Highlights
-
•
Significance of LAMP method in rapid disease diagnosis is highlighted.
-
•
Different detection methods for amplicon visualization are explained.
-
•
Advancements in LAMP technique for disease identification are summarized.
-
•
Trends in development of LAMP disease diagnosis are discussed.
Keywords: LAMP, Detection, Diagnosis, Rapid, Point-of-care
Abstract
Loop-mediated isothermal amplification (LAMP) method has been demonstrated to bea reliable and robust method for detection and identification of viral and microbial pathogens. LAMP method of amplification, coupled with techniques for easy detection of amplicons, makes a simple-to-operate and easy-to-read molecular diagnostic tool for both laboratory and on-field settings. Several LAMP-based diagnostic kits and assays have been developed that are specifically targeted against a variety of pathogens. With the growing needs of the demanding molecular diagnostic industry, many technical advances have been made over the years by combining the basic LAMP principle with several other molecular approaches like real-time detection, multiplex methods, chip-based assays.This has resulted in enhancing thethe sensitivity and accuracy of LAMP for more rigorous and wide-ranging pathogen detection applications. This review summarizes the current developments in LAMP technique and their applicability in present and future disease diagnosis.
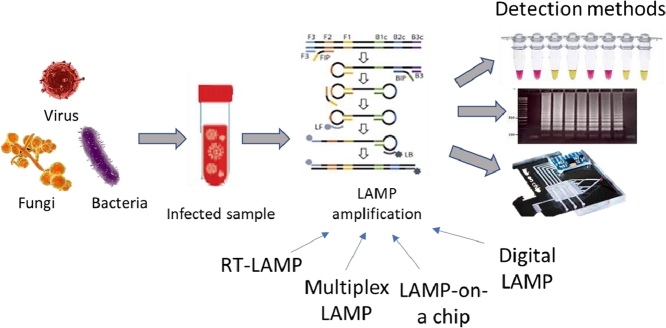
Graphical abstract
Introduction
Nucleic acid amplification methods have been very effectively utilized in the diagnosis of a variety of infectious diseases. Since nucleic acids (NA) are considered to be important biomarkers of pathogen-mediated diseases, techniques to detect their presence are used routinely to accurately detect and identify the causative organism. Polymerase chain reaction (PCR) is widely regarded as the gold standard in NA-based molecular diagnostic applications. PCR leverages the pathogenic NAs present in clinical samples and amplifies specific target DNA sequencesin an exponential mannerwhich can be detected by gel electrophoresis. To increase the specificity and sensitivity of detection, several modifications were introduced like fluorescent probes for real-time amplification detection, reverse-transcriptase PCR, multiplex PCR, nested PCR, etc. Despite these advancements in PCR techniques, it still presents certain challenges in resource-poor and non-laboratory settings. Significant drawbacks related to requirement of expensive and sophisticated thermocycler equipment, trained technical personnel and extended reaction times limits its potential for being a rapid point-of-care diagnostic technique. Also, non-specific annealing of primers and increased risk of false positives result in low specificity which further hinders the development of PCR-based techniques for field-based diagnostics. (Yang and Rothman, 2004; Demeke and Jenkins, 2010)
Isothermal amplification methods have been developed for nucleic acid detection in clinical samples to circumvent the problems associated with regular PCR. (Zanoli and Spoto, 2012; Ding et al., 2015; Zou et al., 2020; Keikha, 2018) Loop-mediated isothermal amplification (LAMP) methods have been the most popular techniques for NA amplification and detection. (Mori and Notomi, 2009) This technique utilizes three primer pairs for specific binding in presence of a DNA polymerase with high strand displacement capability. LAMP has proven its superiority over PCR by specific amplification of target DNA even in the co-presence of non-target sequences while eliminating the need for multiple temperature cycles, lengthy reaction times and sophisticated laboratory settings which are responsible for amplification errorsand longer turn-over times. (Foo et al., 2020; Khan et al., 2018) However, LAMP also suffers from non-specific binding through formation of form primer dimers. The use of multiple primers increases the risk of primer–primer hybridizations resulting in template‐free amplification, leading to false‐positive results. (Rolando et al., 2020) Several advances have been made over the years to enhance the efficiency of LAMP assay. It has been combined with various molecular approaches like real-time and multiplex detection methods in combination with various colorimetric and visual-detection methods for easy identification of positive samples to convert it to a fast, field-deployable and easy-to-operate sample screening technique. (Wong et al., 2018) With the state-of-art revolutions in diagnostic applications, LAMP technique has been modified to develop tools for point-of-care testing with enhanced efficiency and rapidity. This review comprises the advanced LAMP based techniques that have been utilized for sensitive, high-end molecular diagnostic purposes.
Search strategy and selection Criteria
Data for this review was identified by searches of MEDLINE, NCBI, PubMed, and references from relevant articles, using the search terms “LAMP diagnosis”, “LAMP amplification”, “RT-LAMP”, “Multiplex-LAMP” LAMP detection methods and “Digital and chip-based LAMP”. Articles published in the last 5 years were majorly referred.
LAMP detection Techniques
The basic method of LAMP was first developed by Notomi et al. (2000). Major components of the assay reaction mix are nucleotides, Bst DNA polymerase, primer sets and reaction buffer containing magnesium ions. The principle relies on the recognition of six specific sequences on target DNA by four sets of primers viz. outer forward (F3), inner forward (FIP), outer backward (B3) and inner backward (BIP)primers, in the presence of DNA polymerase having high strand displacement activity. The process is enhanced by an additional loop-primer pair which hybridizes with the stem-loop structures and provides increased number of starting points for exponential target amplification. The whole process is done at a single temperature using simple heating equipments like a water bath or heating block and the reaction takes less than an hour. (Nagamine et al., 2002)
Detection of the amplified products can be done by simple agarose gel electrophoresis or using fluorescent DNA intercalating dyes like SYBR green I, propidium iodide or Picogreen. It can also be done by monitoring magnesium pyrophosphate precipitation through visualization of turbidity by naked eye or using a turbidimeter. (Parida et al., 2005) The increase in amplification products in the reaction mix generates pyrophosphate ions which in the presence of magnesium ions form insoluble complexes to make the solution turbid, with the intensity of turbidity being a measure of the strength of amplification reaction. Real-time turbidity monitoring can also be done for quantitative assays. (Mori et al., 2004)
For colorimetric observations, several types of fluorescent dyes have been used for detection of notable color change. Calcein dye, used for fluorescent detection of amplified products, gives a color change from yellowto green in presence of magnesium ions (Safavieh et al., 2016) Additionally, metal ion indicators like hydroxy naphthol blue are employed for colorimetric reading of LAMP amplicons. (Goto et al., 2009)Researchers have demonstrated a pH shift at the end point and have used pH-sensitive dyes like phenol red, neutral red, cresol red and m-cresol for visual color change for amplicon detection. (Tanner et al., 2015)
The colorimetric reading of these methods may be variable and subjective to field-settings; therefore, lateral-flow dipsticks (LFD) have been employed for point-of-care testing as they are generally user-friendly and affordable. Here, the biotin-labelled amplicons are hybridized with FITC that get attached to gold-anti-FITC biotin binding proteins to yield red stripe. This forms the basis of gold nanoparticles biosensors. (Li et al., 2019) LFD methods are regarded as more practical to use as compared to the colorimetric, turbidimetric or gel-electrophoresis methods owing to their ease of use and rapidity of detection. Electrochemical chips containing DNA probes immobilized on silica or glass were used for real-time voltametric monitoring for LAMP amplicons. (Hashimoto et al., 2017)This method is more applicable in high-throughput automated system where detection is simpler to read.
