Abstract
The onset of human disease by infection with SARS-CoV-2 causing COVID-19 has revealed risk factors for disease severity. There are four identified factors that puts one at high-risk for infection and/or mortality creating a disparity: age, comorbidities, race/ethnicity, and gender. Data indicate that the older a person is, and/or the presence of obesity and diabetes, cardiovascular disease, and chronic kidney disease place one at higher risk for COVID-19. In the United States, specific race/ethnicities, particularly African Americans and Native Americans, are strong COVID-19 risk components. Male gender has also emerged as a severity risk factor. For age and racial/ethnicities, the accumulation of health comorbidities are common precipitating mechanisms. In particular, underlying socioeconomic structures in the United States likely drive development of comorbidities, putting affected populations at higher risk for severe COVID-19. Sudden cardiac death triggered by a common sodium channel variant in African Americans with COVID-19 has not been evaluated as a cause for racial disparity. There is no evidence that racial/ethnic differences for COVID-19 are caused by ABO blood groups, use of angiotensin converting enzyme (ACE) inhibitors, or from amino acid substitutions in the SARS-CoV-2 spike protein. There is growing evidence that androgen-enabled expression of ACE2 receptors and the serine protease TMPRSS2, two permissive elements engaging the SARS-CoV-2 spike protein for infection, may contribute to severe COVID-19 in men. Overall, COVID-19 has generated disparities for who is infected and the severity of that infection. Understanding the mechanisms for the disparity will help nullify the differences in risk for COVID-19.
Keywords: COVID-19, SARS-CoV-2, disparity, African American, Hispanic/Latinx, race, ethnicity, diabetes, hypertension, chronic kidney disease, co-morbidity, age, ACE2, androgen, TMPRSS2
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the infecting viral agent that causes coronavirus disease 2019 (COVID-19), a predominant pulmonary syndrome consisting of pneumonitis and vascular endothelialitis with thrombosis [1,2]. SARS-COV-2 gains access to cells by binding the angiotensin converting enzyme 2 (ACE2) receptor enriched in nasal epithelial cells of the upper respiratory track, which then may spread to infect the ACE2-expressing type II alveolar cells in the lung [3,4]. While SARS-CoV-2 has its principle tropism for cells in the nasal passages and the lung, it can infect any cell that may express ACE2, including the liver, heart, and kidney [5]. The vascular endothelialitis observed with SARS-CoV-2 infection is more severe than that observed for H1N1 influenza and non-specific interstitial pneumonia, indicating an archetype unique for COVID-19 [2]. However, infection with SARS-CoV-2 can range from asymptomatic (probably the most common) to severe COVID-19 disease and death [6].
As SARS-CoV-2 caused a worldwide pandemic and COVID-19 cases increased across the globe including into the United States (U.S.), there have been 4 major identified factors that puts a person a higher risk for infection and COVID-19. Age is a strong risk factor for COVID-19, with the older a person is, the higher the risk for severe disease and death. The presence of health co-morbidities, including obesity and diabetes, cardiovascular disease, hypertension, asthma and chronic obstructive pulmonary disease (COPD), and chronic kidney disease place one a higher risk for infection and COVID-19 [7]. In the U.S. and the United Kingdom (UK), specific races and ethnicities, particularly those of African descent and Native Americans, appear to be strong risk factors for infection and COVID-19 [8–12]. Additionally, male gender has emerged as a prominent risk factor for severe COVID-19 [7,12]. This review touches on these 4 risk factors for COVID-19 and the epidemiology and biology behind them for this new disease.
Age and health co-morbidities
As of October 2020, the U.S. has the highest number of reported COVID-19 cases in the world, with approximately 25% of all documented infections in a country with under 5% of the world’s population [13]. The U.S. Center for Diseases Control and Prevention (CDC) reports that all age groups can get infected, with 62% of all infections in the U.S. occur in people less than 50 years of age [7]. Children under the age of 18 years are a growing recognized reservoir for the virus, with African American and Latinx children making up the bulk of this reservoir [7,12,14]. Some children may exhibit a described Multisystem Inflammatory Syndrome that demonstrates dermatologic, gastrointestinal, kidney, and cardiac manifestations, likely from the combination of the virus infection and localized and systemic cytokine release [15,16]. Deaths from COVID-19 are vastly different from the age where the majority are infected, with 95% of all U.S. COVID-19 deaths occur in people over the age of 50 years [7,12]. A striking age trend for death is apparent, with the number of deaths in patients aged 85 years or older higher than those aged 75–84 years, and those 75–84 years of age demonstrating more deaths than those aged 65–74 years, which show more deaths than those aged 55–64 years, and this last age group showing more deaths than those aged 45–54 years [12].
The CDC has posted several medical conditions that place a person at increased risk for COVID-19, including chronic kidney disease, COPD, immunocompromised state, obesity, cardiovascular disease, sickle cell disease, and type II diabetes mellitus [17]. Other co-morbid conditions may place one at increased risk and include asthma, cystic fibrosis, hypertension, cerebrovascular disease, dementia, liver disease, among others [17]. A knowledge-based network of COVID-19 morbidities crafted from literature curation show strong association with hypertension, chronic renal insufficiency, cardiovascular diseases, autoimmune diseases, and pulmonary hypertension, with relationships with genes such as interleukin-6, vascular endothelial growth factor alpha, and ACE2, all of which was linked to patients with four or more of the co-morbidities [18].
The best data on age and co-morbidities to date for COVID-19 come from studies in skilled nursing facilities. Evidence shows rapid transmission to residents of a skilled nursing facility within a 2-week period, and also involving infection of some healthcare personnel and visitors to the facility [19]. The average age of residents was 83 years, with most having one or more comorbidities, including 67% with hypertension, 60% with cardiac disease, 41% with renal disease, 32% with diabetes, 31% with obesity and 32% with existing pulmonary disease, that put them at increased risk for SARS-CoV-2 infection [19]. There were 33.7% deaths for residents, compared to 6.2% for visitors and 0% for healthcare staff [19]. The extent of residents’ co-morbidities was in contrast to that for healthcare personnel (8% hypertension, 8% cardiac disease, 10% diabetes, 6% obesity, 0% renal disease) and visitors (12.5% hypertension, 19% cardiac disease, 18% obesity, 12.5% pulmonary disease, 6% diabetes, 12.5% renal disease) [19]. In another study which compared skilled nursing facility residents who became SARS-Co-V-2 positive versus residents who remained negative for the virus, residents who became virus positive were older (mean age 78.6 years vs 73.8 years) and had higher frequencies of co-morbidities, including chronic lung disease (38% vs 29%), cardiovascular disease (81% vs 61%), cerebrovascular accident (40% vs 29%), and cognitive impairment (58% vs 46%) [6]. This study demonstrated that 56% of residents who tested positive were either pre-symptomatic up to 6 days with the infection prior to onset of symptoms, or remained asymptomatic, contributing to rapid spread in the facility [6].
