Abstract
Aims
To determine the association between registered mental illness and type 2 diabetes mellitus treatment targets, while taking into account the effects of health expenditure and social determinants of health.
Methods
This observational cross‐sectional study was based on routine primary care data, linked to socio‐economic and medical claims data. The main outcomes, analysed by multivariate logistic regression, were achieving primary care guideline treatment targets for HbA1c, systolic blood pressure (SBP) and LDL‐cholesterol in 2017. We examined the association with diagnosed mental illness registered by the general practitioner (GP) or treated via specialist’ mental healthcare between 2016 and 2018, adjusting for, medication use, body mass index, co‐morbidity, smoking, and additionally examining effect‐modification of healthcare expenditures, migration status, income and demographics.
Results
Overall (N = 2862), 64.0% of participants achieved their treatment targets for HbA1c, 65.1% for SBP and 53.0% for LDL‐cholesterol. Adjusted for migrant background, income and care expenditures, individuals <65 years of age with mental illness achieved their HbA1c treatment target more often than those without (OR (95% CI)): treatment by GP: 1.46 (1.01, 2.11), specialist care: 1.61 (1.11, 2.34), as did men with mental illness for SBP: GP OR 1.61 (1.09, 2.40), specialist care OR 1.59 (1.09, 2.45). LDL‐cholesterol target was not associated with mental illness. A migrant background or low income lowered the likelihood of reaching HbA1c targets.
Conclusions
People with registered mental illness appear comparable or better able to achieve diabetes treatment targets than those without. Achieving HbA1c targets is influenced by social disadvantage.
Keywords: diabetes mellitus, type 2; electronic health record data; healthcare expenditures; mental disorders; observational data; social determinants of health; treatment targets
What’s new?
Type 2 diabetes and mental illness exhibit a bi‐directional relationship in terms of disease control as well as prevalence. Social determinants of health are associated with both diseases.
In terms of reaching treatment targets, adults with diabetes and registered mental illness were comparable or better than those without mental illness. Income or a migrant background was independently associated with disease regulation, whereas healthcare expenditure was not.
When mental illness is registered (thus diagnosed) and diabetes is monitored in people with diabetes, diabetes treatment targets can be achieved. Diabetes care providers should therefore consider recognition and prevention of mental illness.
1. INTRODUCTION
Type 2 diabetes mellitus and mental illness often co‐occur, 1 and are associated with decreased quality of life and co‐morbidity. 2 People with type 2 diabetes are not only more likely to develop a mental illness; the causal link is bi‐directional. 3 , 4 In people with mental illness, decreased self management, use of psychiatric medication and genetic predisposition may increase diabetes risk, and (when already present) influence diabetes regulation, 2 , 5 , 6 whereas diabetes itself can lead to depressive symptoms and cognitive decline. 3 , 4 , 7
To reduce the risk of cardiovascular complications, diabetes treatment aims to control HbA1c, blood pressure and cholesterol levels, and for this purpose, personalised treatment targets have been advised by the Dutch primary care guidelines. Existing research on the effect of mental illness on diabetes regulation is inconclusive. Rather than poorer glycaemic control and diabetes monitoring, 2 , 6 it has been observed that people with type 2 diabetes with hospital‐diagnosed depression or antidepressant treatment are actually more likely to achieve HbA1c treatment targets compared with people without. 8 This could be a consequence of beneficial antidepressant medication or the more intensive delivery of healthcare in patients with diabetes receiving treatment for depression. Compared with people not affected by mental illness, Smith et al. 9 found similar mean HbA1c regulation but improved systolic blood pressure (SBP) regulation in individuals with severe mental illness. However, it should be noted that severe mental illness was associated with more extreme risk values (very high or very low) for HbA1c.
Due to their need for both mental and physical care, individuals with diabetes and mental illness make greater use of healthcare and require higher healthcare expenditure. 10 , 11 , 12 , 13 In the Netherlands, type 2 diabetes care is well organised within care groups and is mainly delivered by general practitioners (GPs) and their teams. 14 GPs are also the gatekeeper for mental healthcare and low‐intensity conditions are mainly treated in GP practice centres, whereas people with greater treatment demands are treated in specialist care. The diverse treatment needs of people with multiple morbidities may lead to fragmented care, with possible adverse effects on outcomes. If diabetes treatment targets are not reached despite intensive use of healthcare, there may be room for improvement regarding the delivery of healthcare. Conversely, when treatment targets are reached, efforts may be justified despite high expenditure.
The prevalence and persistence of both mental illness and type 2 diabetes is associated with social determinants of health such as income, employment, migrant background and access to healthcare delivery. 5 , 15 Previous research examining the association between lower SES and healthcare delivery 16 produced mixed results, as any association is highly dependent on the specifics of local healthcare organisation. 17 , 18 A lower socio‐economic status (SES) is associated with poorer achievement of treatment targets for HbA1c, SBP and lipids in people with diabetes. 18 , 19 Social determinants of health should therefore be considered when examining the quality of chronic care delivery in socio‐economic diverse populations.
Although individual impacts of social determinants of health on the prevalence and outcomes of mental illness or type 2 diabetes are well established, the association of mental illness with achieving diabetes targets in relation to social determinants of health remains unclear. Furthermore, healthcare expenditures, as a measure of delivered care, may play a role in achieving targets. We, therefore, investigated associations between mental illness and achieving diabetes treatment targets for HbA1c, SBP, and LDL‐cholesterol. As healthcare expenditures and/or social determinants of health may be associated with mental illness or the above outcomes, we also investigated effect modification and adjusted our analysis in relation to these determinants.
2. METHODS
In this observational cross‐sectional study, we used pseudonymised routine GP care data from The Hague area that were derived from our ‘Extramural Academic Network of the Leiden University Medical Centre’ data warehouse (data of approximately 180,000 citizens from 1 January 2007 to 31 December 2019). 20 Included individuals were informed about use of their data for research purposes and could withdraw via an informed opt‐out procedure. Healthcare data were linked to socio‐economic data within the Social Statistical Datasets from Statistics Netherlands. 21 These datasets cover longitudinal microdata on several domains (demographics, socio‐economic details, including migration background, residence, income, and statutory basic health insurance claims data from all Dutch health insurers) for registered residents of the Netherlands. International Classification of Primary Care (ICPC) codes, body measurements and metformin prescriptions (7‐digit Anatomical Therapeutic Chemical Classification System code (ATC)) were derived from the GP data. Demographics, social determinants of health, death records, medication use and healthcare expenditures were derived from the Social Statistical Datasets for the year 2017. Medication use was derived from claims data, aggregated on an annual basis per person using a 4‐digit ATC. A code book with a detailed description of the determinants is available on request. The ethics Committee Leiden‐The Hague‐Delft exempted this study.
