Abstract
In the pathophysiology of COVID-19, immunomodulatory factors play a vital role. Viruses have epigenetic effects on various genes, particularly methylation. Explaining the changes in immunological factor methylation levels during viral infections requires substantial consideration. HLA-C is a crucial determinant of immune function and NK cell activity and is primarily implicated in viral infections. This research focused on studying HLA-C methylation in COVID-19 patients with different severity. Peripheral blood samples were collected from 470 patients (235 men and 235 women) with RT-qPCR-confirmed COVID-19 test and classified into moderate, severe, and critical groups based on WHO criteria. Also, one hundred (50 men and 50 women) healthy subjects were selected as the control group. Peripheral blood mononuclear cells were used for DNA extraction, and the methylation-specific PCR (MSP) method and gel electrophoresis were used to determine the methylation status of the HLA-C. Significant statistical differences in HLA-C methylation were observed among cases and controls and various stages of the disease. HLA-C methylation in men and women has decreased in all stages (p < 0.05). In comparison with control, HLA-C methylation in both genders were as follows: moderate (women: 41.0%, men: 52.33%), severe (women: 43.42%, men: 64.86%), critical (women: 42.33%, men: 60.07%), and total patients (women: 45.52%, men: 56.97%). Furthermore, the methylation levels in men were higher than in women in all groups (p < 0.05). Significantly, among all groups, the severe group of men participants showed the highest methylation percentage (p < 0.05). No significant differences were detected for different disease severity in the women group (p > 0.1). This study found that HLA-C methylation was significantly lower in COVID-19 patients with different disease severity. There were also significant differences in HLA-C methylation between men and women patients with different severity. Therefore, during managing viral infections, particularly COVID-19, it is critical to consider patient gender and disease severity.
Keywords: HLA-C, Methylation, COVID-19, Epigenetic
Introduction
Since December 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has extremely infected many people and resulted in the COVID-19 pandemic (Cucinotta and Vanelli 2020). It has been established that SARS-CoV-2 invades several organs, including the lungs, gut, heart, testis, kidneys, bladder, esophagus, liver, and brain, since various cells and tissues types express the SARS-CoV-2 receptor (ACE2: angiotensin-converting enzyme 2) (Jafarzadeh et al. 2021a, b; Sharif-zak et al. 2022). COVID-19 has numerous consequences ranging from minor to severe and possible mortality in severe cases. One of the most challenging conditions is acute respiratory distress syndrome (ARDS) development with different disease severity. According to World Health Organization (WHO), four severity stages for COVID-19 patients are defined as mild, moderate, severe, and critical concerning symptoms (Table 1). The classification is based on common, less common, and serious symptoms of patients (Jafarzadeh et al. 2021a, b). Most COVID-19 cases are mild, and the clinical manifestations emerge after a five-day latent period (Organization 2021). On the other hand, some individuals progress to the moderate, severe, and critical stages, which can result in various organ dysfunctions, including ARDS (Organization 2021).
Table 1.
Classification of patients according to their COVID-19 patterns
| Patients | Moderate | Severe | Critical |
|---|---|---|---|
| Symptoms | Pneumonia with cough, fever, dyspnea, fast breathing) and SpO2 ≥ 90% | Severe pneumonia with cough, dyspnea, fever, fast breathing), respiratory rate > 30 breaths/min; severe respiratory distress; or SpO2 < 90% | Acute respiratory distress syndrome (ARDS), Chest imaging with bilateral opacities, lobar or lung collapse, or nodules, and Oxygenation impairment |
| Male (n = 235) | 106 | 96 | 33 |
| Female (n = 235) | 130 | 70 | 35 |
| Total (n = 470) | 236 | 166 | 68 |
Several investigations have revealed different approaches for treating COVID-19 and its severity minimizing. For instance, in some severe COVID-19 cases, combining Traditional Chinese Therapy (TCM) with Western medicine appears to be a promising treatment. (Zaman et al. 2020). Lianhuaqingwen with Arbidol Hydrochloride has also been reported to be an effective treatment for COVID-19 at different stages (Khan et al., 2020). Nevertheless, the most effective treatments include antiviral drugs, supportive treatment, extracorporeal membrane oxygenation (ECMO), and plasma and oxygen therapy (Zhang et al. 2020).
Many inflammatory cells (monocytes, macrophages, lymphocytes, and neutrophils) infiltrate the respiratory system during the COVID-19 progression (Jafarzadeh et al. 2021a, b). Hyper-inflammatory responses and cytokine storm play a fundamental role in COVID-19-related complications (Choustermanet al. 2017; Mitra et al. 2020). Furthermore, several genes play a role in organ inflammatory and antiviral defense processes, influencing the clinical outcome (Pairo-Castineira et al. 2021). The human leukocyte antigen (HLA) system plays a vital role in the immune response (Cho and Gregersen 2011; Heidecker et al. 2011). HLA-C presents various antigens to cytotoxic T lymphocytes (CTLs) and may prevent the lysis of natural killer (NK) cells and CTLs, which are primary cells in viral and inflammatory diseases (Kulpa and Collins 2011).
