Abstract
Aim
This prospective cohort study evaluates clinical and radiographical outcomes of endodontic pulp revitalization (PR) of traumatized necrotic incisors.
Methodology
Pulp revitalization was performed in 75 traumatized necrotic immature incisors from 71 patients. The radiographic outcome measures were continued root formation (width and length), root resorption, apex closure, periapical index, and root development stage. The clinical outcome measures were percussion pain, palpation pain, pathological tooth mobility, swelling, sinus tract, ankylosis, crown discolouration, response to pulp sensitivity test, and subjective pain. Treatment outcomes were categorized as a success based on the absence of clinical symptoms and when radiographic evidence was present for apical healing and continued root development. The performed statistical tests were repeated measures anova, pairwise comparisons of interactions (t‐test), McNemar's test, and linear regression model.
Results
In 45 of 75 teeth (60%), PR was successful with the resolution of clinical and radiographic signs and continued root development. PR failed due to the absence of bleeding (n = 19) and persistent infection (n = 11). PR showed statistically significant increases in root length (11%), and dentinal wall thickness (30%), root maturation (pre‐operative 3.38 [CI 1.88; 4.88]; post‐operative 4.04, [CI 2.56; 5.52]) apical closure (71.4%), healing of pre‐operative apical periodontitis (100%), and healing of pre‐operative inflammatory root resorptions (100%). Three predictive variables for continued root maturation were identified – root development stage at entry (p = .0001, β 0.649), [CI 0.431; 0.867], trauma to the soft tissues (p = .026, β −0.012), [CI −0.0225; −0.015], and pre‐operative dentinal wall thickness (p = .009, β −0.001); [CI −0.001; 0.0001].
Conclusions
Our findings indicate that PR provides satisfactory clinical and radiographical outcomes in traumatized necrotic incisors. The failed cases were related to lack of bleeding and persistent infections, indicating that new techniques are needed to improve the predictability of PR.
Keywords: children, dental trauma, incisors, pulp necrosis, Regenerative endodontics
INTRODUCTION
In Sweden, the incidence rate for dental trauma has not decreased over the past 20 years and maxillary incisors are overrepresented in the prevalence of dental trauma in permanent dentition (Glendor, 2008). In school children, dental trauma reaches its highest prevalence between 8 and 9 years of age when the root development is still not completed (Oldin et al., 2015).
Trauma is the main reason for pulp necrosis in immature permanent incisors when severe dental trauma injuries such as avulsion or intrusive luxation are present (Diogenes & Hargreaves, 2017), and may, in the long term, cause ankylosis and risk for early tooth extraction (Andreasen, 2003). A serious sequela of early tooth loss is the reduction of volume of the alveolar bone process, which leads to difficulties in future prosthodontic treatment planning.
Adequate root canal procedures aim to eliminate root canal infection and to prevent the re‐establishment of a new infection as well as to produce good long‐term outcomes (Haapasalo et al., 2005). However, treatment of infected permanent incisors with open apices is an endodontic challenge, regardless of the treatment modality (Ridell et al., 2006). This challenge is due to differences from the treatment modality for their mature counterparts. The fact that immature teeth are characterized by open apices with short roots, wide root canals, and thin dentine walls makes it complicated to perform an adequate antibacterial endodontic treatment. Minimal mechanical instrumentation is recommended to avoid additional weakening of the tooth.
In recent years, much effort has been made to find alternatives to conventional endodontic treatment since it does not lead to further root formation, leaving the tooth fragile and susceptible to root fracture (AAE 2016; Cvek, 1992; Rafter, 2005). Regenerative endodontic treatment, also called pulp revitalization (PR), has been proposed as an alternative to conventional treatment techniques such as the apexification technique with multiple applications of calcium hydroxide or the apical mineral trioxide aggregate (MTA) plug technique (Bose et al., 2009; Torabinejad et al., 2017). PR appears to promote continued root development by stem cells (Banchs & Trope, 2004; Frisk, 2007; Petersson et al., 1991; Xuan et al., 2018). However, one of the main challenges in PR is persistent microorganisms in the pulpal space even after disinfection regimens (Petridis et al., 2018), a condition that may adversely affect stem cell functions (Diogenes & Hargreaves, 2017).
Dentists, in general, might have a widespread reliance on the technical performance of endodontic treatment and may have a problem eliminating microorganisms in infected root canals (Malmberg et al., 2019). Consequently, the outcome of PR will be jeopardized as no periapical healing will take place in the presence of residual intra‐radicular infection (Verma et al., 2017).
At present, there exists little evidence for the overall efficacy of PR for the treatment of traumatized immature teeth with pulp necrosis and infection (Cvek, 1992; Pereira et al., 2020; Rafter, 2005; Trope, 2010; Wikström et al., 2021), consisting mostly of case reports which indicate that the vast majority of reported failures (there is apparently considerable underreporting) are related to persistent infections (Almutairi et al., 2019; Bezgin et al., 2015; Botero et al., 2017; Ding et al., 2009). There is a knowledge gap about the composition of the microbiota in immature teeth with pulp necrosis and infection. In addition, the inclusion criteria and treatment protocols in the numerous reports of endodontic revitalization treatment of all kinds vary considerably.
In this study, we evaluate the clinical and radiographical outcome of traumatized permanent incisors treated with PR with a follow‐up of at least 24 months.
MATERIALS AND METHODS
Study population and selection of participants
This study was designed as a prospective longitudinal observational trial and was conducted according to the ethical principles of the Declaration of Helsinki (World Medical, 2008). The study has been independently reviewed and approved by the Stockholm Central Ethical Board, Swedish Ethical Review Authority (Dnr: 2018/692‐31). The study protocol was registered at ClinicalTrials.gov (NCT04000854). Before treatment, both spoken and written information about the study was given and written consent was signed by both the parents and their children. The study was conducted according to ICMJE recommendations for best practice and ethical standards in the conduct and reporting of research (ICMJE www.icmje.org/journals‐following‐the‐icmje‐recommendations/).
The study population was comprised of children with one or more traumatized permanent incisors with immature root development with pulp necrosis and/or apical periodontitis. When apical periodontitis was not present radiographically, in addition to the negative response to cold and electric sensibility tests, teeth were included only when clinical signs and symptoms of pulp necrosis were observed (swelling, sinus tract, pain to percussion and palpation, and self‐reported pain).
The participants were referred to specialist clinics in endodontics and paediatric dentistry at the Eastman Institute in Stockholm, Sweden and to the Department of Endodontics, Umeå University, Sweden. The treatment was performed by two of the authors of this study (AW and NRV), both specialists in endodontics with experience in the treatment of traumatized immature teeth in young patients. The patient data were coded and stored according to principles of the general data protection regulation. The inclusion and exclusion criteria are presented in Table 1.
TABLE 1.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| Age 6–22 years | No history of dental trauma |
| Good general health | Medically compromised patients |
| All types of traumatic injuries to permanent incisors | All types of traumatic injuries to canines, premolars and molars |
| Open apex >1 mm | Open apex <1 mm |
| Immature root development (RDS 1–4) | Mature root development (RDS 5–6) |
| Pulp necrosis and/or apical periodontitis | Pre‐operative root fracture |
| No history of endodontic treatment on the particular tooth (no previous access preparation) | |
|
Combined endo‐perio lesions (probing depth ≥6 mm) Allergy to medicaments used during endodontic treatment Decision to choose the different treatment modality | |
| Pulp space not needed for post or core restoration | |
| Good compliance of patient and guardians | |
| Adequate coronal restoration with no signs of caries |
If a patient was referred for more than one tooth, those teeth were also allocated for PR, provided that the inclusion criteria were met. A total of 82 incisors were enrolled in the study between May 2017 and December 2018 (Figure 1). A total of 75 teeth were treated as seven cases were excluded because they were not available for completion of the treatment.
FIGURE 1.
