Abstract
Aim
To evaluate the cardiovascular (CV) efficacy of liraglutide and semaglutide in patients with type 2 diabetes (T2D) and peripheral artery disease (PAD).
Materials and Methods
LEADER and SUSTAIN 6 trials investigated subcutaneous liraglutide (≤1.8 mg/day) and semaglutide (0.5 or 1.0 mg/week), respectively, versus placebo in patients with T2D and high CV risk (median follow‐up: 3.8 and 2.1 years, respectively). The primary outcome was a composite of CV death, non‐fatal myocardial infarction or non‐fatal stroke (major adverse CV event [MACE]) according to the presence of PAD at baseline.
Results
Overall, 1184/9340 (12.7%) patients in LEADER and 460/3297 (14.0%) in SUSTAIN 6 had PAD at baseline. Patients with PAD were at an ~35% increased risk of MACE versus those without (LEADER: hazard ratio [HR] 1.36, 95% confidence interval [CI] 1.17‐1.58; SUSTAIN 6: HR 1.33, 95% CI 0.94‐1.83). The effects of both therapies on MACE were consistently beneficial in patients with PAD (liraglutide: HR 0.77, 95% CI 0.58‐1.01; semaglutide: 0.61, 0.33‐1.13) and without (liraglutide: HR 0.89, 95% CI 0.79‐1.00; semaglutide: HR 0.77, 95% CI 0.58‐1.01; P interaction = .34 for liraglutide and .49 for semaglutide). Absolute risk reductions for MACE were higher in patients with PAD (liraglutide: 4.13%‐point, 95% CI −0.15‐8.42; semaglutide: 4.63%‐point, 95% CI −0.58‐9.84) versus without (liraglutide:1.42%‐point, 95% CI −0.03‐2.87; semaglutide: 1.90%‐point, 95% CI 0.00‐3.80).
Conclusion
Both liraglutide and semaglutide reduce MACE with consistent CV efficacy regardless of PAD status.
Keywords: cardiovascular disease, GLP‐1, macrovascular disease, peripheral artery disease, receptor agonists, type 2 diabetes
1. INTRODUCTION
Peripheral artery disease (PAD), a manifestation of atherosclerosis, 1 is one of the most common initial presentations of cardiovascular disease (CVD) in patients with type 2 diabetes (T2D). 2 Patients with PAD frequently have concomitant T2D. 3 Furthermore, patients with PAD have a high CV risk, including higher rates of CV death, myocardial infarction, stroke and the composite of these outcomes (major adverse CV events [MACE]), compared with those without PAD. 4 , 5 This risk profile is significantly worsened in the setting of concomitant diabetes. 6 Therefore, patients with both diabetes and PAD represent a population with a particularly serious atherothrombotic phenotype and need efficacious risk‐reduction strategies. Although there are few well‐powered trials of drugs to reduce MACE specifically in patients with PAD, subgroup analyses of broader trials have been performed to evaluate consistency in this subgroup. 5 , 7 Some analyses of patients with diabetes by history of PAD have indicated heterogeneity, particularly for safety. 8
Trials of glucagon‐like peptide‐1 (GLP‐1) receptor agonists versus placebo have generally shown reductions of MACE in patients with T2D. 9 , 10 , 11 , 12 , 13 The benefits of this drug class 14 have resulted in widespread change in clinical practice guidelines. 15 , 16 , 17 , 18 The GLP‐1 receptor agonists liraglutide and semaglutide each significantly reduced the risk of MACE compared with placebo in patients with T2D and high‐to‐very‐high CV risk in the LEADER (NCT01179048) and SUSTAIN 6 (NCT01720446) trials, respectively. 9 , 10 , 19 The efficacy and safety data for liraglutide and semaglutide in patients with PAD have not, however, been described previously. We hypothesized that (a) patients in LEADER and SUSTAIN 6 with PAD would be at a higher risk of MACE, and (b) the relative benefits of liraglutide and semaglutide would be consistent in patients with and without PAD, with greater absolute benefits in those with PAD.
2. MATERIALS AND METHODS
The LEADER (NCT01179048) and SUSTAIN 6 (NCT01720446) trial designs have been described previously. 9 , 10 Both trials were randomized, double‐blind, multicentre, placebo‐controlled, CV outcome trials. The trial protocols were reviewed and approved by the institutional review board or ethics committee at each participating site. All patients gave written informed consent before participation.
2.1. Patients
In LEADER, a total of 9340 patients with T2D, HbA1c of 7.0% or higher and high‐to‐very‐high CV risk were randomized to receive subcutaneous liraglutide (1.8 mg/day or the maximum tolerated dose of ≤1.8 mg/day) or placebo, both in addition to standard of care, and followed up for 3.5‐5 years. 10 In SUSTAIN 6, a total of 3297 patients with T2D, HbA1c of 7.0% or higher and high‐to‐very‐high CV risk were randomized to receive subcutaneous semaglutide (0.5 or 1.0 mg/week) or placebo, both added to standard of care, and followed up for 2 years. 9 Patients in both trials were included if they were aged 50 years or older with either established CVD or chronic kidney disease, or aged 60 years or older with at least one additional CV risk factor. 9 , 10 Complete lists of the inclusion and exclusion criteria have been published previously. 9 , 10
The investigators of both trials reported patients as having a history of PAD in the lower extremities at the time of screening if they had an ankle‐brachial index of less than 0.9 or if the diagnosis had been confirmed by ultrasonography or angiography (conventional, computed tomography or magnetic resonance imaging). They also reported whether patients had claudication, more than 50% stenosis of the peripheral arteries and/or prior revascularization at baseline.
2.2. Patient subgroups
In these post hoc analyses, we compared the efficacy and safety of liraglutide or semaglutide versus placebo in patients with and without PAD at baseline. PAD status was based on known history. In additional exploratory analyses, patients with PAD were further subgrouped by the presence or absence of concomitant coronary artery disease (CAD) and/or cerebrovascular disease. Patients with PAD and CAD and/or cerebrovascular disease were defined as those with polyvascular disease. Cerebrovascular disease included prior ischaemic or haemorrhagic stroke, or transient ischaemic attack.
