Abstract
Background
The life expectancy of patients with follicular lymphoma (FL) has improved considerably since the introduction of rituximab. This study examined the proportion of deaths from progressive lymphoma and the impact of FL on survival compared with that in the general population.
Methods
Altogether, 749 patients with grades 1 and 2 FL in 9 institutions between 1997 and 2016 were enrolled. Competing risk models were used to estimate the cumulative incidences of deaths from progressive lymphoma and from other reasons. Excess mortality was analyzed with respect to the corresponding background populations standardized for age and sex using the excess mortality model based on the penalized spline approach.
Results
The median follow‐up duration was 69 months (range, 0‐226 months). The estimated 10‐year overall, disease‐specific, and net survival rates were 72.4%, 86.6%, and 86.4%, respectively. The cumulative incidence of deaths from progressive lymphoma was slightly smaller than that of other causes in the study population (estimated 10‐year cumulative incidences: 12.3% [95% CI, 9.6%‐15.3%] and 15.4% [95% CI, 12.2%‐18.8%], respectively). Excess mortality was observed for up to 10 years after diagnosis, and it slightly increased with time.
Conclusions
Deaths from progressive lymphoma are nearly as common as deaths from other causes in FL patients during the rituximab era. Despite the improvements in survival, there was evidence of excess mortality resulting from FL for at least 10 years after diagnosis.
Keywords: cause of death, follicular lymphoma, mortality, survival, survival rate
Short abstract
Deaths from progressive lymphoma are nearly as common as those from other reasons in patients with follicular lymphoma (FL) in the rituximab era. Despite the improvements in survival, there is evidence of excess mortality from FL for at least 10 years after diagnosis.
Introduction
Follicular lymphoma (FL) is the second most common lymphoma subtype, accounting for approximately one‐fifth of all lymphoma cases in Western countries. The clinical course of FL is heterogeneous. Before the introduction of rituximab, the 10‐year overall survival (OS) in FL was 40% to 56%. 1 , 2 , 3 , 4 Advances in FL care have led to improved survival in both randomized studies and population‐based analyses, 3 , 4 , 5 , 6 leading to an almost 80% 10‐year OS. 7 , 8 , 9
Three previous studies showed that, despite the improved survival rate, most of the FL deaths were attributed to lymphoma‐related causes. 4 , 8 , 10 In recent years, the estimated relative 10‐year survival after first‐line treatment has been 76% to 83%. 3 , 4 , 11 However, in 1 study, mortality rates for patients with FL were lower than those for the general population more than 10 years after the diagnosis, 12 and in 2 other studies, the life expectancy of patients with FL was similar to that of the general population if they exhibited complete response at 24 or 30 months after diagnosis. 7 , 13
In the present study, we used a real‐life data set of 749 patients to examine the mortality among patients with FL. The purpose of this study was to evaluate 1) the proportion of deaths from progressive lymphoma and 2) the impact of FL on expected survival compared with that in the general population.
Materials and Methods
Patients
This retrospective registry study was reviewed and approved by the Regional Ethics Committee of the Northern Ostrobothnia Hospital District and the principles of the Declaration of Helsinki were followed. Clinical data were collected from 4 Finnish and 2 Spanish university hospitals and 3 Finnish central hospitals. All patients (1045) diagnosed with FL between 1997 and 2016 were included in this study: 344 from Spain and 701 from Finland. Patients with primary cutaneous lymphomas, FL grade 3 or unknown grade, composite histology at diagnosis, or histological transformation before any treatment, or whose survival status or cause of death was not identified or who were lost to follow‐up were excluded. Duodenal lymphomas were included. Finally, 749 patients were included. In patients with watchful waiting as the first‐line approach, any proceeding therapy was considered the first‐line treatment. Patient characteristics, treatment information, disease progression, possible retreatments, and death (classified as deaths from progressive lymphoma or other causes) were verified through an extensive review of medical records (Supporting File 1).
Statistical Analysis
Overall survival was calculated from the date of diagnosis to death from any cause or the last follow‐up. Disease‐specific survival (DSS) was calculated from the date of diagnosis to death from progressive lymphoma or the last follow‐up. Progression‐free survival was calculated from the first day of treatment to the first relapse, death from any cause, or the last follow‐up, whichever occurred first. The subsequent survival rates were calculated in a similar manner but considering the follow‐up duration from the first day of the second‐ and third‐line treatments. Net survival was considered the ratio between the proportion of observed survivors in a cohort of cancer patients and the proportion of expected survivors in a comparable set of cancer‐free individuals. Excess mortality was defined as the difference between mortality in patients with FL in relation to mortality in the general population. The excess mortality hazard and its corresponding net survival were analyzed with respect to corresponding Finnish and Spanish background populations standardized for age and sex. 14 All patients (including those in watchful waiting) were included in the survival analysis, which started from the diagnosis.
