Abstract
Objectives
Although psychiatric disorders have been found to be associated with increased risk of dementia, previous findings are mixed, and the nature of these relationships remains poorly understood. We examined longitudinal associations between depression, anxiety, post‐traumatic stress disorders (PTSD), bipolar disorder (BPD), psychotic disorders and subsequent dementia.
Methods
We searched three databases for longitudinal, population‐based studies investigating associations between psychiatric disorders and dementia (PROSPERO registration: CRD42020209638). We conducted narrative synthesis, and random‐effects meta‐analyses to obtain pooled estimates. We used meta‐regression and stratified analyses to examine variation by sex, age‐at‐onset and follow‐up time.
Results
Fifty‐seven citations met eligibility criteria. Most studies focussed on depression (n = 33), which was associated with subsequent all‐cause dementia (pooled relative risk [RR]: 1.96, 95% confidence interval [CI]: 1.59–2.43; I 2 = 96.5%), Alzheimer's Disease (pooled RR: 1.9, 95% CI: 1.52–2.38; I 2 = 85.5%), and Vascular Dementia (pooled RR: 2.71, 95% CI: 2.48–2.97; I 2 = 0). Associations were stronger in studies with shorter follow‐up periods and for severe and late‐onset depression. Findings regarding anxiety were mixed, and we did not find evidence of an overall association (pooled RR: 1.18, 95% CI: 0.96–1.45; I 2 = 52.2%, n = 5). Despite sparse evidence, psychotic disorders (pooled RR: 2.19, 95% CI: 1.44–3.31; I 2 = 99%), PTSD and BPD were associated with subsequent dementia.
Conclusions
People with psychiatric disorders represent high‐risk groups for dementia, highlighting the importance of ongoing symptom monitoring in these groups. Findings regarding temporality and age‐at‐onset indicate that depression symptoms could reflect prodromal dementia for some individuals. Further longitudinal research is required to determine whether psychiatric disorders represent causal risk factors or early markers of dementia neuropathology.
Keywords: Alzheimer's disease, anxiety, bipolar disorder, dementia, depression, geriatric and old age psychiatry, post‐traumatic stress disorder, psychotic disorders, schizophrenia
Key points
Psychiatric disorders are associated with increased risk of subsequent dementia.
Most studies focused on depression, while there was limited longitudinal evidence regarding anxiety, post‐traumatic stress disorder, bipolar disorder or psychotic disorders.
Severe and late‐onset depression showed stronger associations with dementia than earlier‐onset and mild‐to‐moderate depression.
Associations between depression and dementia were stronger in studies with shorter follow‐up periods, indicating that depression symptoms could be markers of prodromal dementia for some individuals.
1. INTRODUCTION
Psychiatric disorders, including depression, anxiety, post‐traumatic stress disorder (PTSD), and psychotic disorders, have been associated with increased dementia risk. 1 , 2 , 3 , 4 However, the nature of these associations remains under‐examined and unclear. Improved understanding of relationships between psychiatric disorders and dementia could have important implications for dementia prevention, which is a major priority for public health.
Although the Lancet Dementia Commission identified late‐life depression as one of 12 modifiable risk factors which could be targeted to prevent dementia, 5 other psychiatric disorders were not identified. This may reflect a scarcity of high‐quality longitudinal evidence, with most previous studies limited by small and unrepresentative samples, and cross‐sectional designs. In addition, previous findings have been mixed, particularly regarding anxiety 6 , 7 and PTSD, 8 , 9 which may reflect heterogeneity in study characteristics including sample, study design, follow‐up time, and exposure and outcome definition.
The causal direction of the association between psychiatric disorders and dementia remains unclear. Psychiatric symptoms may be risk factors for future dementia or alternatively represent early markers of dementia neuropathology. Most previous studies investigating associations between psychiatric disorders and dementia have only included relatively short follow‐up periods (i.e., <10 years). Dementia's long preclinical period of accumulating neuropathological damage over several decades before cognitive and functional decline are apparent, 10 , 11 means that symptoms detected near to dementia diagnosis may represent early dementia symptoms. Therefore, studies with longer follow‐up periods—ideally spanning several decades—are required to determine whether psychiatric disorders represent causal risk factors, or preclinical changes in dementia.
To clarify the nature of these relationships, we synthesised longitudinal evidence on associations between depression, anxiety, PTSD, psychotic disorders, bipolar disorder, and subsequent dementia. We examined whether associations varied by psychiatric disorder and dementia subtype, sex, age‐at‐onset, psychiatric disorder severity, and follow‐up time. We estimated population attributable fractions (PAFs) to indicate the proportion of new dementia cases that would theoretically be preventable through preventing psychiatric disorders in the population.
2. METHOD
We followed PRISMA guidelines (Table S1), 12 including pre‐registering our protocol (http://www.crd.york.ac.uk/PROSPERO, registration number: CRD42020209638).
2.1. Search strategy
We searched PubMed, PsycINFO and Web of Science for terms (listed in Table S2) relating to:
Psychiatric disorders: depression, anxiety, psychotic disorders, bipolar disorder or PTSD
Dementia: all‐cause dementia, Alzheimer's disease (AD) or vascular dementia (VaD)
Study design: cohort, case‐cohort or nested case‐control studies
We restricted our search to peer‐reviewed English language papers published in academic journals, with no restrictions on publication dates. We hand‐searched preprint servers (bioRxiv, medRiv and PsyArXiv) and bibliographies of included papers.
Our inclusion criteria were as follows:
Longitudinal population‐based cohort studies (including case‐cohort or nested case‐control studies). We excluded studies of clinical samples or high‐risk populations, as we judged these to be atypical, with controls unlikely to be representative of the general population.
Participants aged 18 years or older without dementia or mild cognitive impairment (MCI) at baseline.
Dementia diagnosed using validated diagnostic criteria.
Psychiatric disorders meeting diagnostic criteria assessed clinically or via validated screening tool, or attainment of a clinically significant threshold on a validated measure.
2.2. Screening and data extraction
Two researchers independently screened 50% of titles and abstracts, and an overlapping randomly selected 10% (inter‐rater agreement: 99.9%, Cohen's k = 0.96, p = <0.001). Both researchers independently screened all full texts. Discrepancies were resolved by consensus agreement. We extracted data on study characteristics, exposures, outcomes, covariate information, and measures of effect (hazard ratio [HR], incidence rate ratio (IRR), or odds ratio (OR) with 95% CI).
2.3. Study quality
One reviewer assessed risk of bias using the Newcastle‐Ottawa Scale. 13 A second reviewer assessed a randomly selected 10% of citations (inter‐rater agreement: 92.2%, Cohen's k = 0.65, p = 0.01) and we discussed and resolved discrepancies. We examined small study effects using funnel plots and Egger's test of bias. 14
2.4. Data analysis
We conducted narrative synthesis and meta‐analysis, where five or more comparable citations were available, to examine associations between psychiatric disorders and dementia. Where multiple studies reported analyses of the same cohort, we only included the analysis with a longer follow‐up duration in the meta‐analysis, to avoid duplication and reduce risk of reverse causation bias. We prioritised adjusted effect estimates where possible. We estimated pooled relative risks (RR) using random‐effects meta‐analysis in Stata version 16, which accounts for heterogeneity between studies and allows HRs and ORs to be incorporated into the same analysis. 15 , 16 Heterogeneity was assessed using the Q‐test (quantified using I‐squared). Where possible, we conducted a priori subgroup analyses using meta‐regression and stratified analyses to examine variation in associations by sex, follow‐up period, age‐at‐onset, psychiatric disorder severity, and study quality. We estimated PAFs for dementia in relation to each psychiatric disorder using the Levin formula 17 based on pooled RR estimates and prevalence estimates from the Adult Psychiatric Morbidity Survey (APMS). 18
3. RESULTS
3.1. Study characteristics
We identified 7849 citations, of which 57 met eligibility criteria (Figure 1). Studies were published between 1996 19 and 2021 20 (Table 1). Most citations focused on depression (n = 33), others involved anxiety (n = 6), psychotic disorders (n = 7), bipolar disorder (n = 4), PTSD (n = 3) or mixed conditions (n = 4).
FIGURE 1.
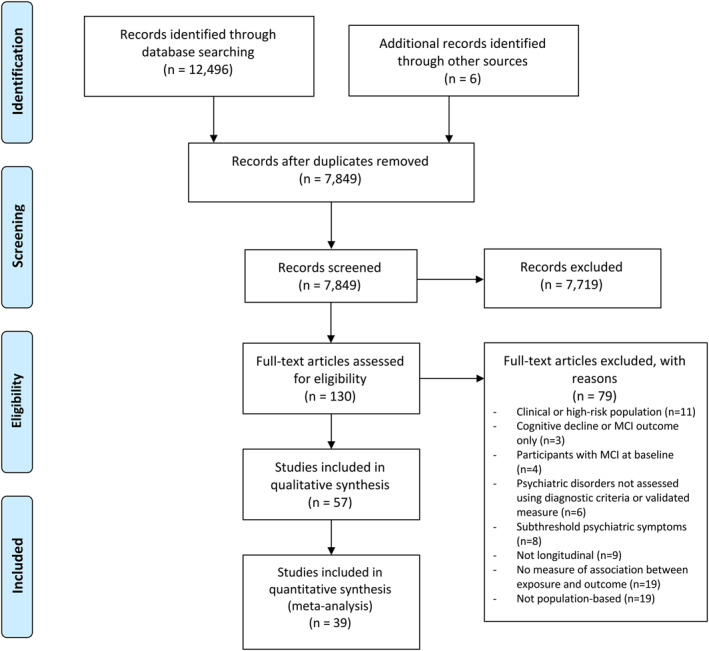
PRISMA flow diagram
TABLE 1.