2.3. Outcomes
The primary outcome was time to first occurrence of CV death, non‐fatal myocardial infarction or non‐fatal stroke (MACE). Prespecified, key secondary outcomes included revascularization (LEADER: coronary revascularization; SUSTAIN 6: coronary or peripheral revascularization) and hospitalization for unstable angina or heart failure in addition to MACE (termed ‘expanded MACE’). Prespecified secondary endpoints were time to first event of the individual components of the composite outcomes and death from any cause. All of these outcomes were adjudicated by an independent, blinded event adjudication committee, with the exception of peripheral revascularization in SUSTAIN 6 that was included in expanded MACE, which was site‐reported. 9 , 10
2.4. Statistical analysis
The time from randomization to the first occurrence of the outcome of interest was analysed using a Cox proportional hazards model. Hazard ratios (HRs) and P values for comparisons between groups were estimated with adjustment for treatment allocation (liraglutide vs. placebo; semaglutide vs. placebo). For SUSTAIN 6 data, the Cox proportional hazards model was also stratified by factors used for randomization: CVD status, insulin treatment and estimated glomerular filtration rate. 9 Additional analyses were performed with multivariable adjustment for baseline variables. Kaplan–Meier curves evaluated the association between treatment groups within subgroups and the outcome of interest. Changes from baseline in HbA1c, blood pressure and body weight at 3 years in LEADER and at 2 years in SUSTAIN 6 were analysed by PAD subgroup using a linear mixed model, which accounted for repeated measures within patients using an unstructured residual covariance matrix. The model included interaction between visit and treatment group, baseline value of the variable of interest, sex, region and antihyperglycaemic therapy at baseline as fixed effects, and interaction between visit and baseline value of interest and baseline age as covariates. A P interaction value of less than .05 was taken to indicate a significant difference in the treatment HRs or estimated treatment contrasts across patients with versus without PAD. No adjustments for multiplicity were performed. All randomized patients were included in the analyses from the time of randomization until death or end of follow‐up. Absolute risk reductions and corresponding number needed to treat (NNT) were calculated using a Cox proportional hazard model 20 with treatment as a fixed factor predicting the probability of an event in the two treatment arms at the median time of follow‐up in LEADER (3.8 years) and SUSTAIN 6 (2.1 years). 9 , 10
3. RESULTS
3.1. Demographics and baseline characteristics
In LEADER and SUSTAIN 6, 1184 (12.7%) and 460 (14.0%) patients had known PAD at baseline, respectively; thus a total of 1644 patients with known PAD were included in the current analysis. In both trials, patients with PAD tended to have a longer diabetes duration than patients without, and higher proportions of patients with PAD were receiving insulin at baseline and were current/previous smokers (Tables S1 and S2). Use of antiplatelet agents and antithrombotic medication was balanced between PAD subgroups in LEADER; however, in SUSTAIN 6, there was a greater use of antiplatelet agents in patients with versus without PAD (Tables S1 and S2). Figure S1A,B shows the distribution and overlap of PAD relative to CAD and/or cerebrovascular disease.
3.2. Risk of CV events and mortality in patients with PAD regardless of treatment
In LEADER, incidence rates of MACE were 4.7 per 100 person‐years of observation (PYO) in patients with a history of PAD and 3.5 per 100 PYO in those without PAD (Table 1). Over a median follow‐up of 3.8 years in the LEADER trial, the occurrence of MACE in those with a history of PAD was higher than in those without (occurring in 209/1184 patients with PAD [17.7%] vs. 1093/8156 patients without PAD [13.4%]; estimated HR without adjustment for baseline variables 1.36, 95% confidence interval [CI] 1.17‐1.58; P < .0001; Figure 1A). Similar results were obtained for expanded MACE when patients with PAD (incidence rate 6.9 per 100 PYO, 304/1184 [25.7%]) were compared with those without (incidence rate 5.5 per 100 PYO, 1706/8156 [20.9%]; HR 1.27, 95% CI 1.12‐1.43; P = .0001; Table 1).
TABLE 1.
Rates of CV events and mortality in LEADER and SUSTAIN 6 trial participants by history of PAD
| LEADER | SUSTAIN 6 | |||||||
|---|---|---|---|---|---|---|---|---|
| Incidence rate (event per 100 person‐years of observation) | HR [95% CI] for PAD versus no PAD, without adjustment for baseline variables | HR [95% CI] for PAD versus no PAD, adjusted for baseline variables | Incidence rate (event per 100 person‐years of observation) | HR [95% CI] for PAD versus no PAD, without adjustment for baseline variables | HR [95% CI] for PAD versus no PAD, adjusted for baseline variables | |||
| PAD at baseline (N = 1184) | No PAD at baseline (N = 8156) | PAD at baseline (N = 460) | No PAD at baseline (N = 2837) | |||||
| MACE | 4.7 | 3.5 | 1.36 [1.17‐1.58] | 1.27 [1.09‐1.48] | 4.5 | 3.6 | 1.33 [0.94‐1.83] | 1.24 [0.88‐1.72] |
| Expanded MACE | 6.9 | 5.5 | 1.27 [1.12‐1.43] | 1.23 [1.09‐1.39] | 9.8 | 6.3 | 1.71 [1.36‐2.14] | 1.66 [1.31‐2.08] |
| CV death | 2.2 | 1.3 | 1.73 [1.39‐2.16] | 1.57 [1.25‐1.97] | 1.0 | 1.4 | 0.80 [0.39‐1.47] | — |
| Non‐fatal myocardial infarction | 1.9 | 1.6 | 1.17 [0.93‐1.47] | 1.14 [0.90‐1.44] | 2.3 | 1.5 | 1.59 [0.97‐2.49] | 1.54 [0.93‐2.45] |
| Non‐fatal stroke | 1.1 | 0.9 | 1.24 [0.92‐1.67] | 1.16 [0.85‐1.57] | 1.4 | 1.0 | 1.42 [0.74‐2.52] | — |
| Coronary revascularization | 2.8 | 2.3 | 1.21 [1.00‐1.47] | 1.24 [1.02‐1.51] | 3.1 | 2.4 | 1.32 [0.87‐1.93] | — |
| Hospitalization for unstable angina pectoris | 0.9 | 0.7 | 1.28 [0.91‐1.82] | 1.42 [1.00‐2.01] | 0.9 | 0.7 | 1.38 [0.62‐2.71] | 1.65 [0.74‐3.35] |
| Hospitalization for heart failure | 1.9 | 1.2 | 1.58 [1.25‐2.00] | 1.48 [1.17‐1.88] | 2.0 | 1.6 | 1.26 [0.75‐2.03] | 1.23 [0.72‐1.99] |
| All‐cause death | 3.3 | 2.2 | 1.53 [1.28‐1.83] | 1.34 [1.12‐1.61] | 1.6 | 1.8 | 0.89 [0.50–1.49] | — |
Note: Some adjusted data are not shown because of convergence issues arising attributed to few events. In SUSTAIN 6, expanded MACE included peripheral revascularization, which was site‐reported.
Abbreviations: CV, cardiovascular; CI, confidence interval; HR, hazard ratio; MACE, major adverse cardiovascular event; PAD, peripheral artery disease.
FIGURE 1.
