Abstract
Electroencephalography was used to investigate the effects of extrastimulation and preterm birth on the development of visual motion perception during early infancy. Infants receiving extra motor stimulation in the form of baby swimming, a traditionally raised control group, and preterm born infants were presented with an optic flow pattern simulating forward and reversed self‐motion and unstructured random visual motion before and after they achieved self‐produced locomotion. Extrastimulated infants started crawling earlier and displayed significantly shorter N2 latencies in response to visual motion than their full‐term and preterm peers. Preterm infants could not differentiate between visual motion conditions, nor did they significantly decrease their latencies with age and locomotor experience. Differences in induced activities were also observed with desynchronized theta‐band activity in all infants, but with more mature synchronized alpha–beta band activity only in extrastimulated infants after they had become mobile. Compared with the other infants, preterm infants showed more widespread desynchronized oscillatory activities at lower frequencies at the age of 1 year (corrected for prematurity). The overall advanced performance of extrastimulated infants was attributed to their enriched motor stimulation. The poorer responses in the preterm infants could be related to impairment of the dorsal visual stream that is specialized in the processing of visual motion.
Keywords: baby swimming, development of visual motion perception, electroencephalography, extra motor stimulation, infants, locomotor experience, prematurity
1. INTRODUCTION
1.1. Visual motion perception and its development
When navigating through the dynamic environment, the gathering of perceptual information allows for controlling and guiding future actions so that accidents might be avoided and goals are reached. Visual motion perception is crucial for successfully navigating the environment and provides essential information for self‐motion, orientation and heading direction, control of posture, and locomotion (Agyei et al., 2015; Vaina & Rushton, 2000). This information, termed optic flow, is the pattern of visual motion available to the eye when we are moving relative to our environment (Gibson, 2015). Considering the relevance of these abilities to everyday life, it is important to understand the developmental processes underlying how infants learn to make use of relevant visual information for motion perception (Agyei et al., 2016a).
There has been considerable progress in understanding the anatomy and electrophysiology of the parts of the visual system processing complex motion in monkeys and normal adults, yet little is known about the development and processing of this fundamental type of information in infants (Gilmore et al., 2004). Measurements of visual evoked potentials (VEPs) as a function of optic flow have demonstrated a progression toward more advanced processing of visual motion information during the first year of life (Vilhelmsen et al., 2019). As indexed by significantly shorter N2 latencies in response to visual motion with age, infants show rapid improvements in their optic flow processing between 3–4 and 11–12 months of age (Agyei et al., 2015). Moreover, electroencephalography (EEG) studies measuring VEPs have shown that normally developing infants at 11–12 months of age, similar to adults (Van der Meer et al., 2008), can differentiate between different types of visual motion and process forward optic flow faster than they do reversed optic flow (Agyei et al., 2015). This is possibly because humans have more experience with forward optic flow in their everyday lives (Agyei et al., 2015; Van der Meer et al., 2008). The ability to perceive visual motion in infants seems to depend on an interaction between the experience of self‐generated active locomotion and neurobiological development (Vilhelmsen et al., 2019). Self‐generated active locomotion appears to improve and expedite the infant's development of visual motion perception (Agyei et al., 2016a; Higgins et al., 1996).
EEG studies measuring visual evoked responses have proven to be complementary techniques for the study of the neural basis of motion perception in the millisecond time scale (Rosander et al., 2007; Van der Meer et al., 2008). In EEG recordings, VEPs reflect the activity of postsynaptic neurons in direct relation to a visual stimulus (Webb et al., 2005), and are dominated by a motion‐sensitive N2 component thought to be generated in human hMT+/V5 (Ahlfors et al., 1999; Kuba et al., 2007). Support is provided because motion perception is impaired by transcranial magnetic stimulation when applied over MT+ at 130−150 ms after onset of the motion stimulus (Sack et al., 2006). Motion VEP waveforms in EEG have been found to be dominated by a negativity (N2) with its origin assumed to be in area MT+ that occurs in adults at latencies around 130–150 ms (Heinrich et al., 2005; Probst et al., 1993) and in 8‐month‐old infants around 180−220 ms poststimulus (Van der Meer et al., 2008). These latency differences are believed to imply changes in visual motion processing, with shorter values indicating faster processing (Agyei et al., 2015).
In addition to the use of VEPs in EEG studies, growing attention has been given to the time–frequency domain, which allows computing the temporal dynamics of EEG oscillations with a technique called temporal spectral evolution (TSE). Modulations in oscillatory activity may be observed as either an increase (i.e., event‐related synchronization, ERS) or a decrease (i.e., event‐related desynchronization, ERD), indicating more or less synchrony in the rhythmic activity of the underlying neuronal populations (Pfurtscheller, 2001). Different classes of oscillations have been distinguished over the years: delta‐band (1–4 Hz), theta‐band (4–7 Hz), alpha‐band (7–13 Hz), beta‐band (13–30 Hz), and gamma‐band (30–150 Hz), with each rhythm assumed to reflect neurophysiological processes that manifest functionally different roles (Buzsáki & Draguhn, 2004; Engel & Fries, 2010; Fries, 2005; Ganzetti & Mantini, 2013; Saby & Marshall, 2012).
Studies using time–frequency analysis of the ongoing EEG in infants have found that brain activity in low‐frequency oscillations, especially in the theta‐alpha range, undergoes systematic development from early childhood to adulthood (De Haan, 2013; Stroganova et al., 1999). Contrary to infants, optic flow studies in adults have found beta band ERS and beta band ERD activity in response to visual motion compared with a static control scene, respectively (Van der Meer et al., 2008; Vilhelmsen et al., 2019). This high‐frequency activation in adults has been attributed to functional responses involving fewer but more specialized neuronal assemblies, reflecting a fully developed motion perception system (Agyei et al., 2015; Van der Meer et al., 2008; Vilhelmsen et al., 2019). The frequency differences between infants and adults from lower to higher frequencies are considered a sign of maturation in various psychophysiological studies (Hudspeth & Pribram, 1992; Stroganova et al., 1999).
1.2. Preterm infants and their perception of visual motion
The human brain is an organized dynamic network of interconnected neurons and associated synapses that work together such that dysfunctions within the network can have unfortunate effects on behavioral patterns (Agyei et al., 2016a). Infants born preterm have been found to be more at risk of neurological deficits and developmental disorders. An infant is defined as preterm when s/he is born before 37 completed weeks of gestation. With increasing numbers of preterm infants surviving, the impact of preterm birth on later cognitive development has been given considerable attention over the years. Magnetic resonance imaging studies have demonstrated that being born preterm causes differential brain development, leading to abnormalities in the microstructure of tissues and in cerebral morphology (Counsell & Boardman, 2005). Some of the dysfunctions of preterm birth have been related to cognitive and behavioral impairments (Aarnoudse‐Moens et al., 2009; Bhutta et al., 2002; De Jong et al., 2012; Delobel‐Ayoub et al., 2009; Johnson, 2007; Salt & Redshaw, 2006). Among the major functions affected by preterm birth, visual cognition is one (Atkinson & Braddick, 2007).
Studies on the perception of visual motion suggest that the cluster of deficits seen in children born prematurely may be related to networks involving the cortical dorsal stream and its connections to parietal, frontal, and hippocampal areas (Atkinson & Braddick, 2007). These findings suggest a possible vulnerability of the dorsal visual processing stream in preterm infants, contrary to findings in normally developing infants (Agyei et al., 2016a; Braddick et al., 2003). Differences in dorsal stream functions, but not in ventral stream functions, have been found between preterm and full‐term infants (Hammarrenger et al., 2007; Tremblay et al., 2014). The dorsal visual stream is developed in the last weeks of fetal life, and premature birth can thus interfere with this development. Guzzetta et al. (2009) reported that preterm children appeared to perform worse than full‐term controls in global motion (optic flow) perception, irrespective of the presence of brain damage. These findings suggest impairment of the dorsal stream during visual processing in preterm children with and without brain damage (Guzzetta et al., 2009; Taylor et al., 2009). Considering the importance of processing visual motion information in several everyday tasks, a dorsal stream vulnerability can have a number of implications for preterm infants’ development.
1.3. Plasticity of the brain
An intriguing feature of the brain is its capacity for structural and functional modification in response to external stimuli. We can now identify an extensive range of neural changes associated with experience. These include increases in brain size, cortical thickness, neuron size, dendritic branching, spine density, synapses per neuron, and glial numbers (Kolb & Whishaw, 1998). This plasticity of the nervous system has been the focus of research efforts for decades (Mohammed et al., 2002). However, this research has long been limited to the adult nervous system. One cardinal principle in developmental psychology is that early experience profoundly affects human development (Fox & Rutter, 2010). A corollary to this principle is that there are certain periods in early development when experiences have a more significant effect than others, called critical or sensitive periods. These periods are often thought of as a window of opportunity where certain types of experience have a fundamental effect on skill development (Greenough et al., 1987). The importance of early experience has been strengthened with advances in neuroscience. Greenough and colleagues (1987) introduced the term “experience‐expectant plasticity” to refer to the role of experience in brain development during early sensitive periods. The developing brain depends on external stimuli to shape neural circuitry patterns via mechanisms of synaptic competition, in which the most effectively activated neural connections are selectively maintained and matured, and those less well‐activated are eliminated (Greenough et al., 1987). At birth, the human brain is equipped with 100 billion neurons, a few of which are connected. During the first years of life, millions of connections are made through an interplay of life experience and maturation, forming complex networks of neurons specialized in processing different kinds of information rapidly. Considering that optimal brain plasticity occurs in the first few months of life (Bonnier, 2008), it is reasonable to suggest infant stimulation can profoundly influence how the brain will develop and how the child will interact with the world throughout life.
Whether, and to what extent, plasticity can compensate for failure of cognitive functions to develop within the first few years of life has generated considerable attention (Bonnier, 2008). Interventions related to developmental disorders generally start later in life, despite being relatively late from the point of view of the brain's plasticity. The crucial significance of developmental processes in the first years of life has received support from data on the modulation of neuronal death, synaptic stabilization, axonal reorientation, axonal and dendritic budding, and recruitment of transient projections (Huttenlocher & Bonnier, 1991; Kolb & Whishaw, 1989; Stanfield et al., 1982), all of which depend on individual experience.
1.4. Extra stimulation
Apart from the rapid maturation of the cortex, increased attention has been given to the link between locomotor experience and development in advancing psychological functions in infancy (Bertenthal & Campos, 1990; Gilmore et al., 2004; Higgins et al., 1996; James & Swain, 2011; Kermoian & Campos, 1988; Uchiyama et al., 2008; Ueno et al., 2018; Walle & Campos, 2014). In addition to EEG studies providing evidence of cortical reorganization after the onset of self‐produced locomotion as good examples of brain plasticity in early development (Bell & Fox, 1996; Corbetta et al., 2014), several lines of evidence have demonstrated the onset of self‐produced locomotion to bring about developmental change (e.g., Bertenthal & Campos, 1990; Campos et al., 2000). When infants acquire the first locomotor skill, typically crawling, it dramatically changes the relationship between the infant and the environment. This opens a sea of exploration of opportunities for the infants, which provides new perspectives and experiences that can drive changes in a host of different psychological phenomena. However, infants need opportunity to explore and interact with the environment independently to develop the competence of crawling. The onset of locomotion can be accelerated with the appropriate stimulation (Zelazo et al., 1972). In contrast to the unidirectional traditional maturational view of brain development, more and more neurologically focused empirical work suggests that locomotion is not merely a maturational antecedent to these changes. Instead, the changes are a function of the specific experiences that accompany moving oneself through the environment (Anderson et al., 2013). Enriched stimulation is further associated with an earlier onset of motor behaviors and to have immediate as well as long‐lasting developmental effects (e.g., Lee & Galloway, 2012; Lobo & Galloway, 2008, 2012).
