Abstract
Bleeding and thrombosis are major clinical problems with high morbidity and mortality. Treatment modalities for these diseases have improved in recent years, but there are many clinical questions remaining and a need to advance diagnosis, management, and therapeutic options. Basic research plays a fundamental role in understanding normal and disease processes, yet this sector has observed a steady decline in funding prospects thereby hindering support for studies of mechanisms of disease and therapeutic development opportunities. With the financial constraints faced by basic scientists, the ISTH organized a basic science task force (BSTF), comprising Scientific and Standardization Committee subcommittee chairs and co‐chairs, to identify research opportunities for basic science in hemostasis and thrombosis. The goal of the BSTF was to develop a set of recommended priorities to build support in the thrombosis and hemostasis community and to inform ISTH basic science programs and policy making. The BSTF identified three principal opportunity areas that were of significant overarching relevance: mechanisms causing bleeding, innate immunity and thrombosis, and venous thrombosis. Within these, five fundamental research areas were highlighted: blood rheology, platelet biogenesis, cellular contributions to thrombosis and hemostasis, structure–function protein analyses, and visualization of hemostasis. This position paper discusses the importance and relevance of these opportunities and research areas, and the rationale for their inclusion. These findings have implications for the future of fundamental research in thrombosis and hemostasis to make transformative scientific discoveries and tackle key clinical questions. This will permit better understanding, prevention, diagnosis, and treatment of hemostatic and thrombotic conditions.
Keywords: hemorrhage, hemostasis, innate immunity, platelet, thrombosis
1. INTRODUCTION
The International Society on Thrombosis and Haemostasis (ISTH) is the leading global organization dedicated to the advancement of understanding, prevention, diagnosis, and treatment of conditions related to thrombosis and hemostasis. As part of the recent strategic planning for the Society, ISTH Council leadership created a working group to review the current state of fundamental research into thrombosis and hemostasis. Funding directed toward discovery research in thrombosis and hemostasis has seen a significant reduction in the last 8–10 years. In the same period there has been a steady decline in the number of basic science abstracts submitted to the ISTH congresses (Figure 1). Based on these findings, the Council working group recommended the creation of a basic science roadmap to prioritize areas of research that the Society could support to address the current scientific challenges in the field of thrombosis and hemostasis. As a first step, the ISTH collaborated with the American Heart Association to develop a joint statement focused on venous thromboembolism. 1 The ISTH would then follow up with a roadmap that would incorporate hemorrhagic, thrombotic, and platelet disorders to cover the entire field. The Scientific and Standardization Committee (SSC) of the ISTH was identified as the ideal body to create a new SSC task force to develop the ISTH roadmap. Members of the basic science task force (BSTF) were chosen from the SSC Executive Committee and from leadership in SSC subcommittees with a primary focus on basic science. The BSTF was asked to prioritize the topic areas that would benefit from increased emphasis over the next 3–5 years.
FIGURE 1.
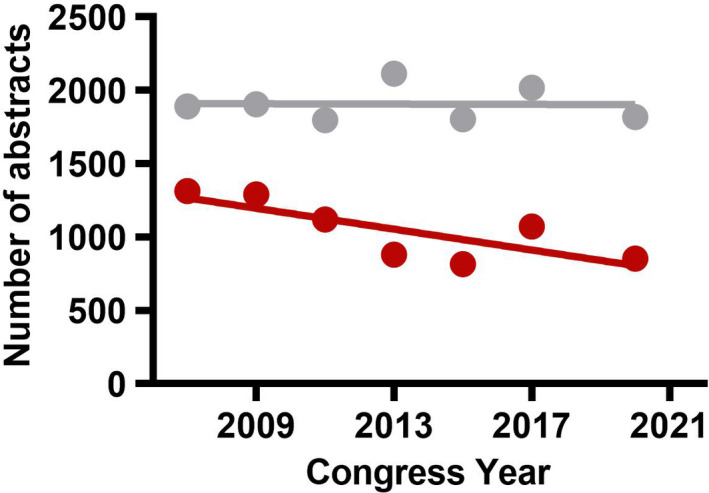
Abstract submission to the ISTH Congresses. Linear regression analysis of clinical (gray) and basic abstracts (red) submission to the ISTH congresses from 2007–2020. The data from 2019 (ISTH Melbourne) were considered an outlier and not included due to the lower number of total submission of abstracts (774 basic and 1363 clinical). Source of data: ISTH Headquarters
Hemostasis is a vital physiological mechanism that has two distinct but simultaneously occurring functions: (1) to stop the bleeding when disruption of the vessel integrity occurs; this process involves formation of hemostatic plugs comprised of platelets and fibrin; and (2) to maintain blood fluidity within the blood vessel so there is an adequate and constant blood circulation within the body. These two apparently contradictory functions of the coagulation system can only be performed when there is a balance between a constant basal level of coagulation activation and a continuous but low level of anticoagulation. Any pathological deviation of this system can lead to excessive blood clot formation (thrombosis) or to bleeding (hemorrhage).
Thrombosis is one of the most frequent causes of mortality worldwide, as it is the common etiology of ischemic heart diseases, ischemic stroke, and venous thromboembolism (VTE). The development of new antithrombotic therapies and strategies has revolutionized the medical management of patients with thrombosis. However, with the currently available agents there still is an accompanying risk of bleeding complications.
Bleeding complications, arising due to an imbalance in the hemostatic system, also pose a major medical issue. Bleeding can occur in patients with (genetic) coagulation defects, in patients with a normal coagulation system who experience severe postoperative bleeding, in those that undergo surgical procedures known to be associated with major blood loss, and in major trauma. Furthermore, bleeding can be a side effect of anticoagulant therapy. For example, the annual rate of major bleeding when direct acting oral anticoagulants (DOACs) are used in atrial fibrillation patients is between 2% and 3%. 2 To understand the crucial balance between coagulation and anticoagulation, fundamental research into the field of thrombosis and hemostasis is essential. The current position paper provides insights into areas of basic science in the field of thrombosis and hemostasis that merit attention in the coming years.
2. METHODS
The BSTF first convened in May 2020 and set to work developing a clear scope for the project. The project scope, agreed in June 2020, was as follows:
To develop a set of recommended priorities for basic science in thrombosis and hemostasis, communicate these priorities to build support in the thrombosis and hemostasis community, and use the priorities to inform ISTH basic science programs and policy.
Of note is the use of the term “priorities” in the initial scope of the project. As the project progressed, BSTF members chose to reframe topics as “opportunity areas,” to communicate that while the opportunities discussed in this article are deemed important by the BSTF, they are not necessarily of higher priority relevant to the many other basic science topics in thrombosis and hemostasis.
