Abstract
Trial‐based cost‐effectiveness analyses (CEAs) are an important source of evidence in the assessment of health interventions. In these studies, cost and effectiveness outcomes are commonly measured at multiple time points, but some observations may be missing. Restricting the analysis to the participants with complete data can lead to biased and inefficient estimates. Methods, such as multiple imputation, have been recommended as they make better use of the data available and are valid under less restrictive Missing At Random (MAR) assumption. Linear mixed effects models (LMMs) offer a simple alternative to handle missing data under MAR without requiring imputations, and have not been very well explored in the CEA context. In this manuscript, we aim to familiarize readers with LMMs and demonstrate their implementation in CEA. We illustrate the approach on a randomized trial of antidepressants, and provide the implementation code in R and Stata. We hope that the more familiar statistical framework associated with LMMs, compared to other missing data approaches, will encourage their implementation and move practitioners away from inadequate methods.
Keywords: cost‐effectiveness analysis, missing data, mixed‐effects, randomized trial, repeated measures model
1. INTRODUCTION
Cost‐effectiveness analyses (CEAs) conducted alongside randomized controlled trials are an important source of information for decision‐makers in the process of technology appraisal (Ramsey et al., 2015). The analysis is based on healthcare outcome data and health service use, typically collected at multiple time points and then combined into overall measures of effectiveness and cost (Drummond et al., 2005). The derivation of these measures is problematic in the presence of missing outcome data, which are a common issue in trials, with on average only 60%–75% of randomized participants having complete CEA data (Leurent et al., 2020; Noble et al., 2012). A popular approach to handle missingness is to discard the participants with incomplete observations (complete case analysis (CCA)), allowing for derivation of the overall measures based on the completers alone. We note that slightly different definitions of CCA are possible, depending on the form of the model of interest, the type of missingness and the inclusion of observed covariates. Without loss of generality, throughout the paper, we refer to CCA as to when only individuals with fully‐observed effect and cost data (i.e., the completers) are included in the analysis, thus requiring the removal of all cases with partially‐observed outcome data. This approach, although appealing by its simplicity, has well‐recognized limitations including loss of efficiency and an increased risk of bias (Carpenter & Kenward, 2012; Faria et al., 2014; Rubin, 1987). In recent years there has been an increment in the uptake of more appropriate statistical methods, such as multiple imputation (MI) (Leurent et al., 2018b) or Bayesian methods (Gabrio et al., 2019), which improve efficiency, rely on less restrictive missingness assumptions, and facilitate the task of conducting sensitivity analysis to alternative missingness assumptions (Gabrio et al., 2020; Leurent et al., 2020). Among these, Missing At Random [MAR ; Rubin (1987)] often provides a desirable starting point as it implies that valid inferences can be drawn based on the observed data. Both CCA and MI are generally valid under some form of MAR assumptions. When estimating a difference between arms adjusted for baseline covariates a CCA will be valid if being a complete‐case is only dependent on these covariates. Multiple imputation will be valid under less restrictive assumptions, as missingness could also depend on the observed outcome at the other time‐points and other variables not in the analysis model. Although MI has become more frequent and accessible in CEAs, its' implementation in routine analyses remains limited compared to CCA (Gabrio et al., 2017; Leurent et al., 2018a), perhaps because of a lack of familiarity, computational time, or the analytical difficulties of the approaches. Indeed, it requires particular care when combined with other statistical procedures, such as taking into account clustering (R. Gomes et al., 2012), or using bootstrap re‐sampling (Brand et al., 2019).
We propose the use of linear mixed effects models (LMMs) as an alternative approach under MAR. Linear mixed effects models are commonly used for the modeling of dependent data (e.g., repeated‐measures) and belong to the general class of likelihood‐based methods. Linear mixed effects models have been occasionally used in CEA under the denomination of multilevel or hierarchical models (Manca et al., 2005; Rice & Jones, 1997) to account for the dependence between observations with a hierarchical structure (e.g., cluster‐randomized trials). Repeated‐measures also follow a hierarchical structure since data within each individual are correlated, and LMM are increasingly used to analyze longitudinal outcome in trials. However, LMMs appear surprisingly uncommon for the analysis of repeated measures in trial‐based CEA, perhaps because of a lack of awareness or familiarity with fitting LMMs. To our knowledge, only (Faria et al., 2014) briefly examined the use of LMMs in trial‐based CEA as an alternative to MI, and concluded that they offer a valid approach under MAR.
In this letter, we aim to familiarize readers with the implementation of LMMs in trial‐based CEA using standard software and summarize the statistical and economic results from a case study. Finally, we discuss the proposed approach and provide some suggestions for future work.
2. METHODS
2.1. Linear mixed effects models for repeated measurements
Linear mixed effects model extends the usual linear model framework by the addition of “random effect” terms, which can take into account the dependence between observations. A simple model for a repeated measure can be written as
| (1) |
where Y ij denotes the outcome repeatedly collected for each individual i = 1,…,N at multiple times j = 1,…,J. The model parameters commonly referred to as fixed effects include an intercept β 1 and the coefficients (β 2 ,…,β P+1 ) associated with the predictors X i1 ,…,X iP , while ω i and ϵ ij are two random terms: ϵ ij is the usual error term and ω i is a random intercept which captures variation in outcomes between individuals. The random terms are typically assumed to be normally distributed such that and . Equation (1) treats the data as having a 2‐level structure, where and capture the variance of the responses within (level 1) and between (level 2) individuals, respectively. The models can be extended to deal with more complex structures, for example, by allowing the effect of the covariates to vary across individuals (random slope) or a different covariance structure of the errors. Linear mixed effects models can be fitted even if some outcome data are missing and provide correct inferences under MAR (Schafer & Graham, 2002). We refer interested readers to the available LMM literature for an in‐depth description of the methods (Molenberghs et al., 2004).
