Abstract
Ex situ normothermic machine perfusion (NMP) is increasingly used for viability assessment of high‐risk donor livers, whereas dual hypothermic oxygenated machine perfusion (DHOPE) reduces ischemia‐reperfusion injury. We aimed to resuscitate and test the viability of initially‐discarded, high‐risk donor livers using sequential DHOPE and NMP with two different oxygen carriers: an artificial hemoglobin‐based oxygen carrier (HBOC) or red blood cells (RBC). In a prospective observational cohort study of 54 livers that underwent DHOPE‐NMP, the first 18 procedures were performed with a HBOC‐based perfusion solution and the subsequent 36 procedures were performed with an RBC‐based perfusion solution for the NMP phase. All but one livers were derived from extended criteria donation after circulatory death donors, with a median donor risk index of 2.84 (IQR 2.52–3.11). After functional assessment during NMP, 34 livers (63% utilization), met the viability criteria and were transplanted. One‐year graft and patient survival were 94% and 100%, respectively. Post‐transplant cholangiopathy occurred in 1 patient (3%). There were no significant differences in utilization rate and post‐transplant outcomes between the HBOC and RBC group. Ex situ machine perfusion using sequential DHOPE‐NMP for resuscitation and viability assessment of high‐risk donor livers results in excellent transplant outcomes, irrespective of the oxygen carrier used.
Keywords: clinical research / practice, donation after circulatory death (DCD), donors and donation, liver transplantation / hepatology, organ perfusion and preservation
Short abstract
Sequential end‐ischemic hypothermic and normothermic machine perfusion results in safe transplantation of previously discarded, high‐risk, human donor livers, irrespective of the oxygen carrier used for normothermic perfusion.
Abbreviations
- (D)HOPE
(dual) hypothermic oxygenated machine perfusion
- COR
controlled oxygenated rewarming
- DBD
donation after brain death
- DCD
donation after circulatory death
- DRI
donor risk index
- HBOC
hemoglobin‐based oxygen carrier
- IQR
interquartile range
- MPS
machine perfusion solution
- NAS
non‐anastomotic strictures
- NMP
normothermic machine perfusion
- RBC
red blood cell
- SCS
static cold storage
- UW
University of Wisconsin
1. INTRODUCTION
Although liver transplantation is the treatment of choice for patients with end‐stage liver disease, its applicability is limited by the shortage of suitable donor organs, resulting in significant mortality on the waiting lists worldwide. On the other hand, the discard rate of deceased donor livers is expected to increase from 22% in 2010, up to 56% in 2030 in the United States, as a result of the raising age of the general population, the increasing incidence of comorbidities such as diabetes mellitus and obesity, as well as an increase in donation after circulatory death (DCD). 1
Static preservation of donor livers submersed in an ice‐cold preservation solution has been the gold standard for organ preservation for decades. While ischemic static cold storage (SCS) is a sufficient preservation method for optimal donor livers, this method has proven to be insufficient for suboptimal donor livers with an increased risk to develop ischemia‐reperfusion injury‐related complications. Among these, primary non‐function and post‐transplant biliary complications, including non‐anastomotic stricture (NAS) of the bile ducts, are a frequent cause of post‐transplant morbidity, early graft loss and patient death. 2 , 3 , 4 , 5 , 6 , 7
To overcome the limitations of SCS, dynamic preservation of donor livers using ex situ machine perfusion has recently entered into clinical practice. Two different types of ex situ liver machine perfusion are currently applied clinically: (dual) hypothermic oxygenated machine perfusion ([D]HOPE) and normothermic machine perfusion (NMP). 8 , 9 , 10 (D)HOPE (<12°C) has been shown to reduce ischemia‐reperfusion injury, especially of the donor bile ducts. 11 , 12 , 13 , 14 The clinical efficacy of DHOPE was recently demonstrated in a multicenter, randomized clinical trial, which revealed a 68% reduction in the risk of NAS and a 39% reduction in early allograft dysfunction after transplantation of DCD livers that underwent DHOPE, compared to SCS alone. 15 In contrast to DHOPE, NMP (35–37°C) allows for biliary viability testing of (high‐risk) donor livers prior to transplantation. 16 , 17 , 18 , 19 However, when applied after static cold storage (end‐ischemic or “back‐to‐base”), NMP is associated with ischemia‐reperfusion injury of the donor liver. This has been postulated to explain the relatively high rate of post‐transplant cholangiopathy in recipients of DCD livers that underwent end‐ischemic NMP. 10 , 17 , 18 , 19 For this reason, it has been proposed that end‐ischemic NMP should best be preceded by a short period of DHOPE. 20 , 21 The first clinical trial of combined, sequential DHOPE and NMP (DHOPE‐NMP trial) for high‐risk DCD livers indeed demonstrated excellent results and a low rate of post‐transplant cholangiopathy. 9 , 22
Contrary to DHOPE, the machine perfusion solution used for NMP should contain an oxygen carrier to supply sufficient oxygen to the metabolically fully active liver. Therefore, either red blood cells (RBC) or an artificial hemoglobin‐based oxygen carrier (HBOC) are used in perfusion solutions for NMP. 16 While HBOC can be used from hypothermia to normothermia, RBC are at risk of hemolysis in hypothermic conditions; hence, in the previously performed clinical trial on DHOPE‐NMP an HBOC was used. 9 However, HBOC has not yet been approved for organ machine perfusion by competent authorities, which limits the wider international dissemination of the protocol. To allow wider application of the combined protocol of sequential DHOPE‐NMP, we have modified the perfusion protocol by using RBC instead of HBOC as an oxygen carrier during the NMP phase, and by using University of Wisconsin (UW) machine perfusion solution (MPS) for the DHOPE‐phase. We hypothesized that ex situ machine perfusion using sequential DHOPE‐NMP for resuscitation and viability assessment of high‐risk human donor livers results in safe transplantation, irrespective of the type of oxygen carrier used. In this study, we compare the outcomes of two consecutive prospective cohorts in which HBOC or RBC were used as oxygen carrier in sequential DHOPE‐NMP of previously declined, high‐risk donor livers.
2. METHODS
2.1. Study cohorts
In 2017, the prospective observational DHOPE‐NMP study was started after Medical Ethical Committee approval, registration in Netherlands Trial Register (NTR5972), and publication of the study protocol. 23 After completion of the clinical trial and assessment of the study endpoints, 9 the DHOPE‐NMP protocol was implemented as standard procedure for initially discarded human livers in our center. The HBOC used in this study (HBOC‐201, Hemopure, HBO2 therapeutics, Souderton, PA) could not be used outside a clinical trial due to the lack of a formal registration. After completion of the trial, we therefore switched to an alternative oxygen carrier for the NMP‐phase, to enable continuation of the initially successful protocol in a prospective observational study. Based on extensive pre‐clinical experience, an RBC‐based perfusion solution was used instead. 24 , 25 , 26 The same predefined endpoints as in the DHOPE‐NMP trial were used. The primary outcome was graft survival at 3 months after transplantation. Secondary endpoints included patient and graft survival at 7 days, and 3, 6, and 12 months after transplantation, as well as occurrence of primary non‐function (PNF), early allograft dysfunction (EAD) and development of NAS. 23 All patients provided informed consent, in compliance with the Declaration of Helsinki principles. The clinical trial with an HBOC‐based perfusion fluid was performed between January 1, 2017, and January 1, 2019 (HBOC‐group) and in all subsequent sequential DHOPE‐NMP procedures, performed between January 1, 2019, and March 1, 2021, an RBC‐based perfusion solution was used (RBC‐group). All patients had follow‐up until August 1, 2021.