Fluorescent resonance energy transfer (FRET) based LAMP assay has been demonstrated for target amplicon-specific detection in which FRET probes were used to hybridize single stranded LAMP products at the loop region, to provide real-time monitoring of the FRET-bound region. (Chou et al., 2011; Hardinge and Murray, 2019)LAMP in combination with enzyme-linked immunosorbent assay (ELISA) has been developed for easy, sensitive and specific diagnosis. It works by incorporating labelled nucleotides during the amplification process, followed by hybridization with specific probes and finally, detection of the captured probe by immunoassay. ((Ravan and Yazdanparast, 2012; Sun et al., 2017), (Becherer et al., 2020)Similarly, DNA functionalized gold nanoparticles (GNP) have also been used to hybridize with the targeted amplicons and prevent the aggregation of GNP in high salt concentration. The negative samples, on the contrary, cause GNP aggregation, leading to a color shift from red to blue, making this method an easy-to-read colorimetric LAMP amplicon detection method. (DehghanEsmatabadi et al., 2015) – (Wachiralurpan et al., 2018) Recent studies have been focused on developing rapid,enhanced and specific detection methods of LAMP assay and several modifications are being made to improve the molecular diagnostic parameters. LAMP technique has been proven to be a versatile and flexible method applied in diagnostic methods of diseases caused by several microorganisms including bacteria (Huang et al., 2018), virus (Parida et al., 2005) and parasites (Avendaño and Patarroyo, 2020).
Diagnostic applications of conventional LAMP
Right from its development, LAMP has been one of the most widely applied amplification methods in molecular diagnostic tools. It has shown to detect a wide range of pathogens from simple Escherichia.coli (Teh et al., 2014) to the newest SARS-CoV-2 (Chaouch, 2021)
From the past two decades, it has gained enormous popularity as a rapid and cost-effective detection method for numerous infectious diseases. Its utility and efficacy have been well demonstrated by various researchers in neglected tropical diseases (NTD), a group of around 20 diseases caused by bacteria, virus, fungi or parasites and mostly prevalent in the poorest regions of the world (WHO 2021). These diseases are often linked with the social stigma and poverty which makes the reach of their diagnosis and treatment further difficult. Due to poor resources and limited lab settings in these areas, LAMP has proven to be a revolutionary technique for therapy monitoring, early disease diagnosis and contact tracing Table 1. briefly summarizes recent studies of the successful detection of NTD causative organisms through LAMP method.
Table 1.
LAMP detection studies for NTD diagnosis.
| Disease | Pathogen | Marker | Sensitivity | References |
|---|---|---|---|---|
| Trypanosomiasis | Trypanosomacruzi | Satellite repetitive sequence | 5fg CL, 50fg DM28 | (Ordóñez et al., 2020) |
| 18S rRNA | 50 parasites/mL | (Rivero et al., 2017) | ||
| Echinococcosis | Echinococcusgranulosus complex | cox1 | 10–100fg | (Avila et al., 2020) |
| Trematodiases | Fasciola hepatica | IGS | 1 egg | (Ghodsian et al., 2019) |
| African sleeping sickness | Trypanosoma brucei gambiense, T. b. rhodesiense | 18S rRNA | 72%, 80% | (Hayashida et al., 2020) |
| Leishmaniasis | Leishmaniasiamensis | 18S rRNA | 94.4% | (Sukphattanaudomchoke et al., 2020) |
| Filariasis | Wuchereriabancrofti, Brugiamalayi | WbLDR, Hha I repeat (BmHha I) | 1/5000th of microfilaria, 1/100th of microfilaria (1 pg) | (Poole et al., 2017) |
| Onchocerciasis | Onchocerca volvulus | O-150 | 1pg | (Alhassan et al., 2016) |
| Schistosomiasis |
Schistosomamansoni S. intercalatum S. haematobium S. bovis |
ITS-1 | S. mansoni and S.intercalatum: 1 pg S. haematobium: 0.1 pg S. bovis: 10 pg |
(Fernández-Soto et al., 2020) |
| Helminthiases | Trichuris trichiura | ITS-2 | Single egg | (Ngari et al., 2020) |
| Scabies | Sarcoptesscabiei | ITS-2 | 0.02ng | (Fraser et al., 2018) |
Mycobacterium leprae, causing leprosy, shares close genomic similarity with Mycobacterium tuberculosis. LAMP assay has been demonstrated to specifically amplify the 16S rRNA target sequence of M. leprae in the presence of M. tuberculosis bearing the same gene. (Garg et al., 2021)This proved the applicability of LAMP to differentiate between closely related species and avoid risk of misdiagnosis and false positives. (Selvarajah et al., 2020) suggested that LAMP-based assays can detect even low levels of Plasmodium parasite in malaria. Almost all the agents of neglected parasitic diseases have been shown to be detected with high sensitivity and specificity through conventional LAMP. LAMP for Mycobacterium tuberculosis (TB-LAMP) in the form of a diagnostic kit, has been recommended by WHO (WHO 2021). It requires less than an hour for the assay to be completed and results can be visualized under UV light. Rapid LAMP diagnostic assays have been recently used in human respiratory diseases like pneumonia caused by Pseudomonas aeruginosa, Acinetobacter baumannii, Klebsiella pneumoniae, Staphylococcus aureus and Stenotrophomonas maltophilia. (Vergara et al., 2020) Additionally, LAMP was also developed for pathogens causing food-borne diseases such as E.coli (Ramezani et al., 2018), Salmonella typhi (Zhuang et al., 2014), Clostridium perfringens (Priya et al., 2018), Campylobacter jejuni (Romero and Cook, 2018), Enterococcus faecalis (Martzy et al., 2017)and Entamoeba histolytica (Mwendwa et al., 2017)
In the current COVID-19 pandemic situation all over the globe, rapid testing and accurate contact tracing are essential for detection and tracking of the virus. Since its outbreak, a number of researchers have demonstrated LAMP as the most effective diagnostic tool for coronavirus infection. (Dong et al., 2021), (Huang et al., 2020) Similarly, LAMP has shown the potential to detect a number of other viruses, for instance, adenovirus (Yuan et al., 2019), influenza A (Poon et al., 2005), herpes virus, varicella zoster (Kaneko et al., 2005) and cytomegalovirus (Wang et al., 2015).
The regular pathological screening of fungal infections is time-consuming and expensive. LAMP offers a rapid and high throughput alternative for detection of several types of disease-causing fungi viz. Candida albicans, Cryptococcus neoformans and Mucor racemosus (Nakayama et al., 2017), Trichosporonasahii (Zhou et al., 2015), Aspergillus fumigatus (Jiang et al., 2021), Pythium insidiosum (Htun et al., 2020), etc. These studies have shown the requirement of a very low amount of DNA for LAMP assay which shows the high sensitivity of this method.
