Abstract
The role of marine primary producers in capturing atmospheric CO2 has received increased attention in the global mission to mitigate climate change. Yet, our understanding of carbon sequestration performed by macroalgae has been limited to a relatively small number of studies that have estimated the ultimate fate of macroalgal‐derived carbon. This systematic review was conducted to provide a timely synthesis of the methods used to determine the fate of macroalgal carbon in this rapidly expanding research area. It also aimed to provide suggestions for more effective future research. We found that the most common methods to estimate the fate of macroalgal carbon can be categorized into groups based on those that quantify: (i) export of macroalgal carbon to other environments—known as horizontal transport; (ii) sequestration of macroalgal carbon into deep‐sea sediments—known as vertical transport; (iii) burial of macroalgal carbon directly beneath a benthic community; (iv) the loss of macroalgal carbon as particulate carbon or dissolved carbon to the water column; (v) the loss of macroalgal carbon to primary consumers; and finally (vi) those studies that combined multiple methods in one location. Based on this review, several recommendations for future research were formulated, which require the combination of multiple methods in a whole system analysis approach.
Keywords: biomass, blue carbon, kelp, macroalgae, seaweed, sequestration
Abbreviations
- DIC
dissolved inorganic carbon
- DOC
dissolved organic carbon
- DOM
dissolved organic matter
- eDNA
environmental DNA
- IPCC
Intergovernmental Panel on Climate Change
- IUCN
International Union for Conservation of Nature
- NPP
net primary productivity
- POC
particulate organic carbon
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses
- RDOC
refractory dissolved organic carbon
- SOM
sediment organic matter
Blue carbon is the carbon stored in aquatic sediments and biomass, whether living or dead (Mcleod et al. 2011). Despite only covering a small area of the total ocean (~7%; Borges et al. 2005), coastal macrophytic systems, such as seagrass meadows, macroalgae forests, salt marshes, and mangroves capture as much as 70% of marine organic carbon (Smith 1981, Duarte et al. 2005). This makes these marine systems intense blue carbon sinks. A dramatic increase in blue carbon research is illustrated by comparing the number of scientific articles with “blue” and “carbon” in the title, which was 12 in 2001 compared to 313 in 2021 (based on Web of Science). This increase is associated with the publication of two key reports in 2009, which highlighted the magnitude of carbon sequestration potential by coastal macrophytic systems worldwide (IUCN 2009, Nellemann et al. 2009).
Macroalgae in particular are the most extensive and productive of all the blue carbon vegetated coastal habitats, estimated to cover 2–6.8 million km2 globally and exporting approximately 43% of their primary production globally as biomass, dissolved organic carbon (DOC), and particulate organic carbon (POC; Duarte et al. 2013, Krause‐Jensen and Duarte 2016, Jayathilake and Costello 2020). Furthermore, it has been estimated that 24% of this macroalgal carbon exported from the area of productivity is sequestered in deep‐sea sediments (Ortega et al. 2019). These figures suggest that macroalgae play a key role in blue carbon systems, however, it must also be considered that many of these estimates are highly variable and are based on low sample numbers. Despite this caveat, owing to their extent, export, and high productivity, macroalgae have long been considered important contributors to the global carbon sink (Smith 1981). In addition, oil deposits derived from macroalgae dating back ~500 million years provide evidence that macroalgae do conduct carbon sequestration on geologically relevant timescales (Sun et al. 2013). Generally, once macroalgal carbon has entered the deep sea it is considered sequestered, regardless of the fate of the carbon, because whether it is buried, grazed, mineralized, or suspended in a nepheloid layer, it is assumed that this carbon will not return to the atmosphere for centuries (Krause‐Jensen and Duarte 2016).
Despite the growing focus on blue carbon and the carbon sink, which coastal marine macrophytes represent, the process of macroalgal carbon burial is not well understood (Pedersen et al. 2020). As most macroalgae grow on rocky substrata, do not have root systems, and do not accumulate carbon‐rich sediments directly beneath the community, the contribution of macroalgae to carbon sequestration has probably been overlooked (Hill et al. 2015, Krause‐Jensen et al. 2018). Previous reviews have highlighted the importance of carbon sequestration by macroalgae (Appendix S1 in the Supporting Information). Yet the ultimate fate of macroalgal carbon remains unknown in most coastal ecosystems which prevents their inclusion in national carbon budgets and accounting (e.g., Cott et al. 2021).
This fundamental information is essential to constrain accurate global carbon models and to justify the conservation of macroalgal forests, particularly as macroalgae are under threat from warming, eutrophication, coastal development, sediment contamination, chemical pollution, and invasive species (Wernberg et al. 2011, Smale et al. 2013, Beaumont et al. 2014, Duarte 2014, Davidson et al. 2018). There is an additional novel threat of large‐scale mechanical macroalgal harvesting across Europe (McMonagle and Morrison 2020). If macroalgal systems are significantly impaired by these stressors and are subsequently degraded they may ultimately convert from carbon sinks to carbon sources (Pendleton et al. 2012, Cullen‐Unsworth and Unsworth 2013).
This study aimed to systematically review the experimental methods employed to date to quantify the fate of macroalgal carbon in marine ecosystems. Once identified, the caveats and potential improvements to each method are discussed to ensure that the most appropriate data are available to inform ecosystem models. Carbon sequestration is defined here as the effective removal of carbon from the atmosphere for hundreds to thousands of years, without the likelihood of it being immediately re‐released (Osman‐Elasha et al. 2005). It is hoped that this review will serve as a beneficial overview and give direction to those designing future experiments on the fate of macroalgal carbon.
MATERIALS AND METHODS
A systematic review was completed using Web of Science (1864–present) according to the 2020 PRISMA statement (Page et al. 2021) and PRISMA‐EcoEvo, a recent extension designed for systematic reviews and meta‐analyses within ecological and evolutionary biology research fields (O’Dea et al. 2021). All databases available on Web of Science were searched using the field “topic” which searches the title, abstract, author keywords, and Keywords Plus® for search terms. An initial scoping review was conducted to develop the first set of search terms. Subsequently an additional root term of “kelp” was added prior to commencing the systematic review. Thirty‐two search term combinations were applied as described in Table 1 using the Boolean operator “AND.” Selection criteria for inclusion in the review were clearly defined a priori and were designed to select novel primary research articles, that present empirical data and quantitative outputs, in English (Table 2).
Table 1.
Search term combinations used to query the databases stored within Web of Science
| Root term | First additional term | Second additional term |
|---|---|---|
| Carbon | Macroalgae/Macroalgal/Seaweed/Kelp | Pool/Storage/Fate |
| Sequester | Macroalgae/Macroalgal/Seaweed/Kelp | |
| Sink | Macroalgae/Macroalgal/Seaweed/Kelp | |
| Detritus | Macroalgae/Macroalgal/Seaweed/Kelp | |
| Export | Macroalgae/Macroalgal/Seaweed/Kelp |
Table 2.
Criteria for inclusion or exclusion from the review
| Inclusion/exclusion criterion | Rationale for inclusion/exclusion |
|---|---|
| Must contain novel research about the fate of carbon from naturally occurring macroalgal communities. | Cultivated macroalgae are negligible in scale in comparison to naturally occurring macroalgal communities. Theoretical models and other reviews were excluded in favor of primary research. |
| Must not contain research conducted solely in laboratory settings. | Laboratory experiments alone cannot be used to determine whole ecosystem productivity or the fate of that productivity. |
| Must report quantitative data on macroalgal carbon displacement, ideally constrained by time and area. | Quantitative information on carbon flux between different pools is required for the comparison of macroalgae to other communities and for integration into large‐scale carbon models. |
| Must contain information on the fate of productivity and not productivity alone. | Information on productivity alone is not sufficient to determine the fate of this productivity. |
| Must be in English | There was no capacity to translate publications into languages other than English. |
Two hundred and fifty‐two publications were identified by the search process for assessment and 48 were selected that met the stated criteria for review (Fig. 1). Plot digitization software was used to include data from figures (https://apps.automeris.io/wpd/). Publications excluded for review were predominantly other reviews; studies on macroalgal productivity alone; studies using tracer molecules to investigate carbon flow without quantification; and mesocosm or microcosm studies (Table 3, Appendix S1).
Fig. 1.
