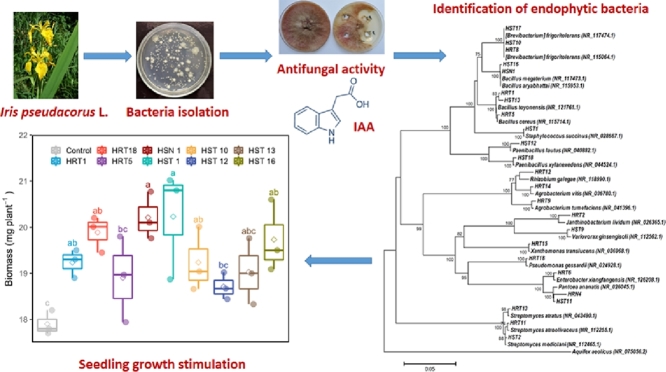
Abstract
This study reports the diversity of cultivable endophytic bacteria associated with yellow iris (Iris pseudacorus L.) by using 16S rRNA gene analysis and their plant beneficial traits. The 16S rRNA sequence similarities of endophytic bacteria isolated from the leaves and roots of yellow iris showed that the isolates belonged to the genera Staphylococcus, Streptomyces, Variovorax, Pantoea, Paenibacillus, Bacillus, Janthinobacterium, Enterobacter, Brevibacterium, Agrobacterium, Rhizobium, Xanthomonas translucens, and Pseudomonas. The endophytic bacteria Pseudomonas gessardii HRT18, Brevibacterium frigoritolerans HRT8, Streptomyces atratus HRT13, and Bacillus toyonensis HST13 exhibited antimicrobial activity against five plant pathogenic fungi Fusarium, Rhizoctonia, Botrytis, Pythium, and Alternaria. They also demonstrated the capability to produce chitinase, protease, glucanase, lipase, HCN, and indole-3-acetic acid (IAA). Thirteen isolates (46%) produced IAA, and the most active IAA producers were Bacillus cereus, Agrobacterium tumefaciens, Agrobacterium vitis, Bacillus megaterium, and Bacillus aryabhattai. The IAA producing bacterial isolates stimulated root and shoot growth of garden cress. Our findings suggest that medicinal plants could be a promising source for isolating plant-beneficial bacteria that can be used to enhance the growth and protect plants against soil-borne pathogens.
Keywords: Yellow iris, Endophytes, Plant pathogenic fungi, Plant beneficial traits
Graphical abstract
1. Introduction
Yellow iris (Iris pseudacorus L.) is a common perennial flowering plant cultivated in many regions of Europe, western Asia, and North America (Sutherland, 1990; Katharine, 2009). There are many reports about antimicrobial activity of different species of Iris: Iris pseudacorus (Ramtin et al., 2013), Iris germanica (Uzair et al., 2016), Iris nigricans (Al-Khateeb and Finjan, 2013), Iris planifolia (Chikhi et al., 2012), Iris pseudopumila (Rigano et al., 2006), Iris aitchisonii (Ajaib et al., 2013) etc. The methanolic, butanolic (Machalska-Gdak et al., 2009), and hydroalcoholic extracts (Tikhomirova et al., 2017) of Iris pseudacorus L. roots showed antimicrobial activity. It has also been observed that leaves and rhizomes of Iris pseudacorus accumulate mainly phenolic substances such as flavanoids, and isoflavonoids (Boland and Donnelly, 1998). The essential oils derived from Iris pseudacorus inhibited both gram-positive and gram-negative bacteria (Ramtin et al., 2014). Earlier, Tamilarasi et al. (2008) observed that the chemical composition of plant exudates affects the microbial activity associated with the plant. For example, the endophytic bacteria associated with medicinal plants with antimicrobial activity such as Matricaria chamomilla, Calendula officinalis and other medicinal plants exhibited higher antimicrobial properties (Köberl et al., 2013, 2014; Rustamova et al., 2020). It assumed that endophytes colonizing plant tissue play an important role in synthesizing of biologically active compounds and protecting plants from soil-borne disease (Cho et al., 2015). The diversity of endophytic microbes associated with medicinal plant species such as Aloe vera, Mentha arvensis, Dracaena cochinchinensis, Hedycium acuminatum, Armoracia rusticana, Hypericum perforatum and others, and their biological activity were reported (Salam et al., 2017; Hastuti et al., 2002; Egamberdieva et al., 2017, 2020a; Shurigin et al. 2020a). The bacterial genera such as Aeromonas, Bacillus, Enterobacter, Chryseobacterium, Cronobacter, Klebsiella, Macrococcus, Pseudomonas, Pantoea, Providencia, Sphingobacterium, and Shigella were isolated from the root of Aloe vera, whereas Bacillus and Pseudomonas were identified as the dominant members of the indigenous bacteria (Akinsanya et al., 2015). Many endophytic bacteria are known to improve fitness through the production of metabolites, e.g. Bacillus megaterium, Pseudomonas sp., and Acinetobacter calcoaceticus associated with Plectranthus tenuiflorus synthesized cell wall degrading enzymes and showed antagonistic activity against pathogenic microbes (El-Deeb et al., 2013). However, to date there are just a few studies on the endophytes of Iris pseudacorus plant. Calheiros et al. (2018) observed 13 different bacterial genera associated with Iris pseudacorus, whereas Pseudomonas, Bacillus, and Rahnella were the most represented genera. Although several studies reported on the phytochemical constituents and biological activity of yellow iris (Iris pseudacorus L.), there have been no reports of plant-associated bacteria of yellow iris, and their plant beneficial properties. Here we report on endophytic bacteria associated with yellow iris from the natural reserve of Uzbekistan identified using 16S rRNA gene analysis and their plant beneficial properties. The knowledge of the medicinal plant-associated endophytic bacteria and their physiological activities within plant tissue will help us understand endophytes’ role in plant development.
2. Materials and Methods
2.1. Plant collection and storage
Iris pseudacorus L. was collected in 2017 from the undisturbed natural reserve area of Uzbekistan, which is considered unique with many endemic plant species. Six individual plants at a distance of 20-30 m were collected as a whole and stored in zip-lock plastic bags in a cold room. The plant material was identified in Department of Botany, Faculty of Biology, National University of Uzbekistan. The root system of plants was carefully separated from leaves, and soil attached to the roots was washed with sterile water.
2.2. Isolation of endophytic bacteria
Plant roots and leaves were sterilized with 99.9% ethanol and 10% NaClO, and rinsed several times in sterile distilled water (Coombs and Franco, 2003). 10 g fresh weight of root and leaves were macerated using a sterile mortar and transferred into plastic tubes with 9 ml sterile phosphate-buffered saline (Mora-Ruiz et al., 2015). A 100 µl aliquots from dilutions (101–105) were spread on Tryptic Soy Agar (TSA) (BD, Difco Laboratories, Detroit, USA) supplemented with nystatin 50 µg/ml, and plates were incubated for four days at 28°C. The bacteria were isolated from different colonies and streaked on nutrient agar plates. The sterility of the root and leaves were tested, placing them onto agar plates.