Reverse transcription lamp (RT-LAMP)
Like RT-PCR, RT-LAMP has also been developed for a number of applications to identify the RNA of the disease-causing pathogen. This has opened a new domain for disease diagnosis through LAMP, using reverse transcriptase enzyme to make complementary DNA from RNA which is then amplified and detected. This assay is practically very simple and effective as the whole reaction is done in a single step: all the reagents including both enzymes (reverse transcriptase and DNA polymerase) are incubated in a single tube at a constant temperature. (Notomi et al., 2000) RT-LAMP has been established as a potential technique for clinical diagnosis and surveillance of several disease outbreaks in developing countries. Successful LAMP diagnostic methods have been developed for various viruses. Currently quantitative RT-PCR (qRT-PCR) is the gold standard for diagnosing viral diseases. However, its high cost and low sensitivity prohibit its usage in surveillance programs where large number of testing is required. Therefore, RT-LAMP has been used as a superior alternative for cheaper and faster detection Table 2. shows recent developments in LAMP assay for detection of highly virulent viruses which have been responsible for some of the deadliest viral outbreaks and caused severe morbidities. Several commercial kits have been developed by companies, some of which have been officially recommended for routine screening and surveillance programs of the diseases.
Table 2.
Recent LAMP assay development for human viruses.
| Pathogen | Time | Sensitivity | Detection Method | References |
|---|---|---|---|---|
| Zika | 20 min | 10−5 PFU | Colorimetric | (Calvert et al., 2017) |
| Ebola | 15–20 min | 256 copies | Real-time fluorometer | (Oloniniyi et al., 2017) |
| HIV | 60 min | 104 RNA copies/mL | RT Quencher probe | (Rudolph et al., 2015) |
| Influenza | 60 min | 0.1 RNA copies | Phenol-red pH indicator | (Ahnet al. et al., 2019) |
| Hepatitis B | 15 min | 2.2fg/µl | Fluorometer | (Quoc et al., 2018) |
| Dengue | 15 min | 0.8 fg/µl | Colorimetric (SYBR Green I) | (Mendes et al., 2019) |
| HPV | 60 min | 10 copies | Colorimetric (SYBR Green I) | (Mudhigeti et al., 2019) |
| H1N1 | <40 min | 10 copies RNA | Real-time turbidimeter, UV | (Kubo et al., 2010) |
| Encephalitis | 70 min | 50 pg RNA | Lateral flow dipstick | (Deng et al., 2015) |
| MERS-CoV | 60 min | 4 RNA copies | Colorimetric (EvaGreen) | (Lee et al., 2017) |
| West Nile virus | 17 min | 0.1 PFU | Real-time turbidimeter | (Parida et al., 2004) |
Several of the viral disease outbreaks and epidemics witnessed in the last few decades occurred in the impoverished and under-developed regions of the world. Without the presence of vaccines, chemotherapeutic agents or advanced healthcare facilities, these types of outbreaks can only be controlled by early, rapid and accurate field diagnosis. RT-LAMP methods have been developed which played a major role in rapid detection of new viruses at point-of-care without the need of elaborate lab facilities. For instance, RT-LAMP was developed by several researchers for rapidly detecting Ebola virus Oloniniyi et al. (2017).demonstrated a RT-LAMP technique for Ebola that has a short turnaround time and can produce results in 15 min. It also has high sensitivity and specificity. Recently, Silva et al. (2019)developed a fast one-step RT-LAMP method which can detect ZIKA in mosquitoes in around 20 min without the need of RNA isolation. The results could be easily read by naked eye through colorimetric observation and are highly sensitive, detecting very low amounts of viral nucleic acid. Similarly, dengue, causing seasonal attacks, is still prevalent in large parts of the world, and needs early diagnosis before the multiplication of the virus. Mendes et al. (2019) developed RT-LAMP which can produce results in 15 min with high sensitivity (in femtograms). The detection can be done simultaneously using colorimetric agents like SYBR GreenI.
Apart from the human viruses, LAMP method for detection of several plant viruses (tobacco mosaic virus, banana streak virus, cauliflower mosaic virus, tomato spotted wilt virus, yellow mosaic virus, potato virus Y to name a few) and animal viruses (foot and mouth disease virus, duck enteritis virus, monkey pox virus, Newcastle disease virus, classical swine fever, etc.) have also been developed. (Saharan et al., 2013)
A major breakthrough the RT-LAMP has given is in the diagnosis of coronavirus SARS-CoV-2 responsible for the recent pandemic. Many researchers from across the world have demonstrated RT-LAMP as an efficient point-of-care rapid molecular diagnostic tool for this highly infectious disease. (Dong et al., 2021), (Huang et al., 2020), (NEB; Park et al., 2020; Schermer et al., 2020) (Thi et al., 2020)Research is being done continuously to improve the current diagnostics and time of exposure during testing. (Alekseenko et al., 2021) have developed a method for direct detection of the virus without performing the sample purification steps and extraction of nucleic acid. They used unextracted nasopharyngeal samples directly from the infected patients and demonstrated the successful detection of positive samples by RT-LAMP without RNA purification steps. They have compared and developed a method which used inexpensive alternatives to the commercial LAMP enzymes and reagents.
Multiplex LAMP
The specificity in diagnosing a particular microbial strain is many-a-times compromised by the co-presence of closely related species sharing a common evolutionary lineage. The overlap of similar genomic sequences impedes the designing of non-specific primers for detecting the NA of a particular species. This leads to erroneous results, leading to misdiagnosis. Multiplex LAMP (mLAMP) utilizes more than one gene to detect multiple target sequences simultaneously, which enhances the specificity and accuracy of this technique. It has also been applied to differentiate between divergent strains or serotypes of the same pathogen. Major merits of the method are short analysis time (<30 min), quantifying a large dynamic range, ability to use unprocessed samples (saliva, tissue), high sensitivity (1–10 copies), compatibility with advanced technological devices (smartphones).
Recently Kim et al. (2021) reported a mLAMP assay to differentiate between tuberculosis (MTB) and non-tuberculosis Mycobacterium (NTM) species by designing primers for IS6110 genes that are specific for MTB and rpoB genes that are common to both. This assay was validated using clinical samples containing both types of mycobacteria with 98.97% analytical sensitivity and 100% specificity. This method coupled with a fluorescent-based detection system showed superiority over the PCR method or traditional LAMP method.
A study by Mahony et al. (2013) for the detection of Influenza A/H1, A/H3 and B demonstrated the superior performance of the mLAMP assay - having 40 min total turnover time using the mLAMP method. With amplification time of 12 min and specimen preparation time of 10 min, the mLAMP time was way faster than PCR (90–120 min).They targeted the matrix genes for Influenza H1 and H3 and NS1 gene for Influenza B and the sensitivity of the test was shown to be one genome equivalent. A few recent mLAMP diagnostic techniques for identifying various pathogens are listed in Table 3.
Table 3.