The intermix of older age and the high prevalence of co-morbidities in this older population are difficult to disentangle as independent COVID-19 risk factors. One could hypothesize that the elderly population possesses weaker immunity contributing towards higher morbidity with COVID-19 when compared to younger populations [20]. Skilled nursing facilities, particularly early in the pandemic, lacked that availability of viral testing and lacked personalized protective equipment such as masks to stem viral transmission [21]. On the other hand, the presence of co-morbidities might be the key factor for SARS-CoV-2 infection in the elderly given its higher prevalence in this population. Isolating the effect of age from age-dependency of co-morbid factors using meta-regression of twelve COVID-19 studies indicates a 2.7% increased risk for disease severity and 0% increased risk of death per year of age, compared to 5.2% increased risk for disease severity and 13.4% increased risk of death per year of age for crude analysis without adjusting for co-morbidities [22].
How might co-morbidities be a COVID-19 risk factor? Analysis of over 700 lung transcriptome samples of patients with severe COVID-19 and co-morbidities revealed higher ACE2 expression in those individuals compared to controls [18]. Another study in asthma patients revealed higher levels of ACE2 and the serine protease TMPRSS2, a permissive factor for the SARS-CoV-2 spike protein, significantly elevated in collected sputum cells from male, African American, and diabetes patients, and patients with more than one co-morbidity [23]. Bioinformatic approaches indicate that tissue from patients using tobacco and/or have COPD, patients with pulmonary arterial hypertension, and patients with lung adenocarcinoma all reveal higher ACE2 expression as compared to normal tissue [18]. These studies strongly suggest that co-morbidities increase risk for SARS-CoV-2 infection and COVID-19 by increasing the expression of ACE2 and/or TMPRSS2 on host lung cells, heightening the permissiveness of viral infection and the chance for progressing to severe disease.
Race and ethnicity
The distribution of COVID-19 cases vary by race and ethnicity, with higher number of cases for African Americans and Latinx as compared to their population proportion in the U.S for 2020 [8]. Racial/ethnic distribution of COVID-19 deaths vary geographically across the 50 U.S. states, with an overall standardize mortality ratio of 3.57 (95% CI 2.84,4.48) comparing African Americans with White Americans, and 1.88 (95% CI 1.61, 2.19) comparing Latinx vs White [24]. Examining COVID-19 deaths as a rate show similar data, with African American deaths at a rate of 88/100,000 population, Native American deaths at 73/100,000, Latinx deaths at 54/100,000, and White Americans at 40/100,000 [25]. When compared to White Americans and adjusted for age, African Americans are 3.6 times more likely, Native Americans 3.4 times more likely, and Latinx 3.2 times more likely to die from COVID-19 [25,26]. This disparity in COVID-19 deaths extends to all age groupings by decade. Even at younger ages when overall deaths from COVID-19 are low, African Americans and Latinx patients exhibit higher death rates (e.g. age 25–34 years, African Americans and Latinx patients die 6.4 and 4.3 times higher than White Americans) [26].
What drives this disparity? At present, there is no evidence for genetic or immune predisposition for COVID-19 by race or ethnicity. One study suggests that counties in the U.S. with higher and more diverse populations (higher percentages of Latinx, Asians, African Americans) have a higher rate of SARS-CoV-2 infection, whereas counties with grocery mobility (lack of food deserts) and work mobility associate with lower rates of infection [27]. Death from COVID-19 also varied by county, with lower deaths in counties with people who are college-educated, hold medical insurance, and have high incomes, and higher mortality among counties with higher poverty prevalence, Medicaid as health insurance, poor grocery mobility, and medical disability [27]. A pre-print study demonstrates the unequal distribution of pre-existing health co-morbidities by race and ethnicity as risk factors for COVID-19 [28]. The prevalence of co-morbidities increases with age, with African Americans showing the highest prevalence of multiple co-morbidities compared to Latinx and White Americans [28]. African Americans aged 45–64 years had 27%, 31%, 20% and 23% of 0, 1, 2, and 3 co-morbid risk factors, respectively, compared to White Americans that had 41%, 31%, 15%, and 13%, respectively [28]. For ages 65 years and older, African Americans had 9%, 27%, 25% and 40% of 0, 1, 2, and 3 co-morbid risk factors, respectively, compared to White Americans that had 20%, 28%, 23%, and 29%, respectively, and Latinx that had 10%, 30%, 33%, and 28%, respectively [28]. Thus, based on high risk co-morbidities, African Americans seem to possess the higher risk for adverse outcomes from COVID-19 [29]. In a study involving nearly 6 million U.S. veterans, African American veterans were twice as likely to test positive for the SARS-CoV-2 even after adjusting for urban versus rural residence [30]. Several co-morbid conditions were associated with hospitalization and use of intensive care, but were not independently associated after adjusting for vital signs and laboratory data (suggesting that co-morbid conditions may be less prognostic than overall evidence of acute on chronic tissue injury based on laboratory abnormalities) [30].
The odds ratio for hospitalization for COVID-19 is about twice for African Americans compared to White Americans after adjustment for age, gender, residence, insurance plan, obesity, and Charlson Comorbidity Index score [9]. However, once hospitalized, the hazard ratio for death was similar between African Americans and White Americans [9]. Additional data evaluating 11,210 hospitalized COVID-19 patients, of which 4,180 were African American, show African American patients were younger, more likely to have Medicaid insurance, and have higher scores on the Neighborhood Deprivation Index and Elixhauser Comorbidity Index; however, there was no difference in in-hospital mortality between African American and White American COVID-19 patients with adjustment for age, gender, insurance, co-morbidities, neighborhood deprivation, and site of care (HR 0.93, 95% CI, 0.80, 1.09) [10]. These data suggest that while more African Americans may get infected and contract COVID-19, once hospitalized, the risk of death is no different than White Americans in similar hospital settings. Parallel data from the UK Biobank demonstrate Black patients showed increased hospitalization for COVID-19 over White patients (OR 2.4; 95% CI 1.5,3.7) after adjusting for socioeconomic factors and cardiorespiratory co-morbidities [11]. UK Biobank participants with a greater Townsend Deprivation Index (composite of employment status, car or home ownership, and household crowding) were at substantially higher risk for COVID-19 hospitalization, similar to that observed with lower self-reported household income [11].