The BSTF met via teleconference throughout 2020 and 2021 to discuss potential opportunity areas for inclusion in this project. Opportunity areas were listed comprehensively, and then collaboratively refined and combined where deemed appropriate to develop a complete inventory for scoring and prioritization. The BSTF work was conducted using a modified Delphi process which included a quantitative decision matrix scoring method described below (and within supporting information).
The BSTF members independently identified the top three to five criteria by which candidate basic science opportunity areas would be ranked. These criteria were discussed and refined by the BSTF who then scored the criteria based on importance. Four criteria were determined by which candidate opportunity areas would be assessed to determine their inclusion in this project. The BSTF then agreed on scoring weights for each of the criteria to be used in prioritization of candidate opportunity areas. The final criteria and their scoring weights are shown in Table 1.
TABLE 1.
Criteria by which basic science opportunity areas were ranked by the BSTF
| Criteria | Definition | Weight |
|---|---|---|
| Originality and innovation | The potential for novel and high‐impact research to be conducted in a research priority. | 40% |
| Addresses unmet clinical needs or knowledge gaps | The degree to which a research priority has the potential to drive changes in clinical practice, or address areas of medical knowledge that are currently uncertain or unexplored. | 30% |
| Translational research opportunities | The degree to which a research priority may encourage the development of translational research studies and initiatives. | 15% |
| Research community interest | The level of interest and enthusiasm within the research community regarding new research within a research priority. | 15% |
Abbreviation: BSTF, basic science task force.
Each candidate opportunity area was scored across the defined criteria (Table 1) by each BSTF member. In the first scoring round, candidate opportunity areas were scored relative to each other, and in the second round, the opportunity areas were scored independently. The BSTF evaluated the score results, and examined overall scores for candidate opportunity areas as well as the scores for individual criteria. Special attention was paid to outlier scores, with discussion to determine the cause behind outliers and determination of their significance. Three of the seventeen opportunity areas identified by the BSTF were deemed to encompass the majority of the other opportunity areas. These three areas, mechanisms causing bleeding, innate immunity and thrombosis, and venous thrombosis, were used to group all the other opportunity areas (Table 2).
TABLE 2.
Opportunity areas for basic science in thrombosis and hemostasis
| Opportunity areas | Mechanisms causing bleeding | Innate immunity and thrombosis | Venous thrombosis |
|---|---|---|---|
| Assessing and visualizing hemostasis | ✓ | ✓ | ✓ |
| Blood rheology‐driven vascular effects | ✓ | ✓ | |
| Cancer‐associated thrombosis | ✓ | ||
| Cellular contributions to thrombosis and hemostasis | ✓ | ✓ | ✓ |
| Major hemorrhage | ✓ | ||
| Obesity‐related thrombotic complications | ✓ | ✓ | |
| Pathogen‐induced effects (e.g., COVID−19, sepsis, etc.) | ✓ | ✓ | ✓ |
| Platelet production and function | ✓ | ||
| Rare bleeding diseases | ✓ | ||
| Rare thrombotic diseases | ✓ | ✓ | |
| Safe anticoagulants | ✓ | ✓ | ✓ |
| Structure‐function of hemostatic proteins | ✓ | ✓ | ✓ |
| Trauma‐induced coagulopathy | ✓ | ✓ | |
| Universal prohemostatics | ✓ | ✓ |
Fourteen opportunity areas were identified and subsequently organized into three overarching categories that umbrella many of the various topics.
3. OPPORTUNITY AREAS FOR THROMBOSIS AND HEMOSTASIS FUNDAMENTAL SCIENCE
The 14 opportunity areas identified (Table 2) were subdivided into clinical and translational themes that relate to five key fundamental research topics including (1) blood rheology‐driven vascular effects, (2) platelet production and function, (3) cellular contributions to thrombosis and hemostasis, (4) structure–function of hemostatic proteins, (5) and assessing and visualizing hemostasis (Figure 2). Below, we outline each of these fundamental research topics, and identify their clinical and translational opportunities and implications for the future of discovery research in thrombosis and hemostasis.
FIGURE 2.
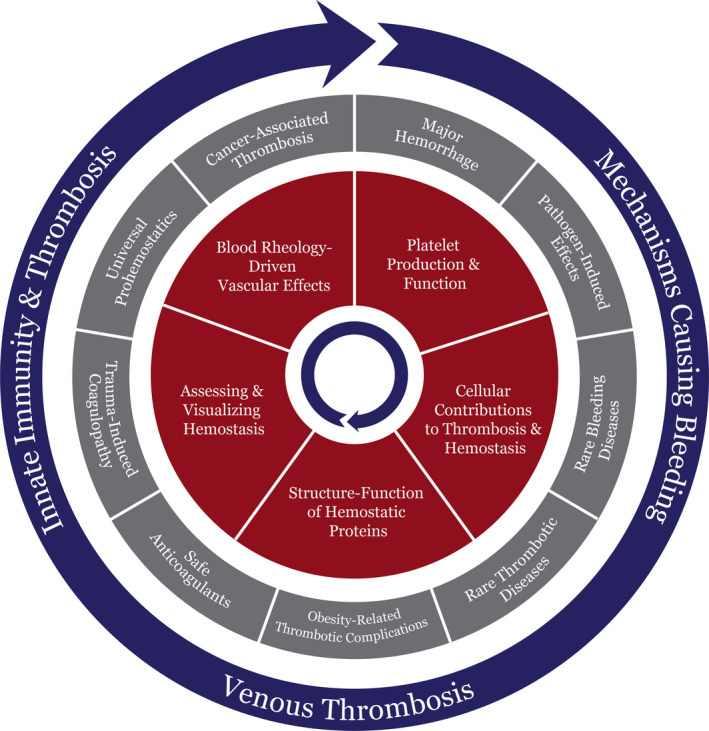
Opportunity areas in fundamental science in thrombosis and hemostasis. The 14 opportunity areas identified by the basic science task force were divided in key fundamental research topics (red) that can address the clinical and translational themes (gray) and their relationship with the three overarching categories (blue). The red and blue circles spin to align and address several opportunity areas in the static gray circle. The fundamental research topics are essential to drive new discoveries in clinical and translational research to enhance our understanding, diagnosis, and treatment of thrombotic and hemostatic disorders
3.1. Key fundamental research topics
3.1.1. Blood rheology–driven vascular effects
Hemodynamics play a central role in hemostasis and arterial thrombosis, affecting all aspects of platelet function and coagulation. Thrombus formation is triggered by platelet–platelet and platelet‐vessel wall interactions facilitated by blood flow, making rheology an important consideration in (1) platelet receptor function, such as von Willebrand factor (VWF) binding to glycoprotein (GP)Ib‐IX‐V; (2) thrombus stability, such as the physical removal/embolization of platelet aggregates; (3) the modulation of shear‐sensitive receptors, and metalloproteinase function; (4) fibrin deposition and structure; (5) the onset of fibrinolysis; and (6) the delivery of antithrombotic and thrombolytic therapeutics. Clinical evaluation of platelet function and coagulation pathways largely rely on systems that exclude flow and shear stress considerations, and a major opportunity exists to develop new approaches, such as microfluidic devices that include dynamic rheology as a contributing factor.