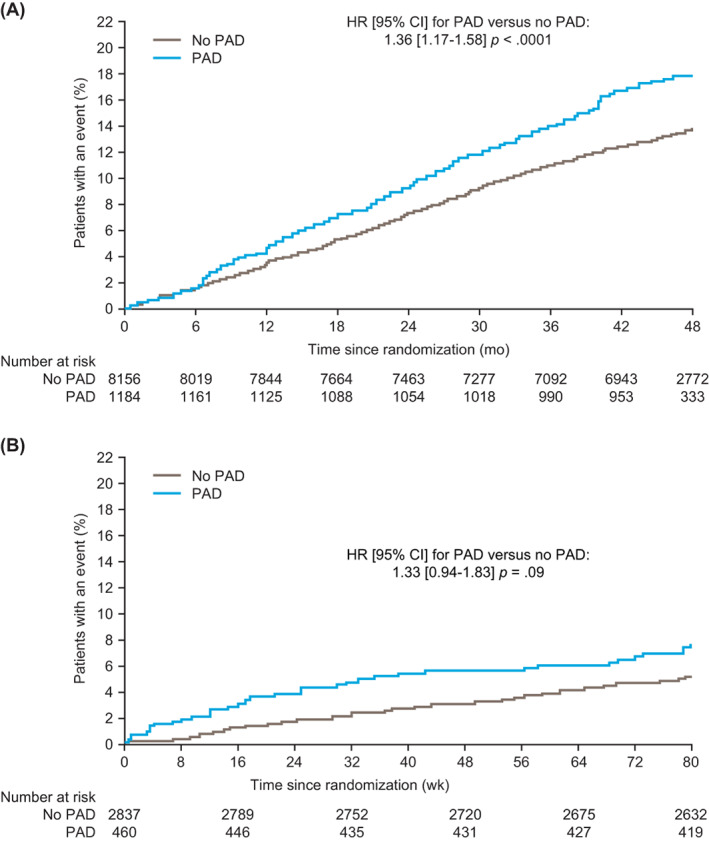
Time to first MACE by history of PAD at baseline. A, LEADER; B, SUSTAIN 6. Data without adjustment for baseline variables are shown. HR, hazard ratio; CI, confidence interval; MACE, major adverse cardiovascular event; PAD, peripheral artery disease
In SUSTAIN 6, incidence rates of MACE were 4.5 per 100 PYO in patients with a history of PAD and 3.6 per 100 PYO in those without PAD (Table 1). Over a median follow‐up of 2.1 years in the SUSTAIN 6 trial, the occurrence of MACE in those with a history of PAD was higher than in those without (43/460 patients with PAD [9.3%] vs. 211/2837 patients without PAD [7.4%]; estimated HR without adjustment for baseline variables 1.33, 95% CI 0.94‐1.83; P = .09; Figure 1B). There was a higher risk of expanded MACE in patients with PAD (incidence rate 9.8 per 100 PYO, 94/460 [20.4%]) compared with those without (incidence rate 6.3 per 100 PYO, 369/2837 [13.0%]; HR 1.71, 95% CI 1.36‐2.14; P < .0001; Table 1).
Table 1 summarizes incidence rates and HRs, both with and without adjustment for baseline variables, for CV outcomes and all‐cause death, in those with PAD versus without PAD.
In exploratory analyses, we evaluated the risk of CV events in patients with polyvascular disease versus those with PAD alone. In LEADER, there was a higher risk of primary MACE (HR 2.23, 95% CI 1.61‐3.08; incidence rate 5.99 vs. 2.8 per 100 PYO) and CV death (HR 1.62, 95% CI 1.04‐2.52; incidence rate 2.6 vs. 1.6 per 100 PYO) in patients with polyvascular disease versus those with PAD alone. Similar results were observed in SUSTAIN 6 for primary MACE (HR 2.42, 95% CI 1.00‐7.06; incidence rate 5.7 vs. 2.2 per 100 PYO in patients with polyvascular disease versus PAD alone, respectively). There were insufficient CV death data to draw statistical comparisons between the subgroups in SUSTAIN 6.
3.3. Effects of liraglutide and semaglutide on CV events in patients with versus without a history of PAD
In LEADER, liraglutide was shown to reduce the occurrence of MACE both in patients with PAD (15.5% of patients with liraglutide vs. 19.6% with placebo; HR without adjustment for baseline variables 0.77, 95% CI 0.58‐1.01) and in those without a history of PAD (12.7% of patients with liraglutide vs. 14.1% with placebo; HR 0.89, 95% CI 0.79‐1.00; P interaction = .34; Figures 2A and 3A). Across PAD subgroups, the effects of liraglutide were consistent for primary MACE, the individual components of primary MACE (except for non‐fatal myocardial infarction), hospitalization for heart failure, expanded MACE and all‐cause death (Figure 3A; Table S3). Liraglutide was associated with a numerically greater absolute risk reduction in those with versus without PAD (absolute risk reduction for the primary endpoint [95% CI], 4.13 [−0.15‐8.42] percentage points in those with PAD vs. 1.42 [−0.03‐2.87] percentage points in those without PAD at 3.8 years). The corresponding NNT to prevent first MACE was 24 in those with PAD and 70 in those without PAD. The relative risk for primary MACE with liraglutide versus placebo was consistent in patients with PAD only and in patients with polyvascular disease (HRs 0.69, 95% CI 0.39‐1.24 and 0.80, 95% CI 0.58‐1.09, respectively; P interaction = .68; Figure 4A).
FIGURE 2.
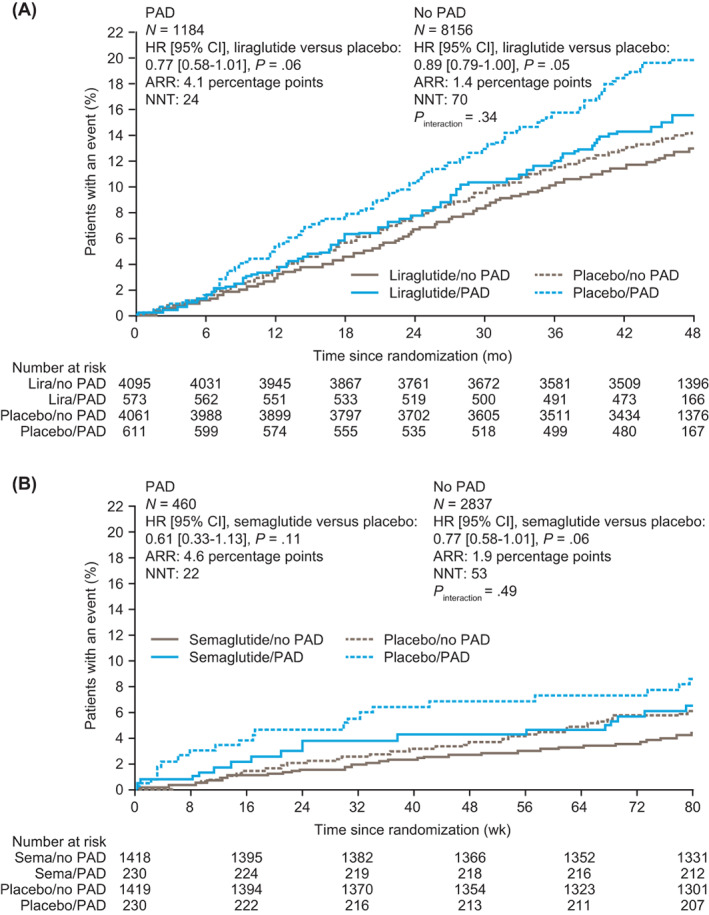
Time to first MACE by history of PAD at baseline and randomized treatment group. A, LEADER; B, SUSTAIN 6. Data without adjustment for baseline variables are shown. ARR, absolute risk reduction at median follow‐up (3.8 years for LEADER; 2.1 years for SUSTAIN 6). CI, confidence interval; HR, hazard ratio; lira, liraglutide; MACE, major adverse cardiovascular event; N, number of patients; NNT, number needed to treat at median follow‐up (3.8 years for LEADER; 2.1 years for SUSTAIN 6); PAD, peripheral artery disease; sema, semaglutide
FIGURE 3.
