Abstract
Background
Left atrial posterior wall isolation (LAPWI) is often performed in addition to pulmonary vein isolation (PVI) in the setting of persistent atrial fibrillation (AF) ablation.
The aim of this study was to evaluate the feasibility and safety of a new cryoballoon ablation system in achieving PVI + LAPWI isolation.
Methods
The study was a prospective, non‐randomized, single center study. Forty consecutive patients, undergoing PVI + LAPWI with the novel POLARx™, were compared to 40 consecutive patients who underwent the same procedure with the established Arctic Front Advance PRO™.
Results
Acute isolation was achieved in all PVs in both groups and left posterior wall isolation (LAPWI) was achieved in 38 patients (95%) in the POLARx group and in 36 patients (90%) in Arctic Front group. Procedural outcomes were similar between both groups, except for lower temperatures during cryoenergy in the POLARx group, for both pulmonary vein isolation (PVI) and LAPWI.
Conclusion
LAPWI + PVI with the novel POLARx™ Cryoballoon is feasible and safe; the results are comparable with the Arctic Front Advance PRO™ system.
Keywords: cryoballoon, left atrial posterior wall ablation, persistent atrial fibrillation, POLARx, pulmonary vein isolation
1. INTRODUCTION
Pulmonary vein isolation (PVI) is the cornerstone of atrial fibrillation (AF) ablation. 1 , 2 Left atrial posterior wall isolation (LAPWI) in addition to PVI by the means of the Arctic Front (Medtronic, Arctic Front Advance) cryoballoon (CB‐A) has shown promising results in patients presenting with persistent (PersAF). 3 , 4 , 5 , 6 , 7 , 8
Recently, a novel Cryoballoon ablation system (POLARx™; Boston Scientific) has been launched on the market. 9
Although there are initial safety and efficacy data for PVI, 10 , 11 , 12 only one case report on LAPWI with this new technology is reported till now. 13
The aim of the study is to evaluate the feasibility and safety of the new POLARx™ Cryoballoon ablation system for PVI + LAPWI, comparing it with the Medtronic Arctic Front Advance™ balloon platform.
2. METHODS
2.1. Study population
The study was a prospective, non‐randomized, single center study. All consecutive patients undergoing PVI + LAPWI, between November 2020 and September 2021, with the novel POLARx™ were prospectively enrolled. They were compared with all consecutive patients who underwent PVI + LAPWI with the established CB‐A platform (Arctic Front Advance PRO™; Medtronic, minneapolis, USA) in the same period. The same operators with comparable experience performed the ablation procedures with both systems in equal proportions.
Patients with symptomatic persistent AF, undergoing first AF ablation procedure, were included in the study. The exclusion criteria were the following: previous AF ablation or cardiac surgery, paroxysmal AF, congenital heart disease, non‐treated coronary artery disease, intracavitary thrombus, significant valvular disease, contraindications to general anesthesia.
The study protocol was carried out in accordance with the ethical principles established by the Declaration of Helsinki and was approved by the local ethics committee of our institution. All patients provided written informed consent.
2.2. Procedure
All patients underwent PVI + LAPWI with the POLARx™ or with the Arctic Front Advance PRO™ CB‐A system.
The POLARx™ platform includes novel tools such as: (1) a dedicated console with pedal control, allowing full and autonomous control by the operator; (2) a 28 mm balloon catheter, designed to maintain the same volume during application of cryoenergy; and (3) a dedicated inner lumen mapping catheter (POLARMap, Boston Scientific).