2.2. Procurement and organ preparation
All donor livers were procured by one of the regional multi‐organ procurement teams in the Netherlands, using a standardized procedure, as described previously. 13 Both extended criteria DCD livers and donation after brain death (DBD) livers were accepted, as long as the liver were nationwide declined for regular transplantation based on medical reasons (Table S1). For DCD livers, a mandatory 5‐minute no‐touch period was applied after determination of circulatory death, and no heparin was administered prior to circulatory arrest. After procurement, grafts were transported using SCS in UW static cold storage solution. Upon arrival in our center, the portal vein and supratruncal aorta were cannulated (25Fr cannulas) at the back‐table. An 8Fr catheter was carefully secured in the common bile duct for bile collection and subsequent analysis. In addition, a 12Fr infusion line was inserted into the inferior vena cava to allow venous sampling (Figure S1). Before insertion of the bile duct catheter, a bile duct biopsy was taken from the distal end of the common bile duct for blinded assessment of the bile duct injury severity score, using light microscopy after hematoxylin & eosin staining. 9 , 27
2.3. Machine perfusion
For the machine perfusion procedures, a Liver Assist device (XVIVO, Gothenburg, Sweden) was used. All livers underwent one hour of DHOPE, with a portal venous pressure of ≤5 mmHg and arterial pressure of ≤25 mmHg. During DHOPE, 1L of 100% O2 was supplied. In the HBOC‐group, a combination of DHOPE, controlled oxygenated rewarming (COR), and NMP was performed without the requirement for a perfusate switch. In the RBC‐group, UW MPS (PumpProtect, Carnamedica, Warshaw, Poland) was used during the DHOPE‐phase and this perfusion fluid was replaced by an RBC‐containing perfusion fluid for the COR‐NMP phase (Figure 1). Apart from the oxygen carrier, there were no major differences between the two groups for the other components of the perfusion solutions used in the COR‐NMP phase (Table S2).
FIGURE 1.
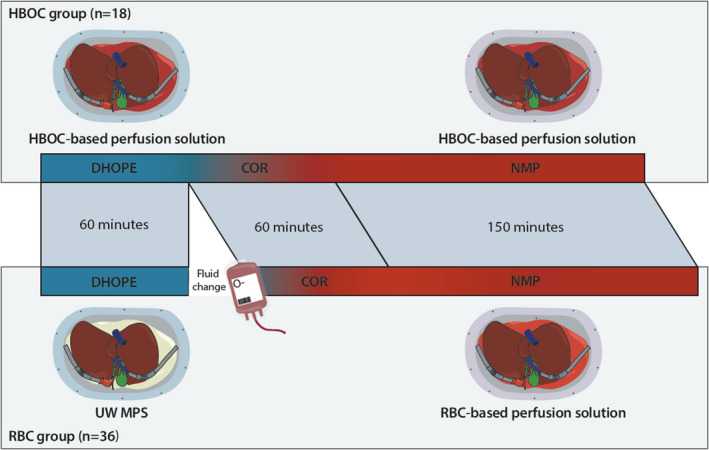
Schematic overview of combined DHOPE and NMP procedures. Initially discarded donor livers either underwent the combination of DHOPE, controlled oxygenation rewarming and NMP with a HBOC or RBC‐based perfusion solution. In the RBC group, a switch of perfusion solution was performed between DHOPE and the rewarming phase, whereas in the HBOC group no switch was required. COR, controlled oxygenated rewarming; DHOPE, dual hypothermic oxygenated machine perfusion; HBOC, hemoglobin‐based oxygen carrier; NMP, normothermic machine perfusion; UW MPS, UW machine perfusion solution [Color figure can be viewed at wileyonlinelibrary.com]
2.4. Procedure with HBOC‐based perfusion solution
Donor livers were flushed at the backtable with 2 L of cold saline prior to connection to the perfusion device for DHOPE. After 1 h of DHOPE, livers underwent one hour of COR, followed by NMP, as described previously. 9 , 22 Details of the perfusion solutions are shown in Table S2. 9 , 22
2.5. Procedure with RBC‐based perfusion solution
After one hour of DHOPE with UW MPS, the liver was briefly disconnected from the perfusion device and placed in a sterile bowl with preservation solution and ice. The circuit was quickly drained and rinsed with at least 3 L of sterile water, using surgical suction tubes. Next, the perfusion machine was primed at 20°C with the perfusion solution for COR and NMP. In the meantime, the donor liver was flushed with 2 L of cold saline to rinse out the potassium‐rich UW MPS. After performing a blood gas analysis to check oxygenation and fluid composition, the liver was reconnected to the Liver Assist device. Replacement of the perfusion fluid required approximately 15 to 30 min. Once the liver was reconnected and rewarmed to 20°C, the temperature and pressures were slowly increased to 37°C at a similar pace as in the HBOC protocol. 22 , 23
2.6. Perfusate and bile analyses
During COR and NMP, oxygen supply was adjusted, by changing the FiO2, based on arterial PO2 (target range 10.0–13.3 kPa) and venous saturation (target range 55%–75%). During NMP, every 30 min arterial, venous, and bile samples were collected for gas analysis (ABL 90 Flex blood gas meter, Radiometer, Brønhøj, Denmark). For the analysis of bile pH, bicarbonate, and glucose, bile was collected under mineral oil (Sigma Aldrich, Darmstadt, Germany) in an Eppendorf tube (2 ml tubes, Sarstedt, Nümbricht, Germany). 27
2.7. Hepatobiliary viability assessment
The hepatobiliary viability was assessed during the first 2.5 h of NMP. The liver was considered suitable for transplantation if the following criteria were met at any time point during the first 2.5 h of NMP꞉ arterial lactate <1.7 mmol/L, arterial pH between 7.35 to 7.45, bile production >10 ml, and bile pH >7.45. 23 In addition, the difference (delta) between bile and perfusate pH, bicarbonate and glucose was determined to assess alkalization of the bile and glucose reabsorption by the biliary epithelium (Table 1). 9 , 28 The importance of these “secondary” viability criteria became obvious with increasing experience and after post‐transplant cholangiopathy had developed in one recipient. 9 The ultimate decision on whether or not to transplant a liver was made by the surgeon supervising the perfusion procedure and the surgeon who was going to perform the transplant procedure. This discussion and decision included the medical condition and urgency of the potential recipient. When a liver did not meet the viability criteria after 2.5 h of NMP, the perfusion was stopped and the liver was secondarily discarded. When the liver met the viability criteria and was accepted for transplantation, NMP of the liver continued during the recipient hepatectomy. When the hepatectomy was finished, the donor liver was disconnected from the perfusion device and subsequently flushed with 2 L of UW cold storage preservation solution and placed in a sterile bowl with UW preservation solution and ice for transfer to the recipient. Implantation was performed using either the piggyback technique or classical implantation. All grafts underwent reperfusion through the portal vein first, and the first 500 ml of flushed recipient blood was discarded prior to the release of the inferior vena cava clamp.
TABLE 1.