Citation characteristics
| First author, year (n) | Sample, setting and study type | Baseline age (years [SD]) a | Total follow‐up (years) | Mean (SD) or median (IQR) follow‐up in years a | Psychiatric disorder or clinically significant symptoms b , c | Dementia diagnosis (N cases) c , d | Quality e | Summary of findings on association between psychiatric disorder and dementia f |
|---|---|---|---|---|---|---|---|---|
| Anxiety (n = 6) | ||||||||
| de Bruijn 2014 | Rotterdam Study, The Netherlands, prospective | Sample 1: 68.6 (8.5), sample 2: 75.5 (6.2) | 18 | Sample 1: 11.8 (5)Sample 2: 5.8 (1.9) | Sample 1: HADS ≥8 | Dementia (DSM‐III‐R, n = 358); AD (NINCDS‐ADRDA, n = 291) | Good | No evidence of association between anxiety disorder and dementia (HR: 0.81, 95% CI: 0.50–1.30) |
| Sample 1 n = 2708 | Sample 2: DSM‐IV | |||||||
| Sample 2 n = 3069 | ||||||||
| Gallacher 2009 n = 1160 | Caerphilly Prospective Study, Wales, prospective | 56.1 (4.4) | 20 | 17.3 (1.3) | STAI≥31 | Dementia (DSM‐IV, n = 69) | Good | Anxiety associated with increased odds of dementia (OR: 2.89, 95% CI: 1.27–6.54) |
| Mortamais 2018 n = 5234 | Three‐City (3C) Study, France, prospective | 73.4 (5.2) | 10 | / | STAI≥44 | Dementia (DSM‐IV, n = 378)AD (NINCDS‐ADRDA, n = 259), VaD (NINDS‐AIREN, n = 23) | Good | High trait anxiety associated with a higher rate of dementia (HR: 1.26, 95% CI: 1.01–1.57) |
| Petkus 2016 n = 1082 | Swedish adoption Twin Study of Ageing, prospective | 60.86 (11.15) | 28 | Years to dementia: 14.65 (6.7) | 1 + standard deviation above mean STPI | Dementia (DSM‐III‐R/IV, n = 172) | Good | Anxiety associated with increased risk of dementia after adjustment for confounders, including depression (HR: 1.48; 95% CI: 1.01–2.18) |
| Santabárbara 2019 n = 4057 | Zaragoza Dementia and Depression study (ZARADEMP), Spain, prospective | No AD: 72.83 (9.03) | 4.5 | Median: 4.4 (IQR: 3.0–4.9) | GMS AGECAT ≥3 | AD (DSM‐IV, n = 87) | Poor | AD associated with previous anxiety (SHR: 3.9, 95% CI: 1.59–9.6), but not anxiety ‘subcases’ (SHR: 1.19, 95% CI: 0.75–1.88). |
| AD: 83.72 (7.13) | ||||||||
| Santabárbara 2020 n = 4057 | ZARADEMP, Spain, prospective | VaD (men: 80.3 (8.2), women: 79.8 (7.9)); No VaD (men: 71.7 (9.1), women: 72.3 (9.1)) | 4.5 | Median: 4.4 (IQR: 3.0–4.9) | GMS AGECAT ≥3 | VaD (DSM‐IV, n = 14) | Poor | Anxiety associated with VaD in men (IRR: 3.24, 95% CI: 1.13–9.35), but not women (IRR: 0.68, 95% CI: 0.19–2.23) |
| Post‐traumatic stress disorder (n = 3) | ||||||||
| Flatt 2018 n = 499,844 | Kaiser permanente Northern California health system, United States, register‐based | 71.1 (7.9) | 13 | 8 (4.6) | ICD‐9: 309.81 | Dementia (ICD‐9, n = 59,127) | Good | PTSD associated with higher rate of dementia (female HR: 1.59, 95% CI: 1.3–1.95; male HR: 1.96, 95% CI: 1.51–2.55) |
| Gradus 2019 n = 279,188 | Danish population register | 51 | 17 | Stress cohort median: 6.1; Comparison: 6.8 | Stress disorders: ICD‐10: F43.x | Dementia (ICD‐10, stress cohort n = 1364) | Good | Those with PTSD had 2 times the rate of dementia compared to those without PTSD (95% CI: 1.3–3.2) |
| Wang 2016 n = 8750 | Nationwide Health Insurance Research Database (NHIRD), Taiwan, register‐based | PTSD: 55.44 (9.20)No PTSD: 55.42 (9.22) | 11 | Years to dementia: PTSD: 4.68 (2.31); No PTSD: 6.96 (2.66) | ICD‐9‐CM: 309.81 | Dementia (ICD‐9‐CM, n = 135) | Good | PTSD (HR: 4.37, 95% CI: 2.53–7.55) and depression (HR: 2.16, 95% CI: 1.28–3.66) associated with subsequent dementia, with a dose‐response relationship by PTSD severity |
| Non‐affective psychotic disorder (n = 7) | ||||||||
| Almeida 2018 n = 38,173 | Health in Men Study (HIMS), Western Australia, prospective | 72.5 (4.6) | 17.7 | / | Non‐affective psychotic disorders: ICD‐8/9: 295, 297; ICD‐10: F20, F22, F23, F25, F28, F29 | Dementia (ICD‐8/9/10, n = 8068) | Good | Psychotic disorders associated with subsequent dementia (SHR: 2.67, 95% CI: 2.3–3.09). Stronger association for shorter duration of psychosis. No variation by age‐at‐onset |
| Kodesh 2020 n = 94,120 | Health maintenance organisation data, Israel, register‐based | 68.9 (7.1) | 4.83 | / | VLOSLP: ICD‐9: 295–299; ICD‐10: F20–F29 | Dementia (ICD‐9/10, n = 6026) | Good | Very late‐onset schizophrenia associated with subsequent dementia (HR: 2.67, 95% CI: 1.82–3.91). |
| Kørner 2009 n = 12,616 | Danish population register | Median LOS: 53.56, comparison: 65.13, VLOSLP and comparison: 71.19 | 4.58 | Median (IQR) LOS: 3.15 (1.56–3.50)VLOSLP: 3 (1.25–4.78) | LOS and VLOSLP: ICD‐10: F20‐F20.9 | Dementia (ICD‐10, LOS n = 20, comparison n = 160; VLOSLP n = 18, comparison n = 157). | Good | LOS and VLOSLP had higher dementia rates than osteoarthritis patients (LOS rate ratio (RR): 3.47, 95% CI: 2.19–5.5; VLOSLP RR: 3.15, 95% CI: 1.93–5.14) and general population (LOS RR: 2.36, 95% CI: 1.54–3.62; VLOSLP RR: 2.21, 95% CI: 1.39–3.5). |
| Lin 2018 n = 30,200 | NHIRD, Taiwan, register‐based | / | 14 | Schizophrenia: 9.4 (11.8), control: 9.5 (10.1) | Schizophrenia: ICD‐9‐CM: 295 | Dementia (ICD‐9‐CM, n = 1237), AD (ICD‐9‐CM, n = 147), VaD (ICD‐9‐CM, n = 162) | Good | Patients with schizophrenia had higher rates of dementia (HR: 2.01, 95% CI: 1.42–2.59), AD (HR: 2.27, 95% CI: 1.99–3.51), and VaD (HR: 2.01, 95% CI: 1.36–2.22) than controls |
| Ribe 2015 n = 2,845,440 | Danish population register | 58.7 (11.2) | 18 | 11 (6) | Schizophrenia: ICD‐8/9: 295 (except 295.79); ICD‐10: F20; Schizoaffective disorder: ICD‐8/9: 295.79, 296.8; ICD‐10: F25 | Dementia (ICD‐8/9/10, n = 136,012) | Good | Schizophrenia associated with incident dementia (IRR: 1.71, 95% CI: 1.6–1.82) |
| Stafford 2021 n = 169,499 | Swedish population register | 70.31 (7.2) | 30 | 8.6 (6.68) | VLOSLP: ICD‐10: F20‐F29 (or ICD‐8/9 equivalent) | Dementia (ICD‐8/9/10, n = 13,610) | Good | VLOSLP associated with higher rate of dementia (HR: 4.22, 95% CI: 4.05–4.41). Association attenuated over time but was present for up to 20 years |
| Stroup 2021 n = 8,011,773 | Medicare beneficiaries, US, register‐based | 74 (8.2) | 11 | / | Schizophrenia: ICD‐8/9/10: F25 | Dementia (ICD‐9/10, n = 1,129,646) | Good | Schizophrenia associated with elevated rate of dementia (age 66 dementia rate – schizophrenia: 52.5 (95% CI: 50.1–54.9), controls: 4.5 (95% CI: 4.4–4.6) per 1000 person‐years at‐risk |
| Bipolar disorder (BPD) (n = 4) | ||||||||
| Almeida 2018 n = 38,173 | Western Australian Data Linkage System, register‐based | 72.5 (4.6) | 17.6 | 12.8 (5.3) | ICD‐8: 296.1, 296.3; ICD‐9: 296.0, 296.1, 296.4, 296.5, 296.6, 296.7, 296.80, 296.81; ICD‐10: F30, F31 | Dementia (ICD‐8/9/10, n = 423) | Good | Late‐onset (≥60 years HR: 2.99, 95% CI: 2.17–4.12) and younger onset BPD associated with dementia (<60 years HR: 2.31, 95% CI: 1.77–3.01) |
| Almeida 2016 n = 37,768 | HIMS, Western Australia, prospective | 72.5 (4.6) | 13 | / | ICD‐8: 296.1 and 296.3; ICD‐9: 296.0, 296.1, 296.4, 296.5, 296.6, 296.7, 296.80, 296.81 | Dementia (ICD‐8/9, n = 4925) | Good | BPD associated with higher dementia rates (HR: 2.3, 95% CI: 1.80–2.94). Stronger association for shorter duration of BPD, or illness onset after 70 years old |
| Lin 2020 n = 102,675 | NHIRD, Taiwan, register‐based | BPD: 55.31 (8.48), comparison: 55.25 (8.55) | 9 | BPD: 6.56 (2.97); comparison: 6.86 (2.85) | ICD‐9‐CM: 296 except 296.2x, 296.3x, 296.9x, and 296.82 | Dementia (ICD‐9‐CM, n = 2122), AD (ICD‐9‐CM, n = 1353), VaD (ICD‐9‐CM, n = 447) | Good | BPD associated with incident dementia (HR: 7.52, 95% CI: 6.86–8.25), AD (HR: 13.16, 95% CI: 11.58–14.96) and VaD (HR: 5.5, 95% CI: 4.53–6.69) |
| Wu 2013 n = 64,804 | NHIRD, Taiwan, register‐based | 74.1 (8.6) | 9 | / | ICD‐9‐ CM: 296.0x, 296.1x, 296.4x, 296.5x, 296.6x, 296.7x, 296.80, 296.81, 296.89 | Dementia (ICD‐9‐CM, n = 9304) | Good | BPD associated with increased odds of dementia (OR: 4.32, 95% CI: 3.21–5.82) |
| Multiple psychiatric diagnoses (n = 4) | ||||||||
