Summary
Among tropical forests, lianas are predicted to have a growth advantage over trees during seasonal drought, with substantial implications for tree and forest dynamics. We tested the hypotheses that lianas maintain higher water status than trees during seasonal drought and that lianas maximize leaf cover to match high, dry‐season light conditions, while trees are more limited by moisture availability during the dry season.
We monitored the seasonal dynamics of predawn and midday leaf water potentials and leaf phenology for branches of 16 liana and 16 tree species in the canopies of two lowland tropical forests with contrasting rainfall regimes in Panama.
In a wet, weakly seasonal forest, lianas maintained higher water balance than trees and maximized their leaf cover during dry‐season conditions, when light availability was high, while trees experienced drought stress. In a drier, strongly seasonal forest, lianas and trees displayed similar dry season reductions in leaf cover following strong decreases in soil water availability.
Greater soil moisture availability and a higher capacity to maintain water status allow lianas to maintain the turgor potentials that are critical for plant growth in a wet and weakly seasonal forest but not in a dry and strongly seasonal forest.
Keywords: canopy, lianas, light, Panama, phenology, seasonality, trees, water potential
Introduction
Lianas (woody vines) are an important plant lifeform in tropical forests, and are second only to trees in terms of biomass and contribution to leaf area (Gentry, 1992; Schnitzer & Bongers, 2002). Lianas use trees for support and access to high‐light positions in the forest canopy. Lianas compete intensely with trees for light and soil resources (Schnitzer, 2005; Toledo‐Aceves, 2015; Rodríguez‐Ronderos et al., 2016) and reduce tree regeneration (Schnitzer & Carson, 2010), growth (van der Heijden et al., 2015, 2019), reproduction (Wright et al., 2015; García León et al., 2018) and survival (Ingwell et al., 2010; Wright et al., 2015). Among tropical forests, liana abundance and diversity increase, both in absolute terms and relative to trees, with decreasing mean annual precipitation and increasing strength of seasonal drought (DeWalt et al., 2010; Parolari et al., 2020). Schnitzer (2005) advanced the seasonal growth advantage hypothesis (SGA) as a potential explanation for the pattern of increasing abundance and diversity of lianas with decreasing moisture availability. The SGA hypothesis posits that lianas maintain a more favorable water balance and thus grow more than co‐occurring trees during the drier season, when water availability is low (Schnitzer, 2005, 2018). Consequently, lianas are expected to accumulate additional annual growth in seasonal tropical forests, ultimately resulting in higher liana abundance than in wet, aseasonal forests, where lianas lack a seasonal growth advantage (Schnitzer, 2005, 2018). Indeed, lianas appear to grow more rapidly than trees during seasonal drought (Schnitzer & van der Heijden, 2019); however, the mechanisms responsible for the higher degree of growth observed in lianas than in trees during seasonal drought are not well understood.
In tropical forests, seasonal light availability is hypothesized to limit productivity and influence vegetative phenology (Van Schaik et al., 1993; Wright & Van Schaik, 1994). Above the canopy, light availability increases as cloud cover decrease from wet to dry season in most tropical forests (Windsor, 1990; Graham et al., 2003). Tropical tree species with access to dry‐season water supplies often regulate the production of leaves to match peaks of high, dry‐season irradiance (Wright & Van Schaik, 1994). Similarly, leaf production and forest productivity (i.e. photosynthesis) peak in the high‐light dry season in evergreen Amazônian and Asian monsoon forests (Elliott et al., 2006; Huete et al., 2006; Morton et al., 2014; Wu et al., 2016). Plants optimize photosynthetic carbon gain by producing new leaves and increasing total leaf area when irradiance is maximal (Field & Mooney, 1983; Kikuzawa, 1995; Kitajima et al., 1997; Doughty & Goulden, 2008). Correlations between leaf production, productivity, and irradiance suggest that light limits many tropical forest trees (Graham et al., 2003; Nemani et al., 2003). Nevertheless, not all trees and forests benefit from the abundant light availability during seasonal drought. In drier seasonal forests (< 2000 mm yr−1), tree species with different drought sensitivities show contrasting phenologies (Grubb, 1998; Eamus, 1999; Guan et al., 2015). Drought‐tolerant trees maintain hydraulic integrity via deep rooting and access to sufficient soil water and are predicted to produce leaves in the dry season (Van Schaik et al., 1993; Nepstad et al., 1994; da Rocha et al., 2004). Drought‐sensitive trees are predicted to produce leaves in the wet season because low water availability in the dry season induces strong water stress and limits plant function. The seasonal light availability hypothesis (SLA) predicts greater leaf production in the season of lowest cloud cover and highest irradiance among plants in forests with adequate water supplies (Van Schaik et al., 1993; Wright & Van Schaik, 1994).
The SGA and SLA hypotheses together suggest that lianas tend to have more access to dry‐season water supplies than trees, allowing lianas to maintain turgor potentials and regulate leaf production to match peaks of high, dry season irradiance. A more active leaf area during seasonal drought in lianas may optimize carbon gain (Field & Mooney, 1983, p. 198; Kikuzawa, 1995; Kitajima et al., 1997; Doughty & Goulden, 2008) and enable high growth rates (Borchert, 1999; Worbes, 1999; Schongart et al., 2002; O’Brien et al., 2008). The available evidence supports more favorable water status and higher physiological activity in lianas than in trees during seasonal drought. Lianas show higher leaf water potentials and photosynthetic rates than trees in the drier season in a seasonal tropical forest in China (Cai et al., 2009; Zhu & Cao, 2009; Chen et al., 2015) and in a common garden experiment in central Panama (Smith‐Martin et al., 2019) (Smith‐Martin et al., 2019). Lianas also allocate a higher proportion of their carbon to leaves (vs the stems) than trees, resulting in a relatively high photosynthetic tissue mass : plant mass ratio (Wyka et al., 2013; Medina‐Vega et al., 2021b), which, combined with high physiological activity during drought, may lead to increasingly high growth rates in dry periods. In central Panama, liana species tend to be evergreen and to produce new leaves during most of the year (Putz & Windsor, 1987), liana saplings grow faster in terms of height than tree saplings in the dry season (Schnitzer, 2005), and canopy lianas grow more rapidly in diameter than canopy trees during the dry season (Schnitzer & van der Heijden, 2019). These findings of these studies – that higher growth rates are observed in lianas than in trees during seasonal drought – support the fundamental premise of the SGA. However, the mechanisms that allow lianas to maintain hydraulic integrity and grow better than co‐occurring trees during dry periods remain unclear.
Previous studies suggest several possible mechanisms. Lianas may have deep (Holbrook & Putz, 1996; Restom & Nepstad, 2004; Andrade et al., 2005; Toledo‐Aceves & Swaine, 2008) and/or shallow lateral roots (De Deurwaerder et al., 2018; Smith‐Martin et al., 2019). Deep roots may enable lianas to access deep soil water (Schnitzer, 2005) and shallow lateral roots to efficiently capture dry‐season precipitation (De Deurwaerder et al., 2018), resulting in high hydraulic integrity and growth during seasonal drought (Schnitzer, 2005). High stem water storage (i.e. capacitance) resulting from abundant nonlignified parenchyma could also allow lianas to maintain hydraulic integrity during dry periods (Angyalossy et al., 2012; Isnard & Feild, 2015). Low resistance to hydraulic flow (i.e. high water transport capacity) resulting from their wide and long xylem elements (Ewers et al., 1991) may allow lianas to reduce water potential differences between soil and leaves, enabling high tissue water potentials and positive turgor potentials during drought. High water use efficiency resulting from strong stomatal control (Cai et al., 2009; Chen et al., 2015) may allow lianas to maximize gas exchange and growth while preventing excessive dehydration (see also Mumbanza et al., 2021). Additionally, a high accumulation of nonstructural carbohydrates for osmoregulation and hydraulic function (O’Brien et al., 2014; Martínez‐Vilalta et al., 2016) and reductions in leaf area (Lambers & Oliveira, 2019) could also explain the maintenance of high water status and turgor potentials in lianas during seasonal drought. Most of these possible mechanisms are mutually compatible and could reinforce one another.
Here, we explore predictions of the SGA and SLA hypotheses for the seasonal dynamics of leaf water status and leaf phenology of lianas and trees in the canopy of two lowland tropical forests on opposite sides of the Isthmus of Panama. One forest is relatively dry, strongly seasonal, and largely dry‐season deciduous. The second forest is wetter, weakly seasonal, and evergreen. We quantify the effects of seasonality on the hydraulic status and leaf phenology of eight liana and eight tree species in each forest (32 species in total). We tested three nonmutually exclusive hypotheses that address the fundamental premises of the SGA and SLA hypotheses. The first hypothesis is that predawn leaf water potentials (Ψpd), which are indicative of access to soil water (Sala et al., 1981; Williams & Araujo, 2002; Santesteban et al., 2019), are higher in lianas than in trees during seasonal drought. The second hypothesis is that midday leaf water potentials (Ψmd), which would provide the positive turgor potential necessary for leaf development (see Boyer & Silk, 2004), are higher in lianas than in trees during seasonal drought. The third hypothesis is that the seasonal timing of leaf development differs between lianas and trees and that lianas tend to maximize leaf cover to match high, dry‐season light conditions, while trees tend to be more limited by moisture availability during the dry season. We test these hypotheses in each forest and discuss the potential mechanisms that might allow lianas to maintain hydraulic integrity and thus to grow better than co‐occurring trees during seasonal drought.
Materials and Methods
Study sites
We collected data from two canopy cranes located in the Parque Natural Metropolitano (PNM, lat. 8°59′41.55″N, long. 79°32′35.22″W, 30 m above sea level (asl)) near Panama's Pacific coast and the Bosque Protector San Lorenzo (BPSL, lat. 9°16′51.71″N, long. 79°58′28.27″W, 130 m asl) near Panama’s Caribbean coast (Fig. 1a). Both cranes are equipped with a gondola suspended by cables from a trolley connected to a rotating jib (Fig. 1b). The 42‐m tall PNM crane had a 51 m jib and accessed 0.81 ha of forest. The 52‐m tall BPSL crane had a 54 m jib and accessed 0.91 ha of forest.
Fig. 1.
