Abstract
Management of worsening heart failure (WHF) has traditionally been hospital‐based, but with the rising burden of heart failure (HF), the pressure on healthcare systems exerted by this disease necessitates a different strategy than long (and costly) hospital stays. A strategy for outpatient intravenous (IV) diuretic treatment of WHF has been developed in certain American centres in the past 10 years, whereas European centres have been mostly favouring ‘classic’ in‐hospital management of WHF. Embracing novel, outpatient approaches for treating WHF could substantially reduce the burden on healthcare systems while improving patient's satisfaction and quality of life. The present article is intended to provide essential knowledge and practical guidelines aimed at helping clinicians implement these new ambulatory approaches using day hospital and/or at‐home hospitalization. The topics addressed by our group of HF experts include the pathophysiological background of diuretic therapy, the most suitable profile of WHF that may be managed in an ambulatory setting, the pharmacological protocols that can be used, as well as a detailed description of healthcare structures that can be proposed to deliver these ambulatory care interventions. The practical aspects of day hospital and hospital‐at‐home IV diuretic administration are specifically emphasized. The algorithm provided along with the practical IV diuretic protocols should assist HF clinicians in implementing this new approach in their local clinical setting.
Keywords: Heart failure, Diuretics, Cardiac congestion, Cardiovascular diseases, Ambulatory management
Introduction
Heart failure (HF) is widely recognized as a major global public health burden. HF affects 2%–3% of the adult population in Western countries 1 , 2 , 3 , 4 and is the most common cardiovascular cause of hospital admission over the age of 60 years. It is estimated that HF consumes approximately 9 billion euros/year of the European healthcare budget.
Congestion, related to pressure and/or volume overload, is fundamental to the pathophysiology, presentation and prognosis of HF, 5 , 6 irrespectively of its typology (i.e. HF with reduced [HFrEF] vs. preserved ejection fraction [HFpEF]). Specifically, increased fluid filtration due to elevated pulmonary capillary pressure leads to an increase in extravascular lung water resulting in pulmonary congestion and severe breathlessness in patients with HF. 7 , 8 Consequently, systemic congestion may also occur, often causing intestinal oedema and reduced absorption of guideline‐directed medical therapies (GDMT) for HF, including loop diuretics. It usually presents – clinically – with various degree of lower limb oedema and substantial weight gain. Worsening symptomatic congestion is one of the main causes leading to urgent HF hospitalization and subsequent poor patient outcomes. 5 , 9 The cornerstone of worsening HF (WHF) treatment is intravenous (IV) loop diuretic, usually administered during hospital stays of 5–15 days (usually less in the US) according to the severity of the congestive episode and the regional differences in healthcare structure. Importantly, patients hospitalized for WHF have a high‐risk of readmission, reaching up to 50% at 6 months. 10 , 11 Iterative hospital admissions have a major impact on quality of life. 12 In addition, hospitalizations by themselves can be associated with iatrogenic and nosocomial complications including malnutrition, infections, dependency, etc., especially in elderly patients. If they could choose, many patients with chronic illnesses, including HF, would prefer to be treated at home, particularly during, and presumably after, the COVID‐19 pandemic. 13
Given the projected substantial rise in HF burden in Western populations over the next decades, the ensuing pressure on healthcare systems exerted by HF will undoubtedly increase. In Europe, the median length of hospital stay is approximately 8 days in units with 24/7 trained nurses and physicians, which largely explains the high cost of WHF management. An ambulatory management strategy for WHF has been developed in American centres in the past 10 years (even if used heterogeneously across centres/regions), 14 whereas European centres (along with a number of American centres) have been mostly favouring ‘classic’ in‐hospital management of WHF. Embracing new approaches for managing WHF could substantially reduce the ‘burden’ of the diagnosis on European healthcare systems while improving quality of life. We believe that there is a common interest for both patients and healthcare providers to favour these ambulatory approaches to WHF. The present review article is intended to provide essential knowledge and practical guidelines aimed at helping clinicians implement these new ambulatory approaches using day hospital and/or at‐home hospitalization.
Herewith, our panel of HF experts will review the pathophysiological background of diuretic therapy, describe the most suitable profile of WHF which may be managed in an ambulatory setting as well as the pharmacological protocols that can be used, and detail the healthcare setting that can be proposed to deliver these ambulatory care interventions.
Part I: Pathophysiological background relevant to diuretics
Patients with WHF typically have clinically significant congestion with volume overload due to chronic retention of sodium and water in the intravascular and extravascular compartments. 15 In this context, diuretics are used to relieve congestion through increased renal natriuresis and diuresis. In an ambulatory setting, it is therefore important to have a sound knowledge on how to use and combine the different diuretic classes together, 16 keeping in mind that only a few small trials have provided, so far, evidence for their best use. 17
As recently presented in a position paper of the Heart Failure Association (HFA), 16 the goal of diuretic therapy is to (i) obtain efficient decongestion, and (ii) maintain sufficient renal and organ perfusion pressures.
An important facet when using diuretics in WHF patients is to understand their pharmacokinetics. In routine practice, many patients with WHF have HF GDMT down‐titrated or discontinued due to a mistaken interpretation of apparent worsening renal function (WRF) 18 without an attempt to increase – substantially – or combine diuretics to achieve complete decongestion. It is hence important to (i) define the optimal diuretic loading dose, (ii) rapidly assess the patient's diuretic response, and (iii) upgrade doses in instances of insufficient response with possible use of sequential nephron blockade. 16 , 19 Patients with WHF usually present with a combination of volume overload accompanied by interstitial and bowel oedema, kidney dysfunction and altered organ perfusion, which impairs both bioavailability of, and response to, diuretic therapy. Furthermore, activation of the renin–angiotensin–aldosterone system (RAAS) renders the patient further resistant to oral diuretics; rising blood urea nitrogen – an indirect measure of RAAS activation – also may occur, masquerading as WRF.
The principal pharmacological properties (including site of action) of the various diuretic classes are presented in Table 1 and Figure 1 . In this review, we will not address the use of acetazolamide and amiloride, being less documented, not routinely available in clinical practice in many countries and recently overviewed in detail somewhere else. 16
Table 1.
Principal pharmacological properties of drugs with diuretic effects
| Natriuretic effect when used in monotherapy (FENa%) | Time to peak efficacy | Half‐life | Bioavailability | Side effects | |
|---|---|---|---|---|---|
| Loop diuretics | 25%–30% | PO: 0.5–1 h IV: 5–10 min | 3 h | Highly variable for oral furosemide 90% for bumetanide/torsemide | Important RAAS activation |
| Diuretic resistance induced by compensatory DCT hypertrophy | |||||
| Hypokalaemia | |||||
| Hypomagnesaemia | |||||
| Hyperuricaemia | |||||
| Gout | |||||
| Thiazide‐like diuretics | 10% Loop diuretics potentializing ++++ | PO: 1–6 h IV: only chlorothiazide with onset of 30 min | HCTZ: 6–15 h Metolazone: 6–20 h Chlortalidone: 45–60 h | HCTZ: 65%–75% Metolazone: 60%–65% Chlortalidone: NA | RAAS activation |
| Hypokalaemia | |||||
| Hyponatraemia | |||||
| Hyperuricaemia | |||||
| Gout | |||||
| Hypercalcaemia | |||||
| Hypomagnesaemia | |||||
| MRAs | 2% | PO: 48–72 h IV: potassium canreonate: 2.5 h | Eplerenone: 3–6 h Canrenone: 17 h | Spironolactone: 90% Eplerenone: 70% | Hyperkalaemia |
| Average increase in serum potassium: 0.4 mmol/L | |||||
| SGLT2i | 3% Loop diuretics potentializing ++ | PO: 1.5–2 h | 12 h | 80%–90% | Keto‐acidosis (in patients treated with insulin) |
| Acetazolamide | Heavily depends on subsequent tubular segments | PO: 2 h | 6 h | >90% | Hypokalaemia |
| Metabolic acidosis | |||||
| Hyponatraemia | |||||
| Amiloride | 2% | PO: 6 h | 6–9 h | 50% | Hyperkalaemia |
| Hyponatraemia |
DCT, distal convoluted tubule; FENa, fractional excretion of sodium; HCTZ, hydrochlorothiazide; IV, intravenous; MRA, mineralocorticoid receptor antagonist; NA, not available; PO, per os; RAAS, renin–angiotensin–aldosterone system; SGLT2i, sodium–glucose cotransporter 2 inhibitor.