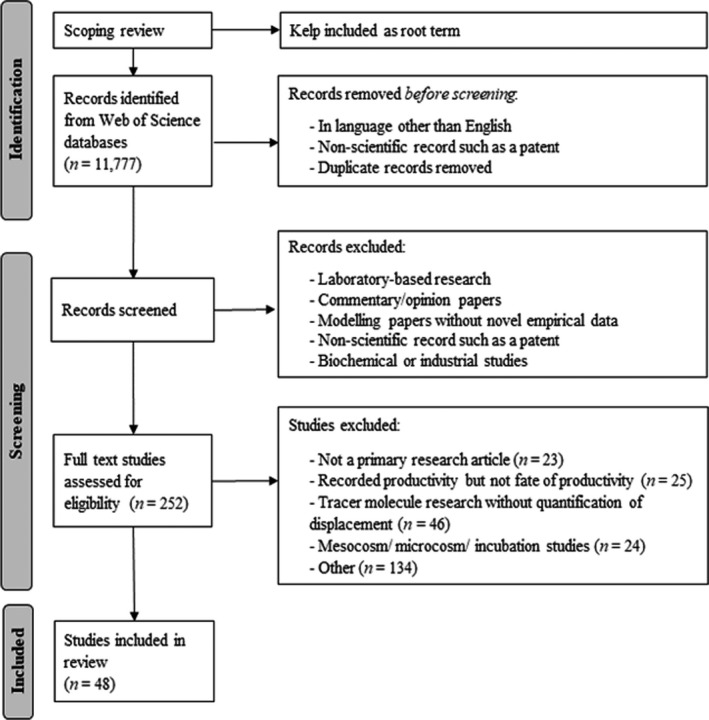
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) style flowchart of the systematic review methodology used.
Table 3.
Most common types of paper identified by the initial search but excluded from review and the rationale for exclusion
| Type of paper | Description and reason for exclusion | Number of studies identified |
|---|---|---|
| Other reviews | Commented on or reviewed some aspects of macroalgal carbon but excluded in favor of primary quantitative research. | 23 |
| Productivity alone | Described community respiration and productivity within macroalgal‐dominated ecosystems without describing the fate of the macroalgal carbon. | 25 |
| Tracer molecules | Identified movement of macroalgal carbon from its source but without quantification of the fate of the carbon. | 46 |
| Microcosm or mesocosm | Measured macroalgal productivity and carbon sequestration in the laboratory without the possibility of scaling these rates up. | 24 |
See Appendix S1 for more detail and citations.
The 48 reviewed studies experimentally determined either: (i) horizontal transport, that is the export of macroalgal carbon to other environments; (ii) vertical export and sequestration of macroalgal carbon in the deep sea; (iii) burial of macroalgal carbon directly beneath the community; (iv) loss as particulate carbon or dissolved carbon to the water column; (v) loss of carbon to primary consumers; or (vi) a combination of these approaches (Figs. 2 and 3).
Fig. 2.
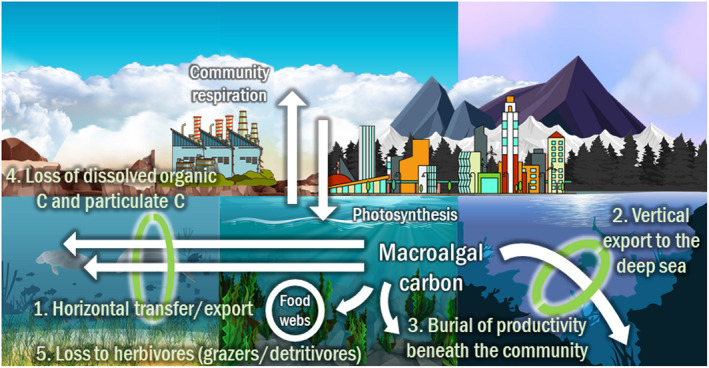
Carbon flux from and within macroalgae systems.
Fig. 3.
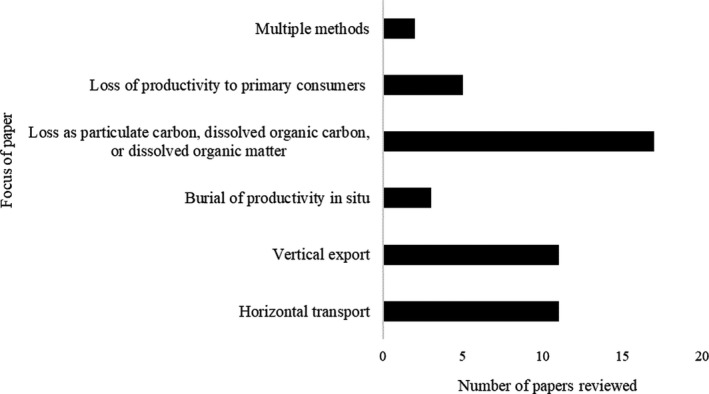
Studies estimating the fate of macroalgal carbon included in this review.
RESULTS AND DISCUSSION
The following results are not an exhaustive list of papers on macroalgal carbon but what was captured by a representative systematic review, using pre‐determined search terms and inclusion criteria (Tables 1, 2, 3). Given that there was no capacity for translation, there was an obvious bias toward papers from countries in which scientists regularly publish their results in English. Of the 48 studies reviewed, ten were conducted in the United States of America, seven in Norway, six in Japan, six in Canada, four in Australia, two in Antarctica, two in Great Britain, and the remaining were each from a different single location.
Horizontal transport
Eleven studies estimated horizontal transport from macroalgal communities, which is the export of detached macroalgal biomass beyond the community boundaries to other environments by physical agents, such as currents and waves. This macroalgae is not necessarily deposited in shallow systems but in sediments as deep as 45 m. With this type of approach, the most that can be done is to identify a macroalgal carbon source, sink, and pathway of export, quantify the displacement of macroalgal carbon from the source, and quantify the deposition of macroalgal biomass in the sink environment. Of the studies reviewed, two described a macroalgal source, sink, and pathway of export but did not quantify displacement (Wernberg et al. 2006, Filbee‐Dexter and Scheibling 2012). Although in these cases displacement is not quantified, the transport pathway has now been recorded and consequently can be studied in more detail. The occurrence of extreme climate events in areas of long‐term monitoring is prime opportunity for this type of study. For example, Filbee‐Dexter and Scheibling (2012) recorded a post‐hurricane pulse of detrital Phaeophyta biomass from macroalgal forests to deeper waters, a reduction of canopy cover from 71% to 39%. This reduction could be updated to estimate biomass or carbon loss if a further survey would relate canopy cover to biomass at the same site.
Three studies quantified the export of macroalgal biomass to an unknown sink (Quartino and Boraso De Zaixso 2008, Wilmers et al. 2012, Pessarrodona et al. 2018). For example, the seasonal loss of Laminaria hyperborea fronds per area was recorded as 187.8 ± 165 g C · m−2 · y−1 lost in cold waters and 101.7 ± 59.6 g C · m−2 · y−1 lost in warm waters (Pessarrodona et al. 2018). These data would be more informative on the fate of macroalgal carbon if a follow‐up experiment was conducted to track the dispersal of biomass using tagged or artificial blades.
Owing to the relative ease of shore sampling, the five studies that quantified deposition of macroalgal carbon in a sink environment described marine to terrestrial pathways (Harrold and Lisin 1989, Kotwicki et al. 2005, Dugan et al. 2011, Lastra et al. 2014, Orr et al. 2014). Considering that <3% of macroalgal production is estimated to strand ashore (Lastra et al. 2014), there may be an over‐representation of macroalgal carbon transport to shores as opposed to subtidal sinks in the literature.
Most studies describing the export of macroalgal biomass to shorelines measure the deposition of fresh weight per area accumulated over time from an unknown donor site in varying units of mass and time. For example, Orr et al. (2005) estimated daily deposition of up to 140 mg dry wt · km−1 to shores in British Columbia, while Dugan et al. (2011) calculated the macrophyte deposition on the shoreline at Santa Barbara, California to be on average 1.7 kg wet wt · m−1 · d−1. This loading rate was adjusted to account for feeding by the abundant talitrid amphipod populations at the same study beach, yielding a more refined estimated annual macroalgal transport rate of 548 kg wet wt · m−1 · year−1. One of these studies demonstrated that it is possible to determine the origin of deposited macroalgal carbon on shores by tagging naturally occurring rafts of Macrocystis pyrifera with radio transmitters along with the shoreline of Monterey Peninsula, California (Harrold and Lisin 1989). This approach gave a more ecosystem‐scale perspective than other shore deposition studies and allowed the authors to estimate that 130,000 tons of wet weight of M. pyrifera are exported from the macroalgal forests at this site to nearby shorelines each year (Harrold and Lisin 1989).