A multidimensional penalized hazard model was used to estimate the hazards and different survivals. 15 The technique is based on multiple penalized splines and allows the estimation of excess hazard and net survival (Supporting File 1). The same method was used to analyze a competing risk situation in which the cumulative incidences of deaths from progressive lymphoma and deaths from other causes were estimated. Pearson χ2 test was used to evaluate the differences between the age groups. Statistical significance was set at P < .05.
Statistical analyses were performed using IBM SPSS Statistics (version 27; IBM Corp, Armonk, NY) and R software (version 4.0.3; R Foundation for Statistical Computing, Vienna, Austria). 16
Results
Patient Characteristics
Patient characteristics are presented in Table 1 and treatment patterns in Supporting Table 1. The median follow‐up duration was 69 months (range, 0‐226 months); 58.3% of the patients were followed for more than 5 years and 16.8% for more than 10 years. Information regarding the line of treatment, disease progression, and mortality is presented in the flowchart in Figure 1.
TABLE 1.
Patient Characteristics
| Variable | No. = 749 |
|---|---|
| Age at diagnosis, y | |
| Median (range) | 60 (18‐100) |
| <60 | 366 (49.2%) |
| 60‐70 | 224 (30.1%) |
| >70 | 154 (20.7%) |
| Sex | |
| Female | 380 (50.8%) |
| Male | 368 (49.2%) |
| NA | 1 |
| Stage | |
| I‐II | 243 (32.4%) |
| III‐IV | 472 (63.0%) |
| NA | 34 |
| FLIPI | |
| 0‐1 | 247 (36.8%) |
| 2 | 196 (29.2%) |
| 3‐5 | 229 (34.1%) |
| NA | 77 |
| LDH level | |
| Normal | 421 (73.1%) |
| Elevated | 155 (26.9%) |
| NA | 173 |
| Hb, g/dL | |
| <12 | 95 (13.8%) |
| ≥12 | 594 (86.2%) |
| NA | 60 |
| B‐symptoms a | |
| Yes | 124 (17.1%) |
| No | 602 (82.9%) |
| NA | 23 |
| Initial treatment b | |
| Immunochemotherapy | 497 (70.9%) |
| Chemotherapy without rituximab | 69 (9.8%) |
| Anthracycline‐containing regimens | 378 (53.9%) |
| Bendamustine | 61 (8.7%) |
| Other chemotherapy | 127 (18.1%) |
| Rituximab monotherapy | 28 (4.0%) |
| Radiation therapy only | 93 (13.3%) |
| Surgery only | 14 (2.0%) |
| Maintenance with rituximab after first‐line therapy | 209 (29.8%) |
Abbreviations: FLIPI, Follicular Lymphoma International Prognostic Index; Hb, hemoglobin; LDH, lactate dehydrogenase; NA, not available.
B‐symptoms include systemic symptoms (eg, unexplained weight loss, fever, night sweats).
Of the patients who received first‐line therapy (n = 701).
Figure 1.
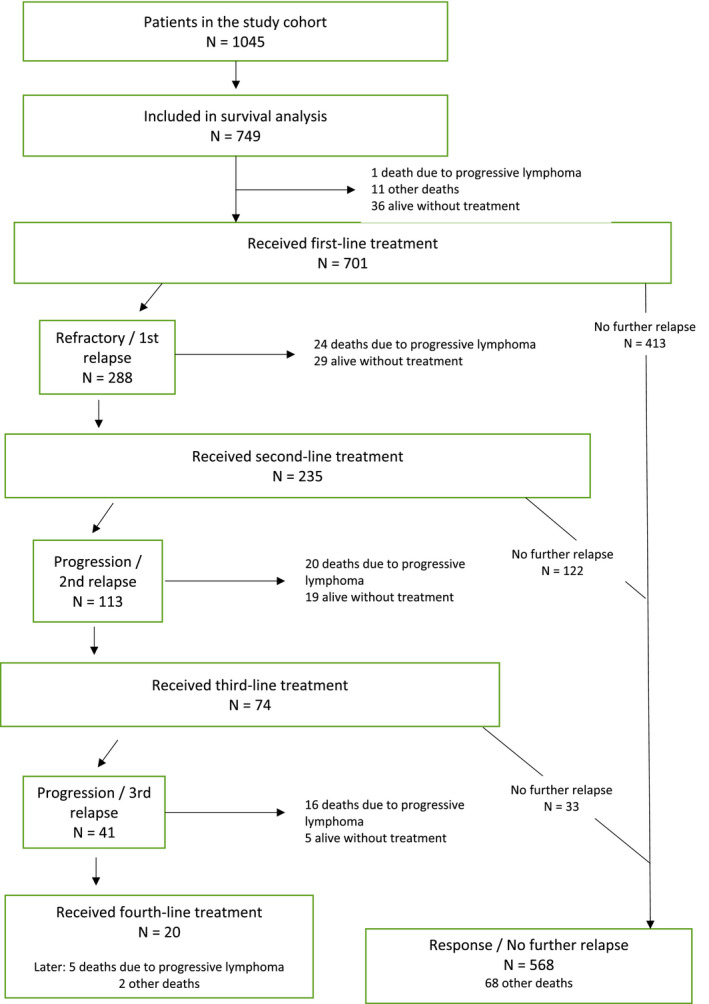
Flow chart of the 749 patients with follicular lymphoma.