Adapted from Mullens et al. 16
Figure 1.
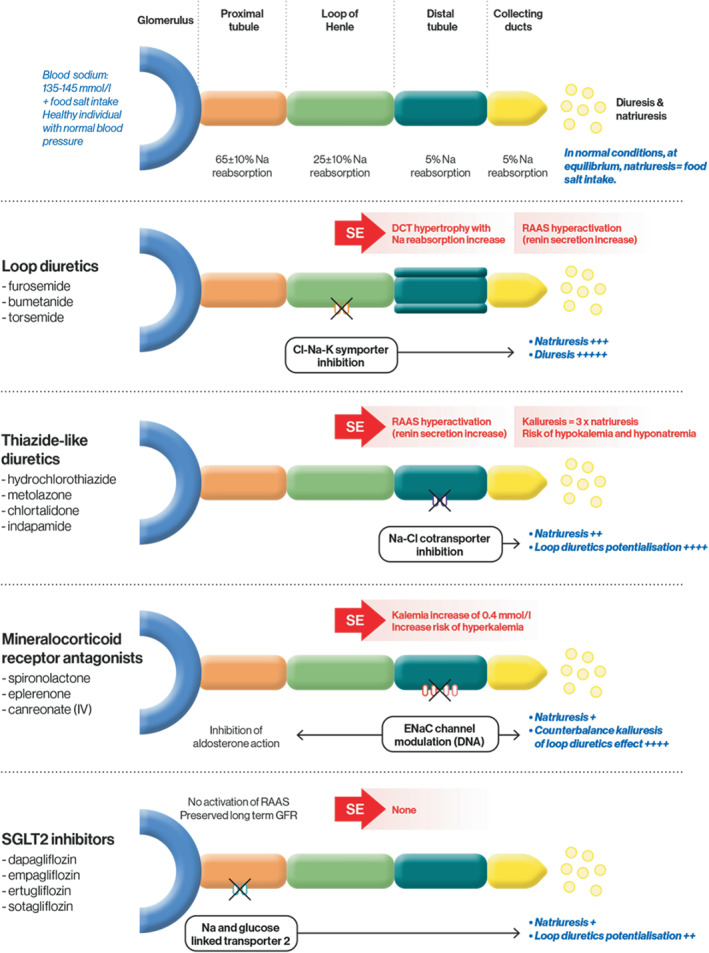
Nephron sites and target ion channels with approximate natriuresis/diuresis effect of the various diuretic classes. Cl, chloride; ENaC, epithelial sodium channel; GFR, glomerular filtration rate; K, potassium; IV, intravenous; Na, sodium; RAAS, renin–angiotensin–aldosterone system; SE, side effect; SGLT2, sodium–glucose cotransporter 2.
The most potent diuretic class includes loop diuretics, with the effect of WHF on their dose–response curve presented in Figure 2 . 20 In the context of WHF and the consequent reduced bioavailability of oral loop diuretics, guidelines recommend the use of IV loop diuretics 5 to rapidly and more efficiently achieve a natriuretic ceiling. 20 Different loop diuretics exist and they have different pharmacokinetics and bioavailability 17 ; a large (∼6000 participants to be enrolled) ongoing trial is comparing oral torasemide to furosemide as treatment for congestion at the time of discharge from an episode of HF (TRANSFORM‐HF, NCT03296813 21 ) following the favourable effect of torasemide on HF hospitalizations suggested in observational studies and the meta‐analysis of small trials. 22
Figure 2.
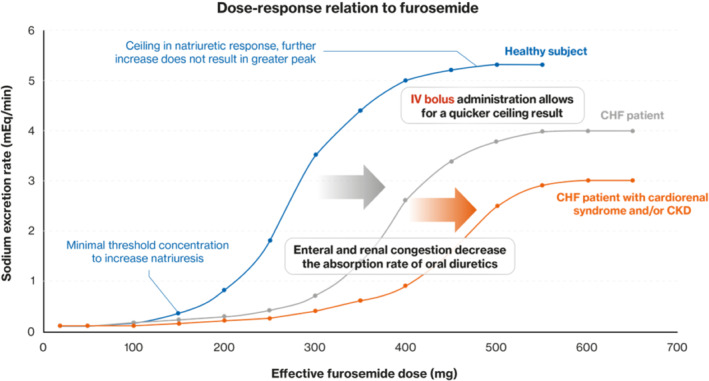
Shift in dose–response relation to furosemide in patients with chronic heart failure (CHF). Renal function (as measured by estimated glomerular filtration rate) has an important impact on the dose–response curve to furosemide: Higher dosing is needed for lower estimated glomerular filtration rate. This is the underlying reason of the right shift observed for patients with CHF and cardiorenal syndrome and/or chronic kidney disease (CKD) (orange curve). IV, intravenous. Adapted from Brater 20
Thiazide‐like diuretics can be used in severe HF patients who responded poorly to loop diuretics in a sequential nephron blockade strategy. These diuretic agents potentialize the natriuretic effect of loop diuretics (Figure 1 ) and in the case of oral and IV metolazone remain effective with reduced filtration rate (<30 ml/min/1.73 m2). 23 This effect can significantly increase diuresis and should be closely monitored with serum potassium and sodium measurements.
