Abstract
In humans, the placenta provides the only fetomaternal connection and is essential for establishing a pregnancy as well as fetal well-being. Additionally, it allows maternal physiological adaptation and embryonic immunological acceptance, support, and nutrition. The placenta is derived from extra-embryonic tissues that develop rapidly and dynamically in the first weeks of pregnancy. It is primarily composed of trophoblasts that differentiate into villi, stromal cells, macrophages, and fetal endothelial cells (FEC). Placental differentiation may be closely related to perinatal diseases, including fetal growth retardation (FGR) and hypertensive disorders of pregnancy (HDP), and miscarriage. There are limited findings regarding human chorionic villous differentiation and placental development because conducting in vivo studies is extremely difficult. Placental tissue varies widely among species. Thus, experimental animal findings are difficult to apply to humans. Early villous differentiation is difficult to study due to the small tissue size; however, a detailed analysis can potentially elucidate perinatal disease causes or help develop novel therapies. Artificial induction of early villous differentiation using human embryonic stem (ES) cells/induced pluripotent stem (iPS) cells was attempted, producing normally differentiated villi that can be used for interventional/invasive research. Here, we summarized and correlated early villous differentiation findings and discussed clinical diseases.
Keywords: placenta, iPS cells, fetal growth retardation, hypertensive disorders of pregnancy, pregnancy, gestational diabetes
1. Placental Function
The placenta is an organ limited to the gestational period that is essential for fetal development in the uterus. Primary functions of this organ include (1) exchange of metabolic substances and gas between the mother and fetus; (2) maintenance of pregnancy via hormone production and secretion; (3) protection of the fetus from the maternal immune system (immunological tolerance).
2. Villous Differentiation and Placental Formation
2.1. From Villi Differentiation to Early Placental Formation
The fertilized egg becomes a blastocyst on the fifth day post-fertilization. This blastocyst consists of an inner cell mass (ICM) and a trophectoderm (TE), the former being the fetal part and the latter being the placenta. In the second embryonic week (4th week of gestation), the blastocyst attached to the endometrium progressively implants into the endometrium, where the TE differentiates into the syncytiotrophoblast (ST) and cytotrophoblast (CT). Around the ninth embryonic day (3 weeks and 2 days of gestation), vacuoles appear in the ST, which eventually fuse to form lacunae. On the other hand, around the 12th embryonic day (3 weeks and 5 days of gestation), capillaries become hyperemic and dilate in the endometrium at the implantation site, forming sinusoids. Then, the ST further invades and erodes the sinusoids, finally leading to a connection between the ST and the sinusoidal endothelial cells where early uteroplacental circulation is formed [1].
After the 13th embryonic day (3 weeks 6 days gestation), the CT invades the ST and forms the primary villi. At the third embryonic week (5th week of gestation), the CT penetrates into the ST and invades the endometrium. Adjacent primary villi also fuse to form a cell layer or the cytotrophoblastic shell, which covers the entire contact surface of the endometrium. Inside the villi, mesodermal cells on the outer wall of the embryo or from the CT invade the central axis to form secondary villi, from which a large number of villous branches ending in the intervillous spaces develop. At the end of the third embryonic week (5th week of gestation), the blood and vascular system differentiate into villous capillaries in the mesodermal villi core. This vasculature eventually connects to the chorionic plate and the capillaries that occur within the connecting stalk, as well as the fetal heart and blood vessels that begin beating at the fourth embryonic week (6th week of gestation), leading to the fetal-placental circulation system. After the fetal placental circulatory system is established, maternal blood and fetal blood are separated from the maternal surface by the intervening cells and tissues, such as the ST-CT-villi interstitial connective tissue, villi capillary endothelium, fetal blood, and maternal blood [2].
2.2. Formation of the Trophoblast
Trophoblasts can be broadly divided into two types—the trophoblast at the tip that invades the endometrium and the trophoblast that exists in the villous space due to the infiltration. The former is a trophoblast that infiltrates the endometrium, known as extravillous trophoblast (EVT). EVTs infiltrate deeply from the trophoblastic basement membrane toward the endometrium while forming a columnar cell column that causes remodeling of the uterine spiral artery. EVTs further infiltrate and temporarily close the arterioles; eventually, the vascular endothelial cells are completely replaced by trophoblasts. Furthermore, since the smooth muscle layer of the blood vessel wall is also replaced by trophoblasts, the proximal arteriole of the spinal artery endometrium is constantly in a dilated state, ensuring an abundant blood supply to the intervillous space.
The latter trophoblasts form villi; hence, they are termed villous trophoblast cells. These cells are maintained as a continuous single layer of CT on the basement membrane of the trophoblast. As the villi develop in the cavity, they become responsible for the formation of the ST that is in contact with maternal blood and covers the outermost surface of the villi. The main role of the ST is to facilitate gas and nutrient exchange between the mother and the fetus [3]. ST also secretes several hormones to maintain pregnancy, such as the human chorionic gonadotropin (hCG) and human placental lactogen (hPL). The proportion of CT decreases with the progression of pregnancy, and ST is mainly observed in the placenta at the end of labor.
2.3. Placental Blood Vessel Development
At around 20 embryonic days (5 weeks gestation), the basic structure of the villi is completed, which is then followed by placental villi angiogenesis. This angiogenesis is not the invasion of fetal blood vessels into the placenta but the formation of new capillaries. At this point, the villi exist as primary or secondary villi, with mesenchymal cells at the center. Hofbauer cells derived from mesenchymal cells appear when the first blood vessels are formed in the secondary villi, and the expression of angiogenic factors from these cells and the surrounding stromal cells leads to angiogenesis in the placenta. In addition, decidual cells and macrophages on the maternal side also express angiogenic factors.
Around the sixth week of gestation, a vascular basement membrane is formed. By the end of the 13th week of gestation, blood vessels in the villi repeat their formation and branching, with no functional differentiation and numerical increase. Then, myofiber cells develop around the blood vessels around the 15th week of gestation, completing their basic structure. The formation of blood vessels continues until the middle of gestation as the placenta grows. During the third trimester, blood vessel branching does not occur in the villi, and a villus morphology suitable for gas exchange is formed [4,5].
3. Placental Formation and Hypoxia
In early pregnancy, blastocysts are exposed to severe hypoxia (2–3% or low PO2 of 15–20 mmHg) in the uterus 6 days after conception [6]. From the 10th to 12th weeks of gestation, the trophoblast occludes the spiral artery and prevents the influx of maternal blood into the trophoblastic space. Therefore, the placenta and fetus are placed under hypoxic conditions. In fact, from 8 to 10 weeks of gestation, the oxygen tensions of the placenta and endometrium were 17.9 mmHg and 39.6 mmHg, respectively. It has been reported that the placental oxygen tension at 11 weeks of gestation is about 1/4 of that of the term decidua [6]. From the 12th to 13th week of gestation, the trophoblastic obstruction in the spiral arteries becomes loose, allowing the maternal blood inflow into the trophoblastic space. This makes the placental oxygen tension equivalent to the endometrial oxygen tension [7]. In the early stages of gestation, the placenta and fetus develop under hypoxic conditions. This hypoxic-ischemia-reoxygenation process is normal, physiological, and essential for normal fetal and placental development [8,9]. As mentioned above, hypoxic conditions are not always harmful to cells and can sometimes be beneficial and protective.
Trophoblastic infiltration into the maternal tissue is similar to that of tumor cells, but unlike tumor cells that infiltrate indefinitely and randomly, trophoblastic infiltration occurs within a controlled range [10]. For example, in invasive tumors, a hypoxic environment induces the expression of vascular endothelial growth factor (VEGF) and angiogenesis [11]. It is postulated that this series of reactions is greatly involved in cell migration and infiltration [12]. Among invasive trophoblasts in vivo, only trophoblasts located near the fetal side of villi proliferate [13]. Furthermore, trophoblasts have a lower proliferative ability and higher infiltration ability as they move away from the fetal side of the placental villi. As trophoblasts infiltrate, they become exposed to a high oxygen environment, which halts cell proliferation and causes the continuation of the trophoblast infiltration [14,15]. This phenomenon is controlled spatiotemporally and is extremely important for the continuation of pregnancy [16].
The hypoxic environment in early pregnancy plays an important role in controlling the proliferation and infiltration of these precise trophoblasts. It is speculated that the proliferative nature of trophoblasts under hypoxic conditions may contribute largely to the development of the placenta before fetal development in the early stages of gestation [17,18]. Furthermore, physiological hypoxia during the early stages of pregnancy is thought to enhance the angiogenic activity of the fetal placental endothelium [19]. At 8–9 weeks of gestation, the expression of reactive oxygen species (ROS) scavenging enzymes in the trophoblast cytoplasm and mitochondria is low, making the trophoblasts susceptible to damage by oxidative stress. The expression of the ROS scavenging enzymes increases after 10 weeks of gestation and gradually allows resistance to oxidative stress [20,21,22]. Thus, the relationship between placental formation and oxygen tension is important, and its abnormalities are closely related to the pathogenesis of HDP and FGR.
Various factors influence the characteristic properties of trophoblasts under hypoxic conditions. Among them, hypoxia-inducible factor (HIF)-1 is a typical transcription factor activated by hypoxia. It has a basic helix-loop-helix-PAS (bHLH-PAS) region consisting of HIF-1α and HIF-1β subunits. HIF-1 regulates the transcription of various genes in a cell-specific manner under hypoxic conditions. Under normal oxygen levels, the proline residues of HIF-1 are hydroxylated by proline hydroxylase (PHD), inducing ubiquitination and proteasome degradation by the ubiquitin ligase Von Hippel Lindau (VHL) disease-causing gene product, pVHL. However, under hypoxic conditions, PHD is inhibited, thereby allowing HIF-1 to have transcriptional activity. HIF-1 is also known to induce a transcriptional response to hypoxic stimulation by binding to hypoxia response elements (HRE) present in the promoters and enhancers of genes involved in the glycolytic pathway, sugar transport, and angiogenesis [23,24]. Downstream enzymes of HIF-1, such as the vascular endothelial growth factor (VEGF), glucose transporter-1 (GLUT1), transforming growth factor β3 (TGFβ3), etc., also affect placental formation [25,26,27]. Mice lacking VHL or HIF-1β undergo intrauterine fetal death due to placental dysfunction [28,29]. Furthermore, VHL and HIF-1β are reported to be involved in the differentiation and invasion of human and mouse trophoblasts [30,31].
TGFβ3 inhibits EVT infiltration by inducing the switch from integrin α1 to integrin α5 under hypoxic conditions and promoting the expression of TGFβ3 [32]. TGFβ3 is highly expressed in the placenta from 6 to 9 weeks of gestation and suppresses trophoblastic invasion. Normally, the expression of TGFβ3 decreases around the ninth week of pregnancy. If the expression is increased after 9 weeks, the placenta becomes shallowly infiltrated by trophoblasts and suppresses the expression of angiogenic genes, causing HDP [33,34,35]. TGFβ3 also regulates genes involved in the cell cycle and is involved in FGR morbidity [36]. In addition, the insulin-like growth factor 2 (IGF-II) has also been reported to affect the trophoblastic infiltration under hypoxia [37].