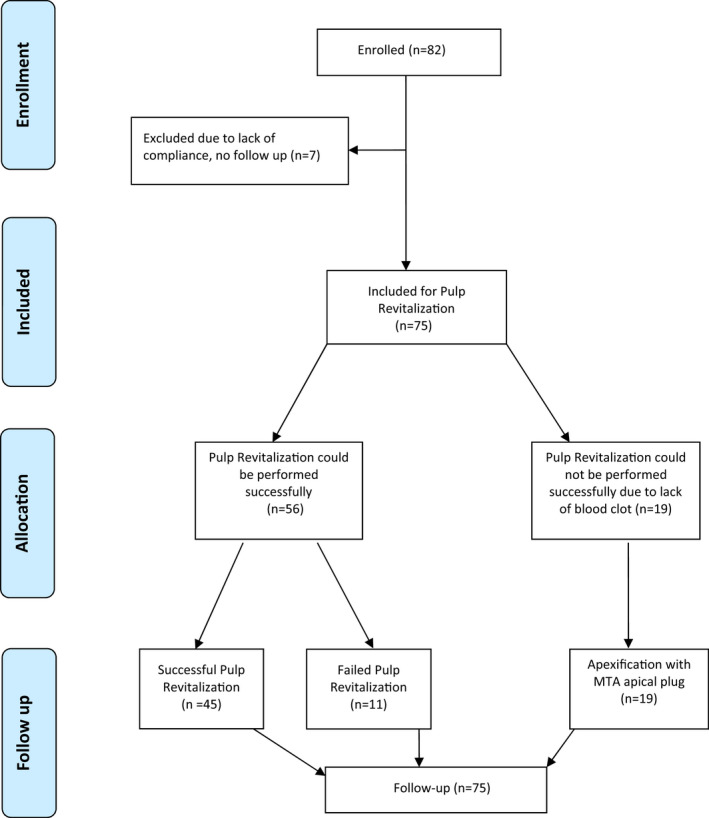
PRISMA 2009 flow diagram
Treatment protocol
Pulp necrosis was verified by cold pulp sensibility test (Endo Ice; Roeko; Endo Frost, Coltene Whaledent Roeko), electric test (Pulp Tester; Analytic Technology), and/or radiographic evidence of periapical lesion. The root canal dressings were either a calcium hydroxide paste (Calasept®, Directa AB) or 2% chlorhexidine digluconate gel (Gluco‐CHeX 2% gel, PPH Cerkamed). All the treatments were performed with the aid of a dental operating microscope (Leica TM, M320 and OPMI Pico, Carl Zeiss).
Clinical procedures at the first visit
Local anaesthesia was applied with conventional infiltration (1.8 ml Citanest Dental Octapressin 3% +0.54% mg/ml prilocain hydrochloride + felypressin, Dentsply Pharmaceutical Limited). A rubber dam was applied, and an access preparation was initiated only through the enamel using a high‐speed burr. Surface disinfection was performed with H2O2 (30%) with either chlorhexidine‐ethanol solution (5%) or iodine tincture (5%). Next, the pulp chamber was accessed through dentine with a sterile burr.
After the radiographic establishment of the working length, the root canal was irrigated with 20 ml 0.5% buffered sodium hypochlorite for 5 min, 2 mm short of the working length (Dakin's solution, APL, Apoteket AB). To prevent further weakening of the immature root, no or only slight mechanical instrumentation was used in the root canal. Instead, the irrigating solutions were agitated with passive ultrasonic tips (IRRI S, IRRI K, Endomark Dental AB). Further irrigation with 5‐ml ethylenediaminetetraacetic acid was applied for 5 min (EDTA, 15.3% APL, Apoteket AB) followed by irrigation with saline solution. Finally, the root canal was dried with sterile paper points, and dressing material was applied with a sterile syringe and distributed with spiral Lentulo filler. The root canal orifice and the access preparation were temporized with a minimum of 4 mm of temporary filling consisting of zinc oxide eugenol cement (Labservice AB) or Coltosol®F (temporary filling material, Colténe/Whaledent AG) and glass ionomer (GC Fuji TM IX GP Fast, GC Corporation). A second appointment was scheduled for approximately 4 weeks after this procedure was performed.
Clinical procedures at the second visit
Any clinical symptoms of persisting infection were evaluated at the second appointment. If clinical symptoms were present, the chemo‐mechanical disinfection protocol was repeated according to the technique described above. Provided the tooth was symptom‐free and no clinical signs of infection were detected, the pulp revitalization procedure was initiated. Local infiltration anaesthesia was applied without vasoconstrictors or with reduced vasoconstrictors (1.8 ml Carbocain Dental, 30 mg/ml, Dentsply Pharmaceuticals DeTrey GmbH, Surrey, UK or 1.8 ml Citanest Dental Octapressin 3% +0.54% mg/ml prilocain hydrochloride + felypressin, Dentsply Pharmaceutical Limited). The temporary filling with glass ionomer was removed and a rubber dam was applied and disinfected with the same technique used at the first treatment visit. The residual temporary material was removed using a sterile burr and the root canal dressing was removed with the application of sodium thiosulfate for 1 min and additional irrigation was performed with sterile saline (Natriumklorid Microspol 9 mg/ml, APL, Apoteket AB).
The root canal was then irrigated with 5‐ml 0.5% buffered sodium hypochlorite (Dakin's solution, APL, Apoteket AB) and 15‐ml EDTA for 5 min (15.3% APL, Apoteket AB). The final irrigation consisted of 2‐ml sterile saline and the root canal was dried with sterile paper points.
Bleeding from the periapical tissue was induced with a pre‐curved file (Kerr 15 or Hedström 30) until the root canal was filled with blood to the level of the cementoenamel junction.
The bleeding was monitored for approximately 15 min to secure blood clot stability. A resorbable collagen scaffold was placed over the blood clot (PARASORB® Cone, Resorba Medical GmbH or Collacone®, Botiss Biomaterials GmbH) and a minimum of 2‐mm bioceramic material was placed at the level of cementoenamel junction: white MTA Pro Root, Dentsply, Maillefer, Switzerland, or Biodentine (Septodont®) or Bioceramic Root Repair material condensable putty (EndoSequence® BC RRM™; Brasseler). Zinc oxide eugenol cement (Labservice AB) or glass ionomer (GC Fuji TM IX GP Fast, GC Corporation) was placed over the bioceramic material and restoration with resin restorative material was made during the same visit.
Data collection and outcome measures
The clinical and radiographic variables were registered at baseline and during the last follow‐up (at least 24 months after treatment). The clinical variables were percussion pain, palpation pain, pathological tooth mobility, swelling, sinus tract, ankylosis with infraposition, crown discolouration, response to pulp sensitivity test, and subjective pain. The radiographic variables were periapical index, root development stage, continued root formation in width and length, root resorption, and apex closure. After completed treatment, follow‐up examinations were performed at 6‐months intervals in all the included patients.
Radiographic analysis
Before radiographic measurements, one observer was calibrated for the use of a PAI score with the standard calibration program containing 100 radiographs. Apical periodontitis was classified as healed (PAI ≤2, no symptoms) or diseased (the presence of symptoms or clinical findings, PAI ≥3) (Orstavik et al., 1986). Teeth were recorded as functional when the absence of any signs or symptoms were noted independently of the PAI score (Friedman & Mor, 2004). Digital radiographs were taken with the aid of a standardized X‐ray positioning holder and using paralleling technique (Forsberg & Halse, 1994). The radiographs were calibrated (the horizontal dimension set to be 26 mm and the vertical dimension 34 mm), aligned, and measured with the tools available in the computer software (Planmeca Romexis 4.6.2 dental imaging software). The reproducibility and reliability of the radiographical assessments of PAI scores were done by repeated measurements on days 1, 2, and 5. A Cohen's Cappa value for intraobserver reproducibility of 0.94 was achieved (percent agreement, weighted kappa, p < .01).
Measurements of the root length and width and apex closure were made at baseline (pre‐operative radiograph) and 24–52 months after treatment (post‐operative radiograph) as previously described (Bose et al., 2009). Apex closure was determined as radiographic evidence of hard tissue deposition at the position of the radiographic apex. The radiographic measurements were classified into three groups: closed apex, where radiographic evidence of complete hard tissue deposition at the radiographic apex could be observed; partially closed apex, where radiographic evidence of incomplete hard tissue deposition at the radiographic apex could be observed; and open apex, where no radiographic evidence of hard tissue deposition at the radiographic apex could be observed (Figure 2).
FIGURE 2.