Mineralocorticoid receptor antagonists (MRAs) are seldom used in the acute setting, although their introduction at this phase should nonetheless be considered. Together with their direct inhibition of aldosterone and their potassium‐sparing effect, they can partially offset significant side‐effects of loop and thiazide diuretics. 24 In addition, MRAs initiated in acute settings have been shown to have natriuretic effects. 25 Since the action of the MRA spironolactone occurs 48–72 h after oral intake (Table 1 ), eplerenone or canrenoate potassium could be favoured in the setting of WHF. 26 In the ATHENA‐HF trial, spironolactone use at 100 mg/day was deemed safe and did not result in hyperkalaemia or WRF (but did not improve outcome either possibly because of the short follow‐up and slow pharmacokinetics of spironolactone). 27
Finally, assessment of diuretic response is critical in routine practice. Clinical signs of decongestion, diuresis, weight loss and renal function have limited sensitivity for guiding diuretic therapy. 28 Urinary sodium monitoring is a simple indicator of diuretic response that is associated with prognosis and has the potential of becoming a useful tool for routine practice guidance of diuretic therapy. 16 , 29 Serial measurement of natriuretic peptide concentrations are commonly utilized in US institutions for monitoring trajectory of decongestion. Given the stability of N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP), even home‐based phlebotomy with centralized measurement may be performed. The use of ultrasound, including serial assessment of lung B‐lines, inferior vena cava or jugular vein diameter, or intra‐renal venous flow have also shown potential to be useful in this setting and is currently under evaluation. 30
Diuretic resistance
As noted, diuretic resistance (defined as the need for progressive dosage up‐titration in order to achieve a net fluid balance) is common in those with WHF, and corresponds to an impaired sensitivity to diuretics resulting in reduced natriuresis. Diuretic resistance translates into a rightward shift of the dose–response curve of sodium excretion rate with a lowering of the sodium ceiling excretion rate (Figure 2 ). Furthermore, WRF‐associated diuretic resistance is an indicator of advanced HF (as poor perfusion can favour WRF) and the need for more advanced therapies. 31 Loop and thiazide diuretics may provide immediate symptom relief, albeit without necessarily decreasing patient mortality. Conversely, consistent data have established that large doses of diuretics 32 , 33 and/or the need for intensification of diuretic treatments 34 are associated with increased mortality in HF, reflecting the severity of congestion and advanced cardiac dysfunction as drivers of poor outcomes. 35
The impact of guideline‐directed medical therapies on congestion
Both persistent congestion and excessive decongestion can lead to down‐titration of GDMT, mostly through renal function‐ and blood pressure‐related effects. 36 , 37 Several studies have focused on the interaction between use of diuretics and these life‐saving HFrEF medications. In a post‐hoc analysis of the EPHESUS trial, patients taking eplerenone had their loop diuretic dose significantly reduced during follow‐up while eplerenone benefit was not dependent on diuretic dose. 38 In a post‐hoc analysis of the PARADIGM‐HF trial, patients randomized to sacubitril/valsartan had a lower subsequent use of diuretics, with fewer loop diuretic dose increases and more frequent dose reductions compared with those taking enalapril. 39 The treatment effect of sacubitril/valsartan to reduce hospitalizations appeared within 30 days, 40 and among those patients hospitalized during PARADIGM‐HF, readmissions were lower among those treated with sacubitril/valsartan. 41 Natriuresis is also potentialized by sacubitril through the natriuretic peptide pathway. 42 These results prompted the recommendation in all subsequent trials evaluating sacubitril/valsartan (including PIONEER‐HF) to consider a dose reduction in loop diuretics. Moreover, in those patients with acute HF randomized to receive sacubitril/valsartan in the PIONEER‐HF trial, rehospitalization for HF was substantially reduced (hazard ratio [HR] 0.61; p = 0.02). 43 This impact of sacubitril/valsartan on congestive complications may have to do with a modest effect of sacubitril to promote natriuresis through boosting of natriuretic peptide concentrations. 42 Supporting the indirect results from PARADIGM‐HF, Desai and colleagues reported early, significant reduction in pulmonary artery pressures among those treated with sacubitril/valsartan (n = 96) compared to a matched control group of 406 patients (−2.9 mmHg; p < 0.001); the reduction of mean pulmonary artery pressure was greatest among those with a baseline pressure ≥30 mmHg. 44 No predicate data exist to suggest sacubitril/valsartan has a diuretic effect on ambulatory outpatients with WHF; however, these recent analyses are in keeping with a meta‐analysis of older trials focusing on RAAS inhibitors showing that these latter drugs have a significant decongestion effect 45 and with likely benefit on those with congestive complications. 46
In the last 2 years, sodium–glucose cotransporter 2 (SGLT2) inhibitors have been shown to improve HF‐related outcomes. 47 These medications induce significant natriuresis and glucosuria, particularly when combined with loop diuretics (Figure 1 ), resulting in reduction in blood and plasma volume. Importantly, SGLT2 inhibitors have a proximal effect, leading to a shift of tubular fluid from the proximal to the distal segments of the nephron. This shift can increase natriuresis depending on the sodium‐retaining capacity of these distal segments (i.e. volume status, neurohumoral activation, use of diuretics). This natriuretic effect is not associated with any significant electrolyte wasting, renal dysfunction or neurohormonal activation. 48 , 49 Importantly, the efficacy, tolerability and safety profile of SGLT2 inhibitors is unaffected by concomitant treatment (or dosage) with a conventional diuretic. 50 Most patients in the DAPA‐HF trial did not undergo a change in diuretic dose during follow‐up, and the mean daily dose of diuretics did not differ between the dapagliflozin and placebo groups. 51 In line with these results, in a moderately‐sized trial study, Boorsma et al. 15 reported that empagliflozin at a daily dose of 10 mg increased plasma osmolarity without affecting fractional sodium, chloride excretion, or urinary osmolality. However, two studies conversely reported favourable decongestion effects with higher doses of empagliflozin. 48 , 52 Despite natriuresis being unchanged following empagliflozin initiation in the aforementioned studies, diuresis did increase in the study by Mordi et al. 52 whereas fractional sodium excretion increased in the Griffin et al. study. 48 Lastly, in keeping with the impact of neprilysin inhibition on pulmonary artery pressures, Nassif and colleagues similarly reported an average 12‐week treatment effect of −1.7 mmHg (p = 0.02) on mean pulmonary artery pressures among patients treated with empagliflozin versus placebo. 53 Trials testing use of SGLT2 inhibitors in patients with diuretic resistance are ongoing (DAPA‐RESIST, NCT04860011). In addition, SGLT2 inhibitors were reported recently to be favourably associated with better overall clinical outcome in the setting of WHF in the EMPULSE trial. 54 This positive result in acute HF, in which congestion is a key issue, parallels the results reported in the SOLOIST‐WHF trial, where the rate of the primary event in patients randomized to sotagliflozin was 30% lower than in the placebo group (HR 0.67; 95% confidence interval 0.52–0.85; p < 0.001). 55 The efficacy and safety of dapagliflozin in acute HF will further be evaluated in the ongoing DICTATE‐AHF trial. 56 Of note, SGLT2 inhibitors appear effective in patients with HFpEF: in the EMPEROR‐Preserved trial, an impressive 27% reduction in the total number of hospitalizations for HF was reported in the empagliflozin group. 57
Importantly, all of these observations are derived from protocolized clinical and biological monitoring allowing to titrate loop diuretic doses to patient status and no predicate data exist focusing on use of SGLT2 inhibitors specifically in patients with WHF, however their safety/efficacy among those with congestion appears promising.
Part II: Identification of patients eligible for intravenous diuretic therapy
The identification of patients with WHF who are more likely to benefit from outpatient treatment with IV diuretics remains insufficiently documented to date. We propose an approach to the use of IV diuretics in an ambulatory setting (Figure 3 ) along with factors to be considered to select eligible patients (Table 2 ). 58 , 59 , 60 , 61 , 62 , 63
Figure 3.
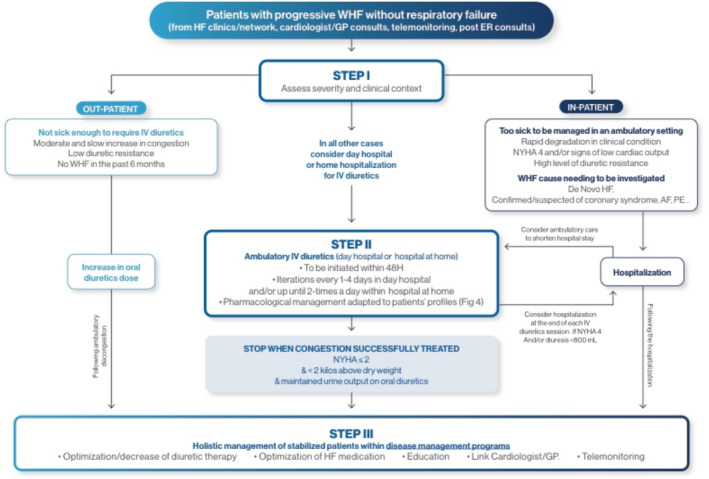
Framework for ambulatory intravenous (IV) diuretics use. AF, atrial fibrillation; ER, emergency room; GP, general practitioner; HF, heart failure; NYHA, New York Heart Association; PE, pulmonary embolism; WHF, worsening heart failure.
Table 2.
Factors to be considered to select patients to be treated with intravenous diuretics in an ambulatory setting
| In favour | Against | |
|---|---|---|
| Clinical scenarios |
Progressive worsening HF 58 , 59 HR: 50–120 bpm 61 SBP >100 mmHg 61 SaO2 >92% 61 Alert from remote HF monitoring 61 |
First episode of HF 60 Critical trigger 61 (rapid arrhythmias, acute coronary syndrome) NYHA class IV 61 |
| HF profiles | Cardiac amyloidosis 62 | Very high dose of oral diuretics (500 mg or more furosemide/day) |
| Comorbidities |
Frailty 63 Palliative care 63 (especially for hospital at home) |
Severely impaired eGFR (i.e. <25 ml/min/1.73 m2) 61 Severe dysnatraemia, dyskalaemia or anaemia 61 |
| Social criteria |
Patient preference 63 Adequate living support 63 |
Difficult/unsanitary living conditions 63 (for at home hospital) |
eGFR, estimated glomerular filtration rate; HF, heart failure; HR, heart rate; NYHA, New York Heart Association; SaO2, arterial oxygen saturation; SBP, systolic blood pressure.
