Abstract
Attenuation of adverse effects of aflatoxin (AFB1) in brains of B1 rats by extracts of leaves of artichoke was studied. The active ingredients in extracts of leaves of artichoke, Cynara scolymus L., were determined by HPLC analysis. In the 42-day experiment, rats were exposed to either sterile water, 4% DMSO, 100 mg artichoke leaf extract/kg body mass, 72 μg aflatoxin B1/kg body mass, or AFB1 plus artichoke leaf extract. Neurotoxicity of AFB1 was determined by an increase in profile of lipids, augmentation of plasmatic glucose and concentrations of insulin, oxidative stress, increased activities of cholinergic enzymes, and a decrease in activities of several antioxidant enzymes and pathological changes in brain tissue. Extracts of artichoke leaf significantly reduced adverse effects caused by AFB1, rescuing most of the parameters to values similar to unexposed controls, which demonstrated that adverse, neurotoxic effects caused by aflatoxin B1 could be significantly reduced by simultaneous dietary supplementation with artichoke leaf extract, which itself is not toxic.
1. Introduction
Mycotoxins are toxic fungal products that thrive in human food and animal feed, causing disease and death in humans and animals, when consumed in concentrations exceeding the limits [1, 2]. These toxigenic fungi contaminating agricultural grains have been divided into two groups. The first are those that invade seed crops and are known as “field” fungi and include species of the genera Alternaria, Fusarium, and Cladosporium, which reputedly gain access to seeds during plant development. The second class is described as “storage” fungi and includes species from the genera Penicillium and Aspergillus that proliferate during storage [3]. Both Aspergillus flavus and Aspergillus parasiticus produce aflatoxins (AFs) that can infect crops before harvesting. Contamination of field crops with aflatoxins causes losses of agricultural commodities in many zones of the world [4]. Therefore, it is necessary to control AF contamination in food and feed to ensure food safety and, more importantly, to safeguard human and animal health.
Aflatoxin B1 (AFB1) is considered the most significant risk factor among mycotoxins and the most frequently found aflatoxin in contaminated foods and feed [5–7]. Due to its stability and heat-resistant nature, not all food processing methods are efficient at removing AFB1. Therefore, public health can be affected following dietary exposure to AFB1. On a worldwide basis, the safe limits of aflatoxin B1 range from 4 to less than 30 μg/kg for human consumption [8]. AFB1 may increase lipid peroxidation, which contributes to cytotoxic damage in many organs, especially the brain, leading to neurodegenerative diseases and weakening of this organ's function [9, 10]. Furthermore, it has been documented that prenatal administration of AFB1 in rat offspring can delay development of reflex responses, learning ability, motor activity, locomotor coordination, and exploratory behavior [11]. It can also cause tumors in both the central and peripheral nervous systems and various nonepithelial neurogenic tumors. In the central nervous system, neurons have a high metabolic rate and a low capacity for anaerobic metabolism. As a result, when the oxygen supply to the brain is insufficient, the neuronal brain cells degenerate within minutes. AFB1 has been reported to alter concentrations of neurotransmitters in brains of rats [12]. While drug-based chemoprotection strategies can be useful in mitigating toxicity of aflatoxins, due to the large numbers of persons potentially affected, practical implementation is limited. Therefore, it is imperative to identify alternative treatments where patients could benefit from fewer complications and a better quality of life. In recent years, renewed interest in natural products has spotlighted drug discovery and cancer research [13, 14]. In the present study, an approach to counteract neurotoxicity caused by AFB1 was investigated in which diets were supplemented with an entirely natural, plant-based product, extracts of leaves of artichoke. The alternative therapy if effective would be cost-effective and result in fewer side effects.
The artichoke, Cynara scolymus L., is a member of the Asteraceae family and a native plant of the Mediterranean. Because of its nutritional benefits and medicinal properties, C. scolymus L. is cultivated worldwide. Officially, medicinal artichoke products consist of dried leaves. It is a rich source of polyphenolic compounds, mainly caffeoylquinic acids, and flavonoids, isolated from the plant polar extracts, together with the polysaccharide inulin, fibers, and minerals. The lipophilic fraction comprises fatty acids, triterpenes, and sesquiterpenes as major metabolites [15]. Using the International Union of Pure and Applied Chemistry (IUPAC) nomenclature, the plant contains up to 6% phenolic acids, including 5-O-caffeoylquinic acid (chlorogenic acid), which is the most abundant substance (39%), followed by 1,5-O-dicaffeoylquinic acid (21%) and 3,4-O-dicaffeoylquinic acid (11%), based on total caffeoylquinic acid content. The artichoke also contains up to 5% sesquiterpene lactones, with cynaropicrin being the primary component. Other ingredients are dehydrocynaropicrin, grosheimin and their derivatives, and 0.35-0.75% flavonoids, including scolymoside, cynaroside, and cyanotrioside [16]. Furthermore, the 1,3-O-dicaffeoylquinic acid (cynarin) content in methanolic extracts of the artichoke is small, about 1.5%, the majority of which is located in the pulp of the leaves, although the dried leaves and stems of artichoke also contain this compound [15].
Phenolic substances contained in artichoke have an essential scavenging activity against reactive oxygen species (ROS) and free radicals and perform as a protective shield against oxidative damage to biological molecules, such as proteins, lipids, and DNA [17]. Gebhardt [18] reported that 1,3-dicaffeoylquinic acid and flavonoids were considered choleretic, anticholestatic, and diuretic, reducing cholesterol. While the plant and its preparations are commonly used to treat dyspepsia, they are also used traditionally to prevent and treat atherosclerosis and kidney dysfunction (diuretic) [19].
Effects of artichoke leaf extract (ALE) after 42 days of exposure in male rats were investigated. General pathological characteristics and specific brain plasma markers were evaluated. Besides, lipid profile, antioxidant enzyme activities, and free radicals were analyzed in the brain to elucidate the potential neuroprotective effects of ALE. Furthermore, the histopathological architecture of the brain was evaluated. In this study, the following hypothesis was tested: adverse neurotoxic effects caused by aflatoxin B1 can be significantly reduced by simultaneous dietary supplementation with extracts of leaves of artichoke.
2. Materials and Methods
2.1. Chemicals
AflatoxinB1 powder (C17H12O6) from Aspergillus flavus was purchased from Sigma-Aldrich, France (CAS Number 1162-65-8, Product Number A6636-10MG, purity 99.7% and formula mass: 312.27). Commercial ALE (super artichoke, capsules) was produced by Western Pharmaceutical Industries, Cairo, Egypt, and used in this study. All reagents and chemicals were of analytical grade.
2.2. Animals
Twenty-five 12-week-old male albino rats were used for this research. The rats were obtained from the Medical Research Institute (Center of Medical Technology), Alexandria University, Egypt. They were housed in clean cages with hardwood bedding. They had access to feed and clean tap water ad libitum. Animals were maintained in a controlled atmosphere at a temperature of 25 ± 5°C and 50-70% humidity, and they were subjected to standard 12 h light and dark cycles.
2.3. The Active Ingredient of the Artichoke, Cynara scolymus L., Leaf Extract
HPLC analysis was carried out using an Agilent 1260 series. Separations were conducted by use of an Eclipse C18 column (4.6 mm × 250 mm, 5 μm). Water and 0.05% trifluoroacetic acid in acetonitrile were used at a flow rate 1 mL/min as mobile phases A and B, respectively. The gradient was programmed consecutively in a linear manner as follows: 0 min (82% A), 0–5 min (80% A), 5-8 min (60% A), 8-12 min (60% A), 12-15 min (82% A), 15-16 min (82% A), and 16-20 (82% A). The multiwavelength detector was monitored at 280 nm. The injection volume was 5 μL for each of the sample solutions. The column temperature was maintained at 40°C.
2.4. Treatment Protocol
Rats were allowed to acclimatize to their environment for two weeks before the experiment started. They were randomly divided into five treatment groups of five rats each and treated with sterile water, control group; 4% DMSO (1 mL/kg body mass), which is the solvent for AFB1, DMSO group; ALE (100 mg/kg, bm), ALE group; AFB1 at the concentration of 72 μg/kg, bm (as 1/100 of LD50% dose: 7.2 mg/kg, bm), AFB1 group; and AFB1 plus ALE, ALE+AFB1 group. Animals were orally gavaged daily with the respective doses for 42 consecutive days. The initial and final body masses of male rats were recorded in each treatment group, and they did not show any significant change among the treated groups.