2.3. Identification of bacteria
The heat treatment method used for extraction of DNA described by Dashti et al. (2009) and the existence of DNA was confirmed by horizontal Gel electrophoresis and quantified with NanoDrop™ One (ThermoFisher Scientific). The 16S rRNA genes were amplified with PCR using the following primers: 16SF 5’-GAGTTTGATCCTGGCTCAG-3’ (Sigma-Aldrich, St. Louis, MO) and 16SR 5’-GAAAGGAGGTGATCCAGCC (Sigma-Aldrich, St. Louis, MO) (Shurigin et al., 2020b). Moreover, RFLP analysis of 16S rRNA products and digital gel imaging system (Gel-Doc XR TM+, Bio-Rad) were used to reduce siblings among bacterial isolates as described by Jinneman et al. (Jinneman et al., 1996). The PCR products were purified with the USB® ExoSAP-IT® PCR Product Cleanup Kit (Affymetrix, USB® Products, USA) and sequencing was performed using ABI PRISM BigDye 3.1 Terminator Cycle Sequencing Ready Reaction Kit (Applied Biosystems) according to the protocol of the manufacturer. The data were evaluated using Chromas (v. 2.6.5) software and EMBOSS Explorer (http://emboss.bioinformatics.nl/). The sequences were identified using the BLAST and comparisons with the GenBank nucleotide data bank from the National Centre for Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov/). The 16S rRNA sequences were deposited to GenBank under the accession numbers MH197373 - MH197385 for root endophytes and MH197386 - MH197396 for leaves endophytes. The phylogenetic tree was constructed using ClustalX 2.1 software and that FASTA format file (Saitou and Nei, 1987). The optimal tree with the sum of branch length = 1.24404154 is shown (Felsenstein, 1985). The Maximum Composite Likelihood method (Tamura et al., 2004) and MEGA6 (Tamura et al., 2013) were used for computing the evolutionary distances.
2.4. In vitro screening for plant beneficial traits
The bacterial isolates were tested for their antifungal activities against Alternaria alternata, Botrytis cinerea, Fusarium solani, F. culmorum, F. oxysporum, Pythium ultimum, and Rhizoctonia solani. The fungal pathogens were obtained from the Culture Collection of the National University of Uzbekistan. The cell-free supernatant was extracted from bacterial suspension incubated for 72 h in TSB medium by solvent extraction procedure using ethyl acetate (Elissawy et al., 2019). Plant extraction was performed using the Soxhlet apparatus described in Rojsanga et al. (2006). The yellow iris plants were dried at room temperature and 10 g plant powder were extracted two times with 200 mL of 80% aqueous ethanol solution at 80°C, 2 h for each time. The extract was filtered, evaporated to dryness, and the residue was cooled in a desiccator for 30 min and then accurately weighed for analysis. The pathogenic fungi were cultivated on peptone dextrose agar (PDA) medium at 27°С within 5-7 days. Agar disks with grown fungi were cut on small squares (with the side size 7 mm) and placed in the center of plates with fresh PDA medium. The plant extract and cell free suspension with endophytes isolates were poured into agar wells. The plates incubated at 27°С until the fungi fully covered control plates without bacteria. The zone of inhibitions was estimated by measuring the diameter of zone between wells and fungal growth.
The HCN production by bactetrial isolates were determined using sterile filter paper saturated with solution of 1% picric acid and 2% sodium carbonate placed to inner surface of Petri plate cover. Petri plate covered with parafilm and incubated at 27°С for 3 days. The change of paper color from yellow to dark blue indicated on HCN release (Castric, 1975).
The siderophore production ability of bacterial isolates was measured by method of Schwyn and Neilands (1987). Protease secretion was revealed by growing isolates on TSA/20 amended with skimmed milk to a final concentration 5%. The halo appeared around colonies indicated the presence of extracellular protease (Brown and Foster, 1970). The method described in Walsh et al. (1995) was used to determine β-1,3 glucanase production, and method of Malleswari and Bagyanarayana (2013) to determine chitinase production by bacterial isolates. The lipase activity of bacterial isolated was evaluated using the Tween lipase indicator assay (Howe and Ward, 1976). The production of IAA (indole 3-acetic acid) by endophytic isolates was studied using the method described by Bano and Musarrat (2003). The bacteria were grown in TSB medium for 72 h at 28°C and mixed with 10 mM orthophosphoric acid and 2 ml of Salkowski reagent. The IAA production was evaluated following pink color developed after 30 min and calculated by using a calibration curve of pure IAA as a standard.
Garden cress (Lepidium sativum L.) was used as the model to determine the plant growth stimulation ability of bacterial inoculants. Seeds were sterilized with 5% sodium hypochlorite (NaClO) for 3 min and were rinsed with sterilized water. Sterilized seeds were germinated in sterile Petri dishes on one layer of filter paper moistened with 3 ml of distilled water at 26°C. Bacterial isolates were cultured in a nutrient broth medium (Difco Lab, Detroit, MI, USA) for three days, and bacterial suspensions were adjusted to an optical density at 620 nm of 0.1 (OD620 = 0.1). The germinated seeds were placed in the bacterial suspension with sterile forceps and shaken gently for 5 min. Twenty healthy and uniform germinated seeds were sown into each Petri plate with three replications. Sterilized distilled water was used as a control treatment. After six days of incubation in a plant growth chamber at 26°C, the lengths of roots and shoots of the seedlings and fresh weight were measured and recorded.
2.5. Data analyses
All statistical analyses were performed by the open-source statistical language R (R Studio v1.4.1717, Boston, MA, USA). The analysis of variance between treatments were performed using Duncan's test (LSD, P=0.05). The figures were plotted using the package “ggplot2”. The mean values of IAA production, antimicrobial activity, and the standard deviation were extracted for each observation.
3. Results
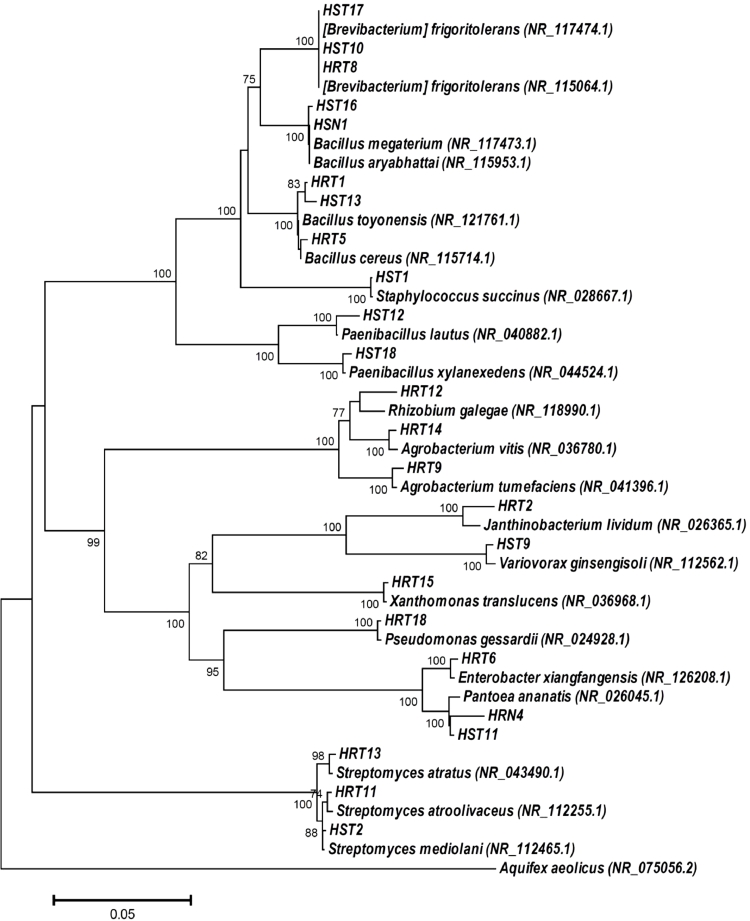
3.1. The isolation and identification of endophytic bacteria
A total of 45 bacterial isolates were isolated from Iris pseudacorus L. plant tissues, and after eliminating siblings, only 13 isolates of endophytic bacteria were left from roots and 11 isolates from leaves. The 16S rRNA gene sequences similarities of bacterial isolates with sequences from GenBank are shown in Table 1 (root endophytes) and Table 2 (leaves endophytes). A phylogenetic tree using the Neighbor-Joining method was constructed, showing the closest relatives of the isolates (Fig. 1). Most bacteria were representatives of phyla Proteobacteria (10 isolates) and Firmicutes (8 isolates). The third phylum Actinobacteria was represented by six isolates. Looking deeply into the systematics of the isolates, it should be noted that phyla Firmicutes includes classes Bacilli and Cocci. Class Bacilli in its turn contains genera Bacillus (Bacillus cereus HRT1, Bacillus toyonensis HRT5, Bacillus toyonensis HST13, Bacillus megaterium HST16, and Bacillus aryabhattai HSN1) and Paenibacillus (Paenibacillus lautus HST12, and Paenibacillus xylanexedens HST18). Class Cocci was represented by genus Staphylococcus (Staphylococcus succinus HST1).