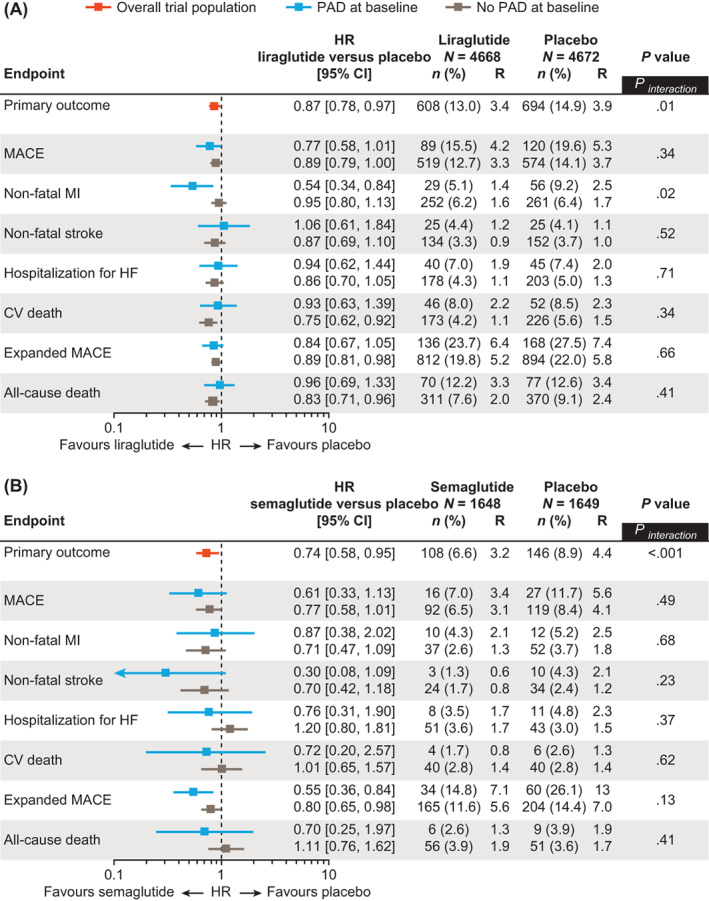
Risk of CV events and mortality by history of PAD at baseline and randomized treatment group. A, LEADER; B, SUSTAIN 6. Data without adjustment for baseline variables are shown. The primary outcome in both trials was a composite outcome including first occurrence of CV death, non‐fatal MI, or non‐fatal stroke. 9 , 10 In SUSTAIN 6, expanded MACE included peripheral revascularization, which was site‐reported. CI, confidence interval; CV, cardiovascular; HF, heart failure; HR, hazard ratio; MACE, major adverse cardiovascular event; MI, myocardial infarction; n, number of patients with an event; PAD, peripheral artery disease; R, rate (events per 100 patient‐years of observation)
FIGURE 4.
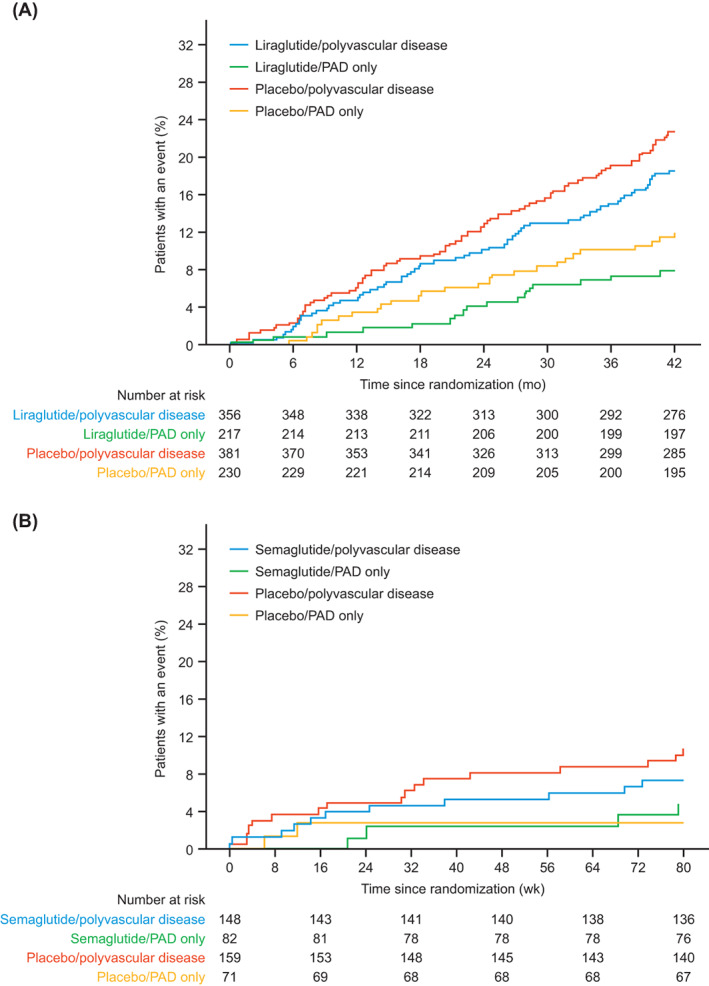
Time to first MACE by treatment arm and PAD only versus polyvascular disease (PAD and CAD and/or cerebrovascular disease) at baseline: A, LEADER; B, SUSTAIN 6. Kaplan–Meier plots have been truncated at A, 42 months for the LEADER trial and B, 80 weeks for the SUSTAIN 6 trial. CAD, coronary artery disease; MACE, major adverse cardiovascular event; PAD, peripheral artery disease
In SUSTAIN 6, semaglutide was associated with a lower occurrence of MACE both in patients with PAD (7.0% of patients with semaglutide vs. 11.7% with placebo; HR without adjustment for baseline variables 0.61, 95% CI 0.33‐1.13) and in those without PAD (6.5% of patients with semaglutide vs. 8.4% with placebo; HR 0.77, 95% CI 0.58‐1.01; P interaction = .49; Figures 2B and 3B). Across PAD subgroups, the effects of semaglutide were consistent for primary MACE, the individual components of primary MACE, hospitalization for heart failure, expanded MACE and all‐cause death (P interaction > .05 for all; Figure 3B; Table S3). Semaglutide was associated with a numerically greater absolute risk reduction in those with versus without PAD (absolute risk reduction for the primary endpoint [95% CI], 4.63 [−0.58‐9.84] percentage points in those with PAD vs. 1.90 [0.00‐3.80] percentage points in those without PAD at 2.1 years). The corresponding NNT to prevent first MACE was 22 in those with PAD and 53 in those without PAD. The relative risk for primary MACE with semaglutide versus placebo was consistent in patients with PAD only and in patients with polyvascular disease (HRs 1.18, 95% CI 0.26‐5.28 and 0.49, 95% CI 0.25‐0.99, respectively; P interaction = .30; Figure 4B).