2.1. Study population
Adults (18–80 years of age) with an active ICPC‐code for type 2 diabetes (T90 and T90.02) and alive in the year 2017 were included. Exclusion criteria included a diabetes duration of <1 year (as diabetes outcomes may be unregulated in the first year after diagnosis 22 ), likely type 1 diabetes, Latent Autoimmune Disease of the Adult, Maturity‐Onset Diabetes of the Young, gestational diabetes or dementia (being more of a neurological aetiology and occurring at older age) Those with data missing on all three treatment targets were excluded, indicating that individuals included attended at least one annual diabetes monitoring visit.
2.2. Mental illness groups
Three mental illness groups were defined: (1) no mental illness; (2) mental illness in 2017 registered in GP data (i.e., individuals with ICPC‐code for mental illness, but no expenditures via specialised mental healthcare) and (3) mental illness identified via mental healthcare expenditure data (i.e. specialist mental healthcare between 2016–2018).
2.3. Treatment targets
Mean HbA1c, SBP and LDL‐cholesterol were calculated from all registered measurements in 2017. Treatment targets were defined by the 2017 Dutch treatment guidelines for primary care. 13 , 23 HbA1c targets were ≤7.0% (≤53 mmol/mol) for those aged <70 or aged ≥70 years without medications or metformin monotherapy and ≤7.5% (≤58 mmol/mol) for those aged ≥70 years with additional blood glucose lowering medication or insulin. 23 The treatment target for SBP was <140 mmHg. 13 The target level for LDL‐cholesterol was ≤2.5 mmol/L, but only for individuals with an indication for primary or secondary prevention of cardiovascular disease (CVD). Indications were secondary prevention of manifest CVD and primary prevention for individuals without CVD but >20% risk of 10‐year fatal and non‐fatal CVD risk score defined by the Systematic Coronary Risk Evaluation function. 13 Reimbursed lipid‐lowering medication in 2017 was defined as an additional indication.
2.4. Medical determinants
Diabetes duration was identified using the first ICPC‐code registration date and was categorised into tertiles of 12–50, 50–85, or 85–120 months. As data were available from 2007 on, the maximum diabetes duration was 10 years. Smoking status was defined as ‘non‐smoker’, ‘previous smoker’ or ‘current smoker’ in 2016–2018. Mean body mass index (BMI (kg/m2)) was calculated from all BMI registered in 2016–2018.
Diabetes‐related vascular co‐morbidity was defined as the presence of one or more ICPC‐codes for manifest micro‐ or macrovascular disease. Other chronic co‐morbidity was defined as the presence of one or more chronic diseases selected based on their high prevalence among the Dutch population. 24
Three diabetes treatment categories were defined: (1) lifestyle advice only (no reimbursed glucose‐lowering medication), (2) oral blood glucose‐lowering medication or GLP‐1 agonists (ATC A10B, no A10A) and (3) use of insulin (ATC A10A). Metformin monotherapy was identified from prescription data on ATC‐7 level from the GP data for people >70 years, to allow their treatment target for HbA1c to be determined. Other medication groups were categorised as ‘yes’ and ‘no’ regarding usage of psychiatric/anti‐hypertensive/lipid‐lowering medication.
2.5. Social determinants
Demographic determinants included sex and age on 1 January 1 2017; social determinants of health included migration status and income. Migration status was clustered into two groups, ‘Dutch origin’ or ‘migrant background’, based on the individual's or parents’ country of origin. Standardised disposable household income was used, which represents the net amount a household can spend on an annual basis, adjusted for household size and composition and divided into percentiles on a population‐wide national level: low (0–33 percentile), middle (33–66 percentile), high (66–100 percentile). Job status was defined by the main source of household income and classified into two groups: (1) income from wages or pension benefits, and (2) income from social security benefits.
2.6. Healthcare expenditures
Total reimbursed healthcare expenditures minus expenditures for mental healthcare were used as an indicator of healthcare utilisation and analysed as tertiles. For baseline characteristics, total expenditures were further grouped into GP expenditure, medical specialist expenditure and pharmacy expenditure.
2.7. Missing data
Missing data were handled using multiple imputation generating 10 imputed datasets (supplementary material (SM) A). 25 Variables in the imputation procedure included all predictors and the outcome variables of the final analysis, plus a two‐digit postal code. Pooled results were calculated using Rubin's rules.
2.8. Statistical analysis
All analyses were performed using SPSS version 25. Descriptive statistics for all determinants were provided in n (%), mean ± standard deviation (SD) or median (interquartile range (IQR)), as appropriate. Continuous data were normally distributed, except for income and healthcare expenditures, which were categorised for the main analyses.
The main analyses were multivariate logistic regression models with the outcomes being achieved treatment targets for (1) HbA1c (2) SBP and (3) LDL‐cholesterol.
The models were built using the following steps: for each outcome variable a basic model was performed including only demographic and medical determinants. Interaction terms for sex and age were added to examine effect modification with mental illness. Second, (1) migration status and (2) income were added and effect modification with mental illness was examined. Finally, total healthcare expenditures were added and effect modification examined. When effect modification was present, models were stratified into appropriate groups. All final models included demographics (sex, age), medical factors (mental illness group, diabetes‐related co‐morbidity, other chronic co‐morbidity, BMI, diabetes duration, psychiatric medication, diabetes treatment category (HbA1c), use of anti‐hypertensives (SBP), use of lipid lowering medication (LDL model)), social factors (migration status, income) and healthcare expenditures.
To assess the associations of migration status, income and healthcare expenditures with mental illness, multinomial multivariate regression analysis was performed on the full population, adjusted for all relevant confounders.