Table 1.
. 16S rRNA gene sequence similarities of endophytic bacteria isolated from root system of Iris pseudacorus L. with sequences registered in GenBank
| Bacterial isolates sequences deposited to GenBank | Closest match (16S ribosomal RNA genes) (GenBank) | ||||
|---|---|---|---|---|---|
| Bacterial isolate | Length (bp) | Accession number | Reference strains | Accession number | Percent identity |
| HRT1 | 1465 | MH197373 | Bacillus cereus | NR_115714.1 | 99 |
| HRT2 | 1446 | MH197374 | Janthinobacterium lividum | NR_026365.1 | 98 |
| HRT5 | 1476 | MH197375 | Bacillus toyonensis | NR_121761.1 | 99 |
| HRT6 | 1409 | MH197376 | Enterobacter xiangfangensis | NR_126208.1 | 99 |
| HRT8 | 1466 | MH197377 | [Brevibacterium] frigoritolerans | NR_117474.1 | 99 |
| HRT9 | 1409 | MH197378 | Agrobacterium tumefaciens | NR_041396.1 | 99 |
| HRT11 | 1443 | MH197379 | Streptomyces atroolivaceus | NR_112255.1 | 99 |
| HRT12 | 1401 | MH197380 | Rhizobium galegae | NR_118990.1 | 97 |
| HRT13 | 1431 | MH197381 | Streptomyces atratus | NR_043490.1 | 99 |
| HRT14 | 1401 | MH197382 | Agrobacterium vitis | NR_036780.1 | 99 |
| HRT15 | 1464 | MH197383 | Xanthomonas translucens | NR_036968.1 | 99 |
| HRT18 | 1462 | MH197384 | Pseudomonas gessardii | NR_024928.1 | 99 |
| HRN4 | 1461 | MH197385 | Pantoea ananatis | NR_026045.1 | 98 |
Table 2.
16S rRNA gene sequence similarities of endophytic bacteria isolated from leaves of Iris pseudacorus L. with sequences registered in GenBank
| Bacterial isolates sequences deposited to GenBank | Closest match (16S ribosomal RNA genes) (GenBank) | ||||
|---|---|---|---|---|---|
| Bacterial isolate | Length (bp) | Accession number | Reference strains | Accession number | Percent identity |
| HST1 | 1473 | MH197386 | Staphylococcus succinus | NR_028667.1 | 99 |
| HST2 | 1451 | MH197387 | Streptomyces mediolani | NR_112465.1 | 99 |
| HST9 | 1456 | MH197388 | Variovorax ginsengisoli | NR_112562.1 | 99 |
| HST10 | 1474 | MH197389 | [Brevibacterium] frigoritolerans | NR_115064.1 | 99 |
| HST11 | 1461 | MH197390 | Pantoea ananatis | NR_026045.1 | 99 |
| HST12 | 1478 | MH197391 | Paenibacillus lautus | NR_040882.1 | 99 |
| HST13 | 1468 | MH197392 | Bacillus toyonensis | NR_121761.1 | 99 |
| HST16 | 1474 | MH197393 | Bacillus megaterium | NR_117473.1 | 99 |
| HST17 | 1470 | MH197394 | [Brevibacterium] frigoritolerans | NR_117474.1 | 99 |
| HST18 | 1470 | MH197395 | Paenibacillus xylanexedens | NR_044524.1 | 99 |
| HSN1 | 1471 | MH197396 | Bacillus aryabhattai | NR_115953.1 | 99 |
Fig. 1.
Phylogenetic tree of bacteria endophytes isolated from Iris pseudacorus L. and their closest relatives from GenBank.
Proteobacteria included representatives of classes Alphaproteobacteria (order Rhizobiales), Betaproteobacteria (order Burkholderiales) and Gammaproteobacteria (orders Pseudomonadales, Enterobacterales, and Xanthomonadales). Order Rhizibales includes isolates: Agrobacterium tumefaciens HRT9, Agrobacterium vitis HRT14, Rhizobium galegae HRT12, and Agrobacterium vitis HRT14. Order Burkholderiales contains isolates Janthinobacterium lividum HRT2, and Variovorax ginsengisoli HST9. Order Pseudomonadales contains isolate Pseudomonas gessardii HRT18. Order Enterobacterales include isolates of 2 families - Enterobacteriaceae (Enterobacter xiangfangensis HRT6), and Erwiniaceae (Pantoea ananatis HRN4, and Pantoea ananatis HST11). Order Xanthomonadales represented by Xanthomonas translucens HRT15.
Actinobacteria includes 2 genera – Brevibacterium (Brevibacterium frigoritolerans HRT8, Brevibacterium frigoritolerans HST10, and Brevibacterium frigoritolerans HST17), and Streptomyces (Streptomyces atroolivaceus HRT11, Streptomyces mediolani HST2, and Streptomyces atratus HRT13).
3.2. Plant beneficial traits
The plant extract and cell-free suspensions of endophytic bacterial isolates were tested against plant pathogenic fungi Fusarium solani, F. culmorum, F. oxysporum, Botrytis cinerea, Pythium ultimum, Rhizoctonia solani, and Alternaria alternata (Table 3).
Table 3.