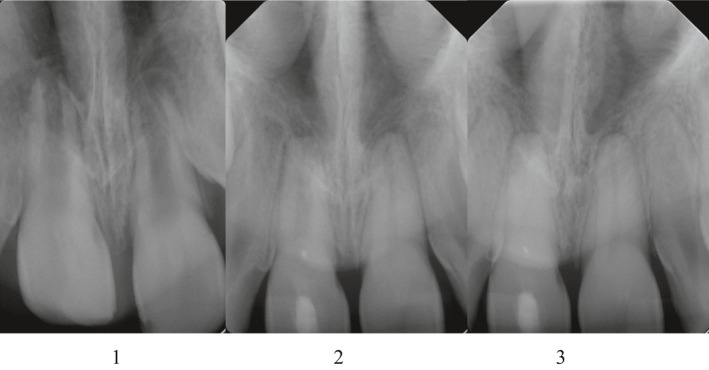
Degrees of apical closure (courtesy of AW, with the permission of Eastman Institute, Stockholm). The radiograph shows tooth 11 before endodontic pulp revitalization (1), during the follow‐ups (2) and at the time of the last follow up, 24 months after the treatment (3). 1: open apex; 2: partially closed apex; 3: closed apex
Radiographic assessment of the root development stage was made according to the method described by Tsilingaridis et al. (2016) (Figure 3). One observer performed the radiographic analyses. Blinding was not possible as the observer who performed the radiographic analyses (AW) also performed most of the treatments.
FIGURE 3.
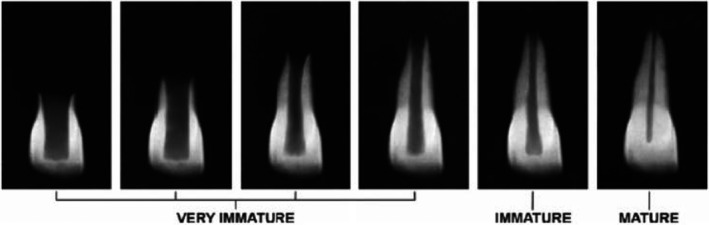
Root development stages by Tsilingaridis et al., 2016. 1–4‐ very immature; 5‐ immature; 6‐ mature. The stage of root development was classified as: 1 = one‐quarter root formation, 2 = one‐half root formation, 3 = threequarters root formation, 4 = full root formation, open apex, 5 = full root formation, half‐closed apex and 6 = full root formation, apex closed
Definition of success and failure
Success was defined as successfully completed PR cases without any clinical symptoms (no subjective pain, sinus tract, swelling, or sensitivity on palpation or percussion) and with radiographic signs of healing of apical periodontitis as well as evidence of continued root development (i.e., continued root formation in length and width and apex closure). Failure was defined as the presence of clinical symptoms after the completion of PR, such as subjective pain, sinus tract, swelling, or sensitivity on palpation or percussion. Radiographical signs defined as a failure were persisting apical periodontitis, the absence of continued root formation in length and width, or no apex closure, all assessed at the final follow‐up. As this study included only traumatized teeth (i.e., trauma sequela shortened survival time), failure was also defined as persisting or progressing resorptions (inflammatory root resorption or replacement resorption with signs of infraposition) or root fracture. In addition, failure was also defined as unsuccessful completion of PR due to lack of bleeding from the periapical tissues at the time of PR.
Statistical analysis
Statistical analysis was performed using the commercially available software program IBM SPSS (version 26, IBM Corporations). A p‐value of <.05 was considered significant for all tests at a statistical level of 95%. A one‐way repeated measures anova was used to evaluate the effect of healing of apical periodontitis on increases in root length and dentinal wall thickness. Measurements were performed at two time‐points, prior to treatment and at the time of the last follow‐up. Mauchly's test was performed to check the assumption of sphericity.
One‐way repeated anova was used to evaluate the effect AP healing had on root length and dentinal wall thickness and post‐hoc testing was used to check whether the assumptions were accurate. A pairwise test of interaction effects was performed (paired t‐test) for the numerical continuous variables and McNemar's test for a paired comparison of the nonparametric categorical variables. In addition, a linear regression model was performed to identify predictor variables on continued root maturation. The reproducibility scores for radiographic measurements were assessed using Cohen Kappa statistics with a minimum coefficient of 0.61 (Landis & Koch, 1977).
RESULTS
Table 2 summarizes the patients' demographics and pre‐operative characteristics. Most patients (80%) were between 6 and 11 years old, 64% of whom were male. The mean age distribution was 10.4 years (range 6–22) for successful PR, and 9.5 years (range 7–11) for unsuccessful PR due to lack of bleeding.
TABLE 2.
Participants' characteristics at baseline
| Treated with PR | Treated with MTA apical plug (due to no bleeding) | |
|---|---|---|
| N | 56 (74.7%) | 19 (25.3%) |
| Age means in years | 10.4 ± 2.8 (range 6–22) | 9.5 ± 1.5 (range 7–11) |
| Sex | ||
| Male | 36 (64.3%) | 9 (47.4%) |
| Female | 20 (35.7%) | 10 (52.6%) |
| Tooth number | ||
| 11 | 23 (41.1%) | 6 (31.6%) |
| 12 | 4 (7.1%) | 0 (0%) |
| 21 | 24 (42.9%) | 10 (47.4%) |
| 22 | 4 (7.1%) | 2 (10.5%) |
| 31 | 0 (0%) | 1 (5.3%) |
| 32 | 1 (1.8%) | 0 (0%) |
| Trauma type | ||
| Concussion | 5 (8.9%) | 1 (5.3%) |
| Subluxation | 6 (10.7%) | 3 (15.8%) |
| Lateral luxation | 24 (42.9%) | 5 (26.3%) |
| Intrusion | 4 (7.1%) | 1 (5.3%) |
| Avulsion | 10 (17.9%) | 2 (10.5%) |
| Enamel‐dentin fracture | 39 (69.6%) | 16 (84.2%) |
| Combination trauma | 35 (62.5%) | 9 (47.4%) |
| Unknown | 3 (5.4%) | 0 (0%) |
| Bone diagnosis at baseline | ||
| Healthy periapical tissues | 6 (10.7%) | 0 (0%) |
| Apical periodontitis | 50 (89.3%) | 19 (100%) |
| Pre‐operative sinus tract | 24 (42.9%) | 5 (26.3%) |
| Pulp diagnosis | ||
| Pulp necrosis | 56 (100%) | 19 (100%) |
| Pre‐operative root development stage | ||
| Stage 2 | 4 (7.1%) | 0 (0%) |
| Stage 3 | 19 (33.9%) | 5 (26.3%) |
| Stage 4 | 29 (51.8%) | 8 (42.1%) |
| Stage 5 | 5 (8.9%) | 6 (31.6%) |
| Stage 6 | 0 (0%) | 0 (0%) |
| Pre‐operative root development stage (mean; ±SD) | 3, 38 (0.75) | [1.88; 4.88] |
| Pre‐operative root resorption | 21 (37.5%) | 3 (15.8%) |
| Pre‐operative symptoms | ||
| Yes | 20 (35.7%) | 2 (10.5%) |
| No | 36 (64.3%) | 17 (89.5%) |
| Pre‐operative crown discoloration | 0 (0%) | 0 (0%) |
| Pre‐operative PAI | ||
| Score 1 and 2 | 9 (16.1%) | 2 (10.5%) |
| Score 3 | 15 (26.8%) | 5 (26.3%) |
| Score 4 | 16 (28.6%) | 5 (26.3%) |
| Score 5 | 16 (28.6%) | 7 (36.8%) |
| Pre‐operative root length (mean; ±SD) | 11.5 (2.15) | [6.9; 16.2] |
| Pre‐operative root width (mean; ±SD) | 2.53 (1.32) | [1.1; 6.5] |
| Total number of teeth | 75 | |
Over half (58.7%) of the included teeth were subject to trauma to both hard tissues (enamel and dentine fractures) and soft tissues (involving the periodontal ligament, such as lateral luxations, concussions, subluxations, extrusive luxations, intrusions, and avulsions). The most frequent type of hard tissue trauma was enamel‐dentin fracture (73%), and the most frequent type of soft tissue trauma was lateral luxation (39%). Severe trauma, such as avulsion and intrusion, was present in 23% of the cases.