Baby swimming is seen as a great opportunity to overcome the movement constraints in very young babies. Due to the antigravity property of water, young infants are able to experience self‐produced and self‐guided locomotion, even prior to the onset of crawling or walking. Baby swimming is therefore suggested to have a positive effect on the infant's cognitive and motor development (Sigmundsson & Hopkins, 2010). Considering previous research on the positive effects of visuomotor experience on the developmental trajectories (Agyei et al., 2015; Anderson et al., 2013; Gilmore et al., 2004; Gilmore & Rettke, 2003), it is plausible to assume that extraordinary motor stimulation in the form of baby swimming courses may also facilitate the development of visual motion perception in infants in their first year of life.
1.5. The present study
The present study explored the effects of receiving extra motor stimulation in early infancy on the development of visual motion perception. Understanding the functional development of the brain and whether extrastimulation accelerates the development of visuo‐cognitive systems is important to ensure early intervention in both healthy infants and at‐risk infants such as preterm infants. Using EEG data in a longitudinal design during the first year of life, we investigated the development of visual motion perception by comparing three groups of infants, that is, infants receiving extra motor stimulation in the form of baby swimming classes, a control group of infants who received a traditional Western upbringing, and preterm infants, at the ages of 4–5 months and 9–12 months. VEP and time–frequency analyses were applied to investigate whether there are any significant differences in brain responses to visual motion between the three infant groups. Previous optic flow studies have shown N2 latencies to decrease with age and experience with self‐produced locomotion during the first year of life, with the shortest latencies observed for forward optic flow, followed by reversed optic flow, and the longest latencies observed for random visual motion. A developmental progression from induced activities at low frequencies to higher‐frequency oscillations has further been reported during the first postnatal year. Given the association between experience and developmental advancements, it was expected for infants receiving extrastimulation to have had greater opportunities for actively engaging with their environment compared with their control and preterm peers, and therefore to show an accelerated development of visual motion perception. Thus, it was hypothesized that extrastimulated infants would display overall shorter latencies of VEPs in addition to induced activities at higher frequencies than the other two infant groups. Based on research indicating impaired dorsal stream functioning, the preterm infants in this study were expected to show delayed development of visual motion processing during the course of the first year of life compared with their peers.
2. METHODS
2.1. Participants
A total of 30 infants, of which 10 had received extrastimulation, 10 belonged to a control group receiving a traditional Western upbringing, and 10 were born preterm, took part in this longitudinal study. The extrastimulated group comprised full‐term infants that had participated in baby swimming classes from an early age (mean weeks of swimming experience at first testing 9, SD = 4.4). Extrastimulated infants had a mean gestational age of 39.8 weeks (SD = 1.2, range = 38.1–41.4), and mean birth weight of 3576 g (SD = 540, range = 3095–4565). All infants in the control group were born full term, with a mean gestational age of 40.1 weeks (SD = 1.1, range = 38.3–41.9), and mean birth weight of 3571 g (SD = 425, range = 3085–4400). The preterm infants (moderate to very preterm) were born at a mean gestational age of 31 weeks (SD = 1.7, range = 28–33 weeks). Their mean birth weight was 1570 g (SD = 285, range = 1000−2080). The preterm infants did not have any major neurological deficits including severe brain damage, retinopathy of prematurity, and other perinatal issues requiring serious medical interventions that may lead to abnormal development.
All infants were Caucasian and received the same Western upbringing. They were from similar socioeconomic backgrounds, as Norway is one of the most egalitarian countries in the world. All infants were born healthy without any neurological deficits as determined by parental report.
Recruitment for the extrastimulated infants (five boys and five girls) entailed contacting parents directly at baby swimming classes held at Pirbadet in Trondheim, or by using a snowball technique. The control group (five boys and five girls) were recruited by contacting parents following birth announcements in the local newspaper or simply by word of mouth. The preterm infants (five boys and five girls) were recruited through the Neonatal Intensive Care Unit at St. Olav's University Hospital in Trondheim.
Infants were tested at two time points in a longitudinal design. First, infants were tested at 4–5 months followed by a second testing when the infants had some crawling experience or at 9–12 months of age. The first test was always used, and the other tests were either at 9–10 months or 11–12 months of age, depending on the infant's crawling experience. The criterion for using the data collected at 9–10 months of age was that the infant should have been crawling for at least 9 weeks. The three groups of infants were matched according to sex and experience with self‐produced locomotion. Experience with self‐produced locomotion was documented for both testing sessions, with self‐produced locomotion being defined as commando crawling, crawling on all fours, walking, or in any other way achieving independent locomotion. At the first testing, the mean age of the extrastimulated infants was 4 months and 19 days (SD = 7.2 days). They had been attending baby swimming classes once a week for between 1.5 and 4 months at the time of the first testing. The classes focused on face‐to‐face communication in the warm water while the parent held the infant in upright and prone positions. Some diving and independent standing with straight legs in the hand of the instructor were also practiced. None of the extrastimulated infants had experience with crawling at the first testing, but eight could roll over from back to stomach. At the time of the second testing, five of the extrastimulated infants had already started advanced swimming classes, and three were about to start soon. At the second testing, the mean age was 9 months and 29 days (SD = 53 days) and all extrastimulated infants could commando crawl and had been doing so for a minimum of 9 weeks (mean weeks of self‐produced locomotion 13). In addition, one of the infants was walking with help, and four were walking independently at the second testing.
Infants in the control group were tested first at a mean age of 4 months and 10 days (SD = 20 days) and then again at a mean age of 11 months and 10 days (SD = 28 days). Four of the infants in the control group had some experience with rolling over from back to stomach at the first testing session, but no experience with self‐produced locomotion. At the time of the second testing, all control infants were crawling and had been able to do so for at least 7 weeks (mean weeks of self‐produced locomotion 14). Three of the infants took some steps alone or with help from furniture, and three were walking independently at the second session.
Onset of self‐produced locomotion for all infants was documented with parental video records, and locomotor status was confirmed at the time of the second testing in the laboratory. Extrastimulated infants were significantly younger when they started to crawl at 29.7 weeks (SD = 7.2) than control infants who on average crawled at 34.4 weeks (SD = 4.5), t(18) = −1.77, p < .05 (one‐tailed).
In order to ensure valid matching, the preterm infants’ age was corrected for prematurity. For the first session, the mean age of the preterm infants was 4 months and 25 days (SD = 7 days). At the second testing, the mean age of the preterm infants was 12 months (SD = 12 days). At the first testing session, three of the preterm infants had some experience with rolling over from back to stomach, but no experience with crawling or other self‐produced locomotion. At the second testing session, all of the infants were commando crawling and had been able to do so for a minimum of 6 weeks (mean weeks of self‐produced locomotion 17). In addition, two of the infants had mastered independent walking and two could walk with help.
EEG recording is a noninvasive method that causes no known harm or physical pain to the participant. Parents gave their informed consent and had the right to withdraw from the testing at any time before or during the experiments. The Norwegian Regional Ethics Committee and the Norwegian Data Services for the Social Sciences approved the study.
2.2. Experimental stimuli
Stimuli were generated with the psychological software tool E‐prime, and projected onto a large monitor (108 cm wide, 70.5 cm high), placed approximately 70 cm away in front of the infant. Three experimental conditions were employed, that is, forward optic flow, reversed optic flow and random motion, as well as a static nonflow control condition used in the time–frequency analysis. For a graphic representation of the experimental set‐up and the visual motion information the infants were presented with, see Figure 1 and Video S1. Duration of presentation for each motion trial was 1500 ms, with the order randomly chosen. To reduce motion adaptation, each motion pattern was followed by a static trial occurring for 1500 ms. To simulate movement in space, 100 black dots were programmed to move on a white background, with the dots being 5 mm in virtual radius. The dots increased or decreased in size at a rate of 0.025 pixels per pixel with reference to the position of the fixation point such that the particles appeared small when far away from the eye in virtual space and large when closer. For forward optic flow, the dots moved outward from the center of the screen, making the dots appear to move toward the infant. For reversed optic flow, dots coherently moved in the opposite direction, that is, toward the center of the screen. For random motion, dots moved in random directions on the screen. Stimuli were presented as a uniform dot distribution to avoid accretion of dots at the center or edges of the screen. Dots that moved off the screen were automatically resized and repositioned on the screen, with an equal probability of placement.
FIGURE 1.

Showing the experimental set‐up. Experimental room with a 9‐month‐old infant sitting in a baby car seat secured to an adjustable chair, wearing an electrode net consisting of 128 sensors. Moving dots simulating forward and reversed self‐motion through optic flow and random visual motion, appeared on the large monitor in front of the infant (see also Video S1). The eye tracker was placed on the desk, between the participant and the screen, to monitor gaze. A parent and assistant were present during the entire session
In the first testing session, extrastimulated infants contributed on average 46 (SD = 16) motion trials, whereas full‐term infants in the control group and premature infants contributed on average 60 (SD = 15) and 54 (SD = 18) motion trials, respectively. In the second testing session, mean motion trial contributions for extrastimulated infants were 60 (SD = 8), whereas for control and premature infants they were on average 54 (SD = 21) and 61 (SD = 19), respectively.
2.3. Data acquisition
EEG activity was recorded with a Geodesic Sensor Net 200 consisting of an array of 128 sensors that were evenly distributed on the infant's head. Amplified EEG signals were recorded with a sampling rate of 500 Hz with Net Station software on a Macintosh computer. To control the infant's visual attention, eye movements were recorded with a TobiiX50‐eye tracking camera and processed with Clear View software on a HP computer. The infant's behavior during the experiment was also recorded by two digital video cameras placed at different angles. Recorded data were subsequently stored for offline analyses.
2.4. Procedure
Parents arrived with their infant some time before the experiment so that the infant could get comfortable with the laboratory surroundings and the parents could sign the informed consent form. An assistant measured the circumference of the infant's head to determine the appropriate size of the EEG net. The net was soaked in a saline electrolyte solution to ensure optimal electrical conductivity, and afterward partially dried with a towel. The infant sat on the parent's lap and was distracted with soap bubbles and small toys as the net was placed on the head. After the net was in place, the parent carried the infant into a dimly lit experimental room, where the infant was placed in a baby car seat. The parent was seated right next to the infant during the whole experiment to reduce stress in the infant (see Figure 1). Research assistants moved into the control room where they managed the data acquisition and stimulus presentation, while one assistant was present in the experimental room to monitor the experiment and help the baby concentrate. The net was connected to the amplifier, and impedance of the electrodes was checked while electrode contact was improved if necessary.
After the infant's eye movements were calibrated in virtual space to the eye tracker, the experimental session began. When the infant showed signs of boredom or loss of interest, the session was paused, and the assistant and the parent played with the infant for a short period to revive the level of interest. The experiment was ended if no further interest could be obtained or the infant showed considerable level of tiredness or frustration.
2.5. Brain data analysis
All brain analyses were carried out in BESA (Brain Electrical Source Analysis) version 7.0. The procedure was the same for all three groups at both testing sessions. Initial preprocessing entailed segmenting and exporting the EEG recordings as raw data files using Net Station software. All bad channels and artifact‐contaminated channels resulting from head or body movements were visually inspected and either removed from further analyses or interpolated manually. In scanning for artifacts, threshold values for gradient and low signal were set at 75 and 0.1 μV, respectively, whereas maximum amplitude was set at 200−230 μV. Averaged window was from 200 to 800 ms at a baseline definition of −100 to 0 ms. The notch filter was set at 50 Hz to remove power line interference from the data. A low cut‐off filter was set at 1.6 Hz to remove slow drift in the data, and a high cut‐off filter was set at 60 Hz.