Clinical situations unlikely to fit an ambulatory intravenous diuretic programme
We suggest that patients with severe de novo HF (i.e. patients with markedly reduced left ventricular ejection fraction [LVEF] and New York Heart Association [NYHA] class ≥III) should be managed in an in‐hospital setting. Indeed, these patients usually require further diagnostic and prognostic workup typically performed in hospital. 60
Secondly, patients presenting with signs of shock/low cardiac output, low oxygen saturation levels (i.e. peripheral oxygen saturation <92%) and/or symptoms at rest (NYHA class IV) should be also managed in hospital. The clinical suspect, or identification, of specific and potentially fatal triggers of clinical deterioration, for instance a rapid conduction of supraventricular arrhythmias, pulmonary embolism or acute coronary syndromes 14 , 58 should also prompt in‐hospital management.
Explosive/rapid worsening does not leave an adequate window for intervening in an outpatient setting. Nevertheless, this clinical scenario does not exclude early discharge with subsequent ambulatory IV diuretic management.
Clinical situations most likely to fit an ambulatory intravenous diuretic programme
Episodes of progressive WHF are very common, and represent the most frequent causes of a HF hospitalization (65% of cases in the EuroHeart Failure Survey II64). Refractory symptoms and signs of congestion despite high doses of oral loop diuretics are major concerns for patients with severe HF. Indeed, these patients represent the main target population of ambulatory treatment with IV diuretics, as they spend a substantial amount of time in hospital (‘frequent flyers’), which impairs their quality of life further and is associated with substantial costs. This particular phenotype corresponds to the population originally targeted by the Brigham and Women's Hospital group in their initial experience of ambulatory IV diuretics: most (80%) had mild–moderate symptoms, with a median maintenance diuretic dose of 240 mg oral furosemide. 58
Similarly, this approach has been proposed in patients with cardiac amyloidosis, 62 a population who also experiences multiple episodes of WHF and poor quality of life.
It is likely that these ambulatory IV diuretic programmes could be especially efficient in patients implanted with a CardioMEMS device, 65 as it allows an early detection of increased pulmonary pressure.
Importantly, a number of patients do not feel sufficiently ill to require in‐hospital admission. Yet their management based solely on oral diuretics is troublesome. There is thus a ‘grey zone’ of patients too ill to be managed with oral diuretics but not sufficiently ill to necessitate an emergency room visit. In these ‘grey‐zone’ scenarios, local availability of an ambulatory IV diuretics service can be widely accepted and effective to improve congestion before it reaches the threshold for in‐hospital admission.
In all instances, the clinical response to IV diuretic sessions should be carefully assessed, through a dedicated local disease management protocol, involving one or several of the following healthcare professionals: HF nurses, local nurses, general practitioners and treating cardiologists.
Part III: Diuretics in ambulatory settings – how to proceed?
The protocol published by Buckley et al. 58 (adapted in Figure 4 ) has been used in more than 250 patients 14 , 59 and sets a good foundation for implementation strategies. This protocol has also been used, with some local adjustments/amendments, 66 by our group on a regular basis in the last few years.
Figure 4.
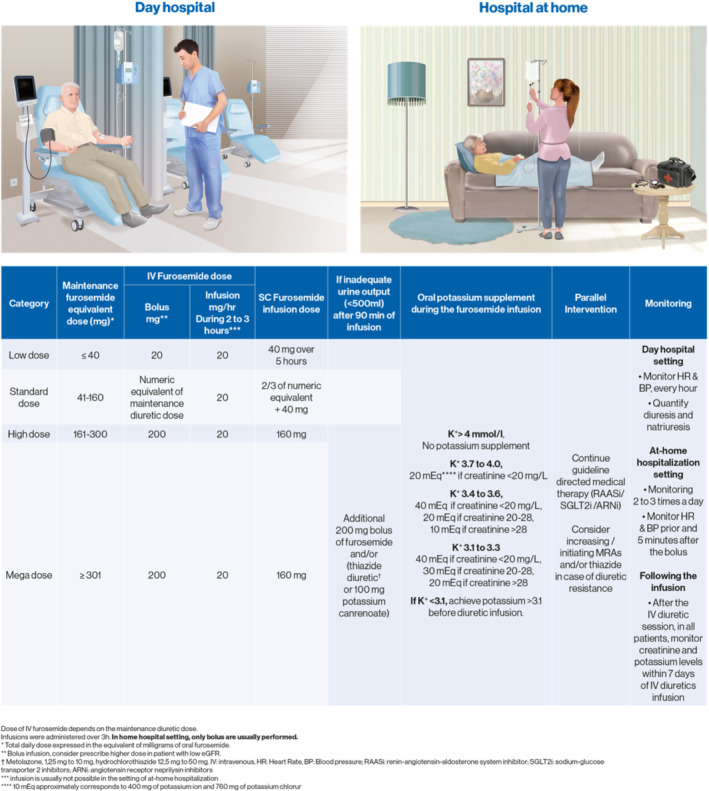
Ambulatory intravenous (IV) diuretics protocol according to maintenance loop diuretic dose. These doses are general guidelines that need to be adapted to renal function. Patients with estimated glomerular filtration rate <30 ml/min/1.73 m2 usually need higher (usually doubled) diuretic dose. ARNi, angiotensin receptor–neprilysin inhibitor; BP, blood pressure; HR, heart rate; MRA, mineralocorticoid receptor antagonist; RAASi, renin–angiotensin–aldosterone inhibitor; SGLT2i, sodium–glucose cotransporter 2 inhibitor.
Briefly, diuretic sessions consist in a 3‐h IV diuretic infusion, performed the same day or next‐day treatment. The pharmacological approach is contingent on the maintenance diuretic dose: patients with the highest oral diuretic maintenance dose would receive the highest IV diuretics (and possibly co‐diuretics such as thiazides). Generally, hydrochlorothiazide doses between 12.5 to 50 mg/session are used; metolazone 2.5 to 5 mg may be vey effective in those with estimated glomerular filtration rate <30 ml/min/1.73 m2. In such patients, IV metolazone or chlorothiazide may also be given; however the advantage of oral thiazide administration makes it preferred. If sequential nephron blockade is utilized with an oral thiazide, the oral medication is administered 30 min prior to the IV loop diuretic. Thiazide has occasionally been replaced by a high‐dose IV MRA by some members of our group in similar settings given the natriuretic and diuretic properties of MRAs, 67 including IV MRAs. 26 The main advantage of MRA in this setting is to offset the primum movens of diuretic resistance, i.e. the key involvement of RAAS – in sharp contrast with thiazide that further increases RAAS activation. Acetazolamide is another option to consider in multi‐site nephron blockade. 68 In the setting of ‘hospital at home’ (HaH), only IV boluses of loop diuretics are typically used, singly or divided to twice a day.
Importantly, the subcutaneous form of furosemide has been developed in recent years. 69 These subcutaneous injections are particularly useful in at‐home hospital settings as they are logistically demanding and do not require the hurdle/inconvenience of repeated venous puncture.
Careful consideration should be paid to dyskalaemia following the IV diuretic session. Briefly, with this protocol, only patients with prior IV diuretic potassium >4 mEq/L (regardless of renal function) or patients with K+ >3.7 if creatinine is 2.0 mg/dl (175 µmol/L) or above will not receive potassium supplements during the IV diuretic sessions (Figure 4 ). In our experience, these patients with low serum potassium should receive MRAs whenever possible. Indeed, patients receiving IV diuretic sessions are likely to have increased doses of oral diuretics in the following weeks, which could further decrease serum potassium levels.