3.4. Effects of liraglutide and semaglutide on HbA1c, blood pressure and weight in patients with versus without a history of PAD
Tables S4 and S5 show changes from baseline in HbA1c, systolic blood pressure, diastolic blood pressure and weight in LEADER and SUSTAIN 6 participants by history of PAD and treatment group. Except for a borderline significantly greater HbA1c reduction in patients with PAD compared with those without PAD in SUSTAIN 6 (P = .047), there was no statistically significant interaction between history of PAD at baseline and treatment group for these endpoints (P interaction > .05).
3.5. Serious adverse events and non‐serious medical events of special interest
Serious adverse events and non‐serious medical events of special interest are summarized in Tables S6 and S7. Overall, these events occurred in 67.5% of LEADER participants with a history of PAD and in 60.7% of those without a history of PAD, with no notable differences within subgroups between those assigned to liraglutide or placebo (Table S6). Corresponding results for the PAD and treatment groups in SUSTAIN 6 were qualitatively similar to those described above for LEADER (events occurred in 49.8% of patients with a history of PAD and 42.6% of those without a history of PAD; Table S7).
4. DISCUSSION
In this post hoc analysis of the LEADER and SUSTAIN 6 trials, we made two key observations. First, even among these overall high CV risk patients in these trials, those with T2D and PAD were at a higher risk of CV events compared with participants with T2D without PAD, as shown in Table 1. This risk was even higher in those with T2D who had polyvascular disease, consistent with previous data on the increasing ischaemic risk in patients with more than one arterial bed involved. 21 , 22 , 23 Second, the significant reduction in MACE associated with liraglutide and semaglutide in the overall population (liraglutide: HR 0.87, 95% CI 0.78‐0.97, P = .01; semaglutide: HR 0.74, 95% CI 0.58‐0.95, P < .001) was consistent for people with T2D and PAD and for those with T2D without PAD. However, because the absolute risk was higher in patients with T2D and PAD, liraglutide and semaglutide treatments were associated with numerically higher absolute risk reductions in LEADER (absolute risk reduction of 4.1% points; HR 0.77 [95% CI 0.58‐1.01], P = .06) and SUSTAIN 6 (absolute risk reduction of 4.6% points; HR 0.61 [95% CI 0.33‐1.13], P = .11) for the primary outcome in patients with versus without PAD, at the median follow‐up times of 3.8 and 2.1 years, respectively. NNTs of just 24 and 22 were observed in patients with PAD at 3.8 years in LEADER and at 2.1 years in SUSTAIN 6, respectively. Furthermore, it may be argued that the numerically greater absolute risk reductions observed in patients with T2D and PAD were a result of greater glucose lowering, blood pressure reduction or weight loss. However, except for change in HbA1c in SUSTAIN 6, baseline and changes in HbA1c, blood pressure and weight were similar or consistent in patients with and without PAD in both trials.
There has been a paucity of vascular protective strategies and dedicated clinical trials in individuals with diabetes and PAD, and antihyperglycaemic therapies have provided mixed results on CV outcomes in patients with T2D. Of the completed sodium‐glucose co‐transporter‐2 inhibitor cardiorenal outcome trials, the PAD subgroup analysis from EMPA‐REG OUTCOME has been published, which confirmed the higher CV risk of this population relative to those without PAD. 5 Empagliflozin was associated with absolute risk reductions of ~1.6 and ~1.7 percentage points in those with and without PAD, respectively, regarding MACE during EMPA‐REG OUTCOME (median observation time 3.1 years; HR for empagliflozin vs. placebo 0.84, 95% CI 0.62‐1.14 in patients with PAD at baseline and 0.86, 95% CI 0.73‐1.02 in patients without PAD at baseline). 5 , 24 Studies suggest that empagliflozin may increase the circulating number of pro‐vascular regenerative progenitor cells in diabetes, an effect that could contribute to enhanced vascular health. 25 In terms of GLP‐1 receptor agonist results, there was no significant reduction in MACE observed with exenatide compared with placebo in the EXSCEL trial, irrespective of PAD status. Similarly, no treatment effect was observed on rates of lower extremity amputations in either patient group. 26 In another study, the use of GLP‐1 receptor agonists was found to lower the risk of major adverse limb events by reducing the rate of amputations needed. 27
Regarding low‐density lipoprotein cholesterol (LDL‐C) reduction, in the FOURIER trial, patients with PAD who received evolocumab (a proprotein convertase subtilisin kexin type 9 [PCSK9] inhibitor) had absolute risk reductions of 3.5% both for the primary composite MACE outcome of CV death, myocardial infarction, stroke, hospital admission for unstable angina or coronary revascularization, and for the key secondary composite MACE outcome of CV death, myocardial infarction or stroke. 7 While the subgroup of patients with T2D and concomitant PAD was not reported, it would be reasonable to suggest that intensive LDL‐C lowering would have at least a similar absolute benefit on these MACE outcomes. Other emerging approaches to vascular protection in patients with PAD involve dual thrombotic pathway inhibition with low‐dose rivaroxaban and aspirin, as studied in the COMPASS trial. 28 This regimen resulted in an ~2 percentage point absolute risk reduction for the primary composite MACE outcome of CV death, myocardial infarction or stroke in the patients with PAD versus aspirin alone, with an increase in non‐fatal bleeding. 28 Similar benefit in terms of MACE was observed in the diabetes subgroup of this trial (absolute risk reduction ~4 percentage points vs. aspirin alone; P interaction = .97 for diabetes vs. no diabetes). 28 Therefore, for patients with PAD, particularly those with T2D, we now have several atheroprotective strategies that appear to work via complementary mechanisms.