The follow‐up period varied from 24 to 52 months. Each case was recalled with 6‐months intervals, 4–8 times post‐operatively depending on the length of the follow‐up period.
Clinical and radiographical outcomes
Table 3 lists the participants' characteristics at follow‐up. No statistically significant differences between trauma severity and clinical and radiographic outcomes could be detected.
TABLE 3.
Participants' characteristics at follow‐up of PR (n = 45)
| Post‐operative clinical signs and symptoms | |
| Yes | 0 |
| No | 45 |
| Post‐operative root resorption | |
| Yes | 0 |
| No | 45 |
| Healing of apical periodontitis | |
| Yes | 45 |
| No | 0 |
| Post‐operative root development stage (mean; ±SD) | 4.04 (0.74) [2.56; 5.52] |
| Post‐operative apex closure | |
| Closed apex | 33 (73.3%) |
| Partially closed apex | 9 (20%) |
| Open apex | 3 (6.7%) |
| Post‐operative pulp canal obliteration | |
| Yes | 10 (22.2%) |
| No | 35 (77.8%) |
| Post‐operative crown discoloration | |
| Yes | 8 (17.8%) |
| No | 37 (82.2%) |
| Post‐op PAI | |
| Scores 1 and 2 | 45 (100%) |
| Score 3 | 0 (0%) |
| Score 4 | 0 (0%) |
| Score 5 | 0 (0%) |
| Post‐operative root length (mean; ±SD) | 12.7 (2.48) [7.7; 19.2] |
| Post‐operative root width (mean; ±SD) | 3.2 (1.16) [1.7; 6.1] |
| Total follow‐up mean (in months) | 27.1 |
| Follow‐up of failed PR (persisting symptoms, n = 11) | 27.5 |
| Follow‐up of failed PR (no bleeding, n = 19) | 25.1 |
| Follow‐up of successful PR (n = 45) | 28 |
Clinical signs and symptoms include pain to percussion and palpation, swelling, sinus tract, pathological tooth mobility, ankylosis tone with signs of infraposition, pathological probing depth.
Abbreviation: PR, pulp revitalization treatment.
Successful PR
Pulp revitalization was judged successful in 45 cases (60%). During the whole observation period, the clinical symptoms resolved thoroughly, and no additional endodontic intervention was required. No response to pulp sensibility tests (cold and electric pulp tests) were observed in any of the PR cases.
Failed PR
The 30 cases classified as failures consisted of two distinct groups. The first group classified as early failures included the cases whose PR was unsuccessful due to lack of bleeding and clot formation. That is, in 19 cases (25.3%), despite efforts to stimulate bleeding from the periapical tissues, the treatment failed. These efforts included leaving a calcium hydroxide dressing in the root canals for a prolonged time (mean 4.6 months, range 1–12 months) when circumstances warranted (e.g., parents rescheduling the appointments several times, patients with behaviour management problems, and/or dental anxiety). At the time of PR, hard tissue deposits were observed at the apical area (apexification) in 14 cases and no hard tissue deposits were observed at the apical area in five cases, so all these cases were treated with the MTA apical plug technique. At the follow‐ups, the teeth were symptom‐free with the resolution of clinical signs and radiographic periapical healing with PAI scores of 1 and 2. The overall periapical healing in this group was 100%. In two cases, the affected teeth were extracted, and an appliance was used to close the orthodontic space due to an uncertain long‐term prognosis.
The second group classified as late failures consisted of 11 cases (14.7%) with persistent clinical symptoms within the group that did not receive PR. Table 4 lists the late failed PR cases.
TABLE 4.
Late PR failures, participants characteristics
| Age | Sex | Tooth | Trauma | Post‐op symptoms | Alternative treatment | Survival (months) |
|---|---|---|---|---|---|---|
| 9 | M | 21 | ED, LL | Persisting apical periodontitis | Retreated (wMTA‐apical plug) | 52 |
| 16 | M | 11 | ED, LL | Pathologic periodontal pocket and mobility grade 3 | Extracted and treated prosthodontically | 13 |
| 8 | M | 21 | ED, Avulsion with extra oral time >3 h. Dry storage | Ankylosis tone and infraposition | Decoronated due to replacement resorption. Treated prosthodontically | 24 |
| 10 | F | 11 | ED, LL | No continued root formation after PR | Retreated (long‐term dressing with CaOH2) | 24 |
| 10 | F | 11 | ED, Avulsion with extra oral time >1 h. Dry storage | Persisting clinical symptoms after PR | Retreated (root filled with Biodentine) | 32 |
| 14 | M | 11 | ED, LL | Persisting clinical symptoms after PR | Retreated (wMTA‐apical plug) | 36 |
| 8 | M | 21 | Avulsion with unknown extra oral time. Dry storage | Ankylosis tone and infraposition | Decoronated due to replacement resorption. Treated prosthodontically | 9 |
| 8 | M | 21 | ED, LL | No continued root formation after PR | Retreated (wMTA‐apical plug) | 24 |
| 9 | F | 21 | ED, LL | Persisting clinical symptoms after PR | Retreated (root filled with Biodentine) | 24 |
| 12 | F | 11 | Unknown | Persisting clinical symptoms after PR | Extracted and treated prosthodontically | 6 |
| 10 | F | 21 | Unknown | No continued root formation after PR | Retreated (wMTA‐apical plug) | 36 |
Abbreviations: ED, enamel dentine fracture; LL, lateral luxation; PR, pulp revitalization treatment; wMTA, white MTA cement.
These cases were characterized by severe trauma. The following outcomes were observed: ankylosis with signs of infraposition (n = 2); persisting apical periodontitis and clinical symptoms and or no signs of continued root development nor apex closure (n = 8); and pathologic periodontal pocket and apical‐marginal communication and mobility grade 3 (n = 1). Six of these teeth were retreated and after resolution of clinical symptoms, they were treated with the MTA apical plug technique. The two teeth with progressing replacement resorption and infraposition were decoronated. One tooth with pathologic periodontal pocket (≥9 mm) and mobility (≥3 mm horizontally and vertically) was extracted and one tooth was retreated and a long‐term dressing with calcium hydroxide was applied. Persisting clinical signs and symptoms were present in seven of the cases. Four cases showed persisting apical periodontitis with PAI scores 3 and 4. Thus, the overall periapical healing in this group was 63.6%.
Crown discolouration
Crown discolouration was the most frequently observed post‐operative adverse effect in both the PR group and the MTA apical plug group (15%). None of the cases presented pre‐operative crown discolouration. Those cases showed crown discolouration after the application of white MTA (8 PR cases; 3 MTA apical plug cases).
Flare‐ups
The second most frequent adverse effect after the first treatment was a flare‐up (6.7%). Three of the cases with very low root development (stages 2 and 3) where PR was performed had a transient adverse effect, which manifested clinically as pain or swelling or persisting sinus tract. These teeth became symptom‐free after re‐entering the root canals and new chemo‐mechanical treatment and re‐dressing with calcium hydroxide or chlorhexidine gluconate gel. The clinical symptoms resolved and no post‐operative pain after completion of the pulp revitalization procedure was reported.
Radiographic measurements
Radiographic images of the 45 teeth successfully treated with PR were considered for the measurements of the continued root development. At baseline, the pre‐operative overall mean value for root development stages (RDS) was 3.38 (CI 1.88; 4.88), and the pre‐operative distribution for RDS was as follows: RDS 2 was 12.5%; RDS 3 was 41%; RDS 4 was 43%; and RDS 5 was 3.6%. Continued root maturation with more mature root development stages was observed post‐operatively and the changes were statistically significant (p = .001). The post‐operative overall mean value for RDS was 4.04 (CI 2.56; 5.52), and the post‐operative distribution for RDS was as follows: RDS 2 was 2%; RDS 3 was 18%; RDS 4 was 57%; RDS 5 was 21.4%; and RDS 6 was 2%. The post‐operative changes in root dimensions (in millimetres) showed increases in root length and dentinal wall thickness and the findings were statistically significant (p = .001). The increase in root length was 11% (mean 1.17 mm), and the increase in dentinal wall thickness was 30% (mean 0.83 mm).
Figure 4 shows the mean differences between pre‐operative and post‐operative variables at 95% CI at the time of the last follow‐up.
FIGURE 4.