2.6. VEP peak analysis
The motion‐sensitive N2 component has its origin in the MT+ region of the cortex. Maximum activity when identifying the N2 components was therefore expected in occipito‐parietal areas. The analysis software computed 3D spherical spline whole‐head voltage maps of EEG scalp signal distributions such that selected N2 peak latencies could be visualized clearly with maximum N2 activity localized in occipito‐parietal regions. Together with the N2 component usually appearing as the most dominant wave in the VEP waveforms, the 3D distribution map was used as further EEG measure when selecting the appropriate N2 peak amplitude and latency.
In identifying the N2 component in each individual subject using the above criteria, grand average waveforms for each group of infants were used. Grand average waveforms estimated the approximate time intervals for the respective N2 components at the various electrode sites. Grand averages were obtained from combining the individual averages for the three infant groups per testing session. From these grand average waveforms, four occipito‐parietal electrodes that showed the highest activation to the most easily recognizable of the experimental conditions (i.e., forward optic flow) were selected. To specifically avoid any bias in selecting the electrodes, especially because of differences in individual infants with respect to their VEPs, the four electrodes selected from the grand average waveforms were the same electrodes chosen for each individual infant during individual analysis of the VEPs. The time intervals from the grand average VEPs served to guide the selection of individual N2 components at different electrode sites. The electrode with the highest activation value among these four electrodes for each individual infant was used for further analyses. The chosen electrode from individual infants could differ from one infant to the next because of the individual subject differences, but the electrode was always one of the four selected electrodes from the grand average. Values for peak latencies and peak amplitudes of the individual averages were recorded for the analyses. Peak latencies were measured as the time from stimulus onset to the peak of each scalp N2 component, whereas peak amplitudes represented maximum amplitudes of the N2 component relative to the prestimulus baseline.
VEP peak analysis was carried out using individual averages. Individual EEG data from each infant were averaged and interpolated into standard 81‐electrode configuration of the 10–10 international standard system after rereferencing to an artificial reference calculated from the average potentials over the scalp. Individual averages for infants in each of the two sessions were combined into a grand average for each session, allowing VEP peak analysis for approximate time intervals for the individual N2 components at selected electrode sites. The 3D spherical spline whole‐head voltage maps of EEG scalp signal distributions were used in aid of visualizing N2 activity in occipital–parietal areas. Values for peak latencies and peak amplitudes of the individual averages were recorded, with peak latencies measured as the time from stimulus onset to the peak of each scalp N2 component. Peak amplitudes represented maximum amplitudes of the N2 component relative to the prestimulus baseline. The values were then subjected to further VEP analyses.
2.7. Time–frequency analysis in brain space
Time–frequency analysis was performed in brain space using predefined multiple source dipoles that modeled activities in the visual areas of the parietal and visual cortices. There is a wide distribution of focal brain activity at the scalp due to the smearing effect of the volume conduction in EEG and the nature of dipole fields. Since the resulting scalp waveforms have mixed contributions from underlying brain sources, source montages derived from a multiple source model were used to obtain optimal separation of focal activity (Scherg & Berg, 1991).
The analysis involved occipital and parietal areas, as these areas are found to be active during motion stimuli presentation (Probst et al., 1993; Zeki et al., 1991). The source montage consisted of 17 sources that modeled activities in the visual pathway and residual activities in other areas of the brain. Of these sources, visual cortex bilateral left (VCbL), visual cortex radial left (VCrL), parietal midline (PM), and visual cortex radial right (VCrR), believed to be active in the visual processing of motion stimuli (Probst et al., 1993; Van der Weel & Van der Meer, 2009; Zeki et al., 1991), were further analyzed (Figure 2). To analyze brain activities using these sources, a four‐shell ellipsoidal head model (Berg & Scherg, 1994; Hoechstetter et al., 2004; Scherg et al., 2002) with the source dipoles inserted was created for each infant where the artifact‐corrected coordinate files were appended. Bone thickness was adjusted for infants at 3.0 mm and conductivity at .02σ as recommended for infants (Grieve et al., 2003; BESA information). Settings for epoch filters and average parameters were the same as in the VEP analyses.
FIGURE 2.
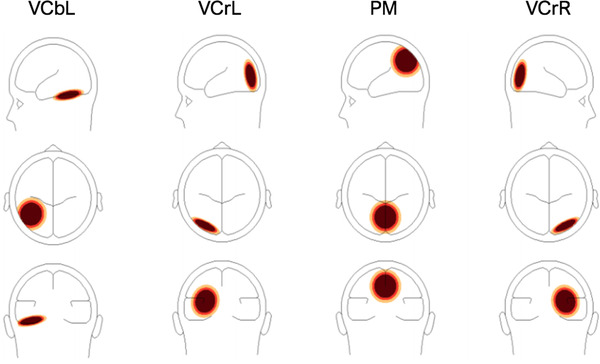
Head model with associated visual cortical areas, from left to right VCbL, VCrL, PM, VCrR. The signal magnitude reflects the estimated source activity in the related brain region if one brain region is active
The resulting time–frequency displays represented the change in amplitude over time (TSE) in the regional sources. Each displayed graph was a plot of spectral amplitude density of one montage channel over time and frequency normalized to the baseline of each frequency. Comparisons between motion and static conditions were computed. Significance (α = .05) was tested with a bootstrapping method in each TSE plot for each of the participants. TSE displays were set to frequency cut‐off of 4−40 Hz at frequency and time sampling of 1 Hz, 50 ms.
Paired sample t‐tests were carried out using BESA Statistics 2.0 (BESA, GmbH) to test for significance in amplitude values and frequency ranges between the TSEs of the motion conditions and the static condition for all infants in each testing session. The multiple comparisons problem was addressed using a combination of permutation testing and data clustering techniques (Maris & Oostenveld, 2007). Cluster alpha, which determines the significance level for building clusters in time and/or frequency, was set at .005. The comparisons allowed observations of significantly dominant oscillatory activities in the regional sources of interest.
3. RESULTS
3.1. VEP responses
The four grand average channels were selected for each group and each testing session based on showing the highest mean N2 amplitudes for forward optic flow. For infants receiving extrastimulation, the selected channels were POz, Pz, Oz, and O1 for the first testing session. For the control infants, the corresponding electrodes were Pz, PO4, Oz, and O2, whereas electrodes for preterm infants at the first testing were PO4, PO8, Oz, and O2. Selected channels at the second session for extrastimulated infants were PO4, POz, Oz, and O2. For the control infants at the second testing they were PO4, Pz, POz, and Oz; and for the preterm infants, P1, Pz, PO8, and POz were used. Latency values from the electrode with the highest N2 amplitude in the forward optic flow condition were used in the ANOVA, with Bonferroni correction used to adjust for multiple comparisons. Thus, the chosen electrode varied across infants and testing sessions, but was always one of the four stated above and was the same for the three motion conditions in each infant. Figure 3 displays the grand average VEPs for the three visual motion conditions for each infant group and each testing session.
FIGURE 3.
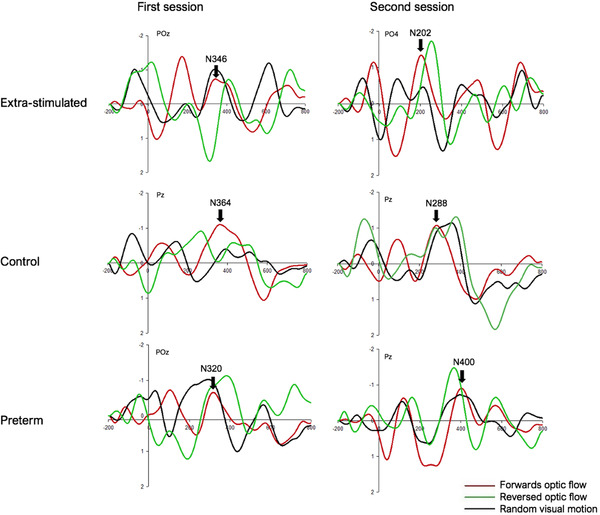
Grand average motion VEPs with epoch set from −200 to 800 ms. Amplitudes (μV) are on the y‐axis and latencies (ms) on the x‐axis. From left to right and top to bottom: the first and second session for infants receiving extrastimulation (POz and PO4), control infants (both Pz), and preterm infants (POz and Pz). Motion conditions are illustrated with colored waveforms and are as follows: forward optic flow (in red), reversed optic flow (in green), and random visual motion (in black). Vertical arrows indicate actual N2 latencies for forward optic flow
3.2. VEP analysis
Mean N2 peak latency for extrastimulated infants at the first session for the three visual motion conditions forward optic flow, reversed optic flow, and random motion was 344 ms (SD = 44), 345 ms (SD = 57), and 380 ms (SD = 43), respectively. Mean N2 latency for forward optic flow for infants in the control group at the first session was 382 ms (SD = 54), with corresponding values for reversed optic flow and random motion at 425 ms (SD = 73) and 434 ms (SD = 73). For the preterm infants at the first session corrected for prematurity, the mean N2 latency for the three motion conditions was 323 ms (SD = 73), 362 ms (SD = 70), and 374 ms (SD = 78), respectively.
The mean N2 latency for the three motion conditions for extrastimulated infants at the second session was reduced to 183 ms (SD = 34), 269 ms (SD = 46), and 328 ms (SD = 36), respectively. For the control group at the second testing, the mean latencies were 289 ms (SD = 54), 348 ms (SD = 53), and 430 ms (SD = 80) for forward optic flow, reversed optic flow, and random visual motion, respectively. For the preterm infants at the second session, the mean N2 latency for the corresponding motion conditions was 386 ms (SD = 49), 388 ms (SD = 51), and 414 ms (SD = 93), respectively (Figure 4).
FIGURE 4.
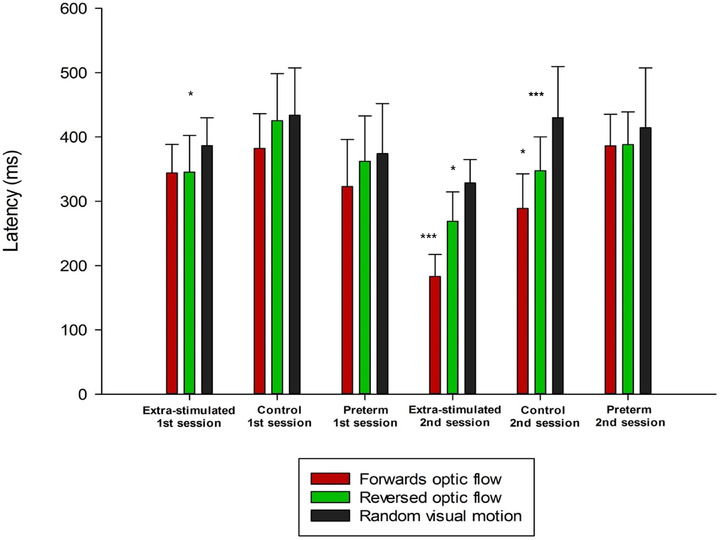
Illustration of group means with standard deviation bars of N2 peak latencies for forward optic flow, reversed optic flow, and random visual motion for infants receiving extrastimulation, infants in the control group, and preterm infants at both testing sessions. In the first testing session, none of the infant groups were able to significantly discriminate between the three forms of visual motion, but extrastimulated infants showed significantly shorter latencies for forward (p < .05) and reversed optic flow (p < .05) compared with random visual motion. In addition, both extrastimulated and preterm infants had significantly shorter overall latencies than the control group (p < .05). From the first to the second testing, extrastimulated infants and control infants significantly improved their latencies, while preterm infants did not show any improvement in latency. In the second session, only extrastimulated infants and control infants were able to differentiate between the three forms of visual motion with the shortest latencies for forward optic flow, followed by reversed optic flow, and the longest latencies for random visual motion. Overall mean latencies were significantly shorter for extrastimulated infants compared with both control (p < .001) and preterm infants (p < .001) in the second session, indicating faster processing of visual motion for extrastimulated infants. Unlike extrastimulated and control infants, preterm infants did not decrease their latencies for visual motion during the course of the first year, and they did not show any evidence of being able to differentiate between forward and reversed optic flow, and random visual motion. ***p < .001, *p < .05
Latencies of the VEPs were analyzed separately using repeated‐measures ANOVAs. The within‐group factor was visual motion condition (forward optic flow, reversed optic flow, random visual motion) and testing session (prelocomotor, self‐produced locomotor experience), whereas between‐groups factor was infant group (extrastimulated, control, preterm).