One particular type of LMMs commonly used in the analysis of repeated measures in clinical trials is referred to as a Mixed Model for Repeated Measurement (MMRM; Wolfinger, 1993). The model includes a categorical effect for time, an interaction between time and treatment arm, and allows errors to have different variance and correlation over time (i.e., unstructured covariance structure). Consider the case where we want to model health related quality of life data (i.e., utilities) collected at three times (baseline and two follow‐ups). The model can then be expressed as:
| (2) |
where U ij is the utility measured for patient i at time j, TIME j and TRT i are the indicators for the time and treatment arm, and TIME j TRT i are the interaction terms between time and treatment arm at each follow‐up (j ≥ 2). Within this model, β 1 , represents the mean utility at baseline, β 2 and β 4 represent the mean utility for the control arm (TRT i = 0) at the first and second follow‐up, while β 3 and β 5 capture the mean difference between the arms at the two follow‐ups. The errors at each time follow a multivariate normal distribution ϵ i ∼ N(0, Σ) with an unstructured covariance matrix Σ, that is, errors are allowed to have different variance and correlation between them. Note that no treatment parameter was included at baseline, this makes sense as no treatment effect is expected at baseline, but is also important to obtain β 3 and β 5 parameters adjusted for baseline utility (see Section 2.2). The model can be easily extended to handle additional time‐points, or to model any other repeatedly measured continuous outcome such as cost.
Incremental (between‐group) or marginal (within‐group) estimates for aggregated outcomes over the trial period, such as quality‐adjusted life years (QALYs) or total costs can be retrieved as linear combinations of the parameter estimates from Equation (2). For example, the mean difference in total cost is obtained by summing up the estimated differences at each follow‐up point, while differences on a QALYs scale can be obtained as weighted linear combinations of the coefficient estimates of the utility model (see Appendix C).
2.2. Adjusting for baseline variables
It is standard practice to adjust the analysis for imbalances in some baseline characteristics, to control for potential imbalance and as well as to increase precision (Manca et al., 2005) while also strengthening the plausibility of the MAR assumption (Little et al., 2012). We note that, differences in baseline outcome values between arms are already adjusted for within the model specification in Equation (2). Other baseline variables can be adjusted for by adding them as predictors to the model, although LMMs require covariates to be completely observed. However, in randomized controlled trials, missing baseline data can be usually addressed by implementing single imputation techniques (e.g., mean‐imputation) to obtain complete data prior to fitting the model, without loss of validity or efficiency (White & Thompson, 2005).
2.3. Assessing cost‐effectiveness
Once estimates for the average effectiveness and total cost differences are obtained, uncertainty can be assessed through resampling methods (e.g., bootstrapping). Results are then often summarized using a Cost‐Effectiveness Plane (CEP; Black [1990]) and a Cost‐Effectiveness Acceptability Curve (CEAC; Van Hout et al. [1994]).
3. TRIAL OVERVIEW
3.1. Overview
The Health Technology Assessment Study of the Use of Antidepressants for Depression in Dementia (SADD, ISRCTN88882979) was a placebo‐controlled randomized trial of participants from old‐age psychiatry services in England (Banerjee et al., 2011). A total of n = 326 participants were enrolled and randomized to receive placebo, or either one of two antidepressants (sertraline and mirtazapine) together with treatment as usual, and followed for 9 months. Outcomes were collected at baseline, 3 and 9 months, including EQ‐5D‐3L which was converted into utility (Dolan, 1997), and resource use for 0–3 months and 3–9 months which were converted into costs (Romeo et al., 2013). Details about the objective and conclusions from the trial as well as the study CEA are reported elsewhere (Banerjee et al., 2013). We restrict our attention to the 9 months cost‐utility analysis of placebo (n 1 = 111) versus mirtazapine (n 2 = 108) from the health and social care cost perspective.
Table 1 shows the missing data patterns of the utility and cost data (U j ,C j ) at baseline (j = 1), 3 months (j = 2) and 9 months (J = 3) follow‐up.
TABLE 1.
Missingness patterns for the utility and cost variables in the SADD study
| Missing data patterns | Placebo (N 1 = 111) | Mirtazapine (N 2 = 108) | Total (N = 219) |
|---|---|---|---|
| U 1 U 2 U 3 C 1 C 2 C 3 | N 1 (%) | N 2 (%) | N(%) |
| ‐ ‐ ‐ ‐ ‐ ‐ | 54 (48%) | 47 (43%) | 101 (46%) |
| ‐ X X ‐ X X | 12 (11%) | 17 (16%) | 29 (13%) |
| ‐ X X ‐ ‐ ‐ | 15 (13%) | 14 (13%) | 29 (13%) |
| ‐ ‐ X ‐ ‐ ‐ | 11 (10%) | 13 (12%) | 24 (11%) |
| ‐ ‐ X ‐ ‐ X | 9 (8%) | 8 (7%) | 17 (8%) |
| ‐ X ‐ ‐ ‐ ‐ | 4 (4%) | 4 (4%) | 8 (4%) |
| ‐ X X ‐ ‐ X | 2 (2%) | 2 (2%) | 4 (2%) |
| ‐ ‐ ‐ ‐ X X | 2 (2%) | 3 (3%) | 5 (2%) |
| ‐ ‐ ‐ ‐ ‐ X | 2 (2%) | 0 (0%) | 2 (1%) |
Note: For each pattern and treatment group the corresponding number and proportions of participants are reported. U j and C j indicate utility or cost variable at time j, while ‐ and X indicate an observed or missing variable, respectively.