Nineteen studies were identified, which did not meet the selection criteria but investigated the degradation of deposited macroalgal carbon in a sink environment. This common experimental method is conducted in situ using naturally occurring macroalgal deposits (Trimmer et al. 2000, Sutula et al. 2014) or more often by “detrital enrichment,” that is using artificially loaded or experimentally buried macroalgal biomass in mesh bags (Alkemade and Van Rijswijk 1993, Mews et al. 2006, Rossi 2007, Olabarria et al. 2010, Rossi et al. 2011, 2013, Dufour et al. 2012, Eereveld et al. 2013, Lastra et al. 2014, 2018, Gómez et al. 2018, Rodil et al. 2019, Haram et al. 2020). These types of experiments are conducted mostly on sandy shore ecosystems, but similar degradation experiments have been conducted in benthic subtidal habitats (de Bettignies et al. 2020, Pedersen et al. 2021), estuarine sandflats (Gladstone‐Gallagher et al. 2016), and in specific seabed hollows where algal blades are known to accumulate (Norkko et al. 2004).
Detached macroalgal biomass, which has been exported and deposited subtidally, cannot necessarily be treated as detritus because it has been found to be actively respiring and growing without degrading or contributing carbon to the underlying sediments (Frontier et al. 2021). In these experiments, the amount of mass lost from the detritus deposited over time, or the change in concentration or amount of carbon mass in the underlying sediment or pore water (Barreiro et al. 2013) serves as a measure of carbon transferred through abiotic decay or detritivore activity. Rarely are results provided as a mass of carbon transferred per unit of time, which prevents comparison to other environments or integration into carbon models. Species‐specific and ecosystem‐specific degradation rates such as these should be paired with anticipated or measured loading rates to estimate carbon flux from detritus to sediment (Pedersen et al. 2021).
Vertical export
Eleven reviewed studies described vertical export. In contrast to horizontal transport, which is the movement from one site to another, vertical export papers measured the arrival of macroalgal carbon material into a deep‐sea location (>1,000 m) from another, more shallow, site. Despite consumption and transformation, once in the deep sea, macroalgal carbon can be considered to be sequestered from the atmosphere as it is removed from the ocean‐atmosphere boundary for geologically relevant amounts of time. Vertical export was originally investigated using cameras attached to remote vehicles which were used to identify and estimate the amount of macroalgal detritus on some deep‐sea floors (Alongi 1990, Harrold et al. 1998, Vetter and Dayton 1999, Britton‐Simmons et al. 2012, Filbee‐Dexter and Scheibling 2014, Filbee‐Dexter et al. 2018, Ramirez‐Llodra et al. 2021). In most studies, results were presented in rough quantitative terms such as percentage cover (Vetter and Dayton 1999) or the number of pieces of drift macrophytes or drift piles per film segment (Britton‐Simmons et al. 2012). Once the presence of macroalgal detritus has been identified, however, this information can be combined with previously determined turnover rates and standing stock data of nearby macroalgal forests to estimate displacement (Ramirez‐Llodra et al. 2021), for example, 45.2 mg C · m−2 · d−1 of Macrocystis pyrifera exported to the Carmel Submarine Canyon benthos (Harrold et al. 1998). More recently, video surveys have been combined with dive observations and tagged samples have been tracked to confirm a suspected source of production (Filbee‐Dexter et al. 2018).
Alternatively, vertical export to the deep sea has been studied using stationary drift nets at canyon mouths, as these act as the conduits through which macroalgal detritus reaches deep‐sea plains (Josselyn et al. 1983). The link between a macroalgal carbon source to deep‐sea sink has also been established using powerful global observation methods which should be further pursued (Dierssen et al. 2009, Kokubu et al. 2019). For example, the striking loss of 588 km2 Colopmenia sp. detritus on the eastern part of the Great Exuma Bank to the nearby Tongue of the Ocean (>1,800 m) was hypothesized using satellite imagery before and after a Langmuir supercell event (Dierssen et al. 2009). It was estimated that this represents a pulsed export of >7 × 1010 g of macroalgal carbon exported to the deep sea, approximately the same amount of the carbon sequestered daily by sinking phytoplankton carbon across the entire subtropical North Atlantic.
The most information a vertical export study can provide is the source of the macroalgal tissue exported to the deep sea, the exact area to which it is exported, and the amount of carbon per area of macroalgal forest which is directed to deep‐sea sediment. For example, benthic sediment cores were collected at depths from 70 to 262 m along the Norwegian coast and distant Laminaria hyperborea forests were identified as a source of carbon to the sediments given the similarity of the carbohydrate and phenolic content of the organic matter within the cores to the L. hyperborea (Abdullah et al. 2017). The observed rate of organic deposition in the area (approximately 0.46 kg C · m−1 · year−1) and the high productivity of the donor L. hyperborea (3 kg C · m−2 · y−1) gave an indication of the amount of L. hyperborea carbon which was sequestered in these deep‐sea sediments. The use of cores is primarily used to study in situ macroalgal burial, with some examples below.
Burial of productivity in situ
Given that macroalgae tend to grow on rocky substrata, it is rare that macroalgal productivity is buried directly beneath the canopy. In some cases, however, macroalgae communities overlie soft sandy or muddy sediments, allowing the displacement of carbon to the sediment beneath them to be measured (Atwood et al. 2018, Gorain et al. 2018, Sfriso et al. 2020). In a study of organic carbon storage in the Great Barrier Reef, Atwood et al. (2018) measured 2.05 mg organic carbon (OC) · ha−1 in the upper 0–5 cm layer of sediment (corresponding to the last 40 y) and 2.04 mg OC · ha−1 in the sediment 5–14 cm deep (corresponding to the last 40–111 y).
Macroalgal blooms often occur in shallow waters such as bays and estuaries, where overlying soft sediment can be sampled using cores. Gorain et al. (2018) measured the amount of organic carbon from macroalgal blooms directed to underlying “muck,” ranging from just 7.6 g · m−2 · month−1 for Pithophora oedogonia blooms to 135.4 g · m−2 · month−1 for Rhizoclonium tortuosum, a small proportion of the biomass yields of these blooms. There are high biomass turnover rates in macroalgal blooms, and they are rapidly degraded through bacterial assimilation, meaning blooms do not act as long‐term carbon sequestration, and direct little or no carbon to the sediment below in the form of organic matter (e.g., Corzo et al. 2009, Lanari et al. 2017).
Studies that describe the carbon in the sediment below a macroalgal community, without associated burial rates, or studies that evaluate “carbon stocks” referring to the carbon in standing biomass will be of less value for building marine carbon models or informing management strategies. For example, based on the accumulation of CaCO3 crusts, microcalcareous epiphytic seaweeds in Italian transitional water systems were estimated to have the capacity to bury between 0.7 and 2.5 tons of CO2 per hectare per year as oxidized surface sediments (Sfriso et al. 2020). Valuable data on in situ carbon burial in sediment with a macroalgal origin (e.g., Gilson and Davies 2020) would be greatly advanced if combined with complementary studies on burial rates or carbon dating.
The IPCC Wetlands Guidance recommends that sediment carbon stocks are standardized to a depth of 1 m below 1 m2, but of the studies screened for review, sampling depths were much shallower (max 15 cm; IPCC 2014). To standardize a 10 cm deep sample to 1 m by multiplying by 10 would unrealistically assume a uniform distribution of carbon in the sediment profile. The systems in which macroalgal burial can be quantified in situ have low sediment carbon content and if compared against cores from other blue carbon systems may be a poor representation of macroalgal carbon burial.
Loss as particulate carbon, dissolved carbon, or dissolved organic matter
Carbon, either in particulate or in a dissolved form, is released rapidly from marine macroalgae which are actively photosynthesizing. Seventeen studies in total were identified that quantified the release of macroalgal carbon as POC, DOC, or dissolved organic matter. Six of these studies measured DOC and POC loss by observation of biomass change as a result of chronic blade erosion, often alongside measurements of loss through entire plant dislodgement (Dean and Hurd 2007, Krumhansl and Scheibling 2011a, de Bettignies et al. 2013, Halat et al. 2015, Pessarrodona et al. 2018, Pedersen et al. 2020). Of these, only Dean and Hurd (2007) did not quantify export in terms of ecosystem area but rather calculated an average erosion rate per Undaria pinnatifida sporophyte of 0.24 cm · d−1 to 0.79 cm · d−1. This would require accompanying information on sporophyte density to estimate carbon lost from the community. The quantification of these export rates is hugely valuable, and they could be beneficial in answering the question of the fate of macroalgal carbon if combined with tracer studies to track the dispersal of this carbon once released into the environment. For example, Wada and Hama (2013) used the humic‐like fluorophore components of suspended dissolved organic matter released from Ecklonia cava to trace its dispersal throughout and beyond Oura Bay, Japan (Wada and Hama 2013).