For latency, a significant two‐way interaction, F(2,24) = 15.01, p < .001, between group and session was found, indicating that overall latencies were significantly shorter for infants receiving extrastimulation than for control and preterm infants in the second session. Overall latencies for forward optic flow in the second session for the extrastimulated infants were approximately 100 ms shorter than for control infants, and 200 ms shorter than for preterm infants.
At the time of the first session, neither control nor preterm infants could significantly differentiate between the three motion conditions. Extrastimulated infants, on the other hand, showed significantly longer latencies for random visual motion compared with forward (p < .05) and reversed (p < .05) optic flow. Across visual motion conditions, extrastimulated and preterm infants showed significantly shorter latencies than control infants (p < .05 for both comparisons) in the first session.
Further, a significant three‐way interaction was found, F(4,54) = 2.80, p < .05 (see Figure 4), showing that only extrastimulated infants (forward‐reversed, p < .001; forward‐random, p < .001; reversed‐random, p < .05) and control infants (forward‐reversed, p < .05; forward‐random, p < .001; reversed‐random, p < .001) were able to significantly differentiate between the three visual motion conditions at the second testing session, with shortest latencies for forward optic flow, followed by reversed optic flow, and longest latencies for random motion with approximately 70 ms between each visual motion condition. Posthoc analyses confirmed that in the second session, extrastimulated infants had significantly shorter latencies across motion conditions than both control (p < .001) and preterm infants (p < .001), and that preterm infants had significantly longer overall latencies than control infants (p < .05) and extrastimulated infants (p < .001). Preterm infants were not able to differentiate between visual motion conditions at the second session, and they did not decrease their N2 latencies for motion during the course of the first year.
3.3. TSE analysis
A time–frequency analysis was carried out for all infants separately for the three visual motion conditions and the static control condition. Subsequent statistical comparisons showed no significant differences between the motion conditions when they were individually compared with one another. The motion conditions were therefore combined into a single motion condition for further analysis.
Figure 5 shows the results of the permutation test displaying the average for infants in each testing session when TSEs of the combined motion condition were compared with the static condition. The permutation test showed significant negative clusters (indicating significantly smaller values in the motion condition than the static condition) in at least one of the visual areas of interest in all three groups. For each group and in each session, significant negative clusters were found in at least one of the four regional sources of interest. The results of the permutation test for the comparison of the combined motion condition and static condition showed negative clusters in the visual areas of interest that appeared to be dominated by activity within the theta‐band range in all three infant groups at both sessions. This prevalent theta‐band activity occurred over relatively longer periods of time when infants were younger (Figures 5(a), 5(c), and 5(e)) compared with shorter periods of time when infants in each respective group were older (Figures 5(b), 5(d), and 5(f)). In addition, the results showed that theta‐band activity was more prevalent and widespread in the first session for control infants (Figure 5(b)) compared with extrastimulated and preterm infants (Figures 5(a) and 5(c)). However, preterm infants showed more widespread theta‐activity (Figure 5(f)) compared with extrastimulated (Figure 5(d)) and control infants (Figure 5(e)) in the second session.
FIGURE 5.
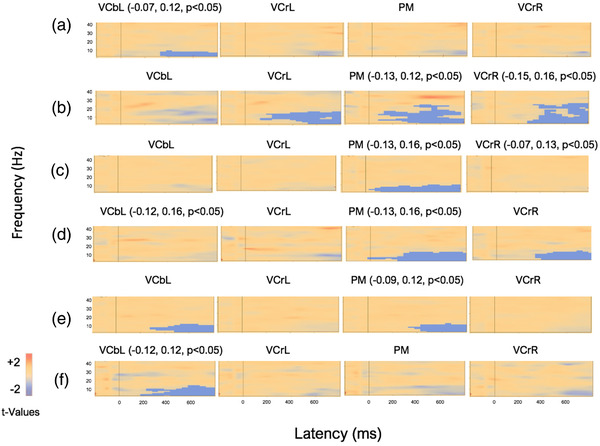
Average visualization of significant data clusters in the visual sources of interest when the combined motion condition was compared with the static condition in extrastimulated infants (a and d), control infants (b and e), and preterm infants (c and f) at the first and second session, respectively. Light blue colors represent negative clusters (i.e., combined motion condition had smaller t‐values than static control condition) and positive clusters are marked with light red colors (i.e., motion condition had larger t‐values than static condition). Significant negative clusters in the visual areas of interest (VCbL, VCrL, PM, VCrR) are marked with light blue voxel marks. Vertical line marks stimulus onset, and epoch is from −200 to 800 ms. Each visual area is dominated by activity in the theta‐band, but over longer periods of time and more prevalent when infants in all groups were younger. The results showed that theta‐band activity was more prevalent and widespread in the first session for control infants compared with both extrastimulated and preterm infants. However, preterm infants showed more widespread theta‐activity compared with extrastimulated and control infants in the second session
The prevalent theta‐band activity appeared as desynchronized oscillatory activity in the TSEs of all groups of infants in both testing sessions when the combined motion condition was compared with the static condition (Figure 6). Further, frequencies of extrastimulated infants and control infants had increased to include expression of desynchronized beta band frequencies (Figures 6(d) and 6(e)), whereas preterm infants still showed desynchronized oscillatory activities in the theta and alpha range (Figure 6(f)). Synchronization in the beta‐band frequency, however, was observed in extrastimulated infants at both first (Figure 6(a)) and second session (Figure 6(d)).
FIGURE 6.
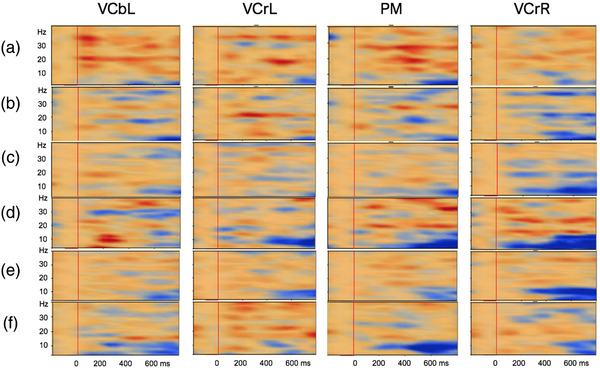
TSE plots across brain regions (VCbL, VCrL, PM, VCrR) when the combined motion condition was compared with the static control condition for a typical extrastimulated infant (a and d), control infant (b and e), and preterm infant (c and f) at the first and second testing session, respectively. Epoch length is −200 to 800 ms, with a baseline of −100 to 0 ms. The red vertical lines indicate stimulus onset at 0 ms. In the TSE plots, induced synchronized and desynchronized activities are shown in red and blue contours, respectively. Induced theta‐band desynchronized activities were observed in all visual areas of interest at both first (a, b, and c) and second (d, e, and f) testing sessions. Synchronization in the beta‐band frequency, however, was observed in the extrastimulated infant at both the first (a) and second testing session (d)
4. DISCUSSION
The present longitudinal study aimed to examine the effects of receiving extrastimulation on the development of functional cortical responses to visual motion in the first year of life. Infants receiving extrastimulation in the form of baby swimming, infants receiving a traditional Western upbringing, and preterm born infants were presented with visual motion on a large screen simulating forward optic flow, reversed optic flow, and random visual motion. VEP and TSE analyses were applied on infants’ evoked and induced electrical brain responses, respectively, to investigate whether extrastimulation was associated with enhanced development of visual motion perception in early infancy, and whether preterm infants showed an abnormal development of visual motion perception compared with their full‐term peers, indicating a possible dorsal stream vulnerability.
4.1. Extra motor stimulation and a greater improvement in visual motion perception
The VEP analysis revealed that during the course of the first postnatal year, developmental improvements in visual motion perception were only observed in infants receiving extra motor stimulation and infants in the control group. This is in line with previous longitudinal studies, which have demonstrated normally developing infants to have faster responses to visual motion stimuli toward the end of the first year of life (Agyei et al., 2015; Rasulo et al., 2021; Vilhelmsen et al., 2019). The ongoing maturation of neuroanatomical structures (Agyei et al., 2015) could partly lead to the relatively faster processing of visual motion and to the shorter latencies found in older infants in the extrastimulated and control group. However, infants receiving extrastimulation showed a greater improvement in visual motion perception during the first year than infants in the control group. Thus, brain maturation is not likely the only factor in the development of visual motion perception, suggesting a close link between self‐generated actions and improved optic flow processing (Agyei et al., 2016a, 2016b; James & Swain, 2011).
Despite genetic factors known to mediate these developmental processes, external influences have been suggested to greatly affect the developing neocortical architecture of the brain (Baroncelli et al., 2010; Berardi et al., 2015; Dubois et al., 2006; Johnson, 2001; Paus et al., 2001). A large number of experiments have shown how rodents raised in stimulating environments show an increase in cortical thickness (Bennett et al., 1964; Forgays & Forgays, 1952; Sirevaag et al., 1988). Several studies on experience‐dependent changes in the cortex have been using animals like cats and monkeys as well, and in general, these studies have found similar results (e.g., Beaulieu & Colonnier, 1987; Floeter & Greenough, 1979; Stell & Riesen, 1987). Among other things, the increase in cortical thickness has been attributed to an enhanced rate of synaptogenesis and myelination of white matter fibers (Markham & Greenough, 2004; Rampon et al., 2000; Sirevaag et al., 1988; Sirevaag & Greenough, 1987), increased complexity in synapse morphology (Sirevaag & Greenough, 1987), and an increase in non‐neuronal metabolic components (Oliet et al., 2001), all of which advance neuronal functions and connectivity. The magnitude of these changes should not be underestimated.