Recent Studies using Multiplex-LAMP.
| Pathogen | Targets | Sensitivity | Specificity | References |
|---|---|---|---|---|
| colistin-resistant bacteria | mcr-1 to mcr-5 | 104 copies/µL for mcr-1, mcr-2, mcr-4 and mcr-5, and105 copies/µL for mcr-3 | 100% | (Zhong et al., 2019) |
| SARS-CoV-2 | open reading frame 1b (ORF1b) and N (nucleocapsid) genes | 105 copies | 100% | (Kim et al., 2019) |
| Foot-and-mouth disease virus | O, A, Asia1, SAT1, SAT2, SAT3 | 98.0% | 98.1% | (Yamazaki et al., 2013) |
| Salmonella spp. And shigella spp. In milk samples | nvA of Salmonella spp. and ipaH of Shigella spp. | 100 fg | 100% | (Shao et al., 2011) |
| Influenza A (A/H1 and A/H3) and influenza B | segment 7 of influenza A and the nucleoprotein gene of influenza B | 94.62% for influenza A and 97.50% for influenza B | 100% | (Jang et al., 2020) |
| Acinetobacter baumannii | recA an oxa-23 | 102 CFU μl−1 | 100% | (Yang et al., 2018) |
Nurul Najian et al. (2016) fabricated a mLAMP based biosensor to detect pathogenic Leptospira using gold nanoparticle-based detection in a lateral flow dipstick. This biosensor simultaneously detected the target DNA, LAMP internal control and a chromatographic control. Positive results showed all three red lines while negative showed only two. The detection limit was found to be as low as 3.95 × 10−1 genomic equivalent ml−1.
Real-Time LAMP
The need for large scale sample analysis demands quantitative results in minimum time, preferably without the additional steps of elaborate steps of sample preparation or amplicon quantification. Traditionally, the quantification of LAMP products relies on end-point analysis using detection methods like non-specific dyes incorporation or turbidity visualization. This can sometimes give ambiguous results. For more precise results, real-time quantitative and colorimetric LAMP can be established on an economical and portable diagnostic device, the use of which requires minimal skill or training.
Real-time LAMP was optimized (Lopez-Jimena et al., 2018) in a single-tube for specific detection of dengue serotypes using different primer mixes extensively searched in available viral genome sequences. The assay was undergone quality control for molecular diagnostics and was found to be 100% reproducible and specific for each serotype. It did not cross-detect other flaviviruses and showed high sensitivity (95.8%).
A portable hand-held biomedical device was fabricated (Papadakis et al., 2020) for performing real-time quantitative colorimetric LAMP that can be operated with a smartphone application. A mini-digital camera was introduced to monitor the color change during amplification which could be subjected to digital image analysis. The device was clinically validated using SARS-CoV-2 samples. False positives and non-specific binding are sometimes regarded as the major disadvantages of the LAMP method. Another approach for real-time detection and quantitative measurements of amplicons is the application of self-quenching and dequenching fluorogenic probes in the LAMP primers. Here, a labelled fluorogenic primer-probe, quenched in an unbound state, produces fluorescence when bound to its target. Therefore, this improved technique is referred to as Fluorescence of Loop Primer upon Self Dequenching-LAMP (FLOS-LAMP). It allows for more specific detection of target sequence in realtime. (Gadkar et al., 2018) used FLOS-LAMP for rapid detection of Varicella-zoster virus using clinical samples. The clinical sensitivity and specificity of the target was found to be 96.8% and 100%, respectively. This method eliminates non-specific and false results and makes the detection more sensitive. Furthermore, Hardinge and Murray (2019) demonstrated the robustness, flexibility and increased sensitivity of LAMP by using different quenched fluorescent primer labels.
FRET (Fluorescence Resonance Energy Transfer) LAMP is another approach for real-time quantification of the amplification process. It employs probes containing a fluorescent dye and a quenching dye. Upon irradiation, the fluorescent dye, like SYBR Green I, transfers its energy to the quenching dye molecules, producing a non-fluorescent substrate Chou et al. (2011). and Severi et al. (2020) used FRET LAMP analysis to detect white spot syndrome virus with a sensitivity of 102 copies.
LAMP-on-a-chip
Extensive sample testing is required to control the transmission and execute contact tracing of a highly contagious infectious disease. This may prove to be tedious work and cause delayed results, further leading to the spread of the disease. The consumption of high amounts of reagents also makes the sample processing expensive. Also, insufficient quantity of sample material hinders the diagnosis. High throughput microarray systems based on miniature chips have been designed for amplification methods which require minimal sample and reagents, and can perform multiple tests at the same time. Several miniaturized LAMP techniques, for instance, microfluidic, electrochemical, paper-based and digital methods, have been developed for rapid sample processing. (Zhang et al., 2019)
Microfluidic LAMP
Micro/nanofluidics has emerged as a highly acceptable platform for manipulating single molecule and nano volume fluidic control. A chip-based microfluidic device for LAMP was fabricated (Trinh and Lee, 2018) for direct detection of foodborne pathogens through paper embedded LAMP reagents and fluorescence detection. The reagents and sample were loaded in the reaction chambers of the chip where the target amplification took place. The device was shown to be sensitive, and cost-effective. Recently Zhou et al. (2020) developed a RT-LAMP microfluidic chip for on-field detection of porcine coronaviruses which showed high sensitivity and specificity, along with short reaction time (40 min).
Digital LAMP
Digital DNA amplification relies on the partitioning of a sample into several individual chambers. A droplet array microfluidic chip was fabricated by Ma et al. (2018) which was capable of executing LAMP in an array of several trapped uniform droplets in nanolitres with a detection limit of single molecule. In another study, a polycarbonate membrane was developed which was track-etched for droplet formation. (Lin et al., 2019) One-step digital LAMP was performed on the membrane. This was shown as a simple alternative to the complex chip fabrication. It offers an advantage for point-of-care detection and analysis in a flexible, simple and rapid way.
Conclusion
For precise disease management, the diagnosis of a disease can only be done by accurate detection of the causative pathogen. Since its discovery, LAMP has evolved with the demanding diagnostic needs of the healthcare system. The original LAMP protocol has often been combined with several other molecular approaches like reverses transcription, real-time quantification and multiplex amplification system to improve its performance. It has shown compatibility with various amplification detection techniques like real-time fluorometry, turbidimetry and colorimetry. Though there have been certain limitations like complex fabrication, cross-contamination or variability of results related to the advanced LAMP methods, further research and development work are in progress to address these issues. The applicability of LAMP has been demonstrated for diagnosis of a wide variety of pathogens, including bacteria, viruses, parasites as well as allergens. LAMP has been used in developing mobile operated biosensors and integrated on-chip models. Studies have shown the technique to have potential in sensitive, specific and rapid detection and analysis of pathogenic agents. Briefly, LAMP is a promising tool for disease identification in developing regions of the world due to its robustness, ease of operation and cost-effectiveness, and satisfies the ASSURED criteria of diagnostics as proposed by WHO.
CRediT authorship contribution statement
Nupur Garg: Writing – original draft, Resources. Farhan Jalees Ahmad: Conceptualization, Writing – review & editing. Sudeshna Kar: Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors acknowledge the support received by Department of Science and Technology, Ministry of Science and Technology, Government of India through DST-PURSE and DST WOS-A schemes.