| Chen 2015 n = 4582 | NHIRD, Taiwan, register‐based | Control: 65.34 (7.47); major depressive disorder: 65.45 (7.53); BPD: 64.72 (7.08) | 13 | Years to dementia: controls: 5.62 (3.23), MDD: 4.01 (3.02); BPD: 4.38 (3.60) | MDD: ICD‐9‐CM: 296.2x, 296.3x; BPD: ICD‐9‐CM: 296.0x, 296.1x, 296.4x, 296.5x, 296.6x, 296.7x, 296.80, 296.81, 296.89 | Dementia (ICD‐9‐CM, n = 547) | Good | BPD (HR: 5.58, 95% CI: 4.26–7.32) and MDD (HR: 3.02, 95% CI: 2.46–3.7) associated with higher rates of dementia |
| Kessing 1999 n = 13,852 | Danish population register | Neurosis: 41.2 (13.8), depression: 51.3 (16.0), schizophrenia: 31.7 (15.4) | 24.7 | Median: 21.6 | Depressive episode: ICD‐8: 296.09, 296.29, Schizophrenia: ICD‐8: 295, Neurosis: ICD‐8: 300 | Dementia (ICD‐8/9/10, n = 424) | Poor | Rates of dementia were increased 14.7‐fold (95% CI: 9.1–22.4) in those with schizophrenia, 13.7‐fold (95% CI: 12.1–15.4) for affective disorder, and 11.2‐fold (95% CI: 9.6–12.9) for neurosis |
| Tapiainen 2017 n = 55,896 | Medication use and Alzheimer's disease study, Finland, register‐based nested case‐control | 79.7 (6.8) | 33 | Time to AD: AD cases: 18.1 years (8.9), controls: 19.4 (9.0) | Psychotic disorders: ICD‐10: F20–F29; mood disorders: ICD‐10: F32–F39; neurotic disorders: ICD‐10: F40–F48 | AD (NINCDS‐ADRDA and DSM‐IV, n = 27,948) | Good | Depression associated with increased odds of AD with 5‐year (OR: 1.17, 95% CI: 1.05–1.3), but not 10‐year interval between diagnoses (OR: 1.08, 95% CI: 0.96–1.23). Anxiety and psychotic disorders were not associated with AD regardless of interval |
| Zotcheva 2018 n = 28,916 | The Nord‐Trøndelag Health Study, Norway, prospective | Midlife moderate‐to‐vigorous physical activity (MVPA): 52.3,No MVPA: 54.6 | 16.3 | 15.2 | Psychological distress: ADI‐4 ≥88th percentile | Dementia (ICD‐10, n = 920) | Good | Psychological distress associated with higher dementia rates (HR: 1.34, 95% CI: 1.03–1.74) |
| Depression (n = 33) | ||||||||
| Almeida 2017 n = 4922 | HIMS, Western Australia, prospective | 77.2 (3.7) | 14.3 | 8.9 | Short version GDS‐15 score ≥7 | Dementia (ICD‐8/9/10, n = 903) | Good | Depression associated with dementia (past depression SHR: 1.3, 95% CI: 1.0–1.6, baseline SHR: 1.5, 95% CI: 1.2–2.0) |
| Becker 2009 n = 288 | Cardiovascular Health Study‐Cognition Study, US, prospective | 77.52 (3.65) | 9 | 7.1 | CES‐D score ≥10 | Dementia (DSM‐IV, n = 48) | Poor | No evidence of association between persistent depression and dementia (HR: 1.33, 95% CI: 0.49–3.65) |
| Berger 1999 n = 222 | The Kungsholmen Project, Sweden, prospective | Incident AD: 85.53 (4.95); no dementia: 83.18 (4.77) | 3 | 3.08 (0.58) | CPRS | AD (DSM‐III‐R, n = 34) | Poor | Depression associated with subsequent AD, particularly for motivation‐related symptoms (OR: 1.4, 95% CI: 1.04–1.89) |
| Buntinx 1996 n = 19,103 | Family‐practice‐based registration network, The Netherlands, register‐based | 50+ | 10 | / | ICPC‐classification: P76 | Dementia (ICPC: n = 137) | Poor | Depression among older people associated with increased risk of dementia (HR: 2.55, 95% CI: 1.19–5.47) |
| Chan 2020 n = 16,725 | NHIRD, Taiwan, register‐based | 41.5 (15.9) | 12 | Time to dementia: control: 10.71 (1.16); MDD: 10.45 (1.86) | ICD‐9‐CM: 296.2x and 296.3x | Dementia (ICD‐9‐CM, n = 508), AD (ICD‐9‐CM, n = 458) | Good | Patients with MDD had increased risk of dementia and AD. Risk highest among difficult‐to‐treat patients |
| Chen 2008UK n = 3,341,China n = 1254 | MRC–Ageing in Liverpool Project Health Aspects (MRC‐ALPHA), UK; Hefei cohort, China | 65+ | MRC‐alpha: 2‐4Hefei‐ 1 | / | GMS‐AGECAT score ≥3 | Dementia (GMS‐AGECAT: Hefei n = 75, MRC‐ALPHA, n = 382 | Poor | Incident dementia associated with severe, but not milder, depression (Hefei cohort HR: 5.44, 95% CI: 1.67–17.8; MRC‐ALPHA, 4‐year follow‐up HR: 2.62, 95% CI: 1.18–5.8). |
| Deckers 2018 n = 278 | The Cambridge City over‐75s cohort (CC75 C), UK, prospective | 87.8 (3.1) | 18 | / | CAMDEX Depressive Symptoms Scale score ≥6 | Dementia (CAMDEX, post‐mortem, cause of death, n = 76) | Good | Depression not associated with incident dementia (OR: 0.78, 95% CI: 0.34–1.78). |
| Ezzati 2019 n = 1219 | The Einstein Ageing Study, US, prospective | 78.3 (5.3) | 17.2 | 4.5 (3.5) | GDS score ≥6 | Dementia (DSM‐IV, n = 132) | Good | Depression associated with dementia in longer‐term (>3 years HR: 1.13, 95% CI: 1.01–1.26), but not short‐term follow‐up (<3 years HR: 1.09, 95% CI: 0.99–1.2). |
| Gatz 2005 n = 766 | Manitoba Study of Health and Ageing, Canada, prospective | 74.5 (6) | 5 | / | CES‐D score ≥16 | Dementia (DSM‐III‐R, n = 56), AD (NINCDS‐ADRDA, n = 36) | Poor | Depression associated with dementia (CES‐D ≥16 HR: 2.37, 95% CI: 1.02–5.54) and AD (OR: 2.75, 95% CI: 1.04–7.24). |
| Geerlings 2000 n = 3147 | Amsterdam Longitudinal Study of the Elderly, The Netherlands, prospective | Depression: 73.6 (5.7), no depression: 73.7 (5.7) | 4 | 3.2 | GMS‐AGECAT score ≥3 | AD (DSM‐III‐R, n = 53) | Good | Depression associated with increased risk of AD (OR: 1.67, 95% CI: 0.76–3.63). |
| Gracia‐Garcia 2015 n = 3864 | ZARADEMP, Spain, prospective | Depression: 73.6 (9.3),No depression: 71.5 (8.9) | 4.5 | / | GMS‐AGECAT score ≥3 | AD (DSM‐IV, n = 70) | Poor | Severe depression associated with subsequent AD (SHR: 4.3, 95% CI: 1.39–13.33). |
| Heser 2013 n = 2663 | German Study on Ageing, Cognition, and Dementia in Primary Care Patients, prospective | 81.2 | 8 | / | CIDI | Dementia (DSM‐IV and ICD‐10, n = 308), AD (DSM‐IV, n = 152) | Good | Very late‐onset depression and current depressive symptoms both predicted all‐cause dementia |
| Heser 2020 n = 97,110 | German health insurance provider, Allgemeine Ortskrankenkasse | 74.7 (6.6) | 9 | 5.82 | ICD‐10: F32 | Dementia (ICD‐10, n = 20,779) | Good | Depression associated with dementia (IRR: 1.58, 95% CI: 1.51–1.64). Stronger association found for shortest interval, men, and younger participants |
| Holmquist 2020 n = 3,341,010 | Swedish population register | Matched cohort: 63.79 (11.89); Sibling cohort‐ depression: 59.10 (8.85), no depression: 59.97 (8.91) | 35 | 10.41 (6.89) | ICD‐10: F32, F33ICD‐8/9: 311, 296B | Dementia (ICD‐8/9/10, n = 9802), AD (ICD‐8/9/10, n = 4201), VaD (ICD‐8/9/10, n = 2329) | Good | Depression associated with dementia (OR: 2.47, 95% CI: 2.35–2.58), VaD (OR: 2.68, 95% CI: 2.44–2.95), and AD (OR: 1.79, 95% CI: 1.68–1.92). Association strongest in first 6 months after depression diagnosis but persisted for more than 20 years |
| Irie 2008 n = 1932 | Honolulu‐Asia Ageing Study, Japan, prospective | 76.3 (3.6) | 6 | / | CES‐D score ≥9 | Dementia (DSM‐III‐R, n = 98) | Good | Depression associated with higher rate of dementia (HR: 2.2, 95% CI: 1.3–3.7) and AD (HR: 2.9, 95% CI: 1.4–5.9), but not VaD (HR: 1.3, 95% CI: 0.3–5.8) |
| Karlsson 2015 n = 2404 | The Swedish Twin Registry | Dementia: 80.1 (6.6), no dementia: 78.9 (6.6) | >10 years | / | ICD‐7: 314.99; ICD‐8: 296.00, 298.00, 300.40–41, 790.20; ICD‐9: 296 C/D/W, 298A, 300E, 309 A/B, 311X; ICD‐10: F32, F33, F34.1, F41.2; Self‐report or anti‐depressant use; CES‐D score ≥20 | Dementia (DSM‐III‐R/IV, n = 804), AD (NINCDS‐ADRDA, n = 469) | Good | Depression associated with increased risk of dementia (HR: 3.41, 95% CI: 2.72–4.27). Strongest association in the 10 years before dementia diagnosis and for late‐onset depression |
| Köhler 2015 n = 35,791 | The Dutch Registration of Family Practices, Limburg, The Netherlands, register‐based | 65+ | 13 | / | ICPC code: P76 | Dementia (ICPC, n = 1680) | Good | Depression associated with increased risk of dementia (HR: 2.03, 95% CI: 1.56–2.64) |
| Köhler 2011 n = 771 | The Maastricht Ageing Study, The Netherlands, prospective | 67.1 (7.3) | 9 | / | Scores in upper quartile of SCL‐90 (revised version). | Dementia (DSM‐III‐R/IV, n = 37), AD (NINCDS‐ADRDA, n = 26), VaD (NINDS‐AIREN, n = 11) | Fair | Depression associated with all‐cause dementia (OR: 2.06, 95% CI: 1.01–4.22), but not AD (OR: 1.81, 95% CI: 0.78–4.23) or VaD (OR: 3.03, 95% CI: 0.86–10.64) |