Traits possibly involved in biocontrol and/or plant growth-promoting activity of bacterial endophytes from Iris pseudacorus L.
| Strain | Antagonistic activity against phytopathogenic fungi(cm) | HCN | Siderophores | Production of exoenzymes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F. solani | F. culmorum | F. oxysporum | B. cinerea | P. ultimum | R. solani | A. alternata | Lipase | Protease | Chitinase | Glucanase | Production ofIAA (µg/ml) | |||
| Bacillus cereus HRT1 | - | - | - | 0.7±0.1 | 0.9±0.1 | 0.8±0.1 | 0.6±0.1 | - | + | - | + | + | + | 7.8±0.2 |
| Janthinobacterium lividum HRT2 | - | - | 1.1±0.1 | 1.2±0.2 | - | 1.0±0.1 | 0.7±0.1 | + | + | - | + | + | - | - |
| Bacillus toyonensis HRT5 | 1.1±0.2 | 1.0±0.2 | - | - | 0.9±0.1 | - | - | + | + | - | + | - | + | 6.9±0.3 |
| Enterobacter xiangfangensis HRT6 | - | - | - | - | - | - | - | - | - | + | - | - | - | - |
| Brevibacterium frigoritolerans HRT8 | 0.9±0.1 | 0.9±0.2 | 0.7±0.1 | 0.8±0.1 | - | - | 0.6±0.1 | + | + | - | + | + | - | - |
| Agrobacterium tumefaciens HRT9 | - | - | - | - | - | - | - | - | - | - | - | - | - | 8.3±0.3 |
| Streptomyces atroolivaceus HRT11 | 0.7±0.1 | 0.8±0.1 | 0.8±0.1 | - | - | 0.7±0.1 | - | + | + | + | + | - | + | - |
| Rhizobium galegae HRT12 | - | - | - | - | - | - | - | - | - | - | - | - | - | 6.5±0.2 |
| Streptomyces atratus HRT13 | 0.7±0.1 | 0.7±0.1 | - | 0.8±0.1 | 1.0±0.1 | 0.6±0.1 | - | + | + | - | + | + | - | - |
| Agrobacterium vitis HRT14 | - | - | - | - | - | - | - | - | - | - | - | - | - | 7.2±0.3 |
| Xanthomonas translucens HRT15 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Pseudomonas gessardii HRT18 | 1.0±0.1 | 1.1±0.1 | 1.0±0.2 | 0.9±0.1 | 0.9±0.2 | 1.0±0.2 | 0.8±0.1 | + | + | + | + | + | + | 6.8±0.3 |
| Pantoea ananatis HRN4 | - | - | - | 0.8±0.1 | - | 0.7±0.1 | - | - | + | - | + | - | + | - |
| Staphylococcus succinus HST1 | - | - | - | - | - | - | - | - | - | - | - | - | - | 5.5±0.2 |
| Streptomyces mediolani HST2 | 0.8±0.1 | 0.9±0.1 | 0.7±0.1 | - | - | - | - | - | + | + | - | + | - | - |
| Variovorax ginsengi soli HST9 | - | - | - | - | - | - | - | - | - | + | - | - | - | |
| Brevibacterium frigoritolerans HST10 | - | - | - | 0.8±0.1 | 0.9±0.1 | - | - | + | + | - | + | - | + | 6.1±0.3 |
| Pantoea ananatis HST11 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Paenibacillus lautus HST12 | 1.0±0.1 | - | - | - | 0.8 ±0.1 | - | 0.7±0.1 | + | + | + | + | + | - | 5.5±0.3 |
| Bacillus toyonensis HST13 | 0.6±0.1 | 0.8±0.1 | 0.7±0.1 | - | - | 0.6±0.1 | 0.5±0.1 | + | + | + | + | + | - | - |
| Bacillus megaterium HST16 | - | - | - | - | - | - | - | - | - | - | + | - | - | 8.4±0.3 |
| Brevibacterium frigoritolerans HST17 | - | - | - | 0.7±0.1 | - | 0.9±0.1 | - | - | + | - | + | - | + | 5.4±0.3 |
| Paenibacillus xylanexedens HST18 | - | - | - | - | - | - | - | - | - | - | + | - | - | 4.5±0.2 |
| Bacillus aryabhattai HSN1 | - | - | 0.8±0.1 | - | 0.7±0.1 | 0.9±0.1 | - | + | + | - | + | + | - | 8.9±0.3 |
| Plant extract | 0.5±0.1 | 0.4±0.1 | 0.4±0.1 | - | 0.4±0.1 | 0.5±0.1 | - | - | - | - | - | - | - | - |
“+” positive; “-” negative
Some bacterial isolates showed antifungal activity against wide spectrum of fungi. The isolate Pseudomonas gessardii HRT18 was active against all seven tested fungi. Three isolates Brevibacterium frigoritolerans HRT8, Streptomyces atratus HRT13, and Bacillus toyonensis HST13 were active against five fungi. Three isolates Bacillus cereus HRT1, Janthinobacterium lividum HRT2, and Streptomyces atroolivaceus HRT11 were active against four fungi. Four isolates Bacillus toyonensis HRT5, Streptomyces mediolani HST2, Paenibacillus lautus HST12, and Bacillus aryabhattai HSN1 were active against three tested fungi. Ten bacterial isolates did not have any antifungal activity. The Iris pseudacorus plant extract had antifungal activity against five out of seven tested fungi. Table 3 shows the traits possibly involved in biocontrol and/or plant growth-promoting activity of bacterial endophytes associated with Iris pseudacorus L. The bacterial isolates were tested for production of HCN, siderophores, cell wall degrading enzymes (lipase, protease, chitinase, and glucanase), and indole-3-acetic acid (IAA). Ten out of twenty four isolates produced HCN, fourteen isolates produced siderophores. Several bacterial isolates produced lipase, protease, chitinase, and glucanase (Table 3).
Thirteen isolates (46%) produced phytohormone IAA. The most active IAA producers were Bacillus cereus HRT1 (7.8±0.2 µg/ml), Agrobacterium tumefaciens HRT9 (8.3±0.3 µg/ml), Agrobacterium vitis HRT14 (7.2±0.3 µg/ml), Bacillus megaterium HST16 (8.4±0.3 µg/ml), and Bacillus aryabhattai HSN1 (8.9±0.3 µg/ml). The bacterial isolate Pseudomonas gessardii HRT18 was positive for all specific PGPR traits.