Ten reviewed studies measured the contribution of a macroalgal community to the DOC pool by scaling up mass‐specific estimates of DOC release rates from short‐term incubations (Fankboner and de Burgh 1977, Abdullah and Fredriksen 2004, Wada et al. 2008, Wada and Hama 2013, Yorke et al. 2013, Barrón et al. 2014, Ruiz‐Halpern et al. 2014, Reed et al. 2015, Egea et al. 2020, Weigel and Pfister 2020). During an incubation experiment, blades or parts of a macroalgal sample are enclosed in a chamber or plastic bag and the change in concentration of DOC is measured either directly or by proxy. DOC release may then be standardized by net primary productivity (NPP) and scaled up using published rates of community values NPP, which is particularly accessible if the incubation experiment is completed in the context of a longer research program (e.g., Reed et al. 2005). Incubations such as these offer more control and greater resolution than measuring carbon release via changes in biomass, although the small size of incubation chambers presents an issue for taking representative samples of macroalgal communities. If a single dominant species of macroalgae is incubated to measure DOC loss and export, this data alone is not representative of the true value of macroalgal contribution to the DOC pool, as the release of DOC from other elements of the community and from degrading detrital biomass is not accounted for.
Furthermore, the short duration of incubation experiments in comparison to long‐term monitoring may incur error as they are understandably conducted in daylight hours and calm weather suitable for fieldwork, although more recent examples have conducted control experiments at night (e.g., Weigel and Pfister 2020). Tracer molecules, such as radiolabelled sodium bicarbonate, have been used in incubation studies since at least the 1970s, wherein the incubated sample is experimentally enriched with a tracer molecule and its emission monitored (Fankboner and de Burgh 1977). This method has yielded results of low DOC release, however, equivalent to 0.002% of blade primary productivity. More recent incubation experiments of Nereocystis luetkeana and Macrocystis pyrifera have shown that of total exuded DOC, <20% originated from recently fixed labeled carbon, suggesting that only reporting exuded DOC which is labeled will underestimate DOC release rates (Weigel and Pfister 2020).
Newly produced DOC is highly labile and is quickly consumed by bacteria, but a large proportion of this consumed DOC is thus transformed into refractory material (Barrón et al. 2014). The refractory DOC pool in the water column has a turnover period of hundreds to thousands of years (Watanabe et al. 2020) and if exported below the mixing layer of the water column it may be stored for a geologically relevant period of time. One paper that was reviewed monitored the release of dissolved inorganic carbon (DIC) and DOC through incubation, and also conducted subsequent degradation experiments to determine the proportion of DOC released which was refractory DOC (RDOC; Watanabe et al. 2020). RDOC was defined as that DOC that is not remineralized 150 d after release. Net release of DOC from the Sargassum horneri community was equivalent to 35% and 6% of net community productivity (NCP) in February and March respectively and it was concluded that the S. horneri community exported 5–20% of its productivity as RDOC. An experimental design of this nature indicates the proportion of exuded DOC, which has carbon sequestration potential at a regional scale, may be scalable to a limited extent.
Loss of productivity to primary consumers
Most quantification of the primary consumption of macroalgae occurs exclusively in a laboratory setting where consumers can be studied in detail (e.g., Ito et al. 2019, Gilson et al. 2021), which excluded these studies from review. Five studies were reviewed, however, which directly measured the loss of macroalgal biomass to herbivores in the field, with consumption rates (of grazers, detritivores, shredders, and others) defined in terms of both area and time (Itoh et al. 2007, Krumhansl and Scheibling 2011b, Norderhaug and Christie 2011, Filbee‐Dexter et al. 2020, Gutow et al. 2020). These studies quantified the macroalgal carbon directed to primary consumption in the field by combining in situ measurements with either laboratory experiments or previous data sets. For example, Norderhaug and Christie (2011) determined that in the case of Norwegian Laminaria hyperborea forests, secondary production by L. hyperborea consumers was 3% of primary productivity at low wave exposure, and 8% at medium and high wave exposure. They combined their novel data on secondary production rates, based on the community composition of mobile macrofauna, with primary production data from a separate recent survey at the same site (Norderhaug and Christie 2011).
Studies of primary consumers may be motivated by observations of a particularly voracious grazer. For example, the stipe‐burrowing herbivorous amphipods Sunamphitoe lessoniophila and Bircenna sp. were estimated to cause a maximum loss of 24–44% of biomass from the Lessonia berteroana kelp forests in northern‐central Chile (Gutow et al. 2020). Less dramatically, grazing rates of gastropod Lacuna vincta on Saccharina longicruris and Laminaria digitata in Nova Scotia were measured as a maximum of 1% and 1.5% of blade area respectively (Krumhansl and Scheibling 2011b). The type of results from these grazing loss studies could be converted into carbon fluxes if scaling relationships were to be established between blade area or mass and carbon content, and the density of blades per area. This analysis is particularly feasible if a grazing survey is part of a larger study on macroalgal production and erosion at one site. The urchins Strongylocentrotus droebachiensis and Echinus esculentus were studied in Malangen Fjord, Northern Norway and it was calculated that between 1.3 and 10.8 kg of fresh biomass are shredded annually per square metre of macroalgal forest (Filbee‐Dexter et al. 2020). The rate of macroalgal detritus produced in the area was required for this calculation, which was available in Pedersen et al. (2020), which again demonstrates how useful multiple studies in the same site can be for a comprehensive understanding of the carbon flow in a macroalgal community.
Whole system analysis
Two studies which were reviewed combined multiple approaches to quantify macroalgal carbon sequestration (Takai et al. 2010, Queirós et al. 2019). Queirós et al. (2019) used environmental DNA (eDNA) sequencing and Bayesian Stable Isotope Mixing Modeling to identify sources of detrital macroalgal biomass on the seabed 13 km south‐southwest of Plymouth, UK, Station L4. The export of POC from identified macroalgal carbon sources was measured according to detritus production rates in the same area, published by Pessarrodona et al. (2018). This carbon export pathway was combined with benthic‐pelagic process measurements derived using incubated box cores to determine the fate of detrital macroalgal carbon once deposited on the seabed. The incubation of box core samples from the sediment allowed the direct measurement of processes such as POC burial, POC flushing (bioirrigation), POC burial through bioturbation, and the production of DIC, suggesting average annual carbon sequestration of 4.89 ± 5.50 mol · m−2 · y−1, 0.73 ± 0.82 mol · m−2 · y−1 of which was macroalgal carbon sequestration (Queirós et al. 2019). Twenty‐three mesocosm or microcosm studies were excluded from this review, all of which described carbon flow from macroalgal detritus to the water column, sediments, or consumers (Appendix S1). If these mesocosms were representative of a certain ecosystem and the mass processes of macroalgal import and export from that ecosystem were known, the carbon sequestration of that ecosystem type could be calculated as per Queirós et al. (2019).
Despite using less advanced technology, Takai et al. (2010) also used a whole system analysis to establish a transport link between macroalgal carbon source and sink, quantified that link, and indicated the fate of the macroalgal carbon which was deposited. The amount of macroalgal detritus on the seafloor around the Izu Peninsula, Japan was measured by dredging and trawling. The source and transport pathway of the macroalgal detritus was determined based on similarities in species compositions. This was verified using carbon and nitrogen stable isotope ratios. Stable isotope analysis was conducted on the sediment organic matter beneath the detrital accumulations, which indicated that macroalgae contribute relatively little to the carbon in the sediment.
The use of multiple methods does not necessarily mean a study on macroalgal carbon will be informative on carbon sequestration. For example, Pfister et al. (2019) used a remarkable number of different methods to compare the seawater chemistry inside and outside Nereocystis luetkeana and Macrocystis pyrifera forests along the Olympic Peninsula of Washington state. Within each forest and 200–400 m away, the carbonate content and DOC content of the water were measured, productivity was estimated based on chl a concentration, and both eDNA and isotopic investigations were conducted. These comparative observations gave strong evidence that macroalgae contribute to nearshore carbon cycling, but the experimental design did not facilitate the quantification of this cycling.
CONCLUSIONS
Once a macroalgal carbon source‐sink pair has been identified, measurements of carbon sequestration can be scaled up to all national habitats of the same type. These figures can then be integrated into global carbon models and used by decision‐makers for effective management and conservation of carbon stocks. Multiple approaches, either combined in the same study (Takai et al. 2010, Queirós et al. 2019), in separate studies on the same site (Harrold and Lisin 1989, Norderhaug and Christie 2011, Queirós et al. 2019, Filbee‐Dexter et al. 2020), or in one study in the scope of a larger project (Reed et al. 2015, Atwood et al. 2018) offer the most comprehensive insight into the fate of macroalgal carbon.