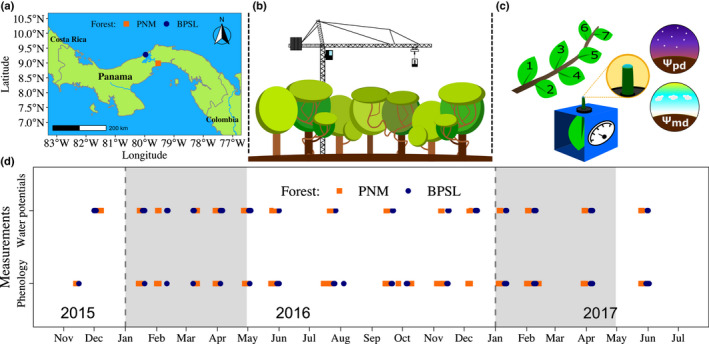
Location of the study sites and overview of the study design. (a) The location of the dry Parque Natural Metropolitano (PNM, orange square) and wet Bosque Protector San Lorenzo (BPSL, dark blue circle). (b) Graphical representation of the canopy cranes towering above the forest canopy, which were used at both study sites. (c) Graphical representation of a sample branch, with its leaves numbered, and a pressure chamber used to measure leaf water potential before dawn (predawn; Ψpd) and during the hour following midday (midday; Ψmd) for both lianas and trees in both forests. (d) Plot showing the timing of vegetative phenology and leaf water potential data collection for both lianas and trees from the canopy cranes in PNM (orange squares) and BPSL (dark‐blue circles). Gray shading identifies the January‐to‐April dry season.
The study sites experience contrasting rainfall regimes (Fig. 2). There is a drier season from mid–late December to mid–late April throughout Panama; however, the Caribbean sources most rain, and there is a strong Caribbean‐to‐Pacific rainfall gradient. At the BPSL and PNM sites, annual rainfall averages 3292 and 1864 mm, respectively, January‐through‐April rainfall averages 336 and 153 mm, and minimum cumulative dry‐season water deficits average −347 and −593 mm (Fig. 2). A stream at the base of the BPSL crane ceases to flow in some years, but streamside pools have retained water since the crane was installed in May 1997. The different dry‐season severities and soil conditions shape the different tree species compositions at both sites (Condit et al., 2013). Seasonality is much stronger at the PNM, where c. 50% of the canopy tree species are dry‐season deciduous (Wright, 2020). Seasonal changes in cloud cover cause seasonal changes in atmospheric transmissivity and solar radiation reaching both forests (Fig. 2). Mean temperatures are 25.4 and 26.1°C at the BPSL and PNM sites, respectively. The PNM site will hereafter be referred to as the ‘dry’ forest and the BPSL site as the ‘wet’ forest (Holdridge, 1967; Murphy & Lugo, 1986).
Fig. 2.
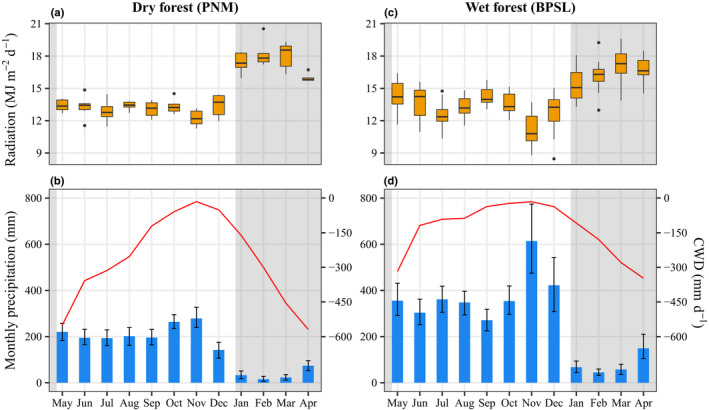
Environmental variables for the dry Parque Natural Metropolitano (PNM) and wet Bosque Protector San Lorenzo (BPSL) forests. Upper panels: Mean monthly solar radiation in the dry (a) and wet (c) forest. Box plots in (a, c) indicate (from bottom to top) the minimum (lower whiskers), lower quartile (Q1), median (horizontal lines), upper quartile (Q3), and maximum (upper whiskers) recorded monthly solar radiation. The points outside the range of the box plots represent outliers. Lower panels: Mean monthly precipitation (blue bars) and average minimum cumulative water deficits (CWD, red lines) for the dry (b) and wet (d) forests. Error bars in (b, d) indicate bootstrap ±SE. Gray shading identifies the January‐to‐April dry season. Horizontal axes indicate the month. Measurements were made at the cranes. Solar radiation is for 2012–2017 for the PNM and 2002, 2003, and 2008–2017 for the BPSL. Precipitation is for 1995–2017 for the PNM and 1997–2017 for the BPSL. Cumulative water deficit is for 2012–2017 for both forests. Cumulative water deficit is the cumulative difference between precipitation and potential evapotranspiration (PET). Data provided by the Physical Monitoring Program of the Smithsonian Tropical Research Institute.
Species selection and census protocol
We randomly selected eight liana and eight tree species at each site from species with two or more canopy individuals present. The 32 species included 22 families, with no species overlap between sites (Supporting Information Table S1). We selected two individuals of each species and four healthy branches of each individual. To minimize the variation associated with light levels, we selected branches in full sunlight and prevented other branches from overtopping them. The branches initially ranged from 30 to 70 cm in length. We numbered every leaf on each branch (Fig. 1c) with a permanent marker in November 2015 and returned 13 times through to May 2017 to record leaf water status and leaf births (Fig. 1d; Table S2). To record leaf births, we numbered new, fully expanded leaves at each census. We included leaves on all new branches that developed within the originally selected branches. If an entire branch died, we selected a replacement branch, so every individual had at least four branches throughout the study. Branch death occurred due to the movement of the gondolas and other biotic factors (i.e. sloths and monkeys feeding and/or hanging from the branches or wind‐thrown trees and lianas).
Measurements of leaf water potential
We used predawn leaf water potential (Ψpd) as an indicator of access to soil moisture (Sala et al., 1981; Williams & Araujo, 2002; Santesteban et al., 2019) and midday leaf water potential (Ψmd) as an indicator of leaf hydration status when evaporative demand is maximal (Williams & Araujo, 2002; Marechaux et al., 2018). We monitored Ψpd and Ψmd during each leaf census (Fig. 1d) and made two additional measurements for all species in December 2015 and December 2016 (Table S2). We collected five leaves from the top of each census individual, avoiding census branches, and placed them in a sealable plastic bag (from which the air had been expelled) with wet paper towels to limit transpiration. We placed the bagged leaves inside a cooler with cooling gel packs to keep temperatures relatively low and further limit transpiration. We measured water potentials within 30 min of collection for three randomly selected leaves using a Scholander pressure chamber (Model 1000; PMS Instruments, Albany, OR, USA). We recorded Ψpd (MPa) before sunrise from 04:30 h to 5:30 h and Ψmd (MPa) from 12:00 h to 13:00 h (Fig. 1c). When deciduous species lost their leaves, we collected and measured the tip of a branch following the same procedure.
Environmental variables
At both cranes, precipitation (mm), solar radiation (MJ m−2 d−1), air temperature (°C), and relative humidity (%) were recorded by permanent weather stations via the Physical Monitoring Program of the Smithsonian Tropical Research Institute (STRI). An electronic tipping bucket (Model TB4; Campbell Scientific Inc., Logan, UT, USA) recorded precipitation at 15 min intervals. Pyranometers (Li‐Cor Model LI200X Silicon Pyranometer; Campbell Scientific Inc.) recorded global solar radiation every 10 s and logged mean, minimum and maximum values every 15 min. The tipping buckets and pyranometers were located on top of the canopy cranes. Temperature and relative humidity probes (Model CS215; Campbell Scientific Inc.) located 25 m above the forest floor recorded air temperature and relative humidity values on the same schedule. We calculated the vapor pressure deficit (VPD, kPa) from relative humidity and temperature as described by Jones (2013). We calculated cumulative water deficit (CWD) as CWD n = CWD n −1 + (P n − PET n ), where n, n th day of the year; P, precipitation (mm); PET, potential evapotranspiration (mm); and positive CWD n −1 is reset to zero. CWD n potentially ranges from zero when P has been consistently larger than PET (i.e. soil saturation) to large negative values during intense dry periods. We calculated PET as described by Penman (1948) using the package evapotranspiration v.1.14 (Guo et al., 2016, 2019) in R v.4.0.3 (R Core Team, 2021) (Methods S1). We assumed soil saturation (i.e. CWD = 0) at the wettest time of the year (November; Fig. 2) and started the calculation of CWD.
Assessment of the seasonal dynamics of leaf water potentials
We quantified the seasonal changes in Ψpd and Ψmd for each site as a function of lifeform (liana vs tree) and CWD for each census using multilevel models with normally distributed errors. A square‐root transformation was used to normalize the absolute values of the response variables, Ψpd and Ψmd. We completed the transformation by multiplying the transformed values by –1 to retain the original direction of the response, with more negative values indicating more negative leaf water potentials. We standardized CWD to Z‐scores, dividing the difference between each observation and its mean value by its SD. Higher standardized (or scaled) values of CWD indicate a higher degree of soil saturation and lower values indicate drier conditions. To test hypotheses one and two, we included the interaction between lifeform and CWD in the models. The models included individuals nested within species as random intercepts to account for the nested structure of the design, which includes repeated censuses on individuals. We included CWD as a random slope to allow the community‐level coefficient (fixed effect) of the covariate to vary among individuals and species. In a preliminary analysis, we observed that the intercept varied consistently from census to census (Notes S1). We thus included the categorical variable ‘census’ as an additional random intercept to absorb variation in the intercept that was unexplained by species and individual identity (Methods S2).
Assessment of the seasonal dynamics of leaf phenology
We quantified the changes in the proportion of leaf cover on branches of lianas and trees during a full annual leaf phenological cycle. This approach allowed us to isolate seasonal variation while controlling variation associated with branch size and allocation differences. For each species, a full annual leaf phenological cycle started with the first census with increasing leaf cover in year one and ended at the census preceding the first census with increasing leaf cover in year two. We then standardized the number of leaves present on each branch by the maximum observed number of leaves present for that same branch during the annual cycle. Branches included in the standardization were surveyed at least four times (i.e. for newly added branches), with a maximum of 10 surveys and a median and a mode of eight surveys. The resulting proportional leaf cover puts all branches on the same scale, with values ranging from 0 (fully deciduous) to 1 (maximum observed leaf cover for that branch).