Previous research has established a link between HLA genetic variants and a variety of viral disease symptoms, including the influenza virus SARS-CoV-1 (Lin et al. 2003), H1N1 (Dutta et al. 2018), and Hantaan virus (Ma et al. 2012). HLA-B has been reported to exacerbate SARS-CoV-2 infection (Nguyen et al. 2020), and it has also been found to promote patients with SARS-COV-1 to severe stages (Gonzalez-Galarza et al. 2010). Therefore, HLA alleles are one of the suspected factors that may be implicated in aggravating the condition of COVID-19 patients. In humans, HLA-C was formed by duplication of the HLA-C homologs and HLA-B gene (Carter 2011). In immunity defense and NK cell activity, HLA-C is a significant determinant associated with many diseases, especially viral infections (Apps et al. 2013; Israeli et al. 2014; Kulkarni et al. 2013 McINTYRE and FAUL 1983).
Epigenetics strongly influences gene expression and function, and DNA methylation is one of the most significant epigenetic alterations (Lande-Diner et al. 2007). Viral infections cause unwanted DNA methylation, which is a fundamental event in such diseases (Okamoto et al. 2014). Furthermore, DNA methylation of specific genes, especially immunomodulatory genes such as HLA-C, might reflect the viral disease severity (Okamoto et al. 2014).
Earlier research on the HLA-C gene found a substantial link between inflammatory conditions and irregular DNA methylation (Mervis and McGee 2020). Since COVID-19 is widely recognized as a severe inflammatory disease that causes multi-organ damage, it is worthwhile to evaluate the potential role of SARS-COV-2 in epigenetic changes. Given the importance of HLA-C in the induction and regulation of inflammatory responses, this case study evaluated the methylation levels of HLA-C in the peripheral blood samples collected from COVID-19 patients. In addition, this study approach clarifies the association of HLA-C methylation levels with disease severity and gender in COVID-19 patients.
Material and Methods
Subjects
This case–control study was conducted on 470 subjects (235 men and 235 women; aged 20–80 years) with quantitive real-time PCR (RT-qPCR)-confirmed COVID-19 who were referred to Imam Khomeini hospital of Jiroft (a city located in the southeast of Iran) in July 2021. Exclusion factors for the patients were a history of other inflammatory diseases, negative RT-qPCR, or personal dissatisfaction. These patients were divided into three subgroups according to the WHO guidance concerning the COVID-19-related clinical symptoms and management, including moderate, severe, and critical patients (Table 1). In addition, we selected 100 healthy individuals (50 men and 50 women) without any inflammatory diseases as the control group. Peripheral blood samples were collected from participants for DNA extraction. The Ethics Committee of Kerman University of Medical Sciences evaluated and approved the research protocol registered with IR.KMU.REC.1400.411.
DNA Extraction and Methylation-Specific PCR
According to the manufacturer's instructions, we used Favorgen Kit (Cat No. FABGK001) for DNA extraction from peripheral blood samples. To determine the quantity and purity of the extracted DNA, the absorbance of the DNA samples was measured at 260 nm and 280 nm using a NanoDrop (Thermo Scientific NanoDrop 1000 spectrophotometer). PCR assay was performed in a Biometra TOne Gradient 96 Thermal Cycler (AnalyticJena) using the Yekta Tajhiz PCR master mix kit (Cat No. YT 1551).
We used methylation-specific PCR (MS-PCR) for the methylation study, which is based on DNA reaction with sodium hydrogen sulfite (sodium bisulfite). Sodium bisulfite converts cytosine to uracil and subsequently converts to thymidine during PCR, while methylated cytosine remains unmodified (Paydar et al. 2019). Hence, extracted DNA was treated with sodium bisulfite based on the Tiwari et al. method (Tiwari et al. 2009). Then, PCR was performed to amplify modified DNA sequences with specific methylated and unmethylated primers (Table 2). Methylated primers detect unchanged cytosines. Thymines, derived from modified cytosines, are identified by unmethylated primers. PCR products were loaded on 1.6% agarose gel to perform the electrophoresis. At the end, the results were interpreted based on detecting the presence or absence of the relevant band in agarose gel. The intensity of electrophoresis bands was calculated using ImageJ software.
Table 2.