We conclude that many macroalgal carbon studies could be greatly and easily expanded by complementary experiments that describe the source, sink, pathway of displacement, or quantity of macroalgae displaced within the same system. For example, existing species‐specific and ecosystem‐specific detritus degradation rates could be paired with deposition rates in a sink environment to estimate carbon flux from detritus to sediment. Similarly, sediment deposition rates or sediment dating could be combined with previous studies of sediment carbon content to determine carbon burial rates. Rates of macroalgal import to an ecosystem can be combined with representative mesocosm studies to estimate carbon sequestration of that ecosystem type. For reference, Table 4 summarizes the common tools used to estimate macroalgal carbon sequestration and the respective advantages and shortcomings of these methods.
Table 4.
Common methods used to estimate macroalgal carbon (MC) sequestration and the respective advantages and shortcomings of these methods
| Method | Description | Advantages | Limitations |
|---|---|---|---|
| Tracer molecule | Use of radiolabelled isotopes, stable isotope ratios, environmental DNA, sterols and n‐alkanols, lignin, lipids, carotenoids, alkanes, amino acids, or others to trace MC dispersal from a known source. | There is flexibility in the range of possible tracer molecules to use. This approach provides good supporting evidence that an environment is an MC sink. | Many tracer molecules are appropriate only for the identification of MC in a sink or source but not quantification of the MC present or movement over time. |
| Survey of deposited material | The systematic and randomized survey of terrestrial, tidal, or sub‐aquatic deposited macroalgae. | Observation may be completed inexpensively. Volunteers may participate if given detailed instructions. May be done over a short period. | There is likely to be an over‐representation of shores as MC sinks owing to the ease of sampling. For surveys using SCUBA diving, training and equipment costs will be high. |
| Repeated sub‐aquatic surveys | Regularly repeated surveys of macroalgal communities to monitor the difference in macroalgal biomass or another feature such as density, erosion, or grazing. | Repeated surveys at the same site can be supplemented with scaling relationships. These can map area decrease, or blade decrease from grazers to MC loss. Easily supplemented with tagging experiments (see below). | Good quality sub‐aquatic surveys require training of surveyors, costly equipment, and a site that can be repeatedly accessed. |
| Degradation of deposited algae (natural or artificial enrichment) | The burial of packages of macroalgal matter, and subsequent measurement of the change in mass of these buried packages. May also measure the increase of MC in the surrounding sediment. | A simple and cost‐effective method to use. Gives species‐specific and ecosystem‐specific degradation rates. Easily paired with loading rates to the system to estimate carbon flux from detritus to sediment. | Results are rarely provided as the mass of carbon transferred per unit of time, which prevents comparison or integration into carbon models. Describes sink dynamics but cannot inform on the MC source or pathway of displacement. |
| Tagging | The tagging and tracking of macroalgal blades/rafts/artificial blades or other macroscopic macroalgal material. May or may not require recovery or direct observation of movement. | Results give very strong evidence of a pathway of displacement. This is particularly useful if the effects of a well‐studied source on the surrounding ecosystem are of interest. | It may be time consuming to recover tags, even if they have a bright color or are radio/satellite‐tracked (GPS). If recovery is required, there may be a high failure rate and many replicates required. |
| Cameras and remote vehicles | The observation of macroalgae deposited in a deep‐sea location using remotely operated cameras. | This is strong supporting evidence that a deep‐sea location is an MC sink. | This observation data only allows for the rough quantification of MC deposition, for example, percentage cover not biomass. |
| Stationary drift nets | The positioning of stationary drift nets in the suspected pathway of displacement between an MC source and sink. | May be relatively cheap and simple to conduct and repeat. Allows for the quantification of MC displacement in a pathway from a source to sink. | Requires that an MC source‐sink pathway is already suspected or identified. Placement of the net may affect results. May be most useful for comparative/seasonal studies. |
| Satellite imagery capturing movement | The observation of MC displacement or deposition from satellite imagery. | May record large fluxes of MC which would otherwise be overlooked. Satellite data are recorded for years and so this approach may be used to study the effects of past extreme weather events. | It is unlikely that the displacement of MC observed from satellite data will be well‐quantified unless it can be related to recent relevant field data. |
| Cores | The use of a corer of any kind to take a sample of the sediment where MC has been deposited. | Can be easily paired with burial rates based on accretion or carbon dating at the same site or derived from an associated mesocosm study. Allows comparison to cores from other blue carbon systems such as seagrass meadows, salt marshes, and mangroves. | MC sediment cores are limited in depth and have been too shallow for carbon accounting schemes as it is difficult to take deeper subtidal or intertidal cores. Cores are not applicable for many macroalgal systems which overly bedrock or coral. |
| Bag or tube incubations | The containment of any macroalgal component in a bag or tube and measurement of the change in DOC, DIC, PIC, or POC of the surrounding water. | Has some control and replication power with the benefit of accounting for in situ effects. The release of MC may be scaled up to ecosystem or regional estimates. | Often incubations are conducted only during the day, cover a small area, and may not be representative of the community as they consider only one component of the living ecosystem. |
| Mesocosm and microcosm studies | The study of DOC, DIC, PIC, POC, or POM moving between organisms, the water column, or the sediment in a container with controlled abiotic conditions. | Offers extensive control over the system, allows for the study of specific conditions of interest, and allows for the precise study of specific nutrient flows. Can be combined with in situ import/export MC data from a site of interest to model the likely fate of MC in a sink. | Laboratory experiments cannot be used alone to predict the behavior of ecosystems in situ. It is difficult to replicate the conditions and complexity of a macroalgal community in such artificial experimental conditions. |
| Benthic chambers | The incubation of a macroalgal system in a benthic chamber flush with the surface below. CO2 consumption or O2 generation is measured as a proxy for community respiration. | Unlike bag or tube incubations, benthic chamber incubations account for community productivity, i.e., the organisms associated with the macroalgal system which may affect net community productivity. Can be scaled by area to ecosystem productivity if the largest component member fits in the benthic chamber. | Benthic chambers may be used to study the release of MC but often study productivity as opposed to the fate of that productivity. |
| Aquatic eddy covariance (AEC) | The measurement of oxygen flux above the canopy of a sub‐aquatic system as a proxy for community production and respiration. | Gives a holistic view of the community productivity, accounting for every constituent organism within. | Informs on the net release of MC from the macroalgal community to the locality but cannot provide information on the fate of this carbon. |
| Fluorometry | The measurement of macroalgal community net productivity using pulse amplitude modulated (PAMs) fluorometry to determine the productivity of constituent members. | Gives detailed, site‐specific, and species‐specific information about productivity. This approach gives information about species interactions such as crowding and shading. | Provides information on the net release of MC from the macroalgal community to the locality but cannot provide information on the fate of this carbon. |
Thank you to the reviewers and to Isabel Jorgensen for their invaluable feedback on this review. Open access funding provided by IReL.
AUTHOR CONTRIBUTIONS
J. Dolliver: Conceptualization (equal); Data curation (equal); Formal analysis (equal); Funding acquisition (equal); Investigation (equal); Methodology (equal); Project administration (equal); Visualization (equal); Writing – original draft (equal); Writing – review & editing (equal). N. O'Connor: Conceptualization (equal); Funding acquisition (equal); Supervision (lead); Writing – review & editing (equal).
CONFLICT OF INTEREST
The authors declare no competing interests.
Supporting information
Appendix S1. Full text papers exlcuded from review at final selection stage.