The proportion of leaf cover takes values from the closed unit interval (0, 1), is possibly skewed, and has nonzero probability at zero and one (Fig. S1). To analyze this nonnormal zero and one inflated distribution, we used zero/one inflated beta regression (ZOIB) (Ospina & Ferrari, 2010; Liu & Eugenio, 2018). The ZOIB model takes data in the closed unit interval (0, 1) and has two components: the beta distribution on (0, 1) and the Bernoulli distribution for the binary 0 and 1 responses. The ZOIB model has four parameters, the mean (μ) and precision (ν) for the beta distribution (continuous response), and α and γ for the zero and one inflation (discrete responses), respectively. μ and ν determine the location, skew, and spread of the beta distribution in the (0, 1) interval, α is the probability that zero or one occurs (zero‐one‐inflation probability), and γ is the probability that one occurs rather than zero (conditional one‐inflation probability). An advantage of the ZOIB model is that covariate effects can be estimated for either or both of the continuous (μ and ν) and discrete responses (α and γ). In our analyses, we quantified covariate effects for both the continuous and discrete responses.
We quantified changes in standardized leaf cover as a function of lifeform, CWD for each census date, and mean solar radiation for the 20 d before each census (Srad). In a preliminary analysis, we tested the association of standardized leaf cover with mean solar radiation for the 10, 20, and 30 d before each census. The results were robust using the three time windows, but models using either 20 or 30 d showed an improved predictive quality. We thus used 20 d. To test hypothesis three, we included the interactions between CWD and lifeform and between Srad and lifeform. Lianas and trees differed in the number of axillary shoots, with trees having multiple branching events and a more complex branching structure than lianas (Medina‐Vega et al., 2021b). Multiple branching affects leaf production (Yaish et al., 2010). To control for different numbers of axillary shoots at each census, we added the covariate number of axillary shoots per branch (sb). To control for differences in water supply, we added the covariates Ψpd (MPa) and Ψmd (MPa) as a proxy for access to soil moisture (Sala et al., 1981; Williams & Araujo, 2002; Santesteban et al., 2019) and leaf hydration status (Williams & Araujo, 2002; Marechaux et al., 2018), respectively. Precipitation and VPD were strongly collinear with CWD, Srad, Ψpd, and Ψmd, with variance inflation factors > 3.0 (Zuur et al., 2010), and were therefore excluded. We did not consider air temperature because it was relatively constant across seasons and sites and because it was included in the estimation of PET, CWD, and VPD.
Following the analyses of the seasonal dynamics of leaf water potentials, we standardized the continuous covariates to Z‐scores. Models included branches nested within individuals and species as random intercepts to represent the nested structure of the design and account for dependence among repeated censuses of each branch. We also included random slopes at the species level for CWD and Srad. These random slopes allowed us to account for interspecific variability in the responses as a function of CWD and Srad. We evaluated alternative models, including the main effects of lifeform, CWD, Srad, Ψpd, Ψmd, sb, and the interaction between CWD and lifeform and between Srad and lifeform (Methods S3).
Prior specifications, model selection, evaluation, and inference
We fitted all models in ‘Stan’ (Carpenter et al., 2017), which fits models using the Hamiltonian Monte Carlo (HMC) algorithm, with its interface to R (v.4.0.3; R Core Team, 2021) via rstan (v.2.21.2; Stan Development Team, 2020) using the package brms (v.2.14.4; Bürkner, 2017, 2018). We opted for the Bayesian approach because, to our knowledge, it provides more practical tools for the building and assessment of the ZOIB model, via the R package brms, than the frequentist approach. See Notes S2, S3 for a description, justification, and sensitivity assessment of the prior probability distributions for the models that best fitted the seasonal dynamics of leaf water potential and leaf phenology, respectively.
For each analysis, we constructed candidate models by removing model terms that did not contribute to the quality of the models, and the best fit model was selected using leave‐one‐out cross‐validation. We estimated each model using four chains of 5000 iterations, each with a warm‐up fraction of ½. We monitored Markov Chain mixing properties and checked parameter convergence graphically via traceplots of the estimated coefficients (Notes S4, S5) and by checking the Rhat metric (Gelman et al., 2013). We inspected the goodness‐of‐fit of the best fit models via posterior predictive model checks (Gabry et al., 2019), where predictions from the best fit model were compared to the observed data (Notes S6, S7). This process allowed us to assess any obvious discrepancies between the final model and the observed data. The results are presented using the median, and the uncertainty in the estimates are summarized using the 89% credible intervals (CIs) computed using the highest density interval (HDI) of posterior distributions, which favors probable over central values, and is recommended for nonsymmetric posterior distributions (Kruschke, 2014; McElreath, 2020).
Results
We tracked the development of 13 764 leaves during a full annual phenological cycle of branches representing 32 species, including 6861 leaves in the dry forest and 6903 leaves in the wet forest.
The seasonal dynamics of leaf water potentials
For the dry forest, the seasonal dynamics of Ψpd were similar between lianas and trees, but the seasonal dynamics of Ψmd differed between lifeforms (Fig. 3a). Ψpd increased consistently with increasing scaled values of CWD for both lifeforms (Table 1: β2 in Model A; Fig. S2a). The hypothesis that lianas have higher Ψpd than trees (hypothesis one, see the Introduction section) is rejected for the dry forest. Ψmd in trees consistently increased with increasing scaled values of CWD (Table 1: β3 in Model C), while Ψmd in lianas did not change with changes in CWD (Table 1: β2 in Model C; Fig. S2b). The hypothesis that lianas have higher Ψmd than trees (hypothesis two, see the Introduction section) is accepted for the dry forest. The best fit models for Ψpd and Ψmd explained 69% (25% , 69% ) and 79% (7% , 79% ) of the variation in Ψpd and Ψmd in the dry forest, respectively, with and representing the variation explained by fixed effects only and by fixed and random effects (Table S3) together, respectively.
Fig. 3.
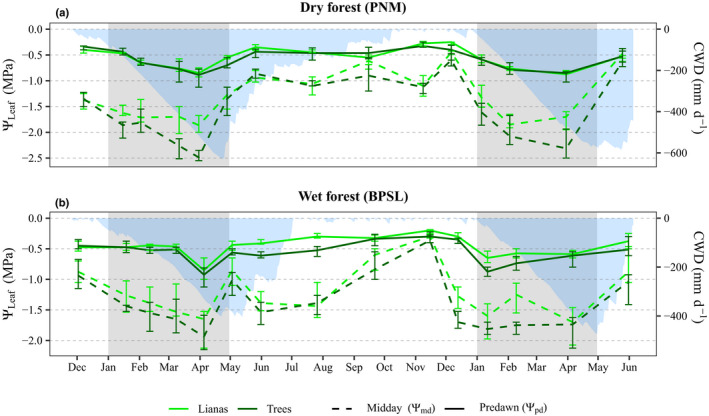
Median predawn (Ψpd, solid lines) and median midday (Ψmd, dashed lines) leaf water potentials for lianas (light green) and trees (dark green) for the dry (PNM) (a) and wet (BPSL) (b) forest. Vertical bars show the 89% confidence interval of the lifeform‐level (i.e. lianas vs trees) median calculated from species‐level median values (i.e. raw data) using 1000 nonparametric bootstraps and the highest density interval method. Light blue areas in the background show cumulative water deficit (CWD) for reference. Gray shading indicates the January‐to‐April dry season. Vertical axes differ between panels and the original scales (–MPa) are used. Horizontal axes represent the time of the month at which the measurements were made.
Table 1.
Summary of the fixed effects coefficients for the models that best fitted predawn (Ψpd) and midday leaf water potentials (Ψmd) in the dry (PNM) and wet (BPSL) forests.
| Fixed effects – coefficients | Dry forest – PNM | Wet forest – BPSL | ||||
|---|---|---|---|---|---|---|
| Median | 89CI lower | 89CI upper | Median | 89CI lower | 89CI upper | |
| Predawn leaf water potential Ψpd | Model A | Model B | ||||
| β0 – Interceptsic | −0.745 | −0.801 | −0.686 | −0.662 | −0.719 | −0.601 |
| β1 – Lifeform treesic | – | – | – | −0.08 | −0.133 | −0.025 |
| β2 – CWDsic | 0.087 | 0.043 | 0.136 | 0.088 | 0.037 | 0.136 |
| β3 – Lifeform treesic : CWDsic | – | – | – | – | – | – |
| Midday leaf water potential Ψmd | Model C | Model D | ||||
| β0 – Interceptsic | −1.106 | −1.242 | −0.973 | −1.065 | −1.172 | −0.962 |
| β1 – Lifeform treesic | −0.088 | −0.187 | 0.007 | −0.089 | −0.154 | −0.025 |
| β2 – CWDsic | 0.034 | −0.068 | 0.139 | 0.048 | −0.048 | 0.147 |
| β3 – Lifeform treesic : CWDsic | 0.017 | 0.005 | 0.029 | – | – | – |
A square‐root transformation was used to normalize the absolute values of the response variables Ψpd and Ψmd. We then multiplied the transformed values by –1 to recover the original direction of the response, with more negative values indicating more negative leaf water potentials. Median estimates that do not include zero within their credible intervals are in bold. Credible intervals were computed using the highest density interval (HDI) of posterior distributions, which is recommended for nonsymmetric (posterior) distributions (Kruschke, 2014). 89CI lower, lower 89% credible interval limit; 89CI upper, upper 89% credible interval limit; β 0…3 estimated coefficients from Equation 1 in Supporting Information Methods S2; c, census; CWDsic, cumulative water deficit at the time of observation; i, individual; s, species. For each forest, we constructed candidate models by removing model terms (fixed and random effects) that did not contribute to the quality of the models, and the best fit model was selected using leave‐one‐out cross‐validation. Covariates that did not contribute to the model are indicated by a dash (‘–’). A coefficient that contains zero within the CIs indicates a negligible association between the covariate and the response variable at the community level (fixed effects; this table), but suggests an important variation in the response at the species level and/or individual level (see random effects in Table S3).
For the wet forest, lianas had higher Ψpd and Ψmd than trees (Table 1: β1 in Models B and D; Fig. S3). Hypotheses one and two are accepted for the wet forest. The best fit models for Ψpd and Ψmd explained 58% (27% , 58% ) and 65% (7% , 65% ) of the variation in Ψpd and Ψmd in the wet forest, respectively. See Figs S4–S7 for the species‐level estimates for Ψpd and Ψmd in the dry and wet forests.