Survival
The 5‐year OS was 86.6% (95% CI, 84.0%‐88.8%). The 5‐year DSS was 93.8% (95% CI, 91.8%‐95.3%). The estimated 10‐year rates were 72.4% (95% CI, 68.3%‐76.1%) and 86.6% (95% CI, 83.2%‐89.3%), respectively (Supporting Fig. 1).
Net Survival and the Impact of Lymphoma on Life Expectancy
Figure 2 shows the total and excess mortality of FL patients over 10 years. Excess mortality was observed during the entire study period, and it slightly increased with time (Fig. 2A). The 5‐year net survival rate was 94.6% (95% CI, 92.0%‐96.3%) and the estimated 10‐year net survival was 86.4% (95% CI, 81.7%‐89.9%) (Fig. 2B).
Figure 2.
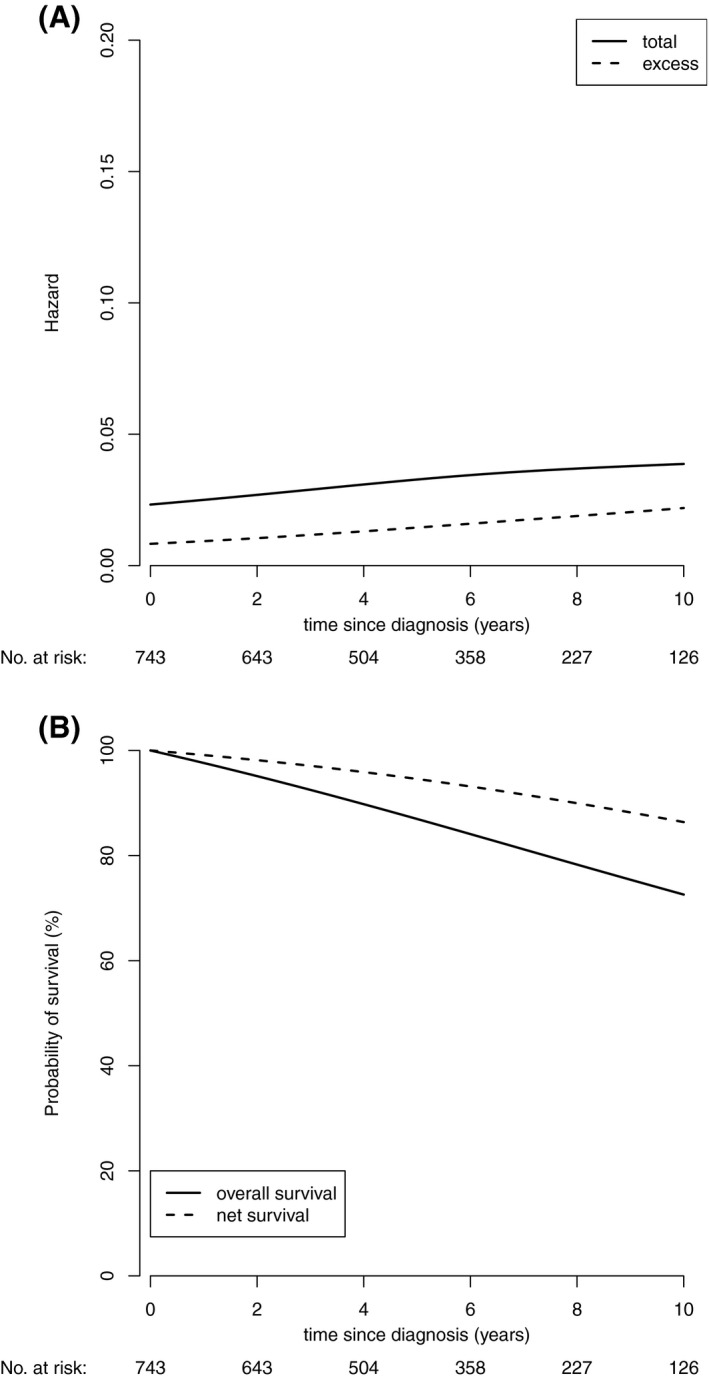
(A) The total and excess mortality of patients with follicular lymphoma. (B) The overall and net survival of patients with follicular lymphoma.
Survival Estimates After Second‐ and Third‐Line Treatments
DSS and OS after the second‐ and third‐line treatments are shown in Figure 3. The 2‐year OS after the second‐line treatment was 87.8% (95% CI, 82.9%‐91.3%) and the 5‐year OS was 73.8% (95% CI, 66.7%‐79.7%). The respective 2‐year DSS was 92.9% (95% CI, 88.2%‐95.8%) and the 5‐year DSS was 79.9% (95% CI, 72.2%‐85.7%) (Fig. 3A). The 2‐year OS after the third‐line treatment was 74.1% (95% CI, 62.8‐82.4%) and the 2‐year DSS was 79.1% (95% CI, 68.0%‐86.8%) (Fig. 3B).