Primer sequences used for methylation-specific PCR
| Gene name | Primer sequence (5’ → 3’) sense | Primer sequence (5’ → 3’) antisense | TM (°C) | Product size |
|---|---|---|---|---|
| HLA-C Methylated | GATCGGAGAGAGTTTTAGTCG | GCTAAATAATCTAAACCGCGA | 53 | 240 |
| HLA-C Unmethylated | GATTGGAGAGAGTTTTAGTTG | AACTTACACTAAATAATCTAAACCACAA | 51 | 260 |
Statistical Analysis
All statistical analyses were carried out using SPSS software version 21 (Chicago, IL). Data were analyzed by independent-samples T-test and one-way ANOVA test following Tukey post hoc test. P values less than 0.05 were considered significant.
Results and Discussion
HLA-C Methylation Levels in Total Patients
The results were interpreted for moderate, severe, and critical groups based on the presence or absence of the relevant band in the agarose gel and represented in Fig. 1. The results highlighted that the methylation of HLA-C in the peripheral blood samples from all COVID-19 patients was significantly lower than that in the healthy control group (p < 0.05) (Fig. 2).
Fig. 1.
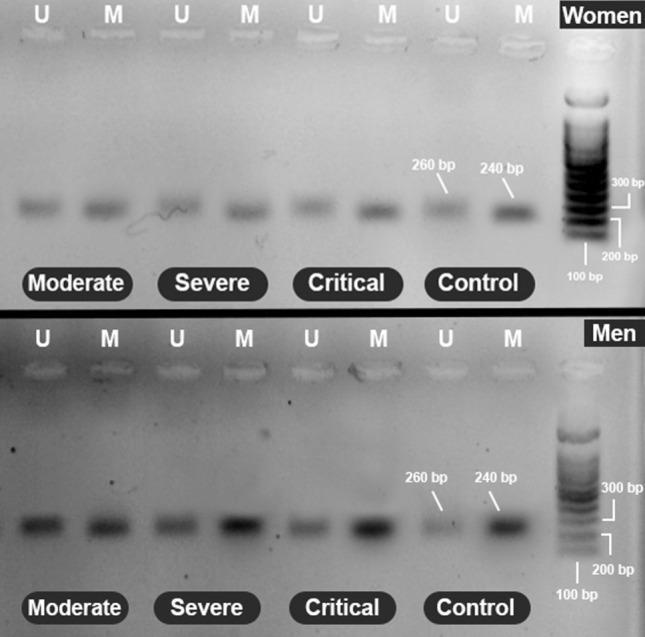
Electrophoresis picture of the methylation statuses of the HLA-C gene in women and men. The methylated band is 240 bp, and the unmethylated band is 260 bp. M: methylated band; U: unmethylated band
Fig. 2.
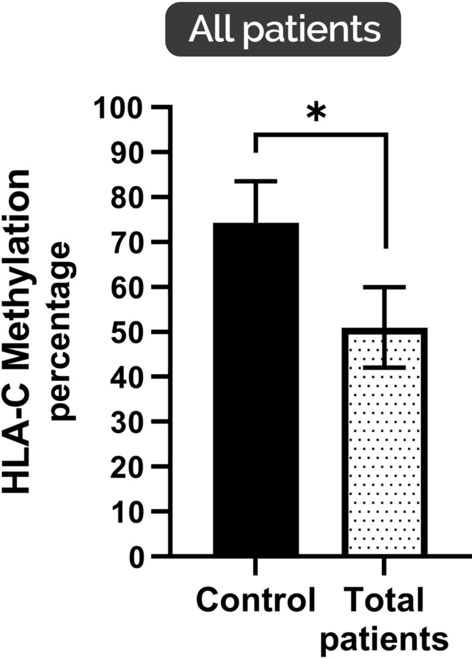
HLA-C average methylation percentage in total patients with COVID-19. Lower HLA-C methylation levels were found for COVID-19 patients than in the control group (*p < 0.05). * indicates significant difference in different groups (*p < 0.05); values represent means ± SEM
From the beginning of the COVID-19 pandemic, the relationship between genetic host factors and SARS-CoV-2 infection has been considered a serious issue. The association between severity forms of COVID-19 patients and molecular changes is crucial. Alteration in the different genes' methylation levels is a remarkable aspect of diseases and malignancies (Abolhassani et al. 2019). It is widely acknowledged that epigenetic changes and alterations in the HLA-C methylation levels occur in response to multifactors including immune response, environmental factors, etc. (Foley et al. 2009). The current study detected a significant decrease in HLA-C methylation in the DNA extracted from peripheral blood samples of all COVID-19 patients compared to the healthy control group. Different studies have shown that HLA-C in immunity defense and NK cell activity is a major determinant that is highly expressed and is associated with many diseases, especially viral infections (Papúchová et al. 2019). Overall, the studies have provided reasonably consistent evidence of an association between HLA-C and viral diseases such as hepatitis C virus (HCV) and human immunodeficiency virus (HIV), which is in line with our study (de Wit et al. 2016; Walter and Ansari 2015). It is noteworthy that HLA-C is a ligand for killer immunoglobulin-like receptors (KIRs) and, thereby, is associated with natural killer (NK) cells that are great interferons producers and implicated in both innate and acquired immunity (Chen et al. 2016; Yawata et al. 2006).