The seasonal dynamics of leaf cover
The study branches were c. 30 m above ground level in the outermost canopy and thus supported the leaves with the longest path length from the root to the crown. These branches also experienced more direct sunlight, faster wind speeds, and greater evaporative demand than lower branches and leaves. Nonetheless, the observed phenological patterns in the sun‐lit branches were consistent with branches at lower levels within the same crown (J.A. Medina‐Vega, pers. obs.).
For the dry forest, the seasonal dynamics of leaf cover were similar for lianas and trees (Fig. 4a). Trees maintained a larger proportion of leaf cover than lianas (Fig. 5a). We observed a high degree of interspecific variation within lifeforms, broadly overlapping variation between lifeforms (error bars in Fig. 4a). Both lianas and trees were under strong hydraulic stress during seasonal drought due to their limited access to soil moisture (Figs 3a, 5b) and, presumably, high vapor pressure deficit. The hypothesis that lianas tend to maximize leaf cover to match high, dry‐season light conditions while trees tend to be more limited by moisture availability during the dry season (hypothesis three, see the Introduction section) is rejected for the dry forest (refer to Notes S8).
Fig. 4.
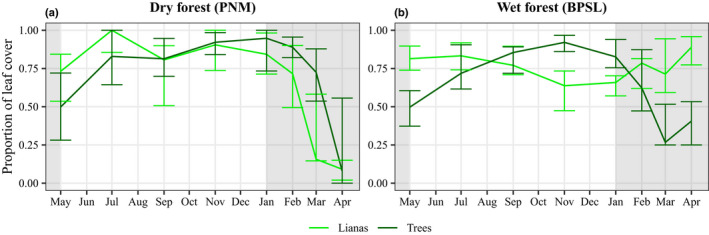
The (median) proportion of leaf cover in branches of lianas (light green) and trees (dark green) for the dry (PNM) (a) and wet (BPSL) (b) forests. Vertical bars show the 89% confidence interval of the lifeform‐level (i.e. lianas vs trees) median calculated from species‐level median values (i.e. raw data) using 1000 nonparametric bootstraps and the highest density interval method. Gray shading indicates the January‐to‐April dry season. Vertical axes represent the proportion of leaf cover. Horizontal axes represent the time of the month at which the measurements were made.
Fig. 5.
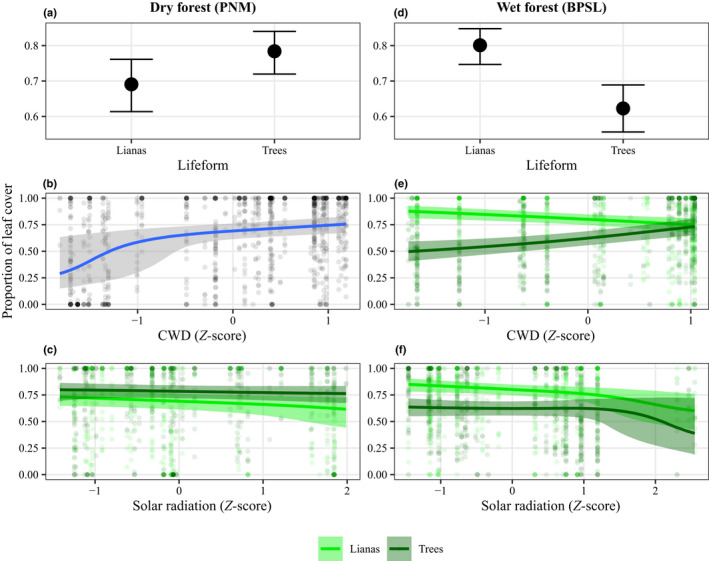
Conditional effects of lifeform (i.e. lianas vs trees) (a, d), cumulative water deficit (CWD) (b, e) and solar radiation (c, f) on the proportion of leaf cover of lianas and trees for the dry (PNM; left column) and wet (BPSL; right column) forests. The y‐axes show the predicted proportion of leaf cover. In (a) and (d), the x‐axes represent the levels of the categorical variable ‘lifeform’; in (b) and (e), they represent the (standardized) Z‐score of CWD (mm day−1); and in (c) and (f), they represent the (standardized) Z‐score of solar radiation (MJ m−2 d−1). The conditional effects for each predictor were calculated by setting the other (continuous) predictors to their mean values. The dots in (a) and (d) indicate the predicted median proportion of leaf cover for lianas and trees calculated from the posterior predictive distribution of the best‐fit model. The blue line in (b) indicates the predicted median proportion of leaf cover for both lianas and trees. The light green and dark green lines in (c), (e), and (f) indicate the predicted median proportion of leaf cover for lianas and trees, respectively. The error bars around the predicted median dot in (a) and (d), and the shadow(s) around the predicted median line(s) in (b), (c), (e) and (f) are represent the 89% credible intervals of the median estimate.
For the wet forest, the seasonal dynamics of leaf cover differed between lianas and trees (Figs 4b, 5e). Trees responded more strongly than lianas to changing light levels and water conditions (Fig. 5e,f). Lianas produced and maintained a higher proportion of leaf cover for longer, including during drier periods (Figs 4b, 5d,e). Hypothesis three is accepted for the wet forest.
For both forests, the covariate to control for multiple branching events (sb) made a consistent contribution to the quality of the models, and the best fit model for each forest explained a considerable amount of the variation in the proportion of leaf cover. An increase in the number of branching events was related to a consistent increase in the proportion of leaf cover (Table S4: β6 in Model A and B for the dry forest and the wet forest, respectively) and to a higher probability of observing a fully covered branch for both lianas and trees in both forests (Table S4:β6 in Model G and H for the dry forest and the wet forest, respectively). The fixed effects of the best fit model from the dry and wet forests explained 27% and 18% of the variation in the proportion of leaf cover (marginal R 2), and including random effects (Table S5), the explained variation increased to 52% and 47% (conditional R 2), respectively. (See Figs S8–S11 for the species‐level estimates of the proportion of leaf cover for lianas and trees in both the dry and wet forests.)
Discussion
The SGA hypothesis and the SLA hypothesis are intimately related and together suggest that higher hydraulic integrity in lianas than in trees during dry periods allows lianas to maintain turgor potentials, which are critical for plant growth (Hsiao & Acevedo, 1974; Boyer & Silk, 2004), and to regulate leaf production to match peaks of high dry‐season irradiance. We found support for the SGA and SLA hypotheses in a wet, weakly seasonal, evergreen forest, but not in a dry, strongly seasonal, largely deciduous forest.
Plant hydraulics support a liana seasonal growth advantage for a wet tropical forest
In the wet forest, lianas maintained higher water status than trees regardless of the season. Here, lianas had higher Ψpd than trees, which indicates greater access to soil water (Sala et al., 1981; Williams & Araujo, 2002; Santesteban et al., 2019) and may be particularly relevant in the dry season. The observed higher Ψpd in lianas than in trees in the wet forest is in line with other studies in a seasonal tropical forest in China (Zhu & Cao, 2009; Chen et al., 2015) and a common garden study in central Panama (Smith‐Martin et al., 2019) and with the hypothesis that lianas have a more efficient root architecture than trees (Schnitzer, 2005), by including deeper roots (Restom & Nepstad, 2004; Chen et al., 2015) and/or by having more shallow lateral roots (Johnson et al., 2013; De Deurwaerder et al., 2018). Lianas may thus benefit more from large volumes of potentially accessible soil water than trees (Smith‐Martin et al., 2019).
The higher Ψmd in lianas in the wet forest indicates that they have lower hydric stress compared to trees (Williams & Araujo, 2002). Similar observations for lianas compared to trees in the dry season have been reported in a common garden study in central Panama (Smith‐Martin et al., 2019). For the wet forest, two independent studies, one using the same set of species (Medina‐Vega et al., 2021a) and another using a number of additional species (De Guzman et al., 2021), reported indistinguishable conductivities between terminal branches of lianas and trees. Moreover, it was reported that the branches of lianas and trees in this wet forest study site did not differ in terms of leaf area : sapwood area ratio (Medina‐Vega et al., 2021b). The lower hydric stress in lianas can thus not be attributed to a higher stem hydraulic conductivity, which in theory could allow lianas to benefit more from available soil water. Lianas and trees also did not differ in terms of hydraulic architecture, at least not in branches (see Medina‐Vega et al., 2021a), which are considered to be the bottlenecks in the hydraulic pathway of large plants (Anfodillo & Olson, 2021). These results contrast with those of multiple other studies, which showed that lianas had a higher xylem conductivity than trees (Ewers, 1985; Gartner et al., 1990; Ewers et al., 1991). It has been reported, in contrast to our findings, that canopy lianas have thinner stem diameters relative to the amount of foliage they supply than trees (Putz, 1983; Wyka et al., 2013). Such conflicting results may partially be explained by differences in sampling (see Rosell & Olson, 2014), since some of the studies reporting larger conductivities and higher leaf area : sapwood area ratios in lianas than in trees used more proximal stem segments (e.g. Ewers, 1985; Gartner et al., 1990; Ewers et al., 1991). While we cannot rule out the possibility that such differences in more proximal stem segments would also be observed for the lianas and trees of our study, the observed similarity in hydraulic conductivity and architecture between liana and tree branches (Medina‐Vega et al., 2021a,b) implies a remarkable convergence of hydraulic conductivity and architecture and cannot explain the difference in Ψmd between lianas and trees in the wet forest.
The higher leaf water potentials in lianas suggest stronger stomatal control compared to trees, which allows lianas to reduce the variation in leaf water potentials by limiting transpiration (E) through reduced stomatal conductance (Gs) (Tyree & Sperry, 1988; Cochard et al., 2002; Sperry et al., 2002). Since such stronger stomatal control may lead to a higher water use efficiency (the ratio of biomass accumulation to water lost) (Sinclair et al., 1984; Tardieu, 2013; Lawson & Blatt, 2014), this may allow the lianas under study here to maintain leaf productivity while preventing excessive dehydration under drier environmental conditions (Sinclair et al., 1984; Mumbanza et al., 2021). Such mechanisms may thus appear in the liana and tree communities under study here, as well as in liana communities in other forests (Andrade et al., 2005; Chen et al., 2015).
Another mechanism allowing lianas to achieve higher leaf productivity during dry periods would be increased osmoregulation, which has been observed in a liana–tree comparison in a tropical forest in French Guiana (Marechaux et al., 2017). Stronger osmoregulation would allow lianas to actively control their osmotic cell pressure by decreasing their osmotic potential (i.e. by accumulating osmotic solutes (glycinebetaine, sorbitol, and proline) (Lambers & Oliveira, 2019)) (Morgan, 1984; Hartmann & Trumbore, 2016), leading to the maintenance of turgor with decreasing water availability (Chaves et al., 2003; Lambers & Oliveira, 2019). In our study we did not measure proxies for osmoregulation, but in addition to a strong stomatal control, more active osmoregulation could also contribute to the maintenance of higher leaf water potentials, as observed for lianas compared to trees.
