Abstract
Aims
No consensus exists on the clinical value of tumour regression grading (TRG) systems for therapy effects of neoadjuvant chemoradiotherapy (nCRT) in oesophageal adenocarcinoma. Existing TRG systems lack standardization and reproducibility, and do not consider the morphological heterogeneity of tumour response. Therefore, we aim to identify morphological tumour regression patterns of oesophageal adenocarcinoma after nCRT and their association with survival.
Methods and results
Patients with oesophageal adenocarcinoma, who underwent nCRT followed by surgery and achieved a partial response to nCRT, were identified from two Dutch upper‐gastrointestinal (GI) centres (2005–18; test cohort). Resection specimens were scored for regression patterns by two independent observers according to a pre‐defined three‐step flowchart. The results were validated in an external cohort (2001–17). In total, 110 patients were included in the test cohort and 115 in the validation cohort. In the test cohort, two major regression patterns were identified: fragmentation (60%) and shrinkage (40%), with an excellent interobserver agreement (κ = 0.87). Here, patients with a fragmented pattern had a significantly higher pathological stage (stages III/IV: 52 versus 16%; P < 0.001), less downstaging (48 versus 91%; P < 0.001), a higher risk of recurrence [risk ratio (RR) = 2.9, 95% confidence interval (CI) = 1.5–5.6] and poorer 5‐year overall survival (30 versus 80% respectively, P = 0.001).
Conclusions
The validation cohort confirmed these findings, although had more advanced cases (case‐stages = III/IV 91 versus 73%, P = 0.005) and a higher prevalence of fragmented‐pattern cases (80 versus 60%, P = 0.002). When combining the cohorts in multivariate analysis, the pattern of response was an independent prognostic factor [hazard ratio (HR) = 1.76, 95% CI = 1.0–3.0]. In conclusion, we established an externally validated, reproducible and clinically relevant classification of tumour response.
Keywords: neoadjuvant chemoradiation treatment, oesophageal cancer, patterns of response, response
![]()
Introduction
The increasing use of neoadjuvant chemoradiotherapy (nCRT) as standard of care for oesophageal cancer has initiated discussions regarding organ‐preserving treatment strategies using active surveillance if complete clinical, endoscopic and radiological response is achieved. 1 , 2 Currently, pathological assessment according to ypTNM remains the gold standard for prognostic response evaluation and staging. 3 , 4 Pathologically complete response after nCRT is achieved in 23% of potentially curable oesophageal adenocarcinomas 5 and is associated with a favourable disease‐free survival. 6 , 7 , 8 The majority of patients, however, have pathologically incomplete response with residual tumour cells at the site of the primary tumour and/or in resected regional lymph nodes, which is associated with a less favourable outcome. 8
Attempts to differentiate between responders and non‐responders by histological assessment of regressive changes following nCRT originated with Mandard in 1994 proposing a five‐tier tumour regression grading (TRG) system in oesophageal cancer. 9 In this model, lower gradings (TRG 1–3 versus TRG 4–5) were associated with better disease‐free survival. Since then, multiple variants for histopathological TRGs have been proposed. 10 , 11 , 12 , 13 , 14 , 15 In general, these grading systems fall into two categories; those that assess the balance between therapy‐induced fibrosis in relation to residual tumour and those that assess the percentage of remaining viable tumour cells within the presumably original tumour bed.
While considerable work has been conducted to classify tumour regression and therapy‐induced stromal changes based on the percentage of residual tumour cells, there is no consensus regarding which TRG system should be used for oesophageal cancer. 16 , 17 The implementation of TRG in daily clinical practice has faced many challenges, including large variations in definitions and classifications and concerns regarding reproducibility between pathologists. 18 Another important limitation of existing TRGs is that most pathologists only incorporate the grade of tumour regression rather than the pattern of response, i.e. the morphology of the tumour. In rectal cancer, studies have indicated that tumours can fragment or shrink in response to neoadjuvant treatment, 13 , 19 , 20 , 21 stressing the importance of a morphological tumour characterization based on the spatial distribution and the architectural arrangement of residual tumour cells. Tumour shrinkage refers to the situation in which the tumour mass downsizes, most preferably towards the lumen. 13 Tumour fragmentation implies disintegration of the tumour mass in differently sized and shaped fragments, which may still reach the initial tumour borders. This fragmentation pattern is reported in 40–80% of rectal cancer patients and is associated with poor response. 19 , 20 , 22 A full understanding of the whole spectrum of tumour regression is essential to further improve the standard of care and to explore new treatment paradigms.
In view of the clinical and biological similarities between different gastrointestinal tumours, we hypothesise that different tumour regression patterns may exist in oesophageal cancer after nCRT. The aim of this study is to identify and characterize these patterns and to evaluate their prognostic impact on survival.
Patients, materials and methods
TEST COHORT
A review of the local pathology databases from two upper gastrointestinal (GI) centres in the Netherlands (the Radboud University Medical Centre and the Canisius Wilhelmina Hospital in Nijmegen) was performed (ethical approval case number: 2018–4039). All patients with a potentially curable adenocarcinoma of the oesophagus and gastro‐oesophageal junction (cT1‐4aN0‐3 M0) who received nCRT followed by oesophagectomy during the period 2005–18 were identified. Patients received nCRT according to the ChemoRadiotherapy for oesophageal cancer followed by Surgery Study (CROSS), 5 i.e. all five cycles of carboplatin and paclitaxel with concurrent radiotherapy (41.4 Gy in 23 fractions). Patients were included when they achieved a partial pathological response. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation at the Radboud UMC and with the Helsinki Declaration of 1964 and later versions.