- Abdullah, M. I. & Fredriksen, S. 2004. Production, respiration and exudation of dissolved organic matter by the kelp Laminaria hyperborea along the west coast of Norway. J. Mar. Biol. Assoc. 84:887–94. [Google Scholar]
- Abdullah, M. I. , Fredriksen, S. & Christie, H. 2017. The impact of the kelp (Laminaria hyperborea) forest on the organic matter content in sediment of the west coast of Norway. Mar. Biol. Res. 13:151–60. [Google Scholar]
- Alkemade, R. & Van Rijswijk, P. 1993. Path analyses of the influence of substrate composition on nematode numbers and on decomposition of stranded seaweed at an Antarctic coast. Neth. J. Sea Res. 31:63–70. [Google Scholar]
- Alongi, D. M. 1990. Bacterial growth rates, production and estimates of detrital carbon utilization in deep‐sea sediments of the Solomon and Coral Seas. Deep Sea Res. Part I Oceanogr. Res. 37:731–46. [Google Scholar]
- Atwood, T. B. , Madin, E. M. P. , Harborne, A. R. , Hammill, E. , Luiz, O. J. , Ollivier, Q. R. , Roelfsema, C. M. , Macreadie, P. I. & Lovelock, C. E. 2018. Predators shape sedimentary organic carbon storage in a coral reef ecosystem. Front. Ecol. Evol. 6:1–11. [Google Scholar]
- Barreiro, F. , Gómez, M. , López, J. , Lastra, M. & de la Huz, R. 2013. Coupling between macroalgal inputs and nutrients outcrop in exposed sandy beaches. Hydrobiologia 700:73–84. [Google Scholar]
- Barrón, C. , Apostolaki, E. T. & Duarte, C. M. 2014. Dissolved organic carbon fluxes by seagrass meadows and macroalgal beds. Front. Mar. Sci. 1:1–11. [Google Scholar]
- Beaumont, N. J. , Jones, L. , Garbutt, A. , Hansom, J. D. & Toberman, M. 2014. The value of carbon sequestration and storage in coastal habitats. Estuar. Coast. Shelf Sci. 137:32–40. [Google Scholar]
- de Bettignies, F. , Dauby, P. , Thomas, F. , Gobet, A. , Delage, L. , Bohner, O. , Loisel, S. & Davoult, D. 2020. Degradation dynamics and processes associated with the accumulation of Laminaria hyperborea (Phaeophyceae) kelp fragments: an in situ experimental approach. J. Phycol. 56:1481–92. [DOI] [PubMed] [Google Scholar]
- de Bettignies, T. , Wernberg, T. , Lavery, P. S. , Vanderklift, M. A. & Mohring, M. B. 2013. Contrasting mechanisms of dislodgement and erosion contribute to production of kelp detritus. Limnol. Oceanogr. 58:1680–8. [Google Scholar]
- Borges, A. V. , Delille, B. & Frankignoulle, M. 2005. Budgeting sinks and sources of CO2 in the coastal ocean: diversity of ecosystem counts. Geophys. Res. Lett. 32:1–4. [Google Scholar]
- Britton‐Simmons, K. H. , Rhoades, A. L. , Pacunski, R. E. , Galloway, A. W. E. , Lowe, A. T. , Sosik, E. A. , Dethier, M. N. & Duggins, D. O. 2012. Habitat and bathymetry influence the landscape‐scale distribution and abundance of drift macrophytes and associated invertebrates. Limnol. Oceanogr. 57:176–84. [Google Scholar]
- Corzo, A. , Bergeijk, S. V. & García‐Robledo, E. 2009. Effects of green macroalgal blooms on intertidal sediments: net metabolism and carbon and nitrogen contents. Mar. Ecol. Prog. Ser. 380:81–93. [Google Scholar]
- Cott, G. , Beca‐Carretero, P. & Stengel, D. B. 2021. Blue Carbon and Marine Carbon Sequestration in Irish Waters and Coastal Habitats. Foras Na Mara, 42 pp.
- Cullen‐Unsworth, L. & Unsworth, R. 2013. Seagrass meadows, ecosystem services, and sustainability. Environ. Sci. Policy 55:14–28. [Google Scholar]
- Davidson, I. C. , Cott, G. M. , Devaney, J. L. & Simkanin, C. 2018. Differential effects of biological invasions on coastal blue carbon: a global review and meta ‐ analysis. Glob. Change Biol. 24:1–13. [DOI] [PubMed] [Google Scholar]
- Dean, P. R. & Hurd, C. L. 2007. Seasonal growth, erosion rates, and nitrogen and photosynthetic ecophysiology of Undaria pinnatifida (Heterokontophyta) in southern New Zealand. J. Phycol. 43:1138–48. [Google Scholar]
- Dierssen, H. M. , Zimmerman, R. C. , Drake, L. A. & Burdige, D. J. 2009. Potential export of unattached benthic macroalgae to the deep sea through wind‐driven Langmuir circulation. Geophys. Res. Lett. 36:1–5. [Google Scholar]
- Duarte, C. M. 2014. Global change and the future ocean: a grand challenge for marine sciences. Front. Mar. Sci. 1:1–16. [Google Scholar]
- Duarte, C. M. , Losada, I. J. , Hendriks, I. E. , Mazarrasa, I. & Marbà, N. 2013. The role of coastal plant communities for climate change mitigation and adaptation. Nat. Clim. Change 3:961–8. [Google Scholar]
- Duarte, C. M. , Middelburg, J. J. & Caraco, N. 2005. Major role of marine vegetation on the oceanic carbon cycle. Biogeosci. 2:1–8. [Google Scholar]
- Dufour, C. , Probert, P. K. & Savage, C. 2012. Macrofaunal colonisation of stranded Durvillaea antarctica on a southern New Zealand exposed sandy beach. N. Z. J. Mar. Freshwater Res. 46:369–83. [Google Scholar]
- Dugan, J. E. , Hubbard, D. M. , Page, H. M. & Schimel, J. P. 2011. Marine macrophyte wrack inputs and dissolved nutrients in beach sands. Estuaries Coast 34:839–50. [Google Scholar]
- Eereveld, P. , Hübner, L. , Schaefer, G. & Zimmer, M. 2013. Herbivory on macro‐algae affects colonization of beach‐cast algal wrack by detritivores but not its decomposition. Oceanologia 55:339–58. [Google Scholar]
- Egea, L. G. , Jiménez‐Ramos, R. , Hernández, I. & Brun, F. G. 2020. Differential effects of nutrient enrichment on carbon metabolism and dissolved organic carbon (DOC) fluxes in macrophytic benthic communities. Mar. Environ. Res. 162:105179. [DOI] [PubMed] [Google Scholar]
- Fankboner, P. V. & de Burgh, M. E. 1977. Diurnal exudation of 14C‐labelled compounds by the large kelp Macrocystis integrifolia Bory. J. Exp. Mar. Biol. Ecol. 28:151–62. [Google Scholar]
- Filbee‐Dexter, K. , Foldager Pedersen, M. , Fredriksen, S. , Magnus Norderhaug, K. , Rinde, E. , Kristiansen, T. , Albretsen, J. & Wernberg, T. 2020. Carbon export is facilitated by sea urchins transforming kelp detritus. Oecologia 192:213–25. [DOI] [PubMed] [Google Scholar]
- Filbee‐Dexter, K. & Scheibling, R. E. 2012. Hurricane‐mediated defoliation of kelp beds and pulsed delivery of kelp detritus to offshore sedimentary habitats. Mar. Ecol. Prog. Ser. 455:51–64. [Google Scholar]
- Filbee‐Dexter, K. & Scheibling, R. E. 2014. Detrital kelp subsidy supports high reproductive condition of deep‐living sea urchins in a sedimentary basin. Aquat. Biol. 23:71–86. [Google Scholar]
- Filbee‐Dexter, K. , Wernberg, T. , Norderhaug, K. M. , Ramirez‐Llodra, E. & Pedersen, M. F. 2018. Movement of pulsed resource subsidies from kelp forests to deep fjords. Oecologia 187:291–304. [DOI] [PubMed] [Google Scholar]
- Frontier, N. , de Bettignies, F. , Foggo, A. & Davoult, D. 2021. Sustained productivity and respiration of degrading kelp detritus in the shallow benthos: detached or broken, but not dead. Mar. Environ. Res. 166:105277. [DOI] [PubMed] [Google Scholar]
- Gladstone‐Gallagher, R. V. , Lohrer, A. M. , Lundquist, C. J. & Pilditch, C. A. 2016. Effects of detrital subsidies on soft‐sediment ecosystem function are transient and source dependent. PLoS ONE 11:e0154790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez, M. , Barreiro, F. , López, J. & Lastra, M. 2018. Effect of upper beach macrofauna on nutrient cycling of sandy beaches: metabolic rates during wrack decay. Mar. Biol. 165:1–12. [Google Scholar]