Figure 3.
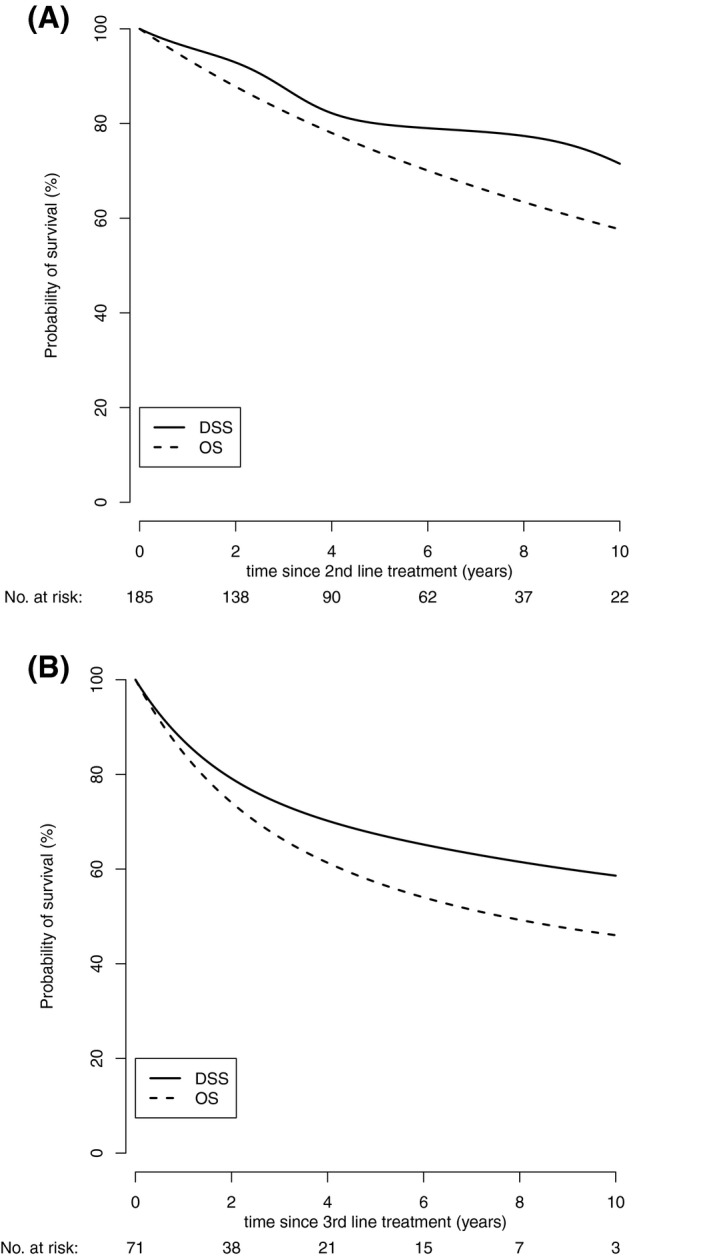
(A) DSS and OS after second‐line treatment. (B) DSS and OS after third‐line treatment. DSS indicates disease‐specific survival; OS, overall survival.
Treatment and Relapse Patterns and Causes of Death
During the follow‐up period, 147 deaths occurred. Among these, 66 (44.9%) were due to progressive lymphoma and 81 (55.1%) were due to other causes. The proportion of patients with disease progression and death from progressive lymphoma increased after each line of treatment. After the first‐line treatment, 24 deaths from progressive lymphoma were noted (3.4%). After the second‐ and third‐line treatments, 20 (8.5%) and 16 (21.6%) deaths were noted, respectively, from progressive lymphoma. The risk of death from progressive lymphoma over time remained relatively stable for 10 years after diagnosis (Fig. 4). The cumulative incidence of deaths from other causes was a little higher than that of progressive lymphoma in the study population (the estimated 10‐year cumulative incidences were 15.4% [95% CI, 12.2%‐18.8%] and 12.3% [95% CI, 9.6%‐15.3%], respectively) (Fig. 5A and Supporting Table 2).
Figure 4.