Even though the literature on similar effects in humans remains relatively scarce (Jacobs et al., 1993), enriched stimulation in early infancy has proven beneficial for facilitating brain development and, in particular, visual development in preterm infants (Guzzetta et al., 2009). The VEP analysis in the present study revealed that developmental improvement in visual motion perception appeared to be greater in extrastimulated infants during the first postnatal year than in control and preterm infants. Extrastimulated infants showed significantly shorter N2 latencies for visual motion than both control and preterm infants in the second testing session at 9–12 months, with latencies approximately 100 ms shorter than the control group and 200 ms shorter than the preterm infants. This major difference in brain responses to visual motion indicates that receiving extra motor stimulation during early infancy may accelerate brain development of dorsal stream functions. Interestingly, these results were found despite extrastimulated infants being significantly younger than their full‐term control peers when tested for the second time. Extrastimulated and full‐term control infants were invited for a second testing session between 9 and 12 months depending on the number of weeks they had been crawling. Extrastimulated infants in the present study started to locomote under their own steam at a younger age and were therefore on average almost 5 weeks younger at the time of the second testing. These results reflect the findings of Lobo and Galloway (2012), who found that infants receiving enhanced handling and positioning had a richer perceptual‐motor history than traditionally‐raised infants. Enriched stimulation has been associated with less time spent in a stationary position (Adolph & Hoch, 2019), and an accelerated onset of motor behaviors such as crawling, standing, independent walking, and improved postural control (Adolph & Hoch, 2019; Karasik et al., 2010; Karasik et al., 2015; Lobo & Galloway, 2012; Zelazo et al.,1972). In turn, such behaviors give rise to greater amounts of self‐generated optic flow, which is argued by Gilmore and Rettke (2003) to provide the foundation from which perceptual information becomes functionalized. The current results may therefore suggest that extrastimulated infants were more experienced in processing different patterns of visual motion than their peers, likely due to having received more opportunities to interact with their surroundings, through enhanced handling and activities, for example, baby swimming. Activities such as baby swimming are “enhanced” because they involve behaviors that are not typical of daily life for young infants born into Western cultures, where infants spend considerable time being placed in supine positions by caregivers (Guzzetta et al., 2009). Descriptive studies have linked greater experience in multiple positions in the first months after birth with better development in the first year for healthy infants and infants born preterm and at risk for delays in development (Fetters & Huang, 2007). Considering that optimal brain plasticity occurs in the first few months of life (Bonnier, 2008), the considerably faster brain responses to visual motion in extrastimulated infants in the present study may therefore indicate that receiving stimulation in an upright or prone position during early infancy may increase processing speed by enhancing brain development of dorsal stream functions.
Our findings suggest that the effect of extra motor stimulation is indirect. Infants who are allowed from birth to spend time in an upright or prone position will have opportunity to train their antigravity muscles, which in turn will lead to improved head–eye and eye–hand coordination as well as an earlier emergence of self‐produced locomotion, ultimately enhancing visual motion perception. We propose that parents who take their newborns to baby swimming classes once a week will, in general, also provide a more stimulating (motor) environment through enhanced handling and positioning. Precisely such daily handling practices are likely to be responsible for the earlier onset of locomotor abilities in the extrastimulated infants. We argue that the mechanism associated with extrastimulation is not so much direct via attending baby swimming classes from an early age, but rather indirect because parents who take their babies swimming will be generally more inclined to stimulate their babies’ motor activity, ultimately resulting in the earlier onset of self‐produced locomotion and leading to enhanced cortical processing of visual motion.
4.2. Preferential sensitivities to expanding stimuli
Previous studies have noted the vital role of visuomotor experiences in favoring certain visual stimuli (Anderson et al., 2013; Bell & Fox, 1996; Gilmore et al., 2007). Our VEP analysis also showed that extrastimulated and control infants could significantly differentiate between visual motion conditions at the second testing session, with the shortest latencies for forward optic flow, followed by reversed optic flow, and longest latencies for random visual motion with approximately 70 ms between each visual motion condition. These results corroborate earlier findings (Giaschi et al., 2007; Gilmore et al., 2007; Imura et al., 2008; Shirai et al., 2004) that found preferential sensitivities to expanding as against contracting stimuli in infants, which could be attributed to infants’ experience with locomotion since humans typically move in a forward manner (Agyei et al., 2015; Shirai et al., 2009; Van der Meer et al., 2008). In addition to maturation, optic flow studies have suggested the ability to differentiate between different forms of visual motion to be related to the onset of self‐produced locomotion (Agyei et al., 2015; Rasulo et al., 2021; Vilhelmsen et al., 2019). Extrastimulated infants with self‐produced locomotor experience showed mean latencies for the N2 peak of forward optic flow at just 183 ms after stimulus onset. Considering previous studies that reported N2 latencies to occur at around 130–150 ms after stimulus onset in adults (Probst et al., 1993; Van der Meer et al., 2008), the short latencies observed in older extrastimulated infants suggests a progression toward almost adult‐like responses to optic flow. Further, the faster processing, as indicated by shorter latencies, of forward optic flow in extrastimulated infants compared with control and preterm infants supports the proposition of enriched stimulation acting to accelerate brain development through providing infants with active visuomotor experiences. The faster detection of optic flow than random visual motion could further reflect the general importance of optic flow for effective self‐navigation during infancy (c.f., Warren et al., 2001 ).
4.3. Preterm infants and extrastimulated infants
Interestingly, preterm infants showed significantly shorter latencies at the first session regardless of visual motion condition compared with control infants at 4–5 months of age. The faster perceptual response can be explained by the fact that preterm infants were tested corrected for prematurity, and therefore had up to 3 months longer exposure and experience to real‐world visual flow than their full‐term peers. The current results may therefore suggest that by the time of the first testing session, extrastimulated infants and preterm infants were more experienced in processing different patterns of flow than full‐term infants in the control group. For extrastimulated infants, it is likely due to being given more opportunities to interact with their surroundings (e.g., baby swimming classes), whereas for preterm infants, it is likely to be related to longer experience outside the womb. Both explanations give, in turn, support to the notion of how experience can affect the development of visual motion perception during the first year of life.
4.4. Preterm infants did not show a similar development as seen in their peers
Even though preterm infants had, in fact, more self‐produced locomotion experience than their extrastimulated and full‐term control peers at the time of the second testing, they did not differentiate between the three visual motion conditions nor did their latencies decrease as they got older. Studies have noted that preterm infants at corrected age of 2–3 months are delayed several weeks compared with full‐term infants when differentiating between changes of direction (Birtles et al., 2007; Braddick et al., 2005). One could expect that, when age is corrected for prematurity, preterm infants would follow a developmental path similar to that of full‐term infants. However, in line with our earlier findings, preterm infants in the current study did not show a similar development regarding visual motion processing as seen in their full‐term peers (Agyei et al., 2016a). These findings could indicate a lack of specialization in, and development of, the dorsal visual processing stream. The developmental period of the dorsal stream is believed to be concentrated around the third trimester of pregnancy (Hammarrenger et al., 2007; Klaver et al., 2011), and the cells of the dorsal pathways need high levels of polyunsaturated fatty acids for optimal functioning of physiological processes (Sabel et al., 2009; Stein, 2001), and is, therefore, more prone to be disrupted by the effects of preterm birth. Thus, it is possible that the unimproved latencies observed in preterm infants at the end of the first year corrected for prematurity, indicate a dorsal stream deficit. However, it is also possible that the unimproved latencies and the inability to differentiate between different forms of visual motion in preterm infants at the second session, indicate a normal delay related to premature birth that may be recovered at a later age (Agyei et al., 2016a). A follow‐up study to monitor the developmental progress into school age of the preterm group could help ascertain the validity of this presumption.
4.5. Infant EEG dominated by low‐frequency activity
Induced responses when perceiving visual motion were further examined in the present study. When comparing the TSEs of the combined visual motion condition with those of the static control condition, induced expressions of theta‐band desynchronization were seen in all three infant groups. The finding corroborates earlier studies showing infant EEG to be dominated by low‐frequency activity with larger amplitudes during processing of visual motion (Agyei et al., 2015; Başar et al., 2001; Klimesch, 1999; Van der Meer et al., 2008). Such low‐frequency rhythms typically appear as widespread patterns of cortical activity across the scalp, suggesting greater compositions of neurons to be implicated in slower‐ compared with faster‐oscillating cell assemblies (Orekhova et al., 2006). In light of this, Agyei et al. (2016a) suggested that low‐frequency activation in infants is likely to reflect the employment of larger and less specialized cortical networks and cells when perceiving visual motion. The widespread theta‐band activities observed in the present study may therefore be interpreted as a general sign of immaturity.
4.6. Widespread activities when infants were younger
Further, more prevalent and widespread desynchronized theta‐band activities occurred when infants were young compared with when they were older in each group. A previous study used the same stimulus with forward optic flow, reversed optic flow, and random visual motion, and found that full‐term infants at both 4–5 and 8–11 months displayed theta‐ and alpha‐band oscillations in response to visual motion (Agyei et al., 2016a). The present study corroborates these findings, where extrastimulated and control infants showed low‐frequency oscillatory brain activity in response to visual motion, with an increase from theta‐ to alpha‐band activity as they got older. Alpha‐ and beta‐band oscillations have been suggested to be important for cross‐network functional connectivity (Ganzetti & Mantini, 2013). Moreover, enhancement in beta rhythm synchronization is suggested to serve as an integrative agent for long‐range communication between neuronal populations residing in different cortical regions (Pfurtscheller et al., 1997). This progression may become more evident as infants become adults since studies have observed increased gamma‐band power in the visual cortex during motion processing in adults (Hoogenboom et al., 2006; Krishnan et al., 2005).
Surprisingly, induced responses also indicated that extrastimulated and preterm infants showed less widespread theta‐band activity compared with control infants at the time of the first testing. As such, these findings could suggest that fewer but more specialized neurons were employed during visual motion processing in extrastimulated infants and preterm infants. This can be explained by the fact that infants who received extrastimulation and preterm infants that were tested corrected for prematurity have had more experience with visual motion processing. This is in line with the VEP analysis from the first testing session, showing extrastimulated and preterm infants to have significantly shorter latencies across motion conditions compared with full‐term control infants. These findings further support the idea of enhanced stimulation and experience in early infancy to act as a facilitating agent in advancing brain development.
4.7. Induced responses in extrastimulated infants
In addition to induced alpha‐band desynchronization, synchronized activities in the beta‐band frequency could be seen in the TSE maps for extrastimulated infants in the second testing session. The finding is in accordance with earlier observations by Agyei et al. (2016a), showing beta‐band synchronization in response to visual motion toward the end of the first year of life. Compared with alpha‐band frequency, beta‐band rhythms are implied to involve fewer but more specialized neurons (Pfurtscheller et al., 1997). As these activities have been reported in response to optic flow stimuli in adults (Van der Meer et al., 2008), expressions of synchronized beta‐band oscillations in extrastimulated infants, and the absence of these in the control and preterm infants, suggest that induced responses were developmentally advanced in extrastimulated infants. Observation of beta‐band oscillations in the extrastimulated infants at the second session could further explain the significantly shorter latencies for VEPs displayed by extrastimulated infants when they were older and had more experience with self‐produced locomotion.
4.8. Preterm infants and disrupted dorsal visual stream development
Preterm infants at the second session showed no synchronized oscillatory activities in the alpha‐beta range, but more widespread theta‐band desynchronization when the TSEs of the combined motion condition were compared with the static control pattern. The absence of high‐frequency activity observed in preterm infants at the second session, combined with the longer VEP latencies, indicates that preterm infants have not yet developed sufficiently specialized networks for rapid processing of visual motion information. These findings reflect previous studies, which have found that preterm infants have less myelinated cortical white matter (Hüppi et al., 1998; Mewes et al., 2006), and demonstrate slower cortical growth (Kapellou et al., 2006) than their full‐term peers.
Preterm infants in the present study did not show a similar progression when it comes to visual motion processing with age and locomotor experience as their full‐term peers. Existing interventions aimed at minimizing disabilities and improving capabilities in children with, or at risk of, neurodevelopmental disorders have been suggested to be most effective when focusing on enhancing caregiver–infant interactions and advancing general motor development (Blauw‐Hospers & Hadders‐Algra, 2005; Dusing et al., 2013; Heathcock et al., 2008).
4.9. Conclusion and future research
The present longitudinal study demonstrated a strong link between receiving extra motor stimulation in early infancy and accelerated developmental improvements of visual motion perception as observed with high‐density EEG. The study confirmed that during their first year, infants receiving extra motor stimulation showed an overall greater sensitivity to visual motion than their traditionally‐raised peers and preterm infants, as reflected by shorter latencies in response to visual motion and oscillatory activities at higher frequencies. The greater improvement in extrastimulated infants was attributed to their caregivers’ overall handling patterns, including enriched activities such as baby swimming. The poorer responses in the preterm infants were associated with impairment of the dorsal visual stream specialized in the processing of visual motion.