EXTERNAL VALIDATION COHORT
A separate external cohort from the Erasmus University Medical Centre in Rotterdam, the Netherlands, was used as a validation cohort for our proposed classification. Patients were selected from a study cohort in which consecutive patients were included between 2001 and 2017 who had pretreatment biopsies and the resection specimen available (MEC‐2021‐0410). Comparably to the test cohort, all included patients presented with a potentially curable adenocarcinoma of the oesophagus and gastro‐oesophageal junction (cT1‐4aN0‐3M0) and completed nCRT according to the CROSS regimen followed by oesophagectomy.
COLLECTION OF CLINICAL DATA AND HISTOLOGICAL REVIEW
Clinical and follow‐up information such as demographic data, clinical tumour–node–metastases (TNM) staging (8th edn), 23 vital status and date of locoregional or distant disease recurrence were collected from individual medical records. Additional macroscopy and microscopy data relevant for assessment, such as pathological stage, were retrieved from the institutional pathology databases. Exclusion criteria were histology other than adenocarcinoma, Type III tumours of the gastro‐oesophageal junction according to the Siewert classification, 24 pathologically complete response or no response to nCRT upon histological review or no follow‐up data available. Tumours with a Mandard stage 5 were classified as non‐responders. Part of the Chirieac grade 4 cases were included if they showed some response. Additionally, adenocarcinomas showing squamous, signet ring cells or mucinous components [World Health Organization (WHO) 5th edn] 25 upon histological review were excluded because of their different biological behaviour, which could interfere in the way these patients respond to therapy.
ASSESSMENT OF TUMOUR RESPONSE
TRG assessment was performed by a pathologist (S.K.O.) according to the five‐tiered Mandard 9 and four‐tiered Chirieac systems. 14 The first is based on the residual tumour‐to‐fibrosis ratio starting at TRG 1, where there is no residual cancer (pathological complete response), to TRG 5, which contains tumours without signs of regression. The Chirieac system measures the percentage of residual tumour cells present, ranging from no residual cancer in TRG 1 to >50% residual cancer in TRG 4. After confirmation of residual tumour in the resection specimen, a minimum of two and a maximum of five representative haematoxylin and eosin (H&E)‐stained slides of the surgical specimen were selected per patient and digitalised for histomorphological assessment of the regression pattern.
PATTERN OF RESPONSE ASSESSMENT AND DEVELOPMENT OF THE FLOWCHART
Based on an initial histomorphological assessment of a subset of cases by a dedicated gastrointestinal pathology team (C.G.M., S.K.O., I.D.N., R.S.P.), five variations of the two main patterns of response, i.e. tumour shrinkage and fragmentation, were found (Figure 1). After setting definitions for each and establishing an order of importance of their defining characteristics, a flowchart was developed to standardize the determination of the response pattern (Figure 2A). Histological sections were reviewed individually by two observers (C.G.M. & S.K.O.) and patterns of response were scored according to the proposed flowchart. A total of 281 slides were examined and a conclusion per patient was given. Doubtful cases were individually discussed among the same researchers. When consensus could not be established, these cases were decided by agreement of two expert GI pathologists (I.D.N. and R.S.P.).
Figure 1.
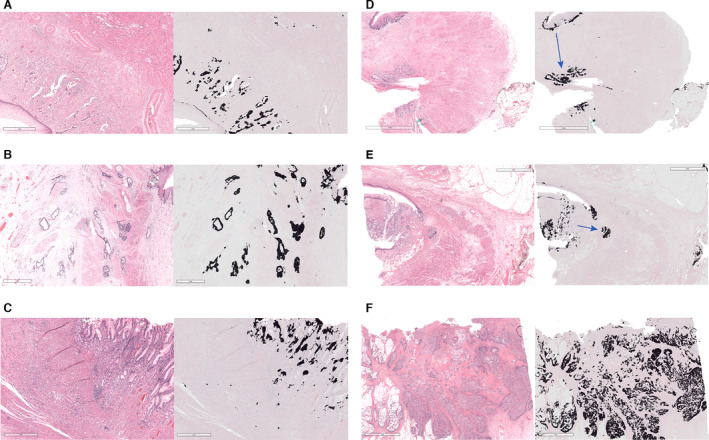
Variations of the two main patterns of response found. A–C, Examples of variations found on the fragmented pattern of response. D,E, Examples of variations of the shrinkage pattern of response. F, No‐response case added as a reference for comparison. On the left of each image there is a haematoxylin and eosin (H&E) snapshot of the pattern of response found. On the right, the corresponding mask created with an artificial intelligence tissue segmentation algorithm to facilitate distinguishing stroma (grey) from tumour (black). Blue arrows show the direction in which the tumour bulk shrunk. Of note, the scale bar is 1 mm for A–C and 2 mm for D–F, in order to facilitate tumour localisation.
Figure 2.