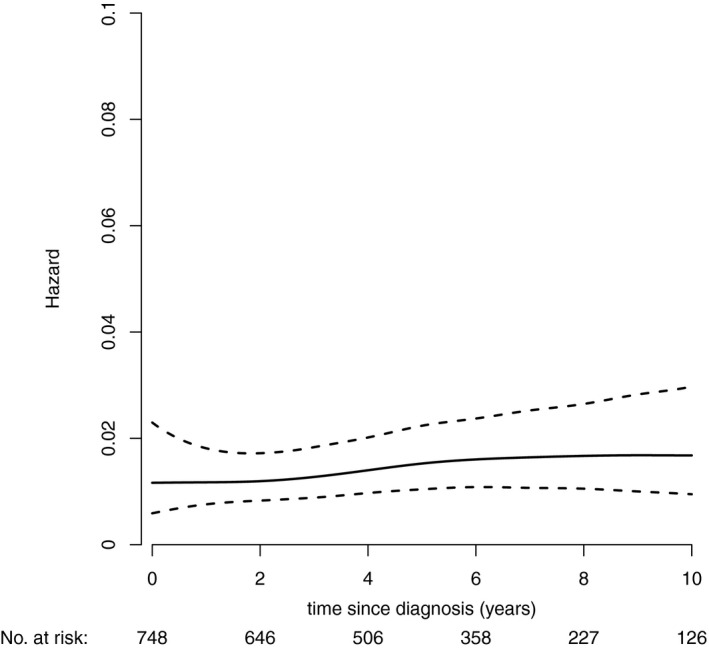
The risk of death from progressive lymphoma with time. Hazard indicates probability for death from progressive lymphoma in certain times on the condition that the event has not yet occurred.
Figure 5.
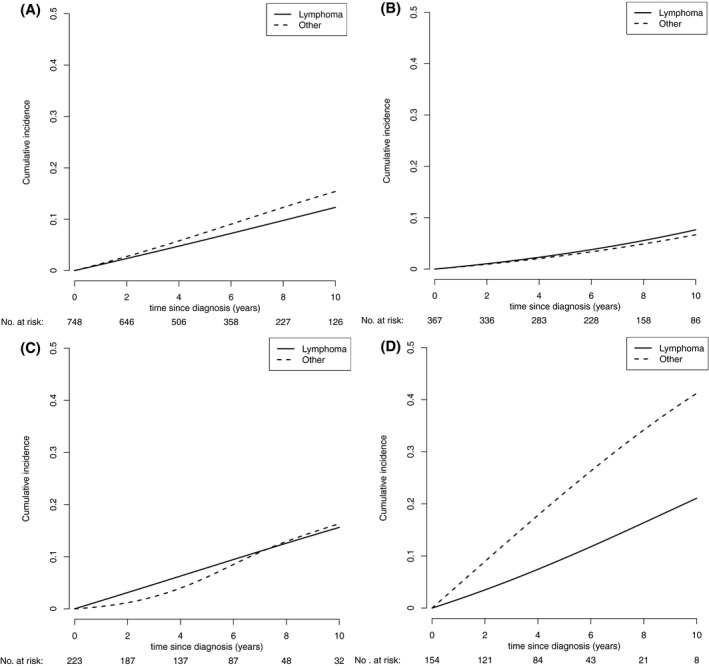
Cumulative incidence for the competing risks of cause of death. (A) Cumulative incidence by cause of death for the entire study population. (B) Cumulative incidence by cause of death for patients aged <60 years. (C) Cumulative incidence by cause of death for patients aged 60 to 69 years. (D) Cumulative incidence by cause of death for patients aged ≥70 years.
Prognostic Impact of Age
Patients aged >70 years were left without treatment more frequently than were younger patients (10.4% vs 5.4%, P = .025). The former group received less immunochemotherapy in general (58.4% vs 68.3%, P = .021), anthracycline‐containing regimens (33.1% vs 54.9%, P < .001), and rituximab maintenance (19.5% vs 30.3%, P = .008) but more less‐intensive therapies (20.1% vs 9.8%, P < .001) than the latter.
The cumulative incidences of both causes of death increased with age. In patients aged <60 years and 60 to 69 years at diagnosis, the cumulative incidences of deaths from the studied causes were virtually similar (estimated 10‐year cumulative incidence for patients aged <60 years: progressive FL, 7.6% [95% CI, 4.7%‐10.9%] vs other causes. 6.7% [95% CI, 3.9%‐10.6%]; 60‐69 years: 15.6% [95% CI, 9.5%‐22.9%] vs 16.3% [95% CI, 9.5%‐23.1%], respectively) (Fig. 5B,C). However, in patients aged older than 70 years at diagnosis, the cumulative incidence of deaths from other causes surpassed that of deaths from progressive lymphoma (10‐year cumulative incidence: 41.2% [95% CI, 29.8%‐52.9%] vs 21.1% [95% CI, 12.2%‐31.8%], respectively) (Fig. 5D).
Discussion
In this study, we analyzed patient survival in a real‐life, unselected data set of 749 patients with FL. The probability of death from progressive lymphoma was slightly smaller than that of other reasons. However, age had a major impact on the results. Deaths from other reasons dominated among patients aged more than 70 years, whereas among younger patients the probability of death from both studied causes was similar. We also observed that FL shortened the lifespan of patients compared with the general population, with a net survival of 86% at 10 years after diagnosis.
