Abstract
(1) Background: Villous atrophy is an indication for small bowel capsule endoscopy (SBCE). However, SBCE findings are not described uniformly and atrophic features are sometimes not recognized; (2) Methods: The Delphi technique was employed to reach agreement among a panel of SBCE experts. The nomenclature and definitions of SBCE lesions suggesting the presence of atrophy were decided in a core group of 10 experts. Four images of each lesion were chosen from a large SBCE database and agreement on the correspondence between the picture and the definition was evaluated using the Delphi method in a broadened group of 36 experts. All images corresponded to histologically proven mucosal atrophy; (3) Results: Four types of atrophic lesions were identified: mosaicism, scalloping, folds reduction, and granular mucosa. The core group succeeded in reaching agreement on the nomenclature and the descriptions of these items. Consensus in matching the agreed definitions for the proposed set of images was met for mosaicism (88.9% in the first round), scalloping (97.2% in the first round), and folds reduction (94.4% in the first round), but granular mucosa failed to achieve consensus (75.0% in the third round); (4) Conclusions: Consensus among SBCE experts on atrophic lesions was met for the first time. Mosaicism, scalloping, and folds reduction are the most reliable signs, while the description of granular mucosa remains uncertain.
Keywords: small bowel atrophy, video-capsule enteroscopy, consensus
1. Introduction
Capsule endoscopy (CE) has opened up a new chapter in small bowel (SB) examination by providing direct visualization of the entire SB mucosa [1].
Currently, small bowel capsule endoscopy (SBCE) and device-assisted endoscopy (DAE) are valuable tools for evaluating and characterizing SB mucosal changes. SBCE has been successfully adopted to identify SB bleedings and chronic anaemia of unknown origin. However, in the last decade, indications for its use have increased. Currently, it is employed for diagnosing and monitoring Crohn’s disease, as well as for identifying the extent and the possible malignant complications of unresponsive coeliac disease (CeD) [2,3,4,5,6]. Of note, preliminary studies suggest that SBCE can be used as a complementary technique to the histological assessment of CeD and seronegative villous atrophy (SNVA) [7].
Mucosal atrophy itself is an important endoscopic finding and, beyond its role as a hallmark of CeD, it can be identified in other inflammatory disorders of the SB. Scalloping, nodularity (also known as granular mucosa), loss of mucosal folds, and a mosaic pattern are the most commonly recognised endoscopic markers of villous atrophy [8]. These endoscopic findings suggest the presence of SB mucosal atrophy that is usually, but not always, associated with CeD [9]. However, the features of SB atrophy at SBCE are not described uniformly, and currently atrophy signs are mainly identified and verified by experts [10].
Interobserver agreement for atrophic signs is reported to vary significantly between SBCE readers. In a study by Rondonotti et al., findings from 32 patients with CeD and 11 patients with normal duodenal mucosal biopsies were examined by 4 reviewers. Kappa values for interobserver agreement ranged between 0.56 and 0.87 [11]. In contrast, Biagi et al. found higher interobserver variability, with kappa values of 0.49, 0.67, and 0.70 between 3 reviewers reading 32 SBCEs with 26 confirmed CeD, suggesting that experience is needed in interpreting SBCEs [12]. In a study by Petroniene et al. that examined SBCEs for 10 CeD patients and 10 controls, interobserver agreement was excellent (κ = 1.00) when only experienced reviewers were considered, whereas agreement decreased when reviewers with little prior experience were considered (κ = 0.20) [13]. Interobserver agreement may also vary according to different CeD characteristics. For example, Murray et al. showed that overall interobserver agreement was highest (κ = 0.77) for the mosaic pattern in 38 patients with CeD who underwent SBCE, followed by scalloping (κ = 0.59), fissuring (κ = 0.41), and villous atrophy (κ = 0.37) [14].
Therefore, as suggested by Jang et al., expertise in reading SBCE with the adoption of structured terminology for capsule findings would likely demonstrate an improvement in reader agreement [15,16].
The aim of our study was to reach international consensus among experts regarding the nomenclature and the description of the main atrophic lesions in SBCE. This effort is part of a wider initiative of the International Capsule endoscopy REsearch (I CARE) Group to reach agreement on the nomenclature and the descriptions of CE findings with a Delphi method-based approach [17,18,19].