Of importance, guideline‐recommended drugs should not be withheld during this IV diuretic period, unless symptomatic hypotension occurs. Indeed, as already emphasized above, RAAS is a key driver of sodium/water retention.
There are sparse data assessing the optimal number of diuretic sessions. Very early follow‐up overviewed by a physician should be undertaken to assess the clinical efficacy of the IV sessions (ideally within 48–72 h). Additional sessions should be performed if substantial congestion persists. Clinical deterioration or a decrease in diuretic efficacy should prompt in‐hospital admission in most instances. Our general threshold to discontinue iterative ambulatory IV diuretics is reported in Figure 3 , and includes a mixture of clinical response, objective evaluation, and laboratory testing (which can include natriuretic peptide quantification).
Part IV: How to organize healthcare in order to successfully perform ambulatory intravenous diuretics?
Hospital at home
‘Hospital at home’ or ‘home hospitalization’ may be used following in‐hospital care. This approach can favour the reduction in length of in‐hospital stay, as patients will be closely followed in this home setting following a short initial in‐hospital admission. During HaH, in addition to the use of IV diuretics, high‐risk behaviours limiting HF care efficacy can be identified (since home is the setting of care). 70 , 71
Not all patients are eligible for this HaH approach. Patient selection begins with a geriatric and social assessment, involving patient, caregiver(s), attending physician and, if applicable, nurses and social workers. The second step is to determine who will be responsible for the patient's treatment – either the patient's general practitioner or a cardiologist.
In our experience, the success of HaH following in‐hospital management of WHF relies on daily nursing assessment (weight, blood pressure, heart rate, oximetry and symptoms), home biology and electrocardiogram, remote access to online patient chart, secured remote prescription, close nursing duty, medical on‐call service and a process for urgent consultation or rehospitalization.
The efficacy of HaH for HF has been recently systematically reviewed by Qaddoura et al. 72 The conclusion of this meta‐analysis was that only a limited number (three trials totalizing 203 patients and three cohorts totalizing 329 patients) of modest‐quality studies were available, in which HaH appears to increase time to readmission, reduce index costs, and improve quality of life. 72 Notwithstanding the latter, larger trials such as the SAFE‐HOME (NCT03156686) and FIL‐EAS (NCT04878263) trials are needed to accumulate definitive evidence.
Day hospital
Much of the published clinical experience regarding ambulatory IV diuretics is currently derived from a day‐hospital setting. 58 This outpatient venue enables delivering 3‐h IV diuretic infusion, which is obviously not possible in a consultation setting and requires dedicated space and resources. A nurse would be devoted to the surveillance of the patient's vitals every hour, as well as verify diuresis and natriuresis, and perform blood tests if needed. In a day‐hospital setting, early low diuresis can be offset using thiazide or MRA during the second half of the infusion session, thanks to the close monitoring of the nurse.
At the end of the session, the patient's status is reassessed to determine whether the IV infusion was sufficiently effective for the patient to be discharged and plans are made for subsequent infusions. Typically, two–three infusions during the first week of treatment for WHF are needed to achieve decongestion and improve clinical status. In most severe cases, a continuous infusion of IV diuretics for 6 h may be required, which will be perceived much less negatively by the patient than regular hospitalizations requiring overnight stay. In the next days, an ambulatory short‐term telemonitoring is useful to verify the favourable impact of the diuretic assault. Repeated natriuretic peptide measurements can also be useful to monitor decongestion.
A day‐hospital setting has additional benefits. For instance, it can favour a multidisciplinary approach and, during the day‐hospital management, patients can be cared for by therapeutic education nurses, dieticians, and other healthcare professionals for additional counselling and re‐evaluation aiming at preventing further episodes of decongestion.
In order to be efficiently used, as in the case of home hospitalization, this facility should be easily accessible to eligible patients. Typically, day‐hospital sessions should be performed within 24 to 48 h of the identification of moderate WHF during a consultation at an HF clinic, within disease management programmes or home monitoring. To maximize efficacy, the coordinative link between the day hospital and these other facilities should be optimal, possibly included in the same parent organization/network. This integration is crucial since a close follow‐up is needed following the IV infusions to determine whether additional sessions are required. As for ‘classical’ WHF requiring ward hospitalizations, congestion episodes tend to recur. These relapses need to be quickly managed in additional day‐hospital sessions, within an organized healthcare network.
Part V: Management following ambulatory intravenous diuretic treatment
Background HFrEF treatment optimization
Given the significant decongestion effect of RAAS inhibitors 45 and rapid improvement in outcome after initiation of contemporary GDMT, every attempt should be made to optimize the non‐loop diuretic HF GDMT after the resolution of congestive episodes following ambulatory IV diuretic therapy. The up‐titration of drugs (initiation/switch to sacubitril/valsartan, as well as initiation of MRA and SGLT2 inhibitors) could be perceived as difficult to achieve in this context given that WRF is often observed during or following WHF. A practical approach to HF treatment management in the setting of WRF 18 and low blood pressure 37 has moreover been published. Importantly, in the month following the IV diuretic session, we recommend a weekly assessment by an HF physician or nurse practitioner in order to adjust the diuretic dose and optimize/up‐titrate the GDMT.
HFpEF and HFmrEF treatment optimization
Importantly, patients with HFpEF and HFrEF are similarly affected by WHF and diuretic strategies in ambulatory clinics appear to have similar benefit for those with HFpEF as it did for HFrEF. 58 Medical optimization is advisable for patients with HFpEF (LVEF ≥50%) or HF with mildly reduced ejection fraction (HFmrEF; LVEF 41%–49%) following decongestion. Initiation/up‐titration of MRAs 73 in this setting may be associated with better outcome and should be considered in patients with HFpEF. In the PARAGON‐HF study, use of sacubitril/valsartan for patients with LVEF ≥45% was associated with reduction of HF hospitalizations in patients with LVEF <55% and women. 74 In the EMPEROR‐Preserved trial, empagliflozin reduced HF hospitalizations by 27% (p < 0.001) in patients with LVEF >40%. 57
How to down‐titrate diuretics after a temporary increase in ambulatory patients?
Despite the importance of loop diuretics in HF management and related adverse events, little effort has focused on ‘optimizing’ diuretics. A number of tools could help optimize diuretic treatment, including natriuretic peptide monitoring, 75 pulmonary arterial pressure remote monitoring, 65 or serial ultrasound imaging of the inferior vena cava, but in routine practice only clinical assessment is used in the vast majority of cases. Importantly, the first step should be to establish whether the previous dose should be considered as the correct posology or whether a higher dose is required. As a general rule, if the previous dose did not prevent WHF, a higher dose should be maintained for at least 3–4 weeks. Reinforcing GDMT will eventually help the down‐titration of loop diuretics.
Part VI: Barriers to the implementation of these healthcare organizations
There are several existing barriers to overcome in the application of these new patient management pathways:
Limited clinical trial evidence (despite a larger use in observational studies 14 ) to document the efficacy or non‐inferiority of these alternative therapeutic approaches.
Further validation of the protocols for the use of diuretics and associated drugs: dose validation, IV access, periodicity, clinical and laboratory monitoring.
Formal endorsement by healthcare insurance providers: these pathways need to be funded (and possibly promoted) by the health insurance sector.
Formalized approach to medical responsibility. In an ambulatory setting, determining who is the physician in charge of the patient's management may be less straightforward than in an inpatient setting.
Formalized process 24/7 for ‘rescue’ management by emergency departments.
Health data transfer/exchange between the different healthcare providers.