Criteria used to determine viability during 2.5 h of NMP [Color table can be viewed at wileyonlinelibrary.com]
| Parameter | Green zone | Orange zone | Red zone | |
|---|---|---|---|---|
| Hepatocytes | Bile production (ml) | ≥ 10 a | 5 to 10 | < 5 |
| Perfusate lactate (mmol/L) | < 1.7 | 1.7 to 4.0 | > 4.0 | |
| Perfusate pH | 7.35–7.45 | 7.25 to 7.35 | < 7.25 | |
| Cholangiocytes | Bile pH | > 7.45 | 7.40 to 7.45 | < 7.40 |
| ΔpH | > 0.10 | 0.05 to 0.10 | < 0.05 | |
| ΔHCO3 − (mmol/L) | > 5.0 | 3.0 to 5.0 | < 3.0 | |
| ΔGlucose (mmol/L) | < −5.0 | −3.0 to −5.0 | > −3.0 |
Viability criteria that needed to be reached within 2.5 h of normothermic machine perfusion. The green zone includes the four original viability criteria (perfusate pH, lactate, bile production, and bile pH) that had to be reached at any time point within 2.5 h after initiation of NMP. The other criteria were secondary criteria that emerged with increasing experience. Orange zone represents potentially acceptable values which are “on the border” and that could be accepted if the other viability criteria are “green.” Red zone indicates values that do not meet the viability criteria.
Abbreviations: NMP; normothermic machine perfusion.
Of which ≥4 ml in the last hour. ∆ indicates the bile value minus the perfusate value.
2.8. Post‐transplant care
Post‐transplant immunosuppression was based on induction with basiliximab and triple therapy using tacrolimus, mycophenolic acid, and a rapid taper of steroids. Doppler ultrasound of the liver was performed routinely on post‐operative day 1, 4, and 7. All patients with signs of cholestasis (icterus or pruritus, or increasing serum gamma glutamyl transferase, alkaline phosphatase, or bilirubin levels) underwent magnetic resonance cholangiography to assess potential intra‐ or extrahepatic biliary strictures.
2.9. Definitions
Static cold ischemia time was defined as the time between initiation of in situ cold flushing in the donor and either graft reperfusion in the recipient or the start of machine perfusion. Recipient warm ischemia time was the time between the liver taken out of ice for implantation and graft reperfusion in the recipient. Post‐reperfusion syndrome was defined as a drop in mean arterial blood pressure of over 30% within the first 10 min of reperfusion, in comparison to the mean arterial blood pressure 5 min before reperfusion. 29 Primary non‐function was defined as liver graft failure leading to patient death or requiring retransplantation within 7 days of transplantation without an identifiable cause. NAS was defined as narrowing of the donor bile ducts on magnetic resonance cholangiography or endoscopic retrograde cholangiography at any other location than the anastomosis in a patient with signs of cholestasis or cholangitis within the first year after transplantation, in the presence of a patent hepatic artery. Anastomotic biliary stricture was defined as narrowing of the bile duct at the biliary anastomosis. Acute rejection was defined as biopsy‐proven rejection with the requirement for steroid bolus treatment. Chronic rejection was based on persistent laboratory abnormalities and histological confirmation. Graft survival was defined as the time interval between transplantation and re‐transplantation or death due to graft dysfunction, with censoring for death with a functioning graft.
2.10. Data collection and statistical analysis
All perioperative data, including hemodynamics, perioperative and postoperative laboratory values, as well as all clinical outcome parameters, were prospectively collected and stored in a secure database according to the data and privacy policy of our hospital. Statistical analyses were performed with SPSS, version 23 (IBM, Armonk, New York, NY). Continuous variables were compared using the two‐sided Mann‐Whitney‐U test and presented as median with interquartile range (IQR). Categorical variables were compared using two‐sided Fisher's exact, or chi‐square test when appropriate. Actuarial one‐year graft and patient survival were analyzed using Kaplan Meier method with a log‐rank test for comparison of groups. Statistical significance was determined at p < .05.
3. RESULTS
3.1. Donor characteristics
The median donor age of all perfused livers was 66 years (56–70 years), and the median donor risk index (DRI) was 2.84 (2.52–3.11) (Table 2). All but one livers were derived from DCD type III or V donors. Between the 34 transplanted and 20 non‐transplanted livers, the only significant differences were cold ischemia time (271 min [245–293] vs. 289 min [271–346], p = .007) and donor risk index (2.73 [2.47–2.93] vs. 2.96 [2.64–3.37], p = .013).
TABLE 2.
Donor characteristics
| Variable | All livers (n = 54) | Transplanted (n = 34) | Non‐transplanted (n = 20) | p‐value transplanted vs non‐transplanted |
|---|---|---|---|---|
| Donor characteristics | ||||
| Age (years) | 66 (56–70) | 63 (55–68) | 68 (58–74) | .103 |
| Body mass index (kg/m2) | 26 (24–28) | 26 (23–27) | 26 (24–30) | .483 |
| Gender | .163 | |||
| Male | 36 (67%) | 25 (74%) | 11 (55%) | |
| Female | 18 (33%) | 9 (27%) | 9 (45%) | |
| Cause of death | .148 | |||
| Trauma | 10 (19%) | 6 (18%) | 4 (20%) | |
| Cerebrovascular attack | 21 (39%) | 12 (35%) | 9 (45%) | |
| Anoxia | 18 (33%) | 14 (41%) | 3 (15%) | |
| Other | 5 (9%) | 2 (6%) | 4 (20%) | |
| Donor type | 1.000 | |||
| DBD | 1 (2%) | 1 (3%) | 0 | |
| DCD | 53 (98%) | 33 (97%) | 20 (100%) | |
| Time from withdrawal of life support to circulatory arrest (min) | 16 (11–20) | 15 (11–19) | 18 (10–22) | .403 |
| Time from circulatory arrest to cold perfusion (min) | 16 (15–18) | 16 (15–18) | 17 (14–19) | .308 |
| Functional donor warm ischemia time b | 30 (25–34) | 29 (25–32) | 32 (26–35) | .155 |
| Last sodium (mmol/L) | 143 (140–146) | 143 (140–146) | 142 (140–148) | .780 |
| Last AST (U/L) | 50 (27–104) | 57 (28–101) | 31 (25–126) | .479 |
| Last ALT (U/L) | 37 (16–100) | 37 (18–100) | 25 (16–118) | .858 |
| Last GGT (U/L) | 46 (18–93) | 53 (19–93) | 35 (14–169) | .548 |
| Last ALP (U/L) | 71 (58–95) | 70 (55–95) | 71 (62–153) | .372 |
| Static cold ischemia time (min) | 279 (255–302) | 271 (245–293) | 289 (271–346) | .007 |
| ET‐DRI a | 2.98 (2.63–3.25) | 2.91 (2.60–3.16) | 3.12 (2.63–3.38) | .105 |
| DRI a | 2.84 (2.52–3.11) | 2.73 (2.47–2.93) | 2.96 (2.64–3.37) | .013 |
Continuous data are presented as median (IQR), categorical data as number (percentage).
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; DBD, donation after brain death; DCD, donation after circulatory death; DRI, donor risk index; ET‐DRI, Eurotransplant donor risk index; GGT, gamma glutamyltransferase.
Time from donor saturation <80% or mean arterial pressure <60 mmHg to initiation of in situ cold flushing in the donor. 33 Static cold ischemia time was defined as the time between initiation of in situ cold flushing in the donor and start of DHOPE. The statistical tests were not powered due to small sample size, these results should be interpreted with caution.
3.2. Machine perfusion characteristics
During NMP, all but one liver (53 of 54, 98%) cleared lactate sufficiently, stabilized perfusate pH and produced bile >10 ml, and thereby met the hepatocellular viability criteria (Table 3). Thirty‐four livers (63%) also met the predefined cholangiocellular viability criteria and were subsequently accepted for transplantation. Machine perfusion characteristics are shown in Figure 2. The livers that met the hepatobiliary viability criteria cleared lactate and normalized pH rapidly in the perfusate. Additionally, the bile pH and delta pH, bicarbonate and glucose were higher for the transplanted livers in comparison to the livers that did not meet the viability criteria. Moreover, the bile duct injury severity score was lower in the livers that were transplanted, compared to the livers that were secondary discarded (3.7 [3.4–4.2] vs. 4.4 [3.9–4.9], p = .001).