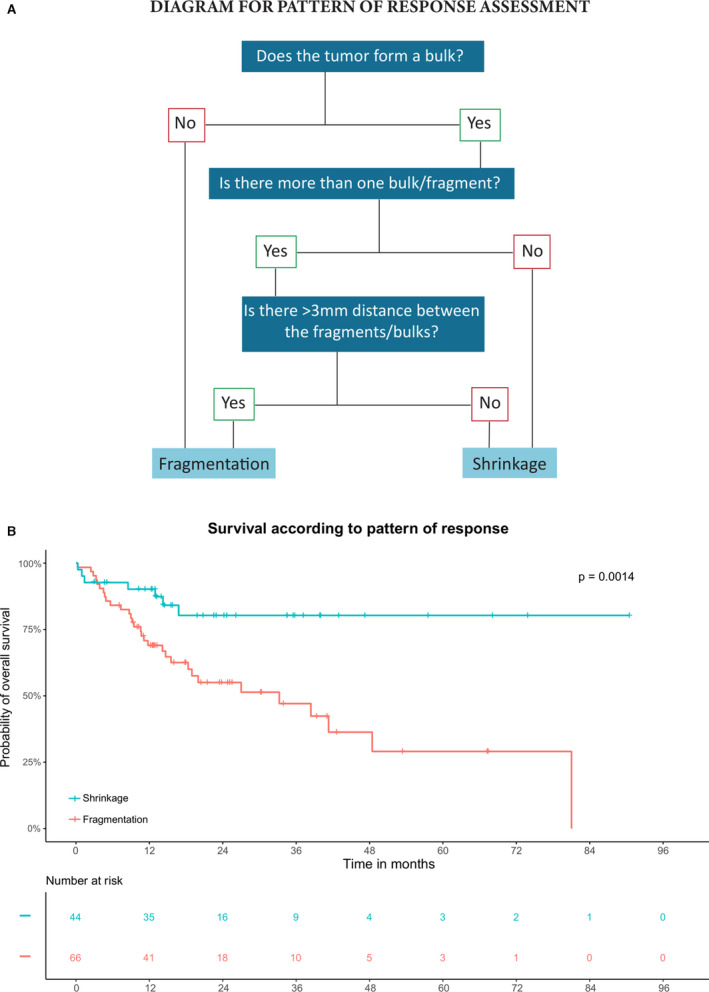
Diagram for pattern of response assessment and the relevance of these patterns on overall survival in the test cohort. A, Flow diagram for histopathological assessment of tumour patterns of response. By following the flowchart answering the questions below, a reproducible general conclusion between two patterns of response can be reached. B, Overall survival analysis of the two main patterns of response in the test cohort. Kaplan–Meier curves showing patients with a shrinkage pattern have a significantly better survival compared to patients with a fragmented pattern.
STATISTICAL ANALYSIS
Baseline characteristics were assessed according to the two main patterns of response. Pearson's χ2 test was used for qualitative measurements and analysis of variance (ANOVA) for quantitative comparisons. Spearman's ρ rank correlation test was used to determine the correlation between patterns of response and pathological stage. Cohen's κ was used to measure interobserver agreement for the patterns of response classification. A κ score between 0.60 and 0.79 was considered moderate agreement, while a κ above 0.80 was considered strong interobserver agreement. Survival time was defined as time from surgical resection to death or end of follow‐up. Recurrence was defined as the time from surgical resection to disease recurrence or end of follow‐up. Overall survival and conditional recurrence‐free survival were estimated with the Kaplan–Meier method and compared with log‐rank testing. Univariate and multivariate Cox regression analyses were performed to identify clinicopathological variables associated with survival in both the test cohort and the external validation cohort to analyse if results of the test cohort were generalisable. A backward stepwise regression was used in multivariate analysis. The two cohorts were combined in order to grant sufficient statistical power to allow differences to appear within the subtypes of the main patterns of response.
For all analyses, a P < 0.05 (two‐sided) was considered statistically significant. Hazard ratios (HRs) and risk ratios (RRs) are presented with a 95% confidence interval (CI). RStudio [RStudio Team (2020); Integrated Development for R. RStudio, PBC, Boston, MA, USA] was used for all analyses and results were confirmed by a second researcher using SPSS statistics version 25.0 (IBM Corporation, Armonk, NY, USA).
DATA AVAILABILITY
The raw clinical data discussed in this study are not publicly available due to patient privacy guidelines, but are available upon reasonable request from the corresponding author. Other data generated in this study are available within the article and the Supporting information data files.
Results
In the test cohort, from the initial 134 patients follow‐up data were available for 117 patients. Upon further histological review, five cases with no response and two patients with complete response after nCRT were excluded. A total of 110 cases with potentially curable adenocarcinoma with a partial pathological response after CROSS nCRT were included. Clinicopathological characteristics according to the two major patterns of response are shown in Table 1.
Table 1.
Clinicopathological characteristics of included patients
| Variable | Test cohort (n = 110) | Validation cohort (n = 115) | Test versus validation | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Shrinkage, n = 44 (40%) | Fragmentation, n = 66 (60%) | n (%) | P | Shrinkage, n = 23 (20%) | Fragmentation, n = 92 (80%) | n (%) | P | P | |
| Age, median (IQR) | 65 (36–83) | 64 (43–82) | 0.74 | 65 (37–74) | 64 (39–83) | 0.44 | 0.95 | ||
| Gender | 0.67 | 0.22 | 0.01 | ||||||
| Male | 33 (75%) | 53 (80%) | 86 (78%) | 23 (100%) | 82 (89%) | 105 (91%) | |||
| Female | 11 (25%) | 13 (20%) | 24 (22%) | 0 (0%) | 10 (11%) | 10 (9%) | |||
| Medical centre | 0.69 | ||||||||
| Centre 1 | 31 (70%) | 50 (76%) | – | – | |||||
| Centre 2 | 13 (30%) | 16 (24%) | – | – | |||||
| Clinical stage | *(n = 65) | 0.78 | *(n = 91) | 0.21 | 0.005 | ||||
| I | 0 (0%) | 1 (2%) | 1 (1%) | 0 | 0 | 0 (0%) | |||
| II | 11 (25%) | 17 (26%) | 28 (26%) | 1 (4%) | 9 (10%) | 10 (9%) | |||
| III | 24 (55%) | 37 (57%) | 61 (56%) | 15 (65%) | 68 (75%) | 83 (73%) | |||
| IV | 9 (20%) | 10 (15%) | 19 (17%) | 7 (31%) | 14 (15%) | 21 (18%) | |||
| Pathological stage | <0.001 | 0.003 | 0.78 | ||||||
| I | 19 (43%) | 6 (9%) | 25 (23%) | 10 (43.5%) | 12 (14%) | 22 (19%) | |||
| II | 18 (41%) | 26 (39%) | 44 (40%) | 10 (43.5%) | 38 (41%) | 48 (42%) | |||
| III | 7 (16%) | 27 (41%) | 34 (31%) | 3 (13%) | 37 (40%) | 40 (35%) | |||
| IV | 0 (0%) | 7 (11%) | 7 (6%) | 0 | 5 (5%) | 5 (4%) | |||
| Recurrence | *(n = 43) | *(n = 63) | <0.001 | *(n = 91) | 0.01 | 0.095 | |||
| Yes | 8 (19%) | 34 (54%) | 42 (40%) | 6 (26%) | 53 (58%) | 59 (52%) | |||
| No | 35 (81%) | 29 (46%) | 64 (60%) | 17 (74%) | 38 (42%) | 55 (48%) | |||
| Downstaging | *(n = 65) | <0.001 | *(n = 91) | 0.005 | 0.97 | ||||
| Yes | 40 (91%) | 31 (48%) | 71 (65%) | 21 (91%) | 52 (57%) | 73 (64%) | |||
| No | 4 (9%) | 34 (52%) | 38 (35%) | 2 (9%) | 39 (43%) | 41 (36%) | |||