We performed several sensitivity analyses. First, using multilevel logistic regression we investigated to what extent differences in outcome variables could be attributed to between‐GP practice differences. Furthermore, we examined the associations of job status as a substitute for income, and mental illness duration <1 year versus >1 year as a substitute for a mental illness group. Lastly, we substituted total healthcare expenditures with pharmacy expenditures only.
Area‐under‐the‐curves (c‐statistic) were calculated to assess internal validity of the main analysis.
3. RESULTS
Of the 5992 living adults with type 2 diabetes identified in the data warehouse in 2017, 2862 were included in the analyses (Figure 1). Most individuals were excluded based on diabetes duration <1 year and missing data on all three outcome measurements. Almost a quarter of all included participants (23%: n = 644) had a mental illness diagnosis, of which 323 received specialist care. Half of the population had a low income (50%) and half (52%) had a migrant background. Median total healthcare expenditures in 2017 were 2560 (IQR 1324–5855) euros (Table 1). Compared with those without mental illness, individuals with mental illness were younger and were more likely to have/more often had a lower income, receive social benefits, and generate higher healthcare expenditures (Table 2, Figure 3, SM B Table S1).
FIGURE 1.
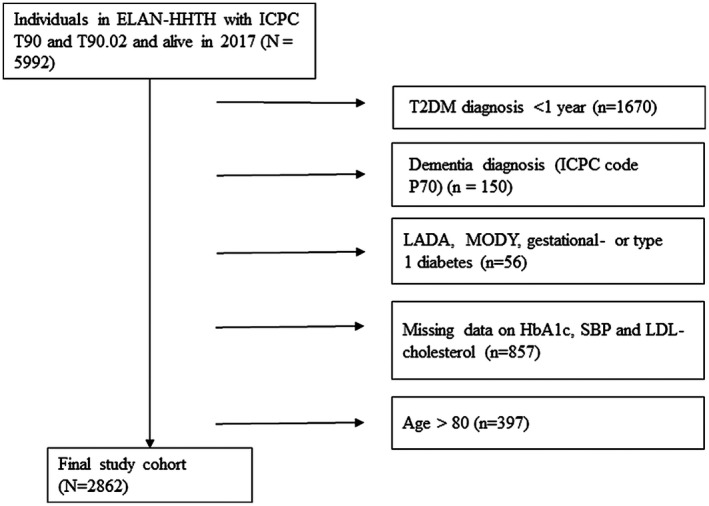
Flowchart of patient inclusion
TABLE 1.
Baseline characteristics
| Baseline characteristics of the total study population and numbers reaching treatment targets | ||||
|---|---|---|---|---|
| On target HbA1c | On target systolic blood pressure | On target LDL‐cholesterol | Total n, (% of total N) | |
| n = 1831 (64%) | n = 1863 (65%) | n = 1516 (53%) | N = 2862 | |
| Women | 851 (66%) | 854 (66%) | 636 (49%) | 1290 (45%) |
| Men | 980 (62%) | 1009 (64%) | 880 (56%) | 1572 (55%) |
| Age (years) | 63.6 ± 10.0 | 60.4 ± 10.8 | 61.7 ± 11.1 | 61.8 ± 10.6 |
| Mental illness | ||||
| No mental illness | 1428 (64%) | 1407 (63%) | 1153 (52%) | 2218 (77%) |
| Mental illness registration GP | 208 (65%) | 217 (67%) | 177 (55%) | 321 (11%) |
| Specialist mental healthcare | 195 (60%) | 240 (74%) | 186 (57%) | 323 (11%) |
| Medical determinants | ||||
| Diabetes duration (months) | 65 (40–91) | 67.8 (41–93) | 71 (44–95) | 68 (41–93) |
| BMI (kg/m2) | 29.9 ± 5.3 | 29.9 ± 5.2 | 30.3 ± 5.5 | 30.2 ± 5.4 |
| HbA1c mmol/mol; (%) | 47 ± 5 (6.4 ± 2.6%) | 54 ± 14 (7.1 ± 3.4%) | 54 ± 14 (7.1 ± 3.4%) | 54 ± 4 (7.1 ± 3.4%) |
| LDL‐cholesterol (mmol/l) | 2.6 ± 0.9 | 2.6 ± 0.9 | 2.1 ± 0.5 | 2.7 ± 0.9 |
| SBP (mmHg) | 136 ± 13.7 | 128 ± 8.5 | 135 ± 13.7 | 136 ± 14.3 |
| Medication usage (n%) | ||||
| Diabetes medication | ||||
| None | 624 (94%) | 438 (66%) | 297 (45%) | 663 |
| Oral blood‐glucose‐lowering drugs or GLP‐1 agonists | 1132 (61%) | 1209 (65%) | 1006 (54%) | 1850 |
| Insulin use | 75 (21%) | 217 (62%) | 213 (61%) | 349 |
| Psychiatric medication | 530 (64%) | 556 (67%) | 446 (54%) | 831 |
| Anti‐hypertensives | 1296 (66%) | 1165 (59%) | 1106 (56%) | 1964 |
| Lipid‐lowering medication | 1316 (63%) | 1375 (661%) | 1238 (60%) | 2082 |
| Smoking | ||||
| Never | 679 (63%) | 740 (68%) | 564 (52%) | 1084 |
| Before | 737 (68%) | 654 (60%) | 593 (55%) | 1088 |
| Current | 415 (60%) | 470 (68%) | 359 (52%) | 691 |
| Co‐morbid conditions | ||||
| No | 543 (62%) | 615 (70%) | 465 (53%) | 882 |
| Micro‐ or macrovascular | 516 (62%) | 515 (62%) | 491 (59%) | 836 |
| Other chronic | 1172 (65%) | 1131 (63%) | 949 (53%) | 1791 |
| Total healthcare expenditures tertiles and healthcare expenditures median (IQR) | ||||
| 1st tertile | 670 (66%) | 670 (66%) | 490 (48%) | 1013 |
| 2nd tertile | 635 (66%) | 647 (67%) | 512 (53%) | 967.2 |
| 3rd tertile | 526 (60%) | 547 (62%) | 514 (58%) | 882 |
| Total minus mental healthcare expenditures | 1914 (961–4219) | 1974 (998–4329) | 2212 (1105–5044) | 2018 (1006–4550) |
| Migration status | ||||
| Dutch origin | 965 (70%) | 827 (60%) | 726 (53%) | 1373 |
| Migrant background | 866 (58%) | 1036 (70%) | 790 (53%) | 1489 |
| Job status | ||||
| Salary or pension benefit | 1546 (66%) | 1497 (64%) | 1232 (52%) | 2349 |
| Social security benefit | 285 (56%) | 366 (71%) | 284 (55%) | 513 |
| Standardized household income in percentiles | ||||
| Low (0–33) | 869 (61%) | 927 (66%) | 791 (56%) | 1416 |
| Middle (33–66) | 487 (64%) | 494 (65%) | 401 (53%) | 764 |
| High (66–100) | 475 (70%) | 442 (65%) | 324 (48%) | 682 |
Data represent n (% of total per category), mean ± SD or median (interquartile range).