- Gorain, P. C. , Sengupta, S. , Satpati, G. G. , Paul, I. , Tripathi, S. & Pal, R. 2018. Carbon sequestration in macroalgal mats of brackish‐water habitats in Indian Sunderbans: potential as renewable organic resource. Sci. Total Environ. 626:689–702. [DOI] [PubMed] [Google Scholar]
- Gutow, L. , Poore, A. G. B. , Díaz Poblete, M. A. , Villalobos, V. & Thiel, M. 2020. Small burrowing amphipods cause major damage in a large kelp. Proc. Royal Soc. B 287:20200330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halat, L. , Galway, M. E. , Gitto, S. & Garbary, D. J. 2015. Epidermal shedding in Ascophyllum nodosum (Phaeophyceae): Seasonality, productivity and relationship to harvesting. Phycologia 54:599–608. [Google Scholar]
- Haram, L. E. , Sotka, E. E. & Byers, J. E. 2020. Effects of novel, non‐native detritus on decomposition and invertebrate community assemblage. Mar. Ecol. Prog. Ser. 643:49–61. [Google Scholar]
- Harrold, C. , Light, K. & Lisin, S. 1998. Organic enrichment of submarine‐canyon and continental‐shelf benthic communities by macroalgal drift imported from nearshore kelp forests. Limnol. Oceanogr. 43:669–78. [Google Scholar]
- Harrold, C. & Lisin, S. 1989. Radio‐tracking rafts of giant kelp: local production and regional transport. J. Exp. Mar. Biol. Ecol. 130:237–51. [Google Scholar]
- Hill, R. , Bellgrove, A. , Macreadie, P. I. , Petrou, K. , Beardall, J. , Steven, A. & Ralph, P. J. 2015. Can macroalgae contribute to blue carbon? An Australian perspective. Limnol. Oceanogr. 60:1689–706. [Google Scholar]
- Ito, M. , Scotti, M. , Franz, M. , Barboza, F. R. , Buchholz, B. , Zimmer, M. , Guy‐Haim, T. & Wahl, M. 2019. Effects of temperature on carbon circulation in macroalgal food webs are mediated by herbivores. Mar. Biol. 166:1–11. [Google Scholar]
- Itoh, H. , Aoki, M. N. , Tsuchiya, Y. , Sato, T. , Shinagawa, H. , Komatsu, T. , Mikami, A. & Hama, T. 2007. Fate of organic matter in faecal pellets egested by epifaunal mesograzers in a Sargassum forest and implications for biogeochemical cycling. Mar. Ecol. Prog. Ser. 352:101–12. [Google Scholar]
- IUCN 2009. The management of natural coastal carbon sinks. Gland, Switzerland, 64 pp. [Google Scholar]
- Jayathilake, D. R. M. & Costello, M. J. 2020. A modelled global distribution of the kelp biome. Biol. Conserv. 252:108815. [Google Scholar]
- Josselyn, M. N. , Cailliet, G. M. , Niesen, T. M. , Cowen, R. , Hurley, A. C. , Connor, J. & Hawes, S. 1983. Composition, export and faunal utilization of drift vegetation in the salt river submarine canyon. Estuar. Coast. Shelf Sci. 17:447–65. [Google Scholar]
- Kokubu, Y. , Rothäusler, E. , Filippi, J. B. , Durieux, E. D. H. & Komatsu, T. 2019. Revealing the deposition of macrophytes transported offshore: evidence of their long‐distance dispersal and seasonal aggregation to the deep sea. Sci. Rep. 9:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotwicki, L. , Wesławski, J. M. , Raczyńska, A. & Kupiec, A. 2005. Deposition of large organic particles (macrodetritus) in a sandy beach system (Puck Bay, Baltic Sea). Oceanologia 47:181–99. [Google Scholar]
- Krause‐Jensen, D. & Duarte, C. M. 2016. Substantial role of macroalgae in marine carbon sequestration. Nat. Geosci. 9:737–42. [Google Scholar]
- Krause‐Jensen, D. , Lavery, P. , Serrano, O. , Marba, N. , Masque, P. & Duarte, C. M. 2018. Sequestration of macroalgal carbon: the elephant in the Blue Carbon room. Biol. Lett. 14:20180236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumhansl, K. A. & Scheibling, R. E. 2011a. Detrital production in Nova Scotian kelp beds: patterns and processes. Mar. Ecol. Prog. Ser. 421:67–82. [Google Scholar]
- Krumhansl, K. A. & Scheibling, R. E. 2011b. Spatial and temporal variation in grazing damage by the gastropod Lacuna vincta in Nova Scotian kelp beds. Aquat. Biol. 13:163–73. [Google Scholar]
- Lanari, M. , Kennedy, H. , Copertino, M. S. , Wallner‐Kersanach, M. & Coelho Claudino, M. 2017. Dynamics of estuarine drift macroalgae: growth cycles and contributions to sediments in 2 shallow areas. Mar. Ecol. Prog. Ser. 570:41–55. [Google Scholar]
- Lastra, M. , López, J. & Rodil, I. F. 2018. Warming intensify CO2 flux and nutrient release from algal wrack subsidies on sandy beaches. Glob. Chang. Biol. 24:3766–79. [DOI] [PubMed] [Google Scholar]
- Lastra, M. , Rodil, I. F. , Sánchez‐Mata, A. , García‐Gallego, M. & Mora, J. 2014. Fate and processing of macroalgal wrack subsidies in beaches of Deception Island, Antarctic Peninsula. J. Sea Res. 88:1–10. [Google Scholar]
- McMonagle, M. & Morrison, L. 2020. The seaweed resources of Ireland: a twenty‐first century perspective. J. Appl. Phycol. 32:1287–300. [Google Scholar]
- Mcleod, E. , Chmura, G. L. , Bouillon, S. , Salm, R. , Björk, M. , Duarte, C. M. , Lovelock, C. E. , Schlesinger, W. H. & Silliman, B. R. 2011. A blueprint for blue carbon: toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2 . Front Ecol. Environ. 9:552–60. [Google Scholar]
- Mews, M. , Zimmer, M. & Jelinski, D. E. 2006. Species‐specific decomposition rates of beach‐cast wrack in Barkley Sound, British Columbia, Canada. Mar. Ecol. Prog. Ser. 328:155–60. [Google Scholar]
- Nellemann, C. , Corcoran, E. , Duarte, C. M. , Valdés, L. , De Young, C. , Fonseca, L. & Grimsditch, G. 2009. Blue carbon: A Rapid Response Assessment. United Nations Environment Programme, GRID‐Arendal, Birkeland, Norway, 80 p. www.grida.no [Google Scholar]
- Norderhaug, K. M. & Christie, H. 2011. Secondary production in a Laminaria hyperborea kelp forest and variation according to wave exposure. Estuar. Coast. Shelf Sci. 95:135–44. [Google Scholar]
- Norkko, A. , Thrush, S. F. , Cummings, V. J. , Funnell, G. A. , Schwarz, A.‐M. , Andrew, N. L. & Hawes, I. 2004. Ecological role of Phyllophora antarctica drift accumulations in coastal soft‐sediment communities of McMurdo Sound, Antarctica. Polar Biol. 27:482–94. [Google Scholar]
- O’Dea, R. E. , Lagisz, M. , Jennions, M. D. , Koricheva, J. , Noble, D. W. A. , Parker, T. H. , Gurevitch, J. , Page, M. J. , Stewart, G. , Moher, D. & Nakagawa, S. 2021. Preferred reporting items for systematic reviews and meta‐analyses in ecology and evolutionary biology: a PRISMA extension. Biol. Rev. 96:1695–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olabarria, C. , Incera, M. , Garrido, J. & Rossi, F. 2010. The effect of wrack composition and diversity on macrofaunal assemblages in intertidal marine sediments. J. Exp. Mar. Biol. Ecol. 396:18–26. [Google Scholar]
- Orr, K. K. , Wilding, T. A. , Horstmeyer, L. , Weigl, S. & Heymans, J. J. 2014. Detached macroalgae: its importance to inshore sandy beach fauna. Estuar. Coast. Shelf Sci. 150:125–35. [Google Scholar]
- Orr, M. , Zimmer, M. , Jelinski, D. E. & Mews, M. 2005. Wrack deposition on different beach types: spatial and temporal variation in the pattern of subsidy. Ecology 86:1496–507. [Google Scholar]
- Ortega, A. , Geraldi, N. R. , Alam, I. , Kamau, A. A. , Acinas, S. G. , Logares, R. , Gasol, J. M. , Massana, R. , Krause‐Jensen, D. & Duarte, C. M. 2019. Important contribution of macroalgae to oceanic carbon sequestration. Nat. Geosci. 12:748–54. [Google Scholar]
- Osman‐Elasha, B. , Pipatti, R. , Agyemang‐Bonsu, W. K. , Al‐Ibrahim, A. M. , Lopez, C. , Marland, G. , Shenchu, H. & Tailakov, O. 2005. Implications of carbon dioxide capture and storage for greenhouse gas inventories and accounting. In Metz, B. , Davidson, O. , de Coninck, H. , Loos, M. & Meyer, L. [Eds.] IPCC Special Report on Carbon dioxide Capture and Storage. Cambridge University Press, Cambridge, pp. 363–79. [Google Scholar]
