the insulin receptor-insulin complex by single-particle cryo-EM analysis. Nature. 2018;556:122–125. doi: 10.1038/nature26153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McKern N.M., Lawrence M.C., Streltsov V.A., Lou M.Z., Adams T.E., Lovrecz G.O., Elleman T.C., Richards K.M., Bentley J.D., Pilling P.A., et al. Structure of the insulin receptor ectodomain reveals a folded-over conformation. Nature. 2006;443:218–221. doi: 10.1038/nature05106. [DOI] [PubMed] [Google Scholar]
- 41.Gutmann T., Kim K.H., Grzybek M., Walz T., Coskun U. Visualization of ligand-induced transmembrane signaling in the full-length human insulin receptor. J. Cell Biol. 2018;217:1643–1649. doi: 10.1083/jcb.201711047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uchikawa E., Choi E., Shang G., Yu H., Bai X.C. Activation mechanism of the insulin receptor revealed by cryo-EM structure of the fully liganded receptor-ligand complex. Elife. 2019;8:e48630. doi: 10.7554/eLife.48630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gutmann T., Schafer I.B., Poojari C., Brankatschk B., Vattulainen I., Strauss M., Coskun U. Cryo-EM structure of the complete and ligand-saturated insulin receptor ectodomain. J. Cell Biol. 2020;219:e201907210. doi: 10.1083/jcb.201907210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weis F., Menting J.G., Margetts M.B., Chan S.J., Xu Y., Tennagels N., Wohlfart P., Langer T., Muller C.W., Dreyer M.K., et al. The signalling conformation of the insulin receptor ectodomain. Nat. Commun. 2018;9:4420. doi: 10.1038/s41467-018-06826-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whittaker J., Whittaker L. Characterization of the functional insulin binding epitopes of the full-length insulin receptor. J. Biol. Chem. 2005;280:20932–20936. doi: 10.1074/jbc.M411320200. [DOI] [PubMed] [Google Scholar]
- 46.Menting J.G., Whittaker J., Margetts M.B., Whittaker L.J., Kong G.K., Smith B.J., Watson C.J., Zakova L., Kletvikova E., Jiracek J., et al. How insulin engages its primary binding site on the insulin receptor. Nature. 2013;493:241–245. doi: 10.1038/nature11781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chrudinova M., Zakova L., Marek A., Socha O., Budesinsky M., Hubalek M., Picha J., Machackova K., Jiracek J., Selicharova I. A versatile insulin analog with high potency for both insulin and insulin-like growth factor 1 receptors: Structural implications for receptor binding. J. Biol. Chem. 2018;293:16818–16829. doi: 10.1074/jbc.RA118.004852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kavran J.M., McCabe J.M., Byrne P.O., Connacher M.K., Wang Z., Ramek A., Sarabipour S., Shan Y., Shaw D.E., Hristova K., et al. How IGF-1 activates its receptor. Elife. 2014;3:e03772. doi: 10.7554/eLife.03772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu Y., Kong G.K., Menting J.G., Margetts M.B., Delaine C.A., Jenkin L.M., Kiselyov V.V., De Meyts P., Forbes B.E., Lawrence M.C. How ligand binds to the type 1 insulin-like growth factor receptor. Nat. Commun. 2018;9:821. doi: 10.1038/s41467-018-03219-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yunn N.-O., Park M., Noh J., Ryu S.H. Stepwise autophosphorylation regulates biased agonism of the insulin receptor. Faseb. J. 2019;34:s1.08794. doi: 10.1096/fasebj.2020.34.s1.08794. [DOI] [Google Scholar]
- 51.Hernandez-Sanchez C., Mansilla A., Pablo F.d., Zardoya R. Evolution of the insulin receptor family and receptor isoform expression in vertebrates. Mol. Biol. Evol. 2008;25:1043–1053. doi: 10.1093/molbev/msn036. [DOI] [PubMed] [Google Scholar]
- 52.Li J., Park J., Mayer J.P., Webb K.J., Uchikawa E., Wu J.Y., Liu S., Zhang X.W., Stowell M.H.B., Choi E., et al. Synergistic activation of the insulin receptor via two distinct sites. Nat. Struct. Mol. Biol. 2022;29:357–368. doi: 10.1038/s41594-022-00750-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Das D., Arur S. Conserved insulin signaling in the regulation of oocyte growth, development, and maturation. Mol. Reprod. Dev. 2017;84:444–459. doi: 10.1002/mrd.22806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lawrence M.C., McKern N.M., Ward C.W. Insulin receptor structure and its implications for the IGF-1 receptor. Curr. Opin. Struct Biol. 2007;17:699–705. doi: 10.1016/j.sbi.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 55.Sim C., Denlinger D.L. Insulin signaling and the regulation of insect diapause. Front. Physiol. 2013;4:189. doi: 10.3389/fphys.2013.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hall C., Yu H., Choi E. Insulin receptor endocytosis in the pathophysiology of insulin resistance. Exp. Mol. Med. 2020;52:911–920. doi: 10.1038/s12276-020-0456-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cai W., Sakaguchi M., Kleinridders A., Gonzalez-Del Pino G., Dreyfuss J.M., O’Neill B.T., Ramirez A.K., Pan H., Winnay J.N., Boucher J., et al. Domain-dependent effects of insulin and IGF-1 receptors on signalling and gene expression. Nat. Commun. 2017;8:14892. doi: 10.1038/ncomms14892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Okada Y., Katsuki M., Okamoto N., Fujioka H., Okada K. A specific type of insulin-like peptide regulates the conditional growth of a beetle weapon. PLoS Biol. 2019;17:e3000541. doi: 10.1371/journal.pbio.3000541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Laustsen P.G., Russell S.J., Cui L., Entingh-Pearsall A., Holzenberger M., Liao R., Kahn C.R. Essential role of insulin and insulin-like growth factor 1 receptor signaling in cardiac development and function. Mol. Cell Biol. 2007;27:1649–1664. doi: 10.1128/MCB.01110-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nassel D.R., Vanden Broeck J. Insulin/IGF signaling in Drosophila and other insects: Factors that regulate production, release and post-release action of the insulin-like peptides. Cell Mol. Life Sci. 2016;73:271–290. doi: 10.1007/s00018-015-2063-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Inoue H. Central insulin-mediated regulation of hepatic glucose production. Endocr. J. 2016;63:EJ15-0540. doi: 10.1507/endocrj.EJ15-0540. [DOI] [PubMed] [Google Scholar]
- 62.Nijhout H.F., Smith W.A., Schachar I., Subramanian S., Tobler A., Grunert L.W. The control of growth and differentiation of the wing imaginal disks of Manduca sexta. Dev. Biol. 2007;302:569–576. doi: 10.1016/j.ydbio.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 63.Chen C.H., Pan J., Di Y.Q., Liu W., Hou L., Wang J.X., Zhao X.F. Protein kinase C delta phosphorylates ecdysone receptor B1 to promote gene expression and apoptosis under 20-hydroxyecdysone regulation. Proc. Natl. Acad. Sci. USA. 2017;114:E7121–E7130. doi: 10.1073/pnas.1704999114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ajaha A., Bouayad N., Aarab A., Rharrabe K. Effect of 20-hydroxyecdysone, a phytoecdysteroid, on development, digestive, and detoxification enzyme activities of Tribolium castaneum (Coleoptera: Tenebrionidae) J. Insect Sci. 2019;19:18. doi: 10.1093/jisesa/iez097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu Q., Brown M.R. Signaling and function of insulin-like peptides in insects. Annu. Rev. Entomol. 2006;51:1–24. doi: 10.1146/annurev.ento.51.110104.151011. [DOI] [PubMed] [Google Scholar]
- 66.Nagasawa H., Kataoka H., Isogai A., Tamura S., Suzuki A., Ishizaki H., Mizoguchi A., Fujiwara Y., Suzuki A. Amino-terminal amino acid sequence of the silkworm prothoracicotropic hormone: Homology with insulin. Science. 1984;226:1344–1345. doi: 10.1126/science.226.4680.1344. [DOI] [PubMed] [Google Scholar]
- 67.Siddle K. Molecular basis of signaling specificity of insulin and IGF receptors: Neglected corners and recent advances. Front. Endocrinol. 2012;3:34. doi: 10.3389/fendo.2012.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boucher J., Kleinridders A., Kahn C.R. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb Perspect Biol. 2014;6:a009191. doi: 10.1101/cshperspect.a009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Versteyhe S., Blanquart C., Hampe C., Mahmood S., Christeff N., Meyts1 P.D., Gray S.G., Issad T. Insulin receptor substrates-5 and -6 are poor substrates for the insulin receptor. Mol. Med. Rep. 2010;3:189–193. doi: 10.3892/mmr_00000239. [DOI] [PubMed] [Google Scholar]