Before the rituximab era, most of the deaths among FL patients were related to progressive lymphoma and the 10‐year OS was 40% to 56%. 1 , 2 , 3 , 4 In the 21st century, rituximab has provided a remarkable survival advantage. In our study, the 5‐year OS was 87%, the estimated 10‐year OS was 72%, and the 10‐year net survival was 86%. A recent report from the Memorial Sloan Kettering Cancer Center involving 1088 patients with FL reported a 5‐year OS of 92% and a 10‐year OS of 80%, which are higher than our findings. 17 Although that study included patients with grade 1 through 3A FL, there might be differences in patient selection because Memorial Sloan Kettering Cancer Center is a private center and the population setting might be different from our real‐life, multicenter setting involving public university hospitals and central hospitals. Junlén et al studied the survival of 2641 patients with FL with any grade disease diagnosed between 2000 and 2010. In the rituximab era (2003‐2010), the 10‐year OS was 59% and the 10‐year relative survival was 76%, 3 which are lower than the rates observed in our study. Inclusion of patients with any grade of FL might have an impact on the difference in the results.
Three recent studies have shown that, despite the improved survival of FL patients, most of the deaths are lymphoma related. 4 , 8 , 10 A recent single‐center study by Mozas et al including 727 patients with grade 1 through 3A FL reported that, although the proportion of deaths from progressive FL had decreased over the decades, it was still higher than other‐cause mortality. 4 Hester et al studied cause‐specific mortality among 6703 elderly patients with FL (aged ≥66 years) of any grade and found that the cumulative incidence of FL‐specific mortality was slightly higher compared with other‐cause mortality at 5 years (21.8% vs 15.7%). 10 Sarkozy et al reported an even greater proportion of lymphoma‐related deaths. After 10 years of follow‐up, lymphoma progression was still the most common cause of death (including 140 of 248 deaths) in their cohort of 1654 patients with FL grade 1 through 3A. 8 Similar findings were observed even among the oldest patients (aged >70 years). In our study, nearly one‐half of the deaths (45%) during a median follow‐up of 69 months were caused by progressive lymphoma and the rest were from other reasons, including treatment‐related deaths. The cumulative incidence of deaths from progressive lymphoma was comparably a little lower than that of other reasons for at least 10 years. Although we observed a higher proportion of competing causes of death compared with the previous studies, our results strengthen the understanding that progressive lymphoma remains an important cause of death, even in the rituximab era and among patients with low‐grade FL.
In our study, the risk of death from progressive lymphoma increased with age, which is consistent with previous findings. 3 , 8 Junlén et al showed that, although the relative survival rates had improved in all age groups, patients aged older than 70 years maintained higher excess mortality compared with others. 3 Moreover, Sarkozy et al reported that the cumulative incidence of lymphoma‐related deaths increased with age at diagnosis and was higher than that of non–lymphoma‐related deaths in all age groups. 8 In our study population, among patients aged more than 70 years, the cumulative incidence of other‐cause mortality was remarkably higher than that from progressive lymphoma, which is in contrast to the findings presented in the previous studies. 8 , 10 In our material, however, older patients were treated with less‐intensive regimens and left without treatment more frequently, indicating that an adverse prognosis among elderly patients was associated with treatment selection and treatment‐related toxicities rather than tumor biology. Because the median age of patients with FL at the time of diagnosis is approximately 60 years, 3 , 6 , 18 , 19 the impact of non–lymphoma‐related mortality is evidently increasing.
The major strengths of our study are the relatively large sample size, detailed data collection, and the appropriate and relatively new methodology used to study the excess mortality of patients with FL. All patients had low‐grade lymphoma, ensuring the homogeneity of the material.
Our study has some limitations. There are quite a lot of data indicating that the cause‐of‐death statements are unreliable in cancer patients. 20 , 21 , 22 , 23 , 24 This causes doubt on the value of this assessment for patients who have multiple potential factors contributing to death. For this reason, we decided to classify the deaths only to those associated with progressive FL and others, and treatment‐related deaths per se were not considered a separate entity but were included among other causes. However, net survival considers all excess mortality, including treatment‐related mortality, compared with the age‐ and sex‐adjusted general population. The results concerning secondary malignancies in this data set have been previously published. 25 , 26 The risk of secondary hematologic malignancies was low, but it increased with multiple lines of therapy. There was no increased risk of solid cancers compared with the general population. Possible transformations were not systematically documented; therefore, their roles cannot be examined from the available data. The follow‐up time was relatively short, considering the indolent nature of FL. However, taking into account the large number of patients included, there were still 126 patients alive at the 10‐year follow‐up. Indeed, because of the long life expectancy of most patients, acquiring true observational data would take at least 10 to 20 years. With the rapid evolution of treatment, such data would already be outdated when they become available. The 69‐month follow‐up provided a good balance between follow‐up and an up‐to‐date treatment paradigm that can still inform care for present‐day patients. Additionally, we acknowledge that uncertainty increases with increasing lines of therapy, and because of the limited follow‐up, patients with an adverse outcome dominate.