Our standard pre‐procedural management and CB‐A ablation has been previously described in detail. 5 , 14 All procedures were performed under general anesthesia and under esophageal temperature monitoring. Cryoenergy applications were interrupted in case of luminal esophageal temperatures (LET) < 15°C. After having obtained LA access, through a steerable sheath (FlexCath Advance Medtronic Inc. 15Fr; POLARSHEATH™ Boston Scientific 15.5 Fr), a 28 mm CB‐A catheter (Arctic Front Advance PRO, Medtronic Inc.; POLARx Balloon Catheter ST 28 mm) was advanced in the LA for PVI and an inner lumen mapping catheter (Achieve, Medtronic Inc.; POLARMap Boston Scientific) was positioned in each PV ostium. The CB‐A was advanced, inflated, and positioned at each PV ostium. Optimal vessel occlusion was defined by selective contrast injection. Once vessel occlusion was deemed satisfactory, delivery of cryoenergy to allow freezing was commenced. Standard cryothermal applications lasted 180 s. Our target temperature was −40°C within the first 60 s. If the temperature did not attain this value, an extra freeze was delivered. The ablation sequence was: left superior PV (LSPV), left inferior PV (LIPV), right inferior PV (RIPV), and right superior PV (RSPV). In order to avoid phrenic nerve palsy (PNP), diaphragmatic stimulation was achieved by pacing the phrenic nerve during septal PVs ablation.
In order to achieve the LAPWI, the lumen mapping catheter was placed deeply in the LSPV to stabilize the CB‐A. As per our standard protocol, cryothermal lesions lasted 120 s. 5 , 6 , 7 , 8 , 14 The first cryoapplication was performed partially overlapping LSPV ostium. By a slight clockwise rotation and progressive “pullback” of the sheath while keeping the CB in contact with the posterior wall, consecutive overlapping freezes were applied along the LAPW. The same maneuver was performed from the right superior pulmonary vein (RSPV) and from inferior PVs for the inferior portion of LAPW. At the end of the procedure, in order to evaluate LAPWI pacing maneuvers to test entrance and exit block were performed. A post ablation 3D electroanatomical map (CARTO 3, Biosense Webster, USA) was performed with a multielectrode mapping catheter (Pentaray, Biosense Webster, USA). Whenever LAPWI could not be achieved using cryoablation alone, point‐by‐point radiofrequency ablation (SmarTouch, Biosense Webster, USA) was used. Radiofrequency was delivered in a power‐controlled mode with a power limit of 30 W and with an ablation index target of 400. In case of LET ≥ 40°C the ablation was interrupted.
During the entire procedure, activated clotting time was maintained over 300 s by supplementing heparin infusion as required.
2.3. Statistical analysis
All variables were tested for normality with Shapiro–Wilk test. Normally distributed variables were described as mean ± standard deviation and the groups were compared through paired or unpaired t‐test as appropriate, while the non‐normally distributed variables were described as median (Inter Quartile Range) and compared by Mann–Whitney test or Wilcoxon signed‐rank test as appropriate. Categorical variables are presented as absolute numbers and percentages. For comparison of categorial data between groups, Pearson χ 2 or Fisher exact test was used. All tests were two‐sided, and a P value of .05 was considered statistically significant. Since no equal previous studies were performed and no sample size calculations were performed. Analyses were performed with SPSS 23.0 statistical software (IBM Company, Chicago, IL, USA). Box plots were drawn with R software version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria).
3. RESULTS
Eighty patients were included in the study, the first 40 patients underwent an index PVI + LAPWI with the Arctic Front Advance PRO™ ablation system and the following 40 patients with the POLARx™. Baseline demographics and clinical data are displayed in Table 1. There was no significant difference between both cohorts, except for dyslipidemia (p =.04). Acute PVI was achieved in all veins in both groups. The mean number of CB‐A applications required for PVI was 5.0 ± 1.3 in the POLARx group and 5.2 ± 1.5 in the Arctic Front group (p =.5). During PVI, the POLARx system reached lower minimum temperature [−59.9 ± 4.02°C in POLARx vs. 49.2 ± 5.2°C in Arctic Front, p = <.001] and lower temperature at time‐to‐isolation [−38.4 ± 11.86°C in POLARx vs. −32.6 ± 4.98°C in Arctic Front, p =.01], (Figure 1). There were no differences in the LET recorded during cryoablation. During PVI, interruption of the application due to LET below 15°C occurred in four patients (10%) in the POLARx group and in two patients (5%) in the Arctic Front group, (p =.34). The complete biophysical ablation parameters for PVI are reported in Table 2.
TABLE 1.