Results are pooled from the results of 10 multiple imputed datasets.
TABLE 2.
Characteristics of individuals with and without mental illness
| Baseline characteristics by mental illness status | |||
|---|---|---|---|
| Characteristic | No mental illness (n = 2218) | Mental illness, monitoring by general practitioner (GP) (n = 321) |
Mental illness, specialist mental healthcare (n = 323) |
| Female sex | 969 (44%) | 163 (51%) | 158 (49%) |
| Age | 62.9 ± 10.4 | 59.8 ± 10.5 | 56.4 ± 10.4 |
| Mental illness duration median years (IQR) | n/a | 5.5 (3.4–8.0) | 5.1 (2.2–8.0) |
|
HbA1c mmol/mol; % |
54 ± 13 7.1 ± 3.4% |
54 ± 14 7.0 ± 3.4% |
56 ± 16 7.2 ± 3.6% |
| SBP mmHg | 137.0 ± 14.2 | 135.4 ± 14.1 | 133.1 ± 14.6 |
| LDL‐cholesterol | 2.67 ± 0.88 | 2.65 ± 0.85 | 2.65 ± 0.93 |
| Healthcare expenditures (Euros) | |||
| Total minus mental healthcare | 1851 (946–4153) | 2217 (1064–5183) | 3036 (1584–6150) |
| General practitioner care | 157 (120–230) | 185 (131–269) | 214 (144–327) |
| Hospital care | 558 (132–1957) | 661 (150–2484) | 765 (203–2963) |
| Pharmaceutical care | 400 (192–916) | 574 (234–1088) | 829 (384–1653) |
| Medication usage | |||
| Diabetes medication | |||
| None | 519 (24%) | 83 (26%) | 62 (19%) |
| Oral blood‐glucose lowering drugs or GLP‐1 agonists | 1454 (66%) | 191 (60%) | 206 (64%) |
| Insulin | 245 (11%) | 47 (15%) | 56 (17%) |
| Anti‐hypertensives | 1544 (70%) | 219 (68%) | 202 (62%) |
| Lipid‐lowering medication | 1606 (72%) | 223 (70%) | 253 (78%) |
| Psychiatric medication | 450 (20%) | 141 (44%) | 240 (74%) |
| Antipsychotics | 19 (0.9%) | 12 (3.7%) | 115 (6%) |
| Anxiolytics | 299 (14%) | 83 (26%) | 141.6 (44%) |
| Antidepressants | 158 (7.1%) | 86 (27%) | 136 (42%) |
| Drugs for addictive disorders | 48 (2.2%) | 11 (3.4%) | 24 (7.4%) |
| Social determinants of health | |||
| Migration status | |||
| Dutch origin | 1100 (50%) | 141 (44%) | 132.1 (41%) |
| Migrant background | 1118 (50%) | 180 (56%) | 191.1 (59%) |
| Household income | |||
| Low | 1036 (47%) | 176 (55%) | 204 (63%) |
| Middle | 617 (28%) | 79 (25%) | 68 (21%) |
| High | 565 (26%) | 66 (21%) | 51 (16%) |
| Job status | |||
| Salary or pension benefit | 1929 (87%) | 238 (74%) | 183 (57%) |
| Social benefit | 289 (13%) | 84 (26%) | 141 (44%) |
Data represent n (% of total per mental illness category), mean ± SD or median (interquartile range).
Results are pooled from the results of 10 multiple imputed datasets.
FIGURE 3.
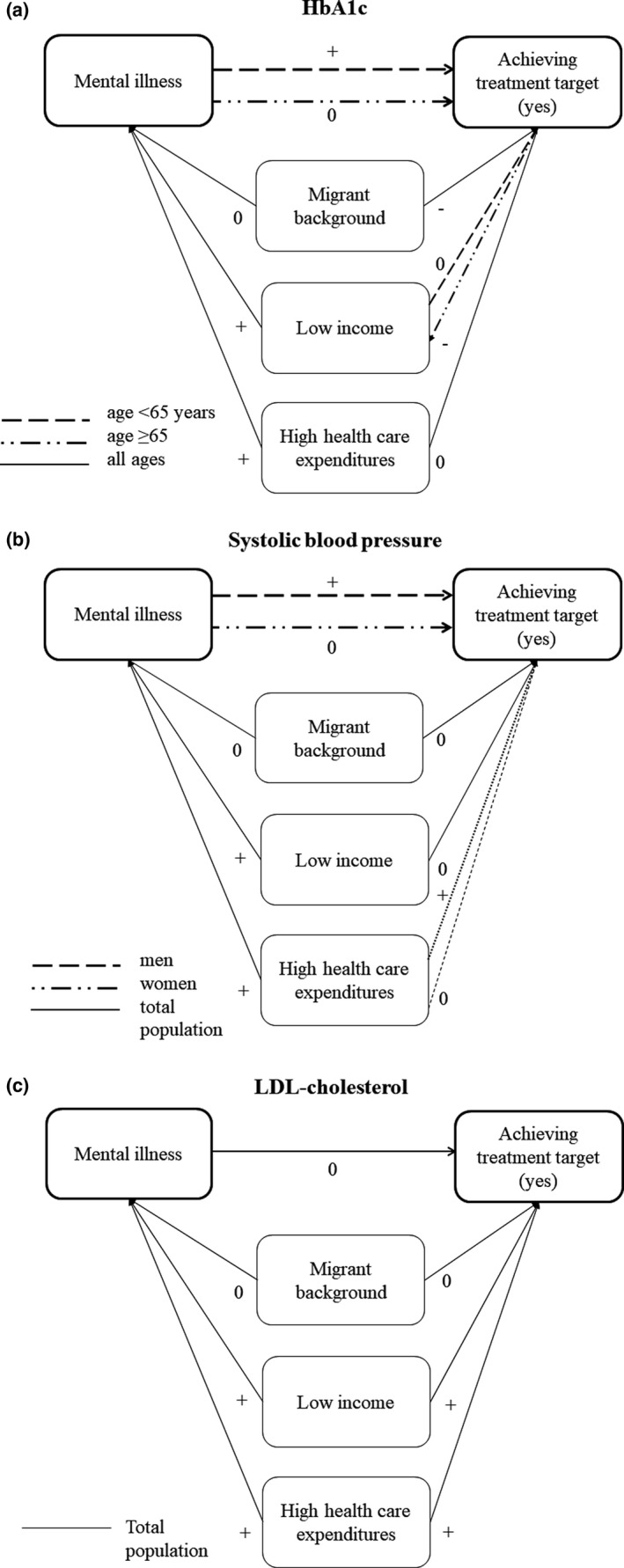
(a–c) Associations of mental illness, social determinants, healthcare expenditures with reaching treatment targets. − Negative association. + Positive association. 0 no association. Associations with treatment targets are adjusted for sex, age, BMI, diabetes duration, co‐morbidities, smoking, income, migration status, healthcare expenditures, as well as use of psychopharmaceuticals, diabetes medication (HbA1c), anti‐hypertensive medication (systolic blood pressure) and lipid‐lowering medication (LDL‐cholesterol). Associations with mental illness are adjusted for sex, age, BMI, diabetes duration, co‐morbidities, income, migration status, smoking, diabetes medication and psychopharmaceuticals (healthcare expenditures). Odds ratios of the associations are displayed in SM B
Except for HbA1c (6.3%), SBP (7.5%), LDL‐cholesterol (13%), smoking (7.7%), BMI (7.7%), the proportion of missing data was less than 1% (SM A).
3.1. Achieving treatment targets
Overall, 64% of participants (n = 1831) achieved their treatment target for HbA1c, 65% (n = 1863) for SBP and 53% (n = 1516) for LDL‐cholesterol.