- 70.Schmidt V., Schulz N., Yan X., Schurmann A., Kempa S., Kern M., Bluher M., Poy M.N., Olivecrona G., Willnow T.E. SORLA facilitates insulin receptor signaling in adipocytes and exacerbates obesity. J. Clin. Investig. 2016;126:2706–2720. doi: 10.1172/JCI84708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rask-Madsen C., Kahn C.R. Tissue-specific insulin signaling, metabolic syndrome, and cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 2012;32:2052–2059. doi: 10.1161/ATVBAHA.111.241919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shi X., Xie X., Jia Y., Li S. Associations of insulin receptor and insulin receptor substrates genetic polymorphisms with polycystic ovary syndrome: A systematic review and meta-analysis. J. Obstet. Gynaecol. Res. 2016;42:844–854. doi: 10.1111/jog.13002. [DOI] [PubMed] [Google Scholar]
- 73.Gralle M., Labrecque S., Salesse C., De Koninck P. Spatial dynamics of the insulin receptor in living neurons. J. Neurochem. 2020;156:88–105. doi: 10.1111/jnc.14950. [DOI] [PubMed] [Google Scholar]
- 74.Ling A.V., Gearing M.E., Semova I., Shin D.J., Clements R., Lai Z.W., Biddinger S.B. FoxO1 is required for most of the metabolic and hormonal perturbations produced by hepatic insulin receptor deletion in male mice. Endocrinology. 2018;159:1253–1263. doi: 10.1210/en.2017-00870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Titone R., Robertson D.M. Insulin receptor preserves mitochondrial function by binding VDAC1 in insulin insensitive mucosal epithelial cells. FASEB J. 2019;34:754–775. doi: 10.1096/fj.201901316RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Oakie A., Zhou L., Rivers S., Cheung C., Li J., Wang R. Postnatal knockout of beta cell insulin receptor impaired insulin secretion in male mice exposed to high-fat diet stress. Mol. Cell Endocrinol. 2020;499:110588. doi: 10.1016/j.mce.2019.110588. [DOI] [PubMed] [Google Scholar]
- 77.Ardon O., Procter M., Tvrdik T., Longo N., Mao R. Sequencing analysis of insulin receptor defects and detection of two novel mutations in INSR gene. Mol. Genet. Metab. Rep. 2014;1:71–84. doi: 10.1016/j.ymgmr.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Greenhill C. Insulin and the insulin receptor regulate gene expression. Nat. Rev. Endocrinol. 2019;15:315. doi: 10.1038/s41574-019-0206-6. [DOI] [PubMed] [Google Scholar]
- 79.Hancock M.L., Meyer R.C., Mistry M., Khetani R.S., Wagschal A., Shin T., Ho Sui S.J., Naar A.M., Flanagan J.G. Insulin receptor associates with promoters genome-wide and regulates gene expression. Cell. 2019;177:722–736. doi: 10.1016/j.cell.2019.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ott R., Melchior K., Stupin J.H., Ziska T., Schellong K., Henrich W., Rancourt R.C., Plagemann A. Reduced insulin receptor expression and altered DNA methylation in fat tissues and blood of women with GDM and offspring. J. Clin. Endocrinol. Metab. 2019;104:137–149. doi: 10.1210/jc.2018-01659. [DOI] [PubMed] [Google Scholar]
- 81.Schellong K., Melchior K., Ziska T., Ott R., Henrich W., Rancourt R.C., Plagemann A. Hypothalamic insulin receptor expression and DNA promoter methylation are sex-specifically altered in adult offspring of high-fat diet (HFD)-overfed mother rats. J. Nutr. Biochem. 2019;67:28–35. doi: 10.1016/j.jnutbio.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 82.Stolzenbach F., Valdivia S., Ojeda-Provoste P., Toledo F., Sobrevia L., Kerr B. DNA methylation changes in genes coding for leptin and insulin receptors during metabolic-altered pregnancies. Biochim. Biophys. Acta Mol. Basis Dis. 2020;1866:165465. doi: 10.1016/j.bbadis.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 83.Pan J., Di Y.Q., Li Y.B., Chen C.H., Wang J.X., Zhao X.F. Insulin and 20-hydroxyecdysone oppose each other in the regulation of phosphoinositide-dependent kinase-1 expression during insect pupation. J. Biol. Chem. 2018;293:18613–18623. doi: 10.1074/jbc.RA118.004891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu Y., Zhou S., Ma L., Tian L., Wang S., Sheng Z., Jiang R.J., Bendena W.G., Li S. Transcriptional regulation of the insulin signaling pathway genes by starvation and 20-hydroxyecdysone in the Bombyx fat body. J. Insect Physiol. 2010;56:1436–1444. doi: 10.1016/j.jinsphys.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 85.Li Y.L., Yao Y.X., Zhao Y.M., Di Y.Q., Zhao X.F. The steroid hormone 20-hydroxyecdysone counteracts insulin signaling via insulin receptor dephosphorylation. J. Biol. Chem. 2021;296:100318. doi: 10.1016/j.jbc.2021.100318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Emlen D.J., Warren I.A., Johns A., Dworkin I., Lavine L.C. A mechanism of extreme growth and reliable signaling in sexually selected ornaments and weapons. Science. 2012;337:860–864. doi: 10.1126/science.1224286. [DOI] [PubMed] [Google Scholar]
- 87.Casasa S., Moczek A.P. Insulin signalling’s role in mediating tissue-specific nutritional plasticity and robustness in the horn-polyphenic beetle Onthophagus taurus. Proc. Biol. Sci. 2018;285:20181631. doi: 10.1098/rspb.2018.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Eichler K., Li F., Litwin-Kumar A., Park Y., Andrade I., Schneider-Mizell C.M., Saumweber T., Huser A., Eschbach C., Gerber B., et al. The complete connectome of a learning and memory centre in an insect brain. Nature. 2017;548:175–182. doi: 10.1038/nature23455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thum A.S., Gerber B. Connectomics and function of a memory network: The mushroom body of larval Drosophila. Curr. Opin. Neurobiol. 2019;54:146–154. doi: 10.1016/j.conb.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 90.Eschment M., Franz H.R., Gullu N., Holscher L.G., Huh K.E., Widmann A. Insulin signaling represents a gating mechanism between different memory phases in Drosophila larvae. PLoS Genet. 2020;16:e1009064. doi: 10.1371/journal.pgen.1009064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Toprak U. The role of peptide hormones in insect lipid metabolism. Front. Physiol. 2020;11:434. doi: 10.3389/fphys.2020.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Smykal V., Pivarci M., Provaznik J., Bazalova O., Jedlicka P., Luksan O., Horak A., Vaneckova H., Benes V., Fiala I., et al. Complex evolution of insect insulin receptors and homologous decoy receptors, and functional significance of their multiplicity. Mol. Biol. Evol. 2020;37:1775–1789. doi: 10.1093/molbev/msaa048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Barbera M., Canas-Canas R., Martinez-Torres D. Insulin-like peptides involved in photoperiodism in the aphid Acyrthosiphon pisum. Insect Biochem. Mol. Biol. 2019;112:103185. doi: 10.1016/j.ibmb.2019.103185. [DOI] [PubMed] [Google Scholar]
- 94.Lin X., Yu N., Smagghe G. Insulin receptor regulates food intake through sulfakinin signaling in the red flour beetle, Tribolium castaneum. Peptides. 2016;80:89–95. doi: 10.1016/j.peptides.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 95.Sang M., Li C., Wu W., Li B. Identification and evolution of two insulin receptor genes involved in Tribolium castaneum development and reproduction. Gene. 2016;585:196–204. doi: 10.1016/j.gene.2016.02.034. [DOI] [PubMed] [Google Scholar]
- 96.Nuss A.B., Brown M.R., Murty U.S., Gulia-Nuss M. Insulin receptor knockdown blocks filarial parasite development and alters egg production in the southern house mosquito, Culex quinquefasciatus. PLoS Negl. Trop. Dis. 2018;12:e0006413. doi: 10.1371/journal.pntd.0006413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ihle K.E., Mutti N.S., Kaftanoglu O., Amdam G.V. Insulin receptor substrate gene knockdown accelerates behavioural maturation and shortens lifespan in honeybee workers. Insects. 2019;10:390. doi: 10.3390/insects10110390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Han B., Zhang T., Feng Y., Liu X., Zhang L., Chen H., Zeng F., Wang M., Liu C., Li Y., et al. Two insulin receptors coordinate oogenesis and oviposition via two pathways in the green lacewing, Chrysopa pallens. J. Insect Physiol. 2020;123:104049. doi: 10.1016/j.jinsphys.2020.104049. [DOI] [PubMed] [Google Scholar]
- 99.Petruzzelli L., Herrera R., Garcia-Arenas R., Rosen O.M. Acquisition of insulin-dependent protein tyrosine kinase activity during Drosophila embryogenesis. J. Biol. Chem. 1985;10:351–353. doi: 10.1016/S0021-9258(17)36202-6. [DOI] [PubMed] [Google Scholar]
- 100.Kremer L.P.M., Korb J., Bornberg-Bauer E. Reconstructed evolution of insulin receptors in insects reveals duplications in early insects and cockroaches. J. Exp. Zool. Part B. 2018;330:305–311. doi: 10.1002/jez.b.22809. [DOI] [PubMed] [Google Scholar]