2. Materials and Methods
2.1. Centres and Design
We adopted the Delphi technique to reach an agreement among a panel of European gastroenterologists working in tertiary referral centres for CE. Particular attention was paid to the selection of CeD specialists with substantial publications on SBCE and CeD, given their particular expertise in atrophic mucosal lesions. Additionally, for each participant, we recorded expertise in CE (e.g., years of activity, number of capsules read per year) as well as their specific expertise in reading CE from coeliac patients.
We employed web-based questionnaires (Google LLC) to collect the responses of the expert panel. In addition, we employed a six-point Likert scale (i.e., strongly disagree, disagree, moderately disagree, moderately agree, agree, strongly agree) to quantify the agreement of the experts on nomenclature, description, and the matching of proposed images of atrophic lesions frequently found on SBCE in coeliac patients.
2.2. Delphi Process and Phases
In the first phase, taking into consideration the relatively low prevalence of SBCE in coeliac patients, we decided to submit the nomenclature and the descriptions of atrophic lesions to a core group of 10 experts with substantial expertise in CeD, with the aim of reaching widespread and shared agreement on these definitions (CB, FB, SCZ, SK, LE, RE, ER, JCS, RS, GET). We set a higher cut-off threshold of 90% of total ‘strongly agree’ and ‘agree’ responses to reach consensus in this core group. This precaution was deemed necessary to strengthen the acquisitions of this first Delphi round. For each Delphi question, we collected the core group comments and suggestions on nomenclature and definitions that were later considered in the following Delphi rounds if the proposed agreement was not reached.
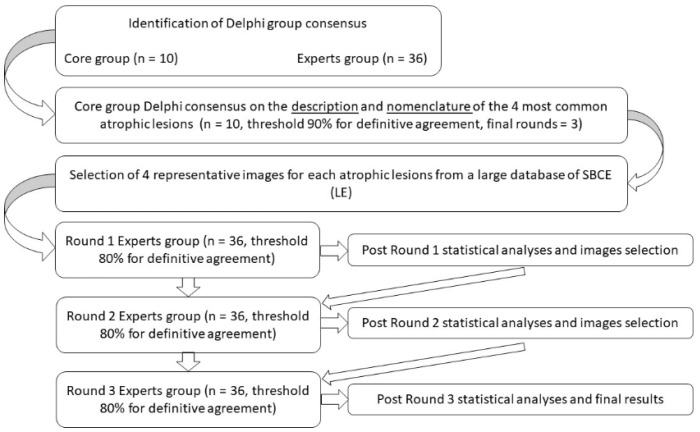
After consensus on nomenclature and description was reached in the core group, we collected a set of images of atrophic lesions from a large database of CE photographs (>1200) of histologically proven atrophic SB mucosa (i.e., Marsh 3 lesions) [20,21]. We submitted the selected images to a group of 36 endoscopists (including the 10 experts of the core group). For each group of images, the experts had to decide whether the images fit the agreed descriptions and nomenclature of the atrophic lesion. In addition, the experts had to decide if any images did not perfectly match the description or if none matched. In this second part of the Delphi process, we set a priori a threshold of 80% of the total ‘strongly agree’ and ‘agree’ responses to meet the definition of consensus. When consensus was not met, we checked if the opinions of the experts regarding replacement of any picture were statistically significant (Figure 1).
Figure 1.
Flowchart of the Delphi process.
2.3. Statistical Analyses
We employed chi-squared analysis to statistically examine our results, considering a random 50:50 probability of choosing between a positive (i.e., one or some images should not be considered) and negative (i.e., all the images are suitable) response. If a majority of the endoscopists gave a positive response, we would consider a 25% probability that a single selected picture would be defined by chance as not suitable and then looked for any picture that statistically emerged as not suitable. The author in charge of the selection of the images (LE) took these results into account and accordingly changed the proposed set of images in the following Delphi round.
Any statements or any set of pictures that did not meet consensus after three Delphi rounds was considered a failure.
We employed R Studio 4.0.0 (R Core Team (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org/) for statistical analyses.