The main limitation of the implementation of the approach proposed herein could be its actual integration within the patient's immediate healthcare environment. Ambulatory care may be impossible or impractical if the local health network is not sufficiently reliable or adapted to perform such management. The minimum requirements for the implementation of ambulatory IV management likely include: (i) clinicians available for clinical reassessment, treatment adjustments, and triage toward hospitalization or urgent visits in HF clinics, (ii) nurses available at home for blood sampling/tests, clinical assessment (weight, vital signs), (iii) social support (meals at home, for instance), and (iv) a direct link of these professionals with a dedicated HF team. All should be efficiently connected through a healthcare coordination framework, ideally available 24/7, but more realistically, operating during regular hours. Importantly, some of these issues could be alleviated by telemonitoring, e.g. for changes in weight or symptoms, and empowering patients and their careers. 76
Conclusion
The rising pressure on healthcare systems will preclude continuing treating all episodes of WHF with lengthy hospital admissions. Therefore, there is a mutual interest for both patients and healthcare providers to favour ambulatory therapeutic approaches that deliver IV diuretics to improve congestion and well‐being, and save costs and resources. Future trials will clarify safety and effectiveness of these models of care and identify patients more likely to gain benefits. It is likely that ambulatory delivery of IV diuretics will soon no longer be an option, but rather a new norm.
Acknowledgements
The authors acknowledge technical support from Novartis for illustrations. Novartis provided financial support for logistic management and was not involved in the preparation/drafting of the manuscript.
Funding
Profs Girerd and Rossignol are supported by a public grant overseen by the French National Research Agency (ANR) as part of the second “Investissements d'Avenir” programme (ANR‐15‐RHU‐0004).
Conflict of interest: N.G. received board fees from Novartis. N.M. declares consultant, honoraria, research, and travel grants from Novartis, Bayer and MSD. J.M.T. received personal fees from Novartis and AstraZeneca; non‐financial support from Novartis, AstraZeneca and Amgen and was principal investigator in trials involving Novartis, Amgen, Servier, Bayer and Merck Sharp Dohme. T.D. received grants and personal fees from Novartis, Vifor, RESMED, Pfizer, Alnylam, Ionsis, Akcea, GSK and personal fees from Prothena. N.L. received personal fees from Novartis, AstraZeneca, Bayer, Boehringer Ingelheim. A.B.G. reports personal fees from Abbott, Novartis, Vifor, Roche Diagnostics, Critical Diagnostics and AstraZeneca, grants, personal fees and non‐financial support from Boehringer Ingelheim, outside the submitted work. P.P. has received travel support from Boehringer Ingelheim and consultation fees from Novartis. J.J. is a trustee of the American College of Cardiology; is a board member of Imbria Pharmaceuticals; has received grant support from Applied Therapeutics, Innolife, Novartis Pharmaceuticals, and Abbott Diagnostics; has received consulting income from Abbott, Janssen, Novartis, and Roche Diagnostics; and has participated in clinical endpoint committees/data safety monitoring boards for Abbott, AbbVie, Amgen, Bayer, CVRx, Janssen, MyoKardia, and Takeda. P.R. reports grants and personal fees from AstraZeneca, Bayer, CVRx, Fresenius, and Novartis, personal fees from Grunenthal, Servier, Stealth Peptides, Vifor Fresenius Medical Care Renal Pharma, Idorsia, NovoNordisk, Ablative Solutions, G3P, Corvidia, Relypsa, and is the cofounder of CardioRenal, a company developing a telemonitoring loop in heart failure (including potassium measurements). F.R. reports grants, personal fees and non‐financial support from Novartis, Servier, Abbott, MSD, Sanofi, Bayer, BMS‐Pfizer, Air Liquide, Abiomed, Resmed and Medtronic. All other authors have nothing to disclose.
References
- 1. Zakeri R, Cowie MR. Heart failure with preserved ejection fraction: controversies, challenges and future directions. Heart. 2018;104:377–84. [DOI] [PubMed] [Google Scholar]
- 2. van Riet EE, Hoes AW, Wagenaar KP, Limburg A, Landman MA, Rutten FH. Epidemiology of heart failure: the prevalence of heart failure and ventricular dysfunction in older adults over time. A systematic review. Eur J Heart Fail. 2016;18:242–52. [DOI] [PubMed] [Google Scholar]
- 3. Groenewegen A, Rutten FH, Mosterd A, Hoes AW. Epidemiology of heart failure. Eur J Heart Fail. 2020;22:1342–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Braunwald E. Heart failure. JACC Heart Fail. 2013;1:1–20. [DOI] [PubMed] [Google Scholar]
- 5. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 6. Harjola VP, Mullens W, Banaszewski M, Bauersachs J, Brunner‐La Rocca HP, Chioncel O, et al. Organ dysfunction, injury and failure in acute heart failure: from pathophysiology to diagnosis and management. A review on behalf of the Acute Heart Failure Committee of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur J Heart Fail. 2017;19:821–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ware LB, Matthay MA. Clinical practice. Acute pulmonary edema. N Engl J Med. 2005;353:2788–96. [DOI] [PubMed] [Google Scholar]
- 8. Gheorghiade M, Follath F, Ponikowski P, Barsuk JH, Blair JE, Cleland JG, et al.; European Society of Cardiology , European Society of Intensive Care Medicine . Assessing and grading congestion in acute heart failure: a scientific statement from the Acute Heart Failure Committee of the Heart Failure Association of the European Society of Cardiology and endorsed by the European Society of Intensive Care Medicine. Eur J Heart Fail. 2010;12:423–33. [DOI] [PubMed] [Google Scholar]
- 9. Van Aelst LNL, Arrigo M, Placido R, Akiyama E, Girerd N, Zannad F, et al. Acutely decompensated heart failure with preserved and reduced ejection fraction present with comparable haemodynamic congestion. Eur J Heart Fail. 2018;20:738–47. [DOI] [PubMed] [Google Scholar]
- 10. Girerd N, Seronde MF, Coiro S, Chouihed T, Bilbault P, Braun F, et al.; INI‐CRCT, Great Network, and the EF‐HF Group . Integrative assessment of congestion in heart failure throughout the patient journey. JACC Heart Fail. 2018;6:273–85. [DOI] [PubMed] [Google Scholar]
- 11. Maggioni AP, Dahlstrom U, Filippatos G, Chioncel O, Crespo Leiro M, Drozdz J, et al. EURObservational research Programme: regional differences and 1‐year follow‐up results of the Heart Failure Pilot survey (ESC‐HF pilot). Eur J Heart Fail. 2013;15:808–17. [DOI] [PubMed] [Google Scholar]
- 12. Ambrosy AP, Khan H, Udelson JE, Mentz RJ, Chioncel O, Greene SJ, et al. Changes in dyspnea status during hospitalization and postdischarge health‐related quality of life in patients hospitalized for heart failure: findings from the EVEREST trial. Circ Heart Fail. 2016;9:e002458. [DOI] [PubMed] [Google Scholar]
- 13. Parekh AK, Goodman RA, Gordon C, Koh HK; HHS Interagency Workgroup on Multiple Chronic Conditions. Managing multiple chronic conditions: a strategic framework for improving health outcomes and quality of life. Public Health Rep. 2011;126:460–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wierda E, Dickhoff C, Handoko ML, Oosterom L, Kok WE, Rover Y, et al. Outpatient treatment of worsening heart failure with intravenous and subcutaneous diuretics: a systematic review of the literature. ESC Heart Fail. 2020;7:892–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boorsma EM, Ter Maaten JM, Damman K, Dinh W, Gustafsson F, Goldsmith S, et al. Congestion in heart failure: a contemporary look at physiology, diagnosis and treatment. Nat Rev Cardiol. 2020;17:641–55. [DOI] [PubMed] [Google Scholar]