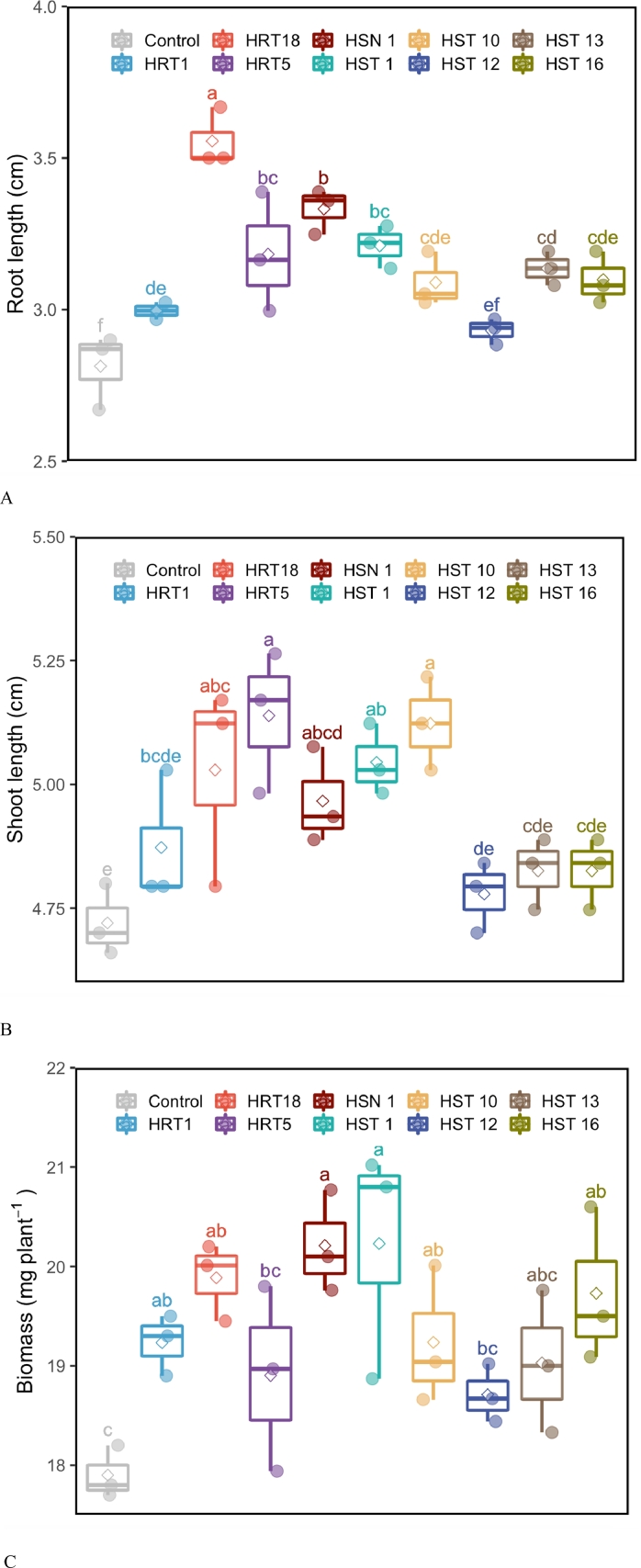
Bacterial isolates which showed one or more PGP traits were evaluated for their plant growth promoting properties. The IAA producing bacterial isolates B. cereus HRT1, B. toyonensis HRT5, P. gessardii HRT18, B. megaterium HST16, B. frigoritolerans HST10, S. succinus HST1, and B. aryabhattai HSN1 significantly stimulated root length by 6, 13, 26, 10, 10, 14, and 18% compared to un-inoculated seedlings (Fig. 2a). The bacterial isolates B. toyonensis HRT5, P. gessardii HRT18, B. frigoritolerans HST10, and S. succinus HST1 stimulated shoot length up to 9% (Fig. 2b). The seedling fresh weight was significantly stimulated by tested bacterial isolates except three isolates B. toyonensis HST13, B. toyonensis HRT5, and P. lautus HST12 (Fig. 2c).
Fig. 2.
Root length (A), shoot length (B) and seedling fresh weight (C) of garden cress (Lepidium sativum L.) after bacterial inoculation. Letters within each column are significantly different at p < 0.05 based on Duncan's test.
4. Discussion
Plant endophytic bacteria have been reported to have an influence on plant growth promotion (Egamberdieva et al., 2017, 2020b; Musa et al., 2020; Shurigin et al., 2020a, 2022).
To the best of our knowledge, this is the first report of endophytic bacteria associated with yellow iris (Iris pseudacorus L.) that proved plant beneficial properties. We have observed Bacillus species both in the roots and the leaves of the yellow iris. Hassan et al. (2018) observed auxin producing bacteria Bacillus cereus that stimulated wheat growth and development. Yanti et al. (2018) used different strains of Bacillus cereus, B. aryabhattai, and B. toyonensis in microbial consortium content, which reduced the development of disease caused by Ralstonia syzygii subsp. indonesiensis and promoted growth and yield of tomato. Xiang et al. (2017) reported Bacillus toyonensis which demonstrated antagonistic activity against Meloidogyne incognita – one of the most important plant-parasitic nematodes affecting cotton in USA. Lee et al. (2012) reported the plant-beneficial traits of B. aryabhattai isolates. In another study Park et al. (2017) observed phytohormone producing B. aryabhattai, which significantly promoted the growth of soybean. Chinnaswamy et al. (2018) reported about nodule endophytic Bacillus megaterium strain NMp082 isolated from Medicago polymorpha enhanced growth and nodulation of Medicago spp.
We have isolated two species of genus Paenibacillus - Paenibacillus lautus HST12 and Paenibacillus xylanexedens HST18 which exhibited plant beneficial traits such as nitrogen fixation, production of the phytohormone, siderophores, and phosphate solubilization. They can also protect plants from soil borne pathogens by triggering a hypersensitive defensive response of a plant, known as induced systemic resistance (ISR) (Grady et al., 2016; Egamberdieva et al., 2019). Orhan (2016) reported that Staphylococcus succinus EN4 isolated from salt-affected soil significantly improved the growth of Triticum aestivum under salt stress (200 mM NaCl). Agrobacterium tumefaciens isolated from peach demonstrated ability to fix atmospheric nitrogen, phosphate solubilisation, production of auxin, and siderophores (Liaqat and Eltem, 2016). The nitrogen-fixing symbionts, tumor producing Agrobacterium species are pathogenic and do not benefit the plant. In our study Janthinobacterium lividum HRT2 was isolated from the root of yellow iris. Kumar et al. (2017) also identified Janthinobacterium lividum from the rhizosphere of Oxyria digyna and Saxifraga oppositifolia in three Arcto-Alpine regions. Koo et al. (2016) reported that bacteria belonging to Janthinobacterium spp. were adapted to cold and dry environments and exhibited strong antimicrobial activity. Bacteria Variovorax ginsengisoli associated with yellow iris was also found in plant tissue of Panax ginseng (Im et al., 2010).
Pantoea is widely distributed bacterial species found in coffee (Nunes and de Melo, 2006), and ginseng (Cho et al., 2007). Jiang et al. (2019) observed a strong inhibition of Ceratocytis fimbriata mycelium growth and spore germination, which cause potato black rot by Pantoea dispersa.
We identified Xanthomonas translucens HRT15, which reported as plant pathogenic bacteria causing bacterial leaf streak (Pesce et al., 2015; Falahi Charkhabi et al., 2017). Three bacterial isolates associated with Iris pseudacorus tissues belong to Brevibacterium frigrotolerans. Similar bacterial species were found in Aloe vera, which showed plant beneficial traits including phytohormone and siderophore production, and phosphate solubilization (Tara and Saharan, 2017). Streptomyces spp. have been reported for their ability to control plant pathogens and produce various antimicrobial compounds such as atramycin and hydrazidomycins (Ueberschaar et al., 2011; Vurukonda et al., 2018). Our results showed that many bacterial isolates demonstrated antifungal properties against plant pathogenic fungi. The isolate Pseudomonas gessardii HRT18 was active against all tested fungi and produced HCN, IAA, siderophores, and cell wall degrading enzymes such as lipase, protease, chitinase, and glucanase. Similar observation reported for plant growth stimulating bacteria Pseudomonas gessardii strain LHRE63 isolated from the endorhizosphere of wheat, which demonstrated plant beneficial traits including synthesis of siderophores, IAA, cellulase, and protease (Sharma et al., 2011). Co-inoculating PGPR Pseudomonas with Rhizobium galegae bv. orientalis improved growth and symbiotic performance of fodder galega (Egamberdieva et al., 2010) through production of IAA and nitrogen fixation.
The isolates Brevibacterium frigoritolerans HRT8, Streptomyces atratus HRT13, and Bacillus toyonensis HST13 were active against five out of seven tested pathogenic fungi and synthesized HCN, siderophores, and cell wall degrading enzymes. This statement was confirmed by the observation of Köberl et al. (2013) for endophytic bacteria isolated from the medicinal plants Matricaria chamomilla, and Calendula officinalis, which showed antifungal activities as their host plant. In another study the higher proportion of endophytic bacteria associated with Hypericum perforatum (Egamberdieva et al., 2017), and Chelidonium majus L. (Goryluk et al., 2009) demonstrated antifungal properties.