The study was conducted in compliance with the Helsinki criteria and all subsequent amendments. The data were collected within the framework of standard patient care. They were treated confidentially, in compliance with the most recent privacy laws at the European and the national level, and anonymized so that those who analysed the data were not able to identify patients (protocol number 137/2021).
3. Results
We recruited 36 SBCE experts from 11 countries (i.e., France, Germany, Greece, Ireland, Israel, Italy, Netherlands, Poland, Spain, Sweden, United Kingdom). Our panel of experts had a median of 13 (IQR 10–19) years’ experience in CE, a mean of 100 (IQR 50–150) CE exams annually and a mean of 5 (IQR 2–11) capsule exams of coeliac patients annually.
The core group identified four main atrophic lesions that are frequently found in SBCE: mosaicism, scalloping, folds reduction, and granular mucosa.
The consensus in the core group (n = 10) on the definitions and the nomenclature of these findings was met in the second round for mosaicism, scalloping, and folds reduction, with an overall agreement of 90%, 100%, and 100%, respectively, and in the third round for granular mucosa, with an overall agreement of 90% (Table 1 and Table 2).
Table 1.
Core group agreement for nomenclature and description of common atrophic lesions.
| Nomenclature and Description | Agreement in Core Group (n = 10) | Number of Rounds |
|---|---|---|
| Mosaicism: loss of villous structure with the presence of non-ulcerated, orthogonally converging fissures of the small bowel mucosa | 9 out of 10 (90%) | 2 |
| Scalloping: presence of multiple incisures on the edge of the small bowel folds (cogwheel appearance) | 10 out of 10 (100%) | 2 |
| Folds reduction: flattening of the mucosa with reduction of the folds (<2 field view) in terms of both height and number | 10 out of 10 (100%) | 2 |
| Granular mucosa: mucosal surface characterized by multiple small nodules, rough villous architecture and edema of the villi | 9 out of 10 (90%) | 3 |
Table 2.
Core group rating on the nomenclature and description of common atrophic lesions.
| Nomenclature and Description | Numerical Scale/Expert Votes | % Agreeing and Strongly Agreeing | Number of Rounds | |||||
|---|---|---|---|---|---|---|---|---|
| Strongly Disagree |
Disagree | Moderately Disagree | Moderately Agree | Agree | Strongly Agree | |||
| Mosaicism | 0 | 0 | 0 | 1 | 5 | 4 | 90 | 2 |
| Scalloping | 0 | 0 | 0 | 0 | 4 | 6 | 100 | 2 |
| Folds Reduction | 0 | 0 | 0 | 0 | 2 | 8 | 100 | 2 |
| Granular Mucosa | 0 | 0 | 0 | 1 | 4 | 5 | 90 | 3 |
Afterward, we collected the responses of the larger panel of experts on the agreement between the established nomenclature and the definitions and the proposed selection of images. The consensus was met in the first round for mosaicism (88.9%), scalloping (97.2%), and fold reduction (94.4%) (Table 3, Figure 2A–C).
Table 3.
Experts group rating on the correspondence between suggested images and core group definition. Consensus was not reached after three rounds for ‘Granular Mucosa’.