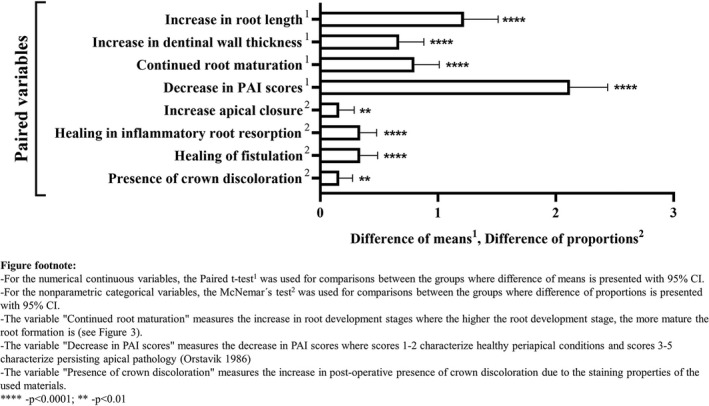
Mean differences between pre‐operative and post‐operative variables at 95% CI
Pre‐operative radiographic appearance of apical periodontitis was observed in 96.5% of the cases. The post‐operative radiographs met the criteria for complete healing of periapical pathology in all the PR cases, with PAI scores representing healthy periapical status (scores 1 and 2). The post‐operative changes in periapical healing were statistically significant (p = .0001). Radiographic evidence of hard tissue deposition at the apical area was evident where complete apical closure was observed (25 cases; 71.4%), where partial apical closure was observed (7 cases; 20%), and when no apical closure was observed (3 cases; 8.6%). The post‐operative changes in apex closure were statistically significant (p = .0001).
Twenty‐four teeth were diagnosed with pre‐operative trauma‐induced infection‐related root resorptions. In these cases, the trauma involved injuries to the periodontal ligament: avulsion (n = 10); intrusion (n = 5); lateral luxation (n = 3); subluxation (n = 2); unknown diagnosis (n = 2), concussion (n = 1); and subluxation (n = 1). In these cases, healing with a total resolution of the radiographic signs of resorption was observed post‐operatively. The post‐operative changes in the healing of inflammatory root resorption were statistically significant (p = .0001). The post‐operative changes in the healing of fistulation and the presence of post‐operative crown discolouration were statistically significant (p = .001 and p = .004, respectively) (Figure 4).
Pulp canal obliteration was observed in eight cases during the follow‐up period (no significance was shown). The post‐operative radiographic analyses of the MTA cases met the criteria for complete healing of periapical pathology in all teeth with PAI scores representing healthy periapical status (scores 1 and 2). There was no statistical difference between the PAI scores of PR and MTA cases.
Statistical outcomes
The statistical significance of the study parameters is presented in Figure 4. PR showed statistical significance in increases in root length and dentinal wall thickness, continued root development and apical closure, and healing of apical periodontitis and inflammatory root resorptions (p = .0001). To investigate this effect, one‐way repeated measures anova test was used. The adjusted multivariate model revealed that several variables had a statistically significant effect on increases in root length and thickness: healing of apical periodontitis (p = .0001); age (p = .0001); trauma to soft and hard tissues (p = .0001); and PAI index (p = .0001). Further analyses with pairwise test (t‐test) confirmed a significant difference between the pre‐operative and post‐operative measurements. The mean differences between pre‐op and post‐op variables at 95% CI for root length increases and dentinal wall thickness was 1.22 (p = .001; CI 0.93; 1.51) and 0.67 (p = .001; CI 0.46; 0.88), respectively. Multivariate tests were performed as Mauchly's test of sphericity was significant.
As the main objective of PR is to achieve continued root maturation, we wanted to predict which variables are influential in this context. Several outcome variables were evaluated with a linear regression model presented in Table 5. The model‐building procedure and the guidelines for reporting regression analysis have previously been described in detail elsewhere (Lang & Secic, 2006).
TABLE 5.
Regression model with dependent variable root development stage at dismissal
| Coefficients a | ||||||
|---|---|---|---|---|---|---|
| Model | Unstandardized coefficients | 95% Confidence interval for B | ||||
| B | Std. Error | Sig. | Lower bound | Upper bound | ||
| 1 | (Constant) | 2.479 | 0.770 | .002 | 0.930 | 4.027 |
| Age in years | −0.006 | 0.029 | .837 | −0.065 | 0.053 | |
| Root development stage entry | 0.649 | 0.108 | .000*** | 0.431 | 0.867 | |
| Trauma to soft tissues | −0.120 | 0.052 | .026* | −0.225 | −0.015 | |
| Trauma to hard tissues | 0.088 | 0.088 | .324 | −0.089 | 0.264 | |
| Pre‐op width | −0.001 | 0.000 | .009** | −0.001 | 0.000 | |
| PAI index pre‐op | −0.073 | 0.072 | .315 | −0.217 | 0.072 | |
| Root resorption entry | −0.044 | 0.186 | .813 | −0.419 | 0.330 | |
The independent variables age (in years), root development stage at entry, pre‐operative width of dentinal walls (in mm), and pre‐operative PAI index (scale 1–5) were entered as a continuous variable. The independent variables root resorption and trauma to soft‐ and hard tissue were entered as categorical variables. The categories presence of pre‐operative inflammatory root resorption and soft tissue trauma were chosen as reference categories. Variables with statistical significance: ***p = .0001‐root development stage entry; **p = .01‐pre‐op width; *p = .05‐trauma to soft tissues.
Dependent variable: root development stage at dismissal.
It was demonstrated that several variables had a statistically significant impact on continued root maturation (adjusted coefficient: R² = 52%). Three predictive variables were identified: root development stage at entry (p = .0001, β 0.649), [CI 0.431; 0.867], trauma to the soft tissues (p = .026, β −0.012), [CI −0.0225; −0.015] and pre‐operative dentine wall thickness (p = .009, β −0.001), [CI −0.001; 0.0001]. Several other variables were excluded from the model as they did not reach statistical significance. These variables were age (p = .83, β −0.006), [CI −0.065; 0.053]; trauma to the hard tissues (p = .32, β 0.088), [CI −0.089; 0.264]; pre‐operative PAI index (p = .31, β −0.073), [CI −0.217; 0.072]; and pre‐operative inflammatory root resorption (p = .81, β −0.044), [CI −0.419; 0.33]. The variable pre‐operative root canal length was excluded from the model due to problems with collinearity (β 13.18).
Our findings indicate that the lower the pre‐operative root development stage, the more root maturation (+0.65 mm) at each level of increase of root development stage, but the post‐operative dentine wall thickness remained lower (−0.01 mm).
DISCUSSION
Clinical outcomes
Our major finding is that pulp revitalization of necrotic traumatized permanent incisors was successful in 60% of the cases. This differs from earlier reports, which report success rates of 80%–90% (Chan et al., 2017; Jeeruphan et al., 2012). One of the reasons for this inconsistency could be that the included cases in previous studies had various aetiology for pulp necrosis. Several authors discuss that dental trauma may lower the success rates of pulp revitalization due to injuries to the periodontal attachment (i.e., after luxation injuries) (Nazzal & Duggal, 2017). As a consequence of trauma, bacterial penetration from the gingival sulcus might cause prolonged healing of the soft tissues and problems with the elimination of bacterial infection (Fouad, 2019). Damage to the apical papilla and Hertwig's epithelial root sheath might cause less favourable outcomes (Lin et al., 2017). Accordingly, severe trauma, for example, avulsion has been found to be a reason for treatment failure (Bukhari et al., 2016; Priya et al., 2016). Also, teeth with lower root development stages are associated with more complications (Alobaid et al., 2014).
Therefore, the lower success rate in our study might be due to dental trauma with combined injuries to soft and hard tissues.
Radiographical outcomes
Our results showed that overall healing of apical periodontitis with PAI scores representing healthy periapical status (scores 1 and 2) occurred in 90% (both PR and MTA apical plug cases). Specifically, in the successful PR cases, resolution of the signs and symptoms of apical periodontitis was achieved in 100%, which is recognized as the primary outcome of pulp revitalization procedures (AAE 2016; Geisler, 2012). However, in the failed PR cases where severe trauma was present (n = 11), apical periodontitis persisted in 63%.