Regarding the HbA1c target, mental illness by age group (<65 years (mean age 54.1 ± 7.7) versus ≥65 years (mean age 71.2 ± 4.1)) showed effect modification. For the SBP target, sex (age men: 62.1±10.4 women: 61.2 ± 10.8) and income showed effect modification. As associations differed between subgroups, all associations are presented for the largest subgroup (age for HbA1c, sex for SBP). No other effect modification was seen (SM B Table S2).
HbA1c Overall, mental illness was not associated with achieving the HbA1c ‐target (OR (95% CI)) GP treatment, 1.26 (0.92, 1.73); specialist care, 1.36 (0.98, 1.88) (SM B, Table S3a). However, adults aged <65 years with mental illness, GP‐registered or in specialist care were more likely to achieve their treatment target for HbA1c compared with those without OR (95% CI) GP treatment, 1.46 (1.01, 2.11); specialist care, 1.61 (1.11, 2.34). Adults aged ≥65 years with higher incomes were also more likely to achieve their HbA1c target. In both age groups, a migrant background and diabetes medication lowered the likelihood of achieving targets (Figures 2a and 3a) compared with native Dutch and those without medication.
FIGURE 2.
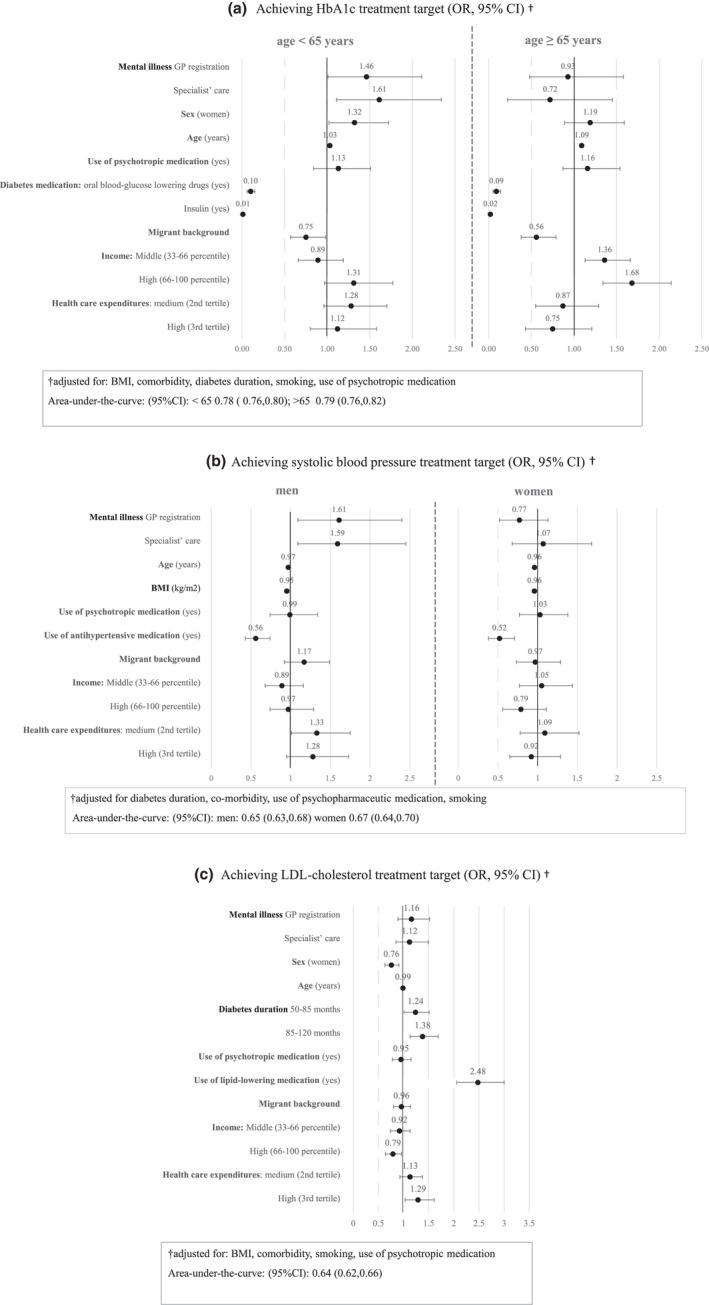
(a–c) Multivariate logistic regression model: achieving cardiovascular treatment targets, mental illness, income, migration status and healthcare expenditures. (a) Achieving the HbA1c target. (b) Achieving the systolic blood pressure target. (c) Achieving the LDL‐cholesterol target. Multivariate logistic regression models of mental illness, income, migration status and healthcare expenditures associated to achieving cardiovascular treatment targets. Not achieving treatment targets was the reference group for the analyses. Results are presented as adjusted odds ratios (OR), with 95% confidence intervals (95% CI), for the pooled results of the imputed data analyses. Exact numbers are displayed in SM B, Table S3. Reference groups for categorical determinants: no mental illness, no anti‐diabetes medication, no anti‐hypertensive medication, no lipid‐lowering medication, diabetes duration 12–50 months, Dutch origin, low income, low healthcare expenditures (first tertile). Odds ratios of the associations are displayed in SM B, Table S3
SBP Overall, mental illness was not associated with the SBP‐target (OR (95% CI)) GP treatment, 1.10 (0.84, 1.46); specialist care, 1.30 (0.94, 1.80) (SM B, Table S3a). However, men with mental illness, GP‐registered or in specialist care, were more likely to achieve the SBP treatment target than men without mental illness OR (95% CI) 1.61 (1.09, 2.40), 1.59 (1.09, 2.45), respectively. For both sexes, higher BMI, higher age, and use of anti‐hypertensive medication compared with those without medication negatively affected SBP targets (Figures 2b and 3b).
LDL‐cholesterol Mental illness was not associated with achieving LDL‐cholesterol targets: OR (95% CI) 1.16 (0.89, 1.52), 1.12 (0.85, 1.49), respectively. Independent from mental illness, use of lipid‐lowering medication, a longer diabetes duration, a lower income and higher healthcare expenditures were all associated with achieving LDL‐cholesterol targets (Figures 2c and 3c).
Regarding area‐under‐the‐curves, the 95% CI for the three main analyses indicated moderate to good internal validity (HbA1c <65: 0.78 (0.76, 0.80) ≥65: 0.79 (0.76, 0.82); SBP men: 0.65 (0.63, 0.68) women: 0.67 (0.64, 0.70); LDL: 0.64 (0.62, 0.66)).