| Kontari 2019 n = 4589 | English Longitudinal Study of Ageing, prospective | 50+ | 10 | / | CES‐D score ≥4 | Dementia (participant or informant reported physician diagnosed dementia, and/or IQCODE score ≥3.5, n = 216) | Poor | Depression associated with higher rate of dementia during follow‐up (HR: 1.82, 95% CI: 1.13–2.95), with attenuation after adjusting for baseline cognitive function (HR: 1.28, 95% CI: 0.78–2.08) |
| Lenoir 2011 n = 7989 | The 3C Study, France, prospective | 74 (5.4) | 4 | / | Self‐reported lifetime treated depression. MDD (DSM‐IV). CES‐D baseline score ≥22 (women), ≥16 (men) | Dementia (DSM‐IV, n = 276), AD (NINCDS‐ADRDA, n = 180), VaD (NINDS‐AIREN, n = 24) | Poor | Dementia associated with baseline depressive symptoms (HR: 1.5, 95% CI: 1.2–2.2), but not self‐reported treated depression or diagnosed MDD |
| Li 2011 n = 3410 | Adult Changes in Thought Study, US, prospective | Significant depressive symptoms: 75.8 (6.2),No depression: 74.8 (6.2) | 15 | 7.1 | CES‐D score ≥16; self‐reported history of depression | Dementia (DSM‐IV, n = 658) | Good | Baseline depression associated with dementia (HR: 1.71, 95% CI: 1.37–2.13), for late‐life (age‐at‐onset ≥50 years) (HR: 1.46, 95% CI: 1.16–1.84), but not early‐life depression (age‐at‐onset <50 years) HR: 1.1, 95% CI: 0.83–1.47) |
| Lin 2017 n = 49,955 | NHIRD, Taiwan, register‐based | Median: 39, IQR: 29–51 | 10 | Depression median: 7.19 (IQR: 5.95–8.48), control: 7.22 (IQR: 6.01–8.51) | ICD‐9‐CM: 296.2x–296.3x, 300.4, 311.x | VaD (ICD‐9‐CM, n = 117) | Good | Depression associated with higher rate of VaD (HR: 3.1, 95% CI: 2.13–4.52) |
| Luppa 2013 n = 1265 | Leipzig Longitudinal Study of the Aged, Germany, prospective | 81.5 | 8 | 4.3 (2.4) | CES‐D score ≥23; MDD diagnosis in DSM‐III‐R | Dementia (DSM‐III‐R/‐IV and ICD‐10, n = 183) | Good | Major depression diagnosis associated with higher rate of dementia (HR: 2.75, 95% CI: 1.01–7.5) |
| Mirza 2014 n = 4393 | Rotterdam Study, The Netherlands, prospective | 72.7 (7.3) | 13.7 | 8.7 (3.5) | CES‐D score ≥16 | Dementia (DSM‐III‐R, n = 582), AD (NINCDS‐ADRDA, n = 489) | Good | Depression associated with dementia in short (HR: 1.08, 95%CI: 1.00–1.17) (0–5 years HR: 1.13, 95%CI: 1.01–1.27) and intermediate (5–10 years HR: 1.14, 95%CI: 1.01–1.29), but not long‐term follow‐up (>10 years HR: 0.83, 95%CI: 0.66–1.04) |
| Richard 2013 n = 1483 | Washington Heights–Inwood Columbia Ageing Project, US, prospective | Depression: 77.7, (7.2), No depression: 76.7 (7.0) | 10.1 | 5.4 | CES‐D score ≥4 | Dementia (DSM‐III‐R, n = 207), AD (NINCDS‐ADRDA, n = 164), VaD (NINDS‐AIREN, n = 33) | Good | Baseline depression associated with higher rate of dementia (HR: 1.8, 95%CI: 1.2–2.7), and AD (HR: 1.9, 95%CI: 1.2–2.9), but not VaD (HR: 1.7, 95% CI: 0.5–5.6) |
| Rolandi 2020 n = 1100 | Brain ageing in Abbiategrasso (InveCe.Ab), Italy, prospective | 70–74 | 8 | 7 | DSM‐IV‐TR MDD or dysthymiaOR 3+: (i) depression history, (ii) depression treatment, (iii) GDS score ≥8, (iv) depressed mood in last week. | Dementia (DSM‐IV‐TR, n = 111) | Poor | Depression not associated with subsequent dementia (SHR: 0.99, 95% CI: 0.4–2.45) |
| Saczynski 2010 n = 949 | The Framingham Heart Study, US prospective | 79 (5) | 17 | 8 | CES‐D score ≥16 | Dementia (DSM‐IV, n = 164)AD (DSM‐IV, n = 136) | Good | Depression associated with increased risk of dementia (HR: 1.72, 95% CI: 1.04–2.84) and AD (HR: 1.76, 95% CI: 1.03–3.01) |
| Singh‐Manoux 2017 n = 10,308 | Whitehall II, UK, prospective | 1985‐ Depression: 44.5 (6), no depression: 45.1 (6); 2003‐ Depression: 60 (6), no depression: 61.5 (6) | 28 | 1985: 26.6 (4.5)1991: 21.7 (3.6)1997: 16.3 (2.7),2003: 11.1 (1.8) | General Health Questionnaire (GHQ‐30) ≥5CES‐D score ≥16 | Dementia (ICD‐10, n = 322) | Good | Dementia associated with depression in late (mean follow‐up 11 years) (HR: 1.67, 95% CI: 1.11–2.49, but not early study phase (mean follow‐up 22 years) (HR: 1.02, 95% CI: 0.72–1.44) |
| Spira 2012 n = 302 | Study of Osteoporotic Fractures, US, prospective | 86.9 (2.1) | 5 | / | GDS score ≥6 | Dementia (DSM‐IV‐R, n = 84) | Poor | Depression associated with increased risk of dementia (OR: 3.15, 95% CI: 1.03–9.65) |
| Tao 2019 n = 8880 | NHIRD, Taiwan, register‐based | 71.55 (5.47) | 7 | 6.94 (0.5) | ICD‐9‐CM: 296.2x at least 2x in 6 months and prescribed antidepressant medication for 90 days and 6 months + after initial diagnosis. | AD (ICD‐9‐CM, n = 155) | Good | Depression associated with increased risk of AD (HR: 2.21, 95% CI: 1.57–3.31) |
| Vilalta 2013 n = 451 | Estudio de Verona, Spain, prospective | 76.9 (5.5) | 5 | / | DSM‐IV diagnosis of major depression | Dementia (DSM‐IV, n = 52), AD (DSM‐IV, n = 30) | Poor | Late‐onset depression associated with increased risk of dementia (HR: 2.64, 95% CI: 1.15–6.0) |
| Wallin 2013 n = 212 | The Umeå 85+/GERDA (GErontologisk Regional DAtabas), Sweden, prospective | Dementia: 88.54 (3.7), no dementia 88.92 (4.35) | 7 | Dementia: 3.82 (1.22) no dementia: 3.24 (1.71) | DSM‐IV diagnosis of depression | Dementia (DSM‐IV, n = 71) | Good | Baseline (OR: 2.91, 95% CI: 1.37–6.16) and follow‐up depression (OR: 1.61, 95% CI: 1.26–2.05) associated with dementia |
| Wu 2020 n = 16,210 | Survey of Health, Ageing and Retirement in Europe (SHARE), 14 countries in Europe | 70.13 | 10 | 7.98 (2.61) | Europe‐depression (EURO‐D) scale score ≥4 | Dementia (participant‐or proxy‐reported physician diagnosis, n = 1030) | Good | Late‐life depression associated with dementia (SHR: 1.52, 95% CI: 1.32–1.75), although only in those below age 80 years. Dose‐response relationship found between depression severity and dementia risk (p for trend <0.001) |
Standard deviation (SD), interquartile range (IQR).
Anxiety and Depression Index (ADI‐4), Center for Epidemiologic Studies‐Depression (CES‐D), Composite International Diagnostic Interview (CIDI), Comprehensive Psychopathological Rating Scale (CPRS), Geriatric Depression Scale (GDS‐15), Hospital Anxiety and Depression Scale (HADS), Spielberger State Trait Anxiety Inventory (STAI), State‐Trait Personality Inventory (STPI), Symptom Checklist‐90‐Revised (SCL‐90).
International Classification of Health Problems in Primary Care (ICPC), The Geriatric Mental State ‐ Automated Geriatric Examination for Computer Assisted Taxonomy (GMS‐AGECAT), Cambridge Diagnostic Examination for the Elderly (CAMDEX).
Alzheimer's disease (AD), Vascular dementia (VaD).
Newcastle‐Ottawa Scale for cohort studies.
Hazard ratio (HR), incidence rate ratio (IRR), odds ratio (OR), subdistribution hazard ratio (SHR).
Sample sizes ranged from 212 21 to 8,011,773 22 (median: 5,234, interquartile range [IQR]: 1483–37,768). 21 studies were register‐based, while 36 involved prospective cohorts. Study quality was assessed as good (n = 42, 74%), fair (n = 1, 1%), or poor (n = 14, 25%). Follow‐up periods ranged from 1 23 to 35 years 24 (median: 12 years, IQR: 8–17.7), and 29 of 57 studies (50.9%) had maximum follow‐up periods longer than 10 years.
3.2. Depression
Thirty‐nine citations reported data on associations between depression and subsequent dementia (Table 1), with studies conducted in Australia, Asia, Europe, and North America. Follow‐up periods ranged from 1 23 to 35 years, 24 and sample sizes were between 212 21 and 3,341,010 individuals. 24 Quality was assessed as good (n = 27), fair (n = 1) or poor (n = 11). Depression was indexed via ICD or DSM criteria, International Classification of Health Problems in Primary Care, GMS‐AGECAT, validated scale or screening tool, or a combined approach (Table 1).
Depression was associated with increased risk of all‐cause dementia in 30 of 33 studies examining this relationship, while only 3 prospective cohort studies conducted in the United Kingdom, Italy and the United States, found no association. 25 , 26 , 27 Quantitative synthesis of 27 comparable estimates from 26 citations demonstrated an association between depression and incident all‐cause dementia (pooled RR: 1.96, 95% CI: 1.59–2.43; I 2 = 96.5%, Figure 2). We did not find evidence of small study effects from visual inspection of a funnel plot (Figure S1) or Egger's test of bias (p = 0.45).
FIGURE 2.