| Images | Numerical Scale/Expert Votes | % Agreeing and Strongly Agreeing | Number of Rounds | |||||
|---|---|---|---|---|---|---|---|---|
| Strongly Disagree |
Disagree | Moderately Disagree | Moderately Agree | Agree | Strongly Agree | |||
| Mosaicism | 0 | 1 | 1 | 2 | 16 | 16 | 88.9 | 1 |
| Scalloping | 0 | 0 | 1 | 0 | 8 | 27 | 97.2 | 1 |
| Folds Reduction | 0 | 1 | 0 | 1 | 18 | 16 | 94.4 | 1 |
| Granular Mucosa | 0 | 3 | 1 | 5 | 20 | 7 | 75.0 | 3 |
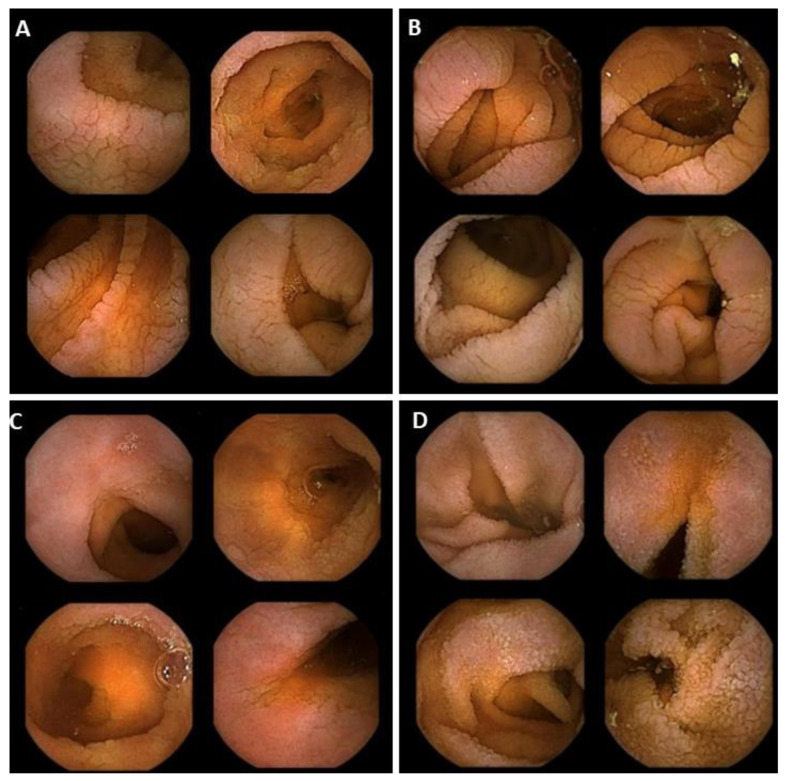
Figure 2.
Agreed images representing the endoscopic findings suggestive for villous atrophy: mosaicism (A), scalloping (B), folds reduction (C), and granular mucosa (D).
Granular mucosa did not reach consensus agreement after three rounds and therefore the Delphi process was halted. In the final round, a global consensus of 75% on the proposed set of images was reached (Figure 2D). In the analysis of the results for this round, we found that the experts pointed out that the left upper quadrant image did not match the given description of granular mucosa (p = 0.001). In post hoc analysis, we looked for any statistically significant association between agreement on the proposed set of images of granular mucosa and overall experience in SBCE, the number of SBCEs per year, and the number of dedicated coeliac SBCEs per year. In particular, we aimed at finding any differences in judgment among the endoscopists in the upper quartile of these categories and the rest of the endoscopists. Of note, we found that years’ experience in SBCE and expertise in reading SBCEs from coeliac patients did not statistically influence decisions on agreement regarding granular mucosa (p = 0.21 and p = 0.28, respectively). On the contrary, the endoscopists in the upper quartile for numbers of SBCEs read per year were more prone to disagreement than the others, regarding the final set of proposed images for granular mucosa (p = 0.03).
4. Discussion
For the first time, this study has established agreement among 36 experts on the definitions of the most common atrophic lesions found in SBCE through a Delphi consensus. This initiative is part of the I CARE Group effort to improve interobserver agreement on CE through the adoption of shared and agreed nomenclature and definitions of CE findings.
We have found agreement on the terminology (i.e., nomenclature and description) of all the atrophic items commonly reported in the literature: mosaicism, scalloping, folds reduction and granular mucosa [8]. Agreement of endoscopic appearance, based on a large database of histologically proven atrophy (i.e., Marsh–Oberhuber 3) photographs of SBCEs, was met for scalloping, mosaicism, and folds reduction. Granular mucosa was the only item that lacked an agreed endoscopic appearance.