Ten-eleven translocation 1 (TET 1), an important gene that has a role in DNA demethylation, affects trophoblast infiltration [37,38]. Thus far, various experimental systems have reported that trophoblasts proliferate under hypoxic conditions. Trophoblasts, which proliferate actively in the body from 6 to 10 weeks of gestation, have decreased mitotic figures from 10 to 12 weeks of gestation when exposed to maternal blood [39]. A study has revealed that under hypoxic conditions, the HTR-8 SVneo cell line of the early human trophoblast has increased proliferative capacity and decreased infiltration capacity into the Matrigel [40]. Trophoblasts isolated from living organisms also had an enhanced proliferative capacity under 2% oxygen concentration compared to those under 20% oxygen concentration [41]. There were also reports of increased VEGF expression mediated by the renin–angiotensin system and increased infiltration ability mediated by the notch signaling under hypoxic conditions [42,43]. On the other hand, recent studies revealed that HIF2α is involved in the placental formation and that HIF2α in the decidua allows the infiltration of the chorion in a mouse model [44,45]. In any case, the relationship between hypoxic conditions and the proliferation and infiltration of trophoblasts is important, and any abnormalities in these processes may be closely related to the pathogenesis of HDP and FGR.
The differentiation between CT and polynuclear ST is performed in a microenvironment with a relatively high oxygen tension. In a study wherein human villous membrane cells were cultured in vitro under different O2 levels, an atmospheric (21%) O2 level promoted natural CTB cell fusion to STB, while low O2 levels (<11%) markedly reduced cell fusion. Low O2 levels were found to downregulate the hormone levels secreted by STB [31,46]. The inhibition of STB differentiation under hypoxic conditions during the first phase of pregnancy is partially dependent on the intact HIF complex. In HIF-mutant mice, CTB differentiates exclusively into STB, suggesting that in the absence of hypoxic conditions or stimulation, CTB, by default, differentiates into STB [47]. HIF-1β deficiency (ARNT) in primary CTB can restore the secretion of STB-producing HCG [31]. Additionally, the expression of trophoblast-specific HIF-1α, which mimics prolonged hypoxia, causes vascular development such as decreased branch morphogenesis, changes in the mesenteric space, and impaired spiral arterial remodeling [48].
4. Formation of the Decidua
Embryo implantation into the endometrium is only possible during the implantation phase, and the sex steroid hormones secreted from the ovary are important for the regulation of endometrial differentiation. The endometrium responds to sex steroid hormones and factors from the embryo, allowing for endometrial changes suitable for embryo implantation. On the 7.5th day post-ovulation, the human embryo is already buried under the epithelium of the endometrium, and the trophoblast cells on the uterine side are enlarged and activated. On the 12th day post-ovulation, lacunar spaces are formed in the trophoblast cell layer, and communication with the maternal blood flow is started. The human chorionic gonadotropin (hCG), which is produced in large quantities by trophoblasts and shares a receptor with LH, reaches the corpus luteum via the maternal bloodstream, which then stimulates progesterone production and maintains embryo implantation.
Immediately after implantation of the blastocyst, the endometrium becomes edematous due to the action of progesterone, the uterine spiral arteries also develop remarkably, and the endometrial stromal cells undergo decidualization. In addition to the mechanism of embryo–maternal implantation induction via the endocrine system, the immune system also plays an important role. In addition, the mother’s immune system develops immune tolerance to the fetus due to the antigen derived from the paternal line and accepts the fetus. One of the immune mechanisms is the proliferation of regulatory T (Treg) cells that specifically recognize the father-derived antigen expressed by the fetus in the mother’s body and suppress the immune response against the fetus [49].
Furthermore, it is thought that normal placental formation is established by the active recognition of fetal-derived trophoblasts by the maternal immune system even during the placental formation stage after implantation. The human placenta is a hemochorial placenta where trophoblasts infiltrate the decidua deeply, allowing for wide contact of fetal and maternal tissues. Immediately after blastocyst implantation, when trophoblasts infiltrate the endometrium, the endometrium becomes the decidua. The number of immune cells is increased at the implantation site in the decidua; the majority are decidual natural killer (dNK) cells, macrophages, and dendritic cells (APCs). It has also become clear that dNK cells and APCs are involved in trophoblast infiltration and placental angiogenesis. From here on, we will outline the role of various immunocompetent cells in the endometrium.
4.1. Natural Killer (NK) Cells
4.1.1. Decidual Natural Killer (dNK) Cells
Initially, NK cells were reported as lymphocytes that were cytotoxic to tumor cells. Later, it was reported that NK cells have both cytotoxicity and cytokine-producing ability in a lymphocyte population separate from T cells and B cells [50]. In humans, NK cells are recognized as CD56+/CD3− cells. There are two populations of NK cells in the blood-cytotoxic NK cells (CD56dim/CD16+) and cytokine-producing NK cells (CD56++/CD16−), with the latter accounting for about 90% of the cell population [51].
The dNK cells in the human endometrium are CD56++/CD16−. However, dNK cells have many granules in the cells and have different properties from CD56++/CD16− NK cells in the blood [52]. Even during non-pregnancy, dNK cells are present in the endometrium; a small number are present in the proliferative phase and early secretory phase, and the number of cells increases in the late secretory phase. In early pregnancy, the number of dNK cells further increases, accounting for about 70% of the total leukocyte count in the decidua during the first trimester and are localized near trophoblasts [53]. Interestingly, dNK cells also increase during delivery [54,55,56]. After the second trimester, dNK cells decrease [54,55]. dNK cells were reported to originate from the following: (1) peripheral blood CD56++/CD16− NK cells, (2) peripheral blood CD56dimCD16+ NK cells, (3) immature NK progenitor cells in the uterus, and (4) hematopoietic stem cells [57,58,59]. In addition, the CXCL12/CXCR4 signaling pathway is used for dNK cell migration to the implantation site [60,61].
4.1.2. Role of dNK Cells
dNK cells regulate the degree of trophoblast invasion into the decidua [62]. dNK cells express HLA receptors, killer cell immunoglobulin-like receptors (KIRs), CD94/NKG2A, and ILT2. These are HLA-C, HLA-E, and HLA-G receptors expressed in trophoblasts, respectively, and contribute to the immunological tolerance of trophoblasts at the fetomaternal interface. dNK cells are also involved in decidua-helicine artery remodeling, increasing the blood flow to the placenta and playing an important process in fetal development [63,64]. In animal models, NK cell-deficient mice were reported to have a reduced blood flow to the placenta [65].
4.2. Decidual Macrophages
Monocytes in the circulating blood are precursors of decidual macrophages. During pregnancy, the phagocytic activity of monocytes decreases, protecting the alloantigen fetus. Decidual macrophages are mainly located in the interstitial site just below the endometrial epithelium and are localized around the fetomaternal interface during the implantation period. Decidual macrophages play a role in transmitting immune information derived from embryonic paternity to the mother and in tissue remodeling of the implantation site.
4.2.1. M1 and M2 Macrophages
Macrophages can be classified into two types—those activated by the classical pathway (M1) and those activated by the alternative pathway (M2) [66]. M1 and M2 have different patterns of surface markers and cytokine secretion [67]. M1 macrophages are associated with inflammation, have a high antigen-presenting ability, secrete a large number of cytokines, including IL-12, IL-23, and ROS, and are involved in the Th1-type response [68]. M2 macrophages secrete cytokines such as IL-4, IL-10, and VEGF, which are involved in tissue remodeling and immunosuppression by promoting Th2-type immune responses. Studies on transcription factors that control macrophage differentiation have indicated that STAT1, C/EBP-α, C/EBP-δ, and NF-κB are involved in M1 macrophage differentiation, while PPARs, STAT3, STAT6, and C/EBP-β are involved in M2 macrophage differentiation [69]. The inflammatory environment in preeclampsia (HDP) increases M1-type digital macrophages [70].
4.2.2. Phenotypes of Decidual Macrophages
M1 macrophages express CD80 and CD86 as surface markers, while M2 macrophages express CD206, CD209, and CD163. After the implantation period during pregnancy, lymphocytes and polymorphonuclear leukocytes do not increase at the implantation site, whereas decidual macrophages increase before delivery. After the complete formation of the placenta, decidual macrophages differentiate mainly to M2 to prevent fetal rejection and allow fetal growth until delivery. Recently, M2 expressing Tim-3 has been attracting attention as an important biomarker for maintaining immune tolerance [71]. Furthermore, decidual macrophages in the first trimester are classified by the expression of CD11c. CD11c-high decidual macrophages had lower expression of phagocytic receptors (CD209 and CD206) than CD11c-low decidual macrophages. CD11c-high decidual macrophages function in lipid metabolism, inflammation, antigen processing, and immunoregulation, while CD11c-low decidual macrophages express genes involved in growth and development regulation and extracellular communication [72].
4.3. Regulatory T (Treg) Cells
Treg cells have the function of suppressing the excessive immune response that underlies autoimmune, inflammatory, and allergic diseases. On the other hand, Treg overactivation may suppress the immune response to cancer and promote its progression. The transcription factor Foxp3 is involved in the regulation of Treg cell development and differentiation. Mutations in the Foxp3 gene were identified as the cause of IPEX (immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome), which is a human autoimmune disease also seen in scurfy mice. Immature T cells in the thymus express Foxp3 upon presentation of a self-antigen by thymic epithelial cells and induce differentiation into Treg [73,74].
Role of Treg Cells
Immune tolerance exists during pregnancy when the fetus is accepted. For example, when a Balb/c female mouse mates with a C57BL/6 male mouse and becomes pregnant, tumor cells derived from C57BL/6 engraft in the pregnant Balb/c female mouse. After delivery, tumor cells derived from C57BL/6 are rejected [75]. This indicates that immune tolerance is achieved in female mice to paternal antigens during pregnancy, which disappears after delivery. Treg cells play an important role in immune tolerance during pregnancy [76]. Decreasing Treg cells during implantation results in implantation failure in allogeneic pregnancies but has no effect in syngeneic pregnancies. In other words, when the number of Treg cells decreases at the time of implantation, embryos expressing an allogeneic antigen are rejected. The expression of Foxp3, a Treg cell marker, is decreased in the endometrium during implantation in unexplained human infertility cases [77]. When Treg cells decrease in the early stages of pregnancy, miscarriages of both male and female fetuses occur in allogeneic pregnancies, but only of male fetuses in syngeneic pregnancies [78,79]. Treg reduction induces an immune tolerance not only to the major histocompatibility complex (MHC) but also to minor histocompatibility antigens (MiHA) such as the male-specific antigen, SRY. These results show that Tregs function to maintain pregnancy.
Th1/Th2/Th17/Treg cell imbalance and abnormal Treg ratios have been reported as the mechanisms involved in implantation failure. In patients with recurrent miscarriages, the ratios of Th1 cells to Th2 cells and of Th17/Treg cells are high, predominantly having Th1 and Th17 cells, respectively [80,81]. There is a close interaction between Treg proliferation and IL-17 secretion. When IL-17 binds to the IL-17 receptor, Treg cells proliferate. Conversely, Treg cells suppress Th17 cell proliferation and IL-17 secretion via Il-10/TGF-β [82]. Administration of IL-17 to mice markedly increased the abortion rate; however, administration of Treg cells to mice significantly increased IL-10 and TGF-β expression and prevented abortion [83]. In addition, a report examining postpartum decidua showed that Treg cell expression was elevated in placenta accreta compared to that in the normal placenta, suggesting that Treg cells may be involved in EVT infiltration [84].