2.5. Blood and Brain Collection
Animals were euthanized at the end of the experimental period, by use of isoflurane (2 mL/kg, bm) by inhalation. Blood was collected by cardiac puncture in test tubes containing heparin. Plasma was obtained from whole blood by centrifugation at 860 × g for 20 min. Blood plasma was kept at −80°C until analysis. Brains were removed, released from adhering fat and connective tissues, washed with chilled saline solution (0.9%), dried on tissue paper, and mass determined. A portion of the brain was immediately kept in 10% formalin for histological studies. Another portion of 2 g of the brain was minced and homogenized (10%, w/v), separately, in ice-cold phosphate buffer (0.25 M, pH 7.4) in a Potter–Elvehjem type homogenizer. Homogenates were centrifuged at 10,000 × g for 20 min at 4°C to pellet cell debris, and the supernatant was collected and stored at −80°C for further analyses.
2.6. Biochemical Analysis
The tests were performed using a UV-visible spectrophotometer.
2.6.1. Plasma Lipid Profile
The plasma lipid profile was performed by measuring concentrations of total lipid (Cat. No. TL20 10), cholesterol (Cat. No. CH12 20), triglycerides (Cat. No. TR20 30), high-density lipoprotein (HDL) (Cat. No. CH12 30), and low-density lipoprotein (LDL) (Cat. No. CH12 31). Analyses were done by colorimetric methods using commercial kits (BioDiagnostic Inc., Cairo, Egypt). Concentrations of VLDL in blood plasma were calculated according to Friedewald's equation [20].
2.6.2. Estimation of Plasma Glucose, Insulin Concentration, and Homeostasis Model Assessment (HOMA)
Concentrations of glucose were quantified by use of a colorimetric method using a commercially available kit (Cat. No. GL13 20, BioDiagnostic Inc., Cairo, Egypt). Concentrations of insulin were measured by ELISA with the assay kit according to the manufacturer's instructions (Bioneovan Ltd., Beijing, China). The homeostasis model assessment of insulin resistance (HOMA-IR) index and the function of the pancreatic β-cells (HOMA-B) provided valid and reliable information based on the fasting plasma glucose level and insulin concentration. HOMA-IR and HOMA-B were calculated with a formula adapted by Matthews et al. [21].
2.6.3. TNF-α, IDO, and TIMP3 Assays
ELISAs were performed for these parameters as per the manufacturer's instructions. The ELISA kits for tumor necrosis factor-alpha (TNF-α), indoleamine-pyrrole 2,3-dioxygenase (IDO), and tissue inhibitor metallopeptidase (TIMP3) were obtained from Bioneovan Ltd., Beijing, China.
2.6.4. Brain Free Radicals and Antioxidant Enzyme Assays
Concentrations of thiobarbituric acid reactive substances (TBARS) (Ref. MD25 29), uric acid (UA) (Ref. UR21 20), and nitric oxide (NO) (Ref. NO25 33) and the activity of superoxide dismutase (SOD) (Ref. SD25 21), glutathione S-transferase (GST) (Ref. GT25 19), and glutathione peroxidase (GPx) (Ref. GP25 24) in brain supernatants were determined spectrophotometrically by use of commercially available assay kits obtained from the BioDiagnostic Company (Cairo, Egypt). Measurements of the above parameters were performed strictly according to the manufacturer's instructions. Urea was measured in the plasma by the urease-Berthelot method [22] (Ref. UR21 20, BioDiagnostic Inc., Cairo, Egypt). Glutathione level (GSH) was determined following the method of Jollow et al. [23], and xanthine oxidase (XO) activity was measured according to Litwack et al. [24].
2.6.5. Estimation of the Activities of Acetylcholinesterase (AChE) and Monoamine Oxidase (MAO)
AChE activity was estimated according to Ellman's method [25]. It was measured spectrophotometrically at 405 nm and expressed as μmol/min/mg protein. MOA activity was determined as described by Hallman et al. [26], where 1 mL of 50 mM sodium phosphate buffer pH 7.4, 150 μL brain supernatant, and 500 μL of 5 mM benzylamine were mixed well. MAO activity (U/l) = ∆A × total volume × 1000/3.2 × brain supernatant volume. The absorbance was measured at 250 nm against air after 30 s and 90 s.
2.7. Histopathology
The brain sections were assessed histologically, using the H&E staining method, to provide general knowledge concerning the potential aflatoxin B1 influence on tissue structure compared to the control and ALE treatment alone. Combined treatment using AFB1 and ALE (ALE+AFB1 group) has also been assessed to evaluate the potential beneficial effects of ALE on the brain. Brain tissues, fixed in 10% formalin immediately after collecting, were processed by cutting pieces in tissue cassettes with an automated tissue processor. They were then embedded in paraffin and, after that, were sectioned with microtome at 4-6 mm thickness. Slides were then stained with hematoxylin and counterstained with eosin for histological examination. Photographs were taken on an Olympus XC30 microscope (Germany) with a digital camera (Olympus UC30 camera). Representative pictures were taken at 20x and 40x magnification from each group.
2.8. Statistical Analysis
Statistical analysis was performed using Statistica 13.1. All biochemical measurements were performed in 5 replicates. The distribution of the data (the Kolmogorov-Smirnov and Shapiro-Wilk tests) and homogeneity of variance (the Levene test) was checked before analysis. The data fulfilled analysis of variance criteria; therefore, parametric tests were used: ANOVA (post hoc LSD test, p < 0.05), and the differences between experimental groups were investigated. All parameters were expressed as the mean ± SD in the figures. Principal component analysis (PCA) was also performed for all measured parameters.
3. Results and Discussion
3.1. Chemical Composition of the Artichoke Leaf Extract
The analysis of artichoke capsule identified 17 components (Figure 1). Apart from some components, such as pyrocatechol, ferulic acid, naringenin, catechin, and gallic acid, the artichoke's highest component concentration was the chlorogenic acid (CGA). CGA has been found to have anti-inflammatory, antioxidant, antiviral, antibacterial, lipid lowering, hypoglycemic, and cardioprotective properties and many other pharmacological benefits [27]. Therefore, CGA may play an important role in health promotion.
Figure 1.
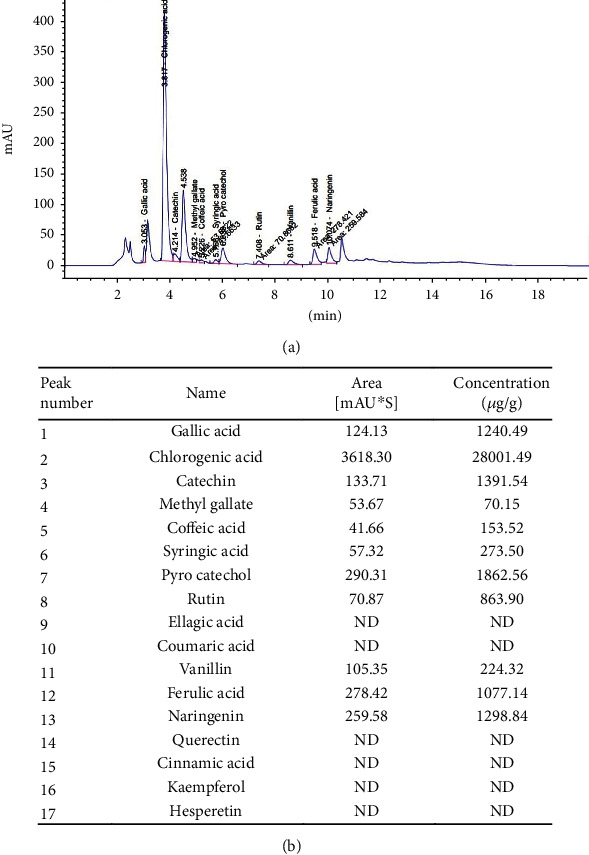
(a) HPLC chromatogram of aqueous extract of the artichoke (Cynara scolymus L.) leaf extract capsule and (b) list of the components of the artichoke capsule and their concentrations.
3.2. Plasma Lipid Profile
Treatment of the rats with ALE for 42 days resulted in a significant (p ≤ 0.05) decrease in total plasma lipids (412.0 ± 12.2 mg/dL), cholesterol (105.3 ± 1.8 mg/dL), triglycerides (139.4 ± 5.8 mg/dL), low-density lipoprotein (LDL; 33.1 ± 12.2 mg/dL), and very-low-density lipoprotein (VLDL; 27.9 ± 1.2 mg/dL) compared to control (562.6 ± 25.9, 160.3 ± 16.9, 152.6 ± 6.5, 96.1 ± 11.9, and 30.5 ± 11.9 mg/dL, respectively). Simultaneously, a significant (p ≤ 0.05) increase in the concentration of high-density lipoproteins (HDL: 44.4 ± 2.9 mg/dL vs. control: 33.8 ± 3.2 mg/dL) in the plasma of rats from the ALE group was found (Figure 2). Compared to all other groups, aflatoxin B1 caused a significant increase in almost all lipid profiles' parameters (total lipids: 705.2 ± 16.3 mg/dL; cholesterol: 211.9 ± 17.2 mg/dL; triglycerides: 173.0 ± 10.2 mg/dL; LDL: 146.1 ± 10.3 mg/dL; and VLDL: 34.6 ± 2.0 mg/dL). The only exception was found for HDL (25.6 ± 2.1 mg/dL), where a significant decrease in concentration in the AFB1 group was noted compared to the rest of the experimental groups (Figure 2(d)). The administration of ALE combined with aflatoxin B1 (ALE+AFB1 group) reduced the concentration of total lipids (537.0 ± 12.6 mg/dL), cholesterol (164.9 ± 12.8 mg/dL), HDL (34.4 ± 2.7 mg/dL), and LDL (107.5 ± 10.7 mg/dL) to the values typical for the control group (Figures 2(a) and 2(b) and 2(d) and 2(e)). Moreover, both factors' combined use resulted in a significant reduction in triglycerides (116.4 ± 13.9 mg/dL) and VLDL (23.3 ± 2.8 mg/dL) concentration compared to the control group (Figures 2(c) and 2(f)).