The present findings may prove beneficial for subsequent attempts to improve the developmental outcome in premature infants who are at risk for abnormal visuomotor and neurological development. Ideally, the study would have included a fourth group of preterm infants that received extrastimulation. The fourth group would help to clarify the potential mechanism(s) behind the current findings. Future and follow‐up studies may reveal whether the accelerated development of visual motion perception observed in extrastimulated children persists through childhood and may prove useful in the early diagnosis of a dorsal stream vulnerability in preterm infants, as well as provide clues for early intervention.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
We would like to thank all babies and their parents for taking part in this study. We are also grateful to Amir Jahanian, Katharina Bock, Stefania Rasulo, and consultant neonatologist Ragnhild Støen for their help with recruitment. We are dedicating this article to Paal Aagaard, baby swimming pioneer in Norway, who passed away in October 2021. For over 40 years, he introduced hundreds of babies to the joys of moving their bodies in the water.
Borge Blystad, J. , & van der Meer, A. L. H. (2022). Longitudinal study of infants receiving extra motor stimulation, full‐term control infants, and infants born preterm: High‐density EEG analyses of cortical activity in response to visual motion. Developmental Psychobiology, 64, e22276. 10.1002/dev.22276
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- Aarnoudse‐Moens, C. S. H. , Weisglas‐Kuperus, N. , van Goudoever, J. B. , & Oosterlaan, J. (2009). Meta‐analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics, 124(2), 717–728. 10.1542/peds.2008-2816 [DOI] [PubMed] [Google Scholar]
- Adolph, K. E. , & Hoch, J. E. (2019). Motor development: Embodied, embedded, enculturated, and enabling. Annual Review of Psychology, 70, 141–164. 10.1146/annurev-psych-010418-102836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agyei, S. B. , Holth, M. , Van der Weel, F. R. , & Van der Meer, A. L. H. (2015). Longitudinal study of perception of structured optic flow and random visual motion in infants using high‐density EEG. Developmental Science, 18(3), 436–451. 10.1111/desc.12221 [DOI] [PubMed] [Google Scholar]
- Agyei, S. B. , Van der Weel, F. R. , & Van der Meer, A. L. H. (2016a). Longitudinal study of preterm and full‐term infants: High‐density EEG analyses of cortical activity in response to visual motion. Neuropsychologia, 84, 89–104. 10.1016/j.neuropsychologia.2016.02.001 [DOI] [PubMed] [Google Scholar]
- Agyei, S. B. , Van der Weel, F. R. , & Van der Meer, A. L. H. (2016b). Development of visual motion perception for prospective control: Brain and behavioral studies in infants. Frontiers in Psychology, 7, 100. 10.3389/fpsyg.2016.00100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlfors, S. , Simpson, G. , Dale, A. , Belliveau, J. , Liu, A. K. , Korvenoja, A. , Virtanen, J. , Huotilainen, M. , Tootell, R. B. H. , Aronen, H. J. , & Ilmoniemi, R. J. (1999). Spatiotemporal activity of a cortical network for processing visual motion revealed by MEG and fMRI. Journal of Neurophysiology, 82(5), 2545–2555. 10.1152/jn.1999.82.5.2545 [DOI] [PubMed] [Google Scholar]
- Anderson, D. I. , Campos, J. J. , Witherington, D. C. , Dahl, A. , Rivera, M. , He, M. , Uchiyama, I. , & Barbu‐Roth, M. (2013). The role of locomotion in psychological development. Frontiers in Psychology, 4, 10.3389/fpsyg.2013.00440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson, J. , & Braddick, O. (2007). Visual and visuocognitive development in children born very prematurely. Progress in Brain Research, 164, 123–149. 10.1016/S0079-6123(07)64007-2 [DOI] [PubMed] [Google Scholar]
- Baroncelli, L. , Braschi, C. , Spolidoro, M. , Begenisic, T. , Sale, A. , & Maffei, L. (2010). Nurturing brain plasticity: Impact of environmental enrichment. Cell Death & Differentiation, 17(7), 1092–1103. 10.1038/cdd.2009.193 [DOI] [PubMed] [Google Scholar]
- Başar, E. , Başar‐Eroglu, C. , Karakaş, S. , & Schürmann, M. (2001). Gamma, alpha, delta, and theta oscillations govern cognitive processes. International Journal of Psychophysiology, 39(2‐3), 241–248. 10.1016/S0167-8760(00)00145-8 [DOI] [PubMed] [Google Scholar]
- Beaulieu, C. , & Colonnier, M. (1987). Effect of the richness of the environment on the cat visual cortex. Journal of Comparative Neurology, 266(4), 478–494. 10.1002/cne.902660404 [DOI] [PubMed] [Google Scholar]
- Bell, M. A. , & Fox, N. A. (1996). Crawling experience is related to changes in cortical organization during infancy: Evidence from EEG coherence. Developmental Psychobiology, 29(7), 551–561 [DOI] [PubMed] [Google Scholar]
- Bennett, E. L. , Diamond, M. C. , Krech, D. , & Rosenzweig, M. R. (1964). Chemical and anatomical plasticity of brain. Science, 146(3644), 610–619. http://www.jstor.org/stable/1714515, 10.1126/science.146.3644.610 [DOI] [PubMed] [Google Scholar]
- Berardi, N. , Sale, A. , & Maffei, L. (2015). Brain structural and functional development: Genetics and experience. Developmental Medicine & Child Neurology, 57, 4–9. 10.1111/dmcn.12691 [DOI] [PubMed] [Google Scholar]
- Berg, P. , & Scherg, M. (1994). A multiple source approach to the correction of eye artifacts. Electroencephalography and Clinical Neurophysiology, 90(3), 229–241. 10.1016/0013-4694(94)90094-9 [DOI] [PubMed] [Google Scholar]
- Bertenthal, B. I. , & Campos, J. J. (1990). A systems approach to the organizing effects of self‐produced locomotion during infancy. Advances in Infancy Research, 6, 1–60. [Google Scholar]
- Birtles, D. B. , Braddick, O. J. , Wattam‐Bell, J. , Wilkinson, A. R. , & Atkinson, J. (2007). Orientation and motion‐specific visual cortex responses in infants born preterm. Neuroreport, 18(18), 1975–1979. 10.1097/WNR.0b013e3282f228c8 [DOI] [PubMed] [Google Scholar]
- Blauw‐Hospers, C. H. , & Hadders‐Algra, M. (2005). A systematic review of the effects of early intervention on motor development. Developmental Medicine & Child Neurology, 47(6), 421–432. 10.1017/s0012162205000824 [DOI] [PubMed] [Google Scholar]
- Bhutta, A. T. , Cleves, M. A. , Casey, P. H. , Cradock, M. M. , & Anand, K. J. (2002). Cognitive and behavioral outcomes of school‐aged children who were born preterm: A meta‐analysis. Jama, 288(6), 728–737. 10.1001/jama.288.6 [DOI] [PubMed] [Google Scholar]
- Bonnier, C. (2008). Evaluation of early stimulation programs for enhancing brain development. Acta Paediatrica, 97(7), 853–858. 10.1111/j.1651-2227.2008.00834.x [DOI] [PubMed] [Google Scholar]
- Braddick, O. , Atkinson, J. , & Wattam‐Bell, J. (2003). Normal and anomalous development of visual motion processing: Motion coherence and “dorsal‐stream vulnerability. Neuropsychologia, 41, 1769–1984. 10.1016/S0028-3932(03)00178-7 [DOI] [PubMed] [Google Scholar]
- Braddick, O. , Birtles, D. , Wattam‐Bell, J. , & Atkinson, J. (2005). Motion‐and orientation‐specific cortical responses in infancy. Vision Research, 45(25‐26), 3169–3179. 10.1016/j.visres.2005.07.021 [DOI] [PubMed] [Google Scholar]
- Buzsáki, G. , & Draguhn, A. (2004). Neuronal oscillations in cortical networks. Science, 304(5679), 1926–1929. 10.1126/science.1099745 [DOI] [PubMed] [Google Scholar]
- Campos, J. J. , Anderson, D. I. , Barbu‐Roth, M. A. , Hubbard, E. M. , Hertenstein, M. J. , & Witherington, D. (2000). Travel broadens the mind. Infancy, 1(2), 149–219. 10.1207/S15327078IN0102_1 [DOI] [PubMed] [Google Scholar]
- Corbetta, D. , Friedman, D. R. , & Bell, M. A. (2014). Brain reorganization as a function of walking experience in 12‐month‐old infants: Implications for the development of manual laterality. Frontiers in Psychology: Cognition, 5, 245. 10.3389/fpsyg.2014.00245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counsell, S. J. , & Boardman, J. P. (2005). Differential brain growth in the infant born preterm: Current knowledge and future developments from brain imaging. Seminars in Fetal and Neonatal Medicine, 10(5), 403–410. 10.1177/0956797613476047 [DOI] [PubMed] [Google Scholar]
- ( De Haan, M. Ed.) (2013). Infant EEG and event‐related potentials. East Sussex, UK: Psychology Press. [Google Scholar]
- De Jong, M. , Verhoeven, M. , & Van Baar, A. L. (2012). School outcome, cognitive functioning, and behaviour problems in moderate and late preterm children and adults: A review. Seminars in Fetal and Neonatal Medicine, 17(3), 163–169. 10.1016/j.siny.2012.02.003 [DOI] [PubMed] [Google Scholar]
- Delobel‐Ayoub, M. , Arnaud, C. , White‐Koning, M. , Casper, C. , Pierrat, V. , Garel, M. , Burguet, A. , Roze, J.‐C. , Matis, J. , Picaud, J.‐C. , Kaminski, M. , & Larroque, B. (2009). Behavioral problems and cognitive performance at 5 years of age after very preterm birth: The EPIPAGE Study. Pediatrics, 123(6), 1485–1492. 10.1542/peds.2008-1216 [DOI] [PubMed] [Google Scholar]
- Dubois, J. , Hertz‐Pannier, L. , Dehaene‐Lambertz, G. , Cointepas, Y. , & Le Bihan, D. (2006). Assessment of the early organization and maturation of infants' cerebral white matter fiber bundles: A feasibility study using quantitative diffusion tensor imaging and tractography. Neuroimage, 30(4), 1121–1132. 10.1016/j.neuroimage.2005.11.022 [DOI] [PubMed] [Google Scholar]
- Dusing, S. C. , Lobo, M. A. , Lee, H. M. , & Galloway, J. C. (2013). Intervention in the first weeks of life for infants born late preterm: A case series. Pediatric Physical Therapy, 25(2), 194. 10.1097/PEP.0b013e3182888b86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel, A. K. , & Fries, P. (2010). Beta‐band oscillations—signaling the status quo? Current Opinion in Neurobiology, 20(2), 156–165. 10.1016/j.conb.2010.02.015 [DOI] [PubMed] [Google Scholar]
- Fetters, L. , & Huang, H. H. (2007). Motor development and sleep, play, and feeding positions in very‐low‐birthweight infants with and without white matter disease. Developmental Medicine & Child Neurology, 49(11), 807–813. 10.1111/j.1469-8749.2007.00807.x [DOI] [PubMed] [Google Scholar]