5. Genes Involved in Villous Differentiation
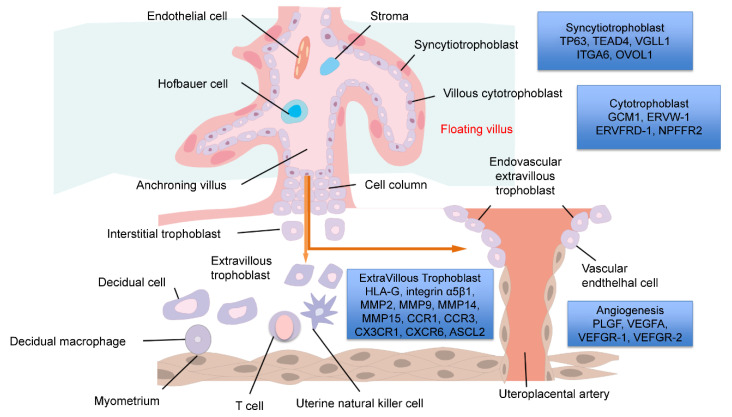
Studies have been conducted to understand villous differentiation using early abortion samples and transgenic mice. In recent years, new experimental tools have become available, such as blastocyst analysis and the above-mentioned induction of trophoblast differentiation using ES cells and iPS cells. Here we describe some known typical genes that contribute to placental differentiation (Figure 1 and Figure 2 and Table 1).
Figure 1.
Placental development immediately before implantation and involved genes in the trophectoderm.
Figure 2.
The fetomaternal interface. The major trophoblast subtypes and maternal cells are illustrated. Genes involved in the formation of the cytotrophoblast, syncytiotrophoblast, extravillous trophoblast, and angiogenesis are described.
Table 1.
Summary of each gene associated with placentation.
| Human First-Trimester Trophoblast Stage and Vascular Development | Gene Symbol | Gene Description |
|---|---|---|
| Trophectoderm (TE) | Caudal Type Homeobox 2 (CDX2) | The interaction of Oct4 and CDX2, which are typical undifferentiated markers that affect the inner cell mass, leads to the differentiation of the trophoblast and the inner cell mass. Although CDX2 is known to be expressed in the trophoblast, the detailed mechanism of trophoblast differentiation is unknown. |
| GATA Binding Protein 3 (GATA3) | GATA3, a transcription factor expressed in trophoblasts, is involved in the differentiation of trophoblasts into villi as well as the infiltration and migration of the villi into the maternal surface. It is thought to play an important role in the placental formation [85,86]. | |
| Cytotrophoblast (CT) | Tumor Protein P63 (TP63) | TP63 is a member of the P53 tumor suppressor family. Through the control of epithelial-mesenchymal transition, cell adhesion, and matrix degradation pathways, TP63 suppresses CT differentiation into EVT and maintains a proliferative CT state [87] |
| TEA Domain Transcription Factor 4 (TEAD4) | Transcription factors of the TEAD family are the ultimate intranuclear effectors of the Hippo pathway. Among these, TEAD4 regulates cell growth, proliferation, and homeostasis in CT [88,89,90]. | |
| Vestigial-Like Family Member 1 (VGLL1) | VGLL1 is a co-transcriptional activator of TEAD4; when VGLL1 expression decreases, the expression of TP63, which is a marker of CT, also decreases. For this reason, VGLL1 is thought to be involved in the maintenance of CT [91,92]. | |
| Integrin Subunit Alpha 6 (ITGA6) | ITGA6 is a cell surface protein that constitutes the major adhesive receptor for laminin. Abundant laminin is present in the stem cell niche, and ITGA6 is involved in cell proliferation and self-renewal [93]. | |
| Ovo-like Transcriptional Repressor 1 (OVOL1) | OVOL1 regulates TP63 expression. OVOL1 is necessary to suppress the differentiation of CT into ST and maintain its state by inhibiting the expression of syncytin1 and syncytin 2 [94]. | |
| Syncytiotrophoblast (ST) |
Glial Cell Missing Transcription Factor 1 (GCM1) | GCM1 is a gene that inhibits the differentiation from CT to EVT and induces differentiation into ST by fusing cells. GCM1 is controlled by GATA3 [95,96]. |
| Neuropeptide FF-Amide Peptide Precursor (NPFF) | The role of neuropeptide FF (NPFF) is well known in the central nervous system. NPFF receptor 2 (NPFFR2) mRNA is abundant in the placenta; however, the function of NPFF-NPFFR2 in placental development is unknown. NPFF acts via NPFFR2, promotes the expression of syncytin 1 and 2 via GCM1, and is involved in ST formation [97]. | |
| Endogenous Retrovirus Group W Envelope Member 1, Envelope (ERVW-1) | ERVW-1, a gene encoding the syncytin-1 protein, is derived from an endogenous retrovirus. Syncytin-1 promotes cell fusion. Genes derived from retroviruses play an indispensable role in placental formation. The expression of ERVW-1 is regulated by GCM-1 [98,99]. | |
| Endogenous Retrovirus Group FRD Member 1, Envelope (ERVFRD-1) | ERVFRD-1 is a gene encoding the syncytin-2 protein. Like ERVW-1, ERVFRD-1 is also derived from an endogenous retrovirus. Syncytin-2 also promotes cell fusion and is also controlled by GCM1 [100,101] | |
| Extravillous trophoblast (EVT) | Major Histocompatibility Complex, Class I, G (HLA-G) | HLA-G is the most representative gene expressed in EVT. It is classified as a human leukocyte antigen, which is a human major histocompatibility complex. HLA-G contributes to immunosuppression to establish pregnancy and allow the fetus to escape maternal immunity [102]. |
| Integrin | Integrin is a protein on the cell surface and is a cell adhesion molecule. It is a heterodimer consisting of two subunits, the α and β chains. Integrin α5β1 is expressed in EVT [103]. Pregnancy-specific glycoproteins (PSGs) are secretory proteins present in the maternal placenta. There are 11 PSG genes in humans, and PSG1 interacts directly with integrin α5β1 [104]. | |
| Matrix Metalloproteinase (MMP) | The MMP family currently has 28 members (MMP 1 to 28), and the expression of MMP2, MMP9, MMP14, and MMP15 has been reported in EVT. MMPs degrade extracellular matrices and proteins expressed on the cell surface [105,106,107,108]. | |
| Chemokines and Chemokine Receptors (CCR) | Chemokines are small-molecule polypeptides involved in cell proliferation, differentiation, apoptosis, angiogenesis, hematopoiesis, tumor promotion, and inflammatory diseases (85,86). Chemokines play an important role in placental function and play a major role in the infiltration of EVT into the maternal decidua, as the chemokine receptors CCR1, CCR3, CX3CR1, and CXCR6 are localized in EVT [109,110,111,112]. | |
| Achaete-Scute Family BHLH Transcription Factor 2 (ASCL2) | ASCL2 is a member of the basic helix-loop-helix (BHLH) family of transcription factors. Its expression is observed in EVT in early pregnancy. While ASCL2 has been reported to be involved in tumor infiltration in breast cancer, it also plays an important role in human EVT formation [31,113]. | |
| Angiogenesis | Placental Growth Factor (PGF) | PGF belongs to the vascular endothelial growth factor (VEGF) family. PGF is expressed in vascular endothelial cells in the placenta and plays a role in vasodilation and angiogenesis [114,115]. |
| Vascular Endothelial Growth Facto A (VEGFA), Fms Related Receptor Tyrosine Kinase 1 (FLT1), and the Kinase Insert Domain Receptor (KDR) | VEGFA, a vascular endothelial growth factor, belongs to the VEGF family and is expressed in trophoblasts and vascular endothelial cells, and is also involved in angiogenesis in the early placenta. VEGFR-1/Flt-1 and VEGFR-2/KDR/Flk-1 have been identified in placental tissues as receptors for the VEGF family [116,117]. |
6. Diseases Involving Placental Dysfunction
6.1. Fetal Growth Restriction (FGR)
Scattered infarcts are characteristic placental histological findings in FGR. However, images of placental infarcts are not FGR-specific and can only be seen in approximately 25% of the FGR placenta. In addition, nodules in ST, CT thickening, fibrinoid deposition, decreased vascular bed, decreased volume of villi, decreased villous space, and non-specific inflammation were observed, which all decreased the blood flow in the fetus-placenta-uterus [118]. Biologically, VEGF-A expression is elevated in FGR placentas. This indicates that a decrease in fetal-placental-uterine blood flow during placental formation induces the expression of angiogenic factors [119]. Furthermore, in FGR, the proportion of uNK cells in the decidua is reduced, as well as the expression of VEGF-A, PGF, interleukin-10, and other angiogenic factors [120,121,122]. In FGR, the expression of HLA-G in EVT is reduced, resulting in the disruption of the fetomaternal immune tolerance and the occurrence of non-specific inflammatory findings. In the placenta of FGR, the mTORC1 activity of Mechanical TOR (mTOR), a serine-threonine kinase protein that regulates cell survival, metabolism, growth, and proliferation, is inhibited [123]. The mTOR pathway regulates the expression and activity of placental transporters responsible for the transport of amino acids, fatty acids, and glucose. Many of these transporters are underexpressed in FGR, resulting in functional loss of the placenta [124,125,126]. Thus, it has been suggested that placental hypoplasia is one of the causes of FGR.
6.2. Hypertensive Disorders of Pregnancy (HDP)
Placental features of preeclampsia include fibrinoid deposition on the vessel wall, macrophage hyperplasia, villus hypoplasia (especially decreased villus count on the maternal surface, decreased villus diameter, and decreased vascular bed), and ST nodules [127,128]. The two-step theory is a well-known cause of preeclampsia [129], which states that a hypoxic state due to vascular remodeling failure secondary to the infiltration of extravillous trophoblasts into the spiral artery results in a decreased blood flow to the villous lumen and an insufficient oxygen supply [130]. Under hypoxic conditions, trophoblasts produce soluble endoglin (sEng) and soluble fms-like tyrosine kinase-1 (sFlt1). sEng constricts blood vessels and restricts blood flow. In addition, sEng and sFlt1 inhibit VEGF and PGF receptors, resulting in the inhibition of angiogenesis and proliferation of vascular endothelial cells. sEng and sFlt1 are expressed transplacentally and are also found in the maternal circulation, inducing maternal vascular endothelial damage along with placental hypoplasia [131,132]. In addition, the heat shock protein 70 (HSP70) released from the placenta, high mobility group box 1, and tissue factor (TF), which promotes coagulation, also contribute to the pathogenesis of HDP [128].