Sensitivity analysis (results available on request) showed small practice variations concerning achieving treatment targets, with intraclass correlation coefficients between 0.5 (0.09%–3.0%)–4.3% (1.7%–10%) for the different models. There was no association of job status with achieving diabetes treatment targets. Individuals with a mental illness duration >1 year were more likely to achieve HbA1c and SBP targets compared with individuals with a mental illness duration of <1 year: OR (CI 95%) HbA1c <65 years: >1 year: 1.56 (1.15, 2.12), <1 year: 1.37 (0.70, 2.66); and SBP men: >1 year: 1.63 (1.17, 2.29), <1 year: 1.42 (0.68, 2.95). Pharmaceutical care appeared to be the primary contributor to the association of healthcare expenditure with achieving treatment targets for SBP (in men) and LDL‐cholesterol: OR (CI 95%) high pharmaceutical expenditure SBP men, 1.55 (1.13, 2.12); high pharmaceutical expenditures LDL‐cholesterol, 1.49 (1.20, 1.86).
4. DISCUSSION
In this study, we explored the complex relationship of mental illness with type 2 diabetes treatment targets in a multi‐ethnic socio‐economically diverse urbanised population. Younger adults (<65 years) with mental illness were more likely to achieve HbA1c treatment targets. Adults with a migrant background, compared with Dutch or older adults with a low income, compared with those with a high income were less likely to achieve HbA1c targets, independent of mental illness. Men with mental illness were more likely to achieve SBP treatment targets. By contrast, mental illness was not associated with achieving LDL‐cholesterol targets. Healthcare expenditure had no confounding or effect‐modifying association with target achievement. A longer duration of mental illness was positively associated with reaching targets, suggesting that disruption due to mental illness can only be improved by recognition and treatment of problems.
Previous studies have reported both impaired 6 , 26 , 27 and improved 8 , 12 , 28 glycaemic control in people with diabetes and depression. Improved HbA1c and SBP control in adults with mental illness might have been influenced by healthcare and self management, as only care users were included in our study. Lister et al. 12 found a positive association between health checks and HbA1c, SBP and LDL‐cholesterol levels in people with severe mental illness. An alternative explanation might be the recognition and treatment of mental illness, as treatment of depression reportedly improves glycaemic control. 28 People with mental illness also received more GP, specialist or pharmaceutical care, as indicated by higher healthcare expenditures, although no evidence was found for effect‐modification through or an independent contribution of healthcare expenditure.
Consistent with previous research, 5 , 12 mental illness was associated with socio‐economic factors, and migrant background and low income were negatively associated with achieving HbA1c targets independent of mental illness. 18 , 19 However, achieving SBP targets was not associated with migration status or income, perhaps due to adequate healthcare delivery or self management in our population. 17 People with a higher income were less likely to achieve LDL‐cholesterol targets, possibly as a result of a lower prevalence of vascular co‐morbidity and less use of lipid‐lowering medication in the high‐income population, both of which are important predictors for achieving LDL targets.
People prescribed anti‐diabetic or anti‐hypertensive medication were less likely to achieve their HbA1c or SBP targets, respectively, which may be explained by a tendency amongst doctors to prescribe medication in response to a failure to reach targets. This suggests that diabetes and cardiovascular risk management was properly initiated, although time or adherence effects were not investigated.