We also found post‐operative increases in root length and dentinal wall thickness after successful PR. The mean changes are similar to the results from earlier studies (Bose et al., 2009; Nawal et al., 2020). It was observed that the more immature root development stage, the more root maturation at each level of increase of each root development stage. This observation is in line with other studies (Chan et al., 2017). Clearly, continued root maturation would be the optimal goal for this treatment strategy as PR aims to strengthen the teeth and provide a better long‐term survival.
In addition, the teeth with trauma‐induced infection‐related root resorption (n = 13) showed healing. An explanation for this is that efficient disinfection of the root canal space was achieved (Heithersay, 2007).
Failed cases
Another major finding is that failure occurred in 40% of our patients due primarily to lack of bleeding (in 14 of the 19 cases) and persistent infection. Induction of bleeding may have been prevented by early apexification with hard tissue deposition as the result of the calcium hydroxide dressings left unintentionally in the root canals for a prolonged period between appointments (Damle et al., 2012; Lin et al., 2016; Pradhan et al., 2006). Although attempts were made to follow the pre‐designed protocol for appointments, prolongation of the application of dressings was necessary with children who had problems with behaviour or compliance or dental anxiety. At the same time, PR is a technique‐sensitive procedure depending both on how experienced the treatment provider is and on the patient's cooperation. Several difficulties—for example, a child's behaviour, proper visualization and stimulation of bleeding, and the ideal placement of the bioceramic sealing material—are linked directly to the rate of success.
Crown discolouration
Several studies have reported that both grey and white MTA induces crown discolouration when placed as a cervical sealing. This effect is compounded in the presence of blood (Felman & Parashos, 2013; Ioannidis et al., 2013). The findings in our study were in line with those previously reported outcomes. As noted, in our study the placement of white MTA at the cementoenamel junction caused crown discolouration, resulting in patients complaining about the appearance of their teeth. Therefore, efforts were made to deal with the issue (i.e., bleaching) and during the continued enrolment in the study, preventive measures were taken to avoid staining by applying another nonstaining formula of bioceramic material.
Flare‐up
As the vast majority of the published studies on PR lack standardized treatment protocol, it is difficult to draw conclusions about the incidence of flare‐ups (Wikström et al., 2021). If we compare PR to conventional endodontic treatment, studies report 2%–3% incidence of post‐operative pain and exacerbation in cases with necrotic pulp and apical periodontitis (Azim et al., 2017; Nair et al., 2017). However, the findings of this study show a 7% incidence of flare‐ups after complete chemo‐mechanical instrumentation. This could be explained by the fact that several cases were characterized by a very low root development stage where difficulties with complete chemo‐mechanical preparation of the root canals could have occurred. No additional treatment was advocated as the patients became symptom‐free. In these cases, another possible risk factor for flare‐ups could be an apical extrusion of infected debris or secondary intraradicular infections. As discussed by Siqueira et al., the presence of bacteria has been correlated to flare‐ups (Siqueira, 2003).
Strengths and limitations
A strength of this study is that it provided a homogenous patient group. The majority of the patients were young children, only immature incisors subjected to traumatic injuries were included. We also standardized the treatment protocol to enable different sites to participate in a multicentre setup. Two specialist clinics in endodontics were involved, which resulted in a large sample size. Opposite to many other studies, this study investigates a large cohort of 75 patients (Torabinejad et al., 2017). As the author mentions, prospective cohort studies provide stronger scientific evidence as they do not rely on existing records (Torabinejad et al., 2005). Moreover, a pre‐defined outcome classification of success and failure was applied to make the observations more conclusive.
Intraoral radiographs were chosen to assess the outcome of PR. Although periapical radiographs have been recognized as the first choice for diagnosing apical periodontitis, their diagnostic accuracy has been debated, and one disadvantage of periapical radiographs is the risk for underdiagnoses (Lofthag‐Hansen et al., 2007). Nevertheless, the radiographs were taken with a standardized X‐ray positioning holder using paralleling technique. This provided minimized changes in angulations between the pre‐operative and post‐operative images. Subsequently, digital measurements were performed in dental imaging software (Planmeca Romexis 4.6.2). Even though only one observer was involved in the measurements, landmarks were used (i.e., straight‐line tool to measure the root length from the cementoenamel junction and dentin thickness measured at the apical third) proposed by Bose et al. (2009). Image alignment software was not applied due to technique‐sensitive procedure that, in our opinion, seems to be suitable for skilled users.
The concern of using different techniques for radiographic measurements has been earlier described in a recent review emphasizing the need for more standard user‐friendly techniques in the future (Nazzal & Duggal, 2017). In addition, even though the observer was calibrated for the use of the PAI index with an available calibration program, it could be discussed that the assignment of the root development stages was not unambiguous. As there is a lack of standardized techniques, the visual assessment was applied for the judgment of the root development stage (Tsilingaridis et al., 2016). As in many other studies, this protocol was also applied in this study (Chen & Chen, 2016; Chueh et al., 2009; Jiang et al., 2017).
This shows that there is a need for standardized radiographic measurement techniques.
Although the root canal dressings (calcium hydroxide and chlorhexidine) were not randomly allocated to the intervention groups and therefore a possible limitation, we believe that ethical considerations make it inappropriate to randomize root canal dressings in traumatized immature teeth as the potential effect of chlorhexidine on trauma‐induced infection‐related root resorption is not well documented. To date, the clinical data on chlorhexidine are only available in the context of its range of antimicrobial effectiveness on root canal pathogens (Mohammadi & Abbott, 2009). On the other hand, calcium hydroxide has a well‐documented long‐term clinical effect on trauma‐induced infection‐related root resorptions (Andreasen et al., 1995; Bakland & Andreasen, 2012; Orstavik & Haapasalo, 1990).
Need for future microbiological research
Little is known about the microflora of immature permanent teeth with root canal infection. There is also a gap of knowledge regarding the persisting bacterial species in failed pulp revitalization cases. Thus, further microbiological research combining culture‐based and molecular approaches with highly sensitive detection methods is needed in this field.
CONCLUSIONS
The summary of the primary and secondary outcomes showed that PR was considered successful in 60% of the cases. When PR was performed successfully, the treatment presented meaningful outcomes, with a resolution of periapical infection, increased root length and width, and apex closure. Two predictive variables for continued root maturation were identified—root development stage at entry and pre‐operative dentine wall thickness. No significant differences in clinical and radiographical outcomes between the calcium hydroxide and chlorhexidine dressings could be detected. The failed cases were related to lack of bleeding and persistent infections, indicating that new techniques are needed to improve the predictability of PR.
CONFLICT OF INTEREST
The authors declare no conflicts of interest related to this study.
AUTHOR CONTRIBUTIONS
AW: Conceptualization; data curation; investigation; project administration; software; and writing the original draft preparation. MB: Conceptualization; methodology; formal analysis; funding acquisition; supervision; validation; writing, reviewing, and editing. NRV: Formal analysis; validation; methodology; writing, reviewing, and editing. OR: Analyses and interpretation of the results; methodology; writing; reviewing, and editing. GT: Conceptualization; formal analysis; supervision; validation; writing, reviewing, and editing.
ETHICAL APPROVAL
This study has been approved by the Stockholm Central Ethical Board, Swedish Ethical Review Authority (Dnr: 2018/692‐31).
ACKNOWLEDGEMENTS
Special thanks to Carina Öhman, Department of Odontology, Umeå University, for her skilful laboratory work. We would also like to thank the dental assistant Eva Jansson, Dept. of Paediatric Dentistry, Folktandvården Eastman Institute, and Eleonor Fowler, Department of Endodontics, Region of Västerbotten, for their assistance with patient registries.
Wikström, A. , Brundin, M. , Romani Vestman, N. , Rakhimova, O. & Tsilingaridis, G. (2022) Endodontic pulp revitalization in traumatized necrotic immature permanent incisors: Early failures and long‐term outcomes—A longitudinal cohort study. International Endodontic Journal, 55, 630–645. Available from: 10.1111/iej.13735
Funding information
This research was funded by the Knut and Alice Wallenberg Foundation, grant number 396168403 and the Region of Västerbotten (Sweden) via TUA, grant numbers 396168402 and 7002665.