Parallel plate flow chambers, glass microcapillaries, and microfluidic flow devices are established tools used extensively in hemostasis and thrombosis research, including mechanistic and pharmacological studies, antiplatelet agent screening, and diagnostic developments. 3 , 4 These tools allow evaluation of thrombotic events under conditions that mimic the variable hemodynamic conditions found throughout the vasculature and in different (patho‐)physiological settings. The next set of challenges will be to fully recapitulate vascular attributes through thrombus formation studies, where thrombin generation is permitted; and through standardized studies of irregular vascular geometries, where contributions from the endothelium, pressure gradients, and deformable basement membrane/matrices are included.
3.1.2. Platelet production and function
Hematopoiesis generates bone marrow‐resident megakaryocytes (MKs) that ensure continuous platelet production of 1011 platelets per day to maintain levels of 150 000–400 000 platelets per microliter of blood. 5 Circulating platelet numbers are tightly regulated and determined by a host of genetic and molecular factors that control MK maturation and platelet release. 6 In healthy humans, platelet levels rarely stray from precise numbers, but the mechanisms governing platelet production remain poorly understood. MKs, the platelet precursor cells, originate from pluripotent hematopoietic stem cells (HSCs) through a specialist series of lineage commitment steps, which are regulated by cytokine and growth factor signaling and differential expression of several transcription factors. 7 MKs undertake a unique series of maturation steps that begin with endomitosis and polyploidization, followed by internal demarcation membrane system development, and finally proplatelet and platelet formation. 8 A role for turbulent flow in proplatelet release 9 has been proposed but, along with other pertinent factors, remains to be fully elucidated. This would be important information for programs focused on maximizing efficient platelet production in vitro for therapeutic applications, including transfusion. 10
Thrombopoietin (TPO) is a hormone produced in the liver. TPO binds to myeloproliferative leukemia protein (cMPL), present on the surface of HSCs, MKs, and platelets, to regulate the differentiation of HSCs to MK precursor cells and enhance the rate of maturation of MKs, accelerating platelet production. However, the TPO/cMPL interaction is just one facet of the thrombopoiesis pathway. 7 There are clearly other molecular players that strongly influence platelet production in both steady‐state and emergency thrombopoiesis, as demonstrated in cMPL‐deficient mice, in which the absence of TPO/cMPL engagement led only to reduced platelet production (by approximately 80%–90%) compared to healthy platelet counts. 11
TPO mimetics are approved for use in only limited clinical situations. With these mimetics, increased platelet counts occur in most recipients after months of treatment; however, responses are often not durable or clinically meaningful. 12 Several studies have identified HSCs with an intrinsic MK bias and propensity to commit directly to the MK lineage, producing MK progenitors, MKs, and platelets bearing the stem cell antigen (Sca)‐1 surface marker through this MK‐biased pathway. 8 Performing detailed analyses of this MK‐biased pathway and identifying other thrombopoietic pathways remain key focuses of the field. Such analyses could reveal mechanisms of thrombopoiesis regulation that could be targeted by therapeutics treating thrombocytopenia in clinical situations in which bone marrow function is ablated.
3.1.3. Cellular contributions to thrombosis and hemostasis
Traditionally, non‐nucleated platelets have been considered the primary cellular component of thrombosis and hemostasis. Platelets are critical for stopping bleeding at injured vessel sites, but they also play an essential role in the development of arterial thrombosis. Depending on the agonist and environment, platelets are known to react in different ways. The existence of subpopulations of platelets, such as aggregating and procoagulant platelets, has become a subject of intensive research in the last decade. 13 Future research on the generation and definition of platelet subpopulations and on their potentially distinct (patho)physiological roles in thrombosis, hemostasis, and inflammation is needed to identify novel therapeutic opportunities in the management of bleeding or thrombotic complications.
As our knowledge expands, other blood cells such as leukocytes and red blood cells (RBCs) are increasingly recognized as active contributors to thrombosis, but the underlying mechanisms are incompletely understood. For example, monocytes, macrophages, and neutrophils are known to regulate coagulation by expressing and releasing coagulation and fibrinolytic factors. 14
Animal models of thrombosis have demonstrated a key role of neutrophils, either directly or after binding to platelets, in venous and arterial thrombosis. 15 An important mechanism by which neutrophils can drive thrombus propagation is via the generation of neutrophil extracellular traps (NETs). 16 The mechanisms via which NETs contribute to thrombosis include tissue factor and factor XII (FXII)‐mediated initiation of coagulation, adhesion of platelets, recruitment of platelet adhesive proteins such as VWF, recruitment of red blood cells, and inhibition of thrombus breakdown. 17 Continued research is required to further unravel the precise temporal and spatial involvement of leukocytes in different pathophysiological contexts, preferentially using physiologically relevant in vivo models of thrombosis. There has been renewed interest in the concept of RBC‐mediated cellular effects on thrombosis and hemostasis. RBCs modulate blood clotting through various mechanisms, such as changing the blood flow viscosity and flow dynamics, forming procoagulant microparticles, interacting with platelets and endothelium, expressing adhesive proteins, and forming an impermeable barrier of tightly packed polyhedrocytes after clot contraction. 18 An important consideration in regulation of thrombosis and hemostasis is the interaction of platelets, leukocytes, and RBCs with the endothelium. Indeed, endothelial cells are also a crucial element of the hemostatic system and their integrity and functionality are critical to maintaining normal hemostasis and preventing thrombosis. Future research should focus on the reciprocal interaction of blood cells with the endothelium and with each other. Better understanding of the fundamental mechanisms of cellular mechanisms that drive thrombosis and hemostasis will lead to the identification of novel potential targets and pharmaceutical interventions for hemostatic and thrombotic disorders, potentially with reduced risks of bleeding. Such knowledge will impact other key areas of development, such as safe anticoagulants, blood rheology‐driven vascular effects, and major hemorrhage.