- 101.Chambers D.B., Androschuk A., Rosenfelt C., Langer S., Harding M., Bolduc F.V. Insulin signaling is acutely required for long-term memory in Drosophila. Front. Neural. Circuits. 2015;9:8. doi: 10.3389/fncir.2015.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tanabe K., Itoh M., Tonoki A. Age-related changes in insulin-like signaling lead to intermediate-term memory impairment in Drosophila. Cell Rep. 2017;18:1598–1605. doi: 10.1016/j.celrep.2017.01.053. [DOI] [PubMed] [Google Scholar]
- 103.Teleman A.A. Molecular mechanisms of metabolic regulation by insulin in Drosophila. Biochem. J. 2009;425:13–26. doi: 10.1042/BJ20091181. [DOI] [PubMed] [Google Scholar]
- 104.Nassel D.R., Kubrak O.I., Liu Y., Luo J., Lushchak O.V. Factors that regulate insulin producing cells and their output in Drosophila. Front. Physiol. 2013;4:252. doi: 10.3389/fphys.2013.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Erion R., Sehgal A. Regulation of insect behavior via the insulin-signaling pathway. Front. Physiol. 2013;4:353. doi: 10.3389/fphys.2013.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ding B.Y., Shang F., Zhang Q., Xiong Y., Yang Q., Niu J.Z., Smagghe G., Wang J.J. Silencing of two insulin receptor genes disrupts nymph-adult transition of alate brown citrus aphid. Int. J. Mol. Sci. 2017;18:357. doi: 10.3390/ijms18020357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Baki A.A., Jung J.K., Kim Y. Alteration of insulin signaling to control insect pest by using transformed bacteria expressing dsRNA. Pest. Manag. Sci. 2020;76:1020–1030. doi: 10.1002/ps.5612. [DOI] [PubMed] [Google Scholar]
- 108.Gulia-Nuss M., Robertson A.E., Brown M.R., Strand M.R. Insulin-like peptides and the target of rapamycin pathway coordinately regulate blood digestion and egg maturation in the mosquito Aedes aegypti. PLoS ONE. 2011;6:e20401. doi: 10.1371/journal.pone.0020401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sim C., Denlinger D.L. Insulin signaling and FOXO regulate the overwintering diapause of the mosquito Culex pipiens. Proc. Natl. Acad. Sci. USA. 2008;105:6777–6781. doi: 10.1073/pnas.0802067105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhao Y.O., Kurscheid S., Zhang Y., Liu L., Zhang L., Loeliger K., Fikrig E. Enhanced survival of Plasmodium-infected mosquitoes during starvation. PLoS ONE. 2012;7:e40556. doi: 10.1371/journal.pone.0040556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Castillo J., Brown M.R., Strand M.R. Blood feeding and insulin-like peptide 3 stimulate proliferation of hemocytes in the mosquito Aedes aegypti. PLoS Pathog. 2011;7:e1002274. doi: 10.1371/journal.ppat.1002274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Silva-Oliveira G., De Paula I.F., Medina J.M., Alves-Bezerra M., Gondim K.C. Insulin receptor deficiency reduces lipid synthesis and reproductive function in the insect Rhodnius prolixus. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2020;1866:158851. doi: 10.1016/j.bbalip.2020.158851. [DOI] [PubMed] [Google Scholar]
- 113.Bui L.T., Ivers N.A., Ragsdale E.J. A sulfotransferase dosage-dependently regulates mouthpart polyphenism in the nematode Pristionchus pacificus. Nat. Commun. 2018;9:4119. doi: 10.1038/s41467-018-05612-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yang C.H., Andrew Pospisilik J. Polyphenism—a window into gene-environment interactions and phenotypic plasticity. Front. Genet. 2019;10:132. doi: 10.3389/fgene.2019.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hoy M.A. Insect Molecular Genetics. Elsevier Science; Amsterdam, The Netherlands: 2019. Molecular genetics of insect behavior; pp. 413–461. [Google Scholar]
- 116.Wheeler D.E., Buck N., Evans J.D. Expression of insulin pathway genes during the period of caste determination in the honey bee, Apis mellifera. Insect Mol. Biol. 2006;15:597–602. doi: 10.1111/j.1365-2583.2006.00681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Azevedo S.V., Hartfelder K. The insulin signaling pathway in honey bee (Apis mellifera) caste development-differential expression of insulin-like peptides and insulin receptors in queen and worker larvae. J. Insect Physiol. 2008;54:1064–1071. doi: 10.1016/j.jinsphys.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 118.Lu H.L., Pietrantonio P.V. Insect insulin receptors: Insights from sequence and caste expression analyses of two cloned hymenopteran insulin receptor cDNAs from the fire ant. Insect Mol Biol. 2011;20:637–649. doi: 10.1111/j.1365-2583.2011.01094.x. [DOI] [PubMed] [Google Scholar]
- 119.Wheeler D.E., Buck N.A., Evans J.D. Expression of insulin/insulin-like signalling and TOR pathway genes in honey bee caste determination. Insect Mol. Biol. 2014;23:113–121. doi: 10.1111/imb.12065. [DOI] [PubMed] [Google Scholar]
- 120.Corona M., Libbrecht R., Wheeler D.E. Molecular mechanisms of phenotypic plasticity in social insects. Curr. Opin. Insect Sci. 2016;13:55–60. doi: 10.1016/j.cois.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 121.Mutti N.S., Dolezal A.G., Wolschin F., Mutti J.S., Gill K.S., Amdam G.V. IRS and TOR nutrient-signaling pathways act via juvenile hormone to influence honey bee caste fate. J. Exp. Biol. 2011;214:3977–3984. doi: 10.1242/jeb.061499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wolschin F., Mutti N.S., Amdam G.V. Insulin receptor substrate influences female caste development in honeybees. Biol. Lett. 2011;7:112–115. doi: 10.1098/rsbl.2010.0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xu H.J., Xue J., Lu B., Zhang X.C., Zhuo J.C., He S.F., Ma X.F., Jiang Y.Q., Fan H.W., Xu J.Y., et al. Two insulin receptors determine alternative wing morphs in planthoppers. Nature. 2015;519:464–467. doi: 10.1038/nature14286. [DOI] [PubMed] [Google Scholar]
- 124.Ye X., Xu L., Li X., He K., Hua H., Cao Z., Xu J., Ye W., Zhang J., Yuan Z., et al. miR-34 modulates wing polyphenism in planthopper. PLoS Genet. 2019;15:e1008235. doi: 10.1371/journal.pgen.1008235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xu H.J., Zhang C.X. Insulin receptors and wing dimorphism in rice planthoppers. Philos. Trans. R Soc. Lond. B Biol. Sci. 2017;372:20150489. doi: 10.1098/rstb.2015.0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li X., Liu F., Wu C., Zhao J., Cai W., Hua H. Decapentaplegic function in wing vein development and wing morph transformation in brown planthopper, Nilaparvata lugens. Dev. Biol. 2019;449:143–150. doi: 10.1016/j.ydbio.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 127.Zhang C.X., Brisson J.A., Xu H.J. Molecular mechanisms of wing polymorphism in insects. Annu. Rev. Entomol. 2019;64:297–314. doi: 10.1146/annurev-ento-011118-112448. [DOI] [PubMed] [Google Scholar]
- 128.Lin X., Gao H., Xu Y., Zhang Y., Li Y., Lavine M.D., Lavine L.C. Cell cycle progression determines wing morph in the polyphenic insect Nilaparvata lugens. iScience. 2020;23:101040. doi: 10.1016/j.isci.2020.101040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhuo J.C., Chen L., Shi J.K., Xu N., Xue W.H., Zhang M.Q., Ren Z.W., Zhang H.H., Zhang C.X. Tra-2 mediates crosstalk between sex determination and wing polyphenism in female Nilaparvata lugens. Genetics. 2017;207:1067–1078. doi: 10.1534/genetics.117.300328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Belgacem Y.H., Martin J.R. Disruption of insulin pathways alters trehalose level and abolishes sexual dimorphism in locomotor activity in Drosophila. J. Neurobiol. 2006;66:19–32. doi: 10.1002/neu.20193. [DOI] [PubMed] [Google Scholar]
- 131.Belgacem Y.H., Martin J.R. Hmgcr in the corpus allatum controls sexual dimorphism of locomotor activity and body size via the insulin pathway in Drosophila. PLoS ONE. 2007;2:e187. doi: 10.1371/journal.pone.0000187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hinke S.A., Cieniewicz A.M., Kirchner T., D’Aquino K., Nanjunda R., Aligo J., Perkinson R., Cooper P., Boayke K., Chiu M.L., et al. Unique pharmacology of a novel allosteric agonist/sensitizer insulin receptor monoclonal antibody. Mol. Metab. 2018;10:87–99. doi: 10.1016/j.molmet.2018.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Changeux J.P., Christopoulos A. Allosteric modulation as a unifying mechanism for receptor function and regulation. Cell. 2016;166:1084–1102. doi: 10.1016/j.cell.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 134.Othman E.M., Kreissl M.C., Kaiser F.R., Arias-Loza P.A., Stopper H. Insulin-mediated oxidative stress and DNA damage in LLC-PK1 pig kidney cell line, female rat primary kidney cells, and male ZDF rat kidneys in vivo. Endocrinology. 2013;154:1434–1443. doi: 10.1210/en.2012-1768. [DOI] [PubMed] [Google Scholar]