TABLE 3.
Donor liver characteristics and actual values of viability markers at 2.5 h of NMP a [Color table can be viewed at wileyonlinelibrary.com]
| Donor | Hepatocellular viability at 2.5 h | Cholangiocellular viability at 2.5 h | Outcomes | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Age | DRI | DWIT | pH | Lactate | Bile > 10 ml | Bile pH | Bile HCO3 | Bile gluc | Delta pH | Delta HCO3 | Delta gluc | Tx | NAS |
| DCD | 63 | 2.92 | 30 | 7.41 | 1.1 | Yes | 7.66 | 28.0 | 13.0 | 0.25 | 15.0 | −14.0 | Yes | No |
| DCD | 53 | 2.39 | 26 | 7.36 | 0.1 | Yes | 7.62 | 26.1 | 8.4 | 0.26 | 14.6 | −11.5 | Yes | No |
| DCD | 52 | 2.31 | 33 | 7.43 | 0.9 | Yes | 7.60 | 29.7 | 13.8 | 0.17 | 12.0 | −8.1 | Yes | No |
| DCD | 72 | 2.90 | 32 | 7.46 | 0.5 | Yes | 7.58 | 27.6 | 6.9 | 0.12 | 7.5 | −9.8 | Yes | No |
| DCD | 61 | 2.51 | 27 | 7.42 | 1.8 | Yes | 7.58 | 24.8 | 12.2 | 0.16 | 6.7 | −5.1 | Yes | No |
| DCD | 69 | 2.53 | 35 | 7.42 | 0.1 | Yes | 7.57 | 22.9 | 10.9 | 0.15 | 8.4 | −13.9 | Yes | No |
| DBD | 61 | 1.89 | ‐ | 7.38 | 0.3 | Yes | 7.57 | 21.0 | 13.6 | 0.19 | 7.0 | −25.4 | Yes | No |
| DCD | 42 | 2.01 | 33 | 7.42 | 0.4 | Yes | 7.55 | 32.0 | 1.6 | 0.13 | 9.6 | −6.3 | Yes | No |
| DCD | 68 | 2.96 | 32 | 7.41 | 0.2 | Yes | 7.55 | 22.6 | 8.2 | 0.14 | 9.5 | −7.7 | Yes | No |
| DCD | 63 | 2.79 | 25 | 7.36 | 0.3 | Yes | 7.55 | 25.0 | 6.5 | 0.19 | 10.6 | −21.5 | Yes | No |
| DCD | 65 | 2.48 | 34 | 7.37 | 0.5 | Yes | 7.55 | 28.7 | 11.8 | 0.18 | 9.3 | −14.2 | Yes | No |
| DCD | 58 | 2.65 | 92 | 7.38 | 0.4 | Yes | 7.54 | 24.0 | 7.2 | 0.16 | 10.4 | −11.5 | Yes | No |
| DCD | 62 | 3.40 | 31 | 7.42 | 0.5 | Yes | 7.50 | 31.0 | 13.8 | 0.08 | 9.0 | −8.3 | Yes | No |
| DCD | 44 | 2.45 | 38 | 7.36 | 1.2 | Yes | 7.49 | 18.0 | 10.1 | 0.13 | 6.0 | −14.2 | Yes | No |
| DCD | 71 | 3.44 | 24 | 7.41 | 0.1 | Yes | 7.48 | 27.5 | 13.2 | 0.07 | 6.5 | −16.8 | Yes | No |
| DCD | 66 | 2.74 | 27 | 7.35 | 0.2 | Yes | 7.46 | 20.9 | 4.5 | 0.12 | 8.2 | −8.4 | Yes | No |
| DCD | 78 | 3.44 | 33 | 7.46 | 1.2 | Yes | 7.63 | 24.3 | 3.1 | 0.17 | 4.4 | −8.6 | Yes | No |
| DCD | 67 | 2.86 | 75 | 7.41 | 0.4 | Yes | 7.51 | 20.0 | 12.5 | 0.10 | 3.8 | −5.6 | Yes | No |
| DCD | 56 | 2.58 | 26 | 7.29 | 0.6 | Yes | 7.55 | 26.8 | 8.5 | 0.26 | 15.2 | −23.5 | Yes | No |
| DCD | 52 | 2.55 | 31 | 7.36 | 0.8 | Yes | 7.46 | 24.9 | 7.4 | 0.10 | 7.9 | −4.4 | Yes | No |
| DCD | 52 | 2.43 | 22 | 7.33 | 1.1 | Yes | 7.46 | 18.5 | 2.9 | 0.14 | 7.6 | −3.1 | Yes | No |
| DCD | 66 | 2.73 | 35 | 7.40 | 1.2 | Yes | 7.68 | 26.1 | 16.6 | 0.28 | 10.6 | −8.3 | Yes | No |
| DCD | 82 | 3.01 | 33 | 7.38 | 0.3 | Yes | 7.61 | 29.4 | 17.0 | 0.23 | 14.2 | −9.0 | Yes | No |
| DCD | 46 | 2.06 | 27 | 7.43 | 1.6 | Yes | 7.70 | 38.9 | 16.8 | 0.27 | 21.3 | −13.2 | Yes | No |
| DCD | 63 | 3.10 | 30 | 7.42 | 0.7 | Yes | 7.51 | 24.8 | 17.7 | 0.09 | 7.1 | −11.3 | Yes | No |
| DCD | 69 | 2.66 | 42 | 7.36 | 0.3 | Yes | 7.45 | 21.9 | 18.7 | 0.09 | 7.0 | −8.3 | Yes | No |
| DCD | 68 | 2.92 | 31 | 7.39 | 0.3 | Yes | 7.46 | 17.7 | 9.1 | 0.07 | 5.2 | −5.0 | Yes | No |
| DCD | 62 | 3.08 | 35 | 7.35 | 1.4 | Yes | 7.56 | 18.0 | 28.0 | 0.21 | 13.3 | −7.0 | Yes | No |
| DCD | 68 | 2.73 | 28 | 7.37 | 0.1 | Yes | 7.52 | 22.5 | 19.4 | 0.16 | 9.9 | −5.0 | Yes | No |
| DCD | 41 | 2.03 | 90 | 7.45 | 1.3 | Yes | 7.51 | 17.0 | 2.5 | 0.06 | 1.7 | −10.6 | Yes c | No |
| DCD | 61 | 2.85 | 33 | 7.42 | 0.4 | Yes | 7.48 | 24.9 | 18.1 | 0.06 | 4.2 | −2.0 | Yes c | No |
| DCD | 69 | 3.18 | 28 | 7.39 | 0.1 | Yes | 7.41 | 21.6 | 12.8 | 0.01 | 3.9 | −3.7 | Yes c | No |
| DCD | 75 | 3.20 | 23 | 7.37 | 0.8 | Yes | 7.53 | 24.7 | 19.6 | 0.16 | 0.9 | −3.0 | Yes c | No |
| DCD | 62 | 2.83 | 25 | 7.46 | 0.4 | Yes | 7.45 | 24.7 | 28.0 | −0.01 | 0.2 | 0.0 | Yes | Yes |
| DCD | 68 | 2.98 | 31 | 7.40 | 0.8 | Yes | 7.49 | 18.8 | 13.3 | 0.09 | 3.6 | −10.8 | No b | |
| DCD | 71 | 3.37 | 35 | 7.40 | 0.9 | Yes | 7.50 | 16.8 | 20.2 | 0.10 | 4.3 | −10.8 | No b | |