Gender Affects the HLA-C Methylation
As illustrated in Fig. 3A, the levels of HLA-C methylation were significantly decreased in women patients with COVID-19 compared to healthy women participants (p < 0.05). The analysis revealed no significant difference among women patients with severe, critical, and moderate forms of COVID-19 for HLA-C methylation (p < 0.05). Figure 3 summarizes the preliminary analysis results. It shows that HLA-C methylation levels in men with moderate, severe, and critical COVID-19 forms were lower than in the control group (p < 0.05) (Fig. 3B). However, in comparison with the healthy group, all different stages showed a significant decrease in HLA-C methylation (p < 0.05) (Fig. 3B). It was conclusively shown that in the men with moderate and critical forms of COVID-19, there are higher levels of HLA-C methylation than in women with the same disease forms (p < 0.05) (Fig. 3C). Also, previous studies suggested that in some viral infections, such as human immunodeficiency virus (HIV), low expression of HLA-C is related to higher disease progression (Apps et al. 2013; Kulpa and Collins 2011).
Fig. 3.
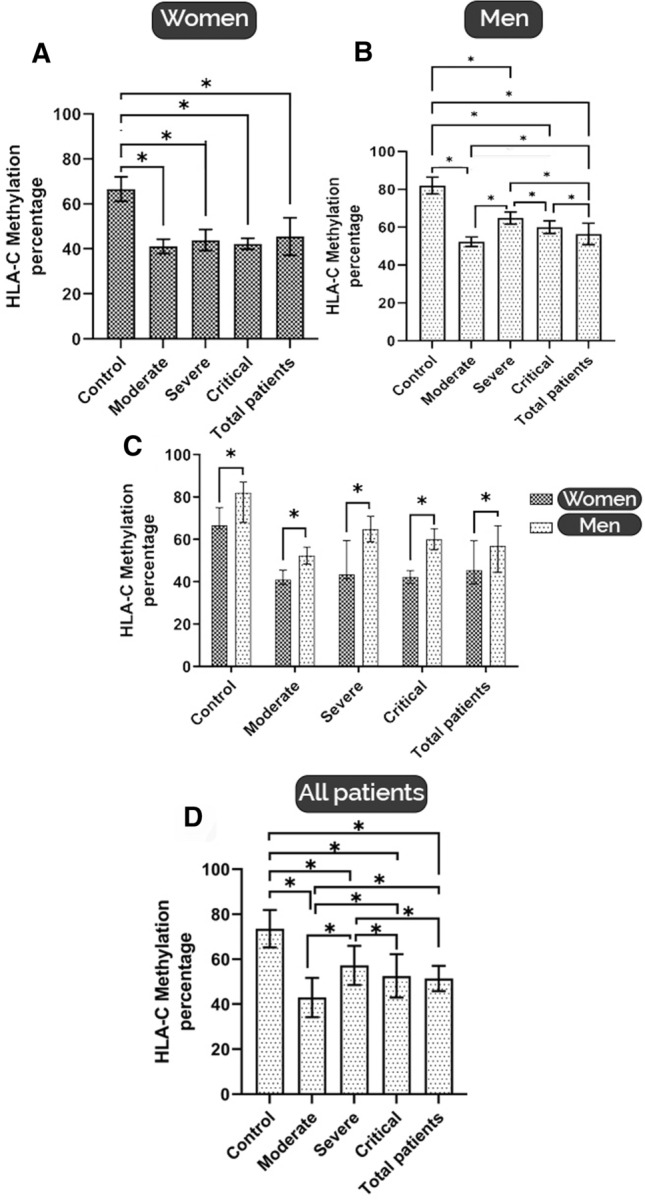
A HLA-C average methylation percentage in women patients with COVID-19 according to disease severity. A significant decrease in HLA-C methylation represents in comparison with the healthy group (*p < 0.05). B HLA-C average methylation percentage in men patients with COVID-19 according to disease severity. A significant decrease in HLA-C methylation represents in comparison with the healthy group, and different methylation was shown in different stages of diseases (*p < 0.05). C) Comparison of HLA-C methylation percentage between women and men patients with various forms of COVID-19. Men patients show higher methylation levels for HLA-C in different stages(*p < 0.05). D HLA-C average methylation percentage in total patients with different COVID-19 severity. Severe stages show higher HLA-C methylation levels. * indicates significant difference in different groups (*p < 0.05); values represent means ± SEM
Our findings confirm that in both women and men patients with COVID-19, HLA-C methylation significantly decreased in different forms of COVID-19. HLA-C methylation in men showed higher levels than in women in moderate, severe, critical, and total patients (p < 0.05) (Fig. 3C). Since the female sex in COVID-19 disease is associated with better outcomes and less mortality (Peckham et al. 2020), It could support our findings that HLA-C methylation is higher in men with all forms of disease severity than in women, especially in severe cases (Fig. 3C). Moreover, as reported, our evidence proves that in men, the severe form has more HLA-C methylation than the mild forms of the disease. These findings could point to additional possible variables that have a role in developing different forms of COVID-19 severity. The immune system has critical mediators such as cytokines and chemokines. They are striking interveners in antiviral immunity that excessively increase during COVID-19, known as the cytokine storm. Cytokine storm is widely accepted as the major cause of severe form in patients and disease progression (Ye et al. 2020).