Figure 2.
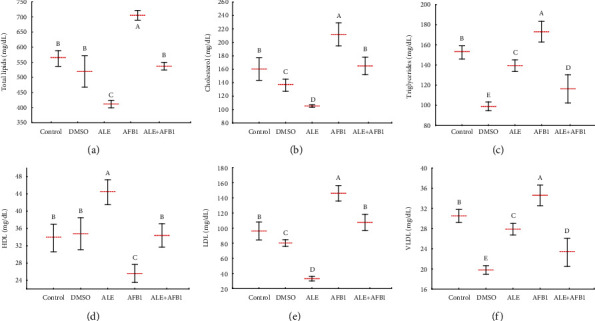
Mean ± SD of total lipids (a), cholesterol (b), triglycerides (c), HDL (d), LDL (e), and VLDL (f) in plasma of male rats treated with AFB1, ALE, and their combination for 42 days. Abbreviations: control: animals treated with water; DMSO: animals treated with DMSO (solvent); ALE: animals treated with artichoke leaves extract (100 mg/kg body weight); AFB1: animals treated with Aflatoxin B1 (72 μg/kg body weight); ALE+AFB1: animals treated with a combination of artichoke extract and aflatoxin B1; LDL: low-density lipoprotein; VLDL: very low-density lipoprotein; HDL: high-density lipoprotein. The same letter (A, B, C, D, and E) denote no significant difference among experimental groups (p < 0.05; n = 5; LSD, ANOVA test).
When studying the brain, lipids are of particular interest because they account for more than 50% of its dry mass. They are the main components of cell membranes and are repositories of chemical energy. The crucial role of lipids in brain physiology and cell signaling has been demonstrated by neurologic disorders and several neurodegenerative diseases in which lipid metabolism is altered [28]. Previously, lipid content of the brain was considered relatively resistant to change induced by dietary intake [29, 30]. However, results presented here show that ALE consumed simultaneously with aflatoxin B1 for 42 days downregulated almost all lipid profile parameters except HDL, which was upregulated following exposure to AFB1 alone. In brief, it is evident that ALE could positively alter lipid content and function of the rat brain. The positive effect of ALE is undoubtedly related to the interaction of the active ingredients of the extract, mainly chlorogenic acid. It is known that some derivatives of CGA may affect lipid metabolism significantly by decreasing the oxidation of low-density lipoproteins (LDL) and total cholesterol. A reduction of cholesterol and triacylglycerols concentrations by 44% and 58% was observed in obese, hyperlipidemic, and insulin-resistant Zucker rats, infused with CGA at a dose of 5 mg/kg body weight/day for three weeks [31]. One of the first hypotheses explaining the action of CGA was that of its cations' chelation properties resulting in oxidative stress inhibition [32]. More recent studies show that this compound can lower cholesterol and positively affect lipid metabolism by upregulating gene expression of a peroxisome proliferation-activated receptor (PPAR-α). This nuclear receptor acts as a transcription factor regulating the expression of genes related to metabolism including lipid metabolism [33]. Our research shows that ALE, containing CGA as its main component, improves lipid metabolism when disrupted by AFB1.
3.3. Plasma Glucose and Insulin Concentration, HOMA-IR, and HOMA-B Index
Exposure to ALE or AFB1 alone resulted in different responses. Treatment with ALE alone caused no significant effect on insulin concentration (4.4 ± 0.2 μU/mL) and the homeostatic model assessment for insulin resistance (HOMA-IR; 0.9 ± 0.04) index, but the concentration of glucose (84.2 ± 1.0 mg/dL) was slightly less, and the homeostatic model assessment for beta cells (HOMA-B; 18.7 ± 0.04) index was elevated compared to the control group (4.3 ± 0.2, 1.0 ± 0.1, 91.4 ± 8.1, and 16.8 ± 1.5, respectively) (Figure 3). Aflatoxin B1 resulted in significantly greater concentrations of glucose (116.4 ± 4.8 mg/dL) and insulin (4.8 ± 0.1 μU/mL) in blood plasma of rats and the HOMA-IR index (1.38 ± 0.1) compared to rats in all other groups. Furthermore, in rats exposed to AFB1, the HOMA-B index (14.9 ± 0.8) was significantly less than in the other groups. The treatment of AFB1-exposed rats with ALE restored the levels of glucose (97.6 ± 5.6 mg/dL), insulin (4.2 ± 0.2 μU/mL) and HOMA (HOMA-IR: 1.0 ± 0.1 and HOMA-B: 15.7 ± 1.1) indices.
Figure 3.
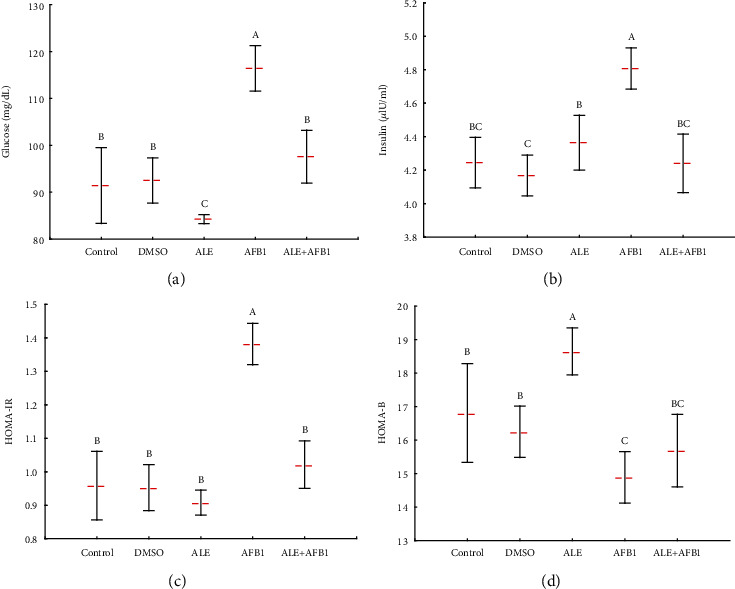
Mean ± SD of glucose (a) and insulin (b) concentration, HOMA-IR (c), and HOMA-B (d) index in plasma of male rats treated with AFB1, ALE, and their combination for 42 days. Abbreviations: HOMA-IR: homeostatic model assessment for insulin resistance; HOMA-B: homeostatic model assessment for beta cells; for other explanations, see Figure 1.
Insulin is pivotal for maintaining glucose metabolism in the periphery and the brain [34]. Alterations in the insulin regulation system lead to hyperinsulinemia, which produces widespread insulin resistance. The latter is a state in which tissues that require glucose have a diminished response to insulin, and the subsequently reduced clearance of glucose from the blood feeds back onto the pancreas to increase secretion of insulin to induce glucose uptake [35]. Results of the current study confirmed previous findings where exposure to AFB1 increased insulin resistance, as shown by the decrease in HOMA-B values associated with elevated HOMA-IR values, which corresponds to decreased insulin effectiveness in regulating plasma glucose. However, simultaneous exposure to ALE and AFB1 positively affects the discussed parameters and brings them to the control level.
The positive effect of artichoke extract can be again associated with the impact of chlorogenic acid. Mentioned above studies by Rodriguez de Sotillo and Hadley [31] on Zucker rats also showed that CGA did not promote sustained hypoglycemia and improved glucose tolerance. Underlying mechanisms include the influence of CGA on the glucose-6-phosphatase activity involved in glucose metabolism [36]. Also, an improved insulin sensitivity after CGA treatment can increase glucose uptake by tissues. It has been shown that phenolic acids improve insulin sensitivity of HepG2 cells by reducing the insulin signaling blockade and modulation of glucose consumption [37]. Therefore, a decrease in insulin and glucose concentrations is connected with decreased synthesis of lipids [31].