The racial/ethnic disparity for COVID-19, as observed in both the U.S. and UK, appear to have its origins in systemic structural disadvantages for this population which set up the development of co-morbidities, with this population ultimately disproportionately acquiring high risk factors for COVID-19 (Figure 1). For instance, African Americans on average have lower college attainment compared to White Americans, and are disproportionately represented among “essential” workers among the food, janitorial, and transportation industries, and were exempted from shelter-in-place mandates during surges of COVID-19 spread [31]. Disadvantages in housing (large number in household for house size, low income neighborhood location, etc.) are more prevalent in African American communities which undermine social distancing efforts, and African Americans are 3 times more likely to use public transportation on a regular basis when compared to White Americans [32]. With lower average household income from “essential” and/or low paying jobs, racial/ethnic minorities are more likely to take a pay cut or lose their job because of the pandemic, and over 70% of Latinx and African Americans do not have any “rainy day” funds to cover 3 months of living expenses compared to 47% of White Americans [33]. Residing in a low-income neighborhood is more associated with grocery store deserts, and accelerates the use of tobacco, alcohol, and low physical activity [34].These consequences, in turn, change the biology and physiology of the person’s health through changes in the microbiome and levels of localized inflammation and compromised immunity, increasing risk for cancers, obesity, diabetes, asthma and COPD, hypertension, cardiovascular disease, and chronic kidney disease (Figure 1). These medical conditions are the exact conditions that have been shown to place one at higher risk for mortality from COVID-19. In turn, COVID-19 has highlighted and exacerbated these inequalities in the U.S. and abroad.
Figure 1. Connections and consequences of socioeconomic disparities in the development of health co-morbidities, making a population at high risk and more susceptible to COVID-19.
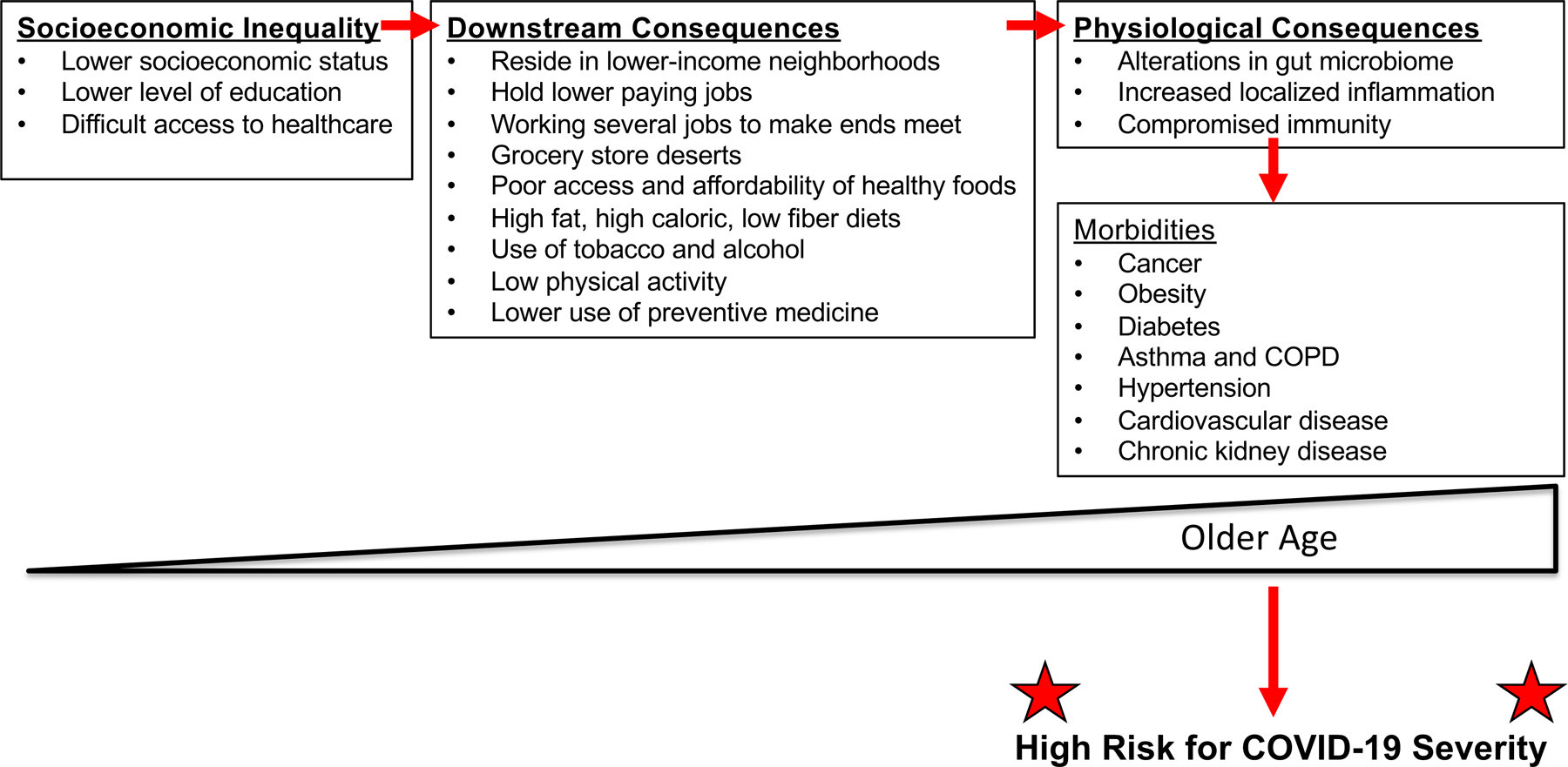
Patterned after Carethers JM and Doubeni CA. Causes of socioeconomic disparities in colorectal cancer and intervention framework and strategies. Gastroenterol 2020;158:354–367.
Use of ACE inhibitors or angiotensin receptor blockers
Hypertension and kidney disease are more prevalent among African Americans potentially making this group more susceptible to severe COVID-19, and this population and others may be medicated with ACE inhibitors or angiotensin-receptor blockers (ARBs) [35]. There have been conflicting studies in animal models and in small patient studies on the effect of ACE inhibition on increasing ACE2 expression, and in theory, provides a potential vehicle for increased SARS-CoV-2 infectivity for patients on ACE inhibition therapy [36]. ACE inhibitors and ARBs in their clinical use affect the activity of ACE1 but there is no real evidence of an effect on ACE2 activity [36]. While the overall use of ACE inhibitors and ARBs are more frequent among COVID-19 patients due to the prevalence of hypertension and cardiovascular disease in the population, two studies show that there is no increased risk for SARS-CoV-2 infection with the use of ACE inhibitors or ARBs, with an adjusted OR of 0.96 (95% CI 0.87–1.07) for ACE inhibitors and 0.95 (95% CI 0.86–1.05) for ARBs [37,38]. Thus, there is no association with use of ACE inhibition or ARBs for COVID-19 infection or severity, and is not a contributor for any racial/ethnic disparity.
Use of hydroxychloroquine and sudden cardiac death
There appears to be no benefit of hydroxychloroquine in treating or preventing COVID-19; it is not recommended for this use [39,40]. However, thousands of patients received hydroxychloroquine in the early parts of the pandemic.