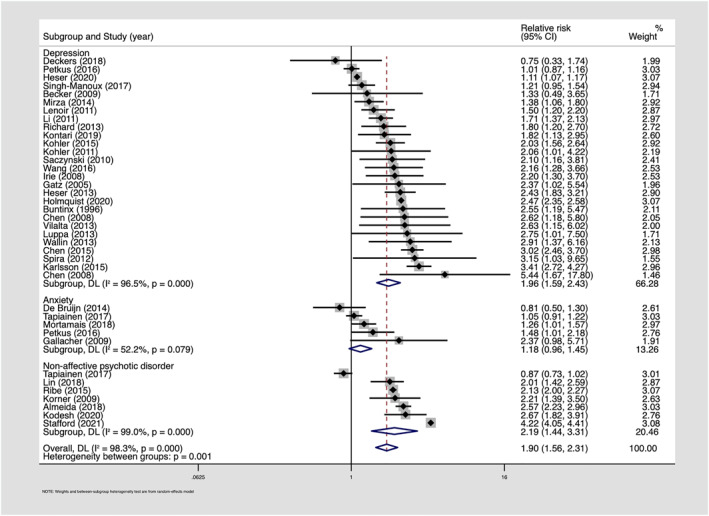
Forest plot—longitudinal associations between depression, anxiety, non‐affective psychotic disorders and subsequent dementia
Similarly, 15 of 17 studies examining depression and subsequent AD reported associations with only two prospective cohort studies finding no association. 28 , 29 Based on 13 comparable estimates, we found evidence of a longitudinal association between depression and AD (pooled RR: 1.90, 95% CI: 1.52–2.38; I 2 = 85.5%, Figure S2). Findings regarding depression and VaD were mixed, with register‐based studies in Taiwan 30 and Sweden, 24 and a prospective cohort study in France 29 finding associations, whereas prospective cohort studies in the United States, 31 The Netherlands, 28 and Japan 32 did not observe associations. In a meta‐analysis of six estimates, we found an overall association between depression and VaD (pooled RR: 2.71, 95% CI: 2.48–2.97; I 2 = 0, Figure S3). As above, we did not find evidence of small study effects (Egger's test: AD p = 0.6, VaD p = 0.5).
3.3. Subgroup analyses
Using random‐effects meta‐regressions, we found no evidence that associations between depression and all‐cause dementia varied by sex, study quality, study type or age‐at‐onset (Table 2, Figures S4–S6), although in stratified analyses we found an association between dementia and late‐onset depression (>60/65 years; pooled RR: 1.92, 95% CI: 1.13–3.24; I 2 = 87.8%; five estimates), but not early or mid‐life depression (<60/65 years pooled RR: 1.17, 95% CI: 0.8–1.73; I 2 = 54.6%; six estimates, Table 2, Figure S7). We found a weaker association between depression and dementia in studies with longer (≥15 years: pooled RR: 1.46, 95% CI: 0.94–2.28; I 2 = 97.1%; meta‐regression p = 0.04) compared with studies with shorter follow‐up periods (≤8 years pooled RR: 2.15, 95% CI: 1.81–2.55; I 2 = 7.3%, Table 2, Figure S8). We observed no differences between studies with medium (9–14 years pooled RR: 1.98, 95% CI: 1.36–2.89; I 2 = 95.5%; meta‐regression p = 0.40) and shorter follow‐up periods.
TABLE 2.
Association between depression and all‐cause dementia: stratified analyses and meta‐regression
| Analysis | N estimates | Pooled RR (95% CI) | Heterogeneity (I2) | Meta‐regression RR (95% CI) |
|---|---|---|---|---|
| All‐cause dementia | 27 | 1.96 (1.59–2.43) | 96.5% | ‐ |
| Dementia subtype | ||||
| AD | 13 | 1.9 (1.52–2.38) | 85.5% | ‐ |
| VaD | 6 | 2.71 (2.48–2.97) | 0% | ‐ |
| Sex | 0.88 (0.57–1.36) | |||
| Male | 5 | 2.15 (1.7–2.71) | 86.8% | |
| Female | 5 | 1.92 (1.34–2.75) | 97.7% | |
| Age‐at‐onset | 1.65 (0.81–3.37) | |||
| Late‐onset | 5 | 1.92 (1.13–3.24) | 87.8% | |
| Early or mid‐life onset | 6 | 1.17 (0.8–1.73) | 54.6% | |
| Follow‐up | ||||
| 1–8 years (reference) | 11 | 2.15 (1.81–2.55) | 7.3% | |
| 9–14 years | 10 | 1.98 (1.36–2.89) | 95.5% | 0.85 (0.57–1.26) |
| >15 years | 6 | 1.46 (0.94–2.28) | 97.1% | 0.64 (0.42–0.99) |
| Quality | 0.85 (0.57–1.28) | |||
| Fair or good | 18 | 1.87 (1.45–2.41) | 97.7% | |
| Poor | 9 | 1.95 (1.56–2.45) | 8.4% | |
| Study type | 1.10 (0.76–1.61) | |||
| Register‐based | 6 | 2.10 (1.32–3.33) | 99.2% | |
| Prospective cohort | 21 | 1.90 (1.53–2.36) | 83% | |
Abbreviations: CI, confidence intervals. RR, relative risk
We also found narrative evidence for stronger associations when intervals between depression and dementia were shorter. In Swedish register data, the association with dementia was strongest in the first 6 months after depression diagnosis (OR: 20.85, 95% CI: 9.63–45.12), then decreased rapidly but persisted for over 20 years (OR: 2.33, 95% CI: 1.32–4.11). 24 In Finnish register data, depression and AD were associated during a 5‐year (OR: 1.17, 95% CI: 1.05–1.3), but not 10‐year interval between diagnoses (OR: 1.08, 95% CI: 0.96–1.23). 33 In Swedish register data, the association was strongest in the 10 years before dementia diagnosis (HR: 4.46, 95% CI: 3.44–5.76), compared with >10 years prior (HR: 1.58, 95% CI: 1.07–2.34). 34 Prospective cohort studies in the Netherlands and Australia also found associations in short‐term (<5 years), but not longer‐term follow‐up (>10 years). 35 In Whitehall II data with 28 years of follow‐up, differences in depressive symptoms only emerged around 11 years before dementia diagnosis, and were not observed in longer‐term follow‐up. 36
We found narrative evidence of stronger associations between dementia and severe, relative to mild or moderate, depression in six of seven studies. Studies involving cohorts in the United Kingdom, China and Spain found that dementia was associated with severe but not milder depression. 23 , 37 Dose response relationships between depression severity and dementia were found in a cohort study involving 14 countries (p for trend <0.001), 38 and a Canadian cohort study. 39 In Swedish register data, dementia was associated with depression at all severity levels, although the association only remained significant for longer than 10 years in moderate and severe depression. 24 In a cohort of men in Western Australia, both severe and mild‐to‐moderate depression were associated with dementia. 35
In a register‐based study from Taiwan which adjusted for comorbid alcohol and substance‐related disorders, the strongest association between depression and dementia was found in difficult‐to‐treat patients who had failed to respond to at least two antidepressant trials (HR: 5.19, 95% CI: 2.56–10.52), with a less pronounced association in those with no antidepressant prescription (HR: 2.37, 95% CI: 1.87–3.01). 40 A cohort study in Western Australia found that the association between depression and dementia did not vary by anti‐depressant medication use, 35 whereas a study conducted in Spain found that untreated depression, but not depression treated with anti‐depressants, was associated with increased risk of AD, although associations attenuated in both groups after adjustment for confounders. 37 Associations between depression and dementia remained present in studies which conducted competing risks regression to account for differing patterns of mortality between groups. 35 , 37 , 38
3.4. Anxiety
We identified seven longitudinal studies conducted in Europe examining associations between anxiety and subsequent dementia (Table 1). In six prospective cohort studies, anxiety was assessed via questionnaires or self‐report measures, while a Finnish register‐based study used ICD‐8 diagnoses. 33 Sample sizes ranged from 1082 41 to 55,896, 33 and study quality was good (n = 5) or poor (n = 2). Mean follow‐up time was between 4.4 42 to 19.4 years, 33 and four of seven studies had follow‐up periods longer than 10 years. 7 , 33 , 41 , 43
Findings were mixed, with four studies finding an association between anxiety and dementia. Associations were observed in a Swedish cohort study with a mean time of 14.7 years to dementia (HR: 1.48, 95% CI: 1.01–2.18), 41 and in the Caerphilly Prospective Study, with a mean follow‐up of 17.3 years (OR: 2.89, 95% CI: 1.27–6.54), 43 including after adjusting for depression. Petkus et al. found that anxiety, but not depression, showed an independent association with dementia. 41 An association was found between anxiety and dementia in the Three‐City Study in France (HR: 1.26, 95% CI: 1.01–1.57), although this attenuated to the null after adjusting for depression symptoms (HR: 1.04, 95% CI: 0.81–1.32). 44 In a prospective cohort study in Spain which incorporated mortality as a competing risk and adjusted for depression, clinically‐significant anxiety (SHR: 3.9, 95% CI: 1.59–9.6), but not subthreshold anxiety (SHR: 1.19, 95% CI: 0.75–1.88), was associated with AD over 4.5 years 42
Conversely, a prospective cohort study in the Netherlands with 18 years of follow‐up, 7 and a Finnish register‐based study with up to 33 years of follow‐up, found no association between anxiety and dementia regardless of the interval between diagnoses. 33 A Spanish cohort study with 4.5 years of follow‐up found an association between anxiety and VaD among men approaching significance (SHR: 2.61, 95% CI: 0.88–7.74), but not among women (SHR: 0.7, 95% CI: 0.25–1.99) using Fine and Grey competing risks regression and after adjustment for depression. 45 We identified five prospective cohort studies, conducted in the Netherlands, Wales, France and Sweden, providing comparable estimates which could be pooled in a meta‐analysis. Sample sizes ranged from 1,082 41 to 55,896 33 and follow‐up periods were between 10 44 and 33 years. 33 All studies were assessed as good quality. We found a pooled RR of 1.18 (95% CI: 0.96–1.45; I 2 = 52.2%) for the association between anxiety and subsequent dementia (Figure 2).