3.4. TNFα, IDO, and TIMP3 in the Brain of Rats
Treatment of rats with ALE had no significant influence on concentrations of tumor necrosis factors α (TNFα; 1302.0 ± 25.5 pg/mg protein), indoleamine-pyrrole 2,3-dioxygenase (IDO; 1563.8 ± 56.4 pg/mg protein), and tissue inhibitor metallopeptidase (TIMP3; 4027.5 ± 259.5 pg/mg protein) (Figure 4). Aflatoxin B1 administered alone for 42 days caused a significant (p < 0.05) increase in TNFα (2387.5 ± 31.8 pg/mg protein) and IDO (2334.5 ± 129.4 pg/mg protein) and, at the same time, a decrease in concentrations of TIMP3 (773.0 ± 45.2 pg/mg protein) compared to controls (1298.5 ± 2.1, 1542.7 ± 41.9, and 3821.0 ± 74.9 pg/mg protein, respectively). These evaluated parameters were generally positively influenced by the administration of AFB1 together with ALE (ALE+AFB1 group). The concentration of TNFα value (1658.5 ± 30.4 pg/mg protein) in rats exposed to ALE+AFB1 was less than that found in the rats exposed to AF1 alone but was still greater than in the control group. The mean IDO value (1726.0 ± 74.7 pg/mg protein) in brains of rats exposed to ALE+AFB1 was similar to that of the control group (1542.7 ± 41.9 pg/mg protein). Concentrations of TIMP3 (2274.0 ± 142.8 pg/mg protein) in brains of rats exposed to AFB1 and ALE simultaneously were greater than in those exposed to AFB1 alone (779.0 ± 45.2 pg/mg protein), but still significantly less compared to unexposed rats in the control group (3821.0 ± 74.9 pg/mg protein).
Figure 4.
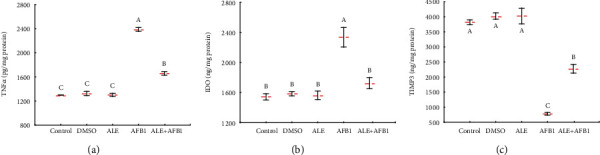
Mean ± SD concentration of TNFα (a), IDO (b), and TIMP3 (c) in the brain of male rats treated with AFB1, ALE, and their combination for 42 days. Abbreviations: TNFα: tumor necrosis factors α; IDO: indoleamine-pyrrole 2,3-dioxygenase; TIMP3: tissue inhibitor metallopeptidase; for other explanations, see Figure 1.
TNF-α is a proinflammatory cytokine that is upregulated in response to various injuries in the hippocampi of the ischemic brain [38] and chronically stressed rats [39]. Consistent with results of previous studies, exposure to AFB1 in the present study affects the concentrations of various inflammatory markers in brains of rats, including proinflammatory cytokines (TNF-α) and tissue inhibitor of metalloproteinases (TIMP3). Results of previous studies have provided evidence that TNF can protect neurons by encouraging the expression of antiapoptotic and antioxidative proteins [40]. Furthermore, experiments with TNF-deficient mice indicate that although TNF has a deleterious effect during the acute response in a traumatized brain, it also plays a crucial part in both the long-term behavioral recovery and the histological repair of the tissues. Alterations of the IDO pathway have also been reported in several neurological diseases, including stroke, multiple sclerosis, Parkinson, Alzheimer, and schizophrenia [41], similar to our experiment. It has been documented that TIMP3 is involved in regulating cellular processes, such as cell proliferation, apoptosis, and angiogenesis [42]. Combined exposure to ALE and aflatoxin B1 (ALE+AFB1) confirmed the beneficial effect of ALE on these parameters by alleviating the toxic damage caused by AFB1 alone and restoring their normal values.
Phenolic acids, also CGA, can reduce tissue inflammation and apoptosis of nerve cells and stimulate brain cell protection [43]. The latest research by Shah et al. [44] showed that CGA could counteract oxidative stress in the ischemic cerebral cortex, lower proinflammatory protein expression, and reduce histopathological changes. The anti-inflammatory effect of CGA was related to the regulation of nuclear factor kappa B (NF-κB), IL-1β, and TNF-α. Our research shows that artichoke extract, rich in phenolic acids, has neuroprotective effects through its influence on pro-inflammatory proteins.
3.5. Oxidative Stress Level and Antioxidant Enzyme Activity
While aflatoxin B1 led to threefold increase in concentration of TBARS (34.8 ± 2.34 nmol/mg protein), ALE caused a significant reduction of this parameter (8.84 ± 0.88 pg/mg protein) in brains of rats compared to control (11.8 ± 0.8 pg/mg protein) (Figure 5(a)). Exposure to a combination of AFB1 and ALE (ALE+AFB1) reduced concentrations of TBARS (16.7 ± 1.4 pg/mg protein), compared to the aflatoxin B1-treated group (34.8 ± 2.34 pg/mg protein). However, the concentration remained greater than in the control group. The xanthine oxidase (XO) activity and concentration of uric acid (UA) were consistent with the TBRAS pattern and were significantly greater in rats exposed to AFB1 (XO: 67.9 ± 2.7 μmol/h/mg protein and UA: 4.7 ± 0.4 mg/mg protein), compared to controls (XO: 39.5 ± 2.9μmol/h/mg protein and UA: 2.3 ± 0.3 mg/mg protein) and all other groups (Figures 4(b) and 4(c)). The treatment of rats by AFB1 and ALE resulted in a reduction of XO activity (40.1 ± 4.1 μmol/h/mg protein) and UA concentration (2.9 ± 0.3 mg/mg protein) compared to the rats from the AFB1 group (XO: 67.9 ± 2.7 μmol/h/mg protein and UA: 4.7 ± 0.4 mg/mg protein).
Figure 5.
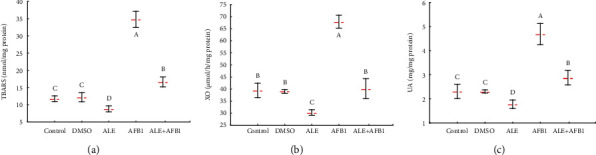
Mean ± SD concentration of TBARS (a), activity of XO (b), and concentration of UA (c) in the brain of male rats treated with AFB1, ALE, and their combination for 42 days. Abbreviations: TBARS: thiobarbituric acid reactive substances; XO: xanthine oxidase; UA: uric acid; for other explanations, see Figure 1.
Exposure of rats to ALE alone caused a significantly greater concentrations of glutathione (GSH: 4.9 ± 0.1 vs. control: 3.9 ± 0.1 μmol/mg protein) and activities of superoxide dismutase (SOD: 11.6 ± 0.8 vs. control: 9.0 ± 0.7 nmol/min/mg protein), glutathione S-transferase (GST: 0.8 ± 0.04 vs. control: 0.6 ± 0.04 nmol/min/mg protein), and glutathione peroxidase (GPx: 0.21 ± 0.006 vs. control: 0.17 ± 0.02 U/mg protein). Aflatoxin B1 had the opposite effect on these parameters. After 42 days of exposure to AFB1, there was significantly less concentration of GSH (2.8 ± 0.2 μmol/mg protein) and lesser activities of SOD (5.7 ± 0.7 nmol/min/mg protein), GST (0.4 ± 0.02 nmol/min/mg protein), and GPx (0.1 ± 0.02 U/mg protein), compared to all other groups. In rats exposed to AFB1 and ALE simultaneously, the concentration of GSH (3.5 ± 0.3 μmol/mg protein) and activities of SOD (8.8 ± 0.6 nmol/min/mg protein), GST (0.6 ± 0.1 nmol/min/mg protein), and GPx (0.16 ± 0.02 U/mg protein) did not differ from values of unexposed rats (GSH: 3.9 ± 0.1 μmol/mg protein; SOD: 9.0 ± 0.7 nmol/min/mg protein; GST: 0.6 ± 0.04 nmol/min/mg protein; and GPx: 0.17 ± 0.02 U/mg protein, respectively) (Figure 6). Consistent with the result of the present study, Surai [45] reported that AFB1 induced damage at both the cellular and tissue levels via the production of free radicals and lipid peroxides. Furthermore, Antonyak and Koval [46] revealed that SOD activity decreased compared to control values following AFB1 administration, while TBARS increased significantly in brain homogenates of experimental animals' group.
Figure 6.