The common SCN51-encoded NAv1.5 sodium channel variant p.Ser1103Tyr-SCN5A is associated with increased risk of ventricular arrhythmias and sudden cardiac death. Hypoxia, an exaggerated immune response, and direct myocardial injury can trigger sudden cardiac death in people with this variant. The p.Ser1103Tyr-SCN5A variant is found almost exclusively in individuals of African descent, with 1 in 13 (8%) of African Americans possessing this variant [41]. This group may be at substantial risk of potentially lethal ventricular arrhythmias due to the coalescence of: (a) intrinsic genetic susceptibility with the p.Ser1103Tyr-SCN5A variant, (b) use of QTc-prolongating drugs such as hydroxychloroquine as a treatment for COVID-19, and (c) generation of hypoxemia and cytokine storm from COVID-19 that in itself elevates arrhythmia risk [41]. While the use of hydroxychloroquine has abated with failed benefit demonstrated by clinical trials, the contribution of the p.Ser1103Tyr-SCN5A variant to any early excess African American COVID-19 deaths has not been studied.
ABO blood groups
A European genome wide association study of 1980 patients with COVID-19 and respiratory failure demonstrated the ABO blood group locus at chromosome 9p34.2 was significantly associated with COVID-19 (P<5×10−8) [42]. Further, blood group specific analyses showed that persons with blood group A was correlated with COVID-19 (OR 1.45, 95% CI 1.2–1.75) whereas persons with blood group O was relatively protected from COVID-19 (OR 0.65, 95% CI 0.53–0.79) [42]. Similarly, a Chinese study demonstrated higher COVID-19 risk with persons of blood group A (OR 1.21, 95% CI 1.02–1.43 for blood group A and OR 1.48, 95% CI 0.97–2.24 for blood group AB), no risk for persons with blood group B (OR 1.09, 95% CI 0.98–1.22) and reduced risk for persons with blood group O (OR 0.67, 95% CI 0.60–0.75) [43]. However, these findings would not explain any racial/ethnic disparity for COVID-19. African Americans have less blood group A and more blood group O than White Americans [44]. Thus, blood group would not be considered a determinant for any racial/ethnic propensity for COVID-19.
SARS-CoV-2 virulence
There is evidence for higher patient fatality from COVID-19 in patients who carry a substitution in the S protein of SARS-CoV-2 (D614G), suggesting an increase in virulence [45]. This and other substitutions and mutations within SARS-CoV-2 have not been evaluated to date for gender, race or ethnicity prevalence, and thus at present does not contribute any evidence towards disparity.
Gender and SARS-CoV-2 infection
Data from Europe and the U.S. indicate that men and women equally are likely to contract COVID-19; however, men are 1.5 times more likely to be hospitalized, are twice likely to be in an intensive care unit, and have 1.7 times the overall rate of death [7,12,46–48]. Specifically, the rate of death for men less than 50 years of age are 2.5 times that of women, and men age 50–59 years have 3.2 times the rate of death of women [48].
What might be driving this gender difference in COVID-19 deaths? There is growing evidence that the interplay between the virus and host expression factors provide a rationale for gender disparity. SARS-CoV-2 utilizes its spike (S) protein to recognize and interact with human ACE2 receptors as the critical step to infecting human cells. To gain entry into human cells, SARS-CoV-2 is benefited by simultaneous human expression of the serine protease TMPRSS2, which primes the S protein for fusogenic activity with the ACE2 receptor, promoting virus entry into host cells [1,3,4]. TMPRSS2 is expressed in several tissues including the prostate, lung, the gastrointestinal tract, and the urinary tract [49,50], and is heavily co-expressed with ACE2 in specific nasal epithelial and respiratory cells lining bronchi and the lung (type II alveolar cells) providing the grounds for COVID-19 to principally be a pulmonary infection syndrome [3,4]. TMPRSS2 is regulated by androgens, raising the hypothesis that the observed male predominance with severe COVID-19 disease could be explained by androgen regulation of TMPRSS2, as the promoter of TMPRSS2 contains an androgen response element [50–52]. This notion has been exemplified in men with prostate cancer where the most frequent somatic alteration identified is fusion of TMPRSS2 with erythroblast transformation-specific (ETS) transcription factors such as ERG (also ETV1, ETV4, ETV5 and FLI1) leading to androgen-dependent overexpression of ERG to drive prostate cancer in the presence of androgens [52]. ACE2 expression can also regulated by androgens, and this regulation of expression of both ACE2 and TMPRSS2 might contribute to the observed gender differences [51]. As an example, specific strains of influenza rely on TMPRSS2 to activate virus hemagglutinin [53] and testosterone modifies influenza activity in animal models [54], providing a theoretical blueprint for this possibility for COVID-19 pathogenesis and severity. In examining total lung tissue as well as individual respiratory cell types, the overall expression of TMPRSS2 was not different between men and women overall or in any cell type [49]. However, there was differential expression for ACE2, with men expressing more in the type II alveolar cells, and showing significant increased co-expression of both TMPRSS2 and ACE2 in type II alveolar cells in men over women [49]. The androgen regulation of these two host virulence factors for SARS-CoV-2 suggests sexual dimorphism for viral entry [46]. The higher expression in TMPRSS2 and ACE2 in male respiratory cells as regulated by androgens may facilitate more opportunity for the virus to take hold, and with infection of type II alveolar cells, set up severe infection in the lung [4]. Clinical trials using already U.S. Food and Drug Administration (FDA)-approved TMPRSS2 inhibitors have been initiated to assess if these drugs prevent or slow SARS-CoV-2 infection [55]. Early reports from men with prostate cancer on androgen deprivation therapy (ADT) compared to men with prostate cancer not on ADT suggests a marked reduction in SARS-CoV-2 infection, with a rate of 8/10,000 infected for men on ADT, and 31/10,000 infected for men not on ADT [51]. This data supports the notion that androgens accentuate SARS-CoV-2 infection. Hyper-androgenic conditions such as patients with acne, androgenetic alopecia, polycystic ovaries, or patients on androgen therapy have not been evaluated to date for their potential higher risk to COVID-19.
It should be noted that while TMPRSS2 expression by androgens might explain gender differences for severe COVID-19, it does not explain any racial or ethnic differences. The highest prevalence of the somatic TMPRSS2-ERG fusion in prostate cancer is among White American (~50%) and Indian Asian men (49%) as compared to men with African (25–30%) or non-Indian Asian (10–20%) ancestry [56–58], although this does not address any germline differences. The androgen response element, the key part of the TMPRSS2 (and other androgen responsive genes) promoter where androgens bind, is polymorphic showing variation in the length of CAG trinucleotide repeats, and this length variation can dictate the transcriptional activity and resistance to androgens [59–61]. Non-Hispanic White men have longer CAG repeat lengths compared to African American men, and the highest risk for advanced stage prostate cancer was with men with shorter repeats [59–61]. The length polymorphism is also associated with male pattern baldness [61,62]. At present, there is no direct evidence for androgen response element length variations and infection of SARS-CoV-2 as this has not been investigated to date as a contribution to both gender and racial variation in severity of COVID-19.