| Mandard score | 0.005 | 0.48 | 0.009 | ||||||
| 2 | 17 (39%) | 8 (12%) | 25 (23%) | 7 (31%) | 16 (17%) | 23 (20%) | |||
| 3 | 21 (48%) | 42 (64%) | 63 (57%) | 15 (65%) | 69 (75%) | 84 (73%) | |||
| 4 | 6 (13%) | 16 (24%) | 22 (20%) | 1 (4%) | 7 (8%) | 8 (7%) | |||
| Chirieac score | <0.001 | <0.001 | 0.15 | ||||||
| 2 | 34 (77%) | 21 (32%) | 55 (50%) | 14 (61%) | 46 (50%) | 60 (52%) | |||
| 3 | 4 (9%) | 31 (47%) | 35 (32%) | 7 (30%) | 37 (40%) | 44 (38%) | |||
| 4 | 6 (14%) | 14 (21%) | 20 (18%) | 2 (9%) | 9 (10%) | 11 (10%) | |||
The P‐value corresponds to Pearson's χ2 test for qualitative measurements and to analysis of variance (ANOVA) test for quantitative measures. The differences in each cohort are calculated in relation to their distribution in said patterns of response, as well as an overall comparison between the two cohorts (last column entitled comparison between cohorts).
Indicates that group size is smaller than patients included because of incomplete data for this variable.
PATTERNS OF TUMOUR REGRESSION
In the test cohort two main patterns were observed: fragmentation (60%) and shrinkage (40%). Fragmentation was characterized by clusters of cells which do not form a bulk or are discontinuous by at least 3 mm distance from the tumour bulk (Figure 1A–C). This fragmented pattern was either characterized by individual cells or groups of up to 10 cells (scattered fragmentation) or cell clusters larger than 10 cells (clustered fragmentation) or a mixed pattern. Shrinkage was characterized as residual tumour in the shape of a bulk which may have individual or separate tumour cell clusters not further than 3 mm away from the main tumour border (Figure 1D,E). Shrinkage may occur towards the lumen (luminal shrinkage) or may be irregular and located in deeper layers (irregular shrinkage).
INTEROBSERVER AGREEMENT
There was full agreement on the tumour regression pattern in 105 of 110 cases (95%) when using the flowchart, as depicted in Figure 2A (κ = 0.872).
CORRELATION BETWEEN TUMOUR REGRESSION PATTERN AND CLINICOPATHOLOGICAL CHARACTERISTICS
Shrinkage was more prominent in pathological stages I–II (84 versus 48% fragmented, P < 0.001). Downstaging, as defined by the decrease in one stage according to the TNM stage (8th edn), 23 was more frequent in shrinkage cases (91 versus 48% fragmented, P < 0.001). When a fragmented pattern was found, tumour remnants were significantly more present in deeper layers: muscularis propria (88 versus 32% in patients with shrinkage; P < 0.001) and subserosa (67 versus 16%; P < 0.001) (Figure S1). Significantly higher TRG scores according to Chirieac and Mandard were seen in fragmented regression pattern (Table 1).
PROGNOSIS
Median follow‐up after surgery was 15 months [interquartile range (IQR) = 10–29] in the test cohort. Patients with a fragmented regression pattern had an increased risk for disease recurrence (RR = 2.9, 95% CI = 1.5–5.6) compared to patients with a shrinkage pattern. Patients with a shrinkage pattern had a better overall survival rate compared to patients with a fragmented pattern (5‐year survival; 80 versus 30%, P = 0.001; Figure 2B). The differences in outcome were even more pronounced when evaluating the estimated conditional survival (5‐year survival when recurrence‐free at 1 year; 95 versus 35%; Figure S2).
In univariate Cox regression analysis, higher pathological stage, a fragmented regression pattern and no downstaging in TNM stage were associated with poor overall survival rate. Spearman's rho rank correlation test revealed a significant correlation between pathological stage and pattern of regression (ρ = 0.40). When estimating the relative importance of each covariate using the relative weight on the total χ2 value, the pattern of regression was the second most important variable after pathological stage.
EXTERNAL VALIDATION
The external validation cohort presented with 132 patients initially, although complete case information was only available for 128 patients. Upon histological review, eight patients were excluded due to presenting a mucinous carcinoma and another five were excluded for showing no response to nCRT. A total of 115 patients were included in the external validation cohort, which comprised relatively more patients with high clinical tumour stages compared to the test cohort (clinical stages III–IV, 91 versus 73%, respectively, P = 0.005), more recurrences (52 versus 40%, respectively, P = 0.095) but with regression similar to the test cohort (Mandard TRG = 3–4, 80 versus 77% and Chirieac 3–4, 48 versus 50%, respectively) (Table 1). In the external validation cohort relatively more fragmented cases were observed compared to the test cohort (80 versus 60%, P = 0.002). Cohen's κ for interobserver agreement was moderate (κ = 0.74) for the scoring of regression patterns. Median follow‐up after surgery was 37 months (IQR = 14–64). The correlation with overall survival in univariate analysis was less strong (HR = 1.72, 95% CI = 0.93–3.20), but the risk of recurrence when a fragmented pattern was present was still striking (RR = 2.21, 95% CI = 1.09–4.49).