Baseline characteristics
| POLARx (n = 40) | Arctic front (n = 40) | p | |
|---|---|---|---|
| Age (years) | 66.6 ± 12.6 | 62.8 ± 11.9 | .16 |
| Gender (male), n (%) | 22 (55) | 26 (65) | .36 |
| Hypertension, n (%) | 31 (77.5) | 28 (70) | .45 |
| Diabetes, n (%) | 12 (30) | 6 (15) | .11 |
| Dyslipidemia, n (%) | 30 (75) | 21 (52.5) | .04 |
| Ischemic heart disease, n (%) | 8 (20) | 5 (12.5) | .4 |
| CHA2DS2VASc score | 2.6 ± 1.4 | 2.2 ± 1.5 | .23 |
| LVEF (%) | 52.5 ± 6.7 | 55 ± 7.5 | .13 |
| LA Volume (ml/m2) | 48.1 ± 9.7 | 49 ± 8.3 | .69 |
| Antiarrhythmic medications | |||
| Class IC, n (%) | 10 (25) | 6 (15) | .26 |
| Class II, n (%) | 17 (52) | 18 (45) | .49 |
| Class III, n (%) | 12 (31) | 14 (35) | .69 |
| Class IV, n (%) | 1 (2.5) | 3 (7.7) | .82 |
Abbreviations: LA, left atrial; LVEF, left ventricular ejection fraction.
FIGURE 1.
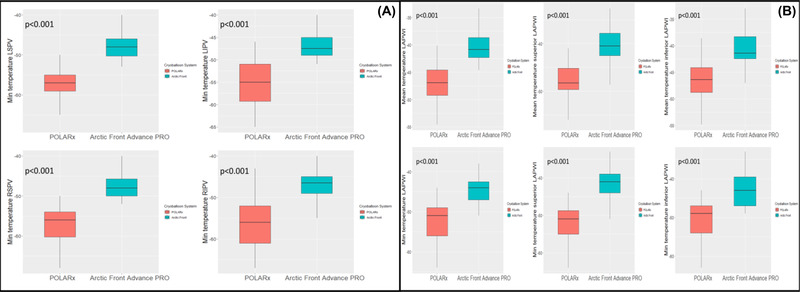
Temperature box plot for the veins (panel A) and for the left atrial posterior wall (panel B) [Colour figure can be viewed at wileyonlinelibrary.com]
TABLE 2.
Procedural characteristics – PVI
| POLARx (n = 40) | Arctic front (n = 40) | p | |
|---|---|---|---|
| Applications to achieve PVI | 5.0 ± 1.3 | 5.2 ± 1.5 | .5 |
| LSPV | 1.3 ± 0.5 | 1.2± 0.5 | .6 |
| LIPV | 1.2 ± 0.6 | 1.3 ± 0.5 | .5 |
| RSPV | 1.3 ± 0.7 | 1.4 ± 0.5 | .5 |
| RIPV | 1.3 ± 0.4 | 1.2 ± 0.5 | .6 |
| Min temperature (°C) | –59.9 ± 4.02 | –49.2 ± 5.2 | < .001 |
| LSPV | –57.3 ± 4.6 | –48.88 ± 5.2 | < .001 |
| LIPV | –55.3 ± 4.9 | –47.9 ± 5 | < .001 |
| RSPV | –56.9 ± 4.7 | –48.5 ± 4.8 | < .001 |
| RIPV | –55.6 ± 5.9 | –47.3 ± 4.3 | < .001 |
| Min LET (°C) | 31.7 ± 1.9 | 32.7 ± 3 | .10 |
| LSPV | 33.7 ± 3.4 | 32.9 ± 4.2 | .43 |
| LIPV | 30.4 ± 5.02 | 31.9 ± 5.6 | .25 |
| RSPV | 32.6 ± 4.6 | 31.1 ± 4.5 | .78 |
| RIPV | 31.1 ± 4.5 | 32.3 ± 5.9 | .38 |
| Duration single application (s) | 191.6 ± 14.5 | 189.7 ± 13.1 | .57 |
| Time to isolation PV (s) | 37.7 ± 9 | 34.1 ± 11.7 | .17 |
| LSPV | 40 ± 16.3 | 35.4 ± 14.3 | .27 |
| LIPV | 34 ± 17.4 | 29.6 ± 11.4 | .33 |
| RSPV | 39 ± 16.7 | 33.75 ± 15.4 | .37 |
| RIPV | 41 ± 15.9 | 38.1 ± 20.2 | .66 |
| Isolation temperature (°C) | –38.4 ± 11.86 | –32.6 ± 4.98 | .01 |
| LSPV | –40.9 ± 12 | –35.7 ± 7.9 | .06 |
| LIPV | –33.6 ± 12.3 | –28.1 ± 8.7 | .13 |
| RSPV | –48.2 ± 9.2 | –31 ± 9.1 | < .01 |
| RIPV | –40 ± 10.5 | –33.9 ± 9.23 | .49 |
Abbreviations: LET, luminal oesophageal temperatures; LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; PVI, pulmonary vein isolation; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein.