Leaf phenology supports a liana seasonal growth advantage for a wet tropical forest
Lianas and trees in the wet forest showed contrasting patterns of leaf cover with seasonality. Lianas maintained their leaf cover during most of the year, while leaf cover in trees was constrained in the dry season (Fig. 4b). This result is consistent with that of another study conducted in central Panama, in which it was reported that liana species tend to be evergreen and produce new leaves during more of the year than tree species (Putz & Windsor, 1987). These results imply that higher leaf water potentials (Figs 3b; S3b,d) allowed the lianas to maintain and produce new leaves during seasonal drought, while the trees reduced their leaf cover during the same periods to avoid water stress (Figs 4b, 5e). The production of new leaf cover during seasonal drought may provide wet forest lianas with two advantages: they benefit from high dry‐season light levels (Fig. 2c) and they benefit from higher assimilation rates from younger than older leaves (Field & Mooney, 1983; Kikuzawa, 1995; Kitajima et al., 1997; Doughty & Goulden, 2008). A higher proportion of leaf cover (Fig. 4b) and higher water status in lianas than in trees during seasonal drought (Figs 3b; S3b,d) are consistent with both the SGA and SLA hypotheses.
Hydraulics, vegetative phenology, and the seasonal growth advantage in a dry forest
In contrast to the wet forest, lianas and trees in the dry forest exhibited a similar response in Ψpd (Fig. 3a) and in changes in leaf cover with increasing drought intensity (Fig. 4a), but differed in terms of their Ψmd responses (Fig. 3a). These results for the dry forest contrast with our expectation that lianas have greater access to soil water and a higher proportion of leaf cover than trees during seasonal drought.
The observation that lianas and trees in the dry forest experienced similar declines in Ψpd during seasonal drought contrasts with observations from the wet forest and with observations from a moist tropical forest in central Panama (Smith‐Martin et al., 2019) and a tropical dry forest in China (Chen et al., 2015). On‐site observations indicate that soils in the dry forest experience strong declines in water potentials (Santiago et al., 2004) and that the water table is deeper than 6 m in the dry season (B. Wolfe and M. Detto, pers. comm.). During seasonal drought, the low soil water supply may induce a strong convergence in Ψpd between lianas and trees in the dry forest.
Lianas in the dry forest experienced lower maximums of hydric stress (i.e. higher Ψmd) than trees in the dry season, implying greater stomatal control (Andrade et al., 2005; Chen et al., 2015). Additionally, increased osmoregulation (Marechaux et al., 2017) may contribute to lower hydric stress (Chaves et al., 2003; Lambers & Oliveira, 2019) in lianas than in trees, but this was not quantified in our study. However, the similar decrease in leaf cover between dry‐forest lianas and trees in the dry season (Fig. 4a) suggests that reducing leaf area is an important mechanism for maintaining hydraulic integrity during seasonal drought (Tyree et al., 1993). This finding is in agreement with those of another study at the same site, in which the cumulative proportion of the canopy surface of both lianas (three species overlap) and trees (six species overlap) was found to be lower in the dry season than in the wet season (Avalos & Mulkey, 1999). The shedding of leaves to avoid drought in tropical dry forests (i.e. mean annual rainfall < 2000 mm; Murphy & Lugo, 1986; Eamus, 1999; Guan et al., 2015) may therefore be a generic phenomenon for both trees and lianas at our dry forest study site (see Fig. S8), which contradicts the SGA and SLA hypotheses.
The maintenance of hydraulic integrity in lianas
Multiple mechanisms may allow lianas to maintain water status within tolerable limits and the turgor potentials critical for plant growth. The water potential of the plant is driven by gradients in water potential (ΔΨ) that result from the interaction between soil water potentials (Ψsoil), which govern water supply, and transpiration (E), which governs water loss (Dixon & Joly, 1895; Pickard, 1981) (Fig. 6). In wetter periods (i.e. high Ψsoil and low VPD), plants maintain smaller gradients in water potentials (low |ΔΨ|) and higher leaf water potentials (Ψleaf), which enable cell turgor and plant growth (Boyer & Silk, 2004; Muller et al., 2011), than in drier periods. In drier periods, Ψsoil drops and leaf‐to‐air vapor pressure differences (D) increase with increasing VPD. High D and low Ψsoil lead to high |ΔΨ| through increased E and reduced conductance (K). High |ΔΨ| leads to lower Ψleaf, reduced cell turgidity, and reduced growth. It has been proposed that lianas may connect strongly to soil water via deep (Restom & Nepstad, 2004) and/or shallow lateral roots (Johnson et al., 2013; De Deurwaerder et al., 2018), and greater K may allow lianas to maintain lower |ΔΨ| by moving available soil water from their roots to their leaves more rapidly than trees (Ewers, 1985; Gartner et al., 1990; Ewers et al., 1991). With increasing drought, greater stomatal control (Andrade et al., 2005; Chen et al., 2015) may allow lianas to reduce variation in ΔΨ by limiting E through reduced stomatal conductance (Gs) (Tyree & Sperry, 1988; Cochard et al., 2002; Sperry et al., 2002) and increased osmoregulation (Marechaux et al., 2017) may allow lianas to maintain turgor potentials and operate at high |ΔΨ| (Chaves et al., 2003; Lambers & Oliveira, 2019). These mechanisms are mutually compatible and could reinforce one another.
Fig. 6.
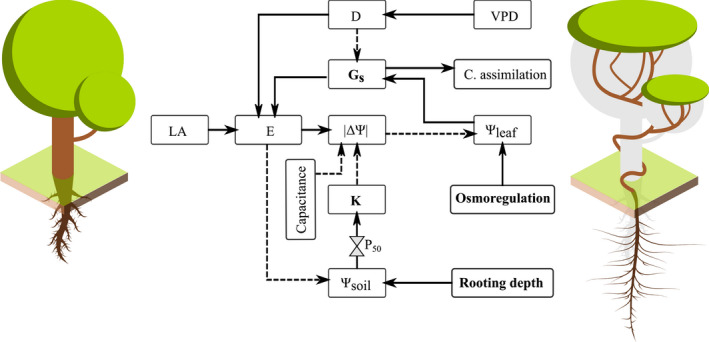
Schematic illustrating some of the variables and the relationships between plant hydraulics and carbon assimilation. Solid lines indicate positive relationships between variables, and dashed lines indicate negative relationships. Water flow and water status in the plant results from gradients in water potentials (ΔΨ) that originate from the interaction between soil water availability (Ψsoil) and transpiration (E). In dry periods, Ψsoil drops and leaf‐to‐air vapor pressure difference (D) increases with increasing vapor pressure deficit (VPD). To maintain low differentials in water potentials (low |ΔΨ|) and physiological activity during seasonal drought, plants may connect strongly with water reserves (i.e. deeper and/or shallower lateral roots, conductance (K) and capacitance), plants may disconnect from the atmosphere with increasing D (i.e. reductions in stomatal conductance (Gs) via stomatal control or reductions in leaf area (LA)), or plants may withstand and operate at high |ΔΨ| (i.e. greater resistance of the xylem to embolism (P50) and increased osmoregulation). Low |ΔΨ| leads to high leaf water potentials, turgor, and growth. Variables in bold are the suggested mechanisms that allow lianas to maintain water status, particularly during seasonal drought, and provide the turgor potentials needed to produce leaves and growth. See ‘The maintenance of hydraulic integrity in lianas’ in the Discussion section for further details.
We discarded mechanisms such as capacitance, which could provide lianas with additional water availability (Ewers & Fisher, 1991; Spicer & Groover, 2010; Isnard & Feild, 2015), and embolism resistance (P50), which could allow lianas to operate under high evaporative demand, because their use is not supported by the literature. Previous studies from our study sites that included individuals from the same set of species as our study reported that the branches of lianas do not have higher capacitance (De Guzman et al., 2017, 2021) or higher embolism resistance than the branches of trees (De Guzman et al., 2021; Medina‐Vega et al., 2021a). Results from these studies suggest that lianas rely primarily on soil water availability and conductive capability (Chen et al., 2016; De Guzman et al., 2017), and in the absence of soil water, liana stems may be highly vulnerable to drought‐induced cavitation (P50) (Hacke et al., 2006; Gutierrez et al., 2009; van der Sande et al., 2013).
Some of the discussed mechanisms (i.e. Gs, K, and osmoregulation (Fig. 6)) that may allow lianas to maintain hydraulic integrity during seasonal drought are not directly tested in this study. Neither does this study directly test the link between high hydraulic integrity during seasonal drought, additional annual growth, and a high or increasing abundance of lianas in seasonally dry tropical forests. Our study does imply, however, that better access to soil water – as implied by the SGA and SLA hypotheses – is not a sufficient explanation for the higher numbers of lianas in drier forests and that the mechanisms that maintain a small |ΔΨ| should be considered from a whole‐plant perspective (Fig. 6). We therefore call for studies that combine multiple physiological observations (see Fig. 6) and the seasonality of those observations (e.g. accumulation of osmotically active compounds), with detailed and more direct measurements of growth (e.g. diameter, height (length), biomass) and for longer time periods. These studies may provide a better picture of the mechanisms that allow lianas to grow more than trees during seasonal drought and explain the higher liana abundances in seasonal tropical forests (Schnitzer, 2005, 2018).
Our study included two forests with no species overlap between sites. Under the Panama rainfall gradient, dry‐season severity and soil P are the main drivers of tree species distribution (Condit et al., 2013), with c. 50% of canopy tree species in the dry forest being dry‐season deciduous (Wright, 2020). Resource availability and life history may also shape the spatial distribution of liana species under this gradient (see Medina‐Vega et al., 2021a,b), and these possibilities require further investigation. Given that the variations in cloud cover (i.e. light), precipitation (Fig. 2) and soil nutrients (Woodring et al., 1980; Santiago et al., 2005) under this gradient are consistent with broader geographical gradients across the tropics, our findings may apply to other dry and wet tropical forests. Remarkably, we found support for the SGA and SLA hypotheses in a wet, weakly seasonal, evergreen forest, but not in a dry, strongly seasonal, largely deciduous forest.