Endophytes colonize inside healthy plant tissues, and provide benefits for plant growth and protect them from soil borne pathogens through producing various secondary metabolites (Raj et al. 2021). For example, the endophytes B. subtilis and B. megaterium isolated from Sophora alopecuroides synthesized IAA, siderophores, and proved antagonistic activity was able to reduce verticillium wilt disease of cotton (Lin et al., 2013). In our study, endophytic bacteria that produce IAA or siderophores, stimulated seedling growth of garden cress. Microbial phytohormone IAA plays an important role in plant physiology, including cell division, and elongation, e.g. IAA producing Stenotrophomona maltophilia stimulated growth of E. globulus root system (Asgher et al., 2015). In conclusion, we demonstrated the diversity of endophytic bacteria isolated from the roots and the leaves of the yellow iris (Iris pseudacorus L.), grown in arid soil in Uzbekistan, and revealed their plant beneficial traits. The isolates belong to the genera Staphylococcus, Streptomyces, Variovorax, Pantoea, Paenibacillus, Bacillus, Janthinobacterium, Enterobacter, Brevibacterium, Agrobacterium, Rhizobium, Xanthomonas translucens, and Pseudomonas. The bacterial isolates associated with yellow iris possessed antifungal activity against plant pathogenic fungi and were able to synthesise cell wall degrading enzymes, phytohormone auxin, and siderohopres. Moreover, plant growth stimulation of garden cress was observed by bacterial inoculants. Our findings suggest that medicinal plants could be a promising source for isolating plant-beneficial bacteria that can be used to enhance the growth and protect plants against soil-borne pathogens.
Declaration of competing interest
The authors declare that there is no conflict of interest.
Acknowledgments
Author Contributions: DE, KD, and TG conceived and designed the experiments. VS and JA performed the laboratory work. DE and VS analyzed the results. All authors assisted in writing the manuscript.
Contributor Information
Vyacheslav Shurigin, Email: slaventus87@inbox.ru.
Jakhongir Alimov, Email: jahongir.alimov@gmail.com.
Kakhramon Davranov, Email: k-davranov@mail.ru.
Tashkhan Gulyamova, Email: gulyamova@gmail.com.
Dilfuza Egamberdieva, Email: dilfuza.egamberdieva@zalf.de, egamberdieva@yahoo.com.
References
- Ajaib M.U., Khan Z., Abbasi M.A., Riaz T. Antimicrobial screening of Iris aitchisonii (Bakar) Boiss. Biologia. 2013;59(1):51–55. [Google Scholar]
- Akinsanya M.A., Goh J.K., Lim S.P., Tinga A.S.Y. Metagenomics study of endophytic bacteria in Aloe vera using next-generation technology. Genomics Data. 2015;6:159–163. doi: 10.1016/j.gdata.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Khateeb E., Finjan S. Isolation and identification of some phytochemical compounds from different parts of Iris nigricans. Eur. Sci. J. 2013;9(6):213–218. [Google Scholar]
- Asgher M., Khan M.I.R., Anjum N.A., Khan N.A. Minimising toxicity of cadmium in plants-role of plant growth regulators. Protoplasma. 2015;252:399–413. doi: 10.1007/s00709-014-0710-4. [DOI] [PubMed] [Google Scholar]
- Bano N., Musarrat J. Characterization of a new Pseudomonas aeruginosa strain NJ-15 as a potential biocontrol agent. Curr. Microbiol. 2003;46:324–328. doi: 10.1007/s00284-002-3857-8. [DOI] [PubMed] [Google Scholar]
- Boland G.M., Donnelly D.M.X. Isoflavonoids and related compounds. Nat. Prod. Rep. 1998;15:241–260. [Google Scholar]
- Brown M.R., Foster J.H. A simple diagnostic milk medium for Pseudomonas aeruginosa. J. Clin. Path. 1970;23:172–177. doi: 10.1136/jcp.23.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calheiros C.S.C., Pereira S.I.A., Castro P.M.L. Culturable bacteria associated to the rhizosphere and tissues of Iris pseudacorus plants growing in a treatment wetland for winery wastewater discharge. Ecol. Eng. 2018;115:67–74. [Google Scholar]
- Castric P.A. Hydrogen cyanide, a secondary metabolite of Pseudomonas aeruginosa. Can. J. Microbiol. 1975;21:613–618. doi: 10.1139/m75-088. [DOI] [PubMed] [Google Scholar]
- Chikhi I., Allali H., Dib M.E., Halla N., Muselli A., Tabti B., Costa J. Free radical scavenging and antibacterial activity of essential oil and solvent extracts of Iris planifolia (Mill) from Algeria. J. Med. Plan. Res. 2012;6(10):1961–1968. [Google Scholar]
- Chinnaswamy A., Pen Coba de la, a T., Stoll A., de la Pena Rojo D., Bravo J., Rincon A., et al. A nodule endophytic Bacillus megaterium strain isolated from Medicago polymorpha enhances growth, promotes nodulation by Ensifer medicae and malleviates salt stress in alfalfa plants. Ann. Appl. Biol. 2018;172(3):295–308. [Google Scholar]
- Cho K.M., Hong S.Y., Lee S.M., Kim Y.H., Kahng G.G., Lim Y.P, et al. Endophytic bacterial communities in ginseng and their antifungal activity against pathogens. Microb. Ecol. 2007;54:341–351. doi: 10.1007/s00248-007-9208-3. [DOI] [PubMed] [Google Scholar]
- Cho S.T., Chang H.H., Egamberdieva D., Kamilova F., Lugtenberg B., Kuo C.H. Genome analysis of Pseudomonas fluorescens PCL1751: a rhizobacterium that controls root diseases and alleviates salt stress for its plant host. PLOS One. 2015;10 doi: 10.1371/journal.pone.0140231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs J.T., Franco C.M. Isolation and identification of actinobacteria from surface-sterilized wheat roots. Appl. Environ. Microbiol. 2003;69:5603–5608. doi: 10.1128/AEM.69.9.5603-5608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashti A.A., Jadaon M.M., Abdulsamad A.M., Dashti H.M. Heat Treatment of Bacteria: A simple method of DNA extraction for molecular techniques. Kuwait Med. J. 2009;41:117–122. [Google Scholar]
- Egamberdieva D., Berg G., Lindström K., Räsänen L.A. Co-inoculation of Pseudomonas spp. with Rhizobium improves growth and symbiotic performance of fodder galega (Galega orientalis Lam.) Eur. J. Soil Biol. 2010;46(3):269–272. [Google Scholar]
- Egamberdieva D., Shurigin V., Alaylar B., Wirth S., Bellingrath-Kimura S.D. Bacterial endophytes from horseradish (Armoracia rusticana G. Gaertn., B. Mey. & Scherb.) with antimicrobial efficacy against pathogens. Plant Soil Environ. 2020;66:309–316. [Google Scholar]