- 16. Mullens W, Damman K, Harjola VP, Mebazaa A, Brunner‐La Rocca HP, Martens P, et al. The use of diuretics in heart failure with congestion – a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2019;21:137–55. [DOI] [PubMed] [Google Scholar]
- 17. Felker GM, Ellison DH, Mullens W, Cox ZL, Testani JM. Diuretic therapy for patients with heart failure: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2020;75:1178–95. [DOI] [PubMed] [Google Scholar]
- 18. Mewton N, Girerd N, Boffa JJ, Courivaud C, Isnard R, Juillard L, et al. Practical management of worsening renal function in outpatients with heart failure and reduced ejection fraction: statement from a panel of multidisciplinary experts and the Heart Failure Working Group of the French Society of Cardiology. Arch Cardiovasc Dis. 2020;113:660–70. [DOI] [PubMed] [Google Scholar]
- 19. Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, et al.; NHLBI Heart Failure Clinical Research Network . Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364:797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brater DC. Pharmacokinetics of loop diuretics in congestive heart failure. Br Heart J. 1994;72(2 Suppl):S40–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eisenstein EL, Sapp S, Harding T, Harrington A, Velazquez EJ, Mentz RJ, et al. Ascertaining death events in a pragmatic clinical trial: insights from the TRANSFORM‐HF trial. J Card Fail. 2022. doi: 10.1016/j.cardfail.2022.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sherif NA, Morra ME, Thanh LV, Elsayed GG, Elkady AH, Elshafay A, et al. Torasemide versus furosemide in treatment of heart failure: a systematic review and meta‐analysis of randomized controlled trials. J Eval Clin Pract. 2020;26:842–51. [DOI] [PubMed] [Google Scholar]
- 23. Agarwal R, Sinha AD. Thiazide diuretics in advanced chronic kidney disease. J Am Soc Hypertens. 2012;6:299–308. [DOI] [PubMed] [Google Scholar]
- 24. Ferreira JP, Santos M, Almeida S, Marques I, Bettencourt P, Carvalho H. Mineralocorticoid receptor antagonism in acutely decompensated chronic heart failure. Eur J Intern Med. 2014;25:67–72. [DOI] [PubMed] [Google Scholar]
- 25. Verbrugge FH, Martens P, Ameloot K, Haemels V, Penders J, Dupont M, et al. Spironolactone to increase natriuresis in congestive heart failure with cardiorenal syndrome. Acta Cardiol. 2019;74:100–7. [DOI] [PubMed] [Google Scholar]
- 26. Girerd N, Aubry M, Lantelme P, Huttin O, Rossignol P. Intravenous mineralocorticoid receptor antagonist use in acutely decompensated heart failure with diuretic resistance. Int Heart J. 2021;62:193–6. [DOI] [PubMed] [Google Scholar]
- 27. Butler J, Anstrom KJ, Felker GM, Givertz MM, Kalogeropoulos AP, Konstam MA, et al.; National Heart Lung and Blood Institute Heart Failure Clinical Research Network . Efficacy and safety of spironolactone in acute heart failure: the ATHENA‐HF randomized clinical trial. JAMA Cardiol. 2017;2:950–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Testani JM, Brisco MA, Kociol RD, Jacoby D, Bellumkonda L, Parikh CR, et al. Substantial discrepancy between fluid and weight loss during acute decompensated heart failure treatment. Am J Med. 2015;128:776–83.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Biegus J, Zymlinski R, Testani J, Marciniak D, Zdanowicz A, Jankowska EA, et al. Renal profiling based on estimated glomerular filtration rate and spot urine sodium identifies high‐risk acute heart failure patients. Eur J Heart Fail. 2021;23:729–39. [DOI] [PubMed] [Google Scholar]
- 30. Pellicori P, Platz E, Dauw J, Maaten JM, Martens P, Pivetta E, et al. Ultrasound imaging of congestion in heart failure: examinations beyond the heart. Eur J Heart Fail. 2021;23:703–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mielniczuk LM, Tsang SW, Desai AS, Nohria A, Lewis EF, Fang JC, et al. The association between high‐dose diuretics and clinical stability in ambulatory chronic heart failure patients. J Card Fail. 2008;14:388–93. [DOI] [PubMed] [Google Scholar]
- 32. Neuberg GW, Miller AB, O'Connor CM, Belkin RN, Carson PE, Cropp AB, et al.; PRAISE Investigators. Prospective Randomized Amlodipine Survival Evaluation . Diuretic resistance predicts mortality in patients with advanced heart failure. Am Heart J. 2002;144:31–8. [DOI] [PubMed] [Google Scholar]
- 33. Domanski M, Norman J, Pitt B, Haigney M, Hanlon S, Peyster E. Diuretic use, progressive heart failure, and death in patients in the Studies of Left Ventricular Dysfunction (SOLVD). J Am Coll Cardiol. 2003;42:705–8. [DOI] [PubMed] [Google Scholar]
- 34. Madelaire C, Gustafsson F, Stevenson LW, Kristensen SL, Køber L, Andersen J, et al. One‐year mortality after intensification of outpatient diuretic therapy. J Am Heart Assoc. 2020;9:e016010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pellicori P, Cleland JG, Zhang J, Kallvikbacka‐Bennett A, Urbinati A, Shah P, et al. Cardiac dysfunction, congestion and loop diuretics: their relationship to prognosis in heart failure. Cardiovasc Drugs Ther. 2016;30:599–609. [DOI] [PubMed] [Google Scholar]
- 36. Rossignol P, Hernandez AF, Solomon SD, Zannad F. Heart failure drug treatment. Lancet. 2019;393:1034–44. [DOI] [PubMed] [Google Scholar]
- 37. Cautela J, Tartiere JM, Cohen‐Solal A, Bellemain‐Appaix A, Theron A, Tibi T, et al. Management of low blood pressure in ambulatory heart failure with reduced ejection fraction patients. Eur J Heart Fail. 2020;22:1357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ferreira JP, Eschalier R, Duarte K, Damman K, Gustafsson F, Schou M, et al. Reduced diuretic dose in patients treated with eplerenone: data from the EPHESUS trial. Circ Heart Fail. 2020;13:e006597. [DOI] [PubMed] [Google Scholar]
- 39. Vardeny O, Claggett B, Kachadourian J, Desai AS, Packer M, Rouleau J, et al. Reduced loop diuretic use in patients taking sacubitril/valsartan compared with enalapril: the PARADIGM‐HF trial. Eur J Heart Fail. 2019;21:337–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Packer M, McMurray JJ, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al.; PARADIGM‐HF Investigators and Coordinators . Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation. 2015;131:54–61. [DOI] [PubMed] [Google Scholar]
- 41. Desai AS, Claggett BL, Packer M, Zile MR, Rouleau JL, Swedberg K, et al.; PARADIGM‐HF Investigators . Influence of sacubitril/valsartan (LCZ696) on 30‐day readmission after heart failure hospitalization. J Am Coll Cardiol. 2016;68:241–8. [DOI] [PubMed] [Google Scholar]
- 42. Wang TD, Tan RS, Lee HY, Ihm SH, Rhee MY, Tomlinson B, et al. Effects of sacubitril/valsartan (LCZ696) on natriuresis, diuresis, blood pressures, and NT‐proBNP in salt‐sensitive hypertension. Hypertension. 2017;69:32–41. [DOI] [PubMed] [Google Scholar]
- 43. Velazquez EJ, Morrow DA, DeVore AD, Duffy CI, Ambrosy AP, McCague K, et al. PIONEER‐HF Investigators. Angiotensin‐neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2019;380:539–48. [DOI] [PubMed] [Google Scholar]
- 44. Desai AS, Heywood JT, Rathman L, Abraham WT, Adamson P, Brett ME, et al. Early reduction in ambulatory pulmonary artery pressures after initiation of sacubitril/valsartan. Circ Heart Fail. 2021;14:e008212. [DOI] [PubMed] [Google Scholar]
- 45. Jobs A, Abdin A, de Waha‐Thiele S, Eitel I, Thiele H, de Wit C, et al. Angiotensin‐converting‐enzyme inhibitors in hemodynamic congestion: a meta‐analysis of early studies. Clin Res Cardiol. 2019;108:1240–8. [DOI] [PubMed] [Google Scholar]