On the other hand, HLA-C has been identified as a significant contributing factor leading to worse outcomes in patients with HIV (Kulpa and Collins 2011). Also, killer immunoglobulin-like receptors (KIRs) are the other implicated mediators in HLA-C action in infectious diseases. Further analysis of our results showed that in men patients, the methylation of HLA-C in severe, critical, and total patients was significantly more than in moderate (p < 0.05) (Fig. 3B).
Effects of Disease Severity on HLA-C Methylation
What stands out in Fig. 3D is the higher rate of HLA-C methylation in severe patients compared to moderate, critical, and total patients (p < 0.05). Both male and female patients had lower methylation levels than healthy volunteers, as seen in Fig. 3. Furthermore, there were substantial differences in HLA-C methylation levels in peripheral blood samples from patients with different COVID-19 patterns based on gender (p < 0.05).
During viral infection, HLA-C surface expression and subtle alterations of its peptidome change strongly by KIRs and natural killer (NK) cells that could be different in various stages of viral and infectious disease (Hilton and Parham 2017), which can be a satisfactory explanation for different levels of methylations in different forms of diseases in the present study. Also, it is observed that prior infections such as influenza impair the NK cell's functions. For instance, a study indicated that influenza infection results in increased superinfection susceptibility due to NK cell response defects in the lung (Small et al. 2010). Such findings could explain why, even though HLA-C methylation is reduced in all COVID-19 patients, they show different degrees of disease severity, as shown in the current study. Moreover, it claimed that genetic changes in some genes, such as HLA-C, are related to susceptibility to infectious diseases such as COVID-19 or result in severe outcomes (Bonaccorsi et al. 2021).
Several studies focused on the level of methylation in the promoters of genes implicated in antiviral responses. (Medina-Gali et al. 2018; Spalluto et al. 2017). It is also demonstrated that the other related respiratory viral infections, including influenza and MERS-CoV, lead to various responses in infected patients through epigenetic modulation (Menachery et al. 2018). In addition, it is indicated that the HLA-C locus is linked to hypnagogic hallucinations and sleep paralysis in post-H1N1 narcolepsy type 1 individuals (NT1) (Juvodden et al. 2020). Despite its long and close relationship with HLA-A and B, HLA-C expression on the cell surface is significantly lower than HLA-A and B (Apps et al. 2015). HLA-C plays a vital role in the adaptive and antigen-specific T cell responses. As a result, it can explain some changes in HLA-C methylation seen in our research.
As described in Fig. 3D, lower rates of HLA-C methylation were detected in moderate form of the disease. Based on current reports, COVID-19 susceptibility or protection is related to particular HLA genotypes (Barquera et al. 2020; Lorente et al. 2021; Shkurnikov et al. 2021). Although our study showed that HLA-C methylation levels were reduced in COVID-19 patients according to the different forms of the disease, it is indicated that HLA-C polymorphic changes are also varied in COVID-19 progression and severity. Hence, Weiner et al. found that COVID-19 patients with the HLA-C*04:01 polymorphism had more severe COVID-19 and a higher chance of SARS-CoV-2 intubation than other patients (Weiner et al. 2021). Moreover, in the case of SARS-COV-1, it has been proved that patients with various HLA polymorphisms have different severity of SARS (Lin et al. 2003). Also, previous studies described that HLA-C is a hetero-trimer with a high polymorphism rate, with approximately 5,600 alleles that could be different in various persons (Snary et al. 1977; Zemmour and Parham 1992).
In addition, a recent investigation in HLA-C genotyping claimed that two types of HLA-C polymorphism have different associations with the various forms of the disease; for instance, HLA-C*06 has a protective effect; on the other hand, HLA-C*17 has a crucial role in increasing the severity of COVID-19 patients (Bonaccorsi et al. 2021). In addition, Sakuraba et al. revealed that HLA-C*05 frequency and the distribution pattern with the KIR2DS4fl as the receptor strongly correlate with COVID-19 severe stages and mortality (Sakuraba et al. 2020).