3.1.4. Structure–function of hemostatic proteins
There are many examples in which the structure–function of proteins has dictated the design and development of therapeutic strategies. Structure–function analysis of proteins can lead to enhanced function of the target or insensitivity to drugs or the human immune system. For example, tenecteplase is a second‐generation thrombolytic agent that was developed from alteplase—a first‐generation agent that is a recombinant form of tissue‐type plasminogen activator (tPA). Protein engineering following structure–function analyses led to the development of tenecteplase, which has improved affinity for fibrin, increased resistance to plasminogen activator inhibitor 1, and augmented half‐life in vivo compared to alteplase. 19 In addition, modifications to ADAMTS‐13 (A Disintegrin And Metalloprotease with ThrombSpondin type 1 motif, 13) render it resistant to autoantibody targeting during thrombotic thrombocytopenic purpura (TTP). 20
Determination of protein structures has been instrumental in the characterization of anticoagulant drugs. For example, the structures of thrombin and factor Xa have defined the interaction between the direct oral anticoagulants (DOACs) and their target enzymes via molecular docking of inhibitors into their target substrates. 21 The antiplatelet drug tirofiban (Aggrastat®) is based on the observation that the Arg‐Gly‐Asp (RGD) triad identified in the parent disintegrin, echistatin (isolated from the venom of the saw‐scaled viper), constitutes a specific recognition element for the platelet integrin αIIbβ3. Medicinal design of this compound resulted in a peptidomimetic, which had a 3000‐fold increase in potency in inhibiting platelet aggregation over its parental compound echistatin. 22 , 23 In addition, elucidation of structural changes that occur during activation of coagulation zymogens, factor XI, and prekallikrein, has provided a structural basis for the design of new and potentially safer anticoagulants, which can specifically target zymogen activation to prevent downstream thrombin generation. 24 , 25
3.1.5. Assessing and visualizing hemostasis
Assessing and visualizing hemostasis represents a fundamental research area in the thrombosis and hemostasis field. The aim is to better understand clot characteristics, including clot formation and degradation processes. This includes examining clot formation at the molecular level, such as assessing associated protein interactions. Evaluation of clot characteristics can also be performed in more complex systems, such as ex vivo studies of plasma or whole blood, including all blood cells; in vitro models that include endothelial cells and blood flow; investigations using material such as obstructed blood vessels from deceased or living patients; and in vivo animal models. Techniques employed to assess and visualize hemostasis are hence extremely diverse. Examples include turbidimetric clot formation and lysis assays performed using plasma 26 , 27 ; Chandler loop 28 , 29 and thromboelastography/rotational thromboelastometry experiments using whole blood 30 ; and microfluidic models that mimic a vessel structure lined with endothelial cells 4 , 31 , 32 , 33 which, when combined with confocal microscopy, allow real‐time clot formation experiments in whole blood under flow conditions. Of these techniques, recent developments include microfluidic models of various vessel geometries (such as bifurcations) and co‐cultures of endothelial cells with vascular smooth muscle cells. 34 , 35 In addition, clot characteristics have been assessed in obstructed blood vessels obtained from deceased patients or from patients undergoing surgical procedures. Intravital microscopy in animal models has allowed visualization and assessment of hemostatic plug formation in real‐time in living organisms, while monitoring therapy outcomes. Animal models for thrombosis include not only the most common mouse models, but also various species from zebrafish to non‐human primates and a variety of triggers such as ferric chloride, laser injury, or mechanical (stasis). 36 , 37 , 38
Methods to assess and visualize hemostasis are critical because they link to all opportunity categories and areas identified in this article. These methods can serve as tools to better understand underlying mechanisms in basic research and in translational research, allowing stratification of patients according to certain characteristics and risk factors. Methods to assess and visualize hemostasis will also be instrumental to approach prominent knowledge gaps, such as differences between the physiological process of hemostasis versus the pathological process of thrombosis, or the factors that determine hemostasis and thrombosis in different vascular beds.
3.2. Overarching clinical and translational opportunity areas
These five fundamental research topics are crucial to our understanding and development of the opportunity areas identified in Table 2. Below, we discuss the overarching opportunity categories that encompass the various clinical and translational themes addressing under‐researched areas in thrombosis and hemostasis (Figure 2).
3.2.1. Mechanisms causing bleeding
Bleeding due to impaired hemostasis and increased antithrombotic activity can have serious consequences; uncontrollable bleeding is often fatal or permanently disabling, and thereby represents a serious medical challenge. Despite advances in identifying targets to potentially improve the efficacies of hemostatic and antithrombotic therapies, evaluation of the safety profile of novel reagents relies on testing of naïve volunteers in clinical trials. This is in part due to a lack of a comprehensive understanding of the mechanisms that cause bleeding in conditions such as trauma‐induced coagulopathy, rare bleeding disorders, and major hemorrhage (Figure 2).
The key mechanisms that drive occlusive thrombus formation have largely been elucidated through in silico, in vitro, ex vivo, and in vivo models of intravascular thrombus formation in diseased vascular beds under shear flow conditions. Experimental models of bleeding that replicate rheological and vascular conditions found in vivo are less common and underdeveloped. The anxiety around assessing safety is largely due to basic scientific and fundamental mechanisms underlying hemostasis remaining undefined. The study of hemostasis in vitro will require development of microfluidic models of hemostatic plug formation, termed “bleeding chips,” 39 to study the spatial dynamics and cell biology of hemostasis under shear flow and samples with variable platelet counts. 39 , 40 , 41 The continued development of techniques such as photolithography to generate relevant vascular bed‐specific geometries that can be endothelialized may be used to study pathways that protect against vascular leakage, to identify targets, and to test agents that enhance the hemostatic function of platelets, endothelial cells, and the coagulation cascade without causing thrombosis. 4 , 31 , 42 Novel in vivo models are required to study bleeding observed in vascular beds with high fibrinolytic activity and variable pressure gradients, which are critical in terms of patient safety and health; these new models would complement the current models of hemostatic plug formation in the setting of experimental trauma (such as punctures, tail clippings, and lacerations to the forearm). 43 , 44
A mechanistic understanding of thrombosis has facilitated the development of antithrombotic agents, targeting either platelets or the coagulation cascade, for use in the prevention and treatment of cardiovascular diseases. A fundamental understanding of the physiological interplay between blood cells and coagulation in the context of the hemodynamic microenvironment of vessels and tissues is required to predict bleeding events and to develop novel therapeutics to treat hemorrhage. This knowledge is requisite for predicting how hemostatic homeostasis may be challenged by on‐ or off‐target effects of drugs, changes in blood cell counts, or pathological challenges (including bacteria and viruses).