Abbreviation: SADD, Study of the Use of Antidepressants for Depression in Dementia.
About half of the trial participants had complete cost‐effectiveness data, with utility values which tended to be more frequently missing than costs.
3.2. Cost‐effectiveness analysis of SADD
We applied the LMM model described above to the SADD data to estimate the difference in QALYs and cost, adjusting for baseline values (utility or cost). A set of 10,000 bootstrap replications were performed to derive cost‐effectiveness results. All models were fitted in R, using the packages and functions described in Appendix A.1. The R and Stata code for implementing the methods are given in Appendix A.2 and Appendix A.3, respectively, and on an online repository (Gabrio, 2022).
4. RESULTS
Table 2 reports the empirical means and standard deviations associated with the utility and cost variables in each treatment group over the study period.
TABLE 2.
Observed means (and standard deviations) of the utility and cost variables at baseline, 3 and 9 months follow‐up for the placebo and mirtazapine group in the SADD trial
| Placebo | (N 1 = 111) | Mirtazapine | (N 2 = 108) | |
|---|---|---|---|---|
| Utilities | ||||
| Baseline | 0.670 (0.268) | N = 111 | 0.688 (0.292) | N = 108 |
| 3 months | 0.733 (0.286) | N = 78 | 0.763 (0.267) | N = 71 |
| 9 months | 0.734 (0.274) | N = 62 | 0.827 (0.195) | N = 64 |
| Costs | ||||
| Baseline | 1514 (3153) | N = 111 | 1191 (2256) | N = 108 |
| 3 months | 1437 (3338) | N = 97 | 1130 (1868) | N = 88 |
| 9 months | 2146 (4401) | N = 84 | 2550 (4288) | N = 78 |
Abbreviation: SADD, Study of the Use of Antidepressants for Depression in Dementia.
People in the mirtazapine group had higher mean utilities at all time‐points, lower mean costs at the first follow‐up and higher mean costs at the last follow‐up.
Table 3 reports the LMM‐estimated marginal means of the utilities and costs at each follow‐up by group, as well as QALYs and total costs. Incremental results, incremental cost‐effectiveness ratio and probability of cost‐effectiveness (at an acceptance threshold of £25,000) between the two groups are also reported.
TABLE 3.
Linear mixed model results for the SADD trial
| Placebo | Mirtazapine | Incremental | ||||
|---|---|---|---|---|---|---|
| Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | |
| U 2 | 0.731 | (0.675; 0.786) | 0.752 | (0.694; 0.810) | 0.021 | (−0.053; 0.096) |
| U 3 | 0.727 | (0.671; 0.783) | 0.781 | (0.721; 0.841) | 0.054 | (−0.024; 0.132) |
| QALYs | 0.540 | (0.510; 0.571) | 0.561 | (0.530; 0.593) | 0.021 | (−0.016; 0.059) |
| C 2 | 1369 | (840; 1899) | 1252 | (695; 1809) | −117 | (−866; 631) |
| C 3 | 2092 | (1226; 2959) | 2760 | (1856; 3664) | 668 | (−544; 1880) |
| Total costs | 3462 | (2236; 4688) | 4012 | (2735; 5289) | 550 | (−1156; 2257) |
| ICER | 57,774 | |||||
| Cost‐effectiveness probability | 34% |
Note: Point estimates and 95% confidence intervals for the marginal means and mean difference of the utilities and costs at 3 (j = 2) and 9 (j = 3) months follow‐up as well as for the quality‐adjusted life years (QALYs) and total costs over the duration of the study. Incremental results are also reported in terms of the incremental cost‐effectiveness ratio (ICER) and cost‐effectiveness probability at k = £25,000 per QALY gained. Costs are expressed in British pounds.
Abbreviation: SADD, Study of the Use of Antidepressants for Depression in Dementia.
The results suggest that the mirtazapine group is associated with higher utilities compared with the placebo group at all follow‐ups, which leads to higher mean QALYs estimates. Although mean costs at 13 weeks are on average higher in the placebo group, the mirtazapine group is associated with relatively higher mean costs at 9 months as well as higher mean total costs. Overall, the mean difference in QALYs was 0.021(95%CI:−0.016;0.059), and difference in cost £550 (95%CI:−1156;2257). We additionally compare the results in terms of mean QALYs and total costs obtained under LMM with those from two alternative analyses: CCA and MI, the latter producing estimates in line with those of LMM with standard errors reduced by approximately13% for QALYs and 25% for total cost estimates compared to CCA (see Appendix B.2).
Figure 1 shows the associated CEP and CEAC. Mirtazapine appears to be slightly more effective and costly, but with important uncertainty, resulting in a probability of being cost‐effective of around 50% at a threshold of £25,000 per QALY gained.
FIGURE 1.