References
- Ahn S.J., et al. Rapid and simple colorimetric detection of multiple influenza viruses infecting humans using a reverse transcriptional loop-mediated isothermal amplification (RT-LAMP) diagnostic platform. BMC Infect. Dis. 2019;19(1):676. doi: 10.1186/s12879-019-4277-8. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alekseenko A., et al. Direct detection of SARS-CoV-2 using non-commercial RT-LAMP reagents on heat-inactivated samples. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-020-80352-8. Art. no. 1, Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhassan A., et al. Comparison of a new visual isothermal nucleic acid amplification test with PCR and skin snip analysis for diagnosis of onchocerciasis in humans. Mol. Biochem. Parasitol. 2016;210(1):10–12. doi: 10.1016/j.molbiopara.2016.07.006. Nov. [DOI] [PubMed] [Google Scholar]
- Avendaño C., Patarroyo M.A. Loop-mediated isothermal amplification as point-of-care diagnosis for neglected parasitic infections. Int. J. Mol. Sci. 2020;21(21) doi: 10.3390/ijms21217981. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila H.G., et al. Development of a copro-LAMP assay for detection of several species of Echinococcus granulosussensulato complex. Vet. Parasitol. 2020;277 doi: 10.1016/j.vetpar.2019.109017. Jan. [DOI] [PubMed] [Google Scholar]
- Becherer L., Borst N., Bakheit M., Frischmann S., Zengerle R., von Stetten F. Loop-mediated isothermal amplification (LAMP) – review and classification of methods for sequence-specific detection. Anal. Methods. 2020;12(6):717–746. doi: 10.1039/C9AY02246E. [DOI] [Google Scholar]
- Calvert A.E., Biggerstaff B.J., Tanner N.A., Lauterbach M., Lanciotti R.S. Rapid colorimetric detection of Zika virus from serum and urine specimens by reverse transcription loop-mediated isothermal amplification (RT-LAMP) PLoS ONE. 2017;12(9) doi: 10.1371/journal.pone.0185340. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaouch M. Loop-mediated isothermal amplification (LAMP): an effective molecular point-of-care technique for the rapid diagnosis of coronavirus SARS-CoV-2. Rev. Med. Virol. 2021:e2215. doi: 10.1002/rmv.2215. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P.-.H., Lin Y.-.C., Teng P.-.H., Chen C.-.L., Lee P.-.Y. Real-time target-specific detection of loop-mediated isothermal amplification for white spot syndrome virus using fluorescence energy transfer-based probes. J. Virol. Methods. 2011;173(1):67–74. doi: 10.1016/j.jviromet.2011.01.009. Apr. [DOI] [PubMed] [Google Scholar]
- DehghanEsmatabadi M.J., et al. Techniques for evaluation of LAMP amplicons and their applications in molecular biology,” Asian Pac. J. Cancer Prev. APJCP. 2015;16(17):7409–7414. doi: 10.7314/apjcp.2015.16.17.7409. [DOI] [PubMed] [Google Scholar]
- Demeke T., Jenkins G.R. Influence of DNA extraction methods, PCR inhibitors and quantification methods on real-time PCR assay of biotechnology-derived traits. Anal. Bioanal. Chem. 2010;396(6):1977–1990. doi: 10.1007/s00216-009-3150-9. Mar. [DOI] [PubMed] [Google Scholar]
- Deng J., Pei J., Gou H., Ye Z., Liu C., Chen J. Rapid and simple detection of Japanese encephalitis virus by reverse transcription loop-mediated isothermal amplification combined with a lateral flow dipstick. J. Virol. Methods. 2015;213:98–105. doi: 10.1016/j.jviromet.2014.12.006. Mar. [DOI] [PubMed] [Google Scholar]
- Ding X., Wu W., Zhu Q., Zhang T., Jin W., Mu Y. Mixed-dye-based label-free and sensitive dual fluorescence for the product detection of nucleic acid isothermal multiple-self-matching-initiated amplification. Anal. Chem. 2015;87(20):10306–10314. doi: 10.1021/acs.analchem.5b02112. Oct. [DOI] [PubMed] [Google Scholar]
- Dong Y., et al. Comparative evaluation of 19 reverse transcription loop-mediated isothermal amplification assays for detection of SARS-CoV-2. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-020-80314-0. Art. no. 1, Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Soto P., et al. Molecular markers for detecting Schistosoma species by loop-mediated isothermal amplification. Dis. Markers. 2020;2020 doi: 10.1155/2020/8042705. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo P.C., et al. Loop-mediated isothermal amplification (LAMP) reaction as viable PCR substitute for diagnostic applications: a comparative analysis study of LAMP, conventional PCR, nested PCR (nPCR) and real-time PCR (qPCR) based on Entamoeba histolytica DNA derived from faecal sample. BMC Biotechnol. 2020;20(1):34. doi: 10.1186/s12896-020-00629-8. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser T.A., Carver S., Martin A.M., Mounsey K., Polkinghorne A., Jelocnik M. A Sarcoptesscabiei specific isothermal amplification assay for detection of this important ectoparasite of wombats and other animals. PeerJ. 2018;6:e5291. doi: 10.7717/peerj.5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadkar V.J., Goldfarb D.M., Gantt S., Tilley P.A.G. Real-time detection and monitoring of loop mediated amplification (LAMP) reaction using self-quenching and de-quenching fluorogenic probes. Sci. Rep. 2018;8(1) doi: 10.1038/s41598-018-23930-1. Art. no. 1, Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg N., Sahu U., Kar S., Ahmad F.J. Development of a Loop-mediated isothermal amplification (LAMP) technique for specific and early detection of Mycobacterium leprae in clinical samples. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-89304-2. Art. no. 1, May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghodsian S., Rouhani S., Fallahi S., Seyyedtabaei S.J., Taghipour N. Detection of spiked fasciola hepatica eggs in stool specimens using LAMP technique. Iran. J. Parasitol. 2019;14(3):387–393. Sep. [PMC free article] [PubMed] [Google Scholar]
- Goto M., Honda E., Ogura A., Nomoto A., Hanaki K.-.I. Colorimetric detection of loop-mediated isothermal amplification reaction by using hydroxy naphthol blue. BioTechniques. 2009;46(3):167–172. doi: 10.2144/000113072. Mar. [DOI] [PubMed] [Google Scholar]
- Hardinge P., Murray J.A.H. Reduced false positives and improved reporting of loop-mediated isothermal amplification using quenched fluorescent primers. Sci. Rep. 2019;9(1) doi: 10.1038/s41598-019-43817-z. Art. no. 1, May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K., Inada M., Ito K. A novel voltammetric approach for real-time electrochemical detection of targeted nucleic acid sequences using LAMP. Anal. Biochem. 2017;539:113–117. doi: 10.1016/j.ab.2017.10.019. Dec. [DOI] [PubMed] [Google Scholar]