- Page, M. J. , McKenzie, J. E. , Bossuyt, P. M. , Boutron, I. , Hoffmann, T. C. , Mulrow, C. D. , Shamseer, L. et al. 2021. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst. Rev. 10:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen, M. F. , Filbee‐Dexter, K. , Norderhaug, K. M. , Fredriksen, S. , Frisk, N. L. , Fagerli, C. W. & Wernberg, T. 2020. Detrital carbon production and export in high latitude kelp forests. Oecologia 192:227–39. [DOI] [PubMed] [Google Scholar]
- Pedersen, M. , Filbee‐Dexter, K. , Frisk, N. , Sárossy, Z. & Wernberg, T. 2021. Carbon sequestration potential increased by incomplete anaerobic decomposition of kelp detritus. Mar. Ecol. Prog. Ser. 660:53–67. [Google Scholar]
- Pendleton, L. , Donato, D. C. , Murray, B. C. , Crooks, S. , Jenkins, W. A. , Sifleet, S. , Craft, C. et al. 2012. Estimating global “blue carbon” emissions from conversion and degradation of vegetated coastal ecosystems. PLoS ONE 7:e43542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessarrodona, A. , Moore, P. J. , Sayer, M. D. J. & Smale, D. A. 2018. Carbon assimilation and transfer through kelp forests in the NE Atlantic is diminished under a warmer ocean climate. Glob. Change Biol. 24:4386–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister, C. A. , Altabet, M. A. & Weigel, B. L. 2019. Kelp beds and their local effects on seawater chemistry, productivity, and microbial communities. Ecology 1–15:e02798. [DOI] [PubMed] [Google Scholar]
- Quartino, M. L. & Boraso De Zaixso, A. L. 2008. Summer macroalgal biomass in Potter Cove, South Shetland Islands, Antarctica: its production and flux to the ecosystem. Polar Biol. 31:281–94. [Google Scholar]
- Queirós, A. M. , Stephens, N. , Widdicombe, S. , Tait, K. , McCoy, S. J. , Ingels, J. , Rühl, S. et al. 2019. Connected macroalgal‐sediment systems: blue carbon and food webs in the deep coastal ocean. Ecol. Monogr. 89:1–21. [Google Scholar]
- Ramirez‐Llodra, E. , Pedersen, T. , Filbee‐Dexter, K. , Hauquier, F. , Guilini, K. , Mikkelsen, N. , Borgersen, G. , Van Gyseghem, M. , Vanreusel, A. & Vilas, D. 2021. Community structure of deep fjord and shelf benthic fauna receiving different detrital kelp inputs in northern Norway. Deep Sea Res. I Oceanogr. Res. 168:103433. [Google Scholar]
- Reed, D. C. , Carlson, C. A. , Halewood, E. R. , Nelson, J. C. , Harrer, S. L. , Rassweiler, A. & Miller, R. J. 2015. Patterns and controls of reef‐scale production of dissolved organic carbon by giant kelp Macrocystis pyrifera . Limnol. Oceanogr. 60:1996–2008. [Google Scholar]
- Rodil, I. F. , Lastra, M. , López, J. , Mucha, A. P. , Fernandes, J. P. , Fernandes, S. V. & Olabarria, C. 2019. Sandy beaches as biogeochemical hotspots: the metabolic role of macroalgal wrack on low‐productive shores. Ecosystems 22:49–63. [Google Scholar]
- Rossi, F. 2007. Recycle of buried macroalgal detritus in sediments: use of dual‐labelling experiments in the field. Mar. Biol. 150:1073–81. [Google Scholar]
- Rossi, F. , Gribsholt, B. , Gazeau, F. , Di Santo, V. & Middelburg, J. J. 2013. Complex effects of ecosystem engineer loss on benthic ecosystem response to detrital macroalgae. PLoS ONE 8:e66650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi, F. , Incera, M. , Callier, M. & Olabarria, C. 2011. Effects of detrital non‐native and native macroalgae on the nitrogen and carbon cycling in intertidal sediments. Mar. Biol. 158:2705–15. [Google Scholar]
- Ruiz‐Halpern, S. , Vaquer‐Sunyer, R. & Duarte, C. M. 2014. Annual benthic metabolism and organic carbon fluxes in a semi‐enclosed Mediterranean bay dominated by the macroalgae Caulerpa prolifera . Front. Mar. Sci. 1:1–10. [Google Scholar]
- Sfriso, A. , Buosi, A. , Adelheid Wolf, M. , Sciuto, K. , Molinaroli, E. , Moro, I. , Mistri, M. , Munari, C. & Augusto Sfriso, A. 2020. Microcalcareous seaweeds as sentinels of trophic changes and CO2 trapping in transitional water systems. Ecol. Indic. 118:106692. [Google Scholar]
- Smale, D. A. , Burrows, M. T. , Moore, P. , O’Connor, N. & Hawkins, S. J. 2013. Threats and knowledge gaps for ecosystem services provided by kelp forests: a northeast Atlantic perspective. Ecol. Evol. 3:4016–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S. V. 1981. Marine macrophytes as a global carbon sink. Science 211:838–40. [DOI] [PubMed] [Google Scholar]
- Sun, Y. G. , Mao, S. Y. , Wang, F. Y. , Peng, P. A. & Chai, P. X. 2013. Identification of the Kukersite‐type source rocks in the Ordovician Stratigraphy from the Tarim Basin, NW China. Sci. Bull. 58:4450–8. [Google Scholar]
- Sutula, M. , Green, L. , Cicchetti, G. , Detenbeck, N. & Fong, P. 2014. Thresholds of adverse effects of macroalgal abundance and sediment organic matter on benthic habitat quality in estuarine intertidal flats. Estuaries Coast 37:1532–48. [Google Scholar]
- Takai, N. , Takatsu, E. , Sawairi, Y. , Kuwae, T. & Yoshihara, K. 2010. Transport and deposition of macrophytes to the dysphotic bottom of coastal waters. Aquat. Bot. 92:289–93. [Google Scholar]
- Trimmer, M. , Nedwell, D. B. , Sivyer, D. B. & Malcolm, S. J. 2000. Seasonal organic mineralisation and denitrification in intertidal sediments and their relationship to the abundance of Enteromorpha sp. and Ulva sp. Mar. Ecol. Prog. Ser. 203:67–80. [Google Scholar]
- Vetter, E. W. & Dayton, P. K. 1999. Organic enrichment by macrophyte detritus, and abundance patterns of megafaunal populations in submarine canyons. Mar. Ecol. Prog. Ser. 186:137–48. [Google Scholar]
- Wada, S. , Aoki, M. N. , Mikami, A. , Komatsu, T. , Tsuchiya, Y. , Sato, T. , Shinagawa, H. & Hama, T. 2008. Bioavailability of macroalgal dissolved organic matter in seawater. Mar. Ecol. Prog. Ser. 370:33–44. [Google Scholar]
- Wada, S. & Hama, T. 2013. The contribution of macroalgae to the coastal dissolved organic matter pool. Estuar. Coast. Shelf Sci. 129:77–85. [Google Scholar]
- Watanabe, K. , Yoshida, G. , Hori, M. , Umezawa, Y. , Moki, H. & Kuwae, T. 2020. Macroalgal metabolism and lateral carbon flows can create significant carbon sinks. Biogeosci. 17:2425–40. [Google Scholar]
- Weigel, B. L. & Pfister, C. A. 2020. The dynamics and stoichiometry of dissolved organic carbon release by kelp. Ecology 102:e03221. [DOI] [PubMed] [Google Scholar]
- Wernberg, T. , Russell, B. D. , Thomsen, M. S. , Gurgel, C. F. D. , Bradshaw, C. J. A. , Poloczanska, E. S. & Connell, S. D. 2011. Seaweed communities in retreat from ocean warming. Curr. Biol. 21:1828–32. [DOI] [PubMed] [Google Scholar]
- Wernberg, T. , Vanderklift, M. A. , How, J. & Lavery, P. S. 2006. Export of detached macroalgae from reefs to adjacent seagrass beds. Oecologia 147:692–701. [DOI] [PubMed] [Google Scholar]
- Wilmers, C. C. , Estes, J. A. , Edwards, M. , Laidre, K. L. & Konar, B. 2012. Do trophic cascades affect the storage and flux of atmospheric carbon? An analysis of sea otters and kelp forests. Front. Ecol. Environ. 10:409–15. [Google Scholar]
- Yorke, C. E. , Miller, R. J. , Page, H. M. & Reed, D. C. 2013. Importance of kelp detritus as a component of suspended particulate organic matter in giant kelp Macrocystis pyrifera forests. Mar. Ecol. Prog. Ser. 493:113–25. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Full text papers exlcuded from review at final selection stage.