LAPWI was achieved in 38 patients (95%) in the POLARx group and in 36 patients (90%) in the Arctic Front group (p =.34), as confirmed by the post ablation 3D electroanatomical map (Figure 2). In the remaining patients, additional RFA was required to complete posterior wall isolation. None of these applications were stopped for LET greater than 40°C. These patients had significantly larger LA compared with patients achieving LAPWI using CB‐A only [59.5 ± 10.8 ml/m2 vs. 47.7 ± 8.1 ml/m2, p =.001] in the absence of differences in the number of cryoapplications [10 ± 2 vs. 10.6 ± 2.3, p =.5].
FIGURE 2.
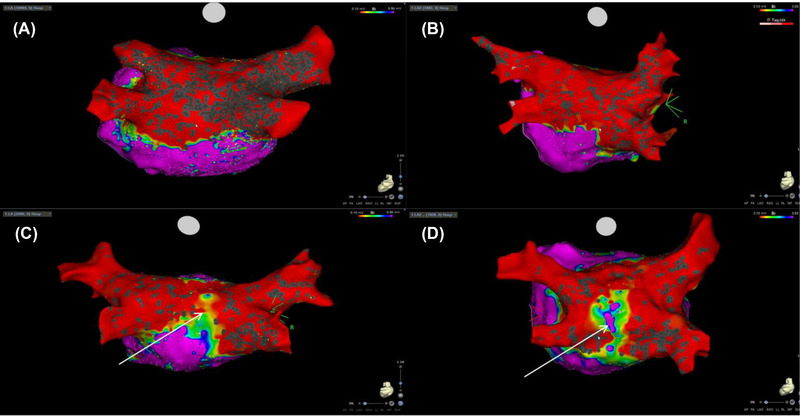
A postablation 3‐dimensional (3‐D) electroanatomic maps (voltage cut‐off: 0.1−0.5 mV) showing pulmonary vein isolation (PVI) + left atrial posterior wall isolation (LAPWI) using the POLARx ™ Cryoballoon ablation system (A) and the Medtronic Arctic Front Advance™ balloon platform (B). In 5% of case in the POLARx group (C) and in 10% in the Arctic Front group (D), a post ablation 3‐D showed the presence of gap (arrow) [Colour figure can be viewed at wileyonlinelibrary.com]
The procedural outcomes are detailed in Table 3. The mean number of CB‐A applications needed for LAPWI was similar between POLARx group and Arctic Front group [10.95 ± 2.3 for POLARx group vs. 10.2 ± 2.4 for Arctic Front group, p =.20]. In particular, for the superior LAPWI segment the mean number of CB‐A applications was 5.3 ± 1.4 in the POLARx group vs. 4.8 ± 1.2 in the Arctic Front group, (p =.15); for the inferior segment of LAPWI the mean number of applications needed was 5.6 ± 1.5 for POLARx vs. 5.1 ± 1.2 for Arctic Front, (p =.12). During LAPWI, POLARx system reached lower mean temperature [−47.2 ± 3.5°C in POLARx vs. −40.4 ± 3.1°C in Arctic Front, p <.001], lower minimum temperature [−52.4 ± 4.7°C in POLARx vs. −44.1 ± 3.8°C in Arctic Front, p <.001], (Figure 1). Nevertheless, the LET were similar between both groups during LAPWI (p =.65). Cryoenergy delivery was interrupted due to a LET below 15°C in three patients (7.5%) in the PolarX group and in two patients (5%) in the Arctic Front group, (p =.34).