- Hayashida K. Development of a bio-inkjet printer LAMP test kit for detecting human African trypanosomiasis. PLoSNegl. Trop. Dis. 2020;14(10) doi: 10.1371/journal.pntd.0008753. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Htun Z.M., et al. Loop-mediated isothermal amplification (LAMP) for identification of pythium insidiosum. Int. J. Infect. Dis. 2020;101:149–159. doi: 10.1016/j.ijid.2020.09.1430. Dec. [DOI] [PubMed] [Google Scholar]
- Huang T.-.T., Liu S.-.C., Huang C.-.H., Lin C.-.J., Huang S.-.T. An Integrated Real-time Electrochemical LAMP Device for Pathogenic Bacteria Detection in Food. Electroanalysis. 2018;30(10):2397–2404. doi: 10.1002/elan.201800382. [DOI] [Google Scholar]
- Huang W.E., et al. RT-LAMP for rapid diagnosis of coronavirus SARS-CoV-2. Microb. Biotechnol. 2020;13(4):950–961. doi: 10.1111/1751-7915.13586. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang W.S., et al. Development of a multiplex isothermal amplification molecular diagnosis method for on-site diagnosis of influenza. PLoS ONE. 2020;15(9) doi: 10.1371/journal.pone.0238615. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L., Gu R., Li X., Mu D. Simple and rapid detection Aspergillus fumigatus by loop-mediated isothermal amplification coupled with lateral flow biosensor assay. J. Appl. Microbiol. 2021;131(5):2351–2360. doi: 10.1111/jam.15092. Nov. [DOI] [PubMed] [Google Scholar]
- Kaneko H., Iida T., Aoki K., Ohno S., Suzutani T. Sensitive and rapid detection of herpes simplex virus and varicella-zoster virus DNA by loop-mediated isothermal amplification. J. Clin. Microbiol. 2005;43(7):3290–3296. doi: 10.1128/JCM.43.7.3290-3296.2005. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keikha M. LAMP method as one of the best candidates for replacing with PCR method,” Malays. J. Med. Sci. MJMS. 2018;25(1):121–123. doi: 10.21315/mjms2018.25.1.15. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M., Wang R., Li B., Liu P., Weng Q., Chen Q. Comparative evaluation of the LAMP assay and PCR-based assays for the rapid detection of alternaria solani. Front. Microbiol. 2018;9 doi: 10.3389/fmicb.2018.02089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.H., Kang M., Park E., Chung D.R., Kim J., Hwang E.S. A simple and multiplex loop-mediated isothermal amplification (LAMP) assay for rapid detection of SARS-CoV. BioChip J. 2019;13(4):341–351. doi: 10.1007/s13206-019-3404-3. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., et al. Development and evaluation of a multiplex loop-mediated isothermal amplification (LAMP) assay for differentiation of Mycobacterium tuberculosis and non-tuberculosis mycobacterium in clinical samples. PLoS ONE. 2021;16(1) doi: 10.1371/journal.pone.0244753. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo T., et al. Development of a reverse transcription-loop-mediated isothermal amplification assay for detection of pandemic (H1N1) 2009 virus as a novel molecular method for diagnosis of pandemic influenza in resource-limited settings. J. Clin. Microbiol. 2010;48(3):728–735. doi: 10.1128/JCM.01481-09. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.H., Baek Y.H., Kim Y.-.H., Choi Y.-.K., Song M.-.S., Ahn J.-.Y. One-pot reverse transcriptional loop-mediated isothermal amplification (RT-LAMP) for detecting MERS-CoV. Front. Microbiol. 2017;7 doi: 10.3389/fmicb.2016.02166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Liu Y., Wang Y., Chen H., Liu C., Wang Y. Lateral flow biosensor combined with loop-mediated isothermal amplification for simple, rapid, sensitive, and reliable detection of Brucella spp. Infect. Drug Resist. 2019;12:2343–2353. doi: 10.2147/IDR.S211644. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X., Huang X., Urmann K., Xie X., Hoffmann M.R. Digital loop-mediated isothermal amplification on a commercial membrane. ACS Sens. 2019;4(1):242–249. doi: 10.1021/acssensors.8b01419. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Jimena B., et al. Development and validation of four one-step real-time RT-LAMP assays for specific detection of each dengue virus serotype. PLoSNegl. Trop. Dis. 2018;12(5) doi: 10.1371/journal.pntd.0006381. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y.-.D., Luo K., Chang W.-.H., Lee G.-.B. A microfluidic chip capable of generating and trapping emulsion droplets for digital loop-mediated isothermal amplification analysis. Lab. Chip. 2018;18(2):296–303. doi: 10.1039/C7LC01004D. Jan. [DOI] [PubMed] [Google Scholar]
- Mahony J., Chong S., Bulir D., Ruyter A., Mwawasi K., Waltho D. Multiplex loop-mediated isothermal amplification (M-LAMP) assay for the detection of influenza A/H1, A/H3 and influenza B can provide a specimen-to-result diagnosis in 40 min with single genome copy sensitivity. J. Clin. Virol. 2013;58(1):127–131. doi: 10.1016/j.jcv.2013.06.006. Sep. [DOI] [PubMed] [Google Scholar]
- Martzy R., et al. A loop-mediated isothermal amplification (LAMP) assay for the rapid detection of Enterococcus spp. in water. Water Res. 2017;122:62–69. doi: 10.1016/j.watres.2017.05.023. Oct. [DOI] [PubMed] [Google Scholar]
- Mendes G.M., et al. Molecular diagnostics of dengue by reverse transcription-loop mediated isothermal amplification (RT-LAMP) in disposable polyester-toner microdevices. J. Braz. Chem. Soc. 2019;30(9):1841–1849. doi: 10.21577/0103-5053.20190092. Sep. [DOI] [Google Scholar]
- Mori Y., Kitao M., Tomita N., Notomi T. Real-time turbidimetry of LAMP reaction for quantifying template DNA. J. Biochem. Biophys. Methods. 2004;59(2):145–157. doi: 10.1016/j.jbbm.2003.12.005. May. [DOI] [PubMed] [Google Scholar]
- Mori Y., Notomi T. Loop-mediated isothermal amplification (LAMP): a rapid, accurate, and cost-effective diagnostic method for infectious diseases. J. Infect. Chemother. 2009;15(2):62–69. doi: 10.1007/s10156-009-0669-9. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudhigeti N., Kalawat U., Hulikal N., Kante M. Evaluation of loop-mediated isothermal amplification assay for detection and typing of human papilloma virus 16 and 18 from endocervical samples. Indian J. Med. Microbiol. 2019;37(2):241–247. doi: 10.4103/ijmm.IJMM_19_58. Apr. [DOI] [PubMed] [Google Scholar]
- Mwendwa F., Mbae C.K., Kinyua J., Mulinge E., Mburugu G.N., Njiru Z.K. Stem loop-mediated isothermal amplification test: comparative analysis with classical LAMP and PCR in detection of Entamoeba histolytica in Kenya. BMC Res. Notes. 2017;10 doi: 10.1186/s13104-017-2466-3. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamine K., Hase T., Notomi T. Accelerated reaction by loop-mediated isothermal amplification using loop primers. Mol. Cell. Probes. 2002;16(3):223–229. doi: 10.1006/mcpr.2002.0415. Jun. [DOI] [PubMed] [Google Scholar]