Other potential COVID-19-driven disparities
Delay of acute care
Data from Italy indicate that during the COVID-19 pandemic surge, the number of out-of-hospital cardiac arrests was 30% lower than identical times the previous year [63]. Similarly, data from California show that about one-third less patients were hospitalized for acute myocardial infarction during the COVID-19 surge as compared to the prior year [64]. These data may indicate that overall there is less presentation to emergency rooms for cardiac conditions out of COVID-19 fear, with avoidance of hospitals and emergency rooms. Similarly, data for stroke evaluations, which may be seen in patients with uncontrolled hypertension and cerebrovascular disease, dropped over 30% during the COVID-19 surge then began to return to baseline after the surge [65]. In total and on top of government-guided reduction in elective medical care, avoidance of acute emergent care also occurred. This aspect has not been examined to date based on race and ethnicity and those medically-underserved; the severity of the avoidance could be more acute and sustained in areas where safety-net services are already challenged and become more financially restrained as a result of the COVID-19 pandemic.
Delay of preventive cancer screening
Screening for cancer is a cornerstone for reducing morbidity and mortality from cancer, as it identifies lesions in a precursor state or early stage when curability is probable [66]. With the COVID-19 surge in March and April 2020 in the U.S., the number of mammograms to screen for breast cancer dropped 89.2%, and the number of colorectal cancer screenings dropped 84.5% [67,68]. This decrease was largely driven by government recommendations to reduce elective non-emergent procedures in order to preserve personalized protective equipment. The long-term consequences of this cancer screening delay depends on how fast providers and the community return to historical screening trends. A conservative estimate based on just a 6-month delay in breast and colorectal cancer screening from pre-COVID-19 trends indicate 10,000 cumulative excess deaths from these two cancers alone over the next decade [69,70]. With a disparity that existed pre-COVID-19 for screening rates for African Americans as compared to White Americans for some cancers [66,71], it is likely that the disparity may be exacerbated with COVID-19 with long-lasting consequences for cancer incidence and mortality [70,72].
Food insecurity
Globally, the poorest households spend up to 70% of income on food. Loss of employment as a result of the pandemic-induced recession can worsen the ability of these households to buy food. Across the world as well in the U.S. with the COVID-19 pandemic, there has been a decline in food production (reduction in labor, need for social distancing) as well as supply chain interruption (e.g. meat packing plants). Furthermore, interruption of public schools, where several low-income students obtain 1 or 2 of their daily meals, may affect the nutrition of students. All of these changes disproportionately affect low-income populations, compounding multiple disparities [73].
Conclusion
The onset of the COVID-19 pandemic has highlighted 4 major risk factors for disease: age, pre-existing health co-morbidities, gender, and race/ethnicity (Table 1). Co-morbidities may be the dominant factor for risk of COVID-19 as one ages, and if one is African American, Latinx, or Native American. For male gender, there is evidence emerging for androgen-driven expression of TMPRSS2 and ACE2 that enable infection and sustained foothold of the SARS-CoV-2 virus. These same host virulence factors appear elevated in patients with co-morbidities, such as COPD, pulmonary hypertension, diabetes, and in African Americans with asthma to put them at higher risk for COVID-19 severe disease.
Table 1.
Summation of COVID-19 major risk factors and their drivers for risk.
| COVID-19 Risk Factor | Attribute for Risk | Driver for Risk |
|---|---|---|
| Age |
|
|
| Co-morbidities |
|
|
| Race/ethnicity |
|
|
| Gender |
|
|
In many ways, the racial/ethnic disparities observed with COVID-19 may start from socioeconomic inequities that enter a single vulnerability cycle starting with the development of co-morbidities that ultimately increase ACE2 and TMPRSS2 expression, both of which elevates one’s susceptibility for COVID-19 that can lead to severe illness or death (Figure 2). If one survives, an already disadvantaged person may become more vulnerable from potential after-effects of COVID-19 with job loss and/or reduction in income, or consequences from other separate acute or chronic illnesses or co-morbid exacerbations such as with cancer, cardiovascular disease and kidney disease, making them more socio-economically disadvantaged fulfilling a more sustained, recurrent and futile cycle (Figure 2). At present, there is no evidence for any genetic or immunologic predisposition for the racial and ethnic disparity for COVID-19, and there is no predilection based on blood groups, use of ACE inhibitors or ARBs, or SARS-CoV-2 virulence. Additional and widened disparities facilitated by COVID-19 may include delays in acute urgent care, food insecurity, and reduced cancer screenings followed by increased deaths from cancer. An understanding of these mechanisms for disparities produced and/or accentuated by the emergence and surge of COVID-19 should aid in strategic approaches to mollifying or nullify these differences.
Figure 2. Schematic for vulnerability cycle for COVID-19 infection and consequences that intertwines and intersects with the vulnerability cycle for socioeconomic inequalities and consequences.