The reason for the occurrence of helical artery hypoplasia has not yet been elucidated, but the interaction of the maternal immune system and the fetal (paternal) major histocompatibility complex (MHC) antigen causes a local fetomaternal immune response. It is believed that promoting tolerance is essential for normal placental formation, and any hindrance to immune tolerance is thought to lead to the development of HDP [133]. Treg cell dysregulation is also involved in the disease. The number of Treg cells and the ratio of Treg/Th17 cells are significantly reduced in preeclampsia [134]. In HDP, the expression of Foxp3, a Treg-specific transcription factor, is decreased, and the expression of the retinoic acid receptor-related orphan nuclear receptor γt (RORγt), a Th17-specific transcription factor, is increased compared to those in healthy pregnant women [135]. As described above, it is suggested that in HDP, the conversion from Treg cells to Th17 cells occurs, resulting in an abnormal immune state in which inflammation is induced. In addition, maternal factors such as obesity, hypertension, and autoimmune diseases increase the risk of developing HDP. Furthermore, the degree of inflammation of maternal blood vessels before pregnancy may contribute to the onset of HDP [136,137,138]. In recent years, it has been reported that autophagy is involved in placental hypoxia and that autophagy failure is associated with the onset of PE [139,140].
6.3. Gestational Diabetes Mellitus (GDM)
The histological findings of the placentae of GDM patients have been reported to include a significant increase in fibrinoid necrosis, infarct image, and immaturity of villi compared to those of the normoglycemic control group. It is also reported that the fetal/placental weight ratio was significantly lower in the diabetic group [141,142]. GDM placentae have increased leptin concentration, increased expression of the TNF-α signaling gene, and increased IL-1 and IL-8 receptor genes compared to normal placentae [143,144,145]. These inflammatory cytokines are speculated to be involved in maternal insulin resistance. In addition to the inflammatory reaction, increased placental oxidative stress and ER stress contribute to the onset of GDM [146,147,148]. In the placenta, glucose is taken up via the glucose transporter (GLUT), and the fetus obtains glucose from the maternal circulation via the placenta. Furthermore, GLUT-1 is expressed in ST and is involved in glucose uptake from the maternal circulatory system. GLUT-1 is upregulated in the placentae of GDM patients, in addition to the upregulated expression of GLUT-3 and GLUT-4 in other organs [149,150].
7. Trophoblast Research Tool
The placenta is involved in the development of various diseases. However, it is difficult to investigate the causes of disease in the human placenta. Although it is possible to obtain the placenta of the diseased patients, it is technically and ethically difficult to reproduce the findings obtained from the placenta in vivo. Various common in vitro models have been used so far. For example, a primary culture using placental tissue after delivery was performed; however, a complete trophoblast was difficult to isolate from the placental tissue, and it was difficult to maintain the culture for a long period, thus making it difficult to use for research. In recent years, single-cell analysis has become possible, bringing new findings to placental research [151,152].
Choriocarcinoma cell lines such as BeWo, JAG-3, and JAR have the advantage of being easily cultured, although their origins are different from those of normal villi [153,154,155]. Choriocarcinoma cell lines are difficult to differentiate from CT to EVT in culture but are easily differentiated from the ST lineage. Therefore, placenta-derived EVT immortalized cell lines such as HTR 8/SVneo and Swan71 are used instead. However, in reality, the phenotype is slightly different from that of the living placenta [156,157]. It is difficult to evaluate any of these differentiation culture systems in terms of whether or not they reflect the state in vivo. In addition, it is difficult to reproduce the research in primary cultures because of the effect of the patient genetic background complicating the culture system. In order to overcome these problems, culture systems using ESCs and iPSCs are established to differentiate into trophoblasts [46,158,159]. We are currently studying this culture system [160]. We focused on KRT7, which is a general surface marker of trophoblasts. We treated iPSCs with BMP4 and reported the results of a comprehensive analysis of KRT7-positive cells [3,161].
In recent years, long-term culture systems of the human CT (monolayer and spheroids) and organoids are produced from the placenta in the early stages of pregnancy without using ESCs or iPSCs [162,163]. There is also a report of a culture system treated with naive iPSCs that was used for research on the differentiation of fertilized eggs into a trophectoderm system [164]. These stem cell and CT culture systems, and organoid production, may be advantageously used to study the process of differentiation from CT to ST and EVT to some extent. However, it is impossible to confirm whether the situation in vivo is reproduced.
8. Conclusions
We have described the morphological development of the placenta and villi and their differentiation from the viewpoint of molecular biological markers, taking into account their relationship with pregnancy-related diseases. The early placental formation is difficult to study from an ethical point of view, and there are many unclear points about its pathophysiology. However, recent studies have reported the development of various culture systems for understanding early placental formation. The issue is how to utilize these new culture systems, which have become feasible in recent years, for investigating the causes of various diseases related to the placenta and developing treatment strategies.
Acknowledgments
We thank our department members for their helpful support.
Author Contributions
Conceptualization, J.K., M.O. and H.N.; Writing—Original Draft Preparation, J.K. and M.O.; Writing—Review and Editing, N.K. and H.N. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Tokyo Medical University (protocol code T2021-0257; 27 January 2022).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the Japan Society for the Promotion of Science Grants-in-Aid for Scientific Research (grant no. 18K09301, 22K09556, and 22K16866).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.James J.L., Carter A.M., Chamley L.W. Human placentation from nidation to 5 weeks of gestation. Part I: What do we know about formative placental development following implantation? Placenta. 2012;33:327–334. doi: 10.1016/j.placenta.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 2.Cuman C., Menkhorst E., Winship A., Van Sinderen M., Osianlis T., Rombauts L.J., Dimitriadis E. Fetal-maternal communication: The role of Notch signalling in embryo implantation. Reproduction. 2014;147:R75–R86. doi: 10.1530/REP-13-0474. [DOI] [PubMed] [Google Scholar]
- 3.Lee C.Q., Gardner L., Turco M., Zhao N., Murray M.J., Coleman N., Rossant J., Hemberger M., Moffett A. What Is Trophoblast? A Combination of Criteria Define Human First-Trimester Trophoblast. Stem Cell Rep. 2016;6:257–272. doi: 10.1016/j.stemcr.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Degner K., Magness R.R., Shah D.M. Establishment of the Human Uteroplacental Circulation: A Historical Perspective. Reprod. Sci. 2017;24:753–761. doi: 10.1177/1933719116669056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saghian R., Bogle G., James J.L., Clark A.R. Establishment of maternal blood supply to the placenta: Insights into plugging, unplugging and trophoblast behaviour from an agent-based model. Interface Focus. 2019;9:20190019. doi: 10.1098/rsfs.2019.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jauniaux E., Watson A., Burton G. Evaluation of respiratory gases and acid-base gradients in human fetal fluids and uteroplacental tissue between 7 and 16 weeks’ gestation. Am. J. Obstet. Gynecol. 2001;184:998–1003. doi: 10.1067/mob.2001.111935. [DOI] [PubMed] [Google Scholar]
- 7.Rodesch F., Simon P., Donner C., Jauniaux E. Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstet. Gynecol. 1992;80:283–285. [PubMed] [Google Scholar]
- 8.Burton G.J., Jauniaux E., Charnock-Jones D.S. The influence of the intrauterine environment on human placental development. Int. J. Dev. Biol. 2010;54:303–312. doi: 10.1387/ijdb.082764gb. [DOI] [PubMed] [Google Scholar]
- 9.Soares M.J., Iqbal K., Kozai K. Hypoxia and Placental Development. Birth Defects Res. 2017;109:1309–1329. doi: 10.1002/bdr2.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Velicky P., Knöfler M., Pollheimer J. Function and control of human invasive trophoblast subtypes: Intrinsic vs. maternal control. Cell Adhes. Migr. 2016;10:154–162. doi: 10.1080/19336918.2015.1089376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varney M.L., Olsen K.J., Mosley R.L., Singh R.K. Paracrine regulation of vascular endothelial growth factor—A expression during macrophage-melanoma cell interaction: Role of monocyte chemotactic protein-1 and macrophage colony-stimulating factor. J. Interferon Cytokine Res. 2005;25:674–683. doi: 10.1089/jir.2005.25.674. [DOI] [PubMed] [Google Scholar]
- 12.Heikkila P., Suojanen J., Pirila E., Vaananen A., Koivunen E., Sorsa T., Salo T. Human tongue carcinoma growth is inhibited by selective antigelatinolytic peptides. Int. J. Cancer. 2006;118:2202–2209. doi: 10.1002/ijc.21540. [DOI] [PubMed] [Google Scholar]
- 13.Irving J.A., Lysiak J.J., Graham C.H., Hearn S., Han V.K., Lala P.K. Characteristics of trophoblast cells migrating from first trimester chorionic villus explants and propagated in culture. Placenta. 1995;16:413–433. doi: 10.1016/0143-4004(95)90100-0. [DOI] [PubMed] [Google Scholar]
- 14.Genbacev O., Joslin R., Damsky C.H., Polliotti B.M., Fisher S.J. Hypoxia alters early gestation human cytotrophoblast differentiation/invasion in vitro and models the placental defects that occur in preeclampsia. J. Clin. Investig. 1996;97:540–550. doi: 10.1172/JCI118447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Genbacev O., Zhou Y., Ludlow J.W., Fisher S.J. Regulation of human placental development by oxygen tension. Science. 1997;277:1669–1672. doi: 10.1126/science.277.5332.1669. [DOI] [PubMed] [Google Scholar]