Several direct and indirect mechanisms have been suggested to mediate the atheroprotective effects of GLP‐1 receptor agonists. 29 , 30 , 31 , 32 , 33 , 34 Experimental studies point towards an effect to modulate endothelial function, vascular smooth muscle cell proliferation, vascular inflammation and lipid deposition. 35 In one study, liraglutide and semaglutide were shown to reduce plaque lesion development in apolipoprotein E‐deficient mice and LDL receptor‐deficient mice. 36 The benefit with semaglutide was associated with favourable regulation of genes in pathways relevant to the pathogenesis of atherosclerosis, including leukocyte recruitment, leukocyte rolling, adhesion/extravasation, cholesterol metabolism, lipid‐mediated signalling, extracellular matrix protein turnover and plaque haemorrhage. 36 The changes in plaque lesion development were observed in a non‐diabetes model of atherosclerosis, and appeared to be partly independent of cholesterol and weight changes. 36 This is consistent with clinical studies showing that the CV benefit of liraglutide was observed independent of baseline LDL‐C and statin use in LEADER. 37 Furthermore, subanalyses from LEADER and SUSTAIN 6 confirm that the CV benefits are observed consistently across all levels of body mass index and blood pressure, and observed in those patients who have an above or below median reduction in these risk factors. 38 , 39
Currently, ongoing trials of semaglutide are investigating outcomes that can have a huge impact on patients living with PAD. The ongoing STRIDE trial aims to assess the effect of subcutaneous once‐weekly semaglutide on walking ability compared with placebo in patients with PAD and T2D. 40 An ongoing, dedicated trial in patients with established CVD, including PAD, and a body mass index of 27 kg/m2 or higher without type 1 diabetes or T2D (SELECT) will provide evidence of whether or not semaglutide injections can be used to reduce CV events in the absence of diabetes. 41 Another ongoing trial studying patients with T2D and established CV or chronic kidney disease (SOUL) will assess the effects of oral semaglutide versus placebo on MACE, with major adverse limb events included as a secondary endpoint. 42 Additionally, a further trial is investigating how subcutaneous once‐weekly semaglutide affects surrogate endpoints for atherosclerosis in patients with T2D and CVD. 43 These trials will add to the existing data on the effect of semaglutide on outcomes relevant to patients with PAD.
4.1. Study limitations
The present study has limitations. As there was no systematic screening for PAD, it relies upon accurate reporting of medical histories of PAD by trial investigators at baseline. As a post hoc analysis, it is not adequately powered to provide robust estimates for each subgroup and for individual components of the primary outcome. While, theoretically, we could have improved the robustness of the estimates by an individual patient pooled analysis of the two trials, the differences in the molecules and duration of follow‐up in each trial precluded us from this approach. Limb events, which are associated with a poor prognosis, 44 were not analysed in this study; because of the protocol and safety data collection methods, it was not possible to analyse all amputations occurring during the trial. 45 We did not evaluate oral semaglutide, which was shown in the PIONEER 6 trial to have a similar trend to a CV benefit as subcutaneous once‐weekly semaglutide. 46 A pooled analysis of oral semaglutide and subcutaneous once‐weekly semaglutide results was not possible because of a different method of assessment of PAD at baseline in the PIONEER 6 trial.
In conclusion, there remains a high unmet medical need in patients with T2D and PAD, attributed in part to the higher CV risk compared with patients with T2D without a history of PAD. Benefits of liraglutide and semaglutide on MACE were consistent in people with and without PAD at baseline. Patients with T2D and PAD may derive greater absolute benefit from these treatments because of their higher risk profile. These data have potential translational implications for atherosclerotic risk reduction in this high‐risk population.
CONFLICT OF INTEREST
SV holds a Tier 1 Canada Research Chair in Cardiovascular Surgery; and reports receiving research grants and/or speaking honoraria from Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Eli Lilly, EOCI Pharmacomm Ltd, HLS Therapeutics, Janssen, Merck, Novartis, Novo Nordisk, Pfizer, PhaseBio, Sanofi, Sun Pharma, and the Toronto Knowledge Translation Working Group. He is the President of the Canadian Medical and Surgical Knowledge Translation Research Group, a federally incorporated not‐for‐profit physician organization. MA‐O has received consulting fees from Amgen and Bayer. LAL has received research funding (to institution), provided continuing medical education on behalf of and/or acted as an adviser to AstraZeneca, Boehringer Ingelheim, Eli Lilly, Lexicon, Merck, Novo Nordisk, Pfizer, Sanofi and Servier. CDM has received consulting fees from Amgen, AstraZeneca, BioAge, Boehringer Ingelheim and PhaseBio. SR is an employee and shareholder of Novo Nordisk A/S. HAS is a former employee of Novo Nordisk A/S. MSR is an employee and shareholder of Novo Nordisk A/S. MPB is the Executive Director of CPC, a non‐profit academic research organization affiliated with the University of Colorado that receives research grant/consulting funding from Abbott, Agios, Alexion Pharma, Alnylam, Amgen, Angionetics, ARCA Biopharma, Array, AstraZeneca, Atentiv, Audentes, Bayer, Better Therapeutics, Brigham and Women's Hospital, Bristol‐Myers Squibb, Cardiol Therapeutics, CellResearch, Cook Medical, Cook, CSL Behring, Eidos Therapeutics, EP Trading Co, Esperion Therapeutics, EverlyWell, Faraday, Fortress. Biotech, HDL Therapeutics, Heartflow, Hummingbird Bioscience, Insmed, Janssen, Kowa Research, Lexicon, Merck, Medtronic, Moderna, Novate Medical, Novo Nordisk, Pfizer, PhaseBio, PPD Development, Prairie Education and Research, Prothena Ciosciences, Regeneron, Regio Biosciences, Sanifit Therapeutics, Sanofi, Smith and Nephew, Stealth, BioTherapeutics, University of Colorado, Worldwide Clinical Trials, Wraser and Yale Cardiovascular Research Group. MPB also reports being a scientific advisor for the STRIDE Trial (supported by Novo Nordisk).
AUTHOR CONTRIBUTIONS
SV and MPB wrote the first draft of the manuscript. LAL was a National Leader of the LEADER and SUSTAIN 6 trials and contributed to the conduct of the studies and acquisition of clinical data. SR performed the statistical analyses. All the authors reviewed and interpreted the data and were involved in revising the manuscript. All the authors approved the final version of the manuscript and take full responsibility for its content. SV had full access to all the data in the study and takes responsibility for its integrity and the data analysis.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14700.
Supporting information
Table S1 Demographics and baseline characteristics of LEADER trial participants by history of PAD.
Table S2 Demographics and baseline characteristics of SUSTAIN 6 participants by history of PAD.
Table S3 Risk of CV events and mortality by history of PAD at baseline and randomized treatment group, adjusted for baseline variables.
Table S4 Changes from baseline in HbA1c, blood pressure and body weight in LEADER trial participants by history of PAD and randomized treatment group.
Table S5 Changes from baseline in HbA1c, blood pressure and body weight in SUSTAIN 6 trial participants by history of PAD and randomized treatment group.