Author contributions
JAM‐V, SJW, FB, SAS and FJS conceived the ideas and designed the methodology. JAM‐V collected and analyzed the data. JAM‐V and SJW interpreted the data. JAM‐V led the writing of the manuscript, with comments from SJW, FB, SAS and FJS. All authors contributed critically to the drafts and gave final approval for publication.
Supporting information
Fig. S1 Frequency distribution for the proportion of leaf cover.
Fig. S2 Conditional effects for the models that best fitted leaf water potentials in the dry forest.
Fig. S3 Conditional effects for the models that best fitted leaf water potentials in the wet forest.
Fig. S4 Species‐level predictions for predawn leaf water potentials – dry forest.
Fig. S5 Species‐level predictions for midday leaf water potentials – dry forest.
Fig. S6 Species‐level predictions for predawn leaf water potentials – wet forest.
Fig. S7 Species‐level predictions for midday leaf water potentials – wet forest.
Fig. S8 Species‐level predictions – proportion of leaf cover as a function of cumulative water deficit in the dry forest.
Fig. S9 Species‐level predictions – proportion of leaf cover as a function of CWD in the wet forest.
Fig. S10 Species‐level predictions – proportion of leaf cover as a function of solar radiation in the dry forest.
Fig. S11 Species‐level predictions – proportion of leaf cover as a function of solar radiation in the wet forest.
Methods S1 Calculation of potential evapotranspiration.
Methods S1 Regression equation – leaf water potential models.
Methods S1 Regression equation – proportion of leaf cover models.
Note S1 Justification for the inclusion of census as a random intercept in the models of leaf water potentials.
Note S2 Prior justification – leaf water potential models.
Note S3 Prior justification – proportion of leaf cover models.
Note S4 Traceplots – leaf water potential models.
Note S5 Traceplots – proportion of leaf cover models.
Note S6 Posterior predictive checks – leaf water potential models.
Note S7 Posterior predictive checks – proportion of leaf cover models.
Note S8 Supplementary results – proportion of leaf cover models.
Table S1 Study species.
Table S2 Census dates.
Table S3 Random effects – leaf water potential models.
Table S4 Fixed effects – proportion of leaf cover models.
Table S5 Random effects – proportion of leaf cover models.
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Acknowledgements
We thank César Gutierrez, Dioselina Vigil, Pieter de Leeuw, Rosa Boschman, Judith de Bree, Felipe Mello, Areli Benito, Yuriani Cozzarelli, Stephany Arizala, Esther Velasquez, Diego Rincón and Eva Gril for their contributions to the data collection, and to Edwin Andrades and Oscar Saldaña for operating the canopy cranes. We thank three anonymous reviewers for their helpful comments on the drafts of this manuscript. This work is part of the research project ‘Explaining the rapid increase in lianas in tropical forest’, with project number 824.14.006, which is financed by the Dutch Research Council (NWO) and also supported by the Smithsonian Tropical Research Institute (STRI).
Data availability
Data and code supporting the results are available from Zenodo: https://doi.org/10.5281/zenodo.6403252 (Medina‐Vega et al., 2022).
References
- Andrade JL, Meinzer FC, Goldstein G, Schnitzer SA. 2005. Water uptake and transport in lianas and co‐occurring trees of a seasonally dry tropical forest. Trees‐Structure and Function 19: 282–289. [Google Scholar]
- Anfodillo T, Olson ME. 2021. Tree mortality: testing the link between drought, embolism vulnerability, and xylem conduit diameter remains a priority. Frontiers in Forests and Global Change 4: 704670. [Google Scholar]
- Angyalossy V, Angeles G, Pace MR, Lima AC, Dias‐Leme CL, Lohmann LG, Madero‐Vega C. 2012. An overview of the anatomy, development and evolution of the vascular system of lianas. Plant Ecology & Diversity 5: 167–182. [Google Scholar]
- Avalos G, Mulkey SS. 1999. Seasonal changes in liana cover in the upper canopy of a neotropical dry forest. Biotropica 31: 186–192. [Google Scholar]
- Borchert R. 1999. Climatic periodicity, phenology, and cambium activity in tropical dry forest trees. Iawa Journal 20: 239–247. [Google Scholar]
- Boyer JS, Silk WK. 2004. Review: hydraulics of plant growth. Functional Plant Biology 31: 761–773. [DOI] [PubMed] [Google Scholar]
- Bürkner P‐C. 2017. brms: an R package for Bayesian multilevel models using Stan. Journal of Statistical Software 80: 1–28. [Google Scholar]
- Bürkner P‐C. 2018. Advanced Bayesian multilevel modeling with the R Package brms. The R Journal 10: 395–411. [Google Scholar]
- Cai ZQ, Schnitzer SA, Bongers F. 2009. Seasonal differences in leaf‐level physiology give lianas a competitive advantage over trees in a tropical seasonal forest. Oecologia 161: 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter B, Gelman A, Hoffman MD, Lee D, Goodrich B, Betancourt M, Brubaker M, Guo J, Li P, Riddell A. 2017. Stan: a probabilistic programming language. Journal of Statistical Software 76: 1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves MM, Maroco JP, Pereira JS. 2003. Understanding plant responses to drought — from genes to the whole plant. Functional Plant Biology 30: 239–264. [DOI] [PubMed] [Google Scholar]
- Chen YJ, Bongers F, Tomlinson K, Fan ZX, Lin H, Zhang SB, Zheng YL, Li YP, Cao KF, Zhang JL. 2016. Time lags between crown and basal sap flows in tropical lianas and co‐occurring trees. Tree Physiology 36: 736–747. [DOI] [PubMed] [Google Scholar]
- Chen YJ, Cao KF, Schnitzer SA, Fan ZX, Zhang JL, Bongers F. 2015. Water‐use advantage for lianas over trees in tropical seasonal forests. New Phytologist 205: 128–136. [DOI] [PubMed] [Google Scholar]
- Cochard H, Coll L, Le Roux X, Ameglio T. 2002. Unraveling the effects of plant hydraulics on stomatal closure during water stress in walnut. Plant Physiology 128: 282–290. [PMC free article] [PubMed] [Google Scholar]
- Condit R, Engelbrecht BM, Pino D, Perez R, Turner BL. 2013. Species distributions in response to individual soil nutrients and seasonal drought across a community of tropical trees. Proceedings of the National Academy of Sciences, USA 110: 5064–5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Deurwaerder H, Hervé‐Fernández P, Stahl C, Burban B, Petronelli P, Hoffman B, Bonal D, Boeckx P, Verbeeck H. 2018. Liana and tree below‐ground water competition—evidence for water resource partitioning during the dry season. Tree Physiology 38: 1071–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Guzman ME, Acosta‐Rangel A, Winter K, Meinzer FC, Bonal D, Santiago LS. 2021. Hydraulic traits of Neotropical canopy liana and tree species across a broad range of wood density: implications for predicting drought mortality with models. Tree Physiology 41: 24–34. [DOI] [PubMed] [Google Scholar]
- De Guzman ME, Santiago LS, Schnitzer SA, Alvarez‐Cansino L. 2017. Trade‐offs between water transport capacity and drought resistance in neotropical canopy liana and tree species. Tree Physiology 37: 1404–1414. [DOI] [PubMed] [Google Scholar]
- DeWalt SJ, Schnitzer SA, Chave J, Bongers F, Burnham RJ, Cai Z, Chuyong G, Clark DB, Ewango CEN, Gerwing JJ et al. 2010. Annual rainfall and seasonality predict pan‐tropical patterns of liana density and basal area. Biotropica 42: 309–317. [Google Scholar]
- Dixon HH, Joly J. 1895. On the ascent of sap. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences 186: 563–576. [Google Scholar]
- Doughty CE, Goulden ML. 2008. Seasonal patterns of tropical forest leaf area index and CO2 exchange. Journal of Geophysical Research Biogeosciences 113: G00B06. [Google Scholar]
- Eamus D. 1999. Ecophysiological traits of deciduous and evergreen woody species in the seasonally dry tropics. Trends in Ecology & Evolution 14: 11–16. [DOI] [PubMed] [Google Scholar]
- Elliott S, Baker PJ, Borchert R. 2006. Leaf flushing during the dry season: the paradox of Asian monsoon forests. Global Ecology and Biogeography 15: 248–257. [Google Scholar]
- Ewers FW. 1985. Xylem’ structure and water conduction in conifer trees, dicot trees, and lianas. Iawa Journal 6: 309–317. [Google Scholar]
- Ewers FW, Fisher JB. 1991. Why vines have narrow stems: histological trends in Bauhinia (Fabaceae). Oecologia 88: 233–237. [DOI] [PubMed] [Google Scholar]
- Ewers FW, Fisher JB, Fichtner K. 1991. Water flux and xylem structure in vines. In: Putz FE, Mooney HA, eds. The biology of vines. Cambridge, UK: Cambridge University Press, 127–160. [Google Scholar]
- Field C, Mooney HA. 1983. Leaf age and seasonal effects on light, water, and nitrogen use efficiency in a California shrub. Oecologia 56: 348–355. [DOI] [PubMed] [Google Scholar]
- Gabry J, Simpson D, Vehtari A, Betancourt M, Gelman A. 2019. Visualization in Bayesian workflow. Journal of the Royal Statistical Society: Series A (Statistics in Society) 182: 389–402. [Google Scholar]
- García León MM, Martínez Izquierdo L, Mello FNA, Powers JS, Schnitzer SA, Edwards D. 2018. Lianas reduce community‐level canopy tree reproduction in a Panamanian forest. Journal of Ecology 106: 737–745. [Google Scholar]
- Gartner BL, Bullock SH, Mooney HA, Brown VB, Whitbeck JL. 1990. Water transport properties of vine and tree stems in a tropical deciduous forest. American Journal of Botany 77: 742–749. [Google Scholar]
- Gelman A, Carlin JB, Stern HS, Dunson DB, Vehtari A, Rubin DB. 2013. Bayesian data analysis. Boca Raton, FL, USA: CRC Press. [Google Scholar]
- Gentry AH. 1992. The distribution and evolution of climbing plants. In: Putz FE, Mooney HA, eds. The biology of vines. Cambridge, UK: Cambridge University Press, 3–50. [Google Scholar]
- Graham EA, Mulkey SS, Kitajima K, Phillips NG, Wright SJ. 2003. Cloud cover limits net CO2 uptake and growth of a rainforest tree during tropical rainy seasons. Proceedings of the National Academy of Sciences, USA 100: 572–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb PJ. 1998. A reassessment of the strategies of plants which cope with shortages of resources. Perspectives in Plant Ecology, Evolution and Systematics 1: 3–31. [Google Scholar]
- Guan K, Pan M, Li H, Wolf A, Wu J, Medvigy D, Caylor KK, Sheffield J, Wood EF, Malhi Y et al. 2015. Photosynthetic seasonality of global tropical forests constrained by hydroclimate. Nature Geoscience 8: 284–289. [Google Scholar]
- Guo D, Westra S, Maier HR. 2016. An R package for modelling actual, potential and reference evapotranspiration. Environmental Modelling & Software 78: 216–224. [Google Scholar]
- Guo D, Westra S, Peterson T. 2019. Evapotranspiration: modelling actual, potential and reference crop evapotranspiration . R package v.1.14. [WWW document] URL https://cran.r‐project.org/web/packages/Evapotranspiration/index.html [accessed 11 September 2019].