- 46. Januzzi JL Jr, Prescott MF, Butler J, Felker GM, Maisel AS, McCague K, et al.; PROVE‐HF Investigators . Association of change in N‐terminal pro‐B‐type natriuretic peptide following initiation of sacubitril‐valsartan treatment with cardiac structure and function in patients with heart failure with reduced ejection fraction. JAMA. 2019;322:1085–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zannad F, Ferreira JP, Pocock SJ, Anker SD, Butler J, Filippatos G, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta‐analysis of the EMPEROR‐Reduced and DAPA‐HF trials. Lancet. 2020;396:819–29. [DOI] [PubMed] [Google Scholar]
- 48. Griffin M, Rao VS, Ivey‐Miranda J, Fleming J, Mahoney D, Maulion C, et al. Empagliflozin in heart failure: diuretic and cardiorenal effects. Circulation. 2020;142:1028–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Berg DD, Jhund PS, Docherty KF, Murphy SA, Verma S, Inzucchi SE, et al. Time to clinical benefit of dapagliflozin and significance of prior heart failure hospitalization in patients with heart failure with reduced ejection fraction. JAMA Cardiol. 2021;6:499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, et al.; DAPA‐HF Trial Committees and Investigators. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 51. Jackson AM, Dewan P, Anand IS, Bělohlávek J, Bengtsson O, de Boer RA, et al. Dapagliflozin and diuretic use in patients with heart failure and reduced ejection fraction in DAPA‐HF. Circulation. 2020;142:1040–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mordi NA, Mordi IR, Singh JS, McCrimmon RJ, Struthers AD, Lang CC. Renal and cardiovascular effects of SGLT2 inhibition in combination with loop diuretics in patients with type 2 diabetes and chronic heart failure: the RECEDE‐CHF trial. Circulation. 2020;142:1713–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nassif ME, Qintar M, Windsor SL, Jermyn R, Shavelle DM, Tang F, et al. Empagliflozin effects on pulmonary artery pressure in patients with heart failure: results from the EMBRACE‐HF trial. Circulation. 2021;143:1673–86. [DOI] [PubMed] [Google Scholar]
- 54. Voors AA, Angermann CE, Teerlink JR, Collins SP, Kosiborod M, Biegus J, et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multinational randomized trial. Nat Med. 2022;28:568–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire D, et al.; SOLOIST‐WHF Trial Investigators . Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2021;384:117–28. [DOI] [PubMed] [Google Scholar]
- 56. Cox ZL, Collins SP, Aaron M, Hernandez GA, McRae AT 3rd, Davidson BT, et al. Efficacy and safety of dapagliflozin in acute heart failure: rationale and design of the DICTATE‐AHF trial. Am Heart J. 2021;232:116–24. [DOI] [PubMed] [Google Scholar]
- 57. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, et al.; EMPEROR‐Preserved Trial Investigators. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451–61. [DOI] [PubMed] [Google Scholar]
- 58. Buckley LF, Carter DM, Matta L, Cheng JW, Stevens C, Belenkiy RM, et al. Intravenous diuretic therapy for the management of heart failure and volume overload in a multidisciplinary outpatient unit. JACC Heart Fail. 2016;4: 1–8. [DOI] [PubMed] [Google Scholar]
- 59. Buckley LF, Stevenson LW, Cooper IM, Knowles DM, Matta L, Molway DW, et al. Ambulatory treatment of worsening heart failure with intravenous loop diuretics: a four‐year experience. J Card Fail. 2020;26:798–9. [DOI] [PubMed] [Google Scholar]
- 60. Greene SJ, Mentz RJ, Felker GM. Outpatient worsening heart failure as a target for therapy: a review. JAMA Cardiol. 2018;3:252–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Verma V, Zhang M, Bell M, Tarolli K, Donalson E, Vaughn J, et al. Outpatient intravenous diuretic clinic: an effective strategy for management of volume overload and reducing immediate hospital admissions. J Clin Med Res. 2021;13:245–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Vaishnav J, Hubbard A, Chasler JE, Lepley D, Cuomo K, Riley S, et al. Management of heart failure in cardiac amyloidosis using an ambulatory diuresis clinic. Am Heart J. 2021;233:122–31. [DOI] [PubMed] [Google Scholar]
- 63. Ahmed FZ, Taylor JK, John AV, Khan MA, Zaidi AM, Mamas MA, et al. Ambulatory intravenous furosemide for decompensated heart failure: safe, feasible, and effective. ESC Heart Fail. 2021;8:3906–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Nieminen MS, Brutsaert D, Dickstein K, Drexler H, Follath F, Harjola VP, et al.; EuroHeart Survey Investigators , Heart Failure Association, European Society of Cardiology . EuroHeart Failure Survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J. 2006;27:2725–36. [DOI] [PubMed] [Google Scholar]
- 65. Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, et al.; CHAMPION Trial Study Group . Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377:658–66. [DOI] [PubMed] [Google Scholar]
- 66. Pacho C, Domingo M, Nunez R, Lupón J, Moliner P, de Antonio M, et al. Early postdischarge STOP‐HF‐Clinic reduces 30‐day readmissions in old and frail patients with heart failure. Rev Esp Cardiol (Engl Ed). 2017;70:631–8. [DOI] [PubMed] [Google Scholar]
- 67. Ferreira JP, Girerd N, Bettencourt Medeiros P, Bento Ricardo M, Almeida T, Rola A, et al. Lack of diuretic efficiency (but not low diuresis) early in an acutely decompensated heart failure episode is associated with increased 180‐day mortality. Cardiorenal Med. 2017;7:137–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Nunez J, Heredia R, Paya A, Sanchis I, Del Prado S, Miñana G, et al. Use of acetazolamide in the treatment of patients with refractory congestive heart failure. Cardiovasc Ther. 2018;36:e12465. [DOI] [PubMed] [Google Scholar]
- 69. Sica DA, Muntendam P, Myers RL, ter Maaten JM, Sale ME, de Boer RA, et al. Subcutaneous furosemide in heart failure: pharmacokinetic characteristics of a newly buffered solution. JACC Basic Transl Sci. 2018;3:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Mendoza H, Martin MJ, Garcia A, Aros F, Aizpuru F, Regalado De Los Cobos J, et al. ‘Hospital at home’ care model as an effective alternative in the management of decompensated chronic heart failure. Eur J Heart Fail. 2009;11:1208–13. [DOI] [PubMed] [Google Scholar]
- 71. Van Spall HGC, Rahman T, Mytton O, Ramasundarahettige C, Ibrahim Q, Kabali C, et al. Comparative effectiveness of transitional care services in patients discharged from the hospital with heart failure: a systematic review and network meta‐analysis. Eur J Heart Fail. 2017;19:1427–43. [DOI] [PubMed] [Google Scholar]
- 72. Qaddoura A, Yazdan‐Ashoori P, Kabali C, Thabane L, Haynes RB, Connolly SJ, et al. Efficacy of hospital at home in patients with heart failure: a systematic review and meta‐analysis. PLoS One. 2015;10:e0129282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Girerd N, Ferreira JP, Rossignol P, Zannad F. A tentative interpretation of the TOPCAT trial based on randomized evidence from the brain natriuretic peptide stratum analysis. Eur J Heart Fail. 2016;18:1411–4. [DOI] [PubMed] [Google Scholar]
- 74. Vaduganathan M, Cunningham JW, Claggett BL, Causland FM, Barkoudah E, Finn P, et al. Worsening heart failure episodes outside a hospital setting in heart failure with preserved ejection fraction: the PARAGON‐HF trial. JACC Heart Fail. 2021;9:374–82. [DOI] [PubMed] [Google Scholar]
- 75. Pruett AE, Lee AK, Patterson JH, Schwartz TA, Glotzer JM, Adams KF Jr. Evolution of biomarker guided therapy for heart failure: current concepts and trial evidence. Curr Cardiol Rev. 2015;11:80–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Cleland JGF, Clark RA, Pellicori P, Inglis SC. Caring for people with heart failure and many other medical problems through and beyond the COVID‐19 pandemic: the advantages of universal access to home telemonitoring. Eur J Heart Fail. 2020;22:995–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