- Floeter, M. K. , & Greenough, W. T. (1979). Cerebellar plasticity: Modification of Purkinje cell structure by differential rearing in monkeys. Science, 206(4415), 227–229. 10.1126/science.113873 [DOI] [PubMed] [Google Scholar]
- Forgays, D. G. , & Forgays, J. W. (1952). The nature of the effect of free environmental experience in the rat. Journal of Comparative and Physiological Psychology, 45(4), 322–328. 10.1037/h0053731 [DOI] [PubMed] [Google Scholar]
- Fox, N. A. , & Rutter, M. (2010). Introduction to the special section on the effects of early experience on development. Child Development, 81(1), 23–27. http://www.jstor.org/stable/40598963, 10.1111/j.1467-8624.2009.01379.x [DOI] [PubMed] [Google Scholar]
- Fries, P. (2005). A mechanism for cognitive dynamics: Neuronal communication through neuronal coherence. Trends in Cognitive Sciences, 9(10), 474–480. 10.1016/j.tics.2005.08.011 [DOI] [PubMed] [Google Scholar]
- Ganzetti, M. , & Mantini, D. (2013). Functional connectivity and oscillatory neuronal activity in the resting human brain. Neuroscience, 240, 297–309. 10.1016/j.neuroscience.2013.02.032 [DOI] [PubMed] [Google Scholar]
- Giaschi, D. , Zwicker, A. , Young, S. A. , & Bjornson, B. (2007). The role of cortical area V5/MT+ in speed‐tuned directional anisotropies in global motion perception. Vision Research, 47(7), 887–898. 10.1016/j.visres.2006.12.017 [DOI] [PubMed] [Google Scholar]
- Gibson, J. J. (2015). The ecological approach to visual perception (Classic edition). East Sussex, UK: Psychology Press. [Google Scholar]
- Gilmore, R. O. , Baker, T. J. , & Grobman, K. (2004). Stability in young infants' discrimination of optic flow. Developmental Psychology, 40(2), 259. 10.1037/0012-1649.40.2.259 [DOI] [PubMed] [Google Scholar]
- Gilmore, R. O. , Hou, C. , Pettet, M. W. , & Norcia, A. M. (2007). Development of cortical responses to optic flow. Visual Neuroscience, 24(6), 845–856. 10.1017/S0952523807070769 [DOI] [PubMed] [Google Scholar]
- Gilmore, R. O. , & Rettke, H. J. (2003). Four‐month‐olds' discrimination of optic flow patterns depicting different directions of observer motion. Infancy, 4(2), 177–200. 10.1207/S15327078IN0402_02 [DOI] [Google Scholar]
- Greenough, W. T. , Black, J. E. , & Wallace, C. S. (1987). Experience and brain development. Child Development, 58, 539–559. 10.2307/1130197 [DOI] [PubMed] [Google Scholar]
- Grieve, P. G. , Emerson, R. G. , Fifer, W. P. , Isler, J. R. , & Stark, R. I. (2003). Spatial correlation of the infant and adult electroencephalogram. Clinical Neurophysiology, 114(9), 1594–1608. 10.1016/S1388-2457(03)00122-6 [DOI] [PubMed] [Google Scholar]
- Guzzetta, A. , Baldini, S. , Bancale, A. , Baroncelli, L. , Ciucci, F. , Ghirri, P. , Putignano, E. , Sale, A. , Viegi, A. , Berardi, N. , Boldrini, A. , Cioni, G. , & Maffei, L. (2009). Massage accelerates brain development and the maturation of visual function. Journal of Neuroscience, 29(18), 6042–6051. 10.1523/JNEUROSCI.5548-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarrenger, B. , Roy, M. S. , Ellemberg, D. , Labrosse, M. , Orquin, J. , Lippe, S. , & Lepore, F. (2007). Developmental delay and magnocellular visual pathway function in very‐low‐birthweight preterm infants. Developmental Medicine & Child Neurology, 49(1), 28–33. 10.1017/s0012162207000084.x [DOI] [PubMed] [Google Scholar]
- Heathcock, J. C. , Lobo, M. , & Galloway, J. C. (2008). Movement training advances the emergence of reaching in infants born at less than 33 weeks of gestational age: A randomized clinical trial. Physical Therapy, 88(3), 310–322. 10.2522/ptj.20070145 [DOI] [PubMed] [Google Scholar]
- Heinrich, S. P. , Renkl, A. E. , & Bach, M. (2005). Pattern specificity of human visual motion processing. Vision Research, 45(16), 2137–2143. 10.1016/j.visres.2005.02.008 [DOI] [PubMed] [Google Scholar]
- Higgins, C. I. , Campos, J. J. , & Kermoian, R. (1996). Effect of self‐produced locomotion on infant postural compensation to optic flow. Developmental Psychology, 32(5), 836–841. 10.1037/0012-1649.32.5.836 [DOI] [Google Scholar]
- Hoechstetter, K. , Bornfleth, H. , Weckesser, D. , Ille, N. , Berg, P. , & Scherg, M. (2004). BESA source coherence: A new method to study cortical oscillatory coupling. Brain Topography, 16(4), 233–238. 10.1023/B:BRAT.0000032857.55223.5d [DOI] [PubMed] [Google Scholar]
- Hoogenboom, N. , Schoffelen, J. M. , Oostenveld, R. , Parkes, L. M. , & Fries, P. (2006). Localizing human visual gamma‐band activity in frequency, time and space. Neuroimage, 29(3), 764–773. 10.1016/j.neuroimage.2005.08.043 [DOI] [PubMed] [Google Scholar]
- Hudspeth, W. J. , & Pribram, K. H. (1992). Psychophysiological indices of cerebral maturation. International Journal of Psychophysiology, 12(1), 19–29. 10.1016/0167-8760(92)90039-E [DOI] [PubMed] [Google Scholar]
- Hüppi, P. S. , Maier, S. E. , Peled, S. , Zientara, G. P. , Barnes, P. D. , Jolesz, F. A. , & Volpe, J. J. (1998). Microstructural development of human newborn cerebral white matter assessed in vivo by diffusion tensor magnetic resonance imaging. Pediatric Research, 44, 584–590. 10.1203/00006450-199810000-00019 [DOI] [PubMed] [Google Scholar]
- Huttenlocher, P. R. , & Bonnier, C. (1991). Effects of changes in the periphery on development of the corticospinal motor system in the rat. Developmental Brain Research, 60(2), 253–260. 10.1016/0165-3806(91)90054-m [DOI] [PubMed] [Google Scholar]
- Imura, T. , Shirai, N. , Tomonaga, M. , Yamaguchi, M. K. , & Yagi, A. (2008). Asymmetry in the perception of motion in depth induced by moving cast shadows. Journal of Vision, 8(13), 10. 10.1167/8.13.10 [DOI] [PubMed] [Google Scholar]
- Jacobs, B. , Schall, M. , & Scheibel, A. B. (1993). A quantitative dendritic analysis of Wernicke's area in humans. II. Gender, hemispheric, and environmental factors. Journal of Comparative Neurology, 327(1), 97–111. 10.1002/cne.903270108 [DOI] [PubMed] [Google Scholar]
- James, K. H. , & Swain, S. N. (2011). Only self‐generated actions create sensori‐motor systems in the developing brain. Developmental Science, 14(4), 673–678. 10.1111/j.1467-7687.2010.01011.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, M. H. (2001). Functional brain development in humans. Nature Reviews Neuroscience, 2(7), 475–483. 10.1038/35081509 [DOI] [PubMed] [Google Scholar]
- Johnson, S. (2007). Cognitive and behavioural outcomes following very preterm birth. Seminars in Fetal and Neonatal Medicine, 12(5), 363–373. 10.1016/j.siny.2007.05.004 [DOI] [PubMed] [Google Scholar]
- Kapellou, O. , Counsell, S. J. , Kennea, N. , Dyet, L. , Saeed, N. , Stark, J. , Maalouf, E. , Duggan, P. , Ajayi‐Obe, M. , Hajnal, J. , Allsop, J. M. , Boardman, J. , Rutherford, F. C. , & Edwards, A. D. (2006). Abnormal cortical development after premature birth shown by altered allometric scaling of brain growth. PLoS One, 3(8), 1382–1390. 10.2174/1389202919666171229144807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasik, L. B. , Adolph, K. E. , Tamis‐LeMonda, C. S. , & Bornstein, M. H. (2010). WEIRD walking: Cross‐cultural research on motor development. Behavioral and Brain Sciences, 33(2‐3), 95–96. 10.1017/S0140525X10000117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasik, L. B. , Tamis‐LeMonda, C. S. , Adolph, K. E. , & Bornstein, M. H. (2015). Places and postures: A cross‐cultural comparison of sitting in 5‐month‐olds. Journal of Cross‐Cultural Psychology, 46(8), 1023–1038. 10.1177/0022022115593803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kermoian, R. , & Campos, J. J. (1988). Locomotor experience: A facilitator of spatial cognitive development. Child Development, 59(4), 908–917. 10.2307/1130258 [DOI] [PubMed] [Google Scholar]
- Klaver, P. , Marcar, V. , & Martin, E. (2011). Neurodevelopment of the visual system in typically developing children. Progress in Brain Research, 189, 113–136. 10.1016/B978-0-444-53884-0.00021-X [DOI] [PubMed] [Google Scholar]
- Klimesch, W. (1999). EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Research Reviews, 29(2‐3), 169–195. 10.1016/S0165-0173(98)00056-3 [DOI] [PubMed] [Google Scholar]
- Kolb, B. , & Whishaw, I. Q. (1989). Plasticity in the neocortex: Mechanisms underlying recovery from early brain damage. Progress in Neurobiology, 32(4), 235–276. 10.1016/0301-0082(89)90023-3 [DOI] [PubMed] [Google Scholar]
- Kolb, B. , & Whishaw, I. Q. (1998). Brain plasticity and behavior. Annual Review of Psychology, 49, 43–64. 10.1146/annurev.psych.49.1.43 [DOI] [PubMed] [Google Scholar]
- Krishnan, G. P. , Skosnik, P. D. , Vohs, J. L. , Busey, T. A. , & O'Donnell, B. F. (2005). Relationship between steady‐state and induced gamma activity to motion. Neuroreport, 16(6), 625–630. 10.1097/00001756-200504250-00022 [DOI] [PubMed] [Google Scholar]
- Kuba, M. , Kubová, Z. , Kremláček, J. , & Langrová, J. (2007). Motion‐onset VEPs: Characteristics, methods, and diagnostic use. Vision Research, 47(2), 189–202. 10.1016/j.visres.2006.09.020 [DOI] [PubMed] [Google Scholar]
- Lee, H.‐M. , & Galloway, J. C. (2012). Early intensive postural and movement training advances head control in very young infants. Physical Therapy, 92(7), 935–947. 10.2522/ptj.20110196 [DOI] [PubMed] [Google Scholar]
- Lobo, M. A. , & Galloway, J. C. (2008). Postural and object‐oriented experiences advance early reaching, object exploration, and means‐end behavior. Child Development, 79(6), 1869–1890. 10.1111/j.1467-8624.2008.01231.x [DOI] [PubMed] [Google Scholar]
- Lobo, M. A. , & Galloway, J. C. (2012). Enhanced handling and positioning in early infancy advances development throughout the first year. Child Development, 83(4), 1290–1302. 10.1111/j.1467-8624.2012.01772.x [DOI] [PubMed] [Google Scholar]
- Maris, E. , & Oostenveld, R. (2007). Nonparametric statistical testing of EEG‐ and MEG‐data. Journal of Neuroscience Methods, 164(1), 177–190. 10.1016/j.jneumeth.2007.03.024 [DOI] [PubMed] [Google Scholar]
- Markham, J. A. , & Greenough, W. T. (2004). Experience‐driven brain plasticity: Beyond the synapse. Neuron Glia Biology, 1(4), 351–363. 10.1017/s1740925x05000219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mewes, A. U. J. , Hüppi, P. S. , Als, H. , Rybicki, F. J. , Inder, T. E. , McAnulty, G. B. , Mulkern, R. V. , Robertson, R. L. , Rivkin, M. J. , & Warfield, S. K. (2006). Regional brain development in serial magnetic resonance imaging of low‐risk preterm infants. Pediatrics, 118(1), 23–33. 10.1542/peds.2005-2675 [DOI] [PubMed] [Google Scholar]