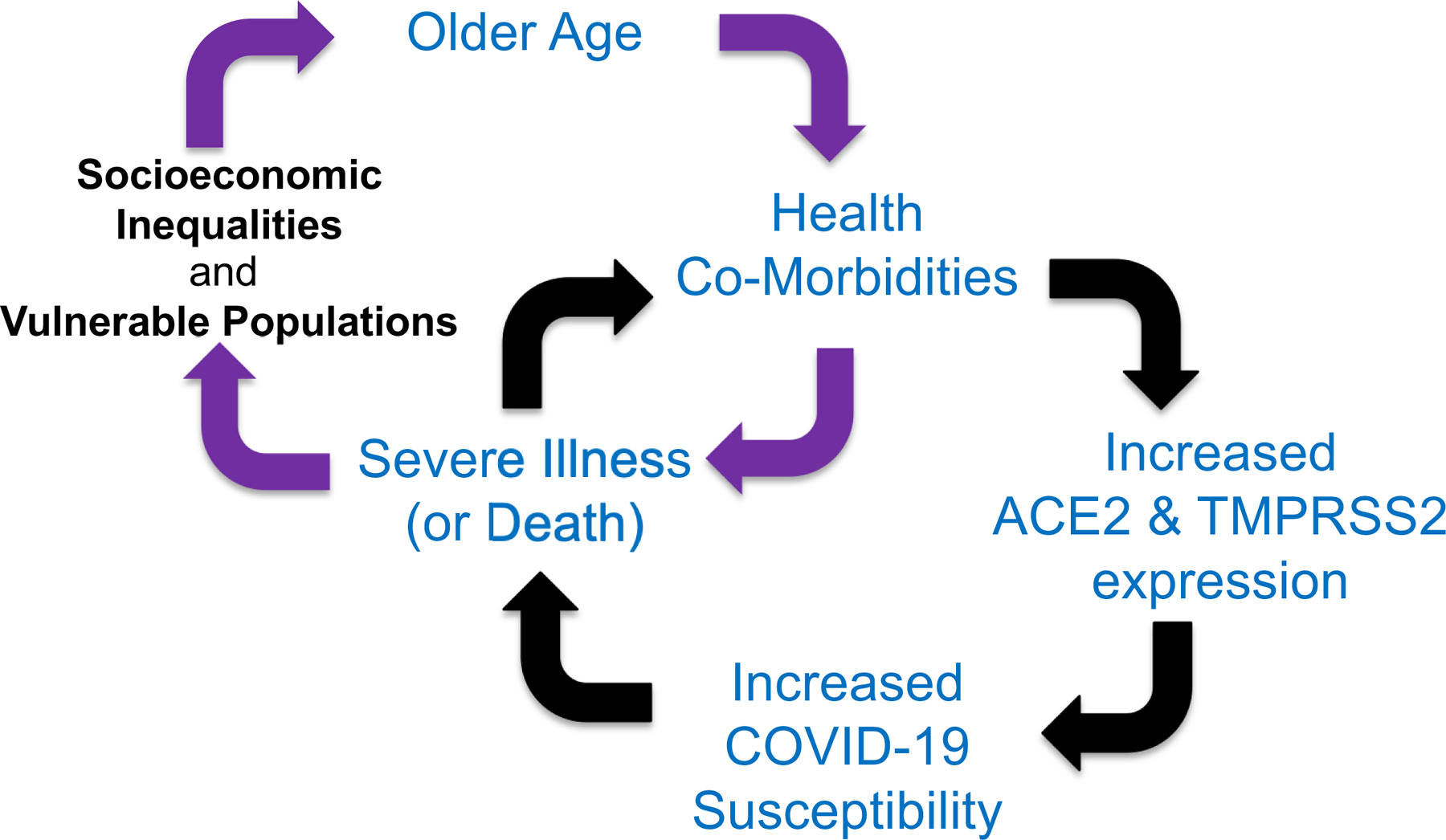
Persistent, prolonged socioeconomic inequalities set up the development of health co-morbidities as one ages, causing increased expression of SARS-CoV-2 host virulence factors that increase one’s susceptibility for severe COVID-19 illness or death as a single cycle (black directional arrows). Survival from COVID-19 may worsen socioeconomic conditions through job loss or reduction in income, further perpetuating a separate cycle that involves ongoing exacerbation of age-related existing co-morbidities that can also sustain or fuel further socioeconomic disadvantage (purple directional arrows).
Acknowledgements:
Supported by the United States Public Health Service (R01 CA206010) and the A. Alfred Taubman Medical Research Institute of the University of Michigan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations:
- COVID-19
coronavirus disease 2019
- ACE
angiotensin converting enzyme
- SARS-CoV-2
severe adult respiratory syndrome-coronovirus-2
- CI
confidence interval
- COPD
chronic obstructive pulmonary disease
- CDC, U.S.
Center for Diseases Control and Prevention
- ARB
angiotensin-receptor blocker
- OR
odds ratio
- CI
confidence interval
- UK
United Kingdom
- U.S.
United States
- ETS
erythroblast transformation-specific transcription factor
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest are disclosed.
References
- 1.Matheson NJ, Lehner PJ. How does SARS-CoV-2 cause COVID-19? Science 2020;369:510–511. [DOI] [PubMed] [Google Scholar]
- 2.Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Eng J Med 2020;383:120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sungnak W, Huang N, Bécavin C, Berg M, Queen R, Litvinukova M, Talavera-López C, Maatz H, Reichart D, Sampaziotis F, Worlock KB, Yoshida M, Barnes JL; HCA Lung Biological Network. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med 2020;26:681–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lukassen S, Chua RL, Trefzer T, Kahn NC, Schneider MA, Muley T, Winter H, Meister M, Veith C, Boots AW, Hennig BP, Kreuter M, Conrad C, Eils R. SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. EMBO J 2020;39:e105114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puelle VG, Lutgehetmann M, Huber TB. Multiorgan and renal tropism of SARS-CoV-2. N Eng J Med 2020;383:590–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arons MM, Hatfield KM, Reddy SC, Kimball A, James A, Jacobs JR, Taylor J, Spicer K, Bardossy AC, Oakley LP, Tanwar S, Dyal JW, Harney J, Chisty Z, Bell JM, Methner M, Paul P, Carlson CM, McLaughlin HP, Thornburg N, Tong S, Tamin A, Tao Y, Uehara A, Harcourt J, Clark S, Brostrom-Smith C, Page LC, Kay M, Lewis J, Montgomery P, Stone ND, Clark TA, Honein MA, Duchin JS, Jernigan JA; Public Health–Seattle and King County and CDC COVID-19 Investigation Team. Presymptomatic SARS-CoV-2 Infections and Transmission in a Skilled Nursing Facility. N Eng J Med 2020;382:2081–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html (accessed August 29, 2020)
- 8.https://covidtracking.com/race (accessed August 29, 2020)
- 9.Price-Haywood EG, Burton J, Fort D, Seone L. Hospitalization and Mortality among Black Patients and White Patients with Covid-19. N Eng J Med 2020;382:2534–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yehia BR, Winegar A, Foegel R, Fakih M, Ottenbacher A, Jesser C, Bufalina A, Huang R-H, Cacchione J. Association of race with mortality among patients hospitalized with coronavirus disease 2019 (COVID-19) at 92 US hospitals. JAMA Network Open 2020;3:e2018039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel AP, Paranjpe MD, Kathiresan NP, Rivas MA, Khera AV. Race, socioeconomic deprivation, and hospitalization for COVID-19 in English participants of a national biobank. Int J Equity Health 2020;19:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.https://www.cdc.gov/nchs/nvss/vsrr/covid_weekly/index.htm (accessed August 29, 2020)
- 13.https://coronavirus.jhu.edu/map.html (accessed August 29, 2020)
- 14.Kim L, Whitaker M, O’Halloran A, Kambhampati A, Chai SJ, Reingold A, et al. Hospitalization rates and characteristics of children aged <18 years hospitalized with laboratory-confirmed COVID-19 – COVID-NET, 14 States, March 1-July 25, 2020. MMWR 2020;69:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dufort EM, Koumans EH, Chow EJ, Rosenthal EM, Muse A, Rowlands J, et al. Multisystem inflammatory syndrome in children in New York State. N Eng J Med 2020;383:347–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Eng J Med 2020;383:334–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/groups-at-higher-risk.html (accessed August 29, 2020)
- 18.Pinto BGG Oliveira AER, Singh Y, Jimenez L, Gonçalves ANA, Ogava RLT, Creighton R, Peron JPS, Nakaya HI. ACE2 Expression is Increased in the Lungs of Patients with Comorbidities Associated with Severe COVID-19. J Infect Dis 2020. Jun 11;jiaa332. doi: 10.1093/infdis/jiaa332. [DOI] [PMC free article] [PubMed]
- 19.McMichael TM, Currie DW, Clark S, Pogosjans S, Kay M, Schwartz NG, Lewis J, Baer A, Kawakami V, Lukoff MD, Ferro J, Brostrom-Smith C, Rea TD, Sayre MR, Riedo FX, Russell D, Hiatt B, Montgomery P, Rao AK, Chow EJ, Tobolowsky F, Hughes MJ, Bardossy AC, Oakley LP, Jacobs JR, Stone ND, Reddy SC, Jernigan JA, Honein MA, Clark TA, Duchin JS; Public Health–Seattle and King County, Evergreen Health, and CDC COVID-19 Investigation Team. Epidemiology of Covid-19 in a Long-Term Care Facility in King County, Washington. N Eng J Med 2020;382:2005–2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alpert A, Pickman Y, Leipold M, Rosenberg-Hasson Y, Ji X, Gaujoux R, et al. A clinically meaningful metric of immune age derived from high-dimensional longitudinal monitoring. Nat Med 2019;25:487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prather KA, Wang CC, Schooley RT. Reducing transmission of SARS-CoV-2. Science 2020;368:1422–1424. [DOI] [PubMed] [Google Scholar]