- Egamberdieva D., Shurigin V., Alaylar B., Ma H., Müller M.E.H., Wirth S., Reckling M., et al. The effect of biochars and endophytic bacteria on growth and root rot disease incidence of Fusarium infested narrow-leafed lupin (Lupinus angustifolius L.) Microorganisms. 2020;8:496. doi: 10.3390/microorganisms8040496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egamberdieva D., Wirth S., Behrendt U., Parvaiz A., Berg G. Antimicrobial activity of medicinal plants correlates with the proportion of antagonistic endophytes. Front. Microbiol. 2017;8:199. doi: 10.3389/fmicb.2017.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egamberdieva D., Wirth S., Bellingrath-Kimura S.D., Mishra J., Arora N.K. Salt-tolerant plant growth promoting rhizobacteria for enhancing crop productivity of saline soils. Front. Microbiol. 2019;10:2791. doi: 10.3389/fmicb.2019.02791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Deeb B., Fayez K., Gherbawy Y. Isolation and characterization of endophytic bacteria from Plectranthus tenuiflorus medicinal plant in Saudi Arabia desert and their antimicrobial activities. J. Plant Inter. 2013;8(1):56–64. [Google Scholar]
- Elissawy A.M., Ebada S.S., Ashour M.A., El-Neketi M., Ebrahim W., Singab AB. New secondary metabolites from the mangrove-derived fungus Aspergillus sp. AV-2. Phytochem. Lett. 2019;29:1–5. [Google Scholar]
- Falahi Charkhabi N., Booher N.J., Peng Z., Wang L., Rahimian H., Shams-Bakhsh M, et al. Complete genome sequencing and targeted mutagenesis reveal virulence contributions of Tal2 and Tal4b of Xanthomonas translucens pv. undulosa ICMP11055 in bacterial leaf streak of wheat. Front. Microbiol. 2017;8:1488. doi: 10.3389/fmicb.2017.01488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Goryluk A., Rekosz-Burlaga H., Blaszczyk M. Isolation and characterization of bacterial endophytes of Chelidonium majus L. Pol. J. Microbiol. 2009;58:355–361. [PubMed] [Google Scholar]
- Grady E.N., MacDonald J., Liu L., Richman A., Yuan Z-Ch. Current knowledge and perspectives of Paenibacillus: a review. Microb. Cell Factories. 2016;15:203. doi: 10.1186/s12934-016-0603-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan T.U., Bano A., Naz I., Hussain M. Bacillus cereus: a competent plant growth promoting bacterium of saline sodic field. Pak. J. Bot. 2018;50(3):1029–1037. [Google Scholar]
- Hastuti U.S., Al-Asna P.M., Rahmawati D. Histologic observation, identification, and secondary metabolites analysis of endophytic fungi isolated from a medicinal plant. Hedichium acuminatum Roscoe. AIP Conf, Proc. 020070. 2002 doi: 10.1063/1.5050166. https://doi.org/ [DOI] [Google Scholar]
- Howe T.G., Ward J.M. The utilization of tween 80 as carbon source by Pseudomonas. J. Gen. Microbiol. 1976;92:234–235. doi: 10.1099/00221287-92-1-234. [DOI] [PubMed] [Google Scholar]
- Im W.T., Liu Q.M., Lee K.J., Kim S.Y., Lee S.T., Yi T.H. Variovorax ginsengisoli sp. nov., a denitrifying bacterium isolated from soil of a ginseng field. Int. J. Syst. Evol. Microb. 2010;60:1565–1569. doi: 10.1099/ijs.0.014514-0. [DOI] [PubMed] [Google Scholar]
- Jiang L., Jeong J.C., Lee J.S., Park J.M., Yang J.-W., Lee M.H., et al. Potential of Pantoea dispersa as an effective biocontrol agent for black rot in sweet potato. Sci. Rep. 2019;9:16354. doi: 10.1038/s41598-019-52804-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinneman K.C., Wetherington J.H., Adams A.M., Johnson J.M., Tenge B.J., Dang N.L. Differentiation of Cyclospora sp. and Eimeria spp. by using the polymerase chain reaction amplification products and restriction fragment length polymorphisms. Food and Drug Admin Lab Information Bulletin LIB. 1996;(4044) [Google Scholar]
- Katharine R.S. Fire Effects Information System. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory (Producer); 2009. Iris pseudacorus.http://www.fs.fed.us/database/feis/plants/forb/iripse/all.html [Google Scholar]
- Köberl M., Ramadan E.M., Adam M., Cardinale M., Hallmann J., Heuer H., et al. Bacillus and Streptomyces were selected as broad-spectrum antagonists against soilborne pathogens from arid areas in Egypt. FEMS Microbiol. Lett. 2013;342:168–178. doi: 10.1111/1574-6968.12089. [DOI] [PubMed] [Google Scholar]
- Köberl M., Schmidt R., Ramadan E.M., Bauer R., Berg G. The microbiome of medicinal plants: diversity and importance for plant growth, quality and health. Front. Microbiol. 2014;4:400. doi: 10.3389/fmicb.2013.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo H., Strope B.M., Kim E.H., Shabani A.M., Kumar R., Crowley M.R, et al. Draft genome sequence of Janthinobacterium sp. Ant5-2-1, isolated from proglacial lake Podprudnoye in the Schirmacher Oasis of East Antarctica. Genome Announc. 2016;4(1):e01600–e01615. doi: 10.1128/genomeA.01600-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Brader G., Sessitsch A., Mäki A., Van Elsas J.D., Nissinen R. Plants assemble species specific bacterial communities from common core taxa in three arcto-alpine climate zones. Front. Microbiol. 2017;8:12. doi: 10.3389/fmicb.2017.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Ka J.O., Song H.G. Growth promotion of Xanthium italicum by application of rhizobacterial isolates of Bacillus aryabhattai in Microcosm Soil. J. Microbiol. 2012;50:45–49. doi: 10.1007/s12275-012-1415-z. [DOI] [PubMed] [Google Scholar]
- Liaqat F., Eltem R. Identification and characterization of endophytic bacteria isolated from in vitro cultures of peach and pear rootstocks. 3 Biotech. 2016;6:120. doi: 10.1007/s13205-016-0442-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T., Zhao L., Yang Y., Guan Q., Gong M. Potential of endophytic bacteria isolated from Sophora alopecuroides nodule in biological control against Verticillium wilt disease. Aust. J. Crop Science. 2013;7:139–146. [Google Scholar]
- Machalska-Gdak A., Los R., Głowniak K., Malm A. Antibacterial activity of Iris pseudacorus L. Planta Med. 2009;75:66. [Google Scholar]
- Malleswari D., Bagyanarayan G. In vitro screening of rhizobacteria isolated from the rhizosphere of medicinal and aromatic plants for multiple plant growth promoting activities. J. Microbiol. Biotechnol. 2013;3(1):84–91. [Google Scholar]
- Mora-Ruiz M.D.R., Font-Verdera F., Díaz-Gil C., Urdiain M., Rodríguez-Valdecantos G., González B. Moderate halophilic bacteria colonizing the phylloplane of halophytes of the subfamily Salicornioideae (Amaranthaceae) Syst. Appl. Microbiol. 2015;38:406–416. doi: 10.1016/j.syapm.2015.05.004. [DOI] [PubMed] [Google Scholar]
- Musa Z., Ma J., Egamberdieva D., Abdelshafy Mohamad O.A., Abaydulla G., Liu Y, et al. Diversity and antimicrobial potential of cultivable endophytic actinobacteria associated with medicinal plant Thymus roseus. Front. Microbiol. 2020;11:191. doi: 10.3389/fmicb.2020.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes F.V., de Melo I.S. Isolation and characterization of endophytic bacteria of coffee plants and their potential in caffeine degradation. Environ. Toxicol. 2006;1:293–297. [Google Scholar]