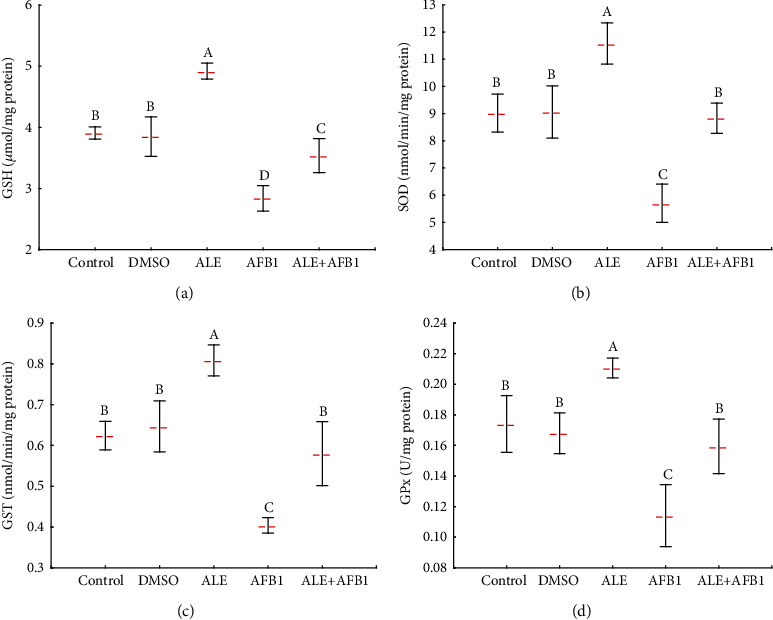
Mean ± SD concentration of GSH (a) and activity of SOD (b), GST (c), and GPx in the brain of male rats treated with AFB1, ALE, and their combination for 42 days. Abbreviations: GSH: glutathione reduced form; SOD: superoxide dismutase; GST: glutathione S-transferase; GPx: glutathione peroxidase; for other explanations, see Figure 1.
Results of the present study were also consistent with those of previous studies that found that treatment with AFB1 leads to increased oxidative stress markers, including TBARS and decreased activities of different antioxidant enzymes, including GSH, SOD, GST, and GPx [47]. Also, oxidative stress catalyzes both hypoxanthine's conversion to xanthine and, in turn, to uric acid [48]. Uric acid is a potent plasma antioxidant that scavenges singlet oxygen, peroxy radicals, and hydroxyl radicals and has been studied extensively in many physiological and pathological systems, including neurodegenerative diseases [49]. Consistent with findings of the study, results of which are reported here, previous studies have found exposure to AFB1 caused harmful effects that cause depletion of the antioxidant defense system leading to oxidative stress and a reduction of amounts of GSH and activities of SOD and GST [50, 51]. Also, AFB1 exposure caused an obvious rise in XO levels.
In this study, exposure to ALE alone antioxidant content TBARS and XO levels was less than those in the control, but exposure to AFB1 resulted in lesser activities of variable antioxidant enzymes in rats. Simultaneous use of ALE together with AFB1 brings the levels of these parameters to control ones. This result is consistent with previous studies revealing that antioxidants may protect tissue from damage by adjusting the detoxification and promoting antioxidant systems [52, 53]. Other studies have shown that phenolic compounds, including CGA, exhibit neuroprotective effects by enhancing the antioxidant defense. This was the case with aluminum-induced [54] or arsenite-induced neurotoxicity in mice [55]. In the experiments, CGA supplementation led to a reduction in the neurotoxicity of the mentioned xenobiotics, which was manifested by an increase in activity of SOD, CAT, GPx, GST, GR, and AChE [54, 55]. In our study, the protective effect of ALE against AFB1 is also due to the enhancement of antioxidative defense, which finally contributes to the neuroprotective effect.
3.6. Acetylcholinesterase and Monoamine Oxidase Activity and Nitric Oxide and Concentrations of Urea
Administration of ALE to rats did not affect acetylcholinesterase (AChE: 7.6 ± 0.6 vs. control: 7.2 ± 0.5 μmol/min/mg protein) and monoamine oxidase (MAO: 11470.0 ± 983.3 vs. control: 11049.1 ± 781.3 U/mg protein) activity. At the same time, aflatoxin B1 caused a significant increase in the activity of both enzymes (AChE: 14.5 ± 2.1 μmol/min/mg protein and MAO: 16435.8 ± 683.6 U/mg protein). AChE (9.4 ± 0.8 μmol/min/mg protein) and MAO (12890.8 ± 1578.8 U/mg protein) activity in the rats from the ALE+AFB1 group was significantly lower than those in rats exposed to ABF1 alone (AChE: 14.5 ± 2.1 μmol/min/mg protein and MAO: 16435.8 ± 683.6 U/mg protein), but still greater than that of the control group (AChE : 7.2 ± 0.5 μmol/min/mg protein and MAO: 11049.1 ± 781.3 U/mg protein) (Figures 7(a) and 7(b)). ALE significantly increased nitric oxide concentration (NO: 6.5 ± 1.0 vs. control: 4.9 ± 0.3 nmol/mg protein) in brains of rats, while exposure to AFB1 alone (4.6 ± 0.8 nmol/mg protein) did not affect this parameter. Exposure to both factors simultaneously (ALE+AFB1) caused a significant increase in concentrations of NO (6.0 ± 0.3 nmol/mg protein), compared to the control group (4.9 ± 0.3 nmol/mg protein) (Figure 7(c)). Neither exposure to AFB1 alone nor ALE had significant had significant effects on concentrations of urea in brains of rat (control: 8.7 ± 1.6; ALE: 8.6 ± 2.0; AFB1: 8.1 ± 1.1; and ALE+AFB1: 9.6 ± 1.1 mg/dL) (Figure 7(d)).
Figure 7.
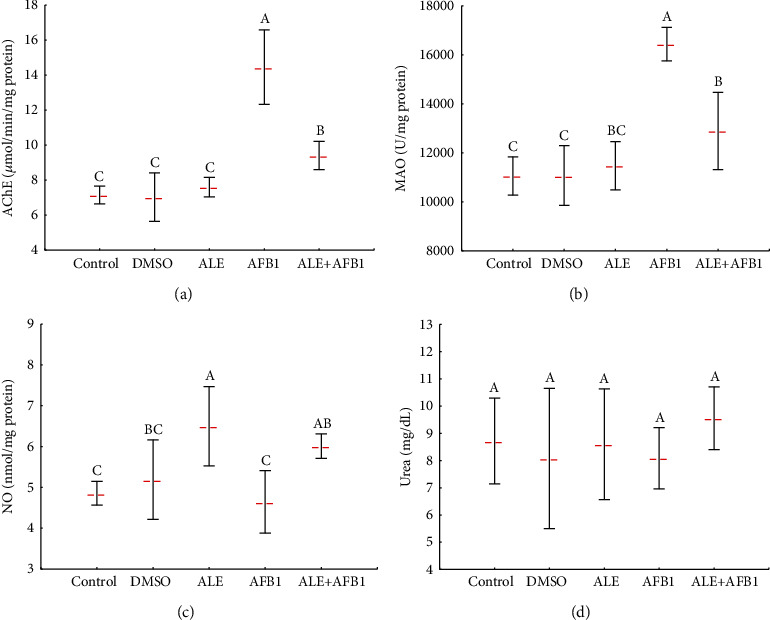
Mean ± SD activity of AChE (a) and MAO (b) and concentration of NO (c) and urea (d) in the brain of male rats treated with AFB1, ALE, and their combination for 42 days. Abbreviations: AChE: acetylcholinesterase; MAO: monoamine oxidase; NO: nitric oxide; for other explanations, see Figure 1.
Cholinergic dysfunction is a prime sign of neurotoxicity. Herein, the toxicity of AFB1 in the brain was characterized by a significant increase in AChE activity, which is in agreement with results of previous studies [56]. Such increase in AChE activity due to exposure to AFB1 can be attributed to increased production of ROS that has been found to enhance the peroxidation of the plasma membrane, thereby affecting the integrity and functionality of the cholinergic system [57]. Exposure of rats to AFB1 significantly enhanced activity of MAO in the brain. MAO was previously shown to augment oxidative stress [58]. Simultaneous exposure to ALE and AFB1 in the present study supports the normalization of both enzyme activity to values close to the control group. The ameliorative effect of ALE may be attributed mainly to its antioxidant action and its ability to scavenge free radicals.
3.7. Relationships among Parameters
To estimate the relationship among all studied parameters, a PCA analysis was performed for all of them together (Figure 8) where the principal component 1 (PC 1) explained nearly 70% of the total variability. PC 1 was strongly created by GSH, HDL, GST, GPx, SOD, and TIMP3 (PC 1 positive values). However, for LDL, MAO, glucose, cholesterol, total lipids, UA, XO, TBARS, HOMA-IR, TNFα, and IDO1, negative PC values 1 were obtained. AChE, insulin, VLDL, and triglycerides had less effect on PC 1, while NO and urea were only weakly related to it. PC2 explained a little more than 10% of the overall variability in the data. Overall, the developed PCA model (PC1+PC2) explained approximately 80% of all parameters' variability (Figure 8(a)).
Figure 8.
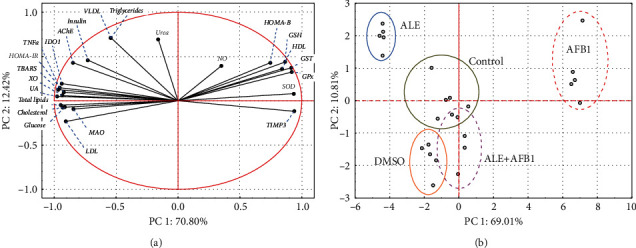
Principal component analysis (PCA) to evaluate similarities among all the parameters measured in the brain or plasma of male rats treated with AFB1 (aflatoxin B1-72 μg/kg body weight), ALE (artichoke leaves extract -100 mg/kg body weight), and their combination for 42 days. (a) PCA analysis of variables. (b) PCA analysis of cases. Abbreviations: see Figures 1–6.