COMBINATION OF THE TWO COHORTS
When combining the two cohorts, both in univariate and multivariate analysis, a fragmented pattern of regression was an independent prognostic marker of overall survival (univariate HR = 2.30, 95% CI =1.41–3.76, multivariate HR = 1.76, 95% CI = 1.04–2.97, Table 2). Furthermore, a fragmentation pattern of response was also correlated with a higher risk for disease recurrence (RR = 2.66, 95% CI = 1.64–4.33) and decreased 5‐year recurrence‐free survival (32 versus 61%, P < 0.001, Figure 3). The statistical power gained by combining the two cohorts allowed differences to appear within the subtypes of the main patterns of response (P < 0.001, Figure 4). Patients with a clustered or mixed fragmentation pattern had the worst overall survival prognosis, while patients with an irregular shrinkage pattern had the best prognosis. To show the independent effect of the pattern of response, we performed stage‐specific survival analysis (Figure S3). The pathological stages stratified to response patterns show the independent effect of patterns of response on survival. Furthermore, we conducted a regression‐specific survival analysis for patients with a TRG 3 and TRG 4 stage (according to the Mandard classification) and according to Chirieac stage 4 (Figure S4). These survival curves show the independent effect of patterns of response on survival, as the curves tend to separate despite referring to the same regression score. When conducting a survival subanalysis dividing Mandard TRG groups 2–4 by pattern of response and comparing them to a group of patients with Mandard TRG 5 the benefit of pattern of response in Mandard groups 2–4 with shrinkage pattern compared to the fragmentation group is striking (Figure S5).
Table 2.
Univariate and multivariate analysis of factors associated with overall survival in both cohorts (test + validation cohort)
| Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|
| Covariate | Number (%) | HR | 95% CI | HR | 95% CI |
| Gender | |||||
| Male | 191 (85%) | 1.18 | (0.67–2.06) | ||
| Female | 34 (15%) | (1.00) | |||
| Age | 1.02 | (0.98–1.00) | |||
| Medical centre | |||||
| Centre 1 | 81 (36%) | (1.00) | |||
| Centre 2 | 29 (13%) | 1.15 | (0.59–2.24) | ||
| Centre 3 | 115 (51%) | 1.14 | (0.72–1.82) | ||
| Clinical stage | n = 223 a | ||||
| I | 1 b (1%) | 2.7 10−7 | (0.00–Inf) b | ||
| II | 38 (17%) | 0.64 | (0.32–1.31) | ||
| III | 144 (64%) | 0.97 | (0.59–1.58) | ||
| IV | 40 (18%) | (1.00) | |||
| Pathological stage | 1.86 | (1.32–2.62) | |||
| I | 47 (21%) | 0.14 | (0.06–0.31) | ||
| II | 92 (41%) | 0.20 | (0.10–0.40) | ||
| III | 74 (33%) | 0.39 | (0.20–0.76) | ||
| IV | 12 (5%) | (1.00) | |||
| Response pattern | 1.76 | (1.04–2.97) | |||
| Shrinkage | 67 (30%) | (1.00) | |||
| Fragmentation | 158 (70%) | 2.30 | (1.41–3.76) | ||
| Downstaging | n = 223 a | 1.19 | (0.70–2.05) | ||
| Yes | 144 (65%) | 0.50 | (0.34–0.72) | ||
| No | 79 (35%) | (1.00) | |||
| Mandard score | |||||
| 2 | 48 (21%) | 0.54 | (0.24–1.21) | ||
| 3 | 147 (65%) | 1.36 | (0.72–2.55) | ||
| 4 | 30 (13%) | (1.00) | |||
| Chirieac score | |||||
| 2 | 115 (51%) | 0.81 | (0.43–1.53) | ||
| 3 | 79 (35%) | 1.38 | (0.73–2.59) | ||
| 4 | 31 (14%) | (1.00) | |||
For each covariate an individual hazard ratio is calculated with a Cox proportional hazard model. HR, Hazard ratio; CI, Confidence interval.
Indicates that group size is smaller than patients included because of incomplete data for this variable.
Calculations marked with an asterisk warrant caution due to the small sample they represent.
Figure 3.
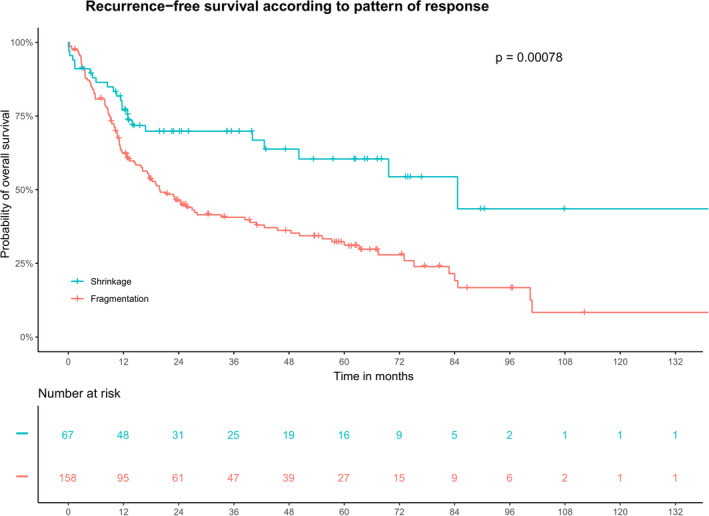
Five‐year recurrence‐free survival analysis for all patients: Kaplan–Meier survival curves representing the beneficial prognosis of shrinkage compared to fragmentation. Data are pooled patients from the test cohort and the validation cohort.