REFERENCES
- American Association of Endodontists (AAE) . (2016) Clinical considerations for a regenerative procedure. Revised 2016. Available from: https://www.aae.org/uploadedfiles/publications_and_research/research/currentregenerativeendodonticconsiderations.pdf [Accessed 6th August 2016]. [Google Scholar]
- Almutairi, W. , Yassen, G.H. , Aminoshariae, A. , Williams, K.A. & Mickel, A. (2019) Regenerative endodontics: a systematic analysis of the failed cases. Journal of Endodontics, 45(5), 567–577. [DOI] [PubMed] [Google Scholar]
- Alobaid, A.S. , Cortes, L.M. , Lo, J. , Nguyen, T.T. , Albert, J. , Abu‐Melha, A.S. et al. (2014) Radiographic and clinical outcomes of the treatment of immature permanent teeth by revascularization or apexification: a pilot retrospective cohort study. Journal of Endodontics, 40(8), 1063–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen, F.M. (2003) Transient root resorption after dental trauma: the clinician's dilemma. Journal of Esthetic and Restorative Dentistry, 15(2), 80–92. [DOI] [PubMed] [Google Scholar]
- Andreasen, J.O. , Borum, M.K. , Jacobsen, H.L. & Andreasen, F.M. (1995) Replantation of 400 avulsed permanent incisors. 4. Factors related to periodontal ligament healing. Endodontics & Dental Traumatology, 11(2), 76–89. [DOI] [PubMed] [Google Scholar]
- Azim, A.A. , Azim, K.A. & Abbott, P.V. (2017) Prevalence of inter‐appointment endodontic flare‐ups and host‐related factors. Clinical Oral Investigations, 21(3), 889–894. [DOI] [PubMed] [Google Scholar]
- Bakland, L.K. & Andreasen, J.O. (2012) Will mineral trioxide aggregate replace calcium hydroxide in treating pulpal and periodontal healing complications subsequent to dental trauma? A review. Dental Traumatology, 28(1), 25–32. [DOI] [PubMed] [Google Scholar]
- Banchs, F. & Trope, M. (2004) Revascularization of immature permanent teeth with apical periodontitis: new treatment protocol? Journal of Endodontics, 30(4), 196–200. [DOI] [PubMed] [Google Scholar]
- Bezgin, T. , Yilmaz, A.D. , Celik, B.N. , Kolsuz, M.E. & Sonmez, H. (2015) Efficacy of platelet‐rich plasma as a scaffold in regenerative endodontic treatment. Journal of Endodontics, 41(1), 36–44. [DOI] [PubMed] [Google Scholar]
- Bose, R. , Nummikoski, P. & Hargreaves, K. (2009) A retrospective evaluation of radiographic outcomes in immature teeth with necrotic root canal systems treated with regenerative endodontic procedures. Journal of Endodontics, 35(10), 1343–1349. [DOI] [PubMed] [Google Scholar]
- Botero, T.M. , Tang, X. , Gardner, R. , Hu, J.C.C. , Boynton, J.R. & Holland, G.R. (2017) Clinical evidence for regenerative endodontic procedures: immediate versus delayed induction? Journal of Endodontics, 43(9S), S75–S81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukhari, S. , Kohli, M.R. , Setzer, F. & Karabucak, B. (2016) Outcome of revascularization procedure: a retrospective case series. Journal of Endodontics, 42(12), 1752–1759. [DOI] [PubMed] [Google Scholar]
- Chan, E.K. , Desmeules, M. , Cielecki, M. , Dabbagh, B. & Ferraz Dos Santos, B. (2017) Longitudinal cohort study of regenerative endodontic treatment for immature necrotic permanent teeth. Journal of Endodontics, 43(3), 395–400. [DOI] [PubMed] [Google Scholar]
- Chen, S.J. & Chen, L.P. (2016) Radiographic outcome of necrotic immature teeth treated with two endodontic techniques: a retrospective analysis. Biomedical Journal, 39(5), 366–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chueh, L.H. , Ho, Y.C. , Kuo, T.C. , Lai, W.H. , Chen, Y.H. & Chiang, C.P. (2009) Regenerative endodontic treatment for necrotic immature permanent teeth. Journal of Endodontics, 35(2), 160–164. [DOI] [PubMed] [Google Scholar]
- Cvek, M. (1992) Prognosis of luxated non‐vital maxillary incisors treated with calcium hydroxide and filled with gutta‐percha. A retrospective clinical study. Endodontics & Dental Traumatology, 8(2), 45–55. [DOI] [PubMed] [Google Scholar]
- Damle, S.G. , Bhattal, H. & Loomba, A. (2012) Apexification of anterior teeth: a comparative evaluation of mineral trioxide aggregate and calcium hydroxide paste. The Journal of Clinical Pediatric Dentistry, 36(3), 263–268. [PubMed] [Google Scholar]
- Ding, R.Y. , Cheung, G.S. , Chen, J. , Yin, X.Z. , Wang, Q.Q. & Zhang, C.F. (2009) Pulp revascularization of immature teeth with apical periodontitis: a clinical study. Journal of Endodontics, 35(5), 745–749. [DOI] [PubMed] [Google Scholar]
- Diogenes, A. & Hargreaves, K.M. (2017) Microbial modulation of stem cells and future directions in regenerative endodontics. Journal of Endodontics, 43(9S), S95–S101. [DOI] [PubMed] [Google Scholar]
- Felman, D. & Parashos, P. (2013) Coronal tooth discoloration and white mineral trioxide aggregate. Journal of Endodontics, 39(4), 484–487. [DOI] [PubMed] [Google Scholar]
- Forsberg, J. & Halse, A. (1994) Radiographic simulation of a periapical lesion comparing the paralleling and the bisecting‐angle techniques. International Endodontic Journal, 27(3), 133–138. [DOI] [PubMed] [Google Scholar]
- Fouad, A.F. (2019) Microbiological aspects of traumatic injuries. Journal of Endodontics, 45(12S), S39–S48. [DOI] [PubMed] [Google Scholar]
- Friedman, S. & Mor, C. (2004) The success of endodontic therapy–healing and functionality. Journal of the California Dental Association, 32(6), 493–503. [PubMed] [Google Scholar]
- Frisk, F. (2007) Epidemiological aspects on apical periodontitis. Studies based on the Prospective Population Study of Women in Goteborg and the Population Study on Oral Health in Jonkoping, Sweden. Swedish Dental Journal Supplement, (189), 11–78, 11 p preceding table of contents. [PubMed] [Google Scholar]
- Geisler, T.M. (2012) Clinical considerations for regenerative endodontic procedures. Dental Clinics of North America, 56(3), 603–626. [DOI] [PubMed] [Google Scholar]
- Glendor, U. (2008) Epidemiology of traumatic dental injuries–a 12 year review of the literature. Dental Traumatology, 24(6), 603–611. [DOI] [PubMed] [Google Scholar]
- Haapasalo, M. , Endal, U. , Zandi, H. & Coil, J.M. (2005) Eradication of endodontic infection by instrumentation and irrigation solutions. Endodontic Topics, 10(1), 77–102. [Google Scholar]
- Heithersay, G.S. (2007) Management of tooth resorption. Australian Dental Journal, 52(1 Suppl), S105–S121. [DOI] [PubMed] [Google Scholar]
- ICMJE . Recommendations for the conduct, reporting, editing and publication of scholarly work in medical journals. Available from: www.icmje.org/journals‐following‐the‐icmje‐recommendations/. 2018.09.01. [PubMed] [Google Scholar]
- Ioannidis, K. , Mistakidis, I. , Beltes, P. & Karagiannis, V. (2013) Spectrophotometric analysis of coronal discolouration induced by grey and white MTA. International Endodontic Journal, 46(2), 137–144. [DOI] [PubMed] [Google Scholar]
- Jeeruphan, T. , Jantarat, J. , Yanpiset, K. , Suwannapan, L. , Khewsawai, P. & Hargreaves, K.M. (2012) Mahidol study 1: comparison of radiographic and survival outcomes of immature teeth treated with either regenerative endodontic or apexification methods: a retrospective study. Journal of Endodontics, 38(10), 1330–1336. [DOI] [PubMed] [Google Scholar]