- Orhan F. Alleviation of salt stress by halotolerant and halophilic plant growth-promoting bacteria in wheat (Triticum aestivum) Braz. J. Microbiol. 2016;47:621–627. doi: 10.1016/j.bjm.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y.-G., Mun B.-G., Kang S.-M., Hussain A., Shahzad R., Seo C.-W. Bacillus aryabhattai SRB02 tolerates oxidative and nitrosative stress and promotes the growth of soybean by modulating the production of phytohormones. PLoS ONE. 2017;12(3) doi: 10.1371/journal.pone.0173203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesce C., Bolot .S, Cunnac S., Portier P., Fischer Le., Saux M., Jacques M.A, et al. High-quality draft genome sequence of the Xanthomonas translucens pv. cerealis pathotype strain CFBP 2541. Genome Announc. 2015;3:e015714–e015774. doi: 10.1128/genomeA.01574-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramtin M., Massiha A., Khoshkholgh-Pahlaviani M.R.M., Issazadeh K., Assmar M., Zarrabi S. In vitro antimicrobial activity of Iris pseudacorus and Urtica dioica. Zahedan J. Res. Med. Sci. 2014;16(3):35–39. [Google Scholar]
- Ramtin M., Pahlaviani M.R.M.K., Massiha A., Issazadeh K., Heidary S. Comparative evaluation of the antibacterial activitites of essential oils of Iris pseudacorus and Urtica dioica native to north Iran. J. Pure Appl. Microbiol. 2013;7:1065–1070. [Google Scholar]
- Rai N., Kumari Keshri P., Verma A., Kamble S.C., Mishra P., Barik S., Kumar Singh S., Gautam V. Plant associated fungal endophytes as a source of natural bioactive compounds. Mycology. 2021;12(3):139–159. doi: 10.1080/21501203.2020.1870579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigano D., Grassia A., Formisano C., Basile A., Sorbo S., Senatore F. Antibacterial and allelopathic activity of methanolic extract from Iris pseudopumila rhizomes. Fitoterapia. 2006;77(6):460–462. doi: 10.1016/j.fitote.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Rojsanga P., Gritsanapan W., Suntornsuk L. Determination of berberine content in the stem extracts of Coscinium fenestratum by TLC densitometry. Med. Princ. Pract. 2006;15(5):373–378. doi: 10.1159/000094272. [DOI] [PubMed] [Google Scholar]
- Rustamova N., Wubulikasimu A., Mukhamedov N., Gao Y., Egamberdieva D., Yili A. Endophytic bacteria associated with medicinal plant Baccharoides anthelmintica diversity and characterization. Curr. Microbiol. 2020;77:1457–1465. doi: 10.1007/s00284-020-01924-5. [DOI] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Salam N., Khieu T.N., Liu M.J., Vu T.T., Chu-Ky S., Quach N.T., et al. Endophytic actinobacteria associated with Dracaena cochinchinensis Lour.: isolation, diversity, and their cytotoxic activities. Biomed. Res. Int. 2017 doi: 10.1155/2017/1308563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwyn B., Neilands J.B. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987;160:45–46. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- Sharma S.K., Johri B.N., Ramesh A., Joshi O.P., Prasad S.V.S. Selection of plant growth-promoting Pseudomonas spp. that enhanced productivity of soybean-wheat cropping system in Central India. J. Microbiol. Biotechnol. 2011;21(11):1127–1142. doi: 10.4014/jmb.1012.12018. [DOI] [PubMed] [Google Scholar]
- Shurigin V., Alikulov B., Davranov K., Ismailov Z. Bacterial endophytes from halophyte black saxaul (Haloxylon aphyllum Minkw.) and their plant growth-promoting properties. J. Appl. Biol. Biotechnol. 2022. 2022;10(01):45–53. [Google Scholar]
- Shurigin V., Egamberdieva D., Alaylar B., Birkeland N.K., Wirth S., Kimura S.D.B. Diversity and biological activity of culturable endophytic bacteria associated with marigold (Calendula officinalis L.) from Chatkal Biosphere Reserve of Uzbekistan. AIMS Microbiology. 2020;7:336–353. doi: 10.3934/microbiol.2021021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shurigin V., Egamberdieva D., Li L., Davranov K., Panosyan H., Birkeland N-K., Wirth S., Bellingrath-Kimura S.D. Endophytic bacteria associated with halophyte Seidlitzia rosmarinus Ehrenb. ex Boiss. from arid land of Uzbekistan and their plant beneficial traits. J. Arid Land. 2020;12:730–740. [Google Scholar]
- Sutherland W.J. Biological flora of the British Isle: Iris pseudacorus L. J. Ecol. 1990. 1990;78:833–848. [Google Scholar]
- Tamilarasi S., Nanthakumar K., Karthikeyan K., Lakshmanaperumalsamy P. Diversity of root associated microorganisms of selected medicinal plants and influence of rhizomicroorganisms on the antimicrobial property of Coriandrum sativum. J. Environ. Biol. 2008;29(1):127–134. [PubMed] [Google Scholar]
- Tamura K., Nei M., Kumar S. Prospects for inferring very large phylogenies by using the neighbor-joining method. PNAS USA. 2004;101:11030–11035. doi: 10.1073/pnas.0404206101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tara M.N., Saharan B.S. Plant growth promoting traits shown by bacteria Brevibacterium frigrotolerans SMA23 Isolated from Aloe vera rhizosphere. Agric. Sci. Digest. 2017;37:226–231. [Google Scholar]
- Tikhomirova E.A., Sorokina A.A., Marakhova A.I. Yellow iris (Iris pseudacorus L.) rhizomes: antimicrobial activity. Pharmacy. 2017;2:4. [Google Scholar]
- Ueberschaar N., Ndejouong B., Le Sage T., Ding L., Maier A., Fiebig H.H., et al. Hydrazidomycins, cytotoxic alkylhydrazides from Streptomyces atratus. Bioorganic Med. Chem. Lett. 2011;21(19):5839–5841. doi: 10.1016/j.bmcl.2011.07.108. [DOI] [PubMed] [Google Scholar]
- Uzair A., Bakht J., Iqbal A., Naveed Kh., Ali N. In vitro antimicrobial activities of different solvent extracted samples from Iris germinica. Pak. J. Pharm. Sci. 2016;29(1):145–150. [PubMed] [Google Scholar]
- Vurukonda SSh.K.P., Giovanardi D., Stefani E. Plant growth promoting and biocontrol activity of Streptomyces spp. as endophytes. Int. J. Mol. Sci. 2018;19(4):952. doi: 10.3390/ijms19040952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh G.A., Murphy R.A., Killeen G.F., Headon D.R., Power R.F. Technical note: Detection and quantification of supplemental fungal b-glucanase activity in animal feed. J. Anim. Sci. 1995;73:1074–1076. doi: 10.2527/1995.7341074x. [DOI] [PubMed] [Google Scholar]
- Xiang N., Lawrence K.S., Kloepper J.W., Donald P.A., McInroy J.A. Biological control of Meloidogyne incognita by spore-forming plant growth-promoting rhizobacteria of cotton. Plant Dis. 2017;101:774–784. doi: 10.1094/PDIS-09-16-1369-RE. [DOI] [PubMed] [Google Scholar]
- Yanti Y., Warnita Reflin, Hamid H. Development of selected PGPR consortium to control Ralstonia syzygii subsp. indonesiensis and promote the growth of tomato. Biodiversitas. 2018;19(6):2073–2078. [Google Scholar]