Subgroups revealed effect differences regarding age and HbA1c, and sex and SBP. People receiving treatment for mental illness were significantly younger, which might explain the effect differences on HbA1c targets between people <65 and ≥65 of age, although underdiagnosis or undertreatment of mental illness in the elderly could also be an explanation. 29 Little is known about sex differences in achieving diabetes treatment targets in people with mental illness. A Norwegian study 30 found that depression and anxiety were associated with higher HbA1c levels but not SBP levels in men. Another study 31 showed that diabetes distress, but not depression, was associated with higher HbA1c levels but only in women. Scores for factors such as well‐being and diabetes quality of life in women were lower in that study, possibly indicating different care demands between genders/sexes.
4.1. Strengths and limitations
Some limitations should be considered. First, the cross‐sectional design precludes identification of causal relationships between mental illness, social determinants of health and healthcare expenditures. However, possible links of these domains to frailty were found. Given the limited geographic study area, our results are only relevant to comparable healthcare systems and socio‐economic compositions.
Another important factor was missing data due to registration and attendance bias. 32 We excluded those with missing data on all three outcome variables, most likely including non‐attenders and those treated via specialist’ healthcare. Care avoiders could not be examined but may be at higher risk of missing targets. Additionally, mental illness registration may reflect GP motivation, and practices with better registration may have better treatment outcomes. However, we found little practice variation in achieving treatment targets, although the small number of practices did limit power. 33
In including persons with reimbursed expenditures of mental illness care in 2018, persons in the ‘mental illness’ group with mental illness treatment after the year of the measured outcomes (2017) may have been included. We included these persons in the main analysis, as the waiting period between application and treatment in specialist’ care are often considerable, and it is likely these persons already have mental (untreated) complaints in 2017. This may have led to underestimation of our results, which was confirmed by our sensitivity analysis that showed that those with mental illness duration <1 year (thus, including those with incident reimbursed expenditures in 2018) in both mental groups were less likely to achieve their targets.
Finally, although treatment location implied severity, we did not actively distinguish between categories of mental illness. This was a clinical decision, as regardless of the diagnosis all mental illnesses interfere with self management skills. Nevertheless, severity of disease might influence diabetes self management. 12
Overall, this study suggests that diabetes targets can be achieved by people with diagnosed mental illness in an urbanised, socio‐economically diverse area. Diabetes monitoring and recognition of mental illness appear to represent the ‘protective key’ in the association of mental illness with achieving treatment targets. All diabetes monitoring visits should therefore address psychological disturbance, and attendance should be encouraged.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
AUTHOR CONTRIBUTION
RV, EN and JS designed the study. JS conducted database management quality assurance. EN carried out data management and data analysis; EN, RV and JS interpreted the data. EN wrote the first draft and revised the manuscript, incorporating contributions from co‐authors. RV, JS, SS and MN critically revised the manuscript. All authors agreed on submission and are joint guarantors of this work.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank all the GPs and participants who provided data for this research.
Nieuwenhuijse EA, Struijs JN, Sutch SP, Numans ME, Vos RC. Achieving diabetes treatment targets in people with registered mental illness is similar or improved compared with those without: Analyses of linked observational datasets. Diabet Med. 2022;39:e14835. doi: 10.1111/dme.14835
Funding information
Funded by a European Foundation for the Study of Diabetes (EFSD) award, supported by Servier: EFSD INTENSE Project 2018.
DATA AVAILABILITY STATEMENT
As data contain personal health information and individual socio‐economic information, data were strictly used under license for the current study and are, therefore, not publicly available.
REFERENCES
- 1. Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes. Diabetes Care. 2001;24(6):1069‐1078. doi: 10.2337/diacare.24.6.1069 [DOI] [PubMed] [Google Scholar]
- 2. De hert M, Correll CU, Bobes J, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10(1):52‐77. doi: 10.1002/j.2051-5545.2011.tb00014.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nouwen A, Adriaanse MC, Dam K, et al. Longitudinal associations between depression and diabetes complications: a systematic review and meta‐analysis. Diabet Med. 2019;36(12):1562‐1572. doi: 10.1111/dme.14054 [DOI] [PubMed] [Google Scholar]
- 4. Golden SH, Lazo M, Carnethon M, et al. Examining a bidirectional association between depressive symptoms and diabetes. JAMA. 2008;299(23):2751‐2759. doi: 10.1001/jama.299.23.2751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lund C, Brooke‐Sumner C, Baingana F, et al. Social determinants of mental disorders and the Sustainable Development Goals: a systematic review of reviews. Lancet Psychiatry. 2018;5(4):357‐369. doi: 10.1016/S2215-0366(18)30060-9 [DOI] [PubMed] [Google Scholar]
- 6. Lustman PJ, Anderson RJ, Freedland KE, de Groot M, Carney RM, Clouse RE. Depression and poor glycemic control: a meta‐analytic review of the literature. Diabetes Care. 2000;23(7):934‐942. doi: 10.2337/diacare.23.7.934 [DOI] [PubMed] [Google Scholar]
- 7. McCrimmon RJ, Ryan CM, Frier BM. Diabetes and cognitive dysfunction. Lancet. 2012;379(9833):2291‐2299. doi: 10.1016/S0140-6736(12)60360-2 [DOI] [PubMed] [Google Scholar]
- 8. Rohde C, Knudsen JS, Schmitz N, Østergaard SD, Thomsen RW. The impact of hospital‐diagnosed depression or use of antidepressants on treatment initiation, adherence and HbA(1c)/LDL target achievement in newly diagnosed type 2 diabetes. Diabetologia. 2021;64(2):361‐374. doi: 10.1007/s00125-020-05303-4 [DOI] [PubMed] [Google Scholar]