3.2.2. Innate immunity and thrombosis
Thrombosis, inflammation, and innate immune responses are closely linked. Various effectors of the hemostatic system are also potent pro‐inflammatory mediators, and several innate immune responses promote the formation of a blood clot. Consequently, the terms “thromboinflammation” and “immunothrombosis” have been coined, emphasizing the close relationship between inflammation and thrombosis and the immune system and thrombosis, respectively. 26 For example, fibrin can stabilize hemostatic plugs to prevent blood loss after vascular injury, while also forming a physical barrier against invading pathogens. Similarly, both platelets and leukocytes actively bridge thrombotic and inflammatory pathways via common molecular amplification loops. 45 Although the concept of immunothrombosis indicates that thrombus formation may have a role in immune defense, uncontrolled escalation of the interplay between immune responses, inflammation, and hemostasis can trigger or aggravate typical immunothrombotic or thromboinflammatory complications, such as disseminated intravascular coagulation (DIC) in sepsis and acute thrombotic events in patients with atherosclerosis. Thrombotic complications in COVID‐19 are another recent example that has been thrown into the limelight. 46 , 47
Research opportunities in the field of innate immunity and thrombosis include understanding the fundamental role of neutrophils, particularly NETs; the cellular and molecular mechanisms of platelet–leukocyte interactions; and the involvement of the complement system. Generated by neutrophils that release their decondensed chromatin as a network of extracellular fibers, NETs form a scaffold that is an important immune strategy against pathogens and is implicated in thrombosis. 48 , 49 Platelets and leukocytes can exert thromboinflammatory effects via direct binding of platelets to innate immune cells (neutrophils and monocytes/macrophages) or via secretion of cytokines/chemokines. 45 , 50 Multiple bidirectional interactions between the complement system and coagulation have been described. 46 The challenge is now to extract those interactions that occur in vivo and are of (patho)‐physiological relevance to identify potential therapeutic targets. Increasing our fundamental understanding of the cellular and molecular aspects constituting the link between innate immunity and thrombosis can be complemented via other opportunity areas, including assessing and visualizing hemostasis, the structure–function relationships of hemostatic proteins, and the development of safe anticoagulants (Table 2).
A better understanding of the fundamental mechanisms underlying immunothrombosis will help to identify new antithrombotic drug targets that do not interfere with normal hemostasis or immune response. Such insights could also become relevant for novel translational research lines that focus on new pharmacological antithrombotic approaches, such as pathogen‐induced effects (such as COVID‐19 and sepsis), obesity‐related thrombotic complications, or rare thrombotic diseases.
3.2.3. Venous thrombosis
Venous thromboembolism is a multifactorial, chronic disorder associated with considerable morbidity and mortality, resulting in a major burden to health care and the economy (Table 2). Every year, there are approximately 10 million cases of VTE worldwide, with approximately 60% of VTE cases manifesting during or after hospitalization. 51 A recent report from the American Heart Association and the ISTH highlights the future research priorities in VTE. 1 VTE increases exponentially with age 52 , 53 , 54 , 55 and is dramatically elevated in individuals over 55 years. 56 VTE is also associated with several disease states, with active cancer accounting for approximately 20% of the overall incidence of VTE. 57 , 58 Importantly, cancer is a major cause of death in VTE patients and vice versa. Obesity is associated with a 2‐ to 5‐fold increase in VTE compared to individuals within the normal body mass index range, 59 , 60 , 61 and similar significant increases in VTE are noted in metabolic syndrome 62 , 63 , 64 , 65 and diabetic patients. 66 , 67 The relationship between VTE and pathogenic infections has been an area of interest for some years but has jostled to the forefront by way of the COVID‐19 pandemic. 68 , 69 , 70 , 71 VTE is now largely considered an immunothrombotic condition that may arise from changes associated with pathogenic infection or during sterile inflammatory states. To manage the burden of VTE in our society, it is imperative that we acquire a solid understanding of pathophysiological mechanisms that underlie this condition.
Elucidating the pathophysiology of VTE has been challenging due to the lack of models and tools available to replicate the complex underlying mechanisms. The German clinician Rudolf Virchow first described three key factors that predispose an individual to VTE, including venous stasis, a hypercoagulable state, and endothelial dysfunction, collectively known as “Virchow’s triad.” Virchow recognized that most diseases are caused by changes at the cellular level, underscoring the importance of fundamental research in this area. Blood rheology very much determines the fate of a (venous) clot, and the strength of the thrombus. With structure–function research of proteins involved in the thrombotic process, novel targets for treatment may be identified. The involvement of platelets in venous thrombosis has gained traction recently. Similarly, the participation of other cells in the venous thrombotic process has received renewed interest, particularly perturbations in the endothelial layer, interactions between RBCs and the fibrin, and the role of NETs.
A major preclinical approach for understanding the mechanisms underpinning VTE is the development of appropriate models to reflect the various elements of the disease. In the past decade, several advances in animal models and biorheology technology have helped shape our understanding of VTE. 72 , 73 , 74 , 75 Use of these models has permitted visualization of initiation, propagation, and stabilization events in deep vein thrombosis (DVT). These models are invaluable tools in exploring the complex etiology of the disease and in identifying novel targets for prevention and treatment of VTE. Combined with our advances in the understanding of cellular contributions to VTE, these models will define novel targets to moderate the hemostatic and inflammatory pathways to negate the burden of VTE on society. Recently, DOACs have proved to be effective in VTE treatment, but it is crucial that we detect the condition at an earlier stage and that we develop safer anticoagulants, such as those that target the contact pathway for effective management of VTE. Similarly, given the immunothrombotic complications in VTE, therapeutic areas to explore may involve targeting the inflammatory and complement pathways to dampen these pathways in vivo and promote more efficient thrombus resolution. To identify novel target areas it is probable that an “omic” approach in large population‐based epidemiological studies will be necessary. Inclusion of biological samples in biorepositories integrated with demographics and clinical and laboratory data will help to tease out the key drivers and shared risk factors for VTE in society.
4. CONCLUDING COMMENTS
Thromboembolic conditions account for one in four deaths worldwide and remain the leading cause of mortality despite increased awareness of the disease and the development of novel treatment and diagnostic options. The rising aging population and the significant burden of metabolic disease and obesity indicate VTE may steadily increase despite our advances. It is apparent that we need to invest significantly in understanding the mechanisms underpinning these processes to decrease the incidence of mortality and disability caused by thrombotic conditions. To tackle this burden, we must employ a holistic approach in which we identify the basic mechanisms and interactions that drive thromboembolic disease. Similarly, the key drivers of hemostatic dysregulation in bleeding and trauma‐induced coagulopathy are poorly understudied and mechanistically undefined. We require the development of novel tools, models, and imaging systems to drive our understanding of these conditions and to develop novel approaches with which to treat patients. This can be achieved by employing a reciprocal “bench to bedside” and “bedside to bench” pipeline (Figure 3), to accelerate our understanding and develop novel therapeutics to treat the myriad hemostatic complications. This was evident during the global COVID‐19 pandemic during which scientists and clinicians worldwide rallied to understand the mechanisms promoting the coagulopathy associated with SARS‐CoV‐2 infection 76 and to define appropriate treatment options. 77 It is commendable to see the importance of fundamental science underscored in this crisis and heartening to see colleagues worldwide work together to resolve issues. It will be important to deploy this approach to tackle other important thrombotic and hemorrhagic conditions.
FIGURE 3.