Table S6 Serious adverse events and non‐serious medical events of special interest in LEADER trial participants by history of PAD.
Table S7 Serious adverse events and non‐serious medical events of special interest in SUSTAIN 6 trial participants by history of PAD.
Figure S1 Venn diagram of number (%) of patients according to PAD, CAD and cerebrovascular disease. (A) LEADER; (B) SUSTAIN 6
ACKNOWLEDGEMENTS
The authors are grateful to the participants, investigators and all of those involved in the conduct of the trials. The authors are also grateful to Ellen Margo Hengeveld, Ofir Frenkel and Zaklina Davicevic‐Elez, Novo Nordisk, for review of and input to the manuscript. Medical writing support (figure development and incorporation of author comments) and editorial support for the development of this manuscript, under the direction of the authors, were provided by Alice Singleton, MSc, and Malgorzata Urbacz, respectively, of Ashfield MedComms, an Ashfield Health company, and were funded by Novo Nordisk.
Verma S, Al‐Omran M, Leiter LA, et al. Cardiovascular efficacy of liraglutide and semaglutide in individuals with diabetes and peripheral artery disease. Diabetes Obes Metab. 2022;24(7):1288‐1299. doi: 10.1111/dom.14700
Parts of these data were previously presented at the American Heart Association's 2019 Scientific Sessions (17 November 2019, Philadelphia, PA, USA) and published in the form of an abstract (Verma S et al. Circulation 2019;140:A11456).
Funding information The trials and this study were sponsored by Novo Nordisk A/S, Søborg, Denmark. LEADER and SUSTAIN 6 are registered with ClinicalTrials.gov (NCT01179048 and NCT01720446).
DATA AVAILABILITY STATEMENT
The patient‐level analysis datasets for the research presented herein are available from the corresponding author upon reasonable request. Individual participant data will be shared in datasets in a deidentified or anonymized format.
REFERENCES
- 1. American Diabetes Association . Peripheral arterial disease in people with diabetes. Diabetes Care. 2003;26:3333‐3341. [DOI] [PubMed] [Google Scholar]
- 2. Shah AD, Langenberg C, Rapsomaniki E, et al. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1.9 million people. Lancet Diabetes Endocrinol. 2015;3:105‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Silbernagel G, Rein P, Saely CH, et al. Prevalence of type 2 diabetes is higher in peripheral artery disease than in coronary artery disease patients. Diab Vasc Dis Res. 2015;12:146‐149. [DOI] [PubMed] [Google Scholar]
- 4. Khoury H, Lavoie L, Welner S, Folkerts K. The burden of major adverse cardiac events and antiplatelet prevention in patients with coronary or peripheral arterial disease. Cardiovasc Ther. 2016;34:115‐124. [DOI] [PubMed] [Google Scholar]
- 5. Verma S, Mazer CD, Al‐Omran M, et al. Cardiovascular outcomes and safety of empagliflozin in patients with type 2 diabetes mellitus and peripheral artery disease: a subanalysis of EMPA‐REG OUTCOME. Circulation. 2018;137:405‐407. [DOI] [PubMed] [Google Scholar]
- 6. Low Wang CC, Blomster JI, Heizer G, et al. Cardiovascular and limb outcomes in patients with diabetes and peripheral artery disease: the EUCLID trial. J Am Coll Cardiol. 2018;72:3274‐3284. [DOI] [PubMed] [Google Scholar]
- 7. Bonaca MP, Nault P, Giugliano RP, et al. Low‐density lipoprotein cholesterol lowering with evolocumab and outcomes in patients with peripheral artery disease: insights from the FOURIER trial (further cardiovascular outcomes research with PCSK9 inhibition in subjects with elevated risk). Circulation. 2018;137:338‐350. [DOI] [PubMed] [Google Scholar]
- 8. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644‐657. [DOI] [PubMed] [Google Scholar]
- 9. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834‐1844. [DOI] [PubMed] [Google Scholar]
- 10. Marso SP, Daniels GH, Brown‐Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double‐blind, randomised placebo‐controlled trial. Lancet. 2019;394:121‐130. [DOI] [PubMed] [Google Scholar]
- 12. Hernandez AF, Green JB, Janmohamed S, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (harmony outcomes): a double‐blind, randomised placebo‐controlled trial. Lancet. 2018;392:1519‐1529. [DOI] [PubMed] [Google Scholar]
- 13. Gerstein HC, Sattar N, Rosenstock J, et al. Cardiovascular and renal outcomes with efpeglenatide in type 2 diabetes. N Engl J Med. 2021;385:896‐907. [DOI] [PubMed] [Google Scholar]
- 14. Sattar N, Lee MMY, Kristensen SL, et al. Cardiovascular, mortality, and kidney outcomes with GLP‐1 receptor agonists in patients with type 2 diabetes: a systematic review and meta‐analysis of randomised trials. Lancet Diabetes Endocrinol. 2021;9:653‐662. [DOI] [PubMed] [Google Scholar]
- 15. American Diabetes Association . 10. Cardiovascular disease and risk management: standards of medical care in diabetes‐2021. Diabetes Care. 2021;44:S125‐S150. [DOI] [PubMed] [Google Scholar]
- 16. Diabetes Canada Clinical Practice Guidelines Expert Committee , Stone JA, Houlden RL, Lin P, Udell JA, Verma S. Cardiovascular protection in people with diabetes. Can J Diabetes. 2018;42(Suppl 1):S162‐S169. [DOI] [PubMed] [Google Scholar]
- 17. Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC guidelines on diabetes, pre‐diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255–323. [DOI] [PubMed] [Google Scholar]
- 18. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2019;140:e596‐e646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Verma S, Bain SC, Buse JB, et al. Occurence of first and recurrent major adverse cardiovascular events with Liraglutide treatment among patients with type 2 diabetes and high risk of cardiovascular events: a post hoc analysis of a randomized clinical trial. JAMA Cardiol. 2019;4:1214‐1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Altman DG, Andersen PK. Calculating the number needed to treat for trials where the outcome is time to an event. BMJ. 1999;319:1492‐1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Verma S, Bhatt DL, Bain SC, et al. Effect of liraglutide on cardiovascular events in patients with type 2 diabetes mellitus and polyvascular disease: results of the LEADER trial. Circulation. 2018;137:2179‐2183. [DOI] [PubMed] [Google Scholar]
- 22. Bonaca MP, Gutierrez JA, Cannon C, et al. Polyvascular disease, type 2 diabetes, and long‐term vascular risk: a secondary analysis of the IMPROVE‐IT trial. Lancet Diabetes Endocrinol. 2018;6:934‐943. [DOI] [PubMed] [Google Scholar]
- 23. Cainzos‐Achirica M, Patel KV, Quispe R, et al. Coronary artery calcium for the allocation of GLP‐1RA for primary prevention of atherosclerotic cardiovascular disease. JACC Cardiovasc Imaging. 2021;14:1470‐1472. [DOI] [PubMed] [Google Scholar]