Therefore, these reports show that in addition to HLA-C methylation, other related factors are implicated in enhancing the severity of COVID-19, contributing to additional evidence. Moreover, it could be presumed that more severity stages in COVID-19 patients might produce hypermethylation in HLA-C instead of hypomethylation with an unexplained mechanism. It may explain why some patients with a lower methylation level showed higher disease severity in the present study. Hence, more research is required due to the multiple reasons for the intricacy of HLA-C and its methylation.
Conclusion
In summary, our research has highlighted the significant relationship between the methylation of HLA-C and different forms of COVID-19. HLA-C methylation was substantially decreased in COVID-19 patients with moderate, severe, and critical forms of the disease. The most significant finding from this study is that the changes in HLA-C methylation levels are gender-related. Regarding HLA-C methylation, there were significant differences between men and women patients with various disease severity. These findings imply that when managing COVID-19, the gender of the patients and the severity of the disease should be considered appropriately. Further exploration of the HLA-C gene expression and epigenetic changes is required to confirm its specific advantages or disadvantages for such viral infectious disease outcomes.
Acknowledgements
The authors are grateful to all the specimen donations and patients’ participation in this study. The current research was financially supported by Kerman University of Medical Sciences, Kerman, Iran. This study was part of a Ph.D. thesis (Ethics code: IR.KMU.REC.1400.411).
Author contribution
GA was involved in conceptualization, methodology and supervision. MSZ did research, performed experiments, wrote the original draft and carried out the statistical analysis. ZaSG performed experiments and carried out the statistical analysis. MAJ collected the sample. MRJ wrote and edited the manuscript. HR was responsible for consulting and sample collection.
Funding
This study was funded by Kerman University of medical sciences.
Declarations
Conflict of interest
The authors have declared that they have no conflicts of interest to disclose concerning this manuscript.
References
- Abolhassani M, Asadikaram G, Paydar P, et al. Organochlorine and organophosphorous pesticides may induce colorectal cancer; a case-control study. Ecotoxicol Environ Saf. 2019;178:168–177. doi: 10.1016/j.ecoenv.2019.04.030. [DOI] [PubMed] [Google Scholar]
- Apps R, Meng Z, Del Prete G, et al. Relative expression levels of the HLA class-I proteins in normal and HIV-infected cells. J Immunol. 2015;194:3594–3600. doi: 10.4049/jimmunol.1403234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps R, Qi Y, Carlson JM, et al. Influence of HLA-C expression level on HIV control. Science. 2013;340:87–91. doi: 10.1126/science.1232685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barquera R, Collen E, Di D, et al. Binding affinities of 438 HLA proteins to complete proteomes of seven pandemic viruses and distributions of strongest and weakest HLA peptide binders in populations worldwide. Hla. 2020;96:277–298. doi: 10.1111/tan.13956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaccorsi I, Carrega P, Venanzi Rullo E, et al. HLA-C*17 in COVID-19 patients: hints for associations with severe clinical outcome and cardiovascular risk. Immunol Lett. 2021;234:44–46. doi: 10.1016/j.imlet.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter AM. Comparative studies of placentation and immunology in non-human primates suggest a scenario for the evolution of deep trophoblast invasion and an explanation for human pregnancy disorders. Reproduction. 2011;141:391–396. doi: 10.1530/REP-10-0530. [DOI] [PubMed] [Google Scholar]
- Chen M, Wang Y, Yao X, et al. Hypermethylation of HLA-C may be an epigenetic marker in psoriasis. J Dermatol Sci. 2016;83:10–16. doi: 10.1016/j.jdermsci.2016.04.003. [DOI] [PubMed] [Google Scholar]
- Cho JH, Gregersen PK. Genomics and the multifactorial nature of human autoimmune disease. New Engl J Med. 2011;365:1612–1623. doi: 10.1056/NEJMra1100030. [DOI] [PubMed] [Google Scholar]
- Chousterman BG, Swirski FK, Weber GF. Cytokine storm and sepsis disease pathogenesis. Sem Immunopathol. 2017;39:517–528. doi: 10.1007/s00281-017-0639-8. [DOI] [PubMed] [Google Scholar]
- Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Bio Medica: Atenei Parmensis. 2020;91:157. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta M, Dutta P, Medhi S, et al. Polymorphism of HLA class I and class II alleles in influenza A (H1N1) pdm09 virus infected population of Assam, Northeast India. J Med Virol. 2018;90:854–860. doi: 10.1002/jmv.25018. [DOI] [PubMed] [Google Scholar]