A case-based PCA analysis identified three clusters (Figure 8(b)). The first cluster was created by data obtained from aflatoxin B1-treated rats. The second cluster, located on the opposite side of the scale and visibly separated from the others, included data from the group treated with ALE. The third cluster, however, quite scattered in the 2D plot space, was made up of data from control, DMSO, and ALE+AFB1 groups. There was no clear division between these three groups.
Results of the PCA analysis confirm that exposure of rats to AFB1 significantly influences the parameters discussed. The use of ALE also has a significant effect on animals, and this effect is opposite to that caused by aflatoxin. It can be assumed that the organism's functions are improved in AFB1+ALE-treated rats that showed reestablishment of almost all studied parameters. The combined exposure to AFB1 and ALE caused a return to a state similar to the control group.
3.8. Brain Histology
The brain sections were assessed histologically using the hematoxylin and eosin (H&E) staining method to determine the effects of AFB1, ALE alone, and in combination. Representative pictures were taken at 4x, 20x, and 40x magnification from each treated group. The assessment focused on the histopathological changes in the hippocampus and its corresponding dentate gyrus (DG). The hippocampus consists of the twisted dentate gyrus with an open concave part directed to the hippocampus. The hippocampus is divided into three subfields: cornu ammonis 1 (CA1), cornu ammonis 2 (CA2), and cornu ammonis 3 (CA3) (Figure 8 – control group). The CA1 region of the hippocampus consists of three layers: stratum pyramidalis (Sp), stratum radiatum (Sr), and stratum oriens (So). The primary cell type of striatum pyramidalis is composed of pyramidal cells that are characterized by well-demarcated rounded nuclei and prominent nucleoli (Figure 9 – control group). Unexposed rats and those exposed to ALE histological structures of the hippocampus and dentate gyrus were similar and determined to be normal, while the group treated with AFB1 exhibited cellular disorganization and degeneration manifested by darkly stained nuclei and extensive vacuolations with pyknotic hyperchromatic eccentric nuclei (Figure 9 – AFB1 group). Significantly, the treatment of AFB1-exposed rats with artichoke extract relieved these pathological changes (Figure 9 – ALE+AFB1 group).
Figure 9.
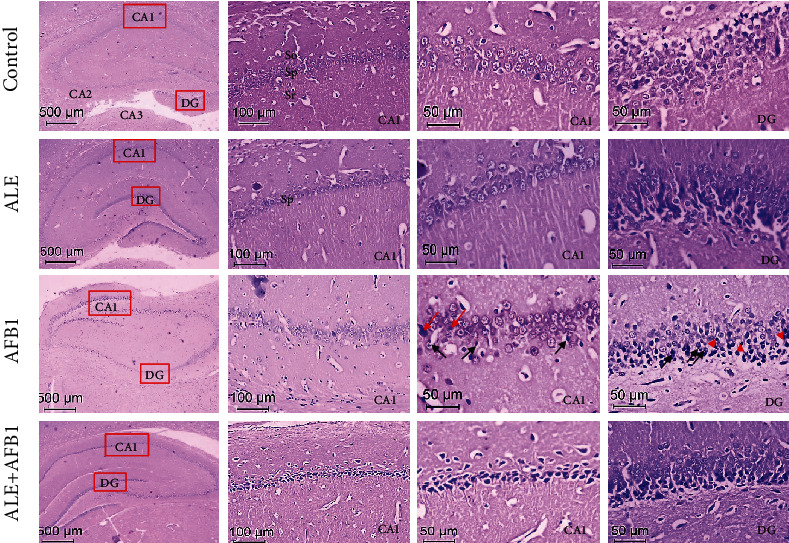
Representative H&E-stained brain samples of male rats from the control group, ALE group (artichoke leaves extract -100 mg/kg body weight), AFB1 group (aflatoxin B1 -72 μg/kg body weight), and ALE+AFB1 group (artichoke leaves extract -100 mg/kg body weight+aflatoxin B1-72 μg/kg body weight). Abbreviations: CA1, 2, 3: cornu ammonis 1, 2, 3; DG: dentate gyrus; Sp: stratum pyramidalis; Sr: stratum radiatum; So: stratum oriens; for other explanations, see Figure 1.
Histopathological assessment of the dentate gyrus in rats exposed to AFB1 exhibited a decrease in the dentate gyrus's granule cell layer thickness compared to the control group. Such a decrease in thickness of the dentate gyrus's main layer results mainly from the degenerative changes of nerve cells in this area. Moreover, glial proliferation, where microglia cells' infiltration was observed, was well manifested in the sections after exposure to AFB1 exposure. The ALE+AFB1 group revealed an improvement compared to the group treated with Aflatoxin B1 alone.
Herein, the AFB1-induced neurotoxicity as manifested by free radicals' augmentation, cholinergic enzymes, lipid profile, TNF-α, and IDO, finds its confirmation in histopathological architecture. Degenerative changes in the hippocampus's pyramid cells and nerve cells in the dentate gyrus were observed. Moreover, inflammatory infiltrations of microglia cells and macrophages were reported. Simultaneous use of ALE with AFB1 (ALE+AFB1 group) attenuates almost all histopathological alterations induced by a single exposure to AFB1. Results of previous studies are consistent with the results observed in this study [59–61].
To sum up, differential concentration of a variety of phenolic compounds has been reported in the artichoke leaf extract capsule in this study. CGA, which is the highly concentrated component in the extract, has been found previously to have several pharmacological properties including antioxidant, anti-inflammatory, lipid lowering, hypoglycemic, antibacterial, antiviral, cardioprotective, anticancer, and immunomodulatory. Therefore, the attenuation of the adverse effects of AFB1 as well as health promotion could be attributable to the phenolic composition of the artichoke leaf extract.
4. Conclusions
Results of the study presented here demonstrate adverse effects of AFB1 on brain function. Overproduction of free radicals manifests AFB1-induced neurotoxicity, dysregulation of lipid profile, glucose level, and insulin concentration, HOMA-IR, TNF-α, and IDO, increased activity of AChE and MAO, and inhibition of the antioxidant enzymes, HOMA-B, and TIMP3. These processes intensify oxidative stress. The factors used in this study did not have a significant effect on the urea concentration in brains of rats. Moreover, changes in histology of brain are visible after exposure to AFB1. ALE, a potent antioxidant, reduced free radical production and increased antioxidant enzyme activity. These ALE-mediated effects are significant in overcoming oxidative stress. Moreover, joint treatment using ALE and AFB1 alleviates the histopathological features induced by the exposure to AFB1. ALE can be successfully considered as a possible nominee for controlling neurotoxicity resulting from food and feed contaminated with AFB1, which causes numerous harmful effects on humans and animals' health. The phenolic compounds present in natural, balanced proportions in ALE have beneficial effects on the brain. The neuroprotective effect of ALE involves the regulation of lipid metabolism and the alleviation of inflammation and oxidative stress.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research, King Saud University, for funding through Vice Deanship of Scientific Research Chairs and Research Chair of Medical and Molecular Genetics. The authors are grateful to Dr. Nashwa W. Yassa at the Bioscreening and Preclinical Trial Lab, Biochemistry Department, Faculty of Science, Alexandria University, Egypt, and the Biochemistry Department, Faulty of Science, Alexandria University, Egypt, for her support and collaboration during this study. Prof. Giesy was supported by the Canada Research Chair program, a Distinguished Visiting Professorship in the Department of Environmental Sciences, Baylor University in Waco, TX, USA.
Data Availability
All data related to the MS are provided in this work.
Ethical Approval
All animal procedures were conducted in accordance with the local Guiding Principles for the Care and Use of Laboratory Animals as adopted and promulgated by Alexandria University.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
Authors' Contributions
Enas A. Ibrahim was responsible for methodology, data curation, formal analysis, investigation, and writing of original draft. Mokhtar I. Yousef performed conceptualization, project administration, validation, formal analysis, investigation, and supervision and reviewed the draft. Doaa A. Ghareeb performed methodology, formal analysis, and supervision and revised the draft. Maria Augustyniak was responsible for validation and formal statistical analysis, wrote the original draft, and reviewed and edited the draft. John P. Giesy wrote, reviewed, and edited the manuscript. Mourad A. M. Aboul-Soud wrote, reviewed, and edited the manuscript. Abeer El Wakil carried out conceptualization, validation, formal analysis, investigation, and supervision, wrote the original draft, and reviewed and edited the draft.