| DCD | 52 | 2.93 | 18 | 7.42 | 2.2 | Yes | 7.48 | 13.8 | 19.2 | 0.06 | 0.7 | −3.1 | No | |
| DCD | 56 | 2.04 | 36 | 7.34 | 1.7 | Yes | 7.28 | 18.3 | 20.4 | −0.06 | 2.1 | −6.6 | No | |
| DCD | 70 | 3.67 | 38 | 7.39 | 1.9 | Yes | 7.43 | 19.7 | 21.7 | 0.04 | 3.1 | −9.3 | No | |
| DCD | 64 | 3.10 | 79 | 7.42 | 0.4 | Yes | 7.43 | 14.8 | 11.1 | 0.01 | 1.4 | −3.9 | No | |
| DCD | 71 | 2.88 | 30 | 7.45 | 1.3 | Yes | 7.40 | 20.0 | 26.0 | −0.05 | 3.9 | −3.0 | No | |
| DCD | 72 | 3.16 | 33 | 7.41 | 0.9 | Yes | 7.43 | 21.0 | 18.4 | 0.02 | 3.3 | −3.4 | No | |
| DCD | 75 | 2.77 | 24 | 7.42 | 0.6 | Yes | 7.42 | 21.0 | 24.6 | 0.00 | 1.5 | −1.4 | No | |
| DCD | 68 | 2.56 | 44 | 7.39 | 1.4 | Yes | 7.43 | 18.3 | 20.9 | 0.04 | 1.0 | −2.1 | No | |
| DCD | 51 | 2.55 | 95 | 7.40 | 1.7 | Yes | 7.42 | 14.2 | 2.0 | 0.02 | 2.0 | −3.9 | No | |
| DCD | 67 | 3.36 | 37 | 7.44 | 0.8 | Yes | 7.40 | 11.0 | 14.1 | −0.04 | 1.0 | −1.4 | No | |
| DCD | 63 | 2.94 | 72 | 7.38 | 0.3 | Yes | 7.39 | 16.9 | 13.3 | 0.01 | 1.3 | −1.0 | No | |
| DCD | 74 | 3.20 | 39 | 7.42 | 1.5 | Yes | 7.38 | 14.0 | 17.3 | −0.04 | 3.6 | −7.6 | No | |
| DCD | 74 | 3.67 | 26 | 7.35 | 1.7 | Yes | 7.43 | 17.0 | 31.0 | 0.08 | 3.0 | 3.0 | No | |
| DCD | 74 | 3.37 | 33 | 7.38 | 0.4 | Yes | 7.43 | 15.9 | 17.5 | 0.05 | 2.3 | −3.3 | No | |
| DCD | 67 | 3.36 | 35 | 7.34 | 0.2 | Yes | 7.36 | 15.0 | 27.0 | 0.02 | 2.0 | −4.0 | No | |
| DCD | 47 | 2.50 | 30 | 7.25 | 3.5 | Yes | 7.32 | 13.2 | 20.2 | 0.07 | 3.1 | −4.3 | No | |
| DCD | 75 | 3.51 | 40 | 7.39 | 0.7 | Yes | 7.33 | 13.0 | 18.6 | −0.06 | 2.0 | −0.7 | No | |
| DCD | 51 | 2.60 | 29 | 7.29 | 1.3 | Yes | 7.22 | 8.0 | 22.6 | −0.07 | −2.0 | −0.4 | No | |
The grey marked cases underwent NMP with a HBOC‐based solution, the blanc cases underwent NMP with a RBC‐based solution. Lactate, (delta) HCO3 and (delta) glucose are presented as mmol/L. Donor warm ischemia time was defined as the time between withdrawal of life support and start of in situ cold perfusion.
Abbreviations: DBD, donation after brain death; DCD, donation after circulatory death; DRI, donor risk index 34 ; DWIT, donor warm ischemia time; gluc, glucose; NAS, non‐anastomotic biliary strictures; Tx, transplantation.
Donor age is denoted in years, DWIT in minutes.
Presented are the actual values measured at 2.5 h of NMP. In some cases, the values of (secondary) criteria were higher or lower than the threshold selection value that was already met at any time point during those 2.5 h. This explains why the values of some (secondary) criteria in transplanted livers are marked as orange or red.
In retrospect, we would consider these livers suitable for transplantation.
Delta HCO3 and glucose were considered ‘borderline’, but post‐transplant magnetic resonance cholangiography imaging displayed no evidence of NAS.
FIGURE 2.
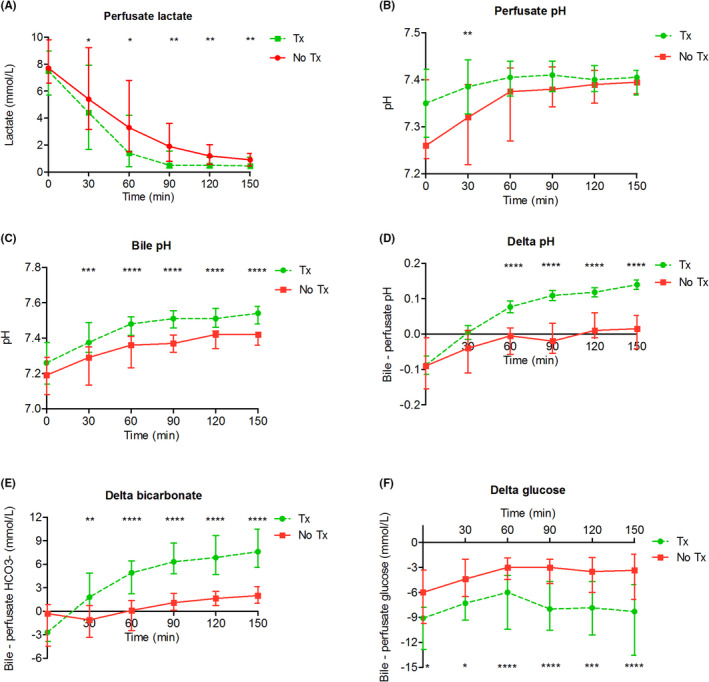
Biochemical viability parameters during NMP. (A) Perfusate lactate levels were significantly lower after 30 min of NMP in the transplanted livers. (B) Perfusate pH was normalized more rapidly in the transplanted livers. (C‐F) Bile pH, delta pH, delta HCO3 −, and delta glucose were higher in the transplanted livers. Delta for pH, HCO3 −, and glucose was calculated between the bile and the arterial value, at the same time point. * Indicates a statistically significant difference p < .05. **p < .01, ***p < .001, **** p < .0001. The statistical tests were not powered due to small sample size, these results should be interpreted with caution. NMP, normothermic machine perfusion; Tx, transplanted; No Tx, not transplanted [Color figure can be viewed at wileyonlinelibrary.com]
3.3. Recipient and transplant characteristics
In total, 34 patients underwent liver transplantation with a donor liver after sequential DHOPE‐NMP (Table 4; Table S3). Hepatocellular carcinoma and cirrhosis based on non‐alcoholic steatohepatitis were the most common indications for transplantation.
TABLE 4.