- 135.Lefever E., Vliebergh J., Mathieu C. Improving the treatment of patients with diabetes using insulin analogues: Current findings and future directions. Expert. Opin. Drug Saf. 2021;20:155–169. doi: 10.1080/14740338.2021.1856813. [DOI] [PubMed] [Google Scholar]
- 136.Kasia J., Lipska M.D. Insulin analogues for type 2 diabetes. Jama-J Am. Med. Assoc. 2019;321:350–351. doi: 10.1001/jama.2018.21356. [DOI] [PubMed] [Google Scholar]
- 137.Kramer C.K., Retnakaran R., Zinman B. Insulin and insulin analogs as antidiabetic therapy: A perspective from clinical trials. Cell Metab. 2021;33:740–747. doi: 10.1016/j.cmet.2021.03.014. [DOI] [PubMed] [Google Scholar]
- 138.Mohammadiarani H., Vashisth H. Insulin mimetic peptide S371 folds into a helical structure. J. Comput Chem. 2017;38:1158–1166. doi: 10.1002/jcc.24746. [DOI] [PubMed] [Google Scholar]
- 139.Jensen M., Palsgaard J., Borup R., de Meyts P., Schaffer L. Activation of the insulin receptor (IR) by insulin and a synthetic peptide has different effects on gene expression in IR-transfected L6 myoblasts. Biochem. J. 2008;412:435–445. doi: 10.1042/BJ20080279. [DOI] [PubMed] [Google Scholar]
- 140.Schaffer L., Brand C.L., Hansen B.F., Ribel U., Shaw A.C., Slaaby R., Sturis J. A novel high-affinity peptide antagonist to the insulin receptor. Biochem. Biophys. Res. Commun. 2008;376:380–383. doi: 10.1016/j.bbrc.2008.08.151. [DOI] [PubMed] [Google Scholar]
- 141.Vikram A., Jena G. S961, an insulin receptor antagonist causes hyperinsulinemia, insulin-resistance and depletion of energy stores in rats. Biochem. Biophys. Res. Commun. 2010;398:260–265. doi: 10.1016/j.bbrc.2010.06.070. [DOI] [PubMed] [Google Scholar]
- 142.Knudsen L., Hansen B.F., Jensen P., Pedersen T.A., Vestergaard K., Schaffer L., Blagoev B., Oleksiewicz M.B., Kiselyov V.V., Meyts P.D. Agonism and antagonism at the insulin receptor. PLoS ONE. 2012;7:e51972. doi: 10.1371/journal.pone.0051972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Taylor S.I., Accili D., Cama A., Imano E., Kadowaki H., Kadowaki T. Unusual forms of insulin resistance. Annu. Rev. Med. 1991;42:373–379. doi: 10.1146/annurev.me.42.020191.002105. [DOI] [PubMed] [Google Scholar]
- 144.Bhaskar V., Goldfine I.D., Bedinger D.H., Lau A., Kuan H.F., Gross L.M., Handa M., Maddux B.A., Watson S.R., Zhu S., et al. A fully human, allosteric monoclonal antibody that activates the insulin receptor and improves glycemic control. Diabetes. 2012;61:1263–1271. doi: 10.2337/db11-1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Issafras H., Bedinger D.H., Corbin J.A., Goldfine I.D., Bhaskar V., White M.L., Rubin P., Scannon P.J. Selective allosteric antibodies to the insulin receptor for the treatment of hyperglycemic and hypoglycemic disorders. J. Diabetes Sci. Technol. 2014;8:865–873. doi: 10.1177/1932296814529886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Corbin J.A., Bhaskar V., Goldfine I.D., Bedinger D.H., Lau A., Michelson K., Gross L.M., Maddux B.A., Kuan H.F., Tran C., et al. Improved glucose metabolism in vitro and in vivo by an allosteric monoclonal antibody that increases insulin receptor binding affinity. PLoS ONE. 2014;9:e88684. doi: 10.1371/journal.pone.0088684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Corbin J.A., Bhaskar V., Goldfine I.D., Issafras H., Bedinger D.H., Lau A., Michelson K., Gross L.M., Maddux B.A., Kuan H.F., et al. Inhibition of insulin receptor function by a human, allosteric monoclonal antibody: A potential new approach for the treatment of hyperinsulinemic hypoglycemia. Mabs. 2014;6:262–272. doi: 10.4161/mabs.26871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Johnson K.W., Neale A., Gordon A., Roessig J., Bezwada P., Vukelich S., Goldfine I., Rubin P. Attenuation of insulin action by an allosteric insulin receptor antibody in healthy volunteers. J. Clin. Endocrinol. Metab. 2017;102:3021–3028. doi: 10.1210/jc.2017-00822. [DOI] [PubMed] [Google Scholar]
- 149.Cieniewicz A.M., Kirchner T., Hinke S.A., Nanjunda R., D’Aquino K., Boayke K., Cooper P.R., Perkinson R., Chiu M.L., Jarantow S., et al. Novel monoclonal antibody is an allosteric insulin receptor antagonist that induces insulin resistance. Diabetes. 2017;66:206–217. doi: 10.2337/db16-0633. [DOI] [PubMed] [Google Scholar]
- 150.Tuerk C., Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990;249:505–510. doi: 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]
- 151.Sefah K., Shangguan D., Xiong X., O’Donoghue M.B., Tan W. Development of DNA aptamers using Cell-SELEX. Nat. Protoc. 2010;5:1169–1185. doi: 10.1038/nprot.2010.66. [DOI] [PubMed] [Google Scholar]
- 152.Yunn N.-O., Koh A., Han S., Lim J.H., Park S., Lee J., Kim E., Jang S.K., Berggren P.O., Ryu S.H. Agonistic aptamer to the insulin receptor leads to biased signaling and functional selectivity through allosteric modulation. Nucleic. Acids Res. 2015;43:7688–7701. doi: 10.1093/nar/gkv767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Park M., Yunn N.-O., Ryu S.H. Sensitizing aptamer INSR-A43 enhances insulin sensitivity and sensitizes insulin receptor signaling pathways. Faseb J. 2020;34:S1. doi: 10.1096/fasebj.2020.34.s1.01971. [DOI] [Google Scholar]
- 154.Yunn N.-O., Lee J., Lee H.S., Oh E.J., Park M., Park S., Jin S.Y., Shin E., Lee J.W.Y., Kim Y., et al. An aptamer agonist of the insulin receptor acts as a positive or negative allosteric modulator, depending on its concentration. Exp. Mol. Med. 2022;54:531–541. doi: 10.1038/s12276-022-00760-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Iaboni M., Fontanella R., Rienzo A., Capuozzo M., Nuzzo S., Santamaria G., Catuogno S., Condorelli G., Franciscis V.D., Esposito C.L. Targeting insulin receptor with a novel internalizing aptamer. Mol. Ther-Nucl. Acids. 2016;5:e365. doi: 10.1038/mtna.2016.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yunn N.-O., Park M., Park S., Lee J., Noh J., Shin E., Ryu S.H. A hotspot for enhancing insulin receptor activation revealed by a conformation-specific allosteric aptamer. Nucleic Acids Res. 2021;49:700–712. doi: 10.1093/nar/gkaa1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Desbuquois B., Carre N., Burnol A.F. Regulation of insulin and type 1 insulin-like growth factor signaling and action by the Grb10/14 and SH2B1/B2 adaptor proteins. FEBS J. 2013;280:794–816. doi: 10.1111/febs.12080. [DOI] [PubMed] [Google Scholar]
- 158.Yin Y., Chen C., Chen J., Zhan R., Zhang Q., Xu X., Li D., Li M. Cell surface GRP78 facilitates hepatoma cells proliferation and migration by activating IGF-IR. Cell Signal. 2017;35:154–162. doi: 10.1016/j.cellsig.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 159.Gu X., Reagan A.M., McClellan M.E., Elliott M.H. Caveolins and caveolae in ocular physiology and pathophysiology. Prog. Retin. Eye. Res. 2017;56:84–106. doi: 10.1016/j.preteyeres.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Fernandes I.P.G., Oliveira-Brett A.M. Caveolin proteins electrochemical oxidation and interaction with cholesterol. Bioelectrochemistry. 2020;133:107451. doi: 10.1016/j.bioelechem.2019.107451. [DOI] [PubMed] [Google Scholar]
- 161.Kwon H., Lee J., Jeong K., Jang D., Pak Y. Fatty acylated caveolin-2 is a substrate of insulin receptor tyrosine kinase for insulin receptor substrate-1-directed signaling activation. Biochim. Biophys. Acta. 2015;1853:1022–1034. doi: 10.1016/j.bbamcr.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 162.Kwon H., Choi M., Ahn Y., Pak Y. N-myristoylation regulates insulin-induced phosphorylation and ubiquitination of Caveolin-2 for insulin signaling. Biochem. Biophys. Res. Commun. 2020;532:535–540. doi: 10.1016/j.bbrc.2020.08.072. [DOI] [PubMed] [Google Scholar]