References
- 1.Al Ayoubi M., Solfrizzo M., Gambacorta L., Watson I., El Darra N. Risk of exposure to aflatoxin B1, ochratoxin A, and fumonisin B1 from spices used routinely in Lebanese cooking. Food and Chemical Toxicology . 2021;147, article 111895 doi: 10.1016/j.fct.2020.111895. [DOI] [PubMed] [Google Scholar]
- 2.Andrade P. D., Dias J. V., Souza D. M., et al. Mycotoxins in cereals and cereal-based products: incidence and probabilistic dietary risk assessment for the Brazilian population. Food and Chemical Toxicology . 2020;143, article 111572 doi: 10.1016/j.fct.2020.111572. [DOI] [PubMed] [Google Scholar]
- 3.Legan J. D. Cereals and cereal products. The Microbiological Safety and Quality of Food . 2000;1:759–783. [Google Scholar]
- 4.Mir S. A., Dar B. N., Shah M. A., et al. Application of new technologies in decontamination of mycotoxins in cereal grains: challenges, and perspectives. Food and Chemical Toxicology . 2021;148, article 111976 doi: 10.1016/j.fct.2021.111976. [DOI] [PubMed] [Google Scholar]
- 5.Li Y., Wang J., Li J., Sun X. An improved overall risk probability-based method for assessing the combined health risks of chemical mixtures: an example about mixture of aflatoxin B1 and microcystin LR by dietary intake. Food and Chemical Toxicology . 2020;146, article 111815 doi: 10.1016/j.fct.2020.111815. [DOI] [PubMed] [Google Scholar]
- 6.Rushing B. R., Selim M. I. Aflatoxin B1: a review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food and Chemical Toxicology . 2019;124:81–100. doi: 10.1016/j.fct.2018.11.047. [DOI] [PubMed] [Google Scholar]
- 7.Zhihua L., Gong Y., Holmes M., et al. Geospatial visualisation of food contaminant distributions: polychlorinated naphthalenes (PCNs), potentially toxic elements (PTEs) and aflatoxins. Chemosphere . 2019;230:559–566. doi: 10.1016/j.chemosphere.2019.05.080. [DOI] [PubMed] [Google Scholar]
- 8.Wu F. Mycotoxin reduction in Bt corn: potential economic, health, and regulatory impacts. Transgenic Research . 2006;15(3):277–289. doi: 10.1007/s11248-005-5237-1. [DOI] [PubMed] [Google Scholar]
- 9.Gugliandolo E., Peritore A. F., D’Amico R., Licata P., Crupi R. Evaluation of neuroprotective effects of quercetin against aflatoxin B1-intoxicated mice. Animals . 2020;10(5):p. 898. doi: 10.3390/ani10050898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sultana R., Perluigi M., Butterfield D. A. Protein oxidation and lipid peroxidation in brain of subjects with Alzheimer‘s disease: insights into mechanism of neurodegeneration from redox proteomics. Antioxidants & Redox Signaling . 2006;8(11-12):2021–2037. doi: 10.1089/ars.2006.8.2021. [DOI] [PubMed] [Google Scholar]
- 11.Wu T. S., Cheng Y. C., Chen P. J., Huang Y. T., Yu F. Y., Liu B. H. Exposure to aflatoxin B1 interferes with locomotion and neural development in zebrafish embryos and larvae. Chemosphere . 2019;217:905–913. doi: 10.1016/j.chemosphere.2018.11.058. [DOI] [PubMed] [Google Scholar]
- 12.Parmar H., Sharma P., Anerao I., Roy H., Roy H. Aflatoxin B1 induced developmental nephrotoxicity in Rir egg. Pelagia Res Libr Adv Appl Sci Res . 2016;7:23–27. [Google Scholar]
- 13.El-Ashram S., El-Samad L. M., Basha A. A., El Wakil A. Naturally-derived targeted therapy for wound healing: beyond classical strategies. Pharmacological Research . 2021;170, article 105749 doi: 10.1016/j.phrs.2021.105749. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y., Yang S., Wang K., et al. Cellular senescence and cancer: focusing on traditional Chinese medicine and natural products. Cell Proliferation . 2020;53(10, article e12894) doi: 10.1111/cpr.12894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lattanzio V., Kroon P. A., Linsalata V., Cardinali A. Globe artichoke: a functional food and source of nutraceutical ingredients. Journal of Functional Foods . 2009;1(2):131–144. doi: 10.1016/j.jff.2009.01.002. [DOI] [Google Scholar]
- 16.Salem M. B., Affes H., Ksouda K., et al. Pharmacological studies of artichoke leaf extract and their health benefits. Plant Foods for Human Nutrition . 2015;70(4):441–453. doi: 10.1007/s11130-015-0503-8. [DOI] [PubMed] [Google Scholar]
- 17.Ceccarelli N., Curadi M., Picciarelli P., Martelloni L., Sbrana C., Giovannetti M. Globe artichoke as a functional food. Mediterranean Journal of Nutrition and Metabolism . 2010;3(3):197–201. doi: 10.3233/s12349-010-0021-z. [DOI] [Google Scholar]
- 18.Gebhardt R. Anticholestatic activity of flavonoids from artichoke (Cynara scolymus L.) and of their metabolites. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research . 2001;7:316–320. [PubMed] [Google Scholar]
- 19.Mahady G. B. Medicinal plants for the prevention and treatment of coronary heart disease. Ethnopharmacology . 2009;2:75–99. [Google Scholar]
- 20.Friedewald W. T., Levy R. I., Fredrickson D. S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clinical Chemistry . 1972;18(6):499–502. doi: 10.1093/clinchem/18.6.499. [DOI] [PubMed] [Google Scholar]
- 21.Matthews D. R., Hosker J. P., Rudenski A. S., Naylor B. A., Treacher D. F., Turner R. C. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia . 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 22.Fawcett J. K., Scott J. E. A rapid and precise method for the determination of urea. Journal of Clinical Pathology . 1960;13(2):156–159. doi: 10.1136/jcp.13.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jollow D. J., Mitchell J. R., Zampaglione N., Gillette J. R. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology . 1974;11(3):151–169. doi: 10.1159/000136485. [DOI] [PubMed] [Google Scholar]
- 24.Litwack G., Bothwell J. W., Williams J. N., Jr., Elvehjem C. A. A colorimetric assay for xanthine oxidase in rat liver homogenates. Journal of Biological Chemistry . 1953;200(1):303–310. [PubMed] [Google Scholar]
- 25.Ellman G. L., Courtney K. D., Andres V., Jr., Featherstone R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochemical Pharmacology . 1961;7(2):88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 26.Hallman J., Oreland L., Edman G., Schalling D. Thrombocyte monoamine oxidase activity and personality traits in women with severe premenstrual syndrome. Acta Psychiatrica Scandinavica . 1987;76(3):225–234. doi: 10.1111/j.1600-0447.1987.tb02890.x. [DOI] [PubMed] [Google Scholar]
- 27.Naveed M., Hejazi V., Abbas M., et al. Chlorogenic acid (CGA): a pharmacological review and call for further research. Biomedicine & Pharmacotherapy . 2018;97:67–74. doi: 10.1016/j.biopha.2017.10.064. [DOI] [PubMed] [Google Scholar]
- 28.Roux A., Muller L., Jackson S. N., et al. Mass spectrometry imaging of rat brain lipid profile changes over time following traumatic brain injury. Journal of Neuroscience Methods . 2016;272:19–32. doi: 10.1016/j.jneumeth.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenwood C. E., Young S. N. Dietary fat intake and the brain: a developing frontier in biological psychiatry. Journal of Psychiatry and Neuroscience . 2001;26(3, article 182) [PMC free article] [PubMed] [Google Scholar]
- 30.Kalman J., Kudchodkar B. J., Krishnamoorthy R., Dory L., Lacko A. G., Agarwal N. High cholesterol diet down regulates the activity of activator protein-1 but not nuclear factor-kappa B in rabbit brain. Life Sciences . 2001;68(13):1495–1503. doi: 10.1016/S0024-3205(01)00955-9. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez de Sotillo D. V., Hadley M. Chlorogenic acid modifies plasma and liver concentrations of: cholesterol, triacylglycerol, and minerals in (fa/fa) Zucker rats. The Journal of Nutritional Biochemistry . 2002;13(12):717–726. doi: 10.1016/s0955-2863(02)00231-0. [DOI] [PubMed] [Google Scholar]
- 32.Kono Y., Kashine S., Yoneyama T., Sakamoto Y. Iron chelation by chlorogenic acid as a natural antioxidant. Bioscience, Biotechnology, And Biochemistry . 1998;62(1):22–27. doi: 10.1271/bbb.62.22. [DOI] [PubMed] [Google Scholar]