Figure 4.
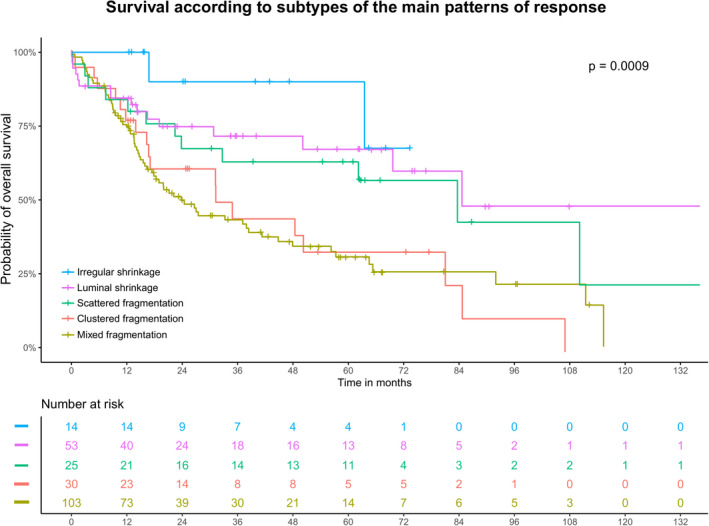
Overall survival analysis of the subtypes of patterns of response observed when combining both test and validation cohorts. Kaplan–Meier curves depict a better prognosis for patients presenting an irregular shrinkage pattern and a poorer prognosis for those presenting a clustered or mixed fragmentation pattern.
Discussion
In this explorative study we show that two major histomorphological tumour regression patterns emerge after nCRT for oesophageal adenocarcinoma. A fragmented pattern was observed in 60% of oesophagectomy specimens of patients in the test cohort, whereas 40% had a shrinkage pattern. The fragmentation pattern was associated with a relatively poor overall and conditional recurrence‐free survival in the test cohort. In the external validation cohort, a significant association was found only with disease recurrence. In multivariate analysis of the combined cohorts a fragmented pattern of response was an independent predictor of poor survival and was associated with a higher risk of disease recurrence. Furthermore, 5‐year recurrence‐free survival showed a favourable prognosis when a shrinkage pattern of response was present (Figure 3).
Compared to well‐established TRG classification systems, the proposed patterns of response classification system appeared to be a better prognostic factor. In both univariate and multivariate analysis, none of these former methods was significantly associated with overall survival. As pathological stage and response pattern are different assessments of tumour regression, we hypothesise that they might interfere with each other. A correlation was indeed found between both variables (ρ = 0.40). This confounding could explain a potential underestimation of the true prognostic strength of the presented classification system. To show the independent effect of the pattern of response, we performed stage‐specific survival analysis (Figure S3). In both early and advanced disease stages there is a considerably worse prognosis in fragmented cases compared to shrinkage cases, stressing the independent effect of patterns of response as a prognostic factor. Moreover, this tendency was identified in regression‐specific survival when we looked at the importance of the pattern of response versus residual tumour gradings following nCRT (Figure S4). The correlation between fragmentation and pathological or TRG stage is not surprising, considering the higher likelihood of tumour cell distribution in deeper layers of the oesophageal wall. In fact, when conducting a subanalysis including Mandard TRG groups 2–4 stratified by pattern of response and comparing them to a group of patients with Mandard TRG 5 the benefit of pattern response in Mandard groups 2–4 with shrinkage pattern compared to the fragmentation group is striking (Figure S5). Furthermore, the Kaplan–Meier curves for Mandard groups 2–4 fragmentation and Mandard group 5 are quite close to each other, stressing the poor survival of patients with a fragmented pattern of response.
Patients with a fragmented pattern of response had an increased risk of recurrence (in test cohort RR = 2.9 and in validation cohort RR = 2.2) compared to patients with a shrinkage pattern. In addition, we aimed to look at a more clinically relevant estimate of risk over time. Conditional survival analyses showed that patients with a shrinkage regression pattern had a better survival compared to patients with a fragmented pattern of regression (Figure S2). This highlights the potential clinical relevance of including the pattern of regression in oesophageal cancer staging.
One main challenge during histological assessment was the heterogeneity of fragment size and distribution. While a minority of the cases purely consisted of either individually dispersed tumour glands (scattered fragmentation) or grouping tumour glands (clustered fragmentation), most of the fragmented tumours displayed a mixture of these two morphologies (mixed fragmentation). The clinical impact of these morphological variants was only possible to investigate when combining both cohorts, allowing for sufficient statistical power. Indeed, differences were found within the subtypes of the main patterns of response (P < 0.001). Patients with a clustered fragmentation or mixed fragmentation pattern had the worst overall survival prognosis while patients with an irregular shrinkage pattern had the best prognosis (Figure 4). These results should be further validated in larger cohorts before they can be applied in clinical decision‐making with respect to adjuvant therapy.
Currently, little is known on the factors that influence the development of these patterns. It has been previously stated that, after irradiation, tumour neoantigens from dying tumour cells are released and subsequently captured by antigen‐presenting cells. 26 Through activation of cytotoxic T cells, remaining vital tumour cells are directly and indirectly targeted by dendritic, B or T helper cells. To date, the link between the anti‐tumour immune response and tumour regression has focused upon high or low immune infiltrates predicting patient outcome. 27 , 28 As therapy interplays with the tumour immune landscape, it seems plausible that the immune response could influence the pattern of response. Further studies on these specific immune cell subsets and their possible link to patterns of response are therefore needed. Moreover, recognition and early clinical evaluation of these patterns of response correlated to (future) imaging modalities may contribute to improved patient selection for further treatment. New pathological approaches focusing upon tumour response to predict patient survival have been recently explored, 29 , 30 exemplifying the applicability and reliance of these data. These studies in particular aimed to assess the clinical relevance of regression changes such as fibrosis, mucinous lakes, keratin pearls and foreign‐body giant cell reactions, as well as the presence of tumour remnants. Therefore, their main focus lies in the stromal changes, while ours is in the morphological tumour response. By so doing, we aimed to focus upon the morphology of the tumour remnant as well as the depth, which has been shown to have additional clinical value beyond TNM staging.