- 163.Kwon H., Jang D., Choi M., Lee J., Jeong K., Pak Y. Alternative translation initiation of Caveolin-2 desensitizes insulin signaling through dephosphorylation of insulin receptor by PTP1B and causes insulin resistance. Biochim. Biophys. Acta Mol. Basis Dis. 2018;1864:2169–2182. doi: 10.1016/j.bbadis.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 164.Zhao N., Liu C.C., Qiao W., Bu G. Apolipoprotein E, receptors, and modulation of Alzheimer’s disease. Biol. Psychiatry. 2018;83:347–357. doi: 10.1016/j.biopsych.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chan E.S., Shetty M.S., Sajikumar S., Chen C., Soong T.W., Wong B.S. ApoE4 expression accelerates hippocampus-dependent cognitive deficits by enhancing Abeta impairment of insulin signaling in an Alzheimer’s disease mouse model. Sci. Rep. 2016;6:26119. doi: 10.1038/srep26119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhao N., Liu C.C., Ingelgom A.J.V., Martens Y.A., Linares C., Knight J.A., Painter M.M., Sullivan P.M., Bu G.J. Apolipoprotein E4 impairs neuronal insulin signaling by trapping insulin receptor in the endosomes. Neuron. 2017;96:115–129. doi: 10.1016/j.neuron.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ong Q.R., Chan E.S., Lim M.L., Cole G.M., Wong B.S. Reduced phosphorylation of brain insulin receptor substrate and Akt proteins in apolipoprotein-E4 targeted replacement mice. Sci. Rep. 2014;4:3754. doi: 10.1038/srep03754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ussar S., Bezy O., Bluher M., Kahn C.R. Glypican-4 enhances insulin signaling via interaction with the insulin receptor and serves as a novel adipokine. Diabetes. 2012;61:2289–2298. doi: 10.2337/db11-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Liao P.Y., Lo H.Y., Liu I.C., Lo L.C., Hsiang C.Y., Ho T.Y. A gastro-resistant peptide from Momordica charantia improves diabetic nephropathy in db/db mice via its novel reno-protective and anti-inflammatory activities. Food Funct. 2022;13:1822–1833. doi: 10.1039/D1FO02788C. [DOI] [PubMed] [Google Scholar]
- 170.Brown J.E., Onyango D.J., Ramanjaneya M., Conner A.C., Patel S.T., Dunmore S.J., Randeva H.S. Visfatin regulates insulin secretion, insulin receptor signalling and mRNA expression of diabetes-related genes in mouse pancreatic beta-cells. J. Mol. Endocrinol. 2010;44:171–178. doi: 10.1677/JME-09-0071. [DOI] [PubMed] [Google Scholar]
- 171.Mitrofanova A., Mallela S.K., Ducasa G.M., Yoo T.H., Rosenfeld-Gur E., Zelnik I.D., Molina J., Varona Santos J., Ge M., Sloan A., et al. SMPDL3b modulates insulin receptor signaling in diabetic kidney disease. Nat. Commun. 2019;10:2692. doi: 10.1038/s41467-019-10584-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Prabhakar P.K., Sivakumar P.M. Protein tyrosine phosphatase 1B inhibitors: A novel therapeutic strategy for the management of type 2 diabetes mellitus. Curr. Pharm. Des. 2019;25:2526–2539. doi: 10.2174/1381612825666190716102901. [DOI] [PubMed] [Google Scholar]
- 173.Verma M., Gupta S.J., Chaudhary A., Garg V.K. Protein tyrosine phosphatase 1B inhibitors as antidiabetic agents—A brief review. Bioorg Chem. 2017;70:267–283. doi: 10.1016/j.bioorg.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 174.Petersen M.C., Madiraju A.K., Gassaway B.M., Marcel M., Nasiri A.R., Butrico G., Marcucci M.J., Zhang D., Abulizi A., Zhang X.M., et al. Insulin receptor Thr1160 phosphorylation mediates lipid-induced hepatic insulin resistance. J. Clin. Investig. 2016;126:4361–4371. doi: 10.1172/JCI86013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Xi Z.H., Fang L., Xu J., Li B.S., Zuo Z.H., Lv L.J., Wang C.G. Exposure to Aroclor 1254 persistently suppresses the functions of pancreatic beta-cells and deteriorates glucose homeostasis in male mice. Environ. Pollut. 2019;249:822–830. doi: 10.1016/j.envpol.2019.03.101. [DOI] [PubMed] [Google Scholar]
- 176.Gorbunov E.A., Nicoll J., Kachaeva E.V., Tarasov S.A., Epstein O.I. Subetta increases phosphorylation of insulin receptor beta-subunit alone and in the presence of insulin. Nutr. Diabetes. 2015;5:e169. doi: 10.1038/nutd.2015.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lawrence C.F., Margetts M.B., Menting J.G., Smith N.A., Smith B.J., Ward C.W., Lawrence M.C. Insulin mimetic peptide disrupts the primary binding site of the insulin receptor. J. Biol. Chem. 2016;291:15473–15481. doi: 10.1074/jbc.M116.732180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sharma P., Kumar S. S961, a biosynthetic insulin receptor antagonist, downregulates insulin receptor expression & suppresses the growth of breast cancer cells. Indian J. Med. Res. 2018;147:545–551. doi: 10.4103/ijmr.IJMR_403_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Oakie A., Wang R. Beta-cell receptor tyrosine kinases in controlling insulin secretion and exocytotic machinery: C-kit and insulin receptor. Endocrinology. 2018;159:3813–3821. doi: 10.1210/en.2018-00716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ward C.W., Menting J.G., Lawrence M.C. The insulin receptor changes conformation in unforeseen ways on ligand binding: Sharpening the picture of insulin receptor activation. Bioessays. 2013;35:945–954. doi: 10.1002/bies.201300065. [DOI] [PubMed] [Google Scholar]
- 181.Gherzi R., Andraghetti G., Versari G., Cordera R. Effect of insulin receptor autophosphorylation on insulin receptor binding. Mol. Cell Endocrinol. 1986;45:247–252. doi: 10.1016/0303-7207(86)90154-1. [DOI] [PubMed] [Google Scholar]
- 182.Ross J., Miron C.E., Plescia J., Laplante P., McBride K., Moitessier N., Moroy T. Targeting MYC: From understanding its biology to drug discovery. Eur. J. Med. Chem. 2020;213:113137. doi: 10.1016/j.ejmech.2020.113137. [DOI] [PubMed] [Google Scholar]
- 183.Schlein M., Ludvigsen S., Olsen H.B., Andersen A.S., Danielsen G.M., Kaarsholm N.C. Properties of small molecules affecting insulin receptor function. Biochemistry. 2001;40:13520–13528. doi: 10.1021/bi015672w. [DOI] [PubMed] [Google Scholar]
- 184.Strekalova T., Costa-Nunes J.P., Veniaminova E., Kubatiev A., Lesch K.P., Chekhonin V.P., Evans M.C., Steinbusch H.W. Insulin receptor sensitizer, dicholine succinate, prevents both Toll-like receptor 4 (TLR4) upregulation and affective changes induced by a high-cholesterol diet in mice. J. Affect. Disord. 2016;196:109–116. doi: 10.1016/j.jad.2016.02.045. [DOI] [PubMed] [Google Scholar]
- 185.Cline B.H., Costa-Nunes J.P., Cespuglio R., Markova N., Santos A.I., Bukhman Y.V., Kubatiev A., Steinbusch H.W., Lesch K.P., Strekalova T. Dicholine succinate, the neuronal insulin sensitizer, normalizes behavior, REM sleep, hippocampal pGSK3 beta and mRNAs of NMDA receptor subunits in mouse models of depression. Front. Behav. Neurosci. 2015;9:37. doi: 10.3389/fnbeh.2015.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kim W.J., Lee W., Jung Y., Jang H.J., Kim Y.K., Kim S.N. PPARbeta/delta agonist GW501516 inhibits TNFalpha-induced repression of adiponectin and insulin receptor in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2019;510:621–628. doi: 10.1016/j.bbrc.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 187.He K., Chan C.B., Liu X., Jia Y., Luo H.R., France S.A., Liu Y., Wilson W.D., Ye K. Identification of a molecular activator for insulin receptor with potent anti-diabetic effects. J. Biol. Chem. 2011;286:37379–37388. doi: 10.1074/jbc.M111.247387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Marsilje T.H., Pei W., Chen B., Lu W., Uno T., Jin Y., Jiang T., Kim S., Li N., Warmuth M., et al. Synthesis, structure-activity relationships, and in vivo efficacy of the novel potent and selective anaplastic lymphoma kinase (ALK) inhibitor 5-chloro-N2-(2-isopropoxy-5-methyl-4-(piperidin-4-yl)phenyl)-N4-(2-(isopropylsulfonyl)phenyl)pyrimidine-2,4- diamine (LDK378) currently in phase 1 and phase 2 clinical trials. J. Med. Chem. 2013;56:5675–5690. doi: 10.1021/jm400402q. [DOI] [PubMed] [Google Scholar]
- 189.Van Erp A.E.M., Hillebrandt-Roeffen M.H.S., van Houdt L., Fleuren E.D.G., van der Graaf W.T.A., Versleijen-Jonkers Y.M.H. Targeting anaplastic lymphoma kinase (ALK) in rhabdomyosarcoma (RMS) with the second-generation ALK inhibitor ceritinib. Target. Oncol. 2017;12:815–826. doi: 10.1007/s11523-017-0528-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Vewinger N., Huprich S., Seidmann L., Russo A., Alt F., Bender H., Sommer C., Samuel D., Lehmann N., Backes N., et al. IGF1R is a potential new therapeutic target for HGNET-BCOR brain tumor patients. Int. J. Mol. Sci. 2019;20:3027. doi: 10.3390/ijms20123027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Li Y.S., Kim J., Li J., Liu F., Liu X.Q., Himmeldirk K., Ren Y.L., Wagner T.E., Chen X.Z. Natural anti-diabetic compound 1,2,3,4,6-penta-O-galloyl-D-glucopyranose binds to insulin receptor and activates insulin-mediated glucose transport signaling pathway. Biochem. Biophys. Res. Commun. 2005;336:430–437. doi: 10.1016/j.bbrc.2005.08.103. [DOI] [PubMed] [Google Scholar]
- 192.Cao Y., Himmeldirk K.B., Qian Y., Ren Y., Malki A., Chen X. Biological and biomedical functions of penta-O-galloyl-D-glucose and its derivatives. J. Nat. Med. 2014;68:465–472. doi: 10.1007/s11418-014-0823-2. [DOI] [PubMed] [Google Scholar]