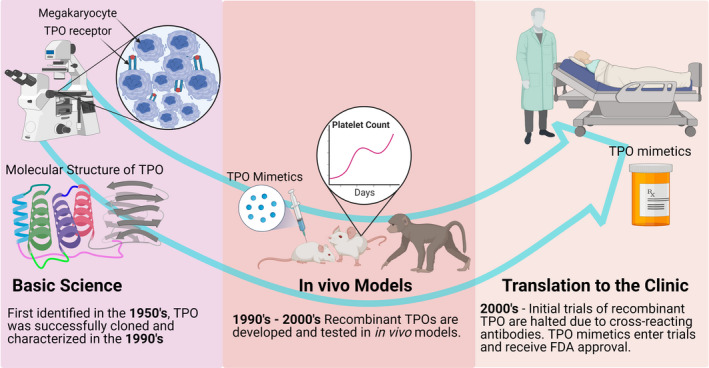
An example of a pipeline from discovery research through to a therapeutic with clear clinical outcomes. The existence of a hormone that regulated platelet production was first proposed in 1958, 78 but was not purified until 1994. 79 In the intervening years, dedicated discovery research was able to define the cellular pathway by which platelets were produced in the bone marrow by megakaryocytes, with careful cell culture and microscopy, protein purification techniques, and animal models of thrombocytopenia. Decades of work demonstrated that megakaryocytes respond to thrombocytopenia by increasing their number, size, and DNA ploidy, and from this fundamental research platform, the TPO mimetics were created, tested, and released for clinical use. FDA, US Food and Drug Administration; TPO, thrombopoietin
The SSC of the ISTH has played a vital role in evolving our understanding of hemostasis and thrombosis and in defining novel and appropriate models and diagnostic tools. The subcommittees thread together all aspects of hemostasis and thrombosis from fundamental research to translational approaches and clinical issues. However, it is evident in this setting that the funding available to discovery scientists to tackle key scientific questions has significantly dwindled in the past two decades thereby providing major challenges to researchers in the field. This is a call to action to challenge these financial constraints faced by fundamental scientists to foster new discoveries, identify novel targets, and develop sensitive therapeutics. This will promote interplay among all parties to tackle the key clinical and fundamental science questions in our field and improve diagnosis, treatment, and prevention of hemostatic and thrombotic conditions.
CONFLICTS OF INTEREST
NJM, EEG, OJTM, SFDM, VS, and JCMM declare no conflicts of interest. SW serves as a paid consultant to ISTH.
AUTHOR CONTRIBUTIONS
All authors were members of the ISTH Basic Sciences Task Force and were involved in the identification and grading of the priorities. All authors contributed to the writing of the manuscript and approved the final version. NJM and JCMM edited and revised the manuscript.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
We thank Drs. Hari Hara Sudhan Lakshmanan and Sven Olson for illustrative assistance and design.
Mutch NJ, Walters S, Gardiner EE, et al. Basic science research opportunities in thrombosis and hemostasis: Communication from the SSC of the ISTH. J Thromb Haemost. 2022;20:1496–1506. doi: 10.1111/jth.15718
Contributor Information
Nicola J. Mutch, Email: n.j.mutch@abdn.ac.uk.
Joost C. M. Meijers, Email: j.c.meijers@amsterdamumc.nl.
REFERENCES
- 1. Cushman M, Barnes GD, Creager MA et al. Venous thromboembolism research priorities: a scientific statement from the American Heart Association and the International Society on Thrombosis and Haemostasis. Circulation. 2020;142:e85–e94. [DOI] [PubMed] [Google Scholar]
- 2. Malik AH, Yandrapalli S, Aronow WS, Panza JA, Cooper HA. Meta‐analysis of direct‐acting oral anticoagulants compared with warfarin in patients >75 years of age. Am J Cardiol. 2019;123:2051‐2057. [DOI] [PubMed] [Google Scholar]
- 3. Colace TV, Tormoen GW, McCarty OJ, Diamond SL. Microfluidics and coagulation biology. Annu Rev Biomed Eng. 2013;15:283‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zilberman‐Rudenko J, Sylman JL, Garland KS, et al. Utility of microfluidic devices to study the platelet‐endothelium interface. Platelets. 2017;28:449‐456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grozovsky R, Giannini S, Falet H, Hoffmeister KM. Regulating billions of blood platelets: glycans and beyond. Blood. 2015;126:1877‐1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kaushansky K. Historical review: megakaryopoiesis and thrombopoiesis. Blood. 2008;111:981‐986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Behrens K, Alexander WS. Cytokine control of megakaryopoiesis. Growth Factors. 2018;36:89‐103. [DOI] [PubMed] [Google Scholar]
- 8. Noetzli LJ, French SL, Machlus KR. New insights into the differentiation of megakaryocytes from hematopoietic progenitors. Arterioscler Thromb Vasc Biol. 2019;39:1288‐1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abbonante V, Di Buduo CA, Malara A, Laurent PA, Balduini A. Mechanisms of platelet release: in vivo studies and in vitro modeling. Platelets. 2020;31:717‐723. [DOI] [PubMed] [Google Scholar]
- 10. Evans AL, Dalby A, Foster HR, et al. Transfer to the clinic: refining forward programming of hPSCs to megakaryocytes for platelet production in bioreactors. Blood Adv. 2021;5:1977‐1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gurney AL, Carver‐Moore K, de Sauvage FJ, Moore MW. Thrombocytopenia in c‐mpl‐deficient mice. Science. 1994;265:1445‐1447. [DOI] [PubMed] [Google Scholar]
- 12. Ghanima W, Cooper N, Rodeghiero F, Godeau B, Bussel JB. Thrombopoietin receptor agonists: ten years later. Haematologica. 2019;104:1112‐1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aslan JE. Platelet proteomes, pathways, and phenotypes as informants of vascular wellness and disease. Arterioscler Thromb Vasc Biol. 2021;41:999‐1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Swystun LL, Liaw PC. The role of leukocytes in thrombosis. Blood. 2016;128:753‐762. [DOI] [PubMed] [Google Scholar]
- 15. Lisman T. Platelet‐neutrophil interactions as drivers of inflammatory and thrombotic disease. Cell Tissue Res. 2018;371:567‐576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fuchs TA, Brill A, Duerschmied D, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci USA. 2010;107:15880‐15885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhou Y, Tao W, Shen F, Du W, Xu Z, Liu Z. The emerging role of neutrophil extracellular traps in arterial, venous and cancer‐associated thrombosis. Front Cardiovasc Med. 2021;8:786387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weisel JW, Litvinov RI. Red blood cells: the forgotten player in hemostasis and thrombosis. J Thromb Haemost. 2019;17:271‐282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Keyt BA, Paoni NF, Refino CJ, et al. A faster‐acting and more potent form of tissue plasminogen activator. Proc Natl Acad Sci USA. 1994;91:3670‐3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Graca NAG, Ercig B, Carolina Velasquez Pereira L, et al. Modifying ADAMTS13 to modulate binding of pathogenic autoantibodies of patients with acquired thrombotic thrombocytopenic purpura. Haematologica. 2020;105:2619‐2630. 10.3324/haematol.2019.226068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fischer PM. Design of small‐molecule active‐site inhibitors of the S1A family proteases as procoagulant and anticoagulant drugs. J Med Chem. 2018;61:3799‐3822. [DOI] [PubMed] [Google Scholar]