TABLE 3.
Procedural characteristics – LAPWI
| POLARx (n = 40) | Arctic front (n = 40) | p | |
|---|---|---|---|
| Total applications | 10.95 ± 2.3 | 10.2 ± 2.4 | .20 |
| Superior applications | 5.3 ± 1.4 | 4.8 ± 1.2 | .15 |
| Inferior applications | 5.6 ± 1.5 | 5.1 ± 1.2 | .12 |
| Duration single application (s) | 119.8 ± 0.8 | 119.9 ± 0.62 | .56 |
| Min temperature (°C) | –52.4 ± 4.7 | –44.1 ± 3.8 | < .001 |
| Min temperature (superior applications) (°C) | 51.7 ± 4.5 | –41.5 ± 4.1 | < .001 |
| Min temperature (inferior applications) (°C) | –50.6 ± 5 | –42.8 ± 4.2 | < .001 |
| Mean temperature (°C) | –47.2 ± 3.5 | –40.4 ± 3.1 | < .001 |
| Mean temperature (superior applications) (°C) | –47.8 ± 3.85 | –40.4 ± 4 | < .001 |
| Mean temperature (inferior applications) (°C) | –46.7 ± 3.8 | –40.9 ± 3.4 | < .001 |
| Min LET (°C) | 29.9 ± 7.8 | 30.7 ± 5.4 | .61 |
| Min LET (superior applications) (°C) | 30.5 ± 5.05 | 31.9 ± 5.4 | .47 |
| Min LET (inferior applications) (°C) | 30.8 ± 7.2 | 31 ± 5.3 | .81 |
| Procedure time (min) | 107.9 ± 51.2 | 101.59 ± 32.5 | .56 |
| Fluoroscopy time (min) | 29.4 ± 14 | 24.7 ± 8 | .11 |
| Left atrial dwell time (min) | 87.8 ± 27.3 | 77.9 ± 29.9 | .18 |
Abbreviation: LET, luminal oesophageal temperatures.
The total procedure time [107.9 ± 51.2 min in the POLARx group vs. 101.59 ± 32.5 min in the Arctic Front group, p =.56], the mean fluoroscopy time [29.4 ± 14 min in the POLARx group vs. 24.7 ± 8 min in the Arctic Front group, p =.11] and the left atrial dwell time]87.8 ± 27.3 min in the POLARx group vs. 77.9 ± 29.9 min in the Arctic Front group, p =.18] were similar between the two groups. Transient phrenic palsy occurred in three patients in the POLARx group (7.5%) and in four patients (10%) in Arctic Front group, (p =.69), with complete resolution before the end of the procedure. One pericardial effusion in the POLARx group was observed. The latter was treated conservatively without the need of further intervention. No deaths, cerebrovascular events, or groin vascular complications occurred in the peri‐procedural period.
4. DISCUSSION
To the best of our knowledge, this is the first study comparing PVI + LAPWI isolation using the POLARx CB‐A and the Arctic Front Advance CB‐A for the treatment of PersAF. The main results are: (1) the POLARx CB‐A is effective and safe for both PVI and LAPWI; (2) acute procedural outcomes and complications of this novel technology are comparable with the established Arctic Front platform; and (3) the POLARx is associated with lower measured temperatures for both PVI and LAPWI.
PVI + LAPWI was successfully achieved in all patients with the POLARx CB‐A. Procedure time, left atrial dwell time, and fluoroscopy time were similar to Arctic Front Advance CB‐A. Previous studies comparing both technologies for PVI showed higher median total procedural time, left atrial dwell time, and fluoroscopy times with the POLARx 10 , 11 ; our results can be explained by the fast learning curve with this new technology for both PVI and LAPWI. This is not surprising, since both technologies share a similar workflow.