Although there is an existing SBCE terminology [16,17,18,19,22], there is still a lack of consensus among experts on the definition and the nomenclature of the most common features of atrophy. With the increasing use of video CE in coeliac patients, especially those with refractory disease, the need for standardization of SBCE reading, reporting, nomenclature and definitions, and the typical appearance of atrophic small bowel lesions has become apparent [23]. This particular need is going to become even more important in the next few years, as the use of artificial intelligence (AI) software for endoscopy diagnosis is expected to markedly increase. Large CE databases with solid and shared findings between experts are keystones for effective machine learning and for building precise and trustworthy AI programs [24,25]. The results of our work have shown that the main features of atrophic lesions are mosaicism, scalloping, and folds reduction, and they have rapidly found consensus among readers of SBCEs, even among those who read only a few CEs of coeliac patients per year. This finding was relatively expected since they are the most recognizable hallmarks of atrophy [26]. In our study, scalloping showed the highest level of agreement for nomenclature, definition, and endoscopic appearance. This is in line with the results of other studies that suggested a strong specificity (99%) and a positive predictive value (97%) for villous atrophy [27]. For mosaicism and folds reduction, the current literature is less clear. However, they demonstrated a high diagnostic accuracy for CeD (98.1%) when associated with scalloping [26]. In our experience, folds reduction shows a slightly higher rate of agreement for description and endoscopic appearance than mosaicism. Notably, during the Delphi process, some doubts were raised about the possibility of finding only a mosaicism pattern without scalloping.
Conversely, the finding ‘granular mucosa’ achieved agreement on its nomenclature and description in the core group of 10 experts but failed to achieve validation in the expert group regarding its assignment to 4 typical images. As mentioned earlier, we found no statistically significant association between agreement on the proposed images and expertise in SBCE in CeD or years’ experience in SBCE. A theoretical general lack of specificity and the presence of similar findings in other small bowel diseases (e.g., oedema in inflammatory bowel disease) may be the main factors that prevented general agreement on this finding [17]. Notably, in the final Delphi discussion for ‘granular mucosa’, the panel of experts presented some concerns about the proposed images. They stated that mucosal changes could be easily mistaken with inflammatory changes or not specific mucosal alterations. Moreover, this finding shows that expertise in coeliac SBCE is not the key factor for identifying the most common atrophic lesions, hinting at the possible ease of reproducibility of our work. With the statistically proven lower agreement in the upper quartile of endoscopists by numbers of SBCEs, it becomes clear that the main features of atrophy may be identifiable by experts in SBCE but not necessarily trained in coeliac SBCE.
There are some limitations to this study. The selection of images from one single database may represent potential subjective bias. However, on the other hand, this particular feature of the study may be balanced by the verified histological confirmation of atrophy for each image, which was usually obtained in a specific tertiary referral center. The web-based process could have a limited discussion and debate on the images. Moreover, the consensus aimed at providing nomenclature for and definitions of hallmarks of mucosal atrophy rather than esteeming their clinical relevance in terms of severity of atrophy. Furthermore, a potential limitation in a real-life scenario is that more than a finding can be present in the same still frame, hindering the possible reproducibility of our work in the hands of non-expert readers. However, the proposed and accepted images could support non-expert readers to recognize single hallmarks of atrophy at SBCE.
In conclusion, our international group of SBCE experts has reached consensus on the nomenclature, description, and identification of atrophic lesions in SBCE. The current study is an important contribution to achieving higher quality SBCE reading and reporting; and, it provides a common technique for evaluating coeliac patients—particularly those with refractory disease—although it must be underlined that atrophy does not always mean CeD [9]. We strongly suggest the adoption of mosaicism, scalloping, and folds reduction when describing atrophy in SBCE. The description of granular mucosa remains unspecific, and it should only be used with caution in centers with a very high volume of SBCEs.
Acknowledgments
The authors are members of the International CApsule endoscopy REsearch (I-CARE) Group, an independent international research group promoting multicenter studies on capsule endoscopy.