- Gutierrez M, San Miguel‐Chavez R, Terrazas T. 2009. Xylem conductivity and anatomical traits in diverse lianas and small tree species from a tropical forest of Southwest Mexico. International Journal of Botany 5: 279–286. [Google Scholar]
- Hacke UG, Sperry JS, Wheeler JK, Castro L. 2006. Scaling of angiosperm xylem structure with safety and efficiency. Tree Physiology 26: 689–701. [DOI] [PubMed] [Google Scholar]
- Hartmann H, Trumbore S. 2016. Understanding the roles of nonstructural carbohydrates in forest trees – from what we can measure to what we want to know. New Phytologist 211: 386–403. [DOI] [PubMed] [Google Scholar]
- van der Heijden GMF, Powers JS, Schnitzer SA. 2015. Lianas reduce carbon accumulation and storage in tropical forests. Proceedings of the National Academy of Sciences, USA 112: 13267–13271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heijden GMF, Powers JS, Schnitzer SA. 2019. Effect of lianas on forest‐level tree carbon accumulation does not differ between seasons: results from a liana removal experiment in Panama. Journal of Ecology 107: 1890–1900. [Google Scholar]
- Holbrook NM, Putz FE. 1996. Physiology of tropical vines and hemiepiphytes: plants that climb up and plants that climb down. In: Mulkey SS, Chazdon RL, Smith AP, eds. Tropical forest plant ecophysiology. Boston, MA, USA: Springer US, 363–394. [Google Scholar]
- Holdridge LR. 1967. Life zone ecology. San José, Costa Rica: Tropical Science Center. [Google Scholar]
- Hsiao TC, Acevedo E. 1974. Plant responses to water deficits, water‐use efficiency, and drought resistance. Agricultural Meteorology 14: 59–84. [Google Scholar]
- Huete AR, Didan K, Shimabukuro YE, Ratana P, Saleska SR, Hutyra LR, Yang WZ, Nemani RR, Myneni R. 2006. Amazon rainforests green‐up with sunlight in dry season. Geophysical Research Letters 33: L06405. [Google Scholar]
- Ingwell LL, Joseph Wright S, Becklund KK, Hubbell SP, Schnitzer SA. 2010. The impact of lianas on 10 years of tree growth and mortality on Barro Colorado Island, Panama. Journal of Ecology 98: 879–887. [Google Scholar]
- Isnard S, Feild TS. 2015. The evolution of angiosperm lianescence: a perspective from xylem structure‐function. In: Schnitzer SA, Bongers F, Burnham RJ, Putz FE, eds. Ecology of lianas. Chichester, UK: John Wiley & Sons, 221–238. [Google Scholar]
- Johnson DM, Domec JC, Woodruff DR, McCulloh KA, Meinzer FC. 2013. Contrasting hydraulic strategies in two tropical lianas and their host trees. American Journal of Botany 100: 374–383. [DOI] [PubMed] [Google Scholar]
- Jones HG. 2013. Plants and microclimate. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Kikuzawa K. 1995. Leaf phenology as an optimal strategy for carbon gain in plants. Canadian Journal of Botany 73: 158–163. [Google Scholar]
- Kitajima K, Mulkey S, Wright S. 1997. Decline of photosynthetic capacity with leaf age in relation to leaf longevities for five tropical canopy tree species. American Journal of Botany 84: 702. [PubMed] [Google Scholar]
- Kruschke J. 2014. Doing Bayesian data analysis: a tutorial with R, JAGS, and Stan. Amsterdam, the Netherlands: Academic Press. [Google Scholar]
- Lambers H, Oliveira RS. 2019. Plant physiological ecology. Cham, Switzerland: Springer. [Google Scholar]
- Lawson T, Blatt MR. 2014. Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiology 164: 1556–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Eugenio EC. 2018. A review and comparison of Bayesian and likelihood‐based inferences in beta regression and zero‐or‐one‐inflated beta regression. Statistical Methods in Medical Research 27: 1024–1044. [DOI] [PubMed] [Google Scholar]
- Marechaux I, Bartlett MK, Iribar A, Sack L, Chave J. 2017. Stronger seasonal adjustment in leaf turgor loss point in lianas than trees in an Amazonian forest. Biology Letters 13: 20160819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maréchaux I, Bonal D, Bartlett MK, Burban B, Coste S, Courtois EA, Dulormne M, Goret J‐Y, Mira E, Mirabel A et al. 2018. Dry‐season decline in tree sapflux is correlated with leaf turgor loss point in a tropical rainforest. Functional Ecology 32: 2285–2297. [Google Scholar]
- Martínez‐Vilalta J, Sala A, Asensio D, Galiano L, Hoch G, Palacio S, Piper FI, Lloret F. 2016. Dynamics of non‐structural carbohydrates in terrestrial plants: a global synthesis. Ecological Monographs 86: 495–516. [Google Scholar]
- McElreath R. 2020. Statistical rethinking: a Bayesian course with examples in R and Stan. Boca Raton, FL, USA: CRC Press. [Google Scholar]
- Medina‐Vega JA, Bongers F, Poorter L, Schnitzer SA, Sterck FJ. 2021a. Lianas have more acquisitive traits than trees in a dry but not in a wet forest. Journal of Ecology 109: 2367–2384. [Google Scholar]
- Medina‐Vega JA, Bongers F, Schnitzer SA, Sterck FJ. 2021b. Lianas explore the forest canopy more effectively than trees under drier conditions. Functional Ecology 35: 318–329. [Google Scholar]
- Medina‐Vega JA, Wright SJ, Bongers F, Schnitzer SA, Sterck FJ. 2022. Data from: Vegetative phenologies of lianas and trees in two Neotropical forests with contrasting rainfall regimes. Zenodo. doi: 10.5281/zenodo.6403252 [accessed 24 March 2022]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. 1984. Osmoregulation and water stress in higher plants. Annual Review of Plant Physiology 35: 299–319. [Google Scholar]
- Morton DC, Nagol J, Carabajal CC, Rosette J, Palace M, Cook BD, Vermote EF, Harding DJ, North PRJ. 2014. Amazon forests maintain consistent canopy structure and greenness during the dry season. Nature 506: 221–224. [DOI] [PubMed] [Google Scholar]
- Muller B, Pantin F, Génard M, Turc O, Freixes S, Piques M, Gibon Y. 2011. Water deficits uncouple growth from photosynthesis, increase C content, and modify the relationships between C and growth in sink organs. Journal of Experimental Biology 62: 1715–1729. [DOI] [PubMed] [Google Scholar]
- Mumbanza FM, Bauters M, Meunier F, Boeckx P, Cernusak LA, De Deurwaerder HPT, Demol M, Meeussen C, Sercu B, Verryckt LT et al. 2021. Lianas and trees exhibit divergent intrinsic water‐use efficiency along elevational gradients in South American and African tropical forests. Global Ecology and Biogeography 30: 2259–2272. [Google Scholar]
- Murphy PG, Lugo AE. 1986. Ecology of tropical dry forest. Annual Review of Ecology and Systematics 17: 67–88. [Google Scholar]
- Nemani RR, Keeling CD, Hashimoto H, Jolly WM, Piper SC, Tucker CJ, Myneni RB, Running SW. 2003. Climate‐driven increases in global terrestrial net primary production from 1982 to 1999. Science 300: 1560–1563. [DOI] [PubMed] [Google Scholar]
- Nepstad DC, Decarvalho CR, Davidson EA, Jipp PH, Lefebvre PA, Negreiros GH, Dasilva ED, Stone TA, Trumbore SE, Vieira S. 1994. The role of deep roots in the hydrological and carbon cycles of Amazonian forests and pastures. Nature 372: 666–669. [Google Scholar]
- O’Brien JJ, Oberbauer SF, Clark DB, Clark DA. 2008. Phenology and stem diameter increment seasonality in a Costa Rican wet tropical forest. Biotropica 40: 151–159. [Google Scholar]
- O’Brien MJ, Leuzinger S, Philipson CD, Tay J, Hector A. 2014. Drought survival of tropical tree seedlings enhanced by non‐structural carbohydrate levels. Nature Climate Change 4: 710–714. [Google Scholar]
- Ospina R, Ferrari SLP. 2010. Inflated beta distributions. Statistical Papers 51: 111–126. [Google Scholar]
- Parolari AJ, Paul K, Griffing A, Condit R, Perez R, Aguilar S, Schnitzer SA. 2020. Liana abundance and diversity increase with rainfall seasonality along a precipitation gradient in Panama. Ecography 43: 25–33. [Google Scholar]
- Penman HL. 1948. Natural evaporation from open water, hare soil and grass. Proceedings of the Royal Society of London. Series A, Mathematical and Physical Sciences 193: 120–145. [DOI] [PubMed] [Google Scholar]
- Pickard WF. 1981. The ascent of sap in plants. Progress in Biophysics and Molecular Biology 37: 181–229. [Google Scholar]
- Putz FE. 1983. Liana biomass and leaf area of a “Tierra Firme” forest in the Rio Negro basin, Venezuela. Biotropica 15: 185–189. [Google Scholar]
- Putz FE, Windsor DM. 1987. Liana phenology on Barro‐Colorado island, Panama. Biotropica 19: 334–341. [Google Scholar]
- R Core Team . 2021. R: a language and environment for statistical computing, v.4.0.3. Vienna, Austria: R Foundation for Statistical Computing. [WWW document] URL https://www.r‐project.org/ [accessed 1 November 2020]. [Google Scholar]
- Restom TG, Nepstad DC. 2004. Seedling growth dynamics of a deeply rooting liana in a secondary forest in eastern Amazonia. Forest Ecology and Management 190: 109–118. [Google Scholar]
- da Rocha HR, Goulden ML, Miller SD, Menton MC, Pinto LDVO, de Freitas HC, e Silva Figueira AM. 2004. Seasonality of water and heat fluxes over a tropical forest in eastern Amazonia. Ecological Applications 14: 22–32. [Google Scholar]
- Rodríguez‐Ronderos ME, Bohrer G, Sánchez‐Azofeifa A, Powers JS, Schnitzer SA. 2016. Contribution of lianas to plant area index and canopy structure in a Panamanian forest. Ecology 97: 3271–3277. [DOI] [PubMed] [Google Scholar]