- 22. Peerschke EI, Reid KB, Ghebrehiwet B. Identification of a novel 33‐kDa C1q‐binding site on human blood platelets. J Immunol. 1994;152:5896‐5901. [PubMed] [Google Scholar]
- 23. Egbertson MS, Chang CT, Duggan ME, et al. Non‐peptide fibrinogen receptor antagonists. 2. Optimization of a tyrosine template as a mimic for Arg‐Gly‐Asp. J Med Chem. 1994;37:2537‐2551. [DOI] [PubMed] [Google Scholar]
- 24. Li C, Voos KM, Pathak M, et al. Plasma kallikrein structure reveals apple domain disc rotated conformation compared to factor XI. J Thromb Haemost. 2019;17:759‐770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bar Barroeta A, van Galen J, Stroo I, Marquart JA, Meijer AB, Meijers JCM. Hydrogen‐deuterium exchange mass spectrometry highlights conformational changes induced by factor XI activation and binding of factor IX to factor XIa. J Thromb Haemost. 2019;17:2047‐2055. 10.1111/jth.14632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pieters M, Philippou H, Undas A, de Lange Z, Rijken DC, Mutch NJ. Subcommittee on Factor X, Fibrinogen, the Subcommittee on F. An international study on the feasibility of a standardized combined plasma clot turbidity and lysis assay: communication from the SSC of the ISTH. J Thromb Haemost. 2018;16:1007‐1012. [DOI] [PubMed] [Google Scholar]
- 27. Longstaff C. Measuring fibrinolysis. Hamostaseologie. 2021;41:69‐75. [DOI] [PubMed] [Google Scholar]
- 28. Mutch NJ, Thomas L, Moore NR, Lisiak KM, Booth NA. TAFIa, PAI‐1 and alpha‐antiplasmin: complementary roles in regulating lysis of thrombi and plasma clots. J Thromb Haemost. 2007;5:812‐817. [DOI] [PubMed] [Google Scholar]
- 29. Mutch NJ, Koikkalainen JS, Fraser SR, et al. Model thrombi formed under flow reveal the role of factor XIII‐mediated cross‐linking in resistance to fibrinolysis. J Thromb Haemost. 2010;8:2017‐2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Drumheller BC, Stein DM, Moore LJ, Rizoli SB, Cohen MJ. Thromboelastography and rotational thromboelastometry for the surgical intensivist: a narrative review. J Trauma Acute Care Surg. 2019;86:710‐721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zilberman‐Rudenko J, McCarty OJT. Utility and development of microfluidic platforms for platelet research. Platelets. 2017;28:425‐426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hesh CA, Qiu Y, Lam WA. Vascularized microfluidics and the blood‐endothelium interface. Micromachines. 2020;11(1):18. 10.3390/mi11010018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mangin PH, Gardiner EE, Nesbitt WS, et al. Subcommittee on B. In vitro flow based systems to study platelet function and thrombus formation: Recommendations for standardization: communication from the SSC on Biorheology of the ISTH. J Thromb Haemost. 2020;18:748‐752. [DOI] [PubMed] [Google Scholar]
- 34. Akbari E, Spychalski GB, Rangharajan KK, Prakash S, Song JW. Flow dynamics control endothelial permeability in a microfluidic vessel bifurcation model. Lab Chip. 2018;18:1084‐1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nguyen N, Thurgood P, Sekar NC, et al. Microfluidic models of the human circulatory system: versatile platforms for exploring mechanobiology and disease modeling. Biophys Rev. 2021;13:769‐786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stalker TJ. Mouse laser injury models: variations on a theme. Platelets. 2020;31:423‐431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Albadawi H, Witting AA, Pershad Y, Wallace A, Fleck AR, Hoang P, Khademhosseini A, Oklu R. Animal models of venous thrombosis. Cardiovasc Diagn Ther. 2017;7(S3):S197–S206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jagadeeswaran P, Cooley BC, Gross PL, Mackman N. Animal models of thrombosis from zebrafish to nonhuman primates: use in the elucidation of new pathologic pathways and the development of antithrombotic drugs. Circ Res. 2016;118:1363‐1379. [DOI] [PubMed] [Google Scholar]
- 39. Poventud‐Fuentes I, Kwon KW, Seo J, et al. A Human vascular injury‐on‐a‐chip model of hemostasis. Small. 2021;17:e2004889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jigar Panchal H, Kent NJ, Knox AJS, Harris LF. Microfluidics in haemostasis: a review. Molecules. 2020;25(4):833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Caruso C, Lam WA. Point‐of‐care diagnostic assays and novel preclinical technologies for hemostasis and thrombosis. Semin Thromb Hemost. 2021;47:120‐128. [DOI] [PubMed] [Google Scholar]
- 42. Zilberman‐Rudenko J, White RM, Zilberman DA, et al. Design and utility of a point‐of‐care microfluidic platform to assess hematocrit and blood coagulation. Cell Mol Bioeng. 2018;11:519‐529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tomaiuolo M, Matzko CN, Poventud‐Fuentes I, Weisel JW, Brass LF, Stalker TJ. Interrelationships between structure and function during the hemostatic response to injury. Proc Natl Acad Sci USA. 2019;116:2243‐2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ivanciu L, Stalker TJ. Spatiotemporal regulation of coagulation and platelet activation during the hemostatic response in vivo. J Thromb Haemost. 2015;13:1949‐1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Iba T, Levy JH. Inflammation and thrombosis: roles of neutrophils, platelets and endothelial cells and their interactions in thrombus formation during sepsis. J Thromb Haemost. 2018;16:231‐241. [DOI] [PubMed] [Google Scholar]
- 46. Garred P, Tenner AJ, Mollnes TE. Therapeutic targeting of the complement system: from rare diseases to pandemics. Pharmacol Rev. 2021;73:792‐827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Martinod K, Deppermann C. Immunothrombosis and thromboinflammation in host defense and disease. Platelets. 2021;32:314‐324. [DOI] [PubMed] [Google Scholar]
- 48. Laridan E, Martinod K, De Meyer SF. Neutrophil extracellular traps in arterial and venous thrombosis. Semin Thromb Hemost. 2019;45:86‐93. [DOI] [PubMed] [Google Scholar]