- Mohammed, A. H. , Zhu, S. W. , Darmopil, S. , Hjerling‐Leffler, J. , Ernfors, P. , Winblad, B. , Diamond, M. C. , Eriksson, P. S. , Bogdanovic, N. , & Bogdanovic, N. (2002). Environmental enrichment and the brain. Progress in Brain Research, 138, 109–133. 10.1016/S0079-6123(02)38074-9 [DOI] [PubMed] [Google Scholar]
- Oliet, S. H. , Piet, R. , & Poulain, D. A. (2001). Control of glutamate clearance and synaptic efficacy by glial coverage of neurons. Science, 292(5518), 923–926. 10.1126/science.1059162 [DOI] [PubMed] [Google Scholar]
- Orekhova, E. , Stroganova, T. , Posikera, I. , & Elam, M. (2006). EEG theta rhythm in infants and preschool children. Clinical Neurophysiology, 117(5), 1047–1062. 10.1016/j.clinph.2005.12.027 [DOI] [PubMed] [Google Scholar]
- Paus, T. , Collins, D. L. , Evans, A. C. , Leonard, G. , Pike, B. , & Zijdenbos, A. (2001). Maturation of white matter in the human brain: A review of magnetic resonance studies. Brain Research Bulletin, 54(3), 255–266. 10.1016/s0361-9230(00)00434-2 [DOI] [PubMed] [Google Scholar]
- Pfurtscheller, G. (2001). Functional brain imaging based on ERD/ERS. Vision Research, 41(10‐11), 1257–1260. 10.1016/s0042-6989(00)00235-2 [DOI] [PubMed] [Google Scholar]
- Pfurtscheller, G. , Stancak, Jr, A. , & Edlinger, G. (1997). On the existence of different types of central beta rhythms below 30 Hz. Electroencephalography and Clinical Neurophysiology, 102(4), 316–325. 10.1016/S0013-4694(96)96612-2 [DOI] [PubMed] [Google Scholar]
- Probst, T. , Plendl, H. , Paulus, W. , Wist, E. , & Scherg, M. (1993). Identification of the visual motion area (area V5) in the human brain by dipole source analysis. Experimental Brain Research, 93(2), 345–351. 10.1007/BF00228404 [DOI] [PubMed] [Google Scholar]
- Rampon, C. , Jiang, C. H. , Dong, H. , Tang, Y. P. , Lockhart, D. J. , Schultz, P. G. , …Hu, Y. (2000). Effects of environmental enrichment on gene expression in the brain. Proceedings of the National Academy of Sciences of the United States of America, 97(23), 12880–12884. 10.1073/pnas.97.23.12880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasulo, S. , Vilhelmsen, K. , Van der Weel, F. R. , & Van der Meer, A. L. H. (2021). Development of motion speed perception from infancy to early adulthood: A high‐density EEG study of simulated forward motion through optic flow. Experimental Brain Research, 239, 3143–3154. 10.1007/s00221-021-06195-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosander, K. , Nyström, P. , Gredebäck, G. , & Von Hofsten, C. (2007). Cortical processing of visual motion in young infants. Vision Research, 47(12), 1614–1623. 10.1016/j.visres.2007.03.004 [DOI] [PubMed] [Google Scholar]
- Sabel, K. G. , Lundqvist‐Persson, C. , Bona, E. , Petzold, M. , & Strandvik, B. (2009). Fatty acid patterns early after premature birth, simultaneously analysed in mothers' food, breast milk and serum phospholipids of mothers and infants. Lipids in Health and Disease, 8(1), 1–15. 10.1002/icd.353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saby, J. N. , & Marshall, P. J. (2012). The utility of EEG band power analysis in the study of infancy and early childhood. Developmental Neuropsychology, 37(3), 253–273. 10.1080/87565641.2011.614663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack, A. T. , Kohler, A. , Linden, D. E. J. , Goebel, R. , & Muckli, L. (2006). The temporal characteristics of motion processing in hMT/V5+: Combining fMRI and neuronavigated TMS. Neuroimage, 29(4), 1326–1335. 10.1016/j.neuroimage.2005.08.027 [DOI] [PubMed] [Google Scholar]
- Salt, A. , & Redshaw, M. (2006). Neurodevelopmental follow‐up after preterm birth: Follow up after two years. Early Human Development, 82(3), 185–197. 10.1016/j.earlhumdev.2005.12.015 [DOI] [PubMed] [Google Scholar]
- Scherg, M. , & Berg, P. (1991). Use of prior knowledge in brain electromagnetic source analysis. Brain Topography, 4(2), 143–150. 10.1007/BF01132771 [DOI] [PubMed] [Google Scholar]
- Scherg, M. , Ille, N. , Bornfleth, H. , & Berg, P. (2002). Advanced tools for digital EEG review: Virtual source montages, whole‐head mapping, correlation, and phase analysis. Journal of Clinical Neurophysiology, 19(2), 91–112. 10.1097/00004691-200203000-00001 [DOI] [PubMed] [Google Scholar]
- Shirai, N. , Birtles, D. , Wattam‐Bell, J. , Yamaguchi, M. K. , Kanazawa, S. , Atkinson, J. , & Braddick, O. (2009). Asymmetrical cortical processing of radial expansion/contraction in infants and adults. Developmental Science, 12(6), 946–955. 10.1111/j.1467-7687.2009.00839.x [DOI] [PubMed] [Google Scholar]
- Shirai, N. , Kanazawa, S. , & Yamaguchi, M. K. (2004). Asymmetry for the perception of expansion/contraction in infancy. Infant Behavior and Development, 27(3), 315–322. 10.1016/j.infbeh.2003.12.004 [DOI] [Google Scholar]
- Sigmundsson, H. , & Hopkins, B. (2010). Baby swimming: Exploring the effects of early intervention on subsequent motor abilities. Child: Care, Health and Development, 36(3), 428–430. 10.1111/j.1365.2214.2009.00990.x [DOI] [PubMed] [Google Scholar]
- Sirevaag, A. M. , Black, J. E. , Shafron, D. , & Greenough, W. T. (1988). Direct evidence that complex experience increases capillary branching and surface area in visual cortex of young rats. Developmental Brain Research, 43(2), 299–304. 10.1016/0165-3806(88)90107-1 [DOI] [PubMed] [Google Scholar]
- Sirevaag, A. M. , & Greenough, W. T. (1987). Differential rearing effects on rat visual cortex synapses.: III. Neuronal and glial nuclei, boutons, dendrites, and capillaries. Brain Research, 424(2), 320–332. 10.1016/0006-8993(87)91477-6 [DOI] [PubMed] [Google Scholar]
- Stanfield, B. B. , O'Leary, D. D. , & Fricks, C. (1982). Selective collateral elimination in early postnatal development restricts cortical distribution of rat pyramidal tract neurones. Nature, 298(5872), 371–373. 10.1038/298371a0 [DOI] [PubMed] [Google Scholar]
- Stein, J. (2001). The magnocellular theory of developmental dyslexia. Dyslexia, 7(1), 12–36. 10.1002/dys.186 [DOI] [PubMed] [Google Scholar]
- Stell, M. , & Riesen, A. (1987). Effects of early environments on monkey cortex neuroanatomical changes following somatomotor experience: Effects on layer III pyramidal cells in monkey cortex. Behavioral Neuroscience, 101(3), 341–346. 10.1037/0735-7044.101.3.341 [DOI] [PubMed] [Google Scholar]
- Stroganova, T. A. , Orekhova, E. V. , & Posikera, I. N. (1999). EEG alpha rhythm in infants. Clinical Neurophysiology, 110(6), 997–1012. 10.1016/S1388-2457(98)00009-1 [DOI] [PubMed] [Google Scholar]
- Taylor, N. M. , Jakobson, L. S. , Maurer, D. , & Lewis, T. L. (2009). Differential vulnerability of global motion, global form, and biological motion processing in full‐term and preterm children. Neuropsychologia, 47(13), 2766–2778. 10.1016/j.neuropsychologia.2009.06.001 [DOI] [PubMed] [Google Scholar]
- Tremblay, E. , Vannasing, P. , Roy, M.‐S. , Lefebvre, F. , Kombate, D. , Lassonde, M. , Lepore, F. , McKerral, M. , & Gallagher, A. (2014). Delayed early primary visual pathway development in premature infants: High density electrophysiological evidence. PLoS One, 9(9), 1–9. 10.1371/journal.pone.0107992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama, I. , Anderson, D. I. , Campos, J. J. , Witherington, D. , Frankel, C. B. , Lejeune, L. , & Barbu‐Roth, M. (2008). Locomotor experience affects self and emotion. Developmental Psychology, 44(5), 1225–123. 10.1037/a0013224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno, M. , Uchiyama, I. , Campos, J. J. , Anderson, D. I. , He, M. , & Dahl, A. (2018). Crawling experience relates to postural and emotional reactions to optic flow in a virtual moving room. Journal of Motor Learning and Development, 6(s1), S63–S75. 10.1123/jmld.2016-0063 [DOI] [Google Scholar]
- Vaina, L. M. , & Rushton, S. K. (2000). What neurological patients tell us about the use of optic flow. International Review of Neurobiology, 44, 293–313. 10.1016/S0074-7742(08)60747-3 [DOI] [PubMed] [Google Scholar]
- Van der Meer, A. L. H. , Fallet, G. , & Van der Weel, F. R. (2008). Perception of structured optic flow and random visual motion in infants and adults: A high‐density EEG study. Experimental Brain Research, 186(3), 493–502. 10.1007/s00221-007-1251-2 [DOI] [PubMed] [Google Scholar]
- Van der Weel, F. R. , & Van der Meer, A. L. H. (2009). Seeing it coming: Infants’ brain responses to looming danger. Die Naturwissenschaften, 96(12), 1385–1391. 10.1007/s00114-009-0585-y [DOI] [PubMed] [Google Scholar]
- Vilhelmsen, K. , Agyei, S. B. , Van der Weel, F. R. , & Van der Meer, A. L. H. (2019). A high‐density EEG study of differentiation between two speeds and directions of simulated optic flow in adults and infants. Psychophysiology, 56(1), e13281. 10.1111/psyp.13281 [DOI] [PubMed] [Google Scholar]
- Walle, E. , & Campos, J. (2014). Infant language development is related to the acquisition of walking. Developmental Psychology, 50(2), 336–348. 10.1037/a0033238 [DOI] [PubMed] [Google Scholar]
- Warren, W. H. , Kay, B. A. , Zosh, W. D. , Duchon, A. P. , & Sahuc, S. (2001). Optic flow is used to control human walking. Nature Neuroscience, 4(2), 213–216. 10.1038/84054 [DOI] [PubMed] [Google Scholar]
- Webb, S. J. , Long, J. D. , & Nelson, C. A. (2005). A longitudinal investigation of visual event‐related potentials in the first year of life. Developmental Science, 8(6), 605–616. 10.1111/j.1467-7687.2005.00452.x [DOI] [PubMed] [Google Scholar]
- Zeki, S. , Watson, J. D. , Lueck, C. J. , Friston, K. J. , Kennard, C. , & Frackowiak, R. S. (1991). A direct demonstration of functional specialization in human visual cortex. Journal of Neuroscience, 11(3), 641–649. 10.1523/JNEUROSCI.11-03-00641.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelazo, P. R. , Zelazo, N. A. , & Kolb, S. (1972). “Walking” in the newborn. Science, 176(4032), 314–315. 10.1126/science.176.4032.314 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