- 33.Wan C. W., Wong C. N., Pin W. K., et al. Chlorogenic acid exhibits cholesterol lowering and fatty liver attenuating properties by up-regulating the gene expression of PPAR-α in hypercholesterolemic rats induced with a high-cholesterol diet. Phytotherapy Research . 2013;27(4):545–551. doi: 10.1002/ptr.4751. [DOI] [PubMed] [Google Scholar]
- 34.De La Monte S. M. Metabolic derangements mediate cognitive impairment and Alzheimer's disease: role of peripheral insulin-resistance diseases. Panminerva Medica . 2012;54(3):171–178. [PMC free article] [PubMed] [Google Scholar]
- 35.Luchsinger J. A. Adiposity, hyperinsulinemia, diabetes and Alzheimer's disease: an epidemiological perspective. European Journal of Pharmacology . 2008;585(1):119–129. doi: 10.1016/j.ejphar.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hemmerle H., Burger H. J., Below P., et al. Chlorogenic acid and synthetic chlorogenic acid derivatives: novel inhibitors of hepatic glucose-6-phosphate translocase. Journal of Medicinal Chemistry . 1997;40(2):137–145. doi: 10.1021/jm9607360. [DOI] [PubMed] [Google Scholar]
- 37.Chen L., Teng H., Cao H. Chlorogenic acid and caffeic acid from Sonchus oleraceus Linn synergistically attenuate insulin resistance and modulate glucose uptake in HepG2 cells. Food and Chemical Toxicology . 2019;127:182–187. doi: 10.1016/j.fct.2019.03.038. [DOI] [PubMed] [Google Scholar]
- 38.Chen S., Yin Z. J., Jiang C., et al. Asiaticoside attenuates memory impairment induced by transient cerebral ischemia-reperfusion in mice through anti-inflammatory mechanism. Pharmacology, Biochemistry, and Behavior . 2014;122:7–15. doi: 10.1016/j.pbb.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 39.You Z., Luo C., Zhang W., et al. Pro- and anti-inflammatory cytokines expression in rat's brain and spleen exposed to chronic mild stress: involvement in depression. Behavioural Brain Research . 2011;225(1):135–141. doi: 10.1016/j.bbr.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 40.Scherbel U., Raghupathi R., Nakamura M., et al. Differential acute and chronic responses of tumor necrosis factor-deficient mice to experimental brain injury. Proceedings of the National Academy of Sciences . 1999;96(15):8721–8726. doi: 10.1073/pnas.96.15.8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fatokun A. A., Hunt N. H., Ball H. J. Indoleamine 2,3-dioxygenase 2 (IDO2) and the kynurenine pathway: characteristics and potential roles in health and disease. Amino Acids . 2013;45(6):1319–1329. doi: 10.1007/s00726-013-1602-1. [DOI] [PubMed] [Google Scholar]
- 42.Qi J. H., Ebrahem Q., Moore N., et al. A novel function for tissue inhibitor of metalloproteinases-3 (TIMP3): inhibition of angiogenesis by blockage of VEGF binding to VEGF receptor-2. Nature Medicine . 2003;9(4):407–415. doi: 10.1038/nm846. [DOI] [PubMed] [Google Scholar]
- 43.Tian L., Su C. P., Wang Q., et al. Chlorogenic acid: a potent molecule that protects cardiomyocytes from TNF-α-induced injury via inhibiting NF-κB and JNK signals. Journal of Cellular and Molecular Medicine . 2019;23(7):4666–4678. doi: 10.1111/jcmm.14351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shah M. A., Kang J. B., Park D. J., Kim M. O., Koh P. O. Chlorogenic acid alleviates cerebral ischemia-induced neuroinflammation via attenuating nuclear factor kappa B activation. Neuroscience Letters . 2022;773, article 136495 doi: 10.1016/j.neulet.2022.136495. [DOI] [PubMed] [Google Scholar]
- 45.Surai P. F. Natural Antioxidants in Avian Nutrition and Reproduction . Nottingham: Nottingham University Press; 2002. [Google Scholar]
- 46.Antonyak H., Koval N. Effects of aflatoxin B1 on lipid peroxidation and activities of antioxidant enzymes in rat organs and erythrocytes. Visnyk of Lviv University. Biological Series . 2015;69:41–48. [Google Scholar]
- 47.Rotimi O. A., Rotimi S. O., Oluwafemi F., Ademuyiwa O., Balogun E. A. Oxidative stress in extra-hepatic tissues of rats co-exposed to aflatoxin B1 and low protein diet. Toxicological Research . 2018;34(3):211–220. doi: 10.5487/TR.2018.34.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hille R., Nishino T. Xanthine oxidase and xanthine dehydrogenase. The FASEB Journal . 1995;9(11):995–1003. doi: 10.1096/fasebj.9.11.7649415. [DOI] [PubMed] [Google Scholar]
- 49.Settle T., Klandorf H. The role of uric acid as an antioxidant in selected neurodegenerative disease pathogenesis: a short review. Brain Disorders Therapy . 2014;3(3, article 129) doi: 10.4172/2168-975X.1000129. [DOI] [Google Scholar]
- 50.Aleissa M. S., Alkahtani S., Abd Eldaim M. A., et al. Fucoidan ameliorates oxidative stress, inflammation, DNA damage, and hepatorenal injuries in diabetic rats intoxicated with aflatoxin B1. Oxidative Medicine and Cellular Longevity . 2020;2020:10. doi: 10.1155/2020/9316751.9316751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alsayyah A., ElMazoudy R., Al-Namshan M., Al-Jafary M., Alaqeel N. Chronic neurodegeneration by aflatoxin B1 depends on alterations of brain enzyme activity and immunoexpression of astrocyte in male rats. Ecotoxicology and Environmental Safety . 2019;182, article 109407 doi: 10.1016/j.ecoenv.2019.109407. [DOI] [PubMed] [Google Scholar]
- 52.Rawal S., Kim J. E., Coulombe R., Jr. Aflatoxin B1 in poultry: toxicology, metabolism and prevention. Research in Veterinary Science . 2010;89(3):325–331. doi: 10.1016/j.rvsc.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 53.Song S., Zhao L., Feng L., Wang W., Cong T., He H. Using aging rats model to investigate anti-oxidative ability of artichoke (Cynara scolymus L.) leaf extract. Acta Horticulturae . 2012;944, article 113 [Google Scholar]
- 54.Wang X. M., Xi Y., Zeng X. Q., Zhao H. D., Cao J. K., Jiang W. B. Effects of chlorogenic acid against aluminium neurotoxicity in ICR mice through chelation and antioxidant actions. Journal of Functional Foods . 2018;40:365–376. doi: 10.1016/j.jff.2017.11.013. [DOI] [Google Scholar]
- 55.Metwally D. M., Alajmi R. A., El-Khadragy M. F., et al. Chlorogenic acid confers robust neuroprotection against arsenite toxicity in mice by reversing oxidative stress, inflammation, and apoptosis. Journal of Functional Foods . 2020;75, article 104202 doi: 10.1016/j.jff.2020.104202. [DOI] [Google Scholar]
- 56.Chtourou Y., Gargouri B., Kebieche M., Fetoui H. Naringin abrogates cisplatin-induced cognitive deficits and cholinergic dysfunction through the down-regulation of AChE expression and iNOS signaling pathways in hippocampus of aged rats. Journal of Molecular Neuroscience . 2015;56(2):349–362. doi: 10.1007/s12031-015-0547-0. [DOI] [PubMed] [Google Scholar]
- 57.Melo J. B., Agostinho P., Oliveira C. R. Involvement of oxidative stress in the enhancement of acetylcholinesterase activity induced by amyloid beta-peptide. Neuroscience Research . 2003;45(1):117–127. doi: 10.1016/s0168-0102(02)00201-8. [DOI] [PubMed] [Google Scholar]
- 58.Ooi J., Hayden M. R., Pouladi M. A. Inhibition of excessive monoamine oxidase A/B activity protects against stress-induced neuronal death in Huntington disease. Molecular Neurobiology . 2015;52(3):1850–1861. doi: 10.1007/s12035-014-8974-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hussein Y. Histological and immunohistochemical changes in the hippocampus of the adult male albino rats treated with amethopterin and the possible protective role of Moringa leaves extract. Egyptian Journal of Anatomy . 2017;40(2):294–300. doi: 10.21608/EJANA.2018.16907. [DOI] [Google Scholar]
- 60.Kaler S., Dhar P., Bhattacharya A., Mehra R. D. Preliminary morphological and immunohistochemical changes in rat hippocampus following postnatal exposure to sodium arsenite. Toxicology International . 2013;20(2):160–169. doi: 10.4103/0971-6580.117259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rosińczuk J., Dymarek R., Całkosiński I. Histopathological, ultrastructural, and immunohistochemical assessment of hippocampus structures of rats exposed to TCDD and high doses of tocopherol and acetylsalicylic acid. BioMed Research International . 2015;2015:13. doi: 10.1155/2015/645603.645603 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data related to the MS are provided in this work.