3.5. PTSD
We identified three population‐based cohort studies examining associations between PTSD and all‐cause dementia in the Unites States, Denmark and Taiwan (Table 1). ICD‐9/10 diagnoses of PTSD and dementia were obtained from medical records. Sample sizes ranged from 8750 46 to 499,844, 47 and total follow up periods were 11, 46 13 47 and 17 years, 48 respectively. Although it was not possible to conduct quantitative synthesis due to an insufficient number of citations, all identified studies observed associations between PTSD and dementia (HR: 1.70 [95% CI: 1.45–2.00], HR: 2 [95% CI:1.3–3.2], 48 HR: 4.37 [95% CI: 2.53–7.55]). 46
In routine data from California, the association was found in both men (HR: 1.96, 95% CI: 1.51–2.55) and women (HR: 1.59, 95% CI: 1.3–1.95), with a more pronounced association among those with comorbid depression. 47 In register data from Taiwan, associations were found in women (HR: 4.8, 95% CI: 2.52–9.12) and men (HR: 3.33, 95% CI: 1.22–9.06). 46 A dose‐response relationship was found between dementia and PTSD severity, indexed by frequency of visits to psychiatric clinics per year for PTSD (<5 visits HR: 2.81, 95% CI: 1.5–5.25; >10 visits HR: 18.13, 95% CI: 9.13–36.00). 46 Associations remained present after adjusting for depression, and two studies found that the relationship between PTSD and dementia was stronger among those with comorbid depression. 46 , 47
3.6. Bipolar disorder
We identified five studies examining associations between bipolar disorder and dementia (Table 1). Two studies ascertained diagnoses from the Western Australian Data Linkage System, 49 , 50 and three used register data from Taiwan. 51 , 52 , 53 Sample sizes ranged from 4582 51 to 102,675, 52 and follow‐up periods were between 9 53 and 17.6 years. 49 All studies were assessed as good quality. Given several studies used the same datasets, it was not possible to pool estimates. Nonetheless, there was consistent evidence of associations between bipolar disorder and dementia. In register data from Taiwan, a strong association was found between bipolar disorder and dementia in men (OR: 4.01, 95% CI: 2.53–6.35) and women (OR: 4.55, 95% CI: 3.07–6.73). 53 In a cohort in Western Australia, dementia was associated with late‐onset (≥60 years HR: 2.99, 95% CI: 2.17–4.12) and younger‐onset bipolar disorder in men (<60 years HR: 2.31, 95% CI: 1.77–3.01), 49 and with recent (<5 years HR: 3.23, 95% CI: 2.03–5.14) and long‐standing bipolar disorder (≥15 years HR: 3.09, 95% CI: 2.16–4.43). 50 Compared to those with bipolar disorder with no psychiatric admissions, the rate of dementia increased by 2.4‐fold in those with 1‐2 psychiatric admissions per year (95% CI: 1.85–3.11) versus 5.72‐fold for those with >2 admissions (95% CI: 4.8–6.81), 52 suggesting that the association may vary by severity. Associations between bipolar disorder and dementia were consistent in studies using Fine and Grey models to examine the competing risk of mortality, 50 , 52 and remained present in studies which adjusted for alcohol and substance‐related disorders. 49 , 50 , 51 , 53
3.7. Psychotic disorders
Nine studies conducted in Australia, Asia, Europe and North America reported associations between psychotic disorders and dementia (Table 1). Sample sizes ranged from 12,616 54 to 8,011,773, 22 and follow‐up periods were between 4 55 to 33 years. 33 Studies were good (n = 8) or poor (n = 1) quality. ICD‐8‐10 diagnoses were obtained from medical records. Seven studies, conducted in Australia, Israel, Taiwan, Denmark, Sweden, and Finland, provided comparable estimates to pool in a meta‐analysis, demonstrating an association between psychotic disorders and dementia (pooled RR: 2.19, 95% CI: 1.44–3.31; I 2 = 99%; Figure 2). Two further studies, which could not be pooled in the meta‐analysis due to a lack of comparable data, also found associations between psychotic disorders and dementia. Using routine data from the United States, Stroup et al. found that schizophrenia was associated with incident dementia across a wide range of ages, 22 and Kessing et al. 56 found an association between schizophrenia and dementia in Danish register data (SMR: 14.7, 95% CI: 9.1–22.4).
All four of the studies focussed specifically on very late‐onset schizophrenia‐like psychosis (VLOSLP) observed associations with dementia, 20 , 54 , 55 , 57 with HRs ranging from 2.21 (95% CI: 1.39–3.5) 54 in Danish register data to 4.22 (95% CI: 4.05–4.41) in Swedish register data. 20 In a Western Australian cohort, the association between psychotic disorders and dementia was stronger with a shorter duration of psychosis (<5 years HR: 4.25, 95% CI: 2.71–6.67; ≥10 years HR: 2.42, 95% CI: 2.10–2.8). 57 Similarly, in Swedish register data the association between psychotic disorders and dementia was strongest in the first year following VLOSLP diagnosis and attenuated over time, despite remaining present for up to 20 years of follow‐up. 20 Patterns of association between psychotic disorders and dementia remained consistent in studies which used competing risks regression to examine whether findings could be explained by differing patterns of mortality between groups. 20 , 54 , 55 , 57
3.8. PAFs
Using pooled RRs obtained in this review and prevalence estimates of psychiatric disorders from the APMS, we estimated PAFs, indicating the proportion of dementia cases theoretically preventable through elimination of psychiatric disorders in the population, assuming associations were causal: depression: 3.1%, anxiety: 1.1%, and psychotic disorders: 0.8% (Table 3).
TABLE 3.
Population attributable fractions (PAF)
| Psychiatric disorder | Prevalence (%) a | RR b | PAF |
|---|---|---|---|
| Depression | 3.3 | 1.96 | 3.07 |
| Anxiety | 5.9 | 1.18 | 1.05 |
| Psychotic disorder | 0.7 | 2.19 | 0.83 |
Abbreviations: PAF, population attributable fraction, RR, relative risk;
Prevalence estimates obtained from the Adult Psychiatric Morbidity Survey.
RRs obtained from pooled estimates in systematic review.
4. DISCUSSION
4.1. Summary of findings and comparison with previous literature
We found that depression, PTSD, bipolar disorder and psychotic disorders were associated with increased risk of subsequent all‐cause dementia. Building on previous meta‐analyses, 3 , 4 we focused only on longitudinal, population‐based studies and clinically‐significant psychiatric symptoms or diagnoses. Findings were similar in studies which took mortality into account as a competing risk, increasing confidence that the results were not attributable to selective attrition. Associations between depression and dementia were stronger in studies with shorter follow‐up periods, where the interval between diagnoses was shorter, and for severe and late‐onset depression, suggesting that the association may at least partly reflect reverse causation. Few studies examined the role of psychiatric comorbidities, although there was some evidence that comorbid depression was associated with increased risk of dementia in PTSD. 46 , 47
Importantly, most identified studies focused on depression, whereas the evidence‐base was considerably weaker for other psychiatric disorders, limiting the possibility for quantitative synthesis. Nonetheless, we found that psychotic disorders were associated with increased risk of dementia, with narrative evidence of a stronger association when the interval between diagnoses was shorter. In contrast, we did not observe an overall association between anxiety and dementia. This corresponds with a previous meta‐analysis which did not find a clear association in analyses including only adjusted estimates (pooled RR: 1.68, 95% CI: 0.94–3.02). 4 However, the review included studies with unrepresentative samples and participants with MCI at baseline, and all except one study had follow‐up periods shorter than 3.8 years. Our finding of an association between PTSD and dementia corresponds with results from a previous review (HR: 1.61, 95% CI: 1.43–1.81), 8 while another review did not find evidence of a significant pooled association (OR: 2.55, 95% CI: 0.43–15.12). 9 In contrast, our review only included studies focused on the general population, rather than veteran or clinical samples.
4.2. Strengths and limitations
Strengths include pre‐registration, carefully defined eligibility criteria to reduce selection bias, and a comprehensive search strategy. In contrast to previous reviews, we focused on longitudinal, population‐based epidemiological studies, and clinically significant psychiatric symptoms or diagnoses. We also note several limitations. Our strict eligibility criteria resulted in relatively few citations, limiting our ability to conduct quantitative syntheses and subgroup analyses. In addition, our focus on clinical psychiatric diagnoses may have favoured retrospective studies of routine data and resulted in exclusion of prospective cohort studies. In addition, retrospective studies tend to have larger sample sizes and therefore often receive larger weights in meta‐analyses. This may have influenced results in that retrospective studies tend to have longer follow‐up periods and may be more prone to biases such as exposure and outcome misclassification and residual confounding. Nonetheless, subgroup analyses for depression did not indicate significant differences in the association between depression and dementia for prospective versus retrospective studies, although it was not possible to examine these differences for other psychiatric diagnoses due to the small number of identified studies. Given high levels of heterogeneity between estimates, pooled estimates should be interpreted cautiously and alongside narrative synthesis. Heterogeneity was lower for AD and VaD outcomes, indicating more consistent effect sizes compared with the all‐cause dementia outcome, where higher heterogeneity could partly reflect differing proportions of AD and VaD across different samples. Limiting our search to English language papers could have led to exclusion of relevant papers in other languages. Only half of included studies had follow‐up periods longer than 10 years, although our pre‐planned subgroup analyses allowed us to consider the effect of this limitation. Further, most studies did not report mean follow‐up time, or interval between diagnoses, limiting our ability to examine temporal relationships.
4.3. Meaning of findings
Associations between psychiatric disorders and dementia are compatible with several explanations. First, psychiatric disorders are associated with poor cardiovascular health 58 , 59 , 60 and negative health behaviours, 61 , 62 , 63 which could, in turn, increase risk for dementia. 64 , 65 , 66 Systemic inflammation and cerebrovascular disorder have been implicated in the pathophysiology of dementia 67 , 68 and psychiatric disorders, 69 , 70 and has been suggested as a candidate pathway in explaining these relationships. 71 Associations could be driven by cognitive reserve, 72 as some individuals with psychiatric disorders could have lower levels of baseline cognitive functioning and may therefore require less neuropathology before meeting clinical thresholds for dementia diagnosis. 73 Psychiatric disorders are associated with high levels of stress 74 , 75 and it is possible that related neurobiological pathways, including hypothalamic‐pituitary‐adrenal axis dysfunction, could increase risk of dementia. 76 , 77 This is an important area for future research, given that few studies have directly tested these potential mechanisms.
Conversely, it is possible that, rather than risk factors, psychiatric symptoms could represent early manifestations of AD neuropathology, which begins to accumulate in the brain over several decades before cognitive and functional decline are apparent. 11 Correspondingly, we found stronger associations between dementia and depression in studies with shorter follow‐up periods, and for late‐onset depression. Another potential explanation is that people with psychiatric disorders have more contact with clinical services, 78 allowing easier detection of dementia, whereas missed or delayed diagnoses may be more likely in those without psychiatric illness. However, our exclusion of clinical samples reduced the likelihood of this measurement error affecting our results.
Our findings demonstrate that patients with psychiatric disorders are at increased risk for dementia, highlighting the importance of ongoing monitoring for functional and cognitive decline in these groups. Importantly, our results demonstrate a need for further high‐quality longitudinal evidence, particularly regarding anxiety, PTSD and bipolar disorder, where evidence was sparse or mixed. Further, studies examining patterns of association over time are required to establish whether associations are present up to 20–30 years before dementia, where bias due to preclinical neurodegeneration is small, or only emerge close to dementia diagnosis, consistent with reverse causation. Studies should also investigate the effect of treatment of psychiatric disorders on dementia risk, given the potential implications for clinical practice and public health. In addition, further research is needed to examine the role of psychiatric comorbidities, which are pervasive and were found to be associated with increased risk of dementia in PTSD. Establishing whether psychiatric disorders are causal risk factors for dementia would have important implications for dementia prevention and treatment and management of psychiatric disorders in mid‐ and late‐life.