- 193.Cao Y., Li Y., Kim J., Ren Y., Himmeldirk K., Liu Y., Qian Y., Liu F., Chen X. Orally efficacious novel small molecule 6-chloro-6-deoxy-1,2,3,4-tetra-O-galloyl-alpha-D-glucopyranose selectively and potently stimulates insulin receptor and alleviates diabetes. J. Mol. Endocrinol. 2013;51:15–26. doi: 10.1530/JME-12-0171. [DOI] [PubMed] [Google Scholar]
- 194.Lan Z.J., Lei Z., Yiannikouris A., Yerramreddy T.R., Li X., Kincaid H., Eastridge K., Gadberry H., Power C., Xiao R., et al. Non-peptidyl small molecule, adenosine, 5′-Se-methyl-5′-seleno-, 2′,3′-diacetate, activates insulin receptor and attenuates hyperglycemia in type 2 diabetic Lepr(db/db) mice. Cell Mol. Life Sci. 2020;77:1623–1643. doi: 10.1007/s00018-019-03249-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Chen J., Sun J., Prinz R.A., Li Y., Xu X. Gingerenone A sensitizes the insulin receptor and increases glucose uptake by inhibiting the activity of p70 S6 kinase. Mol. Nutr. Food Res. 2018;62:e1800709. doi: 10.1002/mnfr.201800709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kunkel S.D., Suneja M., Ebert S.M., Bongers K.S., Fox D.K., Malmberg S.E., Alipour F., Shields R.K., Adams C.M. mRNA expression signatures of human skeletal muscle atrophy identify a natural compound that increases muscle mass. Cell Metab. 2011;13:627–638. doi: 10.1016/j.cmet.2011.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.He Y., Li W., Li Y., Zhang S., Wang Y., Sun C. Ursolic acid increases glucose uptake through the PI3K signaling pathway in adipocytes. PLoS ONE. 2014;9:e110711. doi: 10.1371/journal.pone.0110711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Flory J., Lipska K. Metformin in 2019. JAMA. 2019;321:1926–1927. doi: 10.1001/jama.2019.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Spiering M.J. The mystery of metformin. J. Biol. Chem. 2019;294:6689–6691. doi: 10.1074/jbc.CL119.008628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Xie J., Wang S., Ma P., Ma F., Li J., Wang W., Lu F., Xiong H., Gu Y., Zhang S., et al. Selection of small molecules that bind to and activate the insulin receptor from a DNA-encoded library of natural products. iScience. 2020;23:101197. doi: 10.1016/j.isci.2020.101197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhang B., Salituro G., Szalkowski D., Li Z.H., Zhang Y., Royo I., Vilella D., Diez M.T., Pelaez F., Ruby C., et al. Discovery of a small molecule insulin mimetic with antidiabetic activity in mice. Science. 1999;284:974–977. doi: 10.1126/science.284.5416.974. [DOI] [PubMed] [Google Scholar]
- 202.Diesel B., Kulhanek-Heinze S., Ho¨ltje M., Brandt B., Ho¨ltje H.-D., Vollmar A.M., Kiemer A.K. α-Lipoic acid as a directly binding activator of the insulin receptor: Protection from hepatocyte apoptosis. Biochemistry. 2007;46:2146–2155. doi: 10.1021/bi602547m. [DOI] [PubMed] [Google Scholar]
- 203.Qiang G., Xue S., Yang J.J., Du G., Pang X., Li X., Goswami D., Griffin P.R., Ortlund E.A., Chan C.B., et al. Identification of a small molecular insulin receptor agonist with potent antidiabetes activity. Diabetes. 2014;63:1394–1409. doi: 10.2337/db13-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Manchem V.P., Goldfine I.D., Kohanski R.A., Cristobal C.P., Lum R.T., Schow S.R., Shi S.Y., Spevak W.R., Laborde E., Toavs D.K., et al. A novel small molecule that directly sensitizes the insulin receptor in vitro and in vivo. Diabetes. 2001;50:824–830. doi: 10.2337/diabetes.50.4.824. [DOI] [PubMed] [Google Scholar]
- 205.Pender C., Goldfine I.D., Manchem V.P., Evans J.L., Spevak W.R., Shi S.Y., Rao S., Bajjalieh S., Maddux B.A., Youngren J.F. Regulation of insulin receptor function by a small molecule insulin receptor activator. J. Biol. Chem. 2002;277:43565–43571. doi: 10.1074/jbc.M202426200. [DOI] [PubMed] [Google Scholar]
- 206.Cheng M., Chen S., Schow S.R., Manchem V.P., Spevak W.R., Cristobal C.P., Shi S., Macsata R.W., Lum R.T., Goldfine I.D., et al. In vitro and in vivo prevention of HIV protease inhibitor-induced insulin resistance by a novel small molecule insulin receptor activator. J. Cell Biochem. 2004;92:1234–1245. doi: 10.1002/jcb.20150. [DOI] [PubMed] [Google Scholar]
- 207.Wu M., Dai G., Yao J., Hoyt S., Wang L., Mu J. Potentiation of insulin-mediated glucose lowering without elevated hypoglycemia risk by a small molecule insulin receptor modulator. PLoS ONE. 2015;10:e0122012. doi: 10.1371/journal.pone.0122012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Robinson L., Bajjalieh S., Cairns N., Lum R.T., Macsata R.W., Manchem V.P., Park S.J., Rao S., Schow S.R., Shi S., et al. 5-Substituted isophthalamides as insulin receptor sensitizers. Bioorg. Med. Chem. Lett. 2008;18:3492–3494. doi: 10.1016/j.bmcl.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 209.Girnita A., Girnita L., Prete F.D., Bartolazzi A., Larsson O., Axelson M. Cyclolignans as inhibitors of the insulin-Like growth factor-1 receptor and malignant cell growth. Cancer Res. 2004;64:236–242. doi: 10.1158/0008-5472.CAN-03-2522. [DOI] [PubMed] [Google Scholar]
- 210.Rodon J., DeSantos V., Ferry R.J., Kurzrock R. Early drug development of inhibitors of the insulin-like growth factor-I receptor pathway: Lessons from the first clinical trials. Mol. Cancer Ther. 2008;7:2575–2588. doi: 10.1158/1535-7163.MCT-08-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Liu X., Xie H., Luo C., Tong L., Wang Y., Peng T., Ding J., Jiang H., Li H. Discovery and SAR of thiazolidine-2,4-dione analogues as insulin-like growth factor-1 receptor (IGF-1R) inhibitors via hierarchical virtual screening. J. Med. Chem. 2010;53:2661–2665. doi: 10.1021/jm901798e. [DOI] [PubMed] [Google Scholar]
- 212.Ji Q.S., Mulvihill M.J., Rosenfeld-Franklin M., Cooke A., Feng L., Mak G., O’Connor M., Yao Y., Pirritt C., Buck E., et al. A novel, potent, and selective insulin-like growth factor-I receptor kinase inhibitor blocks insulin-like growth factor-I receptor signaling in vitro and inhibits insulin-like growth factor-I receptor dependent tumor growth in vivo. Mol. Cancer Ther. 2007;6:2158–2167. doi: 10.1158/1535-7163.MCT-07-0070. [DOI] [PubMed] [Google Scholar]
- 213.Mulvihill M.J., Cooke A., Rosenfeld-Franklin M., Buck E., Foreman K., Landfair D., O’Connor M., Pirritt C., Sun Y.C., Yao Y., et al. Discovery of OSI-906: A selective and orally efficacious dual inhibitor of the IGF-1 receptor and insulin receptor. Future Med. Chem. 2009;1:1153–1171. doi: 10.4155/fmc.09.89. [DOI] [PubMed] [Google Scholar]
- 214.Zeng X., Zhang H., Oh A., Zhang Y., Yee D. Enhancement of doxorubicin cytotoxicity of human cancer cells by tyrosine kinase inhibition of insulin receptor and type I IGF receptor. Breast. Cancer Res. Tr. 2012;133:117–126. doi: 10.1007/s10549-011-1713-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wittman M., Carboni J., Attar R., Balasubramanian B., Balimane P., Brassil P., Beaulieu F., Chang C., Clarke W., Dell J., et al. Discovery of a (1H-benzoimidazol-2-yl)-1H-pyridin-2-one (BMS-536924) inhibitor of insulin-like growth factor I receptor kinase with in vivo antitumor activity. J. Med. Chem. 2005;48:5639–5643. doi: 10.1021/jm050392q. [DOI] [PubMed] [Google Scholar]
- 216.Haluska P., Carboni J.M., Loegering D.A., Lee F.Y., Wittman M., Saulnier M.G., Frennesson D.B., Kalli K.R., Conover C.A., Attar R.M., et al. In vitro and in vivo antitumor effects of the dual insulin-like growth factor-i/insulin receptor inhibitor, BMS-554417. Cancer Res. 2006;66:362–371. doi: 10.1158/0008-5472.CAN-05-1107. [DOI] [PubMed] [Google Scholar]
- 217.Hou X., Huang F., Macedo L.F., Harrington S.C., Reeves K.A., Greer A., Finckenstein F.G., Brodie A., Gottardis M.M., Carboni J.M., et al. Dual IGF-1R/InsR inhibitor BMS-754807 synergizes with hormonal agents in treatment of estrogen-dependent breast cancer. Cancer Res. 2011;71:7597–7607. doi: 10.1158/0008-5472.CAN-11-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Schwartz G.K., Dickson M.A., LoRusso P.M., Sausville E.A., Maekawa Y., Watanabe Y., Kashima N., Nakashima D., Akinaga S. Preclinical and first-in-human phase I studies of KW-2450, an oral tyrosine kinase inhibitor with insulin-like growth factor receptor-1/insulin receptor selectivity. Cancer Sci. 2016;107:499–506. doi: 10.1111/cas.12906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Umehara H., Maekawa Y., Koizumi F., Shimizu M., Ota T., Fouad T.M., Willey J., Kaito H., Shiraishi N., Nakashima D., et al. Preclinical and phase I clinical studies of KW-2450, a dual IGF-1R/IR tyrosine kinase inhibitor, in combination with lapatinib and letrozole. Ther. Adv. Med. Oncol. 2018;10:1–14. doi: 10.1177/1758835918786858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Smith D.C., Britten C., Clary D.O., Nguyen L.T., Woodard P., Hurwitz H.I. A phase I study of XL228, a potent IGF1R/AURORA/SRC inhibitor, in patients with solid tumors or hematologic malignancies. J. Clin. Oncol. 2009;27:3512. doi: 10.1200/jco.2009.27.15_suppl.3512. [DOI] [Google Scholar]