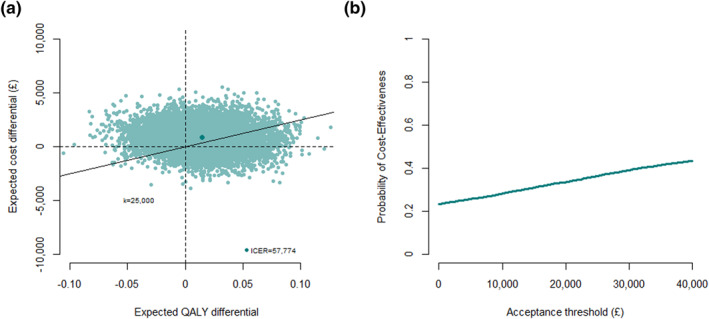
Cost‐Effectiveness Plane (CEP) and Cost‐Effectiveness Acceptability Curve from the Study of the Use of Antidepressants for Depression in Dementia based on a linear mixed effects model fitted on 10,000 bootstrap replications. In the CEP, an acceptance threshold of k = £25,000 per quality‐adjusted life year (QALY) gained is used and the position of the incremental cost‐effectiveness ratio (ICER) on the plot is denoted with a darker colored dot. (a) Cost‐effectiveness plane. (b) Cost‐effectiveness acceptability curve
5. DISCUSSION
In this article we proposed the use of LMMs as an alternative approach to conduct trial‐based CEAs Although repeatedly criticized in the literature, analysts commonly handle missingness in QALYs and total costs via CCA (Gabrio et al., 2017; Leurent et al., 2018a), which causes some loss of information and potentially biases the inferences. The use of imputation methods, especially MI, have been recommended in the literature since they can obtain valid inferences using all observed responses (Faria et al., 2014). However, there may be practical obstacles to the spread of these methods among practitioners. First, analysts may be unfamiliar with the implementation of MI, and feel more confident using simpler approaches. Second, MI can be time‐consuming, particularly when combined with bootstrapping, for which there are alternative implementation strategies possible (Brand et al., 2019).
We believe LMMs represent an alternative approach which can overcome some of these limitations. First, practitioners may be more comfortable with the standard regression framework. Second, LMMs can be tailored to address other data features (e.g., cluster‐randomized trials or non‐normal distribution) while also easily combined with bootstrapping. Third, LMMs do not rely on imputation, and results are therefore deterministic and easily reproducible, whereas the Monte Carlo error associated with MI may cause results to vary from one imputation to another, unless the number of imputations is sufficiently large.
There are also limitations associated with the use of the proposed methods. First, LMM estimates are valid under MAR conditional on the observed outcome and the baseline variables in the analysis model. Multiple‐imputation offers a more flexible framework allowing for inclusion of auxiliary variables not adjusted for in the analysis. Second, while MAR is often a reasonable starting point, sensitivity analysis under non‐at‐random assumptions should be considered (Gabrio et al., 2020; Leurent et al., 2020). While these can be conducted within a LMM framework (Daniels & Hogan, 2008), a MI or Bayesian framework are particularly well suited for this type of analyses (M. Gomes et al., 2019; Mason et al., 2018). Third, the LMM approach works well for repeatedly‐measure outcomes, but addressing missingness within each component of the outcomes (e.g., for disaggregated cost components) increases the complexity of the model. Depending on the amount of observed data and time points, the suggested model may not achieve convergence, and simpler covariance structures could be considered.
Further work can be done to extend the proposed methods to tackle some of the typical features of CEA data. An interesting extension would be to fit a bivariate LMM model to model simultaneously the costs and utilities. Furthermore, while LMMs are robust to non‐normally distributed data in large sample, generalized linear model specifications could be applied to improve the fit to the data using non‐normal distributions (Nixon & Thompson, 2005). A possible solution is to explore the use of multivariate generalized LMMs to account for the correlation and characteristics of the data while also providing a coherent modeling framework based on the same number of individuals for both outcomes.
To conclude, we have shown how LMMs can be used for the analysis of trial‐based CEAs Although the methodology illustrated is already known, particularly in the area of statistical analyses, to our knowledge LMMs have rarely been applied to health economic data collected alongside randomized trials. We believe the proposed methods is preferable to a complete‐case analysis when CEA data are incomplete, and that it can offer an interesting alternative to imputation methods.
CONFLICT OF INTEREST
Sube Banerjee reports grants from NIHR HTA Program, during the conduct of the study. Personal fees from Otsuka, Lundbeck, Lilly, Hamad Medical Services, Nutricia, Axovant, and grants from ESRC, NIHR, UKRI, outside the submitted work. Baptiste Leurent reports personal fees from GAIA AG, outside the submitted work. Andrea Gabrio and Catrin Plumpton declare that they have no conflict of interest.
ACKNOWLEDGEMENTS
We would like to thank Dr. Renee Romeo and Prof. Martin Knapp for their support in using the SADD study data, the winter 2021 HESG attendees for their comments on an initial version of this letter, and Professor Chris Frost for his insight on mixed models. The SADD study was funded by the National Institute for Health Research Health Technology Assessment (NIHR HTA) programme (project number: 04/11/02). The International Statistics and Epidemiology Group is jointly funded by the UK Medical Research Council (MRC) and the UK Foreign, Commonwealth and Development Office (FCDO) under the MRC/FCDO Concordat agreement and is also part of the EDCTP2 programme supported by the European Union. Grant Ref: MR/R010161/1.
APPENDIX A. Implementation
A.1. Software detail
All models can be fitted in R or Stata using different types of pre‐defined functions and commands. In R, we use the function lme from the package nlme, which is specifically designed for fitting LMMs and produces standard statistical outputs, such as fixed and random effect estimates and their standard errors. Estimates for all model parameters are derived based on observed utility and cost responses under MAR using maximum likelihood methods. The function emmeans from the package emmeans can then be used to calculate point estimates and uncertainty measures for linear combinations of the model parameters, including the treatment effects as well as the marginal mean QALYs and total costs by treatment group. In Stata, we use the mixed command to fit the models and then use the post‐processing commands lincom and margins to derive linear combinations of parameter estimates with associated uncertainty measures.