- 24. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117‐2128. [DOI] [PubMed] [Google Scholar]
- 25. Hess DA, Terenzi DC, Trac JZ, et al. SGLT2 inhibition with empagliflozin increases circulating provascular progenitor cells in people with type 2 diabetes mellitus. Cell Metab. 2019;30:609‐613. [DOI] [PubMed] [Google Scholar]
- 26. Badjatiya A, Merrill P, Buse JB, et al. Clinical outcomes in patients with type 2 diabetes mellitus and peripheral artery disease: results from the EXSCEL trial. Circ Cardiovasc Interv. 2019;12:e008018. [DOI] [PubMed] [Google Scholar]
- 27. Lin DS, Lee JK, Chen WJ. Major adverse cardiovascular and limb events in patients with diabetes treated with GLP‐1 receptor agonists vs DPP‐4 inhibitors. Diabetologia. 2021;64:1949‐1962. [DOI] [PubMed] [Google Scholar]
- 28. Anand SS, Bosch J, Eikelboom JW, et al. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double‐blind, placebo‐controlled trial. Lancet. 2018;391:219‐229. [DOI] [PubMed] [Google Scholar]
- 29. Drucker DJ. The cardiovascular biology of glucagon‐like peptide‐1. Cell Metab. 2016;24:15‐30. [DOI] [PubMed] [Google Scholar]
- 30. Terenzi DC, Trac JZ, Teoh H, et al. Vascular regenerative cell exhaustion in diabetes: translational opportunities to mitigate cardiometabolic risk. Trends Mol Med. 2019;25:640‐655. [DOI] [PubMed] [Google Scholar]
- 31. Giblett JP, Clarke SJ, Dutka DP, Hoole SP. Glucagon‐like peptide‐1: a promising agent for cardioprotection during myocardial ischemia. JACC Basic Transl Sci. 2016;1:267‐276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Giblett JP, Axell RG, White PA, et al. Glucagon‐like peptide‐1‐mediated cardioprotection does not reduce right ventricular stunning and cumulative ischemic dysfunction after coronary balloon occlusion. JACC Basic Transl Sci. 2019;4:222‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Marx N, Libby P. Cardiovascular benefits of GLP‐1 receptor agonism: Is inflammation a key? JACC Basic Transl Sci. 2018;3:858‐860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Garg V, Verma S, Connelly K. Mechanistic insights regarding the role of SGLT2 inhibitors and GLP1 agonist drugs on cardiovascular disease in diabetes. Prog Cardiovasc Dis. 2019;62:349‐357. [DOI] [PubMed] [Google Scholar]
- 35. Sharma A, Verma S. Mechanisms by which glucagon‐like‐peptide‐1 receptor agonists and sodium‐glucose Cotransporter‐2 inhibitors reduce cardiovascular risk in adults with type 2 diabetes mellitus. Can J Diabetes. 2020;44:93‐102. [DOI] [PubMed] [Google Scholar]
- 36. Rakipovski G, Rolin B, Nohr J, et al. The GLP‐1 analogs liraglutide and semaglutide reduce atherosclerosis in ApoE(‐/‐) and LDLr(‐/‐) mice by a mechanism that includes inflammatory pathways. JACC Basic Transl Sci. 2018;3:844‐857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Verma S, Leiter LA, Mazer CD, et al. Liraglutide reduces cardiovascular events and mortality in type 2 diabetes mellitus independently of baseline Low‐density lipoprotein cholesterol levels and statin use. Circulation. 2018;138:1605‐1607. [DOI] [PubMed] [Google Scholar]
- 38. Verma S, McGuire DK, Bain SC, et al. Effects of glucagon‐like peptide‐1 receptor agonists liraglutide and semaglutide on cardiovascular and renal outcomes across body mass index categories in type 2 diabetes: results of the LEADER and SUSTAIN 6 trials. Diabetes Obes Metab. 2020;22:2487‐2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Leiter LA, Bain SC, Bhatt DL, et al. The effect of glucagon‐like peptide‐1 receptor agonists liraglutide and semaglutide on cardiovascular and renal outcomes across baseline blood pressure categories: analysis of the LEADER and SUSTAIN 6 trials. Diabetes Obes Metab. 2020;22:1690‐1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. A/S NN A research study to compare a medicine called semaglutide against placebo in people with peripheral arterial disease and type 2 diabetes (STRIDE). 2020. https://clinicaltrials.gov/ct2/show/NCT04560998. Accessed 04 October 2021.
- 41. A/S NN Semaglutide effects on heart disease and stroke in patients with overweight or obesity (SELECT). 2019. https://clinicaltrials.gov/ct2/show/NCT03574597. Accessed 04 October 2021.
- 42. A/S NN A heart disease study of semaglutide in patients with type 2 diabetes (SOUL). 2019. https://clinicaltrials.gov/ct2/show/NCT03914326. Accessed 04 October 2021.
- 43. A/S NN A research study of how semaglutide works in people with disease affecting the heart and/or blood vessels and type 2 diabetes. 2019. https://clinicaltrials.gov/ct2/show/NCT04032197. Accessed 04 October 2021.
- 44. Anand SS, Caron F, Eikelboom JW, et al. Major adverse limb events and mortality in patients with peripheral artery disease: the COMPASS trial. J Am Coll Cardiol. 2018;71:2306‐2315. [DOI] [PubMed] [Google Scholar]
- 45. Dhatariya K, Bain SC, Buse JB, et al. The impact of liraglutide on diabetes‐related foot ulceration and associated complications in patients with type 2 diabetes at high risk for cardiovascular events: results from the LEADER trial. Diabetes Care. 2018;41:2229‐2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Husain M, Birkenfeld AL, Donsmark M, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381:841‐851. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Demographics and baseline characteristics of LEADER trial participants by history of PAD.
Table S2 Demographics and baseline characteristics of SUSTAIN 6 participants by history of PAD.
Table S3 Risk of CV events and mortality by history of PAD at baseline and randomized treatment group, adjusted for baseline variables.
Table S4 Changes from baseline in HbA1c, blood pressure and body weight in LEADER trial participants by history of PAD and randomized treatment group.
Table S5 Changes from baseline in HbA1c, blood pressure and body weight in SUSTAIN 6 trial participants by history of PAD and randomized treatment group.
Table S6 Serious adverse events and non‐serious medical events of special interest in LEADER trial participants by history of PAD.
Table S7 Serious adverse events and non‐serious medical events of special interest in SUSTAIN 6 trial participants by history of PAD.
Figure S1 Venn diagram of number (%) of patients according to PAD, CAD and cerebrovascular disease. (A) LEADER; (B) SUSTAIN 6
Data Availability Statement
The patient‐level analysis datasets for the research presented herein are available from the corresponding author upon reasonable request. Individual participant data will be shared in datasets in a deidentified or anonymized format.