- 9. Smith R, Han LU, Ali S, et al. Glucose, cholesterol and blood pressure in type II diabetes: a longitudinal observational study comparing patients with and without severe mental illness. J Psychiatr Ment Health Nurs. 2019;26(9‐10):347‐357. doi: 10.1111/jpm.12546 [DOI] [PubMed] [Google Scholar]
- 10. Brüne M, Linnenkamp U, Andrich S, et al. Health care use and costs in individuals with diabetes with and without comorbid depression in Germany: results of the cross‐sectional DiaDec study. Diabetes Care. 2021;44(2):407‐415. doi: 10.2337/dc19-2487 [DOI] [PubMed] [Google Scholar]
- 11. van Dijk CE, Hoekstra T, Verheij RA, et al. Type II diabetes patients in primary care: profiles of healthcare utilization obtained from observational data. BMC Health Serv Res. 2013;13:7. doi: 10.1186/1472-6963-13-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lister J, Han LU, Bellass S, et al. Identifying determinants of diabetes risk and outcomes for people with severe mental illness: a mixed‐methods study. Health Serv Delivery Res. 2021;9(10):1‐194. doi: 10.3310/hsdr09100 [DOI] [PubMed] [Google Scholar]
- 13. Nederlands Huisartsengenootschap . NHG‐Standaard Cardiovasculair risicomanagement (eerste herziening). Huisarts Wet. 2012;55(1):14‐28. [Google Scholar]
- 14. Struijs JN, Baan CA. Integrating care through bundled payments–lessons from The Netherlands. N Engl J Med. 2011;364(11):990‐991. doi: 10.1056/NEJMp1011849 [DOI] [PubMed] [Google Scholar]
- 15. Hill‐Briggs F, Adler NE, Berkowitz SA, et al. Social determinants of health and diabetes: a scientific review. Diabetes Care. 2021;44(1):258‐279. doi: 10.2337/dci20-0053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee W, Lloyd JT, Giuriceo K, Day T, Shrank W, Rajkumar R. Systematic review and meta‐analysis of patient race/ethnicity, socioeconomics, and quality for adult type 2 diabetes. Health Serv Res. 2020;55(5):741‐772. doi: 10.1111/1475-6773.13326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brown AF, Gregg EW, Stevens MR, et al. Race, ethnicity, socioeconomic position, and quality of care for adults with diabetes enrolled in managed care: the Translating Research Into Action for Diabetes (TRIAD) study. Diabetes Care. 2005;28(12):2864‐2870. doi: 10.2337/diacare.28.12.2864 [DOI] [PubMed] [Google Scholar]
- 18. van Bruggen S, Kasteleyn MJ, Bonten TN, Chavannes NH, Numans ME, Rauh SP. Socioeconomic status is not associated with the delivery of care in people with diabetes but does modify HbA1c levels: an observational cohort study (Elzha‐cohort 1). Int J Clin Pract. 2021;75(5):e13962. doi: 10.1111/ijcp.13962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schroeder EB, Hanratty R, Beaty BL, Bayliss EA, Havranek EP, Steiner JF. Simultaneous control of diabetes mellitus, hypertension, and hyperlipidemia in 2 health systems. Circ Cardiovasc Qual Outcomes. 2012;5(5):645‐653. doi: 10.1161/circoutcomes.111.963553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ardesch F. A data‐driven population health management approach: the Extramural LUMC Academic Network data warehouse infrastructure. unpublished. 2021. [DOI] [PubMed]
- 21. Bakker BFM, van Rooijen J, van Toor L. The System of social statistical datasets of Statistics Netherlands: an integral approach to the production of register‐based social statistics. Stat J IAOS. 2014;30:411‐424. doi: 10.3233/SJI-140803 [DOI] [Google Scholar]
- 22. Rathmann W, Schwandt A, Hermann JM, et al. Distinct trajectories of HbA1c in newly diagnosed Type 2 diabetes from the DPV registry using a longitudinal group‐based modelling approach. Diabet Med. 2019;36(11):1468‐1477. doi: 10.1111/dme.14103 [DOI] [PubMed] [Google Scholar]
- 23. Rutten GEHMDGW, Nijpels G, Houweling ST, et al. NHG‐standard diabetes mellitus type 2 (derde herziening) [in Dutch]. Huisarts Wet. 2013;56(10):525. [Google Scholar]
- 24. van Oostrom SH, Picavet HS, van Gelder BM, et al. Multimorbidity and comorbidity in the Dutch population ‐ data from general practices. BMC Public Health. 2012;12:715. doi: 10.1186/1471-2458-12-715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sterne JAC, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dirmaier J, Watzke B, Koch U, et al. Diabetes in primary care: prospective associations between depression, nonadherence and glycemic control. Psychother Psychosom. 2010;79(3):172‐178. doi: 10.1159/000296135 [DOI] [PubMed] [Google Scholar]
- 27. Jung A, Du Y, Nübel J, et al. Are depressive symptoms associated with quality of care in diabetes? Findings from a nationwide population‐based study. BMJ Open Diabetes Res Care. 2021;9(1):e001804. doi: 10.1136/bmjdrc-2020-001804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van der Feltz‐Cornelis C, Allen SF, Holt RIG, Roberts R, Nouwen A, Sartorius N. Treatment for comorbid depressive disorder or subthreshold depression in diabetes mellitus: systematic review and meta‐analysis. Brain Behav. 2021;11(2):e01981. doi: 10.1002/brb3.1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li C, Ford ES, Zhao G, Balluz LS, Berry JT, Mokdad AH. Undertreatment of mental health problems in adults with diagnosed diabetes and serious psychological distress: the behavioral risk factor surveillance system, 2007. Diabetes Care. 2010;33(5):1061‐1064. doi: 10.2337/dc09-1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Naicker K, Øverland S, Johnson JA, et al. Symptoms of anxiety and depression in type 2 diabetes: associations with clinical diabetes measures and self‐management outcomes in the Norwegian HUNT study. Psychoneuroendocrinology. 2017;84:116‐123. doi: 10.1016/j.psyneuen.2017.07.002 [DOI] [PubMed] [Google Scholar]
- 31. Rossi MC, Lucisano G, Pintaudi B, et al. The complex interplay between clinical and person‐centered diabetes outcomes in the two genders. Health Qual Life Outcomes. 2017;15(1):41. doi: 10.1186/s12955-017-0613-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wells BJ, Chagin KM, Nowacki AS, Kattan MW. Strategies for handling missing data in electronic health record derived data. EGEMS. 2013;1(3):1035. doi: 10.13063/2327-9214.1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moineddin R, Matheson FI, Glazier RH. A simulation study of sample size for multilevel logistic regression models. BMC Med Res Methodol. 2007;7(1):34. doi: 10.1186/1471-2288-7-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
As data contain personal health information and individual socio‐economic information, data were strictly used under license for the current study and are, therefore, not publicly available.