CONFLICT OF INTEREST
None.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
James B. Kirkbride was supported by a Sir Henry Dale Fellowship (Wellcome Trust/Royal Society grant number: 101272/Z/13/Z); Andrew Sommerlad was supported by the UCL/Wellcome Trust Institutional Strategic Support Fund (grant number: 204841/Z/16/Z), Andrew Sommerlad and Jean Stafford were supported by Postdoctoral Bridging Fellowships from the National Institute for Health Research, University College London Hospital, Biomedical Research Centre; Jean Stafford/Andrew Sommerlad/James B. Kirkbride/Robert Howard were supported by the National Institute for Health Research, University College London Hospital, Biomedical Research Centre. Sponsors did not contribute to study design, data analysis, interpretation of data, writing of the report, or the decision to submit the article for publication.
Stafford J, Chung WT, Sommerlad A, Kirkbride JB, Howard R. Psychiatric disorders and risk of subsequent dementia: systematic review and meta‐analysis of longitudinal studies. Int J Geriatr Psychiatry. 2022;1‐22. 10.1002/gps.5711
James B. Kirkbride and Robert Howard are joint senior authors.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Zilkens RR, Bruce DG, Duke J, Spilsbury K, Semmens JB. Severe psychiatric disorders in mid‐life and risk of dementia in late‐life (age 65–84): a population based case‐control study. Curr Alzheimer Res. 2014;11:681‐693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cai L, Huang J. Schizophrenia and risk of dementia: a meta‐analysis study. Neuropsychiatric Dis Treat. 2018;14:2047‐2055. 10.2147/NDT.S172933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Diniz BS, Butters MA, Albert SM, Dew MA, Reynolds CF, 3rd . Late‐life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta‐analysis of community‐based cohort studies. Br J Psychiatry. 2013;202(5):329‐335. 10.1192/bjp.bp.112.118307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gulpers B, Ramakers I, Hamel R, Köhler S, Voshaar RO, Verhey F. Anxiety as a predictor for cognitive decline and dementia: a systematic review and meta‐analysis. Am J Geriatric Psychiatry. 2016;24(10):823‐842. 10.1016/j.jagp.2016.05.015 [DOI] [PubMed] [Google Scholar]
- 5. Livingston G, Huntley J, Sommerlad A, et al. The Lancet commissions dementia prevention, intervention, and care: 2020 report of the Lancet commission. The Lancet. Published online. 2020;396:413‐446. 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Becker E, Orellana Rios CL, Lahmann C, Rücker G, Bauer J, Boeker M. Anxiety as a risk factor of Alzheimer’s disease and vascular dementia. Br J Psychiatry. 2018;213(5):654‐660. 10.1192/bjp.2018.173 [DOI] [PubMed] [Google Scholar]
- 7. De Bruijn R, Direk N, Mirza SS, et al. Anxiety is not associated with the risk of dementia or cognitive decline: the Rotterdam study. Am J Geriatric Psychiatry. 2014;22(12):1382‐1390. 10.1016/j.jagp.2014.03.001 [DOI] [PubMed] [Google Scholar]
- 8. Günak MM, Billings J, Carratu E, Marchant NL, Favarato G, Orgeta V. Post‐traumatic stress disorder as a risk factor for dementia: systematic review and meta‐analysis. Br J Psychiatry. 2020;217:1‐608. Published online. 10.1192/bjp.2020.150 [DOI] [PubMed] [Google Scholar]
- 9. Kuring JK, Mathias JL, Ward L. Risk of dementia in persons who have previously experienced clinically‐significant depression, anxiety, or PTSD: a systematic review and meta‐analysis. J Affect Disord. 2020;274(May):247‐261. 10.1016/j.jad.2020.05.020 [DOI] [PubMed] [Google Scholar]
- 10. Amieva H, Le Goff M, Millet X, et al. Prodromal Alzheimer’s disease: successive emergence of the clinical symptoms. Ann Neurol. 2008;64(5):492‐498. 10.1002/ana.21509 [DOI] [PubMed] [Google Scholar]
- 11. Villemagne VL, Burnham S, Bourgeat P, et al. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol. 2013;12:357‐367. [DOI] [PubMed] [Google Scholar]
- 12. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wells G, Shea B, O’Connell D, et al. The Newcastle‐Ottawa Scale (NOS) for Assessing the quality of Nonrandomized Studies in meta‐analysis bias and Confounding Newcastle‐Ottawa Scale. Published 2011. http://www.evidencebasedpublichealth.de/download/Newcastle_Ottowa_Scale_Pope_Bruce.pdf [Google Scholar]
- 14. Egger M, Smith GD. Meta‐analysis. Potentials and promise. BMJ. 1997:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dersimonian R, Laird N. Meta‐analysis in clinical trials. Contr Clin Trials. 1986;7:177‐188. [DOI] [PubMed] [Google Scholar]
- 16. Fu R, Gartlehner G, Grant M, et al. Conducting quantitative synthesis when comparing medical interventions: AHRQ and the Effective Health Care Program. J Clin Epidemiol. 2011;64(11):1187‐1197. [DOI] [PubMed] [Google Scholar]
- 17. Benichou J. A review of adjusted estimators of attributable risk. Stat Methods Med Res. 2001;10(3):195‐216. [DOI] [PubMed] [Google Scholar]
- 18. McManus S, Bebbington P, Jenkins R, Brugha T, eds. Mental Health and Wellbeing in England: adult Psychiatric Morbidity Survey 2014. NHS Digital, Government Statistical Service. Published online 2016. [Google Scholar]
- 19. Buntinx F, Kester A, Bergers J, Knottnerus JA. Is depression in elderly people followed by dementia? A retrospective cohort study based in general practice. Age ageing. 1996;25(3):231‐233. 10.1093/ageing/25.3.231 [DOI] [PubMed] [Google Scholar]
- 20. Stafford J, Dykxhoorn J, Sommerlad A, Dalman C, Kirkbride JB, Howard R. Association between risk of dementia and very late‐onset schizophrenia‐like psychosis: a Swedish population‐based cohort study. Psychol Med. 2021;44(0):1‐28. 10.31234/osf.io/nq7eg [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wallin K, Boström G, Kivipelto M, Gustafson Y. Risk factors for incident dementia in the very old. Int Psychogeriatr. 2013;25(7):1135‐1143. 10.1017/S1041610213000409 [DOI] [PubMed] [Google Scholar]
- 22. Stroup TS, Olfson M, Huang C, et al. Age‐specific prevalence and incidence of dementia diagnoses among older US adults with schizophrenia. JAMA Psychiatr. 2021;78:10032. 10.1001/jamapsychiatry.2021.0042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen R, Hu Z, Wei L, Qin X, McCracken C, Copeland JR. Severity of depression and risk for subsequent dementia: cohort studies in China and the UK. Br J Psychiatry. 2008;193(5):373‐377. 10.1192/bjp.bp.107.044974 [DOI] [PubMed] [Google Scholar]
- 24. Holmquist S, Nordström A, Nordström P. The association of depression with subsequent dementia diagnosis: a Swedish nationwide cohort study from 1964 to 2016. PLoS Med. 2020;17(1):1‐16. 10.1371/journal.pmed.1003016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Becker JT, Chang YF, Lopez OL, et al. Depressed mood is not a risk factor for incident dementia in a community‐based cohort. Am J geriatric psychiatry. 2009;17(8):653‐663. 10.1097/jgp.0b013e3181aad1fe [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Deckers K, Köhler S, van Boxtel M, Verhey F, Brayne C, Fleming J. Lack of associations between modifiable risk factors and dementia in the very old: findings from the Cambridge City over‐75s cohort study. Ageing Ment Health. 2018;22(10):1272‐1278. 10.1080/13607863.2017.1280767 [DOI] [PubMed] [Google Scholar]
- 27. Rolandi E, Zaccaria D, Vaccaro R, et al. Estimating the potential for dementia prevention through modifiable risk factors elimination in the real‐world setting: a population‐based study. Alzheimer's Res Ther. 2020;12(1). 10.1186/s13195-020-00661-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Köhler S, Van Boxtel M, Jolles J, Verhey F. Depressive symptoms and risk for dementia: a 9‐year follow‐up of the Maastricht ageing Study. Am J Geriatric Psychiatry. 2011;19(10):902‐905. 10.1097/JGP.0b013e31821f1b6a [DOI] [PubMed] [Google Scholar]
- 29. Lenoir H, Dufouil C, Auriacombe S, et al. Depression history, depressive symptoms, and incident dementia: the 3C Study. J Alzheimer’s Dis. 2011;26(1):27‐38. 10.3233/JAD-2011-101614 [DOI] [PubMed] [Google Scholar]
- 30. Lin WC, Hu LY, Tsai SJ, Yang AC, Shen CC. Depression and the risk of vascular dementia: a population‐based retrospective cohort study. Int J geriatric psychiatry. 2017;32(5):556‐563. 10.1002/gps.4493 [DOI] [PubMed] [Google Scholar]
- 31. Richard E, Reitz C, Honig LH, et al. Late‐life depression, mild cognitive impairment, and dementia. JAMA Neurol. 2013;70(3):374‐382. 10.1001/jamaneurol.2013.603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Irie F, Masaki KH, Petrovitch H, et al. Apolipoprotein E epsilon4 allele genotype and the effect of depressive symptoms on the risk of dementia in men: the Honolulu‐Asia Ageing Study. Archives general psychiatry. 2008;65(8):906‐912. 10.1001/archpsyc.65.8.906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tapiainen V, Hartikainen S, Taipale H, Tiihonen J, Tolppanen AMM. Hospital‐treated mental and behavioral disorders and risk of Alzheimer’s disease: a nationwide nested case‐control study. Eur Psychiatry. 2017;43:92‐98. 10.1016/j.eurpsy.2017.02.486 [DOI] [PubMed] [Google Scholar]