Post‐transplant outcomes of recipients of DHOPE‐NMP livers
| Variable | All livers (n = 34) | HBOC (n = 12) | RBC (n = 22) | p‐value HBOC versus RBC | |
|---|---|---|---|---|---|
| Post‐transplant outcomes | |||||
| Actuarial graft survival | .785 | ||||
| 3 months | 97% | 92% | 100% | ||
| 6 months | 97% | 92% | 100% | ||
| 12 months | 94% | 92% | 95% | ||
| Retransplantation for | |||||
| Chronic rejection | 1 (3%) | 0 | 1 (5%) | 1.000 | |
| Venous outflow tract obstruction | 1 (3%) | 1 (8%) | 0 | .353 | |
| Actuarial patient survival | 1.000 | ||||
| 12 months | 100% | 100% | 100% | ||
| Peak ALT (U/L) | 685 (401–987) | 685 (296–930) | 693 (546–942) | .534 | |
| Peak AST (U/L) | 1030 (635–1757) | 900 (501–1757) | 1042 (762–1374) | .606 | |
| Bilirubin day 7 (µmol/L) | 13 (8–37) | 16 (9–39) | 12 (8–31) | .709 | |
| INR day 7 | 1.0 (1.0–1.1) | 1.0 (1.0–1.0) | 1.0 (1.0–1.1) | .367 | |
| GGT (IU/L) | |||||
| Day 30 | 107 (65–251) | 100 (39–377) | 136 (69–228) | .298 | |
| Day 90 | 62 (21–173) | 67 (15–278) | 60 (25–108) | .933 | |
| Day 180 | 92 (22–183) | 147 (18–191) | 78 (22–224) | .860 | |
| Alkaline phosphatase (U/L) | |||||
| Day 30 | 156 (86–302) | 87(65–277) | 188 (117–311) | .154 | |
| Day 90 | 162 (84–397) | 168 (70–464) | 155 (91–314) | .923 | |
| Day 180 | 143 (104–253) | 136 (84–282) | 146 (105–353) | .681 | |
| Biliary complications | |||||
| Non‐anastomotic strictures | 1 (3%) | 1 (8%) | 0 | .353 | |
| Anastomotic stricture | 12 (35%) | 4 (33%) | 8 (36%) | 1.000 | |
| Bile leakage | 4 (12%) | 1 (8%) | 3 (14%) | .317 | |
| Primary non‐function | 0 | 0 | 0 | ||
| Hepatic artery thrombosis | 0 | 0 | 0 | ||
| Acute rejection | 3 (9%) | 2 (8%) | 1 (5%) | .279 | |
| Chronic rejection | 2 (6%) | 1 (8%) | 1 (5%) | 1.000 | |
| Relaparotomy for: | .667 | ||||
| Bleeding | 3 (9%) | 1 (8%) | 2 (9%) | ||
| Bile leakage | 4 (12%) | 1 (8%) | 3 (14%) | ||
| Other | 4 (12%) | 2 (17%) | 2 (9%) | ||
| ICU stay (days) | 2 (1–3) | 2 (1–4) | 2 (1–3) | .606 | |
| Hospital stay (days) | 18 (14–25) | 17 (15–24) | 18 (14–25) | .696 | |
Continuous data are presented as median (IQR), categorical data as number (percentage). The statistical tests were not powered due to small sample size, these results should be interpreted with caution.
Abbreviations: HBOC, hemoglobin‐based oxygen carrier; HCC, hepatocellular carcinoma; ICG, indocyanine green; ICU, intensive care unit; RBC, red blood cell.
3.4. Oxygen carrier comparison
There were no significant differences in donor characteristics between the two oxygen carrier groups (Table S4). At the start of NMP, perfusate lactate values were significantly higher in the HBOC‐group, compared to the RBC‐group (Figure 3). This initial difference, which is explainable by the fact that HBOC is dissolved in Ringers lactate solution, disappeared after 1 h of NMP. During NMP, there were no significant differences in arterial pH and delta pH, bicarbonate, and glucose between the HBOC and RBC group, and therefore the utilization rate was similar as well (Figure 3B–F).
FIGURE 3.
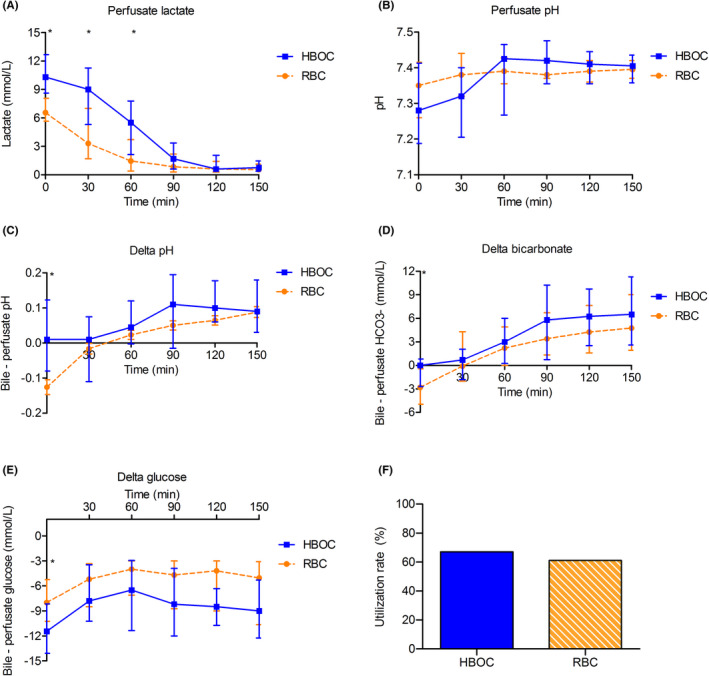
Machine perfusion characteristics. (A) Perfusate lactate levels were significantly lower at the start of NMP in the RBC perfused livers. After 90 min of NMP, the difference was no longer observed. (B‐E) Perfusate pH, delta pH, delta HCO3, and delta glucose were all comparable between both groups. Delta for pH, HCO3, and glucose was calculated between the arterial and bile value, at the same time point. (F) Utilization rate for both groups was similar (67% vs. 61% p = .693). * Indicates a statistically significant difference p < .05. ** p < .01, *** p < .001, **** p < .0001. The statistical tests were not powered due to small sample size, these results should be interpreted with caution. HBOC‐201, hemoglobin‐based oxygen carrier 201; RBC, red blood cells [Color figure can be viewed at wileyonlinelibrary.com]
3.5. Post‐operative results
When comparing the HBOC and RBC group, no differences were observed in post‐transplant outcomes (Table 4; Figure S2). The median follow‐up was 38 months (34–41) in the HBOC group and 17 months (13–25) in the RBC group. Post‐operative results are shown in Table 4. After DHOPE‐NMP, post‐reperfusion syndrome was observed in four (12%) patients. Actuarial 1 ‐year graft survival was 94%, 1‐year patient survival was 100%. In total, two grafts were lost in the first year. One patient in the HBOC group required retransplantation 14 days after the first transplant, because of a hepatic venous outflow tract obstruction. In the RBC group, one patient, who underwent transplantation for cirrhosis due to auto‐immune hepatitis, required retransplantation ten months after the primary transplant because of severe chronic rejection. No primary non‐function was observed after DHOPE‐NMP. One patient in the HBOC group developed NAS, but none developed NAS in the RBC group.
4. DISCUSSION
We present the outcomes of sequential ex situ end‐ischemic DHOPE and NMP for high‐risk donor livers, comparing two protocols with two different oxygen carriers in the perfusion fluid. Transplantation of these initially discarded livers after reconditioning and assessment using DHOPE‐NMP led to excellent results. We found no differences in donor liver utilization rate, post‐operative graft‐related complications or general post‐transplant outcomes between RBC‐ and HBOC‐perfused donor livers.