A.2. R code
R code for fitting the utility and cost LMMs and derive the estimates for the CEA target quantities based on the data from the SADD study.
# load the R packages
library(nlme)
library(emmeans)
# pre‐process data
# load the dataset (in long format)
data_long_SADD <‐ readRDS("data_long_SADD.rds")
# variables: time (time indicator), trt (treatment indicator), u (utility values)
# 3 time points (baseline, 13 and 39 weeks follow‐up)
# define treatment effect indicator at each follow‐up
data_long_SADD$time_2<‐ifelse(data_long_SADD$time = = 2,1,0)
data_long_SADD$time_3<‐ifelse(data_long_SADD$time = = 3,1,0)
data_long_SADD$trt_time_2<‐data_long_SADD$trt*data_long_SADD$time_2
data_long_SADD$trt_time_3<‐data_long_SADD$trt*data_long_SADD$time_3
# define time as a factor
data_long_SADD$time <‐ factor(data_long_SADD$time)
# LMM for utilities
# fit the model using the lme function and use unstructured covariance
LMM_u <‐lme(u ∼ −1 + time + trt_time_2 + trt_time_3, random = ∼ 1 | id,
data = data_long_SADD, method = "ML",
correlation = corSymm(form = ∼ as.numeric(time)|id),
weights = varIdent(form = ∼1|as.numeric(time)),
na.action = na.omit)
# look at parameter estimates and get confidence intervals (CI)
# fixed effects of interactions are the treatment effects at follow‐ups summary(LMM_u)
intervals(LMM_u, level = 0.95, which = "fixed")
# derive marginal means for the utilities in each group at each time point using the emmeans function
mu_u <‐ emmeans(LMM_u, ∼ −1 + time + trt_time_2 + trt_time_3)
# get confidence intervals
confint(mu_u)
# derive the marginal means for the QALYs in each group using the contrast function
# assign time weights to the marginals utility means to obtain correct mean QALYs
# (0.125 for baseline, 0.375 for 13 weeks follow‐up, 0.25 for 39 weeks follow‐up)
mu_e <‐ contrast(mu_u, list(mu0 = c(13/104,13/104 + 26/104,26/104,0,0,0,0,0,0,0,0,0),
mu1 = c(13/104,0,0,0,13/104 + 26/104,0,0,0,26/104,0,0,0)))
# derive mean differences between groups for the QALYs in a similar way
delta_e <‐ contrast(mu_e, list(diff = c(0,‐13/104 ‐ 26/104,‐26/104,0,13/104 + 26/104,0,0,0,26/104,0,0,0)))
# get confidence intervals
confint(mu_e)
confint(delta_e)
#LMM for costs
# fit the model using the lme function and use unstructured covariance
LMM_c <‐ lme(c ∼ −1 + time + trt_time_2 + trt_time_3, random = ∼ 1 | id,
data = data_long_SADD, method = "ML",
correlation = corSymm(form = ∼ as.numeric(time)|id),
weights = varIdent(form = ∼1|as.numeric(time)),
na.action = na.omit)
# look at parameter estimates and get CI
# fixed effects of interactions are the treatment effects at follow‐ups summary(LMM_c)
intervals(LMM_c, level = 0.95, which = "fixed")
# derive marginal means for the costs in each group at each time point using the emmeans function
mu_c <‐ emmeans(LMM_c, ∼ −1 + time + trt_time_2 + trt_time_3)
# get confidence intervals
confint(mu_c)
# derive the marginal means for the total costs in each group using the contrast function
mu_tc <‐ contrast(mu_c, list(mu0 = c(0,1,1,0,0,0,0,0,0,0,0,0),
mu1 = c(0,0,0,0,1,0,0,0,1,0,0,0)))
# derive mean differences between groups for the total costs in a similar way
delta_tc <‐ contrast(mu_tc, list(diff = c(0,‐1,‐1,0,1,0,0,0,1,0,0,0)))
# get confidence intervals
confint(mu_tc)
confint(delta_tc)
A.3. Stata code
Stata code for fitting the utility and cost LMMs and derive the estimates for the CEA target quantities based on the data from the SADD study. ** Open data use "data_long_SADD.dta", clear gen trtp = trt*(time>1) //Treatment arm indicator, for follow‐up visits only ** LMM for utilities mixed u i.time i.time#i.trtp || id:, res(unstructured, t(time)) // || id: = random effect for each participant // res(unstructured, t(time)) = unstructured covariance, for each timepoint // i.time#i.trtp = interactions between time and treatment *Incremental utility at each time point: lincom 2.time#1.trtp //Treatment effect (trtp = 1) at 3 months (time = 2) lincom 3.time#1.trtp //Treatment effect at 9 months *Incremental QALYs lincom 0.375*2.time#1.trtp + 0.25*3.time#1.trtp *Utility per arm at each time‐point margins i.time#i.trtp *QALYs per arm margins i.time#i.trtp, post lincom 0.125*1.time#0.trtp + 0.375*2.time#0.trtp + 0.25*3.time#0.trtp lincom 0.125*1.time#0.trtp + 0.375*2.time#1.trtp + 0.25*3.time#1.trtp ** LMM for costs mixed c i.time i.time#trtp || id:, res(unstructured, t(time)) *Incremental cost at each time point: lincom 2.time#1.trtp //Treatment effect at time 2 lincom 3.time#1.trtp //Treatment effect at time 3 *Incremental total cost lincom 2.time#1.trt + 3.time#1.trt *Cost per arm at each time‐point margins i.time#trtp *Total cost per arm margins i.time#trtp, post lincom 2.time#0.trt + 3.time#0.trt lincom 2.time#1.trt + 3.time#1.trt
APPENDIX B. Results
B.1. Model estimates from the application to the SADD study
Table A1 shows the parameters (and their 95% confidence intervals) from the linear mixed model fitted to the SADD trial data. These parameters can be used to derive the marginal and incrementals utilities and costs reported in Table 2. For example, the treatment arm marginal utility at time 3 (9 months) can be estimated by 0.727 + 0.054 = 0.781.