Author Contributions
Conceptualization, L.E., R.S., C.B., F.B., G.E.T., S.C.Z., S.K., R.E., E.R. and J.C.S.; methodology, L.E. and A.R. (Alessandro Rimondi); software, A.R. (Alessandro Rimondi); validation, formal analysis, investigation, L.E., B.M., R.S., C.B., F.B., G.E.T., S.C.Z., S.K., R.E., E.R., J.C.S., M.B., J.B., S.C., F.C., X.D., P.E., I.F.U., M.K., U.K., A.K., R.L., P.B., H.B., C.M., D.M., A.M., A.N., E.P.C.R., G.P., G.R., M.E.R., A.N., A.R. (Alexander Robertson), C.S., E.T., K.T., G.W.J. and A.R. (Alessandro Rimondi); data curation, L.E., B.M. and A.R. (Alessandro Rimondi); writing—original draft preparation L.E., B.M. and A.R. (Alessandro Rimondi); writing—review and editing, L.E., B.M., R.S., C.B., F.B., G.E.T., S.C.Z., S.K., R.E., E.R., J.C.S., M.B., J.B., S.C., F.C., X.D., P.E., I.F.U., M.K., U.K., A.K., R.L., P.B., H.B., C.M., D.M., A.M., A.N., E.P.C.R., G.P., G.R., M.E.R., A.N., A.R. (Alexander Robertson), C.S., E.T., K.T., G.W.J. and A.R. (Alessandro Rimondi); supervision, L.E., B.M., R.S., C.B., F.B., G.E.T., S.C.Z., S.K., R.E., E.R., J.C.S. and A.R. (Alessandro Rimondi). All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and it was approved by the Institutional Review Board (or Ethics Committee) of Comitato Etico Milano Area 2 (protocol code 137/2021 02/02/2021) for studies involving humans.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available by correspondence to the Corresponding Author.
Conflicts of Interest
X.D. and R.L. are cofounders and shareholders of Augmented Endoscopy. A.K. has the following declarations to make—Co-founder of AJM MED-I-CAPS Ltd.; co-director of iCERV Ltd.; Consultancy fees from Jinshan Ltd.; travel support from Jinshan, Aquilant, and DrFalkPharma; research support (grant) from ESGE/Given Imaging Ltd. and (material) IntroMedic/SynMed; honoraria from DrFalkPharmaUK, Ferring, Jinshan and Medtronic. Consultancy in Advisory board meetings for Dr FalkPharma UK, Tillots, ANKON. E.T. has the following declarations to make -Medtronic and Olympus (consultancy fee), Ankon (study material).
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pennazio M. Diagnosis of small-bowel diseases in the era of capsule endoscopy. Expert Rev. Med. Devices. 2005;2:587–598. doi: 10.1586/17434440.2.5.587. [DOI] [PubMed] [Google Scholar]
- 2.Branchi F., Ferretti F., Orlando S., Tontini G.E., Penagini R., Vecchi M., Elli L. Small-bowel capsule endoscopy in patients with celiac disease, axial versus lateral/panoramic view: Results from a prospective randomized trial. Dig. Endosc. 2020;32:778–784. doi: 10.1111/den.13575. [DOI] [PubMed] [Google Scholar]
- 3.Elli L., Casazza G., Locatelli M., Branchi F., Ferretti F., Conte D., Fraquelli M. Use of enteroscopy for the detection of malignant and premalignant lesions of the small bowel in complicated celiac disease: A meta-analysis. Gastrointest. Endosc. 2017;86:264–273.e1. doi: 10.1016/j.gie.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Lewis S.K., Semrad C.E. Capsule Endoscopy and Enteroscopy in Celiac Disease. Gastroenterol. Clin. N Am. 2019;48:73–84. doi: 10.1016/j.gtc.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Perez-Cuadrado-Robles E., Lujan-Sanchis M., Elli L., Juanmartinena-Fernandez J.-F., Garcıa-Lledo J., Ruano-Dıaz L., Egea-Valenzuela J., Jimenez-Garcıa V.-A., Arguelles-Arias F., Juan-Acosta M.S., et al. Role of capsule endoscopy in alarm features and non-responsive celiac disease: A European multicenter study. Dig. Endosc. 2018;30:461–466. doi: 10.1111/den.13002. [DOI] [PubMed] [Google Scholar]