In 2019, the first clinical experiences of DHOPE‐NMP with the use of a HBOC‐based perfusion solution were presented with an actuarial one‐year graft survival of 100%. 9 However, the unavailability of HBOC outside experimental studies hindered wider acceptance of the combined DHOPE and NMP protocol in other centers. A direct comparison of HBOC‐based versus RBC‐based perfusion solutions for DHOPE‐NMP, as performed in the current study, was therefore necessary. The current study demonstrates that sequential end‐ischemic DHOPE and NMP of initially declined donor livers can be performed with similar good results when an RBC‐based perfusion solution is used instead of a HBOC‐based perfusion solution. These findings may facilitate wider application of this dynamic preservation protocol in clinical practice.
One difference between the two protocols, which is relevant for machine perfusion procedures, is the simplicity of the HBOC‐based procedure in comparison to DHOPE‐NMP with UW MPS and subsequently RBC‐based perfusion. The absence of a perfusate switch makes a perfusion with a HBOC‐based perfusion solution somewhat easier. However, we noted that the perfusion fluid switch can be performed in 15–30 minutes and does not have an impact on the clinical efficacy of the combined DHOPE‐NMP procedure. Since the disposable set of tubing and liver reservoir was not replaced, this modification did also not increase the costs. Another difference between the two protocols is the presence of an oxygen carrier during DHOPE. In the HBOC‐protocol, the HBOC was present, whereas in the RBC protocol there was no oxygen carrier was present in the UW MPS. However, at low temperatures, high amounts of oxygen can be dissolved in a solution, resulting in a high pO2, and therefore no oxygen carrier is required for hypothermic machine perfusion. 16 On the other hand, it did not cause harm either.
In a recently published large multicenter DCD benchmark study, 1‐ year graft survival was 87.3% after transplantation of low‐risk DCD livers and 75.9% after transplantation of high‐risk DCD livers. 33 In the current study using high‐risk livers, we observed an overall 1‐year graft survival of 94%, which supports the benefits of the DHOPE‐NMP protocol for this type of extended criteria donor livers.
Following the multicenter, randomized controlled trial on end‐ischemic oxygenated DHOPE for regular DCD livers, we now use oxygenated DHOPE as standard of care for regular DCD livers. 15 In contrast to DHOPE, end‐ischemic NMP may result in ischemia‐reperfusion injury of the biliary tree and thereby might not reduce the risk of NAS after transplantation. 12 , 17 , 18 , 19 In the current situation in the Netherlands, the largest group of donor livers that are frequently declined for transplantation are those from older (>60 years) DCD donors. The risk of early graft loss due to ischemia‐reperfusion injury related complications, such as primary non‐function or NAS, is generally considered too high in these livers. The application of sequential ex situ end‐ischemic DHOPE and NMP protects these livers against ischemia‐reperfusion injury (DHOPE phase) and enables viability assessment (NMP phase) prior to transplantation, resulting in excellent outcome after transplantation. Most importantly, this innovative dynamic preservation method enables a safe expansion of the number of donor livers for transplantation.
In another clinical study on end‐ischemic NMP for high‐risk donor livers, the VITTAL study, livers underwent NMP for hepatocellular viability testing without a preceding period of DHOPE. 10 The post‐transplant occurrence of NAS in DCD livers was 10 times higher in the VITTAL study, compared to the current results after sequential DHOPE‐NMP. These findings suggest that the short period of DHOPE prior to NMP, as well as biliary viability testing, are important aspects that may have contributed to the low occurrence of NAS in the current cohort. 35 It appears that only livers that have experienced too much ischemia of the bile ducts during the DCD procedure and subsequent SCS are no longer salvageable with DHOPE and are, therefore, did not meet the biliary viability criteria during NMP.
It is obvious that selection of donor livers during machine perfusion is rapidly developing and we are all still learning. As was well discussed in a recent editorial by Quintini et al., clinical studies on NMP for (initially discarded) high‐risk donor livers have been designed with patient safety in mind. 36 Consequently, very few failures have been reported. It is therefore not possible to perform statistical analyses for identification of (new) thresholds and boundaries for viability markers. 36 Only by slowly extending the viability criteria after sufficient confidence has been obtained in the NMP procedure, we and other centers have slowly started to gather more insight in this. With increasing experience and confidence in the protocol, we gradually started to accept livers with some more ‘orange’ and ‘red’ values (Tables 1 and 3), as long as the original selection criteria (lactate, arterial pH, bile production, bile pH) had been met at any point during the first 150 minutes of NMP. In the interest of scientific transparency, we show the data of all individual livers separately in Table 3, so the results of the learning curve of the protocol can be observed. In addition to our original four viability criteria, we tend to rely more on the delta pH, bicarbonate and glucose, with their optimal values as depicted in Table 1. 35
The low rates of NAS and post‐reperfusion syndrome in the current study are in line with the results of the recently published European multicenter, randomized controlled trial on DHOPE in regular DCD liver transplantation. 15 This trial demonstrated that a short period of end‐ischemic DHOPE prior to transplantation results in a 64% reduction of NAS, 57% reduction in post‐reperfusion syndrome, and 39% reduction in early allograft dysfunction after transplantation.
A limitation of the current study is the non‐randomized design between HBOC and RBC perfusions. However, donor and recipient characteristics in the two groups were similar at baseline and no changes were made in the clinical transplant protocols during the study period, making it unlikely that other factors have had an impact on the results.
In conclusion, the combination of end‐ischemic ex situ DHOPE and NMP resulted in safe transplantation of high‐risk donor livers with excellent results. This indicates that this protocol of ex situ machine perfusion provides an effective tool to increase of the number of suitable donor organs for transplantation. No differences were observed in liver utilization rate, graft survival, or post‐transplant complications between livers that underwent sequential DHOPE‐NMP with either a HBOC‐ or RBC‐based perfusion solution. The use of an RBC‐based perfusion solution may facilitate the wider application of the sequential DHOPE‐NMP protocol as a dynamic preservation strategy by other centers.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting information
Fig S1‐S2
Table S1‐S5
ACKNOWLEDGMENT
Funding was obtained by the Netherlands Ministry of Health, Welfare and Sport, and the Jan Kornelis de Cock Foundation, Groningen, the Netherlands.
van Leeuwen OB, Bodewes SB, Lantinga VA, et al. Sequential hypothermic and normothermic machine perfusion enables safe transplantation of high‐risk donor livers. Am J Transplant. 2022;22:1658–1670. doi: 10.1111/ajt.17022
Otto B. van Leeuwen and Silke B. Bodewes share first authorship.
Vincent E. de Meijer and Robert J. Porte share senior authorship.
Contributor Information
Vincent E. de Meijer, Email: v.e.de.meijer@umcg.nl.
Robert J. Porte, Email: r.j.porte@umcg.nl.