In recent years, artificial intelligence (AI) has shown promising results in pathology. 31 Integration of AI algorithms in future research could help classifying response and might also be able to detect smaller tumour clusters leading to a more objective pattern of response assessment, i.e. by segmenting and highlighting tumour areas as shown in Figure 1. In a multidisciplinary setting where not all medical and research professionals are trained to interpret pathological slides, a colour‐coded AI overlay of the H&E slide could enable less‐trained professionals to assess patterns of response. Furthermore, it could be transferred to other tissues by training of the algorithm, providing a valuable tool in staging.
The present study has also limitations. First, retrospective data analyses may have resulted in selection bias. Secondly, an important limitation of our study was the sample size, which did not allow for further investigation of the clinical relevance of different variations (subpatterns) within the two main patterns. This was overcome by inclusion of an external validation cohort.
In conclusion, we have established a reproducible and replicable classification for patterns of response with prognostic implications for patients with potentially curable oesophageal adenocarcinoma. Our proposed classification includes two main tumour response patterns that are independent predictors of survival. A fragmented pattern of regression following nCRT was associated with less downstaging, worse overall survival and a higher risk of recurrence, compared with the shrinkage regression pattern. The findings of this study suggest that integration of tumour regression pattern in response assessment should be considered. This may have important clinical implications in terms of individual patient prognosis and the development of more personalized and effective treatment regimens.
Conflicts of interest
The authors declare no potential conflicts of interest.
Supporting information
Figure S1. Distribution of residual tumor patterns in each layer of the esophageal wall according to pattern of response.
Figure S2. Estimated conditional survival if recurrence‐free at 1 year in patients with fragmentation and shrinkage pattern.
Figure S3. Stage‐specific survival analysis: Kaplan‐Meier survival curves in separate pathological stage groups according to pattern of response.
Figure S4. Regression‐specific survival analysis: Kaplan‐Meier survival curves in separate TRG groups for patterns of response.
Figure S5. Survival according to Mandard TRG score and pattern of response.
Acknowledgements
We would like to thank the Tiniakos family for granting us the ‘George Tiniakos Award’ for the best oral presentation in gastrointestinal, liver and pancreas pathology at the 33rd European Congress of Pathology 2021. Furthermore, we would like to thank Dr Steven Teerenstra for his assistance with the statistical analyses. This work was supported by an Alpe d'HuZes/KWF programme grant (KWF UL 2013‐6311)].
References
- 1. Noordman BJ, Spaander MCW, Valkema R et al. Detection of residual disease after neoadjuvant chemoradiotherapy for oesophageal cancer (preSANO): a prospective multicentre, diagnostic cohort study. Lancet Oncol. 2018; 19; 965–974. [DOI] [PubMed] [Google Scholar]
- 2. Eyck BM, van der Wilk BJ, Noordman BJ et al. Updated protocol of the SANO trial: a stepped‐wedge cluster randomised trial comparing surgery with active surveillance after neoadjuvant chemoradiotherapy for oesophageal cancer. Trials 2021; 22; 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lordick F, Mariette C, Haustermans K, Obermannova R, Arnold D. Oesophageal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann. Oncol. 2016; 27; v50–v57. [DOI] [PubMed] [Google Scholar]
- 4. Ajani JA, D'Amico TA, Bentrem DJ et al. Esophageal and esophagogastric junction cancers, version 2.2019. NCCN clinical practice guidelines in oncology. J. Natl. Compr. Canc. Netw. 2019; 17; 855–883. [DOI] [PubMed] [Google Scholar]
- 5. van Hagen P, Hulshof MC, van Lanschot JJ et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med. 2012; 366; 2074–2084. [DOI] [PubMed] [Google Scholar]
- 6. Nygaard K, Hagen S, Hansen HS et al. Pre‐operative radiotherapy prolongs survival in operable esophageal carcinoma: a randomized, multicenter study of pre‐operative radiotherapy and chemotherapy. The second Scandinavian trial in esophageal cancer. World J. Surg. 1992; 16; 1104–1109. discussion 10. [DOI] [PubMed] [Google Scholar]
- 7. Blum Murphy M, Xiao L, Patel VR et al. Pathological complete response in patients with esophageal cancer after the trimodality approach: the association with baseline variables and survival – The University of Texas MD Anderson Cancer Center experience. Cancer 2017; 123; 4106–4113. [DOI] [PubMed] [Google Scholar]
- 8. Al‐Kaabi A, van der Post RS, van der Werf LR et al. Impact of pathological tumor response after CROSS neoadjuvant chemoradiotherapy followed by surgery on long‐term outcome of esophageal cancer: a population‐based study. Acta Oncol. 2021; 60; 497–504. [DOI] [PubMed] [Google Scholar]
- 9. Mandard AM, Dalibard F, Mandard JC et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 1994; 73; 2680–2686. [DOI] [PubMed] [Google Scholar]
- 10. Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int. J. Colorectal Dis. 1997; 12; 19–23. [DOI] [PubMed] [Google Scholar]
- 11. Puetz K, Bollschweiler E, Semrau R, Mönig SP, Hölscher AH, Drebber U. Neoadjuvant chemoradiation for patients with advanced oesophageal cancer – which response grading system best impacts prognostic discrimination? Histopathology. 2019; 74; 731–743. 10.1111/his.13811 [DOI] [PubMed] [Google Scholar]