- Nakayama T., et al. Detection of fungi from an indoor environment using loop-mediated isothermal amplification (LAMP) method. Biocontrol Sci. 2017;22(2):97–104. doi: 10.4265/bio.22.97. [DOI] [PubMed] [Google Scholar]
- Ngari M.G., et al. Development and evaluation of a loop-mediated isothermal amplification (LAMP) diagnostic test for detection of whipworm, Trichuris trichiura, in faecal samples. J. Helminthol. 2020;94:e142. doi: 10.1017/S0022149X2000022X. Apr. [DOI] [PubMed] [Google Scholar]
- Notomi T. Loop-mediated isothermal amplification of DNA. Nucleic. Acids. Res. 2000;28(12):e63. doi: 10.1093/nar/28.12.e63. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurul Najian A.B., Engku Nur Syafirah E.A.R., Ismail N., Mohamed M., Yean C.Y. Development of multiplex loop mediated isothermal amplification (m-LAMP) label-based gold nanoparticles lateral flow dipstick biosensor for detection of pathogenic Leptospira. Anal. Chim. Acta. 2016;903:142–148. doi: 10.1016/j.aca.2015.11.015. Jan. [DOI] [PubMed] [Google Scholar]
- Oloniniyi O.K., Kurosaki Y., Miyamoto H., Takada A., Yasuda J. Rapid detection of all known ebolavirus species by reverse transcription-loop-mediated isothermal amplification (RT-LAMP) J. Virol. Methods. 2017;246:8–14. doi: 10.1016/j.jviromet.2017.03.011. Aug. [DOI] [PubMed] [Google Scholar]
- Ordóñez D., et al. A trypanosoma cruzi genome tandem repetitive satellite DNA sequence as a molecular marker for a LAMPp assay for diagnosing chagas’ disease. Dis. Markers. 2020;2020 doi: 10.1155/2020/8074314. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEB | SARS “SARS-CoV-2 rapid colorimetric LAMP assay kit .” https://international.neb.com/products/e2019-sars-cov-2-rapid-colorimetric-lamp-assay-kit#Product%20Information (accessed Apr. 28, 2021).
- WHO| Control of Neglected Tropical Diseases “Control of neglected tropical diseases.” https://www.who.int/teams/sexual-and-reproductive-health-and-research/areas-of-work/fertility-care/infertility-definitions-and-terminology/control-of-neglected-tropical-diseases (accessed Apr. 25, 2021).
- G. Papadakis et al., “Real-time colorimetric LAMP methodology for quantitative nucleic acids detection at the point-of-care,” bioRxiv, p. 2020.07.22.215251, Jul. 2020, doi: 10.1101/2020.07.22.215251.
- Parida M., et al. Rapid detection and differentiation of dengue virus serotypes by a real-time reverse transcription-loop-mediated isothermal amplification assay. J. Clin. Microbiol. 2005;43(6):2895–2903. doi: 10.1128/JCM.43.6.2895-2903.2005. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parida M., Posadas G., Inoue S., Hasebe F., Morita K. Real-time reverse transcription loop-mediated isothermal amplification for rapid detection of west Nile virus. J. Clin. Microbiol. 2004;42(1):257–263. doi: 10.1128/JCM.42.1.257-263.2004. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park G.-.S., et al. Colorimetric RT-LAMP methods to detect severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Bio-Protoc. 2020;10(21):e3804. doi: 10.21769/BioProtoc.3804. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole C.B., et al. Colorimetric tests for diagnosis of filarial infection and vector surveillance using non-instrumented nucleic acid loop-mediated isothermal amplification (NINA-LAMP) PLoS ONE. 2017;12(2) doi: 10.1371/journal.pone.0169011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon L.L.M., et al. Detection of human influenza a viruses by loop-mediated isothermal amplification. J. Clin. Microbiol. 2005;43(1):427–430. doi: 10.1128/JCM.43.1.427-430.2005. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priya G.B., et al. Development and evaluation of isothermal amplification assay for the rapid and sensitive detection of Clostridium perfringens from chevon. Anaerobe. 2018;54:178–187. doi: 10.1016/j.anaerobe.2018.09.005. Dec. [DOI] [PubMed] [Google Scholar]
- Quoc N.B., Phuong N.D.N., Chau N.N.B., Linh D.T.P. Closed tube loop-mediated isothermal amplification assay for rapid detection of hepatitis B virus in human blood. Heliyon. 2018;4(3):e00561. doi: 10.1016/j.heliyon.2018.e00561. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramezani R., KardoostParizi Z., Ghorbanmehr N., Mirshafiee H. Rapid and simple detection of Escherichia coli by loop-mediated isothermal amplification assay in urine specimens. Avicenna J. Med. Biotechnol. 2018;10(4):269–272. [PMC free article] [PubMed] [Google Scholar]
- Ravan H., Yazdanparast R. Development and evaluation of a loop-mediated isothermal amplification method in conjunction with an enzyme-linked immunosorbent assay for specific detection of Salmonella serogroup D. Anal. Chim. Acta. 2012;733:64–70. doi: 10.1016/j.aca.2012.04.034. Jul. [DOI] [PubMed] [Google Scholar]
- Rivero R., et al. Rapid detection of Trypanosoma cruzi by colorimetric loop-mediated isothermal amplification (LAMP): a potential novel tool for the detection of congenital Chagas infection. Diagn. Microbiol. Infect. Dis. 2017;89(1):26–28. doi: 10.1016/j.diagmicrobio.2017.06.012. Sep. [DOI] [PubMed] [Google Scholar]
- Rolando J.C., Jue E., Barlow J.T., Ismagilov R.F. Real-time kinetics and high-resolution melt curves in single-molecule digital LAMP to differentiate and study specific and non-specific amplification. Nucleic. Acids. Res. 2020;48(7):e42. doi: 10.1093/nar/gkaa099. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero M.R., Cook N. A Rapid LAMP-based method for screening poultry samples for campylobacter without enrichment. Front. Microbiol. 2018;9 doi: 10.3389/fmicb.2018.02401. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph D.L., Sullivan V., Owen S.M., Curtis K.A. Detection of acute HIV-1 infection by RT-LAMP. PLoS ONE. 2015;10(5) doi: 10.1371/journal.pone.0126609. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safavieh M., et al. Emerging loop-mediated isothermal amplification-based microchip and microdevice technologies for nucleic acid detection. ACS Biomater. Sci. Eng. 2016;2(3):278–294. doi: 10.1021/acsbiomaterials.5b00449. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saharan P., Khatri P., Dingolia S., Duhan J.S., Gahlawat S.K. Rapid detection of viruses using loop-mediated isothermal amplification (LAMP): a review. Biotechnol. Prospects Appl. 2013:287–306. doi: 10.1007/978-81-322-1683-4_21. Oct. [DOI] [Google Scholar]