In conclusion, deaths from progressive lymphoma are as common as deaths from other causes in the rituximab era in patients with FL aged younger than 70 years, whereas deaths from other causes are clearly more common in patients aged older than 70 years. Despite the improvements in progression‐free survival, OS, and DSS over the past decades, there was evidence of excess mortality from FL for at least 10 years after diagnosis.
Funding Support
This work was funded by the Finnish Blood Disease Research Foundation, The Finnish Medical Foundation (Rajamäki Aino) and Thelma Mäkikyrö fund (Kuusisto Milla E.L.).
Conflict of Interest Disclosures
Juan‐Manuel Sancho reports payments from Roche and Gilead for participating on a data safety monitoring board or advisory board. The other authors made no disclosures.
Author Contributions
Aino Rajamäki: Conceptualization, data curation, formal analysis, investigation, methodology, visualization, writing – original draft, and writing – review and editing. Mika Hujo: Formal analysis, methodology, visualization, and writing – original draft. Reijo Sund: Formal analysis, methodology, visualization, and writing – review and editing. Roosa E.I. Prusila: Data curation, investigation, and writing – review and editing. Milla E.L. Kuusisto: Investigation and writing – review and editing. Hanne Kuitunen: Conceptualization, funding acquisition, project administration, and writing – review and editing. Esa Jantunen: Investigation and writing – review and editing. Santiago Mercadal: Investigation and writing – review and editing. Marc Sorigue: Conceptualization, data curation, investigation, methodology, and writing – review and editing. Juan‐Manuel Sancho: Investigation and writing – review and editing. Kaisa Sunela: Conceptualization, formal analysis, methodology, and writing – original draft. Outi Kuittinen: Conceptualization, data curation, formal analysis, funding acquisition, methodology, project administration, resources, supervision, and writing – original draft.
Supporting information
Fig S1
Table S1
Table S2
Supplementary Material
Rajamäki A, Hujo M, Sund R, Prusila REI, Kuusisto MEL, Kuitunen H, Jantunen E, Mercadal S, Sorigue M, Sancho J‐M, Sunela K, Kuittinen O. Mortality among patients with low‐grade follicular lymphoma: A binational retrospective analysis. Cancer. 2022. 10.1002/cncr.34221
The last 2 authors contributed equally to the study.
References
- 1. Swenson WT, Wooldridge JE, Lynch CF, Forman‐Hoffman VL, Chrischilles E, Link BK. Improved survival of follicular lymphoma patients in the United States. J Clin Oncol. 2005;23:5019‐5026. doi: 10.1200/JCO.2005.04.503 [DOI] [PubMed] [Google Scholar]
- 2. Johnson PWM, Rohatiner AZS, Whelan JS, et al. Patterns of survival in patients with recurrent follicular lymphoma: a 20‐year study from a single center. J Clin Oncol. 1995;13:140‐147. doi: 10.1200/JCO.1995.13.1.140 [DOI] [PubMed] [Google Scholar]
- 3. Junlén HR, Peterson S, Kimby E, et al. Follicular lymphoma in Sweden: nationwide improved survival in the rituximab era, particularly in elderly women: a Swedish Lymphoma Registry Study. Leukemia. 2015;29:668‐676. doi: 10.1038/leu.2014.251 [DOI] [PubMed] [Google Scholar]
- 4. Mozas P, Nadeu F, Rivas‐Delgado A, et al. Patterns of change in treatment, response, and outcome in patients with follicular lymphoma over the last four decades: a single‐center experience. Blood Cancer J. 2020;10(3). doi: 10.1038/s41408-020-0299-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tan D, Horning SJ, Hoppe RT, et al. Improvements in observed and relative survival in follicular grade 1‐2 lymphoma during 4 decades: the Stanford University experience. Blood. 2013;122:981‐987. doi: 10.1182/blood-2013-03-491514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mounier M, Bossard N, Belot A, et al. Trends in excess mortality in follicular lymphoma at a population level. Eur J Haematol. 2015;94:120‐129. doi: 10.1111/ejh.12403 [DOI] [PubMed] [Google Scholar]
- 7. Magnano L, Alonso‐Alvarez S, Alcoceba M, et al. Life expectancy of follicular lymphoma patients in complete response at 30 months is similar to that of the Spanish general population. Br J Haematol. 2019;185:480‐491. doi: 10.1111/bjh.15805 [DOI] [PubMed] [Google Scholar]