- Foley D, Craig J, Morley R. Erratum: prospects for epigenetic epidemiology. Am J Epidemiol. 2009;169:1409–1409. doi: 10.1093/aje/kwn380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Galarza FF, Christmas S, Middleton D, et al. Allele frequency net: a database and online repository for immune gene frequencies in worldwide populations. Nucleic Acids Res. 2010;39:D913–D919. doi: 10.1093/nar/gkq1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidecker B, Kittleson MM, Kasper EK, et al. Transcriptomic biomarkers for the accurate diagnosis of myocarditis. Circulation. 2011;123:1174–1184. doi: 10.1161/CIRCULATIONAHA.110.002857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton HG, Parham P. Missing or altered self: human NK cell receptors that recognize HLA-C. Immunogenetics. 2017;69:567–579. doi: 10.1007/s00251-017-1001-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israeli M, Roelen DL, Carrington M, et al. Association between CTL precursor frequency to HLA-C mismatches and HLA-C antigen cell surface expression. Front Immunol. 2014;5:547. doi: 10.3389/fimmu.2014.00547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafarzadeh A, Jafarzadeh S, Nemati M. Therapeutic potential of ginger against COVID-19: is there enough evidence? J Trad Chin Med Sci. 2021;8:267–279. doi: 10.1016/j.jtcms.2021.10.001. [DOI] [Google Scholar]
- Jafarzadeh A, Nemati M, Jafarzadeh S. Contribution of STAT3 to the pathogenesis of COVID-19. Microb Pathog. 2021;154:104836. doi: 10.1016/j.micpath.2021.104836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juvodden HT, Viken MK, Nordstrand SE, et al. HLA and sleep parameter associations in post-H1N1 narcolepsy type 1 patients and first-degree relatives. Sleep. 2020;43:zsz239. doi: 10.1016/j.micpath.2021.104836. [DOI] [PubMed] [Google Scholar]
- Khan S, Ali A, Shi H, et al. COVID-19: Clinical aspects and therapeutics responses. Saudi Pharmaceut J. 2020;28:1004–1008. doi: 10.1016/j.jsps.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni S, Qi Y, O’hUigin C, et al. Genetic interplay between HLA-C and MIR148A in HIV control and Crohn disease. Proc Natl Acad Sci. 2013;110:20705–20710. doi: 10.1073/pnas.1312237110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulpa DA, Collins KL. The emerging role of HLA-C in HIV-1 infection. Immunology. 2011;134:116–122. doi: 10.1111/j.1365-2567.2011.03474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lande-Diner L, Zhang J, Ben-Porath I, et al. Role of DNA methylation in stable gene repression. J Biol Chem. 2007;282:12194–12200. doi: 10.1074/jbc.M607838200. [DOI] [PubMed] [Google Scholar]
- Lin M, Tseng HK, Trejaut JA, et al. Association of HLA class I with severe acute respiratory syndrome coronavirus infection. BMC Med Genet. 2003;4:1–7. doi: 10.1186/1471-2350-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorente L, Martín M, Franco A, et al. HLA genetic polymorphisms and prognosis of patients with COVID-19. Med Intensiva. 2021;45:96–103. doi: 10.1016/j.medin.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Yuan B, Yi J, et al. The genetic polymorphisms of HLA are strongly correlated with the disease severity after Hantaan virus infection in the Chinese Han population. Clin Dev Immunol. 2012 doi: 10.1155/2012/308237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McINTYRE JA, FAUL WP. Recurrent spontaneous abortion in human pregnancy: results of immunogenetical, cellular, and humoral studies. Am J Reprod Immunol. 1983;4:165–170. doi: 10.1111/j.1600-0897.1983.tb00272.x. [DOI] [PubMed] [Google Scholar]
- Medina-Gali R, Belló-Pérez M, Martínez-López A, et al. Chromatin immunoprecipitation and high throughput sequencing of SVCV-infected zebrafish reveals novel epigenetic histone methylation patterns involved in antiviral immune response. Fish Shellfish Immunol. 2018;82:514–521. doi: 10.1016/j.fsi.2018.08.056. [DOI] [PubMed] [Google Scholar]
- Menachery VD, Schäfer A, Burnum-Johnson KE, et al. MERS-CoV and H5N1 influenza virus antagonize antigen presentation by altering the epigenetic landscape. Proc Natl Acad Sci. 2018;115:E1012. doi: 10.1073/pnas.1706928115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mervis JS, McGee JS. DNA methylation and inflammatory skin diseases. Arch Dermatol Res. 2020;312:461–466. doi: 10.1007/s00403-019-02005-9. [DOI] [PubMed] [Google Scholar]