- 16.Norwitz E.R., Schust D.J., Fisher S.J. Implantation and the survival of early pregnancy. N. Engl. J. Med. 2001;345:1400–1408. doi: 10.1056/NEJMra000763. [DOI] [PubMed] [Google Scholar]
- 17.Red-Horse K., Zhou Y., Genbacev O., Prakobphol A., Foulk R., McMaster M., Fisher S.J. Trophoblast differentiation during embryo implantation and formation of the maternal-fetal interface. J. Clin. Investig. 2004;114:744–754. doi: 10.1172/JCI200422991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Douglas R.M., Haddad G.G. Genetic models in applied physiology: Invited review: Effect of oxygen deprivation on cell cycle activity: A profile of delay and arrest. J. Appl. Physiol. 2003;94:2068–2083. doi: 10.1152/japplphysiol.01029.2002. discussion 2084. [DOI] [PubMed] [Google Scholar]
- 19.Zhou C., Zou Q.Y., Jiang Y.Z., Zheng J. Role of oxygen in fetoplacental endothelial responses: Hypoxia, physiological normoxia, or hyperoxia? Am. J. Physiol. Cell Physiol. 2020;318:C943–C953. doi: 10.1152/ajpcell.00528.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watson A.L., Skepper J.N., Jauniaux E., Burton G.J. Susceptibility of human placental syncytiotrophoblastic mitochondria to oxygen-mediated damage in relation to gestational age. J. Clin. Endocrinol. Metab. 1998;83:1697–1705. doi: 10.1210/jc.83.5.1697. [DOI] [PubMed] [Google Scholar]
- 21.Watson A.L., Palmer M.E., Jauniaux E., Burton G.J. Variations in expression of copper/zinc superoxide dismutase in villous trophoblast of the human placenta with gestational age. Placenta. 1997;18:295–299. doi: 10.1016/S0143-4004(97)80064-1. [DOI] [PubMed] [Google Scholar]
- 22.Jauniaux E., Watson A.L., Hempstock J., Bao Y.P., Skepper J.N., Burton G.J. Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am. J. Pathol. 2000;157:2111–2122. doi: 10.1016/S0002-9440(10)64849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giatromanolaki A., Koukourakis M.I., Sivridis E., Turley H., Talks K., Pezzella F., Gatter K.C., Harris A.L. Relation of hypoxia inducible factor 1 alpha and 2 alpha in operable non-small cell lung cancer to angiogenic/molecular profile of tumours and survival. Br. J. Cancer. 2001;85:881–890. doi: 10.1054/bjoc.2001.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramirez-Bergeron D.L., Simon M.C. Hypoxia-inducible factor and the development of stem cells of the cardiovascular system. Stem Cells. 2001;19:279–286. doi: 10.1634/stemcells.19-4-279. [DOI] [PubMed] [Google Scholar]
- 25.Yu N., Wu J.L., Xiao J., Fan L., Chen S.H., Li W. HIF-1alpha regulates angiogenesis via Notch1/STAT3/ETBR pathway in trophoblastic cells. Cell Cycle. 2019;18:3502–3512. doi: 10.1080/15384101.2019.1689481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caniggia I., Mostachfi H., Winter J., Gassmann M., Lye S.J., Kuliszewski M., Post M. Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFbeta(3) J. Clin. Investig. 2000;105:577–587. doi: 10.1172/JCI8316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishi H., Nakada T., Hokamura M., Osakabe Y., Itokazu O., Huang L.E., Isaka K. Hypoxia-inducible factor-1 transactivates transforming growth factor-beta3 in trophoblast. Endocrinology. 2004;145:4113–4118. doi: 10.1210/en.2003-1639. [DOI] [PubMed] [Google Scholar]
- 28.Adelman D.M., Gertsenstein M., Nagy A., Simon M.C., Maltepe E. Placental cell fates are regulated in vivo by HIF-mediated hypoxia responses. Genes Dev. 2000;14:3191–3203. doi: 10.1101/gad.853700a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gnarra J.R., Ward J.M., Porter F.D., Wagner J.R., Devor D.E., Grinberg A., Emmert-Buck M.R., Westphal H., Klausner R.D., Linehan W.M. Defective placental vasculogenesis causes embryonic lethality in VHL-deficient mice. Proc. Natl. Acad. Sci. USA. 1997;94:9102–9107. doi: 10.1073/pnas.94.17.9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Genbacev O., Krtolica A., Kaelin W., Fisher S.J. Human cytotrophoblast expression of the von Hippel-Lindau protein is downregulated during uterine invasion in situ and upregulated by hypoxia in vitro. Dev. Biol. 2001;233:526–536. doi: 10.1006/dbio.2001.0231. [DOI] [PubMed] [Google Scholar]
- 31.Wakeland A.K., Soncin F., Moretto-Zita M., Chang C.W., Horii M., Pizzo D., Nelson K.K., Laurent L.C., Parast M.M. Hypoxia Directs Human Extravillous Trophoblast Differentiation in a Hypoxia-Inducible Factor-Dependent Manner. Am. J. Pathol. 2017;187:767–780. doi: 10.1016/j.ajpath.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao H., Jiang Y., Cao Q., Hou Y., Wang C. Role of integrin switch and transforming growth factor Beta 3 in hypoxia-induced invasion inhibition of human extravillous trophoblast cells. Biol. Reprod. 2012;87:47. doi: 10.1095/biolreprod.112.099937. [DOI] [PubMed] [Google Scholar]
- 33.Caniggia I., Grisaru-Gravnosky S., Kuliszewsky M., Post M., Lye S.J. Inhibition of TGF-beta 3 restores the invasive capability of extravillous trophoblasts in preeclamptic pregnancies. J. Clin. Investig. 1999;103:1641–1650. doi: 10.1172/JCI6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farrell A., Alahari S., Ermini L., Tagliaferro A., Litvack M., Post M., Caniggia I. Faulty oxygen sensing disrupts angiomotin function in trophoblast cell migration and predisposes to preeclampsia. JCI Insight. 2019;4:e127009. doi: 10.1172/jci.insight.127009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yinon Y., Nevo O., Xu J., Many A., Rolfo A., Todros T., Post M., Caniggia I. Severe intrauterine growth restriction pregnancies have increased placental endoglin levels: Hypoxic regulation via transforming growth factor-beta 3. Am. J. Pathol. 2008;172:77–85. doi: 10.2353/ajpath.2008.070640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen T.P.H., Yong H.E.J., Chollangi T., Brennecke S.P., Fisher S.J., Wallace E.M., Ebeling P.R., Murthi P. Altered downstream target gene expression of the placental Vitamin D receptor in human idiopathic fetal growth restriction. Cell Cycle. 2018;17:182–190. doi: 10.1080/15384101.2017.1405193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pringle K.G., Kind K.L., Thompson J.G., Roberts C.T. Complex interactions between hypoxia inducible factors, insulin-like growth factor-II and oxygen in early murine trophoblasts. Placenta. 2007;28:1147–1157. doi: 10.1016/j.placenta.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 38.Zhu J., Wang K., Li T., Chen J., Xie D., Chang X., Yao J., Wu J., Zhou Q., Jia Y., et al. Hypoxia-induced TET1 facilitates trophoblast cell migration and invasion through HIF1alpha signaling pathway. Sci. Rep. 2017;7:8077. doi: 10.1038/s41598-017-07560-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tedde G., Piras A.T. Mitotic index of the Langhans’ cells in the normal human placenta from the early stages of pregnancy to the term. Cells Tissues Organs. 1978;100:114–119. doi: 10.1159/000144889. [DOI] [PubMed] [Google Scholar]
- 40.Kilburn B.A., Wang J., Duniec-Dmuchowski Z.M., Leach R.E., Romero R., Armant D.R. Extracellular matrix composition and hypoxia regulate the expression of HLA-G and integrins in a human trophoblast cell line. Biol. Reprod. 2000;62:739–747. doi: 10.1095/biolreprod62.3.739. [DOI] [PubMed] [Google Scholar]
- 41.Jiang B., Kamat A., Mendelson C.R. Hypoxia prevents induction of aromatase expression in human trophoblast cells in culture: Potential inhibitory role of the hypoxia-inducible transcription factor Mash-2 (mammalian achaete-scute homologous protein-2) Mol. Endocrinol. 2000;14:1661–1673. doi: 10.1210/mend.14.10.0539. [DOI] [PubMed] [Google Scholar]
- 42.Delforce S.J., Wang Y., Van-Aalst M.E., Corbisier de Meaultsart C., Morris B.J., Broughton-Pipkin F., Roberts C.T., Lumbers E.R., Pringle K.G. Effect of oxygen on the expression of renin-angiotensin system components in a human trophoblast cell line. Placenta. 2016;37:1–6. doi: 10.1016/j.placenta.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 43.Perlman B.E., Merriam A.A., Lemenze A., Zhao Q., Begum S., Nair M., Wu T., Wapner R.J., Kitajewski J.K., Shawber C.J., et al. Implications for preeclampsia: Hypoxia-induced Notch promotes trophoblast migration. Reproduction. 2021;161:681–696. doi: 10.1530/REP-20-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Colson A., Depoix C.L., Baldin P., Hubinont C., Sonveaux P., Debieve F. Hypoxia-inducible factor 2 alpha impairs human cytotrophoblast syncytialization: New insights into placental dysfunction and fetal growth restriction. FASEB J. 2020;34:15222–15235. doi: 10.1096/fj.202001681R. [DOI] [PubMed] [Google Scholar]
- 45.Matsumoto L., Hirota Y., Saito-Fujita T., Takeda N., Tanaka T., Hiraoka T., Akaeda S., Fujita H., Shimizu-Hirota R., Igaue S., et al. HIF2alpha in the uterine stroma permits embryo invasion and luminal epithelium detachment. J. Clin. Investig. 2018;128:3186–3197. doi: 10.1172/JCI98931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horii M., Li Y., Wakeland A.K., Pizzo D.P., Nelson K.K., Sabatini K., Laurent L.C., Liu Y., Parast M.M. Human pluripotent stem cells as a model of trophoblast differentiation in both normal development and disease. Proc. Natl. Acad. Sci. USA. 2016;113:E3882–E3891. doi: 10.1073/pnas.1604747113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maltepe E., Krampitz G.W., Okazaki K.M., Red-Horse K., Mak W., Simon M.C., Fisher S.J. Hypoxia-inducible factor-dependent histone deacetylase activity determines stem cell fate in the placenta. Development. 2005;132:3393–3403. doi: 10.1242/dev.01923. [DOI] [PubMed] [Google Scholar]
- 48.Albers R.E., Kaufman M.R., Natale B.V., Keoni C., Kulkarni-Datar K., Min S., Williams C.R., Natale D.R.C., Brown T.L. Trophoblast-Specific Expression of Hif-1alpha Results in Preeclampsia-Like Symptoms and Fetal Growth Restriction. Sci. Rep. 2019;9:2742. doi: 10.1038/s41598-019-39426-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rowe J.H., Ertelt J.M., Xin L., Way S.S. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature. 2012;490:102–106. doi: 10.1038/nature11462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vivier E., Tomasello E., Baratin M., Walzer T., Ugolini S. Functions of natural killer cells. Nat. Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 51.Faas M.M., de Vos P. Uterine NK cells and macrophages in pregnancy. Placenta. 2017;56:44–52. doi: 10.1016/j.placenta.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 52.Trundley A., Moffett A. Human uterine leukocytes and pregnancy. Tissue Antigens. 2004;63:1–12. doi: 10.1111/j.1399-0039.2004.00170.x. [DOI] [PubMed] [Google Scholar]