- 12. Shapiro J, ten Kate FJ, van Hagen P, Biermann K, Wijnhoven BP, van Lanschot JJ. Residual esophageal cancer after neoadjuvant chemoradiotherapy frequently involves the mucosa and submucosa. Ann. Surg. 2013; 258; 678–688; discussion 88–89. [DOI] [PubMed] [Google Scholar]
- 13. Gosens MJ, Klaassen RA, Tan‐Go I et al. Circumferential margin involvement is the crucial prognostic factor after multimodality treatment in patients with locally advanced rectal carcinoma. Clin. Cancer Res. 2007; 13; 6617–6623. [DOI] [PubMed] [Google Scholar]
- 14. Chirieac LR, Swisher SG, Ajani JA et al. Posttherapy pathologic stage predicts survival in patients with esophageal carcinoma receiving preoperative chemoradiation. Cancer 2005; 103; 1347–1355. [DOI] [PubMed] [Google Scholar]
- 15. Schneider PM, Baldus SE, Metzger R et al. Histomorphologic tumor regression and lymph node metastases determine prognosis following neoadjuvant radiochemotherapy for esophageal cancer: implications for response classification. Ann. Surg. 2005; 242; 684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Karamitopoulou E, Thies S, Zlobec I et al. Assessment of tumor regression of esophageal adenocarcinomas after neoadjuvant chemotherapy: comparison of 2 commonly used scoring approaches. Am. J. Surg. Pathol. 2014; 38; 1551–1556. [DOI] [PubMed] [Google Scholar]
- 17. Lerttanatum N, Tharavej C, Chongpison Y, Sanpavat A. Comparison of tumor regression grading system in locally advanced esophageal squamous cell carcinoma after preoperative radio‐chemotherapy to determine the most accurate system predicting prognosis. J. Gastrointest. Oncol. 2019; 10; 276–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nagtegaal ID, Glynne‐Jones R. How to measure tumour response in rectal cancer? An explanation of discrepancies and suggestions for improvement. Cancer Treat. Rev. 2020; 84; 101964. [DOI] [PubMed] [Google Scholar]
- 19. Hav M, Libbrecht L, Geboes K et al. Prognostic value of tumor shrinkage versus fragmentation following radiochemotherapy and surgery for rectal cancer. Virchows Arch. 2015; 466; 517–523. [DOI] [PubMed] [Google Scholar]
- 20. Fernandez‐Acenero MJ, Estrada Munoz L, Sastre Varela J et al. Prognostic influence of histopathological regression patterns in rectal adenocarcinoma receiving neoadjuvant therapy. J. Gastrointest. Oncol. 2017; 8; 49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nagtegaal ID, Quirke P. What is the role for the circumferential margin in the modern treatment of rectal cancer? J. Clin. Oncol. 2008; 26; 303–312. [DOI] [PubMed] [Google Scholar]
- 22. Perez RO, Habr‐Gama A, Smith FM et al. Fragmented pattern of tumor regression and lateral intramural spread may influence margin appropriateness after TEM for rectal cancer following neoadjuvant CRT. J. Surg. Oncol. 2014; 109; 853–858. [DOI] [PubMed] [Google Scholar]
- 23. Brierley JD, Gospodarowicz MK, Wittekind C. Classification of malignant tumours. 8th ed. Hoboken, NJ: Wiley‐Blackwell/Union for International Cancer Control, 2017. [Google Scholar]
- 24. Siewert JR, Hölscher AH, Becker K, Gössner W. Cardia cancer: attempt at a therapeutically relevant classification. Chirurg 1987; 58; 25–32. [PubMed] [Google Scholar]
- 25. Nagtegaal ID, Odze RD, Klimstra D et al. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020; 76; 182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lhuillier C, Rudqvist NP, Elemento O, Formenti SC, Demaria S. Radiation therapy and anti‐tumor immunity: exposing immunogenic mutations to the immune system. Genome Med. 2019; 11; 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Glynne‐Jones R, Hall M, Nagtegaal ID. The optimal timing for the interval to surgery after short course preoperative radiotherapy (5 × 5 Gy) in rectal cancer – are we too eager for surgery? Cancer Treat. Rev. 2020; 90; 102104. [DOI] [PubMed] [Google Scholar]
- 28. Angell HK, Bruni D, Barrett JC, Herbst R, Galon J. The immunoscore: colon cancer and beyond. Clin. Cancer Res. 2020; 26; 332–339. [DOI] [PubMed] [Google Scholar]
- 29. Shapiro J, Biermann K, van Klaveren D et al. Prognostic value of pretreatment pathological tumor extent in patients treated with neoadjuvant chemoradiotherapy plus surgery for esophageal or junctional cancer. Ann. Surg. 2017; 265; 356–362. [DOI] [PubMed] [Google Scholar]
- 30. Brinkmann S, Noordman BJ, Hölscher AH et al. External validation of pretreatment pathological tumor extent in patients with neoadjuvant chemoradiotherapy plus surgery for esophageal cancer. Ann. Surg. Oncol. 2020; 27; 1250–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Litjens G, Kooi T, Bejnordi BE et al. A survey on deep learning in medical image analysis. Med. Image Anal. 2017; 42; 60–88. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Distribution of residual tumor patterns in each layer of the esophageal wall according to pattern of response.
Figure S2. Estimated conditional survival if recurrence‐free at 1 year in patients with fragmentation and shrinkage pattern.
Figure S3. Stage‐specific survival analysis: Kaplan‐Meier survival curves in separate pathological stage groups according to pattern of response.
Figure S4. Regression‐specific survival analysis: Kaplan‐Meier survival curves in separate TRG groups for patterns of response.
Figure S5. Survival according to Mandard TRG score and pattern of response.
Data Availability Statement
The raw clinical data discussed in this study are not publicly available due to patient privacy guidelines, but are available upon reasonable request from the corresponding author. Other data generated in this study are available within the article and the Supporting information data files.