- 6.Tomba C., Elli L., Elli L., Juanmartinena-Fernandez J.-F., Garcıa-Lledo J., Ruano-Dıaz L., Egea-Valenzuela J., Jimenez-Garcıa V.-A., Arguelles-Arias F., Juan-Acosta M.S., et al. Enteroscopy for the early detection of small bowel tumours in at-risk celiac patients. Dig. Liver Dis. 2014;46:400–404. doi: 10.1016/j.dld.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 7.Chetcuti Zammit S., Bull L.A., Sanders D.S., Galvin J., Dervilis N., Sidhu R., Worden K. Towards the Probabilistic Analysis of Small Bowel Capsule Endoscopy Features to Predict Severity of Duodenal Histology in Patients with Villous Atrophy. J. Med. Syst. 2020;44:195. doi: 10.1007/s10916-020-01657-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barada K., Habib R.H., Malli A., Hashash J.G., Halawi H., Maasri K., Tawil A., Mourad F., Sharara A.I., Soweid A., et al. Prediction of celiac disease at endoscopy. Endoscopy. 2014;46:110–119. doi: 10.1055/s-0033-1359200. [DOI] [PubMed] [Google Scholar]
- 9.Elli L., Branchi F., Sidhu R., Guandalini S., Assiri A., Rinawi F., Shamir R., Das P., Makharia G.K. Small bowel villous atrophy: Celiac disease and beyond. Expert Rev. Gastroenterol. Hepatol. 2017;11:125–138. doi: 10.1080/17474124.2017.1274231. [DOI] [PubMed] [Google Scholar]
- 10.Chetcuti Zammit S., McAlindon M.E., Sanders D.S., Sidhu R. Assessment of disease severity on capsule endoscopy in patients with small bowel villous atrophy. J. Gastroenterol. Hepatol. 2021;36:1015–1021. doi: 10.1111/jgh.15217. [DOI] [PubMed] [Google Scholar]
- 11.Rondonotti E., Spada C., Cave D., Pennazio M., Riccioni M.E., De Vitis I., Schneider D., Sprujevnik T., Villa F., Langelier J., et al. Video capsule enteroscopy in the diagnosis of celiac disease: A multicenter study. Am. J. Gastroenterol. 2007;102:1624–1631. doi: 10.1111/j.1572-0241.2007.01238.x. [DOI] [PubMed] [Google Scholar]
- 12.Biagi F., Rondonotti E., Campanella J., Villa F., Bianchi P.I., Klersy C., de Franchis R., Corazza G.R. Video capsule endoscopy and histology for small-bowel mucosa evaluation: A comparison performed by blinded observers. Clin. Gastroenterol. Hepatol. 2006;4:998–1003. doi: 10.1016/j.cgh.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Petroniene R., Dubcenco E., Baker J., Ottaway C., Tang S.-J., Streutker C., Gardiner G., Warren R., Jeejeebhoy K.N. Given capsule endoscopy in celiac disease: Evaluation of diagnostic accuracy and interobserver agreement. Am. J. Gastroenterol. 2005;100:685–694. doi: 10.1111/j.1572-0241.2005.41069.x. [DOI] [PubMed] [Google Scholar]
- 14.Murray J.A., Rubio-Tapia A., van Dyke C.T., Brogan D.L., Knipschield M.A., Lahr B., Rumalla A., Zinsmeister A.R., Gostout C.J. Mucosal atrophy in celiac disease: Extent of involvement, correlation with clinical presentation, and response to treatment. Clin. Gastroenterol. Hepatol. 2008;6:186–193. doi: 10.1016/j.cgh.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jang B.I., Lee S.H., Moon J.-S., Cheung D.Y., Lee I.S., Kim J.O., Cheon J.H., Park C.H., Byeon J.-S., Park S.Y., et al. Inter-observer agreement on the interpretation of capsule endoscopy findings based on capsule endoscopy structured terminology: A multicenter study by the Korean Gut Image Study Group. Scand. J. Gastroenterol. 2010;45:370–374. doi: 10.3109/00365520903521574. [DOI] [PubMed] [Google Scholar]
- 16.Korman L.Y., Delvaux M., Gay G., Hagenmuller F., Keuchel M., Friedman S., Weinstein M., Shetzline M., Cave D., de Franchis R. Capsule endoscopy structured terminology (CEST): Proposal of a standardized and structured terminology for reporting capsule endoscopy procedures. Endoscopy. 2005;37:951–959. doi: 10.1055/s-2005-870329. [DOI] [PubMed] [Google Scholar]