- Mitra P, Misra S, Sharma P. COVID-19 pandemic in India: what lies ahead. Indian J Clin Biochem. 2020;35:257–259. doi: 10.1007/s12291-020-00886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen A, David J, Maden S. Human leukocyte antigen susceptibility map for SARS-CoV-2. J Virol. 2020;94:00510–00520. doi: 10.1128/JVI.00510-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto Y, Shinjo K, Shimizu Y, et al. Hepatitis virus infection affects DNA methylation in mice with humanized livers. Gastroenterology. 2014;146:562–572. doi: 10.1053/j.gastro.2013.10.056. [DOI] [PubMed] [Google Scholar]
- Organization WHO (2021) COVID-19 clinical management: living guidance, 25 January 2021: World Health Organization
- Pairo-Castineira E, Clohisey S, Klaric L, et al. Genetic mechanisms of critical illness in Covid-19. Nature. 2021;591:92–98. doi: 10.1038/s41586-020-03065-y. [DOI] [PubMed] [Google Scholar]
- Papúchová H, Meissner TB, Li Q, et al. The dual role of HLA-C in tolerance and immunity at the maternal-fetal interface. Front Immunol. 2019 doi: 10.3389/fimmu.2019.02730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paydar P, Asadikaram G, Nejad HZ, et al. Epigenetic modulation of BRCA-1 and MGMT genes, and histones H4 and H3 are associated with breast tumors. J Cell Biochem. 2019;120:13726–13736. doi: 10.1002/jcb.28645. [DOI] [PubMed] [Google Scholar]
- Peckham H, de Gruijter NM, Raine C, et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat Commun. 2020;11:1–10. doi: 10.1038/s41467-020-19741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuraba A, Haider H, Sato T. Population difference in allele frequency of HLA-C*05 and its correlation with COVID-19 mortality. Viruses. 2020;12:1333. doi: 10.3390/v12111333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif-zak M, Abbasi-jorjandi M, Asadikaram G, et al. CCR2 and DPP9 expression in the peripheral blood of COVID-19 patients: influences of the disease severity and gender. Immunobiology. 2022;227:152184. doi: 10.1016/j.imbio.2022.152184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shkurnikov M, Nersisyan S, Jankevic T, et al. Association of HLA class I genotypes with severity of coronavirus disease-19. Front Immunol. 2021;12:423. doi: 10.3389/fimmu.2021.641900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small CL, Shaler CR, McCormick S, et al. Influenza infection leads to increased susceptibility to subsequent bacterial superinfection by impairing NK cell responses in the lung. J Immunol. 2010;184:2048–2056. doi: 10.4049/jimmunol.0902772. [DOI] [PubMed] [Google Scholar]
- Snary D, Barnstable C, Bodmer W, et al. Molecular structure of human histocompatibility antigens: the HLA-C series. Eur J Immunol. 1977;7:580–585. doi: 10.1002/eji.1830070816. [DOI] [PubMed] [Google Scholar]
- Spalluto CM, Singhania A, Cellura D, et al. IFN-γ influences epithelial antiviral responses via histone methylation of the RIG-I promoter. Am J Respir Cell Mol Biol. 2017;57:428–438. doi: 10.1165/rcmb.2016-0392OC. [DOI] [PubMed] [Google Scholar]
- Tiwari S, Manoj G, Prasanth K, et al. Simplified and versatile method for bisulfite-based DNA methylation analysis of small amounts of DNA. J Clin Lab Anal. 2009;23:172–174. doi: 10.1002/jcla.20314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter L, Ansari AA. MHC and KIR polymorphisms in rhesus macaque SIV infection. Front Immunol. 2015 doi: 10.3389/fimmu.2015.00540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner J, Suwalski P, Holtgrewe M, Rakitko A, et al. Increased risk of severe clinical course of COVID-19 in carriers of HLA-C*04:01. EClinicalMedicine. 2021;40:101099. doi: 10.1016/j.eclinm.2021.101099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit J, Borghans JA, Kesmir C, et al. Editorial: role of HLA and KIR in viral infections. Front Immunol. 2016;7:286. doi: 10.3389/fimmu.2016.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yawata M, Yawata N, Draghi M, et al. Roles for HLA and KIR polymorphisms in natural killer cell repertoire selection and modulation of effector function. J Exp Med. 2006;203:633–645. doi: 10.1084/jem.20051884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q, Wang B, Mao J. The pathogenesis and treatment of the `Cytokine Storm' in COVID-19. J Infect. 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman W, Saqib S, Ullah F, et al. COVID-19: Phylogenetic approaches may help in finding resources for natural cure. Phytother Res PTR. 2020;34:2783–2785. doi: 10.1002/ptr.6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemmour J, Parham P. Distinctive polymorphism at the HLA-C locus: implications for the expression of HLA-C. J Exp Med. 1992;176:937–950. doi: 10.1084/jem.176.4.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XY, Huang HJ, Zhuang DL, et al. Biological, clinical and epidemiological features of COVID-19, SARS and MERS and AutoDock simulation of ACE2. Infect Dis Poverty. 2020;9:10–20. doi: 10.1186/s40249-020-00691-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