- 53.Smith S.D., Dunk C.E., Aplin J.D., Harris L.K., Jones R.L. Evidence for immune cell involvement in decidual spiral arteriole remodeling in early human pregnancy. Am. J. Pathol. 2009;174:1959–1971. doi: 10.2353/ajpath.2009.080995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Williams P.J., Searle R.F., Robson S.C., Innes B.A., Bulmer J.N. Decidual leucocyte populations in early to late gestation normal human pregnancy. J. Reprod. Immunol. 2009;82:24–31. doi: 10.1016/j.jri.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 55.Rieger L., Segerer S., Bernar T., Kapp M., Majic M., Morr A.K., Dietl J., Kammerer U. Specific subsets of immune cells in human decidua differ between normal pregnancy and preeclampsia--a prospective observational study. Reprod. Biol. Endocrinol. 2009;7:132. doi: 10.1186/1477-7827-7-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pique-Regi R., Romero R., Tarca A.L., Sendler E.D., Xu Y., Garcia-Flores V., Leng Y., Luca F., Hassan S.S., Gomez-Lopez N. Single cell transcriptional signatures of the human placenta in term and preterm parturition. eLife. 2019;8:e52004. doi: 10.7554/eLife.52004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Keskin D.B., Allan D.S., Rybalov B., Andzelm M.M., Stern J.N., Kopcow H.D., Koopman L.A., Strominger J.L. TGFbeta promotes conversion of CD16+ peripheral blood NK cells into CD16- NK cells with similarities to decidual NK cells. Proc. Natl. Acad. Sci. USA. 2007;104:3378–3383. doi: 10.1073/pnas.0611098104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vacca P., Vitale C., Montaldo E., Conte R., Cantoni C., Fulcheri E., Darretta V., Moretta L., Mingari M.C. CD34+ hematopoietic precursors are present in human decidua and differentiate into natural killer cells upon interaction with stromal cells. Proc. Natl. Acad. Sci. USA. 2011;108:2402–2407. doi: 10.1073/pnas.1016257108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu S., Diao L., Huang C., Li Y., Zeng Y., Kwak-Kim J.Y.H. The role of decidual immune cells on human pregnancy. J. Reprod. Immunol. 2017;124:44–53. doi: 10.1016/j.jri.2017.10.045. [DOI] [PubMed] [Google Scholar]
- 60.Tao Y., Li Y.H., Piao H.L., Zhou W.J., Zhang D., Fu Q., Wang S.C., Li D.J., Du M.R. CD56(bright)CD25+ NK cells are preferentially recruited to the maternal/fetal interface in early human pregnancy. Cell Mol. Immunol. 2015;12:77–86. doi: 10.1038/cmi.2014.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lu H., Jin L.P., Huang H.L., Ha S.Y., Yang H.L., Chang R.Q., Li D.J., Li M.Q. Trophoblast-derived CXCL12 promotes CD56(bright) CD82(−) CD29(+) NK cell enrichment in the decidua. Am. J. Reprod. Immunol. 2020;83:e13203–e13215. doi: 10.1111/aji.13203. [DOI] [PubMed] [Google Scholar]
- 62.Lash G.E., Robson S.C., Bulmer J.N. Review: Functional role of uterine natural killer (uNK) cells in human early pregnancy decidua. Placenta. 2010;31:S87–S92. doi: 10.1016/j.placenta.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 63.Moffett A., Colucci F. Uterine NK cells: Active regulators at the maternal-fetal interface. J. Clin. Investig. 2014;124:1872–1879. doi: 10.1172/JCI68107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Choudhury R.H., Dunk C.E., Lye S.J., Aplin J.D., Harris L.K., Jones R.L. Extravillous Trophoblast and Endothelial Cell Crosstalk Mediates Leukocyte Infiltration to the Early Remodeling Decidual Spiral Arteriole Wall. J. Immunol. 2017;198:4115–4128. doi: 10.4049/jimmunol.1601175. [DOI] [PubMed] [Google Scholar]
- 65.Zhang J., Adams M.A., Croy B.A. Alterations in maternal and fetal heart functions accompany failed spiral arterial remodeling in pregnant mice. Am. J. Obstet. Gynecol. 2011;205:485.e1–485.e16. doi: 10.1016/j.ajog.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wynn T.A., Chawla A., Pollard J.W. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Italiani P., Boraschi D. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation. Front. Immunol. 2014;5:514. doi: 10.3389/fimmu.2014.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martinez F.O., Gordon S., Locati M., Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: New molecules and patterns of gene expression. J. Immunol. 2006;177:7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 69.Lawrence T., Natoli G. Transcriptional regulation of macrophage polarization: Enabling diversity with identity. Nat. Rev. Immunol. 2011;11:750–761. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- 70.Medeiros L.T., Peracoli J.C., Bannwart-Castro C.F., Romao M., Weel I.C., Golim M.A., de Oliveira L.G., Kurokawa C.S., Medeiros Borges V.T., Peracoli M.T. Monocytes from pregnant women with pre-eclampsia are polarized to a M1 phenotype. Am. J. Reprod. Immunol. 2014;72:5–13. doi: 10.1111/aji.12222. [DOI] [PubMed] [Google Scholar]
- 71.Chabtini L., Mfarrej B., Mounayar M., Zhu B., Batal I., Dakle P.J., Smith B.D., Boenisch O., Najafian N., Akiba H., et al. TIM-3 regulates innate immune cells to induce fetomaternal tolerance. J. Immunol. 2013;190:88–96. doi: 10.4049/jimmunol.1202176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Houser B.L., Tilburgs T., Hill J., Nicotra M.L., Strominger J.L. Two unique human decidual macrophage populations. J. Immunol. 2011;186:2633–2642. doi: 10.4049/jimmunol.1003153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hori S., Nomura T., Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 74.Fontenot J.D., Gavin M.A., Rudensky A.Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 75.Tilburgs T., Scherjon S.A., van der Mast B.J., Haasnoot G.W., Voort-Maarschalk M.V.V., Roelen D.L., van Rood J.J., Claas F.H. Fetal-maternal HLA-C mismatch is associated with decidual T cell activation and induction of functional T regulatory cells. J. Reprod. Immunol. 2009;82:148–157. doi: 10.1016/j.jri.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 76.Lissauer D., Piper K., Goodyear O., Kilby M.D., Moss P.A. Fetal-specific CD8+ cytotoxic T cell responses develop during normal human pregnancy and exhibit broad functional capacity. J. Immunol. 2012;189:1072–1080. doi: 10.4049/jimmunol.1200544. [DOI] [PubMed] [Google Scholar]
- 77.Jasper M.J., Tremellen K.P., Robertson S.A. Primary unexplained infertility is associated with reduced expression of the T-regulatory cell transcription factor Foxp3 in endometrial tissue. Mol. Hum. Reprod. 2006;12:301–308. doi: 10.1093/molehr/gal032. [DOI] [PubMed] [Google Scholar]
- 78.Shima T., Sasaki Y., Itoh M., Nakashima A., Ishii N., Sugamura K., Saito S. Regulatory T cells are necessary for implantation and maintenance of early pregnancy but not late pregnancy in allogeneic mice. J. Reprod. Immunol. 2010;85:121–129. doi: 10.1016/j.jri.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 79.Kahn D.A., Baltimore D. Pregnancy induces a fetal antigen-specific maternal T regulatory cell response that contributes to tolerance. Proc. Natl. Acad. Sci. USA. 2010;107:9299–9304. doi: 10.1073/pnas.1003909107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee S.K., Kim J.Y., Hur S.E., Kim C.J., Na B.J., Lee M., Gilman-Sachs A., Kwak-Kim J. An imbalance in interleukin-17-producing T and Foxp3(+) regulatory T cells in women with idiopathic recurrent pregnancy loss. Hum. Reprod. 2011;26:2964–2971. doi: 10.1093/humrep/der301. [DOI] [PubMed] [Google Scholar]
- 81.Wang W.J., Hao C.F., Qu Q.L., Wang X., Qiu L.H., Lin Q.D. The deregulation of regulatory T cells on interleukin-17-producing T helper cells in patients with unexplained early recurrent miscarriage. Hum. Reprod. 2010;25:2591–2596. doi: 10.1093/humrep/deq198. [DOI] [PubMed] [Google Scholar]
- 82.Wu L., Li J., Xu H.L., Xu B., Tong X.H., Kwak-Kim J., Liu Y.S. IL-7/IL-7R signaling pathway might play a role in recurrent pregnancy losses by increasing inflammatory Th17 cells and decreasing Treg cells. Am. J. Reprod. Immunol. 2016;76:454–464. doi: 10.1111/aji.12588. [DOI] [PubMed] [Google Scholar]
- 83.Wang W.J., Liu F.J., Xin L., Hao C.F., Bao H.C., Qu Q.L., Liu X.M. Adoptive transfer of pregnancy-induced CD4+CD25+ regulatory T cells reverses the increase in abortion rate caused by interleukin 17 in the CBA/JxBALB/c mouse model. Hum. Reprod. 2014;29:946–952. doi: 10.1093/humrep/deu014. [DOI] [PubMed] [Google Scholar]
- 84.Schwede S., Alfer J., von Rango U. Differences in regulatory T-cell and dendritic cell pattern in decidual tissue of placenta accreta/increta cases. Placenta. 2014;35:378–385. doi: 10.1016/j.placenta.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 85.Krendl C., Shaposhnikov D., Rishko V., Ori C., Ziegenhain C., Sass S., Simon L., Muller N.S., Straub T., Brooks K.E., et al. GATA2/3-TFAP2A/C transcription factor network couples human pluripotent stem cell differentiation to trophectoderm with repression of pluripotency. Proc. Natl. Acad. Sci. USA. 2017;114:E9579–E9588. doi: 10.1073/pnas.1708341114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Home P., Kumar R.P., Ganguly A., Saha B., Milano-Foster J., Bhattacharya B., Ray S., Gunewardena S., Paul A., Camper S.A., et al. Genetic redundancy of GATA factors in the extraembryonic trophoblast lineage ensures the progression of preimplantation and postimplantation mammalian development. Development. 2017;144:876–888. doi: 10.1242/dev.145318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li Y., Moretto-Zita M., Leon-Garcia S., Parast M.M. p63 inhibits extravillous trophoblast migration and maintains cells in a cytotrophoblast stem cell-like state. Am. J. Pathol. 2014;184:3332–3343. doi: 10.1016/j.ajpath.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lin K.C., Park H.W., Guan K.L. Regulation of the Hippo Pathway Transcription Factor TEAD. Trends Biochem. Sci. 2017;42:862–872. doi: 10.1016/j.tibs.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Haider S., Meinhardt G., Saleh L., Fiala C., Pollheimer J., Knofler M. Notch1 controls development of the extravillous trophoblast lineage in the human placenta. Proc. Natl. Acad. Sci. USA. 2016;113:E7710–E7719. doi: 10.1073/pnas.1612335113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Saha B., Ganguly A., Home P., Bhattacharya B., Ray S., Ghosh A., Rumi M.A.K., Marsh C., French V.A., Gunewardena S., et al. TEAD4 ensures postimplantation development by promoting trophoblast self-renewal: An implication in early human pregnancy loss. Proc. Natl. Acad. Sci. USA. 2020;117:17864–17875. doi: 10.1073/pnas.2002449117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pobbati A.V., Chan S.W., Lee I., Song H., Hong W. Structural and functional similarity between the Vgll1-TEAD and the YAP-TEAD complexes. Structure. 2012;20:1135–1140. doi: 10.1016/j.str.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 92.Soncin F., Khater M., To C., Pizzo D., Farah O., Wakeland A., Arul Nambi Rajan K., Nelson K.K., Chang C.W., Moretto-Zita M., et al. Comparative analysis of mouse and human placentae across gestation reveals species-specific regulators of placental development. Development. 2018;145:dev156273. doi: 10.1242/dev.156273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Krebsbach P.H., Villa-Diaz L.G. The Role of Integrin alpha6 (CD49f) in Stem Cells: More than a Conserved Biomarker. Stem Cells Dev. 2017;26:1090–1099. doi: 10.1089/scd.2016.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Renaud S.J., Chakraborty D., Mason C.W., Rumi M.A., Vivian J.L., Soares M.J. OVO-like 1 regulates progenitor cell fate in human trophoblast development. Proc. Natl. Acad. Sci. USA. 2015;112:E6175–E6184. doi: 10.1073/pnas.1507397112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Baczyk D., Drewlo S., Proctor L., Dunk C., Lye S., Kingdom J. Glial cell missing-1 transcription factor is required for the differentiation of the human trophoblast. Cell Death Differ. 2009;16:719–727. doi: 10.1038/cdd.2009.1. [DOI] [PubMed] [Google Scholar]