- Schermer B., et al. Rapid SARS-CoV-2 testing in primary material based on a novel multiplex RT-LAMP assay. PLoS ONE. 2020;15(11) doi: 10.1371/journal.pone.0238612. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvarajah D., Naing C., Htet N.H., Mak J.W. Loop-mediated isothermal amplification (LAMP) test for diagnosis of uncomplicated malaria in endemic areas: a meta-analysis of diagnostic test accuracy. Malar. J. 2020;19(1):211. doi: 10.1186/s12936-020-03283-9. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severi C., Melnychuk N., Klymchenko A.S. Smartphone-assisted detection of nucleic acids by light-harvesting FRET-based nanoprobe. Biosens. Bioelectron. 2020;168 doi: 10.1016/j.bios.2020.112515. Nov. [DOI] [PubMed] [Google Scholar]
- Shao Y., Zhu S., Jin C., Chen F. Development of multiplex loop-mediated isothermal amplification-RFLP (mLAMP-RFLP) to detect Salmonella spp. and Shigella spp. in milk. Int. J. Food Microbiol. 2011;148(2):75–79. doi: 10.1016/j.ijfoodmicro.2011.05.004. Aug. [DOI] [PubMed] [Google Scholar]
- Sukphattanaudomchoke C., et al. Simplified closed tube loop mediated isothermal amplification (LAMP) assay for visual diagnosis of Leishmania infection. Acta Trop. 2020;212 doi: 10.1016/j.actatropica.2020.105651. Dec. [DOI] [PubMed] [Google Scholar]
- Sun Y.-.L., Yen C.-.H., Tu C.-.F. Immunocapture loop-mediated isothermal amplification assays for the detection of canine parvovirus. J. Virol. Methods. 2017;249:94–101. doi: 10.1016/j.jviromet.2017.08.009. Nov. [DOI] [PubMed] [Google Scholar]
- Tanner N.A., Zhang Y., Evans T.C. Visual detection of isothermal nucleic acid amplification using pH-sensitive dyes. BioTechniques. 2015;58(2):59–68. doi: 10.2144/000114253. Feb. [DOI] [PubMed] [Google Scholar]
- Teh C.S.J., Chua K.H., Lim Y.A.L., Lee S.C., Thong K.L. Loop-mediated isothermal amplification assay for detection of generic and verocytotoxin-producing escherichia coli among indigenous individuals in Malaysia. Sci. World J. 2014;2014 doi: 10.1155/2014/457839. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thi V.L.D., et al. A colorimetric RT-LAMP assay and LAMP-sequencing for detecting SARS-CoV-2 RNA in clinical samples. Sci. Transl. Med. 2020;12(556) doi: 10.1126/scitranslmed.abc7075. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh T.N.D., Lee N.Y. A rapid and eco-friendly isothermal amplification microdevice for multiplex detection of foodborne pathogens. Lab. Chip. 2018;18(16):2369–2377. doi: 10.1039/C8LC00424B. Aug. [DOI] [PubMed] [Google Scholar]
- Vergara A., et al. Assessment of a loop-mediated isothermal amplification (LAMP) assay for the rapid detection of pathogenic bacteria from respiratory samples in patients with hospital-acquired pneumonia. Microorganisms. 2020;8(1) doi: 10.3390/microorganisms8010103. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachiralurpan S., et al. Rapid colorimetric assay for detection of listeria monocytogenes in food samples using LAMP formation of DNA concatemers and gold nanoparticle-DNA probe complex. Front. Chem. 2018;6 doi: 10.3389/fchem.2018.00090. https://www.frontiersin.org/article/10.3389/fchem.2018.00090 Accessed: Feb. 17,2022. (Online).Available. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., et al. Rapid detection of active human cytomegalovirus infection in pregnancy using loop-mediated isothermal amplification. Mol. Med. Rep. 2015;12(2):2269–2274. doi: 10.3892/mmr.2015.3572. Aug. [DOI] [PubMed] [Google Scholar]
- WHO . WHO; 2021. WHO | The use of Loop-Mediated Isothermal Amplification (TB-LAMP) For the Diagnosis of Pulmonary tuberculosis: Policy Guidance.http://www.who.int/tb/publications/lamp-diagnosis-molecular/en/ accessed Apr. 27. [PubMed] [Google Scholar]
- Wong Y.-.P., Othman S., Lau Y.-.L., Radu S., Chee H.-.Y. Loop-mediated isothermal amplification (LAMP): a versatile technique for detection of micro-organisms. J. Appl. Microbiol. 2018;124(3):626–643. doi: 10.1111/jam.13647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki W., et al. Development and evaluation of multiplex RT-LAMP assays for rapid and sensitive detection of foot-and-mouth disease virus. J. Virol. Methods. 2013;192(1):18–24. doi: 10.1016/j.jviromet.2013.03.018. Sep. [DOI] [PubMed] [Google Scholar]
- Yang R., et al. A multiplex loop-mediated isothermal amplification assay for rapid screening of Acinetobacter baumannii and D carbapenemase OXA-23 gene. Biosci. Rep. 2018;38(5) doi: 10.1042/BSR20180425. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., Rothman R.E. PCR-based diagnostics for infectious diseases: uses, limitations, and future applications in acute-care settings. Lancet Infect. Dis. 2004;4(6):337–348. doi: 10.1016/S1473-3099(04)01044-8. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X.-.Y., Wang Y.-.L., Meng K., Zhang Y.-.X., Xu H.-.Y., Ai W. LAMP real-time turbidity detection for fowl adenovirus. BMC Vet. Res. 2019;15(1):256. doi: 10.1186/s12917-019-2015-5. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanoli L.M., Spoto G. Isothermal amplification methods for the detection of nucleic acids in microfluidic devices. Biosensors. 2012;3(1):18–43. doi: 10.3390/bios3010018. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Xu Y., Fohlerova Z., Chang H., Iliescu C., Neuzil P. LAMP-on-a-chip: revising microfluidic platforms for loop-mediated DNA amplification. TrAC Trends Anal. Chem. 2019;113:44–53. doi: 10.1016/j.trac.2019.01.015. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L.-.L., et al. Multiplex loop-mediated isothermal amplification (multi-LAMP) assay for rapid detection of mcr-1to mcr-5 in colistin-resistant bacteria. Infect. Drug Resist. 2019;12:1877–1887. doi: 10.2147/IDR.S210226. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., et al. Development of a loop-mediated isothermal amplification assay for rapid detection of trichosporonasahii in experimental and clinical samples. BioMed Res. Int. 2015;2015 doi: 10.1155/2015/732573. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., et al. Microfluidic-RT-LAMP chip for the point-of-care detection of emerging and re-emerging enteric coronaviruses in swine. Anal. Chim. Acta. 2020;1125:57–65. doi: 10.1016/j.aca.2020.05.034. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang L., et al. Detection of Salmonella spp. by a loop-mediated isothermal amplification (LAMP) method targeting bcfD gene. Lett. Appl. Microbiol. 2014;59(6):658–664. doi: 10.1111/lam.12328. Dec. [DOI] [PubMed] [Google Scholar]
- Zou Y., Mason M.G., Botella J.R. Evaluation and improvement of isothermal amplification methods for point-of-need plant disease diagnostics. PLoS ONE. 2020;15(6) doi: 10.1371/journal.pone.0235216. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]