- Rosell JA, Olson ME. 2014. Do lianas really have wide vessels? Vessel diameter–stem length scaling in non‐self‐supporting plants. Perspectives in Plant Ecology, Evolution and Systematics 16: 288–295. [Google Scholar]
- Sala OE, Lauenroth WK, Parton WJ, Trlica MJ. 1981. Water status of soil and vegetation in a shortgrass steppe. Oecologia 48: 327–331. [DOI] [PubMed] [Google Scholar]
- van der Sande MT, Poorter L, Schnitzer SA, Markesteijn L. 2013. Are lianas more drought‐tolerant than trees? A test for the role of hydraulic architecture and other stem and leaf traits. Oecologia 172: 961–972. [DOI] [PubMed] [Google Scholar]
- Santesteban LG, Miranda C, Marín D, Sesma B, Intrigliolo DS, Mirás‐Avalos JM, Escalona JM, Montoro A, de Herralde F, Baeza P et al. 2019. Discrimination ability of leaf and stem water potential at different times of the day through a meta‐analysis in grapevine (Vitis vinifera L.). Agricultural Water Management 221: 202–210. [Google Scholar]
- Santiago LS, Kitajima K, Wright SJ, Mulkey SS. 2004. Coordinated changes in photosynthesis, water relations and leaf nutritional traits of canopy trees along a precipitation gradient in lowland tropical forest. Oecologia 139: 495–502. [DOI] [PubMed] [Google Scholar]
- Santiago LS, Schuur EAG, Silvera K. 2005. Nutrient cycling and plant–soil feedbacks along a precipitation gradient in lowland Panama. Journal of Tropical Ecology 21: 461–470. [Google Scholar]
- Schnitzer SA. 2005. A mechanistic explanation for global patterns of liana abundance and distribution. The American Naturalist 166: 262–276. [DOI] [PubMed] [Google Scholar]
- Schnitzer SA. 2018. Testing ecological theory with lianas. New Phytologist 220: 366–380. [DOI] [PubMed] [Google Scholar]
- Schnitzer SA, Bongers F. 2002. The ecology of lianas and their role in forests. Trends in Ecology & Evolution 17: 223–230. [Google Scholar]
- Schnitzer SA, Carson WP. 2010. Lianas suppress tree regeneration and diversity in treefall gaps. Ecology Letters 13: 849–857. [DOI] [PubMed] [Google Scholar]
- Schnitzer SA, van der Heijden GMF. 2019. Lianas have a seasonal growth advantage over co‐occurring trees. Ecology 100: e02655. [DOI] [PubMed] [Google Scholar]
- Schongart J, Piedade MTF, Ludwigshausen S, Horna V, Worbes M. 2002. Phenology and stem‐growth periodicity of tree species in Amazonian floodplain forests. Journal of Tropical Ecology 18: 581–597. [Google Scholar]
- Sinclair TR, Tanner CB, Bennett JM. 1984. Water‐use efficiency in crop production. BioScience 34: 36–40. [Google Scholar]
- Smith‐Martin CM, Bastos CL, Lopez OR, Powers JS, Schnitzer SA. 2019. Effects of dry‐season irrigation on leaf physiology and biomass allocation in tropical lianas and trees. Ecology 100: e02827. [DOI] [PubMed] [Google Scholar]
- Sperry JS, Hacke UG, Oren R, Comstock JP. 2002. Water deficits and hydraulic limits to leaf water supply. Plant, Cell & Environment 25: 251–263. [DOI] [PubMed] [Google Scholar]
- Spicer R, Groover A. 2010. Evolution of development of vascular cambia and secondary growth. New Phytologist 186: 577–592. [DOI] [PubMed] [Google Scholar]
- Stan Development Team . 2020. RStan: the R interface to Stan. v.2.21.2. [WWW document] URL http://mc‐stan.org/ [accessed 1 November 2020]. [Google Scholar]
- Tardieu F. 2013. Plant response to environmental conditions: assessing potential production, water demand, and negative effects of water deficit. Frontiers in Physiology 4: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo‐Aceves T. 2015. Above‐ and belowground competition between lianas and trees. In: Schnitzer SA, Bongers F, Burnham RJ, Putz FE, eds. Ecology of lianas. Chichester, UK: John Wiley & Sons, 147–163. [Google Scholar]
- Toledo‐Aceves T, Swaine MD. 2008. Above‐ and below‐ground competition between the liana Acacia kamerunensis and tree seedlings in contrasting light environments. Plant Ecology 196: 233–244. [Google Scholar]
- Tyree MT, Cochard H, Cruiziat P, Sinclair B, Ameglio T. 1993. Drought‐induced leaf shedding in walnut: evidence for vulnerability segmentation. Plant, Cell & Environment 16: 879–882. [Google Scholar]
- Tyree MT, Sperry JS. 1988. Do woody plants operate near the point of catastrophic xylem dysfunction caused by dynamic water stress?: answers from a model. Plant Physiology 88: 574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schaik CP, Terborgh JW, Wright SJ. 1993. The phenology of tropical forests: adaptive significance and consequences for primary consumers. Annual Review of Ecology and Systematics 24: 353–377. [Google Scholar]
- Williams LE, Araujo FJ. 2002. Correlations among predawn leaf, midday leaf, and midday stem water potential and their correlations with other measures of soil and plant water status in Vitis vinifera . Journal of the American Society for Horticultural Science 127: 448–454. [Google Scholar]
- Windsor DM. 1990. Climate and moisture variability in a tropical forest: long‐term records from Barro Colorado Island, Panama. Smithsonian Contributions to the Earth Sciences 29: 1–145. [Google Scholar]
- Woodring W, Stewart R, Stewart J. 1980. Geologic map of the Panama Canal. Reston, VA, USA: US Geological Survey. [Google Scholar]
- Worbes M. 1999. Annual growth rings, rainfall‐dependent growth and long‐term growth patterns of tropical trees from the Caparo Forest Reserve in Venezuela. Journal of Ecology 87: 391–403. [Google Scholar]
- Wright SJ. 2020. The Smithsonian Tropical Research Institute: a century of ecological and applied research. Biological Conservation 252: 108858. [Google Scholar]
- Wright SJ, Sun IF, Pickering M, Fletcher CD, Chen YY. 2015. Long‐term changes in liana loads and tree dynamics in a Malaysian forest. Ecology 96: 2748–2757. [DOI] [PubMed] [Google Scholar]
- Wright SJ, Van Schaik CP. 1994. Light and the phenology of tropical trees. The American Naturalist 143: 192–199. [Google Scholar]
- Wu J, Albert LP, Lopes AP, Restrepo‐Coupe N, Hayek M, Wiedemann KT, Guan K, Stark SC, Christoffersen B, Prohaska N et al. 2016. Leaf development and demography explain photosynthetic seasonality in Amazon evergreen forests. Science 351: 972–976. [DOI] [PubMed] [Google Scholar]
- Wyka TP, Oleksyn J, Karolewski P, Schnitzer SA. 2013. Phenotypic correlates of the lianescent growth form: a review. Annals of Botany 112: 1667–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaish MWF, Guevara DR, El‐Kereamy A, Rothstein SJ. 2010. Axillary shoot branching in plants. In: Pua EC, Davey MR, eds. Plant developmental biology ‐ biotechnological perspectives. Berlin, Germany: Springer, 37–52. [Google Scholar]
- Zhu S‐D, Cao K‐F. 2009. Hydraulic properties and photosynthetic rates in co‐occurring lianas and trees in a seasonal tropical rainforest in southwestern China. Plant Ecology 204: 295–304. [Google Scholar]
- Zuur AF, Ieno EN, Elphick CS. 2010. A protocol for data exploration to avoid common statistical problems. Methods in Ecology and Evolution 1: 3–14. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Frequency distribution for the proportion of leaf cover.
Fig. S2 Conditional effects for the models that best fitted leaf water potentials in the dry forest.
Fig. S3 Conditional effects for the models that best fitted leaf water potentials in the wet forest.
Fig. S4 Species‐level predictions for predawn leaf water potentials – dry forest.
Fig. S5 Species‐level predictions for midday leaf water potentials – dry forest.
Fig. S6 Species‐level predictions for predawn leaf water potentials – wet forest.
Fig. S7 Species‐level predictions for midday leaf water potentials – wet forest.
Fig. S8 Species‐level predictions – proportion of leaf cover as a function of cumulative water deficit in the dry forest.
Fig. S9 Species‐level predictions – proportion of leaf cover as a function of CWD in the wet forest.
Fig. S10 Species‐level predictions – proportion of leaf cover as a function of solar radiation in the dry forest.
Fig. S11 Species‐level predictions – proportion of leaf cover as a function of solar radiation in the wet forest.
Methods S1 Calculation of potential evapotranspiration.
Methods S1 Regression equation – leaf water potential models.
Methods S1 Regression equation – proportion of leaf cover models.
Note S1 Justification for the inclusion of census as a random intercept in the models of leaf water potentials.
Note S2 Prior justification – leaf water potential models.
Note S3 Prior justification – proportion of leaf cover models.
Note S4 Traceplots – leaf water potential models.
Note S5 Traceplots – proportion of leaf cover models.
Note S6 Posterior predictive checks – leaf water potential models.
Note S7 Posterior predictive checks – proportion of leaf cover models.
Note S8 Supplementary results – proportion of leaf cover models.
Table S1 Study species.
Table S2 Census dates.
Table S3 Random effects – leaf water potential models.
Table S4 Fixed effects – proportion of leaf cover models.
Table S5 Random effects – proportion of leaf cover models.
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Data Availability Statement
Data and code supporting the results are available from Zenodo: https://doi.org/10.5281/zenodo.6403252 (Medina‐Vega et al., 2022).