- 96.Chiu Y.H., Chen H. GATA3 inhibits GCM1 activity and trophoblast cell invasion. Sci. Rep. 2016;6:21630. doi: 10.1038/srep21630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhu H., Peng B., Klausen C., Yi Y., Li Y., Xiong S., von Dadelszen P., Leung P.C.K. NPFF increases fusogenic proteins syncytin 1 and syncytin 2 via GCM1 in first trimester primary human cytotrophoblast cells. FASEB J. 2020;34:9419–9432. doi: 10.1096/fj.201902978R. [DOI] [PubMed] [Google Scholar]
- 98.Mi S., Lee X., Li X., Veldman G.M., Finnerty H., Racie L., LaVallie E., Tang X.Y., Edouard P., Howes S., et al. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature. 2000;403:785–789. doi: 10.1038/35001608. [DOI] [PubMed] [Google Scholar]
- 99.Frendo J.L., Olivier D., Cheynet V., Blond J.L., Bouton O., Vidaud M., Rabreau M., Evain-Brion D., Mallet F. Direct involvement of HERV-W Env glycoprotein in human trophoblast cell fusion and differentiation. Mol. Cell. Biol. 2003;23:3566–3574. doi: 10.1128/MCB.23.10.3566-3574.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Esnault C., Priet S., Ribet D., Vernochet C., Bruls T., Lavialle C., Weissenbach J., Heidmann T. A placenta-specific receptor for the fusogenic, endogenous retrovirus-derived, human syncytin-2. Proc. Natl. Acad. Sci. USA. 2008;105:17532–17537. doi: 10.1073/pnas.0807413105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lu X., Wang R., Zhu C., Wang H., Lin H.Y., Gu Y., Cross J.C., Wang H. Fine-Tuned and Cell-Cycle-Restricted Expression of Fusogenic Protein Syncytin-2 Maintains Functional Placental Syncytia. Cell Rep. 2017;21:1150–1159. doi: 10.1016/j.celrep.2017.10.019. [DOI] [PubMed] [Google Scholar]
- 102.Moser G., Drewlo S., Huppertz B., Armant D.R. Trophoblast retrieval and isolation from the cervix: Origins of cervical trophoblasts and their potential value for risk assessment of ongoing pregnancies. Hum. Reprod. Update. 2018;24:484–496. doi: 10.1093/humupd/dmy008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Aplin J.D. Expression of integrin alpha 6 beta 4 in human trophoblast and its loss from extravillous cells. Placenta. 1993;14:203–215. doi: 10.1016/S0143-4004(05)80261-9. [DOI] [PubMed] [Google Scholar]
- 104.Rattila S., Dunk C.E.E., Im M., Grichenko O., Zhou Y., Yanez-Mo M., Blois S.M., Yamada K.M., Erez O., Gomez-Lopez N., et al. Interaction of Pregnancy-Specific Glycoprotein 1 With Integrin Alpha5beta1 Is a Modulator of Extravillous Trophoblast Functions. Cells. 2019;8:1369. doi: 10.3390/cells8111369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jovanovic M., Stefanoska I., Radojcic L., Vicovac L. Interleukin-8 (CXCL8) stimulates trophoblast cell migration and invasion by increasing levels of matrix metalloproteinase (MMP)2 and MMP9 and integrins alpha5 and beta1. Reproduction. 2010;139:789–798. doi: 10.1530/REP-09-0341. [DOI] [PubMed] [Google Scholar]
- 106.Tarrade A., Goffin F., Munaut C., Lai-Kuen R., Tricottet V., Foidart J.M., Vidaud M., Frankenne F., Evain-Brion D. Effect of matrigel on human extravillous trophoblasts differentiation: Modulation of protease pattern gene expression. Biol. Reprod. 2002;67:1628–1637. doi: 10.1095/biolreprod.101.001925. [DOI] [PubMed] [Google Scholar]
- 107.Harris L.K., Smith S.D., Keogh R.J., Jones R.L., Baker P.N., Knofler M., Cartwright J.E., Whitley G.S., Aplin J.D. Trophoblast- and vascular smooth muscle cell-derived MMP-12 mediates elastolysis during uterine spiral artery remodeling. Am. J. Pathol. 2010;177:2103–2115. doi: 10.2353/ajpath.2010.100182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bjorn S.F., Hastrup N., Larsen J.F., Lund L.R., Pyke C. Messenger RNA for membrane-type 2 matrix metalloproteinase, MT2-MMP, is expressed in human placenta of first trimester. Placenta. 2000;21:170–176. doi: 10.1053/plac.1999.0447. [DOI] [PubMed] [Google Scholar]
- 109.Fujiwara H., Higuchi T., Sato Y., Nishioka Y., Zeng B.X., Yoshioka S., Tatsumi K., Ueda M., Maeda M. Regulation of human extravillous trophoblast function by membrane-bound peptidases. Biochim. Biophys. Acta. 2005;1751:26–32. doi: 10.1016/j.bbapap.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 110.Hannan N.J., Jones R.L., White C.A., Salamonsen L.A. The chemokines, CX3CL1, CCL14, and CCL4, promote human trophoblast migration at the feto-maternal interface. Biol. Reprod. 2006;74:896–904. doi: 10.1095/biolreprod.105.045518. [DOI] [PubMed] [Google Scholar]
- 111.Huang Y., Zhu X.Y., Du M.R., Wu X., Wang M.Y., Li D.J. Chemokine CXCL16, a scavenger receptor, induces proliferation and invasion of first-trimester human trophoblast cells in an autocrine manner. Hum. Reprod. 2006;21:1083–1091. doi: 10.1093/humrep/dei436. [DOI] [PubMed] [Google Scholar]
- 112.Drake P.M., Red-Horse K., Fisher S.J. Reciprocal chemokine receptor and ligand expression in the human placenta: Implications for cytotrophoblast differentiation. Dev. Dyn. 2004;229:877–885. doi: 10.1002/dvdy.10477. [DOI] [PubMed] [Google Scholar]
- 113.Varberg K.M., Iqbal K., Muto M., Simon M.E., Scott R.L., Kozai K., Choudhury R.H., Aplin J.D., Biswell R., Gibson M., et al. ASCL2 reciprocally controls key trophoblast lineage decisions during hemochorial placenta development. Proc. Natl. Acad. Sci. USA. 2021;118:e2016517118. doi: 10.1073/pnas.2016517118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li B., Sharpe E.E., Maupin A.B., Teleron A.A., Pyle A.L., Carmeliet P., Young P.P. VEGF and PlGF promote adult vasculogenesis by enhancing EPC recruitment and vessel formation at the site of tumor neovascularization. FASEB J. 2006;20:1495–1497. doi: 10.1096/fj.05-5137fje. [DOI] [PubMed] [Google Scholar]
- 115.Athanassiades A., Lala P.K. Role of placenta growth factor (PIGF) in human extravillous trophoblast proliferation, migration and invasiveness. Placenta. 1998;19:465–473. doi: 10.1016/S0143-4004(98)91039-6. [DOI] [PubMed] [Google Scholar]
- 116.Demir R., Kayisli U.A., Seval Y., Celik-Ozenci C., Korgun E.T., Demir-Weusten A.Y., Huppertz B. Sequential expression of VEGF and its receptors in human placental villi during very early pregnancy: Differences between placental vasculogenesis and angiogenesis. Placenta. 2004;25:560–572. doi: 10.1016/j.placenta.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 117.Shore V.H., Wang T.H., Wang C.L., Torry R.J., Caudle M.R., Torry D.S. Vascular endothelial growth factor, placenta growth factor and their receptors in isolated human trophoblast. Placenta. 1997;18:657–665. doi: 10.1016/S0143-4004(97)90007-2. [DOI] [PubMed] [Google Scholar]
- 118.Bjoro K., Jr. Gross pathology of the placenta in intrauterine growth retardation. Ann. Chir. Gynaecol. 1981;70:316–322. [PubMed] [Google Scholar]
- 119.Barut F., Barut A., Gun B.D., Kandemir N.O., Harma M.I., Harma M., Aktunc E., Ozdamar S.O. Intrauterine growth restriction and placental angiogenesis. Diagn. Pathol. 2010;5:24. doi: 10.1186/1746-1596-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Williams P.J., Bulmer J.N., Searle R.F., Innes B.A., Robson S.C. Altered decidual leucocyte populations in the placental bed in pre-eclampsia and foetal growth restriction: A comparison with late normal pregnancy. Reproduction. 2009;138:177–184. doi: 10.1530/REP-09-0007. [DOI] [PubMed] [Google Scholar]
- 121.Amu S., Hahn-Zoric M., Malik A., Ashraf R., Zaman S., Kjellmer I., Hagberg H., Padyukov L., Hanson L.A. Cytokines in the placenta of Pakistani newborns with and without intrauterine growth retardation. Pediatr. Res. 2006;59:254–258. doi: 10.1203/01.pdr.0000196332.37565.7d. [DOI] [PubMed] [Google Scholar]
- 122.Lash G.E., Schiessl B., Kirkley M., Innes B.A., Cooper A., Searle R.F., Robson S.C., Bulmer J.N. Expression of angiogenic growth factors by uterine natural killer cells during early pregnancy. J. Leukoc. Biol. 2006;80:572–580. doi: 10.1189/jlb.0406250. [DOI] [PubMed] [Google Scholar]
- 123.Roos S., Jansson N., Palmberg I., Saljo K., Powell T.L., Jansson T. Mammalian target of rapamycin in the human placenta regulates leucine transport and is down-regulated in restricted fetal growth. J. Physiol. 2007;582:449–459. doi: 10.1113/jphysiol.2007.129676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cetin I., Alvino G. Intrauterine growth restriction: Implications for placental metabolism and transport. A review. Placenta. 2009;30((Suppl. SA)):S77–S82. doi: 10.1016/j.placenta.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 125.Gaccioli F., Lager S. Placental Nutrient Transport and Intrauterine Growth Restriction. Front. Physiol. 2016;7:40. doi: 10.3389/fphys.2016.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xu J., Wang J., Cao Y., Jia X., Huang Y., Cai M., Lu C., Zhu H. Downregulation of Placental Amino Acid Transporter Expression and mTORC1 Signaling Activity Contributes to Fetal Growth Retardation in Diabetic Rats. Int. J. Mol. Sci. 2020;21:1849. doi: 10.3390/ijms21051849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Boyd P.A., Scott A. Quantitative structural studies on human placentas associated with pre-eclampsia, essential hypertension and intrauterine growth retardation. Br. J. Obstet. Gynaecol. 1985;92:714–721. doi: 10.1111/j.1471-0528.1985.tb01454.x. [DOI] [PubMed] [Google Scholar]