TABLE A1.
Parameters from the linear mixed models (LMM) fitted to the SADD utility and cost data
| Fixed effects | Utilities model | Costs model | ||
|---|---|---|---|---|
| Estimate | 95% CI | Estimate | 95% CI | |
| TIME 1 | 0.674 | (0.637; 0.711) | 1355 | (991; 1720) |
| TIME 2 | 0.731 | (0.675; 0.786) | 1369 | (840; 1899) |
| TIME 3 | 0.727 | (0.671; 0.783) | 2092 | (1226; 2959) |
| TIME 2 TRT | 0.021 | (−0.053; 0.095) | −117 | (−866; 631) |
| TIME 3 TRT | 0.054 | (−0.024; 0.132) | 668 | (−544; 1880) |
Note: Point estimates and 95% confidence intervals (CI).
Abbreviation: SADD, Study of the Use of Antidepressants for Depression in Dementia.
B.2. Parameters estimates under LMM, CCA and MI, fitted to the SADD data
Table A2 compares the SADD cost‐effectivenes estimates (and their standard error) under CCA, MI, and LMM, with the CCA and MI models fitted as described below.
TABLE A2.
Estimates and standard errors (SE) for the mean QALYs and total cost in the SADD trial under complete case analysis (CCA), multiple imputation (MI), and linear mixed model (LMM)
| Placebo | Mirtazapine | Incremental | ||||
|---|---|---|---|---|---|---|
| Estimate | SE | Estimate | SE | Estimate | SE | |
| CCA | ||||||
| QALYs | 0.557 | (0.016) | 0.579 | (0.017) | 0.022 | (0.023) |
| Total costs | 3375 | (774) | 3695 | (831) | 320 | (1148) |
| MI | ||||||
| QALYs | 0.541 | (0.013) | 0.561 | (0.014) | 0.021 | (0.020) |
| Total costs | 3472 | (593) | 4023 | (623) | 552 | (862) |
| LMM | ||||||
| QALYs | 0.540 | (0.016) | 0.561 | (0.016) | 0.021 | (0.019) |
| Total costs | 3462 | (623) | 4012 | (649) | 550 | (868) |
Abbreviation: QALYs, quality‐adjusted life years; SADD, Study of the Use of Antidepressants for Depression in Dementia.
Complete case analysis. The analysis used only individuals with fully‐observe utility and cost data at each time point in the SADD study, that is, the 101 completers as shown in the first row of Table 1. The analysis was conducted using separate standard linear regression models fitted at the level of QALYs and total costs, including treatment and baseline outcome values as predictors to derive adjusted estimates for the incremental mean QALYs and total costs between the groups of the study.
Multiple imputation. The analysis used all available observed utility and cost data at each time point in the SADD study and consisted in two steps. In the first step, imputation for missing utility and cost values was carried out using a MI by chained equation approach (Van Buuren, 2018). We imputed both outcomes under the assumption of normality to ensure a comparable match with the assumptions of LMM under MAR. The imputation models included observed outcome data at any time point as predictors, and imputation was performed by treatment group with a total number of 500 imputed datasets. In the second step, within each imputed dataset, outcome variables were aggregated into QALYs and total cost measures and standard linear regression models, as defined in CCA, were fitted to the imputed data and parameter estimates were derived. Finally, estimates were combined across the imputed datasets via Rubin's rules to correctly quantity missingness uncertainty.
APPENDIX C. QALYs derivation
Figure A1 illustrates how to derive the QALYs defined by the area under the curve (AUC) of the utilities, for example, for an individual with utilities of 0.3, 0.6 and 0.4 at baseline, 3 and 9 months respectively. Assuming a linear increase between utilities, the AUC is traditionally defined by the sum of multiple trapezoids aereas (in blue here). This can be rewriten as a sum of rectangular aereas (in pink here), defined by t 0.1 *u 0 +(t 0.1 +t 1.2 )*u 1 +(t 1.2 )*u 2 , where u 1 is the utility at time j, and t i.j is half of the time (in years) between time‐points i and j. For example, for this individual, AUC = 0.125*0.3 + 0.375*0.6 + 0.25*0.4 = 0.3625.
FIGURE A1.
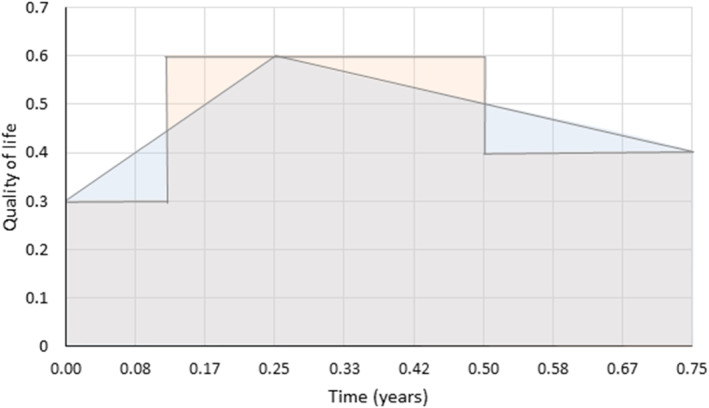
Derivation of QALYs as a weighted average of utilities
The same weights can be used to derive the mean AUC for a group of individuals (based on the group mean utility at each time‐point), or the mean difference in AUC between two groups (based on the mean difference in utility at each time point). Discounting can be applied by reducing the relevant weights, for example, after 1 year. Note that when estimating the difference in QALYs adjusted for baseline utility in trials, there is no need to include the baseline term which is null.