- 221.Bell I.M., Stirdivant S.M., Ahern J., Culberson J.C., Darke P.L., Dinsmore C.J., Drakas R.A., Gallicchio S.N., Graham S.L., Heimbrook D.C., et al. Biochemical and structural characterization of a novel class of inhibitors of the type 1 insulin-like growth factor and insulin receptor kinases. Biochemistry. 2005;44:9430–9440. doi: 10.1021/bi0500628. [DOI] [PubMed] [Google Scholar]
- 222.Yasujima T., Saito K., Moore R., Negishi M. Phenobarbital and insulin reciprocate activation of the nuclear receptor constitutive androstane receptor through the insulin receptor. J. Pharmacol. Exp. Ther. 2016;357:367–374. doi: 10.1124/jpet.116.232140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Li Z., Zhu Y., Huang T., Hisatome I., Yamamoto T., Cheng J. High uric acid induces insulin resistance in cardiomyocytes in vitro and in vivo. PLoS ONE. 2016;11:e0147737. doi: 10.1371/journal.pone.0147737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Masi S., Georgiopoulos G., Alexopoulos G., Pateras K., Rosada J., Seravalle G., Ciuceis C.D., Taddei S., Borghi C., Grassi G., et al. The complex relationship between serum uric acid, endothelial function and small vessel remodeling in humans. J. Clin. Med. 2020;9:2027. doi: 10.3390/jcm9072027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Tassone E.J., Cimellaro A., Perticone M., Hribal M.L., Sciacqua A., Andreozzi F., Sesti G., Perticone F. Uric acid impairs insulin signaling by Promoting enpp1 Binding to insulin receptor in human umbilical vein endothelial cells. Front. Endocrinol. 2018;9:98. doi: 10.3389/fendo.2018.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Wang Y.H., Guan Q.L., Liu G.F., Wu X.Y., Wang X.J., Sheng J. 2′-O-methylperlatolic acid enhances insulin-regulated blood glucose-lowering effect through insulin receptor signaling pathway. J. Diabetes Res. 2022;2022:2042273. doi: 10.1155/2022/2042273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Taochy C., Yu A., Bouche N., Bouteiller N., Elmayan T., Dressel U., Carroll B.J., Vaucheret H. Post-transcriptional gene silencing triggers dispensable DNA methylation in gene body in Arabidopsis. Nucleic Acids Res. 2019;47:9104–9114. doi: 10.1093/nar/gkz636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Schubeler D. Function and information content of DNA methylation. Nature. 2015;517:321–326. doi: 10.1038/nature14192. [DOI] [PubMed] [Google Scholar]
- 229.Edwards J.R., Yarychkivska O., Boulard M., Bestor T.H. DNA methylation and DNA methyltransferases. Epigenet. Chromatin. 2017;10:23. doi: 10.1186/s13072-017-0130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Adam S., Anteneh H., Hornisch M., Wagner V., Lu J., Radde N.E., Bashtrykov P., Song J., Jeltsch A. DNA sequence-dependent activity and base flipping mechanisms of DNMT1 regulate genome-wide DNA methylation. Nat. Commun. 2020;11:3723. doi: 10.1038/s41467-020-17531-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Nishiyama A., Mulholland C.B., Bultmann S., Kori S., Endo A., Saeki Y., Qin W., Trummer C., Chiba Y., Yokoyama H., et al. Two distinct modes of DNMT1 recruitment ensure stable maintenance DNA methylation. Nat. Commun. 2020;11:1222. doi: 10.1038/s41467-020-15006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Chen Y.T., Lin W.D., Liao W.L., Tsai Y.C., Liao J.W., Tsai F.J. NT5C2 methylation regulatory interplay between DNMT1 and insulin receptor in type 2 diabetes. Sci. Rep. 2020;10:16087. doi: 10.1038/s41598-020-71336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Careddu M.G., Allegrini S., Pesi R., Camici M., Garcia-Gil M., Tozzi M.G. Knockdown of cytosolic 5′-nucleotidase II (cN-II) reveals that its activity is essential for survival in astrocytoma cells. Biochim. Biophys. Acta-Mol. Cell Res. 2008;1783:1529–1535. doi: 10.1016/j.bbamcr.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 234.Binayi F., Zardooz H., Ghasemi R., Hedayati M., Askari S., Pouriran R., Sahraei M. The chemical chaperon 4-phenyl butyric acid restored high-fat diet- induced hippocampal insulin content and insulin receptor level reduction along with spatial learning and memory deficits in male rats. Physiol. Behav. 2021;231:113312. doi: 10.1016/j.physbeh.2021.113312. [DOI] [PubMed] [Google Scholar]
- 235.Che Z.P., Tian Y.E., Liu S.M., Jiang J., Hu M., Chen G.Q. Stereoselective synthesis of 4beta-acyloxypodophyllotoxin derivatives as insecticidal agents. J. Asian Nat. Prod. Res. 2019;21:1028–1041. doi: 10.1080/10286020.2018.1490275. [DOI] [PubMed] [Google Scholar]
- 236.Wang R., Zhao Y., Huang Z., Zhou Y., Wang W., Xuan Y., Zhen Y., Ju B., Guo S., Zhang S. Self-assembly of podophyllotoxin-loaded lipid bilayer nanoparticles for highly effective chemotherapy and immunotherapy via downregulation of programmed cell death ligand 1 production. ACS Nano. 2022;16:3943–3954. doi: 10.1021/acsnano.1c09391. [DOI] [PubMed] [Google Scholar]
- 237.Xiao J., Gao M., Sun Z., Diao Q., Wang P., Gao F. Recent advances of podophyllotoxin/epipodophyllotoxin hybrids in anticancer activity, mode of action, and structure-activity relationship: An update (2010–2020) Eur. J. Med. Chem. 2020;208:112830. doi: 10.1016/j.ejmech.2020.112830. [DOI] [PubMed] [Google Scholar]
- 238.Ou K., Kang Y., Chen L., Zhang X., Chen X., Zheng Y., Wu J., Guan S. H2O2-responsive nano-prodrug for podophyllotoxin delivery. Biomater. Sci. 2019;7:2491–2498. doi: 10.1039/C9BM00344D. [DOI] [PubMed] [Google Scholar]
- 239.Zi C.T., Yang L., Xu F.Q., Dong F.W., Yang D., Li Y., Ding Z.T., Zhou J., Jiang Z.H., Hu J.M. Synthesis and anticancer activity of dimeric podophyllotoxin derivatives. Drug Des. Devel. Ther. 2018;12:3393–3406. doi: 10.2147/DDDT.S167382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Davey S.G. Engineering etoposide. Nat. Rev. Chem. 2020;4:63. doi: 10.1038/s41570-020-0166-3. [DOI] [PubMed] [Google Scholar]
- 241.Nerella S., Kankala S., Gavaji B. Synthesis of podophyllotoxin-glycosyl triazoles via click protocol mediated by silver (I)-N-heterocyclic carbenes and their anticancer evaluation as topoisomerase-II inhibitors. Nat. Prod. Res. 2021;35:9–16. doi: 10.1080/14786419.2019.1610958. [DOI] [PubMed] [Google Scholar]
- 242.Lv M., Zhang Y., Wang F., Zhang S., Xu H. Non-food renewable and bioactive forest products for pest management: Valuation of agricultural properties of podophyllotoxin analogs derived from Podophyllum hexandrum as botanical pesticides. Ind. Crop. Prod. 2020;153:112608. doi: 10.1016/j.indcrop.2020.112608. [DOI] [Google Scholar]
- 243.Yu X., Che Z., Xu H. Recent advances in the chemistry and biology of podophyllotoxins. Chemistry. 2017;23:4467–4526. doi: 10.1002/chem.201602472. [DOI] [PubMed] [Google Scholar]
- 244.Huang X., Zhang Y., Wang F., Zhang S., Lv M., Hui X. Renewable forest bioresources for pest management: Semisynthesis of esters containing ferrocene scaffolds of podophyllotoxin from Juniperus sabina L. as botanical pesticides. Ind. Crop. Prod. 2020;152:112510. doi: 10.1016/j.indcrop.2020.112510. [DOI] [Google Scholar]
- 245.Che Z.P., Tian Y., Yang J.M., Liu S.M., Jiang J., Hu M., Chen G.Q. Screening of insecticidal activity of podophyllotoxin analogues against Athetis dissimilis. Nat. Prod. Commun. 2019;14:117–120. doi: 10.1177/1934578X1901400131. [DOI] [Google Scholar]
- 246.Sun Z., Lv M., Yu X., Xu H. Application of sustainable natural bioesources in crop protection: Insight into a podophyllotoxin-derived botanical pesticide for regulating insect vestigial wing of Mythimna separata Walker. ACS Sustain. Chem. Eng. 2017;5:3945–3954. doi: 10.1021/acssuschemeng.6b03145. [DOI] [Google Scholar]
- 247.Wang R., Qu C., Wang Z., Yang G. Cross-resistance, biochemical mechanism and fitness costs of laboratory-selected resistance to pyridalyl in diamondback moth, Plutella xylostella. Pestic. Biochem. Phys. 2020;163:8–13. doi: 10.1016/j.pestbp.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 248.Abbasi-Mojdehia M.R., Hajizadeha J., Zibaeea A., Keyhanianc A.A., Rajaei F. Alterations in some physiological processes of Bactrocera oleae Rossi (Diptera: Tephritidae) following pyridalyl treatment. Pestic. Biochem. Phys. 2019;164:85–90. doi: 10.1016/j.pestbp.2019.12.014. [DOI] [PubMed] [Google Scholar]