- 22.Starke KR, Petereit-Haack G, Schubert M, Kamph D, Schliebner A, Hegewald J, Seidler A. The age-related risk of severe outcomes due to COVID-10 infection: a rapid review, meta-analysis, and meta regression. Int J Environ Res Public Health 2020;17:5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peters MC, Sajuthi S, Deford P, Christenson S, Rios CL, Montgomery MT, et al. COVID-19 related genes in sputum cells in asthma: relationship to demographic features and corticosteroids. Am J Respir Crit Care Med 2020;202:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gross CP, Essien UR, Pasha S, Gross J, Wang S-y, Nunez-Smith M. Racial and ethnic disparities in population-level Covid-19 mortality. J Gen Intern Med 2020;4:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.https://www.apmresearchlab.org/covid/deaths-by-race (accessed August 29, 2020)
- 26.https://usafacts.org/articles/covid-deaths-race-state-age-black-hispanic-white/ (accessed August 29, 2020)
- 27.Abedi V, Olulana O, Avula V, Chaudhary D, Khan A, Shahjouei S, Li J, Zand R. Racial, Economic and Health Inequality and COVID-19 Infection in the United States. J Racial Ethn Health Disparities 2020. Sep 1:1–11. doi: 10.1007/s40615-020-00833-4. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wiemers EE, Abrahams S, AlFakhri M, Hotz VJ, Schoeni RF, Seltzer JA. Disparities in Vulnerability to Severe Complications from COVID-19 in the United States. medRxiv 2020. May 30;2020.05.28.20115899. doi: 10.1101/2020.05.28.20115899. Preprint [DOI] [PMC free article] [PubMed]
- 29.Koi M, Okita Y, Takeda K, Koeppe E, Stoffel EM, Galanko JA, McCoy AN, Keku T, Carethers JM. Co-morbid risk factors and NSAID use among White and Black Americans that predicts overall survival from diagnosed colon cancer. PLoS One 2020;15(10):e0239676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rentsch CT, Kidwai-Khan F, Tate JP, Park LS, King JT, Skanderson M, Hauser RG, Schultze A, Jarvis CI, Holodniy M, Lo Re V, Akgun KM, Crothers K, Taddei TH, Freiberg MS, Justice AC. Patterns of COVID-19 testing and mortality by race and ethnicity among United States veterans: A nationwide cohort study. PLoS Med 2020. Sep 22;17(9):e1003379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Selden TM, Berdahl TA. COVID-19 And Racial/Ethnic Disparities In Health Risk, Employment, And Household Composition. Health Affairs 2020:101377hlthaff202000897. [DOI] [PubMed]
- 32.Anderson M. Who relies on public transportation in the U.S https://www.pewresearch.org/fact-tank/2016/04/07/who-relies-on-public-transit-in-the-u-s/; (accessed August 29, 2020)
- 33.https://www.pewresearch.org/fact-tank/2020/05/05/financial-and-health-impacts-of-covid-19-vary-widely-by-race-and-ethnicity/ (accessed August 29, 2020)
- 34.Carethers JM, Doubeni CA. Causes of socioeconomic disparities in colorectal cancer and intervention framework and strategies. Gastroenterol 2020;158:354–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.https://www.cdc.gov/nchs/products/databriefs/db289.htm (accessed August 29, 2020)
- 36.Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin-angiotensin-aldosterone system inhibitors inpatients with Covid-19. N Eng J Med 2020;382:1653–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin-angiotensin-aldosterone system blockers and the risk of Covid-19. N Eng J Med 2020;382:2431–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reynolds HR, Adhikari S, Pulgarin C, Troxel AB, Iturrate E, Johnson SB, Hausvater A, Newman JD, Berger JS, Bangalore S, Katz SD, Fishman GI, Kunichoff D, Chen Y, Ogedegbe G, Hochman JS. Renin-angiotensin-aldosterone system inhibitors and risk of Covid-19. N Eng J Med 2020;382:2441–2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Geleris J, Sun Y, Platt J, Zucker J, Baldwin M, Hripcsak G, Labella A, Manson DK, Kubin C, Barr RG, Sobieszczyk ME, Schluger NW. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N Eng J Med 2020;382:2411–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boulware DR, Pullen MF, Bangdiwala AS, Pastick KA, Lofgren SM, Okafor EC, Skipper CP, Nascene AA, Nicol MR, Abassi M, Engen NW, Cheng MP, LeBar D, Lother SA, Mackenzie LJ, Drobot G, Marten N, Zarychaski R, Kelly LE, Schwartz IS, McDonald EG, Rajasingham R, Lee TC, Hullsiek KH. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N Eng J Med 2020;383:517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giudicessi JR, Roden DM, Wilde AAM, Ackerman MJ. Genetic susceptibility for COVID-19-associated sudden cardiac death in African Americans. Heart Rhythm 2020;S1547–5271(20)30419–7. [DOI] [PMC free article] [PubMed]
- 42.Ellinghaus D, Degenhardt F, Bujanda L, Buti M, Albillos A, Invernizzi P, et al. Genomewide association study of severe Covid-19 with respiratory failure. N Eng J Med 2020;382 (doi: 10.1056/NEJMoa2020283) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao J, Yang Y, Huang H, Li D, Gu D, Lu X, et al. Relationship between the ABO blood group and the COVID-19 susceptibility. Clin Infect Dis 2020; Aug 4;ciaa1150. doi: 10.1093/cid/ciaa1150. Online ahead of print. [DOI] [PMC free article] [PubMed]
- 44.https://www.redcrossblood.org/donate-blood/blood-types.html (accessed August 29, 2020)