Gabrio, A. , Plumpton, C. , Banerjee, S. , & Leurent, B. (2022). Linear mixed models to handle missing at random data in trial‐based economic evaluations. Health Economics, 31(6), 1276–1287. 10.1002/hec.4510
DATA AVAILABILITY STATEMENT
Data from the SADD trial were provided by the investigators for the purpose of this article. They are available upon reasonable request from the chief investigator (sube.banerjee@plymouth.ac.uk) along with the other trial datasets.
REFERENCES
- Banerjee, S. , Hellier, J. , Dewey, M. , Romeo, R. , Ballard, C. , Baldwin, R. , Bentham, P. , Fox, C. , Holmes, C. , Katona, C. , Knapp, M. , Lawton, C. , Lindesay, J. , Livingston, G. , McCrae, N. , Moniz‐Cook, E. , Murray, J. , Nurock, S. , Orrell, M. , … Burns, A. (2011). Sertraline or mirtazapine for depression in dementia (HTA‐SADD): A randomised, multicentre, double‐blind, placebo‐controlled trial. The Lancet, 378(9789), 403–411. 10.1016/s0140-6736(11)60830-1 [DOI] [PubMed] [Google Scholar]
- Banerjee, S. , Hellier, J. , Romer, R. , Dewey, M. , Knapp, M. , Ballard, C. , Baldwin, R. , Bentham, P. , Fox, C. , Holmes, C. , Katona, C. , Lawton, C. , Lindesay, J. , Livingston, G. , McCrae, N. , Moniz‐Cook, E. , Murray, J. , Nurock, S. , Orrell, M. , … Burns, A. (2013). Study of the use of antidepressants for depression in dementia: The HTA‐SADD trial‐a multicentre, randomised, double‐blind, placebo‐controlled trial of the clinical effectiveness and cost‐effectiveness of sertraline and mirtazapine. Health Technology Assessment, 17(7), 1–166. 10.3310/hta17070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black, W. C. (1990). The CE plane: A graphic representation of cost‐effectiveness. Medical Decision Making, 10(3), 212–214. 10.1177/0272989x9001000308 [DOI] [PubMed] [Google Scholar]
- Brand, J. , van Buuren, S. , le Cessie, S. , & van den Hout, W. (2019). Combining multiple imputation and bootstrap in the analysis of cost‐effectiveness trial data. Statistics in Medicine, 38(2), 210–220. 10.1002/sim.7956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter, J. , & Kenward, M. (2012). Multiple imputation and its application. John Wiley & Sons. [Google Scholar]
- Daniels, M. J. , & Hogan, J. W. (2008). Missing data in longitudinal studies: Strategies for bayesian modeling and sensitivity analysis. Chapman and Hall. [Google Scholar]
- Dolan, P. (1997). Modeling valuations for EuroQol health states. Medical Care, 35(11), 1095–1108. 10.1097/00005650-199711000-00002 [DOI] [PubMed] [Google Scholar]
- Drummond, M. F. , Schulpher, M. J. , Claxton, K. , Stoddart, G. L. , & Torrance, G. W. (2005). Methods for the economic evaluation of health care programmes (3rd ed.). Oxford university press. [Google Scholar]
- Faria, A. , Gomes, M. , Epstein, D. , & White, I. (2014). A guide to handling missing data in cost‐effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics, 32(12), 1157–1170. 10.1007/s40273-014-0193-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrio, A. (2022, March 23). LMM in CEA [Repository]. Github. https://github.com/AnGabrio/Code/tree/master/lmm_in_CEA
- Gabrio, A. , Daniels, M. J. , & Baio, G. (2020). A bayesian parametric approach to handle missing longitudinal outcome data in trial‐based health economic evaluations. Journal of the Royal Statistical Society: Series A, 183(2), 607–629. 10.1111/rssa.12522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrio, A. , Mason, A. , & Baio, G. (2019). A full Bayesian model to handle structural ones and missingness in economic evaluations from individual‐level data. Statistics in Medicine, 38(8), 1399–1420. 10.1002/sim.8045 [DOI] [PubMed] [Google Scholar]
- Gabrio, A. , Mason, A. J. , & Baio, G. (2017). Handling missing data in within‐trial cost‐effectiveness analysis: A review with future recommendations. PharmacoEconomics‐Open, 1(2), 79–97. 10.1007/s41669-017-0015-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes, M. , Radice, R. , Camarena Brenes, J. , & Marra, G. (2019). Copula selection models for non‐Gaussian outcomes that are missing not at random. Statistics in Medicine, 38(3), 480–496. 10.1002/sim.7988 [DOI] [PubMed] [Google Scholar]
- Gomes, R. , Grieve, R. , Nixon, R. , & Edmunds, WJ. (2012). Statistical methods for cost‐effectiveness analyses that use data from cluster randomized trials. Medical Decision Making, 32(1), 209–220. 10.1177/0272989x11407341 [DOI] [PubMed] [Google Scholar]