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Orman ES, Mayorga ME, Wheeler SB, et al. Declining liver graft quality threatens the future of liver transplantation in the United States. Liver Transpl. 2015;21:1040‐1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Callaghan CJ, Charman SC, Muiesan P, et al. Outcomes of transplantation of livers from donation after circulatory death donors in the UK: a cohort study. BMJ Open. 2013;3(9):e003287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Vries Y, von Meijenfeldt FA, Porte RJ. Post‐transplant cholangiopathy: Classification, pathogenesis, and preventive strategies. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1507‐1515. [DOI] [PubMed] [Google Scholar]
- 4. Buis CI, Verdonk RC, van der Jagt EJ, et al. Nonanastomotic biliary strictures after liver transplantation, part 1: radiological features and risk factors for early vs. late presentation. Liver Transpl. 2007;13:708‐718. [DOI] [PubMed] [Google Scholar]
- 5. den Dulk AC, Sebib Korkmaz K, de Rooij BJF, et al. High peak alanine aminotransferase determines extra risk for nonanastomotic biliary strictures after liver transplantation with donation after circulatory death. Transplant Int. 2015;28:492‐501. [DOI] [PubMed] [Google Scholar]
- 6. Dubbeld J, Hoekstra H, Farid W, et al. Similar liver transplantation survival with selected cardiac death donors and brain death donors. Br J Surg. 2010;97:744‐753. [DOI] [PubMed] [Google Scholar]
- 7. O'Neill S, Roebuck A, Khoo E, Wigmore SJ, Harrison EM. A meta‐analysis and meta‐regression of outcomes including biliary complications in donation after cardiac death liver transplantation. Transplant Int. 2014;27:1159‐1174. [DOI] [PubMed] [Google Scholar]
- 8. Schlegel A, Kron P, Dutkowski P. Hypothermic machine perfusion in liver transplantation. Curr Opin Organ Transplant. 2016;21:308‐314. [DOI] [PubMed] [Google Scholar]
- 9. van Leeuwen OB, de Vries Y, Fujiyoshi M, et al. Transplantation of high‐risk donor livers after ex situ resuscitation and assessment using combined hypo‐ and normothermic machine perfusion: a prospective clinical trial. Ann Surg. 2019;270:906‐914. [DOI] [PubMed] [Google Scholar]
- 10. Mergental H, Laing RW, Kirkham AJ, et al. Transplantation of discarded livers following viability testing with normothermic machine perfusion. Nat Commun. 2020;11:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guarrera JV, Henry SD, Samstein B, et al. Hypothermic machine preservation in human liver transplantation: the first clinical series. Am J Transplant. 2010;10:372‐381. [DOI] [PubMed] [Google Scholar]
- 12. Dutkowski P, Schlegel A, de Oliveira M, et al. HOPE for human liver grafts obtained from donors after cardiac death. J Hepatol. 2014;60:765‐772. [DOI] [PubMed] [Google Scholar]
- 13. van Rijn R, Karimian N, Matton APM, et al. Dual hypothermic oxygenated machine perfusion in liver transplants donated after circulatory death. Br J Surg. 2017;104:907‐917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Patrono D, Lavezzo B, Molinaro L, et al. Hypothermic oxygenated machine perfusion for liver transplantation: an initial experience. Exp Clin Transplant. 2018;16:172‐176. [DOI] [PubMed] [Google Scholar]
- 15. van Rijn R, Schurink IJ, de Vries Y, et al. Hypothermic machine perfusion in liver transplantation—a randomized trial. N Engl J Med. 2021;384:1391‐1401. [DOI] [PubMed] [Google Scholar]
- 16. Bodewes SB, van Leeuwen OB, Thorne AM, et al. Oxygen transport during ex situ machine perfusion of donor livers using red blood cells or artificial oxygen carriers. Int J Mol Sci. 2021;22:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mergental H, Perera MTPR, Laing RW, et al. Transplantation of declined liver allografts following normothermic ex‐situ evaluation. Am J Transplant. 2016;16:3235‐3245. [DOI] [PubMed] [Google Scholar]
- 18. Watson CJE, Kosmoliaptsis V, Randle LV, et al. Normothermic perfusion in the assessment and preservation of declined livers before transplantation: hyperoxia and vasoplegia‐important lessons from the first 12 cases. Transplantation. 2017;101:1084‐1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Watson CJE, Kosmoliaptsis V, Pley C, et al. Observations on the ex situ perfusion of livers for transplantation. Am J Transplant. 2018;18:2005‐2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van Leeuwen OB, van Reeven M, van der Helm D, et al. Donor hepatectomy time influences ischemia‐reperfusion injury of the biliary tree in donation after circulatory death liver transplantation. Surgery. 2020;168:160‐166. [DOI] [PubMed] [Google Scholar]
- 21. van Rijn R, van den Berg AP, Erdmann JI, et al. Study protocol for a multicenter randomized controlled trial to compare the efficacy of end‐ischemic dual hypothermic oxygenated machine perfusion with static cold storage in preventing non‐anastomotic biliary strictures after transplantation of liver grafts donated after circulatory death: DHOPE‐DCD trial. BMC Gastroenterol. 2019;19:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Vries Y, Matton APM, Nijsten MWN, et al. Pretransplant sequential hypo‐and normothermic machine perfusion of suboptimal livers donated after circulatory death using a hemoglobin‐based oxygen carrier perfusion solution. Am J Transplant. 2019;19:1202‐1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. de Vries Y, Berendsen TA, Fujiyoshi M, et al. Transplantation of high‐risk donor livers after resuscitation and viability assessment using a combined protocol of oxygenated hypothermic, rewarming and normothermic machine perfusion: study protocol for a prospective, single‐arm study (DHOPE‐COR‐NMP trial). BMJ Open. 2019;9(8):e028596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Matton APM, Burlage LC, van Rijn R, et al. Normothermic machine perfusion of donor livers without the need for human blood products. Liver Transpl. 2018;24:528‐538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Op den Dries S, Karimian S, Westerkamp AC, et al. Ex vivo normothermic machine perfusion and viability testing of discarded human donor livers. Am J Transplant. 2013;13:1327‐1335. [DOI] [PubMed] [Google Scholar]
- 26. Sutton ME, Op den Dries S, Karimian N, et al. Criteria for viability assessment of discarded human donor livers during ex vivo normothermic machine perfusion. PLoS One. 2014;9:e110642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Matton APM, de Vries Y, Burlage LC, et al. Biliary bicarbonate, pH, and glucose are suitable biomarkers of biliary viability during ex situ normothermic machine perfusion of human donor livers. Transplantation. 2019;103:1405‐1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brüggenwirth IMA, de Meijer VE, Porte RJ, Martins PN. Viability criteria assessment during liver machine perfusion. Nat Biotechnol. 2020;38:1260‐1262. [DOI] [PubMed] [Google Scholar]
- 29. Aggarwal S, Kang Y, Freeman JA, Fortunato FL Jr, Pinsky MR. Postreperfusion syndrome: hypotension after reperfusion of the transplanted liver. J Crit Care. 1993;8:154‐160. [DOI] [PubMed] [Google Scholar]
- 30. Laing RW, Bhogal RH, Wallace L, et al. The use of an acellular oxygen carrier in a human liver model of normothermic machine perfusion. Transplantation. 2017;101:2746‐2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Braat AE, Blok JJ, Putter H, et al. The Eurotransplant donor risk index in liver transplantation: ET‐DRI. Am J Transplant. 2012;12:2789‐2796. [DOI] [PubMed] [Google Scholar]
- 32. Feng S, Goodrich NP, Bragg‐Gresham JL, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783‐790. [DOI] [PubMed] [Google Scholar]
- 33. Kalisvaart M, Croome KP, Hernandez‐Alejandro R, et al. Donor warm ischemia time in DCD liver transplantation‐working group report from the ILTS DCD, liver preservation, and machine perfusion consensus conference. Transplantation. 2021;105:1156‐1164. [DOI] [PubMed] [Google Scholar]
- 34. Schlegel A, van Reeven M, Croome K, et al. A multicentre outcome analysis to define global benchmarks for donation after circulatory death liver transplantation. J Hepatol. 2021;S0168–8278(21):02110‐2113. [DOI] [PubMed] [Google Scholar]
- 35. van Leeuwen OB, de Vries Y, de Meijer VE, et al. Hypothermic machine perfusion before viability testing of previously discarded human livers. Nat Commun. 2021;12(1):1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Quintini C, Del Prete L, Diago Uso T, Liu Q. Will this liver work? The "ibis redibis" of machine preservation viability assessment. Liver Transpl. 2022; in press. doi: 10.1002/lt.26411 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1‐S2
Table S1‐S5
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