- 17.Leenhardt R., Buisson A., Bourreille A., Marteau P., Koulaouzidis A., Li C., Keuchel M., Rondonotti E., Toth E., Plevris J.N. Nomenclature and semantic descriptions of ulcerative and inflammatory lesions seen in Crohn’s disease in small bowel capsule endoscopy: An international Delphi consensus statement. United Eur. Gastroenterol. J. 2020;8:99–107. doi: 10.1177/2050640619895864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leenhardt R., Koulaouzidis A., Namara D.M., Keuchel M., Sidhu R., McAlindon M.E., Saurin J.C., Eliakim R., Sainz I.F., Plevris J.N., et al. A guide for assessing the clinical relevance of findings in small bowel capsule endoscopy: Analysis of 8064 answers of international experts to an illustrated script questionnaire. Clin. Res. Hepatol. Gastroenterol. 2021;45:101637. doi: 10.1016/j.clinre.2021.101637. [DOI] [PubMed] [Google Scholar]
- 19.Leenhardt R., Li C., Koulaouzidis A., Cavallaro F., Cholet F., Eliakim R., Fernandez-Urien I., Kopylov U., McAlindon M., Németh A., et al. Nomenclature and semantic description of vascular lesions in small bowel capsule endoscopy: An international Delphi consensus statement. Endosc. Int. Open. 2019;7:E372–E379. doi: 10.1055/a-0761-9742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oberhuber G., Granditsch G., Vogelsang H. The histopathology of coeliac disease: Time for a standardized report scheme for pathologists. Eur. J. Gastroenterol. Hepatol. 1999;11:1185–1194. doi: 10.1097/00042737-199910000-00019. [DOI] [PubMed] [Google Scholar]
- 21.Rostami K., Marsh M.N., Johnson M.W., Mohaghegh H., Heal C., Holmes G., Ensari A., Aldulaimi D., Bancel B., Bassotti G., et al. ROC-king onwards: Intraepithelial lymphocyte counts, distribution & role in coeliac disease mucosal interpretation. Gut. 2017;66:2080–2086. doi: 10.1136/gutjnl-2017-314297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delvaux M., Friedman S., Keuchel M., Hagenmüller F., Weinstein M., Cave D., de Franchis R., Gay G., Korman L.Y. Structured terminology for capsule endoscopy: Results of retrospective testing and validation in 766 small-bowel investigations. Endoscopy. 2005;37:945–950. doi: 10.1055/s-2005-870266. [DOI] [PubMed] [Google Scholar]
- 23.Elli L., Scaramella L., Tontini G.E., Topa M., Conte D., Sidhu R., Rondonotti E., Penagini R., Vecchi M. Clinical impact of videocapsule and double balloon enteroscopy on small bowel bleeding: Results from a large monocentric cohort in the last 19 years. Dig. Liver Dis. 2022;54:251–257. doi: 10.1016/j.dld.2021.07.014. [DOI] [PubMed] [Google Scholar]
- 24.Leenhardt R., Fernandez-Urien Sainz I., Rondonotti E., Toth E., van de Bruaene C., Baltes P., Rosa B.J., Triantafyllou K., Histace A., Koulaouzidis A., et al. PEACE: Perception and Expectations toward Artificial Intelligence in Capsule Endoscopy. J. Clin. Med. 2021;10:5708. doi: 10.3390/jcm10235708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tontini G.E., Rimondi A., Vernero M., Neumann H., Vecchi M., Bezzio C., Cavallaro F. Artificial intelligence in gastrointestinal endoscopy for inflammatory bowel disease: A systematic review and new horizons. Ther. Adv. Gastroenterol. 2021;14:17562848211017730. doi: 10.1177/17562848211017730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brocchi E., Tomassetti P., Misitano B., Epifanio G., Corinaldesi R., Bonvicini F., Gasbarrini G., Corazza G. Endoscopic markers in adult coeliac disease. Dig. Liver Dis. 2002;34:177–182. doi: 10.1016/S1590-8658(02)80190-6. [DOI] [PubMed] [Google Scholar]
- 27.Kasirer Y., Turner D., Lerman L., Schechter A., Waxman J., Dayan B., Bergwerk A., Rachman Y., Freier Z., Silbermintz A. Scalloping is a reliable endoscopic marker for celiac disease. Dig. Endosc. 2014;26:232–235. doi: 10.1111/den.12130. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available by correspondence to the Corresponding Author.