- 128.Redman C.W., Tannetta D.S., Dragovic R.A., Gardiner C., Southcombe J.H., Collett G.P., Sargent I.L. Review: Does size matter? Placental debris and the pathophysiology of pre-eclampsia. Placenta. 2012;33:S48–S54. doi: 10.1016/j.placenta.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 129.Redman C.W. Current topic: Pre-eclampsia and the placenta. Placenta. 1991;12:301–308. doi: 10.1016/0143-4004(91)90339-H. [DOI] [PubMed] [Google Scholar]
- 130.Wang A., Rana S., Karumanchi S.A. Preeclampsia: The role of angiogenic factors in its pathogenesis. Physiology. 2009;24:147–158. doi: 10.1152/physiol.00043.2008. [DOI] [PubMed] [Google Scholar]
- 131.Maynard S.E., Min J.Y., Merchan J., Lim K.H., Li J., Mondal S., Libermann T.A., Morgan J.P., Sellke F.W., Stillman I.E., et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Investig. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Levine R.J., Maynard S.E., Qian C., Lim K.H., England L.J., Yu K.F., Schisterman E.F., Thadhani R., Sachs B.P., Epstein F.H., et al. Circulating angiogenic factors and the risk of preeclampsia. N. Engl. J. Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 133.Redman C.W., Sargent I.L. Immunology of pre-eclampsia. Am. J. Reprod. Immunol. 2010;63:534–543. doi: 10.1111/j.1600-0897.2010.00831.x. [DOI] [PubMed] [Google Scholar]
- 134.Hosseini A., Dolati S., Hashemi V., Abdollahpour-Alitappeh M., Yousefi M. Regulatory T and T helper 17 cells: Their roles in preeclampsia. J. Cell Physiol. 2018;233:6561–6573. doi: 10.1002/jcp.26604. [DOI] [PubMed] [Google Scholar]
- 135.Eghbal-Fard S., Yousefi M., Heydarlou H., Ahmadi M., Taghavi S., Movasaghpour A., Jadidi-Niaragh F., Yousefi B., Dolati S., Hojjat-Farsangi M., et al. The imbalance of Th17/Treg axis involved in the pathogenesis of preeclampsia. J. Cell Physiol. 2019;234:5106–5116. doi: 10.1002/jcp.27315. [DOI] [PubMed] [Google Scholar]
- 136.Duckitt K., Harrington D. Risk factors for pre-eclampsia at antenatal booking: Systematic review of controlled studies. BMJ. 2005;330:565. doi: 10.1136/bmj.38380.674340.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Avagliano L., Bulfamante G.P., Morabito A., Marconi A.M. Abnormal spiral artery remodelling in the decidual segment during pregnancy: From histology to clinical correlation. J. Clin. Pathol. 2011;64:1064–1068. doi: 10.1136/jclinpath-2011-200092. [DOI] [PubMed] [Google Scholar]
- 138.Yoshida K., Yano A., Kusama K., Ishikawa G., Tamura K. Alpha 1 Antitrypsin Regulates Trophoblast Syncytialization and Inflammatory Factor Expression. Int. J. Mol. Sci. 2022;23:1955. doi: 10.3390/ijms23041955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nakashima A., Yamanaka-Tatematsu M., Fujita N., Koizumi K., Shima T., Yoshida T., Nikaido T., Okamoto A., Yoshimori T., Saito S. Impaired autophagy by soluble endoglin, under physiological hypoxia in early pregnant period, is involved in poor placentation in preeclampsia. Autophagy. 2013;9:303–316. doi: 10.4161/auto.22927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Saito S., Nakashima A. A review of the mechanism for poor placentation in early-onset preeclampsia: The role of autophagy in trophoblast invasion and vascular remodeling. J. Reprod. Immunol. 2014;101–102:80–88. doi: 10.1016/j.jri.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 141.Daskalakis G., Marinopoulos S., Krielesi V., Papapanagiotou A., Papantoniou N., Mesogitis S., Antsaklis A. Placental pathology in women with gestational diabetes. Acta Obstet. Gynecol. Scand. 2008;87:403–407. doi: 10.1080/00016340801908783. [DOI] [PubMed] [Google Scholar]
- 142.Madazli R., Tuten A., Calay Z., Uzun H., Uludag S., Ocak V. The incidence of placental abnormalities, maternal and cord plasma malondialdehyde and vascular endothelial growth factor levels in women with gestational diabetes mellitus and nondiabetic controls. Gynecol. Obstet. Investig. 2008;65:227–232. doi: 10.1159/000113045. [DOI] [PubMed] [Google Scholar]
- 143.Radaelli T., Varastehpour A., Catalano P., Hauguel-de Mouzon S. Gestational diabetes induces placental genes for chronic stress and inflammatory pathways. Diabetes. 2003;52:2951–2958. doi: 10.2337/diabetes.52.12.2951. [DOI] [PubMed] [Google Scholar]
- 144.Lappas M., Yee K., Permezel M., Rice G.E. Release and regulation of leptin, resistin and adiponectin from human placenta, fetal membranes, and maternal adipose tissue and skeletal muscle from normal and gestational diabetes mellitus-complicated pregnancies. J. Endocrinol. 2005;186:457–465. doi: 10.1677/joe.1.06227. [DOI] [PubMed] [Google Scholar]
- 145.Magee T.R., Ross M.G., Wedekind L., Desai M., Kjos S., Belkacemi L. Gestational diabetes mellitus alters apoptotic and inflammatory gene expression of trophobasts from human term placenta. J. Diabetes Complicat. 2014;28:448–459. doi: 10.1016/j.jdiacomp.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lappas M., Hiden U., Desoye G., Froehlich J., Hauguel-de Mouzon S., Jawerbaum A. The role of oxidative stress in the pathophysiology of gestational diabetes mellitus. Antioxid. Redox Signal. 2011;15:3061–3100. doi: 10.1089/ars.2010.3765. [DOI] [PubMed] [Google Scholar]
- 147.Zhu C., Yang H., Geng Q., Ma Q., Long Y., Zhou C., Chen M. Association of oxidative stress biomarkers with gestational diabetes mellitus in pregnant women: A case-control study. PLoS ONE. 2015;10:e0126490. doi: 10.1371/journal.pone.0126490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yung H.W., Alnaes-Katjavivi P., Jones C.J., El-Bacha T., Golic M., Staff A.C., Burton G.J. Placental endoplasmic reticulum stress in gestational diabetes: The potential for therapeutic intervention with chemical chaperones and antioxidants. Diabetologia. 2016;59:2240–2250. doi: 10.1007/s00125-016-4040-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gaither K., Quraishi A.N., Illsley N.P. Diabetes alters the expression and activity of the human placental GLUT1 glucose transporter. J. Clin. Endocrinol. Metab. 1999;84:695–701. doi: 10.1210/jc.84.2.695. [DOI] [PubMed] [Google Scholar]
- 150.Dekker Nitert M., Barrett H.L., Kubala M.H., Scholz Romero K., Denny K.J., Woodruff T.M., McIntyre H.D., Callaway L.K. Increased placental expression of fibroblast growth factor 21 in gestational diabetes mellitus. J. Clin. Endocrinol. Metab. 2014;99:E591–E598. doi: 10.1210/jc.2013-2581. [DOI] [PubMed] [Google Scholar]
- 151.Sooranna S.R., Oteng-Ntim E., Meah R., Ryder T.A., Bajoria R. Characterization of human placental explants: Morphological, biochemical and physiological studies using first and third trimester placenta. Hum. Reprod. 1999;14:536–541. doi: 10.1093/humrep/14.2.536. [DOI] [PubMed] [Google Scholar]
- 152.Suryawanshi H., Morozov P., Straus A., Sahasrabudhe N., Max K.E.A., Garzia A., Kustagi M., Tuschl T., Williams Z. A single-cell survey of the human first-trimester placenta and decidua. Sci. Adv. 2018;4:eaau4788. doi: 10.1126/sciadv.aau4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pattillo R.A., Gey G.O. The establishment of a cell line of human hormone-synthesizing trophoblastic cells in vitro. Cancer Res. 1968;28:1231–1236. [PubMed] [Google Scholar]
- 154.Kohler P.O., Bridson W.E. Isolation of hormone-producing clonal lines of human choriocarcinoma. J. Clin. Endocrinol. Metab. 1971;32:683–687. doi: 10.1210/jcem-32-5-683. [DOI] [PubMed] [Google Scholar]
- 155.Pattillo R.A., Hussa R.O., Garancis J.C. Glycogen metabolism in human hormone-producing trophoblastic cells in continuous culture. I. Regulation of glycogen metabolism by glucose. In Vitro. 1971;7:59–67. doi: 10.1007/BF02628263. [DOI] [PubMed] [Google Scholar]
- 156.Straszewski-Chavez S.L., Abrahams V.M., Alvero A.B., Aldo P.B., Ma Y., Guller S., Romero R., Mor G. The isolation and characterization of a novel telomerase immortalized first trimester trophoblast cell line, Swan 71. Placenta. 2009;30:939–948. doi: 10.1016/j.placenta.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Abou-Kheir W., Barrak J., Hadadeh O., Daoud G. HTR-8/SVneo cell line contains a mixed population of cells. Placenta. 2017;50:1–7. doi: 10.1016/j.placenta.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 158.Amita M., Adachi K., Alexenko A.P., Sinha S., Schust D.J., Schulz L.C., Roberts R.M., Ezashi T. Complete and unidirectional conversion of human embryonic stem cells to trophoblast by BMP4. Proc. Natl. Acad. Sci. USA. 2013;110:E1212–E1221. doi: 10.1073/pnas.1303094110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yabe S., Alexenko A.P., Amita M., Yang Y., Schust D.J., Sadovsky Y., Ezashi T., Roberts R.M. Comparison of syncytiotrophoblast generated from human embryonic stem cells and from term placentas. Proc. Natl. Acad. Sci. USA. 2016;113:E2598–E2607. doi: 10.1073/pnas.1601630113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kojima J., Fukuda A., Taira H., Kawasaki T., Ito H., Kuji N., Isaka K., Umezawa A., Akutsu H. Efficient production of trophoblast lineage cells from human induced pluripotent stem cells. Lab. Investig. 2017;97:1188–1200. doi: 10.1038/labinvest.2016.159. [DOI] [PubMed] [Google Scholar]
- 161.Maldonado-Estrada J., Menu E., Roques P., Barre-Sinoussi F., Chaouat G. Evaluation of Cytokeratin 7 as an accurate intracellular marker with which to assess the purity of human placental villous trophoblast cells by flow cytometry. J. Immunol. Methods. 2004;286:21–34. doi: 10.1016/j.jim.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 162.Tsuchida N., Kojima J., Fukuda A., Oda M., Kawasaki T., Ito H., Kuji N., Isaka K., Nishi H., Umezawa A., et al. Transcriptomic features of trophoblast lineage cells derived from human induced pluripotent stem cells treated with BMP 4. Placenta. 2020;89:20–32. doi: 10.1016/j.placenta.2019.10.006. [DOI] [PubMed] [Google Scholar]
- 163.Okae H., Toh H., Sato T., Hiura H., Takahashi S., Shirane K., Kabayama Y., Suyama M., Sasaki H., Arima T. Derivation of Human Trophoblast Stem Cells. Cell Stem Cell. 2018;22:50–63.e6. doi: 10.1016/j.stem.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 164.Turco M.Y., Gardner L., Kay R.G., Hamilton R.S., Prater M., Hollinshead M.S., McWhinnie A., Esposito L., Fernando R., Skelton H., et al. Trophoblast organoids as a model for maternal-fetal interactions during human placentation. Nature. 2018;564:263–267. doi: 10.1038/s41586-018-0753-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.