- Leurent, B. , Gomes, M. , & Carpenter, J. R. (2018a). Missing data in trial‐based cost‐effectiveness analysis: An incomplete journey. Health Economics, 27(6), 1024–1040. 10.1002/hec.3654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leurent, B. , Gomes, M. , Cro, S. , Wiles, N. , & Carpenter, J. R. (2020). Reference‐based multiple imputation for missing data sensitivity analyses in trial‐based cost‐effectiveness analysis. Health Economics, 29(2), 171–184. 10.1002/hec.3963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leurent, B. , Gomes, M. , Faria, R. , Morris, S. , Grieve, R. , & Carpenter, J. (2018b). Sensitivity analysis for not‐at‐random missing data in trial‐based cost‐effectiveness analysis: A tutorial. PharmacoEconomics, 1–13(8), 889–901. 10.1007/s40273-018-0650-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little, R. J. , D’Agostino, R. , Cohen, M. L. , Dickersin, K. , Emerson, S. S. , Farrar, J. T. , Frangakis, C. , Hogan, J. W. , Molenberghs, G. , Murphy, S. A. , Neaton, J. D. , Rotnitzky, A. , Scharfstein, D. , Shih, W. J. , Siegel, J. P. , & Stern, H. (2012). The prevention and treatment of missing data in clinical trials. New England Journal of Medicine, 367(14), 1355–1360. 10.1056/nejmsr1203730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manca, A. , Rice, N. , Sculpher, M. J. , & Briggs, A. H. (2005). Assessing generalisability by location in trial‐based cost‐effectiveness analysis: The use of multilevel models. Health Economics, 14(5), 471–485. 10.1002/hec.914 [DOI] [PubMed] [Google Scholar]
- Mason, A. J. , Gomes, M. , Grieve, R. , & Carpenter, J. R. (2018). A bayesian framework for health economic evaluation in studies with missing data. Health Economics, 27(11), 1670–1683. 10.1002/hec.3793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenberghs, G. , Thijs, H. , Jansen, I. , Beunckens, C. , Kenward, M. G. , Mallinckrodt, C. , & Carroll, R. J. (2004). Analyzing incomplete longitudinal clinical trial data. Biostatistics, 5(3), 445–464. 10.1093/biostatistics/kxh001 [DOI] [PubMed] [Google Scholar]
- Nixon, R. , & Thompson, S. (2005). Methods for incorporating covariate adjustment, subgroup analysis and between‐centre differences into cost‐effectiveness evaluations. Health Economics, 14(12), 1217–1229. 10.1002/hec.1008 [DOI] [PubMed] [Google Scholar]
- Noble, S. , Hollingworth, W. , & Tilling, K. (2012). Missing data in trial‐based cost‐effectiveness analysis: The current state of play. Health Economics, 21(2), 187–200. 10.1002/hec.1693 [DOI] [PubMed] [Google Scholar]
- Ramsey, S. D. , Willke, R. J. , Glick, H. , Reed, S. D. , Augustovski, F. , Jonsson, B. , Briggs, A. , & Sullivan, S. D. (2015). Cost‐effectiveness analysis alongside clinical trials II—an ISPOR good research practices task force report. Value in Health, 18(2), 161–172. 10.1016/j.jval.2015.02.001 [DOI] [PubMed] [Google Scholar]
- Rice, N. , & Jones, A. (1997). Multilevel models and health economics. Health Economics, 6(6), 561–575. [DOI] [PubMed] [Google Scholar]
- Romeo, R. , Knapp, M. , Hellier, J. , Dewey, M. , Ballard, C. , Baldwin, R. , Bentham, P. , Burns, A. , Fox, C. , Holmes, C. , Katona, C. , Lawton, C. , Lindesay, J. , Livingston, G. , McCrae, N. , Moniz‐Cook, E. , Murray, J. , Nurock, S. , O'Brien, J. , … Banerjee, S. (2013). Cost‐effectiveness analyses for mirtazapine and sertraline in dementia: Randomised controlled trial. The British Journal of Psychiatry, 202(2), 121–128. 10.1192/bjp.bp.112.115212 [DOI] [PubMed] [Google Scholar]
- Rubin, D. B. (1987). Multiple imputation for nonresponse in surveys. John Wiley and Sons. [Google Scholar]
- Schafer, J. L. , & Graham, J. W. (2002). Missing data: Our view of the state of the art. Psychological Methods, 7(2), 147–177. 10.1037/1082-989x.7.2.147 [DOI] [PubMed] [Google Scholar]
- van Buuren, S. (2018). Flexible imputation of missing data. Chapman and Hall/CRC. [Google Scholar]
- van Hout, B. A. , Al, M. J. , Gordon, G. S. , & Rutten, F. F. (1994). Costs, effects and c/e‐ratios alongside a clinical trial. Health Economics, 3(5), 309–319. 10.1002/hec.4730030505 [DOI] [PubMed] [Google Scholar]
- White, I. R. , & Thompson, S. G. (2005). Adjusting for partially missing baseline measurements in randomized trials. Statistics in Medicine, 24(7), 993–1007. 10.1002/sim.1981 [DOI] [PubMed] [Google Scholar]
- Wolfinger, R. (1993). Covariance structure selection in general mixed models. Communications in Statistics ‐ Simulation and Computation, 22(4), 1079–1106. 10.1080/03610919308813143 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data from the SADD trial were provided by the investigators for the purpose of this article. They are available upon reasonable request from the chief investigator (sube.banerjee@plymouth.ac.uk) along with the other trial datasets.