The measured temperatures reached by the POLARx CB‐A were 10°C lower compared with Arctic Front Advance CB‐A. This finding is consistent with previous reports. 10 , 11 , 12 Noticeably, the temperatures were also lower during applications on the posterior wall. The temperatures displayed on the console of both systems are commonly referred to as inner balloon temperature. The latter are the result of the return gas temperature from inside the cryoballoon, and do not reflect the values at the tissue balloon interface. The inner balloon strongly depends on independent variables such as the balloon position within the PV ostium and on the LAPW, balloon to PV diameter ratio, and balloon manipulation by the operator. As a result, inner balloon temperature may differ from balloon to balloon, despite similarities in energy delivery and overall approach. In addition, although the position of the thermocouple (measuring the internal balloon gas temperature) is similar between both systems (21.5 mm from injection coil), 12 the more compliant balloon of POLARx may bring the thermocouple closer the cooling area if compared to the stiffer Artic Front. This might explain the lower temperatures recorded with this system.
Moreover, a difference can be found in the gas pressure, that is kept stable and lower in POLARx system (2.5 psi), while it increases to higher values during cryoenergy applications with the Arctic Front Advance PRO. 15 , 16 The ideal gas law states that, for a given constant volume a higher pressure translates into higher temperatures potentially, giving a further physical explanation to the different temperature recordings between both technologies.
These technical differences between the two systems did not translate into changes in the procedural workflow for PVI + LAPWI, as confirmed by procedural data.
Despite lower temperatures, no significantly different LET was recorded, compared to the Arctic Front platform. Future studies including larger cohorts of patients and with longer follow‐up are needed in order to evaluate the influence of lower temperatures on clinical outcome.
The number of applications needed to perform LAPWI was comparable between both technologies. 6 , 13
The complication rate was low, comparable between both technologies; the lower temperatures attained during PVI and LAPWI ablation with the POLARx did not translate in higher rates of PNP or lower LET. Finally, the current study confirms that adding LAPWI to PVI with cryoballoon technology does not increase complication rates.
5. LIMITATIONS
The study was a non‐randomized analysis of consecutive patients conducted in a relatively limited number of patients. Furthermore, it reports a single center experience.
No esophagogastroduodenoscopy was performed after ablation, esophageal damage might have been underestimated. Finally, the lack of a clinical follow‐up or remap prevent from defining any algorithms, such as a temperature‐guided approach, for the new ablation system.
6. CONCLUSIONS
LAPWI + PVI with the novel POLARx™ Cryoballoon is feasible and safe. No differences were found compared to the traditional cryoablation platform in terms of acute efficacy and complications. Long term follow‐up studies are awaited to assess the clinical outcome.
CONFLICTS OF INTEREST
C.d.A. reports speaker fees for Medtronic, Biotronik, Biosense Webster, Abbott and Boston Scientific; teaching honoraria from Medtronic, Biotronik, Abbott and Boston Scientific; proctoring honoraria from Medtronic, Abbott and Biotronik; G.B.C. reports speaker fees for Medtronic, Biotronik, Biosense Webster and Abbott; teaching honoraria from Medtronic and Biotronik; proctoring honoraria from Medtronic; A.B. is consultant for Biotronik; P.B. reports consulting fees and speaker honoraria from Medtronic.
Bisignani A, Pannone L, Miraglia V, et al. Feasibility and safety of left atrial posterior wall isolation with a new Cryoballoon technology in patients with persistent atrial fibrillation. Pacing Clin Electrophysiol. 2022;45:605–611. 10.1111/pace.14495
Antonio Bisignani and Luigi Pannone contributed equally as first author.
Gian‐Battista Chierchia and Carlo de Asmundis contributed equally as senior authors.