- 45.Eaaswarkhanth M, Al Madhoun A, Al-Mulla F. Could the D614G substitution in the SARS-CoV-2 spike (S) protein be associated with higher COVID-19 mortality? Int J Infect Dis 2020;96:459–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gebhard C, Regitz-Zagrosek V, Neuhauser HK, Morgan R, Klein SL. Impact of sex and gender on COVID-19 outcomes in Europe. Biol Sex Diff 2020;11:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salje H, Kiem CT, Lefrancq N, Courtejoi N, Bosetti P, Paireau J, et al. Estimating the burden of SARS-CoV-2 in France. Science 2020;369:208–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.https://www.brookings.edu/blog/up-front/2020/05/15/covid-19-much-more-fatal-for-men-especially-taking-age-into-account/ (accessed August 29, 2020)
- 49.Song H, Seddighzadeh B, Cooperberg MR, Huang FW. Expression of ACE2, the SARS-CoV-2 receptor, and TMPRSS2 in prostate epithelial cells. Eur Urol 2020;78:296–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stopsack KH, et al. TMPRSS2 and COVID-19: Serendipity or opportunity for intervention? Cancer Discovery 2020;10:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wadman M. Sex hormones signal why virus hits men harder. Science 2020;368:1038–1039. [DOI] [PubMed] [Google Scholar]
- 52.Ateeq B, Bhatia V, Goel S. Molecular discriminators of racial disparities in prostate cancer. Trends in Cancer 2016;2:117–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Limburg H, Harbig A, Bestle D, Stein DA, Moulton HM, Jaeger J, et al. TMPRSS2 is the major activating protease of influenza A virus in primary human airway cells and influenza B virus in human type II pneumocytes. J Virol 2019;93:e00649–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.vom Steeg LG, Vermillion MS, Hall OJ, Alam O, McFarland R, Chen H, Zirkin B, Klein SL. Age and testosterone mediate influenza pathogenesis in male mice. Am J Physiol Lung Cell Mol Physiol 2016;311:L1234–L1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoffman M, Keine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020;181:271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lindquist KJ, Paris PL, Hoffmann TJ, Cardin NJ, Kazma R, Mefford JA, Simko JP, Ngo V, Chen Y, Levin AM, Chitale D, Helfand BT, Catalona WJ, Rybicki BA, Witte JS. Mutational landscape of aggressive prostate tumors in African American men. Cancer Res 2016;76:1860–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bastus NC, Boyd LK, Mao X, Stankiewicz E, Kudahetti SC, Oliver RTD, Berney D, Lu Y-J. Androgen-induced TMPRSS2:ERG fusion in nonmalignant prostate epithelial cells. Cancer Res 2010;70:9544–9548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou CK, Young D, Yeboah ED, Coburn SB, Tettey Y, Biritwum RB, et al. TMPRSS2-ERG gene fusions in prostate cancer of West African Men and a meta-analysis of racial differences. Am J Epidemiol 2017;186:1352–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sartor O, Zheng Q, Eastham JA. Androgen receptor gene CAG repeat length varies in a race-specific fashion in men without prostate cancer. Urology 1999;53:378–380. [DOI] [PubMed] [Google Scholar]
- 60.Bennett CL, Price DK, Kim S, Liu D, Jovanovic BD, Nathan D, Johnson ME, Montgomery JS, Cude K, Brockbank JC, Sartor O, Figg WD. Racial variation in CAG repeat lengths within the androgen receptor gene among prostate cancer patients of lower socioeconomic status. J Clin Oncol 2002;20:3599–3604. [DOI] [PubMed] [Google Scholar]
- 61.McCoy J, Wambier CG, Vano-Galvan S, Shapiro J, Sinclair R, Ramos PM, Washenik K, Andrade M, Herrera S, Goren A. Racial variations in COVID-19 deaths may be due to androgen receptor genetic variation associated with prostate cancer and androgenetic alopecia. Are anti-androgens a potential treatment for COVID-19? J Cosmet Dermatol 2020;19:1542–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ellis JA, Stebbing M, Harrap SB. Polymorphisms of the androgen receptor gene is associated with male pattern baldness. J Invest Dermatol 2001;116:452–455. [DOI] [PubMed] [Google Scholar]
- 63.Baldi E, Mare C, Savastano S. Out-of-Hospital cardiac arrest during the Covid-19 outbreak in Italy. N Eng J Med 2020;383:496–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Solomon MD, McNulty EJ, Go AS. The Covid-19 pandemic and the incidence of acute myocardial infarction. N Eng J Med 2020;383:691–693. [DOI] [PubMed] [Google Scholar]
- 65.Kansagra AP, Goyal MS, Hamilton S, Albers GW. Collateral effect of Covid-19 on stroke evaluation in the United States. N Eng J Med 2020;383:400–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carethers JM. Screening for colorectal cancer in African Americans: Determinants and rationale for an earlier age to commence screening. Dig Dis Sci 2015;60:711–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.London JW, Fazio-Eynullayeva E, Palchuk MB, Sankey P, McNair C. Effect of the COVID-19 pandemic on cancer-related patient encounters. JCO Clin Cancer Inform 2020;4:657–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mast C, del Rio AM. Delayed cancer screenings – a second look. Epic Health Research Network (July 17, 2020) https://ehrn.org/delayed-cancer-screenings-a-second-look/ (accessed August 29, 2020)
- 69.Sharpless NE. COVID-19 and cancer. Science 2020;368:1290. [DOI] [PubMed] [Google Scholar]
- 70.Carethers JM, Sengupta R, Blakey R, Ribas A, D’Souza G. Disparities in cancer prevention in the COVID-19 era. Cancer Prev Res 2020. Sep 17:canprevres.0447.2020. doi: 10.1158/1940-6207.CAPR-20-0447. Online ahead of print. [DOI] [PMC free article] [PubMed]
- 71.Carethers JM. Clinical and genetic factors to inform reducing colorectal cancer disparities in African Americans. Front Oncol 2018;8:531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Newman L, Winn R, Carethers JM. Similarities in risk for COVID-19 and cancer disparities. Clin Cancer Res 2020. (accepted manuscript) [DOI] [PMC free article] [PubMed]
- 73.Laborde D, Martin W, Swinnen J, Vos R. COVID-19 risks to global food security. Science 2020;369:500–502. [DOI] [PubMed] [Google Scholar]