- 49. Sorvillo N, Cherpokova D, Martinod K, Wagner DD. Extracellular DNA NET‐works with dire consequences for health. Circ Res. 2019;125:470‐488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Noubouossie DF, Reeves BN, Strahl BD, Key NS. Neutrophils: back in the thrombosis spotlight. Blood. 2019;133:2186‐2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jha AK, Larizgoitia I, Audera‐Lopez C, Prasopa‐Plaizier N, Waters H, Bates DW. The global burden of unsafe medical care: analytic modelling of observational studies. BMJ Qual Saf. 2013;22:809‐815. [DOI] [PubMed] [Google Scholar]
- 52. Anderson FA Jr, Wheeler HB, Goldberg RJ, et al. A population‐based perspective of the hospital incidence and case‐fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch Intern Med. 1991;151:933‐938. [PubMed] [Google Scholar]
- 53. Hansson PO, Welin L, Tibblin G, Eriksson H. Deep vein thrombosis and pulmonary embolism in the general population. ‘The Study of Men Born in 1913'. Arch Intern Med. 1997;157:1665‐1670. [PubMed] [Google Scholar]
- 54. Silverstein MD, Heit JA, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ 3rd. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25‐year population‐based study. Arch Intern Med. 1998;158:585‐593. [DOI] [PubMed] [Google Scholar]
- 55. White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107:I4‐8. [DOI] [PubMed] [Google Scholar]
- 56. Naess IA, Christiansen SC, Romundstad P, Cannegieter SC, Rosendaal FR, Hammerstrom J. Incidence and mortality of venous thrombosis: a population‐based study. J Thromb Haemost. 2007;5:692‐699. [DOI] [PubMed] [Google Scholar]
- 57. Heit JA, Spencer FA, White RH. The epidemiology of venous thromboembolism. J Thromb Thrombolysis. 2016;41:3‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Laporte S, Mismetti P, Decousus H, et al. Clinical predictors for fatal pulmonary embolism in 15,520 patients with venous thromboembolism: findings from the Registro Informatizado de la Enfermedad TromboEmbolica venosa (RIETE) Registry. Circulation. 2008;117:1711‐1716. 10.1161/circulationaha.107.726232 [DOI] [PubMed] [Google Scholar]
- 59. Yang G, De Staercke C, Hooper WC. The effects of obesity on venous thromboembolism: a review. Open J Prev Med. 2012;2:499‐509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lentz SR. Thrombosis in the setting of obesity or inflammatory bowel disease. Hematology Am Soc Hematol Educ Program. 2016;2016:180‐187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. El‐Menyar A, Asim M, Al‐Thani H. Obesity paradox in patients with deep venous thrombosis. Clin Appl Thromb Hemost. 2018;24:986‐992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ay C, Tengler T, Vormittag R, et al. Venous thromboembolism–a manifestation of the metabolic syndrome. Haematologica. 2007;92:374‐380. 10.3324/haematol.10828 [DOI] [PubMed] [Google Scholar]
- 63. Yang Y, Li Z, Liang H, Tian J. Association between metabolic syndrome and venous thromboembolism after total joint arthroplasty: a meta‐analysis of cohort studies. J Orthop Surg Res. 2020;15:570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Stewart LK, Kline JA. Metabolic syndrome increases risk of venous thromboembolism recurrence after acute pulmonary embolism. Ann Am Thorac Soc. 2020;17:821‐828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Stewart LK, Kline JA. Metabolic syndrome increases risk of venous thromboembolism recurrence after acute deep vein thrombosis. Blood Adv. 2020;4:127‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tsai AW, Cushman M, Rosamond WD, Heckbert SR, Polak JF, Folsom AR. Cardiovascular risk factors and venous thromboembolism incidence: the longitudinal investigation of thromboembolism etiology. Arch Intern Med. 2002;162:1182‐1189. [DOI] [PubMed] [Google Scholar]
- 67. Ageno W, Becattini C, Brighton T, Selby R, Kamphuisen PW. Cardiovascular risk factors and venous thromboembolism: a meta‐analysis. Circulation. 2008;117:93‐102. [DOI] [PubMed] [Google Scholar]
- 68. Porfidia A, Valeriani E, Pola R, Porreca E, Rutjes AWS, Di Nisio M. Venous thromboembolism in patients with COVID‐19: Systematic review and meta‐analysis. Thromb Res. 2020;196:67‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Malas MB, Naazie IN, Elsayed N, Mathlouthi A, Marmor R, Clary B. Thromboembolism risk of COVID‐19 is high and associated with a higher risk of mortality: a systematic review and meta‐analysis. EClinicalMedicine. 2020;29:100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tan BK, Mainbourg S, Friggeri A, et al. Arterial and venous thromboembolism in COVID‐19: a study‐level meta‐analysis. Thorax. 2021;76:970‐979. [DOI] [PubMed] [Google Scholar]
- 71. Loo J, Spittle DA, Newnham M. COVID‐19, immunothrombosis and venous thromboembolism: biological mechanisms. Thorax. 2021;76:412‐420. [DOI] [PubMed] [Google Scholar]
- 72. Diaz JA, Saha P, Cooley B, et al. Choosing a mouse model of venous thrombosis: a consensus assessment of utility and application. J Thromb Haemost. 2019;17:699‐707. [DOI] [PubMed] [Google Scholar]
- 73. Campos J, Brill A. By word of mouse: using animal models in venous thrombosis research. Platelets. 2020;31:447‐454. [DOI] [PubMed] [Google Scholar]
- 74. Lehmann M, Schoeman RM, Krohl PJ, et al. Platelets drive thrombus propagation in a hematocrit and glycoprotein VI‐dependent manner in an in vitro venous thrombosis model. Arterioscler Thromb Vasc Biol. 2018;38:1052‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. DeCortin ME, Brass LF, Diamond SL. Core and shell platelets of a thrombus: a new microfluidic assay to study mechanics and biochemistry. Res Pract Thromb Haemost. 2020;4:1158‐1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mizurini DM, Hottz ED, Bozza PT, Monteiro RQ. Fundamentals in Covid‐19‐associated thrombosis: molecular and cellular aspects. Front Cardiovasc Med. 2021;8:785738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Bikdeli B, Madhavan MV, Jimenez D, et al. Global Covid‐19 Thrombosis Collaborative Group EbtINE, the Iua SbtESCWGoPC, Right Ventricular F. COVID‐19 and Thrombotic or Thromboembolic Disease: implications for prevention, antithrombotic therapy, and follow‐up: JACC State‐of‐the‐Art Review. J Am Coll Cardiol. 2020;75:2950‐2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kelemen E, Cserhati I, Tanos B. Demonstration and some properties of human thrombopoietin in thrombocythaemic sera. Acta Haematol. 1958;20:350‐355. [DOI] [PubMed] [Google Scholar]
- 79. Kuter DJ. Milestones in understanding platelet production: a historical overview. Br J Haematol. 2014;165:248‐258. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