- 34. Karlsson IK, Bennet AM, Ploner A, et al. Apolipoprotein E epsilon 4 genotype and the temporal relationship between depression and dementia. Neurobiol Aging. In: Rnes, ed. 2015;36;4:1751‐1756. 10.1016/j.neurobiolaging.2015.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Almeida OP, Hankey GJ, Yeap BB, Golledge J, Flicker L. Depression as a modifiable factor to decrease the risk of dementia. Transl Psychiatry. 2017;7(5):e1117. 10.1038/tp.2017.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Singh‐Manoux A, Dugravot A, Fournier A, et al. Trajectories of depressive symptoms before diagnosis of dementia: a 28‐year follow‐up study. JAMA Psychiatr. 2017;74(7):712‐718. 10.1001/jamapsychiatry.2017.0660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gracia‐Garcia P, De‐la‐Camara C, Santabarbara J, et al. Depression and incident Alzheimer disease: the impact of disease severity. Am J Geriatric Psychiatry. 2015;23(2):119‐129. 10.1016/j.jagp.2013.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wu JJ, Wang HX, Yao W, Yan Z, Pei JJ. Late‐life depression and the risk of dementia in 14 countries: a 10‐year follow‐up study from the Survey of Health, Ageing and Retirement in Europe. J Affect Disord. 2020;274:671‐677. 10.1016/j.jad.2020.05.059 [DOI] [PubMed] [Google Scholar]
- 39. Gatz JL, Tyas SL, . St John P , Montgomery P. Do depressive symptoms predict Alzheimer’s disease and dementia? J Gerontol Ser A, Biol Sci Med Sci. 2005;60(6):744‐747. 10.1093/gerona/60.6.744 [DOI] [PubMed] [Google Scholar]
- 40. Chan YLE, Chen MH, Tsai SJ, et al. Treatment‐resistant depression enhances risks of dementia and Alzheimer’s disease: a nationwide longitudinal study. J Affect Disord. 2020;274(May):806‐812. 10.1016/j.jad.2020.05.150 [DOI] [PubMed] [Google Scholar]
- 41. Petkus AJ, Reynolds CA, Wetherell JL, Kremen WS, Pedersen NL, Gatz M. Anxiety is associated with increased risk of dementia in older Swedish twins. Alzheimer’s Dementia. 2016;12(4):399‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Santabárbara J, Lopez‐Anton R, de la Camara C, et al. Clinically significant anxiety as a risk factor for dementia in the elderly community. Acta Psychiatr Scand. 2019;139(1):6‐14. 10.1111/acps.12966 [DOI] [PubMed] [Google Scholar]
- 43. Gallacher J, Bayer A, Fish M, et al. Does anxiety affect risk of dementia? Findings from the Caerphilly Prospective Study. Psychosom Med. 2009;71(6):659‐666. 10.1097/PSY.0b013e3181a6177c [DOI] [PubMed] [Google Scholar]
- 44. Mortamais M, Abdennour M, Bergua V, et al. Anxiety and 10‐year risk of incident dementia‐an association shaped by depressive symptoms: results of the prospective Three‐City study. Front Neurosci. 2018;12:1‐9. 10.3389/fnins.2018.00248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Santabárbara J, Villagrasa B, Lopez‐anton R, Cámara CDl, Gracia‐García P, Lobo A. Anxiety and risk of vascular dementia in an elderly community sample: the role of sex. Brain Sci. 2020;10(5):1‐11. 10.3390/brainsci10050265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang TYY, Wei HTT, Liou YJJ, et al. Risk for developing dementia among patients with posttraumatic stress disorder: a nationwide longitudinal study. J Affect Disord. 2016;205(May 2013):306‐310. 10.1016/j.jad.2016.08.013 [DOI] [PubMed] [Google Scholar]
- 47. Flatt JD, Gilsanz P, Quesenberry CPJ, Albers KB, Whitmer RA. Post‐traumatic stress disorder and risk of dementia among members of a health care delivery system. Alzheimer’s Dementia. 2018;14(1):28‐34. 10.1016/j.jalz.2017.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gradus JL, Horváth‐Puhó E, Lash TL, et al. Stress disorders and dementia in the Danish population. Am J Epidemiol. 2019;188(3):493‐499. 10.1093/aje/kwy269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Almeida OP, Hankey GJ, Yeap BB, Golledge J, Flicker L. Older men with bipolar disorder: clinical associations with early and late onset illness. Int J geriatric psychiatry. 2018;33(12):1613‐1619. 10.1002/gps.4957 [DOI] [PubMed] [Google Scholar]
- 50. Almeida OP, McCaul K, Hankey GJ, Yeap BB, Golledge J, Flicker L. Risk of dementia and death in community‐dwelling older men with bipolar disorder. Br J Psychiatry. 2016;209(2):121‐126. 10.1192/bjp.bp.115.180059 [DOI] [PubMed] [Google Scholar]
- 51. Chen MH, Li CT, Tsai CF, et al. Risk of subsequent dementia among patients with bipolar disorder or major depression: a nationwide longitudinal study in Taiwan. J Am Med Dir Assoc. 2015;16(6):504‐508. 10.1016/j.jamda.2015.01.084 [DOI] [PubMed] [Google Scholar]
- 52. Lin SH, Cheng CM, Tsai SJ, et al. A population‐based, Nationwide longitudinal study of bipolar disorder with incident dementia in Taiwan. Am J Geriatric Psychiatry. 2020;28(5):530‐541. 10.1016/j.jagp.2019.10.016 [DOI] [PubMed] [Google Scholar]
- 53. Wu KY, Chang CM, Liang HY, et al. Increased risk of developing dementia in patients with bipolar disorder: a nested matched case‐control study. Bipolar Disord. 2013;15(7):787‐794. [DOI] [PubMed] [Google Scholar]
- 54. Kørner A, Lopez AG, Lauritzen L, Andersen PK, Kessing LV. Late and very‐late first‐contact schizophrenia and the risk of dementia—a nationwide register based study. Int J Geriatric Psychiatry. 2009;24(1):61‐67. 10.1002/gps.2075 [DOI] [PubMed] [Google Scholar]
- 55. Kodesh A, Goldberg Y, Rotstein A, et al. Risk of dementia and death in very‐late‐onset schizophrenia‐like psychosis: a national cohort study. Schizophrenia Res. 2020;223:220‐226. 10.1016/j.schres.2020.07.020 [DOI] [PubMed] [Google Scholar]
- 56. Kessing LV, Olsen EW, Mortensen PB, Andersen PK. Dementia in affective disorder: a case‐register study. Acta Psychiatr Scand. 1999;100(3):176‐185. 10.1111/j.1600-0447.1999.tb10843.x [DOI] [PubMed] [Google Scholar]
- 57. Almeida OP, Ford AH, Hankey GJ, Yeap BB, Golledge J, Flicker L. Risk of dementia associated with psychotic disorders in later life: the health in men study (HIMS). Psychol Med. 2019;49(2):232‐242. 10.1017/S003329171800065X [DOI] [PubMed] [Google Scholar]
- 58. Rugulies R. Depression as a predictor for coronary heart disease: a review and meta‐analysis. Am J Prev Med. 2002;23(1):51‐61. [DOI] [PubMed] [Google Scholar]
- 59. Crump C, Winkleby MA, Sundquist K, Sundquist J. Comorbidities and mortality in persons with schizophrenia: a Swedish national cohort study. Am J Psychiatry. 2013;170(3):324‐333. [DOI] [PubMed] [Google Scholar]
- 60. Emdin CA, Odutayo A, Wong CX, Tran J, Hsiao AJ, Hunn BHM. Meta‐analysis of anxiety as a risk factor for cardiovascular disease. Am J Cardiol. 2016;118(4):511‐519. [DOI] [PubMed] [Google Scholar]
- 61. Strine TW, Chapman DP, Kobau R, Balluz L. Associations of self‐reported anxiety symptoms with health‐related quality of life and health behaviors. Soc Psychiatry Psychiatr Epidemiol. 2005;40(6):432‐438. [DOI] [PubMed] [Google Scholar]
- 62. Sullivan LE, Fiellin DA, O’Connor PG. The prevalence and impact of alcohol problems in major depression: a systematic review. Am J Med. 2005;118(4):330‐341. [DOI] [PubMed] [Google Scholar]
- 63. McCreadie RG. Diet, smoking and cardiovascular risk in people with schizophrenia. Br J Psychiatry. 2003;183:534‐539. [DOI] [PubMed] [Google Scholar]
- 64. Viswanathan A, Rocca W, Tzourio C. Vascular risk factors and dementia. How to move forward? Neurology. 2009;72(4):368‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Alagiakrishnan K, McCracken P, Feldman H. Treating vascular risk factors and maintaining vascular health: is this the way towards successful cognitive ageing and preventing cognitive decline? Postgrad Med J. 2006;82(964):101‐105. 10.1136/pgmj.2005.035030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10(9):819‐828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Dziedzic T. Systemic inflammatory markers and risk of dementia. Am J Alzheimer’s Dis other Dementias. 2006;21(4):258‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Taylor WD, Aizenstein HJ, Alexopoulos GS. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Mol Psychiatry. 2013;18(9):963‐974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kirkpatrick B, Miller BJ. Inflammation and schizophrenia. Schizophr Bull. 2013;39(6):1174‐1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Valkanova V, Ebmeier KP, Allan CL. CRP, IL‐6 and depression: a systematic review and meta‐analysis of longitudinal studies. J Affect Disord. 2013;150(3):736‐744. 10.1016/j.jad.2013.06.004 [DOI] [PubMed] [Google Scholar]
- 71. Leonard BE. Inflammation, depression and dementia: are they connected? Neurochem Res. 2007;32(10):1749‐1756. [DOI] [PubMed] [Google Scholar]
- 72. Barnett JH, Salmond CH, Jones PB, Sahakian BJ. Cognitive reserve in neuropsychiatry. Psychol Med. 2006;36(36):1053‐1064. [DOI] [PubMed] [Google Scholar]
- 73. Stern Y. Cognitive reserve and Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20:69‐74. [DOI] [PubMed] [Google Scholar]
- 74. Howes OD, McDonald C, Cannon M, Arseneault L, Boydell J, Murray RM. Pathways to schizophrenia: the impact of environmental factors. Int J Neuropsychopharmacol. 2004;7(Suppl 1):S7‐S13. 10.1017/S1461145704004122 [DOI] [PubMed] [Google Scholar]
- 75. Madsen IEH, Nyberg ST, Magnusson Hanson LL, et al. Job strain as a risk factor for clinical depression: systematic review and meta‐analysis with additional individual participant data. Psychol Med. 2017;47(8):1342‐1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Johansson L, Guo X, Waern M, et al. Midlife psychological stress and risk of dementia: a 35‐year longitudinal population study. Brain. 2010;133(8):2217‐2224. 10.1093/brain/awq116 [DOI] [PubMed] [Google Scholar]
- 77. Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. 2011;7(6):323‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wang TY, Wei HT, Liou YJ, et al. Risk for developing dementia among patients with posttraumatic stress disorder: a nationwide longitudinal study. J Affect Disord. 2016;205(May 2013):306‐310. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