- 249.Abbasi-Mojdehi M.R., Hajizadeh J., Zibaee A., Keyhanian A.A. Effect of pyridalyl on mortality, fecundity and physiological performance of olive fruit fly, Bactrocera oleae Rossi (Diptera: Tephritidae) J. Asia-Pac Entomol. 2019;22:506–512. doi: 10.1016/j.aspen.2019.03.008. [DOI] [Google Scholar]
- 250.Powell G.F., Ward D.A., Prescott M.C., Spiller D.G., White M.R., Turner P.C., Earley F.G., Phillips J., Rees H.H. The molecular action of the novel insecticide, Pyridalyl. Insect Biochem. Mol. Biol. 2011;41:459–469. doi: 10.1016/j.ibmb.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 251.Shao X.H., Lai D., Zhang L., Xu H. Induction of autophagy and apoptosis via PI3K/AKT/TOR pathways by azadirachtin A in Spodoptera litura Cells. Sci. Rep. 2016;6:35482. doi: 10.1038/srep35482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Ha M., Kim V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014;15:509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 253.Tafrihi M., Hasheminasab E. MiRNAs: Biology, biogenesis, their Web-based tools, and databases. MicroRNA. 2019;8:4–27. doi: 10.2174/2211536607666180827111633. [DOI] [PubMed] [Google Scholar]
- 254.Hill M., Tran N. MicroRNAs regulating MicroRNAs in cancer. Trends Cancer. 2018;4:465–468. doi: 10.1016/j.trecan.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 255.Asgari S. MicroRNA functions in insects. Insect Biochem. Mol. Biol. 2013;43:388–397. doi: 10.1016/j.ibmb.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 256.Sun X., Liu J., Xu C., Tang S.C., Ren H. The insights of Let-7 miRNAs in oncogenesis and stem cell potency. J. Cell Mol. Med. 2016;20:1779–1788. doi: 10.1111/jcmm.12861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Nweke E.E., Brand M. Downregulation of the let-7 family of microRNAs may promote insulin receptor/insulin-like growth factor signalling pathways in pancreatic ductal adenocarcinoma. Oncol Lett. 2020;20:2613–2620. doi: 10.3892/ol.2020.11854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Frutos M.F., Galan-Chilet I., Goedeke L., Kim B., Pardo-Marques V., Perez-Garcia A., Herrero J.I., Fernandez-Hernando C., Kim J., Ramirez C.M. MicroRNA 7 impairs insulin signaling and regulates A beta levels through posttranscriptional regulation of the insulin receptor substrate 2, insulin receptor, insulin-degrading enzyme, and liver X receptor pathway. Mol. Cell Biol. 2019;39:e00170-19. doi: 10.1128/MCB.00170-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Ling L., Kokoza V.A., Zhang C., Aksoy E., Raikhel A.S. MicroRNA-277 targets insulin-like peptides 7 and 8 to control lipid metabolism and reproduction in Aedes aegypti mosquitoes. Proc. Natl. Acad. Sci. USA. 2017;114:E8017–E8024. doi: 10.1073/pnas.1710970114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Shang F., Niu J., Ding B.Y., Zhang W., Wei D.D., Wei D., Jiang H.B., Wang J.J. The miR-9b microRNA mediates dimorphism and development of wing in aphids. Proc. Natl. Acad. Sci. USA. 2020;117:8404–8409. doi: 10.1073/pnas.1919204117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Mansour M.A. Ubiquitination: Friend and foe in cancer. Int. J. Biochem. Cell Biol. 2018;101:80–93. doi: 10.1016/j.biocel.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 262.Foot N., Henshall T., Kumar S. Ubiquitination and the regulation of membrane proteins. Physiol. Rev. 2017;97:253–281. doi: 10.1152/physrev.00012.2016. [DOI] [PubMed] [Google Scholar]
- 263.Sun T., Liu Z., Yang Q. The role of ubiquitination and deubiquitination in cancer metabolism. Mol. Cancer. 2020;19:146. doi: 10.1186/s12943-020-01262-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Rui L.Y., Yuan M.S., Frantz D., Shoelson S., White M.F. SOCS-1 and SOCS-3 block insulin signaling by ubiquitin-mediated degradation of IRS1 and IRS2. J. Biol. Chem. 2002;277:42394–42398. doi: 10.1074/jbc.C200444200. [DOI] [PubMed] [Google Scholar]
- 265.Xu X., Sarikas A., Dias-Santagata D.C., Dolios G., Lafontant P.J., Tsai S.-C., Zhu W., Nakajima H., Nakajima H.O., Field L.J., et al. The CUL7 E3 ubiquitin ligase targets insulin receptor substrate 1 for ubiquitin-dependent degradation. Mol. Cell. 2008;30:403–414. doi: 10.1016/j.molcel.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Yi J.S., Park J.S., Ham Y.M., Nguyen N., Lee N.R., Hong J., Kim B.W., Lee H., Lee C.S., Jeong B.C., et al. MG53-induced IRS-1 ubiquitination negatively regulates skeletal myogenesis and insulin signalling. Nat. Commun. 2013;4:2354. doi: 10.1038/ncomms3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Nagarajan A., Petersen M.C., Nasiri A.R., Butrico G., Fung A., Ruan H.B., Kursawe R., Caprio S., Thibodeau J., Bourgeois-Daigneault M.C., et al. MARCH1 regulates insulin sensitivity by controlling cell surface insulin receptor levels. Nat. Commun. 2016;7:12639. doi: 10.1038/ncomms12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Kaksonen M., Roux A. Mechanisms of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2018;19:313–326. doi: 10.1038/nrm.2017.132. [DOI] [PubMed] [Google Scholar]
- 269.Choi E., Kikuchi S., Gao H., Brodzik K., Nassour I., Yopp A., Singal A.G., Zhu H., Yu H. Mitotic regulators and the SHP2-MAPK pathway promote IR endocytosis and feedback regulation of insulin signaling. Nat. Commun. 2019;10:1473. doi: 10.1038/s41467-019-09318-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Choi E., Yu H. Spindle checkpoint regulators in insulin signaling. Front. Cell Dev. Biol. 2018;6:161. doi: 10.3389/fcell.2018.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Hagan R.S., Manak M.S., Buch H.K., Meier M.G., Meraldi P., Shah J.V., Sorger P.K. p31(comet) acts to ensure timely spindle checkpoint silencing subsequent to kinetochore attachment. Mol. Biol. Cell. 2011;22:4236–4246. doi: 10.1091/mbc.e11-03-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Yonei Y., Takabe W. Liver training: Yes or no? Glycative Stress Res. 2015;2:121–128. [Google Scholar]
- 273.Murphy J.E., Padilla B.E., Hasdemir B., Cottrell G.S., Bunnett N.W. Endosomes: A legitimate platform for the signaling train. Proc. Natl. Acad. Sci. USA. 2009;106:17615–17622. doi: 10.1073/pnas.0906541106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Wang J., Chen X., Tong S., Zhou H., Sun J., Gou Y., Wu F., Hu J., Xu J., Ding G. Overexpression of WDFY2 inhibits prostate cancer cell growth and migration via inactivation of Akt pathway. Tumor. Biol. 2017;39:1010428317704821. doi: 10.1177/1010428317704821. [DOI] [PubMed] [Google Scholar]
- 275.Zhang L., Li X., Zhang N., Yang X., Hou T., Fu W., Yuan F., Wang L., Wen H., Tian Y., et al. WDFY2 potentiates hepatic insulin sensitivity and controls endosomal localization of the insulin receptor and IRS1/2. Diabetes. 2020;69:1887–1902. doi: 10.2337/db19-0699. [DOI] [PubMed] [Google Scholar]
- 276.Hayakawa A., Leonard D., Murphy S., Hayes S., Soto M., Fogarty K., Standley C., Bellve K., Lambright D., Mello C., et al. The WD40 and FYVE domain containing protein 2 defines a class of early endosomes necessary for endocytosis. Proc. Natl. Acad. Sci. USA. 2006;103:11928–11933. doi: 10.1073/pnas.0508832103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Evin G., Hince C. BACE1 as a therapeutic target in Alzheimer’s disease: Rationale and current status. Drugs Aging. 2013;30:755–764. doi: 10.1007/s40266-013-0099-3. [DOI] [PubMed] [Google Scholar]
- 278.Rajendran L., Honsho M., Zahn T.R., Keller P., Geiger K.D., Verkade P., Simons K. Alzheimer’s disease β-amyloid peptides are released in association with exosomes. Proc. Natl. Acad. Sci. USA. 2006;103:11172–11177. doi: 10.1073/pnas.0603838103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Obata T., Yokota I., Yokoyama K., Okamoto E., Kanezaki Y., Tanaka Y., Maegawa H., Teshigawara K., Hirota F., Yuasa T., et al. Soluble insulin receptor ectodomain is elevated in the plasma of patients with diabetes. Diabetes. 2007;56:2028–2035. doi: 10.2337/db07-0394. [DOI] [PubMed] [Google Scholar]
- 280.Mesallamy H.O.E., Hamdy N.M., Mostafa D.M., Amin A.I. The serine protease granzyme B as an inflammatory marker, in relation to the insulin receptor cleavage in human obesity and type 2 diabetes mellitus. J. Interf. Cytok. Res. 2014;34:179–186. doi: 10.1089/jir.2013.0059. [DOI] [PubMed] [Google Scholar]
- 281.Dassano A., Loretelli C., Fiorina P. Idebenone and T2D: A new insulin-sensitizing drug for personalized therapy. Pharmacol. Res. 2019;139:469–470. doi: 10.1016/j.phrs.2018.12.008. [DOI] [PubMed] [Google Scholar]