- Jiang, X. , Liu, H. & Peng, C. (2017) Clinical and radiographic assessment of the efficacy of a collagen membrane in regenerative endodontics: a randomized controlled clinical trial. Journal of Endodontics, 43(9), 1465–1471. [DOI] [PubMed] [Google Scholar]
- Landis, J.R. & Koch, G.G. (1977) The measurement of observer agreement for categorical data. Biometrics, 33(1), 159–174. [PubMed] [Google Scholar]
- Lang, T.A. & Secic, M. (2006) How to report statistics in medicine: annotated guideline for authors, editors, and reviewers, 2nd edition, Philadelphia, PA: American College of Physicians. [Google Scholar]
- Lin, J.C. , Lu, J.X. , Zeng, Q. , Zhao, W. , Li, W.Q. & Ling, J.Q. (2016) Comparison of mineral trioxide aggregate and calcium hydroxide for apexification of immature permanent teeth: a systematic review and meta‐analysis. Journal of the Formosan Medical Association, 115(7), 523–530. [DOI] [PubMed] [Google Scholar]
- Lin, J. , Zeng, Q. , Wei, X. , Zhao, W. , Cui, M. , Gu, J. et al. (2017) Regenerative endodontics versus apexification in immature permanent teeth with apical periodontitis: a prospective randomized controlled study. Journal of Endodontics, 43(11), 1821–1827. [DOI] [PubMed] [Google Scholar]
- Lofthag‐Hansen, S. , Huumonen, S. , Gröndahl, K. & Gröndahl, H.G. (2007) Limited cone‐beam CT and intraoral radiography for the diagnosis of periapical pathology. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontics, 103(1), 114–119. [DOI] [PubMed] [Google Scholar]
- Malmberg, L. , Hägg, E. & Björkner, A.E. (2019) Endodontic infection control routines among general dental practitioners in Sweden and Norway: a questionnaire survey. Acta Odontologica Scandinavica, 77(6), 434–438. [DOI] [PubMed] [Google Scholar]
- Mohammadi, Z. & Abbott, P.V. (2009) The properties and applications of chlorhexidine in endodontics. International Endodontic Journal, 42(4), 288–302. [DOI] [PubMed] [Google Scholar]
- Nair, M. , Rahul, J. , Devadathan, A. & Mathew, J. (2017) Incidence of endodontic flare‐ups and its related factors: a retrospective study. Journal of International Society of Preventive & Community Dentistry, 7(4), 175–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawal, R.R. , Utneja, S. , Sharma, V. , Yadav, S. & Talwar, S. (2020) Long‐term follow‐up of traumatized immature necrotic permanent teeth treated with regenerative endodontic protocol using platelet‐rich fibrin: a prospective case series. Journal of Conservative Dentistry, 23(4), 417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazzal, H. & Duggal, M.S. (2017) Regenerative endodontics: a true paradigm shift or a bandwagon about to be derailed? European Archives of Paediatric Dentistry, 18(1), 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldin, A. , Lundgren, J. , Nilsson, M. , Norén, J.G. & Robertson, A. (2015) Traumatic dental injuries among children aged 0–17 years in the BITA study – a longitudinal Swedish multicenter study. Dental Traumatology, 31(1), 9–17. [DOI] [PubMed] [Google Scholar]
- Orstavik, D. & Haapasalo, M. (1990) Disinfection by endodontic irrigants and dressings of experimentally infected dentinal tubules. Endodontics & Dental Traumatology, 6(4), 142–149. [DOI] [PubMed] [Google Scholar]
- Orstavik, D. , Kerekes, K. & Eriksen, H.M. (1986) The periapical index: a scoring system for radiographic assessment of apical periodontitis. Endodontics & Dental Traumatology, 2(1), 20–34. [DOI] [PubMed] [Google Scholar]
- Pereira, A.C. , Oliveira, M.L. , Cerqueira‐Neto, A. , Vargas‐Neto, J. , Nagata, J.Y. , Gomes, B.P.F.A. et al. (2020) Outcomes of traumatised immature teeth treated with apexification or regenerative endodontic procedure: a retrospective study. Australian Endodontic Journal, 47(2), 178–187. [DOI] [PubMed] [Google Scholar]
- Petersson, K. , Håkansson, R. , Håkansson, J. , Olsson, B. & Wennberg, A. (1991) Follow‐up study of endodontic status in an adult Swedish population. Endodontics & Dental Traumatology, 7(5), 221–225. [DOI] [PubMed] [Google Scholar]
- Petridis, X. , van der Sluis, L.W.M. , Dijkstra, R.J.B. , Brinker, M.G.L. , van der Mei, H.C. & Harmsen, M.C. (2018) Secreted products of oral bacteria and biofilms impede mineralization of apical papilla stem cells in TLR‐, species‐, and culture‐dependent fashion. Scientific Reports, 8(1), 12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan, D.P. , Chawla, H.S. , Gauba, K. & Goyal, A. (2006) Comparative evaluation of endodontic management of teeth with unformed apices with mineral trioxide aggregate and calcium hydroxide. Journal of Dentistry for Children, 73(2), 79–85. [PubMed] [Google Scholar]
- Priya, M.H. , Tambakad, P.B. & Naidu, J. (2016) Pulp and periodontal regeneration of an avulsed permanent mature incisor using platelet‐rich plasma after delayed replantation: a 12‐month clinical case study. Journal of Endodontics, 42(1), 66–71. [DOI] [PubMed] [Google Scholar]
- Rafter, M. (2005) Apexification: a review. Dental Traumatology, 21(1), 1–8. [DOI] [PubMed] [Google Scholar]
- Ridell, K. , Petersson, A. , Matsson, L. & Mejàre, I. (2006) Periapical status and technical quality of root‐filled teeth in Swedish adolescents and young adults. A Retrospective Study. Acta Odontologica Scandinavica, 64(2), 104–110. [DOI] [PubMed] [Google Scholar]
- Siqueira, J.F. Jr (2003) Microbial causes of endodontic flare‐ups. International Endodontic Journal, 36(7), 453–463. [DOI] [PubMed] [Google Scholar]
- Torabinejad, M. , Kutsenko, D. , Machnick, T.K. , Ismail, A. & Newton, C.W. (2005) Levels of evidence for the outcome of nonsurgical endodontic treatment. Journal of Endodontics, 31(9), 637–646. [DOI] [PubMed] [Google Scholar]
- Torabinejad, M. , Nosrat, A. , Verma, P. & Udochukwu, O. (2017) Regenerative endodontic treatment or mineral trioxide aggregate apical plug in teeth with necrotic pulps and open apices: a systematic review and meta‐analysis. Journal of Endodontics, 43(11), 1806–1820. [DOI] [PubMed] [Google Scholar]
- Trope, M. (2010) Treatment of the immature tooth with a non‐vital pulp and apical periodontitis. Dental Clinics of North America, 54(2), 313–324. [DOI] [PubMed] [Google Scholar]
- Tsilingaridis, G. , Malmgren, B. , Andreasen, J.O. , Wigen, T.I. , Maseng Aas, A.L. & Malmgren, O. (2016) Scandinavian multicenter study on the treatment of 168 patients with 230 intruded permanent teeth – a retrospective cohort study. Dental Traumatology, 32(5), 353–360. [DOI] [PubMed] [Google Scholar]
- Verma, P. , Nosrat, A. , Kim, J.R. , Price, J.B. , Wang, P. , Bair, E. et al. (2017) Effect of residual bacteria on the outcome of pulp regeneration in vivo. Journal of Dental Research, 96(1), 100–106. [DOI] [PubMed] [Google Scholar]
- Wikström, A. , Brundin, M. , Lopes, M.F. , El Sayed, M. & Tsilingaridis, G. (2021) What is the best long‐term treatment modality for immature permanent teeth with pulp necrosis and apical periodontitis? European Archives of Paediatric Dentistry, 22(3), 311–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Medical Association (WMA) . (2009) Declaration of Helsinki. Ethical principles for medical research involving human subjects. Jahrbuch für Wissenschaft und Ethik, 14(1), 233–238. [Google Scholar]
- Xuan, K. , Li, B. , Guo, H. , Sun, W. , Kou, X. , He, X. et al. (2018) Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. Science Translational Medicine, 10(455), eaaf3227 [DOI] [PubMed] [Google Scholar]