- 8. Sarkozy C, Maurer MJ, Link BK, et al. Cause of death in follicular lymphoma in the first decade of the rituximab era: a pooled analysis of French and US cohorts. J Clin Oncol. 2019;37:144‐152. doi: 10.1200/JCO.18.00400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bachy E, Seymour JF, Feugier P, et al. Sustained progression‐free survival benefit of rituximab maintenance in patients with follicular lymphoma: long‐term results of the PRIMA study. J Clin Oncol. 2019;37:2815‐2824. doi: 10.1200/JCO.19.01073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hester LL, Park SI, Wood WA, Stürmer T, Brookhart MA, Lund JL. Cause‐specific mortality among Medicare beneficiaries with newly diagnosed non‐Hodgkin lymphoma subtypes. Cancer. 2019;125:1101‐1112. doi: 10.1002/cncr.31821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rivas‐Delgado A, Magnano L, Moreno‐Velázquez M, et al. Response duration and survival shorten after each relapse in patients with follicular lymphoma treated in the rituximab era. Br J Haematol. 2019;184:753‐759. doi: 10.1111/bjh.15708 [DOI] [PubMed] [Google Scholar]
- 12. Provencio M, Royuela A, Torrente M, et al. Prognostic value of event‐free survival at 12 and 24 months and long‐term mortality for non‐Hodgkin follicular lymphoma patients: a study report from the Spanish Lymphoma Oncology Group. Cancer. 2017;123:3709‐3716. doi: 10.1002/cncr.30795 [DOI] [PubMed] [Google Scholar]
- 13. Maurer MJ, Bachy E, Ghesquières H, et al. Early event status informs subsequent outcome in newly diagnosed follicular lymphoma. Am J Hematol. 2016;91:1096‐1101. doi: 10.1002/ajh.24492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Max Planck Institute for Demographic Research M . Human Life Table Database. Accessed March 31, 2022. www.lifetable.de/cgi‐bin/data.php
- 15. Remontet L, Uhry Z, Bossard N, et al. Flexible and structured survival model for a simultaneous estimation of non‐linear and non‐proportional effects and complex interactions between continuous variables: performance of this multidimensional penalized spline approach in net survival trend ana. Stat Methods Med Res. 2019;28:2368‐2384. doi: 10.1177/0962280218779408 [DOI] [PubMed] [Google Scholar]
- 16. Fauvernier M, Remontet L, Uhry Z, Bossard N, Roche L. survPen: an R package for hazard and excess hazard modelling with multidimensional penalized splines. J Open Source Softw. 2019;4:1434. doi: 10.21105/joss.01434 [DOI] [Google Scholar]
- 17. Batlevi CL, Sha F, Alperovich A, et al. Follicular lymphoma in the modern era: survival, treatment outcomes, and identification of high‐risk subgroups. Blood Cancer J. 2020;10:74. doi: 10.1038/s41408-020-00340-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Le M, Ghazawi FM, Alakel A, et al. Incidence and mortality trends and geographic patterns of follicular lymphoma in Canada. Curr Oncol. 2019;26:e473‐e481. doi: 10.3747/co.26.4625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Morrison VA, Shou Y, Bell JA, et al. Treatment patterns and survival outcomes in patients with follicular lymphoma: a 2007 to 2015 Humedica Database Study. Clin Lymphoma Myeloma Leuk. 2019;19:e172‐e183. doi: 10.1016/j.clml.2018.12.017 [DOI] [PubMed] [Google Scholar]
- 20. Hoel DG, Ron E, Carter R, Mabuchi K. Influence of death certificate errors on cancer mortality trends. J Natl Cancer Inst. 1993;85:1063‐1068. doi: 10.1093/jnci/85.13.1063 [DOI] [PubMed] [Google Scholar]
- 21. Modelmog D, Rahlenbeck S, Trichopoulos D. Accuracy of death certificates: a population‐based, complete‐coverage, one‐year autopsy study in East Germany. Cancer Causes Control. 1992;3:541‐546. doi: 10.1007/BF00052751 [DOI] [PubMed] [Google Scholar]
- 22. Sehdev AES, Hutchins GM. Problems with proper completion and accuracy of the cause‐of‐death statement. Arch Intern Med. 2001;161:277‐284. doi: 10.1001/archinte.161.2.277 [DOI] [PubMed] [Google Scholar]
- 23. Schaffar R, Rapiti E, Rachet B, Woods L. Accuracy of cause of death data routinely recorded in a population‐based cancer registry: impact on cause‐specific survival and validation using the geneva cancer registry. BMC Cancer. 2013;13. doi: 10.1186/1471-2407-13-609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Izci H, Tambuyzer T, Vandeven J, et al. Cause of death for patients with breast cancer: discordance between death certificates and medical files, and impact on survival estimates. Arch Public Health. 2021;79:1‐8. doi: 10.1186/s13690-021-00637-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Prusila REI, Sorigue M, Jauhiainen J, et al. Risk of secondary haematological malignancies in patients with follicular lymphoma: an analysis of 1028 patients treated in the rituximab era. Br J Haematol. 2019;187:364‐371. doi: 10.1111/bjh.16090 [DOI] [PubMed] [Google Scholar]
- 26. Sorigue M, Prusila REI, Jauhiainen J, et al. Incidence of solid cancer in patients with follicular lymphoma. Acta Oncol (Madr). 2019;58:1564‐1569. doi: 10.1080/0284186X.2019.1643918 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Table S1
Table S2
Supplementary Material