REFERENCES
- 1. Haïssaguerre M, Jaïs P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339(10):659‐666. [DOI] [PubMed] [Google Scholar]
- 2. Pappone C, Rosanio S, Oreto G, et al. Circumferential radiofrequency ablation of pulmonary vein ostia: a new anatomic approach for curing atrial fibrillation. Circulation. 2000;102(21):2619‐2628. [DOI] [PubMed] [Google Scholar]
- 3. Della Rocca DG, Di Biase L, Mohanty S, et al. Targeting non‐pulmonary vein triggers in persistent atrial fibrillation: results from a prospective, multicentre, observational registry. Europace. 2021;23(12):1939‐1949. 10.1093/europace/euab161 [DOI] [PubMed] [Google Scholar]
- 4. Salih M, Darrat Y, Ibrahim AM, et al. Clinical outcomes of adjunctive posterior wall isolation in persistent atrial fibrillation: a Meta‐analysis. J Cardiovasc Electrophysiol. 2020;31(6):1394‐1402. 10.1111/jce.14480. Epub 2020 Apr 20. [DOI] [PubMed] [Google Scholar]
- 5. Bisignani A, Cecchini F, Mugnai G, et al. Single procedural outcomes in the setting of percutaneous ablation for persistent atrial fibrillation: a propensity‐matched score comparison between different strategies. J Interv Card Electrophysiol. 2021. 10.1007/s10840-021-00968-2 [DOI] [PubMed] [Google Scholar]
- 6. Aryana A, Allen SL, Pujara DK, et al. Concomitant pulmonary vein and posterior wall isolation using cryoballoon with adjunct radiofrequency in persistent atrial fibrillation. JACC Clin Electrophysiol. 2021;7(2):187‐196. [DOI] [PubMed] [Google Scholar]
- 7. Aryana A, Baker JH, Espinosa Ginic MA, et al. Posterior wall isolation using the cryoballoon in conjunction with pulmonary vein ablation is superior to pulmonary vein isolation alone in patients with persistent atrial fibrillation: a multicenter experience. Heart Rhythm. 2018;15:1121‐1129. [DOI] [PubMed] [Google Scholar]
- 8. Bisignani A, Pannone L, Bala G, et al. Repeat procedures for recurrent persistent atrial fibrillation: a propensisty‐matched score comparison between left atrial linear ablation with radiofrequency and posterior wall isolation with Cryoballoon. J Arrhythmia. 2021;37:1287‐1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boston Scientific . The POLARx Cryoballoon System. https://www.bostonscientific.com/en‐EU/medical‐specialties/electrophysiology/arrhythmias/single‐shot‐ablation/polarx‐cryoballoon.html. Accessed September 1, 2020.
- 10. Creta A, Kanthasamy V, Schilling RJ, et al. First experience of POLARx™ versus Arctic Front Advance™: an early technology comparison. J Cardiovasc Electrophysiol. 2021;32(4):925‐930. [DOI] [PubMed] [Google Scholar]
- 11. Kochi AN, Moltrasio M, Tundo F, et al. Cryoballoon atrial fibrillation ablation: single‐center safety and efficacy data using a novel cryoballoon technology compared to a historical balloon platform. J Cardiovasc Electrophysiol. 2021;32(3):588‐594. [DOI] [PubMed] [Google Scholar]
- 12. Yap SC, Anic A, Breskovic T, et al. Comparison of procedural efficacy and biophysical parameters between two competing cryoballoon technologies for pulmonary vein isolation: insights from an initial multicenter experience. J Cardiovasc Electrophysiol. 2021; 32(3):580‐587. 10.1111/jce.14915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moltrasio M, Kochi AN, Fassini G, Riva S, Tundo F, Tondo C. High‐density mapping validation of antral pulmonary vein isolation and posterior wall isolation created with a new cryoballoon ablation system: the first reported case. J Cardiovasc Electrophysiol. 2020;31(12):3318‐3321. [DOI] [PubMed] [Google Scholar]
- 14. Bisignani A, Overeinder I, Kazawa S, et al. Posterior box isolation as an adjunctive ablation strategy with the second‐generation cryoballoon for paroxysmal atrial fibrillation: a comparison with standard cryoballoon pulmonary vein isolation. J Interv Card Electrophysiol. 2021;61(2):313‐319. [DOI] [PubMed] [Google Scholar]
- 15.BSC Data on file with Boston Scientific, EP‐639201‐AA.
- 16.Medtronic Cryoconsole ™ Operator's Manual 106E2/106A2‐K, M999956A001.


